Abstract
Following traumatic peripheral nerve injury, adequate restoration of function remains an elusive clinical goal. Recent research highlights the complex role that the immune system plays in both nerve injury and regeneration. Pro-regenerative processes in wounded soft tissues appear to be significantly mediated by cytokines of the type 2 immune response, notably interleukin (IL)-4. While IL-4 signaling has been firmly established as a critical element in general tissue regeneration during wound healing, it has also emerged as a critical process in nerve injury and regeneration. In this context of peripheral nerve injury, endogenous IL-4 signaling has recently been confirmed to influence more than leukocytes, but including also neurons, axons, and Schwann cells. Given the role IL-4 plays in nerve injury and regeneration, exogenous IL-4 and/or compounds targeting this signaling pathway have shown encouraging preliminary results to treat nerve injury or other neuropathy in rodent models. In particular, the exogenous stimulation of the IL-4 signaling pathway appears to promote post-injury neuron survival, axonal regeneration, remyelination, and thereby improved functional recovery. These preclinical data strongly suggest that targeting IL-4 signaling pathways is a promising translational therapy to augment treatment approaches of traumatic nerve injury. However, a better understanding of the type 2 immune response and associated signaling networks functioning within the nerve injury microenvironment is still needed to fully develop this promising therapeutic avenue.
Keywords: Anti-Inflammatory, Inflammation, Interleukin, Peripheral Nerve, Regeneration
Introduction
Every year, over 3 million upper extremity traumas and over 4 million lower extremity traumas are estimated to occur in the US1,2. Of these, approximately 3% and 2%, respectively, involve peripheral nerve injury (PNI)3,4. PNIs can result in severe motor and sensory deficits, as well as neuropathic pain that have devastating impact on quality of life5. In an attempt to ameliorate these symptoms, patients can undergo nerve repair surgery, yet despite these efforts, restoration of function following surgery still produces poor outcomes: in median and ulnar nerve reconstructions, 52% and 43% achieve satisfactory motor and sensory recovery respectively6. These suboptimal surgical outcomes are due to factors that limit nerve regeneration: neuronal death following injury, a decreased capacity for neurons to regenerate axons over time, the slow growth of regenerating axons compounded with long distances between lesion site and target, misdirected axonal regrowth to targets, and potentially irreversible chronic denervation-induced muscle atrophy7–10. Thus, there is a great need to develop therapies that will enhance nerve regeneration and improve functional recovery following PNI.
The immune system plays a crucial and intricate role throughout the wound healing process. Immune cells, while low in quantity within healthy peripheral nerve, are swiftly recruited to nerve tissue in response to injury: first neutrophils, followed by monocytes/macrophages, and then lymphocytes. These immune cells respond to injury initially by phagocytosing debris and secreting cytokines to further attract leukocytes from circulation and to polarize one another towards pro-inflammatory phenotypes. With time this type 1 immune response, mediated by pro-inflammatory “M1” macrophages and type 1 T-helper cells, evolves into a pro-regenerative, healing phenotype mediated by “M2” macrophages and type 2 T-helper cells. This pro-regenerative, type 2 immune response resolves inflammation to facilitate regeneration and ultimately restore functionality of the nerve. Specifically, some cytokines have emerged as interesting candidates that could play important roles in guiding the immune and resident cells in injured tissue towards pro-regenerative processes. Interleukin 4 (IL-4) is of particular interest as it is the principal cytokine governing wound healing in the type 2 immune response. IL-4 signaling in damaged nerve may play an important role in promoting regeneration and restoring function. In this review, we will focus on the role of IL-4 signaling, both on its endogenous role during the cascade of responses that follow traumatic nerve injury and regeneration, and on its role when used as a potential exogenous therapeutic to manage nerve injuries.
Overview of nerve injury and regeneration
At a macroscopic level, nerve includes myelinated axons of motor neurons and large sensory neurons, and the non-myelinated smaller axons of sensory and autonomic neurons, bundled into fascicles to compose a nerve fiber. Following nerve injury, different segments of nerve (axons vs soma) encounter and interact with different environments and cells (Schwann cells, macrophages, fibroblasts, microglial, etc.) in a region-specific manner. As such, nerve injury can be viewed in a compartmentalized fashion (Figure 1), which we first review to provide context for the cells potentially involved in IL-4 signaling.
Figure 1 – Schematic of nerve injury and regeneration.
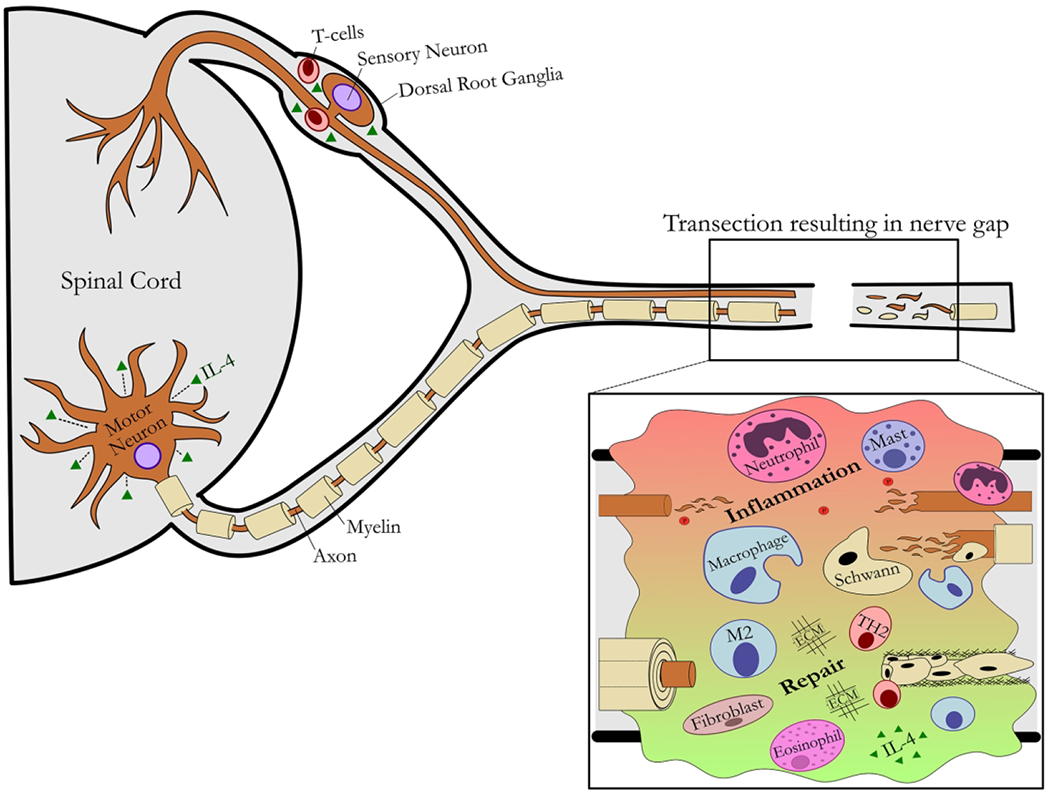
An overview of the affected central and peripheral nervous system compartments is shown in the context of a nerve transection resulting in a nerve gap.
Proximal nerve and neuronal response.
Following injury, a small amount of degeneration occurs in the axons of the proximal nerve, but these axons remain viable, as they are still intact with the neuronal soma. In these proximal axons, cytoskeletal and myelin degeneration occurs only up to the first node of Ranvier. With this, the axons undergo a dormant phase until genes are activated to facilitate axon growth. Proximal to the site of nerve injury, neurons sense compromised axonal function and upregulate a regeneration-associated gene (RAG) program in response (Figure 2). These genes become activated through the axonal trauma, as significant calcium influx, retrograde transport of kinases, and other pathways are triggered leading to activation of the RAG program. The RAG program includes expression of inflammatory signaling pathways, neurotrophic factors, and cytoskeletal elements to promote axon outgrowth from the proximal end of the damaged axon. Additionally, there is release of pro-inflammatory cytokines in the dorsal horn of the spinal cord by injury-activated glial cells, including microglial, where this action is potentiated by increased levels of neurotrophic factors locally11. Only neurons that survive the initial injury will be able to upregulate the RAG program and undergo regeneration. Motor neurons are generally spared from cell death if the axonal injury is distal enough from the cell bodies and the organism is mature12,13, while sensory neuron death can be as high as 40% regardless of injury location and age13–16.
