Abstract
Immune checkpoint inhibitors have revolutionized the treatments of cancers but are also associated with immune related adverse events that can interfere with their use. The types and severity of adverse events vary with checkpoint inhibitors. A single mechanism of pathogenesis has not emerged: postulated mechanisms involve direct effects of the checkpoint inhibitor, emergence of autoantibodies or autoreactive T cells, and destruction by toxic effects of activated T cells. Several host factors such as genotypes, preexisting autoimmune disease, inflammatory responses and others may have predictive value. Ongoing investigations seek to identify ways of modulating the autoimmunity without affecting the anti-tumor response with agents that are specific for the autoimmune mechanisms.
Introduction:
Checkpoint inhibitor (CPI) immunotherapy has altered the landscape of cancer treatment, demonstrating efficacy and improved survival in a growing number of advanced malignancies [1,2]. Monoclonal antibodies (mAbs) targeting immune checkpoint molecules such as programmed cell death 1 (PD-1) or its ligand programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4), release the inhibition of immune cells resulting in tumor cell destruction and control of tumor growth. A consequence of immune system activation by CPI therapy is the development of immune-related adverse events (irAE), which can affect most organ systems and, in some cases, can be life threatening. In this review, we focus on mechanisms, risk factors and predictors of these irAEs and current and emerging treatment options with consideration of their impact on tumor responses.
Overview of CPI-induced irAEs:
The development of irAEs following CPI therapy is relatively common [3]. The incidence of irAEs following anti-CTLA-4 is estimated to be 72% for any irAE and 24–34% for high grade irAE with a dose dependent effect on risk [4–6]. Estimates for anti-PD-1 or anti-PD-L1 related irAEs are 66–74% for any event and 14%−21% for higher grade irAEs [5–7]. With combination therapies, such as anti-CTLA-4 and anti-PD-1, the frequency is higher than with monotherapies (88–94.9% for any event and 41–59% for high grade events) and more often leads to discontinuation of treatment [6,8,9].
The most common irAEs involve the skin, gastrointestinal tract, liver, endocrine organs and lungs [5,9]. However, there are differences in the frequency of the irAEs in individual organs based on type of CPI treatment. For example, hypophysitis, colitis and rash are more frequent with anti-CTLA-4 therapy, but pneumonitis, thyroid dysfunction and diabetes are more frequently seen with anti-PD-1 [5,10–14].
The time to onset of autoimmune complications is variable, depending on the organ affected and the type of treatment. Complications can occur after a single dose of CPI and as early as within days of treatment, but the median time to onset following CPI start is typically within weeks to months. The earliest complications to develop are dermatological, followed by gastrointestinal, hepatic, endocrine, lung and renal complications [9].
In general, the irAEs occur with all tumors suggesting that side effects are dependent on the CPI itself and the host rather than the tumor. One notable exception is CPI-induced vitiligo which develops preferentially in patients receiving CPIs for melanoma [15,16] and may be related to melanocyte antigens shared with tumors. In addition, the risk of CPI-induced colitis/diarrhea is increased in melanoma patients treated with anti-PD-1 compared to patients with non-small cell lung cancer (NSCLC) and renal cell carcinoma (RCC) [13,17].
Fatal irAEs tend to occur early in treatment and the incidence depends on the type of treatment: 0.36% for anti-PD-1, 0.38% for anti-PD-L1, 1.08% for anti-CTLA-4 and 1.23% for combination of anti-PD-1/PD-L1 plus anti-CTLA-4 [18]. The frequencies and type of fatal events depend on treatment modality: Colitis is the most common fatality with anti-CTLA-4, pneumonitis with anti-PD-1/PD-L1, and colitis and cardiac complications with combination therapy [18]. Fatality rates were 39.7% for myocarditis, 5% for colitis and 2% for endocrine events.
Although the clinical features of some irAEs share similarities with their spontaneous autoimmune counterparts, there are often notable differences [3]. For example, diabetic ketoacidosis may be the presenting scenario with CPI-induced diabetes (CPI-DM), similar to type 1 diabetes (T1D), and patients may have low to undetectable C-peptide suggesting beta cell destruction [10]. However, other features are distinguishing. For example, the progression of beta cell failure is more rapid with CPI-DM and autoantibodies are less common [10,14]. Hyperthyroidism may be found at presentation with CPI-induced thyroiditis, but it typically progresses to hypothyroidism rather than remitting, and the kinetics of progression are more rapid compared to other causes of thyroiditis [19]. Other irAEs, such as colitis, are fully reversible, thus differing from inflammatory bowel disease, and the histologic picture is also different [17].
General mechanisms of irAEs:
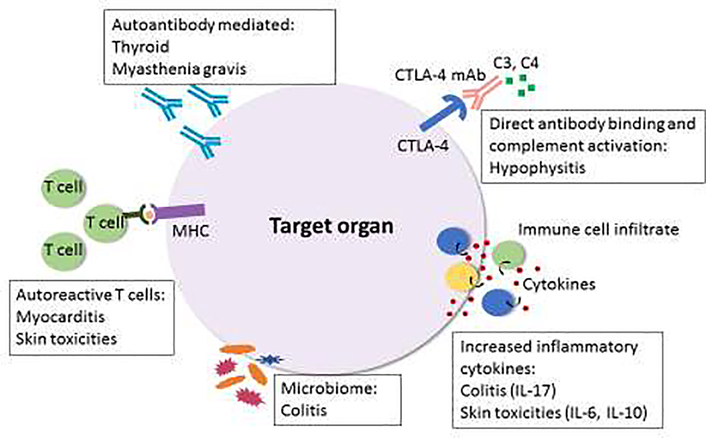
The reasons why some patients develop particular complications and others do not remain largely unknown. Several mechanisms have been proposed to play a role in the development of CPI-induced irAEs, including autoreactive T cells, B cells/autoantibodies, complement, cytokines/chemokines, and the microbiome (Figure 1).
