Abstract
Objective:
The link between marital quality and cellular aging remains underexplored. This study examined how both positive and negative marital quality were associated with salivary telomere length among partnered adults in the United States over the age of 50°years.
Methods:
Data were from the 2008 Health and Retirement Study (N = 3203). Ordinary least squares regression was used to estimate the link between marital quality and telomere length.
Results:
While neither positive nor negative marital quality was significantly associated with telomere length among older women, positive and negative marital quality had an interacting effect on telomere length among men. Specifically, when negative marital quality was low, higher positive marital quality was associated with shorter telomere length, whereas when negative marital quality was high, higher positive marital quality was associated with longer telomere length.
Discussion:
The findings speak to the complex nature of intimate partnerships and the implications of these partnerships for cellular aging processes.
Keywords: marital quality, telomere, aging, intimate relationships, Health and Retirement Study
Introduction
Being married is a critical source of social support; researchers have found that marriage is associated with better mental and physical health and greater longevity (Kiecolt-Glaser & Wilson, 2017). However, the health advantages of marriage depend on the quality of the relationship (Carr & Springer, 2010). Marital quality is also associated with age-related chronic diseases, disease progression, and diverse aspects of physiological functioning (Robles, 2014; Yu & Zhang, 2020). Indeed, marital support (i.e., positive marital quality) confers health benefits both by enhancing cardiovascular, immune, and neuroendocrine systems and by buffering stress-induced physiological insults. Marital strain (i.e., negative marital quality), in contrast, has a direct negative effect on the body’s physiological systems (Robles & Kiecolt-Glaser, 2003). Despite the growing body of evidence about the physiological pathways through which marital quality affects health, there is little research about the connection between marital quality and the cellular aging process. Telomere shortening, a fundamental process of aging, is involved in the development and progression of a variety of chronic diseases (Blackburn & Epel, 2017). This study examines the link between marital quality and salivary telomere length in adults aged 51°years and older in the United States, using data from the 2008 Health and Retirement Study (HRS). Given the long-standing observation of gender differences in the links between marriage and health, we also consider whether the relationship between marital quality and salivary telomeres differs for men and women.
Marital Quality and Telomere Length: Previous Empirical Evidence
To our knowledge, no previous studies have specifically examined telomere length in relation to martial quality. Mainous et al. (2010) reported that adults who were married or cohabiting had significantly longer leukocyte telomere length than those who were unpartnered, but the study did not consider the quality of relationships. Using a probability sample of older adults, Barger and Cribbet (2016) found that those who had no spousal support (but had other social support) had significantly shorter telomeres than those who had any amount of spousal support. However, the study did not assess the level of spousal support.
Studies examining telomere length in relation to social relationships in general (without distinguishing specific types of relationships) have presented mixed evidence. For example, Carroll et al. (2013) reported that greater social support in general (i.e., including from friends and family) was significantly associated with longer leukocyte telomere length among adults aged 65°years and older but not their younger counterparts. In contrast, Uchino et al. (2012) found that in a regional sample of 136 participants, the level of supportive ties in an individual’s social network was not significantly associated with telomere length, whereas having more ambivalent ties, characterized by both high positivity and negativity, was significantly linked with shorter telomere length. Using data from the 2008 HRS, Lincoln et al. (2019) reported that respondents who had more negative interactions with family members had significantly shorter telomere length, and the relationship was especially pronounced among African Americans. In addition, they found that greater social support from other family members was associated with significantly shorter telomere length. Despite this important research, no previous studies have specifically examined whether marital quality is related to telomere length.
A growing number of studies have documented the links between marital quality and other biomarkers of physiological functioning (Robles et al., 2014). For example, Donoho et al. (2013, 2015) analyzed data from the Midlife in the United States (MIDUS) study and found that greater marital support was associated with higher high-frequency heart rate variability and lower levels of inflammation (indexed by interleukin-6 and C-reactive protein), whereas greater marital strain was associated with lower high-frequency heart rate variability and higher levels of inflammation. Gallo et al. (2003) showed that greater marital satisfaction was linked to less atherosclerosis in the carotid arteries and aorta among a regional sample of middle-aged women. Liu and Waite (2014) reported that both increases in marital strain and decreases in marital support were significantly associated with higher cardiovascular risks such as a high level of C-reactive protein and fast heart rate, particularly among older people and women. Overall, this body of research suggests that marital support is associated with better physiological functioning, whereas marital strain is linked to worse physiological outcomes. Based on this prior work, our general hypothesis is that higher levels of positive marital quality (i.e., marital support) are associated with longer salivary telomere length, while higher levels of negative marital quality (i.e., marital strain) are associated with shorter salivary telomere length.
Interaction of Positive and Negative Marital Quality
Theorists of social support have long argued that both positive and negative exchanges and emotions coexist in social relationships (Uchino et al., 2001). For example, it is not uncommon that married couples mixed the contradictory feelings of love and support together with conflict and criticisms with their spouses (Fingerman et al., 2004). More importantly, such co-occurring and synergy of positivity and negativity in a relationship can interact with each other to produce important implications for individuals’ well-being, particularly in close relationships such as marriage (Ross et al., 2019). For example, interpersonal relationships characterized by high positivity and negativity are regarded as a normative feature of social interaction (Lüscher & Pillemer, 1998). This type of relationships tend be more unpredictable and thus can be a source of interpersonal stressors, which have notable health implications (Uchino et al., 2004). Therefore, the health-promoting effect of positive relationship quality is dampened in this type of relationships when negative relationship quality is high (Ross et al., 2019). Consistent with this view, family scholars argued that marital quality should be conceptualized as two correlated and yet distinct dimensions that involve positive and negative appraisals of marriage and that the interplay of these two dimensions have its unique impact on married people’s well-being (Fincham & Linfield, 1997), calling for greater attention to the interactive nature of positive and negative aspects of close relationships and its consequences for health (Ross et al., 2019).
