Abstract
Introduction:
Candida spp. are commensal yeasts capable of causing a wide range of infections such as superficial, oral, vaginal, or even systemic infections. Despite medical advances, the antifungal pharmacopeia remains limited and the development of alternative strategies is needed.
Areas covered:
The authors discuss available treatments for Candida spp. infections, highlighting advantages and limitations related to pharmacokinetics, cytotoxicity, and antimicrobial resistance. Moreover, they present new perspectives to improve the activity of the available antifungals, discussing their immunomodulatory potential and advances on drug delivery carriers. Several new therapeutic approaches are presented including recent synthesized antifungal compounds; drug repurposing using a diversity of antibacterial, antiviral and non-antimicrobial drugs; combination therapies with different compounds or photodynamic therapy; and finally innovations based on nano-particulate delivery systems.
Expert opinion:
With the lack of novel drugs, the available assets must be leveraged to their best advantage through modifications that enhance delivery, efficacy, and solubility. However, these efforts are met with continuous challenges presented by microbes in their infinite plight to resist and survive therapeutic drugs. The pharmacotherapeutic options in development need to focus on new antimicrobial targets. The success of each antimicrobial agent brings strategic insights to the next phased approach in treating Candida spp. infections.
Keywords: Candida spp., antifungal compounds, drug repurposing, drug combination, drug delivery
1. Introduction
Systemic Candida spp. infections present a significant challenge to the medical community. Infections are difficult to diagnose, treat, and sometimes can relapse as persistent infections [1,2]. As part of the natural microbiome, Candida spp. are commensal yeasts found in the skin, oral cavity, and gut microbiota [3]. However, impaired host immune responses or tissue trauma can lead to dysbiosis and allow commensal yeast to transition into opportunistic pathogens [4,5]. Pathogenic Candida spp. can exhibit several virulence factors such as adhesins, morphological switching (transitioning from yeast cells to filamentous forms), secretion enzymes (proteases and phospholipases), and biofilms [4,5]. Many studies reported that Candida biofilms can be formed on the mucosa, skin or medical devices surfaces, and lead to oral, vulvovaginal, wound or systemic infections [6–8]. The ability of Candida spp. to form biofilms results in several clinical implications since the biofilm structure protects Candida cells from host immune system and antifungal drugs [6–8]. Although the biofilm formation on abiotic surfaces and its role in systemic candidiasis is already established, the formation of Candida biofilms on mucosa surfaces remains questionable [9,10]. Previous studies demonstrated that C. albicans form biofilms in vivo on mucosa surfaces in animal models of oral [11] and vulvovaginal candidiasis [12], however Swidsinski et al. [10] did not find biofilms on vaginal biopsies of patients with candidiasis.
Patients at risk for invasive candidiasis are those experiencing prolonged care in intensive care units, patients receiving abdominal surgery, individuals suffering from acute necrotizing pancreatitis, hematologic malignant disease, solid-organ transplantation, solid-organ tumors, patients receiving hemodialysis, low birth weight or preterm infants, recipients of broad-spectrum antibiotics, glucocorticoids, or anti-cancer chemotherapy, patients with central vascular catheter and/or total parenteral nutrition, and individuals with advanced acquired immunodeficiency syndrome (AIDS) [3,13,14]. Thus, there is a significant vulnerable patient population at risk for infection. This diverse population can add to the difficulty of recognizing infections. Further, the spectrum of medications required to treat underlying diseases or conditions within this populations can also present a challenge in effectively treating fungal infection.
The arduous task to effectively diagnose and treat fungal infections within the vulnerable population is further made difficult by the variance of medically significant candidiasis causing species. Candida albicans, Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei are the most frequent pathogens of candidiasis with differing patterns of epidemiology and antifungal susceptibility [3,15]. Although the infections by non-albicans Candida species have significantly increased, C. albicans remains the most prevalent and pathogenic species [16,17]. Recently, a new species has been detected worldwide, Candida auris, and has garnered concern due to antifungal resistance profiles [18].
The treatment of candidiasis is restricted by the limited therapeutic arsenal, high cost, and narrow antifungal drug spectrum of action [19]. Another obstacle is the toxicity profiles from certain available therapeutics due to the similarity between eukaryotic fungal and human cells [20], manifesting as nephrotoxicity and hepatotoxicity. Some drug regimens require hospitalization to monitor for these toxic effects and can require mediation through adjusted dosages, limited use, or eventually discontinuation of therapy [21]. Effective treatment is also hampered by global emergence of resistance (Table 1). Developed resistance puts tension on an already limited drug arsenal. Drug failure can also occur when medications for primary ailments antagonize antifungal agents, creating a maelstrom [22].
Table 1.
Resistance index of Candida strains to antifungal agents in different countries
| Country | Clinical specimen | Resistance index | References |
|---|---|---|---|
| Argentina | Vulvovaginal | Fluconazole (3.55% C. albicans) | [227] |
| Brazil | Saliva and oropharyngeal | Fluconazole (27% C. albicans) | [228] |
| Brazil | blood | Fluconazol (6% C. glabrata; 7.3% C. tropicalis) Voriconazol (4.9% C. tropicalis) |
[229] |
| Canada | blood | Fluconazole (0.6 % C. albicans; 1% C. glabrata; 4.9% C. parapsilosis complex) Micafungin (0.1% C. albicans; 2.5% C. glabrata) |
[230] |
| China | Invasive candidiasis | Fluconazole (20% C. albicans; 11% C. glabrata; 29.7% C. tropicalis; 20% C. parapsilosis) Itraconazole (28.2% C. albicans; 6.8% C. glabrata; 40.5 % C. tropicalis; 33.4% C. krusei; 20% C. parapsilosis) Voriconazole (23.6% C. albicans; 6.8% C. glabrata; 27% C. tropicalis; 25% C. krusei; 20% C. parapsilosis) |
[231] |
| Ghana | vulvovaginal | Fluconazole (50% C. albicans; 12 % C. glabrata; 1% C. parapsilosis) Niatatin (4%C. glabrata; 1% C. krusei) Voriconazole (7% C. albicans; 11% C. glabrata; 1% C. krusei; 1% C. parapsilosis) |
[232] |
| Iran | oropharyngeal | ketoconazole (93.75% C. albicans; 89.28% of Candida non-albicans Candida species) Fluconazole (62.50% C. albicans; 42.85% Candida non-albicans Candida species) |
[233] |
| Ireland | blood | Fluconazole (2% C. albicans; 37 % C. glabrata Itraconazole (5% C. albicans; 21% C. glabrata; Flucytosine (3% C. albicans) Amphotericin B (14% C. glabrata) |
[234] |
| Italy | blood | Fluconazole (1.2% C. albicans; 12% C. glabrata; 6% C. parapsilosis, 10% C. tropicalis) Itraconazole (2% C. albicans; 25% C. glabrata; 0.7% C. parapsilosis, 3.9% C. tropicalis) Voriconazole (1.4% C. albicans; 0.7% C. parapsilosis, 1.9% C. tropicalis) Anidulafungin (0.5% C. glabrata; 1.9% C. tropicalis) Caspofungin (0.5% C. glabrata; 1.9% C. tropicalis) |
[235] |
| Peru | blood | Fluconazole (C. parapsilosis 2,3%) Voriconazole (5% C. albicans) |
[236] |
| Peru | blood | Fluconazole (2.2% C. albicans; 5% C. parapsilosis) | [237] |
| Saudi Arabia | blood | Fluconazole (23% C. albicans; 27.8% C. tropicalis; 51.7% C. glabrata; 48% C. parapsilosis) Voriconazole (17% C. albicans; 12,5% C. tropicalis; 21.7% C. glabrata; 2% C. parapsilosis; 6% C. krusei) Amphotericin B (2% C. albicans; 23.5% C. krusei) Caspofungin (23% C. albicans; 4.2% C. tropicalis; 3.3% C. glabrata; 6% C. krusei) |
[238] |
| Scotland | blood | Fluconazole (5.26% C. glabrata) | [239] |
| Spain | blood | Fluconazole (0.3% C. albicans; 6.6% C. tropicalis; 10.8% C. glabrata; 0.9% C. parapsilosis) Voriconazole (6.6% C. tropicalis) Caspofungin (0.7% C. albicans; 1.08% C. glabrata) Micafungin (0.35% C. albicans) |
[240] |
| Taiwan | blood | Fluconazole (13.9% C. tropicalis; 3.1% C. glabrata; 6.1% C. parapsilosis) Voriconazole (10.7% C. tropicalis) Caspofungina (2.5% C. tropicalis; 2.1% C. glabrata) Micafungina (2.5% C. tropicalis; 5.2% C. glabrata) Anidulafungina (1.6% C. tropicalis; 5.2% C. glabrata) |
[241] |
| Turkey | blood | Fluconazole (2.8 % C. glabrata) | [242] |
| USA | blood | Fluconazole (0.3% C. albicans; 8.6% C. glabrata; 7.6% C. parapsilosis; 4.2% C. tropicalis) Voriconazole (0.1% C. albicans; 2.1% C. parapsilosis; 2.1% C. tropicalis) Echinocandins (0.4% C. albicans; 4.4% C. glabrata; 2.6% C. krusei) |
[243] |
| Pakistan, India, South Africa, and Venezuela | blood, urine, soft tissue and other | Fluconazole (93% C. auris) Amphotericin B (35% C. auris) Echinocandins (7% C. auris) Flucytosine (6% C. auris) |
[115] |
| Southern Asian, South African and Japanese/Korean | Nosocamial infection | Amphotericin B (14.6% C. auris) Fluconazole (100% C. auris) Itraconazole (4.6% C. auris) Voriconazole (48.3% C. auris) Posaconazole (12.8% C. auris) Isavuconazole (5.9% C. auris) Anidulafungin (C. auris 6.8%) Nistatina 3.6% (C. auris) |
[244] |
| USA | blood, urine and extern ear channel | Fluconazole (71.4% C. auris) Voriconazole (14.2 C. auris) Amphotericin B (14.2% C. auris) |
[245] |
| Spain | Urine and blood | Fluconazole (100% C. auris) Voriconazole (17.9% C. auris) Isavuconazole (1.8% C. auris) Anidulafungin (3.6% C. auris) |
[246] |
In this review, we discuss current available treatments licensed for monotherapy against Candida spp. infections (polyenes, azoles, and echinocandins), highlighting advantages and limitations. Additionally, we address potential new therapeutic strategies to provide perspectives for future management of superficial and invasive candidiasis.
2. Current available treatments
2.1. Polyenes
Polyene drugs are very effective at inhibiting Candida spp. and a multitude of other fungal pathogens. Inhibition is enabled through targeting ergosterol, a cholesterol-like substance in the fungal cell membrane, a structure important for maintaining cell integrity [23,24]. Until now, it was postulated that polyenes lead to ergosterol disruption by forming small channels in the fungal membrane and, consequently, promoting leakage of intracellular ions [20,25]. However, recent studies have shown that polyenes also form large extra-membranous aggregates that extract ergosterol from cell membrane lipid bilayers like a sterol sponge [26–28]. Therefore, the polyenes mechanism of action could be primarily attributed to ergosterol removal [26–28]. Polyenes also damage the fungal cell by forming reactive oxygen species (ROS) and by inhibiting the membrane transporters of some amino acids and glucose [29]. The commonly used polyenes for Candida spp. treatment are amphotericin B (AmB) and nystatin.
2.1.1. Amphotericin B
AmB is poorly absorbed in the gastrointestinal tract and parenteral administration is required. Despite its high efficiency, this antifungal causes serious collateral damage including nephrotoxicity, anaphylaxis, and electrolyte abnormalities [30,31]. Side effects can be explained by poor solubility in water and aggregate formation, which are also able to extract cholesterol from mammalian cells causing toxicity [30,32]. Due to low aqueous solubility, AmB needs to be ensconced by a carrier agent, resulting in different pharmaceutical formulations. Commercially available AmB formulations are Fungizone® (original formulation with sodium deoxycholate), Abelcet® (lipid complex formulation), Amphocil® (colloidal dispersion formulation), and AmBisome® (liposomal formulation), all administrated intravenously.
Over the years, many studies have been performed to compare the efficacy and safety of these different formulations. Recently, Steimbach et al. [33] evaluated the treatments with AmB deoxycholate and lipid-based AmB formulations in a systematic review including randomized controlled trials in patients with any degree of immunosuppression and susceptibility to invasive fungal infection. Analyzing several studies performed in the U.S. (9), France (4) and India (3), the authors found that AmB deoxycholate presented the same efficacy of the lipid-based AmB formulations. However, lipid-based formulations showed a safer profile with reductions in adverse effects, including nephrotoxicity, fever, chills, and vomiting.
Despite the lack of oral formulations and adverse effects, AmB continues to be widely used due to its broad spectrum fungicidal activity against yeasts, molds, and dimorphic fungi [34]. Despite the extensive use of polyenes over the past 50 years, the emergence of Candida spp. resistance to this antifungal class is uncommon, possibly because removal of ergosterol alters all cellular processes dependent on membrane ergosterol and simultaneous mutations at these targets are highly improbable [26,28,33–35]. Although resistance is rare, Bailly et al. documented increasing MIC values of C. glabrata strains from patients under AmB treatment, and suggested that reduced susceptibility could be attributed to its haploid genome, making it more prone to phenotypic changes upon genetic mutation. C. lusitaniae, another haploid Candida yeast, is often associated with resistance to AmB [36,37].
2.1.2. Nystatin
Another polyene used to treat Candida spp. infections is nystatin. This compound is not absorbed by the gastrointestinal tract but is also very toxic when provided parenterally and is therefore more commonly used topically [38]. Available in local preparations, its administration has been widely used for treating superficial infections such as oral and vulvovaginal candidiasis [39]. The drug is widely used due to its broad-spectrum antifungal activity [38,40]. Importantly, most Candida strains remain susceptible to nystatin. In a study by Fan et al., researchers found both C. albicans and non-albicans Candida isolates from vulvovaginal candidiasis were susceptible to nystatin [41]. A study evaluating oropharyngeal candidiasis by Yu et al., 100% of isolates showed susceptibility to nystatin with MIC values of ≤0.015–4 μg/mL against C. albicans, 1–4 against C. glabrata, 0.125–2 against C. tropicalis, and 1–2 against C. parapsilosis [42].
Nystatin can present challenges due to drug interactions [43,44]. A recent in vitro study performed by Scheibler et al. proved that nystatin efficacy to treat oral candidiasis is reduced by chlorhexidine, an antiseptic agent widely used to control oral infections in healthy and immunosuppressed individuals [43]. The combination treatment of nystatin and chlorhexidine affected the efficacy of both drugs at inhibiting C. albicans planktonic and biofilms states. The MICs of nystatin and chlorhexidine were higher when these drugs were used sequentially compared to their respective individual MICs. The nystatin-chlorhexidine combination led to hindered reductions in biofilm total biomass compared to individual treatments. HPLC analysis indicated that the concentrations of nystatin and chlorhexidine in mixture were significantly lower than their respective values in single formulations. This was likely a result of increased compound degradation within the mixture formulations.
In spite of drug interaction challenges, nystatin can inhibit fungi as an immunomodulator. Cell membrane lipids play crucial roles in modulating both innate and adaptative immune responses through pathogen recognition, lymphocyte activation, and cytokine signaling [45,46]. Using a vulvovaginal candidiasis model in rats, Zhang et al. demonstrated that nystatin treatment enhanced vaginal mucosa immune responses against C. albicans by up-regulating IFN-γ-related cellular response, the IL-17 signaling pathway, and IgG-mediated immunity [45]. Therefore, the immunoregulatory role of nystatin presents a promising field that needs further exploration to fully recognize its potential.
2.2. Antifungal azoles
Members of the azole class inhibit 14α-lanosterol demethylase, one of the enzymes responsible for ergosterol biosynthesis, leading to fluidity reduction, alterations in the activity of membrane-associated enzymes, and inhibition of growth that result in cell lysis and death [47]. The azoles consist of two subclasses based on the number of nitrogen atoms in the ring: imidazoles, which contain two nitrogen atoms, and triazoles, formed by three nitrogen atoms [48]. Imidazole was the first to be introduced, but the class now includes miconazole, ketoconazole, tioconazole, butoconazole, clotrimazole, econazole, sertaconazole, and terconazole. These compounds present broad-spectrum activity against different Candida spp. with various formulations available [49]; however, due to toxicity, currently, they are recommended for treating superficial candidiasis such as oral and vulvovaginal candidiasis [47,50]. Improvements in the safety profiles were achieved with the creation of the second subclass, triazole. This group includes: fluconazole, itraconazole, voriconazole, and posaconazole [47,51–55].
