Abstract
Objective:
To examine the effects of selenium supplementation on pregnancy out-comes and disease progression among HIV-infected pregnant women in Lagos.
Methods:
A randomized, placebo-controlled trial conducted among HIV-positive pregnant women between September 2018 and August 2019. At enrollment, 90 women were randomly assigned into each treatment arm to receive either a daily tablet of 200 μg elemental selenium or a placebo. Relevant participants’ sociodemographic and clinical data were collected at enrollment and delivery.
Results:
Women in the selenium arm had a significantly lower risk of preterm delivery (relative risk [RR] 0.32, 95% confidence interval [CI] 0.11–0.96) and a non-significant reduction in the risk of delivering term neonates with a low delivery weight (RR 0.24, 95% CI 0.05–1.19). Supplemental selenium does not increase the risk of perinatal death and adverse drug events.
Conclusion:
The study reported a beneficial effect of prenatal selenium supplements on the risk of preterm delivery with no further reduction in risk among HIV-infected women who used the supplements for more than 14 weeks.
Trial registration:
Pan African Clinical Trial Registry (PACTR201809756724274).
Keywords: CD4+ cell count, Lagos, Lagos University Teaching Hospital, low birth weight, preterm delivery, selenium, viral load
1 |. INTRODUCTION
Micronutrient deficiencies are common among pregnant women from economically disadvantaged settings where diets with low content of minerals and vitamins are consumed. Pregnant women and their children under-5 are at the highest risk.1 Selenium, a non-metallic chemical element of great importance to human health,2 is one of the key components of several human selenoproteins that are involved in redox reactions.3 Selenium is an antioxidant that helps to protect the body against the damaging effects of free radicals and it is essential for the activity of the enzyme glutathione peroxidase.4 Glutathione peroxidase is the main intracellular antioxidant that protects against reactive oxygen species and subsequent oxidation-induced cellular damage.4,5 Biochemical selenium deficiency has been associated with accelerated disease progression6 and increased mortality7 among HIV-infected humans. These may be attributed to the role of selenium in antioxidant defense and immunity.8 We previously reported a 20.4% prevalence of selenium deficiency among HIV-positive Nigerian pregnant women, and that low selenium status in HIV-infected women significantly increased the risk of preterm birth and the delivery of low birth weight neonates.9 Several studies have also reported low selenium status in a significant proportion of HIV-infected individuals, and serum selenium concentration has been shown to decline with HIV disease progression.10
Data on the selenium content of Nigerian or African foods is limited. The selenium content of plant foods varies with the selenium content of the soil in a geographical location.11 The Recommended Dietary Intake (RDI) of selenium for pregnant women is 60 μg/day and its upper tolerable intake for adults is set at 400 μg (5.1 μmol) selenium/day.11 Given the high upper tolerable intake and the regulation of body homeostasis through urinary excretion, selenium supplementation is regarded as a safe intervention.11,12 There are currently few studies among pregnant HIV-infected Black African women that have examined the effects of selenium supplementation on pregnancy outcomes. This study is, therefore, aimed to assess the effects of prenatal selenium supplementation on the major pregnancy outcomes (preterm birth and low birth weight) and HIV disease progression among HIV-infected pregnant women at the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria.
2 |. MATERIALS AND METHODS
This is a prospective, randomized, double-blind, placebo-controlled trial involving confirmed HIV-seropositive pregnant women who sought care at the prenatal clinics of LUTH. The Health Research Ethics Committee of LUTH’s Institutional Review Board granted ethical approval and provided the oversight function for the study. The trial protocol was registered, modified, and updated for completion on the Pan African Clinical Trial Registry (PACTR201809756724274). The detailed protocol is published elsewhere13 and some of the details are described below.
Participants were enrolled in the trial between September 1, 2018 and April 31, 2019 (modification of the trial protocol).13 Participation was voluntary and eligible participants enrolled in the study were confirmed HIV-seropositive pregnant women aged 15–49 years with a singleton pregnancy at 14–27 weeks of gestation. Women who had multiple gestations, significant renal and hepatic impairment, those with an expected delivery date beyond July 2019 (modification of the trial protocol),13 those who had received a long course (at least 3 months) of mineral supplements containing selenium in the last 6 months before enrollment, and those who refused consent at enrollment were excluded from the study. Before participant enrollment, ethical approval was obtained from the study hospital’s Health Research Ethics Committee (Approval Number, ADM/DCST/HREC/APP/2438). The ethical principles of the Helsinki Declaration were applied throughout the course of the study. All participants were counseled before enrollment and read and signed an informed consent form. The investigators ensured strict confidentiality of participant information. The study sponsor had no role in the study design, data collection, data analyses, data interpretation, or writing of the report.