Figure 1. Potential mechanisms of CPI-induced immune related adverse events.
Mechanisms of irAEs vary depending on the organ system affected and type of CPI. The pathophysiology of irAEs remains largely unknown but postulated mechanism are shown. Although frequency of autoantibodies is lower than in spontaneous autoimmune disease, autoantibodies may paly a role in some autoimmune complications, including thyroid dysfunction where presence of autoantibodies at baseline increases risk of this complication. Direct CTLA-4 mAb binding to pituitary cells and complement activation may lead to hypophysitis. Inflammatory cytokines also seem to play a role in the development of certain irAEs. T cell mediated processes, in particular autoreactive T cells, may be involved in the development of myocarditis and skin toxicities. The microbiome may impact risk of developing irAEs, such as colitis, and may also be mechanistically important.
Autoreactive T cells have been postulated to play a role in the development of CPI-induced irAEs. In patients with fulminant myocarditis from checkpoint blockade, histopathological analysis of myocardial and skeletal tissue showed CD4+ and CD8+ T cell infiltrate but not CD20+ cells or antibody deposits [20]. In some patients with CPI-induced DM an increase in beta cell antigen reactive CD8+T cells was increased in the circulation[21]. T cell infiltrates have been implicated in several other CPI-induced irAEs including colitis, dermatological complications, nephritis, liver injury, sicca syndrome and pneumonitis, in several cases with a predominance of CD4+ T cells [22,23]. A role for Th1 and Th17 cells has been proposed for CPI-induced colitis [22,24].
There is also evidence for a role for humoral immunity in CPI-induced irAEs. Das et al identified a role for early B cell changes in irAEs in melanoma patients treated with checkpoint blockade [25]. These changes included a reduction in circulating B cells and an increase in CD21lo PD-1+ B cells and plasmablasts with changes preceding and correlated with frequency and timing of adverse events. Autoantibodies implicated in spontaneous autoimmune diseases have been identified in CPI-induced adverse events but their role remains unclear. For example, in patients who develop thyroid dysfunction, autoantibodies (thyroglobulin and/or thyroid peroxidase antibodies) are only detected in 18–70% of patients whereas in Hashimoto’s thyroiditis they are present 90–95% of the time [19,26,27]. There is some evidence that the presence of thyroid autoantibodies at baseline may increase the risk of CPI-induced thyroid dysfunction [28–30]. Similarly, in spontaneous T1D, beta cell reactive autoantibodies are detected in >90% of individuals, but only present in about 50% of patients with CPI-DM [10,14,31]. Approximately half of patients with CPI-induced myasthenia gravis (MG) have anti-acetylcholine receptor antibodies [32]. Patients with CPI-induced inflammatory arthritis are more likely to be rheumatoid factor (RF) and cyclic citrullinated peptide negative compared to rheumatoid arthritis patients [33]. In most cases, there is lack of data regarding whether autoantibodies are present prior to CPI initiation. One study profiled baseline antibodies in melanoma patients treated with CPIs and identified antibody signatures predictive of irAEs, including those targeting TNFα signaling pathways [34]. Another study examining preexisting autoantibodies (RF, antithyroglobulin, thyroid peroxidase, and anti-nuclear antibody) in patients with NSCLC treated with anti-PD-1 found that autoantibodies were more common in patients who developed irAEs[30]. Skin iRAEs, for example, were more frequent in patients who were positive for RF factor at baseline.
Shared antigens between tumor and tissue may lead to stimulation of autoreactive cells. There was evidence of shared T cell clones in the cardiac muscle, skeletal muscle and tumor supporting a role for autoreactive T cells in the development of myocarditis that develop due to shared antigens [20]. In patients with lung cancer who develop skin toxicities, shared T cell antigens were detected in the skin and tumor and were able to stimulate T cells in vitro [35]. Likewise, vitiligo is a frequent event in patients with melanoma treated with CPIs [16].
A unique mechanism has been postulated to lead to hypophysitis in anti-CTLA-4 treated patients. Iwama et al found that human anterior pituitary cells express CTLA-4 and in a mouse model of anti-CTLA-4 induced hypophysitis these cells became sites of anti-CTLA-4 binding and subsequent complement deposition and immune cell infiltration. Patients with CPI-induced hypophysitis developed anti-pituitary antibodies whereas patients without hypophysitis did not [36]. Pathology from patients with CPI-induced hypophysitis confirmed CTLA-4 expression on pituitary cells and implicated both IgG and T cell mediated processes in pituitary destruction. The currently proposed mechanism involves binding of anti-CTLA-4 to pituitary cells, complement activation with macrophage/phagocyte infiltration, enhanced antigen presentation and infiltration of B and T cells, leading to endocrine cell destruction [37].
Certain cytokines and chemokines, have been implicated in tumor response and development of toxicities in patients treated with CPIs. Baseline IL-17 levels were found to be associated with subsequent development of colitis [38]. Increased levels of IL-17 were found in patients with colitis and the rise and fall in levels were temporally correlated with the development and resolution of the complication respectively [39]. Elevated IL-6 and IL-10 have been identified in patients with dermatological irAEs[40]. Elevated IL-1β, IL-2, and GM-CSF at baseline and early decrease in IL-8, G-CSF, and MCP-1 were associated with development of thyroid dysfunction[41].
