Abstract
Incentives are used to improve many health-related behaviors, but evidence is mixed for their effectiveness both during the incentivization period and, even more so, on the persistence of the behavior after incentives are withdrawn. In this paper, we present the results of a randomized controlled trial that successfully uses incentives to improve medication adherence among HIV-infected patients in Uganda over 20 months, and follows the sample for another 6 months to measure the persistence of these behavioral improvements. Our study contributes to the literature on habit formation by identifying a behavioral strategy that is associated with persistently high medication adherence after controlling for observable individual-level characteristics and the receipt of incentives. We find evidence supporting a psychological theory of habits as reflexive context-behavior associations, which suggests new ways of designing incentive-based interventions for better promoting persistent, healthier behaviors.
Keywords: habit formation, incentives, medication adherence, HIV
JEL: I12, D9, O15
I. Introduction
One fourth of all deaths in the United States are attributable to conditions that could be prevented with healthier habits (García 2016; CDC 2016). Unfortunately, existing interventions have been largely unsuccessful at fostering these lifelong behavioral improvements. Incentives are frequently employed to stimulate healthier habits (e.g. Acland and Levy 2015; Loewenstein, Price, and Volpp 2016; Carrera et al. 2018), but even when they are found to initiate healthier behaviors, the behavioral changes rarely persist after incentives are withdrawn and therefore, questions remain about the necessary conditions for habit formation (Wood and Neal 2016). In this study, we identify a behavioral strategy that is associated with persistently high antiretroviral (ARV) medication adherence after controlling for observable individual-level characteristics and the receipt of incentives. Our evidence supports the conceptualization of habits as contextually cued behaviors, and challenges theories of habit formation in the economics and psychology literatures. We present empirical evidence that context matters for developing persistent habits, and incentivizing the performance of a healthier behavior in response to a consistent contextual cue is a promising approach for promoting healthier habits.
This study focuses on promoting consistently high medication adherence, which is necessary for reducing the burden of chronic diseases on health and economic outcomes across the world (Polonsky and Henry 2016; Linnemayr 2017; Abegaz et al. 2017; Dunn et al. 2018). Medication nonadherence among people living with the Human Immunodeficiency Virus (HIV) is particularly harmful because high rates of adherence to ARV medications reduces the likelihood of HIV viral transmission, and thus represents an important step in ending the global HIV epidemic (Abrams and Strasser 2015; Harries et al. 2016). In Uganda, an increase in the availability of free ARV medications has reduced many of the common structural barriers to ARV adherence, but achieving and maintaining high rates of adherence is still hindered by cognitive barriers commonly observed among all populations, such as present-biased time preferences and declining intrinsic motivation (Linnemayr and Stecher 2015; Czaicki et al. 2018; Dilorio et al. 2008). Interventions addressing these psychological barriers are greatly needed to improve medication adherence and reduce the associated healthcare costs for a wide range of chronic health conditions that similarly require consistently high treatment adherence (Cutler et al. 2018).
Incentives have been suggested as a method for combating several of the common cognitive barriers to performing healthier behaviors, but there is mixed evidence for their effectiveness (Rosen et al. 2007; Sorensen et al. 2007; Simoni et al. 2008; Vrijens et al. 2008; Asch et al. 2015; Thirumurthy et al. 2019). In response to this conflicting evidence, recent studies have focused on ways to optimize intervention design parameters for a specific behavioral setting (Thirumurthy, Asch, and Volpp 2019). However, even when incentives are successfully designed for a targeted health behavior and lead to behavioral improvements, few studies have found that incentives lead to persistent, healthier behaviors after incentives are withdrawn (Acland and Levy 2015; Royer, Stehr, and Sydnor 2015; Rohde and Verbeke 2017; Carrera et al. 2018). The prevailing economic theory of habit formation, which began with Pollak (1970) and Ryder and Heal’s (1973) habit stock model,1 cannot explain the lack of behavioral persistence after incentives are withdrawn (Mantzari et al. 2015), which suggests that alternative behavioral mechanisms may underly many habitual behaviors.
The psychology literature offers two alternative behavioral theories to explain the lack of behavioral persistence from the use of incentives. First, Self-Determination Theory posits that extrinsic rewards such as incentives will reduce, or “crowd-out,” intrinsic motivation (Deci, Koestner, and Ryan 1999; Deci and Ryan 2000; Ryan and Deci 2000). This detrimental effect on motivation may explain the lack of behavioral persistence observed in incentive-based interventions since psychologists have also proposed that behavior change will lead to habit formation only when people are sufficiently motivated and prepared to make a change (Ajzen 1985). A separate psychological theory defines habits as “context-behavior associations” (Wood and Rünger 2016; Wood 2017). According to this theory, “habits are automatic behavioral responses to contextual cues … developed through repetition of behavior in consistent contexts” (Wood and Neal 2007; Lally and Gardner 2013; Verplanken, Verplanken, and Ryan 2018). Since extant research has yet to design incentives that also reinforce contextual consistency for a targeted behavior, this theory offers a second explanation for why incentives have not successfully created persistent, healthier habits and suggests ways of designing more effective incentive-based interventions.
