Abstract
There is increasing evidence that novel coronavirus disease 2019 (COVID-19) leads to a significant coagulopathy, a phenomenon termed “COVID-19 associated coagulopathy”. COVID-19 has been associated with increased rates of both venous and arterial thromboembolic events, a source of significant morbidity and mortality in this disease. Further evidence suggests a link between the inflammatory response and coagulopathy associated with COVID-19. This presents a unique set of challenges for diagnosis, prevention, and treatment of thrombotic complications. In this review, we summarize and discuss the current literature on laboratory coagulation disruptions associated with COVID-19 and the clinical effects of thromboembolic events including pulmonary embolism (PE), deep vein thrombosis (DVT), peripheral arterial thrombosis, and acute ischemic stroke in COVID-19. Endothelial injury and augmented innate immune response are implicated in the development of diffuse macro- and microvascular thrombosis in COVID-19. The pathophysiology of COVID-19 associated coagulopathy is an important determinant of appropriate treatment and monitoring of these complications. We highlight the importance of diagnosis and management of dysregulated coagulation in COVID-19 in order to improve outcomes in COVID-19 patients with thromboembolic complications.
Keywords: COVID-19, Coagulopathy, Coagulation, Thrombosis, Thromboembolism, DVT, Pulmonary Embolism
Introduction:
As of September 2020, there have been over 31 million worldwide confirmed cases of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), with over 1 million confirmed deaths (1). In the United States alone, there have been over 7 million cases and over 200,000 deaths from the novel virus. In early retrospective studies, cohorts of COVID-19 patients in Wuhan, China were found to have disruptions in coagulation parameters including elevated D-dimer levels and prothrombin time (PT) (2, 3). These alterations in coagulation were predictive of a high mortality, with 15 of 22 (71.4%) of non-survivors meeting International Society of Thrombosis and Hemostasis (ISTH) criteria for disseminated intravascular coagulation (DIC) in comparison to just 1 of the 162 (0.6%) survivors in one early study (3). By March 2020, ISTH had published an algorithm for recognition and management of “coagulopathy in COVID-19” (4). Markers of coagulation disruption in COVID-19 currently under investigation include D-dimer levels, platelet count, Von Willebrand Factor (VWF), Factor VIII, thromboelastography (TEG), lupus anticoagulant (LAC), antiphospholipid antibodies (APLs), and fibrinogen.
In addition to the disturbances in laboratory markers of coagulation, COVID-19 infection has been associated with both venous and arterial thrombosis (5, 6). Autopsy studies of COVID-19 patients have shown both macro- and microvascular thrombosis (7, 8). The SARS-Cov2 virus uses angiotensin converting enzyme-2 (ACE-2) as its main receptor; this membrane protein is expressed in the blood vessels, lungs, heart, kidneys, and numerous other tissues. It has been hypothesized that SARS-CoV-2 binding to ACE-2 leads to local and systemic inflammatory response, endothelial injury, and an imbalance in pro- and anticoagulant signals, with resultant macro- and microvascular thrombosis (9). Ongoing mechanistically-driven and clinical research seeks to identify the most efficient pharmacologic targets for preventing and treating arterial and venous thrombosis associated with COVID-19 infection. We performed a systematic review of the literature to provide an update on derangements of coagulation laboratory assays, clinical manifestations of coagulopathy, and current recommendations for prevention and treatment of thrombotic complications in patients with COVID-19 infection.
Methods:
This systematic review was carried out with the assistance of a Masters level librarian through Library Services at Mayo Clinic in Rochester, Minnesota. Multiple databases were interrogated including: EMBASE, MEDLINE, Scopus, Web of Science, Cochrane Database of Systematic Reviews, ACP Journal Club, Cochrane Central Register of Controlled Trials (CCTR), Cochrane Clinical Answers (CCA), Cochrane Methodology Register, Database of Abstracts of Reviews and Effects (DARE), Health Technology Assessment Database (HTA) and National Health Service Economic Evaluation Databases (NHSEED). The following keywords were utilized: COVID-19, coagulopathy, Coronavirus, COVID, SARS-COV-2, coagulation, endothelial, thrombosis, anticoagulation, thromboembolism, DVT, hypercoagulable, pulmonary embolism. We restricted our results to articles written or translated in English and the following article types: clinical trials, retrospective reviews, cohort studies, society guidelines, meta-analysis, case control studies and case series. This search yielded 788 individual citations, which were screened based on title and brief review of the abstract by two independent physician reviewers. From these, 590 articles were excluded. Articles were excluded at this stage for the following reasons: Publications that were clearly not relevant to the topic (for example, related to coagulopathy but not of COVID-19, articles related to other viral illnesses, articles related to other aspects of COVID-19), descriptions of future studies or ongoing clinical trials, erratum from studies yielding duplicate results, abstracts without full articles or abstracts in English with associated articles that were not translated, and opinion pieces. This resulted in 198 potentially relevant articles, which we reviewed in depth by two physician reviewers. From these, 75 articles were excluded. Reasons for exclusion at this stage were: Articles were found to not be pertinent on further review (for example, articles focused on other aspects of COVID-19), articles with only in vitro data that did not use any patient samples, articles that described clinical features of COVID-19, but did not expand on coagulopathy, one article that was later retracted, articles found to be opinion pieces on further review, brief communications without sufficient data to assess, review articles or small case series, small meta-analyses in which all or most of the articles were already assessed and incorporated in this review. The remaining 123 articles were then re-evaluated, verified for inclusion and summarized by two independent authors. We also reviewed the bibliographies of the 123 articles and performed thorough search for other relevant background references. This yielded 11 results, for a total of 134 cited references (Figure 1). Additionally, we present some unpublished data within this manuscript from our institution’s experience with COVID-19. Collection of this de-identified data was approved by the Mayo Clinic Institutional Review Board (IRB) and permission to utilize this data was provided to us by the study team.
Figure 1:
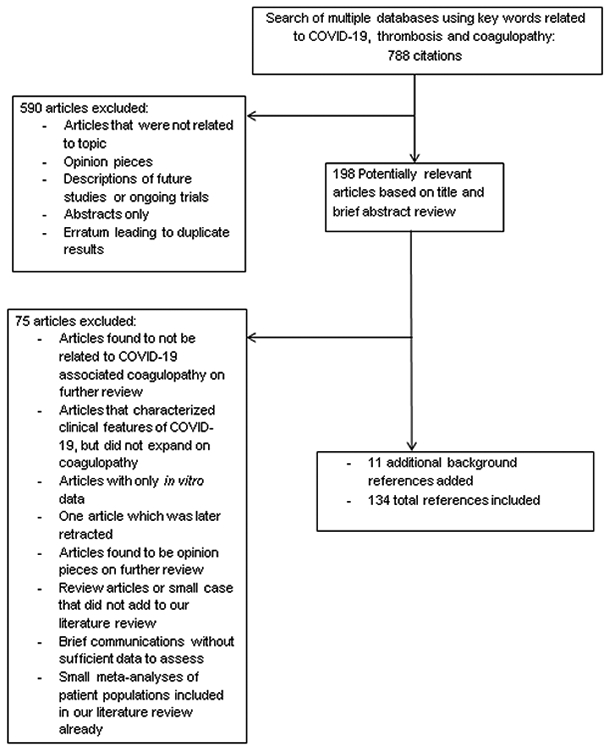
Description of Methods
Laboratory Abnormalities in COVID-19 Patients:
D-dimer:
Early work on COVID-19 associated coagulopathy noted consistently elevated levels of D-dimer, a marker of fibrin degradation by the action of plasmin on stabilized fibrin, in COVID-19 patients (2). Tang et al. showed that patients who died from COVID-19 had significantly greater D-dimer levels, pro-thrombin time (PT) and fibrinogen on admission as compared to survivors of COVID-19 infection (3). In another retrospective analysis of 343 patients from Wuhan, China, a D-dimer level ≥ 2.0 μg/dL was significantly associated with higher mortality (18% vs. 0.4%), with 92.3% sensitivity and 83.3% specificity (10). The dynamic association between D-dimer level and COVID-19 disease prognosis has been repeatedly demonstrated (11-13). Multiple studies also demonstrate a relationship between the trend of D-dimer levels and COVID-19 disease progression (14-17). Initial studies equated the elevation in D-dimer level with DIC, but ongoing work has suggested that COVID-19 associated coagulopathy is a unique entity (3, 4, 18). At our own institution’s special coagulation laboratory, we have measured soluble fibrin monomer levels (SFMC), a sensitive and specific marker of DIC, from patients who had elevated D-dimer levels. Of an initial cohort of 35 COVID-19 patients, D-dimer level ranged from 220-100,000 ng/mL (median 627 ng/mL), and 27 patients (77%) were found to have elevated D-dimer levels. Of these 27 patients, SFMC ranged from 5-1,100 mcg/mL (median 5 mcg/mL), and only 5 patients (18.5%) were found to have elevated SFMC. Additionally only 4 of the 35 (11%) patients included in this study were found to have ISTH DIC score ≥ 5 (cutoff for diagnosis of overt DIC). The low frequency of elevated SFMC and ISTH DIC score ≥ 5 suggests that the elevated D-dimer levels observed in COVID-19 does not represent overt DIC (unpublished Mayo Clinic data).
Elevated D-dimer level is a marker for venous thromboembolism (VTE) in the appropriate clinical setting. In COVID-19 patients, elevated D-dimer levels have been shown to be predictive of VTE development (19-21). A number of studies have sought to quantitatively define the association of D-dimer levels with VTE in COVID-19 (22). In a multicenter retrospective cohort study, Artifoni et al. showed that a D-dimer level < 1.0 μg/ml had a 95% negative predictive value (NPV) for venous thromboembolism (VTE), which encompasses deep vein thrombosis (DVT) and pulmonary embolism (PE), while a D-dimer of > 3.0 μg/mL had an 80% positive predictive value (PPV) for VTE (19). All patients in this study received appropriate weight-based pharmacologic VTE prophylaxis with enoxaparin. Elevated D-dimer levels also correlate with VTE in non-critically ill COVID-19 patients. In one study of 29 non-ICU patients, a D-dimer level of > 5,000 μg/dL was an independent predictor of PE (OR 3.77, 95% CI [1.18-12.16], p = 0.03) Similarly, in another study of 156 hospitalized patients, a D-dimer level of > 1,570 ng/mL was associated with a higher frequency of asymptomatic DVT (OR 9.1, 95% CI [1.1-70.1], PPV 19%), with a D-dimer level less than 1,570 ng/mL having a NPV of 97.5% for asymptomatic DVT (23, 24). Thus, D-dimer level potentially has a role in clinical decision making for patients with COVID-19 infection. It is worthwhile to note that different institutions and laboratories vary in unit of measurements for D-dimer levels, with the most common measurements being ng/mL, μg/ml, and μg/dL. The ISTH has recommended admission testing and monitoring of D-dimer, prothrombin time (PT), platelet count, and fibrinogen in all hospitalized COVID-19 patients (4). Further clinical and laboratory validation will be needed to better understand the roles of these labs in risk stratifying COVID-19 patients for VTE development (4).
