Abstract
Older adults with anxiety have lower gray matter brain volume – a component of accelerated aging. We have previously validated a machine learning model to predict brain age, an estimate of an individual’s age based on voxel-wise gray matter images. We investigated associations between brain age and anxiety, depression, stress, and emotion regulation. We recruited 78 participants (≥50yrs) along a wide range of worry severity. We collected imaging data and computed voxel-wise gray matter images, which was input into an existing machine learning model to estimate brain age. We conducted a multivariable linear regression between brain age and age, sex, race, education, worry, anxiety, depression, rumination, neuroticism, stress, reappraisal, and suppression. We found that greater brain age was significantly associated with greater age, male sex, greater worry, greater rumination, and lower suppression. Male sex, worry and rumination are associated with accelerated aging in late life and expressive suppression may have a protective effect. These results provide evidence for the transdiagnostic model of negative repetitive thoughts, which are associated with cognitive decline, amyloid, and tau.
Keywords: Worry, Rumination, Anxiety, Generalized Anxiety Disorder, Late Life, Brain Age, Accelerated Aging
“Age is an issue of mind over matter. If you don’t mind, it doesn’t matter.”
-Mark Twain
Introduction
In the last decade, several studies have reported an independent effect of anxiety on aging. Clinically, anxiety and its disorders have been described as risk factors for multiple age-related medical conditions(Andreescu and Varon, 2015; Lambiase et al., 2014; Tully et al., 2014; Tully et al., 2013). In particular, pathologic worry has been associated with the development of coronary heart disease(Tully et al., 2013), while a higher burden of anxiety symptoms was associated prospectively with increased risk for incident stroke independent of other risk factors (including depression)(Lambiase et al., 2014). In the Nurses’ Health Study(Okereke and Grodstein, 2013), a 4-year longitudinal study of community-dwelling older women (N=16,351), higher midlife anxiety was related to worse later-life overall cognitive function and verbal memory.
Chronic anxiety has been associated with higher beta amyloid burden(Donovan et al., 2018). Moreover, in individuals with similar beta amyloid burden, participants with chronic anxiety had worse longitudinal cognitive decline compared to those without anxiety(Pietrzak et al., 2015; Pietrzak et al., 2014). In a 2-year observational study, older adults with mildly elevated worry symptoms performed worse on measures of visual learning and memory than older adults with no or minimal worry symptoms(Pietrzak et al., 2012). Multiple animal studies reported impaired neurogenesis in anxiety(Revest et al., 2009), and several human studies described brain structural changes associated with anxiety in midlife (e.g., lower hippocampal volumes and lower gray matter in the amygdala and hippocampus(Ly and Andreescu, 2018)). Our previous reports in a geriatric anxiety sample describe structural grey matter differences such as thinning of the orbital frontal cortex and rostral anterior cingulate cortex in late-life Generalized Anxiety Disorder (GAD) compared with non-GAD older controls(Andreescu et al., 2017) and a potential effect of cerebrovascular burden in impairing emotion regulation in late-life GAD(Karim, H. et al., 2016).
These studies indicate that anxiety and/or worry may contribute to accelerated aging. The putative mechanisms include molecular aging markers (e.g. shortened telomeres(Okereke et al., 2012)) and increased stress-response including chronic inflammatory stress, increased hypothalamic-pituitary-adrenocortical (HPA) activity and excessive autonomic responses(O’Donovan et al., 2010; O’Donovan et al., 2013; Perna et al., 2016). However, research in this area is still in early stages and the pathways linking anxiety or worry with brain aging remain unclear.
Brain age prediction uses machine learning to estimate an individual’s chronological age from neuroimaging data(Cole, 2020; Cole and Franke, 2017; Cole et al., 2015; Ly et al., 2019). Individuals whose brain is estimated to be older than age-matched healthy peers (higher brain age than chronological age) may have experienced a higher cumulative exposure to brain insults or were more impacted by those pathological insults or alternatively reflect non-neurodegenerative processes. Brain age may indicate a potential discrepancy between biological and chronological age, suggesting that pathological neuroprogression (combination of neurodegeneration, neurotoxicity and lowered neuroplasticity) is associated with accelerated aging(Perna et al., 2016). These models have been used recently to demonstrate the association between greater brain age with cognitive impairment, Alzheimer’s disease, traumatic brain injury, and mortality(Cole and Franke, 2017; Cole et al., 2015; Cole et al., 2018; Liem et al., 2017; Ly et al., 2019).
We have previously developed a machine learning model for estimating brain age from neuroimaging scans while accounting for amyloid status in a large cohort (n=757)(Ly et al., 2019). Our brain age prediction model contextualizes whole brain structural information of a test cohort against structural information from a large healthy participant cohort (i.e., without psychiatric disorders, cognitive impairment, or significant brain amyloid) spanning a wide range of ages (20 to 85 years) to generate a machine learning-based prediction of the test participant’s chronological age. In this way, discrepancies between actual chronological age and predicted brain age in test groups may indicate pathological disruption or acceleration of the aging process. We were able to delineate significant differences in brain age relative to chronological age between cognitively normal individuals with and without significant amyloid beta deposition in the brain(Ly et al., 2019).
Most of the studies available regarding the potential effect of late-life anxiety in accelerated aging use heterogenous and often non-specific measures for anxiety. Anxiety and its disorders encompass multiple clinical constructs such as worry, rumination, somatization(Zebb and Beck, 1998) and are highly comorbid with both depression and neuroticism(Clark et al., 1994; Hettema et al., 2006; Hettema et al., 2004). It is thus more difficult to detangle the specific effect of various phenotypes on accelerated aging(Andreescu et al., 2015a). Additionally, the highly heterogenous changes that occur in aging make it difficult to interpret various cross-sectional studies that point toward an association between anxiety and aging.
In the current study, we aimed to test if the different anxiety phenotypes (worry, global anxiety, rumination) as well as their more frequent comorbidities (depression severity, neuroticism) were associated with brain aging. Given the hypothesis regarding the role of increased stress response we also included the Perceived Stress Questionnaire(Cohen et al., 1983) in the model. Given our previous reports regarding emotion regulation deficits in late-life anxiety(Andreescu et al., 2015b; Karim, H. et al., 2016; Karim et al., 2017), we also included in the current model the Emotion Regulation Questionnaire (ERQ), a self-report measure of two emotion regulation strategies (cognitive reappraisal and expressive suppression)(Gross and John, 2003).
Methods
Participants and Study Design
Participants were recruited through the Functional neuroanatomy correlates of worry in older adults (FINA) study (R01 MH108509). We recruited participants (n=78) who were 50 years and older with and without anxiety (generalized anxiety disorder, panic disorder, social phobia, etc.) and/or mood disorders (e.g., major depressive disorder, persistent depressive disorder, or unspecified depressive disorder). Diagnosis was assessed through the structured clinical interview for DSM V (SCID). Our cohort had 23 participants with GAD (29%), 58 with any other anxiety disorder (74%), and 25 with current or lifetime prevalence of major depressive disorder (MDD) (32%). We also report these and other diagnoses in the results. Inclusion of these variable diagnoses allowed for representation of worry severity along a wide spectrum, such that worry was normally distributed. Participants were excluded if they were diagnosed with autism spectrum disorders, intellectual development disorder, or any form of psychosis or bipolar disorder. Other exclusion criteria were: a diagnosis of major neurocognitive disorder (e.g., dementia), a 3MSE (modified mini-mental exam) score < 84, a diagnosis of personality disorder, increased suicide risk, use of antidepressants within the last five to fourteen days, history of drug/alcohol abuse within last six months, use of high doses of benzodiazepines (greater than equivalent to 2mg of lorazepam), uncorrected vision problems that would preclude neuropsychiatric testing, below 6th grade level of reading, clinical diagnosis of cerebrovascular accident, multiple sclerosis, vasculitis or significant head trauma. Participants with ferromagnetic objects in body, claustrophobia, or too large to fit in an MRI scanner were also excluded.