Figure 2 – Focused schematic of the response to peripheral nerve injury within the central compartment and the cells implicated in IL-4 signaling.
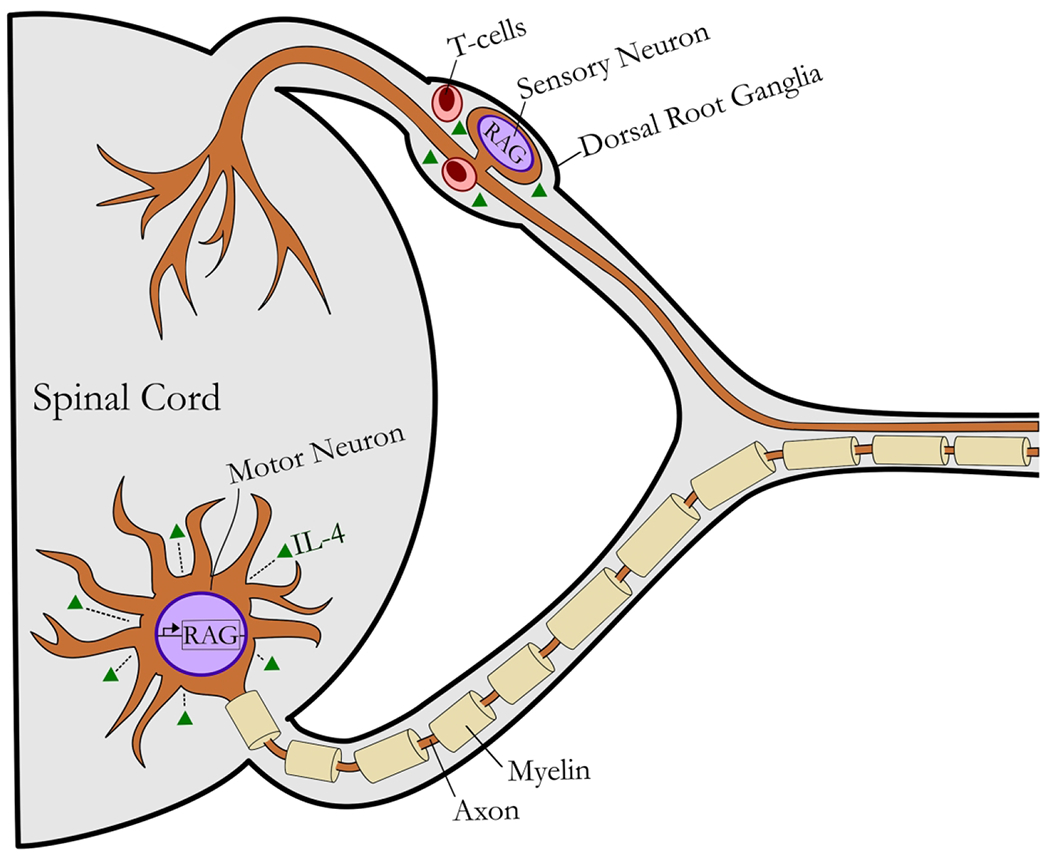
Following axotomy, injury-induced retrograde signals activate a regeneration-associated gene (RAG) program within neurons. This gene program results in release of pro-inflammatory cytokines and axon outgrowth. IL-4 signaling in the ventral horn and DRG further stimulate pro-regenerative processes within neurons, promoting cell survival and recovery. TH2 cells (Effector T cells) have been implicated as a source or regulator for IL-4 signaling post axotomy. IL-4 signaling to motoneurons promotes their survival, although its effects to sensory neurons is less clear despite the known accumulation of T cells within dorsal root ganglia.
Distal nerve response.
Following trauma to axons within a nerve, subsequent axonal degeneration occurs in the distal nerve. This axonal degeneration is necessary for nerve regeneration as it activates and recruits non-neuronal cells that clear the distal stump of debris and prepare it to receive newly sprouted axons from the proximal nerve. Schwann cells (SC) respond to the absence of axonal contact by assuming a dedifferentiated, non-myelinating, phagocytic phenotype. These new pro-regenerative Schwann cells begin expressing neurotrophic and pro-inflammatory cytokines that increase the permeability of the blood-nerve barrier, enabling neutrophils and then macrophages to arrive at the injury site. These hematogenous cells, along with resident macrophages in the nerve, assist Schwann cells in phagocytosing debris and remodeling the extracellular matrix. Once cellular debris in the distal stump has been cleared, Schwann cells begin aligning themselves along the basal lamina of the endoneurium to form hollow tubes, known as bands of Büngner, into which sprouting axons from the proximal nerve stump may grow to reach their end-organ targets8,17. Within nerve, macrophages then experience anti-inflammatory signaling that promotes their conversion to a phenotype that promotes SC maturation. Additionally, axons express neuregulin type 1, which interacts with receptor ErbB2 on SCs, signaling to SCs to mature and myelinate. These processes lead to restoration of function.
Nerve gap response.
In circumstances when nerve is severely injured resulting in a gap between the proximal and distal nerve, the response within the gap area is similar in ways to general, multi-phase, wound healing processes. For most soft tissues, at the site of injury, blood coagulation and transient vasoconstriction occur, filling the tissue gap with a blood clot consisting of platelets, macrophages, and other immune cells, ECM, growth factors, and importantly, chemotactic and pro-inflammatory cytokines. These cytokines attract leukocytes from circulating blood, first neutrophils and then monocytes/macrophages, each phagocytosing cellular debris and enhancing inflammation and repair through cytokine release. Lastly, T lymphocytes arrive and further assist in promoting and ultimately resolving inflammation in the injured tissue. These late pro-regenerative signals promote fibroblast activity, neovascularization and angiogenesis, and long-term tissue remodeling18,19.
Similarly, in nerve gaps, immediately after a nerve has been injured, an array of factors are released including pro-inflammatory chemotactic factors to attract resident and circulating leukocytes (Figure 3). Extrinsic and intrinsic coagulation cascades convert fibrinogen to fibrin, which serves as a dense ECM upon which neutrophils, macrophages, fibroblasts, endothelial cells, and later Schwann cells may migrate to bridge the proximal and distal nerve stumps. Neutrophils and then macrophages arrive at the injury site migrating upon the scaffold and begin phagocytosing debris and remodeling the scaffold in preparation for regeneration. Both tissue resident and later hematogenous macrophages secrete vascular endothelial growth factor (VEGF), a pro-angiogenic factor, recruiting endothelial cells to the nerve gap20,21. As blood vessels form, fibroblasts and dedifferentiated Schwann cells migrate along the endothelial basal lamina, forming cords similar in structure to the bands of Büngner in the distal stump. Concurrently, there is an influx of other myeloid cells (i.e. eosinophils) and T lymphocytes promoting expression of anti-inflammatory cytokines21–23. With these elements in place, the healing nerve gap is prepared to receive and guide sprouting axons from the proximal stump to the distal stump. The newly-formed matrix of Schwann cells, fibroblasts, and blood vessels in the nerve gap conduct the regenerating axons to the distal nerve stump, where processes proceed to facilitate axon growth to their end-organ targets as described previously.
Figure 3 – Focused schematic of the inflammatory and pro-regenerative phases of nerve post-axotomy.