Thrombocytopenia:
Decreased platelet counts or thrombocytopenia appear to be uncommon, but associated with poor prognosis in COVID-19 (15, 25, 26). Multiple studies have shown an association between thrombocytopenia and mortality in COVID-19 (27). A retrospective study of 383 COVID-19 patients in Wuhan, China showed a threefold increased rate of mortality (30.9% vs. 8.9%) in patients with thrombocytopenia (platelet count < 125 x 109/L) on admission as opposed to patients who did not have thrombocytopenia on admission. They additionally performed a Cox proportional hazards regression model that showed a 40% decrease in mortality associated with every 50 x 109/L increased in platelet count (28). Similarly, Yang et al. showed that while thrombocytopenia (platelet count < 125 x 109/L) was present in only 20.7% of 1,476 hospitalized COVID-19 patients, platelet nadir was associated with mortality, even when adjusted for age and gender (29). There are also reports of “delayed phase” thrombocytopenia. A retrospective study from Wuhan, China showed that 32 of 271 (11.8%) patients developed thrombocytopenia at 14 days after symptom onset, and the mean time from symptom onset to thrombocytopenia was 28.3 days (26). The majority of thrombocytopenic patients in this study were older with worse outcomes, including increased hospital length of stay and increased rate of mortality. It has been proposed that COVID-19 leads to thrombocytopenia through bone marrow suppression, either as a direct viral effect or from cytokine release (30-32). This is supported by evidence of impaired megakaryocyte maturation in bone marrow aspirates from thrombocytopenic COVID-19 patients (26). An alternate hypothesis is that thrombocytopenia occurs through a consumptive process of platelet destruction by immune complexes and generation of thrombi and microthrombi in the lungs. Although thrombocytopenia is certainly not present in the majority of COVID-19 patients, it is consistently linked to poor prognosis, at various stages in the disease process. Platelet count should thus be checked on admission and monitored in hospitalized COVID-19 patients (4).
Von Willebrand Factor (VWF):
Studies have characterized a component of “endotheliopathy” in COVID-19 related coagulopathy. Endothelial injury by COVID-19 induced inflammation is thought to contribute to the hypercoagulable state (33, 34). A recent review identified disruption to endothelium and altered hemostasis as the key drivers in individual phenotypes of COVID-19 (35). In a case report, Escher et al showed markedly elevated plasma VWF antigen (VWF:Ag) and Factor VIII (FVIII) activity levels of 555%, 520% and 369% respectively in a critically ill COVID-19 positive patient on hospital day 21, indicative of endothelial cell injury (34). They also demonstrated a decrease in D-dimer levels and liberation from mechanical ventilation after starting heparin (34). Escher et al subsequently published a case series showing elevated levels of VWF, FVIII and fibrinogen in COVID-19 patients despite chemoprophylaxis (36). Persistently elevated levels of VWF have also been shown in both critically ill and non-critically ill COVID-19 patients (33, 37, 38). Additionally, there is a case series showing a relative reduction in levels of ADAMTS13 in COVID-19, further pointing to the potential role of endothelial injury in these patients (38). Although measurement of VWF level and activity are not common practice in the clinical setting, these data support that the coagulopathy of COVID-19 results, in part, from widespread endothelial injury.
Antiphospholipid Antibodies:
More recently, there has been evidence that various anti-phospholipid antibodies (APLs) are present in COVID-19 patients. Several studies have reported presence (45-85%) of lupus anticoagulant (LAC) in the blood of critically ill COVID-19 patients (37, 39-41). Elevation of LAC is not typically measured during acute illness, and expression has been transient and variable in COVID-19 patients, with no definitive association to thrombotic complications (42). This effect may be due to non-specific assay interference with C-reactive protein (CRP) (43). Although the clinical significance of LAC in patients with COVID-19 remains uncertain, high LAC titers are associated with prolonged activated partial thromboplastin time (aPTT), which can be problematic when using aPTT for monitoring of unfractionated heparin therapy (40). As such, several authors advocate using anti-Xa levels to guide heparin treatment in COVID-19 patients (40, 44).
In a cohort of 74 mechanically ventilated COVID-19 patients, anti-cardiolipin IgG and/or IgM and/or anti-B2-glycoprotein-I (anti-B2-GP1) IgG were detected in 9 patients, but did not correlate with thrombosis or coagulopathy (42). In a group of 56 COVID-19 patients, although 45 patients were positive for LAC, anti-cardiolipin or anti-B2-GP1, IgG and IgM were detected in only 5/45 patients (10%) (41). In another study of 66 critically ill COVID-19 patients in China, APLs were present in 31 (47%) patients, with IgG anti-B2-GP1 identified in 19 (28.8%) of these 66 patients (39). This study found that 5 of 15 patients (33%) who had multiple elevated APLs suffered ischemic stroke, compared to 0/25 with normal levels and 0/16 with elevation of a single APL (39). Weakly positive anti-cardiolipin and anti-B2-GP1 IgM (no IgG) were also described in 2 of 24 (8.3%) of patients in a cohort of COVID-19 patients in Madrid with confirmed VTE (45). IgA type APLs, which are not thought to play a role in pathogenesis, have also been described in COVID-19 patients (39).
APLs have been associated with other systemic viral illnesses but typically are not associated with an elevated thrombotic risk (46). COVID-19 infection is associated with an overall hypercoagulable state, but it is unclear whether the presence of APLs contributes to this coagulopathy or represents cross-reactivity between the virus and host cell receptors. It has been proposed that host B2-GP1 domains are exposed as a result of SARS-CoV-2 induced endothelial cell injury and give rise to cross-reactive anti-B2-GP1 antibodies or anti-cardiolipin antibodies (42). Further investigation is needed to elucidate the potential role of APLs within the schematic of COVID-19 associated coagulopathy. Current ISTH guidelines advocate for testing and diagnosis of anti-phospholipid antibody syndrome, if clinically indicated, with confirmatory testing 12 weeks following initial detection, even in patients with multiple positive APLs (47).
Thromboelastography and COVID-19 Coagulopathy:
Thromboelastography (TEG) is a dynamic measure of the viscoelastic properties of coagulation and is increasingly employed in critical care, emergency, and surgical settings, particularly to guide plasma and platelet transfusion in bleeding patients. Several studies have found COVID-19 patients to be hypercoagulable by TEG or rotational thromboelastometry (ROTEM). In a series of 21 COVID-19 ICU patients, 90% (n=19) demonstrated hypercoagulability based on ICU admission TEG parameters (48). In a cohort of 24 intubated COVID-19 patients with a total of 30 TEGs drawn, half the measurements (n=15) had shortened R-times, 90% (n=27) had shortened K-times, and 87% (n=26) had increased maximum amplitude (MA) indicating more rapid clot formation and increased clot strength (49). Other small series of COVID-19 patients admitted to the ICU had similar findings in which ROTEM measurements demonstrated maximum clot firmness (MCF) above the normal range, along with elevated levels of D-dimers and fibrinogen (18, 50). Authors postulate that their findings indicate a widespread shutdown of fibrinolysis, with elevated D-dimer levels driven by local fibrinolysis in the lungs (18). In a cohort of 44 ICU patients, 57% showed evidence of complete “fibrinolysis shutdown” with elevated D-dimer levels and a Ly30 of 0% (51). This group went on to describe a 50% VTE rate and 80% rate of new hemodialysis requirement in patients with both D-dimer level > 2,600 ng/mL and Ly30 of 0% (51). Another small retrospective series of 25 COVID-19 ICU patients demonstrated “fibrinolysis shutdown” (defined by the authors as EXTEM maximum lysis of <3.5%) in 11/21 patients, with 8 of those 11 patients going on to develop a thrombotic event (52). Similarly, others have found elevated D-dimer levels to correlate with specific abnormal parameters on TEG (53). There are others who believe that TEG and ROTEM may not be well suited for identifying decreased fibrinolytic activity, particularly as it may be possible that large amounts of deposited fibrin in COVID-19 patients may be due to overwhelming physiologic response (54). As such, TEG and/or ROTEM, when interpreted in the context of other lab parameters, can be a helpful adjunct test both in understanding an individual’s coagulome and stratifying VTE risk.
COVID-19 Associated Coagulopathy Leads to Increased Venous and Arterial Thrombosis
The clinical phenotype of COVID-19 associated coagulopathy includes macro- and microvascular arterial and venous thrombosis. Evidence of increased thrombotic complications in COVID-19 was first shown by Klok et al. who reviewed 184 patients with proven COVID-19 associated pneumonia from three intensive care units (ICUs.) They found a 31% total rate of venous and arterial thrombotic complications, with 81% of these being PE (5). The only independent predictors of thrombotic events in this initial study were age and coagulopathy defined as pro-thrombin time (PT) prolongation > 3s or activated partial thromboplastin time (aPTT) prolongation > 5s from the upper limits of normal (5). In a follow up study, patients with venous and arterial thrombotic complications related to COVID-19 were found to have greater all-cause mortality (55). Additional single-center studies have shown varying rates of venous and arterial thromboembolic events associated with COVID-19. A single-center retrospective study of 92 ICU patients found that 37 of the 92 (40%) had thrombotic complications, with the majority (n=19) being PEs (6). However, this study also found that 19 patients (21%) had hemorrhagic complications. Of these 19 patients, 16 were on therapeutic anticoagulation with 8 started on therapeutic anticoagulation in the absence of a confirmed thrombotic event (6). Their work emphasizes the importance of carefully considering the risks of therapeutic anticoagulation. An additional retrospective review of 362 hospitalized patients revealed a 7.7% (n=22) rate of arterial and venous thromboembolic events, higher in ICU patients than general care patients, with 57% (n=16) of these being VTE (56).
There have been a number of publications studying VTE specifically in critically ill cohorts of COVID-19 patients. In a retrospective single-center study of 75 ICU patients with COVID-19 associated pneumonia, 25 (33.3%) were found to have thromboembolic complications, with all patients requiring high doses of heparin to achieve therapeutic state (57). In a cohort of 91 critically ill COVID-19 patients, 24 (26%) developed VTE (58). VTE is a significant problem in non-critically ill patents as well. In a retrospective single-center study of 289 consecutively admitted COVID-19 patients on the general care floor, 49 patients (17%) were found to have VTEs, with 42 of these being PE. Patients who developed VTE were more likely to require transfer to ICU, had greater length of stay, had lower hemoglobin levels at discharge, and had greater peak D-dimer levels than those who did not develop VTE. On multivariable analysis, time from symptom onset to admission, international medical prevention registry on venous thromboembolism (IMPROVE) score, leukocyte count at admission, and lack of pharmacologic VTE prophylaxis, were independent predictors of VTE (59).
Al-Samkari et al. performed a multicenter, retrospective study of 400 patients and found a VTE rate of 4.8% (in 19 patients), arterial thrombosis rate of 2.8% (in 11 patients), and an additional 8 patients with recurrent clotting of dialysis circuits, for a total thrombotic complication rate of 9.5%. They additionally identified 21 bleeding complications in 19 patients (25). For each of these endpoints, the rate was greater in ICU patients compared with patients admitted to the general care floor (25). A multicenter prospective study demonstrated a significantly greater rate of arterial and venous thromboembolic events, particularly PE, for patients with COVID-19 related acute respiratory distress syndrome (ARDS) vs. matched patients with non-COVID-19 related ARDS (9 of 77 [11.7%] vs. 7 of 145 [4.8%]) (37). At our own institution, we have collected data from a cohort of 105 hospitalized COVID-19 patients receiving pharmacologic VTE prophylaxis and found a total of 6 (5.9%) arterial and venous thromboembolic events (unpublished Mayo Clinic data), including 3 VTEs, 2 myocardial infarctions, and one ischemic stroke. Additional thrombotic complications reported in literature have been central line associated thrombosis, clotting of continuous renal replacement therapy circuits, and veno-venous extracorporeal membrane oxygenation (ECMO) circuit complications (25, 55). Recently, two meta-analyses have estimated overall VTE risk in COVID-19 patients receiving pharmacologic prophylaxis at 23.9% and 31% (60, 61). It does appear that the elevated VTE risk may continue even after hospital discharge. In a prospective multicenter study of 1,877 discharged COVID-19 patients, the rate of VTE was determined to be 4.8 per 1,000 discharges (with 42 day follow up). The rate from the prior year was 3.1 per 1,000 discharges but this was not a statistically significant difference (62). Further studies of ambulatory and post-discharge COVID-19 patients are needed to better elucidate the potential VTE risks to these patients even outside of the hospital setting. For hospitalized patients, both on the general wards and in the ICU, vigilant monitoring for VTE and timely prophylaxis are recommended.
