Abstract
Objective:
To assess the impact of different types of physical activity types on longitudinal knee joint structural changes over 48-months in overweight and obese subjects.
Materials and Methods:
We included 415 subjects with a BMI ≥ 25kg/m2, Kellgren-Lawrence scores ≤ 3 at baseline and Whole-Organ Magnetic Resonance Imaging Score (WORMS) scores available from the Osteoarthritis Initiative cohort. Regular self-reported participation in six physical activity types was assessed: ball sports, bicycling, jogging/running, elliptical-trainer, racquet sports and swimming. Moreover, they were classified into high and low impact physical activity groups. Evaluation of structural knee abnormalities was performed using WORMS obtained by two independent observers blinded to the subjects’ physical activity and timepoint. Linear regression models were used to assess the associations between participation in different physical activity types and changes in WORMS.
Results:
No significant differences in epidemiological data were found between the groups except for gender composition and there were no significant differences in baseline WORMS. In the cohort as a whole and most exercise groups overall WORMS significantly increased during the observational period. Highest increases compared to the remainder of the group were found in the high impact group (increase in WORMS: 4.65; [95% CI]: [3.94,5.35];p=0.040) and the racquet sports group (6.39; [95% CI]: [5.13,7.60];p≤0.001). Subjects using an elliptical-trainer showed the lowest increase in WORMS (−1.50 [−0.21, 3.22];p=0.002).
Conclusion:
Progression of knee joint degeneration was consistently higher in subjects engaging in high impact and racquet sports while subjects using an elliptical-trainer showed the smallest changes in structural degeneration.
Keywords: Knee osteoarthritis, Knee articular cartilage, MRI, physical activity, obesity
Introduction
Knee osteoarthritis (OA) is worldwide the second most frequent cause of lower extremity disability, and it has a global incidence of 199 cases per 100.000 (1), including over 14 million people with symptomatic knee OA in the US (2). Overweight and obese individuals have a higher incidence of knee OA due to excessive knee joint load. An association between weight loss and less cartilage degeneration has been previously reported (3, 4). However, the association between physical activity and knee OA, has not been systematically addressed in overweight and/or obese subjects and its association seems to be controversial. On the one hand, physical activity has been found to be beneficial in the management knee homeostasis, the physiologic knee joint load providing an optimized chondrocyte cycle of production and destruction (5). Furthermore, mild to non-weight-bearing physical activities such as swimming, bicycling, elliptical trainer are recommended to lose weight and to have a healthy life style (6). On the other hand, excessive fast-paced physical activity with high load-joint torsion such as racquet sports, ball sports and running have been found to have an increased incidence of knee injury compared to mild-moderate exercise such as swimming, bicycling and low-impact aerobics independent of body weight (5, 7). Furthermore, joint injury can lead to a trauma-initiated joint degeneration, which has been found to be associated with 5% of new knee OA cases (7, 8). Previous studies have found associations between physical activities, such as running, soccer, basketball weightlifting and daily recreational activities and knee OA detected with radiographs (9–12); however, the reported correlations may underestimate early grade of cartilage degeneration and mainly did not focus on overweight and/or obese individuals. The effect of different types of physical activity on morphologic degeneration of the knee has never been assessed with MR Imaging, but MRI is the most sensitive modality which can depict early structural alteration of cartilage and knee internal structures (3).
Therefore, the aims of this study were (i) to investigate knee joint structural degeneration over 48-months in overweight and obese subjects who performed high impact physical activities compared to those who performed low impact physical activities, and (ii) to assess how different types of physical activity affect progression of degenerative changes, using semi-quantitative MRI-grading over a period of 48-months.
Materials and Methods
Subject selection
Participants were selected from the Osteoarthritis Initiative (OAI, https://oai.epi-ucsf.org), a multicentric cohort study of individuals with or without risk factors for knee osteoarthritis (OA) as well as individuals with mild to moderate OA, supported by the U.S. National Institutes of Health (NIH). Participants were recruited from February 2004 to May 2006 and longitudinal clinical and imaging data were collected in four different medical centers until January 2015. The entire cohort consists of 4796 men and women, aged 45–79 years at enrollment. The study was approved by the local institutional review boards and informed consent was obtained from all participants; the study was also compliant with the Health Insurance Portability and Accountability Act.
We included overweight and obese subjects with a body mass index (BMI) of ≥ 25 kg/m2 at baseline and during the observational period who reported physical activity during the observation period of the OAI based on self-administered questionnaires. Moreover, the regularity of each activity performed (number of years, numbers of months per year) was evaluated. The participant provided information about the physical activity that they performed at least 20 minutes within a given day for at least 10 times in their life per each age range. Moreover, they were asked to report the 3 activities they performed most frequently. For each of the top 3 activities, they were asked how many years they participated in that physical activity. Subjects who did not report the top 3 activities for at least 5 years were excluded from the analysis. Subjects with a diagnosis of rheumatoid arthritis (n=9) and those that did not have MRI scans available of the right knee at baseline and 48-months follow-up were excluded from the analysis. In addition, subjects who did not have right knee WORMS evaluation at baseline and at 48 months in our database were excluded from the study. The detailed subject selection process is shown in Figure 1. Overall 415 subjects were studied. In all individuals the OAI database provided Kellgren Lawrence (K/L) scores from the interpretation of knee radiographs, information on knee surgery and/or arthroscopy and Knee Injury and Osteoarthritis Outcome Scores (KOOS).
Figure 1:
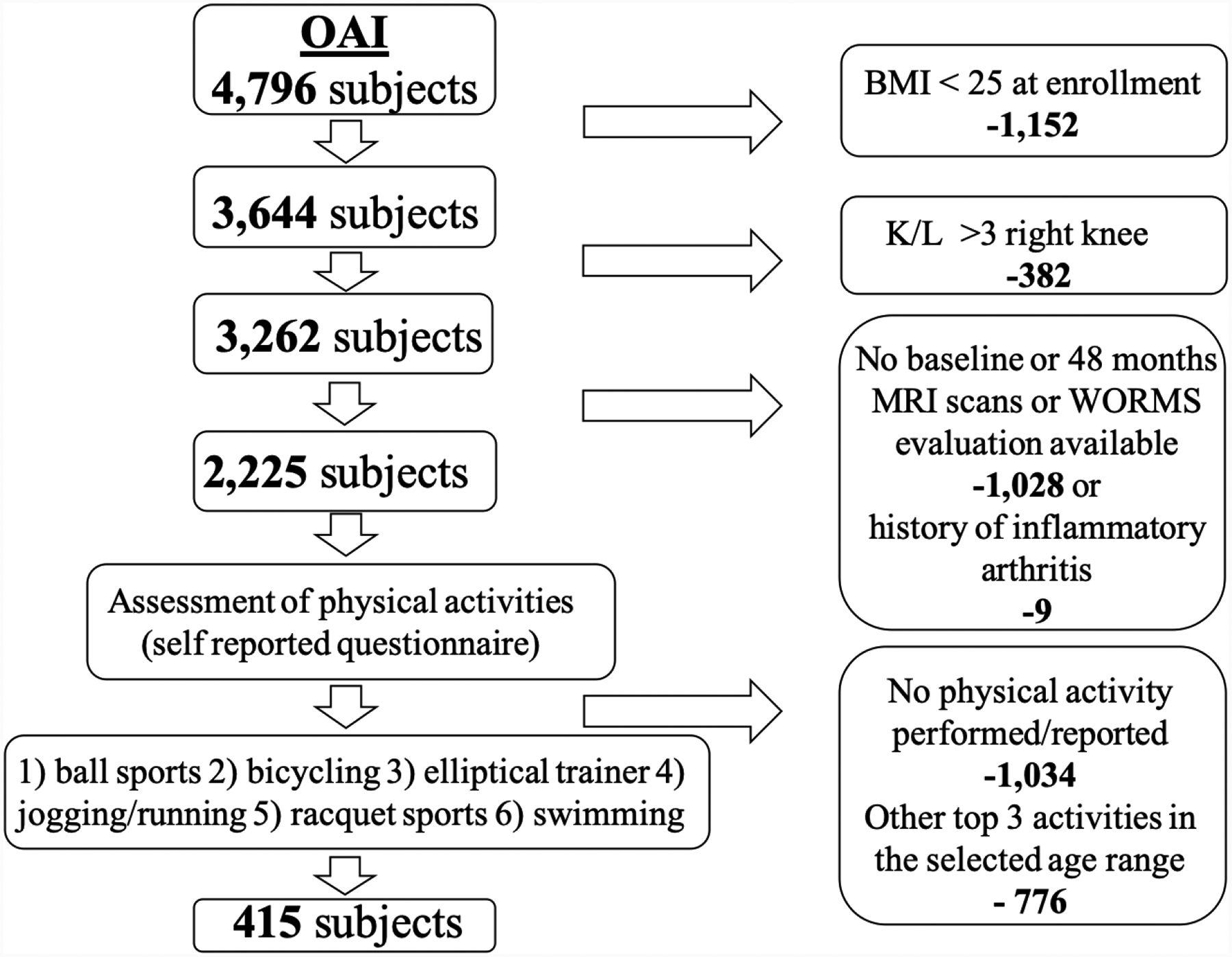
Selection of study subjects. Flow chart illustrating the subject selection from the OAI database. (BMI=Body Mass Index, K/L= X-ray based Kellgren-Lawrence score).