When appropriate, participants underwent an adequate washout on antidepressants determined by the primary psychiatrist on the study (CA). For fluoxetine, the washout interval was 6 weeks. Participants who were prescribed low dose psychotropics for pain, sleep disturbances, and/or medical conditions were allowed to continue them in most circumstances. The following common antidepressants were allowed at low doses due to medical reasons: amitriptyline (50mg/day), doxepin (50mg/day), trazodone (100mg/day), imipramine (50mg/day). There were 7 participants who had taken psychotropics (though tapered off for the scan) but they did not differ in brain age, though this does not necessarily indicate an association or lack thereof with psychotropics – we do not include psychotropics as a covariate in further analysis. Participants were recruited from the Pittsburgh area via Pitt+Me (website resource from the university), in-person recommendations, flyers, and radio/television ads. This study was approved by the University of Pittsburgh Institutional Review Board. All participants gave written informed consent prior to participating in the study.
Assessments
Along with demographic information (age, sex, race, and education), we assessed the following: worry (PSWQ, Penn State Worry Questionnaire)(Meyer et al., 1990), overall anxiety (HARS, Hamilton Anxiety Rating Scale)(Hamilton, 1959), depression (MADRS, Montgomery-Asberg Depression Rating Scale)(Montgomery and Asberg, 1979), rumination (Rumination Subscale from RSQ, Response Styles Questionnaire)(Bagby et al., 2004), neuroticism subscale from the Five Factor Inventory (NEO-FFI)(Costa and McCrae, 1992), perceived stress (PSS, Cohen’s Perceived Stress Scale)(Cohen, 1988), and the habitual use of cognitive reappraisal and suppression subscale (ERQ, Emotion Regulation Questionnaire)(Gross and John, 2003). We also collected data on illness severity (CIRSG, cumulative illness rating scale for geriatrics)(Salvi et al., 2008), and cognitive function (3MSE, modified-mini mental status exam)(Teng and Chui, 1987).
MRI Data Acquisition
MRI scans were obtained at the MR Research Center of the University of Pittsburgh using a 3T Siemens MAGNETOM Prisma scanner and a 32-channel head coil. A sagittal, whole-brain T1-weighted magnetization prepared rapid gradient echo (MPRAGE) was collected with repetition time (TR)=2400ms, echo time (TE)=2.22ms, flip angle (FA)=8deg, field of view (FOV)=320×300 with 208 slices, 0.8mm3 isotropic resolution, 0.4mm slice gap, and GeneRalized Autocalibrating Partial Parallel Acquisition (GRAPPA) with acceleration factor of 2 (total time 6.63min). We used a suboptimal interslice gap of 0.4mm as this allowed for higher isotropic resolution. We now also acknowledge this as a limitation. A sagittal, whole-brain T2-weighted Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE) was collected with TR=3200ms, TE=563ms, FA=120deg, FOV=320×300 with 208 slices, 0.8mm3 isotropic resolution, no slice gap, and GRAPPA with acceleration factor of 2 (total time 5.95min). An axial, whole-brain T2-weighted Fluid Attenuated Inversion Recovery (FLAIR) was collected with TR=10,000ms, TE=91ms, FA=135deg, inversion time (TI)=2,500ms, FOV=320×320 with 104 slices, 0.8mm x 0.8mm x 1.6mm resolution, no slice gap, and GRAPPA with acceleration factor of 2 (total time 5.95min). Participants were in the MR scanner for approximately 45–60 minutes as we also collected functional MRI data as well (not presented).
Structural Processing
Processing was conducted in statistical parametric mapping toolbox (SPM12)(Penny et al., 2011) in MatLab 2018b (MathWorks, Natick, MA). All interpolation was done with a 4th degree B-spline and the similarity metric used for coregistration between different image types was normalized mutual information. The T2-SPACE and FLAIR were first independently coregistered to the MPRAGE. All three were input into a multispectral segmentation that bias corrects each image and segments them into gray matter, white matter, cerebrospinal fluid, skull, soft-tissue, and air(Ashburner and Friston, 2005). Due to the high burden of white matter hyperintensities, we adjusted the number of Gaussians used to identify white matter to two to improve identification of gray and white matter(Karim, H.T. et al., 2016). This ensures an accurate segmentation of the gray matter. The gray and white matter maps are inputs into a process to generate a study-specific template to estimate gray matter images.
We used DARTEL (Diffeomorphic Anatomical Registration using Exponentiated Lie Algebra) to generate a study-specific template(Ashburner, 2007). DARTEL aligns each participant’s gray matter image (along with white matter) to a standard MNI space template using a combination of linear and non-linear registrations. Then an average image is generated across all participants – this is the first template. The gray matter images are aligned again and coregistered again. Then another average image is generated. This process is iterated until an increasingly crisp average template, to which the data are iteratively aligned. DARTEL uses an iterative process of averages across participants and iterative coregistration to improve normalization to a standard anatomic space. Once a study-specific template is generated (an iterative average across participants), each image is normalized and then transformed into a gray matter image that preserves the total amount of grey matter by multiplying by the determinant of the Jacobian of the transformations(Ashburner, 2007). All images are normalized to a 1mm3 isotropic resolution. The gray matter images were smoothed using a Gaussian kernel of full width at half-maximum of 4mm. These gray matter images are input into the brain age estimation model.
Brain Age Estimation
We have previously validated a brain age estimation algorithm that predicts chronological age with gray matter maps(Ly et al., 2019) using the Pattern Recognition for Neuroimaging Toolbox (PRoNTo)(Schrouff et al., 2013). Whole brain, voxel-wise gray matter densities were mean-centered and used to calculate a similarity matrix kernel (dot product)(LaConte et al., 2005) that was input into a Gaussian processes regression to predict chronological age. The training set, which includes 757 adult MRIs of individuals without any psychiatric or neurological disorder as well as Alzheimer’s pathology as measured by positron emission tomography, has been previously described(Ly et al., 2019). This data was from the ADNI, Information eXtraction from Images (IXI), and OASIS-3 – which are all publicly available. The cohort (ADNI, IXI, OASIS-3) is used as a covariate to account for differences in scanner/site/protocol. The current study’s participants were not part of the training set. Using this pre-trained model, we can estimate the brain age of each participant in the current study.
While white matter hyperintensities are likely factors that influence brain aging, our brain age model utilizes primarily gray matter and not white matter data. Thus, our brain age marker more accurately could be stated to be a ‘gray matter’ age marker.