This paper examines both the immediate and persistent effects of using incentives to improve ARV medication adherence among HIV-infected patients in Uganda, and provides preliminary evidence in support of contextual cue-based intervention methods for successfully creating persistent medication adherence habits. By observing ARV medication adherence behavior during both the 20-month incentives-based intervention and 6-month post-incentivization period, a longer period than many incentive-based studies,2 we were able to study the impact of incentives on habit formation more thoroughly than much of the existing behavior change research. In addition, we collected measures of participants’ present-biasedness and intrinsic motivation during the intervention which allowed us to compare the explanatory power of the behavioral economic model of intertemporal choice and alternative psychological theories of habit formation on participants’ observed behavior during and after the intervention. We additionally constructed a novel measure of temporally consistent daily pill-taking based on the observed timing of participants’ daily pill-taking, calculated as the fraction of pills taken close to each participants’ typical pill-taking time. We then combined this measure with survey data to identify participants who successfully used a contextual cue for their ARV adherence, and examined the association between using time-based pill-taking cues and participants’ post-incentives ARV adherence as well as the interaction between using time-based cues and the treatment effects over the 20-month intervention.
Our intervention was implemented among a sample of 155 adult clients of the Mildmay HIV Clinic in Kampala, Uganda who were randomly assigned to either receive incentives conditional on observable medication adherence behaviors, or to a control group. Importantly, incentive for medication adherence were not conditioned on the daily timing of pill-taking but were received for successfully taking the prescribed daily ARV medications at any point between 12 am and 11:59pm. Small in-kind incentives were awarded through a lottery mechanism at each clinic visit, which were pre-scheduled at 3-month intervals for a 20-month period, resulting in roughly 7 potential prize drawings per participant during the intervention. This lottery format and the nonmonetary, in-kind prizes were informed by extensive formative research among the client population, and all participants (including the control group) were also provided the usual standard of care at the Mildmay clinic which includes medication adherence support and free ARV medications.
Our study reveals that carefully selected incentive parameters can produce significant improvements to short-term medication adherence but that persistent, long-term behavioral changes are likely to occur only for the participants with a contextually-cued daily medication routine, which supports the theory of context-dependent behavioral associations as an underlying mechanism of habit formation in this setting. Specifically, the results show that directly incentivizing ARV adherence increased mean adherence by 5.4 percentage points relative to the control group over the 20-month incentivization period. This increase manifested as soon as incentives were offered and there was no statistically significant relationship between present-biased time preferences and adherence during or after incentivization, which cannot be explained by the standard economic theories of habit formation and intertemporal choice. Counter to Self-Determination Theory, we also found that intrinsic motivation increased during the incentivization period, which we explore further in the discussion section. Importantly, intervention effects persisted after incentives were withdrawn only for the participants who used time-based contextual cues for their daily ARV medication pill-taking. Mean adherence declined among all participants by 3.4 p.p. during the 6 months after incentives were withdrawn except among the participants that successfully used time-based adherence cues, such as taking ARV medications after a television show or morning prayers, and this difference remained even after conditioning on observable participant-level characteristics and study group assignment. Finally, heterogenous treatment effect analyses revealed that using time-based cues was associated with 19.5 p.p. higher ARV adherence throughout the 20-month intervention and that our incentives were primarily effective at improving adherence among those who did not use time-based cues, but it is unknown what caused participants, all of whom had documented or self-reported adherence problems, to adopt this behavioral strategy during the intervention. The success of time-based pill-taking routines supports the role of contextual cues for maintaining habitual behaviors, and suggests that future interventions should explore incentivizing the use of contextual cues for establishing persistent, healthier habits.
Our research offers four contributions to the behavior change literature. First, the results show that carefully designed intervention parameters can produce short-term improvements in medication adherence behavior and suggest how incentives may be successfully tailored to improve other health behaviors. Second, we are able to examine whether short-term improvements in medication adherence behavior become persistent behavioral habits by measuring medication adherence during the 6-months after incentive are withdrawn. This stands in stark contrast to existing studies that either do not observe behaviors after incentives are withdrawn or do not find significant predictors of behavioral persistence. Third, we collected measures of participants’ time preferences and intrinsic motivation throughout the intervention which allowed us to test alternative theories of habit formation; neither the accumulation of a habit stock of medication adherence behavior nor declining intrinsic motivation can explain the observed changes in ARV medication adherence behavior during this intervention. The final contribution of this research is the use of detailed contextual information to support the characterization of habits as context-behavior associations. Specifically, taking medications in response to a time-based contextual cue during the intervention strongly predicts persistent medication adherence after incentives are withdrawn.
This paper proceeds as follows. Section II describes competing theories of habit formation from the economics and psychology literatures and the testable predictions from these theories for study participants’ observed ARV mediation adherence behavior. Section III outlines the intervention setting and design, and presents sample descriptive statistics. Section IV contains the paper’s main analytical results, and the concluding section of this paper discusses how these results inform the design of future incentive-based interventions for establishing healthier habits as well as this study’s limitations.
II. Background Literature
The use of incentives for promoting long-term behavior change in economics research is often motivated by the prevailing theory of habit formation that describes the beneficial effect of past consumption on the marginal utility of current and future consumption, otherwise known as state-dependent preferences (Pollak 1970; Ryder and Heal 1973; Becker and Murphy 1988; Adamowicz and Swait 2012). By repeating the same form of consumption, or performing the same behavior, individuals build their “habit stock,” a weighted sum of past actions, which increases their marginal utility of continuing the same action in current and future periods.3 Thus, incentives are designed to promote habit formation by rewarding the performance of a targeted behavior so that a sufficiently large habit stock is built, increasing the desirability of continuing the behavior in current and future periods. This habit formation process may naturally occur for desirable behaviors, as described by the model for rational addiction (Becker and Murphy 1988), but for behaviors that are difficult to initially motivate, incentives may be necessary to initiate the accumulation of a sufficient habit stock. In the case of medication adherence and other health promoting activities that similarly require upfront costs to produce future health benefits, a sufficient behavioral habit stock may not naturally form without the use of incentives to add initial motivation for performing the desired behavior.