Pulmonary Embolism:
A number of studies have focused on incidence and factors predictive of PE for COVID-19 patients in various clinical settings (Table 2). An initial case series of 20 patients revealed that 8 (40%) had findings of peripheral, multifocal PE on CT angiogram (CTA), along with elevated D-dimer and greater Revised Geneva Score (63). A retrospective study of 72 non-hospitalized COVID-19 patients referred to the emergency room for imaging demonstrated an 18% (n=13) rate of PE (64). In this series, patients with PE were found to be significantly older and have significantly greater D-dimer levels than those without PE (64). A larger, single-center study of 1,477 patients admitted with COVID-19 revealed that of the 214 who underwent CT angiography (CTA), 80 were identified to have PEs, thus a 5.9% rate in total patients included (65). Patients with PEs were more likely to have required ICU care and had greater D-dimer and C-reactive protein (CRP) levels in comparison to patients who had negative imaging (65). A retrospective multicenter study of 1,240 COVID-19 patients revealed an 8.3% (n=103) rate of PE (66). Multivariable analysis revealed male sex, administration of anticoagulation at prophylactic or therapeutic dose, elevated CRP, and days from symptom onset to hospitalization to be independent predictors of PE (66). An additional retrospective multicenter study of 135 COVID-19 patients, all of whom underwent chest CTA revealed 32 PEs (24%). Subgroup analysis showed that 12 of 24 patients admitted to the ICU (50%) developed PE. In comparison to patients who did not develop PE, patients who developed PE in this cohort had greater D-dimer levels and were more likely to require ICU admission with mechanical ventilation (67).
Table 2:
Clinical Findings of Venous and Arterial Thrombosis in COVID-19 PatientsΔ
| Endpoint | Study | Country of Origin | Design | Centers | No. of COVID -19 Patients |
Frequency observed |
|---|---|---|---|---|---|---|
| Overall thrombotic complications | Klok et al.* (5) | Netherlands | Retrospective | Single | 184 | 31% |
| Fraisse et al.* (6) | France | Retrospective | Single | 92 | 40% | |
| Al-Samkari et al. (25) | USA | Retrospective | Multi | 400 | 9.5% | |
| Helms et al. (37) | France | Prospective | Multi | 150 | 16.7% | |
| Lodigiani et al. (56) | Italy | Retrospective | Single | 362 | 7.7% | |
| Beun et al. (57) | Netherlands | Retrospective | Single | 75 | 33.3% | |
| Mayo Clinic unpublished | USA | Retrospective | Single | 105 | 5.9% | |
| VTE | Cui et.al. (20) | China | Case series | Single | 81 | 25% |
| Hippensteel et al.* (58) | USA | Retrospective | Single | 91 | 26.1% | |
| Trimaille et al. (59) | France | Retrospective | Single | 289 | 17% | |
| Chi et al. (60) | USA | Meta-analysis | Multi | 1981 | 23.9% | |
| Hasan et al.* (61) | UK, Australia, Malaysia | Meta-analysis | Multi | 899 | 31% | |
| Roberts et al. (62) | UK | Prospective | Multi | 1877 | 4.5% | |
| Pulmonary Embolism | Mestre-Gomez et al. (23) | Spain | Retrospective | Single | 452 | 6.4% |
| Bavaro et al. (63) | Italy | Case series | Single | 20 | 40% | |
| Gervaise et al. (64) | France | Retrospective | Single | 72 | 18% | |
| Whyte et al. (65) | UK | Retrospective | Single | 1477 | 5.9% | |
| Fauvel et al. (66) | France | Retrospective | Multi | 1240 | 8.3% | |
| Bompard et al. (67) | France | Retrospective | Multi | 135 | 24% | |
| Poissy et al.* (68) | France | Case series | Single | 106 | 20.6% | |
| Taccone et al.* (69) | Belgium | Retrospective | Single | 40 | 33% | |
| Mak et al.* (70) | UK | Case series | Single | 51 | 35% | |
| Deep Vein Thrombosis | Demelo-Rodriguez et al.† (24) | Spain | Prospective | Single | 156 | 14.7% |
| Chen Set al.† (71) | China | Retrospective | Single | 88 | 46% | |
| Zhang et al.*† (72) | China | Retrospective | Single | 143 | 46.1% | |
| Rieder et al. (73) | Germany | Prospective | Single | 49 | 6.1% | |
| Santoliquido et al.† (74) | Italy | Prospective | Single | 84 | 11.9% | |
| Middeldorp et al.† (75) | Netherlands | Prospective | Single | 198 | 42% (at 21d) | |
| Trigonis et al.*(76) | USA | Retrospective | Single | 45 | 42.2% | |
| Voicu et al.*† (77) | France | Prospective | Single | 56 | 46% | |
| Acute Cerebrovascular Disease | Li Y et al. (79) | China | Retrospective | Single | 219 | 4.6% |
| Merkler et al. (80) | USA | Retrospective | Multi | 2132 | 1.5% | |
| Yaghi et al. (82) | USA | Retrospective | Multi | 3556 | 0.9% | |
| Tan et al. (84) | Singapore | Meta-analysis | Multi | 4466 | 1.2% | |
| Rothstein et al. (85) | USA | Retrospective | Multi | 844 | 2.4% ischemic 0.9% hemorrhagic |
Table includes studies that provide clinical outcomes
Studies included patients admitted to ICUs only
Studies utilized screening ultrasound for identification of DVT; except in Middeldorp et al. where only 55 of 198 patients underwent screening ultrasound.
Patients requiring ICU care have been the focus of a number of studies on PE in COVID-19. A study of 106 consecutively admitted critically ill patients with COVID-19 revealed 22 (20.6%) patients developed PE (68). Historical controls from the prior year with similar illness severity were found to have a 6.1% (12 of 196) rate of PE (68). A study of 40 critically ill COVID-19 patients at a single center, all of whom required mechanical ventilation and underwent CTA, found 13 PEs (40% rate). Of these 40 patients, 22 received standard dose pharmacologic prophylaxis (4000 IU subcutaneous enoxaparin daily), and of these, 11 (50%) developed PE. The other 18 patients received either high dose pharmacologic prophylaxis (4000 IU enoxaparin twice a day) or therapeutic unfractionated heparin (1500-2200 IU/hr) and only 2 (11%) developed PE. The authors of this study advocate consideration of higher dose regimens in COVID-19 patients (69). Mak et al. were the first to study a cohort of COVID-19 patients requiring ECMO for refractory hypoxemic respiratory failure. Of the 51 patients included in this study, 18 (35%) were found to have evidence of pulmonary artery thrombus, and 28 (54%) were found to have evidence of some pulmonary ischemic changes, typically peripheral wedge shaped low-attenuation areas (70). Meanwhile, looking at 452 consecutively admitted non-critically ill COVID-19 patients at a single-center, Mestre-Gomez et al. found 29 (6.4%) patients with PE despite adequate pharmacologic prophylaxis, with D-dimer > 5,000 μg/dl being the only significant predictor of PE on multivariable analysis (23). Their data also suggest that hyperlipidemia requiring statin use may actually have a potentially protective role in the development of PE in COVID-19 patients (23). This heterogeneous group of studies (case series, retrospective single and multi-center studies) suggests the rate of PE in COVID-19 patients ranges from 5.9%-40%. Important clinical characteristics associated with PE appear to be elevated D-dimer levels, possibly the trend of D-dimer level, and need for ICU care.
Deep Vein Thrombosis:
In the early months of COVID-19, a number of retrospective studies from Wuhan, China showed that hospitalized COVID-19 patients had a DVT rate ranging from 25 - 46% (20, 71, 72). In multiple studies, increased D-dimer levels were significantly associated with DVT (14, 20, 28). In another early study, 49 COVID-19 patients presenting to the ER with signs and symptoms of DVT underwent duplex of lower extremities. When they were compared to 141 COVID-19 negative patients presenting with similar signs and symptoms, there was a 6.1% rate of DVT in COVID-19 patients compared to a 3.5% rate patients without COVID-19 (73). After these initial studies, a number of groups published work in cohorts of COVID-19 patients who underwent screening duplex for DVT. A prospective study of 156 patients with COVID-19 and elevated D-dimer revealed a 14.7% (n=23) rate of asymptomatic DVT but multivariable analysis revealed significantly greater D-dimer levels in patients who developed DVT (24). A similar prospective, single center study of 84 consecutively admitted non-ICU patients with COVID-19, all of whom received pharmacologic prophylaxis (enoxaparin or fondaparinux) and underwent screening lower extremity duplex, found 10 (11.9%) patients with DVT (74). Elevated D-dimer levels, prior or active cancer history, and need for high-flow nasal cannula or non-invasive ventilation all were significantly associated with development of DVT (74). In 198 hospitalized COVID-19 patients followed over time, the prevalence of DVT at 7, 14, and 21 days was 16%, 33%, and 42% (75). This series found higher rates of DVT in patients admitted to the ICU vs. those admitted to the general care floor, and also showed an association of DVT with mortality (75).
Just as with PE and other VTEs, there appears to be a greater rate of DVT in critically ill COVID-19 patients, who have been the focus of numerous studies. In a retrospective review of 45 critically ill COVID-19 patients who received enoxaparin for prophylaxis, required intubation and mechanical ventilation, and underwent lower extremity ultrasound, DVTs were identified in 19 of 42 (42.2%) patients (76). In this cohort, patients who developed DVT were more likely to have greater D-dimer levels and ultrasound performed earlier in hospitalization, though the indications for ultrasound are not elaborated on in the study (76). In another prospective single-center study, Voicu et al. were able to perform screening lower extremity duplex in 56 consecutively admitted critically ill COVID-19 patients at the time of admission and again after 7 days. They found a total of 26 (46% rate) DVTs and patients with DVT were once again found to have greater D-dimer than those who did not develop DVT (77). While the reported rate of DVT associated with COVID-19 has been widely varied (6-46%)(Table 2), DVT is consistently associated with elevated D-dimer levels and ICU admission status.
Cerebrovascular disease:
Acute ischemic stroke is associated with COVID-19 and has been reported as a presenting symptom (78). In an early single-center retrospective study of 219 patients in Wuhan, China, 10 (4.6%) developed acute ischemic stroke (79). Clinical features associated with stroke were age, disease severity, cardiovascular risk factors, elevated CRP and D-dimer levels (79). Merkler et al. performed a retrospective study of 2136 patients presenting to the ER or admitted with COVID-19 vs. 1516 patients with influenza (80). There was a statistically significant increase in acute ischemic stroke (1.5% vs. 0.2%) in patients with COVID-19 as compared to those with influenza, and this difference remained significant when adjusted for age, sex, and race (80). Multiple case series have demonstrated that ischemic stroke can be the presenting symptom of COVID-19 with high rate of mortality and residual morbidity, including paralysis and aphasia (81).