Empirical investigations of the interaction of positive and negative exchanges in social relationships have presented mixed findings. While some empirical studies have indicated that the coexistence of high positivity and negativity in social ties has negative health consequences, others showed benefits of such relationships (Rothman et al., 2017). For example, Uchino et al. (2012) found that individuals who reported more ambivalent relationships (characterized by a high level of both positive and negative emotions) in their social networks had significantly shorter telomere length, whereas Lincoln et al. (2019) reported that the co-occurrence of family support and negative exchanges was associated with longer telomeres. Based on this body of research, we hypothesize that there is a significant interaction between positive and negative marital quality in their effect on salivary telomere length.
Marital Quality and Telomere Length: A Gendered Perspective
Research on both telomere length and the link between marriage and health has identified gender differences. On average, women have longer telomeres than men (Brown et al., 2017). A gendered perspective on marriage suggests that women and men are socialized differently and women are expected to be more attuned to other people’s emotions—particularly in a marital relationship—than men. Because women are more often the primary providers of care and emotional comfort to family members and tend to be more sensitive to relationship atmosphere than men, marital quality affects women’s health more than men’s health (Kiecolt-Glaser & Newton, 2001).
The empirical evidence for this gendered perspective on marriage and health is mixed. For example, Donoho et al. (2013) showed that lower levels of spousal support were significantly associated with greater inflammation among women but not men. Liu and Waite (2014) reported that at older ages, the link between change in marital quality over a 5-year period and cardiovascular risk was more prominent among women than men. Uchino et al. (2012) found a significant association between more ambivalent ties in social relationships (not just marriage) and shorter telomere length among women but not men. In contrast, another body of research has found either no gender differences or a greater impact of marital quality on physical health among men than women. For example, Whisman et al. (2014) reported that a decrease in positive exchanges and an increase in negative exchanges were significantly correlated with diabetes prevalence among men, but not among women. Further,Seeman et al. (2002) demonstrated that greater social integration and emotional support was significantly linked to lower allostatic load only among older men. Yet Barger and Cribbet (2016) found no gender differences in the association between having an unsupportive spouse and telomere length. Given the mixed empirical evidence, we explore gender differences in the link between marital quality and telomere length.
Data and Methods
Sample
Data were drawn from the 2008 HRS, which was sponsored by the National Institute on Aging and administered by the University of Michigan (HRS, 2019; RAND Corporation, 2019). The HRS is a longitudinal study of nationally representative samples of civilian Americans aged 51°years and older. In addition to conducting biennial longitudinal surveys, the HRS has collected information on respondents’ psychosocial characteristics every 4 years since 2006; this information includes respondents’ assessments of the supportive and unsupportive behaviors of their spouses and unmarried cohabiting partners. In 2008, sensitive telomere data were collected from respondents who consented to provide a saliva sample (HRS, 2013). Both psychosocial and telomere data used in this study were collected from respondents that were randomly selected for enhanced face-to-face interviews in 2008. For detailed sample designs of the leave-behind psychosocial modules, we refer readers to the HRS documentation (Smith et al. 2017).
The sample was drawn from the 5808 respondents who provided saliva samples for telomere data in the 2008 HRS. After excluding respondents who had not completed the leave-behind questionnaires or were not currently living with a partner, the final analytic sample included 3203 respondents who reported having a spouse (N = 3049) or unmarried partner (N = 154). We included both married and cohabiting respondents because previous studies have suggested that marriage and cohabitation are relatively similar for older couples (Brown and Wright 2017; King and Scott 2005). Supplemental analyses that excluded cohabitors (results not shown but available upon request) produced findings similar to those reported herein.
Measures
Salivary telomere length.
Past research has found a strong correlation between salivary telomere length and the length of leukocyte telomeres measured from blood samples (Mitchell et al., 2014) and has shown that salivary telomere length is an indicator of a variety of age-related chronic diseases, health behaviors (e.g., smoking and exercise), and hypothalamicpituitary axis responses to stress (Blackburn & Epel, 2017; Savolainen et al., 2015). Saliva samples were obtained directly via Oragene collection kits and were sent immediately to a central laboratory in a −80-degree freezer; upon arrival at the laboratory, DNA was extracted from the samples and stored. The original sample plates were placed back to a −80-degree freezer after DNA extraction. Average telomere length was assayed using quantitative real-time polymerase chain reaction (quantitative PCR), which compares the telomere sequence copy number in each person’s sample (T) to a single-copy gene copy number (S). The resulting ratio, known as the T/S ratio, is proportional to the mean telomere length in the DNA of a reference sample of multiple individuals (Cawthon, 2002; Health and Retirement Study, 2013). Past research has suggested that the T/S ratio is a measure of relative telomere length that is as effective as the traditional measure of terminal restriction fragment lengths (Cawthon, 2002). Because the distribution of telomere length was highly skewed, we converted the T/S ratios to natural logarithmic values.
Marital quality.