This economic model of habit formation makes two predictions about the temporal dynamics of medication adherence behavior induced by incentives. First, behavioral persistence will be observed for all participants who successfully engage in daily pill-taking, regardless of the context or timing of their daily adherence behavior. Second, unless incentives induce perfect adherence, an initial increase in daily pill taking will be followed by further increases in adherence over time as individuals build a new habit stock and thus further increase their marginal utility for medication adherence. The inability of many individuals to naturally initiate this habit formation process for future health promoting behaviors is further exacerbated by present-biased time preferences (Frederick, Loewenstein, and O’Donoghue 2002). For present-biased individuals, their relatively high discount rate for future consumption means that the small immediate costs of performing health promoting behaviors may outweigh the heavily discounted future benefits. Since existing research has documented that present-biased preferences in our specific study population are associated with lower rates of medication adherence (Linnemayr and Stecher 2015), the use of sufficiently large incentives is likely necessary to improve the adherence behavior of all participants in this setting. According to this model of intertemporal choice, we would also expect to see a greater likelihood of behavioral improvements from incentives among those with present-biased preferences, since these are individuals that would otherwise be least likely to self-initiate the habit formation process. Additionally, this theory suggests that habits would be more likely to deteriorate over time among those with high discount rates, since a larger habit stock is needed to raise the marginal utility of future discounted health benefits above the immediately experienced marginal costs. This paper will use the observed dynamics of medication adherence behavior during and after the receipt of medication adherence incentives to directly test the predictions derived from this economic theory of habit formation and model of intertemporal choice.
The psychology literature provides two alternative theories for the cognitive processes that underly habitual behaviors (Deci and Ryan 2000; Deci, Koestner, and Ryan 1999; Ryan and Deci 2000; Wood and Neal 2007; 2016), each of which yield testable hypotheses for the impact of incentives on the creation of medication adherence habits. First, Self-Determination Theory posits that the use of extrinsic rewards such as incentives will reduce intrinsic motivation (Deci and Ryan 2010; 2000; Deci, Koestner, and Ryan 1999; Ryan and Deci 2000). Under this theory, a desired behavior is performed in the absence of extrinsic rewards only when sufficient intrinsic motivation for the behavior exists. The detrimental effect of incentives on intrinsic motivation has thus been offered as a possible explanation for the lack of behavioral persistence observed in most incentivization interventions (Wood and Neal 2016), since psychologists have also proposed that behavior change will lead to habit formation only when people are sufficiently motivated and prepared for making a change (Ajzen 1985). To test this theory, we administered validated measures of intrinsic motivation to all participants four times during the incentivization period. We used the variation in this measure over time and the association between self-reported intrinsic motivation and medication adherence behavior both during incentive provision and once incentives were withdrawn to investigate the explanatory power of Self-Determination Theory in this setting.
A separate psychological theory defines habits as “context-behavior associations” (Wood and Neal 2007). According to this theory, “habits are automatic behavioral responses to contextual cues … developed through repetition of behavior in consistent contexts” (Wood and Neal 2007; Lally and Gardner 2013; Verplanken, Verplanken, and Ryan 2018). This theory emphasizes the importance of the decision-making environment on the reflexive, or subconscious, performance of habitual behaviors, and serves as motivation for the use of Implementation Intentions, or action planning, as an intervention strategy for forming new, healthier habits (Orbell, Hodgkins, and Sheeran 1997; Gollwitzer and Brandstätter 1997; Sheeran and Orbell 1999; Gollwitzer and Sheeran 2006). For implementation intentions, participants create “when-then” action plans for performing the targeted behavior after observing or experiencing a pre-specified contextual cue (Oettingen and Gollwitzer 2010). This intervention strategy has been found to successfully increase physical activity (Arbour and Martin Ginis 2009), getting a flu shot (Milkman et al. 2011), and completing a colonoscopy (Milkman et al. 2013), and evidence from recent studies shows that implementation intentions can lead to persistent behavioral change (Hagger and Luszczynska 2014; Duckworth, Milkman, and Laibson 2018), such as decreased fat intake among adults four months after being asked to form implementation intentions for a low-fat diet (Armitage 2004). The success of this approach highlights the power of contextual cues for maintaining behavioral changes, and suggests that incentivizing healthier behaviors independent of context will not directly facilitate the formation of these important context-behavior associations.
In the current study, the measure of ARV adherence behavior is derived from Medication Event Monitoring System (MEMS) caps, which enable us to directly investigate the role of contextual cues on habit formation. Specifically, the MEMS caps provide data on the exact timing of each pill bottle opening. Participants are also asked to report on their usage of time-based daily pill-taking remainders, such as phone alarms or timing their pill-taking with television shows or daily prayers, on three mid-intervention surveys. We used the observed temporal consistency of daily pill-taking, defined as the percentage of pills taken within a two-hour window of participants’ modal pill-taking time, to identify the participants that successfully used their self-reported time-based contextual cues for their medication adherence, and we estimated the association between persistent ARV adherence after incentives were withdrawn and participants’ use of time-based contextual cues as a test of the context-behavior association theory in this setting.
III. Study Setting and Design
Setting:
This study was conducted in partnership with Mildmay Uganda between March 2013 and February 2016. The Mildmay HIV clinic provides free HIV testing, treatment, and general medical services to over 23,000 HIV-infected clients in Kampala, Uganda. Since Mildmay provides comprehensive health care services for clients free of charge, almost all clients exclusively seek HIV-related treatment from Mildmay as opposed to receiving treatment and HIV medications from multiple sources. Thus, participants’ measured adherence for the HIV drugs provided by Mildmay is likely to capture an accurate measurement of participants’ full HIV medication adherence behavior.