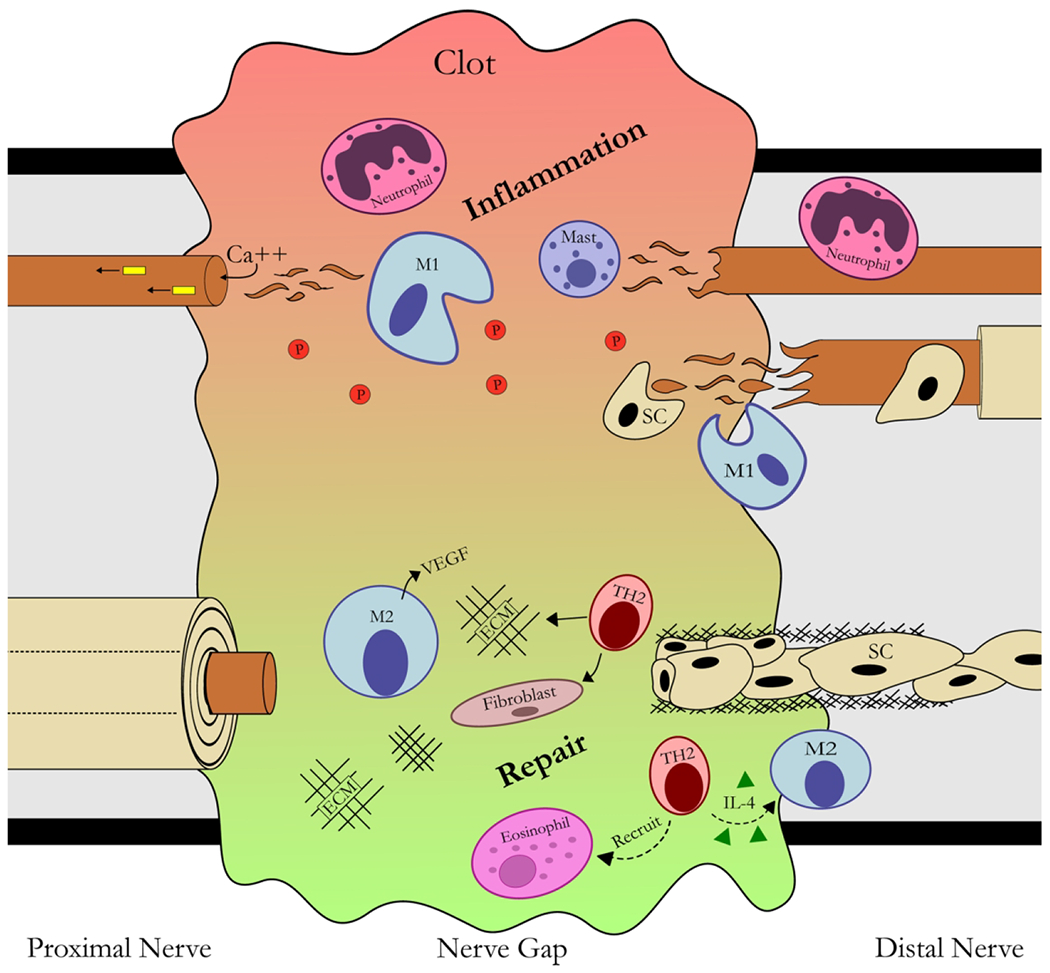
In the proximal nerve, retrograde signals and calcium influx signal neurons to injury activating a RAG program (see figures 1&2). Within the nerve gap, the pro-inflammatory phase of injury involves leukocyte accumulation including platelets (P), neutrophils, macrophages (M), and inflammatory cytokines forming a clot to promote inflammation and a provisional ECM. This inflammation resolves as T-cells arrive in the nerve gap and promote pro-regenerative processes including IL-4 regulation and leukocyte modulation and recruitment. The pro-regenerative phase involves changes to macrophages from Type 2 (i.e. IL-4) cytokines leading to promotion of neovascularization, angiogenesis, ECM remodeling, and then recruitment of aligned Schwann cell (SC) channels within the gap. In the distal nerve, axons undergo Wallerian degeneration, where SCs dedifferentiate into a pro-inflammatory, phagocytic phenotype recruiting other phagocytic leukocytes. Following this inflammatory phase, these dedifferentiated SCs then align themselves into channels (Bands of Bungner) to facilitate axon growth from the proximal nerve.
The role of inflammation and endogenous IL-4 signaling during injury and regeneration
During regeneration, the immune system establishes a balance between pro-inflammatory and anti-inflammatory processes (type 1 and type 2, respectively). The type 1 response is characterized by processes that enhance inflammation, such as the recruitment of leukocytes, expression of pro-inflammatory cytokines, and polarization of macrophages to the M1 phenotype. This inflammation indirectly promotes tissue regeneration by supplying chemotactic cytokines that attract cells required for wound healing. The type 2 response directly promotes tissue regeneration, characterized by the cytokines IL-4 and IL-13. These cytokines classically promote anti-inflammatory, pro-regenerative immune cell phenotypes, such as the M2 macrophage and Type 2 helper T cell (Th2). Considerably more is known regarding type 2 responses and IL-4 signaling in general wound healing, while less is known about these responses and signaling during nerve injury and regeneration, where each compartmentalized region of nerve could have important roles and unique cellular targets.
IL-4 signaling in the context of general wound healing.
IL-4 is a small, globular protein similar in structure to other cytokines. In response to injury, basophils24, eosinophils25, and mast cells26 are classically associated as sources of IL-4 for the innate immune response (Figure 4A). But, other innate immune cells, including NK T cells24 and neutrophils27 have been found to express IL-4 as well. In the adaptive immune response, type 2 helper (Th2 CD4+ T cells) and follicular T helper cells can be principal sources of IL-4 or regulate IL-4 expression from other cells, such as eosinophils28.
Figure 4 – Schematic of interleukin (IL)-4 signaling sources (A) and targets (B).
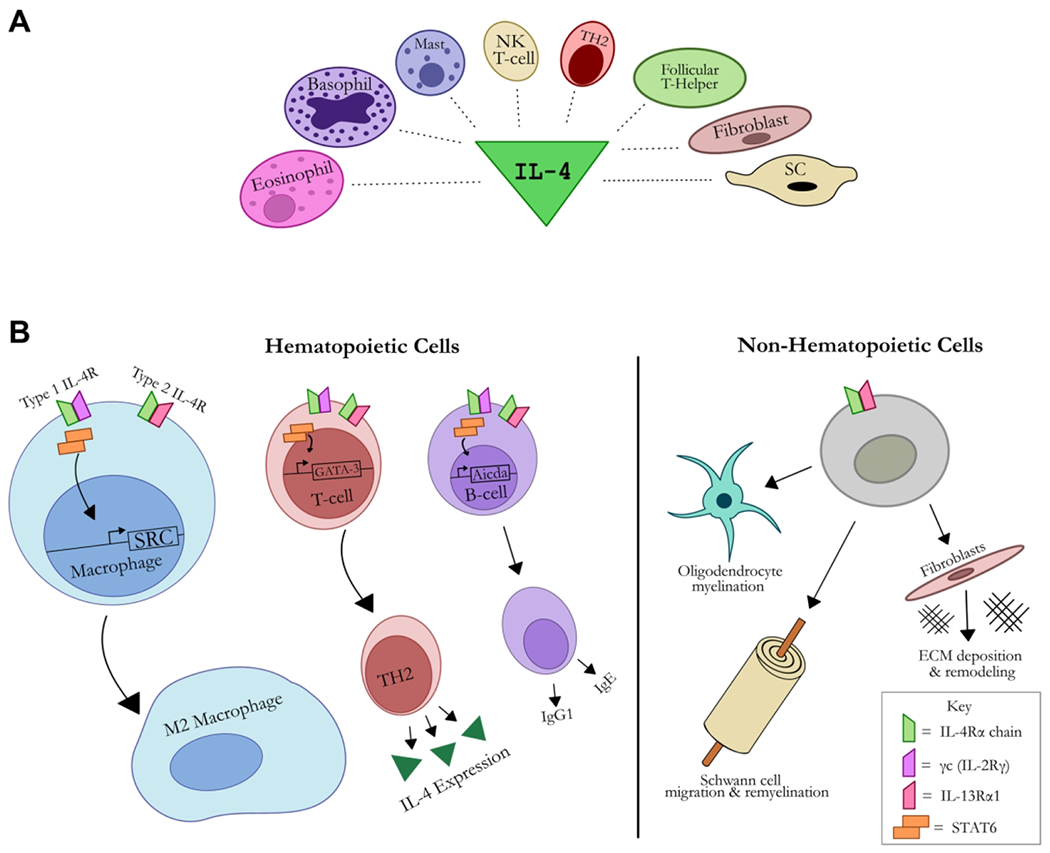
(A) Cell sources of IL-4 signaling include: Type 2 T-helper (TH2) cells, eosinophils, basophils, mast cells, natural killer (NK) T-cells, follicular T-helper cells, fibroblasts, and Schwann cells (SC). IL-4 signals via IL-4R, a complex of either the type I (IL-4Rα and γc) or type II (IL-4Rα and IL-13Rα1) chains. The type I receptor complex activates janus kinases (JAK), whereas type II (IL-13Rα1) activates tyrosine kinase 2 (TYK2) and JAKs, which in turn activate STAT-6 leading to dimerization and transmigration to the nucleus to transduce changes to response genes. (B) Cells of hematopoietic origin (i.e. macrophages, T-cells, B-cells) express both Type I and Type II IL-4 receptors, whereas non-hematopoietic cells (i.e. oligodendrocytes, Schwann cells, fibroblasts) only express the type II IL-4 receptor. This signaling promotes cell-specific gene expression, such as promoting M2 macrophage phenotypes. In non-hematopoietic cells specific to nerve, IL-4 signaling promotes myelination in oligodendrocytes, migration and remyelination in Schwann cells, and extracellular matrix (ECM) deposition and remodeling in fibroblasts.