Perception of marital quality was measured by respondents’ assessments of the supportive and unsupportive behaviors of their spouses or partners. We followed prior research and distinguished between these two dimensions (Fincham & Linfield, 1997). Positive marital quality was evaluated by three questions regarding spouses’ or partners’ supportive behavior: (a) “How much do they really understand the way you feel about things?” (b) “How much can you rely on them if you have a serious problem?” (c) “How much can you open up to them if you need to talk about your worries? ” Negative marital quality was measured by four questions about spouses’ or partners’ unsupportive behaviors: (d) “How much do they make too many demands on you? ” (e) "How much do they criticize you? ” (f) "How much do they let you down when you are counting on them?" (g) “How much do they get on your nerves? ” Responses to these seven questions ranged from 1 (a lot) to 4 (not at all). Scores were reverse coded (i.e., higher scores indicate greater agreement that the spouse/partner engages in the referenced behavior). Per HRS instructions, two summary scores were computed by averaging respondents’ scores across the three items for positive marital quality and the four items for negative marital quality. The final summary indicators range from 1 to 4, with higher values indicating higher positive or negative marital quality. Both the positive (Cronbach’s α = .82) and negative marital quality (Cronbach’s α = .79) indicators have satisfactory psychometric properties (Smith et al., 2017). The bivariate correlation between positive and negative marital quality is −.484 for men and −.598 for women in the analytic sample.
Covariates.
The analytic models controlled for a series of health-related and sociodemographic covariates that have been shown to predict both marital quality and telomere length. Health-related covariates included self-reported health, number of self-reported chronic conditions, and body weight. Self-reported health was measured on a five-point scale from 1 (poor) to 5 (excellent). The number of chronic conditions was a count of the following conditions respondents reported having hypertension, diabetes, heart disease, stroke, arthritis, lung disease, and cancer. Values ranged from 0 to 7. Body weight was recoded into four groups based on respondents’ body mass index (BMI): underweight (BMI < 18.5), normal (18.5 ≤ BMI ≤ 24.9, reference category), overweight (25 ≤ BMI ≤ 29.9), and obese (BMI ≥ 30).
The models included six sociodemographic covariates: age measured in years, race/ethnicity (non-Hispanic white [reference], non-Hispanic Black, non-Hispanic other race, and Latino), immigrant status (no = 0), education (less than high school [reference], high school graduate, some college, and college graduate or above), annual household income (measured in nominal dollars as the sum of all income from the respondent and the spouse, not including other household members), and net household assets (the sum of all wealth components, excluding the second residence minus all debts). Both income and net assets were adjusted for household size and naturally logged in the final models. Missing cases were imputed in the RAND HRS. We also included an indicator for being married or cohabiting.
Analytic approach.
A series of gender-stratified ordinary least squares (OLS) regression models were estimated to analyze the association between marital quality and telomere length. Models were run separately by gender because our additional analyses of gender interaction models suggested that all gender interaction effects including both two-way and three-way interactions of gender, positive and negative marital quality were statistically significant at the p < .05 level (results available upon request), indicting distinct gender patterns in the association of marital quality with telomere length. Model 1 estimated the basic link between positive/negative marital quality and telomere length (main effects model). Model 2 added the interaction term between positive and negative marital quality. Both models controlled for the health-related and sociodemographic covariates described above and were adjusted for the complex survey design of the HRS using PROC SURVEYREG (SAS Institute Inc, 2013). Less than 3% of respondents in the analytic sample had missing data, with 2.03% missing on “how often does spouse criticize you?” and less than 2% missing on the other covariates including the three indicators of positive marital quality, three of the four indicators of negative marital quality, education, immigration status, self-reported health, body weight, drinking, smoking, and physical activity. Missing data were addressed via multiple imputation techniques; 10 multiply imputed datasets were created using PROC MI in SAS 9.4, and the final model results were produced using the PROC MIANALYZE procedure.
Results
Table 1 presents weighted sample characteristics by gender. Mean salivary telomere length is comparable for men and women. Consistent with previous studies (Bulanda, 2011; Umberson & Williams, 2005), women report significantly lower levels of positive marital quality and higher levels of negative marital quality than men. With regard to health profiles, women have better physical health than men on all three measures (self-reported health, number of chronic health conditions, and body weight).
Table 1.
Weighted Sample Characteristics (N = 3203).
| Men (N = 1649) | Women (N = 1554) | Significance | |
|---|---|---|---|
| Salivary telomere length | 1.38 (.022) | 1.38 (.012) | |
| Positive marital quality | 3.58 (.019) | 3.38 (.018) | *** |
| Negative marital quality | 1.90 (.020) | 2.05 (.024) | *** |
| Age | 66.05 (.250) | 65.07 (.244) | *** |
| Race/ethnicity | |||
| Non-Hispanic White | 82.93% | 84.57% | |
| Non-Hispanic Black | 6.32% | 5.50% | |
| Non-Hispanic other races | 2.79% | 2.47% | |
| Latino | 7.96% | 7.46% | |
| % Of immigrants | 9.36% | 9.41% | |
| Educational attainment | |||
| Less than high school | 15.35% | 12.78% | * |
| High school graduates | 28.83% | 38.75% | *** |
| Some college | 24.06% | 25.86% | |
| College graduates or above | 31.76% | 22.61% | *** |
| % Married | 96.20% | 96.64% | |
| Total net assets | 593782.3 (31503.56) | 668264.5 (43908.94) | * |
| Annual household income | 90622.87 (3964.44) | 89355.51 (4788.49) | * |
| Self-reported health | 3.25 (.036) | 3.33 (.045) | * |
| Number of chronic conditions | 1.81 (.039) | 1.66 (.038) | ** |
| Body weight | |||
| Underweight | .55% | 1.15% | |
| Normal | 21.91% | 31.31% | *** |
| Overweight | 44.29% | 34.49% | *** |
| Obese | 33.24% | 33.15% | |
Note.
p < .05
p < .01
p < .001 for two-tailed tests of gender difference. The numbers in parentheses are standard deviations.