Statistical Analysis
We conducted a linear regression analysis in SPSS 26 (IBM, Armonk, NY). We used brain age as the outcome and the following as independent predictors: chronological age, sex, race, education (years), worry (PSWQ), anxiety (HARS), depression severity (MADRS), rumination (RSQ), neuroticism (NEO-FFI), reappraisal (ERQ, reappraisal subscale), suppression (ERQ, suppression subscale), and stress (PSS). The models conducted all had variance inflation factor (VIF) below 5, showed normally distributed standardized residuals (based on a histogram and QQ-plot), and did not violate the assumption of homoscedasticity.
A total of 69 participants (88.5%) had all data available, however there were missing values for: 3MSE (4 not collected), HARS (2 lost questionnaires), MADRS (3 not collected, 2 lost questionnaires), RSQ (1 participant error, 1 not collected), NEO-FFI (2 refused, 4 participant error), ERQ (1 refused, 3 participant error), and PSS (1 refused, 3 participant error). We conducted multiple imputations analysis(Newgard and Haukoos, 2007; Schafer, 1999) (5 imputations) in SPSS to impute missing values using the Markov Chain Monte Carlo method(MacKay and Mac Kay, 2003) and fully conditional specification with linear regression, assuming our values were missing at random with an arbitrary missing pattern(Schunk, 2008). We conducted statistical independent t-tests or χ2 tests where appropriate to identify if the 9 participants with missing data differed significantly on demographic and cognitive factors compared to those without missing data. We found that they did not differ on age, sex, race, and 3MSE, but did find that education was lower by approximately 1 year in those who were missing data.
Every variable used in the regression as well as the outcome (brain age) were used in the imputation model, as this has been shown to improve the imputation and is not a ‘self-fulfilling prophecy,’ but rather “replays the strength of associations between predictors and outcomes present in the complete cases, to enable valid analyses(Moons et al., 2006).” All variables were constrained to their appropriate values (e.g., HARS ranges from 0 to 56 thus values may not be imputed outside this range). We report both the imputed pooled results as well as the estimates from the original model with missing data (n=69). Pooling is computed automatically by SPSS using Rubin’s rules(Rubin, 2004).
Each variable was inspected for outliers and the following variables had some outliers: HARS (n=1), MADRS (n=4), RSQ (n=1), brain age (n=2), and reappraisal ERQ subscale (n=1). We conducted the regression with those participants removed (not shown) and found that the estimates did not differ from when they were included in the model.
Factors that are associated with brain age, may also be associated with chronological age and thus may be a confound. To understand whether factors that were significantly related to brain age or chronological age or both, we conducted independent t-tests or correlations with chronological age.
Exploratory Analysis
Following statistical analysis, we found a significant association between brain age and rumination. Given that the RSQ is increasingly divided into a reflective pondering and ruminative brooding component(Schoofs et al., 2010; Whitmer and Gotlib, 2011), these are largely thought to be adaptive and maladaptive, respectively. We divided the RSQ into those two components and then conducted multiple imputations as well as a similar regression analysis, however replaced RSQ with either reflective pondering (RSQ reflection) or ruminative brooding (RSQ brooding). This helps address whether reflection vs. brooding was significantly associated with brain age.
Results
We report the characteristics of the sample in table 1. Of note, worry is normally distributed around a mean worry severity of 47.6. Demographics match those of the surrounding Pittsburgh area. The mean absolute error between chronological and brain age in our sample was 4.7 with r(76)=0.75 and r2=0.56, which indicates that our model was able to predict chronological age accurately in this sample within the expected tolerance. For characterization, we also report the following diagnoses based on the SCID for DSM V: 23 with GAD (29%), 58 with any other anxiety disorder (74%), 25 with current or lifetime prevalence of MDD (32%), 10 with either current Dysthymic disorder/current or lifetime depressive disorder not otherwise specified/current or lifetime mood disorder due to general medication (13%), 19 with lifetime prevalence of a substance use disorder (24%), and 5 with current/lifetime prevalence of an eating disorder (6%).
Table 1.
Characteristics of the sample
| Variable Name | Number Missing | Imputed Mean (pooled) | ||
|---|---|---|---|---|
| Std. | ||||
| Age, years | 61.2 | 8.5 | 0 | N/A |
| Sex, number female | 53 (68%) | 0 | N/A | |
| Race, W/B/HPI/MR | 63 (81%); 13 (17%); 1 (1%); 1 (1%) | 0 | N/A | |
| Education, years | 15.6 | 2.6 | 0 | N/A |
| Cumulative Illness (CIRSG) | 3.0 | 2.3 | 1 | N/A |
| Worry (PSWQ) | 47.6 | 14.7 | 0 | N/A |
| Anxiety (HARS) | 8.5 | 6.9 | 2 | 8.5 |
| Depression (MADRS) | 8.2 | 8.1 | 5 | 8.6 |
| Rumination (RSQ) | 37.7 | 12.6 | 2 | 37.7 |
| Neuroticism (NEO-FFI) | 19.5 | 10.7 | 6 | 19.9 |
| Reappraisal (ERQ Subscale) | 29.4 | 7.8 | 4 | 29.4 |
| Suppression (ERQ Subscale) | 13.8 | 5.4 | 4 | 13.8 |
| Stress (PSS) | 15.5 | 8.6 | 4 | 15.6 |
| Overall Cognitive (3MSE) | 96.7 | 0.5 | 4 | N/A |
| Brain Age, years | 63.6 | 6.1 | 0 | N/A |
-Means and standard deviations are reported unless otherwise noted
-Means for both the original data and imputed values (see number of missing data) are reported
-CIRSG - Cumulative Illness Rating Scale for Geriatrics; PSWQ - Penn State Worry Questionnaire; HARS - Hamilton Anxiety Rating Scale; MADRS - Montgomery-Asberg Depression Rating Scale; RSQ - Response Styles Questionnaire; NEO-FFI - Neuroticism, Extroversion, Openness to Experience-Five Factor Inventory; ERQ - Emotion Regulation Questionnaire; PSS — Cohen’s Perceived Stress Scale; 3MSE, modified-mini mental status exam
-N/A - not applicable or not imputed
-Race: W - White, B - Black, HPI - Hawaiian or Pacific Islander, and MR - Mixed Race
We found that brain age was significantly associated with several factors that explained 72% of the variance in brain age [F(11,57)=13.3, p<0.001, r2=0.72]. We found the following: (1) for every year, participant’s brain age was greater by 0.57 years (~6.8 months); (2) on average, women’s brains were younger by 3.4 years (~41 months) compared to men; and (3) for every point greater on the RSQ, brain age was greater by 0.14 years (~1.7 months) (see table 2 and figure 1).
Table 2.