The primary end points examined were preterm birth (delivery before 37 completed weeks of pregnancy) and low birth weight in term neonates (baby’s birth weight less than 2500 g) whereas the secondary end points were HIV disease progression (change in CD4+ cell count and viral load), change in hemoglobin concentrations, adverse events such as hepatotoxicity, gastrointestinal disturbances, dermatologic effects (nail and hair loss, and dermatitis), and neurotoxicity, and perinatal mortality (stillbirths and neonatal deaths in the first week of life).
Sample size was calculated using the data derived from our previous study on the effects of selenium status on the pregnancy outcome of HIV-infected pregnant women9 and a randomized controlled trial on the effect of selenium supplementation on pregnancy outcome by Kupka et al.14 To investigate the effect of selenium supplementation on the primary end point (preterm delivery) and the major secondary end point (HIV disease progression), we used an estimated weighted effect size of 0.3 for a two-sided test and type I error rate of 5% to achieve a power of 80%, that is, Zα = 1.96 and Zβ = 0.84, adjusted for a 20% drop-out rate to give a sample size of 179. Therefore, a total sample size of 180 HIV-infected pregnant women was enrolled in the study and they were randomly assigned using a 1:1 block randomization code generated from Random Allocation software version 1.0 (May 2004) to receive a daily oral tablet of 200 μg elemental selenium or placebo for the duration of their pregnancies.
Data collection began on the day a participant was assigned into a treatment arm and continued until the termination of the trial at delivery or until the participant withdrew from the trial at any time for any reason (Figure 1). Using a structured interviewer-administered questionnaire at enrollment, we collected data on the participant’s sociodemographic status and obstetric history, duration of HIV diagnosis (in months), WHO clinical stage of the disease,15 type and duration of antiretroviral treatment use (in months), baseline hemoglobin levels (in g/dl), CD4+ cell count (in cells/mm3), viral load (in copies/ml), and body mass index (BMI; calculated as maternal weight [using the actual pre-gestational or first-trimester measurement] in kilograms divided by the square of height in meters). Gestational age was based on the date of the participant’s last menstrual period, which was obtained at the time of random assignment whereas the participants’ socioeconomic classes were determined using the women’s educational levels and their partners’ occupations as proposed by Olusanya et al.16 The woman’s level of education was scored as: 0, tertiary education; 1, secondary level; and 2, primary education or less; and the husband/partner’s occupation was scored as: 1, professional; 2, semi-skilled; and 3, unskilled. The sum of both scores gave the socio-economic class of the woman. Class 1 represents the highest while Class 5 represents the lowest socioeconomic class with Class 2, 3, and 4 in between. All women received the standard prenatal doses of iron, folic acid, and malarial chemoprophylaxis.
FIGURE 1.