Ethics approval was obtained from RAND’s Human Subjects Protection Committee, the Research Ethics Committee at Mildmay clinic, and the Uganda National Council for Science and Technology.
Eligibility, recruitment and treatment assignment:
Clients of the Mildmay HIV clinic were eligible for participation if they were at least 18 years of age, had documented adherence problems (either missed at least one clinic visit in the last 6 months or self-reported adherence problems), and were on ARV medication for at least 2 years (“treatment-mature” clients). The study was targeted to treatment-mature clients because their continued pursuit of HIV treatment indicated they had overcome many of the structural barriers to clinic care typically discussed in the literature, such as social stigma, transportation costs, and other economic hardships (Rintamaki et al. 2006; Kagee et al. 2011). The remaining barriers to proper medication adherence for these clients are hypothesized to be common psychological barriers, such as present-biased time preferences and declining intrinsic motivation, which have been successfully combated through behavioral incentives in other settings (e.g. Rosen et al. 2007).
Based on a list of all Mildmay clients who satisfied the study eligibility criteria in March 2013, potential participants were recruited on a rolling basis at the time of clinic check-in for their first appointment occurring between March and August 2013. Written informed consent was then obtained in the client’s preferred language, and participants were randomized to either one of two intervention groups or the control group. Consenting participants then completed a 45-minute baseline survey that measured respondents’ demographics, socioeconomic status, health status, intertemporal choice preferences, intrinsic motivation for ARV medication adherence, and planned use of time-based pill-taking cues. Of the initial 201 eligible Mildmay clients approached during clinic check-in, 46 clients were not recruited because of refusal, scheduling problems, or language barriers until a final study sample size of 155 participants was reached. Additional details about the study protocol can be found in a prior publication that described the initial, short-term treatment effects over the first 9 months of the intervention (Linnemayr, Stecher, and Mukasa 2017). This sample size was targeted based on initial power calculations for between-group comparisons of the main outcome, cumulative mean ARV adherence over the 20-month intervention, and the available funds for this pilot research study. Observably random dropout occurred at month 20, when over half of participants had their MEMS caps prematurely collected, so our investigation of adherence behavior over the 6 months post-incentives was conducted on only 60 participants, limiting the statistical power of these analyses. This missing follow-up data is discussed and analyzed in more detail below.
All participants received Mildmay’s standard HIV care, including medication adherence counseling services and free ARV medications, while participants in the two treatment groups were additionally provided with behavioral incentives designed to further promote medication adherence over the 20-month intervention period. Both treatment groups were eligible to win small, in-kind prizes awarded through a lottery mechanism. If eligible, participants in either treatment group would draw a single card from a bag with a 1/6th probability of winning their choice of three different prizes valued at roughly $1.50: an umbrella, a coffee mug, or a thermos. These nonmonetary prizes were chosen based on previous research among Mildmay’s client population that helped to identify both highly desirable and immediately useful items. Additionally, the use of small prizes awarded through a lottery mechanism reduced the overall cost of the intervention, which was an important concern of both Mildmay and other local community partners. By lowering overall costs, this intervention represents a widely scalable tool for promoting medication adherence in a region that urgently needs effective, low-cost tools for combating the HIV epidemic.
The two treatment groups differed in the behavior that determined prize eligibility during the 20-month intervention:
Treatment group 1 was eligible for the lottery if they attended their clinic appointments on their pre-scheduled day.
Treatment group 2 was eligible for the lottery if their mean ARV adherence (percent of daily pill doses taken as prescribed) measured over the 3-month period since their last clinic visit was at least 90%.
Based on these criteria, eligible participants in either treatment group would participate in the prize drawing at the end of each clinic visit. Clinic visits were pre-scheduled at roughly 3-month intervals so each participant had the potential to play the lottery approximately 7 times during the 20-month intervention, ensuring they would win at least one prize in expectation. Mean ARV adherence was measured as the number of observed MEMS pill bottle openings on a given 24-hour day divided by the participant’s prescribed pill regimen (either a once a day or twice a day regimen). If the number of openings exceeded the prescribed amount on a given day, the adherence percentage on that day was capped at 100%. Alternative adherence measures that penalized excessive pill bottle openings or counted excessive openings towards either of the subsequent two day’s totals (to allow for the potential storage of pills for later days) were also tested, and the results were largely unchanged. During the intervention, participants won an average of 1.7 (SD 1.4) times, and no participant won more than 3 times and therefore no one had to choose the same prize twice.
The intervention design parameters for this study were selected to avoid several of the common drawbacks in unsuccessful incentive-based interventions tested in the existing literature, such as infrequent incentive provision and indirect behavioral outcomes (Kamenica 2012; Thirumurthy, Asch, and Volpp 2019). Specifically, prize eligibility was determined every 3 months based on participants’ standard frequency of clinic visits since more frequent rewards would have required participants to make unnecessary and costly clinic visits. As shown in Table 1, the average cost of travelling to the clinic is $4.23, which represents roughly 6.3% of participants’ monthly income. Since prizes were only valued at $1.50, requiring unnecessary clinic visits was expected to undermine the utility benefit of the incentives, so the reward frequency was set to match these 3-month appointments. Additionally, the behaviors determining each treatment group’s prize eligibility were chosen to be directly observable, controllable, and easily understood by the participants. The first treatment group was incentivized to attend clinic appoints according to their pre-schedule appointments, since it has been shown that timely clinic attendance (which coincides with pharmacy refills) is associated with higher engagement in care and medication adherence. This behavior is also easily observable by the clinic, making this a readily scalable intervention method. The second treatment group was incentivized based on their electronically recorded medication adherence behavior, which is the primary behavioral outcome of this intervention. These two behaviors are necessary for patients to properly adhere to ARV medications and attain viral suppression, and were hypothesized to provide a more directly observable and attainable behavioral goal than designing incentives conditional on a health outcome, such as HIV viral load, which is less well-understood and more difficult for individuals to directly control. While the goal of ARV medication adherence is to reach HIV viral suppression, a physiological status that restores immune system health and reduces the risk of viral transmission, this intervention was intentionally designed to promote the intermediate and observable behaviors necessary for achieving viral suppression. These two treatment groups were also designed to provide additional evidence for the appropriate frequency of the incentivized behavior, where a comparison of the treatment effects between these two study groups will help improve the design of future incentive-based interventions.
