Abstract
Many different forms of hormonal contraception are used by millions of women worldwide. These contraceptives differ in the dose and type of synthetic progestogenic compound (progestin) used, as well as the route of administration and whether or not they contain estrogenic compounds. There is an increasing awareness that different forms of contraception and different progestins have different side-effect profiles, in particular their cardiovascular effects, effects on reproductive cancers and susceptibility to infectious diseases. There is a need to develop new methods to suit different needs and with minimal risks, especially in under-resourced areas. This requires a better understanding of the pharmacokinetics, metabolism, serum and tissue concentrations of progestins used in contraception as well as the biological activities of progestins and their metabolites via steroid receptors. Here we review the current knowledge on these topics and identify the research gaps. We show that there is a paucity of research on most of these topics for most progestins. We find that major impediments to clear conclusions on these topics include a lack of standardized methodologies, comparisons between non-parallel clinical studies and variability of data on serum concentrations between and within studies. The latter is most likely due, at least in part, to differences in intrinsic characteristics of participants. The review highlights the importance of insight on these topics in order to provide the best contraceptive options to women with minimal risks.
Keywords: Progestin, pharmacokinetics, metabolism, serum concentration, contraception
1. Introduction
A range of progestins, or synthetic progestogens, is used at different doses in various formulations for endocrine therapy in women (Africander, et al., 2011; Sitruk-Ware, 2004; Sitruk-Ware, et al., 2013), such as menopausal hormonal therapy (MHT) and contraception. Progestins include compounds structurally related to progesterone (P4) or testosterone (Stanczyk, et al., 2013). Although they all have progestational activity, they exhibit a wide range of other properties which can translate into different clinical outcomes and thus cannot be considered as a single class of compound. These differences most likely arise due to different off-target effects via various steroid receptors (SRs) and other steroid-binding proteins, as well as differences in metabolism, pharmacokinetics and pharmacodynamics (Africander, et al., 2011; Hapgood, et al., 2018; Stanczyk, et al., 2013). We will focus on hormonal contraceptives (HCs), although many of the topics are also relevant to MHT. Side-effects of HCs may include effects on susceptibility to infectious diseases, immune function, breast cancer and cardiovascular disease (reviewed in (Africander, et al., 2011; Hapgood, et al., 2018; Marjoribanks, et al., 2017; Stanczyk, et al., 2013)). Increasing interest in these issues, coupled with improved technology and a drive to use lower doses of progestins (Polis, et al., 2018; Shelton & Halpern, 2014) and determine minimum doses for contraceptive efficacy (Callahan, et al., 2015; Cherala, et al., 2016), have led to several new insights on progestin pharmacokinetics and techniques to measure progestins in serum and genital tract samples from women on contraceptives (Blue, et al., 2018; Buckner, et al., 2019; Laszlo, et al., 2019). Issues such as objective measures of contraceptive usage rather than relying on self-reporting by trial participants are becoming crucial to interpretation of clinical trial data (Achilles, Mhlanga, et al., 2018; Heffron, et al., 2017). It is evident that there may be a high degree of inter-individual variability in progestin serum concentrations between women that may depend on multiple intrinsic factors, making determination of in vivo progestin concentrations important. Requirements for access to more diverse contraceptive choices (WHO, 2019) and increased use of HCs together with anti-retroviral (ARV) drugs has increased interest in progestin pharmacokinetics and drug-drug interactions (Achilles, Hendrix, et al., 2018; Chappell, et al., 2017; Cohn, et al., 2007; Heffron, et al., 2014; McNicholas, et al., 2015; Mornar, et al., 2012; Nanda, et al., 2016; Sierra-Ramirez, et al., 2011; Thurman, et al., 2013; Thurman, et al., 2018; Zia, et al., 2019). Here we review the pharmacokinetics, metabolism and serum concentrations of progestins with a focus on those most widely used in HCs worldwide and in sub-Saharan Africa.
2. Commonly used methods of HC
HCs vary in the type and dose of progestin, absence or presence of an estrogenic compound, as well as the method and frequency of administration (Sitruk-Ware, et al., 2013). In low- and middle-income countries, the most common form of contraception is progestin-only injectables (United Nations Department of Economic and Social Affairs Population Division, 2015, 2019), which are highly effective, reversible methods. The most common form is the three-monthly intramuscular (IM) injection of 150 mg of medroxyprogesterone acetate (MPA) Depo-Provera or DMPA-IM), while Sayana Press (DMPA-SQ or DMPA-SC), a three-monthly, lower (104 mg) DMPA dose injectable contraceptive delivered subcutaneously (SC) has also been introduced (Family Planning 2020 (FP2020), 2014; PATH, 2017; Polis, et al., 2018; Schivone, et al., 2016). Another progestin-only injectable widely used in South Africa is Nur-Isterate or Norigest, a two-monthly injection containing 200 mg of norethisterone (NET) enanthate (NET-EN) (Heffron, et al., 2019; National Department of Health & ICF, 2019). Other long-term highly efficient and reversible progestin-only contraception methods include etonogestrel (ETG)-releasing subdermal implants (Implanon, Nexplanon) and intravaginal rings (IVRs) (NuvaRing), as well as levonorgestrel (LNG)-releasing implants (Jadelle, Norplant, Sino-Implant) and intra-uterine devices (IUDs) (Mirena, Skyla, Liletta) (Sitruk-Ware, et al., 2013). LNG is used extensively worldwide in many different HCs (Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium, 2019; Polis, Phillips, et al., 2016; United Nations Department of Economic and Social Affairs Population Division, 2015, 2019). Less widely used progestin-only contraceptives include administration of nestorone (NES) in an IVR or implant (Sitruk-Ware, et al., 2003). Most estrogen-containing contraceptives are administered as combined oral contraceptives (COCs), which currently contain varying doses of the progestins nomegestrol acetate (NoMAc), drospirenone (DRSP), gestodene (GES), dienogest (DNG), norgestimate (NGM), cyproterone acetate (CPA), LNG, ETG or NET (Sitruk-Ware, et al., 2013). Some estrogen-containing contraceptives are administered by other routes, such as IM injection of MPA plus estradiol (E2) cypionate (E2C) (Cycloprovera, Cyclofem or Lunelle) (Kaunitz, 2001) or IVRs containing NES, ETG or NET (Sitruk-Ware, et al., 2013) or a transdermal patch containing norelgestromin (NGMN) (Abrams, et al., 2002), which all also contain ethinyl estradiol (EE). Besides the abovementioned progestins, there are several others, which will not be discussed in this review since there is very little information available and they are not widely used.
3. Progestins and SRs
Progestins exert their biological effects via binding to and activating intracellular SRs, which are ligand-activated transcription factors that regulate transcription of specific target genes by multiple mechanisms (Jacobsen & Horwitz, 2012; Newton, et al., 2010; Oakley & Cidlowski, 2013; Scheschowitsch, et al., 2017). The progestogenic activity of all progestins is due to their actions via the progesterone receptor (PR) (Enfield, et al., 2020). However, some progestins also bind to and activate other members of the SR family to different degrees, including the classical glucocorticoid, androgen and mineralocorticoid receptors (GR, AR and MR, respectively), exerting differential agonist, partial agonist or even antagonistic transcriptional effects via some of these receptors (Africander, et al., 2013; Koubovec, et al., 2005; Louw-du Toit, et al., 2020; Louw-du Toit, et al., 2017; Ronacher, et al., 2009). We have previously comprehensively reviewed the mechanisms of action of progestins via SRs (Africander, et al., 2011; Hapgood, et al., 2014; Hapgood, et al., 2018; Hapgood, et al., 2004).
Established and potential differential actions of progestins via SRs most likely form the basis for their differential clinical outcomes and side-effects, besides differential actions due to pharmacokinetics and metabolism. Of particular interest are the pharmacokinetics and side-effects of DMPA-IM compared to DMPA-SC and intramuscular NET-EN, since DMPA-IM has been associated with increased HIV-1 acquisition compared to NET-EN and condom use or no contraception, although the observational data have limitations (Morrison, et al., 2015; Polis, Curtis, et al., 2016; Ralph, et al., 2015). No such increased risk of HIV-1 acquisition has been detected for COCs, while little information is available for other HCs. Data from a recent randomized open-label trial suggest that DMPA-IM has a 23-29% increased risk, but has less than a 50% increased HIV-1 risk, compared to an LNG implant (Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium, 2019). Notably, MPA, unlike NET and LNG, binds the GR with a relatively high affinity (Hapgood, et al., 2018; Koubovec, et al., 2005; Ronacher, et al., 2009; unpublished data). Although several progestins are discussed in this review, most available data on pharmacokinetics, metabolism and serum concentrations reviewed here are for MPA, NET and LNG.
4. Pharmacokinetics and metabolism of progestins
The concentration of the progestin available to elicit biological actions in target tissues is influenced by factors such as route of administration, metabolism, bioavailability, half-life and availability after binding to steroid-binding proteins. Here, we discuss these factors for select progestins used in contraception (Fig. 1).
Figure 1. Chemical structures of P4 and progestins commonly used in contraception.
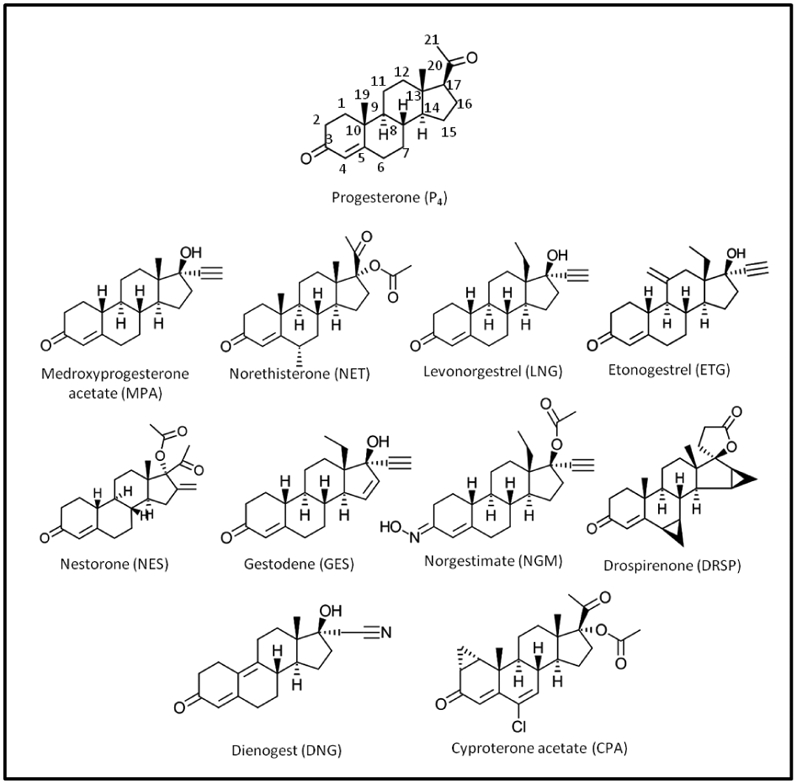
For P4, the letters 1-21 denote the carbon number.
4.1. Route of administration and metabolism
Information on the metabolism of progestins in humans is scant; however, available studies suggest that this is influenced by route of administration i.e. oral or parenteral (IM injections, implants, vaginal gels or rings, IUDs and transdermal patches) (Africander, et al., 2011; Stanczyk, et al., 2013). In contrast to parenterally administered progestins, progestins taken orally undergo hepatic first-pass metabolism, resulting in a significant reduction in progestin concentration (Stanczyk, et al., 2013). Hepatic first-pass progestin metabolism occurs via steroidogenic enzymes like cytochrome P450 enzymes, hydroxysteroid dehydrogenases (HSDs) and reductases in the intestinal mucosa. Subsequently, progestin metabolites and unmetabolized progestins are transported via the portal vein to the liver, where several metabolites, many of which remain unidentified, are produced by steroidogenic enzymes (Edelman, et al., 2010; Stanczyk, et al., 2013). The parent progestin and/or its metabolites, either unconjugated or conjugated, are then released into the blood. While unconjugated compounds are transported to target tissues, conjugated compounds are excreted in the urine and faeces. Conjugated products are formed when the hydroxyl group of the parent progestin and/or progestin metabolites is sulfated or glucuronidated (Stanczyk, 2003; Stanczyk, et al., 2013). These reactions assist with either the transport of compounds by making hydrophobic compounds more water soluble or by inactivating toxic compounds (Schonborn, 2010; Vīna, et al., 2013). Parenterally administered progestins are also significantly metabolized in the liver (Stanczyk, 2003; Stanczyk, et al., 2013). Metabolism may also occur at the site of administration or the target sites expressing steroid-metabolizing enzymes. For example, steroid 5α-reductase and 17β-HSD are not only found in the liver (Jin & Penning, 2001; Narasaka, et al., 2000) but also in the female genital tract (FGT) (endometrium (Dassen, et al., 2007; Konings, et al., 2018), vagina (Berman, et al., 2003), uterus (Konings, et al., 2018) and skin (Cassidenti, et al., 1991; Martel, et al., 1992)).
4.2. P4 and progestin metabolites
Progestins are used instead of P4 in endocrine therapy due to the rapid metabolism of P4 (Hapgood, et al., 2004; Speroff & Darney, 1996; Stanczyk, et al., 2013). Understanding the metabolism of progestogens is important as tissue- and cell-specific metabolites may result in differential beneficial and/or detrimental biological effects. We summarize below the main metabolites identified for P4 and progestins commonly used in contraception (Fig. 1).
4.2.1. P4
P4 metabolism targets the 3-keto and 20-keto groups and the double-bond between carbon 4 and 5 in the A-ring of the steroid structure (Fig. 1) (Kuhl, 2011). A number of enzymes, including reductases, HSDs and cytochrome P450 enzymes have been implicated (Lewis, et al., 2004; Miller & Auchus, 2011; Wiebe, 2006). For example, in human breast tissue, P4 is converted mainly to 4-pregnenes e.g. 3α-dihydroprogesterone, by 3α-hydroxysteroid oxidoreductase (3α-HSO) or 20S-hydroxyprogesterone by 20α-HSO, or to 5α-pregnanes (e.g. 5α-dihydroprogesterone) by 5α-reductase (Wiebe, 2006). Normal breast tissue produces significantly more 4-pregnenes than 5α-pregnanes, while in tumorous breast tissue the production of 5α-reduced metabolites is favoured (Wiebe, 2006; Wiebe, et al., 2000). While the ratio between these P4 metabolites has been suggested to contribute to breast cancer risk (Wiebe, 2006; Wiebe, et al., 2000), a recent study did not show increased risk with the circulating ratio of the 5α-dihydroprogesterone:3α-dihydroprogesterone in postmenopausal women (Trabert, et al., 2020). Both 5α-pregnanes and 5β-pregnanes also occur in the human liver (Jin, et al., 2011; Stanczyk, et al., 2013), while hydroxylated derivatives of P4 are produced in the human brain by cytochrome P450 CYP2D6 (Hiroi, et al., 2001). The precise functions of many of these metabolites are still unknown.
4.2.2. MPA
Although MPA has been used for more than 60 years (Regidor, 2018), information regarding its metabolism is scarce. It has been suggested that the acetate at carbon 21 may limit metabolism (Stanczyk, et al., 2013). Nonetheless, MPA can be hydroxylated at carbons 2, 6 and 21 in humans, with 6β, 21-dihydroxy-MPA (Table 1) being the major metabolite (Fukushima, et al., 1979; Helmreich & Huseby, 1962; Sturm, et al., 1991). Cytochrome P450 3A polypeptide 4 (CYP3A4), highly expressed in the liver (Lynch & Price, 2007; Thummel, 2007), may be involved in this hydroxylation (Kobayashi, et al., 2000; Zhang, et al., 2008). Although MPA itself is likely the active progestogenic compound (Hapgood, et al., 2004), further research is necessary to identify other possible metabolites, their concentrations and possible physiological functions, since MPA is the progestin associated with the most side-effects.
TABLE 1.