Physical Activity
Information on specific types of physical activity was recorded at the 96 months visit of the OAI using a questionnaire (13, 14). This survey had been validated and successfully applied in previous studies (9, 15). Participants were asked the following questions: “What were the top three frequently performed physical activities during the age range 35–49, and in the age range 50 and older listed from above?”. The self-administered questionnaire is provided at the NIAMS OAI website (https://nda.nih.gov/oai/). A participant could have therefore reported from one to six different types of physical activity. Since high impact activities potentially have greater risk for knee degeneration due to higher compression and shear loading (16), participants that reported at least one high impact physical activity within one age range were defined as performing high impact activities. In some cases, a participant performed more than one type of high or low impact physical activity, in these cases we followed the “harmful” principle: they were categorized in the physical activity group with the highest joint load and frictional shear stress (5, 17–19).
A list of 37 different physical activities identified by univocal code was provided in the OAI questionnaire. In our study, we included those activities if a minimum of 40 participants performed this type of activity to ensure sufficient statistical power for analysis. The following activities were included: (1) bicycling (outdoor or individual stationary cycling), (2) elliptical trainer, (3) jogging or running (outdoor or indoor treadmill or track), (4) swimming, (5) sport types using a racquet (including: tennis single or double, badminton, squash, and racquet ball), and finally (6) sports types including a ball (basketball, baseball, volleyball, football, soccer and handball).
In order to assess how different types of physical activity may affect the longitudinal changes in joint structure, we classified the different activities in high and low impact, according to estimated intensity of joint impact and loading (5, 7). Therefore bicycling, elliptical trainer, and swimming were defined as low impact activities, while jogging or running, sport types using racquets (tennis, badminton, squash and racquet ball), and sport types including a ball (baseball, basketball, football, handball, soccer and volleyball) were defined as high impact activities. Concerning the high impact activities, we considered jogging or running as high compressive but low shear physical activity, and ball sports and racquet sports as high compression and high friction shear stress physical activities (18, 20, 21).
MRI protocol and image analysis
All right knee studies were acquired using 3.0-T MRI scanners (Siemens Magnetom Trio, Siemens Healthcare, Erlangen, DE) with quadrature transmit-receive coils (USA Instruments, Aurora, OH, USA) at four different OAI centers (University of Maryland, School of Medicine, Baltimore, MD; University of Pittsburgh, Pittsburgh, PA; Memorial Hospital of Rhode Island, Pawtucket, RI and The Ohio State University, Columbus, OH). The following sequences were analyzed using the Whole Organ Resonance Imaging Score (WORMS): (a) 3D dual echo steady-state (DESS) gradient-echo with water excitation (WE) sequences obtained in the sagittal plane (16.3ms/4.7ms/25°, repetition time (TR) / echo time (TE)/flip angle), (b) 2D proton density-weighted sequences in the coronal plane (3700 ms/29 ms, TR/TE) and (c) 2D intermediate-weighted fat suppressed (FS) sequences (3200 ms/30 ms, TR/TE) obtained in the sagittal plane. Further specification about the MR imaging sequences are provided in the OAI protocol (22).
All readings were performed using the modified WORMS scoring system, that has been validated and applied in multiple previous studies (23–25), independently by two radiologists (SS with 3 years of experience and MP with 11 years of experience) who were blinded to the physical activity information. Readings with divergent findings were adjudicated with an experienced musculoskeletal radiologist (25 years of experience).
In all baseline and 48-month follow-up MR images the following features were evaluated: articular cartilage, meniscus and ligamentous abnormalities, bone marrow edema pattern, subarticular cysts, effusion, loose bodies and popliteal cysts. Cartilage abnormalities, bone marrow edema pattern and subarticular cysts were graded in six joint compartments (patella, trochlea, medial femur condyle, lateral femur condyle, medial tibia and lateral tibia) as previously described (3, 4, 23–26). In addition, signal changes and tears of the anterior horn, body and posterior horn of the medial and lateral menisci as well abnormal signal and structural abnormalities of the anterior and posterior cruciate ligaments (ACL, PCL), medial and lateral collateral ligaments (MCL, LCL), patellar and popliteal tendon were graded. Additional criteria included joint effusion, loose bodies and popliteal cysts. For each feature, a sum score was calculated by adding the lesion scores of all subregions of the knee and an overall WORMS score was obtained by adding these sum scores.
The intra- and interreader reliability of WORMS grading by our group estimated with intraclass correlation coefficients (ICCs) along with 95% confidence intervals calculated for each WORMS imaging feature, has been validated in multiple prior studies(3, 26–30). The ICCs for intrareader agreement were between 0.85 and 0.99 for each of the following WORMS imaging features: cartilage, meniscus, ligaments, bone marrow edema pattern, subarticular cyst, effusion, loos bodies and popliteal cysts. The ICCs for interreader agreement were between 0.75 to 0.97 for each of the following WORMS imaging features: cartilage, meniscus, ligaments, bone marrow edema pattern, subarticular cyst, effusion, loos bodies and popliteal cysts.
Statistical Analysis
Statistical analysis was performed using STATA software (Version 14, College Station, TX, USA: SataCorp LP). Differences in participant characteristics between different types of physical activity groups were assessed using Pearson’s X2-test (categorical variables) and linear regression (continuous variables). Linear regression models were used to assess the differences in changes in WORMS features over 48 months between the high and low impact groups. In addition, linear regression models were used to assess the differences in changes in WORMS features over 48 months between individual types of physical activity groups and the remainder of the cohort. Furthermore, a multivariate analysis was performed. All analyses were adjusted for common risk factors of knee OA such as age, race, sex and baseline BMI. Primary outcomes were defined as 48-month changes in WORMS scores.
Results
Participant Characteristics
In total, 415 participants were included in this study. Subject characteristics are listed in Table 1. There was a statistically significant difference in gender composition (p=<0.001) between subjects in high and low physical activity groups, otherwise no significant differences were found. Among the six different types of physical activity groups, there was a statistically difference in gender composition (p=<0.001), otherwise no significant differences were found. The baseline WORMS scores are summarized in Table 1.
Table 1.