Table 1:
Participation Rates and Demographics for Full and Analytic Samples
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
|---|---|---|---|---|---|---|---|---|
| Analytic Sample | ||||||||
| Full Sample | Analytic Sample | Sample Diff. | P-value | Control Mean | TreatT1 Diff. | TreatT2 Diff. | All Groups Equal P-value | |
| Participation Rate | 0.89 | - | - | - | 0.90 | −0.06 | 0.03 | 0.137 |
| Age | 38.97 | 39.00 | 0.03 | 0.952 | 37.42 | 2.72 | 1.88 | 0.146 |
| Female | 0.63 | 0.64 | 0.01 | 0.691 | 0.62 | −0.01 | 0.05 | 0.831 |
| Primary education | 0.52 | 0.51 | −0.01 | 0.567 | 0.52 | 0.03 | −0.02 | 0.944 |
| Married | 0.49 | 0.50 | 0.01 | 0.494 | 0.42 | 0.17 | 0.04 | 0.331 |
| Household assets | 5.01 | 5.10 | 0.08 | 0.139 | 4.76 | 0.57 | 0.18 | 0.232 |
| Own home | 0.74 | 0.73 | −0.01 | 0.417 | 0.70 | 0.06 | 0.06 | 0.652 |
| Monthly income $ | 65.71 | 66.63 | 0.92 | 0.782 | 52.80 | 19.49 | 18.72 | 0.162 |
| Travel costs $ | 4.04 | 4.23 | 0.19 | 0.131 | 3.90 | 0.87 | −0.43 | 0.457 |
| Household size | 4.71 | 4.80 | 0.09 | 0.305 | 4.31 | 0.81 | 0.40 | 0.262 |
| Health limits physical activity | 0.12 | 0.12 | 0.00 | 0.984 | 0.18 | −0.10 | −0.09 | 0.357 |
| Mental health index | 0.51 | 0.51 | −0.00 | 0.895 | 0.55 | −0.08 | −0.01 | 0.219 |
| Intrinsic motivation index | 0.79 | 0.80 | 0.01 | 0.126 | 0.79 | −0.00 | 0.00 | 0.661 |
| Present-biased preferences | 0.44 | 0.43 | −0.01 | 0.426 | 0.56 | −0.17 | −0.16 | 0.712 |
| Time-based cues | - | 0.13 | - | - | 0.12 | −0.04 | 0.03 | 0.634 |
| Cumulative mean adherence thru M20 | - | 0.84 | - | - | 0.80 | 0.04 | 0.06 | 0.065* |
| Observations | 155 | 138 | 45 | 43 | 50 | |||
Notes: Descriptive statistics for the full sample (N=155) and analytic sample (N=138), as well as descriptive statistics among the three randomized study groups. Wilcoxon rank-sum tests were used to compare the full and analytic sample characteristics (p-values in column 4) and the non-parametric Kruskal Wallis test was used to evaluate the equality of observable characteristics across the three study groups (p-values in column 8), where
p < 0.10,
p < 0.05,
p < 0.01.
Additionally, the top row displays the attrition rate from the full sample to the analytic sample, and the impact of attrition on each study group. The final two rows show how the use of time-based pill-taking cues and cumulative medication adherence over the 20-month intervention differed between each study group. Treatment group 1 (TreatT1) was eligible for the lottery based on timely clinic attendance, and treatment group 2 (TreatT2) was eligible for the lottery if their observed mean ARV adherence was at least 90%. The control group was not eligible for behavioral incentives, and all groups received the usual standard of care.
Summary statistics:
Table 1 presents descriptive statistics for the full sample of 155 clients from the Mildmay HIV clinic who were evenly randomized across the three study groups, as well as the characteristics of the final analytic sample of 138 clients who remained after sample attrition. The first two columns show the mean of several important demographic and psychological measures recorded on the baseline survey among the full and analytic samples, respectively, as well as the study participation rate (the fraction of recruited clients who were retained in the study for the full 20-month intervention period). The third and fourth columns show the difference in these observable characteristics between the full and analytic samples and the p-values from Wilcoxon rank-sum tests of equality. The fifth column shows the demographic and psychological characteristics of the control group in the analytic sample. The next two columns present the difference in means between the control group and each treatment group, and the final column presents p-values testing whether the three study groups have equal means using the non-parametric Kruskal Wallis test (Kruskal and Wallis 1952).
Sample attrition due to moving away from Kampala, MEMS-cap device malfunction, or study fatigue during the RAP program was limited to 11% (17 participants) and equally experienced across study groups (p = 0.14). The final analytical sample contained 138 participants who were on average approximately 39 years old, 64% female, roughly half had at least a primary school education, and 50% were married. Slight differences exist between study groups in participants’ monthly disposable income (sample mean = $66.63) and travel costs to the Mildmay clinic (sample mean = $4.23), but these differences were insignificant as demonstrated by the p-values in the final column of Table 1. The study groups were also balanced in their levels of household assets, home ownership, household size, and measures of both physical and mental health. After the 20-month intervention period, it was planned that all participants would continue to use the MEMS-caps for 6 months to observe the persistence of ARV adherence behavior after incentives were withdrawn. Miscommunication between the research team and project staff led to over half of participants returning their MEMS-caps during their 20-month clinic visit, so post-incentives data are only available for 60 participants. Table 2 shows how the sample composition differs between the participants with complete observations for all 26 months and those who are not observed after their 20-month clinic appointment, and demonstrates that these samples were observationally equivalent.