Metabolites of progestins commonly used in contraception
| Progestin | Metabolites | Active compound | Reference(s) |
|---|---|---|---|
| MPA | 1β-hydroxy-MPA$; 2β-hydroxy-MPA$; 6β-hydroxy-MPA$; 6β, 21-dihydroxy-MPA$ | MPA | (Fukushima, et al., 1979; Helmreich & Huseby, 1962; Sturm, et al., 1991; Zhang, et al., 2008) |
| NET-EN | NET; EE | NET; EE | (Bayer Healthcare Pharmaceuticals Inc, 2011; Stanczyk & Roy, 1990) |
| NET | 5α-NET; 5β-NET; 3α,5α-NET; 3β,5α-NET; 3α,5β-NET; 3β,5β-NET; EE | 5α-NET; 3α,5α-NET; 3β,5α-NET; NET; EE | (Blom, et al., 2001; Chu, et al., 2007; Chwalisz, et al., 2012; Garcia-Becerra, et al., 2004; Kuhl, 2005; Kuhl & Wiegratz, 2007; Kuhnz, et al., 1997; Larrea, et al., 2001; Lemus, et al., 2009; Reed, et al., 1990; Schoonen, et al., 2000; Stanczyk, 2003; Stanczyk & Roy, 1990) |
| LNG | 5α-LNG; 5β-LNG; 3α,5α-LNG; 3β,5α-LNG; 3α,5β-LNG; 3β,5β-LNG; 2α-hydroxy-LNG; 16β-hydroxy-LNG; 16β-hydroxy-3α,5β-tetrahydro-LNG | 5α-LNG; 3α,5α-LNG; 3β,5α-LNG; LNG | (Garcia-Becerra, et al., 2002; Garcia-Becerra, et al., 2004; Larrea, et al., 2001; Schoonen, et al., 2000; Stanczyk, 2003; Stanczyk & Roy, 1990) |
| GES | 5α-GES; 3α,5α-GES; 3β,5α-GES; 1β-hydroxy-GES; 6α-hydroxy-GES; 11α-hydroxy-GES; 11β-hydroxy-GES | 5α-GES; 3α,5α-GES; 3β,5α-GES; GES | (Garcia-Becerra, et al., 2004; Larrea, et al., 2001; Lemus, et al., 2001; Stanczyk & Roy, 1990; Ward & Back, 1993) |
| NGM | Norelgestromin; Norgestrel (LNG) | Norelgestromin; LNG | (Juchem, et al., 1993; Madden, et al., 1990; McGuire, et al., 1990; Phillips, et al., 1990; Prifti, et al., 2004; Stanczyk, 2003) |
| ETG | 6β-hydroxy-11,22-epoxy-ETG*; 11,22-epoxy-ETG*; 10β-hydroxy-ETG*; 6β-hydroxy-ETG*; 14α-hydroxy-ETG* | ETG | (Baydoun, et al., 2016) |
| NES | 17α-deacetyl-NES#; 4,5-dihydro-17α-deacetyl-NES# | NES | (Kumar, et al., 2017; Prasad, et al., 2010) |
| DRSP | 4,5-dihydro-DRSP-3-sulfate; acid form of DRSP; 6β,7β,15β,16β-dimethylene-11α-hydroxy-3-oxo-l7α-pregn-4-en-21,17-carbolactone**; 6β,7β,15β,16β-dimethylene-11α-hydroxy-3-oxo-l7β-pregn-4-en-21,17-carbolactone**; 6β,7β,15β,16β-dimethylene-11β-hydroxy-3-oxo-l7α-pregn-4-en-21,17-carbolactone**; 6β,7β,15β,16β-dimethylene-2β-hydroxy-3-oxo-l7α-pregn-4-en-21,17-carbolactone** | DRSP | (Bachmann & Kopacz, 2009; Krattenmacher, 2000; Quintana, et al., 2013; Wiesinger, et al., 2015) |
| DNG | 6β-hydroxy-DNG$ | DNG | (Shin, et al., 2013) |
| CPA | 15β-hydroxy-CPA; 3α-hydroxy-CPA | 15β-hydroxy-CPA; CPA | (Bhargava, et al., 1977; Kerdar, et al., 1995; Schindler, et al., 2003) |
Activity of metabolites unknown.
ETG hydroxylation in fungi (Cunninghamella blakesleeana and C. echinulate). Presence and physiological relevance in humans not known.
NES metabolism in rats administered by subcutaneous injection. Presence and physiological relevance in humans not known.
DRSP is metabolised in fungal cells (Absidia corymbifera, BAFC 1072, A. coerulea and Syncephalastrum racemosum). Presence and physiological relevance in humans not known.
4.2.3. NET
NET-A and NET-EN are metabolized to the progestogenic compound NET. In the liver, NET undergoes extensive metabolism in its A-ring structure when given orally, producing dihydro- (5α-NET and 5β-NET) and tetrahydro- (3α,5α-NET, 3β,5α-NET, 3α,5β-NET and 3β,5β-NET) metabolites (Fig. 2, Table 1). The dihydro-metabolites are formed after reduction of the double bond between carbon 4 and 5 in the A-ring and after addition of hydrogen to both carbons, while the addition of a hydroxyl group to carbon 3 results in the formation of the tetrahydro-metabolites (Edelman, et al., 2010). The major metabolite in serum of women receiving 2 mg NET is 3α,5α-NET sulfate, while lower concentrations of 3α,5β-NET sulfate and 3β,5β-NET sulfate are also present (Stanczyk & Roy, 1990). In contrast, 3α,5β-NET sulfate is the major metabolite in women receiving 25 mg NET, while lower concentrations of 3α,5α-NET sulfate and glucuronidated 3α,5β-NET are also present (Stanczyk & Roy, 1990). Notably, high concentrations of unmetabolized NET are still present in the serum of women receiving either dosage (Stanczyk & Roy, 1990). The predominant metabolites in urine of women receiving 25 mg NET are 3α,5β-NET sulfate and 3α,5β-NET glucuronide. Metabolism of NET to the 5α-reduced metabolites 5α-NET, 3α, 5α-NET and 3β, 5α-NET by 3β-HSD and/or 5α-reductase occurs in the uterus, vagina and aorta of rats (Blom, et al., 2001). These metabolites are likely also produced in women using NET, as 3β-HSD and/or 5α-reductase are expressed in the these and other human FGT tissues (Andersson, et al., 2008; Berman, et al., 2003; Gibson, et al., 2013; Konings, et al., 2018).
Figure 2. Structures of (A) NET, (B) GES, (C) NGM and LNG, and their respective metabolites.
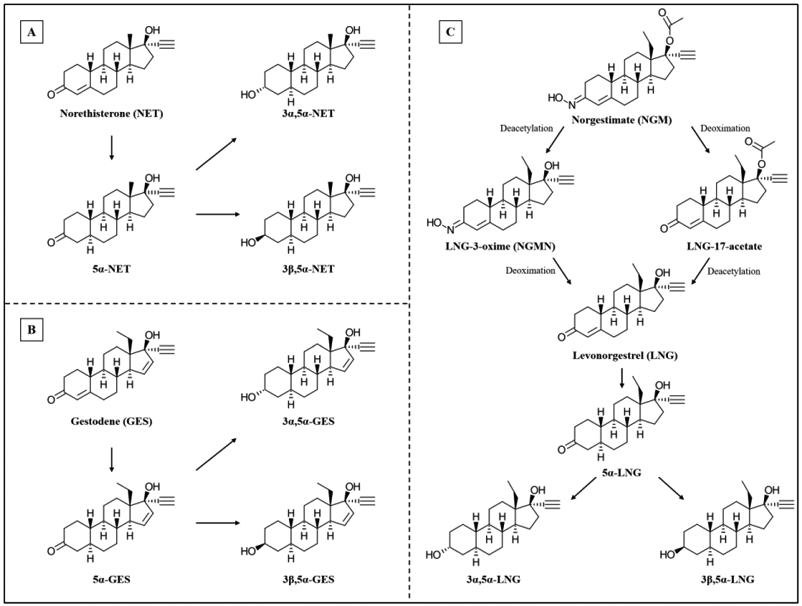
(A and B are redrawn from (Larrea, et al., 2001); C is adapted from (Garcia-Becerra, et al., 2002; Juchem, et al., 1993)).
NET and its acetate form, NET-A, can also be converted to the potent estrogen EE when taken orally by women (Chu, et al., 2007; Kuhnz, et al., 1997). While some studies suggest that this conversion is catalyzed by cytochrome P450 aromatase (CYP19A1) (Barbieri, et al., 1983; Yamamoto, et al., 1986), others suggest that other enzymes are involved (Kuhl, 2005; Kuhl & Wiegratz, 2007). EE displays estrogenic activity via both ER subtypes (Perkins, et al., 2017), while 5α-NET, 3α,5α-NET and 3β,5α-NET only display estrogenic activity via ER-α (Larrea, et al., 2001). Although 5α-NET is also an AR and PR agonist, it is more potent than NET via the AR, but less potent than NET via PR-A and PR-B, (Garcia-Becerra, et al., 2004; Larrea, et al., 2001). Interestingly, 5α-NET is more potent via PR-A than PR-B, while 3α,5α-NET is a partial agonist for PR-B but not PR-A (Larrea, et al., 2001).
4.2.4. NGM, NGMN and LNG
NGM is metabolized to LNG-17-acetate and NGMN, also known as 17-deacetyl-noregestimate or LNG-3-oxime (Juchem, et al., 1993; McGuire, et al., 1990), with NGMN being the main progestogenic metabolite (Fig. 2, Table 1). Both NGM and NGMN elicit progestogenic and androgenic activity (Juchem, et al., 1993; Phillips, et al., 1990; Prifti, et al., 2004). In the human liver, NGMN undergoes metabolism to form LNG (Fig. 2).
LNG is reduced to form dihydro- (5α-LNG and 5β-LNG) and tetrahydro- (3α,5α-LNG, 3β,5α-LNG, 3α,5β-LNG and 3β,5β-LNG) metabolites (Fig. 2, Table 1). However, hydroxylated metabolites of LNG, e.g. 2α-hydroxy-LNG, 16α-hydroxy-LNG, 16β-hydroxy-LNG and 16β-hydroxy-3α,5β-tetrahydro-LNG are also detected (Stanczyk & Roy, 1990). Only one study appears to have determined the concentration of LNG metabolites in serum and urine (Stanczyk & Roy, 1990). It showed that LNG is still present in its unmetabolized form in serum following an oral dose of 1.5 mg, while sulfated-, glucuronidated- and unconjugated 3α,5β-LNG metabolites are also present. Although glucuronidated 3α,5β-LNG is the major metabolite in urine, some sulfated 3α,5β-LNG and glucuronidated 16β-hydroxy-3α,5β-tetrahydro-LNG also occur. Both 3α,5β-LNG and 3α,5α-LNG are present in the serum of women (Jadelle® (LNG-releasing implant) and Mirena® (LNG-releasing IUD package leaflets) (Bayer Healthcare Pharmaceuticals Inc, 2014, 2016). These metabolites display similar, but significantly lower potencies than 5α-LNG and LNG for PR-A and PR-B, while 3β,5α-LNG is estrogenic via ERα, but not ERβ (Garcia-Becerra, et al., 2002). Although 5α-LNG, like LNG, activates both the PR and AR, 5α-LNG is equipotent to LNG via the AR (Garcia-Becerra, et al., 2004), but less potent than LNG via the PR.
4.2.5. GES
Similar to LNG, dihydro- (5α-GES), tetrahydro- (3α,5α-GES, 3β,5α-GES) and hydroxylated metabolites of GES (1β-hydroxy-GES, 6α-hydroxy-GES, 11α-hydroxy-GES and 11β-hydroxy-GES) have been identified (Fig. 2, Table 1). GES hydroxylation in the human liver is reportedly catalysed by CYP3A4 (Ward & Back, 1993). GES, 5α-GES, 3α,5α-GES and 3β,5α-GES all display agonist activity via both PR isoforms (Garcia-Becerra, et al., 2004; Larrea, et al., 2001); however, GES is more potent. In contrast, GES and 5α-GES display similar potencies via the AR (Garcia-Becerra, et al., 2004), while the androgenic properties of 3α,5α-GES and 3β,5α-GES remain unknown. While neither GES nor its metabolites display activity via ERβ, both 5α-GES, 3α,5α-GES and 3β,5α-GES, unlike GES, display estrogenic properties via ERα (Larrea, et al., 2001).
4.2.6. ETG
ETG, also referred to as 3-keto-desogestrel, is the main progestogenic metabolite of the orally-administered progestin desogestrel (DSG) (Stanczyk, 2003; Verhoeven, et al., 1998; Viinikka, et al., 1976), and elicits stronger progestogenic activity than DSG itself (Viinikka, et al., 1976). CYP3A4 has been implicated in the metabolism of ETG to form hydroxylated metabolites in humans (Gentile, et al., 1998; Korhonen, et al., 2005) (Table 1). However, ETG is shown to be metabolized by fungi to form 6β-hydroxy-11,22-epoxy-ETG, 10β-hydroxy-ETG, 14α-hydroxy-ETG, 11,22-epoxy-ETG and 6β-hydroxy-ETG (Baydoun, et al., 2016). The latter two metabolites can inhibit the activity of β-glucuronidase (Naz, et al., 2013). Whether any of these metabolites are produced in humans, and have physiological relevance, remains to be determined.
4.2.7. NES
NES is biologically inactive when taken orally due to its rapid metabolism in the liver (Heikinheimo, et al., 1994; Kumar, et al., 2000; Noe, et al., 1993; Schindler, et al., 2008; Sitruk-Ware, 2006). Although it appears that NES metabolites have not been identified in humans, some have been identified in rodents following subcutaneous injection (Kumar, et al., 2017; Prasad, et al., 2010). One study identified 17α-deacetyl-NES and 4,5-dihydro-17α-deacetyl-NES (Prasad, et al., 2010) in serum and urine (Table 1), while another identified 5α-dihydronestorone (5α-DHNES), 20α-dihydronestorone (20α-DHNES), 3α, 5α-tetrahydronestorone (3α, 5α-THNES) and 3β, 5α-tetrahydronestorone (3β, 5α-THNES) in serum and the brain (Kumar, et al., 2017). Both 5α-DHNES and 3α, 5α-THNES display weaker progestogenic potencies than NES, while the activity of the other metabolites is not known.
4.2.8. DRSP
DRSP likely undergoes extensive metabolism, as very low levels of DRSP are observed in human urine and faeces (Wiesinger, et al., 2015), and 20 minor, inactive metabolites of DRSP are excreted mostly as glucuronidated and sulfated conjugates (Krattenmacher, 2000). Two inactive metabolites of DRSP, 4,5-dihydro-DRSP-3-sulfate and an acid form of DRSP (Fig. 3), have also been identified in human plasma (Bachmann & Kopacz, 2009; Krattenmacher, 2000; Wiesinger, et al., 2015). While DRSP is also metabolized to four other metabolites by fungal cells (Table 1) (Quintana, et al., 2013), their biological activities and occurrence in humans remain unknown.
Figure 3. Structure of DRSP and its metabolites, an acid form of DRSP and 4,5-dihydro-DRSP-3-sulfate.
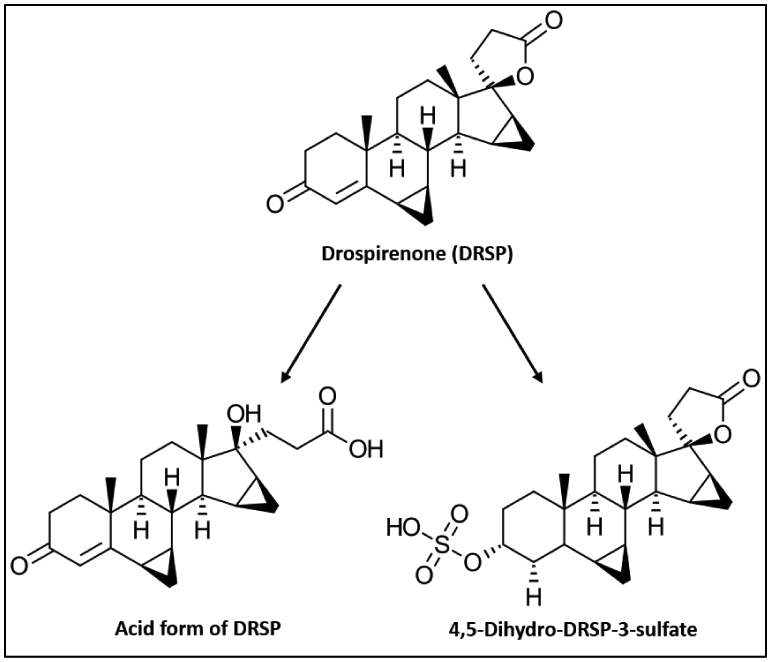
(redrawn from (Krattenmacher, 2000)).
4.2.9. DNG
Little is known about the metabolism of DNG. While it is predominantly found in serum in its unchanged form following oral administration (Wellington & Perry, 2002), DNG may be metabolized by CYP3A4 to various inactive metabolites which are rapidly eliminated from serum and excreted in urine (McCormack, 2010). Although the majority of these metabolites are unknown, at least one has been identified as 6β-hydroxy-DNG (Shin, et al., 2013) (Table 1), the biological function of which is unknown.
4.2.10. CPA
CPA is metabolized to 15β-hydroxy-CPA (Bhargava, et al., 1977) in humans, dogs and rhesus monkeys, and to 3α-hydroxy-CPA in rats (Kerdar, et al., 1995) (Table 1). Although the biological activity of 3α-hydroxy-CPA is unknown, 15β-hydroxy-CPA elicits similar anti-androgenic activity to CPA, but significantly weaker progestogenic activity (Kuhl, 2011).
4.3. Bioavailability
The terms availability and bioavailability are often confused. The former refers to how much of the drug is accessible to the cells (discussed later), while the latter refers to how much of the administered drug reaches the bloodstream after metabolism (Edelman, et al., 2010; Stanczyk, et al., 2013). Limited available data show that for all progestins except NES, most of the administered dose is available in circulation following metabolism (Table 2). The very low bioavailability of oral NES is presumably the reason why it is administered parenterally (Fraser, et al., 2005; Fraser, et al., 2007; Sitruk-Ware, 2006). Whether the bioavailability of NES or any of the other progestins is influenced by the route of delivery remains unknown.
TABLE 2.
Reported bioavailabilities and half-lives of progestins commonly used in contraception
ND – not determined. The information in the brackets indicates the type and concentration of the estrogen component used in the progestin-estrogen combined contraceptive.
Initial release rate of 20 μg per day reduced to ~10 μg per day after 5 years.
Initial release rate of two rods, each containing 75 mg, is ~100 μg per day. After 12 months it decreases to ~40 μg per day, and after 24 months to ~30 μg per day.
Initial release rate of 60 -70 μg/day (week 5 – 6), decreases to ~35 -45 μg per day after 1 year, ~30 – 40 μg per day after 2 years and ~25 – 30 μg per day after 3 years.
Release rate of ~0.120 mg ETG and ~0.015 mg EE per day for three weeks.
Release rate of ~0.150 mg NGMN and ~0.020 mg EE per 24 hours.
Large standard deviation (SD) observed in t1/2 values (70.7 ± 50.4 hours).
Large SD observed in t1/2 values (59.4 ± 26.6 hours).