Subject characteristics and baseline WORMS scores of high and low physical activity and types of physical activity.
| Subject Characteristics | High Impact (N=244) | Low Impact (N=171) | p-value | Ball sports (N=70) | Bicycling (N=74) | Elliptical trainer (N=41) | Jogging/running (N=94) | Racquet sports (N=80) | Swimming (N=56) | p-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 59.2 (±8.9) | 59.4 (±8.9) | 0.885 | 57.8 (±8.9) | 57.6 (±8.9) | 56.0 (±9.0) | 58,5 (±8.8) | 61.4 (±8.9) | 64.1 (±8.9) | 0.501 |
| BMI (kg/m3) | 29.5 (±3.4) | 30.1 (±3.5) | 0.069 | 29.7 (±3.4) | 30.0 (±3.5) | 30.6 (±3.5) | 29.3 (±3.4) | 29.6 (±3.4) | 30.0 (±3.5) | 0.501 |
| Sex | F=84 (34) M=160 (66) |
F=99 (58) M=72 (42) |
≥0.001 | F=10 (14) M=60 (86) |
F=42 (57) M=32 (43) |
F=21 (51) M=20 (49) |
F=43 (48) M=49 (52) |
F=29 (36) M=51 (64) |
F=36 (64) M=20 (36) |
≤0.001 |
| K/L score | 0=82 (34) 1=51 (21) 2=75 (11) 3=36 (15) |
0=56 (33) 1=36 (21) 2=56 (33) 3=23 (13) |
0.965 | 0=20 (28.5) 1=20 (28.5) 2=19 (27) 3=11 (16) |
0=25 (34) 1=17 (23) 2=20 (27) 3=12 (16) |
0=13 (32) 1=5 (12) 2=18 (44) 3=5 (12) |
0=38 (40) 1=19 (20) 2=27 (29) 3=10 (11) |
0=24 (30) 1=12 (15) 2=29 (36) 3=15 (19) |
0=18 (32) 1=14 (25) 2=18 (32) 3=6 (11) |
0.507 |
| Ethnicity | Caucasian=198 (81) African/American=43 (18) Asian=3 (1) |
Caucasian=150 (88) African/American=19 (11) Asian=2 (1) |
0.185 | Caucasian=56 (80) African/American=14 (20) Asian=0 |
Caucasian=67 (90.5) African/American=7 (9.5) Asian=0 |
Caucasian=33 (80.5) African/American=8 (19.5) Asian=0 |
Caucasian=73 (78) African/American=20 (21) Asian=1 (1) |
Caucasian=69 (86.2) African/American=9 (11.2) Asian=2 (2.5) |
Ceucasian=50 (89) African/American=4 (7) Asian=2 (4) |
0.099 |
| Knee surgery/arthroscopy | Y=6 (2) N=231 (95) *=7 (3) |
Y=2 (1) N=166 (97) *=3 (2) |
0.339 | Y=2 (3) N=65 (93) *=3 (4) |
Y=0 N=73 (99) *=1 (1) |
Y=1 (1) N=40 (99) |
Y=1 (1) N=89 (95) *=4 (4) |
Y=3 (4) N=77 (96) |
Y=1 (2) N=53 (95) *=2 (3) |
0.620 |
| KOOS pain | 86 (±15.4) | 88 (±15.1) | 0.467 | 82.4 (±15.5) | 86.5 (±15.2) | 89.1 (±15.3) | 88.2 (±15.4) | 87.9 (±15.4) | 87.8 (±15.2) | 0.501 |
| KOOS symptoms | 88 (±13.5) | 89 (±13.2) | 0.319 | 86.1 (±13.5) | 87.5 (±13.2) | 91.2 (±13.3) | 88.2 (±13.6) | 88.2 (±13.5) | 89.1 (±13.2) | 0.501 |
| WORMS Baseline Scores | High Impact vs Low Impact | p-value | Ball Sports | p-value | Bicycling | p-value | Elliptical trainer | p-value | Jogging/running | p-value | Racquet sports | p-value | Swimming | p-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall Worms score | 1.83 [−0.56,4.22] | 0.13 | 1.25 [−1.51,4.02] | 0.37 | 0.05 [−2.51,2.63] | 0.96 | −1.91[−5.45,1.63] | 0.28 | −0.46 [−2.62,1.68] | 0.67 | 1.54[−1.10,4.19] | 0.25 | −1.14 [−3.82,1.52] | 0.40 |
| Cartilage | ||||||||||||||
| Cartilage Sum | 0.88 [−0.15,1.93] | 0.095 | 0.80 [−0.43,2.03] | 0.20 | −0.12 [−1.27,1.10] | 0.82 | −0.47[−2.05,1.09] | 0.55 | −0.50 [−1.46,0.45] | 0.30 | 1.47[0.30,2.64] | 0.014 | −0.37 [−1.56,0.81] | 0.53 |
| Meniscus | ||||||||||||||
| Meniscus Sum | 0.46[−0.50,1.43] | 034 | 0.45 [−0.68,1.58] | 0.43 | −0.10[1.15,0.94] | 0.84 | −0.84 [−2.29,0.60] | 0.25 | −0.07[−0.95,0.80] | 0.86 | −0.35[−1.43,0.72] | 0.52 | −0.38 [−1.47,0.70] | 0.49 |
The Table shows the subjects characteristics and WORMS scores at baseline. Continuous data are expressed as mean ± SD. Categorical data are presented in numbers with percentage in parenthesis. P values are for differences between participants with different types of physical activity and were calculated using either Pearson’s X2 test (categorical variables) or analysis of variance (parametric testing). Baseline WORMS scores are listed as average WORMS score adjusted for common risk factor of knee OA age, sex, race and baseline BMI, with [95% Confidence interval]. Significant statistical results (p=≤0.050) are in bold.
Longitudinal WORMS findings related to different types of physical activity
Longitudinal change results including overall WORMS sum score, cartilage sum score and subscores (medial femur and medial tibia), meniscus scores, were the primary outcomes of this study and are summarized by high and low impact and types of physical activity in Tables 2, 3, 4 and 5.
Table 2.
WORMS grade longitudinal changes for high and low impact physical activity over 48 months.
| Worms Parameters | High impact | P-value |
|---|---|---|
| Overall Worms score | 4.65 [3.94,5.35] | 0.040 |
| Cartilage | ||
| Global Sum | 2.25 [1.88,2.61] | 0.13 |
| Patella | 0.44 [0.31,0.56] | 0.97 |
| Trochlea | 0.37 [0.26,0.48] | 0.56 |
| Medial femur | 0.48 [0.35,0.60] | 0.034 |
| Medial tibia | 0.39 [0.27,0.52] | 0.006 |
| Lateral femur | 0.34 [0.22,0.45] | 0.46 |
| Lateral tibia | 0.23 [0.13,0.34] | 0.058 |
| Meniscus | ||
| Global sum | 0.91 [0.68,1.15] | 0.066 |
| Worms Parameters | Low impact | P-value |
|---|---|---|
| Overall Worms score | 3.46 [2.61,4.32] | 0.040 |
| Cartilage | ||
| Global Sum | 1.80 [1.36,2.24] | 0.13 |
| Patella | 0.43 [0.28,0.58] | 0.97 |
| Trochlea | 0.32 [0.19,0.43] | 0.56 |
| Medial femur | 0.26 [0.10,0.41] | 0.034 |
| Medial tibia | 0.10 [−0.04,0.26] | 0.006 |
| Lateral femur | 0.27 [0.13,0.40] | 0.46 |
| Lateral tibia | 0.40 [0.27,0.53] | 0.058 |
| Meniscus | ||
| Global sum | 0.56 [0.27,0.84] | 0.066 |
Data are listed as adjusted means, adjusted for common risk factors of knee OA age, sex, race and baseline BMI, with [95% Confidence Intervals] and calculated as the numerical means for each different type of physical activity. Significant statistical results (p= ≤0.050) are in bold.
Table 3.