Table 2:
Sample Composition Between M20 and M26 and Characteristics of M20 Attrition
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
|---|---|---|---|---|---|---|---|---|
| Only M20 Sample | ||||||||
| Complete M26 | Only M20 | Sample Diff. | P-value | Control Mean | TreatT1 Diff. | TreatT2 Diff. | All Groups Equal P-value | |
| Control group | 0.40 | 0.27 | −0.13 | 0.255 | 1.00 | - | - | - |
| T1 Treatment | 0.33 | 0.29 | −0.04 | 0.612 | - | 1.00 | - | - |
| T2 Treatment | 0.27 | 0.44 | 0.17 | 0.041 | - | - | 1.00 | - |
| Age | 39.57 | 38.56 | −1.00 | 0.635 | 36.76 | 1.85 | 2.89 | 0.646 |
| Female | 1.62 | 1.65 | 0.04 | 0.654 | 1.57 | 0.12 | 0.11 | 0.442 |
| Primary education | 0.52 | 0.51 | −0.00 | 0.964 | 0.62 | −0.10 | −0.18 | 0.601 |
| Married | 0.53 | 0.47 | −0.06 | 0.494 | 0.38 | 0.14 | 0.12 | 0.114 |
| Household assets | 5.25 | 4.99 | −0.26 | 0.490 | 4.62 | 1.16 | 0.06 | 0.117 |
| Own home | 0.77 | 0.71 | −0.06 | 0.420 | 0.52 | 0.17 | 0.30 | 0.284 |
| Monthly income $ | 71.44 | 62.74 | −8.70 | 0.128 | 43.77 | 37.53 | 18.02 | 0.2S3 |
| Travel costs $ | 4.30 | 4.17 | −0.13 | 0.984 | 3.78 | 1.74 | −0.29 | 0.612 |
| Household size | 4.92 | 4.71 | −0.20 | 0.274 | 4.10 | 1.12 | 0.64 | 0.779 |
| Health limits physical activity | 0.13 | 0.10 | −0.03 | 0.577 | 0.14 | −0.06 | −0.05 | 0.548 |
| Mental health index | 0.57 | 0.47 | −0.10 | 0.388 | 0.45 | −0.03 | 0.08 | 0.821 |
| Intrinsic motivation index | 0.81 | 0.78 | −0.03 | 0.247 | 0.81 | −0.04 | −0.03 | 0.664 |
| Present- biased preferences | 0.47 | 0.40 | −0.07 | 0.417 | 0.48 | −0.08 | −0.12 | 0.577 |
| Time- based cues | 0.13 | 0.13 | −0.01 | 0.930 | 0.15 | −0.03 | −0.02 | 0.472 |
| Cumulative mean adherence thru M20 | 0.84 | 0.83 | −0.00 | 0.679 | 0.81 | 0.02 | 0.03 | 0.288 |
| Observations | 60 | 78 | 21 | 23 | 34 | |||
Notes: Descriptive statistics for the sample with complete observations during the 20-month intervention and 6 months post-intervention (N=60), “Complete M26,” compared to the sample who were only observed for the 20-month intervention (N=78), “Only M20.” Wilcoxon rank-sum tests were used to compare the Complete M26 and Only M20 sample characteristics (p-values in column 4), where * p < 0.10, ** p < 0.05, *** p < 0.01. Columns 5 – 8 compare the characteristics of the Only M20 sample across all three study groups, and the non-parametric Kruskal Wallis test was used to evaluate the equality of observable characteristics across the three study groups (p-values in column 8).
The first column of Table 2 shows the average study group assignment, demographics, psychological measures, and adherence behavior for the 60 participants with complete post-incentives observations. The next three columns present the same descriptive statistics for the sample with incomplete post-incentives observations, followed by the difference in means between these two groups and the p-values associated with Wilcoxon rank-sum tests of equality. The only significant compositional difference was that the full 26-month sample contained a smaller fraction of participants in the second treatment group (reward eligibility conditional on mean ARV adherence of at least 90%). To examine how the differential rates of missing follow-up data by study group after month 20 may impact the results, columns 5 – 8 of Table 2 compare the observed characteristics of the participants in each study group who had their MEMS-cap collected in month 20. The insignificant differences between these groups, particularly in regards to their adherence behavior, such as the use of time-based cues and their cumulative mean adherence during the 20-month intervention, suggests that the analyses of post-incentives behavior do not suffer from observable sources of sample selection bias.