The microbiome has been suggested to play a role in both tumor response and the development of irAE [42–44]. Patients treated with anti-CTLA-4 for melanoma with baseline gut microbiome enriched for Faecalibacterium and other Firmicutes had better tumor response and longer progression-free survival and to a lesser extent overall survival. These subjects also had more frequent colitis whereas those enriched for Bacteroidetes had less frequent colitis [43]. A prospective study of patients with melanoma undergoing anti-CTLA-4 treatment, also found that patients with abundant pre-inflammation Bacteroidetes phylum were less likely to develop CPI-induced colitis [44]. Similarly, tumor response to anti-PD-L1 can also be modulated by the microbiome [42]. Although the anti-tumor efficacy has been linked, there have not been clear ties between the microbiome and irAEs.
There is an emerging interest in tissue-resident memory T (Trm) cells in cancer and evidence that these cells may be targets of CPIs in the tumor microenvironment and influence anti-tumor response as well as irAEs [45]. Finally, CTLA-4 on Tregs is an important modulator of Treg function [46]. Although there is mixed data on the effects of anti-CTLA-4 on eliminating or modulating Tregs in the tumor microenvironment [47], given that Tregs represent an important gatekeeper for the prevention of autoimmunity, a potential role for Tregs in CPI-induced irAEs warrants further investigation.
Risk factors/predictors of autoimmunity (Table 1):
Table 1:
Predictors of adverse events following checkpoint inhibitor therapy
| Genotype | • High risk T1D HLA alleles, such as HLA-DR4, in patients with CPI-induced DM [10, 14] • HLA-DRB1*04:05 association with CPI-induced inflammatory arthritis [33] • HLA-DRB1*11:01 association with CPI-induced pruritis [48] |
| History of autoimmune disease | • irAEs may be more frequent and occur sooner in patients with underlying autoimmune disease [49, 50] |
| Baseline autoantibodies | • Presence of thyroid autoantibodies at baseline increase risk of thyroid dysfunction [28–30] • Skin irAEs may be more frequent in patients with positive RF at baseline [30] • Baseline autoantibody signatures, such as those targeting TNFα signaling pathways may be predictive of irAEs [34] |
| Cytokine levels | • Baseline IL-17 levels may predict CPI-induced colitis [38] • IL-1β, IL-2, and GM-CSF at baseline and early decreases in IL-8, G-CSF, and MCP-1 predict thyroid dysfunction [41] • Cytokine toxicity score predictive of severe irAEs [93] |
| Immune cell changes | • Reduction in circulating B cells, increase in CD21lo PD-1+ B cells and plasmablasts precede adverse events [25] • Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio may predict appearance of irAEs [94] |
| Microbiome | • Baseline microbiome enriched for Faecalibacterium predictive of colitis [43] • Abundance of Bacteroidetes phylum may be protective for development of colitis [44] |
| Tumor burden | • High tumor burden in NSCLC associated with higher risk of severe irAEs [95] |
Abbreviations: DM: diabetes, G-CSF: granulocyte colony-stimulating factor, GM-CSF: granulocyte-macrophage colony-stimulating factor, HLA: human leukocyte antigen, irAE: immune-related adverse event, MCP-1: monocyte chemoattractant protein-1, NSCLC: non-small cell lung cancer, RF: rheumatoid factor, T1D: type 1 diabetes
Genetic factors may affect the development of irAEs following CPIs. A number of studies have identified a significant HLA-DR4 predominance in patients who develop CPI-DM [10,14]. Other organ specific HLA associations include HLA-DRB1*04: 05 in patients with CPI-induced inflammatory arthritis [33] and HLA-DRB1*11:01 and pruritis [48]. There may be an interaction between HLA genotypes, primary cancer, and the CPI.
Cancer patients with underlying autoimmune disease were not initially included in clinical trials and therefore their risk of developing irAEs or exacerbation of their underlying autoimmune disease were not well understood. irAEs may be more frequent in patients with underlying autoimmune disease [49,50]. There is evidence that onset of irAEs may occur faster in patients with underlying autoimmune disease [50] suggesting that close monitoring of these patients early in the treatment course is warranted. Although most autoimmune flares and irAEs could be managed without discontinuation of therapy, fatalities are possible [51]. Studies regarding risk of autoimmune flares or irAEs in patients whose autoimmune disease is active vs inactive at the start of CPI have been conflicting [49,51]. In general, it is advisable that patients with underlying autoimmunity, especially when the exacerbation of which could produce significant morbidity and even mortality (ie inflammatory bowel disease (IBD)) should be managed by multidisciplinary teams with careful consideration of alternative therapies, risks and benefits.
Adverse events and treatment response:
Studies have thus far been conflicting regarding whether the development of irAEs and/or their severity may predict tumor response. Although not all have shown an association of irAEs with improved tumor response [52], several reports suggest that patients who develop irAEs, including for example endocrine complications and vitiligo in patients with melanoma, have better tumor response rates and improved survival [12,53–56]. Importantly, in large, randomized trials, patients who discontinue CPIs due to toxicity are more likely to respond than patients without clinically meaningful irAEs [57]. Our analysis in patients with CPI-DM showed that the majority of patients had at least partial tumor response but numbers were too small to make statistical inferences[10].
Management:
Management of irAEs depends on the type of adverse event, its mechanism, and severity. Due to lack of trials to guide management, treatment decisions are primarily based on data from retrospective studies and expert consensus [12]. In general, low grade irAEs do not require cessation of therapy and may not require immunosuppressive therapy whereas higher grade adverse events may require both.
Glucocorticoids are the most frequent first line anti-inflammatory agent used in patients with CPI-induced irAEs and are effective in most cases. In deciding to treat patients with glucocorticoids it is important to consider potential side effects of steroid treatment and potential impact on anti-tumor response. Several studies have demonstrated that response rates and/or survival are not affected by the need for steroids to treat irAEs [52,53,58]. A recent systematic review and meta-analysis found that steroids used to mitigate irAEs, as opposed to symptomatic or supportive care, did not negatively impact survival [58]. However, steroids at the start of or early in the course of CPI treatment may negatively impact outcomes [59–62]. Patients with lung cancer treated with anti-PD-1 on >10mg daily of prednisone at baseline had worse outcomes compared to patients on low dose steroids at baseline, although steroid indication seems to play a role [59,63].