WORMS grade longitudinal changes over 48 months for subjects in the racquet ball group compared to the remainder of the cohort.
| Worms Parameters | Racquet ball | P-value |
|---|---|---|
| Overall Worms score | 6.39 [5.13,7.60] | ≤0.001 |
| Cartilage | ||
| Global Sum | 3.00 [2.37,3.63] | 0.003 |
| Patella | 0.54 [0.33,0.76] | 0.39 |
| Trochlea | 0.48 [0.29,0.68] | 0.12 |
| Medial femur | 0.66 [0.44,0.89] | 0.015 |
| Medial tibia | 0.57 [0.34,0.81] | 0.019 |
| Lateral femur | 0.43 [0.25,0.61] | 0.084 |
| Lateral tibia | 0.31 [0.11,0.51] | 0.99 |
| Meniscus | ||
| Global sum | 1.21 [0.79,1.64] | 0.029 |
Data are listed as adjusted means, adjusted for common risk factors of knee OA age, sex, race and baseline BMI, with [95% Confidence Intervals] and calculated as the numerical means for each different type of physical activity. Significant statistical results (p= ≤0.050) are in bold.
Table 4.
WORMS grade longitudinal changes over 48 months for subjects in the elliptical trainer group compared to the remainder of the cohort.
| Worms Parameters | Elliptical trainer | P-value |
|---|---|---|
| Overall Worms score | 1.50 [−0.21,3.22] | 0.002 |
| Cartilage | ||
| Global Sum | 0.83 [−0.03,1.71] | 0.003 |
| Patella | 0.39 [0.09,0.69] | 0.66 |
| Trochlea | 0.36 [−0.17,0.35] | 0.048 |
| Medial femur | 0.14 [−0.16,0.45] | 0.080 |
| Medial tibia | −0.12 [−0.45,0.20] | 0.007 |
| Lateral femur | 0.02 [−0.22,0.27] | 0.030 |
| Lateral tibia | 0.31 [0.03,0.58] | 0.97 |
| Meniscus | ||
| Global sum | 0.22 [−0.36,0.81] | 0.059 |
Data are listed as adjusted means, adjusted for common risk factors of knee OA age, sex, race and baseline BMI, with [95% Confidence Intervals] and calculated as the numerical means for each different type of physical activity. Significant statistical results (p= ≤0.050) are in bold.
Table 5.
WORMS grade longitudinal changes over 48 months for subjects in bicycling group, swimming group, jogging/running group and sport ball group compared to the remainder of the cohort respectively.
| Worms Parameters | Bicycling | P-value | Worms Parameters | Swimming | P-value |
|---|---|---|---|---|---|
| Overall Worms score | 3.75 [2.54,4.96] | 0.43 | Overall Worms score | 3.99 [2.73,5.26] | 0.73 |
| Cartilage | Cartilage | ||||
| Global Sum | 2.04 [1.43,2.66] | 0.81 | Global Sum | 1.93 [1.29,2.58] | 0.56 |
| Patella | 0.50 [0.30,0.71] | 0.61 | Patella | 0.35 [0.13,0.56] | 0.30 |
| Trochlea | 0.38 [0.19,0.56] | 0.67 | Trochlea | 0.40 [0.21,0.59] | 0.53 |
| Medial femur | 0.34 [0.12,0.55] | 0.50 | Medial femur | 0.25 [0.02,0.47] | 0.14 |
| Medial tibia | 0.16 [−0.06,0.38] | 0.15 | Medial tibia | 0.08 [−0.15,0.32] | 0.045 |
| Lateral femur | 0.30 [0.12,0.47] | 0.89 | Lateral femur | 0.38 [0.20,0.56] | 0.25 |
| Lateral tibia | 0.37 [0.18,0.56] | 0.53 | Lateral tibia | 0.46 [0.26,0.66] | 0.11 |
| Meniscus | Meniscus | ||||
| Global sum | 0.60 [0.19,1.01] | 0.38 | Global sum | 0.75 [0.32,1.18] | 0.91 |
| Worms Parameters | Jogging/running | P-value | Worms Parameters | Sport ball | P-value |
| Adjusted means changes in WORMS over 48 months [95% CI] | Jogging/running vs other groups | Adjusted means changes in WORMS over 48 months [95% CI] | Sport ball vs other groups | ||
| Overall Worms score | 4.50 [3.54,5.46] | 0.49 | Overall Worms score | 3.90 [2.60,5.20] | 0.62 |
| Cartilage | Cartilage | ||||
| Global Sum | 2.29 [1.81,2.78] | 0,40 | Global Sum | 2.11 [1.45,2.77] | 0.99 |
| Patella | 0.49 [0.32,0.65] | 0.64 | Patella | 0.54 [0.32,0.77] | 0.40 |
| Trochlea | 0.38 [0.23,0.52] | 0.60 | Trochlea | 0.26 [0.06,0.46] | 0.38 |
| Medial femur | 0.49 [0.32,0.67] | 0.24 | Medial femur | 0.36 [0.12,0.59] | 0.66 |
| Medial tibia | 0.39 [0.21,0.57] | 0.32 | Medial tibia | 0.21 [−0.03,0.46] | 0.39 |
| Lateral femur | 0.35 [0.20,0.34] | 0.33 | Lateral femur | 0.42 [0.24,0.61] | 0.11 |
| Lateral tibia | 0.20 [0.05,0.35] | 0.11 | Lateral tibia | 0.30 [0.10,0.51] | 0.91 |
| Meniscus | Meniscus | ||||
| Global sum | 0.71 [0.39,1.04] | 0.71 | Global sum | 0.91 [0.46,1.35] | 0.52 |
Data are listed as adjusted means, adjusted for common risk factors of knee OA age, sex, race and baseline BMI, with [95% Confidence Intervals] and calculated as the numerical means for each different type of physical activity. Significant statistical results (p= ≤0.050) are in bold.
Overall WORMS score
In the longitudinal analysis, the overall WORMS scores increased significantly more in the high impact physical activity group compared the low impact group (Adjusted means changes in WORMS score [95% CI]:4.65 [3.94,5.35]; p=0.040) (Table 2). With regard to the specific physical activities, the overall WORMS scores increased significantly more in participants in the racquet sports group compared to the remainder of the cohort (Adjusted means changes in WORMS score [95% CI]:6.39 [5.13,7.60]; p=≤0.001) (Table 3). Moreover, the lowest increase of overall WORMS scores compared to the remainder of the group was found in the elliptical trainer group (Adjusted means changes in WORMS score [95% CI]:1.50 [−0.21,3.22]; p=0.002) (Table 4). A graphic summary of longitudinal adjusted means by physical activity groups compared to each other is shown in Figure 2. No significant differences were found for longitudinal change in overall WORMS scores for participants in the other physical activity groups compared to the remainder of the cohort (Table 5).