Serum concentration of LNG measured using GC-MS and pharmacokinetic parameters evaluated using software package TOPFIT (Thomae GmbH, Germany).
Serum concentration of LNG measured using RIA and pharmacokinetic parameters evaluated using WinNonLin (v 5.2; Pharsight, Moutain View, CA).
4.4. Half-life
The contraceptive efficacy of a progestin is not only influenced by its bioavailability, but also by the time the progestin is present in the body to elicit a biological effect. This is reflected by half-life of a progestin, which refers to the time it takes for the maximum serum concentration to decrease to 50% (Stanczyk, et al., 2013). Progestins used in contraceptives exhibit a range of half-life values (Table 2). For example, the oral intake of NET results in a shorter half-life (2.5 – 12 hours) than NET administered intramuscularly (~278 hours) (Table 2). Similarly, a shorter half-life (24 hours) occurs for oral versus IM injection (~1200 hours) for MPA (Table 2). Interestingly, the half-lives for IM and SC administration of MPA in combination with E2 did not significantly differ (~577 vs 742 hours) (Sierra-Ramirez, et al., 2011).
Although variable half-life values have also been reported for LNG, these do not appear to be significantly affected by dose or route of administration, or the presence of EE (Table 2). However, the half-life is much longer in obese than normal weight women using COCs containing LNG and EE (52.1 vs 25.6 hours; 73.6 vs 37.6 hours) (Edelman, et al., 2009; Westhoff, Torgal, Mayeda, Stanczyk, et al., 2010). Whether factors such as body weight also affect the half-life of other progestins is largely unknown. However, body weight does not appear to influence the half-life of MPA following administration of DMPA-SC (Jain, et al., 2004).
The serum concentration of ETG is often measured in women using an oral contraceptive containing DSG and appears to not be influenced by route of administration, unlike limited data for NES (Table 2). Half-life values of GES are not influenced by dose (Table 2). Similar half-life values are obtained for DRSP and DNG, respectively, whether they are used alone or in combination with an estrogen (Table 2). Due to a paucity of data, similar conclusions could not be drawn for NGM and CPA (Table 2).
Taken together, the current data suggest that half-life is progestin-specific, and only sometimes dependent on dose, route of administration, whether estrogens are co-administered, and body weight. However, very limited data is available for most progestin formulations, and several have not been investigated for effects of all these variables. Finally, failure to detect differences in half-lives may be due to high inter-individual variability and resulting insufficient power of the studies.
4.5. Serum binding proteins
Availability of a specific concentration of progestin to the cells is dependent on its interaction with serum binding proteins such as corticosteroid binding globulin (CBG) and sex hormone binding globulin (SHBG). CBG preferentially binds and transports cortisol in the blood, while SHBG is a carrier protein for testosterone and/or estrogen (Pugeat, et al., 1981; Siiteri, et al., 1982). Steroid hormones bound to CBG and SHBG are unavailable to target tissues, while the unbound (free) steroids are available to elicit their biological effects in cells of target tissues.
Binding of progestins to CBG or SHBG not only influences the concentration of progestin available to target tissues, but may also result in the displacement of endogenous steroids from these proteins, thus increasing the availability of endogenous hormones free to elicit a biological response in target tissues. For example, NET, LNG, GES and ETG, unlike MPA, NGM, NES, DRSP, DNG and CPA, bind to SHBG to varying degrees (Table 3), suggesting that these progestins may compete with testosterone and/or estrogen for binding to SHBG and that only a fraction of bioavailable NET, LNG, GES and ETG will be available to target tissues. None of the progestins discussed in this review bind to CBG (Table 3), suggesting that progestins may not modulate the amount of cortisol available to target tissues via this mechanism.
TABLE 3.
Percentage binding of progestins to steroid-binding proteins present in blood and percentage of the progestin available to target tissues
| Progestin | SHBG- bound (%) |
CBG-bound (%) |
Albumin- bound (%) |
Free (%) |
Available (%) |
Reference(s) |
|---|---|---|---|---|---|---|
| MPA | 0 | 0 | 88 | 12 | 100 | (Kuhl, 2011; Schindler, et al., 2003) |
| NET | 35.5 | 0 | 60.8 | 3.7 | 64.5 | (Hammond, et al., 1982; Kuhl, 2011) |
| LNG | 47.5 | 0 | 50 | 2.5 | 52.5 | (Fotherby, 1990; Hammond, et al., 1982; Kuhl, 2011; Kuhnz, al-Yacoub, & Fuhrmeister, 1992; Kuhnz, Blode, et al., 1994; Kuhnz, Staks, et al., 1994; Stanczyk, 2003) |
| 64.1 - 68.3* | 30.5 - 34.6 | 1.1 - 1.3 | 31.6 - 35.9 | |||
| 55 - 73.6# | 25.5 - 43.6 | 0.9 - 1.4 | 26.4 - 45 | |||
| GES | 57 – 81.5 | 0 | 17.9 – 41.3 | 0.6 – 1.8 | 18.5 – 43.1 | (Kuhnz, Baumann, et al., 1993; Kuhnz, Schutt, et al., 1992) |
| NGM/NGMN | 0 | 0 | 97.2 | 2.8 | 100 | (Hammond, et al., 2003; Kuhl, 2011) |
| ETG | 15 | 0 | 63.5 | 4.5 | 68 | (Fotherby, 1990; Kuhl, 2011; Kuhnz, et al., 1990) |
| 32 | ||||||
| NES | 0 | 0 | 87 | 13 | 100 | (Fotherby, 1990; Kuhl, 2011) |
| DRSP | 0 | 0 | 95 - 97 | 3 - 5 | 100 | (Kuhl, 2011; Schindler, et al., 2003) |
| DNG | 0 | 0 | 90 | 10 | 100 | (Foster & Wilde, 1998; Kuhl, 2011; McCormack, 2010) |
| CPA | 0 | 0 | 93 | 7 | 100 | (Kuhl, 2011; Schindler, et al., 2003) |
Increased binding of LNG to SHBG in women using Microgynon® 30 (150 μg LNG and 30 μg EE) for 3 months.
Increased binding of LNG to SHBG in women using Triquilar® ED (day 1-6: 50 μg LNG and 30 μg EE; day 7-11: 75 μg LNG and 40 μg EE; day 12-21: 125 μg LNG and 30 μg EE) for 3 months.
Cortisol levels could however be influenced by progestins modulating the levels of CBG. Indeed, clinical studies indicate that oral contraceptive doses of NET (van der Vange, et al., 1990), LNG (Wiegratz, et al., 2003), DSG (Jung-Hoffmann, et al., 1992; Kuhl, et al., 1995), GES (Wiegratz, et al., 1995) and NGM (Wiegratz, et al., 1995) used in combination with EE, increase the serum concentration of CBG in healthy users. In contrast, 1 μM MPA, in the absence and presence of E2, decreased CBG mRNA expression in a human endometrial cancer cell line (Misao, et al., 1998b).
MPA can also increase or decrease SHBG levels in a concentration-dependent manner in an endometrial cancer cell line. For example, low concentrations of MPA (0.1 nM) in combination with 10 nM E2 increase the mRNA expression of SHBG, while higher concentrations of MPA (1 – 10 μM), in the absence or presence of 10 nM E2, decrease SHBG mRNA expression (Misao, et al., 1998a). Furthermore, use of the injectable contraceptives DMPA-IM (Jeppsson, et al., 1982) and NET-EN (Zhao, et al., 1992) is associated with decreased SHBG levels in women. Interestingly, in women using COCs containing LNG and EE, the EE component increases SHBG levels, which leads to increased binding of LNG to SHBG, and decreased free LNG (Kuhnz, al-Yacoub, & Fuhrmeister, 1992; Kuhnz, Blode, et al., 1994). Combinations of EE with other progestins also elevate SHBG levels in women, for instance, in a vaginal ring containing ETG and EE (Fleischer, et al., 2009) as well as in COCs containing EE and DSG (Jung-Hoffmann, et al., 1992), GES (Wiegratz, et al., 1995), NGM (Wiegratz, et al., 1995), DNG (Oettel, et al., 1997), or DRSP (Batukan & Muderris, 2006). SHBG levels also increase in COCs containing DNG and E2V (Di Carlo, et al., 1983). To the best of our knowledge, no information is available on binding of progestin metabolites to CBG and SHBG, or whether these metabolites can regulate the expression of these binding proteins.
Clearly there is a paucity of research on the influence of progestins used in contraception on SHBG and CBG levels. More research is needed to understand how the modulation of SHBG and/or CBG levels influence the freely available endogenous steroid hormone levels, as well as the concentrations of progestins freely available to elicit their biological effects in target cells or tissues. Moreover, as the majority of the above-mentioned studies focus on COCs rather than progestin-only contraceptives, further studies are needed to establish the relative roles of EE and/or specific progestins on regulation of serum binding protein levels.
5. Progestin concentrations in serum
Maintaining a concentration of progestin sufficient for contraceptive efficacy is essential for the duration of treatment. However, depending on the progestin, dose, method and route of administration, concentrations much higher than those required to maintain contraceptive efficacy are frequently attained and remain for variable lengths of time for some contraceptives. Concerns have arisen as to possible side-effects of such high concentrations (Hapgood, et al., 2018). To inform on these issues, maximal concentrations (Cmax) are frequently reported, as well as the time taken to reach Cmax (tmax), and concentrations at varying time intervals after first administration (Table 4). Long-acting injectable contraceptives typically exhibit a sharp peak in serum progestin levels a few days after injection, with concentrations much higher than required for contraceptive efficacy, which decrease with variable kinetics, and then remain fairly constant at levels just above contraceptive efficacy for a few months (Fig. 4) (Kirton & Cornette, 1974; Polis, et al., 2018). As the progestin serum half-life is shorter for COCs (Tamassia, et al., 1982), tmax is shorter for oral than injectable contraceptives. Hence daily administration is required to maintain levels above contraceptive efficacy. Long-acting progestins used in implants generally exhibit Cmax values a few weeks after implantation due to slow release, but maintain levels of contraceptive efficacy for months or years (Sivin, et al., 2001; Wenzl, et al., 1998). Intravaginally administered contraceptives generally exhibit Cmax values within hours of administration which decline and remain fairly constant over months or years, above levels required for contraceptive efficacy (Dogterom, et al., 2005; Timmer & Mulders, 2000). Table 4 summarizes some of these key pharmacokinetic parameters of progestins used in different HCs. A key issue, especially for contraceptives administered intravaginally, is the progestin concentrations in local tissue such as the FGT, where tissue-specific side-effects may occur. There is, however, little data on progestin levels within target tissues. A variety of methods have been used to measure progestin serum concentrations, including radioimmunoassay (RIA) and various liquid chromatography (LC) methods such as high performance liquid chromatography (HPLC) (Milano, et al., 1982; Read, et al., 1985), ultra-performance liquid chromatography (UPLC) (Thomas, et al., 2013; Westhoff, et al., 2012), either alone or coupled to a mass spectrometry instrument with one (LC-MS) or two mass analysers (LC-MS/MS). Gas chromatography (GC) or GC coupled to mass spectrometry (GC-MS) have also been used (Dikkeschei, et al., 1985; Jarvinen, et al., 1989; Kaiser, et al., 1974; Rossi, et al., 1979). Mass spectrometry methods are emerging as the method of choice for clinical samples as they offer the advantage of high sensitivity and specificity while being able to multiplex and measure levels of several progestins simultaneously (Abujrais, et al., 2019; Blue, et al., 2018; Buckner, et al., 2019; Cirrincione, et al., 2018; Laszlo, et al., 2019; Soldin & Soldin, 2009; Stanczyk & Clarke, 2010).
TABLE 4:
Systemic concentrations of progestins.
Reported Cmax values were measured at different time points and using various methods.
The concentrations at 1 year for long-term contraceptives are 0.434-0.864 nM (LNG IUD), 0.509-1.605 nM (LNG 75 mg implant), 0.618-1.986 nM (LNG 36 mg implant), 0.54-0.837 nM (ETG implant) and 0.06-0.221 nM (NES implant).
Data are summarized from detailed tables of published concentrations, available at Mendeley Data (http://dx.doi.org/10.17632/5sck77c9b9.1).
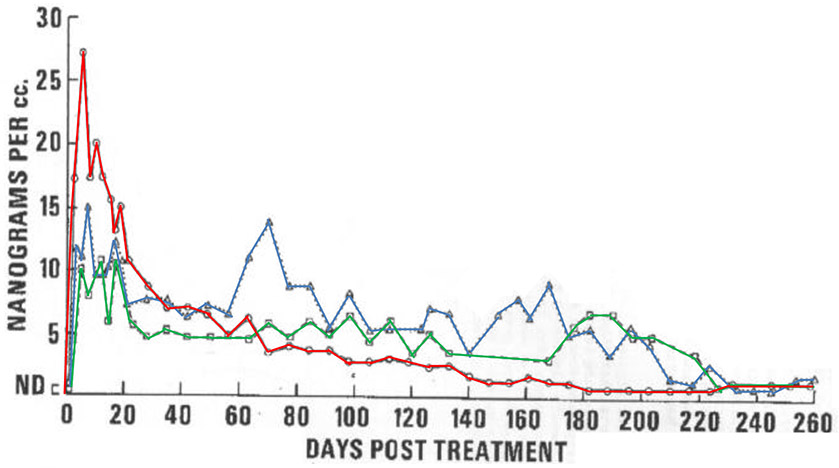
Figure 4. Large inter-individual variability occurs for Cmax, steady-state values and shape of the pharmacokinetic profile in DMPA-IM users.
Serum MPA levels in 3 women (red, green and blue lines) over 260 days following a single injection of DMPA-IM, as measured by direct RIA (adapted with permission from (Kirton & Cornette, 1974)).
Although the pharmacokinetic parameters between different HCs (Table 4) depend on route of administration, progestin and dose, it is unclear to what extent differences are also affected by non-parallel investigations and differences in study design, including number of participants, their demographic characteristics (lactation, ethnicity, race, body mass index (BMI), weight and metabolism), duration on HC, and time and frequency of measurements. More strikingly, large inter-individual and inter-study variation in progestin Cmax levels is generally reported for the same HC method. Whether these are due to some of the above-mentioned factors and/or different methods of quantification and/or steps prior to quantification is unclear from the literature. These large inter-study variations highlight the need for standardized methodologies, including specifications on solvents used for extraction of different progestins, methods of analysis and reporting of data (Stanczyk & Clarke, 2010; Stanczyk, et al., 2007).
Extensive tables showing data and sources from a comprehensive PubMed search of the literature on serum concentrations of progestins commonly used in contraception is available at Mendeley Data (http://dx.doi.org/10.17632/5sck77c9b9.1) [dataset]. Table 4 summarized these, while key points are discussed below.
5.1. Variations in serum progestin concentrations
5.1.1. MPA
Cmax and tmax values for MPA in DMPA-IM users have been reported for only a few studies where sampling was conducted at frequent intervals p.i. (Kirton & Cornette, 1974; Ortiz, et al., 1977; U.S. Food and Drug Administration, 2003). These suggest that tmax is 3-6 days p.i. Studies not sampling in this interval may have underreported Cmax values and incorrectly assigned tmax. A wide inter-study range of Cmax values from 2.6-30 nM up to 65-100 nM is reported for 150 mg DMPA-IM (Table 4) [dataset]. More consistent values are reported for a plateau concentration of about 2.6 nM, which is maintained for 3 months (Mishell, 1996; Ortiz, et al., 1977). Besides large inter-study variability, large inter-individual variation occurs for MPA serum levels with DMPA-IM use, especially immediately after injection, as well as the shape of the pharmacokinetic profile. This is shown graphically for 3 women over 260 days following injection (Kirton & Cornette, 1974). Several studies reporting similar Cmax values suggest that variation cannot be explained by different quantification methods (i.e. RIA versus LC-MS or GC-MS) (Bonny, et al., 2014; Fotherby, Saxena, et al., 1980; Shrimanker, et al., 1978).
For the three-monthly 104 mg MPA injection DMPA-SC, three studies measuring serum MPA levels over time report a tmax between 8-21 days after injection with a Cmax range of 0.52-6.73 nM (Table 4) [dataset]. However, as for DMPA-IM, frequency of sampling times may not accurately reflect tmax or Cmax if they occur within 0-8 days. Whether DMPA-SC is likely to have fewer side-effects than DMPA-IM due to the lower dose is unclear. Only two studies directly compare the two MPA formulations. One study assessed the levels of other reproductive hormones (E2, P4, follicle stimulating hormone) in parallel but not MPA levels (Jain, et al., 2004); the other study only directly compared MPA levels at 6 months, 1 year and 2 years (Kaunitz, et al., 2009), making it impossible to reliably compare their tmax and Cmax values. To date, only one study has attempted measurement of MPA levels in cervical secretions. This study in DMPA-SC users had limited success, with only 4/8 samples giving detectable values (Buckner, et al., 2019).
5.1.2. NET
For the 200 mg IM injection of NET-EN, reported tmax values are 3-10 days and Cmax ranged from 2.44-86 nM, with an upper range at 117 nM in one study (Table 4) [dataset]. The Cmax values differ up to 48-fold, which is similar to the 38-fold range (2.6-99.6 nM) in Cmax for MPA (Fotherby, Saxena, et al., 1980; Koetsawang, 1977; Smit, et al., 2004), and are consistent with high inter-individual differences in NET levels. However, as for MPA, chosen sampling times (earliest at 3 days) may not accurately capture tmax and Cmax values. Reported steady-state NET levels of 0.39-14.6 nM are more variable than the 2.6 nM for DMPA-IM, which may in part be due to different sampling times for NET (30-120 days) (Fotherby, et al., 1978a, 1978b; Goebelsmann, et al., 1979). Serum NET levels after oral doses of ethynodiol acetate, which is metabolized to NET, also show wide ranges of serum NET levels (Cooke, et al., 1985; Vose, et al., 1979; Walls, et al., 1977), reflecting large biological variation. Limited data on NET levels in reproductive tissues show that NET is detectable in cervix, endometrium, myometrium (Reed, et al., 1973) and cervical mucus (Fels, et al., 2013).