IL-4 regulates target cell function and transcriptional activity through cell surface receptors (Figure 4B). Assembly of the IL-4 receptor complex is initiated when IL-4 binds the extracellular domain of the IL-4Rα chain. This high-affinity association between receptor and ligand enables the IL-4Rα chain to bind either the common gamma chain (γc, also known as the IL-2Rγ) or the IL-13Rα1 chain respectively – forming functional type 1 IL-4R or the type 2 IL-4R complexes29. The IL-4Rα and IL-13Rα1 chains are expressed in both hematopoietic and non-hematopoietic cells. Contrastingly, the γc chain is expressed primarily in hematopoietic cells and is mostly absent in non-hematopoietic cells. In myeloid cells, such as macrophages, eosinophils, and mast cells, IL-13Rα1 and the γc chain are equally expressed whereas in lymphocytes, there is increased expression of the γc chain compared to IL-13Rα129.
Once an IL-4-receptor complex has formed, intracellular Jak kinases become activated leading to downstream activation of STAT629. STAT6 molecules dimerize and translocate to the nucleus, activating transcriptional machinery with diverse effects for each cell type. For example, IL-4-STAT6 signaling in macrophages upregulates Src kinase expression, promoting M2 phenotype differentiation30; in B cells the Aicda gene encoding AID (activation-induced cytidine deaminase) is upregulated which is required for class switching31; and in T cells the GATA-3 transcription factor is upregulated which promotes the Th2 cell phenotype32. Overall, IL-4 associated downstream signaling can be quite diverse based on the cell targeted.
IL-4 signaling in the context of nerve injury and regeneration.
While the immune system is regarded as a major source providing IL-4 within nerve, IL-4 is indeed expressed within cells specific to nerve after injury (Figure 4A). Systemically, blood plasma levels of IL-4 do not change following sciatic nerve crush in a rodent model33. But, locally within nerve, an inflammatory and regenerative milieu has been implicated to produce IL-4. In both neuropathic and morphologically normal human sural nerves, immunohistochemical staining for IL-4 were found in myelinating Schwann cells, immunoreactive lymphocytes, and putative macrophages and fibroblasts34.
IL-4 signaling associated with cell populations specific to nerve after injury and regeneration are still sources of ongoing investigations, but there is increasing evidence that IL-4 targets these cells (Figure 4B). In the central nervous system, which also contains select neuronal bodies of peripheral nerves such as motor neurons, many neuronal populations, astrocytes, and microglia have been found to express IL-4R. Additionally, IL-4R has been found not only on neuron cell bodies, but also axons of CNS neurons35. The role of IL-4 on injured neurons and their axons has primarily been studied in the CNS. IL-4 mediates CNS neuroprotection and regeneration following injury through inducing microglia and macrophages to a pro-regenerative phenotype36,37 and additionally promotes oligodendrocyte differentiation and subsequent remyelination38. But also, in a series of studies relevant for peripheral nerve injuries, Jones’ group elucidated a critical role for IL-4 in nerve regeneration following peripheral injury affecting cranial nerves. Using facial nerve transection models in mice, they showed that Th2 cells are necessary for facial motor nucleus (FMN) survival post axotomy and that these immune cells’ neuroprotective effects are dependent on IL-4 expression39–44. Walsh et al found similar results in the CNS, showing an MHCII-independent neuroprotective role for a subset of IL-4 producing T cells45. For spinal nerves, the presence of T-cells (and therefore, potentially IL-4) in the DRG and sympathetic ganglia has been reported following sciatic nerve transection46–49 (Figure 2). But, the role of T cells and IL-4 in promoting survival of sensory and sympathetic neurons, as well as spinal motor neurons, has yet to be elucidated. Furthermore, how T cells affect the CNS to activate motoneurons is yet not clear, but could involve their actions within the meninges50,51, as demonstrated for IL-17 affecting brain52.
IL-4 is directly involved in macrophage M2 polarization within nerve53, where these polarized M2 macrophages have a clear role in directing SC regenerative responses. Increased Schwann cell proliferation and migration were seen in response to IL-4 induced M2 macrophage-derived microvesicles54. Similar results were found with IL-4-stimulated M2 macrophages in an in vitro SC culture55. During the pro-regenerative phase of nerve repair, IL-4 signaling has been shown to increase Schwann cell mobility, conducing them to form the cellular cords previously mentioned that guide regenerating axons56. And finally, during nerve regeneration across a nerve gap, IL-4 signaling was associated with promoting SC myelination within the regenerated nerve gap22,23. However, while these responses involved IL-4 signaling, it is not yet clear whether direct IL-4 signaling via SCs was involved, as SCs also express the IL-4Rα chain57. From these studies, there is potential that IL-4 signaling within SCs was partly involved in observed responses, rather than all effects being mediated indirectly to SCs via IL-4 signaling to cells of the immune system (i.e. macrophages).
Mesenchymal cells within nerve, such as stromal cells and fibroblasts, play a central role in tissue injury and regeneration. These cells are also receptive to cytokines, stimulating them to take on a variety of phenotypes depending on the tissue and circumstance. In the wound-healing response, type II cytokines play an important role influencing fibroblasts to deposit ECM to enable tissue repair. Early in this process, IL-4 has been shown to specifically induce expression of tenascin by fibroblasts. Tenascin is important in wound-healing as it precedes collagen deposition and cellular migration58. Furthermore, IL-4 signaling induces increased migration and ECM protein deposition by fibroblasts59. However, when type II signaling and fibroblast activity is extended beyond the normal wound-healing timeline, as in allergic diseases, tissue scarring and fibrosis can occur. Whether these processes are recapitulated within nerve is not yet known, but overall, IL-4 signaling within cell populations specific to nerve could clearly be instrumental to injury and regenerative responses.
The potential of exogenous IL-4 as a therapy
Type 2 immunity and therapies targeting IL-4/IL-13 might play a significant role in overcoming severe nerve injuries that do not regenerate, or regenerate to a degree but yield inadequate recovery. In Table 1, the findings from studies of exogenous IL-4 and its effects on rodent models of neuropathies are presented. While many of these studies are not specific to traumatic peripheral nerve injury (PNI), the results suggest some findings could be extrapolated and hold promise in the context of PNI. The studies were broadly divided into two categories – systemically versus locally administered IL-4 treatments – and into a third category for resveratrol, an IL-4R-modulating drug.