Regression model explaining variance in brain age.
| Variable | ß | B (SE) | 95% CI | t-statistic, p-value |
|---|---|---|---|---|
| Constant | 27.834 (5.76) | (16.295, 39.373) | 4.832, p<0.001 | |
| Age | 0.81 | 0.567 (0.058) | (0.451, 0.684) | 9.745, p<0.001 |
| Sex (Male Reference) | −0.26 | −3.242 (1.044) | (−5.333, −1.151) | −3.106, p<0.005 |
| Race (White Reference) | −0.04 | −0.643 (1.323) | (−3.293, 2.007) | −0.486, p=0.629 |
| Education | −0.02 | −0.06 (0.198) | (−0.456, 0.336) | −0.304, p=0.763 |
| Worry (PSWQ) | 0.17 | 0.068 (0.053) | (−0.037, 0.174) | 1.295, p=0.201 |
| Anxiety (HARS) | −0.10 | −0.098 (0.145) | (−0.389, 0.193) | −0.677, p=0.501 |
| Depression (MADRS) | 0.09 | 0.065 (0.109) | (−0.154, 0.284) | 0.593, p=0.556 |
| Rumination (RSQ) | 0.29 | 0.143 (0.06) | (0.024, 0.263) | 2.407, p<0.05 |
| Neuroticism (FFI-N) | −0.15 | −0.088 (0.08) | (−0.248, 0.071) | −1.11, p=0.272 |
| Reappraisal (ERQ) | 0.00 | −0.001 (0.065) | (−0.131, 0.129) | −0.016, p=0.987 |
| Suppression (ERQ) | −0.08 | −0.092 (0.092) | (−0.277, 0.093) | −1.001, p=0.321 |
| Stress (PSS) | −0.09 | −0.06 (0.085) | (−0.231, 0.111) | −0.707, p=0.482 |
ß values indicate standardized coefficients while B indicate unstandardized coefficients. We also report 95% confidence intervals and indicate significant associations in bold.
Figure 1.
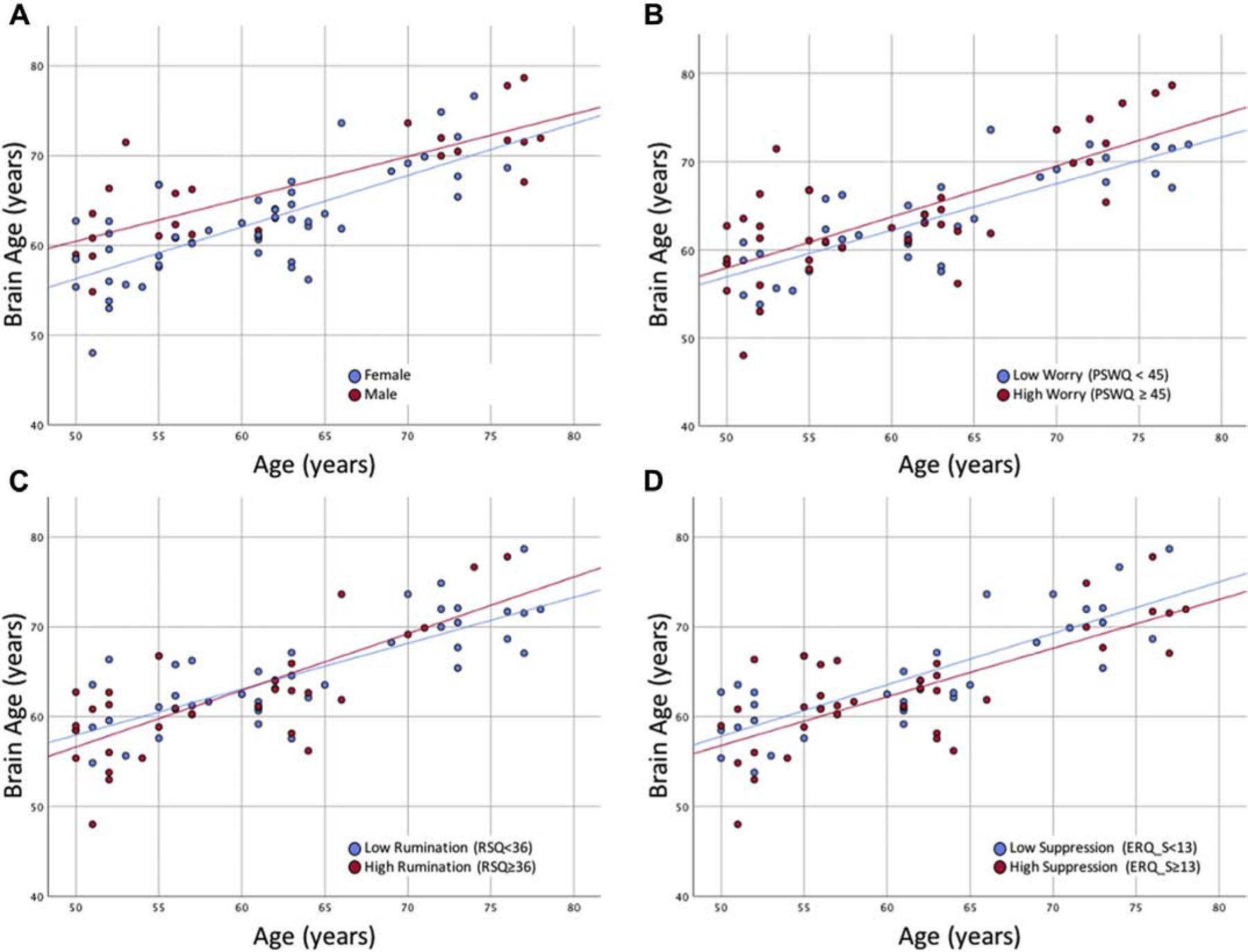
Significant associations between brain age and sex (A), worry (B), rumination (C), and suppression (D) adjusting for chronological age. Cutoffs for PSWQ, RSQ, and ERQ suppression were based off of the medians of the sample as these are meant to illustrate associations that utilized continuous measures. Greater brain age is associated with greater age, being male (compared to female), greater worry, greater rumination, and lower suppression.
After imputing values that were missing for 9 participants (see table 1), we reconducted our regression and found the following (pooled results): (1) for every year, participant’s brain age was greater by approximately 0.53 years (~6.4 months); (2) on average, women’s brains were younger by 4.1 years (~49 months) compared to men; (3) for every point greater on the PSWQ, brain age was greater by 0.11 years (~1.3 months); (4) for every point greater on the RSQ, brain age was greater by 0.11 years (~1.3 months); (5) for every point greater on the ERQ suppression scale, brain age was lower by 0.17 years (~2.0 months).
The imputed models explained 68 to 72% (range) of the variance in brain age across imputations (variance is not a pooled metric). We showed associations between these factors in figure 1 using non-imputed data.
We found that men and women did not differ by chronological age [t(76)=0.9, p=0.346]. We found that greater chronological age was correlated with lower rumination [RSQ, r(75)=−0.37, p<0.005], but not with worry [PSWQ, r(77)=−0.21, p=0.069] or suppression [ERQ suppression, r(76)=−0.05, p=0.685]. Pooled results did not differ.
Exploratory Results
When replacing RSQ with RSQ reflection, we found no significant association between RSQ reflection and brain age in the imputed [t=0.9, p=0.360, B (SE)=0.173 (0.189), ß=0.025, CI=(−0.199, 0.546)] or original data [t=1.2, p=0.238, B (SE)=0.232 (0.194), ß=0.113, CI=(−0.158, 0.621)]. When replacing RSQ with RSQ brooding, we found a significant association between RSQ brooding and brain age in the imputed [t=2.2, p<0.05, B (SE)=0.514 (0.233), ß=0.228, CI=(0.057, 0.972)] and original data [t=2.6, p<0.05, B (SE)=0.605 (0.235), ß=0.303, CI=(0.135, 1.075)].