Depending on severity, duration, and response of adverse events to steroids, other immunosuppressive agents may be required. In cases of severe irAEs refractory to steroids, infliximab, a mAb targeting TNFα, has emerged as an alternate therapeutic agent that has been used successfully to treat several irAEs (e.g., colitis, rashes, pneumonitis, ocular complications, myocarditis, among others)[12]. Infliximab, which has been used in the treatment of IBD, has been particularly useful in cases of CPI-induced colitis refractory to glucocorticoids [17,64,65]. In murine models, prophylactic TNFα blockade also eliminated colitis without affecting anti-tumor response [66]. In vitro studies suggest that infliximab had a minor impact on the activation of tumor specific tumor-infiltrating lymphocytes (TILs) whereas even low doses of steroids had a negative impact on anti-tumor effects of TILs [67]. Notably, the efficacy of infliximab in treating colitis points to a potential role for TNFα in CPI-induced colitis. (Table 2)
Table 2:
Therapies for checkpoint inhibitor related adverse events
| Drug name | Mechanism of action | Examples of efficacy in reversing irAE | Effect on tumor response | Clinical trial |
|---|---|---|---|---|
|
Routinely used immunosuppressants: | ||||
| Glucocorticoids | Anti-inflammatory agent | • 1st line therapy for most irAEs. • Not effective for reversing endocrinopathies. |
• Some studies suggest that tumor response and survival are not affected by steroids [52, 53, 58] whereas others show possible negative impact [54, 59–63]. | |
| • In vitro data suggests negative impact on anti-tumor effects of TILs [67]. | ||||
| Infliximab | TNFα mAb | • Colitis [17, 64] • May be safer and result in faster resolution of symptoms compared to steroids for colitis [64,65]. • Others: Myocarditis, pneumonitis, uveitis [12] |
• No known negative impact on tumor response [64,65]. • Preclinical studies suggest that anti-tumor response is maintained [66,67]. |
|
|
Emerging and potential future therapeutic options: | ||||
| Vedolizumab | Gut specific integrin α4β7 mAb | • Colitis [68–70] • Potential benefit in preventing autoimmune flares in patients with IBD? [71] |
Clinical outcomes favorable [69, 71]. | NCT04407247: Comparison of vedolizumab vs infliximab for clinical remission/response of CPI-induced diarrhea/colitis |
| Alemtuzumab | CD52 mAb | Myocarditis [78] | Unknown | |
| Abatacept | CTLA-4 agonist | • Myocarditis [79] • CPI-induced worsening of myasthenia gravis (MG) [80] |
No tumor progression at 1 month [79] | |
| Rituximab | CD20 mAb | Neurological complications (eg encephalitis and MG), bullous pemphigoid-like skin disease, renal vasculitis, hematological complications [12, 40, 82–85] | Mixed: progression, partial and complete responses reported [82–84] | NCT03719131: Comparison of irAEs in patients on CPIs with or without rituximab |
| Tocilizumab | IL-6 receptor antagonist | • Pneumonitis, serum sickness/SIRS, cerebritis, colitis, pancreatitis and coagulopathy [86] • Inflammatory arthritis [87] |
• No statistically significant difference in overall survival but limited by small size and with trend towards worse survival with increased doses of tocilizumab [86]. • 1/3 patients maintained durable anti-tumor response [87]. |
NCT03601611: Benefit of tocilizumab on diarrhea and/ or colitis and/ or arthritis induced by CPIs |
| Secukinumab | IL-17A mAb | • Psoriatic rash and colitis [88] • Psoriasiform dermatological complication [89] |
Tumor progression occurred in one patient [88] but in the other there was no impact on tumor response [89]. | |
| Fecal transplant | Possibly due to increased Tregs and decreased effector T cells [90] | Colitis [90] | Unknown | • NCT04038619: Phase I trial of fecal transplant for CPI-induced colitis/ diarrhea • NCT04163289: Preventing irAEs using fecal transplant |
Not included are other immunosuppressants occasionally used/recommended for treatment of irAEs such as mycophenolate mofetil, tacrolimus, cyclophosphamide, intravenous immunoglobulin, and anti-thymocyte globulin [12].
Abbreviations: IBD: inflammatory bowel disease, irAE: Immune related adverse event, mAb: monoclonal antibody, MG: myasthenia gravis, SIRS: systemic inflammatory response syndrome, TIL: tumor-infiltrating lymphocytes
While most CPI-induced irAEs resolve, endocrine adverse events are unique in that they are largely irreversible. Glucocorticoids have not been shown to reverse endocrine complications and in the case of hypophysitis, high dose steroids may worsen clinical outcomes [54,73]. One case report suggested that infliximab may be efficacious in reversing CPI-DM. Reversal of diabetes occurred in the setting of infliximab treatment for oligoarthritis but the patient had been on steroids, mixed meal tolerance test showed both impaired insulin secretion and peripheral resistance, and the patient was not documented to be insulinopenic [74]. Thus, it is not clear that this was a typical case of CPI-DM and the potential benefit of infliximab for this complication remains uncertain. Inflammatory arthritis is also unique in that the complication often persists and requires long term immunosuppression [72]. Treatment modalities include steroids in the majority of patients and in some cases conventional synthetic or biological disease-modifying antirheumatic drugs.
The decision to resume CPI treatment following an irAE depends on the severity of the complication, the stage of the malignancy and availability of alternate therapies. Studies to date show that it is possible to retreat with CPIs following complications. However, there should be careful consideration of risks and benefits given patients can have recurrence or development of new toxicities and some cases of fatality have been reported with retreatment [1,75]. Furthermore, some patients who have had treatment success and require cessation of therapy due to irAEs may not need to restart CPIs due to ongoing benefit from therapy [76].