Table 1:
Study findings of exogenous IL-4 and its effects on rodent models of neuropathies
| Animal Model | Intervention and Dosing | Administration and Duration | Outcome Model and Endpoint | Findings | |
|---|---|---|---|---|---|
| Systemically Administered IL-4: | |||||
| Deretzi et al, 1998 | Male Lewis rat (150-180 g) – induced experimental autoimmune neuritis (EAN) | Rat recombinant IL-4 1 μg/rat/day or 0.1 μg/rat/day |
Intranasally administered once daily for six consecutive days, beginning on day 5 post-inoculation (pi) | • Clinical assessment of paresis severity – day 80 pi • Immunohistochemical analysis of sciatic nerve – day 15 pi • Lymphocyte proliferation assay of mononuclear cell culture |
• IL-4 treatment at both high and low dose significantly reduced EAN symptoms beginning at day 11 pi. IL-4 treatment also decreased the duration of EAN symptoms compared to control. •IL-4 treatment reduced macrophage, lymphocyte, and granulocyte infiltration in pi affected nerve. Treatment also reduced regional demyelination and inflammation measured in sciatic nerve. •IL-4 treatment suppressed T-cell proliferation and autoantigen reactivity. |
| Lima et al, 2017 | Wistar Han female rats (14 weeks old, 210-260 g) – severe contusive spinal cord injury | Rat recombinant IL-4 0.35 μg/kg/day |
Intraperitoneally administered every 12h for 7 days, beginning on day of surgery | • Basso, Beattie and Bresnahan (BBB) locomotor scale – week 7 • Serum cytokine analysis – day 7 •Histological immunofluorescence analysis of spinal cord – week 8 |
• BBB showed no difference between treatment and untreated (saline) after spinal cord injury; however, 100% of IL-4 treatment group recovered ability to support weight compared to 33% of untreated group. • IL-4 treatment increased serum IL-10 2-fold at day 7. • IL-4 treatment decreased the area of the injured spinal cord region occupied by macrophages and the number of iNOS-positive cells. The untreated group resulted in decreased numbers of oligodendrocytes and ventral horn motor neurons compared to IL-4 treatment group. |
| Vogelaar et al, 2018 | Three experimental autoimmune encephalomyelitis (EAE) mouse models of multiple sclerosis | Mouse recombinant IL-4 1μg/day |
Injected intrathecally or sprayed nasally every other day for 14 days | • Clinical score, IL-4Rα KO model – day 35 • CatWalk-automated gait analysis – day 35 • Corticospinal tract immunofluorescence analysis – day 35 • Fluorescence-activated cell sorting (FACS) analysis of IL-4 treated EAE mice – day 37 • In vitro cortical neuron explants and multiple molecular experiments to explore IL-4 signaling in neurons |
• Intrathecal IL-4 treatment significantly improved clinical score independent of MS model. Intranasal IL-4 treatment also markedly improved clinical score in the model. IL-4Rα KO reversed this trend. • Gait analysis showed that IL-4 treated mice models displayed no further deterioration of base of support and also experienced complete recovery of stride length. • IL-4 treatment from day 20 to day 35 halted progressive axonal pathology compared to untreated (PBS). Intranasal administration was more effective than intrathecal administration at reducing axonal swellings. • Mice treated with IL-4 during disease chronic phase ameliorated progressive disease without affecting inflammation. If treated early in disease progression, inflammation was reduced along with clinical score. •In cortical neuron culture models, IL-4 acted directly on IL-4R via IRS1-PI3K-PKC signaling with GAP-43 and CaM as regulators of F-actin. |
| Locally Administered IL-4: | |||||
| Mokarram et al, 2012 | Adult Lewis male rats (250-300 g) – tibial branch transection with 15mm nerve gap | Rat recombinant IL-4 1 μg/mL |
IL-4 and agarose hydrogel-filled, semi-permeable, polysulfone tube bridging the nerve defect | • Immunohistochemical analysis of macrophage populations in nerve sections – 3 weeks •Immunohistochemical analysis of Schwann cells in nerve sections – 3 weeks • Immunohistochemical analysis of axons in nerve sections – 3 weeks |
• Both IL-4 and IFN-γ-filled conduits increased total macrophage numbers within conduit compared to unmodified (agarose alone), The IL-4 treatment group produced significantly higher ratios of M2a and M2c macrophages compared to other groups. •IL-4 treatment enhanced Schwann cell migration toward the middle of the conduit compared to other groups. • IL-4 treatment produced greater (~20 times) numbers of axons in the distal conduit compared to other groups. |
| Kiguchi et al, 2015 | Male ICR mice (age 4-5 weeks) – partial sciatic nerve ligation | Mouse recombinant IL-4 10ng/day, 30ng/day, or 100ng/day |
Injected perineurally four times during the first week or during the second week post-surgery | • Von Frey test for tactile allodynia and Hargreaves test for thermal hyperalgesia – day 21 • Immunofluorescence and qRT-PCR analysis of ex vivo sciatic nerve and mouse macrophage cultures – day 7 |
• IL-4 treatment attenuated neuropathic pain. This effect was reversed when animals were pre-treated with the STAT6 inhibitor AS1517499. • IL-4 treatment shifted macrophage phenotype from M1 to M2 in a STAT6-mediated fashion within nerve. |
| Liao et al, 2019 | Adult Sprague-Dawley rats – sciatic nerve transection with 15mm nerve gap | Rat recombinant IL-4 1 μg/mL |
IL-4 and collagen-filled silicone rubber conduits bridging the nerve defect | • Gross examination of silicone chambers – 6 weeks • Nerve macrophage immunohistochemistry – 6 weeks • Electrophysiology, fluorogold retrograde labeling, spinal cord immunohistochemistry |
• IL-4 treatment revealed higher rates of successful axon regeneration across the conduit compared to unmodified conduits and IFN-γ conduits. • IL-4 treatment resulted in significantly higher density of macrophages recruited to the regenerating nerve. • IL-4 treatment was not different than untreated conduits in other regenerative outcomes including: electrophysiological measurements, number of fluorogold labeled DRG neurons regenerating axons, or calcitonin gene-related peptide (CGRP) levels in the spinal cord dorsal horn. |
| Celik et al, 2020 | Male C57BL/6J mice (22-30g, 6-13 weeks old) – chronic constriction injury, sciatic nerve | Mouse recombinant IL-4 200 ng/day |
Injected perineurally daily for seven days, beginning on day 14 post injury | • Von Frey test for tactile allodynia – day 26 • Hargreaves test for thermal hyperalgesia – day 26 • FISH, qRT-PCR and enzyme immunoassays for quantifying opioid peptide expression by macrophages isolated from injured nerves – day 22 |
• IL-4 treatment attenuated neuropathy-induced mechanical allodynia. This effect was reversed with anti-IL-4Rα antibody administration during IL-4 treatment. • IL-4 treatment did not change neuropathy-induced heat hypersensitivity. • IL-4 treatment induced macrophages to M2 phenotype, producing increased levels of opioid peptides compared to control groups. Treated macrophages produced analgesic effect after transfer to newly injured mice. |
| Ahmed Ali et al, 2020 | Adult Sprague-Dawley rats – facial nerve transection with 5mm nerve gap | IL-4-encoding lentivirus | Multichannel bridges loaded with IL-4 lentivirus | • Quantitative histomorphometric analysis of facial nerve – 10 weeks • Electrophysiological analysis (EMG) of facial nerve and vibrissal musculature – 10 weeks |
• IL-4 treatment produced greater total nerve fibers, fiber density, and percent neural tissue distal to the repair site compared to the empty conduit group and gap defect (unrepaired) group. • IL-4 treatment compared to the gap defect group resulted in greater amplitude (CMAP). IL-4 treatment produced shorter latency times compared to empty conduit and gap defect groups. |
| IL-4 Modulating Treatments: | |||||
| Xu et al, 2018 | Male Sprague Dawley rats (200-250 g) – Chronic constriction injury, sciatic | Resveratrol 200 mg/kg |
Intraperitoneally administered once daily for 14 days, beginning on day of surgery | • Mechanical withdrawal threshold and thermal withdrawal latency tests – day 14 • RT-qPCR of ipsilateral dorsal lumbar spinal cord – day 14 • siRNA IL-4Ra knockdown in neuronal cell culture and RT-PCR |
• Resveratrol treatment attenuated mechanical allodynia and thermal hyperalgesia. • Resveratrol treatment enhanced anti-inflammatory signaling, upregulating expression of IL-4Rα in the dorsal spinal cord. • IL-4Rα knockdown reversed resveratrol-induced transcription of anti-inflammatory genes in neuronal culture model. |
Systemic treatment using exogenous IL-4 was associated with improved regenerative outcomes in rodent neuropathy models. Studies using autoimmune models of neuropathy reported significantly reduced severity of symptoms with exogenous IL-4, and that treatment attenuated neuronal inflammation and progressive demyelination35,60. Vogelaar et al found that IL-4 therapy ameliorated disease progression equally whether treatment begun in early or late stages of disease. Interestingly, when IL-4 therapy was administered at an early phase, symptoms and inflammation from the disease model were both reduced. However at a late phase, symptoms were reduced while no change in inflammation was observed – suggesting a potential nonimmune mechanism for IL-4 during chronic demyelinating diseases like MS35. Finally, systemic IL-4 used in a spinal cord contusive injury model demonstrated decreased inflammation throughout the spinal cord and increased numbers of oligodendrocytes and motor neurons in the ventral horn compared to untreated models61. While additional research is needed to confirm the relevance of these findings to PNI, these findings suggest that an IL-4 therapy may need to target neuronal populations to promote regeneration.