A multicenter study of 3,556 patients hospitalized with COVID-19, found acute ischemic strokes that were verified by imaging in 32 (0.9%) patients (82). In comparison to contemporary controls without COVID-19, who developed acute ischemic stroke, these patients had greater frequency of “cryptogenic” strokes, greater initial NIH stroke scale, greater peak D-dimer level, and higher mortality (82). Sweid et.al performed a retrospective case series of 22 patients with COVID-19 who developed acute stroke, largely thrombotic or embolic in nature (83). Many of these patients had involvement of both arterial and venous vasculature and multi-vessel involvement. Of the 16 of 22 patients who required mechanical thrombectomy, many of the procedures were described as complicated due to clot burden and consistency (83). Finally, a meta-analysis of 39 studies with a total of 135 cases of acute ischemic stroke in patients with COVID-19 describes a pooled frequency of 1.2% (84). Laboratory and imaging features associated with the occurrence of acute ischemic stroke include elevated D-dimer and fibrinogen, presence of antiphospholipid antibodies, and large vessel thrombosis, embolism, or stenosis (84). Acute stroke was associated with 38% overall mortality (84).
With ongoing studies, it is becoming evident that COVID-19 patients are also prone to intracerebral hemorrhage (ICH) due to disruptions in the microvascular circulation. In a retrospective multicenter cohort with diverse patient population (52% female, 68% black), Rothstein et al. identified 20 ischemic strokes (2.4% rate) in 844 patients, but also found 8 patients with hemorrhagic stroke (0.9%) (85). Of 4,131 COVID-19 patients admitted to three centers, 115 underwent brain MRI and 25 (overall rate 0.6%) had findings consistent with leukoencephalopathy or cerebral micro-bleeds. In comparison to patients who had negative MRIs, these 25 patients had lower Glasgow Coma Scale (median 6 vs. 14), imaging later in hospital course, greater peak D-dimer level, lower nadir platelet count, greater international normalized ratio (INR), longer duration of ventilator support, longer hospitalization, and higher mortality (20% vs. 9%) (86). A case series of 5 COVID-19 patients with ICH showed that 4 of these were in lobar territories. The authors comment that typically lobar ICH only represents 15 - 30% of cases and is associated with vascular anomalies; however none of the 5 patients in this series had identifiable vascular anomalies. The authors postulate that mechanisms contributing to ICH in COVID-19 may include direct and indirect endothelial injury or disruption of renin-angiotensin system (87). After identifying 2 COVID-19 patients on ECMO who suffered fatal frontal intraparenchymal hemorrhage in the absence of laboratory derangements, Heman-Ackah and colleagues have advocated for routine imaging in patients in whom routine neurologic exams are unreliable due to sedation and paralysis (88). Though these studies describe < 5% rate of acute cerebrovascular events in COVID-19 patients, these events are characterized by unique pathologic features and high mortality, and there is increasing evidence of association between COVID-19 and ICH. It is clear that imaging studies are necessary to determine treatment course, but ongoing prospective studies are necessary to determine individual risk stratification and need for imaging.
Peripheral Arterial Thromboembolism:
In addition to acute ischemic strokes, COVID-19 has been associated with peripheral arterial disease. As with ischemic stroke, acute lower extremity arterial occlusion has been seen as a presenting symptom of COVID-19 in patients with no prior history of hypercoagulability or peripheral vascular disease (89). A single-center study was published after the authors noted an increase in the number of procedures necessitated by acute limb ischemia; from January through March 2020, 16.3% (23/141) of the vascular procedures were done for acute limb ischemia, and 87% (20/23) were positive for COVID-19 (90). As a comparison, only 1.8% (3/163) vascular interventions performed in the previous year had been done for acute limb ischemia (90). This phenomenon was further characterized in a propensity matched study of 16 COVID-19 positive and 32 COVID-19 negative patients who underwent lower extremity CT angiography (CTA) after presenting with symptoms of acute limb ischemia (91). All 16 of the COVID-19 patients were found to have evidence of at least one level of arterial thrombosis while 69% of COVID-19 negative patients had clot burden. Additionally, the COVID-19 patients had greater “clot score” by three different measures (91). While the rate of surgical intervention did not differ between the two groups, the patients with COVID-19 had a 38% limb amputation rate (compared to 3% for COVID-19 negative patients) and a 25% mortality (compared to 3% for COVID-19 negative patients) (91). These initial studies suggest that peripheral arterial thromboembolism contributes to increased morbidity and mortality in COVID-19 patients.
Post-Mortem Studies:
Autopsy reports confirm the increased frequency of thromboembolic events observed in patients with COVID-19. In a series of the first 80 autopsies performed for patients who died of COVID-19 in Hamburg, Germany, 32 were found to have DVTs including 17 with concurrent PE (7). Similarly, a case series of 10 autopsies done in Austria showed some degree of small and medium vessel pulmonary thrombosis in all patients (92). Further post-mortem clinicopathologic examinations have revealed a prominence of diffuse alveolar hemorrhage, severe pulmonary capillary congestion, and peripheral and alveolar microvessel thrombosis in deceased COVID-19 patients (93, 94). In a case series of 7 autopsies, platelet rich microthrombi were found not only in the pulmonary vasculature but also in hepatic, cardiac, and renal vasculature (8). This study also suggested greater than usual numbers of megakaryocytes in pulmonary and cardiac tissues (8). Another post-mortem case series showed co-localization of SARS-CoV2 envelope proteins with C4b and C5b-9 in pulmonary microvasculature along with alveolar microvessel thrombosis, suggesting a role of complement in COVID-19 associated coagulopathy (95). Microvessel thrombi have also recently been demonstrated in vivo using hand held video capillaroscopy of sublingual vessels in a series of 13 critically ill COVID-19 patients requiring mechanical ventilation (96).
A number of novel techniques have been used in autopsy studies of COVID-19 patients. Of 86 consecutive COVID-19 deaths in one series, 68 were described as unexpected, in comparison to 70 of 334 deaths at the same center the year prior. Of these, 64 of 68 patients underwent post mortem computed tomography and 15 (23%) were found to have proximal PE, in comparison to just 5 of 70 (7.1%) from the prior year (97). Ultrasound-guided minimally invasive autopsy was performed in the first 10 fatal cases of COVID-19 in Brazil and demonstrated all patients to have diffuse alveolar damage with high density of megakaryocytes and 8 of the 10 patients to have fibrinous thrombi in alveolar arterioles (98). To investigate the interactions between inflammatory response and pulmonary microangiopathy, Li et al. used high resolution microscopy to create 3D reconstructions of lung tissue samples from patients who died from COVID-19 infection (99). Their 3D reconstructions revealed extensive small vessel thrombosis, distinct from diffuse alveolar hemorrhage, mature megakaryocytes within the small vessels of the lung, and fibrin deposition with inflammatory cell attachment, which were much more extensive than appreciated in 2D histology (99). Together, the emerging evidence from post-mortem studies suggests that COVID-19 is a disease of pulmonary microangiopathy with involvement of inflammation and coagulation.
Innate Immune Response and Endothelial Injury Contribute to COVID-19 Associated Coagulopathy
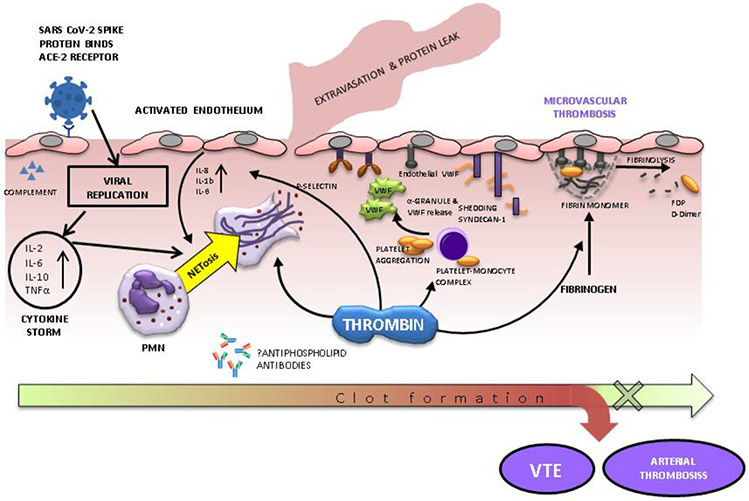
The complex interactions between inflammation and coagulation have been implicated in sepsis, DIC, ischemia-reperfusion, trauma-induced coagulopathy, and many other systemic disorders (44). There is mounting evidence for an inflammatory component of COVID-19 associated coagulopathy, with one study even showing elevated leukocyte count on admission to be an independent predictor of VTE (59). Proposed contributing factors include endothelial injury, cytokine storm, complement activation, particularly the alternative pathway which has recently been shown to trigger a hypercoagulable state, and formation of neutrophil extracellular traps (Figure 2) (100, 101). Fogarty et.al compared 33 critically ill and non-survivors of COVID-19 to 50 non-critically ill survivors (102). The 33 patients who required ICU stay or died had comparatively greater CRP, D-dimer, and fibrinogen at day 1 and 4, suggesting both inflammatory and coagulation disruption correlate with poor prognosis (102). The Global COVID-19 Thrombosis Collaborative Group describes “thromboinflammation” as an area of ongoing interest for identifying pharmacologic targets to prevent thrombosis (44). Elevated levels of cytokines including IL-2, IL-6, IL-10, TNF-alpha, and diffuse alveolar inflammation with fibrin deposition form the basis for their investigation (44). It is also thought that cytokine release may be a driver of direct and systemic damage related to COVID-19 (103). Immunomodulators targeting the complement cascade, JAK-kinase, or IL-6, with anti-thrombotic properties are potential preventative options in patients who have not yet developed thrombosis (44).
Figure 2:
Proposed mechanisms by which SARS Cov-2 infection leads to micro- and macrovascular thrombosis
Endothelial injury may be an inciting insult for inflammatory mediated thrombosis (Figure 2). Case series of critically ill COVID-19 patients have shown persistent severe elevation in VWF, fibrinogen, and factor VII, all of which are suggestive of endothelial injury (34, 36). However, unlike patients with TTP or DIC, these patients have normal platelet counts and many have normal ADAM-TS13 activity (36). This has been further investigated with other markers of endothelial injury. In a single-center study, 40 ICU patients with COVID-19 were found to have significantly greater levels of soluble P-selectin, a marker of endothelial cell and platelet activation, in comparison to 10 COVID-19 positive patients who did not require ICU care (33). Furthermore, the authors found that levels of thrombomodulin, another marker of endothelial cell activation, were associated with in-hospital mortality (33). In another single-center study, endothelial cell adhesion markers, including vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular adhesion protein-1 (VAP-1), were elevated in 39 COVID-19 patients in comparison to 32 COVID-19 negative controls, and levels of elevation had a correlation to disease severity (104). Based on their findings, the author proposed that COVID-19 infection leads to release of pro-inflammatory cytokines which cause endothelial injury. Endothelial injury subsequently leads to altered expression of pro and anti-thrombotic factors and thrombosis (104). An alternate proposed theory is that SARS-CoV-2 viral infection leads to damage of the endothelial cell plasma membrane resulting in oxidative stress and release of reactive oxygen species which prime vessels for thrombosis (105). Interestingly, circulating endothelial cells (CECs) were found to be elevated in 66 COVID-19 patients when compared to 30 COVID-19 negative patients, but treatment with therapeutic anticoagulation led to significant decrease in CECs (106). Ongoing studies are necessary to further define the connections between endotheliopathy and coagulopathy in COVID-19.