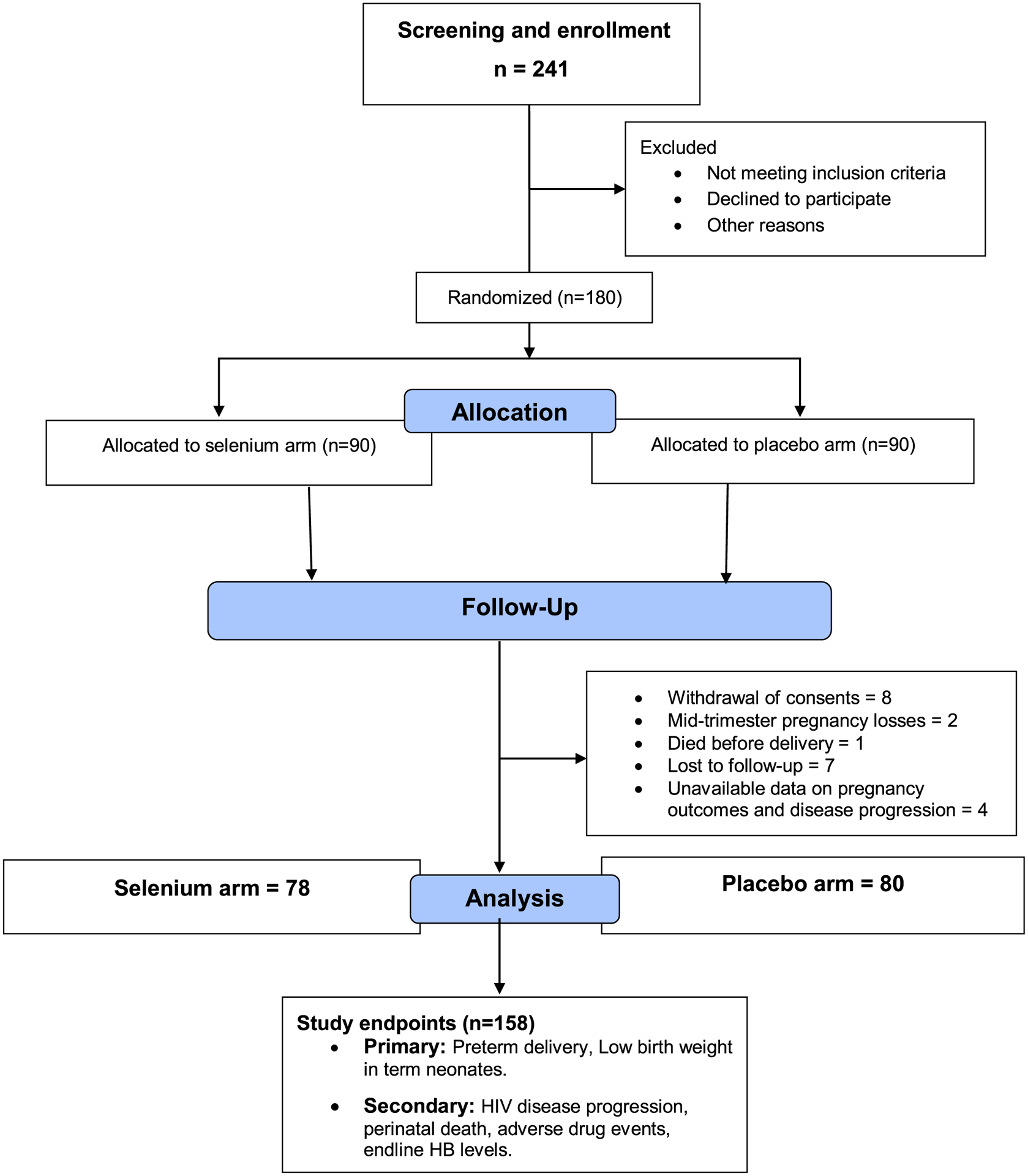
Flowchart showing selection of the study population.
Allocation concealment was as described in the study protocol.13 The randomization codes were generated from Random Allocation software version 1.0 (May 2004) by the study statistician. The active drug (selenium) and placebo tablets were indistinguishable in shape, size, and color, and were packed in identical coded transparent dispensing sachets by the on-site pharmacy technician, who then stored the coded randomization list in sequentially numbered, opaque, sealed envelopes that were kept in a locked filing cabinet at the study site until the end of the study. At every 2-weekly follow-up visit, a newly filled dispensing sachet with the same numeric code was given to each woman and pills remaining in used sachets were counted to assess compliance. The study investigators, clinic staff, and participants were blinded to the treatment arms.
At delivery, data were collected from the participant’s case notes on the most recent CD4+ cell count and viral load (performed within the last 6 weeks), and the most recent hemoglobin levels. The total duration of supplement intake—selenium or placebo—in weeks, level of compliance with use of the intervention (good compliance level is defined as usage of at least 10 of the 16, 2-weekly prescribed tablets), gestational age at delivery (in weeks), and the baby’s birth weight (in grams) were also recorded. Original data were transferred to an electronic database system located in a guarded facility at the trial site by the research assistant. Access to the study data was restricted with the principal investigator having access to only the final data set. An independent steering committee monitored and ensured strict compliance with the study protocol.13
Statistical analyses were carried out using SPSS version 23.0 for Windows (IBM) and the intention-to-treat principle was used in the outcome data analyses. Quantitative data were tested for normality using the Kolmogorov-Smirnov test with Lilliefors significance correction and log transformation (log10) was performed on maternal CD4+ cell counts and viral load. The associations between any two groups of continuous variables were tested using the independent sample t-test (normal distribution) or the Mann-Whitney U test (skewed data) and that of two groups of categorical variables with χ2 or Fisher exact test where appropriate. Multivariate analyses using the multinomial logistic regression model were used to assess the association between the treatment and the study end points. Variables with p less than 0.2 were included in the final multivariate analyses and their interaction effects on the study end points were estimated as relative risk (RR). The mean differences for maternal hemoglobin concentration, CD4+ cell counts, and viral load in between treatment arms were estimated using the linear mixed models for repeated measurements. Point estimates of post-randomization change in values and 95% confidence intervals (CIs) directly modelled the difference between repeated measures. The level of significance was reported at P less than 0.05.