2.2.1. Fluconazole
Fluconazole and itraconazole are first generation compounds within the triazole subclass. Fluconazole can be provided via oral or parenteral administration, making it amenable for treating a variety of candidiasis infections, such as oropharyngeal, esophageal, peritoneal, vaginal, and disseminated [47]. Fluconazole has high gastrointestinal absorption with similar concentration obtained by endogenous administration. Since fluconazole can reach cerebrospinal fluid and its concentration in urine is higher than serum, fluconazole is used to treat central nervous system and symptomatic cystitis Candida spp. infections [56,57]. The use of fluconazole during pregnancy, mainly, in the first trimester should be avoided due to the risk of spontaneous abortion and malformations [58,59]. Taking this into account, the FDA does not recommend using fluconazole at any stage of pregnancy. Another limitation of fluconazole is the pharmacological interaction with certain drugs that can result in decreased efficacy and increased toxicity for one or both administered drugs [60]. Fluconazole-drug interactions have already been described with several drug classes, including tacrolimus, cyclosporine, cisapride, and warfarin [61,62].
The efficacy of fluconazole as well as its convenience and patient tolerance makes this antifungal the first option for the treatment of oral [63] and vulvovaginal candidiasis [63,64]. In a recent meta-analysis study, involving 4,042 participants with oral candidiasis, Fang et al. concluded that fluconazole had better mycological cure rate compared to other antifungal drugs: itraconazole, miconazole, clorimazole, ketoconazole, nystatin, and amphotericin B. However, the incidence of recurrence rate and adverse effects were not evaluated by these authors [63]. Qin et al. [65] performed a meta-analysis review to compare the effectiveness of different treatments for vulvovaginal candidiasis, including 41 randomized controlled clinical trials. Nine antifungal drugs showed more effectiveness than placebo in the treatment of patients. Fluconazole was the best antifungal drug, followed by clotrimazole, miconazole, itraconazole, ketoconazol, econazole, butoconazole, terbinafine and terconazole. Denison et al. showed that the efficacy of oral or topical azole treatments was similar in relation to clinical cure of uncomplicated vulvovaginal candidiasis, however the oral treatment cleared yeast from the vagina better than topical ones [66].
Fluconazole has been shown to be effective in the treatment of 70–80% of Candida strains and therapeutic failure is usually associated with susceptibility profile of each Candida strain [64]. C. albicans and mainly non-albicans Candida species are becoming increasingly resistant to fluconazole [64,66]. Therefore, an accurate diagnosis should be performed to guide the selection of the appropriate treatment, including culture confirmation, species identification techniques, and in vitro tests of susceptibility to antifungal drugs [64,67]. Nystatin can be used as a treatment option in the case of oral and vulvovaginal candidiasis resistant to fluconazole [67,68]. Other alternatives include flucytosine and AmB creams for vulvovaginal candidiasis and itraconazole, or posaconazole and AmB deoxycholate suspensions for oral candidiasis [57].
In summary, fluconazole has been available for almost three decades; this compound is generic, inexpensive and largely safe outside of pregnancy. Limitations are few in terms of toxicity, but shortcomings for treating mucosal infections can occur due to resistance in non-albicans Candida species and more recently C. albicans.
2.2.2. Itraconazole
Like fluconazole, itraconazole is also available for oral and intravenous use; however, it has poor aqueous solubility and low bioavailability [69]. Thus, it is recommended for fluconazole refractory Candida infections [57]. In 2018, a new oral formulation, SUBA itraconazole capsules (SUper BioAvailability), was approved by the Food and Drug Administration (FDA). This new formulation is based on a polymeric matrix that controls itraconazole release in the duodenum, improving dissolution and absorption [70,71]. In prophylaxis for stem cell transplantation and treatment for hematological malignancies patients, the SUBA®- itraconazole formulation presented more rapid therapeutic levels and less inter-patient variability in comparison to the traditional itraconazole formulation [71].
2.2.3. Voriconazole and Posaconazole
Voriconazole and posaconazole are members of the second generation triazoles, mainly developed to address emergent fluconazole and itraconazole resistance. These compounds are considered fungicidal, have a broader spectrum of activity compared to the earlier azoles, and are indicated for oropharyngeal and invasive candidiasis [57,72]. They have demonstrated in vitro activity against most Candida species [73]. Rodrigues et al. verified that voriconazole was notably more effective than fluconazole against C. glabrata biofilms, in which voriconazole had better diffusion through the biofilms and higher cell penetration capacity [54]. Although voriconazole and posaconazole demonstrates fungicidal activity and broader spectrum than fluconazole in in vitro studies, there is little comparative clinical data among these antifungals. El-Ghmmaz et al. [74] compared the effectiveness of voriconazole and fluconazole in preventing invasive fungal infections in 70 patients undergoing hematopoietic stem cell transplantation. The prophylaxis with voriconazole did not differ from fluconazole regarding the prevention of invasive fungal infections and overall survival. Devanlay et al. [75] analyzed the posaconazole and fluconazole as primary prophylactic antifungal agents in 91 patients with acute myeloid leukemia. The results did also not distinguish any difference between posaconazole and fluconazole prophylaxis. However, mycological examination of stools showed an increased colonization by non-albicans Candida species in patients treated with fluconazole, suggesting a selection pressure on Candida growth by this antifungal.
Voriconazole and posaconazole are available in oral and intravenous formulation [57,72]; however, their pharmacokinetics represent a therapeutic challenge [52,76]. Voriconazole has good oral bioavailability, but its absorption can be influenced by food [57]. Posaconazole provides a delayed-release tablet formulation with improvements in absorption [77], reaching more serum drug concentration and higher efficacy than oral suspension formulation [77,78]. Both antifungals exhibit highly variable inter- and intra-patient pharmacokinetics, and numerous factors have been associated with their variability in plasma levels, such as altered intestinal absorption, drug interactions, diarrhea, chemotherapy, age, and weight [76].
In a recent study, Hachem et al. compared the safety and efficacy of voriconazole and posaconazole as prophylactic drugs for invasive fungal infection in 200 patients with hematological malignancies in the U.S. [79]. The efficacy was very similar between the groups with comparable mortality rates. However, symptomatic adverse effects were more frequent in the voriconazole group, while posaconazole was better tolerated by patients. Unfortunately, liver function abnormalities were more common in the posaconazole group. Other specific adverse effects have been reported for these antifungals. The administration of voriconazole was associated with skin photosensitivity [80] and increased risk for cutaneous squamous cell carcinoma in patients who underwent lung and hematopoietic cell transplants [81–83]. Parkers et al. reported visual hallucinations and neurological disturbances in a patient with high posaconazole concentration in serum, suggesting that patients treated with new formulations of posacanozole should be monitored for visual and central nervous system (CNS) alterations [84].
2.2.4. Isavuconazole, Ravuconazole, and Albaconazole
More recently, additional triazole agents were developed, including isavuconazole, ravuconazole, and albaconazole [47,85]. Isavuconazole is available in oral or intravenous administrations and use for invasive candidiasis was investigated in a recent Phase 3 clinical trial (Clinicaltrials.gov, NCT00413218), in which the treatment with isavuconazole showed similar efficacy and safety to the treatment with caspofungin followed by voriconazole [86]. In another clinical trial (Phase 2), isavuconazole also exhibited comparable efficacy and safety to fluconazole for the treatment of esophageal candidiasis [87]. Ravuconazole and albaconazole are undergoing clinical trials for use in intravenous and oral formulations, respectively [88]. Both antifungals present promising effects for the treatment of fungal infections caused by fluconazole and itraconazole resistant strains [88].
2.2.5. Azole resistance
Due to the numerous advantages of the azoles, this antifungal class has been used as a gold standard therapy for over 50 years; however, this has resulted in the global emergence of resistant strains [89–92]. Azole resistance mechanisms developed by Candida spp. have widely been investigated, and currently three main mechanisms are described: (1) overexpression of membrane transporters [85,93,94]; (2) alterations of ergosterol biosynthesis [95,96]; and more recently, (3) alterations in sterol import [97].
Overexpression of membrane transporters is an important resistance mechanism of Candida spp. to azole agents. Two classes of membrane transporters in Candida cell membrane have been associated to azole resistance: ATP binding cassette (ABC) and major facilitator superfamily (MFS). Both classes are integral cell membrane proteins with different mechanisms of obtaining energy to drive efflux of substrates as azoles. The ABC proteins are primary active transporters that employ energy from the hydrolysis of ATP, while MFS proteins are secondary active transporters that use a proton gradient from the cell membrane as an energy source to efflux drugs [94,98]. The increased expression of ABC and MFS transport proteins have been correlated, respectively, with the overexpression of Candida spp. drug resistance genes (CDR1 and CDR2) and multi-drug resistant genes (MDR1 and MDR2) [98]. The overexpression of CDR and MDR genes was correlated with decreased azole susceptibility in several Candida spp., such as C. albicans [99], C. glabrata [100], C. parapsilosis [101], and C. auris [102].
Azoles also exert less efficacy when there are alterations to ergosterol biosynthesis, leading to decreased affinity to the fungal cell target [95]. Erg11 is an essential enzyme that regulates the ergosterol biosynthesis pathway. Mutations in ERG11 cause amino acid substitutions that result in proteins being unable to binds to azoles, consequently generating azole resistance [94]. Flower et al. sequenced ERG11 for 63 C. albicans fluconazole resistant isolates and observed that 87% presented at least one mutation in the ERG11 gene [96]. A number of amino acids substitutions have been correlated to azole resistance: A114S, Y132H, Y132F, K143R, Y257H, K143Q, F145L, S405F, D446E, G448E, F449V, G450E, and G464S [95,96]. In a recent multicenter study, Chowdhary et al. found 90% of fluconazole resistant C. auris isolates among 350 strains were caused by amino acid substitutions Y132 and K143 in ERG11 [103].
Fungal cells exposed to azoles must synthesize more endogenous ergosterol or import exogenous sterol to survive antifungal treatment [94,97]. In 2018, Lin et al. showed that the presence of exogenous cholesterol or ergosterol in growth media made C. glabrata strain highly resistant to fluconazole and voriconazole. In contrast, C. glabrata mutant strain lacking the AUS1 gene that encodes a sterol influx transporter exhibited hypersensitivity to azoles [97]. Therefore, Candida spp. can scavenge free sterols for the cell membrane as cholesterol, acquiring resistance to azole antifungals.
2.3. Echinocandins
Echinocandins inhibit (1,3) β-D glucan synthase (encoded by FKS genes) that are responsible for the biosynthesis of glucan, the major polysaccharide present in the fungal cell wall. The absence of this target polysaccharide in human cells results in low toxicity. Other advantages of this antifungal class includes low propensity for drug-drug interactions and rapid fungicidal activity against Candida spp., including triazoles-resistant clinical isolates. The available echinocandins are caspofungin, micafungin, and anidulafungin. All the echinocandins have large molecular weight and are available in intravenously formulations; thus, the absence of an oral formulation is a limitation of this antifungal class [104–107].
Candida spp. resistant to echinocandins are uncommon; varying from 1 to 10% of the total isolates depending on the species analyzed [108–110]. Recent data indicate that the resistance rates to echinocandins have remained low for most Candida isolates: C. albicans (0–0.1%), C. tropicalis (0.5–0.7%), and C. krusei (0–1.7%) [89]. C. lusitaniae, C. parapsilosis, and C. guilliermondii are known to have reduced susceptibility to echinocandins, and C. glabrata is considered the most resistant species associated with treatment failures [111–113]. C. glabrata has shown an increase in resistance in recent years, reaching rates of 8 to 11% [89]. Resistant C. glabrata isolates tended to be non-susceptible/resistant to at least two echinocandins [89] and can exhibit cross-resistance to fluconazole [113]. Candida auris, also well known for fluconazole resistance, exhibits variable susceptibility to echinocandins with resistant rates reported at 2–7% in India, 4% in the U.S., and 7% among isolates collections from Pakistan, South Africa, and Venezuela [103,114,115].
Candida spp. resistance to echinocandins occurs due to mutations in FKS genes (FKS1 and FKS2), which are responsible for the expression of the (1,3) β-D glucan synthase. Hot spot mutation regions in FKS1 have been observed for C. albicans, C. krusei, C. parapsilosis, and C. auris [103,110,116–120], while mutations on both FKS1 and FKS2 were reported for C. glabrata [118]. Acquired resistance to echinocandins has also been associated with a compensatory increase in cell wall chitin in response to inhibition of (1,3) β-D glucan by this antifungal class [112,113]. Walker et al. demonstrated that the in vitro treatment of C. albicans with a sub-MIC level of caspofungin led to an increase in chitin content, that resulted in a reduction of susceptibility to caspofungin [112]. Using a mouse model of systemic candidiasis, Lee et al. verified that after 48 h post-infection, caspofungin treatment induced an increase in chitin in C. albicans cells recovered from kidneys [121]. In addition, some of the recovered clones had acquired a point mutation in FKS1. The authors suggested that two non-exclusive mechanisms can affect echinocandins sensitivity: acquisition of FKS1 mutations and elevation of chitin levels via the stimulation of cell wall integrity pathways. In 2020, Walker et al. showed that increased chitin exposure in C. albicans cells, in response to caspofungin treatment, altered cytokine production by macrophages, suggesting that cell wall remodeling influences host immune responses [113]. Therefore, the understanding about the gene mutations and wall remodeling processes during Candida spp. infection can provide new perspectives to improve the drug efficacy [121].
Efficacy among Candida isolates and low toxicity profiles means echinocandins are recommended as the first line defense for treatment of suspected or documented candidemia and invasive candidiasis [57,122]. This recommendation is supported by clinical trials that reported echinocandins class superiority compared to other antifungal classes when treating invasive Candida infections [123,124]. Additionally, recent studies demonstrated that echinocandins are capable of eradicating Candida biofilms, providing an interesting therapy option for catheter-related Candida biofilm infections [125–127]. Basas et al. compared the efficacy of anidulafungin with amphotericin B (L-AmB) in the treatment of C. albicans and C. glabrata biofilms, using in vitro and in vivo models [126]. Anidulafungin exhibited greater inhibitory activity than L-AmB against biofilms formed in vitro on silicone discs. The minimum biofilm eradication concentration for 90% (MBEC90) of anidulafungin was > 100-fold and > 1000-fold more effective than L-AmB for C. glabrata and C. albicans strains, respectively. In the in vivo study, central venous catheters were inserted into rabbits infected by Candida strains. Antifungal lock therapy with L-AmB and anidulafungin were able to reduce fungal burden at a similar capacity against C. albicans; however, anidulafungin was more effective than L-AmB against C. glabrata.
3. Therapeutic approaches in development
3.1. New antifungal compounds
To overcome the limitations with currently available treatments new synthetic and natural antifungal compounds have been investigated [128]. For all types of compounds, the main challenges involve the identification of substances with broader antimicrobial spectra and action mechanisms that limit the emergence of resistant strains, while maintaining good pharmacokinetic and low toxicity [129–131].
3.1.1. Structure modifications to existing antifungal classes
3.1.1.1. Modified amphotericin B
Many of the new synthetic compounds focus on structural modifications in the already available drugs, including polyenes, azoles, and echinocandins. Among them, enchochleated amphotericin B (Coch-AmB) is a new formulation that includes this polyene in cochleates (phospholipid spiral multilayered structure), providing oral administration of amphotericin B. The efficacy of Coch-AmB was demonstrated in systemic candidiasis mouse model [132], and the compound is currently undergoing safety and efficacy evaluation in patients with vulvovaginal candidiasis in a phase 2 clinical trial [129].
3.1.1.2. Modified Azoles
A large number of antifungal compounds have been developed based on modified azoles structures [133,134]. Shrestha et al. modified fluconazole by replacing one of the triazole rings with a linear alkyl chain and by adding different structures on the phenyl ring, generating compounds active against Candida spp. and less cytotoxic to mammalian cells according to in vitro assays [133]. Xie et al. synthesized twenty-nine novel triazole analogues of ravuconazole and isavuconazole, verifying that most of them showed in vitro antifungal activity, including activity against C. albicans, C. glabrata, and C. parapsilosis strains [134]. Based on the structure-activity relationships (SAR) analyses, these authors concluded that the most effective compounds were achieved by replacing the 4-cyanophenylthioazole moiety of ravuconazole with fluorophenylisoxazole [134]. Other researchers designed tetrazoles with metal-binding groups, resulting in molecules more selective for fungal sterol biosynthesis and with longer half-lives, designated as tetrazoles VT-1598, VT-1129, and VT-1161 [135,136]. All of them showed promising results in preclinical studies, and VT-1161 (Oteseconazole) is currently in a phase 3 clinical trial for the treatment of vulvovaginal candidiasis [137]. Tetrazoles have showed potent in vitro and in vivo inhibitory activity against azole-and echinocandin-resistant Candida isolates, including C. glabrata, C. krusei and C. auris [137–139]. Furthermore, VT-1161 demonstrated excellent efficacy and safety in a murine model of vulvovaginal candidiasis, with high volume of distribution, high oral absorption, long half-life, and rapid penetration into vaginal tissues [140].