Monocytes and neutrophils are two innate immune cells of particular interest in “thromboinflammation” or “immunothrombosis” associated with COVID-19. A recent prospective study of 35 patients with severe COVID-19 infections found that markers of platelet activation (P-selectin and thromboxane B2) correlates with elevated CRP (32). The group went on to perform immunofluorescence assays that showed increased platelet-monocyte aggregates in patients with severe COVID-19 infection in comparison to mild and asymptomatic patients and COVID-19 negative controls (32). They further discovered that tissue factor (TF), a major activator of the extrinsic pathway of coagulation, was expressed by monocytes, especially those monocytes in complex with platelets (32). This suggests that the interaction between cell types was necessary to induce coagulation cascade. Ex vivo studies revealed that platelets isolated from COVID-19 patients were able to induce TF expression by monocytes (32). Additionally, plasma from COVID-19 patients incubated with platelets isolated from healthy controls resulted in platelet activation, suggesting that circulating markers can lead to platelet activation (32). Their experiments suggest bi-directional signaling between platelets and monocyte TF expression leading to activated inflammation and hypercoagulability in COVID-19.
Neutrophils, known as first responders in innate immunity, have been well described in the last two decades to form neutrophil extracellular traps (NETs) by which they extrude chromatin studded with histones and other damage-associated molecular patterns or DAMPs. NETs have since been described to play a role in sepsis, autoimmune disorders, cancer, and a plethora of other systemic inflammatory disorders (107-109). In particular NETs contribute to micro- and macrovascular thrombi in a number of models (109, 110). The first pilot study looking at NETs in COVID-19 was performed at a single-center comparing 50 hospitalized COVID-19 patients with 30 healthy controls (111). Markers of NET formation, including cell free DNA, myeloperoxidase-DNA complexes (MPO-DNA), and citrullinated histone-3 (citH3), were elevated in the COVID-19 patients in comparison to healthy controls (111). Furthermore, there was an increasing trend of all three markers of NET formation with worsening oxygenation (111). In vitro studies combining serum from COVID-19 patients with neutrophils isolated from healthy controls, led to NET formation, suggesting that circulating markers are able to induce NET formation (111). Several groups have confirmed elevated levels of circulating NET degradation products including myeloperoxidase-DNA (MPO) with autopsy studies showing NETs present in microvasculature of lungs, liver, and kidney (101, 112, 113). In a study of 33 COVID-19 patients with 17 matched healthy controls, the COVID-19 patients were identified to have greater circulating platelet-neutrophil aggregates by flow cytometry. The 3 COVID-19 patients who died in this cohort were found to have co-localization of NETs with platelets in lung tissue (101). At our institution we have recently begun collecting samples from COVID-19 patients to assess for markers of NET formation, complement activation, and fibrinolysis. This data may pave the way to individualized VTE risk stratification in COVID-19 patients.
It has recently been proposed that hypoxia itself is a significant driver of thrombosis associated with COVID-19 (114). Prior studies have shown that the vascular response to hypoxia is controlled by hypoxia inducible transcription factors (HIFs), particularly at high altitudes. HIF-1α has recently been shown to play a critical role in the acute inflammatory response associated with acute respiratory distress syndrome (ARDS) associated with trauma and hemorrhagic shock (115). HIFs, including HIF-1α are known to promote thrombosis through plasminogen activator inhibitor (PAI) 1 and TF (116). Additionally, HIF-2 has a pro-thrombotic effect by inhibiting the action TF inhibitor (116). HTF-1α is expressed in alveolar epithelial cells and has been shown to trigger cellular inflammation with release of pro-inflammatory and potentially pro-thrombotic cytokines, including TNFα and interleukins such as IL-6 (103). Additionally, hypoxia is thought to have direct pro-thrombotic effects on the endothelium, including suppression of thrombomodulin and reduction of fibrinolytic potential (116). It has been theorized that targeting HIFs may ameliorate both COVID-19 induced endothelial damage and thrombosis (117). Further work is needed to determine the mechanisms by which hypoxia leads to thromboembolic events in COVID-19. Hypoxia induced transcription factors, activation of neutrophil extracellular traps, monocytes, complement, and pro-inflammatory cytokines are all potential contributors to “thromboinflammation” with COVID-19 and may represent pharmacologic targets for prevention treatment of thrombosis.
COVID-19 Associated Coagulopathy: Recommendations for Prevention and Treatment
COVID-19 has presented the world with the unique challenge of discovering the basics of the pathophysiology and managing the disease at the same time. A number of societies have released guidance for the prevention and treatment of COVID-19 associated coagulopathy, while many groups have also released case studies detailing off label uses of pharmacologic agents based on clinical circumstance (118). The ISTH currently recommends that all hospitalized COVID-19 patients receive prophylactic dose low-molecular weight heparin in the absence of contraindications (4, 118). Other groups echo this guidance, and some state that consideration should be given to prophylaxis in high risk ambulatory patients as well as post-discharge patients with laboratory evidence of persistently elevated levels of D-dimer (62, 118). Studies have been performed on the safety of utilizing intermediate or therapeutic dose anticoagulation in a “prophylactic manner” for COVID-19 patients; however at this time, in the absence of documented thromboembolism, there is no conclusive guidance on using intermediate or therapeutic anticoagulation in COVID-19 (4, 119, 120). Tang et al. found that use of heparin for seven days was associated with a decrease in mortality in patients with a sepsis induced coagulopathy (SIC) score > 4, and a D-dimer level greater than six times the upper limit of normal (121). There is limited evidence that heparin may interact with the spike S1 protein domain on SARS COV-2 giving it both antiviral and anti-thrombotic properties (44, 122). It is recommended that anti-Xa be monitored to guide heparin treatment, rather than aPTT as discussed above due to the high prevalence of LAC and other factors making the aPTT unreliable in many COVID-19 patients (44).
Although institutional policies vary, many medical centers do not routinely screen for VTE, in the absence of clinical suspicion. Given the high burden of VTE seen in COVID-19 patients, questions regarding screening or empiric treatment with therapeutic anticoagulation have been proposed. D-dimer level has been shown to correspond to VTE risk in COVID-19 patients, as discussed above, with different screening thresholds proposed. Studies have advocated for VTE screening with bilateral lower extremity ultrasound for patients with a D-dimer level >2,000 ng/mL, and empiric treatment with therapeutic heparin for a D-dimer > 5,500 ng/mL, while others have shown a D-dimer level > 2,600 ng/mL to be predictive of VTE development, and still others who recommend therapeutic anticoagulation for a D-dimer level > 2,000 ng/mL on ICU admission or an increase greater than six times the admission level (53, 76, 123). Monitoring D-dimer levels may prove to be a helpful adjunct for clinical decision making regarding surveillance and treatment of VTE in COVID-19. With the high rates of VTE observed in patients receiving chemoprophylaxis, there is also discussion about the utility of alternate therapies, such as antiplatelet agents (124). In a single-center case control study, five critically ill COVID-19 patients were given an antiplatelet regimen in addition to heparin, with significant improvement in their pulmonary status as compared to controls (125). However, in a larger retrospective review of 3,772 hospitalized and ambulatory COVID-19 patients, 266 patients on antiplatelet agents at the time of admission were propensity matched to 798 patients not on antiplatelet agents or other anti-coagulants. Patients on antiplatelet agents were not found to have significantly different all-cause mortality (HR 1.029, CI 95% [0.723-1.466], p=0.997) or need for mechanical ventilation (HR 1.239, CI 95% [0.807-1.901], p=0.256) from those who were not on antiplatelet agents or other anti-coagulants (126). Use of anti-platelet agents specifically for the treatment of COVID-19 related coagulopathy is not currently recommended (127).
Fibrinolytic agents including tissue plasminogen activator (tPA), have also been used in a small number of cases. Under certain circumstances, off-label use without overt evidence of VTE has been advocated when it is not possible to obtain a CT PE study, or there is high clinical suspicion of PE with potential signs of PE on bedside echocardiogram (128). In a case series, Goyal et al. described administering low dose tPA to three patients on the verge of intubation due to the suspicion for pulmonary microthrombi, and all three were weaned from oxygen within 3-7 days (129). Several other small case series (between 3-5 patients) have described temporary clinical improvement in after administration of tPA, with descriptions of some patients being extubated and others avoiding intubation after tPA administration (130). However, these case series do not demonstrate a durable response to tPA in all patients, and as always the risks of systemic fibrinolysis, specifically devastating intracranial hemorrhage, need to be considered. Off label use of tPA in COVID-19 is not currently recommended, though it is being studied in an ongoing trial (NCT04357730).
Because of the high frequency of VTE in COVID-19 patients despite chemoprophylaxis, the question of need for greater than prophylactic doses of heparin has been raised (61, 69, 123, 131). Studies have demonstrated that these patients require higher doses of Unfractionated Heparin or Low Molecular Weight Heparin, titrated based on anti-Xa levels (57, 132). A recent review has advocated for using viscoelastic testing, such as TEG and ROTEM, to monitor efficacy of anticoagulant therapy in COVID-19 (133). Additionally, COVID-19 patients on long-term anticoagulation may receive some protective benefit, especially since many COVID-19 patients are admitted with VTEs from the ambulatory setting, suggesting the hypercoagulable phase of COVID-19 starts early in the disease process (123). In a cohort of 70 elderly COVID-19 patients in Italy (> 70 years old) being followed by an outpatient cardiology clinic, chronic direct oral anticoagulant (DOAC) use was independently associated with decreased mortality (134). However, other authors have not found a statistically significant mortality difference between patients on chronic anticoagulation and those who are not (126). Treatment with therapeutic anticoagulation in a “prophylactic” manner against COVID-19 associated coagulopathy is not currently recommended, though there is guidance towards considering chemoprophylaxis in the ambulatory or post-discharge setting if clinically appropriate, as it appears that the hypercoagulable state may persist beyond hospitalization in select patients. As more evidence becomes available, it is likely that a combination of clinical and laboratory factors will be needed to individualize treatment with anticoagulants, tPA, antiplatelet agents, or even recombinant thrombomodulin, in COVID-19 patients suffering thromboembolic events (133).
Conclusion:
COVID-19 associated coagulopathy is a unique clinical and pathophysiologic challenge with a distinct laboratory phenotype that results from complex interactions between regulators of inflammation and coagulation. Resultant venous and arterial thrombosis both in macro- and microvasculature leads to significant morbidity and mortality in COVID-19. Many of the papers reviewed in this manuscript were case series, retrospective single and multi-center studies. It is imperative that ongoing clinical research is undertaken to determine pharmacologic strategies that will lead to improved outcomes. In particular, randomized controlled trials in COVID-19 patients with derangements in coagulation are needed to accurately risk-stratify patients for thrombotic complications and streamline treatment guidelines.