3 |. RESULTS
Initially, 180 HIV-infected pregnant women at 14–27 weeks of singleton pregnancies were enrolled and randomly assigned to the treatment arms. At the time of random allocation, the mean age of the participating women was 29.7 ± 6.0 years and the mean length of pregnancy was 19.5 ± 4.0 weeks. There were no significant differences in the baseline characteristics of the two treatment arms (Table 1).
TABLE 1.
Baseline characteristics of participants in the treatment armsa
| Characteristic | Total (n = 180) | Selenium (n = 90) | Placebo (n = 90) | P value |
|---|---|---|---|---|
| Age, years | 29.7 ± 6.0 | 29.3 ± 6.3 | 30.1 ± 5.8 | 0.356 |
| Pregnancy duration at enrollment, weeks | 19.5 ± 4.0 | 20.5 ± 3.9 | 19.7 ± 4.0 | 0.208 |
| Body mass index | 26.2 ± 5.2 | 26.7 ± 5.6 | 25.6 ± 4.7 | 0.176 |
| Prior pregnancies | ||||
| 0 | 28 (15.6) | 14 (15.6) | 14 (15.6) | 0.943 |
| 1–3 | 143 (79.4) | 72 (80.0) | 71 (78.9) | |
| >3 | 9 (5.0) | 4 (4.4) | 5 (5.6) | |
| Tribe | ||||
| Yoruba | 86 (47.8) | 42 (46.7) | 44 (48.9) | 0.917 |
| Ibo | 42 (23.3) | 23 (25.6) | 19 (21.1) | |
| Hausa | 33 (18.3) | 16 (17.8) | 17 (18.9) | |
| Others | 19 (10.6) | 9 (10.0) | 10 (11.1) | |
| Level of education | ||||
| Unschooled | 13 (7.2) | 8 (8.9) | 5 (5.6) | 0.803 |
| Primary education | 52 (28.9) | 27 (30.5) | 25 (27.8) | |
| Secondary education | 82 (45.6) | 39 (43.3) | 43 (47.8) | |
| Tertiary education | 33 (18.3) | 16 (17.8) | 17 (18.9) | |
| Occupation | ||||
| Housewife | 31 (17.2) | 14 (15.6) | 17 (18.9) | 0.630 |
| Trader | 87 (48.3) | 42 (46.7) | 45 (50.0) | |
| Artisan | 19 (10.6) | 12 (13.3) | 7 (7.8) | |
| Civil servant | 43 (23.9) | 22 (24.4) | 21 (23.3) | |
| Social class | ||||
| Class 1 | 29 (16.1) | 13 (14.4) | 16 (17.8) | 0.564 |
| Class 2 | 13 (7.2) | 7 (7.8) | 6 (6.7) | |
| Class 3 | 72 (40.0) | 35 (38.9) | 37 (41.1) | |
| Class 4 | 54 (30.0) | 31 (34.4) | 23 (25.6) | |
| Class 5 | 12 (6.7) | 4 (4.4) | 8 (8.9) | |
| WHO disease stage | ||||
| Stage 1 | 110 (61.1) | 55 (61.1) | 55 (61.1) | 0.938 |
| Stage 2 | 61 (33.9) | 30 (33.3) | 31 (34.4) | |
| Stage 3 | 9 (5.0) | 5 (5.6) | 4 (4.4) | |
| Time since HIV diagnosis, months | ||||
| <12 | 56 (31.1) | 27 (30.0) | 29 (32.2) | 0.9480.948 |
| 12–60 | 110 (61.1) | 56 (62.2) | 54 (60.0) | |
| ≥60 | 14 (7.8) | 7 (7.8) | 7 (7.8) | |
| ART duration, months | ||||
| <6 | 101 (56.1) | 50 (55.6) | 51 (56.7) | 0.881 |
| ≥6 | 79 (43.9) | 40 (44.4) | 39 (43.3) | |
| Hemoglobin, g/dl | 10.8 ± 1.7 | 10.9 ± 1.5 | 10.7 ± 1.8 | 0.352 |
| CD4+ cells count (log)b | 2.5 ± 0.3 | 2.6 ± 0.2 | 2.5 ± 0.3 | 0.513 |
| Viral load (log)b | 3.7 ± 1.6 | 3.6 ± 1.5 | 3.8 ± 1.6 | 0.540 |
Abbreviations: ART, antiretroviral treatment; BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters).
Values are given as mean ± SD, or number (percentage) unless indicated otherwise.
A base-10 scale was used.