5.1.3. LNG
LNG is commonly administered in many different HCs (Table 4) [dataset]. LNG administered in the IUD Mirena (52 mg LNG) generates serum LNG levels of 275-2430 pM at shorter time points (1-3 months), but slightly lower levels of 58-1620 pM after long-term use (1-8 years). However, there is little information on tmax and Cmax for sampling times less than 1 month. LNG administered in the subdermal implant Norplant (36 mg LNG) has a reported tmax at 24 hours after insertion and a Cmax of 0.8-11.3 nM. Later sampling times show lower serum LNG levels ranging from 0.4-1.3 nM (1 month-6 years) (Table 4). In COCs, LNG is administered at variable doses (100, 150, 250 μg or 1.5 mg) in combination with variable doses of EE (30 or 50 μg), resulting in large variation in reported serum LNG levels. For the 150 μg LNG + 30 μg EE COC (Nordette, Seasonique, Levora, Oralcon), tmax is reached at 1-2 hours and Cmax is between 4.2-30 nM (Table 4).
LNG is one of the few progestins with literature available on levels in FGT tissue [dataset]. High but variable LNG levels were reported for the endometrium (808 ± 511 ng/g) of 4 women receiving an IUD releasing 30 μg/day for 36-49 days (Nilsson, et al., 1982). In the same study, the authors measured 3.5 ng/g LNG in the endometria of two women receiving an oral dose of 250 μg/day LNG for 7 days, i.e. about 100-400 times lower than the IUD. Within the myometrium, fallopian tubes and fat tissue, LNG levels were comparable (1-5 ng/g) for both routes of administration (Nilsson, et al., 1982). Serum LNG levels were lower in the IUD group (647 ± 326 pM) compared to the oral group (1790 ± 669 pM). The differences in endometrial and serum LNG levels between the two groups may reflect differences in daily doses, routes for LNG to enter the blood for the different routes of administration, and likely accumulation of LNG in the endometrium. These results are consistent with a recent study showing higher LNG levels in cervical fluid compared to serum in Mirena users (Buckner, et al., 2019). However, more research on tissue LNG levels is required to make clearer conclusions.
5.1.4. ETG
ETG Cmax levels for the implant Implanon rise from 820 ± 249 pM at 8 hours to 0.6-3.7 nM at 6 days, and a level of 2.76 nM in the first few weeks (Table 4) [dataset]. After long-term use of 3 months-3 years, serum ETG levels range from 481-2470 pM. In a study comparing Implanon (ETG) and Norplant (LNG), serum LNG showed greater individual variation (0.3-6 nM) compared to serum ETG (0.9-3.7 nM), especially within the first 7 days after insertion (Makarainen, et al., 1998). Serum ETG levels after oral administration of 150 μg DSG together with 30 μg EE, range from 4.62-19.1 nM depending on time of sampling (1.5 hours-21 days) (Table 4) [dataset]. Likewise, ETG serum levels following insertion of the NuvaRing IVR are also dependent on time of sampling (2 days-5 weeks) and range from 2.1-7.76 nM (Table 4) [dataset].
5.1.5. NES
Serum NES levels after IVR insertion range from 99-134 pM (15 days-6 months) for the 50 μg IVR, and 250-350 pM (21 days-25 weeks) for the 150 μg IVR, also containing 15 μg EE (Table 4) [dataset]. For implants containing around 80 mg NES, serum levels range from 60-259 pM depending on time of sampling (1-12 months) (Table 4) [dataset].
5.1.6. Other progestins
Very little information is available for serum levels of the less-commonly used progestins (Table 4) [dataset]. While there are more studies for some progestins (GES, DRSP, DNG) than others (NoMAc, megestrol acetate (MA), NGMN, CPA), collectively the available studies indicate that a wide range of variation in serum levels also occurs for these less well-studied progestins. For instance, levels of NoMAc administered in the COC containing 2.5 mg NoMAc plus 1.5 mg E2 reach a Cmax of 19.5-33.2 nM (Table 4), while a Cmax range of 6.8-48.3 nM is reached following administration of COC containing 75 μg GES plus 30 μg EE (Table 4). DRSP, typically administered in a COC containing 3 mg DRSP plus 20-30 μg EE (Yaz, Yasmin), generates serum levels of 2-298 nM DRSP, although this variation appears to depend on time of sampling (Table 4). DNG administered in a COC containing 2 mg (with or without 2 mg E2V or 30 μg EE) generated serum DNG levels ranging from 73.9-206 nM (Table 4). For most COCs, tmax is reached within the first 3 hours post administration (Table 4). Together, the abovementioned data again highlight the variability of serum progestin levels between studies, different routes of administration and timing of sample measurements.
5.2. Effects of duration on contraceptives on serum progestin levels
The length of time on HC may be relevant to interpreting Cmax serum progestin values, since these may be influenced by progestin accumulation, rate of clearance, bioavailability and metabolism. However, studies stratifying contraceptive users based on different durations on contraception and longitudinal studies are lacking. Oral MPA is reported to accumulate due to its slow elimination half-life of 2.5 days (Pollow, et al., 1989). More extensive literature on DMPA-IM is, however, contradictory. One study suggests that MPA levels after 12 weeks p.i do not vary with the number of injections over 4.4-10.6 years (Jeppsson, et al., 1982), similar to results from another study comparing the first and fifth injection cycles of DMPA-IM (Schwallie, 1974). However, another study showed a trend of slightly increased MPA levels after longer (4-5 years: 0.5-1.6 ng/ml) compared to shorter (1 year or less: 0.3-1.5 ng/ml) duration of use, although there was wide inter-individual variability (Smit, et al., 2004). Other studies on DMPA-IM also suggest slightly higher levels occur in serum after repeated injections (Koetsawang, et al., 1979; U.S. Food and Drug Administration, 2003). For the lower dose monthly injectable Cyclofem (25 mg MPA + 5 mg E2C), serum MPA levels were higher in women who had received 31-45 injections (3.41 ± 0.19 nM) compared to those who had received only one (2.65 ± 0.13 nM) (Koetsawang, et al., 1979). A recent study, however, showed steady state MPA levels of 1.11 nM after two months (Thurman, et al., 2013). In agreement with another study (Zhou, et al., 1998), no accumulation was observed once steady state was attained. For example, women receiving multiple doses of NET-EN (5-11 injections) required a longer mean time for serum NET levels to fall below detection or 0.2 nM (152 days) than women who received only a single dose (107 days) (Fotherby, et al., 1978b). In this study, mean NET levels were higher at 90 days (2.44 nM) in multiple-injection users compared to levels at 70 days (1.22 nM) in single-dose users (Fotherby, et al., 1978b). Together these results suggest that progestin serum levels may change with duration of contraceptive use for the injectables DMPA-IM and NET-EN. However, limited data on cumulative effects exists for most HCs, while for the available data it is unclear to what extent detected differences are due to unmatched populations, given the high inter-individual variability in serum concentrations.
5.3. Effects of demographic/intrinsic factors on serum progestin levels
Studies on progestin serum levels usually record the BMI of the women in their cohort. Recent reviews on the efficacy of contraceptives in obese women (Simmons & Edelman, 2016; Stanczyk, et al., 2018) conclude that, in general, contraceptive efficacy is not affected in obese women. It does not, however, follow that progestin concentrations are not affected by BMI, since many HCs use progestin doses above the threshold needed for contraceptive efficacy (Cherala, et al., 2016). Use of BMI as an index has limitations including its inability to distinguish between muscle and fat mass and “body type”. For example, Asian women are generally smaller in height and lower in body weight compared to European women and could have similar BMIs but smaller “body type”. Comparisons based on ethnicity thus may also be comparing differences in “body type” for similar BMIs. Comparisons between ethnic groups may also be influenced by population pharmacogenetics and pharmacogenomics, such as variations in allelic frequencies of drug metabolism enzymes (Kobayashi, et al., 2000). Consistent with this, large variations in drug metabolizing enzyme allelic frequencies have been reported in African populations (Dandara, et al., 2011; Ikediobi, et al., 2011),
Results of studies investigating the relationship between progestin serum levels in women and their BMI vary for different HCs. Several studies have found a link between higher BMI and/or weight and lower progestin concentrations for women on HCs such as the ETG implant (Implanon), LNG rod (Norplant) and LNG IUD (Mirena) (Huber & Wenzl, 1998; Mornar, et al., 2012). However, studies on ETG implants in American women of different ethnicities (McNicholas, et al., 2015) and in a population of primarily Hispanic women (Morrell, et al., 2016) did not detect such a link. For women on NET-EN, DMPA-IM or DMPA-SC, most studies also find no link between BMI and progestin concentration (Fotherby & Koetsawang, 1982; Jain, et al., 2004; Lan, et al., 1984; Nanda, et al., 2016; Smit, et al., 2004). Limited data do, however, suggest a possible link between ethnicity and/or "body type” and progestin concentrations. Higher initial NET but not MPA serum levels were observed in Indian women compared to Swedish women, although sample sizes were small, and body weight, but not height or BMI, were reported (Fotherby, Saxena, et al., 1980). Since Indian women have BMIs 1-2.5 kg/m2 lower than the global average (Finucane, et al., 2011), higher serum NET levels may correlate with lower BMI. Many of the studies reporting the upper range of DMPA-IM Cmax values were carried out in Thai women (Fotherby & Koetsawang, 1982; Koetsawang, 1977; Shrimanker, et al., 1978), suggesting that women of smaller “body type” may be exposed to higher levels of progestin, with possible higher side-effect risks. However, to our knowledge there are no studies comparing Cmax for DMPA-IM in Thai and non-Thai women. Together, most studies do not show a link between BMI and progestin levels. However, there are some inconsistencies in the literature and more data is thus required. Apart from the abovementioned studies observing serum levels in relation to weight or population for select progestins, most studies have failed to address the effect of “body type” on serum progestin levels. The question whether lactation has an effect on serum progestin levels has also arisen. For MPA, two studies using RIA report lactating women receiving DMPA-IM show very high serum MPA levels of 78 nM in Chinese women (range 42-78 nM) (Fang, et al., 2004) and up to 99.6 nM in Thai women (range 24.3-99.6 nM) (Koetsawang, 1977) after the first week p.i. Interestingly, another study in non-lactating Thai women reported lower serum MPA levels of 4.4-23.3 nM one week after injection, also using RIA (Shrimanker, et al., 1978). However, to date, no direct comparison between lactating and non-lactating women of the same ethnicity receiving MPA or other progestin contraceptives has been made within the same study, using the same method of quantification. It is therefore unclear whether lactating women have different progestin serum levels than non-lactating women.
6. Conclusions and Research Gaps
Understanding metabolism and pharmacokinetics of progestogens in women is important for understanding dose requirements for contraceptive efficacy, and the potentially beneficial or detrimental effects of progestogens and/or their metabolites in women. Evidence is emerging that metabolism is influenced by the route of administration i.e. orally or parenterally, that different steroid-metabolising enzymes are expressed in peripheral tissues, that some progestins generate more metabolites than others, and that several progestin metabolites have biological activities via the PR and/or other members of the SR family. Interestingly, some progestins also generate estrogenic metabolites that may provide additional beneficial or detrimental effects. It is clear from the relatively little information available that more research is required on the identification of cell- and tissue-specific progestin metabolites, metabolizing enzymes and the biological activities of metabolites. This may be particularly relevant for susceptibility to STIs for progestins administered in the genital tract as well as for reproductive tissue cancers for all progestins and methods of administration. Most progestins have similar bioavailabilities when delivered orally but the effect of route of administration remains underexplored. Substantial data are available on progestin half-lives, which appear to sometimes depend on type and dose of progestin, route of administration, whether it is co-administered with an estrogen and body weight. Whether differences in half-life are masked by inter-individual differences are however underexplored. While some information is available on differential effects of COCs on the regulation of CBG and SHBG levels as well as endogenous steroid levels via binding to these proteins, data are scant for progestins, especially as used in progestin-only contraceptives, and no information is available for progestin metabolites. Substantial information is available about serum concentrations of some but not other progestins used in different forms of contraception. Several HCs result in serum progestin concentrations above those required for contraceptive efficacy and development of HCs using lower doses for minimal side-effects requires further investigation. Determination of real differences in serum concentrations between methods is hampered by high inter-individual variability within studies for some methods, different sampling times and methods of detection, different demographic characteristics of study populations, varying number of participants in the studies being compared, and a tendency to compare results of non-parallel studies. What is clear, however, is that high inter-individual variability in serum concentrations for some methods of contraception suggest that side-effects may be different for different women using the same method, given that SR responses are highly dose-dependent. Moreover, very little information is available about progestins or their metabolite concentrations in tissue or FGT fluids, which requires more investigation, since the relative levels may be very different and progestin-dependent. While there is evidence that duration of contraceptive use affects serum levels for some methods, the effects of intrinsic participant factors on serum levels are unclear from the literature. BMI does not appear to detectably influence MPA serum levels in injectable DMPA users, while the effect for other methods is less clear. Research gaps include addressing the effects of intrinsic factors such as body size, height and lactation on serum progestin levels. This may be particularly important for injectable contraceptives such as DMPA-IM, where side-effects may depend critically on initial peak serum concentrations which may be determined by such intrinsic factors. The identification of important questions to be investigated will depend on the particular contraceptive method and its known clinical risk profile. Basic mechanistic research to establish proof of concepts and hypotheses for testing on clinical samples would be invaluable in this regard. Minimizing confounding intrinsic factors of study participants and standardization of methodologies for sample collection and detection by high throughput methods should enable many of the above research gaps to be filled.
Acknowledgements:
The authors wish to thank the Interlibrary Loans department at the University of Cape Town for sourcing many references for A.J.B, and to Meghan Cartwright for re-drawing Figures 1-3. This work was supported by the U.S. National Institutes of Health and South African Medical Research Council through its U.S.-SA Program for Collaborative Biomedical Research (R01HD083026 and R01AI152118) to JPH. The content and findings reported herein are the sole deduction, view and responsibility of the researchers and do not reflect the official position and sentiments of the NIH and SAMRC.
Abbreviations
- BMI
body mass index
- CBG
corticosteroid binding globulin
- COC
combined oral contraceptive
- CPA
cyproterone acetate
- DNG
dienogest
- DRSP
drospirenone
- DSG
desogestrel
- E2
estradiol
- E2C
estradiol cypionate
- E2V
estradiol valerate
- EE
ethinyl estradiol
- ETG
etonogestrel
- GES
gestodene
- HC
hormonal contraception
- IM
intramuscular
- IVR
intravaginal ring
- LNG
levonorgestrel
- MA
megestrol acetate
- MPA
medroxyprogesterone acetate
- MS
mass spectrometry
- NGM
norgestimate
- NGMN
norelgestromin
- NES
nestorone
- NET
norethisterone/norethindrone
- NET-A
NET acetate
- NET-EN
NET enanthate
- NoMAc
nomegestrol acetate
- P4
progesterone
- p.i.