Table 1:
Summary of Relevant Lab Findings in COVID-19*
| End point | Study | Country of Origin |
Design | Centers | No. of COVID D-19 Patients |
Relevant Findings |
|---|---|---|---|---|---|---|
| D-dimer level | Jin et al. (2) | China | Meta-analysis | Multi | 4889 | ↑ D-dimer in non-survivors |
| Tang et al. (3) | China | Retrospective | Single | 183 | ↑ D-dimer in non-survivors | |
| Zhang et al. (10) | China | Retrospective | Single | 343 | ↑ Mortality with D-dimer ≥ 2.0 μg/dL | |
| Yu et al. (11) | China | Retrospective | Single | 1,561 | D-dimer > 0.6 μg/mL on admission associated w/ severe COVID-19 | |
| Chilmuri et al. (12) | USA | Retrospective | Single | 375 | D-dimer > 1,000 ng/mL on admission associated increased odds of in-hospital mortality | |
| Gayam et al. (13) | USA | Retrospective | Single | 408 | D-dimer is independent predictor of mortality in hospitalized African American patients | |
| Li Y et al. (14) | China | Prospective | Multi | 279 | Dynamic relationship between D-dimer and disease progression | |
| DiMinno et al (15) | Italy | Meta-analysis | Multi | 1,511 | ↑ D-dimer in non-survivors | |
| Liu J et al. (16) | China | Retrospective | Single | 1,190 | ↑ D-dimer on admission independent predictor of in-hospital death | |
| Li C et al. (17) | China | Retrospective | Single | 749 | Normal day 1 and day 3 D-dimer strongly associated with survival | |
| Artifoni et al. (19) | France | Retrospective | Multi | 71 | D-dimer level ≥ 3.0 μg/ml with 80% positive predictive value for VTE | |
| Cui et al. (20) | China | Retrospective | Single | 81 | ↑ D-dimer in patients with VTE, ↑ D-dimer with anticoagulation | |
| Martin-Rojas et al. (21) | Spain | Retrospective | Single | 206 | ↑ D-dimer independent predictor of thrombotic event | |
| Cho et al. (22) | USA | Retrospective | Single | 158 | D-dimer > 6,494 ng/mL associated with increased risk of DVT, NPV 88.0% | |
| Mestre-Gomez et al. (23) | Spain | Retrospective | Single | 91 | D-dimer ≥ 5000 ≥g/dL independent predictor of PE | |
| Demelo-Rodriguez et al. (24) | Spain | Prospective | Single | 156 | D-dimer ≥ 1570 ng/mL associated with DVT | |
| Platelet count | Di Minno et al. (15) | Italy | Meta-Analysis | Multi | 1,511 | Thrombocytopenia associated with disease severity and mortality |
| Liu Y et al. (28) | China | Retrospective | Single | 383 | Dose dependent association of platelet count with mortality | |
| Al-Samkari et al. (25) | USA | Retrospective | Multi | 429 | Admission ↑ platelets predictive of thrombotic complications Thrombocytopenia predictive of major bleeding event |
|
| Chen W et al. (26) | China | Retrospective | Single | 272 | 11.8% “delayed phase” thrombocytopenia with poor outcomes | |
| Yang et al. (29) | China | Retrospective | Single | 1476 | 20.7% with thrombocytopenia, platelet nadir correlates to mortality | |
| Chen R et al. (30) | China | Retrospective | Multi | 548 | Lower platelet count on admission and ↓ trend through hospitalization in non-survivors | |
| Zhao et al. (31) | China | Retrospective | Single | 532 | Lower mean platelet count at multiple time points in non-survivors | |
| Hottz et al. (32) | Brazil | Prospective | Single | 35 | ↑ Platelet activation in severe COVID-19 | |
| VWF | Goshua et al. (33) | USA | Retrospective | Single | 68 | ↑ VWF activity in ICU and non-ICU |
| Escher et al. (34) | Switzer land | Case series | Single | 3 | ↑ VWF and Factor VIII with normal ADAMSTS13 and platelet count | |
| Blasi et al. (38) | Spain | Case series | Single | 23 | ↑VWF and ↓ADAMSTS13 in COVID patients vs. healthy controls | |
| Anti-phospholipid antibodies | Helms et al. (37) | France | Prospective | Multi | 150 | 85% of tested with increased lupus anticoagulant |
| Anti-phospholipid antibodies (cont) | Xiao et al. (39) | China | Cross-section | Single | 79 | APLs seen in 47% of critically ill COVID-19 patients |
| Bowles et al. (40) | UK | Prospective | Single | 216 | Lupus anticoagulant detected in 91% with prolonged aPTT | |
| Harzallah et al. (41) | France | Prospective | Single | 56 | Lupus anticoagulant (+) in 45% | |
| Siguret et al. (42) | France | Prospective | Single | 74 | 85% with lupus anticoagulant, 12% with other APLs | |
| Galeano-Valle et al. (45) | Spain | Case series | Single | 24 | 2 patients with weakly positive anti-cardiolipin and anti-B2-GP1 IgM. | |
| TEG/ROT EM | Ibanez et al. (18) | Spain | Cross-section | Single | 12 | ↑ Clot firmness on ROTEM |
| Mortus et al. (48) | USA | Case series | Single | 21 | ↑MA associated with thrombotic events | |
| Panigada et al. (49) | Italy | Cross-section | Single | 24 | ↓R,K,Ly30; ↑MA in COVID-19 | |
| Pavoni et al. (50) | Italy | Retrospective | Single | 40 | Hypercoagulable on ROTEM | |
| Wright et al. (51) | USA | Retrospective | Single | 44 | Fibrinolysis shutdown associated with VTE | |
| Creel-Bulos (52) | USA | Case series | Single | 25 | Fibrinolysis shutdown associated with thrombosis | |
| Yuriditsky et al. (53) | USA | Retrospective | Single | 64 | TEG not correlated to VTE |
Studies included are those which specify laboratory abnormalities seen in COVID-19 patients
Table 3:
Summary of Findings Pertaining to Prevention, Treatment, and Diagnosis of Thromboembolic Events associated with COVID-19
| Endpoint | Study | Country of Origin |
Design | Centers | No. of COVID D-19 Patients |
Relevant Findings |
|---|---|---|---|---|---|---|
| Outpatient Anti coagulation | Beun et al. (57) | Netherlands | Retrospective | Single | 75 | VTE patients required unusually high dose UFH > 35,000 IU/day for goal aPTT |
| Taccone et al. (69) | Belgium | Retrospective | Single | 40 | High dose chemoprophylaxis associated with ↓ PE | |
| Roberts et al. (85) | UK | Retrospective | Single | 1,877 | ↑ Post-discharge VTE (OR 1.6) | |
| Tang et al. (121) | China | Retrospective | Single | 449 | Lower mortality in patients with a SIC score ≥ 4 treated with heparin for ≥ 7 days | |
| Maatman et al. (123) | USA | Retrospective | Multi | 109 | Critically ill patients with D-dimer level > 2,600 ng/mL should get screening duplex | |
| Tremblay et al. (126) | USA | Retrospective | Multi | 3,772 | No difference in survival or time to mechanical ventilation with prior anticoagulant or anti-platelet | |
| White et al. (132) | UK | Retrospective | Single | 69 | ↑ Heparin dosing needed to achieve therapeutic levels in 15 patients | |
| Rossi et al. (134) | Italy | Retrospective | Single | 70 | Chronic DOAC use independently predicts survival | |
| VTE Screening Thresholds | Yuriditsky et al. (53) | USA | Retrospective | Single | 64 | Recommend therapeutic anticoagulation for ICU patients w/D-dimer level > 2,000 ng/mL or ↑ 6x-10x admission level |
| Trigonis et al. (76) | USA | Retrospective | Single | 45 | Recommend ultrasound for D-dimer level > 2,000 ng/mL & consider therapeutic anticoagulation for D-dimer level > 5,500 ng/mL | |
| Use of Anti-Platelet Agents | Russo et al. (124) | Italy | Retrospective | Multi | 192 | No change in ARDS or in-hospital mortality associated with anti-platelet or anticoagulation |
| Viecca et al. (125) | Italy | Case Series | Single | 5 | Hypoxia improvement in ICU patients treated with specific anti-platelet regimen | |
| Use of Fibrinolytic Agents | Bona et al. (128) | Italy | Case Series | Single | 4 | Clinical improvement in 3 of 4 patients treated with tPA for bedside diagnosis of PE |
| Goyal et al. (129) | India | Case Series | Single | 3 | 3 patients treated with tPA for respiratory failure weaned from oxygen within 3-7 days. | |
| Christie et al. (130) | USA | Case Series | Single | 5 | TPA given for worsening respiratory failure; improved respiratory status in all 5 patients |
Key Points:
COVID-19 associated coagulopathy is characterized by a unique set of laboratory disturbances that are associated with poor prognosis, including elevated D-dimer level, thrombocytopenia, and hypercoagulable profile on TEG and ROTEM. Anti-phospholipid antibodies have also been proposed to play a role in COVID-19 related coagulopathy.
Clinical manifestations of disrupted coagulation in COVID-19 patients include DVT, PE, acute ischemic stroke, and peripheral arterial thrombosis.
Microvascular thrombosis has been observed in autopsy studies and likely as a result of “thromboinflammation”. Immune responses, including cytokine release, complement activation, NETosis, and monocyte-platelet aggregation, contribute to the pathophysiology of coagulopathy in COVID-19.
Patients with COVID-19 infection, who require ICU care and have elevated D-dimer levels are at increased risk of developing thromboembolic complications.
Vigilant monitoring of laboratory and clinical risk factors are warranted to guide chemoprophylaxis and treatment of thrombotic events for inpatients and post-discharge.
Treatment with therapeutic anticoagulation in a “prophylactic” manner against COVID-19 associated coagulopathy is not currently recommended.
Acknowledgments
Funding: This project was supported by T32 AG049672 from the National Institute of Aging (NIA) and Robert and Arlene Kogod Center for Aging, Mayo Clinic (JG), R38HL150086 Stimulating Access to Research in Residency (TAM) from the National Heart, Lung, and Blood Institute (NHLBI), R01 GM 126086-03 (MSP) from the National Institute of General Medical Sciences (NIGMS), UM1 HL120877-06 (MSP) by the Trans-Agency Consortium for Trauma-Induced Coagulopathy (TACTIC), 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), the NIH Roadmap for Medical Research and by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCRR.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References:
- 1.: COVID-19 Dashboard by the Center for Systems Science and Engineering at Johns Hopkins University. 2020, p. https://coronavirus.jhu.edu/map.html.