Of the 180 women randomized, eight withdrew their consent during the study for personal reasons, two experienced mid-trimester pregnancy losses, one died before delivery, seven were lost to follow up, and four did not have any available data on their pregnancy outcomes and HIV disease progression (12 in the selenium arm and 10 in the placebo arm; P = 0.649) (Figure 1). The median follow-up period from randomization to delivery among the participants was 17 weeks (interquartile range 14–21 weeks).
Of the randomized women, 19 (10.6%) had preterm deliveries with an incidence of 5.6% (n = 5) in women in the selenium arm compared with 15.6% (n = 14) in women in the placebo arm (P = 0.091). Among the 161 women who had term deliveries, 9 (5.0%) had neonates with a low delivery weight with women who had prenatal selenium supplementation having an incidence of 2.4% (n = 2) compared with 9.2% (n = 7) among those in the placebo arm (P = 0.167). We recorded 4 (2.2%) perinatal deaths and 34 (18.9%) women with various adverse drug events in the study. However, the use of selenium supplements had no statistically significant effects on the risks of perinatal death (P = 0.932) and adverse drug events (P = 0.898) (Table 2).
TABLE 2.
Effects of selenium supplementation on the primary and secondary end points (n = 180)
| Endpoint | Selenium (n = 90) | Placebo (n = 90) | P value |
|---|---|---|---|
| Pregnancy duration at delivery | |||
| Preterm (<37 weeks) | 5 (5.6) | 14 (15.6) | 0.091 |
| Term (≥37 weeks) | 73 (81.1) | 66 (73.3) | |
| Drop-out/incomplete data | 12 (13.3) | 10 (11.1) | |
| Delivery weight for term neonatesb | |||
| <2500 g | 2 (2.4) | 7 (9.2) | 0.167 |
| ≥ 2500 g | 71 (83.5) | 59 (77.6) | |
| Drop-out/incomplete data | 12 (14.1) | 10 (13.2) | |
| Perinatal death | |||
| Dead | 2 (2.2) | 2 (2.2) | 0.932c |
| Alive | 76 (84.4) | 78 (86.7) | |
| Drop-out/incomplete data | 12 (13.3) | 10 (11.1) | |
| Adverse event | |||
| Yes | 17 (18.9) | 17 (18.9) | 0.898 |
| No | 61 (68.7) | 63 (70.0) | |
| Drop-out/incomplete data | 12 (13.3) | 10 (11.1) | |
Abbreviations: CI, confidence interval; RR, relative risks.
Values are given as number (percentage).
n = 161.
Fisher exact test.
On performing the multivariate analysis of all variables at end-line, mothers on prenatal selenium supplements have a statistically significant lower risk of preterm delivery compared with women in the placebo arm (RR 0.32, 95% CI 0.11–0.95; P = 0.039). We also recorded that the use of prenatal supplemental selenium tablets for at least 14 weeks decreased the risk of preterm delivery three-fold (RR 0.32, 95% CI 0.11–0.86; P = 0.025). However, with further interaction analysis between treatment type and duration of use of treatment, the use of selenium supplements for a minimum duration of 14 weeks did not have any influence on the risk of preterm delivery (Table 3). The use prenatal selenium supplements had no statistically significant effect on the risk of delivery of a low birth weight neonate at term (RR 0.24, 95% CI 0.05–1.19; P = 0.080) (Table 4).
TABLE 3.
Relative risks of intervention-covariate effect on preterm delivery (n = 180)
| Intervention and covariates | Estimates of effect on preterm delivery | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| RR (95% CI) | P value | aRR (95% CI) | P value | |
| Intervention | ||||
| Selenium | 0.32 (0.11–0.95) | 0.039 | 0.32 (0.11–0.96) | 0.041 |
| Placebo | 1.00 (ref) | 1.00 (reference) | ||
| Duration of intervention, weeks | ||||
| ≥14 | 0.31 (0.11–0.86) | 0.025 | 0.32 (0.10–0.88) | 0.028 |
| <14 | 1.00 (ref) | 1.00 (reference) | ||
| Compliance to intervention | ||||
| Good | 0.86 (0.26–2.82) | 0.807 | - | - |
| Poor | 1.00 (ref) | - | ||
| Hemoglobin concentration, g/dl | ||||
| ≥10 | 1.56 (0.48–5.06) | 0.459 | - | - |
| <10 | 1.00 (ref) | - | ||
| CD4+ cells count/mm3 | ||||
| ≥350 | 0.79 (0.24–2.58) | 0.692 | - | - |
| <350 | 1.00 (ref) | - | ||
| Viral load, copies/ml | ||||
| ≥1000 | 1.59 (0.59–4.29) | 0.355 | - | - |
| <1000 | 1.00 (ref) | - | ||
Abbreviations: aRR, adjusted relative risk; CI, confidence interval; RR, relative risk.