Figure 2:
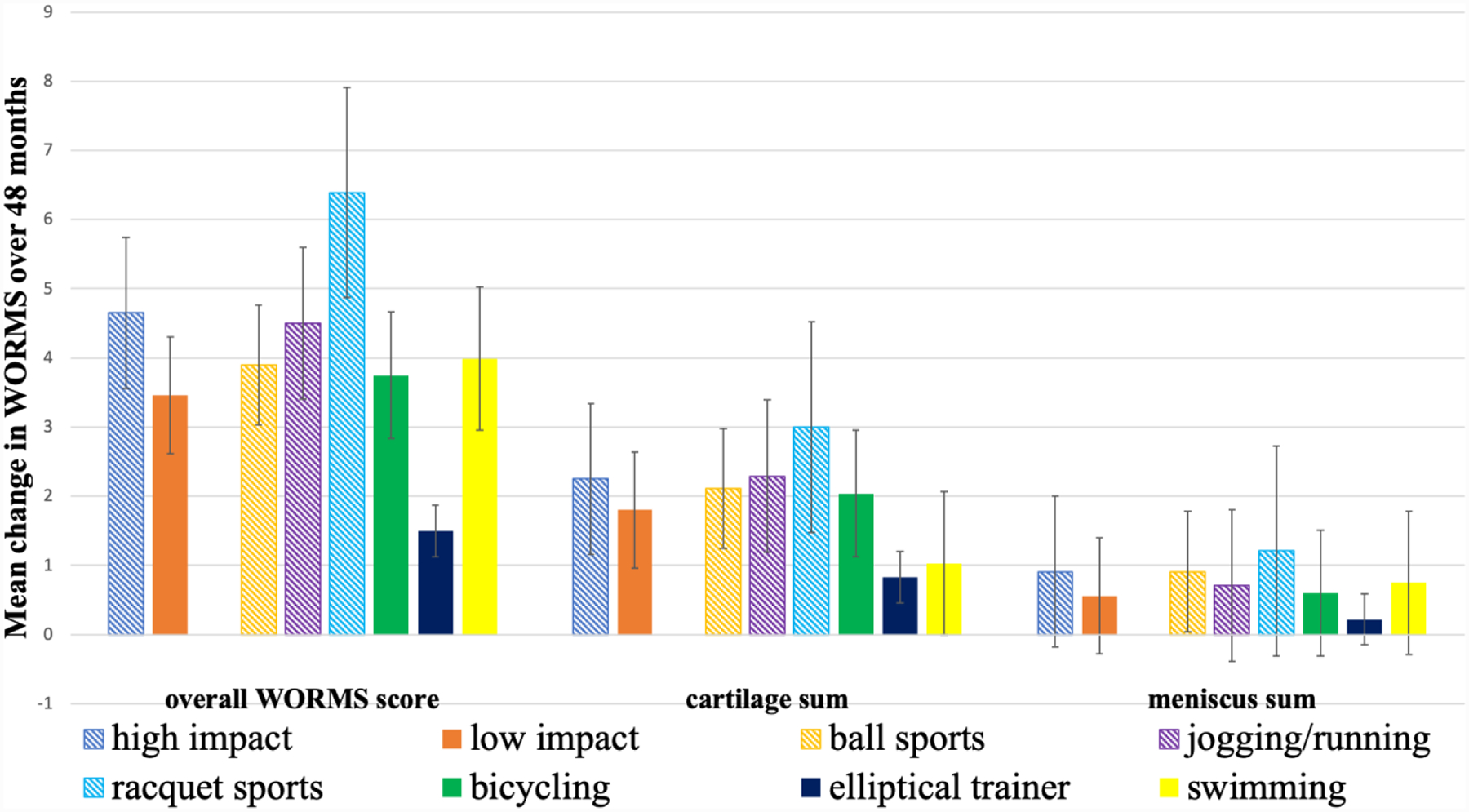
Graphic representation of adjusted mean changes in WORMS (± standard error of the mean) over 48 months in high impact, low impact, ball sports, jogging/running, racquet sports, bicycling, elliptical trainer, and swimming groups. Note that elliptical trainer has the smallest changes compared to the remainder of the cohort (p=0.001). Overall WORMS score; cartilage sum = sum of score of all cartilage compartments; meniscus sum = sum of score of all meniscal compartments.
Cartilage Subscores
In the longitudinal analysis, participants categorized in the high impact sports group showed a higher increase in cartilage sum score, but it was not statistically significant (p=0.13). However, the medial femorotibial joint cartilage compartments explored separately showed a statistically significant increase including the medial femur (p=0.034) and medial tibia (p=0.006) (Fig. 3; Table 2). The cartilage sum score increased significantly more in participants in the racquet sports group compared to the remainder of the cohort (Adjusted means changes in WORMS score [95% CI]: 3.00 [2.37,3.63]; p=0.003), likewise there was a significant difference in the medial femur cartilage compartment (p=0.015) and medial tibial cartilage compartment (p=0.003), respectively (Table 3). Comparison of right knee MR images between two overweight subjects in the racquet sports and elliptical trainer groups are shown in Figure 4. The lowest increase of cartilage sum scores was found in the elliptical trainer group compared to the remainder of the cohort (Adjusted means changes in WORMS score [95% CI 0.83 [−0.03,1.71]; p=0.003), likewise there was a significant difference in the medial tibia cartilage compartment [p=0.007] (Table 4). No significant differences were found for longitudinal WORMS cartilage parameters in the other physical activity groups (Table 5).
Figure 3:
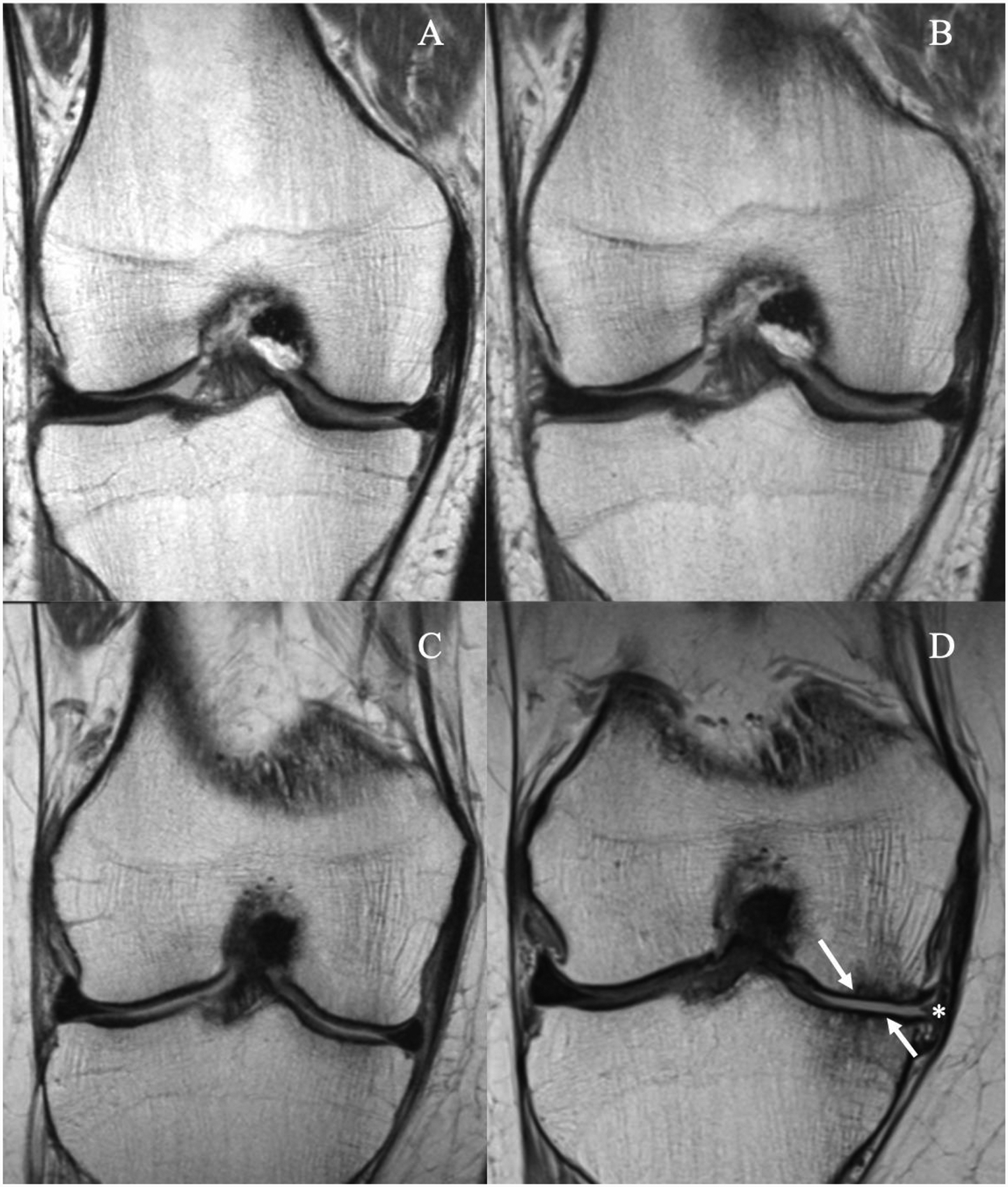
MR images of the right knee obtained with a coronal proton density-weighted sequence (A, B, C, D) at baseline (A, C) and after 48 months (B, D). Obese 76-year-old man in the low impact physical activity group (A-B) and obese 59-year-old woman in the high impact physical activity group (C-D). The woman in the high impact activity group developed full thickness cartilage defects in both medial femur condyle and tibia in D (arrows), with associated extrusion of the meniscus body (asterisk). In contrast, no cartilage defects were seen in the man in the low physical activity group (A, B).