Table 2 reports the results of OLS regressions of salivary telomere length on marital quality for older men and women. Model 1 suggests that among men, while positive marital quality is significantly associated with shorter telomere length (β = −0.042, p value = .029), negative marital quality is not significantly associated with telomere length, despite the expected sign (β = −0.011, p value = .504). Model 2 tests whether there is an interaction between positive and negative marital quality in their associations with telomere length. The results show that both positive and negative marital quality and their interaction are significantly associated with telomere length, suggesting that the relationship between positive marital quality and telomere length is conditional on levels of negative martial quality and vice versa. While the main effect of positive marital quality is significant and negative (β = −0.147, p value = .004), the interaction term is significant and positive (β = 0.460, p value = .028). Similarly, while, as expected, there is a significant and negative association between negative marital quality and telomere length (β = −0.172, p value = .027), this relationship is also modified by levels of positive marital quality.
Table 2.
Linear Regression Models of Logged Salivary Telomere Length on Marital Quality (N = 3203).
| Men |
Women |
|||
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Positive marital quality | −.042* (−.079, −.005) | −.147** (−1.267, −.047) | −.007 (−.032, .018) | .009 (−.079, .097) |
| Negative marital quality | −.011 (−.042, .020) | −.172* (−1.885, −.019) | −.009 (−.031, .013) | .014 (−.100, .128) |
| Positive × negative marital quality | .046* (.005, .087) | −.007 (−.040, .026) | ||
| Age | −.001 (−.003, .001) | −.001 (−.003, .001) | −.002* (−.004, −.00004) | −.002* (−.004, −.00004) |
| Race/ethnicity (non-Hispanic White = 0) | ||||
| Non-Hispanic Black | .111* (.025, .197) | .110* (.026, .194) | .090** (.031, .149) | .090** (.031, .149) |
| Non-Hispanic other races | .063 (−.037, .163) | .055 (−.045, .155) | .042 (−.036, .120) | .043 (−.035, .121) |
| Latino | .029 (−.032, .090) | .025 (−.036, .086) | .022 (−.031, .075) | .024 (−.029, .077) |
| Whether immigrant (US-born = 0) | .026 (−.041, .093) | .031 (−.034, .096) | .047 (−.014, .108) | .047 (−.012, .016) |
| Education (less than high school = 0) | ||||
| High school graduate | −.008 (−.061, .045) | −.004 (−.057, .049) | −.001 (−.048, .046) | −.001 (−.050, .048) |
| Some college | .015 (−.050, .080) | .020 (−.043, .083) | .029 (−.030, .088) | .029 (−.030, .088) |
| College graduate or above | .027 (−.030, .084) | .032 (−.025, .089) | .031 (−.024, .086) | .032 (−.021, .085) |
| Men |
Women |
|||
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Annual household income | .009 (−.013, .031) | .008 (−.014, .030) | .006 (−.006, .018) | .006 (−.006, .018) |
| Total net asset | −.011 (−.040, .018) | −.011 (−.040, .018) | .008 (−.014, .030) | .008 (−.014, .030) |
| Married (cohabiting = 0) | .037 (−.016, .090) | .035 (−.024, .094) | .088** (.025, .151) | .090** (.029, .151) |
| Self-reported health | .009 (−.009, .027) | .010 (−.010, .030) | .004 (−.014, .022) | .004 (−.014, .022) |
| Number of chronic conditions | −.009 (−.025, .007) | −.007 (−.023, .009) | −.005 (−.017, .007) | −.005 (−.017, .007) |
| Body weight (normal = 0) | ||||
| Underweight | −.037 (−.111, .037) | −.041 (−.114, .032) | .038 (−.099, .175) | .038 (−.099, .175) |
| Overweight | .053** (.014, .092) | .053** (.014, .092) | .006 (−.035, .047) | .006 (−.035, .047) |
| Obese | .071** (.026, .116) | .073** (.028, .118) | .023 (−.012, .058) | .022 (−.013, .057) |
| Intercept | .403 (−.030, .836) | .783** (.199, 1.367) | .189 (−.119, .497) | .133 (−.330, .596) |
| R-square | .031 | .035 | .033 | .033 |
| Adjusted R-square | .030 | .034 | .032 | .032 |
Note.
p < .05.
p < .01.
p < .001.
All the models were adjusted for complex survey designs. Household income and net assets were naturally logged and adjusted for the household size. Numbers in parentheses are 95% confidence intervals.