TABLE 4.
Relative risks of intervention-covariate effect on low birth weight in term neonates (n = 161)
| Intervention and covariates | Estimates of effect on low birth weight | |
|---|---|---|
| RR (95% CI) | P value | |
| Intervention | ||
| Selenium | 0.24 (0.05–1.19) | 0.080 |
| Placebo | 1.00 (ref) | |
| Duration of intervention, weeks | ||
| ≥14 | 0.60 (0.12–3.10) | 0.542 |
| <14 | 1.00 (ref) | |
| Compliance to intervention | ||
| Good | 0.79 (0.16–4.06) | 0.780 |
| Poor | 1.00 (ref) | |
| Hemoglobin concentration, g/dl | ||
| ≥10 | 0.83 (0.19–3.48) | 0.794 |
| <10 | 1.00 (ref) | |
| CD4+ cells count/mm3 | ||
| ≥350 | 0.50 (0.12–2.13) | 0.349 |
| <350 | 1.00 (ref) | |
| Viral load, copies/ml | ||
| ≥1000 | 1.37 (0.53–5.34) | 0.649 |
| <1000 | 1.00 (ref) | |
Abbreviations: RR, relative risk.
In Table 5, we report that selenium supplementation had no statistically significant effect on the change in the levels of hemoglobin concentrations between enrollment and delivery (mean difference 0.112, 95% CI −0.380 to 0.605; P = 0.653). Antenatal use of selenium supplements also had no significant effects on the levels of HIV disease markers between enrollment and delivery (CD4+ cell count, P = 0.326 and viral load, P = 0.230).
TABLE 5.
Effects of selenium supplementation on change in haemoglobin levels and HIV disease progression
| Endpoint | Mean value in intervention armsd | Mean difference (95% CI)c | p-valuee | ||
|---|---|---|---|---|---|
| Selenium | Placebo | ||||
| Hb concentration | Baseline | 10.82 ± 1.43 | 10.55 ± 1.75 | 0.27 (−0.23 to 0.77) | 0.653 |
| Endline | 10.98 ± 1.44 | 10.90 ± 1.71 | 0.08 (−0.42 to 0.58) | ||
| Within-group mean difference ± SD | −0.16 ± 2.20 | −0.35 ±2.55 | 0.11 (−0.38 to 0.61)b | ||
| CD4+ cells count (log)a | Baseline | 2.56 ±0.24 | 2.52 ±0.26 | 0.04 (−0.04 to 0.12) | 0.326 |
| Endline | 2.70 ± 0.18 | 2.67 ± 0.20 | 0.03 (−0.03 to 0.09) | ||
| Within-group mean difference ± SD | −0.14 ± 0.13 | −0.16 ±0.13 | 0.03 (−0.03 to 0.09)b | ||
| Viral load (log)a | Baseline | 3.55 ± 1.61 | 3.84 ±1.59 | −0.29 (−0.79 to 0.22) | 0.230 |
| Endline | 3.60 ±1.63 | 2.92 ± 1.67 | −0.31 (−0.83 to 0.20) | ||
| Within-group mean difference ± SD | 0.95 ± 0.94 | 0.92 ± 0.76 | −0.31 (−0.83 to 0.20)b | ||
Abbreviation: CI, confidence interval.
Values are the mean difference between the selenium and placebo arms. The mean differences, 95% CIs directly modelled the differences between repeated measures.
P value obtained from a robust parallel-group analysis with adjustment for baseline variables.
Based on linear mixed models for repeated measurements.
A base-10 scale was used.
4 |. DISCUSSION
In this randomized controlled trial, the use of prenatal selenium supplements in HIV-infected Nigerian women did not have significant effects on HIV disease progression. However, there was a statistically significant decrease in the risk of preterm delivery in women who received selenium supplements in pregnancy and this effect was further accentuated by the use of the supplements for a minimum duration of 14 weeks.
We reported only one maternal death in the course of this study. There were also four (2.2%) perinatal deaths reported, equivalent to 25.3 deaths per 1000 total births, and this is significantly lower than the national perinatal mortality rate of 41 deaths per 1000 births in 2013.17 These findings were, however, not surprising because all the women randomized in the study were booked prenatal clients of the hospital, who were most likely exposed to routine effective prenatal interventions such as health promotion, risk assessment, and prevention and prompt treatment of all pregnancy-related conditions. We could not attribute these reduced maternal and perinatal deaths to the intervention assignment as our study was not powered primarily to detect these effects.