Figure 4:
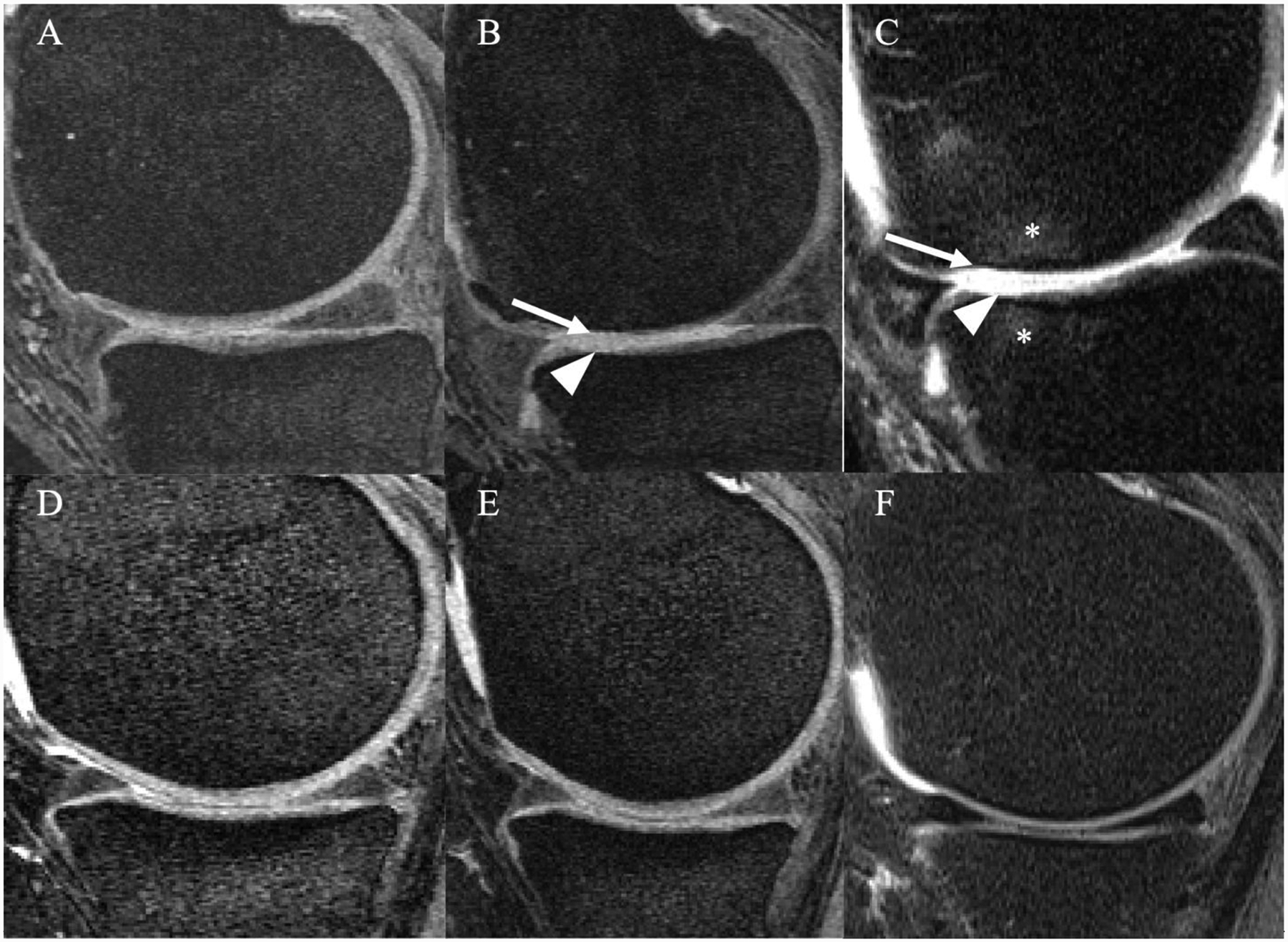
MR images of the right knee obtained with a sagittal dual-echo steady state (DESS) (A,B,D,E) and intermediate-weighted fat suppressed sequence (C,F) at baseline (A, D) and after 48 months (B, C, E, F). Overweight 64-year-old man in the racquet sports group (A-C) and overweight 47-year-old woman in the elliptical trainer group (D-F). The man in the racquet sports group developed full thickness cartilage defects in both medial femur condyle and tibia in B-C (arrow and arrowhead) with associated bone marrow edema like lesions (asterisks). In contrast, no cartilage defects were seen in the woman in the elliptical trainer group (D, E or F).
Meniscus
In the longitudinal analysis, participants in the high impact sports group showed a higher increase in meniscus sum score, but it was not statistically significant (p=0.066). Meniscus sum scores increased significantly more in participants in the racquet sports group compared to the remainder of the cohort (1.21 [0.79,1.64]; p=0.029), (Table 3). No significant differences were found for longitudinal WORMS menisci parameters for participants in the other physical activity groups.
Multivariate analysis of WORMS subgroup
Multivariate models were built to understand which WORMS subgroup scores account for the changes in overall WORMS scores. For the racquetball analysis, the two terms: change in medial cartilage sum scores and change in lateral meniscus sum scores were driving the relationship between racquetball and change in WORMS. The coefficient for racquetball in the analysis adjusted for age, race, gender, and BMI was 2.49 (p<0.001). When adding the two variables (change in medial cartilage sum score and change in lateral meniscus sum score) the relationship between racquetball and delta overall WORMS became non-significant (coefficient = 0.84, p = 0.07). Similarly, for the elliptical trainer analysis, medial cartilage sum score and change in lateral meniscus sum score were driving the relationship between elliptical and delta WORMS. The coefficient for elliptical trainer in the unadjusted analysis adjusted for age, race, gender, and BMI was −2.98 (p=0.002). When adding the two variables (change in medial cartilage sum score and change in lateral meniscus sum score) the relationship between elliptical and delta overall WORMS became non-significant (coefficient = −0.83, p = 0.18).
Discussion
This study investigated structural degeneration of the knee joint, using a semi-quantitative MRI grading, in overweight and obese individuals who performed high impact physical activity and compared them to individuals who performed low impact physical activity. We also compared structural degeneration in individuals performing different types of physical activities. While we found that overall WORMS scores significantly increased in the entire cohort and most exercise groups during the observational period, the high impact physical activity group, showed greater progression in particular in the medial cartilage compartments (medial femur and medial tibia) over 48 months. Moreover, the type of physical activity performed, especially racquet sports, was significantly associated with degree of progression of overall knee joint and cartilage defects.
Subjects who played racquet sports (high compression and high shear) showed significantly greater overall knee joint degeneration when compared to the remainder of the cohort. These findings suggest that a fast-paced and high shear load to the knee joint is more harmful for cartilage than is mild or soft joint load or shear stress. Furthermore, the racquets sport group showed elevated cartilage degeneration in the medial tibia, which supports the hypothesis that high impact activities, in particular with high compression and high shear are more damaging in the medial compartment (32). Studies by Anandacoomarasamy et. al and Eckstein et. al have shown that the medial compartment generally displays higher rates of cartilage loss in overweight and obese subjects with OA, which could be attributed to a greater proportion of load transmitted through the medial tibiofemoral compartment (20, 33). Hence, high impact physical activity with elevated load and high shear forces may trigger and accelerate this process. Moreover, subjects who played racquet sports showed significantly more meniscal degeneration when compared to the remainder of the cohort. Our hypothesis is that in overweight and obese individuals the joint mechanics are impaired with a harmful joint overload and triggering increased contact stress on the meniscus.