Discussion
Our results indicate that worry and rumination in late life are associated with an accelerated brain aging process. Surprisingly, there was no effect of perceived stress and the propensity to use suppression seems to have had a protective effect on brain aging in this sample. Brain age in men was greater compared to brain age in women.
Worry and rumination share common phenomenological features such as difficult to control, repetitive negative thinking. However, worry and rumination have been typically been described as two distinct processes, with worry being usually associated with prospective negative thinking and generalized anxiety disorder and rumination with retrospective negative thinking and depression(Nolen-Hoeksema, 2000). Classically, rumination is triggered by sad mood and maintains depressive symptoms by promoting negative cognitive biases(Just and Alloy, 1997). Similarly, classic worry theoretical models such as Borkovec’s cognitive avoidance model, posit that worry serves as a cognitive avoidance strategy that inhibits the emotional processing of highly anxiogenic material(Borkovec, 1994). However, newer theories propose a transdiagnostic approach that 1) includes both worry and rumination under the umbrella of repetitive negative thoughts (RNT) and 2) describe the detrimental effect of RNT throughout multiple categorical diagnoses including major depression, GAD, Social Phobia, Bipolar disorder, Obsessive compulsive Disorder, Eating disorders and Post-Traumatic Stress Disorder (PTSD)(Aldao et al., 2010; Ehring, 2008). Several authors have proposed RNT as the core of anxiety-depression comorbidity(Gustavson et al., 2018; McEvoy et al., 2013), while others emphasized the association of RNT with worse psychological, physical and cognitive health in older adults(Segerstrom et al., 2010).
Recently, RNTs have been “imported” into the aging and dementia field. As such, in 2015, Marchant & Howard advanced a model of Cognitive Debt that would involve certain symptoms/disorders actively depleting cognitive reserve and increase vulnerability to Alzheimer’s Disease (AD)(Marchant and Howard, 2015). Thus, there is building evidence that depression, anxiety, sleep disorders, neuroticism and PTSD increase the risk for AD and the authors suggest that RNT are the shared process which may drive the acquisition of Cognitive Debt through diverting cognitive and emotional resources to distressing thought processes(Marchant and Howard, 2015). The neurobiological signature of Cognitive Debt and AD might rely on the relationship between hippocampus, prefrontal cortex (PFC), and amygdala with the HPA stress response(Marchant and Howard, 2015). More recently, RNT were cross-sectionally associated with cognitive decline, beta amyloid deposits and entorhinal tau(Marchant et al., 2020).
Our results, that single out both worry and rumination as predictors of accelerated aging, would fit well into the overall model of RNT as contributing to increased Cognitive Debt. These results also emphasize the need for preventative interventions targeting RNT in older adults (e.g. mindful meditation, cognitive behavioral therapy or positive reappraisal therapy – a newer attempt to incorporate mindful meditation into cognitive therapy(Hanley and Garland, 2014)).
The exploratory analysis regarding subtypes of rumination also rendered relevant results. Treynor, Gonzales and Nolen-Hoeksema (Treynor et al., 2003) differentiated between “reflective pondering” and “brooding” factors of rumination, and subsequent research confirmed that the brooding sub-type of rumination encompasses the more maladaptive aspects of rumination and it is more often associated with mood/anxiety pathology in midlife (Treynor et al., 2003) and late-life (Sutterlin et al., 2012) while reflective pondering may be conducive to problem-solving strategies (Sutterlin et al., 2012). Our preliminary results pointing toward the brooding sub-type as predictive of brain aging suggest that further refinement of RNT phenomenology may be required for both future interventions and mechanistic studies analyzing the biological underpinning of RNT.
Our brain age model was fit from data on participants who were without significant beta amyloid in the brain, which made our brain age measure more sensitive to amyloid (i.e., individuals with significant amyloid had greater brain age)(Ly et al., 2019). Given the link between RNT and dementia as well as beta amyloid, there is some reason to suspect a possible link in our study as well. Future studies should investigate whether and how RNT impacts brain aging with measures of beta amyloid to understand its role in impacting brain aging.
Regarding the protective role of expressive suppression, a response-focused form of emotion regulation that seeks to prevent the outward expression of an already-generated emotion(Gross, 1998), several studies have indicated a positive association between expressive suppression and volumes of the anterior insula, dorsomedial PFC and dorsal anterior cingulate (ACC)(Cutuli, 2014; Giuliani et al., 2011a; Giuliani et al., 2011b; Hermann et al., 2014). Although there is data linking expressive suppression to anxious and depressive symptoms(Gross and John, 2003) as well as memory impairment(Hayes et al., 2010), we may cautiously interpret these results through the use-dependent brain plasticity theory(Classen et al., 1998; Giuliani et al., 2011a) that posits a ‘use it or lose it’ approach. Thus, chronic preferential use of expressive suppression may maintain a higher volume in prefrontal brain regions counterbalancing the thinning effect of aging. An additional explanation involves the age group used in the current study – emotion regulation strategies effective in younger adults may become less effective with age(Urry, 2010), and although older adults report using cognitive reappraisal more than younger adults, it is possible that older adults may rely less on a resource-demanding strategy such as reappraisal and use simpler techniques such as distraction or suppression(Livingstone and Isaacowitz, 2018).
Our results confirm the multiple previous reports indicating that sex differences influence brain morphology and atrophy rates. Past studies have shown greater volume loss in the gray matter in men compared to women(Armstrong et al., 2019). Throughout adulthood, research indicates that the female brain is more youthful than the male brain, with studies in females showing less loss of cerebral blood flow following puberty, more brain glycolysis during young adulthood, and less loss of protein synthesis-related gene expression during aging(Goyal et al., 2019). These young/middle age adult characteristics might provide some degree of resilience to aging-related changes and would apply to observations in large epidemiological studies of aging that associated male sex with worse memory and decreased hippocampal volume among cognitively normal individuals(Jack et al., 2015).
We found that there were no differences in chronological age between men and women in our study and chronological age was not correlated with worry or expressive suppression. This further helps show that brain age is an independent correlate of sex, worry, and expressive suppression. We did find that rumination was negatively correlated with chronological age, but positively correlated with brain age. This may explain the crossing between lines for low/high rumination. Future studies should test whether age has a moderating role on the association between rumination and brain age.