Nutritional deficiencies are common among individuals with HIV infections because of factors such as the oxidative state induced by the virus, malabsorption, metabolic alterations, gut infections, and gut barrier dysfunction produced by chronic HIV infection.18 Our previous study9 and other epidemiological studies19,20 have shown that HIV infection is associated with low selenium status. The lack of effects of prenatal selenium supplements on most of the pregnancy outcomes and HIV disease progression in this study was corroborated by another randomized controlled trial conducted by Kupka et al.14 in Dar es Salaam, Tanzania. This is despite our previous study in the same setting in Lagos reporting a relatively high prevalence of selenium deficiency (20.4%) among HIV-seropositive pregnant women.9 There are several possible explanations for this lack of effects. It is possible that the selenium supplement is only effective among patients with advanced HIV disease but participants in these trials were primarily in the asymptomatic early stage of the disease. The beneficial effects of high selenium supplement use may also be confounded significantly by the optimal markers of HIV disease and progression at enrollment and delivery in this study. However, in contrast to our current study and that of Kupka et al.,14 Hurwitz et al.6 reported in their randomized, placebo-controlled trial conducted in Miami, Florida that the use of daily selenium supplements in HIV-1-seropositive men and non-pregnant women can suppress the progression of HIV-1 through a reduction in viral load and an increase in CD4+ cell count. Also, conversely to our study, a recent Norwegian prospective population-based cohort study by Barman et al.21 in pregnant women regardless of their HIV-status showed that maternal dietary selenium intake, but not supplemental selenium intake, during the first half of pregnancy was significantly associated with a decreased risk of preterm delivery. Prelabor rupture of the membranes is the cause of about one-third of all preterm births,22 so the finding that selenium supplementation (100 μg/day) effectively reduces the incidence of prelabor rupture of the membranes in a 2010 study by Tara et al.23 among primigravid pregnant women was similarly supported by our present study. This indicated an effect that may be attributable to the anti-inflammatory and antioxidant properties of this important trace element.24
Our study reported no further reduction in the risk of preterm delivery among HIV-infected women who took their selenium supplements for more than 14 weeks. This suggests that prolonged use of supplemental selenium in pregnant women has no additional beneficial effect on their risk of having a preterm birth. Therefore, HIV-infected mothers should be encouraged to commence the use of the supplement at any point of presentation in pregnancy. This is particularly important in this part of the world where a vast majority of pregnant women will not book until much later in pregnancy.
We recorded a significant proportion of the participants (18.9%) reporting one or more adverse drug events but there was no statistically significant association with the intervention administered. These are patient-reported symptoms and may be attributed to the concept of “placebo effect”, which is recognized as a genuine psychobiological phenomenon due to the overall therapeutic context in both laboratory and clinical settings.25 These responses may also be due to the participants’ lack of awareness of most unusual pregnancy-related symptoms, which may erroneously be attributed to their use of the intervention agent.
The major limitation of this study was the extreme difficulty encountered in extracting reliable information on the intake of selenium-rich diets or selenium-containing supplements from some of the participants, and this factor could have had some direct or indirect influence on the study findings. The study was also not powered to detect the influence of the study intervention on the secondary end points and this may account for the absence of any significant effect as reported. However, this is the first trial of selenium supplementation conducted among HIV-infected pregnant women of West African descent.
In conclusion, the study reported a beneficial effect of prenatal selenium supplements on preterm delivery among HIV-infected pregnant women, with no further risk reduction when selenium was used for more than 14 weeks. Therefore, there is a justification for providing selenium supplements to HIV-infected pregnant women, irrespective of their selenium status and women should be encouraged to use the supplements at any point of their presentation in pregnancy to reduce their risk of preterm delivery. Given the rise in preterm births over the past three decades and the associated huge public healthcare costs, the findings of this study could have implications for the future evidence-based management of HIV in pregnancy.
ACKNOWLEDGMENTS
The work reported in this publication was supported by the Fogarty International Center and National Institute of Mental Health of the National Institutes of Health under Award Number D43TW010543. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLIC TS OF INTEREST
The authors have no conflicts of interest.