Studies considering the effects of IL-4 on peripheral nerve injury models focused on the effects of locally administered IL-4 at the site of peripheral neuropathy. Overall, these studies found an increase in pro-regenerative processes associated with IL-4 treatment. Nerve conduit repair models showed greater axon regeneration and axon density across a nerve gap when using IL-4-filled conduits compared to no drug within a onduit62,63. Additionally, IL-4 treatment increased Schwann cell migration into the conduit and was associated with increased numbers of macrophages in the nerve. Upon further analysis, this greater macrophage population was composed primarily of M2 macrophages and had a decreased M1 macrophage ratio compared to controls55. Alternatively, perineural injected IL-4 treatment attenuated neuropathic pain in a dose-dependent fashion in models of neuropathic pain64. IL-4 treatment was also associated with increased M2 macrophage populations in the affected nerve. Interestingly, Celik et al reported IL-4-induced M2 macrophages produced increased levels of opioid peptides compared to untreated groups, unveiling a potential mechanism whereby IL-4 treatment reduces neuropathy-associated pain. Compared to the previously discussed systemic IL-4 studies, the findings here carry greater relevance for applications to treat peripheral nerve injury. However, given the heterogeneity of local environments that PNS neuron components project their axons to, and the heterogeneity of the neurons themselves, more research is needed to elucidate the specific effects IL-4 has on the various cells of the PNS environments (nerve gap, proximal and distal stumps, DRG, dorsal horn, ventral horn, etc.) and how these effects might work together to promote nerve repair and regrowth.
Finally, an interesting study was done using resveratrol, as opposed to IL-4. Resveratrol is a naturally-derived polyphenol that is known for its wide-spread beneficial effects (i.e. cardioprotective, antioxidant, anti-inflammatory, anti-tumorigenic, etc.) with myriad proposed mechanisms of action65. Additionally, resveratrol has been shown to improve outcomes of various kinds of nerve injury. Xu et al found that resveratrol attenuates neuropathic pain through IL-4R-mediated signaling. With resveratrol treatment, they observed an upregulation of anti-inflammatory receptors and signaling in the spinal dorsal horn, leading to reduced mechanical allodynia and thermal hyperalgesia. This resveratrol-induced anti-inflammatory signaling was reversed with an IL-4Rα knock out, suggesting an IL-4R-dependent mechanism for resveratrol-mediated alleviation of neuropathic pain66.
Translating an IL-4 therapy
Given the role endogenous IL-4 plays in guiding the regenerative phase of the wound response and the encouraging results from its exogenous use in treating PNI in rodents, there is great clinical translational potential for an IL-4-focused therapy. While exogenous IL-4 attenuates neuropathy and promotes significant nerve regeneration with recovery, the exact cellular mechanisms whereby IL-4 treatment promotes nerve regrowth still needs considerable attention. And, while there seems high potential to translate IL-4 therapies to the clinic given these early results, exogenous IL-4 treatment is not devoid of risks and potential side effects. Although IL-4 is critical for promoting tissue regeneration, chronic IL-4 exposure in tissues promotes excessive tissue remodeling, fibrosis, and chronic inflammation. Chronic inflammatory diseases frequently result in tissue fibrosis and are attributed to a significant proportion of deaths worldwide67,68. Excessive IL-4 signaling has been implicated in various chronic inflammatory conditions like allergic asthma. As such, many therapies have been developed to block IL-4 signaling and diminish its long-term effects on tissue inflammation and scarring29,69. Thus, great care needs to be taken in developing an exogenously applied IL-4 treatment to maintain the desired regenerative effects while attenuating unwanted scarring and potential fibrosis.
Based upon these concerns, some risk could be minimized by IL-4 local delivery at the nerve injury site through injections or nerve conduits, rather than systemic administration. If unwanted side effects pose a serious roadblock for treatment design, having an understanding of IL-4 signaling pathways within specific cells would be critical for potentially developing IL-4 therapies specific to the cells and sites required for stimulating nerve regeneration. Given the structure and distribution of the IL-4R subtypes, it might be possible to accomplish this. Non-hematopoietic cells principally express the type 2 IL-4R, whereas hematopoietic cells express both type 1 and type 2. Attempts to mutate IL-4 to preferably activate the type 1 receptor, preventing fibrotic side effects, has been met with setbacks, but recent results are promising and show potential for developing a hematogenous cell-selective “superkine”29,70. Alternatively, it also might be worthwhile to search for additional therapies that modify or promote IL-4 signaling without administering exogenous IL-4 itself. This method could pose fewer side effects compared to native IL-4 therapy and potentially be easier to develop and administer. The recent results from Xu et al using resveratrol to stimulate IL-4-mediated signaling are encouraging for this form of PNI treatment. However, care needs to be taken as some studies have found negative effects for resveratrol as a pro-oxidizing agent65. Overall, more research will need to be done to ensure treatment safety in the context of peripheral nerve injury, but therapies targeting IL-4 signaling have demonstrated promise to potentially better manage PNIs.
Conclusion
IL-4 is a promising target for managing peripheral nerve injury and enhancing recovery. It is the principal cytokine governing the pro-regenerative type 2 immune response, suppressing inflammation and stimulating immune cells to a healing phenotype. The cells implicated in nerve regeneration – Schwann cells, macrophages, fibroblasts, neurons, etc. – respond to IL-4 signaling, either indirectly via other immune cells or directly, and subsequently promote regrowth. Without IL-4, nerve recovery post-injury is greatly hindered. IL-4 treatments for nerve injury and neuropathy in rodent models have shown encouraging preliminary results and indicate targeting IL-4 signaling as a promising translational therapy for recovery from traumatic nerve injury. More study is needed to characterize the precise signaling network – IL-4 sources, targets, and effects – between the various cells of post-trauma nerve that is necessary for regeneration. With that knowledge, more specific and robust therapies could potentially be developed to optimize nerve regeneration without the unwanted effects of systemically stimulated type II immunity.
Acknowledgements
This work was supported in part by the National Institutes of Neurological Disorders and Stroke of the National Institutes of Health (NIH) under award number R01 NS115960 (M.D.W.) to Washington University.
Funding: This work was supported in part by the National Institutes of Neurological Disorders and Stroke of the National Institutes of Health (NIH) under award number R01 NS115960 (M.D.W.) to Washington University.
Footnotes
The authors have no competing interests to declare.