There is currently no known treatment modality to prevent CPI-induced irAEs. Budesonide treatment was not protective in anti-CTLA-4 induced colitis [77]. While one study showed that baseline steroids prior to CPI start was protective against the development of irAEs, as mentioned previously baseline prednisone at doses >10mg daily may negatively impact survival[59].
Potential emerging/future therapies:
As our understanding of the mechanisms underlying CPI-induced irAEs grows, our ability to offer patients more targeted therapies for these complications with limited impact on tumor responses is essential. Emerging therapies have been shown in some cases to successfully treat irAEs, but in general the efficacy and impact on tumor responses of these therapies remains unknown. Myocarditis has the highest fatality rates amongst irAEs and thus understanding the mechanisms underlying this complication and identifying additional treatment modalities is of great interest. Alemtuzumab (anti-CD52) and abatacept (CTLA-4 agonist) have recently been used to successfully treat glucocorticoid refractory myocarditis[78,79]. A concern with abatacept is potential for infectious complications and possible tumor progression. After one month of treatment with abatacept there was no evidence of tumor progression in the treated patient, but further studies are needed to better understand the efficacy on myocarditis reversal and possible effects on tumor growth. Interestingly, abatacept has also been used successfully to treat CPI-induced worsening of MG [80]. Anti-CD52 leads to T cell depletion (as well as other peripheral immune cells) and although it led to resolution of a potentially fatal complication its impact on tumor response is not known.
Vedolizumab, a gut specific anti-integrin α4β7 antibody, that is used for treatment of IBD is emerging as a promising potential treatment option for patients with CPI-induced colitis. Small studies have shown that vedolizumab leads to colitis remission in patients with CPI-induced colitis that is steroid refractory with favorable outcomes and good safety profiles [68,69]. Furthermore, early introduction of selective immunosuppressive agents such as infliximab and vedolizumab may be associated with more favorable clinical outcomes [70]. A patient with Crohn’s disease was successfully treated for advanced melanoma with concurrent anti-PD-1 and vedolizumab without autoimmune disease flare [71]. Whether selective inhibition of T cell migration to other tissues affected by CPIs is feasible is not yet known.
A preclinical model suggests that B cell targeted therapies may potentially be used to treat irAEs without impacting anti-tumor activity [81]. Rituximab, an antibody targeting CD20, has been used as an alternate or additional therapy in steroid refractory neurological complications of CPIs, such as encephalitis and MG, [82,83], bullous pemphigoid-like skin disease [40], renal vasculitis[84], and hematological complications[85]. Intravenous immunoglobulins have also been used [12,85]. Tocilizumab, an IL-6 receptor antagonist, has been used in patients, mostly with lung cancer, treated with anti-PD-1 and with steroid refractory irAEs[86]. Clinical improvement was noted in 79.4% of patients but further work is necessary to better understand the efficacy and impact on clinical course of this inhibitor. Tocilizumab has also been used to successfully treat severe CPI-induced inflammatory arthritis[87]. A case of a patient with preexisting autoimmunity on anti-PD-1 for colon cancer developed skin and GI complications that were successfully reversed with IL-17 blockade [88]. However, IL-17 blockade led to tumor progression. A patient with melanoma and with anti-PD-1 induced psoriasiform dermatological complication responded to IL-17A blockade without altering the patient’s tumor response [89].
Manipulation of the gut microbiome may be a potential mechanism for preventing the development of colitis. Fecal transplant has been successfully used for the treatment of refractory CPI-induced colitis, possibly through a Treg mediated process [90]. However, there is evidence that higher gut microbiome diversity is associated with improved tumor response [91] and antibiotic consumption is associated with reduced response to anti-PD-1 treatment [92]; microbiome manipulation could potentially impact cancer course.
Conclusions:
Autoimmune side effects of CPIs are frequent and, in some cases, can have significant morbidity and even mortality. The pathophysiology of the irAEs is related to the mechanisms of action of the CPIs but the organ(s) affected vary according to the CPI and other patient factors including HLA. Many of the irAEs resolve or can be treated with brief courses of glucocorticoids. Others, including endocrine and cardiac irAEs may be permanent or have dire consequences. Further studies may identify immediate causes of the irAEs or may identify those individuals at highest risk to avoid morbidity and potentially prevent the onset without affecting the anti-tumor response.
Highlights.
Adverse events are frequent with checkpoint inhibitors and occurs with all tumor types
A single mechanism has not emerged as explaining all of these adverse events
Host factors such as genotype and microbiome may affect risk of the adverse events
Studies are identifying ways to stop adverse events without impeding tumor response
Acknowledgements
Supported by grants R01CA227473, R21AI135562, R43DK116577, R01DK120362, P50CA121974, R01216846, DK0077058, JDRF 3-APF-2019-753-A-N, and from the Parker Institute/JDRF/ Helmsley Foundation.
Conflicts of interest
Drs Herold and Perdigoto have no relevant conflicts of interest to declare. Dr Kluger has received research funding (institutional funding) from Merck, Bristol-Myers Squibb, and Apexigen, and personal fees from Corvus, Nektar, Pfizer, Iovance, Immunocore, Celldex, Array Biopharma, Merck, Bristol-Myers Squibb, Instilbio, Elevate Bio, Clinigen and Shionogi.