- 2.Jin S, Jin Y, Xu B, Hong J, Yang X: Prevalence and Impact of Coagulation Dysfunction in COVID-19 in China: A Meta-Analysis. Thromb Haemost, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang N, Li D, Wang X, Sun Z: Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18(4):844–847, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T: ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 18(5):1023–1026, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. : Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191:145–147, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraisse M, Logre E, Pajot O, Mentec H, Plantefeve G, Contou D: Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care 24(1):275, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edler C, Schroder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, Klein A, Langenwalder F, Lutgehetmann M, Meissner K, et al. : Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med 134(4):1275–1284, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, et al. : Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine 24:100434, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joly BS, Siguret V, Veyradier A: Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med 46(8):1603–1606, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z: D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost 18(6):1324–1329, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu HH, Qin C, Chen M, Wang W, Tian DS: D-dimer level is associated with the severity of COVID-19. Thromb Res 195:219–225, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chilimuri S, Sun H, Alemam A, Mantri N, Shehi E, Tejada J, Yugay A, Nayudu SK: Predictors of Mortality in Adults Admitted with COVID-19: Retrospective Cohort Study from New York City. West J Emerg Med 21(4):779–784, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gayam V, Chobufo MD, Merghani MA, Lamichanne S, Garlapati PR, Adler MK: Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J Med Virol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Zhao K, Wei H, Chen W, Wang W, Jia L, Liu Q, Zhang J, Shan T, Peng Z, et al. : Dynamic relationship between D-dimer and COVID-19 severity. Br J Haematol 190(1):e24–e27, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Minno MND, Calcaterra I, Lupoli R, Storino A, Spedicato GA, Maniscalco M, Di Minno A, Ambrosino P: Hemostatic Changes in Patients with COVID-19: A Meta-Analysis with Meta-Regressions. J Clin Med 9(7), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Zhang S, Wu Z, Shang Y, Dong X, Li G, Zhang L, Chen Y, Ye X, Du H, et al. : Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care 10(1):99, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Hu B, Zhang Z, Qin W, Zhu Z, Zhai Z, Davidson BL, Wang C: D-dimer Triage for COVID-19. Acad Emerg Med 27(7):612–613, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibanez C, Perdomo J, Calvo A, Ferrando C, Reverter JC, Tassies D, Blasi A: High D dimers and low global fibrinolysis coexist in COVID19 patients: what is going on in there? J Thromb Thrombolysis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artifoni M, Danic G, Gautier G, Gicquel P, Boutoille D, Raffi F, Neel A, Lecomte R: Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. J Thromb Thrombolysis 50(1):211–216, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui S, Chen S, Li X, Liu S, Wang F: Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 18(6):1421–1424, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Rojas RM, Perez-Rus G, Delgado-Pinos VE, Domingo-Gonzalez A, Regalado-Artamendi I, Alba-Urdiales N, Demelo-Rodriguez P, Monsalvo S, Rodriguez-Macias G, Ballesteros M, et al. : COVID-19 coagulopathy: An in-depth analysis of the coagulation system. Eur J Haematol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho ES, McClelland PH, Cheng O, Kim Y, Hu J, Zenilman ME, D'Ayala M: Utility of d-dimer for diagnosis of deep vein thrombosis in coronavirus disease-19 infection. J Vasc Surg Venous Lymphat Disord, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mestre-Gomez B, Lorente-Ramos RM, Rogado J, Franco-Moreno A, Obispo B, Salazar-Chiriboga D, Saez-Vaquero T, Torres-Macho J, Abad-Motos A, Cortina-Camarero C, et al. : Incidence of pulmonary embolism in non-critically ill COVID-19 patients. Predicting factors for a challenging diagnosis. J Thromb Thrombolysis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demelo-Rodriguez P, Cervilla-Munoz E, Ordieres-Ortega L, Parra-Virto A, Toledano-Macias M, Toledo-Samaniego N, Garcia-Garcia A, Garcia-Fernandez-Bravo I, Ji Z, de-Miguel-Diez J, et al. : Incidence of asymptomatic deep vein thrombosis in patients with COVID-19 pneumonia and elevated D-dimer levels. Thromb Res 192:23–26, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, et al. : COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 136(4):489–500, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Li Z, Yang B, Wang P, Zhou Q, Zhang Z, Zhu J, Chen X, Yang P, Zhou H: Delayed-phase thrombocytopenia in patients with coronavirus disease 2019 (COVID-19). Br J Haematol 190(2):179–184, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, Gao Y, Cai L, Wang Z, Yin P, et al. : Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol 7(9):e671–e678, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Sun W, Guo Y, Chen L, Zhang L, Zhao S, Long D, Yu L: Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets 31(4):490–496, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, Shang Y: Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost 18(6):1469–1472, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen R, Sang L, Jiang M, Yang Z, Jia N, Fu W, Xie J, Guan W, Liang W, Ni Z, et al. : Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol 146(1):89–100, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, Wang K, Zuo P, Liu Y, Zhang M, Xie S, Zhang H, Chen X, Liu C: Early decrease in blood platelet count is associated with poor prognosis in COVID-19 patients-indications for predictive, preventive, and personalized medical approach. EPMA J:1–7, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hottz ED, Azevedo-Quintanilha IG, Palhinha L, Teixeira L, Barreto EA, Pao CRR, Righy C, Franco S, Souza TML, Kurtz P, et al. : Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood 136(11):1330–1341, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, et al. : Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 7(8):e575–e582, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escher R, Breakey N, Lammle B: Severe COVID-19 infection associated with endothelial activation. Thromb Res 190:62, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osuchowski MF, Aletti F, Cavaillon JM, Flohe SB, Giamarellos-Bourboulis EJ, Huber-Lang M, Relja B, Skirecki T, Szabo A, Maegele M: SARS-CoV-2/COVID-19: Evolving Reality, Global Response, Knowledge Gaps, and Opportunities. Shock 54(4):416–437, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Escher R, Breakey N, Lammle B: ADAMTS13 activity, von Willebrand factor, factor VIII and D-dimers in COVID-19 inpatients. Thromb Res 192:174–175, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, et al. : High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 46(6):1089–1098, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blasi A, von Meijenfeldt FA, Adelmeijer J, Calvo A, Ibanez C, Perdomo J, Reverter JC, Lisman T: In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J Thromb Haemost, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao M, Zhang Y, Zhang S, Qin X, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, et al. : Brief Report: Anti-phospholipid antibodies in critically ill patients with Coronavirus Disease 2019 (COVID-19). Arthritis Rheumatol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, MacDonald V, Green L, Sivapalaratnam S, Pasi KJ, et al. : Lupus Anticoagulant and Abnormal Coagulation Tests in Patients with Covid-19. N Engl J Med 383(3):288–290, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harzallah I, Debliquis A, Drenou B: Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost 18(8):2064–2065, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siguret V, Voicu S, Neuwirth M, Delrue M, Gayat E, Stepanian A, Megarbane B: Are antiphospholipid antibodies associated with thrombotic complications in critically ill COVID-19 patients? Thromb Res 195:74–76, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schouwers SM, Delanghe JR, Devreese KM: Lupus Anticoagulant (LAC) testing in patients with inflammatory status: does C-reactive protein interfere with LAC test results? Thromb Res 125(1):102–4, 2010. [DOI] [PubMed] [Google Scholar]
- 44.Bikdeli B, Madhavan MV, Gupta A, Jimenez D, Burton JR, Der Nigoghossian C, Chuich T, Nouri SN, Dreyfus I, Driggin E, et al. : Pharmacological Agents Targeting Thromboinflammation in COVID-19: Review and Implications for Future Research. Thromb Haemost 120(7):1004–1024, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galeano-Valle F, Oblitas CM, Ferreiro-Mazon MM, Alonso-Munoz J, Del Toro-Cervera J, di Natale M, Demelo-Rodriguez P: Antiphospholipid antibodies are not elevated in patients with severe COVID-19 pneumonia and venous thromboembolism. Thromb Res 192:113–115, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M: The unique characteristics of COVID-19 coagulopathy. Crit Care 24(1):360, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devreese KMJ, Ortel TL, Pengo V, de Laat B, Subcommittee on Lupus Anticoagulant/Antiphospholipid A: Laboratory criteria for antiphospholipid syndrome: communication from the SSC of the ISTH. J Thromb Haemost 16(4):809–813, 2018. [DOI] [PubMed] [Google Scholar]
- 48.Mortus JR, Manek SE, Brubaker LS, Loor M, Cruz MA, Trautner BW, Rosengart TK: Thromboelastographic Results and Hypercoagulability Syndrome in Patients With Coronavirus Disease 2019 Who Are Critically Ill. JAMA Netw Open 3(6):e2011192, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A: Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 18(7):1738–1742, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pavoni V, Gianesello L, Pazzi M, Stera C, Meconi T, Frigieri FC: Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis 50(2):281–286, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, Nydam TL, Moore PK, McIntyre RC Jr.: Fibrinolysis Shutdown Correlation with Thromboembolic Events in Severe COVID-19 Infection. J Am Coll Surg 231(2):193–203 e1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Creel-Bulos C, Auld SC, Caridi-Scheible M, Barker N, Friend S, Gaddh M, Kempton CL, Maier C, Nahab F, Sniecinski R: Fibrinolysis Shutdown and Thrombosis in A COVID-19 ICU. Shock, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuriditsky E, Horowitz JM, Merchan C, Ahuja T, Brosnahan SB, McVoy L, Berger JS: Thromboelastography Profiles of Critically Ill Patients With Coronavirus Disease 2019. Crit Care Med 48(9):1319–1326, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lisman T: Fibrinolytic shutdown in COVID-19 is likely a misnomer. Shock Published Ahead of Print, 2020. [DOI] [PubMed] [Google Scholar]
- 55.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. : Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res 191:148–150, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Alexia B, et al. : Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 191:9–14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A: Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol 42 Suppl 1:19–20, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hippensteel JA, Burnham EL, Jolley SE: Prevalence of venous thromboembolism in critically ill patients with COVID-19. Br J Haematol 190(3):e134–e137, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trimaille A, Curtiaud A, Marchandot B, Matsushita K, Sato C, Leonard-Lorant I, Sattler L, Grunebaum L, Ohana M, Von Hunolstein JJ, et al. : Venous thromboembolism in non-critically ill patients with COVID-19 infection. Thromb Res 193:166–169, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chi G, Lee JJ, Jamil A, Gunnam V, Najafi H, Memar Montazerin S, Shojaei F, Marszalek J: Venous Thromboembolism among Hospitalized Patients with COVID-19 Undergoing Thromboprophylaxis: A Systematic Review and Meta-Analysis. J Clin Med 9(8), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hasan SS, Radford S, Kow CS, Zaidi STR: Venous thromboembolism in critically ill COVID-19 patients receiving prophylactic or therapeutic anticoagulation: a systematic review and meta-analysis. J Thromb Thrombolysis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberts LN, Whyte MB, Georgiou L, Giron G, Czuprynska J, Rea C, Vadher B, Patel RK, Gee E, Arya R: Postdischarge venous thromboembolism following hospital admission with COVID-19. Blood 136(11):1347–1350, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bavaro DF, Poliseno M, Scardapane A, Belati A, De Gennaro N, Stabile Ianora AA, Angarano G, Saracino A: Occurrence of Acute Pulmonary Embolism in COVID-19-A case series. Int J Infect Dis 98:225–226, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gervaise A, Bouzad C, Peroux E, Helissey C: Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whyte MB, Kelly PA, Gonzalez E, Arya R, Roberts LN: Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res 195:95–99, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fauvel C, Weizman O, Trimaille A, Mika D, Pommier T, Pace N, Douair A, Barbin E, Fraix A, Bouchot O, et al. : Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J 41(32):3058–3068, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bompard F, Monnier H, Saab I, Tordjman M, Abdoul H, Fournier L, Sanchez O, Lorut C, Chassagnon G, Revel MP: Pulmonary embolism in patients with COVID-19 pneumonia. Eur Respir J 56(1), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S, et al. : Pulmonary Embolism in Patients With COVID-19: Awareness of an Increased Prevalence. Circulation 142(2):184–186, 2020. [DOI] [PubMed] [Google Scholar]