References
- 1.Ootes D, Lambers KT, Ring DC. The epidemiology of upper extremity injuries presenting to the emergency department in the United States. Hand (N Y). 2012;7(1):18–22. doi: 10.1007/s11552-011-9383-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambers K, Ootes D, Ring D. Incidence of patients with lower extremity injuries presenting to US emergency departments by anatomic region, disease category, and age. Clin Orthop Relat Res. 2012;470(1):284–290. doi: 10.1007/s11999-011-1982-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huckhagel T, Nüchtern J, Regelsberger J, Lefering R. Nerve injury in severe trauma with upper extremity involvement: evaluation of 49,382 patients from the TraumaRegister DGU® between 2002 and 2015. Scand J Trauma Resusc Emerg Med. Published online 2018. doi: 10.1186/s13049-018-0546-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huckhagel T, Nüchtern J, Regelsberger J, Gelderblom M, Lefering R. Nerve trauma of the lower extremity: Evaluation of 60,422 leg injured patients from the TraumaRegister DGU® between 2002 and 2015. Scand J Trauma Resusc Emerg Med. Published online 2018. doi: 10.1186/s13049-018-0502-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciaramitaro P, Mondelli M, Logullo F, et al. Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst. Published online 2010. doi: 10.1111/j.1529-8027.2010.00260.x [DOI] [PubMed] [Google Scholar]
- 6.Ruijs ACJ, Jaquet JB, Kalmijn S, Giele H, Hovius SER. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg. 2005;116(2):484–486. doi: 10.1097/01.prs.0000172896.86594.07 [DOI] [PubMed] [Google Scholar]
- 7.Gordon T, Tyreman N, Raji MA. The basis for diminished functional recovery after delayed peripheral nerve repair. J Neurosci. 2011;31(14):5325–5334. doi: 10.1523/JNEUROSCI.6156-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan D, Mackinnon SE, Wood MD. Advances in the repair of segmental nerve injuries and trends in reconstruction. Muscle and Nerve. 2020;61(6):726–739. doi: 10.1002/mus.26797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood MD, Mackinnon SE. Pathways regulating modality-specific axonal regeneration in peripheral nerve. Exp Neurol. 2015;265:171–175. doi: 10.1016/j.expneurol.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood MDMD, Kemp SWPSWP, Weber C, Borschel GHGH, Gordon T. Outcome measures of peripheral nerve regeneration. Ann Anat. 2011;193(4):321–333. doi: 10.1016/j.aanat.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 11.Bobinski F, Teixeira JM, Sluka KA, Santos ARS. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain. Published online 2018. doi: 10.1097/j.pain.0000000000001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu QG, ForDen J, Walsh SK, Gordon T, Midha R. Motoneuron survival after chronic and sequential peripheral nerve injuries in the rat. J Neurosurg. 2010;112(4):890–899. doi: 10.3171/2009.8.JNS09812 [DOI] [PubMed] [Google Scholar]
- 13.Kemp SWP, Chiang CD, Liu EH, et al. Characterization of neuronal death and functional deficits following nerve injury during the early postnatal developmental period in rats. Dev Neurosci. 2015;37(1):66–77. doi: 10.1159/000368769 [DOI] [PubMed] [Google Scholar]
- 14.Schmalbruch H Loss of sensory neurons after sciatic nerve section in the rat. Anat Rec. 1987;219(3):323–329. doi: 10.1002/ar.1092190314 [DOI] [PubMed] [Google Scholar]
- 15.West CA, McKay Hart A, Terenghi G, Wiberg M. Sensory neurons of the human brachial plexus: a quantitative study employing optical fractionation and in vivo volumetric magnetic resonance imaging. Neurosurgery. 2012;70(5):1183–1194; discussion 1194. doi: 10.1227/NEU.0b013e318241ace1 [DOI] [PubMed] [Google Scholar]
- 16.McKay Hart A, Brannstrom T, Wiberg M, Terenghi G. Primary sensory neurons and satellite cells after peripheral axotomy in the adult rat: timecourse of cell death and elimination. Exp Brain Res. 2002;142(3):308–318. doi: 10.1007/s00221-001-0929-0 [DOI] [PubMed] [Google Scholar]
- 17.Menorca RMG, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29(3):317–330. doi: 10.1016/j.hcl.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35–43. doi: 10.1159/000339613 [DOI] [PubMed] [Google Scholar]
- 19.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. Published online 2011. doi: 10.1017/S1462399411001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cattin AL, Burden JJ, Van Emmenis L, et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell. Published online 2015. doi: 10.1016/j.cell.2015.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan D, Acevedo-Cintrón JA, Sayanagi J, Snyder-Warwick AK, Mackinnon SE, Wood MD. The CCL2/CCR2 axis is critical to recruiting macrophages into acellular nerve allograft bridging a nerve gap to promote angiogenesis and regeneration. Exp Neurol. 2020;331. doi: 10.1016/j.expneurol.2020.113363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan D, Hunter DADA, Schellhardt L, et al. The accumulation of T cells within acellular nerve allografts is length-dependent and critical for nerve regeneration. Exp Neurol. 2019;318:216–231. doi: 10.1016/j.expneurol.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan D, Hunter DA, Schellhardt L, et al. T cells modulate IL-4 expression by eosinophil recruitment within decellularized scaffolds to repair nerve defects. Acta Biomater. 2020;112:149–163. doi: 10.1016/j.actbio.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimoto T The Hunt for the Source of Primary Interleukin-4: How We Discovered That Natural Killer T Cells and Basophils Determine T Helper Type 2 Cell Differentiation In Vivo. Front Immunol. 2018;9:716. doi: 10.3389/fimmu.2018.00716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melo RCN, Spencer LA, Perez SAC, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6(11):1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva EZM, Jamur MC, Oliver C. Mast Cell Function: A New Vision of an Old Cell. J Histochem Cytochem. Published online 2014. doi: 10.1369/0022155414545334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandt E, Woerly G, Younes AB, Loiseau S, Capron M. IL-4 production by human polymorphonuclear neutrophils. J Leukoc Biol. Published online 2000. doi: 10.1189/jlb.68.1.125 [DOI] [PubMed] [Google Scholar]
- 28.Brown MA. IL-4 Production by T Cells: You Need a Little to Get a Lot. J Immunol. Published online 2008. doi: 10.4049/jimmunol.181.5.2941 [DOI] [PubMed] [Google Scholar]
- 29.Junttila IS. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol. Published online 2018. doi: 10.3389/fimmu.2018.00888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu X, Wang H, Han C, Cao X. Src promotes anti-inflammatory (M2) macrophage generation via the IL-4/STAT6 pathway. Cytokine. Published online 2018. doi: 10.1016/j.cyto.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 31.Dedeoglu F, Horwitz B, Chaudhuri J, Alt FW, Geha RS. Induction of activation-induced cytidine deaminase gene expression by IL-4 and CD40 ligation is dependent on STAT6 and NFκB. Int Immunol. Published online 2004. doi: 10.1093/intimm/dxh042 [DOI] [PubMed] [Google Scholar]
- 32.Hosoya T, Maillard I, Engel JD. From the cradle to the grave: Activities of GATA-3 throughout T-cell development and differentiation. Immunol Rev. Published online 2010. doi: 10.1111/j.1600-065X.2010.00954.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells MR, Racis SP, vaidya U, Racis SP Jr., vaidya U. Changes in plasma cytokines associated with peripheral nerve injury. J Neuroimmunol. 1992;39(3):261–268. doi: 10.1016/0165-5728(92)90260-r [DOI] [PubMed] [Google Scholar]
- 34.Deprez M, Lüke U, Verlaet M, Debrus S, Delvenne P, Martin JJ. Detection of cytokines in human sural nerve biopsies: An immunohistochemical and molecular study. Acta Neuropathol. Published online 2001. doi: 10.1007/s004010000300 [DOI] [PubMed] [Google Scholar]