REFERENCES
- 1.Bailey RL, West KP Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(suppl 2):22–33. [DOI] [PubMed] [Google Scholar]
- 2.Mehta S, Fawzi WW. Micronutrient supplementation as adjunct treatment for HIV-infected patients. Clin Infect Dis. 2010;50(12):1661–1663. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger FP, Raman AV, Reeves MA, Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J. 2009;422(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rayman MP. The importance of selenium to human health. Lancet. 2000;356(9225):233–241. [DOI] [PubMed] [Google Scholar]
- 5.Schweizer U, Fradejas-Villar N. Why 21? The significance of seleno-proteins for human health revealed by inborn errors of metabolism. FASEB J. 2016;30(11):3669–3681. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz BE, Klaus JR, Llabre MM, et al. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Arch Intern Med. 2007;167(2):148–154. [DOI] [PubMed] [Google Scholar]
- 7.Kupka R, Msamanga GI, Spiegelman D, et al. Selenium status is associated with accelerated HIV disease progression among HIV-1-infected pregnant women in Tanzania. J Nutr. 2004;134(10):2556–2560. [DOI] [PubMed] [Google Scholar]
- 8.Baum MK, Campa A. Role of selenium in HIV/AIDS. In: Hatfield DL, Berry MJ, Gladyshev VN, eds. Selenium - Its Molecular Biology and Role in Human Health. New York: Springer; 2006:299–310. [Google Scholar]
- 9.Okunade KS, Olowoselu OF, Osanyin GE, John-Olabode S, Akanmu SA, Anorlu RI. Selenium deficiency and pregnancy outcome in pregnant women with HIV in Lagos, Nigeria. Int J Gynaecol Obstet. 2018;142(2):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck KW, Schramel P, Hedl A, Jaeger H, Kaboth W. Serum trace element levels in HIV-infected subjects. Biol Trace Elem Res. 1990;25(2):89–96. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academies Press (US); 2000:7, Selenium. https://www.ncbi.nlm.nih.gov/books/NBK225470/ [PubMed] [Google Scholar]
- 12.Bergamaschi DP, Mariath AB, Abbade JF, Grillo LP, Diniz CSG, Hinnig PF. Selenium supplementation during pregnancy for improving maternal and newborn outcomes. Cochrane Database Syst Rev. 2012;3:CD009673. [Google Scholar]
- 13.Okunade KS, John-Olabode S, Akinsola OJ, Akinajo O, Akanmu SA, Kanki PJ. Effects of selenium supplementation on pregnancy outcome and disease progression in HIV-infected pregnant women in Lagos, Nigeria: study protocol for a randomised, double-blind, placebo-controlled trial. Medicine. 2019;98(3):e12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kupka R, Mugusi F, Aboud S, et al. Randomized, double-blind, placebo-controlled trial of selenium supplements among HIV infected pregnant women in Tanzania: effects on maternal and child out-comes. Am J Clin Nutr. 2008;87(6):1802–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Interim WHO Clinical Staging of HIV/AIDS and HIV/AIDS Case Definitions for Surveillance (African Region). Geneva: WHO; 2005:20–28. [Google Scholar]
- 16.Olusanya O, Okpere E, Ezimokhai M. The importance of social class in voluntary fertility control in a developing country. West Afr J Med. 1985;4(4):205–212. [Google Scholar]
- 17.National Population Commission. Federal Republic of Nigeria: final report on Nigeria Demographic and Health Survey. 2013. http://dhsprogram.com/publications/publication-fr293-dhs-final-reports.Cfm. Accessed September 22, 2020.
- 18.Thuppal SV, Jun S, Cowan A, Bailey RL. The nutritional status of HIV-infected US adults. Curr Dev Nutr. 2017;1(10):e001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogunro PS, Ogungbamigbe TO, Elemie PO, Egbewale BE, Adewole TA. Plasma selenium concentration and glutathione peroxidase activity in HIV-1/ AIDS infected patients: a correlation with the disease progression. Niger Postgrad Med J. 2006;13(1):1–5. [PubMed] [Google Scholar]
- 20.Khalili H, Soudbakhsh A, Hajiabdolbaghi M, et al. Nutritional status and serum zinc and selenium levels in Iranian HIV infected individuals. BMC Infect Dis. 2008;8:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barman M, Brantsæter AL, Nilsson S, et al. Maternal dietary selenium intake is associated with increased gestational length and decreased risk of preterm delivery. Br J Nutr. 2020;123(2):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FIGO Working Group on Good Clinical Practice in Maternal-Fetal Medicine. Good clinical practice advice: prediction of preterm labor and preterm premature rupture of membranes. Int J Gynaecol Obstet. 2019;144(3):340–346. [DOI] [PubMed] [Google Scholar]
- 23.Tara F, Rayman MP, Boskabadi H, et al. Selenium supplementation and premature (pre-labour) rupture of membranes: a ran-domised double-blind placebo-controlled trial. J Obstet Gynaecol. 2010;30(1):30–34. [DOI] [PubMed] [Google Scholar]
- 24.Curran JE, Jowett JBM, Elliott KS, et al. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005;37:1234–1241. [DOI] [PubMed] [Google Scholar]
- 25.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375(9715):686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]