3.1.1.3. Modified Echinocandins
Structural modifications of echinocandins have also been generated. Rezafungin, is a new antifungal derived from anidulafungin that is in phase 2 clinical trial to test efficacy against candidemia and invasive candidiasis. The stability that provides a longer half-life along with a favorable safety profile permits rezafungin to be administered weekly rather than daily. This extended release drug should reduce patient hospitalization times by altering therapeutic regimens. In addition, rezafungin has efficacy for treating less-susceptible fungal pathogens and shows low propensity to induce antifungal resistance [141]. Hager et al. demonstrated that rezafungin was more potent than amphotericin B and micafungin against C. auris, reducing kidneys fungal burden in immunosuppressed mice. In this report, rezafungin (20 mg/kg) was administered on alternating days, while amphotericin B (0.3 mg/kg) and micafungin (5 mg/kg) were given daily. The data suggest that rezafungin can inhibit C. auris even when provided in less frequent doses [142].
3.1.2. Development of new antifungal classes
In addition to developing antifungals derived from polyenes, azoles, and echinocandins, new antifungal classes have been introduced in the last years (Figure 1) [137]. Among them, enfumafungin is an antifungal targeting the cell wall by inhibiting the (1,3)β-D glucan synthase. Although, enfumafungin shares the same drug target with echinocandins, this compound presents a structurally distinct antifungal class [143]. Enfumafungin is a glycosylated fernene-type triterpenoid produced by the fungus Hormonema carpetanum. Due to the potent antifungal activity, enfumafungin is being employed in the development of the antifungal ibrexafungerp (SCY-078) [144]. The advantages of SCY-078 are the possibilities of both intravenous and oral administrations and the fungicidal activity against Candida spp., including strains resistant to azoles and echinocandins [129,145–150]. SCY-078 oral formulation is in clinical trial to treat invasive and vulvovaginal candidiasis [144]. Azie et al. reported that ibrexafungerp has numerous attributes for the treatment of vaginal candidiasis, including oral one-day dose, high tissue penetration, increased activity at low pH, low risk for drug-drug interactions and reduced toxicity [151].
Figure 1:
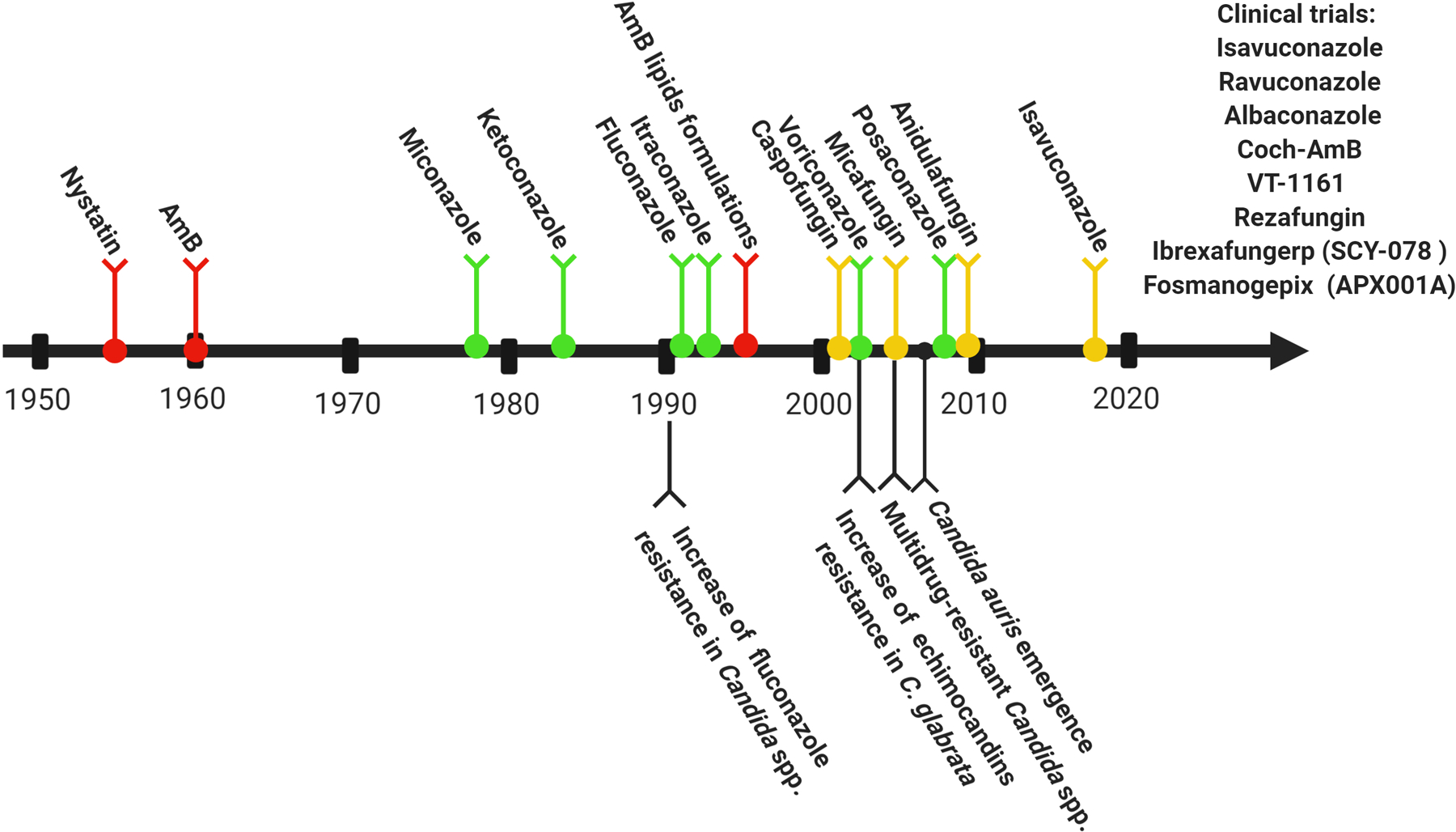
Timeline of antifungal discovery and emergence of drug-resistant Candida isolates. Polyenes are represented in red lines, azoles are represented in green lines and echinocandins are represented in yellow lines. This figure is based on the studies of Ananda-Rajah el al. [263], Perlin et al. [264] and Alexander et al. [265]. Created with BioRender.com.
Another promising antifungal is manogepix (fosmanogepix APX001A), which inhibits Gwt1, an enzyme required in the glycosylphosphatidylinositol biosynthesis pathway, a component present in the yeast cell wall and membrane [52,152]. This compound has exhibited broad-spectrum activity against Candida spp., except for C. krusei [153,154]. Efficacy against C. auris has also been demonstrated in in vitro and in vivo studies and phase 2 clinical trials are ongoing [154–156]. A concern has been brought to light through a new study which identified two efflux-mediated mechanisms in C. albicans and C. parapsilosis that were associated to decreased manogepix susceptibility [157], suggesting the potential for resistance or reduced efficacy that will need to be monitored.
Since the cell wall and plasma membrane are the main targets of antifungal drugs, some researchers have focused on the development of antifungals with alternative target sites, such as arylamidine T-2307 [48,52,158]. Arylamidine mode of action inhibits the respiratory chain, compromising cell energy production [159]. In a study by Mitsuyama et al., arylamidine T-2307 exhibited potent inhibition against C. albicans, including fluconazole resistant strains, and showed efficacy in the treatment of disseminate candidiasis in a mouse model [160]. More recently, Wiederhold et al. demonstrated arylamidine T-2307 inhibitory activity against C. auris in a mouse model, in which treatment improved the survival rate and reduced the kidney fungal burden compared to an untreated group [158].
3.1.3. Identification of new bioactive molecules from natural products
Natural compounds have always been important sources of new antimicrobial drugs [161–163]. Since the first modern antimicrobial agents, many antibacterial and antifungal drugs have taken the form of semi-synthetic derivatives of natural products [162], including two relevant antifungal classes: polyenes and echinocandins [128]. Natural product drug discovery is an intensive labor that requires the isolation and characterization of several bioactive molecules [161,162]. The feasibility of high-throughput screening shifted research focus to synthetic compound libraries, but the current emergence of multi-drug resistant strains is reviving attention for natural compounds in academic and biotechnology sectors [162].
Most natural compounds investigated include extracts from plant or microbial origins [161,164–166]. In a systematic review, Singla et al. showed that plants from the order Lamiales, Apiales, Asterales, Myrtales, Sapindales, Acorales, Poales and Laurales exhibit antifungal activity against Candida spp. [161]. Likewise, antifungal activity has been reported from different microorganisms, such as Lactobacillus spp. [166] and Streptococcus spp. [165,167]. Antifungal activities has been associated with compounds belonging to the terpenoids, phenylpropanoid, alkaloids, flavonoids, polyphenol, naphthoquinone and saponins classes, but their mechanisms of action and possible synergism with antifungal drugs still need to be investigated for complete elucidation [161].
3.1.4. Search for compounds targeting Candida spp. virulence mechanisms.
Another attractive antifungal approach is the identification of compounds targeting specific Candida spp. virulence mechanisms. The advantages to treat candidiasis with anti-virulence agents include the preservation of the host normal microbiome, as well as lower toxicity and reduced selective pressure for developing resistance in relation to drugs that target fungal growth [168–175]. Moreover, anti-virulence strategies can have a substantial impact for both prophylactic and therapeutic management of candidiasis [169]. In this context, many authors have sought inhibitors of hydrolytic enzymes, morphogenesis, adhesion and biofilm formation [171].
To identify new antifungal agents targeting virulence mechanisms, Bonvicini et al. investigated some compounds of chalcones that are precursors of flavonoids. Forty chalcone-based analogues were screened against C. albicans, and two compounds (5 and 7) were capable of weakening its pathogenicity factors. Both compounds inhibited hyphae and biofilm production, indicating a potential use in anti-infective therapeutics [168]. Romo et al. screened 30,000 drug-like small-molecule compounds from the DIVERSet library (ChemBridge Corporation), identifying N-[3-(allyloxy)-phenyl]-4-methoxybenzamide (9029936) as the major compound with inhibitory activity against filamentation and biofilm formation of C. albicans [173]. Prasath et al. investigated the effects of palmitic acid on the virulence factors of C. tropicalis. After 48 h of treatment, palmitic acid decreased the enzymatic activity, leading to a reduction of 53 to 72% of lipase production and a total inhibition of protease production. The authors suggested that palmitic acid can be applied to increase the efficacy of conventional antifungal drugs in the control of non-albicans Candida species [176].
3.2. Drug repurposing
Drug repurposing is an interesting strategy to investigate new uses of existing compounds [177,178]. Two principles support the drug repurposing concept: 1) many active drugs are not fully understood and 2) there are common molecular and genetic factors between different pathologies [179]. Since repurposed candidates have already been evaluated for pharmacokinetic, pharmacodynamic, and toxicological effects, the process is faster and cheaper compared to traditional novel drug discovery [177,179–181]. Within this context, a number of off-patent compound libraries have been screened to find antifungal agents, evaluating effects on fungal growth, morphogenesis, and biofilm formation of Candida spp., as well as synergistic action with antifungal drugs. To date, various compounds with inhibitory activity against Candida spp. have been identified including antimicrobial (antibacterial and antiviral) agents and non-antimicrobial pharmacological classes [182–186].
3.2.1. Antimicrobial agents
Repositioning antibacterial drugs as antifungal is considered a promising strategy for treating candidiasis [182,183], and in some cases, candidate compounds can also be employed for the dual purpose for treating mixed infections caused by bacteria and fungi [182]. Jadhav et al. found that moxifloxacin, an antibacterial fluroquinolone, can decrease both planktonic and biofilm states of C. albicans, as well as inhibit the yeast to hyphal transition [182]. Interestingly, the moxifloxacin antifungal mechanism of action was multi-targeted. Moxifloxacin exhibited good binding to the active sites of C. albicans topoisomerase II, an enzyme associated with DNA replication, and also affected a number of genes involved in C. albicans morphogenesis via MAPK and cAMP-PKA pathways [182].
Among a total of 21 sulfa antibacterial drugs, Eldesouky et al. [183] found 15 compounds with anti-Candida activity. These compounds exhibited synergistic action with fluconazole against C. albicans fluconazole resistant isolates in both in vitro assays and a Caenorhabditis elegans in vivo model. Synergistic activity was attributed to dihydropteroate synthase (DHPS) enzyme inhibition by sulfa drugs, leading to restriction of the Candida ergosterol biosynthesis pathway and, consequently, improving the effect of fluconazole [183].
Boron containing compounds have known antibacterial activity [187] and the boric acid has been clinically used as topical antifungal agent for vulvovaginal candidiasis caused by azole resistant Candida strains [64]. Based on this evidence, recently, Rossoni et al. tested the antifungal effects of surface pre-reacted glass-ionomer (S-PRG) eluate that is used in dental materials to suppress cariogenic bacteria and reduce dental plaque accumulation. S-PRG is a material that releases six types of ions, BO33- (Borate), Na+, Sr2+, SiO33-, Al3+ and F-, in the oral cavity. S-PRG eluate exhibited antifungal activity against C. albicans, C. glabrata, C. krusei, and C. tropicalis, reduced in vitro biofilm formation and protected G. mellonella against experimental candidiasis, demonstrating therapeutic potential for oral candidiasis [188].
Antiviral drugs have also been studied for repurposing potential. Yousfi et al. focused their studies on ribavirin, a guanosine analog with broad-spectrum activity against RNA and DNA viruses that is commonly used for the treatment of hepatitis C virus (HCV) [189]. Ribavirin exhibited antifungal activity against 63 Candida spp. isolates among 100 isolates tested. C. parapsilosis and C. tropicalis demonstrated the greatest susceptibility. Promisingly, ribavirin was effective against multidrug-resistant C. albicans and showed synergistic action with fluconazole, itraconazole, and posaconazole [189].
Other investigational agents for antifungal use include HIV-protease inhibitors, such as indinavir, ritonavir, and saquinavir [184,190,191]. Studies that investigated these compounds note clinical evidences that treatment of HIV positive patients with protease inhibitors results in improved mucosal Candida infections, with direct effect on fungi rather than augmenting host immune status [192]. Cassone et al. demonstrated that indinavir and ritonavir were able to reduce the growth and secretory aspartic proteases (Sap) production by C. albicans, as well as inhibit the development of vaginal candidiasis in rats with an efficacy comparable to fluconazole [190]. Calug et al. reported a significant structural similarity between C. albicans Sap2 and HIV-1 protease, inciting the development of a single inhibitory drug able to interact with both viral and fungal targets [191].
3.2.2. Non-antimicrobial agents
Several studies have reported antifungal properties from pharmacological classes that lacked antimicrobial characterization, including anti-inflammatory, anticancer, antidepressant, antipsychotic, anesthetics, antihyperlipidemic and others (Table 2). As an example, auranofin, an anti-inflammatory drug used to treat rheumatoid arthritis [193], was investigated by Fuchs et al. as a repurposed antibacterial and antifungal agent [185]. Auranofin was capable of inhibiting Gram-positive bacteria, as well as fungal species, such as Candida spp. Auranofin inhibited thioredoxin reductase, part of the thioredoxin system responsible for protecting microbial cell against oxidative stress. Since the thioredoxin system is conserved in both prokaryotic and eukaryotic organisms, auranofin can be the initiate of a future class of antibiotics and antifungals based on this new microbial target [185,194,195].
Table 2.