Our study has several limitations: we do not have longitudinal data to follow-up on the effect of the predictive factors described above; we also do not have any other biological markers of aging to corroborate the current results (e.g., inflammatory cytokines, cortisol levels, cerebral beta-amyloid burden). Most participants had mild if any depressive symptoms; thus, we cannot make inferences about the added effect of clinical depression on accelerated aging. Given the cross-sectional nature of our study, it is unclear whether brain aging is a result of atrophy or damage or differences in non-neurodegenerative processes (e.g., may be due to differences in other inter-individual differences). The use of FWHM of 4mm is based previous brain age models which have utilized 4mm(Cole et al., 2015; Cole et al., 2018; Ly et al., 2019; Smith et al., 2019), this likely can bias the results and is ultimately somewhat arbitrary. In the past, smoothing has been used to boost statistical power (i.e., greater smoothing reduces complexity of multiple comparisons problem), however in brain aging models is largely meant to deal with greater structural variability due to aging. We utilized an MPRAGE with a slice gap of 0.4mm3 due to the high resolution of the sequence (0.8mm3), this may affect segmentation as other images were acquired at 1mm3 isotropic resolution. All images are resolved to a 1mm3 isotropic resolution with a FWHM of 4mm, which help account for small differences. There are differences in the sites, scanners, and sequences for each site including this one which may inadvertently affect the results or conclusions of the current study. Typically, structural MRI scans are adequately harmonized for scanning parameters, however our approach utilized a post-hoc correction. Past studies have shown that this generates reliable brain age estimates(Cole et al., 2017), but this may nonetheless affect the current results. Our sample is relatively well-educated (average 15 years), and since education has been shown to be associated with markers of reserve this may mean that these results may not generalize well to the general population. Future studies should recruit samples with a broader education range and should also measure markers like intelligence quotient (IQ) as this may impact reserve as well. Another limitation is that the participants who were missing some data differed on education by approximately 1 year, which may influence these results, however they did not differ on cognitive function (3MSE). Future studies should investigate these associations in larger samples using approaches that utilize regularization and cross-validation as it is possible that a more parsimonious model (e.g., fewer predictors with maximized variance explained) may be a better fit. The brain age marker used in this analysis utilizes only gray matter maps and reflects a ‘gray matter’ age rather than brain age in general so should be interpreted as such. Our analysis regarding brooding vs. reflection was exploratory and should be interpreted with caution, but future studies should investigate the divergent mechanisms of these two constructs and their effect on brain age.
In conclusion, we present novel data suggesting a deleterious effect on aging of both worry and rumination in older adults as well as a potential protective effect of using expressive suppression. These results also emphasize the role of preventative interventions in reducing accelerated aging by targeting modifiable factors such as worry and rumination in late life.
Table 3.
Regression model explaining variance in brain age using imputed data.
| Variable | ß | B (SE) | 95% CI | t-statistic, p-value |
|---|---|---|---|---|
| Constant | 30.151 (5.606) | (19.163, 41.138) | 5.378, p < 0.001 | |
| Age | 0.742 | 0.534 (0.057) | (0.422, 0.646) | 9.374, p < 0.001 |
| Sex (Male Reference) | −0.322 | −4.186 (1.037) | (−6.218, −2.153) | −4.036, p < 0.001 |
| Race (White Reference) | 0.017 | 0.264 (1.245) | (−2.176, 2.705) | 0.212, p=0.832 |
| Education | −0.047 | −0.111 (0.195) | (−0.493, 0.272) | −0.567, p=0.571 |
| Worry (PSWQ) | 0.258 | 0.107 (0.051) | (0.008, 0.207) | 2.107, p<0.05 |
| Anxiety (HARS) | −0.184 | −0.162 (0.14) | (−0.437, 0.113) | −1.158, p=0.247 |
| Depression (MADRS) | 0.064 | 0.046 (0.109) | (−0.168, 0.259) | 0.419, p=0.675 |
| Rumination (RSQ) | 0.233 | 0.113 (0.06) | (−0.003, 0.23) | 1.901, p=0.057 |
| Neuroticism (FFI-N) | −0.107 | −0.06 (0.083) | (−0.222, 0.102) | −0.726, p=0.468 |
| Reappraisal (ERQ) | 0.077 | 0.06 (0.062) | (−0.061, 0.181) | 0.979, p=0.328 |
| Suppression (ERQ) | −0.149 | −0.169 (0.09) | (−0.345, 0.007) | −1.879, p=0.060 |
| Stress (PSS) | −0.119 | −0.084 (0.086) | (−0.252, 0.085) | −0.97, p=0.332 |
B indicate unstandardized coefficients. We also report 95% confidence intervals and indicate significant associations in bold.
Brain age is a machine learning derived marker of accelerated aging.
Greater brain age associated with more worry and rumination, and less suppression.
Women had lower brain age compared to men, replicating past studies on brain age.
Past work showed that negative repetitive thoughts are related to greater AD risk.
There is need for developing interventions targeting repetitive negative thoughts.
Acknowledgements.
We would like to acknowledge the staff of the Geriatric Psychiatry Neuroimaging Lab for their work and support.
Funding. This work was funded by NIMH R01MH108509, NIMH R01 MH 076079, NIMH R01 MH121619, NIA R01AG023651, R01GM113243, and NIA T32AG055381.
Abbreviations.
- 3MSE
modified-mini mental status exam
- ACC
anterior cingulate
- AD
Alzheimer’s Disease
- CIRSG
Cumulative Illness Rating Scale for Geriatrics
- DARTEL
Diffeomorphic Anatomical Registration using Exponentiated Lie Algebra
- ERQ
Emotion Regulation Questionnaire
- FA
flip angle
- FLAIR
Fluid Attenuated Inversion Recovery
- FOV
field of view
- GAD
generalized anxiety disorder
- GRAPPA
GeneRalized Autocalibrating Partial Parallel Acquisition
- HARS
Hamilton Anxiety Rating Scale
- HPA
hypothalamic-pituitary-adrenocortical activity
- MADRS
Montgomery-Asberg Depression Rating Scale
- MPRAGE
magnetization prepared rapid gradient echo
- NEO-FFI
Neuroticism, Extroversion, Openness to Experience-Five Factor Inventory
- PFC
Prefrontal Cortex
- PSS
Cohen’s Perceived Stress Scale
- PSWQ
Penn State Worry Questionnaire
- PTSD
Post-Traumatic Stress Disorder
- RNT
Repetitive Negative Thoughts
- RSQ
Response Styles Questionnaire
- SPACE
Sampling Perfection with Application optimized Contrasts using different flip angle Evolution
- TE
Echo Time
- TR
Repetition Time
- VIF
Variance Inflation Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest. The authors report no conflicts of interest.
The authors report no conflicts of interest. No authors’ institutions have contracts relating to this research through which it or any other organization may stand to gain financially now or in the future. There are no agreements between any authors or their institutions that could be seen as involving a financial interest in this work.
The data contained in the manuscript being submitted have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
This study was approved by the University of Pittsburgh Institutional Review Board. All participants gave written informed consent prior to participating in the study.
All authors have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data.