Abbreviations:
- CPI
checkpoint inhibitor
- CTLA-4
cytotoxic T-lymphocyte antigen 4
- DM
diabetes
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HLA
human leukocyte antigen
- IBD
inflammatory bowel disease
- irAE
immune-related adverse event
- mAb
monoclonal antibody
- MCP-1
monocyte chemoattractant protein-1
- MG
myasthenia gravis
- NSCLC
non-small cell lung cancer
- PD-1
programmed cell death 1
- PD-L1
programmed cell death ligand 1
- RCC
renal cell carcinoma
- RF
rheumatoid factor
- T1D
type 1 diabetes
- TIL
tumor-infiltrating lymphocytes
- Trm
tissue-resident memory T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Postow MA, Sidlow R, Hellmann MD: Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018, 378:158–168. [DOI] [PubMed] [Google Scholar]
- 2.Ribas A, Wolchok JD: Cancer immunotherapy using checkpoint blockade. Science 2018, 359:1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan KK, Bass AR: Autoimmune complications of immunotherapy: pathophysiology and management. BMJ 2020, 369:m736. [DOI] [PubMed] [Google Scholar]
- 4.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T: Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015, 13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnaud-Coffin P, Maillet D, Gan HK, Stelmes JJ, You B, Dalle S, Peron J: A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer 2019, 145:639–648. [DOI] [PubMed] [Google Scholar]
- 6.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al. : Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 2017, 377:1345–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, Shen C, Duma N, Vera Aguilera J, Chintakuntlawar A, et al. : Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol 2019, 5:1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, Li X, Zhu H, Zhong X, Pan J, et al. : The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): a systematic review and meta-analysis. BMC Cancer 2019, 19:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, Lebbe C, Kirkwood JM, Schachter J, Daniels GA, et al. : Pooled Analysis Safety Profile of Nivolumab and Ipilimumab Combination Therapy in Patients With Advanced Melanoma. J Clin Oncol 2017, 35:3815–3822. [DOI] [PubMed] [Google Scholar]
- 10.Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, Gettinger S, Sznol M, Young A, Rushakoff R, et al. : Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 2018, 67:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM: Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol 2018, 4:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. : Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018, 36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR: Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017, 28:2377–2385. [DOI] [PubMed] [Google Scholar]
- 14.de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, Van der Auwera BJ, Gorus FK, Roep BO, Aspeslagh S, Neyns B, et al. : Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol 2019, 181:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo JA, Fisher DE, Flaherty KT: Prognostic Significance of Cutaneous Adverse Events Associated With Pembrolizumab Therapy. JAMA Oncol 2015, 1:1340–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, et al. : Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016, 54:139–148. [DOI] [PubMed] [Google Scholar]
- 17.Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, Marthey L, Collins M, Chaput N, Robert C, Carbonnel F: Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut 2018, 67:2056–2067. [DOI] [PubMed] [Google Scholar]
- 18.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. : Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018, 4:1721–1728.* This systematic review and meta-analysis highlights that toxicities including endocrine complications can be fatal.
- 19.de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, Bravenboer B: Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated With Pembrolizumab. J Clin Endocrinol Metab 2016, 101:4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, et al. : Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016, 375:1749–1755.** This important paper shows that autoreactive T cells play a role in the development of myocarditis and that shared antigens are involved.
- 21.Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, Herold KC: Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 2015, 38:e55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibraheim H, Perucha E, Powell N: Pathology of immune-mediated tissue lesions following treatment with immune checkpoint inhibitors. Rheumatology (Oxford) 2019, 58:vii17–vii28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warner BM, Baer AN, Lipson EJ, Allen C, Hinrichs C, Rajan A, Pelayo E, Beach M, Gulley JL, Madan RA, et al. : Sicca Syndrome Associated with Immune Checkpoint Inhibitor Therapy. Oncologist 2019, 24:1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bamias G, Delladetsima I, Perdiki M, Siakavellas SI, Goukos D, Papatheodoridis GV, Daikos GL, Gogas H: Immunological Characteristics of Colitis Associated with Anti-CTLA-4 Antibody Therapy. Cancer Invest 2017, 35:443–455. [DOI] [PubMed] [Google Scholar]
- 25.Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, Bacchiocchi A, Kluger H, Wei W, Halaban R, et al. : Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 2018, 128:715–720.** Important paper demonstrating a role for B cells in the development of irAEs and that changes in B cells may predict adverse events.
- 26.Mazarico I, Capel I, Gimenez-Palop O, Albert L, Berges I, Luchtenberg F, Garcia Y, Fernandez-Morales LA, De Pedro VJ, Caixas A, et al. : Low frequency of positive antithyroid antibodies is observed in patients with thyroid dysfunction related to immune check point inhibitors. J Endocrinol Invest 2019, 42:1443–1450. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi I, Yasoda A, Matsumoto S, Sakamori Y, Kim YH, Nomura M, Otsuka A, Yamasaki T, Saito R, Kitamura M, et al. : Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PLoS One 2019, 14:e0216954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maekura T, Naito M, Tahara M, Ikegami N, Kimura Y, Sonobe S, Kobayashi T, Tsuji T, Minomo S, Tamiya A, et al. : Predictive Factors of Nivolumab-induced Hypothyroidism in Patients with Non-small Cell Lung Cancer. In Vivo 2017, 31:1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimbara S, Fujiwara Y, Iwama S, Ohashi K, Kuchiba A, Arima H, Yamazaki N, Kitano S, Yamamoto N, Ohe Y: Association of antithyroglobulin antibodies with the development of thyroid dysfunction induced by nivolumab. Cancer Sci 2018, 109:3583–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, Kawana S, Saito R, Aso M, Tsurumi K, et al. : Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2019, 5:376–383.* One of few studies investigating the role of preexisting autoantibodies in the development in the development of irAEs.