Drug repurposing for candidiasis: compounds active against Candida spp.; its action on virulence mechanism and combinatory effect with antifungal drugs
| Drug classes according to traditional use | Compounds | Antifungal action | Reference |
|---|---|---|---|
| Antibacterial | Sulfa antibacterial drugs | Reverse azole resistance, synergism fluconazole | [183] |
| Clotrimazole | Growth inhibition | [189] | |
| Dequalinium dichloride | Growth inhibition | [189,247] | |
| Ciclopirox ethanolamine | Growth inhibition | [189,247] | |
| Nifuroxime | Growth inhibition | [248] | |
| Nitroxoline | Growth inhibition, anti-biofilm, synergism miconazole | [248,249] | |
| Chlorquinaldol | Growth inhibition | [248] | |
| Octanoic Acid | Growth inhibition | [248] | |
| Antiseptics | Thimerosal | Anti-biofilm | [250] |
| Benzethonium chloride | Growth inhibition, anti-biofilm | [189,247,250] | |
| Alexidine dihydrochloride | Growth inhibition, anti-biofilm | [247,250] | |
| Thonzonium bromide | Growth inhibition, anti-biofilm | [189,247,250] | |
| Chlorhexidine | Growth inhibition, anti-biofilm | [189,250] | |
| Methyl benzethonium chloride | Growth inhibition, anti-biofilm | [189,247,250] | |
| Chloroxine | Growth inhibition anti-biofilm | [189,247,250] | |
| Monensin sodium salt | Anti-biofilm | [250] | |
| Clioquinol | Growth inhibition, anti-biofilm | [189,247,250] | |
| Hexachlorophene | Growth inhibition, anti-biofilm synergism miconazole | [247,249,250] | |
| Boric acid | Biofilm reduction, growth and germination inhibition | [251] | |
| Bacitracin | Anti-biofilm | [250] | |
| Disinfectant | Broxyquinoline | Anti-biofilm, synergism miconazole | [249] |
| Antiviral agent | Ribavirin | Growth inhibition, synergism azole | [189] |
| Indinavir | Growth inhibition, aspartyl protease inhibition | [190] | |
| Ritonavir | Growth inhibition, aspartyl protease inhibition | [190] | |
| Antiparasitic | Pyrvinium pamoate | Growth inhibition, anti-biofilm, anti-biofilm synergism miconazole | [189,247,249,250,252] |
| Pentamidine isethionate | Growth inhibition, anti-biofilm, synergism miconazole | [189,252] | |
| Avermectin B1a | Anti-biofilm | [250] | |
| Dihydroartemisinin | Growth inhibition, anti-biofilm, synergism miconazole | [247,249] | |
| Gentian violet | Anti-biofilm, synergism miconazole | [249] | |
| Bithionate disodium | Anti-biofilm, synergism miconazole | [249] | |
| Artesunate | Anti-biofilm, synergism miconazole | [249] | |
| Pinaverium bromide | Growth inhibition | [189] | |
| Avermectin B1 | Growth inhibition | [189] | |
| Triclabendazole | Growth inhibition | [189] | |
| Emetic | R(−)-Apomorphine hydrochloride Hemihydrate |
Growth inhibition | [252] |
| Antiemetic | Thiethylperazine dimalate | Growth inhibition | [247] |
| Trifluoperazine dihydrochloride | Growth inhibition | [247] | |
| Anti-hypertensive | Amlodipine besilate | Anti-biofilm | [253] |
| Guanadrel sulfate | Growth inhibition | [247] | |
| Nisoldipine | Growth inhibition | [252] | |
| Anestesic | Tramadol | Germ tube formation, adhesion, anti-biofilms | [254] |
| Dimethisoquin hydrochloride | Growth inhibition | [247,252] | |
| Dyclonine hydrochloride | Growth inhibition | [247] | |
| Anticancer | Vinblastine | Anti-biofilm, growth inhibition | [255,256] |
| Vincristine | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Paclitaxel | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Docetaxel | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Oxaliplatin | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Carboplatin | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Cisplatin | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Gemcitabine | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Bleomycin | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Doxorubicin | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| 5-Fluorouracil | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Decarbazine | Anti-biofilm | [255] | |
| Etoposide | Anti-biofilm | [255] | |
| Leucovorin or folinic acid | Anti-biofilm | [255] | |
| Tamoxifen | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Irinotecan | Anti-biofilm, growth and germ tubes inhibition | [255,256] | |
| Daunorubicin | Growth and germ tubes inhibition | [256] | |
| Mitoxantrone | Growth and germ tubes inhibition | [256] | |
| Mitomycin-C | Growth and germ tubes inhibition | [256] | |
| Epirubicin | Growth and germ tubes inhibition | [256] | |
| Dactinomycin | Growth and germ tubes inhibition | [256] | |
| Busulfan | Growth and germ tubes inhibition | [256] | |
| Carmustine | Growth and germ tubes inhibition | [256] | |
| Cyclophosmamide | Growth and germ tubes inhibition | [256] | |
| Ifosfamide | Growth and germ tubes inhibition | [256] | |
| Melphalan | Growth and germ tubes inhibition | [256] | |
| Methotrexate | Growth and germ tubes inhibition | [256] | |
| Hydrooxyurea | Growth inhibition | [256] | |
| Formestane | Growth and germ tubes inhibition | [256] | |
| Etoposide | Growth and germ tubes inhibition | [256] | |
| Leuprolide | Growth and germ tubes inhibition | [256] | |
| Dacarbazine | Growth and germ tubes inhibition | [256] | |
| Melengestrol acetate | Growth inhibition | [257] | |
| Megestrol acetate | Growth inhibition | [257] | |
| Tosedostat | Growth inhibition, morphological changes | [257] | |
| Amonafide | Growth inhibition, morphological changes | [257] | |
| Rapamycin | Growth inhibition, morphological changes | [257] | |
| Thioguanine | Growth inhibition | [189] | |
| Thiosemicarbazone | Growth inhibition, azole synergism | [196] | |
| Antipsychotic | Methiothepin maleate | Growth inhibition | [247,257] |
| Haloperidol | Growth inhibition, morphological changes | [257] | |
| Trifluperidol 2HCl | Growth inhibition, morphological changes | [257] | |
| Bromperidol and derivates | Growth inhibition, azole synergism | [258] | |
| Zotepine | Growth inhibition, anti-biofilm | [247,250] | |
| Prochlorperazine dimaleate | Growth inhibition | [247] | |
| Antiepileptic/ Antidepressant | Diazepam | Growth and germ tubes inhibition, anti-biofilm | [259] |
| Lorazepam | Growth and germ tubes inhibition, anti-biofilm | [259] | |
| Midazolam | Growth and germ tubes inhibition, anti-biofilm | [259] | |
| Phenobarbitone | Growth and germ tubes inhibition and anti-biofilm | [259] | |
| Tamoxifen citrate | Growth inhibition | [247,257] | |
| Sertraline | Growth inhibition, morphological changes and anti-biofilm | [197,247] | |
| Rolipram | Growth inhibition | [247] | |
| Anemia | Stanozolol | Growth inhibition | [257] |
| Anti-inflammatory | Ebselen | Synergism fluconazole/anidulafungin, anti-biofilm | [247,252] |
| Anticoagulant | Argatroban | Growth inhibition | [252] |
| Antipsoriatic | Anthralin | Growth inhibition | [189] |
| Antihyperlipidemic | Fluvastatin | Growth inhibition | [248] |
| Anti-fadigue | Fipexide hydrochloride |
Growth inhibition | [247] |
| Antirheumatic/ analgesic | Auranofin | Growth inhibition, anti-biofilm | [185,189,250,257,260] |
| Antilipemic | Tetra ethylenepentamine pentahydrochloride | Growth inhibition | [189] |
| Broad spectrum of pharmacologic effects | Pilocarpine | Morphological changes and anti-biofilm | [261] |
| Benign prostatic hyperplasia | Finasteride | Morphological changes anti-biofilm and synergism fluconazole | [262] |
| Chelating agent | Pentetic acid | Growth inhibition | [189,252] |
| Coronary dilator, spasmolytic, uricosuric | Benzbromarone | Anti-biofilm | [250] |
| Decongestant | Octodrine | Growth inhibition | [248] |
| Deterrent of alcohol consumption | Disulfiram | Growth inhibition | [189,248,257] |
| Immune-suppression | Mycophenolic acid | Growth inhibition | [248] |
| Mydriatic vasodilator | Yohimbine hydrochloride | Anti-biofilm | [250] |
| Vasodilator/Antiplatelet | Suloctidil | Growth inhibition, voriconazole synergism | [247,252] |
Seeking additional repurposing antifungals, Sun et al. investigated NSC319726, a thiosemicarbazone anticancer agent [196]. This compound had antifungal activity against Candida spp. in the range of 0.1 – 2.0 μg/mL and synergistic action with fluconazole, itraconazole, and voriconazole. Using a high concentration of NSC319726, synergistic activity was also found with caspofungin. Through transcriptome analysis, NSC319726 mechanism of action was correlated to ribosome biogenesis inhibition and induction of oxidative stress [196]. In another study, Gouri et al. investigated the antifungal action of sertraline, a compound used to treat of depression, against three C. auris strains resistant to fluconazole and amphotericin [197]. In additional to significantly inhibiting planktonic cultures, sertraline impaired fungal morphogenesis and biofilm formation. The mechanism of action against C. auris was explained by the binding nature of sertraline to the sterol 14 alpha demethylase, which is involved in ergosterol biosynthesis. Therefore, this compound presents a new, promising antifungal against emergent drug resistant C. auris [197].
3.3. Combination therapy
In contrast to the usual candidiasis treatment with a single antifungal agent, new approaches employing multiple therapeutic agents (a “drug cocktail”) present an opportunity to overcome fungal resistance by engaging multiple targets [198,199]. Developing resistance to multiple deployed agents becomes more difficult because the fungal cells are less capable of compensating for the actions of two or more drugs or acquire mutations in multiple genes without adverse impact to cell survival or fitness [199,200]. Combined therapies seek synergistic effects that enhance the antifungal activity directly and/or adjunct effects that alter the susceptibility of Candida cells to the antifungal drug, reverting resistance status [179,201]. These treatments can employ a combination of two antifungal drugs or a combination of antifungal drug with new compounds [198,200,202].
3.3.1. Available antifungal drug combinations
The combination of antifungal drugs are an interesting approach since the synergistic effects can reduce therapeutic dosages, and therefore, decrease toxicity and side effects caused by high doses of a single drug [198]. Reginatto et al. tested the effects of combining anidulafungin and amphotericin B treatments against Candida biofilms formed in vitro on venous catheter [203]. The combination of these antifungals at lower concentrations had a significant increase in the antifungal activity in relation to the individual agents. The use of anidulafungin (0.5 μg/mL) or amphotericin B (2.5 μg/mL) individually were able to inhibit 37–75% and 49–68% of the biofilms, respectively, while the combination of anidulafungin and amphotericin B, at the same concentrations, reached 94–100% of biofilm inhibition. To obtain similar reductions with anidulafungin alone, it was necessary to use this antifungal at higher concentration (1 μg/mL), indicating that the combined therapy can decrease the toxicity caused by the isolated treatments.
Despite the in vitro evidences, until now, few clinical studies evaluated the efficacy of combined systemic antifungal drugs for candidiasis treatment. Recently, Ahuja et al. reported two cases of endovascular infections caused by C. parapsilosis in patients with prosthetic valves who responded positively to combination antifungal therapy without surgical intervention [204]. One patient was treated with micafungin and fluconazole, while the other one was treated with micafungin, fluconazole, and flucytosine. The use of combination therapies in both patients resulted in successful treatment without the need for surgical intervention, suggesting that they could pose a useful approach for the patients with endovascular infections.
Amphotericin B and 5-fluorocytosine (5-FC) have been used in combination to treat invasive candidiasis. Since the concurrent delivery of these antifungals by intravenous administration is precluded due to drug precipitation, recently Alvarez et al. developed a formulation that facilitates co-delivery of AmB and 5-FC using PEG-lipid poly(ethylene glycol)-distearoylphosphatidylethanolamine (PEG-DSPE) micelles. The formulation developed showed efficacy in reducing the fungal burden of neutropenic mice with disseminated candidiasis [205]. The commercial availability of formulations with combined components can incite future clinical trials.
3.3.2. Combination of antifungal drugs with new compounds and therapies
Studies have also been conducted to identify compounds that can act synergistically with antifungal drugs. Using the natural antimicrobial agent lactoferrin from human mucosal secretions, Fernandes et al. found a synergistic effect with AmB against a diverse range of yeasts, including C. parapsilosis complex, C. dubliniensis, C. tropicalis, and C. albicans [206]. The associated therapy reduced MIC values 8-fold for lactoferrin and 4-fold for AmB. Moreover, the combination of these compounds was effective in G. mellonella infected by C. albicans, prolonging survival in 93% of larvae past 10 days and reducing fungal burden 5-fold compared to amB alone [206].
The known antifungal fluconazole was combined with ginkgolide B extracted from the Ginkgo biloba leaf by Li et al. who verified a synergistic effect against C. albicans in planktonic and biofilm states, and also found the dual application efficacious in the G. mellonella infection model [198]. Ginkgolide B also increased the antifungal effect of fluconazole on azole-resistant C. albicans, reducing the MIC of fluconazole from > 512 μg/mL to 0.25–1 μg/mL, with the fractional inhibitory concentration index (FICI) ranged from 0.06 to 0.25. The synergistic effect resulted in phenotypic effects characterized by reduced filamentation, disruption of intracellular calcium, and inhibition of drug efflux pumps [198].
In addition to traditions chemical interactions, photodynamic chemicals have also exhibited beneficial effects when applied in combination with antifungal agents. Photochemicals are applied as inert compounds that are induced to an excited electron state when light is applied. Interestingly, Chibebe Junior et al. married fluconazole treatment to photodynamic therapy (PDT) using methylene blue to produce reactive oxygen species when excited [207]. For this process, G. mellonella larvae were infected by a fluconazole resistant C. albicans strain, and subsequently, treated with fluconazole in combination with methylene blue activated by red light irradiation. Larvae treated with a combination of PDT and fluconazole showed 50% survival at the end of the experiment (7 days) compared to control groups treated with fluconazole or PDT alone that resulted in 100% mortality. The prolonged survival reached by the combination therapy suggested that permeabilization on the fungal cells caused by PDT make C. albicans more susceptible to fluconazole. The combination of antifungal drug with PDT can be a promising approach in the treatment of mucosal candidiasis.
3.4. Nano-particulate drug delivery systems
Currently, the products obtained from nanotechnology provide interesting physiochemical characteristics enabling its applications in different health-care areas [208,209]. Nanoparticles are nanometer size particles that serve to transport drugs that are dissolved, entrapped, encapsulated, adsorbed, or chemically attached to carriers [210,211]. The use of nanoparticles can improve bioavailability of antifungal drugs because their small size facilitates reaching the vascular system and tissues [209,212]. Additionally, it has been reported that nanoparticles can contribute in overcoming microbial drug-resistance since antifungal compounds loaded in specific formulations avoid drug recognition by efflux pump proteins, keeping the drug inside fungal cells where it can be most effective [213].
Solid lipid nanoparticles (SLN) are a drug delivery system composed of biodegradable lipids prepared from oil-in-water nanoemulsions, which enable higher drug penetration, better contact with the cell target, and controlled liberation of the antifungal [213,214]. Moazeni et al. demonstrated that solid lipid nanoparticles loaded with fluconazole were capable of reducing the MIC values for fluconazole-resistant strains by 4, 8 and 4 folds, respectively for C. albicans, C. parapsilosis, and C. glabrata [213].
Nanostructured lipid carriers (NLC) present another transport option and are synthetized using solid lipids incorporated into liquid lipids, resulting in nanostructures with improved drug incorporation and time release properties. The liquid oil droplets in a solid matrix increase the loading potential in comparison to SLNs [209]. Jansook et al. compared amB-loaded in SLN and NLC with the commercially available amB colloidal dispersion (Fungizone®) [215]. Both nanoparticles formulations had similar in vitro antifungal activity reducing the MIC value by 4 folds compared to Fungizone®. Moreover, the lower hemolytic activity and lower aggregate formation capacity demonstrated that these nanoparticles could be less toxic than Fungizone® [215].
In addition to lipidic nanoparticles, polymeric systems have also been developed for drug delivery [216]. Polymeric nanoparticles are obtained from natural polymers (eg, chitosan, gelatin, and alginate) or synthetic polymers (polylactide, poly lactide-co-glycolide, copolymers, and polyacrylates), and are able to protect drugs from degradation or prevent side effects from toxicity [209,217]. El Rabey et al. used fungal chitosan extracted from Amylomyces rouxii to synthetize nanoparticles, which were later loaded with fluconazole [218]. Fluconazole was slowly released from the synthesis of chitosan nanoparticles in the first hours (3.4% after 3 h and 11.3% after 5 h) followed by a substantial increased after 12 h (94.8%), exhibiting significant antifungal activity against strains of C. albicans, C. parapsilosis, and C. glabrata resistant to fluconazole, itraconazole, and voriconazole [218].
Another promising nano-particulate drug delivery system involves metals that have intrinsic antimicrobial properties such as gold (Au), silver (Ag), and zinc (Zn) [219–222]. Metal-based nanoparticles show unique physicochemical properties that provide magnetic field-controlled drug delivery carriers [222]. Hussain et al. proved the enhanced antifungal efficacy of nystatin or fluconazole after conjugation with silver nanoparticles (NS) [221]. This conjugation increased the inhibition percentage of C. albicans in a dose-dependent manner reaching 90–100% for both nystatin-SN and fluconazole-SN formulations at 200 μg/mL. In addition, no cytotoxicity for human cell lines was observed, suggesting that metal nanoparticle formulations could be a safe and effective alternative to improve the efficacy of the current antifungal treatments [221,223].