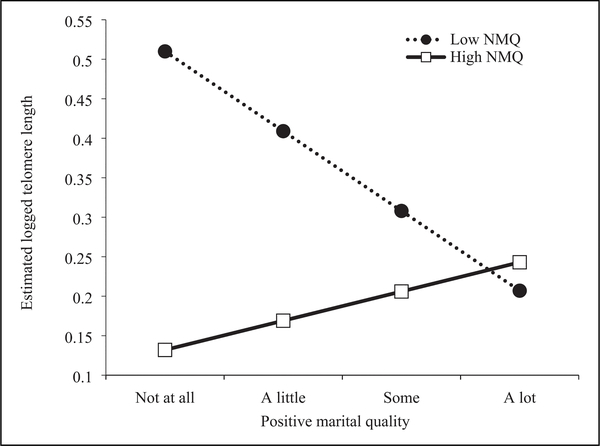
Figures 1 and 2 illustrate these interaction effects based on the results in Model 2 of Table 2. Following previous research (Lincoln et al., 2019), we define low and high positive/negative marital quality as the minimum (1) and maximum (4) scores for the purpose of graphic presentation. Figure 1 shows that higher positive marital quality is associated with longer telomere length only when older men perceive high negative marital quality (solid line), whereas higher positive marital quality is associated with shorter telomere length in the presence of low negative marital quality (dotted line). Similarly, Figure 2 suggests that higher negative marital quality is associated with shorter telomere length only when older men perceive low positive marital quality (solid line), whereas higher negative marital quality is associated with longer telomere length in the presence of high positive marital quality (dotted line).
Figure 1.
Telomere length and levels of positive marital quality among older men: High versus low negative marital quality.
Note. This figure is made based on the results from Model 2 for older men; NMQ = negative marital quality.
Figure 2.
Telomere length and levels of negative marital quality among older men: High versus low positive marital quality.
Note. This figure is made based on the results from Model 2 for older men; PMQ = positive marital quality.
Last, Models 3 and 4 show that neither positive and negative marital quality nor their interaction is significantly associated with telomere length among women.
In terms of the other covariates, the results in Table 2 are largely consistent with previous research. For example, as expected, older age is associated with shorter telomere length (although the relationship is only significant among women), and non-Hispanic Black older adults have significantly longer telomeres than their non-Hispanic white counterparts. These findings align with previous research using an HRS sample (Brown et al., 2017). The effect for education (no high school degree compared to at least some college education) is in the expected direction but is not statistically significant. A recent meta-analysis indicated that the research on the association between socioeconomic status (SES) and telomere length has produced mixed findings—previous studies have found positive, negative, and no associations between SES and telomere length (Robertson et al., 2013). Being married is associated with significantly longer telomere length than cohabiting among women but not men. The associations between health-related covariates and telomere length are largely in the expected directions. Interestingly, obesity and overweight are associated with significantly longer telomere length than normal weight, primarily among older men; a result that is consistent with previous findings shows that body weight and telomere length were positively associated among a sample of middle-aged and older adults (An & Yan, 2017).
Discussion
Martial quality predicts a range of mental and physical health outcomes (Robles, 2014). A growing number of studies have extended the literature on marital quality and health to understand how marital quality is related to various processes of physiological functioning (Kiecolt-Glaser & Wilson 2017; Smith 2019). Yet the association between marital quality and cellular aging remains underexplored. The current study extends this research by examining the links between both positive and negative marital quality and salivary telomere length—a direct measure of cellular aging processes and an informative indicator of physical health (Blackburn & Epel, 2017)—among older Americans. Overall, our results (in particular among men), on the one hand, are consistent with previous findings in the general literature of marital quality and health, and, on the other hand, also adds important nuances to the literature, highlighting the complex nature of marriage and intimate partnerships, which simultaneously include positive and negative exchanges, and the implications of these complex exchanges for biological aging. The identification of an interaction between positive and negative marital quality in their effect on telomere length, in particular among older men, adds empirical evidence to the literature on the health implications of social relationships. Below, we discuss the key findings and their implications.
First, for older men, when negative marital quality is low, positive marital quality is associated with shorter telomere length. While this finding is inconsistent with our hypothesis, it is not totally surprising, given the increasingly mixed empirical evidence on the link between general social support and physical health (Uchino, 2009). For example, Lincoln et al. (2019) reported that greater support from family members other than a spouse or partner was associated with shorter telomere length in the HRS sample. Karlsson et al. (2010) found that greater emotional support predicted increased sickness absence in a random sample of middle-aged and older workers. Moreover, past research on intergenerational support showed that although greater support from adult children was significantly linked with greater positive mood among older adults, particularly the unmarried, after reaching a certain threshold, excessive intergenerational support began to depress positive mood (Silverstein et al., 1996). A study of elderly Singaporeans found that receiving greater social support was significantly associated with more depressive symptoms (indirectly) via the dampening of personal mastery among women (Ang & Malhotra, 2016).
While spousal support may enhance a person’s health and well-being, it may also have unintended negative consequences by hindering an individual’s sense of independence and autonomy (Silverstein et al., 1996), thereby compromising mental and physical health; thus, a negative effect of spousal support could be partially mediated through a decreased sense of personal mastery (Ang & Malhotra, 2016). Alternatively, health selection may be responsible for the inverse relationship between positive marital quality and telomere length; specifically, those with poorer health might solicit more support from their spouses (Lincoln et al., 2019; Uchino, 2009), especially when negative marital quality is low.
Second, we find that for older men, when negative marital quality is high, higher positive marital quality is associated with longer telomere length. This finding is consistent with recent studies reporting that a mix of positive and negative emotions was linked to better psychological and physical health (Adler & Hershfield, 2012; Hershfield et al., 2013). Lincoln et al. (2019) argued that stress buffering may explain why older adults in the United States who reported frequent positive and negative exchanges had longer telomeres than those who reported a supportive relationship with little negativity. Our finding is also in line with the broader literature, which suggests that positive marital quality promotes health (Robles et al., 2014), possibly by reducing psychosocial stressors and encouraging salutary health behaviors, thus promoting healthy cellular aging (Carroll et al., 2013; Robles & Kiecolt-Glaser, 2003).