To investigate the psychological attributes associated with persistent ARV adherence, measures of participants’ intrinsic motivation, intertemporal choice preferences, and pill-taking routines were collected on baseline and month 4, 14, and 20 surveys. Intrinsic motivation was measured using the Intrinsic Motivation Inventory (Deci, Koestner, and Ryan 1999; Ryan and Deci 2000; 2008), where a motivation index is calculated as the percent of questions receiving affirmative responses (either “Agree” or “Strongly agree”) in regard to participants’ perceptions of the importance, value, and usefulness of ARV medications, as well as their perceived competence and social support for adhering to their ARV medication protocol. Present-biased time preferences were identified based on responses to a Multiple Price List (Meier and Sprenger 2015) survey module, where participants were asked to choose between hypothetical pairs of rewards valued from $13.50 to $27.00 received either tomorrow, in one year, or in two years. Those who chose the smaller, sooner reward when deciding between tomorrow or one year, but then displayed inconstant temporal discounting by being willing to wait for the larger reward when deciding between one year or two years for the same pair of rewards were classified as having present-biased time preferences (Andersen et al. 2006; Frederick, Loewenstein, and O’Donoghue 2002). Finally, participants were asked about their use of daily pill-taking reminders on the follow-up surveys in months 4, 14, and 20. These self-reported reminders were categorized as either being “time-based reminders,” such as using an alarm or timing pill-taking with a TV show or daily prayers, or “variably-timed reminders,” such as having a family member remind them or taking pills with dinner or after work. This information was then combined with the observed temporal consistency of participants’ pill bottle openings in order to identify the participants who successfully used time-based pill-taking routines. Specifically, participants’ modal pill-taking time was calculated within the 30 days before and after each pill-taking observation, and temporally consistent pill-taking was defined as taking at least 90% of pills within a 2-hour window of this moving modal pill-taking time over the 20-month intervention period. Among those who reported using time-based reminders, over 96% of their observed pill-taking was within 2 hours of their moving modal pill-taking time, which motivated this definition, and the results are largely unchanged under alternative cutoffs (e.g. 1-hour and 3-hour windows); described below. The participants who both reported using “time-based reminders” and displayed temporal consistency in their pill-taking are believed to have successfully established a time-based pill-taking routine, which is hypothesized to increase the likelihood of maintaining proper adherence after the incentives are withdrawn.
Table 1 demonstrates that the randomization balanced participants on these important psychological dimensions – average intrinsic motivation for ARV adherence was observed to be 0.8 out of 1 and roughly 43% of participants displayed present-biased preferences on the baseline survey. Additionally, 13% of participants displayed time-based pill-taking routines over the 20-month intervention. Importantly, the three study groups were statistically equal in terms of intrinsic motivation, present-biased time preferences, and the use of time-based cues, all of which may influence the observed treatment effects on cumulative mean adherence during the 20-month intervention that are presented at the bottom of Table 1. Table 2 confirms that the dropout between month 20 and month 26 due to project miscommunication was evenly experienced across these psychological dimensions.
Table 3 helps to characterize the 26 participants (19%) who were identified as successfully using time-based pill-taking cues during the intervention period. The first column shows that, on average, those with time-based routines were slightly older and more educated, but the p-values in the fourth column of Table 3 indicate that these differences are not statistically significant. Instead, participants with time-based pill-taking routines were 19% more likely to own a home and earn $34.79 more in monthly income. Better physical and mental health is also significantly associated with establishing time-based pill-taking routines, and the final row of Table 3 shows that the use of time-based routines is associated with a 14% higher mean ARV adherence during the 20-month intervention. The subsequent columns in Table 3 demonstrate the stability of the temporal consistency definition by comparing the observable characteristics among time-based pill-takers identified using a 1-hour and 3-hour time window around participants’ moving modal time. In addition to the small differences in group sizes (22 are considered consistent using the 1-hour definition and 29 are consistent according to the 3-hour definition), non-parametric tests of equality between these alternative definitions confirm that these differences are statistically insignificant. The following regression analyses will be used to estimate the magnitude of the treatment effects on cumulative mean ARV adherence both during and after incentivization, as well as test the significance of the association between time-based routines and the persistence of ARV adherence habits after incentives were withdrawn.
Table 3:
Sample Composition for Time-based Adherence Routines and Stability of Temporal Consistency Definition
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
|---|---|---|---|---|---|---|---|---|
| Stability of Temporal Consistency Definition | ||||||||
| Time-based cues | Irregular timing | Diff. | P-value | 2-hour def. | 1-hour def. Diff. | 3-hour def. Diff. | All Croups Equal P-value | |
| Age | 40.46 | 38.56 | −1.90 | 0.413 | 40.46 | 2.74 | −1.47 | 0.453 |
| Female | 0.62 | 0.65 | 0.03 | 0.754 | 0.62 | 0.02 | −0.04 | 0.534 |
| Primary education | 0.58 | 0.50 | −0.07 | 0.615 | 0.58 | 0.03 | 0.05 | 0.581 |
| Married | 0.50 | 0.50 | −0.00 | 0.906 | 0.50 | 0.01 | −0.01 | 0.928 |
| Household assets | 5.69 | 4.95 | −0.75 | 0.108 | 5.69 | 0.15 | −0.15 | 0.394 |
| Own homo | 0.88 | 0.69 | −0.19 | 0.052* | 0.88 | 0.04 | −0.07 | 0.423 |
| Monthly income $ | 92.19 | 57.40 | −34.79 | 0.016** | 92.19 | 16..S7 | 17.14 | 0.184 |
| Travel costs $ | 3.94 | 4.28 | 0.34 | 0.958 | 3.94 | 0.29 | 0.89 | 0.732 |
| Household size | 5.00 | 4.74 | −0.26 | 0.680 | 5.00 | 0.20 | −0.35 | 0.512 |
| Health limits physical activity | 0.00 | 0.14 | 0.14 | 0.044** | 0.00 | 0.06 | 0.08 | 0.172 |
| Mental hra.lth index | 0.31 | 0.57 | 0.25 | 0.008*** | 0.31 | −0.08 | 0.04 | 0.596 |
| Intrinsic motivation index | 0.85 | 0.79 | −0.06 | 0.114 | 0.85 | 0.09 | −0.07 | 0.402 |
| Present-biased preferences | 0.42 | 0.42 | 0.00 | 0.997 | 0.42 | −0.04 | 0.03 | 0.616 |
| Cumulative mean adherence thru M20 | 0.95 | 0.81 | −0.14 | 0.002*** | 0.95 | 0.02 | −0.05 | 0.166 |
| Observations | 26 | 112 | 26 | 22 | 29 | |||
Notes: Descriptive statistics for the sample that report using time-based cues on the baseline survey and display temporal consistency (defined as pill-taking within 2 hours of moving modal time) in their daily pill-bottle openings during the 20-month intervention (N=26), “Time-based cues,” compared with the sample that did not use time-based adherence cues (N=112), “Irregular timing.” Wilcoxon rank-sum tests were used to compare the Time-based cues and Irregular timing sample characteristics (p-values in column 4), where
p < 0.10,
p < 0.05,
p < 0.01. Columns 5 – 8 compare the characteristics of the Time-based cues sample with alternative definitions of temporal consistency (within 1 hour or 3 hours of moving modal time), and the non-parametric Kruskal Wallis test was used to evaluate the equality of observable characteristics across these alternative definitions (p-values in column 8).