post-injection
- RIA
radioimmunoassay
- SC
subcutaneous
- SHBG
serum hormone binding globulin
- SR
steroid receptor
Footnotes
Conflict of Interest Statement:
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdalla KA, Shabaan MM, & Stanczyk FZ (1992). Interrelationship of serum levonorgestrel and sex hormone-binding globulin levels following vaginal and oral administration of combined steroid contraceptive tablets. Contraception, 45, 111–118. [DOI] [PubMed] [Google Scholar]
- Abrams LS, Skee DM, Natarajan J, Wong FA, & Anderson GD (2002). Pharmacokinetics of a contraceptive patch (Evra/Ortho Evra) containing norelgestromin and ethinyloestradiol at four application sites. Br J Clin Pharmacol, 53, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abujrais S, Olovsson M, Ahnoff M, Rasmusson AJ, Larsson A, Akerfeldt T, et al. (2019). A sensitive method detecting trace levels of levonorgestrel using LC-HRMS. Contraception, 100, 247–249. [DOI] [PubMed] [Google Scholar]
- Achilles SL, Hendrix CW, & Poloyac SM (2018). Safety and pharmacokinetics of dapivirine and levonorgestrel vaginal rings for multipurpose prevention of HIV and pregnancy. In HIV Research for Prevention (HIVR4P). Madrid. [Google Scholar]
- Achilles SL, Mhlanga FG, Musara P, Poloyac SM, Chirenje ZM, & Hillier SL (2018). Misreporting of contraceptive hormone use in clinical research participants. Contraception, 97, 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aedo AR, Landgren BM, Johannisson E, & Diczfalusy E (1985). Pharmacokinetic and pharmacodynamic investigations with monthly injectable contraceptive preparations. Contraception, 31, 453–469. [DOI] [PubMed] [Google Scholar]
- Africander D, Louw R, & Hapgood JP (2013). Investigating the anti-mineralocorticoid properties of synthetic progestins used in hormone therapy. Biochem Biophys Res Commun, 433, 305–310. [DOI] [PubMed] [Google Scholar]
- Africander D, Verhoog N, & Hapgood JP (2011). Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids, 76, 636–652. [DOI] [PubMed] [Google Scholar]
- Andersson S, Minjarez D, Yost NP, & Word RA (2008). Estrogen and progesterone metabolism in the cervix during pregnancy and parturition. J Clin Endocrinol Metab, 93, 2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann G, & Kopacz S (2009). Drospirenone/ethinyl estradiol 3 mg/20 mug (24/4 day regimen): hormonal contraceptive choices - use of a fourth-generation progestin. Patient Prefer Adherence, 3, 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back DJ, Bates M, Breckenridge AM, Hall JM, MacIver M, Orme ML, et al. (1981). The pharmacokinetics of levonorgestrel and ethynylestradiol in women - studies with Ovran and Ovranette. Contraception, 23, 229–239. [DOI] [PubMed] [Google Scholar]
- Back DJ, Breckenridge AM, Crawford FE, McIver M, Orme ML, Rowe PH, et al. (1978). Kinetics of norethindrone in women. II. Single-dose kinetics. Clin Pharmacol Ther, 24, 448–453. [DOI] [PubMed] [Google Scholar]
- Back DJ, Grimmer SF, Rogers S, Stevenson PJ, & Orme ML (1987). Comparative pharmacokinetics of levonorgestrel and ethinyloestradiol following intravenous, oral and vaginal administration. Contraception, 36, 471–479. [DOI] [PubMed] [Google Scholar]
- Back DJ, Grimmer SF, Shenoy N, & Orme ML (1987). Plasma concentrations of 3-keto-desogestrel after oral administration of desogestrel and intravenous administration of 3-keto-desogestrel. Contraception, 35, 619–626. [DOI] [PubMed] [Google Scholar]
- Barbieri RL, Petro Z, Canick JA, & Ryan KJ (1983). Aromatization of norethindrone to ethinyl estradiol by human placental microsomes. J Clin Endocrinol Metab, 57, 299–303. [DOI] [PubMed] [Google Scholar]
- Basaraba CN, Westhoff CL, Pike MC, Nandakumar R, & Cremers S (2017). Estimating systemic exposure to levonorgestrel from an oral contraceptive. Contraception, 95, 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassol S, Garza-Flores J, Cravioto MC, Diaz-Sanchez V, Fotherby K, Lichtenberg R, et al. (1984). Ovarian function following a single administration of depo-medroxyprogesterone acetate (DMPA) at different doses. Fertil Steril, 42, 216–222. [PubMed] [Google Scholar]
- Batukan C, & Muderris II. (2006). Efficacy of a new oral contraceptive containing drospirenone and ethinyl estradiol in the long-term treatment of hirsutism. Fertil Steril, 85, 436–440. [DOI] [PubMed] [Google Scholar]
- Baydoun E, Wahab AT, Shoaib N, Ahmad MS, Abdel-Massih R, Smith C, et al. (2016). Microbial transformation of contraceptive drug etonogestrel into new metabolites with Cunninghamella blakesleeana and Cunninghamella echinulata. Steroids, 115, 56–61. [DOI] [PubMed] [Google Scholar]
- Bayer Healthcare Pharmaceuticals Inc. (1991). Triodene ED Product Sheet. In.
- Bayer Healthcare Pharmaceuticals Inc. (2010a). Femodene® ED Product sheet. In: Bayer Inc. [Google Scholar]
- Bayer Healthcare Pharmaceuticals Inc. (2010b). Visanne Product sheet. In: Bayer Inc. [Google Scholar]
- Bayer Healthcare Pharmaceuticals Inc. (2011). NUR-ISTERATE® Product Sheet. In (pp. 1–7): Bayer Inc. [Google Scholar]
- Bayer Healthcare Pharmaceuticals Inc. (2013). Skyla® Product sheet. In: Bayer Inc. [Google Scholar]
- Bayer Healthcare Pharmaceuticals Inc. (2014). Mirena® Product sheet. In: Bayer Inc. [Google Scholar]
- Bayer Healthcare Pharmaceuticals Inc. (2016). Jadelle® Product sheet. In: Bayer Inc. [Google Scholar]
- Bayer Healthcare Pharmaceuticals Inc. (2000). Mirelle Product Sheet. In. [Google Scholar]
- Bennink HJ (2000). The pharmacokinetics and pharmacodynamics of Implanon, a single-rod etonogestrel contraceptive implant. Eur J Contracept Reprod Health Care, 5 Suppl 2, 12–20. [PubMed] [Google Scholar]
- Bergink W, Assendorp R, Kloosterboer L, van Lier W, Voortman G, & Qvist I (1990). Serum pharmacokinetics of orally administered desogestrel and binding of contraceptive progestogens to sex hormone-binding globulin. Am J Obstet Gynecol, 163, 2132–2137. [DOI] [PubMed] [Google Scholar]
- Berman JR, Almeida FG, Jolin J, Raz S, Chaudhuri G, & Gonzalez-Cadavid NF (2003). Correlation of androgen receptors, aromatase, and 5-alpha reductase in the human vagina with menopausal status. Fertil Steril, 79, 925–931. [DOI] [PubMed] [Google Scholar]
- Bhargava AS, Seeger A, & Gunzel P (1977). Isolation and identification of 15-beta-hydroxy cyproterone acetate as a new metabolite of cyproterone acetate in dog, monkey and man. Steroids, 30, 407–418. [DOI] [PubMed] [Google Scholar]
- Bhaumik UG,A; Mandal U; Chatterjee B; Kanti Sarkar A; Bose A; Kanta Ray K; Kumar Pal T,. (2008). Determination of Drospirenone in Human Plasma by LC–Tandem-MS. Chromatographia, 68, 713–720. [Google Scholar]
- Bick A, Louw-du Toit R, Skosana S, Africander D, & Hapgood J (2020). Circulating concentrations of progestins used in contraception. https://data.mendeley.com/datasets/5sck77c9b9/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blode H, Foidart JM, & Heithecker R (2001). Transfer of drospirenone to breast milk after a single oral administration of 3 mg drospirenone + 30 microg ethinylestradiol to healthy lactating women. Eur J Contracept Reprod Health Care, 6, 167–171. [PubMed] [Google Scholar]
- Blode H, Kowal K, Roth K, & Reif S (2012). Pharmacokinetics of drospirenone and ethinylestradiol in Caucasian and Japanese women. Eur J Contracept Reprod Health Care, 17, 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom MJ, Wassink MG, van Wijk F, Ederveen AG, Kloosterboer HJ, Verhoeven CH, et al. (2001). Metabolism of norethisterone and norethisterone derivatives in rat uterus, vagina, and aorta. Drug Metab Dispos, 29, 976–982. [PubMed] [Google Scholar]
- Blue SW, Winchell AJ, Kaucher AV, Lieberman RA, Gilles CT, Pyra MN, et al. (2018). Simultaneous quantitation of multiple contraceptive hormones in human serum by LC-MS/MS. Contraception, 97, 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonny AE, Lange HL, Rogers LK, Gothard DM, & Reed MD (2014). A pilot study of depot medroxyprogesterone acetate pharmacokinetics and weight gain in adolescent females. Contraception, 89, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgelt LM, & Martell CW (2012). Estradiol valerate/dienogest: a novel combined oral contraceptive. Clin Ther, 34, 37–55. [DOI] [PubMed] [Google Scholar]
- Brache V, Massai R, Mishell DR, Moo-Young AJ, Alvarez F, Salvatierra AM, et al. (2000). Ovarian function during use of Nestorone(R) subdermal implants. Contraception, 61, 199–204. [DOI] [PubMed] [Google Scholar]
- Brache V, Mishell DR, Lahteenmaki P, Alvarez F, Elomaa K, Jackanicz T, et al. (2001). Ovarian function during use of vaginal rings delivering three different doses of Nestorone. Contraception, 63, 257–261. [DOI] [PubMed] [Google Scholar]
- Buckner LR, Drobnis EZ, Augustine MS, Rogers LK, Akers J, Mott PD, et al. (2019). Cervical and systemic concentrations of long acting hormonal contraceptive (LARC) progestins depend on delivery method: Implications for the study of HIV transmission. PLoS One, 14, e0214152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R, Stanczyk F, Taylor D, Steiner M, Kopf G, & Dorflinger L (2015). Measuring total plasma levonorgestrel (LNG) levels among users of contraceptive implants: a comparison of radioimmunoassay and mass spectrometry methods. In Fertility control club hormonal contraception methods: from basic research to clinical practice. Barcelona, Spain. [Google Scholar]
- Carol W K. G; Michels W; Boer J; Pocha C (1991). Studies on the pharmacokinetics of contraceptive steroids under steady-state conditions [in German]. Zentralbl Gynakol, 113, 1298–1303. [PubMed] [Google Scholar]
- Cassidenti DL, Paulson RJ, Serafini P, Stanczyk FZ, & Lobo RA (1991). Effects of sex steroids on skin 5 alpha-reductase activity in vitro. Obstet Gynecol, 78, 103–107. [PubMed] [Google Scholar]
- Chappell CA, Lamorde M, Nakalema S, Chen BA, Mackline H, Riddler SA, et al. (2017). Efavirenz decreases etonogestrel exposure: a pharmacokinetic evaluation of implantable contraception with antiretroviral therapy. AIDS, 31, 1965–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherala G, Edelman A, Dorflinger L, & Stanczyk FZ (2016). The elusive minimum threshold concentration of levonorgestrel for contraceptive efficacy. Contraception, 94, 104–108. [DOI] [PubMed] [Google Scholar]
- Chu MC, Zhang X, Gentzschein E, Stanczyk FZ, & Lobo RA (2007). Formation of ethinyl estradiol in women during treatment with norethindrone acetate. The Journal of Clinical Endocrinology & Metabolism, 92, 2205–2207. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Surrey E, & Stanczyk FZ (2012). The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis. Reprod Sci, 19, 563–571. [DOI] [PubMed] [Google Scholar]
- Cirrincione LR, Penchala SD, Scarsi KK, Podany AT, Winchester LC, Back DJ, et al. (2018). Development, validation and utilization of a highly sensitive LC-MS/MS method for quantification of levonorgestrel released from a subdermal implant in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci, 1084, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn SE, Park JG, Watts DH, Stek A, Hitti J, Clax PA, et al. (2007). Depo-medroxyprogesterone in women on antiretroviral therapy: effective contraception and lack of clinically significant interactions. Clin Pharmacol Ther, 81, 222–227. [DOI] [PubMed] [Google Scholar]
- Cooke ID, Back DJ, & Shroff NE (1985). Norethisterone concentration in breast milk and infant and maternal plasma during ethynodiol diacetate administration. Contraception, 31, 611–621. [DOI] [PubMed] [Google Scholar]
- Croxatto HB (2002). Mechanisms that explain the contraceptive action of progestin implants for women. Contraception, 65, 21–27. [DOI] [PubMed] [Google Scholar]
- Croxatto HB, Diaz S, Brandeis A, Pavez M, & Johansson ED (1985). Plasma levonorgestrel and progesterone levels in women treated with silastic covered rods containing levonorgestrel. Contraception, 31, 643–654. [DOI] [PubMed] [Google Scholar]
- Dandara C, Lombard Z, Du Plooy I, McLellan T, Norris SA, & Ramsay M (2011). Genetic variants in CYP (−1A2, −2C9, −2C19, −3A4 and −3A5), VKORC1 and ABCB1 genes in a black South African population: a window into diversity. Pharmacogenomics, 12, 1663–1670. [DOI] [PubMed] [Google Scholar]
- Darney PD, Taylor RN, Klaisle C, Bottles K, & Zaloudek C (1996). Serum concentrations of estradiol, progesterone, and levonorgestrel are not determinants of endometrial histology or abnormal bleeding in long-term Norplant implant users. Contraception, 53, 97–100. [DOI] [PubMed] [Google Scholar]
- Dassen H, Punyadeera C, Kamps R, Delvoux B, Van Langendonckt A, Donnez J, et al. (2007). Estrogen metabolizing enzymes in endometrium and endometriosis. Hum Reprod, 22, 3148–3158. [DOI] [PubMed] [Google Scholar]
- Depypere HT, Stanczyk FZ, Croubels S, Blondeel PN, Roche NA, Depypere BP, et al. (2019). Breast levonorgestrel concentrations in women using a levonorgestrel-releasing intrauterine system. Contraception. [DOI] [PubMed] [Google Scholar]
- Di Carlo F, Gallo E, Conti G, & Racca S (1983). Changes in the binding of oestradiol to uterine oestrogen receptors induced by some progesterone and 19-nor-testosterone derivatives. J Endocrinol, 98, 385–389. [DOI] [PubMed] [Google Scholar]
- Dibbelt L, Knuppen R, Kuhnz W, & Jutting G (1992). Pharmacokinetics and protein binding of gestodene under treatment with a low-dose combination oral contraceptive for three months. Arzneimittelforschung, 42, 1146–1152. [PubMed] [Google Scholar]
- Dikkeschei LD, van Veelen H, Nagel GT, Willemse PH, & Wolthers BG (1985). Specific and sensitive determination of medroxyprogesterone acetate in human serum by gas chromatography-mass spectrometry. J Chromatogr, 345, 1–10. [DOI] [PubMed] [Google Scholar]
- Dogterom P, van den Heuvel MW, & Thomsen T (2005). Absence of pharmacokinetic interactions of the combined contraceptive vaginal ring NuvaRing with oral amoxicillin or doxycycline in two randomised trials. Clin Pharmacokinet, 44, 429–438. [DOI] [PubMed] [Google Scholar]
- Edelman AB, Carlson NE, Cherala G, Munar MY, Stouffer RL, Cameron JL, et al. (2009). Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception, 80, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman AB, Cherala G, Munar MY, McInnis M, Stanczyk FZ, & Jensen JT (2014). Correcting oral contraceptive pharmacokinetic alterations due to obesity: a randomized controlled trial. Contraception, 90, 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman AB, Cherala G, & Stanczyk FZ (2010). Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review. Contraception, 82, 314–323. [DOI] [PubMed] [Google Scholar]
- Enfield K, Cartwright M, Toit RL, Avenant C, Africander D, & Hapgood JP (2020). Characterisation of progestins used in hormonal contraception and progesterone via the progesterone receptor. Biochem Biophys Res Commun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. (2019). HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet, 394, 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Family Planning 2020 (FP2020). (2014). Novel Agreement Expands Access to Pfizer’s Contraceptive, Sayana® Press, for Women Most in Need in the World’s Poorest Countries. In.