Overall workouts using an elliptical trainer were especially associated with reduced progression of overall knee joint and cartilage defects. A recent study showed that overweight and obesity are associated with greater vertical loading rates (34) and increased risk for total knee replacement surgery, thus restricted weight bearing exercises should be preferred in order to lose weight and reduce the excessive knee joint overload. Our regional analyses, showing a strong association for the medial tibia, support this hypothesis, although our work adds a key component of reducing activities that include cutting and have high shear loads such as racquet sports.
Interestingly, when comparing two different types of high impact physical activity groups i.e. racquets sport and running, presenting opposite shear stress loading: the former high and the latter low, runners showed less overall knee joint and cartilage degeneration. Our results are aligned with previously several studies that have shown the benefit of running especially if mild and not long distance (9, 15). Thus, it has been hypothesized that running as a lifestyle intervention might increase the muscle strength and improve the proprioception (9) and if performed safely should not be avoided. Our findings also showed that when comparing different low impact activities with the remainder of the cohort such as bicycling, elliptical trainer and swimming, the elliptical trainer was associated with the lowest increase in WORMS subscores over 48 months. In previous studies, we found that for subjects at risk for OA or with OA low impact physical activities are most beneficial, however, recommendations were not based on imaging findings but on clinical symptoms and pain relief (32, 35). Thus, the results of our study, which show differences in the structural progression of overall knee joint and cartilage degeneration detected with semi-quantitative morphologic MR imaging are novel compared to previous studies, which analyzed smaller study cohorts and used less sensitive imaging techniques (radiographs vs MRI). Some previous studies assessed the association between obesity on OA progression or even the association between different methods of weight loss including physical activity as weight loss regime (3, 36, 37), however, the literature is inconsistent regarding the effects of different types of physical activity on the knee joint in overweight and obesity subjects. Our findings confirmed that low impact physical activity in particular elliptical trainer is associated with less progression of overall knee joint degeneration and less cartilage defects and emphasized the importance of a healthy lifestyle with minimal joint overload. Moreover, bicycling, jogging/running and swimming groups also showed lower overall knee joint degeneration when compared to the other physical activity groups. Our findings may highlight that the commonly suggested low impact activities to prevent knee OA such as bicycling, and swimming may be reconsidered since the rate of progression was not significantly different from high impact physical activities such as running.
In this study we did not include a control cohort as previous studies have investigated larger cohorts of obese and overweight subjects from the OAI with and without weight loss over a period of 4 years (3, 37). Gersing et al found (3) that in a stable weight group over 4 years global sum cartilage WORMS increased by 2.3 [2.0,2.7] and by 1.6 [1.3,1.9] if subjects lost 5–10% weight; in the current study we found an increase in global sum cartilage WORMS by 2.3 [2.0,2.6] for high impact activities and by 1.9 [1.7, 2.3] for low impact activities. These results suggest that low impact activities have a similarly beneficial impact on joint health as weight loss.
When we looked at the weight changes over 48-months, few participants (22/415) lost weight with their BMI being below the threshold of 25, however, these participants were equally distributed throughout the different physical activity groups and a statistically meaningful analysis of this subcohort was therefore not possible. Please also note, that in the entire cohort the average BMI at baseline and after 48-months did not significantly change (p>0.05), BMI was 30 at both time points.
Our study has several limitations. The information concerning the types of physical activity performed was retrospectively acquired through a self-administered questionnaire and a recall bias could not be excluded. Thus, evidence regarding causation between different types of physical activity performed on progression of overall knee joint and cartilage degeneration is somewhat limited. In some cases, a participant performed more than one physical activity, therefore it was not possible to discriminate which physical activity affected the knee joint degeneration most. This limitation of the OAI study may have introduced a bias to our analyses. In order to strengthen our analysis, we evaluated the regularity of each activity performed (number of years) and we excluded participants who reported to be competitive players, which may have introduced a bias to our analysis, however, we followed the “harmful” principle: subjects were categorized in the physical activity group with the highest joint load and friction shear stress (18, 20, 21). In this study, we focused on only overweight and/or obese adults that were active, therefore we did not have a sedentary cohort because the purpose of our study was to assess how different types of physical activities were associated with knee degenerative changes rather than to assess the relationship between physical activity and knee OA, which was analyzed in previous studies (39–41). Another potential bias could have been introduced by the subjects’ selection process from the OAI cohort. The largest number of potential participants was excluded because they did not report any physical activity or their BMI was not greater than 25. Subsequently, a smaller number of subjects were excluded because they did not have WORMS readings available in our database. We decided to include the most frequently performed physical activities, which are also considered for the management of the knee OA. However, we acknowledge limitations due to recall bias and incomplete evaluation of frequency, duration and intensity of physical activities performed.
In summary our study showed that high impact physical activities, and in particular racquet sports, were significantly associated with increased progression of overall knee joint degeneration and cartilage loss in obese and overweight individuals with risk factors for OA or mild to moderate radiographic evidence of OA. We found that low impact activities such as those performed on an elliptical trainer may be most beneficial for overall knee joint and cartilage health and therefore may be more useful for both losing weight and maintaining a healthy lifestyle in obese and overweight individuals.
Acknowledgements
The analyses in this study were funded by a grant from the National Institutes of Health (NIH/NIAMS R01-AR064771, PI: Thomas M. Link, MD, PhD) and a scholarship grant to Silvia Schirò, MD by the University of Parma, Italy 43106. The Osteoarthritis Initiative (OAI) is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR- 2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: none
Contributor Information
S. Schirò, Department of Radiology and Biomedical Imaging, University of California San Francisco, 185 Berry St, San Francisco, CA 94107; Section of Radiology, Department of Medicine and Surgery (DiMeC), University of Parma, Via Gramsci 14, Parma, Italy 43106
S.C. Foreman, Department of Radiology and Biomedical Imaging, University of California San Francisco, 185 Berry St, San Francisco, CA 94107; Department of Radiology, Technical University of Munich, Munich, Germany
G.B. Joseph, Department of Radiology and Biomedical Imaging, University of California San Francisco, 185 Berry St, San Francisco, CA 94107
R.B. Souza, Department of Radiology and Biomedical Imaging; Department of Physical Therapy and Rehabilitation Science, University of California, San Francisco, USA
C.E. McCulloch, Department of Epidemiology and Biostatistics, University of California, San Francisco, USA
M.C. Nevitt, Department of Epidemiology and Biostatistics, University of California, San Francisco, USA
T.M. Link, Department of Radiology and Biomedical Imaging, University of California San Francisco, 185 Berry St, San Francisco, CA 94107
References:
- 1.Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartilage. 2018;26(3):319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30(8):914–8. [DOI] [PubMed] [Google Scholar]