4. Conclusion
Despite great advances in the medical field, morbidity and mortality rates associated with Candida spp. are still high. Treatments for these infections remain limited to only three major antifungal classes, polyenes, azoles, and echinocandins, which address a large variety of clinical manifestations (superficial, mucosal, systemic and invasive candidiasis), and thus must be deployed in different ways. These antifungal classes have been widely used over many years seeking to reach a counterbalance between their effectiveness and limitations related to pharmacokinetics and cytotoxicity. However, the exhaustive use of these limited antifungals leads to development of drug resistance mechanisms by fungal cells. Motivated by the rapid emergence of resistant strains, several researchers have introduced new antifungal agents and raised attractive therapeutic options for candidiasis, exploring structural modifications on polyenes, azoles and echinocandins, synthesis of different antifungal classes, drug repurposing, combination therapies, and drug delivery systems.
5. Expert opinion
The emergence of antifungal drug resistance is the biggest challenge when trying to treat candidiasis. The prevalence of antifungal resistance varies according to Candida species with greater prominence found among non-albicans Candida species. Recently, the most significant concerns about antifungal resistance has been the emergence of multi-drug resistant C. auris strains. C. auris was first described in Japan (2009) and rapidly spread across the 5 continents [224,225]. Most reports of C. auris infections involve critically ill hospitals patients that result in high mortality rates due to limited treatment options [224,226]. C. auris presents high level of antifungal resistance with reduced susceptibility to azoles, polyenes and even echinocandins. For example, Ostrowsky et al. found 99.7% of C. auris isolates resistant to fluconazole, 63.4% resistant to AmB, and 3.9% resistant to echinocandins from hospitalized patients in USA [114]. During the treatment of these patients with antifungal medications, some C. auris strains became resistant to the three antifungal classes. Troublingly, the progressive isolation of strains resistant to the all three antifungal classes shifts C. auris from multidrug resistance to pan-resistance status. The emergence of pan-resistant C. auris strain is an alarming signal for the necessity of new therapeutic options.
To improve the therapeutic approaches for candidiasis, many researchers have explored alternative means to deploy the available arsenal. Among them, recent studies have investigated different drug delivery systems for polyenes, azoles or echinocandins that provide localized targeted delivery that consequentially results in reductions antifungal dosages. The development of new liposomal, colloidal, and polymeric carriers represents a promising approach to increase the effectiveness and to counteract emerging resistance for all antifungal classes. In addition, new ways of approaching targets are coming into focus, such as the capacity of polyenes to extract sterols from the fungal cell membrane (sterol sponge model) and the ability of echinocandins to increase fungal cell wall chitin exposure. Approaches that remodel fungal cell surfaces suggest that these antifungals can have an important role provoking immune responses so cells are not so stealth in the body. Thus, bolstering antimicrobial chemotherapy with enhanced or altered immune responses.
The most significant impact anticipated in the field is always the introduction of new antifungal compounds, a situation that has been in an extended drought. There are however, a few antifungal compounds in clinical trials, yet the number pales in comparison to other disease indices. Most of candidate drugs include synthetic compounds with mechanisms of action focused on targeting the plasma membrane or cell wall. These compounds include structural modifications of the available antifungal classes (Coch-AmB, tetrazoles VT-1598, VT-1129, VT-1161 and rezafungin) or new antifungal classes (enfumafungin and manogepix). Although manogepix is a promising antifungal candidate for candidiasis, resistance mechanisms associated to efflux pump for this antifungal were already reported. To avoid cross-resistance mechanisms, antifungal agents with novel modes of action need to be urgently developed, as arylamidine T-2307 that targets the inhibition of fungal respiratory chain or N-[3-(allyloxy)-phenyl]-4-methoxybenzamide that acts on Candida spp. virulence mechanisms.
The current arsenal defines a limited number of fungal targets. A potential means of identifying new targets is to reveal what nature has already determined as effective targets. Natural compounds are an attractive source of antifungal agents with different mechanisms of action. To date, several extracts from plants and microorganisms with antifungal activity were identified; however, discovery, isolation, and interrogation can be a laborious, multifaceted processes. Thus, the challenge is best met by close collaborations between academia and the biotechnology sector.
Seeking to accelerate the introduction of new antifungal treatments into clinical practice, repurposing drugs presents an accelerated track. Drug repurposing has entailed screening a vast number of curated compounds from around the word. Most drugs with antifungal activities were identified among antibacterial, anti-parasitic, and anti-cancer classes. However, drug repurposing can present limitation as well. In some cases, the new use of a compound as an antifungal drug requires higher doses than the original defined use, which can result in adverse effects or toxicity. Therefore, pharmacokinetic aspects such as plasma protein binding, half-life, and tissue distribution need to be carefully evaluated for the new drug dosage and treatment regimen [179,181].
Therapy combinations are also an attractive means to multiply the drug arsenal through different amalgamations simultaneously affect multiple fungal targets, reaching synergistic effects that enhance the antifungal activities, reduce the therapeutic dosage, and impair the development of antifungal resistance. Diverse compounds have demonstrated synergistic action with polyenes, azoles, and echinocandins drugs. Photodynamic therapy offers an additional impact that can be deployed with antifungal therapies as a means to enhance antifungal action on superficial candidiasis. In additional, new nano-particulate drug delivery systems have emerged with advantages to traditional antifungal drug delivery systems such as liposomal, colloidal, and polymeric carriers. Currently, promising nano-particulate carriers primary involve lipidic, polymeric and metal nanoparticles.
In summary, scientists continue to stretch limited resources to their maximum potential but Candida spp. fights back by developing new means of resistance. With limited resources, scientists have strategically assembled pharmacotherapeutic options through new compounds, repurposing, combinations, and delivery methods that offer promising future perspectives for candidiasis.
Article highlights.
Enchochleated-Amphotericin B (Coch-AmB), tetrazoles, rezafungin, enfumafungin, manogepix and arylamidine are new promising antifungal drugs.
Drug repurposing using antibacterial, antiviral, and non-antimicrobial drugs can accelerate the introduction of new antifungal agents into clinical practice.
Drug combinations are attractive options that can enhance the antifungal activities and impair development of antifungal resistance.
Innovative drug delivery systems for antifungal compounds have been successfully developed using solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), polymeric nanoparticles, and metal-based nanoparticles.
The success of each antimicrobial agent brings strategic insights to the next phased approach in treating Candida spp. infections.
Funding:
The authors acknowledge support from the National Council for Scientific Development (CNPq): through the Researcher on Productivity PQ-1C award granted to JC Junqueira (award no. 306330/2018–0). L Scorzoni has also received a Coordination for the Improvement of Higher Education Personnel (CAPES) scholarship while E Mylonakis and BB Fuchs were supported by NIH grant P20 GM121344.
Footnotes
Declaration of Interest:
E Mylonakis participates in the Cidara_ ReSTORE multicenter trial and in the past has received grant support from Synexis, Sanofi, T2 Biosystems and Astellas. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Jampilek J How can we bolster the antifungal drug discovery pipeline? Future medicinal chemistry. 2016. August;8(12):1393–7.* An interesting review that describes the current panorama of fungal infection around the word and future perspectives about antifungal treatments.
- 2.Brown GD, Denning DW, Levitz SM. Tackling Human Fungal Infections. Science. 2012;336(6082):647–647. [DOI] [PubMed] [Google Scholar]
- 3.Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015. October;373(15):1445–56. [DOI] [PubMed] [Google Scholar]
- 4.Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013. February 15;4(2):119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sardi JC, Scorzoni L, Bernardi T, et al. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013. January;62(Pt 1):10–24. [DOI] [PubMed] [Google Scholar]
- 6.Chandra J, Mukherjee PK. Candida Biofilms: Development, Architecture, and Resistance. Microbiol Spectr. 2015. August;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulati M, Nobile CJ. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microbes Infect. 2016. May;18(5):310–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inigo M, Del Pozo JL. Fungal biofilms: From bench to bedside. Rev Esp Quimioter. 2018. September;31 Suppl 1:35–38.*This study discribes the clinical relevance of Candida spp. biofilms, antifungal resistance and therapeutic options.
- 9.Noverr MC, Fidel PL Jr. Questions remain regarding the presence of fungal species biofilm in women with vulvovaginal candidiasis. Am J Obstet Gynecol. 2019. August;221(2):169. [DOI] [PubMed] [Google Scholar]
- 10.Swidsinski A, Guschin A, Tang Q, et al. Vulvovaginal candidiasis: histologic lesions are primarily polymicrobial and invasive and do not contain biofilms. Am J Obstet Gynecol. 2019. January;220(1):91 e1–91 e8. [DOI] [PubMed] [Google Scholar]
- 11.Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, et al. Characterization of mucosal Candida albicans biofilms. PLoS One. 2009. November 24;4(11):e7967.** This is a guideline for the management of Candida spp. infections addressed to the treatments of common or specific clinical situations.
- 12.Harriott MM, Lilly EA, Rodriguez TE, et al. Candida albicans forms biofilms on the vaginal mucosa. Microbiology (Reading). 2010. December;156(Pt 12):3635–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arendrup MC, Sulim S, Holm A, et al. Diagnostic issues, clinical characteristics, and outcomes for patients with fungemia. J Clin Microbiol. 2011. September;49(9):3300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lortholary O, Renaudat C, Sitbon K, et al. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensive Care Med. 2014. September;40(9):1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009. March 1;48(5):503–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infec. 2019. October;25(10):1200–1212. [DOI] [PubMed] [Google Scholar]
- 17.Giacobbe DR, Maraolo AE, Simeon V, et al. Changes in the relative prevalence of candidaemia due to non-albicans Candida species in adult in-patients: A systematic review, meta-analysis and meta-regression. Mycoses. 2020. April;63(4):334–342. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhary A, Sharma C, Meis JF. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017. May;13(5):e1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denning DW, Bromley MJ. Infectious Disease. How to bolster the antifungal pipeline. Science. 2015. March;347(6229):1414–6. [DOI] [PubMed] [Google Scholar]
- 20.Perfect JR. The antifungal pipeline: a reality check. Nat Rev Drug Discov. 2017. September;16(9):603–616.** Interesting review about antifungal drug developments for different fungal pathogens, describing novel pathways and targets, agents in development and current challenges.
- 21.Tverdek FP, Kofteridis D, Kontoyiannis DP. Antifungal agents and liver toxicity: a complex interaction. Expert Rev Anti Infect Ther. 2016. August;14(8):765–76. [DOI] [PubMed] [Google Scholar]
- 22.Butts A, Reitler P, Nishimoto AT, et al. A Systematic Screen Reveals a Diverse Collection of Medications That Induce Antifungal Resistance in Candida Species. Antimicrob Agents Chemother. 2019. May;63(5).**This work presents a systematic screen aiming to idenfity antagonistic drug interactions between fluconazole and other approved medications.
- 23.Baginski M, Czub J. Amphotericin B and its new derivatives - mode of action. Curr Drug Metab. 2009. June;10(5):459–69. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues CF, Henriques M. Liposomal and Deoxycholate Amphotericin B Formulations: Effectiveness against Biofilm Infections of Candida spp. Pathogens. 2017. December 1;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odds FC, Brown AJ, Gow NA. Antifungal agents: mechanisms of action. Trends Microbiol. 2003. June;11(6):272–9. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad S, Joseph L, Parker JE, et al. ERG6 and ERG2 Are Major Targets Conferring Reduced Susceptibility to Amphotericin B in Clinical Candida glabrata Isolates in Kuwait. Antimicrob Agents Chemother. 2019. February;63(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohner K Antimicrobial mechanisms: a sponge against fungal infections. Nat Chem Biol. 2014. June;10(6):411–2. [DOI] [PubMed] [Google Scholar]
- 28.Anderson TM, Clay MC, Cioffi AG, et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol. 2014. May;10(5):400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kristanc L, Bozic B, Jokhadar SZ, et al. The pore-forming action of polyenes: From model membranes to living organisms. Biochim Biophys Acta Biomembr. 2019. February 1;1861(2):418–430. [DOI] [PubMed] [Google Scholar]
- 30.Torrado JJ, Espada R, Ballesteros MP, et al. Amphotericin B formulations and drug targeting. J Pharm Sci. 2008. July;97(7):2405–25. [DOI] [PubMed] [Google Scholar]
- 31.Alves D, Vaz AT, Grainha T, et al. Design of an Antifungal Surface Embedding Liposomal Amphotericin B Through a Mussel Adhesive-Inspired Coating Strategy. Front Chem. 2019;7:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caillet J, Bergès J, J. L. Theoretical study of the self-association of amphotericin B. Biochim Biophys Acta 1995;1240:179–195. [DOI] [PubMed] [Google Scholar]
- 33.Steimbach LM, Tonin FS, Virtuoso S, et al. Efficacy and safety of amphotericin B lipid-based formulations-A systematic review and meta-analysis. Mycoses. 2017. March;60(3):146–154.** Systematic review including randomized controlled trials aiming to compare the efficacy and side effects of AmB deoxycholate and lipid-based AmB formulations.
- 34.Gintjee TJ, Donnelley MA, Thompson GR 3rd. Aspiring Antifungals: Review of Current Antifungal Pipeline Developments. J Fungi (Basel). 2020. February 25;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azoulay E, Timsit JF, Lautrette A, et al. Weekly high-dose liposomal amphotericin B (L-AmB) in critically ill septic patients with multiple Candida colonization: The AmBiDex study. PLoS One. 2017;12(5):e0177093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young LY, Hull CM, Heitman J. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob Agents Chemother. 2003. September;47(9):2717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannan A, Asner SA, Trachsel E, et al. Comparative Genomics for the Elucidation of Multidrug Resistance in Candida lusitaniae. mBio. 2019. December 24;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol 2000. 2009. February;49:39–59. [DOI] [PubMed] [Google Scholar]
- 39.Lyu X, Zhao C, Yan ZM, et al. Efficacy of nystatin for the treatment of oral candidiasis: a systematic review and meta-analysis. Drug Des Devel Ther. 2016;10:1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quindos G, Gil-Alonso S, Marcos-Arias C, et al. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med Oral Patol Oral Cir Bucal. 2019. March 1;24(2):e172–e180.* Review of the recent studies on treatment of oral candidiasis, presenting well-designed figures and tables.
- 41.Fan S, Liu X, Wu C, et al. Vaginal nystatin versus oral fluconazole for the treatment for recurrent vulvovaginal candidiasis. Mycopathologia. 2015. February;179(1–2):95–101. [DOI] [PubMed] [Google Scholar]
- 42.Yu SY, Zhang L, Chen S, et al. Candida isolates causing refractory or recurrent oropharyngeal candidiasis in 11 hospitals in China. Infect Drug Resist. 2019;12:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheibler E, da Silva RM, Leite CE, et al. Stability and efficacy of combined nystatin and chlorhexidine against suspensions and biofilms of Candida albicans. Arch Oral Biol. 2018. May;89:70–76. [DOI] [PubMed] [Google Scholar]
- 44.Liu W, Guan X, Yu Z, et al. A Drug-drug Interaction Between Cyclosporine and Nystatin. Clin Ther. 2018. April;40(4):660–662. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Li T, Chen X, et al. Nystatin enhances the immune response against Candida albicans and protects the ultrastructure of the vaginal epithelium in a rat model of vulvovaginal candidiasis. BMC Microbiol. 2018. October 25;18(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim DH, Rhim BY, Eo SK, et al. Differential regulation of CC chemokine ligand 2 and CXCL8 by antifungal agent nystatin in macrophages. Biochem Biophys Res Commun. 2013. August 2;437(3):392–6. [DOI] [PubMed] [Google Scholar]
- 47.Maertens JA. History of the development of azole derivatives. Clin Microbiol Infect. 2004. March;10 Suppl 1:1–10. [DOI] [PubMed] [Google Scholar]
- 48.Lee H, Lee DG. Novel Approaches for Efficient Antifungal Drug Action. J Microbiol Biotechnol. 2018. November 28;28(11):1771–1781. [DOI] [PubMed] [Google Scholar]
- 49.Isham N, Ghannoum MA. Antifungal activity of miconazole against recent Candida strains. Mycoses. 2010. September;53(5):434–7. [DOI] [PubMed] [Google Scholar]
- 50.Gupta AK, Daigle D, Foley KA. Drug safety assessment of oral formulations of ketoconazole. Expert Opin Drug Saf. 2015. February;14(2):325–34. [DOI] [PubMed] [Google Scholar]
- 51.Vanden Bossche H, Koymans L. Cytochromes P450 in fungi. Mycoses. 1998;41 Suppl 1:32–8. [DOI] [PubMed] [Google Scholar]
- 52.Campoy S, Adrio JL. Antifungals. Biochem Pharmacol. 2017. June 1;133:86–96. [DOI] [PubMed] [Google Scholar]
- 53.Lei J, Xu J, Wang T. In vitro susceptibility of Candida spp. to fluconazole, itraconazole and voriconazole and the correlation between triazoles susceptibility: Results from a five-year study. J Mycol Med. 2018. June;28(2):310–313. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigues CF, Goncalves B, Rodrigues ME, et al. The Effectiveness of Voriconazole in Therapy of Candida glabrata’s Biofilms Oral Infections and Its Influence on the Matrix Composition and Gene Expression. Mycopathologia. 2017. August;182(7–8):653–664. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Yang Q, Chen L, et al. Cross-resistance between voriconazole and fluconazole for non-albicans Candida infection: a case-case-control study. Eur J Clin Microbiol Infect Dis. 2017. November;36(11):2117–2126. [DOI] [PubMed] [Google Scholar]
- 56.Thaler F, Bernard B, Tod M, et al. Fluconazole penetration in cerebral parenchyma in humans at steady state. Antimicrob Agents Chemother. 1995. May;39(5):1154–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016. February 15;62(4):e1–50.** This is a guideline for the management of Candida spp. infections. Addresses the treatment for common and particular patients or clinical situations.