- Fang S, Sun D, Jiang H, & Luo H (2004). Concentration changes of medroxyprogesterone acetate in serum and milk in lactating women who used depo geston. J Reprod Contraception, 15, 157–162. [Google Scholar]
- Fels H, Steward R, Melamed A, Granat A, Stanczyk FZ, & Mishell DR Jr. (2013). Comparison of serum and cervical mucus hormone levels during hormone-free interval of 24/4 vs. 21/7 combined oral contraceptives. Contraception, 87, 732–737. [DOI] [PubMed] [Google Scholar]
- Fenton C, Wellington K, Moen MD, & Robinson DM (2007). Drospirenone/ethinylestradiol 3mg/20microg (24/4 day regimen): a review of its use in contraception, premenstrual dysphoric disorder and moderate acne vulgaris. Drugs, 67, 1749–1765. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. (2011). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet, 377, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer K, van Vliet HA, Rosendaal FR, Rosing J, Tchaikovski S, & Helmerhorst FM (2009). Effects of the contraceptive patch, the vaginal ring and an oral contraceptive on APC resistance and SHBG: a cross-over study. Thromb Res, 123, 429–435. [DOI] [PubMed] [Google Scholar]
- Foster RH, & Wilde MI (1998). Dienogest. Drugs, 56, 825–833; discussion 834-825. [DOI] [PubMed] [Google Scholar]
- Fotherby K (1990). Potency and pharmacokinetics of gestagens. Contraception, 41, 533–550. [DOI] [PubMed] [Google Scholar]
- Fotherby K, Howard G, Shrimanker K, Elder M, & Bye PG (1978a). Occurrence of ovulation in women receiving the injectable contraceptive norethisterone oenanthate. Contraception, 18, 535–542. [DOI] [PubMed] [Google Scholar]
- Fotherby K, Howard G, Shrimanker K, Elder M, & Bye PG (1978b). Plasma levels of norethisterone after single and multiple injections of norethisterone oenanthate. Contraception, 18, 1–6. [DOI] [PubMed] [Google Scholar]
- Fotherby K, & Koetsawang S (1982). Metabolism of injectable formulations of contraceptive steroids in obese and thin women. Contraception, 26, 51–58. [DOI] [PubMed] [Google Scholar]
- Fotherby K, Koetsawang S, & Mathrubutham M (1980). Pharmacokinetic study of different doses of Depo Provera. Contraception, 22, 527–536. [DOI] [PubMed] [Google Scholar]
- Fotherby K, Saxena BN, Shrimanker K, Hingorani V, Takker D, Diczfalusy E, et al. (1980). A preliminary pharmacokinetic and pharmacodynamic evaluation of depot-medroxyprogesterone acetate and norethisterone oenanthate. Fertil Steril, 34, 131–139. [DOI] [PubMed] [Google Scholar]
- Fraser IS, Weisberg E, Brache V, Alvarez F, Massai R, Mishell DR Jr., et al. (2005). Serum Nestorone and ethinyl estradiol levels, and ovulation inhibition in women using three different dosage combinations of a Nestorone progestogen-ethinyl estradiol contraceptive vaginal ring on a bleeding-signaled regimen. Contraception, 72, 40–45. [DOI] [PubMed] [Google Scholar]
- Fraser IS, Weisberg E, Kumar N, Kumar S, Humberstone AJ, McCrossin L, et al. (2007). An initial pharmacokinetic study with a Metered Dose Transdermal Systemfor delivery of the progestogen Nestorone as a possible future contraceptive. Contraception, 76, 432–438. [DOI] [PubMed] [Google Scholar]
- Frey R, Unger S, van der Mey D, Becker C, Saleh S, Wensing G, et al. (2016). Pharmacokinetic interaction study between riociguat and the combined oral contraceptives levonorgestrel and ethinylestradiol in healthy postmenopausal women. Pulm Circ, 6, S97–S102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C, Berse M, Klein S, Rohde B, & Hochel J (2018). In Vivo Formation of Ethinylestradiol After Intramuscular Administration of Norethisterone Enantate. J Clin Pharmacol, 58, 781–789. [DOI] [PubMed] [Google Scholar]
- Fukushima DK, levin J, Liang JS, & Smulowitz M (1979). Isolation and partial synthesis of a new metabolite of medroxyrogesterone acetate. Steroids, 34, 57–72. [DOI] [PubMed] [Google Scholar]
- Garcia-Becerra R, Borja-Cacho E, Cooney AJ, Jackson KJ, Lemus AE, Perez-Palacios G, et al. (2002). The intrinsic transcriptional estrogenic activity of a non-phenolic derivative of levonorgestrel is mediated via the estrogen receptor-alpha. J Steroid Biochem Mol Biol, 82, 333–341. [DOI] [PubMed] [Google Scholar]
- Garcia-Becerra R, Cooney AJ, Borja-Cacho E, Lemus AE, Perez-Palacios G, & Larrea F (2004). Comparative evaluation of androgen and progesterone receptor transcription selectivity indices of 19-nortestosterone-derived progestins. J Steroid Biochem Mol Biol, 91, 21–27. [DOI] [PubMed] [Google Scholar]
- Gentile DM, Verhoeven CH, Shimada T, & Back DJ (1998). The role of CYP2C in the in vitro bioactivation of the contraceptive steroid desogestrel. J Pharmacol Exp Ther, 287, 975–982. [PubMed] [Google Scholar]
- Gerrits MG, Schnabel PG, Post TM, & Peeters PA (2013). Pharmacokinetic profile of nomegestrol acetate and 17beta-estradiol after multiple and single dosing in healthy women. Contraception, 87, 193–200. [DOI] [PubMed] [Google Scholar]
- Gibson DA, McInnes KJ, Critchley HO, & Saunders PT (2013). Endometrial Intracrinology--generation of an estrogen-dominated microenvironment in the secretory phase of women. J Clin Endocrinol Metab, 98, E1802–1806. [DOI] [PubMed] [Google Scholar]
- Goebelsmann U, Stanczyk FZ, Brenner PF, Goebelsmann AE, Gentzschein EK, & Mishell DR Jr. (1979). Serum norethindrone (NET) concentrations following intramuscular NET enanthate injection. Effect upon serum LH, FSH, estradiol and progesterone. Contraception, 19, 283–313. [DOI] [PubMed] [Google Scholar]
- Halpern V, Combes SL, Dorflinger LJ, Weiner DH, & Archer DF (2014). Pharmacokinetics of subcutaneous depot medroxyprogesterone acetate injected in the upper arm. Contraception, 89, 31–35. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Abrams LS, Creasy GW, Natarajan J, Allen JG, & Siiteri PK (2003). Serum distribution of the major metabolites of norgestimate in relation to its pharmacological properties. Contraception, 67, 93–99. [DOI] [PubMed] [Google Scholar]
- Hammond GL, Lahteenmaki PL, Lahteenmaki P, & Luukkainen T (1982). Distribution and percentages of non-protein bound contraceptive steroids in human serum. J Steroid Biochem, 17, 375–380. [DOI] [PubMed] [Google Scholar]
- Hapgood JP, Africander D, Louw R, Ray RM, & Rohwer JM (2014). Potency of progestogens used in hormonal therapy: toward understanding differential actions. J Steroid Biochem Mol Biol, 142, 39–47. [DOI] [PubMed] [Google Scholar]
- Hapgood JP, Kaushic C, & Hel Z (2018). Hormonal Contraception and HIV-1 Acquisition: Biological Mechanisms. Endocr Rev, 39, 36–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapgood JP, Koubovec D, Louw A, & Africander D (2004). Not all progestins are the same: implications for usage. Trends Pharmacol Sci, 25, 554–557. [DOI] [PubMed] [Google Scholar]
- Heffron R, Achilles SL, Dorflinger LJ, Hapgood JP, Kiarie J, Polis CB, et al. (2019). Pharmacokinetic, biologic and epidemiologic differences in MPA- and NET-based progestin-only injectable contraceptives relative to the potential impact on HIV acquisition in women. Contraception, 99, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron R, Mugo N, Were E, Kiarie J, Bukusi EA, Mujugira A, et al. (2014). Preexposure prophylaxis is efficacious for HIV-1 prevention among women using depot medroxyprogesterone acetate for contraception. AIDS, 28, 2771–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron R, Parikh UM, Penrose KJ, Mugo N, Donnell D, Celum C, et al. (2017). Objective Measurement of Inaccurate Condom Use Reporting Among Women Using Depot Medroxyprogesterone Acetate for Contraception. AIDS Behav, 21, 2173–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikinheimo O, Noe G, Haukkamaa M, & Lahteenmaki P (1994). The progestin ST 1435--rapid metabolism in man. Contraception, 50, 275–289. [DOI] [PubMed] [Google Scholar]
- Helmreich ML, & Huseby RA (1962). Identification of a 6,21-dihydroxylated metabolite of medroxyprogesterone acetate in human urine. J Clin Endocrinol Metab, 22, 1018–1032. [DOI] [PubMed] [Google Scholar]
- Hidalgo MM, Hidalgo-Regina C, Bahamondes MV, Monteiro I, Petta CA, & Bahamondes L (2009). Serum levonorgestrel levels and endometrial thickness during extended use of the levonorgestrel-releasing intrauterine system. Contraception, 80, 84–89. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Stanczyk FZ, Goebelsmann U, Brenner PF, Lumkin ME, & Mishell DR Jr. (1975). Radioimmunoassay of serum medroxyprogesterone acetate (Provera) in women following oral and intravaginal administration. Steroids, 26, 373–386. [DOI] [PubMed] [Google Scholar]
- Hiroi T, Kishimoto W, Chow T, Imaoka S, Igarashi T, & Funae Y (2001). Progesterone oxidation by cytochrome P450 2D isoforms in the brain. Endocrinology, 142, 3901–3908. [DOI] [PubMed] [Google Scholar]
- Huber J, & Wenzl R (1998). Pharmacokinetics of Implanon. An integrated analysis. Contraception, 58, 85S–90S. [DOI] [PubMed] [Google Scholar]
- Humpel M, Wendt H, Pommerenke G, Weiss C, & Speck U (1978). Investigations of pharmacokinetics of levonorgestrel to specific consideration of a possible first-pass effect in women. Contraception, 17, 207–220. [DOI] [PubMed] [Google Scholar]
- Humpel M, Wendt H, Schulze PE, Dogs G, Weiss C, & Speck U (1977). Bioavailability and pharmacokinetics of cyproterone acetate after oral administration of 2.0 mg cyproterone acetate in combination with 50 micrograms ethinyloestradiol to 6 young women. Contraception, 15, 579–588. [DOI] [PubMed] [Google Scholar]
- Humpel M, W. H; Dogs G; Weis C; Rietz S; Speck U (1977). Intraindividual comparison of pharmacokinetic parameters of d-Norgestrel, lynestrol and cyproterone acetate in 6 women. Contraception, 16, 199–215. [Google Scholar]
- Ikediobi O, Aouizerat B, Xiao Y, Gandhi M, Gebhardt S, & Warnich L (2011). Analysis of pharmacogenetic traits in two distinct South African populations. Hum Genomics, 5, 265–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen BM, & Horwitz KB (2012). Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol, 357, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain J, Dutton C, Nicosia A, Wajszczuk C, Bode FR, & Mishell DR Jr. (2004). Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera. Contraception, 70, 11–18. [DOI] [PubMed] [Google Scholar]
- Jarvinen T, Keinonen T, Auriola S, Peura P, Hirvonen E, & Palva E (1989). Specific and sensitive quantitation of medroxyprogesterone acetate in human serum by gas chromatography-mass spectrometry. J Chromatogr, 495, 13–20. [DOI] [PubMed] [Google Scholar]
- Jensen JT, Edelman AB, Chen BA, Archer DF, Barnhart KT, Thomas MA, et al. (2018). Continuous dosing of a novel contraceptive vaginal ring releasing Nestorone(R) and estradiol: pharmacokinetics from a dose-finding study. Contraception, 97, 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppsson S, Gershagen S, Johansson ED, & Rannevik G (1982). Plasma levels of medroxyprogesterone acetate (MPA), sex-hormone binding globulin, gonadal steroids, gonadotrophins and prolactin in women during long-term use of depo-MPA (Depo-Provera) as a contraceptive agent. Acta Endocrinol (Copenh), 99, 339–343. [DOI] [PubMed] [Google Scholar]
- Jin Y, Mesaros AC, Blair IA, & Penning TM (2011). Stereospecific reduction of 5beta-reduced steroids by human ketosteroid reductases of the AKR (aldo-keto reductase) superfamily: role of AKR1C1-AKR1C4 in the metabolism of testosterone and progesterone via the 5beta-reductase pathway. Biochem J, 437, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, & Penning TM (2001). Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab, 15, 79–94. [DOI] [PubMed] [Google Scholar]
- Juchem M, Pollow K, Elger W, Hoffmann G, & Mobus V (1993). Receptor binding of norgestimate--a new orally active synthetic progestational compound. Contraception, 47, 283–294. [DOI] [PubMed] [Google Scholar]
- Jung-Hoffmann C, Storch A, & Kuhl H (1992). Serum concentrations of ethinylestradiol, 3-keto-desogestrel, SHBG, CBG and gonadotropins during treatment with a biphasic oral contraceptive containing desogestrel. Horm Res, 38, 184–189. [DOI] [PubMed] [Google Scholar]
- Kaiser DG, Carlson RG, & Kirton KT (1974). GLC determination of medroxyprogesterone acetate in plasma. J Pharm Sci, 63, 420–424. [DOI] [PubMed] [Google Scholar]
- Kaunitz AM (2001). Lunelle monthly injectable contraceptive. An effective, safe, and convenient new birth control option. Arch Gynecol Obstet, 265, 119–123. [DOI] [PubMed] [Google Scholar]
- Kaunitz AM, Darney PD, Ross D, Wolter KD, & Speroff L (2009). Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception, 80, 7–17. [DOI] [PubMed] [Google Scholar]
- Kerdar RS, Baumann A, Brudny-Kloppel M, Biere H, Blode H, & Kuhnz W (1995). Identification of 3 alpha-hydroxy-cyproterone acetate as a metabolite of cyproterone acetate in the bile of female rats and the potential of this and other already known or putative metabolites to form DNA adducts in vitro. Carcinogenesis, 16, 1835–1841. [DOI] [PubMed] [Google Scholar]
- Kirton KT, & Cornette JC (1974). Return of ovulatory cyclicity following an intramuscular injection of medroxyprogesterone acetate (Provera). Contraception, 10, 39–45. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Mimura N, Fujii H, Minami H, Sasaki Y, Shimada N, et al. (2000). Role of human cytochrome P450 3A4 in metabolism of medroxyprogesterone acetate. Clin Cancer Res, 6, 3297–3303. [PubMed] [Google Scholar]
- Koetsawang S (1977). Injected long--acting medroxyprogesterone acetate. Effect on human lactation and concentrations in milk. J Med Assoc Thai, 60, 57–60. [PubMed] [Google Scholar]
- Koetsawang S, Shrimanker K, & Fotherby K (1979). Blood levels of medroxyprogesterone acetate after multiple injections of depoprovera or cycloprovera. Contraception, 20, 1–4. [DOI] [PubMed] [Google Scholar]
- Konings G, Brentjens L, Delvoux B, Linnanen T, Cornel K, Koskimies P, et al. (2018). Intracrine Regulation of Estrogen and Other Sex Steroid Levels in Endometrium and Non-gynecological Tissues; Pathology, Physiology, and Drug Discovery. Front Pharmacol, 9, 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook K, Gabelnick H, & Duncan G (2002). Pharmacokinetics of levonorgestrel 0.75 mg tablets. Contraception, 66, 73–76. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Tolonen A, Uusitalo J, Lundgren S, Jalonen J, & Laine K (2005). The role of CYP2C and CYP3A in the disposition of 3-keto-desogestrel after administration of desogestrel. Br J Clin Pharmacol, 60, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubovec D, Ronacher K, Stubsrud E, Louw A, & Hapgood JP (2005). Synthetic progestins used in HRT have different glucocorticoid agonist properties. Mol Cell Endocrinol, 242, 23–32. [DOI] [PubMed] [Google Scholar]
- Krattenmacher R (2000). Drospirenone: pharmacology and pharmacokinetics of a unique progestogen. Contraception, 62, 29–38. [DOI] [PubMed] [Google Scholar]
- Kuhl H (1990). Pharmacokinetics of oestrogens and progestogens. Maturitas, 12, 171–197. [DOI] [PubMed] [Google Scholar]
- Kuhl H (2005). Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric, 8, 3–63. [DOI] [PubMed] [Google Scholar]
- Kuhl H (2011). Pharmacology of Progestogens. J Reproduktionsmed Endokrinol, 8, 157–176. [Google Scholar]
- Kuhl H, Bremser HJ, & Taubert HD (1982). Serum levels and pharmacokinetics of norethisterone after ingestion of lynestrenol: its relation to dose and stage of the menstrual cycle. Contraception, 26, 303–315. [DOI] [PubMed] [Google Scholar]
- Kuhl H, Jung-Hoffmann C, & Fitzner M (1995). Prodrug versus drug effects of 150 micrograms desogestrel or 3-keto-desogestrel in combination with 30 micrograms ethinylestradiol on hormonal parameters: relevance of the peak serum level of 3-keto-desogestrel. Horm Res, 44, 126–132. [DOI] [PubMed] [Google Scholar]
- Kuhl H, Jung-Hoffmann C, & Heidt F (1988a). Alterations in the serum levels of gestodene and SHBG during 12 cycles of treatment with 30 micrograms ethinylestradiol and 75 micrograms gestodene. Contraception, 38, 477–486. [DOI] [PubMed] [Google Scholar]