- 69.Taccone FS, Gevenois PA, Peluso L, Pletchette Z, Lheureux O, Brasseur A, Garufi A, Talamonti M, Motte S, Nobile L, et al. : Higher Intensity Thromboprophylaxis Regimens and Pulmonary Embolism in Critically Ill Coronavirus Disease 2019 Patients. Crit Care Med, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mak SM, Mak D, Hodson D, Preston R, Retter A, Camporota L, Benedetti G: Pulmonary ischaemia without pulmonary arterial thrombus in COVID-19 patients receiving extracorporeal membrane oxygenation: a cohort study. Clin Radiol 75(10):795 e1–795 e5, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen S, Zhang D, Zheng T, Yu Y, Jiang J: DVT incidence and risk factors in critically ill patients with COVID-19. J Thromb Thrombolysis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, Zhang C, Li H, Xia X, Kong S, et al. : Deep Vein Thrombosis in Hospitalized Patients With COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation 142(2):114–128, 2020. [DOI] [PubMed] [Google Scholar]
- 73.Rieder M, Goller I, Jeserich M, Baldus N, Pollmeier L, Wirth L, Supady A, Bode C, Busch HJ, Schmid B, et al. : Rate of venous thromboembolism in a prospective all-comers cohort with COVID-19. J Thromb Thrombolysis 50(3):558–566, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santoliquido A, Porfidia A, Nesci A, De Matteis G, Marrone G, Porceddu E, Camma G, Giarretta I, Fantoni M, Landi F, et al. : Incidence of deep vein thrombosis among non-ICU patients hospitalized for COVID-19 despite pharmacological thromboprophylaxis. J Thromb Haemost, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Muller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, et al. : Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 18(8):1995–2002, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trigonis RA, Holt DB, Yuan R, Siddiqui AA, Craft MK, Khan BA, Kapoor R, Rahman O: Incidence of Venous Thromboembolism in Critically Ill Coronavirus Disease 2019 Patients Receiving Prophylactic Anticoagulation. Crit Care Med 48(9):e805–e808, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Voicu S, Bonnin P, Stepanian A, Chousterman BG, Le Gall A, Malissin I, Deye N, Siguret V, Mebazaa A, Megarbane B: High Prevalence of Deep Vein Thrombosis in Mechanically Ventilated COVID-19 Patients. J Am Coll Cardiol 76(4):480–482, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, et al. : Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med 382(20):e60, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, Wang D, Mao L, Jin H, Hu B: Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, et al. : Risk of Ischemic Stroke in Patients With Coronavirus Disease 2019 (COVID-19) vs Patients With Influenza. JAMA Neurol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lameijer JRC, van Houte J, van Berckel MMG, Canta LR, Yo LSF, Nijziel MR, Krietemeijer GM, Troquay SAM, Buise MP, Hendriks J: Severe arterial thromboembolism in patients with Covid-19. J Crit Care 60:106–110, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. : SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke 51(7):2002–2011, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sweid A, Hammoud B, Bekelis K, Missios S, Tjoumakaris SI, Gooch MR, Herial NA, Zarzour H, Romo V, DePrince M, et al. : Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke:1747493020937189, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan YK, Goh C, Leow AST, Tambyah PA, Ang A, Yap ES, Tu TM, Sharma VK, Yeo LLL, Chan BPL, et al. : COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis 50(3):587–595, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL: Acute Cerebrovascular Events in Hospitalized COVID-19 Patients. Stroke 51(9):e219–e222, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agarwal S, Jain R, Dogra S, Krieger P, Lewis A, Nguyen V, Melmed K, Galetta S: Cerebral Microbleeds and Leukoencephalopathy in Critically Ill Patients With COVID-19. Stroke 51(9):2649–2655, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benger M, Williams O, Siddiqui J, Sztriha L: Intracerebral haemorrhage and COVID-19: Clinical characteristics from a case series. Brain Behav Immun 88:940–944, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heman-Ackah SM, Su YS, Spadola M, Petrov D, Chen HI, Schuster J, Lucas T: Neurologically Devastating Intraparenchymal Hemorrhage in COVID-19 Patients on Extracorporeal Membrane Oxygenation: A Case Series. Neurosurgery 87(2):E147–E151, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ilonzo N, Rao A, Berger K, Phair J, Vouyouka A, Ravin R, Han D, Finlay D, Tadros R, Marin M, et al. : Acute thrombotic events as initial presentation of patients with COVID-19 infection. J Vasc Surg Cases Innov Tech 6(3):381–383, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bellosta R, Luzzani L, Natalini G, Pegorer MA, Attisani L, Cossu LG, Ferrandina C, Fossati A, Conti E, Bush RL, et al. : Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldman IA, Ye K, Scheinfeld MH: Lower extremity arterial thrombosis associated with COVID-19 is characterized by greater thrombus burden and increased rate of amputation and death. Radiology:202348, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M: Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med 173(5):350–361, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, et al. : Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 20(10): 1135–1140, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS: Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 8(7):681–686, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J: Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res 220:1–13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.do Espirito Santo DA, Lemos ACB, Miranda CH: In vivo demonstration of microvascular thrombosis in severe COVID-19. J Thromb Thrombolysis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benzakoun J, Hmeydia G, Delabarde T, Hamza L, Meder JF, Ludes B, Mebazaa A: Excess out-of-hospital deaths during the COVID-19 outbreak: evidence of pulmonary embolism as a main determinant. Eur J Heart Fail 22(6):1046–1047, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duarte-Neto AN MR, da Silva LFF, Malheiros DM, de Oliveira EP, Filho JT, Pinho JRR, Gomes-Gouvea MS, Salles APM, de Oliveira RS, Mauad T, Saldiva PH, Dolhnikoff M: Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guang Li SEF, Brian Summa, Carola Wenk, Aibek Akmatbekov, Harbert Jack L., Vander Heide Richard S. : Multiscale 3-dimensional pathology findings of COVID-19 diseased lung using high-resolution cleared tissue microscopy. bioRxiv, 2020. [Google Scholar]
- 100.Samuels JM, Coleman JR, Moore EE, Bartley M, Vigneshwar N, Cohen M, Silliman CC, Sauaia A, Banerjee A: Alternative Complement Pathway Activation Provokes a Hypercoagulable State with Diminished Fibrinolysis. Shock 53(5):560–565, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, et al. : Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136(10):1169–1179, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fogarty H, Townsend L, Ni Cheallaigh C, Bergin C, Martin-Loeches I, Browne P, Bacon CL, Gaule R, Gillett A, Byrne M, et al. : More on COVID-19 coagulopathy in Caucasian patients. Br J Haematol 189(6):1060–1061, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leyfman Y, Erick TK, Reddy SS, Galwankar S, Nanayakkara PWB, Di Somma S, Sharma P, Stawicki SP, Chaudry IH: Potential Immunotherapeutic Targets for Hypoxia Due to COVI-Flu. Shock 54(4):438–450, 2020. [DOI] [PubMed] [Google Scholar]
- 104.Tong M, Jiang Y, Xia D, Xiong Y, Zheng Q, Chen F, Zou L, Xiao W, Zhu Y: Elevated Expression of Serum Endothelial Cell Adhesion Molecules in COVID-19 Patients. J Infect Dis 222(6):894–898, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Panfoli I: Potential role of endothelial cell surface ectopic redox complexes in COVID-19 disease pathogenesis. Clin Med (Lond) 20(5):e146–e147, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khider L, Gendron N, Goudot G, Chocron R, Hauw-Berlemont C, Cheng C, Rivet N, Pere H, Roffe A, Clerc S, et al. : Curative anticoagulation prevents endothelial lesion in COVID-19 patients. J Thromb Haemost, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Apel F, Zychlinsky A, Kenny EF: The role of neutrophil extracellular traps in rheumatic diseases. Nat Rev Rheumatol 14(8):467–475, 2018. [DOI] [PubMed] [Google Scholar]
- 108.Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H, Tsung A: Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res 76(6):1367–80, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, Jenne CN: Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129(10):1357–1367, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fuchs TA, Brill A, Wagner DD: Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol 32(8):1777–83, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, et al. : Neutrophil extracellular traps in COVID-19. JCI Insight 5(11), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I, Sturzl M, Staats L, Mahajan A, Schauer C, et al. : Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 58:102925, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, et al. : Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation 142(12):1176–1189, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thachil J: Hypoxia - an overlooked trigger for thrombosis in COVID-19 and other critically ill patients. J Thromb Haemost, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li M, Li G, Yu B, Luo Y, Li Q: Activation of Hypoxia-Inducible Factor-1alpha Via Succinate Dehydrogenase Pathway During Acute Lung Injury Induced by Trauma/Hemorrhagic Shock. Shock 53(2):208–216, 2020. [DOI] [PubMed] [Google Scholar]
- 116.Gupta N, Zhao YY, Evans CE: The stimulation of thrombosis by hypoxia. Thromb Res 181:77–83, 2019. [DOI] [PubMed] [Google Scholar]
- 117.Marchetti M: COVID-19-driven endothelial damage: complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematol 99(8):1701–1707, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, Levi M, Samama CM, Thachil J, Giannis D, et al. : Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 18(8):1859–1865, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Langer F, Kluge S, Klamroth R, Oldenburg J: Coagulopathy in COVID-19 and Its Implication for Safe and Efficacious Thromboprophylaxis. Hamostaseologie 40(3):264–269, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, Holley AB, Jimenez D, Le Gal G, Rali P, et al. : Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest 158(3):1143–1163, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z: Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 18(5):1094–1099, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim SY, Jin W, Sood A, Montgomery DW, Grant OC, Fuster MM, Fu L, Dordick JS, Woods RJ, Zhang F, et al. : Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res 181:104873, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maatman TK, Jalali F, Feizpour C, Douglas A 2nd, McGuire SP, Kinnaman G, Hartwell JL, Maatman BT, Kreutz RP, Kapoor R, et al. : Routine Venous Thromboembolism Prophylaxis May Be Inadequate in the Hypercoagulable State of Severe Coronavirus Disease 2019. Crit Care Med 48(9):e783–e790, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Russo V, Di Maio M, Attena E, Silverio A, Scudiero F, Celentani D, Lodigiani C, Di Micco P: Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: A multicenter observational study. Pharmacol Res 159:104965, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Viecca M, Radovanovic D, Forleo GB, Santus P: Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res 158:104950, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tremblay D, van Gerwen M, Alsen M, Thibaud S, Kessler A, Venugopal S, Makki I, Qin Q, Dharmapuri S, Jun T, et al. : Impact of anticoagulation prior to COVID-19 infection: a propensity score-matched cohort study. Blood 136(1):144–147, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ramacciotti E, Macedo AS, Biagioni RB, Caffaro RA, Lopes RD, Guerra JC, Orsi FA, Marques MA, Tafur AJ, Caprini JA, et al. : Evidence-Based Practical Guidance for the Antithrombotic Management in Patients With Coronavirus Disease (COVID-19) in 2020. Clin Appl Thromb Hemost 26:1076029620936350, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bona RD, Valbusa A, Malfa G, Giacobbe DR, Ameri P, Patroniti N, Robba C, Gilad V, Insorsi A, Bassetti M, et al. : Systemic fibrinolysis for acute pulmonary embolism complicating acute respiratory distress syndrome in severe COVID-19: a case series. Eur Heart J Cardiovasc Pharmacother, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goyal A, Saigal S, Niwariya Y, Sharma J, Singh P: Successful use of tPA for thrombolysis in COVID related ARDS: a case series. J Thromb Thrombolysis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Christie DB 3rd, Nemec HM, Scott AM, Buchanan JT, Franklin CM, Ahmed A, Khan MS, Callender CW, James EA, Christie AB, et al. : Early outcomes with utilization of tissue plasminogen activator in COVID-19-associated respiratory distress: A series of five cases. J Trauma Acute Care Surg 89(3):448–452, 2020. [DOI] [PubMed] [Google Scholar]
- 131.Aryal MR, Gosain R, Donato A, Pathak R, Bhatt VR, Katel A, Kouides P: Venous Thromboembolism in COVID-19: Towards an Ideal Approach to Thromboprophylaxis, Screening, and Treatment. Curr Cardiol Rep 22(7):52, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.White D, MacDonald S, Bull T, Hayman M, de Monteverde-Robb R, Sapsford D, Lavinio A, Varley J, Johnston A, Besser M, et al. : Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis 50(2):287–291, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mazzeffi MA, Chow JH, Tanaka K: COVID-19 Associated Hypercoagulability: Manifestations, Mechanisms, and Management. Shock, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rossi R, Coppi F, Talarico M, Boriani G: Protective role of chronic treatment with direct oral anticoagulants in elderly patients affected by interstitial pneumonia in COVID-19 era. Eur J Intern Med 77:158–160, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]