- 31.Bingley PJ: Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab 2010, 95:25–33. [DOI] [PubMed] [Google Scholar]
- 32.Johansen A, Christensen SJ, Scheie D, Hojgaard JLS, Kondziella D: Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: Systematic review. Neurology 2019, 92:663–674. [DOI] [PubMed] [Google Scholar]
- 33.Cappelli LC, Dorak MT, Bettinotti MP, Bingham CO, Shah AA: Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology (Oxford) 2019, 58:476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gowen MF, Giles KM, Simpson D, Tchack J, Zhou H, Moran U, Dawood Z, Pavlick AC, Hu S, Wilson MA, et al. : Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J Transl Med 2018, 16:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berner F, Bomze D, Diem S, Ali OH, Fassler M, Ring S, Niederer R, Ackermann CJ, Baumgaertner P, Pikor N, et al. : Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol 2019, 5:1043–1047.** Another significant paper showing a role for shared antigens in the development of skin toxicities in patients with lung cancer.
- 36.Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P: Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014, 6:230ra245.** This study identifies that direct binding of anti-CTLA-4 to pituitary cells and complement activation may lead to hypophysitis.
- 37.Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, Taverna G, Cosottini M, Lupi I: Hypophysitis Secondary to Cytotoxic T-Lymphocyte-Associated Protein 4 Blockade: Insights into Pathogenesis from an Autopsy Series. Am J Pathol 2016, 186:3225–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, Kirkwood JM: Baseline circulating IL-17 predicts toxicity while TGF-beta1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015, 3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Callahan MK YA, Tandon S, et al. : Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis. J Clin Oncol 2011, 29. [Google Scholar]
- 40.Phillips GS, Wu J, Hellmann MD, Postow MA, Rizvi NA, Freites-Martinez A, Chan D, Dusza S, Motzer RJ, Rosenberg JE, et al. : Treatment Outcomes of Immune-Related Cutaneous Adverse Events. J Clin Oncol 2019, 37:2746–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S, Takeshima K, Furukawa Y, Morita S, Yamamoto Y, et al. : Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci 2020, 111:1468–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al. : Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, et al. : Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017, 28:1368–1379.* One of several studies showing that baseline microbiome may influence the development of colitis.
- 44.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, et al. : Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun 2016, 7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanc C, Hans S, Tran T, Granier C, Saldman A, Anson M, Oudard S, Tartour E: Targeting Resident Memory T Cells for Cancer Immunotherapy. Front Immunol 2018, 9:1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S: CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322:271–275. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Zheng P: How Does an Anti-CTLA-4 Antibody Promote Cancer Immunity? Trends Immunol 2018, 39:953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasan Ali O, Berner F, Bomze D, Fassler M, Diem S, Cozzio A, Jorger M, Fruh M, Driessen C, Lenz TL, et al. : Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur J Cancer 2019, 107:8–14. [DOI] [PubMed] [Google Scholar]
- 49.Haanen J, Ernstoff MS, Wang Y, Menzies AM, Puzanov I, Grivas P, Larkin J, Peters S, Thompson JA, Obeid M: Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol 2020. [DOI] [PubMed] [Google Scholar]
- 50.Danlos FX, Voisin AL, Dyevre V, Michot JM, Routier E, Taillade L, Champiat S, Aspeslagh S, Haroche J, Albiges L, et al. : Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer 2018, 91:21–29. [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME: Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med 2018, 168:121–130. [DOI] [PubMed] [Google Scholar]
- 52.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, Carvajal RD, Dickson MA, D’Angelo SP, Woo KM, et al. : Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015, 33:3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, et al. : Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 2017, 35:785–792. [DOI] [PubMed] [Google Scholar]
- 54.Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, Cohen J, Sullivan RJ: High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018, 124:3706–3714. [DOI] [PubMed] [Google Scholar]
- 55.Kotwal A, Kottschade L, Ryder M: PD-L1 Inhibitor-Induced Thyroiditis Is Associated with Better Overall Survival in Cancer Patients. Thyroid 2020, 30:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, Viollet R, Thomas M, Roy S, Benannoune N, et al. : Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol 2016, 152:45–51. [DOI] [PubMed] [Google Scholar]
- 57.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. : Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015, 373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L, Cabiddu M, Borgonovo K, Dognini G, Brighenti M, et al. : Association of Steroids use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers (Basel) 2020, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, Martinez-Bernal G, Ferrara R, Lai WV, Hendriks LEL, et al. : Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 2018, 36:2872–2878. [DOI] [PubMed] [Google Scholar]
- 60.Scott SC, Pennell NA: Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J Thorac Oncol 2018, 13:1771–1775. [DOI] [PubMed] [Google Scholar]
- 61.Fuca G, Galli G, Poggi M, Lo Russo G, Proto C, Imbimbo M, Ferrara R, Zilembo N, Ganzinelli M, Sica A, et al. : Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019, 4:e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chasset F, Pages C, Biard L, Roux J, Sidina I, Madelaine I, Basset-Seguin N, Viguier M, Madjlessi-Ezr AN, Schneider P, et al. : Single-center study under a French Temporary Authorization for Use (TAU) protocol for ipilimumab in metastatic melanoma: negative impact of baseline corticosteroids. Eur J Dermatol 2015, 25:36–44. [DOI] [PubMed] [Google Scholar]
- 63.Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM: Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol 2019, 37:1927–1934. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, Lum P, Raju G, Shuttlesworth G, Stroehlein J, et al. : Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer 2018, 6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL Jr., Abdel-Wahab N, Anderson J, Davis JE, Joseph J, Uemura M, et al. : Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer 2018, 6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, de Andrea C, Rodriguez-Ruiz ME, Perez-Gracia JL, Marquez-Rodas I, et al. : Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019, 569:428–432.** Important study showing that it is possible to uncouple treatment of irAEs and anti-tumor response.