- Kuhl H, Jung-Hoffmann C, & Heidt F (1988b). Serum levels of 3-keto-desogestrel and SHBG during 12 cycles of treatment with 30 micrograms ethinylestradiol and 150 micrograms desogestrel. Contraception, 38, 381–390. [DOI] [PubMed] [Google Scholar]
- Kuhl H, & Wiegratz I (2007). Can 19-nortestosterone derivatives be aromatized in the liver of adult humans? Are there clinical implications? Climacteric, 10, 344–353. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, al-Yacoub G, & Fuhrmeister A (1992). Pharmacokinetics of levonorgestrel and ethinylestradiol in 9 women who received a low-dose oral contraceptive over a treatment period of 3 months and, after a wash-out phase, a single oral administration of the same contraceptive formulation. Contraception, 46, 455–469. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, al-Yacoub G, Power J, Ormesher SE, Back DJ, & Jutting G (1992). Pharmacokinetics and serum protein binding of 3-keto-desogestrel in women during three cycles of treatment with a low-dose combination oral contraceptive. Arzneimittelforschung, 42, 1142–1146. [PubMed] [Google Scholar]
- Kuhnz W, Baumann A, Staks T, Dibbelt L, Knuppen R, & Jutting G (1993). Pharmacokinetics of gestodene and ethinylestradiol in 14 women during three months of treatment with a new tri-step combination oral contraceptive: serum protein binding of gestodene and influence of treatment on free and total testosterone levels in the serum. Contraception, 48, 303–322. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, Blode H, & Mahler M (1994). Systemic availability of levonorgestrel after single oral administration of a norgestimate-containing combination oral contraceptive to 12 young women. Contraception, 49, 255–263. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, Heuner A, Hümpel M, Seifert W, & Michaelis K (1997). In vivo conversion of norethisterone and norethisterone acetate to ethinyl etradiol in postmenopausal women. Contraception, 56, 379–385. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, Pfeffer M, & al-Yacoub G (1990). Protein binding of the contraceptive steroids gestodene, 3-keto-desogestrel and ethinylestradiol in human serum. J Steroid Biochem, 35, 313–318. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, Schutt B, Power J, & Back DJ (1992). Pharmacokinetics and serum protein binding of gestodene and 3-keto-desogestrel in women after single oral administration of two different contraceptive formulations. Arzneimittelforschung, 42, 1139–1141. [PubMed] [Google Scholar]
- Kuhnz W, Staks T, & Jutting G (1993). Pharmacokinetics of cyproterone acetate and ethinylestradiol in 15 women who received a combination oral contraceptive during three treatment cycles. Contraception, 48, 557–575. [DOI] [PubMed] [Google Scholar]
- Kuhnz W, Staks T, & Jutting G (1994). Pharmacokinetics of levonorgestrel and ethinylestradiol in 14 women during three months of treatment with a tri-step combination oral contraceptive: serum protein binding of levonorgestrel and influence of treatment on free and total testosterone levels in the serum. Contraception, 50, 563–579. [DOI] [PubMed] [Google Scholar]
- Kumar N, Fagart J, Liere P, Mitchell SJ, Knibb AR, Petit-Topin I, et al. (2017). Nestorone(R) as a Novel Progestin for Nonoral Contraception: Structure-Activity Relationships and Brain Metabolism Studies. Endocrinology, 158, 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Koide SS, Tsong Y, & Sundaram K (2000). Nestorone: a progestin with a unique pharmacological profile. Steroids, 65, 629–636. [DOI] [PubMed] [Google Scholar]
- Lahteenmaki P, Toivonen J, & Lahteenmaki PL (1983). Postabortal contraception with norethisterone enanthate injections. Contraception, 27, 553–562. [DOI] [PubMed] [Google Scholar]
- Lahteenmaki P, Weiner E, Lahteenmaki P, Johansson E, & Luukkainen T (1981). Contraception with subcutaneous capsules containing ST-1435. Pituitary and ovarian function and plasma levels of ST-1435. Contraception, 23, 63–75. [DOI] [PubMed] [Google Scholar]
- Lahteenmaki P, Weiner E, Lahteenmaki P, Johansson ED, & Luukkainen T (1982). Pituitary and ovarian function during contraception with one subcutaneous implant releasing a progestin, ST-1435. Contraception, 25, 299–306. [DOI] [PubMed] [Google Scholar]
- Lan PT, Aedo AR, Landgren BM, Johannisson E, & Diczfalusy E (1984). Return of ovulation following a single injection of depo-medroxyprogesterone acetate: a pharmacokinetic and pharmacodynamic study. Contraception, 29, 1–18. [DOI] [PubMed] [Google Scholar]
- Larrea F, Garcia-Becerra R, Lemus AE, Garcia GA, Perez-Palacios G, Jackson KJ, et al. (2001). A-ring reduced metabolites of 19-nor synthetic progestins as subtype selective agonists for ER alpha. Endocrinology, 142, 3791–3799. [DOI] [PubMed] [Google Scholar]
- Laszlo CF, Paz Montoya J, Shamseddin M, De Martino F, Beguin A, Nellen R, et al. (2019). A high resolution LC-MS targeted method for the concomitant analysis of 11 contraceptive progestins and 4 steroids. J Pharm Biomed Anal, 175, 112756. [DOI] [PubMed] [Google Scholar]
- Laurikka-Routti M, & Haukkamaa M (1992). A contraceptive subdermal implant releasing the progestin ST-1435: ovarian function, bleeding patterns, and side effects. Fertil Steril, 58, 1142–1147. [PubMed] [Google Scholar]
- Lemus AE, Enriquez J, Hernandez A, Santillan R, & Perez-Palacios G (2009). Bioconversion of norethisterone, a progesterone receptor agonist into estrogen receptor agonists in osteoblastic cells. J Endocrinol, 200, 199–206. [DOI] [PubMed] [Google Scholar]
- Lemus AE, Santillan R, Damian-Matsumura P, Garcia GA, Grillasca I, & Perez-Palacios G (2001). In vitro metabolism of gestodene in target organs: formation of A-ring reduced derivatives with oestrogenic activity. Eur J Pharmacol, 417, 249–256. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Wiebe JP, & Heathcote JG (2004). Expression of progesterone metabolizing enzyme genes (AKR1C1, AKR1C2, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma. BMC Cancer, 4, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licea-Perez H, Wang S, Bowen CL, & Yang E (2007). A semi-automated 96-well plate method for the simultaneous determination of oral contraceptives concentrations in human plasma using ultra performance liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 852, 69–76. [DOI] [PubMed] [Google Scholar]
- Louw-du Toit R, Hapgood JP, & Africander D (2020). A direct comparison of the transcriptional activities of progestins used in contraception and menopausal hormone therapy via the mineralocorticoid receptor. Biochem Biophys Res Commun, 526, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw-du Toit R, Perkins MS, Hapgood JP, & Africander D (2017). Comparing the androgenic and estrogenic properties of progestins used in contraception and hormone therapy. Biochem Biophys Res Commun, 491, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch T, & Price A (2007). The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician, 76, 391–396. [PubMed] [Google Scholar]
- Madden S, Back DJ, & Orme ML (1990). Metabolism of the contraceptive steroid desogestrel by human liver in vitro. J Steroid Biochem, 35, 281–288. [DOI] [PubMed] [Google Scholar]
- Makarainen L, van Beek A, Tuomivaara L, Asplund B, & Coelingh Bennink H (1998). Ovarian function during the use of a single contraceptive implant: Implanon compared with Norplant. Fertil Steril, 69, 714–721. [DOI] [PubMed] [Google Scholar]
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A, & Lee J (2017). Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev, 1, CD004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel C, Rheaume E, Takahashi M, Trudel C, Couet J, Luu-The V, et al. (1992). Distribution of 17 beta-hydroxysteroid dehydrogenase gene expression and activity in rat and human tissues. J Steroid Biochem Mol Biol, 41, 597–603. [DOI] [PubMed] [Google Scholar]
- Massai MR, Diaz S, Quinteros E, Reyes MV, Herreros C, Zepeda A, et al. (2001). Contraceptive efficacy and clinical performance of Nestorone implants in postpartum women. Contraception, 64, 369–376. [DOI] [PubMed] [Google Scholar]
- Massai R, Diaz S, Jackanicz T, & Croxatto HB (2000). Vaginal rings for contraception in lactating women. Steroids, 65, 703–707. [DOI] [PubMed] [Google Scholar]
- McCormack PL (2010). Dienogest: a review of its use in the treatment of endometriosis. Drugs, 70, 2073–2088. [DOI] [PubMed] [Google Scholar]
- McGuire JL, Phillips A, Hahn DW, Tolman EL, Flor S, & Kafrissen ME (1990). Pharmacologic and pharmacokinetic characteristics of norgestimate and its metabolites. Am J Obstet Gynecol, 163, 2127–2131. [DOI] [PubMed] [Google Scholar]
- McNicholas C, Maddipati R, Zhao Q, Swor E, & Peipert JF (2015). Use of the etonogestrel implant and levonorgestrel intrauterine device beyond the U.S. Food and Drug Administration-approved duration. Obstet Gynecol, 125, 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck and Co. Inc. (2017). Implanon® Product sheet. In (pp. 1–27): Merck & Co. Inc. [Google Scholar]
- Milano G, Carle G, Renee N, Boublil JL, & Namer M (1982). Determination of medroxyprogesterone acetate in plasma by high-performance liquid chromatography. J Chromatogr, 232, 413–417. [DOI] [PubMed] [Google Scholar]
- Miller WL, & Auchus RJ (2011). The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev, 32, 81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misao R, Nakanishi Y, Fujimoto J, & Tamaya T (1998a). Effect of medroxyprogesterone acetate on sex hormone-binding globulin mRNA expression in the human endometrial cancer cell line Ishikawa. Eur J Endocrinol, 138, 574–582. [DOI] [PubMed] [Google Scholar]
- Misao R, Nakanishi Y, Fujimoto J, & Tamaya T (1998b). Effects of sex steroid hormones on corticosteroid-binding globulin gene expression in human endometrial cancer cell line Ishikawa. Ann Clin Biochem, 35 (Pt 5), 637–642. [DOI] [PubMed] [Google Scholar]
- Mishell DR Jr. (1996). Pharmacokinetics of depot medroxyprogesterone acetate contraception. J Reprod Med, 41, 381–390. [PubMed] [Google Scholar]
- Mornar S, Chan LN, Mistretta S, Neustadt A, Martins S, & Gilliam M (2012). Pharmacokinetics of the etonogestrel contraceptive implant in obese women. Am J Obstet Gynecol, 207, 110 e111–116. [DOI] [PubMed] [Google Scholar]
- Morrell KM, Cremers S, Westhoff CL, & Davis AR (2016). Relationship between etonogestrel level and BMI in women using the contraceptive implant for more than 1 year. Contraception, 93, 263–265. [DOI] [PubMed] [Google Scholar]
- Morrison CS, Chen PL, Kwok C, Baeten JM, Brown J, Crook AM, et al. (2015). Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS Med, 12, e1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueck AO, & Sitruk-Ware R (2011). Nomegestrol acetate, a novel progestogen for oral contraception. Steroids, 76, 531–539. [DOI] [PubMed] [Google Scholar]
- Munro CJ, Laughlin LS, VonSchalscha T, Baldwin DM, & Lasley BL (1996). An enzyme immunoassay for serum and urinary levonorgestrel in human and non-human primates. Contraception, 54, 43–53. [DOI] [PubMed] [Google Scholar]
- Nanda K, Callahan R, Taylor D, Wang M, Agot K, Jenkins D, et al. (2016). Medroxyprogesterone acetate levels among Kenyan women using depot medroxyprogesterone acetate in the FEM-PrEP trial. Contraception, 94, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasaka T, Moriya T, Endoh M, Suzuki T, Shizawa S, Mizokami Y, et al. (2000). 17Beta-hydroxysteroid dehydrogenase type 2 and dehydroepiandrosterone sulfotransferase in the human liver. Endocr J, 47, 697–705. [DOI] [PubMed] [Google Scholar]
- National Department of Health, & ICF. (2019). South Africa Demographic and Health Survey 2016. In. Pretoria: National Department of Health - NDoH - ICF. [Google Scholar]
- Naz H, Islam A, Waheed A, Sly WS, Ahmad F, & Hassan I (2013). Human beta-glucuronidase: structure, function, and application in enzyme replacement therapy. Rejuvenation Res, 16, 352–363. [DOI] [PubMed] [Google Scholar]
- Newton R, Leigh R, & Giembycz MA (2010). Pharmacological strategies for improving the efficacy and therapeutic ratio of glucocorticoids in inflammatory lung diseases. Pharmacol Ther, 125, 286–327. [DOI] [PubMed] [Google Scholar]
- Nilsson CG, Haukkamaa M, Vierola H, & Luukkainen T (1982). Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol (Oxf), 17, 529–536. [DOI] [PubMed] [Google Scholar]
- Noe G, Salvatierra A, Heikinheimo O, Maturana X, & Croxatto HB (1993). Pharmacokinetics and bioavailability of ST 1435 administered by different routes. Contraception, 48, 548–556. [DOI] [PubMed] [Google Scholar]
- Oakley RH, & Cidlowski JA (2013). The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol, 132, 1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettel M, Carol W, Graser T, Klinger G, Mellinger U, Moore C, et al. (1997). [Effect of ethinyl estradiol-dienogest combination on serum androgen concentrations]. Zentralbl Gynakol, 119, 597–606. [PubMed] [Google Scholar]
- Oettel M B.-M. S; Elger W; Golbs S; Hobe G; Kaufmann G; Mathieu M; Moore C; Schneider B; Puri C; Ritter P; Redderson G; Schon R; Strauch G; Zimmermann H,. (1995). A 19-norprogestin without a 17a-ethinyl group II: dienogest from a pharmacokinetic point of view. Drugs of Today, 31, 499–516. [Google Scholar]
- Organon USA Inc. (2005). NuvaRing® Product Sheet. In.
- Orme M, Back DJ, Ward S, & Green S (1991). The pharmacokinetics of ethynylestradiol in the presence and absence of gestodene and desogestrel. Contraception, 43, 305–316. [DOI] [PubMed] [Google Scholar]
- Ortho-McNeil-Janssen Pharmaceuticals Inc. (2010). Ortho Evra Product Sheet. In.
- Ortiz A, Hirol M, Stanczyk FZ, Goebelsmann U, & Mishell DR (1977). Serum medroxyprogesterone acetate (MPA) concentrations and ovarian function following intramuscular injection of depo-MPA. J Clin Endocrinol Metab, 44, 32–38. [DOI] [PubMed] [Google Scholar]
- PATH. (2017). Advocacy Pack for Subcutaneous DMPA. In: Washington, DC: PATH. [Google Scholar]
- Perez-Campos EF (2010). Ethinylestradiol/dienogest in oral contraception. Drugs, 70, 681–689. [DOI] [PubMed] [Google Scholar]
- Perez-Lopez FR (2008). Clinical experiences with drospirenone: from reproductive to postmenopausal years. Maturitas, 60, 78–91. [DOI] [PubMed] [Google Scholar]
- Perkins MS, Louw-du Toit R, & Africander D (2017). A comparative characterization of estrogens used in hormone therapy via estrogen receptor (ER)-alpha and -beta. J Steroid Biochem Mol Biol, 174, 27–39. [DOI] [PubMed] [Google Scholar]
- Pfizer. (2005). Noriday Product Sheet. In. [Google Scholar]
- Pfizer. (2016a). Depo-SubQ Provera104 Spec sheet. In: Pharmacia and Upjohn Company. [Google Scholar]
- Pfizer. (2016b). Package leaflet: Information for the user Depo-Provera® 150 mg/ml Suspension for Injection. In: Pfizer Limited. [Google Scholar]
- Phillips A, Demarest K, Hahn DW, Wong F, & McGuire JL (1990). Progestational and androgenic receptor binding affinities and in vivo activities of norgestimate and other progestins. Contraception, 41, 399–410. [DOI] [PubMed] [Google Scholar]
- Polis CB, Achilles SL, Hel Z, & Hapgood JP (2018). Is a lower-dose, subcutaneous contraceptive injectable containing depot medroxyprogesterone acetate likely to impact women's risk of HIV? Contraception, 97, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polis CB, Curtis KM, Hannaford PC, Phillips SJ, Chipato T, Kiarie JN, et al. (2016). An updated systematic review of epidemiological evidence on hormonal contraceptive methods and HIV acquisition in women. AIDS, 30, 2665–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polis CB, Phillips SJ, Hillier SL, & Achilles SL (2016). Levonorgestrel in contraceptives and multipurpose prevention technologies: does this progestin increase HIV risk or interact with antiretrovirals? AIDS, 30, 2571–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollow K, Kreienberg R, & Di Pietro N (1989). Medroxyprogesterone acetate: steady-state pharmacokinetics bioequivalence of two oral formulations. J Cancer Res Clin Oncol, 115, 397–399. [DOI] [PubMed] [Google Scholar]
- Population Council. (2002). Jadelle NDA 20-544. In.