References
- Aldao A, Nolen-Hoeksema S, Schweizer S, 2010. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin Psychol Rev 30(2), 217–237. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Mennin D, Tudorascu D, Sheu LK, Walker S, Banihashemi L, Aizenstein H, 2015a. The many faces of anxiety-neurobiological correlates of anxiety phenotypes. Psychiatry Res 234(1), 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L, Aizenstein H, 2015b. Emotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment. Am J Geriatr Psychiatry 23(2), 200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Tudorascu D, Sheu LK, Rangarajan A, Butters MA, Walker S, Berta R, Desmidt T, Aizenstein H, 2017. Brain structural changes in late-life generalized anxiety disorder. Psychiatry Res 268, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Varon D, 2015. New research on anxiety disorders in the elderly and an update on evidence-based treatments. Current psychiatry reports 17(7), 595. [DOI] [PubMed] [Google Scholar]
- Armstrong NM, An Y, Beason-Held L, Doshi J, Erus G, Ferrucci L, Davatzikos C, Resnick SM, 2019. Sex differences in brain aging and predictors of neurodegeneration in cognitively healthy older adults. Neurobiol Aging 81, 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, 2007. A fast diffeomorphic image registration algorithm. Neuroimage 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2005. Unified segmentation. Neuroimage 26(3), 839–851. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Rector NA, Bacchiochi JR, McBride C, 2004. The stability of the response styles questionnaire rumination scale in a sample of patients with major depression. Cognitive Therapy and Research 28(4), 527–538. [Google Scholar]
- Borkovec TD, 1994. The nature, functions, and origins of worry., in: Tallis GDF (Ed.) Worrying: Perspectives on theory, assessment, and treatment. Wiley & Sons, Sussex, England. [Google Scholar]
- Clark LA, Watson D, Mineka S, 1994. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol 103(1), 103–116. [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG, 1998. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79(2), 1117–1123. [DOI] [PubMed] [Google Scholar]
- Cohen S, 1988. Perceived stress in a probability sample of the United States.
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. Journal of health and social behavior 24(4), 385–396. [PubMed] [Google Scholar]
- Cole JH, 2020. Multimodality neuroimaging brain-age in UK biobank: relationship to biomedical, lifestyle, and cognitive factors. Neurobiol Aging 92, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Franke K, 2017. Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci 40(12), 681–690. [DOI] [PubMed] [Google Scholar]
- Cole JH, Leech R, Sharp DJ, Alzheimer’s Disease Neuroimaging I., 2015. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol 77(4), 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Poudel RPK, Tsagkrasoulis D, Caan MWA, Steves C, Spector TD, Montana G, 2017. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage 163, 115–124. [DOI] [PubMed] [Google Scholar]
- Cole JH, Ritchie SJ, Bastin ME, Valdes Hernandez MC, Munoz Maniega S, Royle N, Corley J, Pattie A, Harris SE, Zhang Q, Wray NR, Redmond P, Marioni RE, Starr JM, Cox SR, Wardlaw JM, Sharp DJ, Deary IJ, 2018. Brain age predicts mortality. Mol Psychiatry 23(5), 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa PT, McCrae RR, 1992. Revised NEO personality inventory (NEO-PI-R) and Neo five-factor inventory (NEO-FFI). Psychological Assessment Resources. [Google Scholar]
- Cutuli D, 2014. Cognitive reappraisal and expressive suppression strategies role in the emotion regulation: an overview on their modulatory effects and neural correlates. Front Syst Neurosci 8, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan NJ, Locascio JJ, Marshall GA, Gatchel J, Hanseeuw BJ, Rentz DM, Johnson KA, Sperling RA, Harvard Aging Brain S., 2018. Longitudinal Association of Amyloid Beta and Anxious-Depressive Symptoms in Cognitively Normal Older Adults. Am J Psychiatry, appiajp201717040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T, & Watkins ER, 2008. Repetitive negative thinking as a transdiagnostic process. International Journal of Cognitive Therapy 1(3), 192–205. [Google Scholar]
- Giuliani NR, Drabant EM, Bhatnagar R, Gross JJ, 2011a. Emotion regulation and brain plasticity: expressive suppression use predicts anterior insula volume. Neuroimage 58(1), 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani NR, Drabant EM, Gross JJ, 2011b. Anterior cingulate cortex volume and emotion regulation: is bigger better? Biol Psychol 86(3), 379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, Benzinger TL, Morris JC, Raichle ME, Vlassenko AG, 2019. Persistent metabolic youth in the aging female brain. Proc Natl Acad Sci U S A 116(8), 3251–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, 1998. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of personality and social psychology 74(1), 224–237. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP, 2003. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of personality and social psychology 85(2), 348–362. [DOI] [PubMed] [Google Scholar]
- Gustavson DE, du Pont A, Whisman MA, Miyake A, 2018. Evidence for Transdiagnostic Repetitive Negative Thinking and Its Association with Rumination, Worry, and Depression and Anxiety Symptoms: A Commonality Analysis. Collabra Psychol 4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1959. The assessment of anxiety states by rating. Br J Med Psychol 32(1), 50–55. [DOI] [PubMed] [Google Scholar]
- Hanley AW, Garland EL, 2014. Dispositional Mindfulness Co-varies with Self-Reported Positive Reappraisal. Pers Individ Dif 66, 146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Morey RA, Petty CM, Seth S, Smoski MJ, McCarthy G, Labar KS, 2010. Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Front Hum Neurosci 4, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Bieber A, Keck T, Vaitl D, Stark R, 2014. Brain structural basis of cognitive reappraisal and expressive suppression. Soc Cogn Affect Neurosci 9(9), 1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS, 2006. A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry 163(5), 857–864. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Kendler KS, 2004. Genetic and environmental sources of covariation between generalized anxiety disorder and neuroticism. Am J Psychiatry 161(9), 1581–1587. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, Lowe V, Senjem ML, Gunter JL, Machulda MM, Gregg BE, Pankratz VS, Rocca WA, Petersen RC, 2015. Age, Sex, and APOE epsilon4 Effects on Memory, Brain Structure, and beta-Amyloid Across the Adult Life Span. JAMA Neurol 72(5), 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just N, Alloy LB, 1997. The response styles theory of depression: tests and an extension of the theory. J Abnorm Psychol 106(2), 221–229. [DOI] [PubMed] [Google Scholar]
- Karim H, Tudorascu DL, Aizenstein H, Walker S, Good R, Andreescu C, 2016. Emotion Reactivity and Cerebrovascular Burden in Late-Life GAD: A Neuroimaging Study. Am J Geriatr Psychiatry 24(11), 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim HT, Andreescu C, MacCloud RL, Butters MA, Reynolds CF, Aizenstein HJ, Tudorascu DL, 2016. The effects of white matter disease on the accuracy of automated segmentation. Psychiatry Research: Neuroimaging 253, 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim HT, Tudorascu DL, Butters MA, Walker S, Aizenstein HJ, Andreescu C, 2017. In the grip of worry: cerebral blood flow changes during worry induction and reappraisal in late-life generalized anxiety disorder. Transl Psychiatry 7(8), e1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaConte S, Strother S, Cherkassky V, Anderson J, Hu X, 2005. Support vector machines for temporal classification of block design fMRI data. Neuroimage 26(2), 317–329. [DOI] [PubMed] [Google Scholar]