- 67.Draghi A, Borch TH, Radic HD, Chamberlain CA, Gokuldass A, Svane IM, Donia M: Differential effects of corticosteroids and anti-TNF on tumor-specific immune responses: implications for the management of irAEs. Int J Cancer 2019, 145:1408–1413. [DOI] [PubMed] [Google Scholar]
- 68.Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, Carneiro A, Marsal J: Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 2017, 66:581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abu-Sbeih H, Ali FS, Alsaadi D, Jennings J, Luo W, Gong Z, Richards DM, Charabaty A, Wang Y: Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J Immunother Cancer 2018, 6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abu-Sbeih H, Ali FS, Wang X, Mallepally N, Chen E, Altan M, Bresalier RS, Charabaty A, Dadu R, Jazaeri A, et al. : Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer 2019, 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frohne CC, Llano EM, Perkovic A, Cohen RD, Luke JJ: Complete response of metastatic melanoma in a patient with Crohn’s disease simultaneously receiving anti-alpha4beta7 and anti-PD1 antibodies. J Immunother Cancer 2019, 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braaten TJ, Brahmer JR, Forde PM, Le D, Lipson EJ, Naidoo J, Schollenberger M, Zheng L, Bingham CO, Shah AA, et al. : Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis 2020, 79:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma C, Hodi FS, Giobbie-Hurder A, Wang X, Zhou J, Zhang A, Zhou Y, Mao F, Angell TE, Andrews CP, et al. : The Impact of High-Dose Glucocorticoids on the Outcome of Immune-Checkpoint Inhibitor-Related Thyroid Disorders. Cancer Immunol Res 2019, 7:1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trinh B, Donath MY, Laubli H: Successful Treatment of Immune Checkpoint Inhibitor-Induced Diabetes With Infliximab. Diabetes Care 2019, 42:e153–e154. [DOI] [PubMed] [Google Scholar]
- 75.Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME, Gambarin-Gelwan M, Wilkins O, Panora E, Halpenny DF, Long NM, et al. : Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol Res 2018, 6:1093–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Chesney J, et al. : Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J Clin Oncol 2017, 35:3807–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, et al. : A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009, 15:5591–5598. [DOI] [PubMed] [Google Scholar]
- 78.Esfahani K, Buhlaiga N, Thebault P, Lapointe R, Johnson NA, Miller WH Jr.: Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N Engl J Med 2019, 380:2375–2376. [DOI] [PubMed] [Google Scholar]
- 79.Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, Kerneis M: Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N Engl J Med 2019, 380:2377–2379. [DOI] [PubMed] [Google Scholar]
- 80.Kumar B, Ballas Z: Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018, 378:1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Damsky W, Jilaveanu L, Turner N, Perry C, Zito C, Tomayko M, Leventhal J, Herold K, Meffre E, Bosenberg M, et al. : B cell depletion or absence does not impede anti-tumor activity of PD-1 inhibitors. J Immunother Cancer 2019, 7:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ito M, Fujiwara S, Fujimoto D, Mori R, Yoshimura H, Hata A, Kohara N, Tomii K: Rituximab for nivolumab plus ipilimumab-induced encephalitis in a small-cell lung cancer patient. Ann Oncol 2017, 28:2318–2319. [DOI] [PubMed] [Google Scholar]
- 83.Crusz SM, Radunovic A, Shepherd S, Shah S, Newey V, Phillips M, Lim L, Powles T, Szlosarek PW, Shamash J, et al. : Rituximab in the treatment of pembrolizumab-induced myasthenia gravis. Eur J Cancer 2018, 102:49–51. [DOI] [PubMed] [Google Scholar]
- 84.Mamlouk OLJ, Abdelrahim M, Tchakarov AS, Glass WF, Selamet U, Buni M, Abdel-Wahab N, Abudayyeh A: Checkpoint inhibitor-related renal vasculitis and use of rituximab. Journal for ImmunoTherapy of Cancer 2020, 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delanoy N, Michot JM, Comont T, Kramkimel N, Lazarovici J, Dupont R, Champiat S, Chahine C, Robert C, Herbaux C, et al. : Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol 2019, 6:e48–e57. [DOI] [PubMed] [Google Scholar]
- 86.Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, Cherukuri S, Parent T, Hardin J, Walker P: Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 2019, 25:551–557. [DOI] [PubMed] [Google Scholar]
- 87.Kim ST, Tayar J, Trinh VA, Suarez-Almazor M, Garcia S, Hwu P, Johnson DH, Uemura M, Diab A: Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 2017, 76:2061–2064. [DOI] [PubMed] [Google Scholar]
- 88.Esfahani K, Miller WH Jr.: Reversal of Autoimmune Toxicity and Loss of Tumor Response by Interleukin-17 Blockade. N Engl J Med 2017, 376:1989–1991. [DOI] [PubMed] [Google Scholar]
- 89.Johnson D, Patel AB, Uemura MI, Trinh VA, Jackson N, Zobniw CM, Tetzlaff MT, Hwu P, Curry JL, Diab A: IL17A Blockade Successfully Treated Psoriasiform Dermatologic Toxicity from Immunotherapy. Cancer Immunol Res 2019, 7:860–865. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez CA, Chang CC, et al. : Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med 2018, 24:1804–1808.* Demonstrates the use of fecal transplant as possible treatment option for refractory CPI-induced colitis.
- 91.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. : Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. : Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359:91–97. [DOI] [PubMed] [Google Scholar]
- 93.Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, Breen EJ, Yang JY, Ghazanfar S, Kefford RF, et al. : Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res 2019, 25: 1557–1563. [DOI] [PubMed] [Google Scholar]
- 94.Paven A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, Guarneri V, Aprile G, Conte P, Bonanno L: Peripheral blood markers identify risk of immune-realted toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. Oncologist 2019, 24: 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakata Y, Kawamura K, Ichikado K, Shingu N, Yasuda Y, Eguchi Y, Anan K, Hisanaga J, Nitawaki T, Iio M, et al. : The association between tumor burden and severe immune-related adverse events in non-small cell lung cancer patients responding to immune-checkpoint inhibitor treatment. Lung Cancer 2019, 130: 159–161. [DOI] [PubMed] [Google Scholar]