- 58.Molgaard-Nielsen D, Svanstrom H, Melbye M, et al. Association Between Use of Oral Fluconazole During Pregnancy and Risk of Spontaneous Abortion and Stillbirth. JAMA. 2016. January 5;315(1):58–67. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Y, Bateman BT, Gray KJ, et al. Oral fluconazole use in the first trimester and risk of congenital malformations: population based cohort study. BMJ. 2020. May 20;369:m1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu DT, Peterson JF, Seger DL, et al. Frequency of potential azole drug-drug interactions and consequences of potential fluconazole drug interactions. Pharmacoepidemiol Drug Saf. 2005. November;14(11):755–67. [DOI] [PubMed] [Google Scholar]
- 61.Nakagita K, Wada K, Terada Y, et al. Effect of fluconazole on the pharmacokinetics of everolimus and tacrolimus in a heart transplant recipient: Case report. Int J Clin Pharmacol Ther. 2018. June;56(6):270–276. [DOI] [PubMed] [Google Scholar]
- 62.Michalets EL, Williams CR. Drug interactions with cisapride: clinical implications. Clin Pharmacokinet. 2000. July;39(1):49–75. [DOI] [PubMed] [Google Scholar]
- 63.Fang J, Huang B, Ding Z. Efficacy of antifungal drugs in the treatment of oral candidiasis: A Bayesian network meta-analysis. J Prosthet Dent. 2020. March 10.** Meta-analysis study that evaluates the efficacy and treatment for oral candidiasis.
- 64.Story K, Sobel R. Fluconazole Prophylaxis in Prevention of Symptomatic Candida Vaginitis. Curr Infect Dis Rep. 2020. January 21;22(1):2. [DOI] [PubMed] [Google Scholar]
- 65.Qin F, Wang Q, Zhang C, et al. Efficacy of antifungal drugs in the treatment of vulvovaginal candidiasis: a Bayesian network meta-analysis. Infect Drug Resist. 2018;11:1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denison HJ, Worswick J, Bond CM, et al. Oral versus intra-vaginal imidazole and triazole anti-fungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database Syst Rev. 2020. August 24;8:CD002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miranda-Cadena K, Marcos-Arias C, Mateo E, et al. Prevalence and antifungal susceptibility profiles of Candida glabrata, Candida parapsilosis and their close-related species in oral candidiasis. Arch Oral Biol. 2018. November;95:100–107. [DOI] [PubMed] [Google Scholar]
- 68.Saxon Lead Author G, Edwards A, Rautemaa-Richardson R, et al. British Association for Sexual Health and HIV national guideline for the management of vulvovaginal candidiasis (2019). Int J STD AIDS. 2020. October;31(12):1124–1144. [DOI] [PubMed] [Google Scholar]
- 69.Kale P, Johnson LB. Second-generation azole antifungal agents. Drugs Today (Barc). 2005. February;41(2):91–105. [DOI] [PubMed] [Google Scholar]
- 70.Lindsay J, Sandaradura I, Wong K, et al. Serum levels, safety and tolerability of new formulation SUBA-itraconazole prophylaxis in patients with haematological malignancy or undergoing allogeneic stem cell transplantation. J Antimicrob Chemother. 2017. December 1;72(12):3414–3419. [DOI] [PubMed] [Google Scholar]
- 71.Nield B, Larsen SR, van Hal SJ. Clinical experience with new formulation SUBA(R)-itraconazole for prophylaxis in patients undergoing stem cell transplantation or treatment for haematological malignancies. J Antimicrob Chemother. 2019. October 1;74(10):3049–3055. [DOI] [PubMed] [Google Scholar]
- 72.Heimann SM, Penack O, Heinz WJ, et al. Intravenous and tablet formulation of posaconazole in antifungal therapy and prophylaxis: A retrospective, non-interventional, multicenter analysis of hematological patients treated in tertiary-care hospitals. Int J Infect Dis. 2019. June;83:130–138.* This study analyzes the effectiveness of posaconazole for therapy or prophylaxis of candidiasis.
- 73.Sanchis M, Guarro J, Sutton DA, et al. Voriconazole and posaconazole therapy for experimental Candida lusitaniae infection. Diagn Microbiol Infect Dis. 2016. January;84(1):48–51. [DOI] [PubMed] [Google Scholar]
- 74.El-Ghammaz AMS, El-Zimaity M, Elafifi AM, et al. Effectiveness and Cost-Effectiveness of Prophylactic Voriconazole and Fluconazole Regarding Prevention of Post-hematopoietic Stem Cell Transplantation Invasive Fungal Infection and Its Related Death: A Single Center Experience. Indian J Hematol Blood Transfus. 2020. October;36(4):680–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Devanlay C, Tavernier-Tardy E, Bourmaud A, et al. Impact of fluconazole versus posaconazole prophylaxis on the incidence of fungal infections in patients receiving induction chemotherapy for acute myeloid leukemia. Biomed J. 2015. May-Jun;38(3):235–43. [DOI] [PubMed] [Google Scholar]
- 76.Yi WM, Schoeppler KE, Jaeger J, et al. Voriconazole and posaconazole therapeutic drug monitoring: a retrospective study. Ann Clin Microbiol Antimicrob. 2017. September 11;16(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pham AN, Bubalo JS, Lewis JS 2nd. Comparison of posaconazole serum concentrations from haematological cancer patients on posaconazole tablet and oral suspension for treatment and prevention of invasive fungal infections. Mycoses. 2016. April;59(4):226–233. [DOI] [PubMed] [Google Scholar]
- 78.Belling M, Kanate AS, Shillingburg A, et al. Evaluation of Serum Posaconazole Concentrations in Patients with Hematological Malignancies Receiving Posaconazole Suspension Compared to the Delayed-Release Tablet Formulation. Leuk Res Treatment. 2017;2017:3460892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hachem R, Assaf A, Numan Y, et al. Comparing the safety and efficacy of voriconazole versus posaconazole in the prevention of invasive fungal infections in high-risk patients with hematological malignancies. Int J Antimicrob Agents. 2017. September;50(3):384–388.* This study compares the safety and efficacy of voriconazole versus posaconazole.
- 80.Mizuno S, Itoh M, Matsuo H, et al. Case of ultraviolet B-mediated photosensitivity during the administration of voriconazole. J Dermatol. 2019. September;46(9):e327–e328. [DOI] [PubMed] [Google Scholar]
- 81.Tang H, Shi W, Song Y, et al. Voriconazole exposure and risk of cutaneous squamous cell carcinoma among lung or hematopoietic cell transplant patients: A systematic review and meta-analysis. J Am Acad Dermatol. 2019. February;80(2):500–507 e10. [DOI] [PubMed] [Google Scholar]
- 82.Hamandi B, Fegbeutel C, Silveira FP, et al. Voriconazole and squamous cell carcinoma after lung transplantation: A multicenter study. Am J Transplant. 2018. January;18(1):113–124. [DOI] [PubMed] [Google Scholar]
- 83.Kolaitis NA, Duffy E, Zhang A, et al. Voriconazole increases the risk for cutaneous squamous cell carcinoma after lung transplantation. Transpl Int. 2017. January;30(1):41–48. [DOI] [PubMed] [Google Scholar]
- 84.Parkes LO, Cheng MP, Sheppard DC. Visual Hallucinations Associated with High Posaconazole Concentrations in Serum. Antimicrob Agents Chemother. 2016. February;60(2):1170–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Allen D, Wilson D, Drew R, et al. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev Anti Infect Ther. 2015. June;13(6):787–98. [DOI] [PubMed] [Google Scholar]
- 86.Kullberg BJ, Viscoli C, Pappas PG, et al. Isavuconazole Versus Caspofungin in the Treatment of Candidemia and Other Invasive Candida Infections: The ACTIVE Trial. Clin Infect Dis. 2019. May 30;68(12):1981–1989.* This study describes a recent clincal trial comparing the use of isavuconazole with caspofungin for candidemia or invasive candidiasis.
- 87.Viljoen J, Azie N, Schmitt-Hoffmann AH, et al. A phase 2, randomized, double-blind, multicenter trial to evaluate the safety and efficacy of three dosing regimens of isavuconazole compared with fluconazole in patients with uncomplicated esophageal candidiasis. Antimicrob Agents Chemother. 2015. March;59(3):1671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peyton LR, Gallagher S, Hashemzadeh M. Triazole antifungals: a review. Drugs Today (Barc). 2015. December;51(12):705–18. [DOI] [PubMed] [Google Scholar]
- 89.Hendrickson JA, Hu C, Aitken SL, et al. Antifungal Resistance: a Concerning Trend for the Present and Future. Curr Infect Dis Rep. 2019. November 16;21(12):47.* Review of epidemiology and antifungal mechanisms of action and resistance of Aspergillus spp. and Candida spp. including C. auris.
- 90.Arendrup MC, Patterson TF. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J Infect Dis. 2017. August 15;216(suppl_3):S445–S451. [DOI] [PubMed] [Google Scholar]
- 91.Hadrich I, Ayadi A. Epidemiology of antifungal susceptibility: Review of literature. J Mycol Med. 2018. September;28(3):574–584. [DOI] [PubMed] [Google Scholar]
- 92.Farmakiotis D, Kontoyiannis DP. Epidemiology of antifungal resistance in human pathogenic yeasts: current viewpoint and practical recommendations for management. Int J Antimicrob Agents. 2017. September;50(3):318–324. [DOI] [PubMed] [Google Scholar]
- 93.Cannon RD, Lamping E, Holmes AR, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009. April;22(2):291–321, Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhattacharya S, Sae-Tia S, Fries BC. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics (Basel). 2020. June 9;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiang MJ, Liu JY, Ni PH, et al. Erg11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS Yeast Res. 2013. June;13(4):386–93. [DOI] [PubMed] [Google Scholar]
- 96.Flowers SA, Colon B, Whaley SG, et al. Contribution of clinically derived mutations in ERG11 to azole resistance in Candida albicans. Antimicrob Agents Chemother. 2015. January;59(1):450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li QQ, Tsai HF, Mandal A, et al. Sterol uptake and sterol biosynthesis act coordinately to mediate antifungal resistance in Candida glabrata under azole and hypoxic stress. Mol Med Rep. 2018. May;17(5):6585–6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prasad R, Rawal MK. Efflux pump proteins in antifungal resistance. Front Pharmacol. 2014;5:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rocha MFG, Bandeira SP, de Alencar LP, et al. Azole resistance in Candida albicans from animals: Highlights on efflux pump activity and gene overexpression. Mycoses. 2017. July;60(7):462–468. [DOI] [PubMed] [Google Scholar]
- 100.Farahyar S, Zaini F, Kordbacheh P, et al. Expression of Efflux Pumps and Fatty Acid Activator One Genes in Azole Resistant Candida Glabrata Isolated From Immunocompromised Patients. Acta Med Iran. 2016. July;54(7):458–64. [PubMed] [Google Scholar]
- 101.de Souza MC, Santos AG, Reis AM. Adverse Drug Reactions in Patients Receiving Systemic Antifungal Therapy at a High-Complexity Hospital. J Clin Pharmacol. 2016. December;56(12):1507–1515. [DOI] [PubMed] [Google Scholar]
- 102.Rybak JM, Doorley LA, Nishimoto AT, et al. Abrogation of Triazole Resistance upon Deletion of CDR1 in a Clinical Isolate of Candida auris. Antimicrob Agents Chemother. 2019. April;63(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chowdhary A, Prakash A, Sharma C, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother. 2018. April 1;73(4):891–899. [DOI] [PubMed] [Google Scholar]
- 104.Denning DW. Echinocandins: a new class of antifungal. J Antimicrob Chemother. 2002. June;49(6):889–91. [DOI] [PubMed] [Google Scholar]
- 105.. Bassetti M, Righi E, Montravers P, et al. What has changed in the treatment of invasive candidiasis? A look at the past 10 years and ahead. J Antimicrob Chemother. 2018. January 1;73(suppl_1):i14–i25.** Interesting review of antifungal drugs, Candida spp. diagnostic, antifungal suceptibility, and treatment limitation. Describes recent treatment guidelines from different contries.
- 106.Denning DW. Echinocandin antifungal drugs. Lancet. 2003. October 4;362(9390):1142–51. [DOI] [PubMed] [Google Scholar]
- 107.Eschenauer G, Depestel DD, Carver PL. Comparison of echinocandin antifungals. Ther Clin Risk Manag. 2007. March;3(1):71–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chapman B, Slavin M, Marriott D, et al. Changing epidemiology of candidaemia in Australia. J Antimicrob Chemother. 2017. April 1;72(4):1103–1108. [DOI] [PubMed] [Google Scholar]
- 109.Kritikos A, Neofytos D, Khanna N, et al. Accuracy of Sensititre YeastOne echinocandins epidemiological cut-off values for identification of FKS mutant Candida albicans and Candida glabrata: a ten year national survey of the Fungal Infection Network of Switzerland (FUNGINOS). Clin Microbiol Infect. 2018. November;24(11):1214 e1–1214 e4. [DOI] [PubMed] [Google Scholar]
- 110.Taj-Aldeen SJ, Salah H, Perez WB, et al. Molecular Analysis of Resistance and Detection of Non-Wild-Type Strains Using Etest Epidemiological Cutoff Values for Amphotericin B and Echinocandins for Bloodstream Candida Infections from a Tertiary Hospital in Qatar. Antimicrob Agents Chemother. 2018. September;62(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rivero-Menendez O, Navarro-Rodriguez P, Bernal-Martinez L, et al. Clinical and Laboratory Development of Echinocandin Resistance in Candida glabrata: Molecular Characterization. Front Microbiol. 2019;10:1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Walker LA, Gow NA, Munro CA. Elevated chitin content reduces the susceptibility of Candida species to caspofungin. Antimicrob Agents Chemother. 2013. January;57(1):146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Walker LA, Munro CA. Caspofungin Induced Cell Wall Changes of Candida Species Influences Macrophage Interactions. Front Cell Infect Microbiol. 2020;10:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ostrowsky B, Greenko J, Adams E, et al. Candida auris Isolates Resistant to Three Classes of Antifungal Medications - New York, 2019. MMWR Morb Mortal Wkly Rep. 2020. January 10;69(1):6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin Infect Dis. 2017. January 15;64(2):134–140.* This study aims to characterize C. auris isolates from 3 continents regarding antifungal suceptibility and genetic analysis.
- 116.Garcia-Effron G, Katiyar SK, Park S, et al. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Agents Chemother. 2008. July;52(7):2305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Diaz-Garcia J, Alcala L, Martin-Rabadan P, et al. Susceptibility of uncommon Candida species to systemic antifungals by the EUCAST methodology. Med Mycol. 2019. November 29. [DOI] [PubMed]
- 118.Spettel K, Barousch W, Makristathis A, et al. Analysis of antifungal resistance genes in Candida albicans and Candida glabrata using next generation sequencing. PLoS One. 2019;14(1):e0210397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kordalewska M, Lee A, Park S, et al. Understanding Echinocandin Resistance in the Emerging Pathogen Candida auris. Antimicrob Agents Chemother. 2018. June;62(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rodrigues CF, Correia A, Vilanova M, et al. Inflammatory Cell Recruitment in Candida glabrata Biofilm Cell-Infected Mice Receiving Antifungal Chemotherapy. J Clin Med. 2019. January 26;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee KK, Maccallum DM, Jacobsen MD, et al. Elevated cell wall chitin in Candida albicans confers echinocandin resistance in vivo. Antimicrob Agents Chemother. 2012. January;56(1):208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reboli AC, Rotstein C, Pappas PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007. June 14;356(24):2472–82. [DOI] [PubMed] [Google Scholar]
- 123.Villaescusa T, Vazquez L, Bergua JM, et al. Micafungin as antifungal prophylaxis in non-transplanted haemotological patients. Rev Esp Quimioter. 2020. February;33(1):44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen Q, Lin MH, Chen ML, et al. Efficacy and safety of micafungin for invasive candida infections: a meta-analysis of randomized controlled trials. Chin Med J (Engl). 2012. January;125(2):345–51. [PubMed] [Google Scholar]
- 125.Fujimoto K, Takemoto K. Efficacy of liposomal amphotericin B against four species of Candida biofilms in an experimental mouse model of intravascular catheter infection. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2018. December;24(12):958–964. *This study demonstrated that liposomal amphotericin B is capable of eradicating Candida biofilms, providing an interesting therapeutic option for catheter-related Candida biofilm infections.