Third, we find that for older men, when positive marital quality was low, higher negative marital quality was associated with shorter telomere length. This result is consistent with the literature on the detrimental health impacts of negative marital quality (Robles et al., 2014). The extant research indicates that psychosocial stressors such as marital strain directly affect physiological functioning, for example, increasing cell stress and inflammation, which are associated with accelerated telomere shortening (Rentscher et al., 2020). In addition, negative marital quality can induce depression and unhealthy behaviors, thus damaging healthy cellular aging (Blackburn & Epel, 2017; Kiecolt-Glaser & Newton, 2001), and this pattern is more likely to emerge in relationships with low levels of support.
Fourth, the results also suggest that for older men, when positive marital quality was high, higher negative marital quality was associated with longer telomere length. Some recent studies among older adults have suggested that marital strain may actually promote certain dimensions of health, such as cognitive ability (Xu et al., 2016) and diabetes control (Liu et al., 2016). The current study adds further evidence to this line of research, suggesting that negative marital quality may promote healthy cellular aging but only when the spouse is supportive. Spouses in supportive relationships may remind each other to engage in health-promoting behaviors (e.g., eating healthy food) and restrain from unhealthy behaviors (e.g., quit smoking), both of which promote healthy cellular aging; these reminders may also be a source of friction, creating strain in the relationship (Liu et al., 2016).
Notably, we found that marital quality was linked to telomere length among men but not women. This gendered finding is consistent with some past research that showed a stronger association between marital quality and other health outcomes (such as risk of diabetes) for men than for women, pointing to greater health benefits of marriage for men (Whisman et al., 2014; see Liu & Waite, 2014 for different gender findings). This finding also speaks to the gendered dynamics of marriage and intimate partnerships and gender role socialization. Complying with healthcare reminders and paying attention to one’s healthcare needs conflict with the traditional culture of masculinity in the United States (Courtenay, 2000), and thus, men are more likely to resist help and care than women when health services are needed and are more likely to delay help-seeking behaviors (Schrock & Schwalbe, 2009; Smith et al., 2007). Given this gender difference, the spousal support and strain that occur in the context of compromised independence may take a greater toll on men’s health than on women’s health. A recent study reported that higher levels of marital support increased negative emotions and decreased feelings of calm among severely impaired men but had the opposite effects among severely impaired women, reducing negative emotions and increasing feelings of calm (Carr et al., 2017). Our gendered findings may also reflect gendered processes of health solicitation. Past research on care provision suggests that relative to men, women are more attuned to family members’ health conditions and therefore more likely to provide care for family members, especially unhealthy spouses, while men are more likely than women to receive care in a relationship (Allen, 1994). Further, the significant associations between marital quality and telomere length among men may also reflect men’s greater tendency to rely exclusively on their spouses/partners for emotional support (England, 2005; Thomeer et al., 2015).
Limitations
This study has several limitations. First, it is likely that both causation and selection contribute to the link between marital quality and health (Carr & Springer, 2010). Telomere data in HRS are cross-sectional, which prevent determination of causality. Recent research showed that chronic physical health conditions prospectively predict change in marital quality (Tracy & Utz, 2020), suggesting a health selection process. Since telomere shortening has been suggested as an important biological mechanism for various age-related chronic health conditions and mortality (Blackburn & Epel, 2017), accelerated shortening of telomere length can be expected to produce physical health decline, which may, in turn, affect marital quality either by increasing marital strain or decreasing marital support. Future research should analyze longitudinal data to better understand the potentially bidirectional relationship between marital quality and telomere length to shed light on causation and selection processes. Second, the measures of marital quality in the current study are limited. For example, the measure of positive marital quality focuses on a single dimension, namely, perceived spousal support. Past research has suggested that different measures of social support, particularly differences in the distinction between perceived and received social support, may have contributed to the mixed findings on the relationship between social support and health (Lincoln et al., 2019; Uchino, 2009). Last, because the HRS did not collect data on gender-relevant information such as gender ideology, masculinity, or femininity, we were not able to examine whether and how traditional gender norms influence the observed gendered patterns in the link between marital quality and telomere length.
Conclusion
Despite the limitations, this study advances the broader literature on social relationships and physical health by examining the link between marital quality and salivary telomere length among older Americans. The findings shed light on the complexity of the link between relationship support and physical health, which may be the result of both causal and selection processes. In addition, the results reveal that marital quality may have an important influence on cellular aging, especially among older men, a link that has been underexplored in previous research. Future research should continue to investigate the specific mechanisms through which marital quality shapes the process of cellular aging (e.g., via the indicator of telomere length) and in particular should use population-based longitudinal data to inform the design and implementation of public programs and policies that effectively promote healthy aging.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Aging (Grant No. R01 AG061118).