- 35.Vogelaar CF, Mandal S, Lerch S, et al. Fast direct neuronal signaling via the IL-4 receptor as therapeutic target in neuroinflammation. Sci TransI Med. 2018; 10(430). doi: 10.1126/scitranslmed.aao2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, López-Vales R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia. Published online 2016. doi: 10.1002/glia.23041 [DOI] [PubMed] [Google Scholar]
- 37.Fenn AM, Hall JCE, Gensel JC, Popovich PG, Godbout JP. IL-4 signaling drives a unique arginase+/IL-1β+ microglia phenotype and recruits macrophages to the inflammatory CNS: Consequences of age-related deficits in IL-4Rα after traumatic spinal cord injury. J Neurosci. Published online 2014. doi: 10.1523/JNEUROSCI.1146-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Psachoulia K, Chamberlain KA, Heo D, et al. IL4I1 augments CNS remyelination and axonal protection by modulating T cell driven inflammation. Brain. 2016;139(Pt 12):3121–3136. doi: 10.1093/brain/aww254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serpe CJ, Tetzlaff JE, Coers S, Sanders VM, Jones KJ. Functional recovery after facial nerve crush is delayed in severe combined immunodeficient mice. Brain Behav Immun. 2002; 16(6):808–812. doi: 10.1016/s0889-1591(02)00017-x [DOI] [PubMed] [Google Scholar]
- 40.Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17(5):393–402. doi: 10.1016/s0889-1591(03)00028-x [DOI] [PubMed] [Google Scholar]
- 41.DeBoy CA, Xin J, Byram SC, Serpe CJ, Sanders VM, Jones KJ. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4+ T cells. Exp Neurol. Published online 2006. doi: 10.1016/j.expneurol.2006.04.028 [DOI] [PubMed] [Google Scholar]
- 42.Byram SC, Serpe CJ, DeBoy CA, Sanders VM, Jones KJ. Motoneurons and CD4+ effector T cell subsets: Neuroprotection and repair. Clin Neurosci Res. Published online 2006. doi: 10.1016/j.cnr.2006.06.001 [DOI] [Google Scholar]
- 43.Xin J, Wainwright DA, Serpe CJ, Sanders VM, Jones KJ. Phenotype of CD4+ T cell subsets that develop following mouse facial nerve axotomy. Brain Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beahrs T, Tanzer L, Sanders VM, Jones KJ. Functional recovery and facial motoneuron survival are influenced by immunodeficiency in crush-axotomized mice. Exp Neurol. Published online 2010. doi: 10.1016/j.expneurol.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh JT, Hendrix S, Boato F, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125(2):699–714. doi: 10.1172/JCI76210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu P, McLachlan EM. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience. Published online 2002. doi: 10.1016/S0306-4522(02)00065-9 [DOI] [PubMed] [Google Scholar]
- 47.Hu P, McLachlan EM. Inflammation in sympathetic ganglia proximal to sciatic nerve transection in rats. Neurosci Lett. Published online 2004. doi: 10.1016/j.neulet.2004.04.077 [DOI] [PubMed] [Google Scholar]
- 48.Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav Immun. Published online 2007. doi: 10.1016/j.bbi.2006.10.013 [DOI] [PubMed] [Google Scholar]
- 49.McLachlan EM, Hu P. Inflammation in dorsal root ganglia after peripheral nerve injury: Effects of the sympathetic innervation. Auton Neurosci Basic Clin. Published online 2014. doi: 10.1016/j.autneu.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 50.Ransohoff RM, Kivisäkk P, Kidd G, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3(7):569–581. doi: 10.1038/nri1130 [DOI] [PubMed] [Google Scholar]
- 51.Engelhardt B, Ransohoff RM. Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. Published online 2012. doi: 10.1016/j.it.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 52.Ribeiro M, Brigas HC, Temido-Ferreira M, et al. Meningeal gammadelta T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci Immunol. 2019;4(40). doi: 10.1126/sciimmunol.aay5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomlinson JE, Žygelyte E, Grenier JK, Edwards MG, Cheetham J. Temporal changes in macrophage phenotype after peripheral nerve injury. J Neuroinflammation. 2018;15(1):185. doi: 10.1186/s12974-018-1219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhan C, Bin Ma C, Yuan HM, Cao BY, Zhu JJ. Macrophage-derived microvesicles promote proliferation and migration of Schwann cell on peripheral nerve repair. Biochem Biophys Res Commun. 2015;468(1–2):343–348. doi: 10.1016/j.bbrc.2015.10.097 [DOI] [PubMed] [Google Scholar]
- 55.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda R V. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33(34):8793–8801. doi: 10.1016/j.biomaterials.2012.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stettner M, Labus S, Weinberger J, et al. Schwann cell locomotion during peripheral nerve inflammation. J Neuroimmunol. 2014;275(1):72. doi: 10.1016/j.jneuroim.2014.08.189 [DOI] [Google Scholar]
- 57.Ozaki A, Nagai A, Lee YB, Myong NH, Kim SU. Expression of cytokines and cytokine receptors in human Schwann cells. Neuroreport. 2008;19(1):31–35. doi: 10.1097/WNR.0b013e3282f27e60 [DOI] [PubMed] [Google Scholar]
- 58.Makhluf HA, Stepniakowska J, Hoffman S, Smith E, LeRoy EC, Trojanowska M. IL-4 upregulates tenascin synthesis in scleroderma and healthy skin fibroblasts. J Invest Dermatol. 1996; 107(6):856–859. doi: 10.1111/1523-1747.ep12331160 [DOI] [PubMed] [Google Scholar]
- 59.Sato T, Liu X, Basma H, et al. IL-4 induces differentiation of human embryonic stem cells into fibrogenic fibroblast-like cells. J Allergy Clin Immunol. 2011; 127(6): 1595–603.e9. doi: 10.1016/j.jaci.2011.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deretzi G, Zou LP, Pelidou SH, et al. Nasal administration of recombinant rat IL-4 ameliorates ongoing experimental autoimmune neuritis and inhibits demyelination. J Autoimmun. Published online 1999. doi: 10.1006/jaut.1998.0259 [DOI] [PubMed] [Google Scholar]
- 61.Lima R, Monteiro S, Lopes JP, et al. Systemic lnterleukin-4 Administration after Spinal Cord Injury Modulates Inflammation and Promotes Neuroprotection. Pharm. 2017; 10(4). doi: 10.3390/ph10040083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao CF, Chen CC, Lu YW, et al. Effects of endogenous inflammation signals elicited by nerve growth factor, interferon-γ, and interleukin-4 on peripheral nerve regeneration. J Biol Eng. 2019; 13:86. doi: 10.1186/s13036-019-0216-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ali SA, Hanks JE, Stebbins AW, et al. Delivery of lnterleukin-4–Encoding Lentivirus Using Multiple-Channel Bridges Enhances Nerve Regeneration. Laryngoscope. Published online 2020. doi: 10.1002/lary.28629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Celik M, Labuz D, Keye J, Glauben R, Machelska H. IL-4 induces M2 macrophages to produce sustained analgesia via opioids. JCI Insight. Published online 2020. doi: 10.1172/jci.insight.133093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salehi B, Mishra AP, Nigam M, et al. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines. 2018;6(3). doi: 10.3390/biomedicines6030091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu M, Cheng Z, Ding Z, Wang Y, Guo Q, Huang C. Resveratrol enhances IL-4 receptor-mediated anti-inflammatory effects in spinal cord and attenuates neuropathic pain following sciatic nerve injury. Mol Pain. 2018;14:1744806918767549. doi: 10.1177/1744806918767549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gieseck RL, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. Published online 2018. doi: 10.1038/nri.2017.90 [DOI] [PubMed] [Google Scholar]
- 68.Milner JD, Orekov T, Ward JM, et al. Sustained IL-4 exposure leads to a novel pathway for hemophagocytosis, inflammation, and tissue macrophage accumulation. Blood. 2010;116(14):2476–2483. doi: 10.1182/blood-2009-11-255174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maes T, Joos GF, Brusselle GG. Targeting interleukin-4 in asthma: lost in translation? Am J Respir Cell Mol Biol. 2012;47(3):261–270. doi: 10.1165/rcmb.2012-0080TR [DOI] [PubMed] [Google Scholar]
- 70.Junttila IS, Creusot RJ, Moraga I, et al. Redirecting cell-type specific cytokine responses with engineered interleukin-4 superkines. Nat Chem Biol. Published online 2012. doi: 10.1038/nchembio.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]