- 126.Basas J, Palau M, Gomis X, et al. Efficacy of liposomal amphotericin B and anidulafungin using an antifungal lock technique (ALT) for catheter-related Candida albicans and Candida glabrata infections in an experimental model. PLoS One. 2019;14(2):e0212426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Swaminathan S, Kamat S, Pinto NA. Echinocandins: Their role in the management of Candida biofilms. Indian J Med Microbiol. 2018. Jan-Mar;36(1):87–92. [DOI] [PubMed] [Google Scholar]
- 128.Alves IA, Savi FM, de Vasconcelos CBJ, et al. The Patenting and Technological Trends in Candidiasis Treatment: A Systematic Review (2014–2018). Curr Top Med Chem. 2019;19(28):2629–2639. [DOI] [PubMed] [Google Scholar]
- 129.Van Daele R, Spriet I, Wauters J, et al. Antifungal drugs: What brings the future? Med Mycol. 2019. June 1;57(Supplement_3):S328–S343. [DOI] [PubMed] [Google Scholar]
- 130.Li D, She X, Calderone R. The antifungal pipeline: the need is established. Are there new compounds? FEMS Yeast Res. 2020. June 1;20(4). [DOI] [PubMed] [Google Scholar]
- 131.PD UMASGGRC. Antifungal Peptides as Therapeutic Agents. Frontiers in Cellular and Infection Microbiology. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Santangelo R, Paderu P, Delmas G, et al. Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob Agents Chemother. 2000. September;44(9):2356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shrestha SK, Garzan A, Garneau-Tsodikova S. Novel alkylated azoles as potent antifungals. Eur J Med Chem. 2017. June 16;133:309–318. [DOI] [PubMed] [Google Scholar]
- 134.Xie F, Ni T, Zhao J, et al. Design, synthesis, and in vitro evaluation of novel antifungal triazoles. Bioorg Med Chem Lett. 2017. May 15;27(10):2171–2173. [DOI] [PubMed] [Google Scholar]
- 135.Hoekstra WJ, Garvey EP, Moore WR, et al. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg Med Chem Lett. 2014. August 1;24(15):3455–8. [DOI] [PubMed] [Google Scholar]
- 136.Warrilow AG, Hull CM, Parker JE, et al. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother. 2014. December;58(12):7121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rauseo AM, Coler-Reilly A, Larson L, et al. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect Dis. 2020. February;7(2):ofaa016.** This review describes new antifungal therapies in clinical development, containing interesting figures and tables.
- 138.Wiederhold NP, Lockhart SR, Najvar LK, et al. The Fungal Cyp51-Specific Inhibitor VT-1598 Demonstrates In Vitro and In Vivo Activity against Candida auris. Antimicrob Agents Chemother. 2019. March;63(3).** This study evaluated in vitro antifungal activity of VT-1598 and in vivo efficacy in neutropenic mice infected by C. auris.
- 139.Silva LN, de Mello TP, de Souza Ramos L, et al. New and Promising Chemotherapeutics for Emerging Infections Involving Drug-resistant Non-albicans Candida Species. Curr Top Med Chem. 2019;19(28):2527–2553. [DOI] [PubMed] [Google Scholar]
- 140.Garvey EP, Hoekstra WJ, Schotzinger RJ, et al. Efficacy of the clinical agent VT-1161 against fluconazole-sensitive and -resistant Candida albicans in a murine model of vaginal candidiasis. Antimicrob Agents Chemother. 2015. September;59(9):5567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sofjan AK, Mitchell A, Shah DN, et al. Rezafungin (CD101), a next-generation echinocandin: A systematic literature review and assessment of possible place in therapy. J Glob Antimicrob Resist. 2018. September;14:58–64.*Systematic review of Rezafungin (CD101) that demontrates its safety, long half-life and potential for the treatment of patients that require the echinocandis treatment.
- 142.Hager CL, Larkin EL, Long LA, et al. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother. 2018. August 1;73(8):2085–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee A, Prideaux B, Zimmerman M, et al. Penetration of Ibrexafungerp (formerly SCY-078) at the site of infection in an Intra-abdominal candidiasis mouse model. Antimicrob Agents Chemother. 2019. December 23. [DOI] [PMC free article] [PubMed]
- 144.Kuhnert E, Li Y, Lan N, et al. Enfumafungin synthase represents a novel lineage of fungal triterpene cyclases. Environ Microbiol. 2018. September;20(9):3325–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 Is Fungicidal against Candida Species in Time-Kill Studies. Antimicrob Agents Chemother. 2017. March;61(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Larkin E, Hager C, Chandra J, et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob Agents Chemother. 2017. May;61(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pfaller MA, Messer SA, Rhomberg PR, et al. Differential Activity of the Oral Glucan Synthase Inhibitor SCY-078 against Wild-Type and Echinocandin-Resistant Strains of Candida Species. Antimicrob Agents Chemother. 2017. August;61(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wring SA, Randolph R, Park S, et al. Preclinical Pharmacokinetics and Pharmacodynamic Target of SCY-078, a First-in-Class Orally Active Antifungal Glucan Synthesis Inhibitor, in Murine Models of Disseminated Candidiasis. Antimicrob Agents Chemother. 2017. April;61(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Arendrup MC, Jorgensen KM, Hare RK, et al. EUCAST in vitro activity of Ibrexafungerp (SCY-078) against C. auris isolates; comparison with activity against C. albicans and C. glabrata and with that of six comparators. Antimicrob Agents Chemother. 2019. December 16. [DOI] [PMC free article] [PubMed]
- 150.Davis MR, Donnelley MA, Thompson GR. Ibrexafungerp: A novel oral glucan synthase inhibitor. Med Mycol. 2020. July 1;58(5):579–592. [DOI] [PubMed] [Google Scholar]
- 151.Azie N, Angulo D, Dehn B, et al. Oral Ibrexafungerp: an investigational agent for the treatment of vulvovaginal candidiasis. Expert Opin Investig Drugs. 2020. September;29(9):893–900.**This study describes the benefits of the glucan synthase inhibitor, Ibrexafungerp, for vulvovaginal candidiasis.
- 152.Kapoor M, Moloney M, Soltow QA, et al. Evaluation of Resistance Development to the Gwt1 Inhibitor Manogepix (APX001A) in Candida Species. Antimicrob Agents Chemother. 2019. December 20;64(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao M, Lepak AJ, VanScoy B, et al. In Vivo Pharmacokinetics and Pharmacodynamics of APX001 against Candida spp. in a Neutropenic Disseminated Candidiasis Mouse Model. Antimicrob Agents Chemother. 2018. April;62(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Arendrup MC, Jorgensen KM. Manogepix (APX001A) displays potent in vitro activity against human pathogenic yeast, but with an unexpected correlation to fluconazole MICs. Antimicrob Agents Chemother. 2020. May 4. [DOI] [PMC free article] [PubMed]
- 155.Wiederhold NP, Najvar LK, Shaw KJ, et al. Efficacy of Delayed Therapy with Fosmanogepix (APX001) in a Murine Model of Candida auris Invasive Candidiasis. Antimicrob Agents Chemother. 2019. November;63(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hager CL, Larkin EL, Long L, et al. In Vitro and In Vivo Evaluation of the Antifungal Activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother. 2018. March;62(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liston SD, Whitesell L, Kapoor M, et al. Enhanced Efflux Pump Expression in Candida Mutants Results in Decreased Manogepix Susceptibility. Antimicrob Agents Chemother. 2020. April 21;64(5).*This study reports the efflux-mediated mechanisms associated with decreased manogepix susceptibility.
- 158.Wiederhold NP, Najvar LK, Jaramillo R, et al. The Novel Arylamidine T-2307 Demonstrates In vitro and In vivo Activity Against Candida auris. Antimicrob Agents Chemother. 2019. December 16.**This study demonstrates the potental of arylamidine T-2307 against C. auris in a mouse model.
- 159.Yamashita K, Miyazaki T, Fukuda Y, et al. The Novel Arylamidine T-2307 Selectively Disrupts Yeast Mitochondrial Function by Inhibiting Respiratory Chain Complexes. Antimicrob Agents Chemother. 2019. August;63(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mitsuyama J, Nomura N, Hashimoto K, et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother. 2008. April;52(4):1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Singla RK, Dubey AK. Molecules and Metabolites from Natural Products as Inhibitors of Biofilm in Candida spp. pathogens. Curr Top Med Chem. 2019;19(28):2567–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jackson N, Czaplewski L, Piddock LJV. Discovery and development of new antibacterial drugs: learning from experience? J Antimicrob Chemother. 2018. June 1;73(6):1452–1459. [DOI] [PubMed] [Google Scholar]
- 163.Futuro DO, Ferreira PG, Nicoletti CD, et al. The Antifungal Activity of Naphthoquinones: An Integrative Review. An Acad Bras Cienc. 2018;90(1 Suppl 2):1187–1214. [DOI] [PubMed] [Google Scholar]
- 164.Zida A, Bamba S, Yacouba A, et al. Anti-Candida albicans natural products, sources of new antifungal drugs: A review. J Mycol Med. 2017. March;27(1):1–19. [DOI] [PubMed] [Google Scholar]
- 165.Barbosa JO, Rossoni RD, Vilela SF, et al. Streptococcus mutans Can Modulate Biofilm Formation and Attenuate the Virulence of Candida albicans. PLoS One. 2016;11(3):e0150457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rossoni RD, de Barros PP, de Alvarenga JA, et al. Antifungal activity of clinical Lactobacillus strains against Candida albicans biofilms: identification of potential probiotic candidates to prevent oral candidiasis. Biofouling. 2018. February;34(2):212–225. [DOI] [PubMed] [Google Scholar]
- 167.Dos Santos JD, Fugisaki LRO, Medina RP, et al. Streptococcus mutans Secreted Products Inhibit Candida albicans Induced Oral Candidiasis. Front Microbiol. 2020;11:1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bonvicini F, Gentilomi GA, Bressan F, et al. Functionalization of the Chalcone Scaffold for the Discovery of Novel Lead Compounds Targeting Fungal Infections. Molecules. 2019. January 21;24(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Romo JA, Pierce CG, Esqueda M, et al. In Vitro Characterization of a Biaryl Amide Anti-virulence Compound Targeting Candida albicans Filamentation and Biofilm Formation. Front Cell Infect Microbiol. 2018;8:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cui J, Ren B, Tong Y, et al. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence. 2015;6(4):362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gizinska M, Staniszewska M, Ochal Z. Novel Sulfones with Antifungal Properties: Antifungal Activities and Interactions with Candida spp. Virulence Factors. Mini Rev Med Chem. 2019;19(1):12–21. [DOI] [PubMed] [Google Scholar]
- 172.Grainha TRR, Jorge P, Perez-Perez M, et al. Exploring anti-quorum sensing and anti-virulence based strategies to fight Candida albicans infections: an in silico approach. FEMS Yeast Res. 2018. May 1;18(3). [DOI] [PubMed] [Google Scholar]
- 173.Romo JA, Pierce CG, Chaturvedi AK, et al. Development of Anti-Virulence Approaches for Candidiasis via a Novel Series of Small-Molecule Inhibitors of Candida albicans Filamentation. mBio. 2017. December 5;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vila T, Romo JA, Pierce CG, et al. Targeting Candida albicans filamentation for antifungal drug development. Virulence. 2017. February 17;8(2):150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ahmad Khan MS, Alshehrei F, Al-Ghamdi SB, et al. Virulence and biofilms as promising targets in developing antipathogenic drugs against candidiasis. Future Sci OA. 2020. February 3;6(2):FSO440.*This review highlights the fungal virulence factors as approach for the identification of promissing compounds. Contains informative and didatic tables.
- 176.Prasath KG, Tharani H, Kumar MS, et al. Palmitic Acid Inhibits the Virulence Factors of Candida tropicalis: Biofilms, Cell Surface Hydrophobicity, Ergosterol Biosynthesis, and Enzymatic Activity. Front Microbiol. 2020;11:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004. August;3(8):673–83. [DOI] [PubMed] [Google Scholar]
- 178.Langedijk J, Mantel-Teeuwisse AK, Slijkerman DS, et al. Drug repositioning and repurposing: terminology and definitions in literature. Drug Discov Today. 2015. August;20(8):1027–34. [DOI] [PubMed] [Google Scholar]
- 179.Farha MA, Brown ED. Drug repurposing for antimicrobial discovery. Nat Microbiol. 2019. April;4(4):565–577.**This review focus on the drug repurposing as approach for the antimicrobial discovery describing its benefits and challenges.