Footnotes
Declaration of Confliicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Adler JM, & Hershfield HE (2012). Mixed emotional experience is associated with and precedes improvements in psychological well-being. PLos One, 7(4), e35633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SM (1994). Gender differences in spousal caregiving and unmet need for care. Journal of Gerontology, 49(4), S187–S195. [DOI] [PubMed] [Google Scholar]
- An R, & Yan H (2017). Body weight status and telomere length in U.S. middle-aged and older adults. Obesity Research & Clinical Practice, 11(1), 51–62. [DOI] [PubMed] [Google Scholar]
- Ang S, & Malhotra R (2016). Association of received social support with depressive symptoms among older males and females in Singapore: Is personal mastery an inconsistent mediator? Social Science & Medicine, 153, 165–173. [DOI] [PubMed] [Google Scholar]
- Barger SD, & Cribbet MR (2016). Social support sources matter: Increased cellular aging among adults with unsupportive spouses. Biological Psychology, 115, 43–49. [DOI] [PubMed] [Google Scholar]
- Blackburn E, & Epel E (2017). The telomere effect: A revolutionary approach to living younger, healthier, longer Grand Central Publishing. [Google Scholar]
- Brown L, Needham B, & Ailshire J (2017). Telomere length among older U.S. adults: Differences by race/ethnicity, gender, and age. Journal of Aging and Health, 29(8), 1350–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, & Wright MR (2017). Marriage, cohabitation, and divorce in later life. Innovation in Aging, 1(2), igx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulanda JR (2011). Gender, marital power, and marital quality in later life. Journal of Women & Aging, 23(1), 3–22. [DOI] [PubMed] [Google Scholar]
- Carr D, Cornman JC, & Freedman VA (2017). Disability and activity-related emotion in later life: Are effects buffered by intimate relationship support and strain? Journal of Health and Social Behavior, 58(3), 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D, & Springer KW (2010). Advances in families and health research in the 21st century. Journal of Marriage and Family, 72(3), 743–761. [Google Scholar]
- Carroll JE, Roux AVD, Fitzpatrick AL, & Seeman T (2013). Low social support is associated with shorter leukocyte telomere length in late life: Multi-ethnic study of atherosclerosis (MESA). Psychosomatic Medicine, 75(2), 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM (2002). Telomere measurement by quantitative PCR. Nucleic Acids Research, 30(10), e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay WH (2000). Constructions of masculinity and their influence on men’s well-being: A theory of gender and health. Social Science & Medicine, 50(10), 1385–1401. [DOI] [PubMed] [Google Scholar]
- Donoho CJ, Crimmins EM, & Seeman TE (2013). Marital quality, gender, and markers of inflammation in the MIDUS cohort. Journal of Marriage and Family, 75(1), 127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho CJ, Seeman TE, Sloan RP, & Crimmins EM (2015). Marital status, marital quality, and heart rate variability in the MIDUS cohort. Journal of Family Psychology, 29(2), 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P (2005). Emerging theories of care work. Annual Review of Sociology, 31, 381–399. [Google Scholar]
- Fincham FD, & Linfield KJ (1997). A new look at marital quality: Can spouses feel positive and negative about their marriage? Journal of Family Psychology, 11(4), 489–502. [Google Scholar]
- Fingerman KL, Hay EL, & Birditt KS (2004). The best of ties, the worst of ties: Close, problematic, and ambivalent social relationships. Journal of Marriage and Family, 66(3), 792–808. [Google Scholar]
- Gallo LC, Troxel WM, Kuller LH, Sutton-Tyrrell K, Edmundowicz D, & Matthews KA (2003). Marital status, marital quality, and atherosclerotic burden in postmenopausal women. Psychosomatic Medicine, 65(6), 952–962. [DOI] [PubMed] [Google Scholar]
- Hershfield HE, Scheibe S, Sims TL, & Carstensen LL (2013). When feeling bad can be good: Mixed emotions benefit physical health across adulthood. Social Psychological and Personality Science, 4(1), 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRS. (2013). Health and Retirement Study, 2008 Telomere data set. Produced and distributed by the University of Michigan with funding from the National Instituteon Aging (grant number NIAU01AG009740)
- HRS. (2019). Health and Retirement Study. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (NIA U01AG009740)
- Karlsson N, Skargren E, & Kristenson M (2010). Emotional support predicts more sickness absence and poorer self assessed work ability: A two-year prospective cohort study. BMC Public Health, 10(1), 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, & Newton TL (2001). Marriage and health: His and hers. Psychological Bulletin, 127(4), 472–503. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, & Wilson SJ (2017). Lovesick: How couples’ relationships influence health. Annual Review of Clinical Psychology, 13, 421–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King V, & Scott ME (2005). A comparison of cohabiting relationships among older and younger adults. Journal of Marriage and Family, 67(2), 271–285. [Google Scholar]
- Lincoln KD, Lloyd DA, & Nguyen AW (2019). Social relationships and salivary telomere length among middle-aged and older african american and white adults. The Journals of Gerontology: Series B, 74(6), 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, & Waite L (2014). Bad marriage, broken heart? age and gender differences in the link between marital quality and cardiovascular risks among older adults. Journal of Health and Social Behavior, 55(4), 403–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Waite L, & Shen S (2016). Diabetes risk and disease management in later life: A national longitudinal study of the role of marital quality. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 71(6), 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher K, & Pillemer K (1998). Intergenerational ambivalence: A new approach to the study of parent-child relations in later life. Journal of Marriage and the Family, 60(2), 413–425. [Google Scholar]