- 3.Gersing AS, Schwaiger BJ, Nevitt MC, Joseph GB, Chanchek N, Guimaraes JB, et al. Is Weight Loss Associated with Less Progression of Changes in Knee Articular Cartilage among Obese and Overweight Patients as Assessed with MR Imaging over 48 Months? Data from the Osteoarthritis Initiative. Radiology. 2017;284(2):508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guimaraes JB, Nevitt MC, McCulloch CE, Schwaiger BJ, Gersing AS, Facchetti L, et al. Association of weight change with progression of meniscal intrasubstance degeneration over 48 months: Data from the Osteoarthritis Initiative. Eur Radiol. 2018;28(3):953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckwalter JA, Lane NE. Athletics and osteoarthritis. Am J Sports Med. 1997;25(6):873–81. [DOI] [PubMed] [Google Scholar]
- 6.Alkatan M, Baker JR, Machin DR, Park W, Akkari AS, Pasha EP, et al. Improved Function and Reduced Pain after Swimming and Cycling Training in Patients with Osteoarthritis. J Rheumatol. 2016;43(3):666–72. [DOI] [PubMed] [Google Scholar]
- 7.Buckwalter JA. Sports, joint injury, and posttraumatic osteoarthritis. J Orthop Sports Phys Ther. 2003;33(10):578–88. [DOI] [PubMed] [Google Scholar]
- 8.Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459–67. [DOI] [PubMed] [Google Scholar]
- 9.Lo GH, Musa SM, Driban JB, Kriska AM, McAlindon TE, Souza RB, et al. Running does not increase symptoms or structural progression in people with knee osteoarthritis: data from the osteoarthritis initiative. Clin Rheumatol. 2018;37(9):2497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaganti RK, Lane NE. Risk factors for incident osteoarthritis of the hip and knee. Curr Rev Musculoskelet Med. 2011;4(3):99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefevre-Colau MM, Nguyen C, Haddad R, Delamarche P, Paris G, Palazzo C, et al. Is physical activity, practiced as recommended for health benefit, a risk factor for osteoarthritis? Ann Phys Rehabil Med. 2016;59(3):196–206. [DOI] [PubMed] [Google Scholar]
- 12.Driban JB, Hootman JM, Sitler MR, Harris KP, Cattano NM. Is Participation in Certain Sports Associated With Knee Osteoarthritis? A Systematic Review. J Athl Train. 2017;52(6):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kriska AM, Sandler RB, Cauley JA, LaPorte RE, Hom DL, Pambianco G. The assessment of historical physical activity and its relation to adult bone parameters. Am J Epidemiol. 1988;127(5):1053–63. [DOI] [PubMed] [Google Scholar]
- 14.Chasan-Taber L, Erickson JB, McBride JW, Nasca PC, Chasan-Taber S, Freedson PS. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. 2002;155(3):282–9. [DOI] [PubMed] [Google Scholar]
- 15.Lo GH, Driban JB, Kriska AM, McAlindon TE, Souza RB, Petersen NJ, et al. Is There an Association Between a History of Running and Symptomatic Knee Osteoarthritis? A Cross-Sectional Study From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2017;69(2):183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vannini F, Spalding T, Andriolo L, Berruto M, Denti M, Espregueira-Mendes J, et al. Sport and early osteoarthritis: the role of sport in aetiology, progression and treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1786–96. [DOI] [PubMed] [Google Scholar]
- 17.Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002(402):21–37. [DOI] [PubMed] [Google Scholar]
- 18.Whitney GA, Jayaraman K, Dennis JE, Mansour JM. Scaffold-free cartilage subjected to frictional shear stress demonstrates damage by cracking and surface peeling. J Tissue Eng Regen Med. 2017;11(2):412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskelinen ASA, Mononen ME, Venalainen MS, Korhonen RK, Tanska P. Maximum shear strain-based algorithm can predict proteoglycan loss in damaged articular cartilage. Biomech Model Mechanobiol. 2019;18(3):753–78. [DOI] [PubMed] [Google Scholar]
- 20.Eckstein F, Wirth W, Hudelmaier MI, Maschek S, Hitzl W, Wyman BT, et al. Relationship of compartment-specific structural knee status at baseline with change in cartilage morphology: a prospective observational study using data from the osteoarthritis initiative. Arthritis Res Ther. 2009;11(3):R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier F, Lewis CG, Pierce DM. The evolving large-strain shear responses of progressively osteoarthritic human cartilage. Osteoarthritis Cartilage. 2019;27(5):810–22. [DOI] [PubMed] [Google Scholar]
- 22.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alizai H, Virayavanich W, Joseph GB, Nardo L, Liu F, Liebl H, et al. Cartilage lesion score: comparison of a quantitative assessment score with established semiquantitative MR scoring systems. Radiology. 2014;271(2):479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bucknor MD, Nardo L, Joseph GB, Alizai H, Srikhum W, Nevitt MC, et al. Association of cartilage degeneration with four year weight gain--3T MRI data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23(4):525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph GB, McCulloch CE, Nevitt MC, Neumann J, Gersing AS, Kretzschmar M, et al. Tool for osteoarthritis risk prediction (TOARP) over 8 years using baseline clinical data, X-ray, and MRI: Data from the osteoarthritis initiative. J Magn Reson Imaging. 2018;47(6):1517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann J, Hofmann FC, Heilmeier U, Ashmeik W, Tang K, Gersing AS, et al. Type 2 diabetes patients have accelerated cartilage matrix degeneration compared to diabetes free controls: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2018;26(6):751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwaiger BJ, Gersing AS, Mbapte Wamba J, Nevitt MC, McCulloch CE, Link TM. Can Signal Abnormalities Detected with MR Imaging in Knee Articular Cartilage Be Used to Predict Development of Morphologic Cartilage Defects? 48-Month Data from the Osteoarthritis Initiative. Radiology. 2016;281(1):158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun D, Neumann J, Joseph GB, Foreman S, Nevitt MC, McCulloch CE, et al. Introduction of an MR-based semi-quantitative score for assessing partial meniscectomy and relation to knee joint degenerative disease: data from the Osteoarthritis Initiative. Eur Radiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan J, Stehling C, Muller-Hocker C, Schwaiger BJ, Lynch J, McCulloch CE, et al. Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T MRI - an analysis from the incidence cohort of the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serebrakian AT, Poulos T, Liebl H, Joseph GB, Lai A, Nevitt MC, et al. Weight loss over 48 months is associated with reduced progression of cartilage T2 relaxation time values: data from the osteoarthritis initiative. J Magn Reson Imaging. 2015;41(5):1272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devereux-Fitzgerald A, Powell R, Dewhurst A, French DP. The acceptability of physical activity interventions to older adults: A systematic review and meta-synthesis. Soc Sci Med. 2016;158:14–23. [DOI] [PubMed] [Google Scholar]
- 32.Bennell KL, Nelligan RK, Kimp AJ, Wrigley TV, Metcalf B, Kasza J, et al. Comparison of weight bearing functional exercise and non-weight bearing quadriceps strengthening exercise on pain and function for people with knee osteoarthritis and obesity: protocol for the TARGET randomised controlled trial. BMC Musculoskelet Disord. 2019;20(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anandacoomarasamy A, Leibman S, Smith G, Caterson I, Giuffre B, Fransen M, et al. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Ann Rheum Dis. 2012;71(1):26–32. [DOI] [PubMed] [Google Scholar]
- 34.Pamukoff DN, Lewek MD, Blackburn JT. Greater vertical loading rate in obese compared to normal weight young adults. Clin Biomech (Bristol, Avon). 2016;33:61–5. [DOI] [PubMed] [Google Scholar]
- 35.Bartels EM, Juhl CB, Christensen R, Hagen KB, Danneskiold-Samsoe B, Dagfinrud H, et al. Aquatic exercise for the treatment of knee and hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:CD005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gersing AS, Schwaiger BJ, Nevitt MC, Zarnowski J, Joseph GB, Feuerriegel G, et al. Weight loss regimen in obese and overweight individuals is associated with reduced cartilage degeneration: 96-month data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2019;27(6):863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gersing AS, Solka M, Joseph GB, Schwaiger BJ, Heilmeier U, Feuerriegel G, et al. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2016;24(7):1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fithian DC, Kelly MA, Mow VC. Material properties and structure-function relationships in the menisci. Clin Orthop Relat Res. 1990(252):19–31. [PubMed] [Google Scholar]
- 39.Kretzschmar M, Lin W, Nardo L, Joseph GB, Dunlop DD, Heilmeier U, et al. Association of Physical Activity Measured by Accelerometer, Knee Joint Abnormalities, and Cartilage T2 Measurements Obtained From 3T Magnetic Resonance Imaging: Data From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2015;67(9):1272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hovis KK, Stehling C, Souza RB, Haughom BD, Baum T, Nevitt M, et al. Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum. 2011;63(8):2248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin W, Alizai H, Joseph GB, Srikhum W, Nevitt MC, Lynch JA, et al. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013;21(10):1558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