- Praditpan P, Hamouie A, Basaraba CN, Nandakumar R, Cremers S, Davis AR, et al. (2017). Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception, 95, 464–469. [DOI] [PubMed] [Google Scholar]
- Prasad KV, Rao BS, Sivakumar B, & Prema K (1979). Pharmacokinetics of norethindrone in Indian Women. Contraception, 20, 77–90. [DOI] [PubMed] [Google Scholar]
- Prasad PV, Bashir M, Sitruk-Ware R, & Kumar N (2010). Single-dose pharmacokinetics of Nestorone, a potential female-contraceptive. Steroids, 75, 252–264. [DOI] [PubMed] [Google Scholar]
- Prifti S, Lelle I, Strowitzki T, & Rabe T (2004). Induction of androgen receptor activity by norgestimate and norelgestromin in MDA-MB 231 breast cancer cells. Gynecol Endocrinol, 19, 18–21. [DOI] [PubMed] [Google Scholar]
- Pugeat MM, Dunn JF, & Nisula BC (1981). Transport of steroid hormones: interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab, 53, 69–75. [DOI] [PubMed] [Google Scholar]
- Pyra M, Lingappa JR, Heffron R, Erikson DW, Blue SW, Patel RC, et al. (2018). Concordance of self-reported hormonal contraceptive use and presence of exogenous hormones in serum among African women. Contraception, 97, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana PG, Romero SM, Vaamonde G, & Baldessari A (2013). New metabolites of drospirenone obtained in Mucorales fungi culture. Journal of Molecular Catalysis B: Enzymatics, 97, 110–117. [Google Scholar]
- Rahimy MH, Cromie MA, Hopkins NK, & Tong DM (1999). Lunelle monthly contraceptive injection (medroxyprogesterone acetate and estradiol cypionate injectable suspension): effects of body weight and injection sites on pharmacokinetics. Contraception, 60, 201–208. [DOI] [PubMed] [Google Scholar]
- Ralph LJ, McCoy SI, Shiu K, & Padian NS (2015). Hormonal contraceptive use and women's risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis, 15, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read J, Mould G, & Stevenson D (1985). Simple high-performance liquid chromatographic method for the determination of medroxyprogesterone acetate in human plasma. J Chromatogr, 341, 437–444. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Fotherby K, Peck JE, & Gordon Y (1973). Localization of norethisterone in the reproductive tract of women. J Endocrinol, 59, 569–577. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Ross MS, Lai LC, Ghilchik MW, & James VH (1990). In vivo conversion of norethisterone to ethynyloestradiol in perimenopausal women. J Steroid Biochem Mol Biol, 37, 301–303. [DOI] [PubMed] [Google Scholar]
- Regidor PA (2018). The clinical relevance of progestogens in hormonal contraception: Present status and future developments. Oncotarget, 9, 34628–34638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronacher K, Hadley K, Avenant C, Stubsrud E, Simons SS Jr., Louw A, et al. (2009). Ligand-selective transactivation and transrepression via the glucocorticoid receptor: role of cofactor interaction. Mol Cell Endocrinol, 299, 219–231. [DOI] [PubMed] [Google Scholar]
- Rossi E, De Pascale A, Negrini P, Frigerio A, Castegnaro E, & Castegnaro E (1979). Quantitative gas-liquid chromatographic determination of medroxyprogesterone acetate in human plasma. J Chromatogr, 169, 416–421. [DOI] [PubMed] [Google Scholar]
- Sambol NC, Harper CC, Kim L, Liu CY, Darney P, & Raine TR (2006). Pharmacokinetics of single-dose levonorgestrel in adolescents. Contraception, 74, 104–109. [DOI] [PubMed] [Google Scholar]
- Sang GW, Fotherby K, Howard G, Elder M, & Bye PG (1981). Pharmacokinetics of norethisterone oenanthate in humans. Contraception, 24, 15–27. [DOI] [PubMed] [Google Scholar]
- Scheschowitsch K, Leite JA, & Assreuy J (2017). New Insights in Glucocorticoid Receptor Signaling-More Than Just a Ligand-Binding Receptor. Front Endocrinol (Lausanne), 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. (2003). Classification and pharmacology of progestins. Maturitas, 46 Suppl 1, S7–S16. [DOI] [PubMed] [Google Scholar]
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. (2008). Classification and pharmacology of progestins. Maturitas, 61, 171–180. [DOI] [PubMed] [Google Scholar]
- Schivone G, Dorflinger L, & Halpern V (2016). Injectable contraception: updates and innovation. Curr Opin Obstet Gynecol, 28, 504–509. [DOI] [PubMed] [Google Scholar]
- Schonborn JL (2010). The role of the liver in drug metabolism. Anaesthesia tutorial of the week 179, 17th May 2010. ATOTW. In. [Google Scholar]
- Schoonen WG, Deckers GH, de Gooijer ME, de Ries R, & Kloosterboer HJ (2000). Hormonal properties of norethisterone, 7alpha-methyl-norethisterone and their derivatives. J Steroid Biochem Mol Biol, 74, 213–222. [DOI] [PubMed] [Google Scholar]
- Schwallie PC (1974). Experience with Depo-provera as an injectable contraceptive. J Reprod Med, 13, 113–117. [PubMed] [Google Scholar]
- Segall-Gutierrez P, Taylor D, Liu X, Stanzcyk F, Azen S, & Mishell DR Jr. (2010). Follicular development and ovulation in extremely obese women receiving depo-medroxyprogesterone acetate subcutaneously. Contraception, 81, 487–495. [DOI] [PubMed] [Google Scholar]
- Selim MF, & Hussein AF (2013). Endothelial function in women using levonorgestrel-releasing intrauterine system (LNG-IUS). Contraception, 87, 396–403. [DOI] [PubMed] [Google Scholar]
- Shelton JD, & Halpern V (2014). Subcutaneous DMPA: a better lower dose approach. Contraception, 89, 341–343. [DOI] [PubMed] [Google Scholar]
- Shi YE, He CH, Gu J, & Fotherby K (1987). Pharmacokinetics of norethisterone in humans. Contraception, 35, 465–475. [DOI] [PubMed] [Google Scholar]
- Shin D, Lee S, Lim KS, Park JS, Shin SG, Jang IJ, et al. (2013). Pharmacokinetic study of single and multiple oral administrations of 2 mg dienogest in healthy Korean women. Contraception, 87, 750–755. [DOI] [PubMed] [Google Scholar]
- Shrimanker K, Saxena BN, & Fotherby K (1978). A radioimmunoassay for serum medroxyprogesterone acetate. J Steroid Biochem, 9, 359–363. [DOI] [PubMed] [Google Scholar]
- Sierra-Ramirez JA, Lara-Ricalde R, Lujan M, Velazquez-Ramirez N, Godinez-Victoria M, Hernadez-Munguia IA, et al. (2011). Comparative pharmacokinetics and pharmacodynamics after subcutaneous and intramuscular administration of medroxyprogesterone acetate (25 mg) and estradiol cypionate (5 mg). Contraception, 84, 565–570. [DOI] [PubMed] [Google Scholar]
- Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, & Kuhn RW (1982). The serum transport of steroid hormones. Recent Prog Horm Res, 38, 457–510. [DOI] [PubMed] [Google Scholar]
- Simmons KB, & Edelman AB (2016). Hormonal contraception and obesity. Fertil Steril, 106, 1282–1288. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R (2004). Pharmacological profile of progestins. Maturitas, 47, 277–283. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R (2006). New progestagens for contraceptive use. Hum Reprod Update, 12, 169–178. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R, Brache V, Maguire R, Croxatto H, Kumar N, Kumar S, et al. (2007). Pharmacokinetic study to compare the absorption and tolerability of two doses of levonorgestrel following single vaginal administration of levonorgestrel in Carraguard gel: a new formulation for "dual protection" contraception. Contraception, 75, 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitruk-Ware R, Nath A, & Mishell DR Jr. (2013). Contraception technology: past, present and future. Contraception, 87, 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitruk-Ware R, Small M, Kumar N, Tsong YY, Sundaram K, & Jackanicz T (2003). Nestorone: clinical applications for contraception and HRT. Steroids, 68, 907–913. [DOI] [PubMed] [Google Scholar]
- Sivakumar B, Prasad KV, Ravinder P, Ramalakshmi BA, & Narasinga Rao BS (1989). Pharmacokinetics of norethisterone from two different combination contraceptive pills in Indian women. Indian J Physiol Pharmacol, 33, 10–14. [PubMed] [Google Scholar]
- Sivin I, Lahteenmaki P, Mishell DR Jr., Alvarez F, Diaz S, Ranta S, et al. (1997). First week drug concentrations in women with levonorgestrel rod or Norplant capsule implants. Contraception, 56, 317–321. [DOI] [PubMed] [Google Scholar]
- Sivin I, Mishell DR Jr., Alvarez F, Brache V, Elomaa K, Lahteenmaki P, et al. (2005). Contraceptive vaginal rings releasing Nestorone and ethinylestradiol: a 1-year dose-finding trial. Contraception, 71, 122–129. [DOI] [PubMed] [Google Scholar]
- Sivin I, Wan L, Ranta S, Alvarez F, Brache V, Mishell DR Jr., et al. (2001). Levonorgestrel concentrations during 7 years of continuous use of Jadelle contraceptive implants. Contraception, 64, 43–49. [DOI] [PubMed] [Google Scholar]
- Smit J, Botha J, McFadyen L, & Beksinska M (2004). Serum medroxyprogesterone acetate levels in new and repeat users of depot medroxyprogesterone acetate at the end of the dosing interval. Contraception, 69, 3–7. [DOI] [PubMed] [Google Scholar]
- Soldin SJ, & Soldin OP (2009). Steroid hormone analysis by tandem mass spectrometry. Clin Chem, 55, 1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speroff L, & Darney PD (1996). Injectable Contraception. In A clinical guide for contraception (2nd ed.). Baltimore: Williams & Wilkins. [Google Scholar]
- Stanczyk FZ (2003). All progestins are not created equal. Steroids, 68, 879–890. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, & Archer DF (2014). Gestodene: a review of its pharmacology, potency and tolerability in combined contraceptive preparations. Contraception, 89, 242–252. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Burke AE, Hong KM, & Archer DF (2018). Morbid obesity: potential effects of hormonal contraception. Contraception, 98, 174–180. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, & Clarke NJ (2010). Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol, 121, 491–495. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Hapgood JP, Winer S, & Mishell DR Jr. (2013). Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev, 34, 171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk FZ, Lee JS, & Santen RJ (2007). Standardization of steroid hormone assays: why, how, and when? Cancer Epidemiol Biomarkers Prev, 16, 1713–1719. [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, & Roy S (1990). Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception, 42, 67–96. [DOI] [PubMed] [Google Scholar]
- Sturm G, Haberlein H, Bauer T, Plaum T, & Stalker DJ (1991). Mass spectrometric and high-performance liquid chromatographic studies of medroxyprogesterone acetate metabolites in human plasma. J Chromatogr, 562, 351–362. [DOI] [PubMed] [Google Scholar]
- Tamassia V, Battaglia A, Ganzina F, Isetta AM, Sacchetti G, Cavalli F, et al. (1982). Pharmacokinetic approach to the selection of dose schedules for medroxyprogesterone acetate in clinical oncology. Cancer Chemother Pharmacol, 8, 151–156. [DOI] [PubMed] [Google Scholar]
- Taneepanichskul S (2005). Norelgestromin/ethinyl estradiol transdermal system. J Med Assoc Thai, 88 Suppl 2, S82–84. [PubMed] [Google Scholar]
- Theron HB, Coetzee C, Sutherland FC, Wiesner JL, & Swart KJ (2004). Selective and sensitive liquid chromatography-tandem mass spectrometry method for the determination of levonorgestrel in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci, 813, 331–336. [DOI] [PubMed] [Google Scholar]
- Thomas T, Petrie K, Shim J, Abildskov KM, Westhoff CL, & Cremers S (2013). A UPLC-MS/MS method for therapeutic drug monitoring of etonogestrel. Ther Drug Monit, 35, 844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel KE (2007). Gut instincts: CYP3A4 and intestinal drug metabolism. J Clin Invest, 117, 3173–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman A, Kimble T, Hall P, Schwartz JL, & Archer DF (2013). Medroxyprogesterone acetate and estradiol cypionate injectable suspension (Cyclofem) monthly contraceptive injection: steady-state pharmacokinetics. Contraception, 87, 738–743. [DOI] [PubMed] [Google Scholar]
- Thurman A, Schwartz J, Brache V, Chen B, Chandra N, Kashuba A, et al. (2018). Effect of Hormonal Contraception on Pharmacokinetics of Vaginal Tenofovir: Increased Tenofovir Diphosphate in Depot Medroxyprogesterone Acetate Users. AIDS Research and Human Retroviruses, 34, Abstract no. P21.03. [DOI] [PubMed] [Google Scholar]
- Timmer CJ, & Mulders TM (2000). Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinet, 39, 233–242. [DOI] [PubMed] [Google Scholar]
- Timmer CJ, Srivastava N, Dieben TO, & Cohen AF (1999). Bioavailability and bioequivalence of etonogestrel from two oral formulations of desogestrel: Cerazette and Liseta. Eur J Drug Metab Pharmacokinet, 24, 335–343. [DOI] [PubMed] [Google Scholar]
- Toh YC, Jain J, Rahnny MH, Bode FR, & Ross D (2004). Suppression of ovulation by a new subcutaneous depot medroxyprogesterone acetate (104 mg/0.65 mL) contraceptive formulation in Asian women. Clin Ther, 26, 1845–1854. [DOI] [PubMed] [Google Scholar]
- Trabert B, Bauer DC, Buist DSM, Cauley JA, Falk RT, Geczik AM, et al. (2020). Association of Circulating Progesterone With Breast Cancer Risk Among Postmenopausal Women. JAMA Netw Open, 3, e203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay D, Gainer E, & Ulmann A (2001). The pharmacokinetics of 750 microg levonorgestrel following administration of one single dose or two doses at 12- or 24-h interval. Contraception, 64, 327–331. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. (2003). Depo-SubQ Provera 104 (Medroxyprogesterone Acetate) Injectable Suspension New Drug Application No.: 021583. In: Center for Drug Evaluation and Research Clinical Pharmacology and Biopharmaceutics Review. [Google Scholar]
- United Nations Department of Economic and Social Affairs Population Division. (2015). Trends in Contraceptive Use Worldwide 2015 (ST/ESA/SER.A/349). In.
- United Nations Department of Economic and Social Affairs Population Division. (2019). Estimates and Projections of Family Planning Indicators 2019. In (Vol. 2019). New York: United Nations. [Google Scholar]
- van der Vange N, Blankenstein MA, Kloosterboer HJ, Haspels AA, & Thijssen JH (1990). Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone. Contraception, 41, 345–352. [DOI] [PubMed] [Google Scholar]
- Verhoeven CH, Krebbers SF, Wagenaars GN, & Vos RM (1998). In vitro and in vivo metabolism of desogestrel in several species. Drug Metab Dispos, 26, 927–936. [PubMed] [Google Scholar]
- Victor A, & Johansson ED (1976). Pharmacokinetic observations on medroxyprogesterone acetate administered orally and intravaginally. Contraception, 14, 319–329. [DOI] [PubMed] [Google Scholar]
- Viinikka L, Ylikorkala O, Nummi S, Virkkunen P, Ranta T, Alapiessa U, et al. (1976). Biological effects of a new and potent progestagen. A clinical study. Acta Endocrinol (Copenh), 83, 429–438. [DOI] [PubMed] [Google Scholar]
- Vīna I, Linde R, Patetko A, & Semjonovs P (2013). Glucuronic acid from fermented beverages: biochemical functions in humans and its role in health protection. IJRRAS, 14, 217–230. [Google Scholar]
- Virutamasen P, Leepipatpaiboon S, Kriengsinyot R, Vichaidith P, Muia PN, Sekadde-Kigondu CB, et al. (1996). Pharmacodynamic effects of depot-medroxyprogesterone acetate (DMPA) administered to lactating women on their male infants. Contraception, 54, 153–157. [DOI] [PubMed] [Google Scholar]
- Vose CW, Butler JK, Williams BM, Stafford JE, Shelton JR, Rose DA, et al. (1979). Bioavailability and pharmacokinetics of norethisterone in women after oral doses of ethynodiol diacetate. Contraception, 19, 119–127. [DOI] [PubMed] [Google Scholar]
- Walls C, Vose CW, Horth CE, & Palmer RF (1977). Radioimmunoassay of plasma norethisterone after ethynodiol diacetate administration. J Steroid Biochem, 8, 167–171. [DOI] [PubMed] [Google Scholar]
- Ward S, & Back DJ (1993). Metabolism of gestodene in human liver cytosol and microsomes in vitro. J Steroid Biochem Mol Biol, 46, 235–243. [DOI] [PubMed] [Google Scholar]
- Warren RJ, & Fotherby K (1974). Radioimmunoassay of synthetic progestogens, norethisterone and norgestrel. J Endocrinol, 62, 605–618. [DOI] [PubMed] [Google Scholar]
- Wellington K, & Perry CM (2002). Estradiol valerate/dienogest. Drugs, 62, 491–504; discussion 505-496. [DOI] [PubMed] [Google Scholar]
- Wenzl R, van Beek A, Schnabel P, & Huber J (1998). Pharmacokinetics of etonogestrel released from the contraceptive implant Implanon. Contraception, 58, 283–288. [DOI] [PubMed] [Google Scholar]
- Westhoff CL, Torgal AH, Mayeda ER, Petrie K, Thomas T, Dragoman M, et al. (2012). Pharmacokinetics and ovarian suppression during use of a contraceptive vaginal ring in normal-weight and obese women. Am J Obstet Gynecol, 207, 39 e31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff CL, Torgal AH, Mayeda ER, Pike MC, & Stanczyk FZ (2010). Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception, 81, 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff CL, Torgal AH, Mayeda ER, Stanczyk FZ, Lerner JP, Benn EK, et al. (2010). Ovarian suppression in normal-weight and obese women during oral contraceptive use: a randomized controlled trial. Obstet Gynecol, 116, 275–283. [DOI] [PubMed] [Google Scholar]
- WHO. (2019). Contraceptive eligibility for women at high risk of HIV. Guidance statement: recommendations on contraceptive methods used by women at high risk of HIV. In (Vol. 2019). Switzerland: World Health Organisation. [PubMed] [Google Scholar]
- Wiebe JP (2006). Progesterone metabolites in breast cancer. Endocr Relat Cancer, 13, 717–738. [DOI] [PubMed] [Google Scholar]
- Wiebe JP, Muzia D, Hu J, Szwajcer D, Hill SA, & Seachrist JL (2000). The 4-pregnene and 5alpha-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion. Cancer Res, 60, 936–943. [PubMed] [Google Scholar]
- Wiegratz I, Jung-Hoffmann C, & Kuhl H (1995). Effect of two oral contraceptives containing ethinylestradiol and gestodene or norgestimate upon androgen parameters and serum binding proteins. Contraception, 51, 341–346. [DOI] [PubMed] [Google Scholar]
- Wiegratz I, Kutschera E, Lee JH, Moore C, Mellinger U, Winkler UH, et al. (2003). Effect of four different oral contraceptives on various sex hormones and serum-binding globulins. Contraception, 67, 25–32. [DOI] [PubMed] [Google Scholar]
- Wiesinger H, Berse M, Klein S, Gschwend S, Hochel J, Zollmann FS, et al. (2015). Pharmacokinetic interaction between the CYP3A4 inhibitor ketoconazole and the hormone drospirenone in combination with ethinylestradiol or estradiol. Br J Clin Pharmacol, 80, 1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyeth Pharmaceuticals. Norplant® Product sheet. In: Wyeth Department of Environment, Health & Safety. [Google Scholar]
- Yamamoto T, Yoshiji S, Yasuda J, Shiroshita K, Kitawaki J, Fujii M, et al. (1986). Aromatization of norethindrone to ethynylestradiol in human adult liver. Endocrinol Jpn, 33, 527–531. [DOI] [PubMed] [Google Scholar]
- Zhang JW, Liu Y, Zhao JY, Wang LM, Ge GB, Gao Y, et al. (2008). Metabolic profiling and cytochrome P450 reaction phenotyping of medroxyprogesterone acetate. Drug Metab Dispos, 36, 2292–2298. [DOI] [PubMed] [Google Scholar]
- Zhao XJ, Shao GX, Zhang J, & Sang GW (1992). [Effects of steroid contraceptive on serum levels of sex hormone binding globulin in women]. Shengzhi Yu Biyun, 12, 13–17. [PubMed] [Google Scholar]
- Zhou XF, Shao QX, Han XJ, Weng LJ, & Sang GW (1998). Pharmacokinetics of medroxyprogesterone acetate after single and multiple injection of Cyclofem in Chinese women. Contraception, 57, 405–411. [DOI] [PubMed] [Google Scholar]
- Zia Y, Tang JH, Chinula L, Tegha G, Stanczyk FZ, & Kourtis AP (2019). Medroxyprogesterone acetate concentrations among HIV-infected depot-medroxyprogesterone acetate users receiving antiretroviral therapy in Lilongwe, Malawi. Contraception. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman H, Thebault JJ, Duvauchelle T, Mignot A, Renoux A, & Gualano V (2000). Pharmacokinetics of estradiol valerate 2mg + dienogest 2mg (climodien(R) 2/2) after single and repeated oral administration in healthy postmenopausal women. Clin Drug Investig, 20, 123–134. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Duvauchelle T, Gualano V, Kaufmann G, Bervoas-Martin S, Breitbarth H (1999). Pharmacokinetics of dienogest as a single drug or in combination with estradiol valerate or ethinylestradiol. Drugs Today, 35, 27–39. [Google Scholar]