- 180.Butts A, Krysan DJ. Antifungal drug discovery: something old and something new. PLoS Pathog. 2012. September;8(9):e1002870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oprea TI, Bauman JE, Bologa CG, et al. Drug Repurposing from an Academic Perspective. Drug Discov Today Ther Strateg. 2011. Winter;8(3–4):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jadhav A, Bansode B, Phule D, et al. The antibacterial agent, moxifloxacin inhibits virulence factors of Candida albicans through multitargeting. World J Microbiol Biotechnol. 2017. May;33(5):96. [DOI] [PubMed] [Google Scholar]
- 183.Eldesouky HE, Mayhoub A, Hazbun TR, et al. Reversal of Azole Resistance in Candida albicans by Sulfa Antibacterial Drugs. Antimicrob Agents Chemother. 2018. March;62(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tsang CS, Hong I. HIV protease inhibitors differentially inhibit adhesion of Candida albicans to acrylic surfaces. Mycoses. 2010. November;53(6):488–94. [DOI] [PubMed] [Google Scholar]
- 185.Fuchs BB, RajaMuthiah R, Souza AC, et al. Inhibition of bacterial and fungal pathogens by the orphaned drug auranofin. Future Med Chem. 2016;8(2):117–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jung EH, Meyers DJ, Bosch J, et al. Novel Antifungal Compounds Discovered in Medicines for Malaria Venture’s Malaria Box. mSphere. 2018. Mar-Apr;3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Silva MP, Saraiva L, Pinto M, et al. Boronic Acids and Their Derivatives in Medicinal Chemistry: Synthesis and Biological Applications. Molecules. 2020. September 21;25(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rossoni RD, de Barros PP, Lopes L, et al. Effects of surface pre-reacted glass-ionomer (S-PRG) eluate on Candida spp.: antifungal activity, anti-biofilm properties, and protective effects on Galleria mellonella against C. albicans infection. Biofouling. 2019. November 11:1–10. [DOI] [PubMed] [Google Scholar]
- 189.Yousfi H, Cassagne C, Ranque S, et al. Repurposing of Ribavirin as an Adjunct Therapy against Invasive Candida Strains in an In Vitro Study. Antimicrob Agents Chemother. 2019. October;63(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cassone A, De Bernardis F, Torosantucci A, et al. In vitro and in vivo anticandidal activity of human immunodeficiency virus protease inhibitors. J Infect Dis. 1999. August;180(2):448–53. [DOI] [PubMed] [Google Scholar]
- 191.Calugi C, Guarna A, Trabocchi A. Insight into the structural similarity between HIV protease and secreted aspartic protease-2 and binding mode analysis of HIV-Candida albicans inhibitors. J Enzyme Inhib Med Chem. 2013. October;28(5):936–43. [DOI] [PubMed] [Google Scholar]
- 192.Hoegl L, Thoma-Greber E, Rocken M, et al. HIV protease inhibitors influence the prevalence of oral candidosis in HIV-infected patients: a 2-year study. Mycoses. 1998. Sep-Oct;41(7–8):321–5. [DOI] [PubMed] [Google Scholar]
- 193.Kean WF, Hart L, Buchanan WW. Auranofin. Br J Rheumatol. 1997. May;36(5):560–72. [DOI] [PubMed] [Google Scholar]
- 194.She P, Liu Y, Wang Y, et al. Antibiofilm efficacy of the gold compound auranofin on dual species biofilms of Staphylococcus aureus and Candida sp. J Appl Microbiol. 2020. January;128(1):88–101. [DOI] [PubMed] [Google Scholar]
- 195.Thangamani S, Maland M, Mohammad H, et al. Repurposing Approach Identifies Auranofin with Broad Spectrum Antifungal Activity That Targets Mia40-Erv1 Pathway. Front Cell Infect Microbiol. 2017;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sun N, Li D, Zhang Y, et al. Repurposing an inhibitor of ribosomal biogenesis with broad anti-fungal activity. Sci Rep. 2017. December 5;7(1):17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gowri M, Jayashree B, Jeyakanthan J, et al. Sertraline as a promising antifungal agent: inhibition of growth and biofilm of Candida auris with special focus on the mechanism of action in vitro. J Appl Microbiol. 2020. February;128(2):426–437. [DOI] [PubMed] [Google Scholar]
- 198.Li Y, Yang J, Li X, et al. The effect of Ginkgolide B combined with fluconazole against drug-resistant Candida albicans based on common resistance mechanisms. Int J Antimicrob Agents. 2020. May 23:106030. [DOI] [PubMed] [Google Scholar]
- 199.Chen X, Ren B, Chen M, et al. ASDCD: antifungal synergistic drug combination database. PLoS One. 2014;9(1):e86499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee Y, Puumala E, Robbins N, et al. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem Rev. 2020. May 22. [DOI] [PMC free article] [PubMed]
- 201.Butts A, Palmer GE, Rogers PD. Antifungal adjuvants: Preserving and extending the antifungal arsenal. Virulence. 2017. February 17;8(2):198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rodrigues CF, Alves DF, Henriques M. Combination of Posaconazole and Amphotericin B in the Treatment of Candida glabrata Biofilms. Microorganisms. 2018. December 4;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Reginatto P, Bergamo VZ, Berlitz SJ, et al. Rational selection of antifungal drugs to propose a new formulation strategy to control Candida biofilm formation on venous catheters. Braz J Microbiol. 2020. February 19. [DOI] [PMC free article] [PubMed]
- 204.Ahuja T, Fong K, Louie E. Combination antifungal therapy for treatment of Candida parapsilosis prosthetic valve endocarditis and utility of T2Candida Panel(R): A case series. IDCases. 2019;15:e00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Alvarez C, Andes DR, Kang JY, et al. Antifungal Efficacy of an Intravenous Formulation Containing Monomeric Amphotericin B, 5-Fluorocytosine, and Saline for Sodium Supplementation. Pharm Res. 2017. May;34(5):1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fernandes KE, Weeks K, Carter DA. Lactoferrin is broadly active against yeasts and highly synergistic with amphotericin B. Antimicrob Agents Chemother. 2020. February 24. [DOI] [PMC free article] [PubMed]
- 207.Chibebe Junior J, Sabino CP, Tan X, et al. Selective photoinactivation of Candida albicans in the non-vertebrate host infection model Galleria mellonella. BMC Microbiol. 2013. October 1;13:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dal Lago V, França de Oliveira L, de Almeida Gonçalves K, et al. Size-selective silver nanoparticles: future of biomedical devices with enhanced bactericidal properties [10.1039/C1JM12297E]. Journal of Materials Chemistry. 2011;21(33):12267–12273. [Google Scholar]
- 209.Prasad M, Lambe UP, Brar B, et al. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed Pharmacother. 2018. January;97:1521–1537. [DOI] [PubMed] [Google Scholar]
- 210.Zajaczkowski T, Wojcicki J, Lawczynski L. [Effect of polyenophosphatidylcholine on the antiparacoagulating activity of the aorta and fibrin monomer complexes in chronic experimental alcoholism]. Pol Tyg Lek. 1975. February 10;30(6):237–8. [PubMed] [Google Scholar]
- 211.Walsh TJ, Viviani MA, Arathoon E, et al. New targets and delivery systems for antifungal therapy. Med Mycol. 2000;38 Suppl 1:335–47. [PubMed] [Google Scholar]
- 212.Davis SS. Biomedical applications of nanotechnology--implications for drug targeting and gene therapy. Trends Biotechnol. 1997. June;15(6):217–24. [DOI] [PubMed] [Google Scholar]
- 213.Moazeni M, Kelidari HR, Saeedi M, et al. Time to overcome fluconazole resistant Candida isolates: Solid lipid nanoparticles as a novel antifungal drug delivery system. Colloids Surf B Biointerfaces. 2016. June 1;142:400–407. [DOI] [PubMed] [Google Scholar]
- 214.Cevc G Lipid vesicles and other colloids as drug carriers on the skin. Adv Drug Deliv Rev. 2004. March 27;56(5):675–711. [DOI] [PubMed] [Google Scholar]
- 215.Jansook P, Pichayakorn W, Ritthidej GC. Amphotericin B-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): effect of drug loading and biopharmaceutical characterizations. Drug Dev Ind Pharm. 2018. October;44(10):1693–1700. [DOI] [PubMed] [Google Scholar]
- 216.Sawant B, Khan T. Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. Biomed Pharmacother. 2017. December;96:1478–1490. [DOI] [PubMed] [Google Scholar]
- 217.Zhang Z, Tsai PC, Ramezanli T, et al. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013. May-Jun;5(3):205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.El Rabey HA, Almutairi FM, Alalawy AI, et al. Augmented control of drug-resistant Candida spp. via fluconazole loading into fungal chitosan nanoparticles. Int J Biol Macromol. 2019. September 6;141:511–516. [DOI] [PubMed] [Google Scholar]
- 219.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009. Jan-Feb;27(1):76–83. [DOI] [PubMed] [Google Scholar]
- 220.Zhang H, Ji Z, Xia T, et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS nano. 2012. May 22;6(5):4349–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hussain MA, Ahmed D, Anwar A, et al. Combination Therapy of Clinically Approved Antifungal Drugs Is Enhanced by Conjugation with Silver Nanoparticles. Int Microbiol. 2019. June;22(2):239–246.* This study demostrates the enhanced antifungal efficacy of nystatin or fluconazole when in conjugation with silver nanoparticles.
- 222.Wnorowska U, Fiedoruk K, Piktel E, et al. Nanoantibiotics containing membrane-active human cathelicidin LL-37 or synthetic ceragenins attached to the surface of magnetic nanoparticles as novel and innovative therapeutic tools: current status and potential future applications. J Nanobiotechnology. 2020. January 2;18(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ahmad A, Wei Y, Syed F, et al. Amphotericin B-conjugated biogenic silver nanoparticles as an innovative strategy for fungal infections. Microb Pathog. 2016. August;99:271–281. [DOI] [PubMed] [Google Scholar]
- 224.Ademe M, Girma F. Candida auris: From Multidrug Resistance to Pan-Resistant Strains. Infect Drug Resist. 2020;13:1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Iguchi S, Itakura Y, Yoshida A, et al. Candida auris: A pathogen difficult to identify, treat, and eradicate and its characteristics in Japanese strains. J Infect Chemother. 2019. October;25(10):743–749. [DOI] [PubMed] [Google Scholar]
- 226.Alfouzan W, Dhar R, Albarrag A, et al. The emerging pathogen Candida auris: A focus on the Middle-Eastern countries. J Infect Public Health. 2019. Jul-Aug;12(4):451–459. [DOI] [PubMed] [Google Scholar]
- 227.Theill L, Dudiuk C, Morano S, et al. Prevalence and antifungal susceptibility of Candida albicans and its related species Candida dubliniensis and Candida africana isolated from vulvovaginal samples in a hospital of Argentina. Rev Argent Microbiol. 2016. Jan-Mar;48(1):43–9. [DOI] [PubMed] [Google Scholar]
- 228.Junqueira JC, Vilela SFG, Rossoni RD, et al. Oral colonization by yeasts in HIV-positive patients in Brazil. Revista do Instituto de Medicina Tropical de São Paulo. 2012;54(1):17–24. [DOI] [PubMed] [Google Scholar]
- 229.Santos ER, Dal Forno CF, Hernandez MG, et al. Susceptibility of Candida spp. isolated from blood cultures as evaluated using the M27-A3 and new M27-S4 approved breakpoints. Rev Inst Med Trop Sao Paulo. 2014. Nov-Dec;56(6):477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fuller J, Dingle TC, Bull A, et al. Species distribution and antifungal susceptibility of invasive Candida isolates from Canadian hospitals: results of the CANWARD 2011–16 study. J Antimicrob Chemother. 2019. August 1;74(Suppl 4):iv48–iv54. [DOI] [PubMed] [Google Scholar]
- 231.Zeng ZR, Tian G, Ding YH, et al. Surveillance study of the prevalence, species distribution, antifungal susceptibility, risk factors and mortality of invasive candidiasis in a tertiary teaching hospital in Southwest China. BMC Infect Dis. 2019. November 7;19(1):939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Waikhom SD, Afeke I, Kwawu GS, et al. Prevalence of vulvovaginal candidiasis among pregnant women in the Ho municipality, Ghana: species identification and antifungal susceptibility of Candida isolates. BMC Pregnancy Childbirth. 2020. May 6;20(1):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jahanshiri Z, Manifar S, Moosa H, et al. Oropharyngeal candidiasis in head and neck cancer patients in Iran: Species identification, antifungal susceptibility and pathogenic characterization. J Mycol Med. 2018. June;28(2):361–366. [DOI] [PubMed] [Google Scholar]
- 234.Ryan P, Motherway C, Powell J, et al. Candidaemia in an Irish intensive care unit setting between 2004 and 2018 reflects increased incidence of Candida glabrata. J Hosp Infect. 2019. July;102(3):347–350. [DOI] [PubMed] [Google Scholar]
- 235.Prigitano A, Cavanna C, Passera M, et al. Evolution of fungemia in an Italian region. J Mycol Med. 2020. April;30(1):100906. [DOI] [PubMed] [Google Scholar]
- 236.Bustamante B, Martins MA, Bonfietti LX, et al. Species distribution and antifungal susceptibility profile of Candida isolates from bloodstream infections in Lima, Peru. J Med Microbiol. 2014. June;63(Pt 6):855–860. [DOI] [PubMed] [Google Scholar]
- 237.Rodriguez L, Bustamante B, Huaroto L, et al. A multi-centric Study of Candida bloodstream infection in Lima-Callao, Peru: Species distribution, antifungal resistance and clinical outcomes. PLoS One. 2017;12(4):e0175172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Alkharashi N, Aljohani S, Layqah L, et al. Candida Bloodstream Infection: Changing Pattern of Occurrence and Antifungal Susceptibility over 10 Years in a Tertiary Care Saudi Hospital. Can J Infect Dis Med Microbiol. 2019;2019:2015692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rajendran R, Sherry L, Deshpande A, et al. A Prospective Surveillance Study of Candidaemia: Epidemiology, Risk Factors, Antifungal Treatment and Outcome in Hospitalized Patients. Front Microbiol. 2016;7:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Falces-Romero I, Romero-Gomez MP, Moreno-Ramos F, et al. Epidemiology of bloodstream Candida species in a Spanish tertiary care hospital as a guide for implementation of T2MR (T2CANDIDA(R)) for rapid diagnosis of candidemia. Med Mycol. 2020. July 7. [DOI] [PubMed]
- 241.Wu PF, Liu WL, Hsieh MH, et al. Epidemiology and antifungal susceptibility of candidemia isolates of non-albicans Candida species from cancer patients. Emerg Microbes Infect. 2017. October;6(10):e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dagi HT, Findik D, Senkeles C, et al. Identification and antifungal susceptibility of Candida species isolated from bloodstream infections in Konya, Turkey. Annals of clinical microbiology and antimicrobials. 2016. May 31;15(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Toda M, Williams SR, Berkow EL, et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia - Four Sites, United States, 2012–2016. MMWR Surveill Summ. 2019. September 27;68(8):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Szekely A, Borman AM, Johnson EM. Candida auris Isolates of the Southern Asian and South African Lineages Exhibit Different Phenotypic and Antifungal Susceptibility Profiles In Vitro. J Clin Microbiol. 2019. May;57(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Vallabhaneni S, Kallen A, Tsay S, et al. Investigation of the First Seven Reported Cases of Candida auris, a Globally Emerging Invasive, Multidrug-Resistant Fungus - United States, May 2013-August 2016. MMWR Morb Mortal Wkly Rep. 2016. November 11;65(44):1234–1237. [DOI] [PubMed] [Google Scholar]
- 246.Ruiz-Gaitan AC, Canton E, Fernandez-Rivero ME, et al. Outbreak of Candida auris in Spain: A comparison of antifungal activity by three methods with published data. Int J Antimicrob Agents. 2019. May;53(5):541–546. [DOI] [PubMed] [Google Scholar]
- 247.de Oliveira HC, Monteiro MC, Rossi SA, et al. Identification of Off-Patent Compounds That Present Antifungal Activity Against the Emerging Fungal Pathogen Candida auris. Front Cell Infect Microbiol. 2019;9:83.* This study identifies possible candidates for Candida auris treatment based on off-patent compounds library.
- 248.Kim K, Zilbermintz L, Martchenko M. Repurposing FDA approved drugs against the human fungal pathogen, Candida albicans. Annals of clinical microbiology and antimicrobials. 2015. June 9;14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.De Cremer K, Lanckacker E, Cools TL, et al. Artemisinins, new miconazole potentiators resulting in increased activity against Candida albicans biofilms. Antimicrob Agents Chemother. 2015. January;59(1):421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Siles SA, Srinivasan A, Pierce CG, et al. High-throughput screening of a collection of known pharmacologically active small compounds for identification of Candida albicans biofilm inhibitors. Antimicrob Agents Chemother. 2013. August;57(8):3681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.De Seta F, Schmidt M, Vu B, et al. Antifungal mechanisms supporting boric acid therapy of Candida vaginitis. J Antimicrob Chemother. 2009. February;63(2):325–36. [DOI] [PubMed] [Google Scholar]
- 252.Wall G, Chaturvedi AK, Wormley FL Jr., et al. Screening a Repurposing Library for Inhibitors of Multidrug-Resistant Candida auris Identifies Ebselen as a Repositionable Candidate for Antifungal Drug Development. Antimicrob Agents Chemother. 2018. October;62(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gupta P, Chanda R, Rai N, et al. Antihypertensive, Amlodipine Besilate Inhibits Growth and Biofilm of Human Fungal Pathogen Candida. Assay Drug Dev Technol. 2016. July;14(5):291–297. [DOI] [PubMed] [Google Scholar]
- 254.Kathwate GH, Karuppayil SM. Tramadol, an Opioid Receptor Agonist: An Inhibitor of Growth, Morphogenesis, and Biofilm Formation in the Human Pathogen, Candida albicans. Assay Drug Dev Technol. 2016. December;14(10):567–572. [DOI] [PubMed] [Google Scholar]
- 255.Wakharde AA, Halbandge SD, Phule DB, et al. Anticancer Drugs as Antibiofilm Agents in Candida albicans: Potential Targets. Assay Drug Dev Technol. 2018. July;16(5):232–246.*This study shows the potential of anticancer drugs in the elimination of C. albicans biofilms.
- 256.Routh MM, Chauhan NM, Karuppayil SM. Cancer drugs inhibit morphogenesis in the human fungal pathogen, Candida albicans. Brazilian journal of microbiology : [publication of the Brazilian Society for Microbiology]. 2013;44(3):855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Stylianou M, Kulesskiy E, Lopes JP, et al. Antifungal application of nonantifungal drugs. Antimicrob Agents Chemother. 2014;58(2):1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Holbrook SYL, Garzan A, Dennis EK, et al. Repurposing antipsychotic drugs into antifungal agents: Synergistic combinations of azoles and bromperidol derivatives in the treatment of various fungal infections. Eur J Med Chem. 2017. October 20;139:12–21. [DOI] [PubMed] [Google Scholar]
- 259.Kathwate GH, Shinde RB, Karuppayil SM. Antiepileptic Drugs Inhibit Growth, Dimorphism, and Biofilm Mode of Growth in Human Pathogen Candida albicans. Assay Drug Dev Technol. 2015. Jul-Aug;13(6):307–12. [DOI] [PubMed] [Google Scholar]
- 260.Wiederhold NP, Patterson TF, Srinivasan A, et al. Repurposing auranofin as an antifungal: In vitro activity against a variety of medically important fungi. Virulence. 2017. February 17;8(2):138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Nile C, Falleni M, Cirasola D, et al. Repurposing Pilocarpine Hydrochloride for Treatment of Candida albicans Infections. mSphere. 2019. January 23;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Chavez-Dozal AA, Lown L, Jahng M, et al. In vitro analysis of finasteride activity against Candida albicans urinary biofilm formation and filamentation. Antimicrob Agents Chemother. 2014. October;58(10):5855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ananda-Rajah MR, Slavin MA, Thursky KT. The case for antifungal stewardship. Curr Opin Infect Dis. 2012. February;25(1):107–15. [DOI] [PubMed] [Google Scholar]
- 264.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017. December;17(12):e383–e392. [DOI] [PubMed] [Google Scholar]
- 265.Alexander BD, Johnson MD, Pfeiffer CD, et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013. June;56(12):1724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]