- Lambiase MJ, Kubzansky LD, Thurston RC, 2014. Prospective study of anxiety and incident stroke. Stroke 45(2), 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem F, Varoquaux G, Kynast J, Beyer F, Kharabian Masouleh S, Huntenburg JM, Lampe L, Rahim M, Abraham A, Craddock RC, Riedel-Heller S, Luck T, Loeffler M, Schroeter ML, Witte AV, Villringer A, Margulies DS, 2017. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage 148, 179–188. [DOI] [PubMed] [Google Scholar]
- Livingstone KM, Isaacowitz DM, 2018. The roles of age and attention in general emotion regulation, reappraisal, and expressive suppression. Psychology and aging 33(3), 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly M, Andreescu C, 2018. Advances and Barriers for Clinical Neuroimaging in Late-Life Mood and Anxiety Disorders. Current psychiatry reports 20(1), 7. [DOI] [PubMed] [Google Scholar]
- Ly M, Yu GZ, Karim HT, Muppidi NR, Mizuno A, Klunk WE, Aizenstein HJ, Alzheimer’s Disease Neuroimaging, I., 2019. Improving brain age prediction models: incorporation of amyloid status in Alzheimer’s disease. Neurobiol Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay DJ, Mac Kay DJ, 2003. Information theory, inference and learning algorithms. Cambridge university press. [Google Scholar]
- Marchant NL, Howard RJ, 2015. Cognitive debt and Alzheimer’s disease. J Alzheimers Dis 44(3), 755–770. [DOI] [PubMed] [Google Scholar]
- Marchant NL, Lovland LR, Jones R, Pichet Binette A, Gonneaud J, Arenaza-Urquijo EM, Chetelat G, Villeneuve S, Group P.-A.R., 2020. Repetitive negative thinking is associated with amyloid, tau, and cognitive decline. Alzheimers Dement. [DOI] [PubMed] [Google Scholar]
- McEvoy PM, Watson H, Watkins ER, Nathan P, 2013. The relationship between worry, rumination, and comorbidity: evidence for repetitive negative thinking as a transdiagnostic construct. Journal of affective disorders 151(1), 313–320. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD, 1990. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther 28(6), 487–495. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br J Psychiatry 134, 382–389. [DOI] [PubMed] [Google Scholar]
- Moons KG, Donders RA, Stijnen T, Harrell FE Jr., 2006. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 59(10), 1092–1101. [DOI] [PubMed] [Google Scholar]
- Newgard CD, Haukoos JS, 2007. Advanced statistics: missing data in clinical research--part 2: multiple imputation. Acad Emerg Med 14(7), 669–678. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, 2000. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol 109(3), 504–511. [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, Malone KM, 2010. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun 24(7), 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Slavich GM, Epel ES, Neylan TC, 2013. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci Biobehav Rev 37(1), 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke OI, Grodstein F, 2013. Phobic anxiety and cognitive performance over 4 years among community-dwelling older women in the Nurses’ Health Study. Am J Geriatr Psychiatry 21(11), 1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke OI, Prescott J, Wong JY, Han J, Rexrode KM, De Vivo I, 2012. High phobic anxiety is related to lower leukocyte telomere length in women. PLoS One 7(7), e40516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, 2011. Statistical parametric mapping: the analysis of functional brain images. Elsevier. [Google Scholar]
- Perna G, Iannone G, Alciati A, Caldirola D, 2016. Are Anxiety Disorders Associated with Accelerated Aging? A Focus on Neuroprogression. Neural Plast 2016, 8457612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Lim YY, Neumeister A, Ames D, Ellis KA, Harrington K, Lautenschlager NT, Restrepo C, Martins RN, Masters CL, Villemagne VL, Rowe CC, Maruff P, Australian Imaging B., Lifestyle Research G., 2015. Amyloid-beta, anxiety, and cognitive decline in preclinical Alzheimer disease: a multicenter, prospective cohort study. JAMA psychiatry 72(3), 284–291. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Maruff P, Woodward M, Fredrickson J, Fredrickson A, Krystal JH, Southwick SM, Darby D, 2012. Mild worry symptoms predict decline in learning and memory in healthy older adults: a 2-year prospective cohort study. Am J Geriatr Psychiatry 20(3), 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Scott JC, Neumeister A, Lim YY, Ames D, Ellis KA, Harrington K, Lautenschlager NT, Szoeke C, Martins RN, Masters CL, Villemagne VL, Rowe CC, Maruff P, Australian Imaging, B., Lifestyle Research, G., 2014. Anxiety symptoms, cerebral amyloid burden and memory decline in healthy older adults without dementia: 3-year prospective cohort study. Br J Psychiatry 204, 400–401. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN, 2009. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry 14(10), 959–967. [DOI] [PubMed] [Google Scholar]
- Rubin DB, 2004. Multiple imputation for nonresponse in surveys. John Wiley & Sons. [Google Scholar]
- Salvi F, Miller MD, Grilli A, Giorgi R, Towers AL, Morichi V, Spazzafumo L, Mancinelli L, Espinosa E, Rappelli A, Dessi-Fulgheri P, 2008. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc 56(10), 1926–1931. [DOI] [PubMed] [Google Scholar]
- Schafer JL, 1999. Multiple imputation: a primer. Stat Methods Med Res 8(1), 3–15. [DOI] [PubMed] [Google Scholar]
- Schoofs H, Hermans D, Raes F, 2010. Brooding and reflection as subtypes of rumination: Evidence from confirmatory factor analysis in nonclinical samples using the Dutch Ruminative Response Scale. Journal of Psychopathology and Behavioral Assessment 32(4), 609–617. [Google Scholar]
- Schrouff J, Rosa MJ, Rondina JM, Marquand AF, Chu C, Ashburner J, Phillips C, Richiardi J, Mourao-Miranda J, 2013. PRoNTo: pattern recognition for neuroimaging toolbox. Neuroinformatics 11(3), 319–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunk D, 2008. A Markov chain Monte Carlo algorithm for multiple imputation in large surveys. AStA Advances in Statistical Analysis 92(1), 101–114. [Google Scholar]
- Segerstrom SC, Roach AR, Evans DR, Schipper LJ, Darville AK, 2010. The structure and health correlates of trait repetitive thought in older adults. Psychology and aging 25(3), 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vidaurre D, Alfaro-Almagro F, Nichols TE, Miller KL, 2019. Estimation of brain age delta from brain imaging. Neuroimage 200, 528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterlin S, Paap MC, Babic S, Kubler A, Vogele C, 2012. Rumination and age: some things get better. J Aging Res 2012, 267327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Chui H, 1987. The modified mini-mental state examination (3MS). Can J Psychiatry 41(2), 114–121. [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S, 2003. Rumination reconsidered: A psychometric analysis. Cognitive therapy and research 27(3), 247–259. [Google Scholar]
- Tully PJ, Cosh SM, Baumeister H, 2014. The anxious heart in whose mind? A systematic review and meta-regression of factors associated with anxiety disorder diagnosis, treatment and morbidity risk in coronary heart disease. J Psychosom Res 77(6), 439–448. [DOI] [PubMed] [Google Scholar]
- Tully PJ, Cosh SM, Baune BT, 2013. A review of the affects of worry and generalized anxiety disorder upon cardiovascular health and coronary heart disease. Psychol Health Med 18(6), 627–644. [DOI] [PubMed] [Google Scholar]
- Urry HL, Gross JJ, 2010. Emotion Regulation in Older Age. Current Dirrections inPsychological Science 19 (6): 352–357. [Google Scholar]
- Whitmer A, Gotlib IH, 2011. Brooding and reflection reconsidered: A factor analytic examination of rumination in currently depressed, formerly depressed, and never depressed individuals. Cognitive Therapy and Research 35(2), 99–107. [Google Scholar]
- Zebb BJ, Beck JG, 1998. Worry versus anxiety. Is there really a difference? Behav Modif 22(1), 45–61. [DOI] [PubMed] [Google Scholar]