- Mainous AG III, Everett CJ, Diaz VA, Baker R, Mangino M, Codd V, & Samani NJ (2010). Leukocyte telomere length and marital status among middle-aged adults. Age and Ageing, 40(1), 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, Garfinkel I, & Notterman D (2014). Social disadvantage, genetic sensitivity, and children’s telomere length. Proceedings of the National Academy of Sciences, 111(16), 5944–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND Corporation. (2019). RAND HRS longtitudinal files 2016 (V1). Produced by the RAND Center for the Study of Aging, with funding from the National Institute on Aging and the Social Security Administration
- Rentscher KE, Carroll JE, & Mitchell C (2020). Psychosocial stressors and telomere length: A current review of the science. Annual Review of Public Health, 41, 223–245. [DOI] [PubMed] [Google Scholar]
- Robertson T, Batty GD, Der G, Fenton C, Shiels PG, & Benzeval M (2013). Is socioeconomic status associated with biological aging as measured by telomere length? Epidemiologic Reviews, 35(1), 98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF (2014). Marital quality and health: Implications for marriage in the 21st century. Current Directions in Psychological Science, 23(6), 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, & Kiecolt-Glaser JK (2003). The physiology of marriage: Pathways to health. Physiology & Behavior, 79(3), 409–416. [DOI] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, & McGinn MM (2014). Marital quality and health: A meta-analytic review. Psychological Bulletin, 140(1), 140–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross KM, Rook K, Winczewski L, Collins N, & Dunkel Schetter C (2019). Close relationships and health: The interactive effect of positive and negative aspects. Social and Personality Psychology Compass, 13(6), e12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman NB, Pratt MG, Rees L, & Vogus TJ (2017). Understanding the dual nature of ambivalence: Why and when ambivalence leads to good and bad outcomes. Academy of Management Annals, 11(1), 33–72. [Google Scholar]
- SAS Institue Inc. (2013). SAS/STAT 13.1 user guide: The SURVEYREG procedure SAS Institute Inc. [Google Scholar]
- Savolainen K, Eriksson JG, Kajantie E, Lahti J, & Räikkönen, ¨ K. (2015). Telomere length and hypothalamic-pituitary-adrenal axis response to stress in elderly adults. Psychoneuroendocrinology, 53, 179–184. [DOI] [PubMed] [Google Scholar]
- Schrock D, & Schwalbe M (2009). Men, masculinity, and manhood acts. Annual Review of Sociology, 35, 277–295. [Google Scholar]
- Seeman TE, Singer BH, Ryff CD, Dienberg Love G, & Levy-Storms L (2002). Social relationships, gender, and allostatic load across two age cohorts. Psychosomatic Medicine, 64(3), 395–406. [DOI] [PubMed] [Google Scholar]
- Silverstein M, Chen X, & Heller K (1996). Too much of a good thing? Intergenerational social support and the psychological well-being of older parents. Journal of Marriage and the Family, 58, 970–982. [Google Scholar]
- Smith TW (2019). Relationships matter: Progress and challenges in research on the health effects of intimate relationships. Psychosomatic Medicine, 81(1), 2–6. [DOI] [PubMed] [Google Scholar]
- Smith JA, Braunack-Mayer A, Wittert G, & Warin M (2007). “I’ve been independent for so damn long!”: Independence, masculinity and aging in a help seeking context. Journal of Aging Studies, 21(4), 325–335. [Google Scholar]
- Smith J, Ryan L, Sonnega A, & Weir D (2017). Psychosocial and lifestyle questionnaire, 2006–2016: Documentation report, core section LB Survey Research Center, Univeristy of Michigan.
- Thomeer MB, Reczek C, & Umberson D (2015). Gendered emotion work around physical health problems in mid-and later-life marriages. Journal of Aging Studies, 32, 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy EL, & Utz RL (2020). For better or for worse: Health and marital quality during midlife. Journal of Aging and Health. Advance online publication doi:10.1177/0898264320948305 [DOI] [PubMed] [Google Scholar]
- Uchino BN (2009). Understanding the links between social support and physical health: A life-span perspective with emphasis on the separability of perceived and received support. Perspectives on Psychological Science, 4(3), 236–255. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cawthon RM, Smith TW, Light KC, McKenzie J, Carlisle M, Gunn H, Birmingham W, & Bowen K (2012). Social relationships and health: Is feeling positive, negative, or both (ambivalent) about your social ties related to telomeres? Health Psychology, 31(6), 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Holt-Lunstad J, Smith TW, & Bloor L (2004). Heterogeneity in social networks: A comparison of different models linking relationships to psychological outcomes. Journal of Social and Clinical Psychology, 23(2), 123–139. [Google Scholar]
- Uchino BN, Holt-Lunstad J, Uno D, & Flinders JB (2001). Heterogeneity in the social networks of young and older adults: Prediction of mental health and cardiovascular reactivity during acute stress. Journal of Behavioral Medicine, 24(4), 361–382. [DOI] [PubMed] [Google Scholar]
- Umberson D, & Williams K (2005). Marital quality, health, and aging: Gender equity? The Journals of Gerontology: Series B, 60(2), S109–S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, Li A, Sbarra DA, & Raison CL (2014). Marital quality and diabetes: Results from the health and retirement study. Health Psychology, 33(8), 832. [DOI] [PubMed] [Google Scholar]
- Xu M, Thomas PA, & Umberson D (2016). Marital quality and cognitive limitations in late life. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 71(1), 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y-L, & Zhang Z (2020). Relationship quality and functional limitations among older adults with cardiovascular disease in the United States of America. Ageing and Society, 40(8), 1694–1717. [Google Scholar]