IV. Results
Primary Treatment Effects:
The pre-registered4 primary analyses examine cumulative mean ARV adherence over the 20-month intervention for the 138 HIV-infected clients of Mildmay HIV Clinic who completed the study. Figure 1a shows the average monthly mean ARV adherence separately among each of the three study groups, as well as for the two treatment groups pooled. Figure 1b shows the cumulative mean ARV adherence for these same study groups, where this cumulative measure was calculated as the average monthly ARV adherence between month 2 and the indicated subsequent month.
Figure 1.
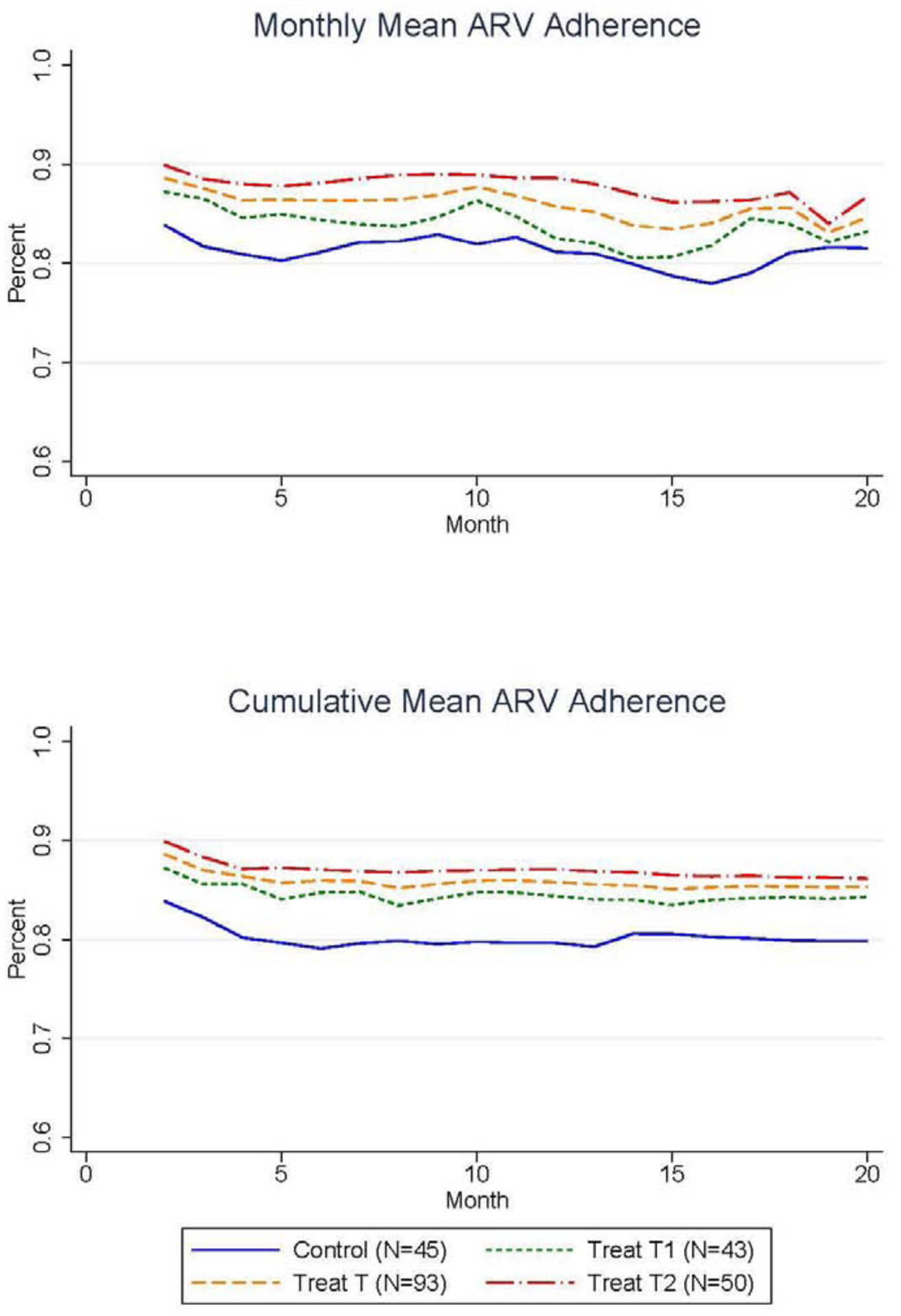
Positive Impact of Incentives on Monthly and Cumulative Mean Adherence During the 20-Month Intervention
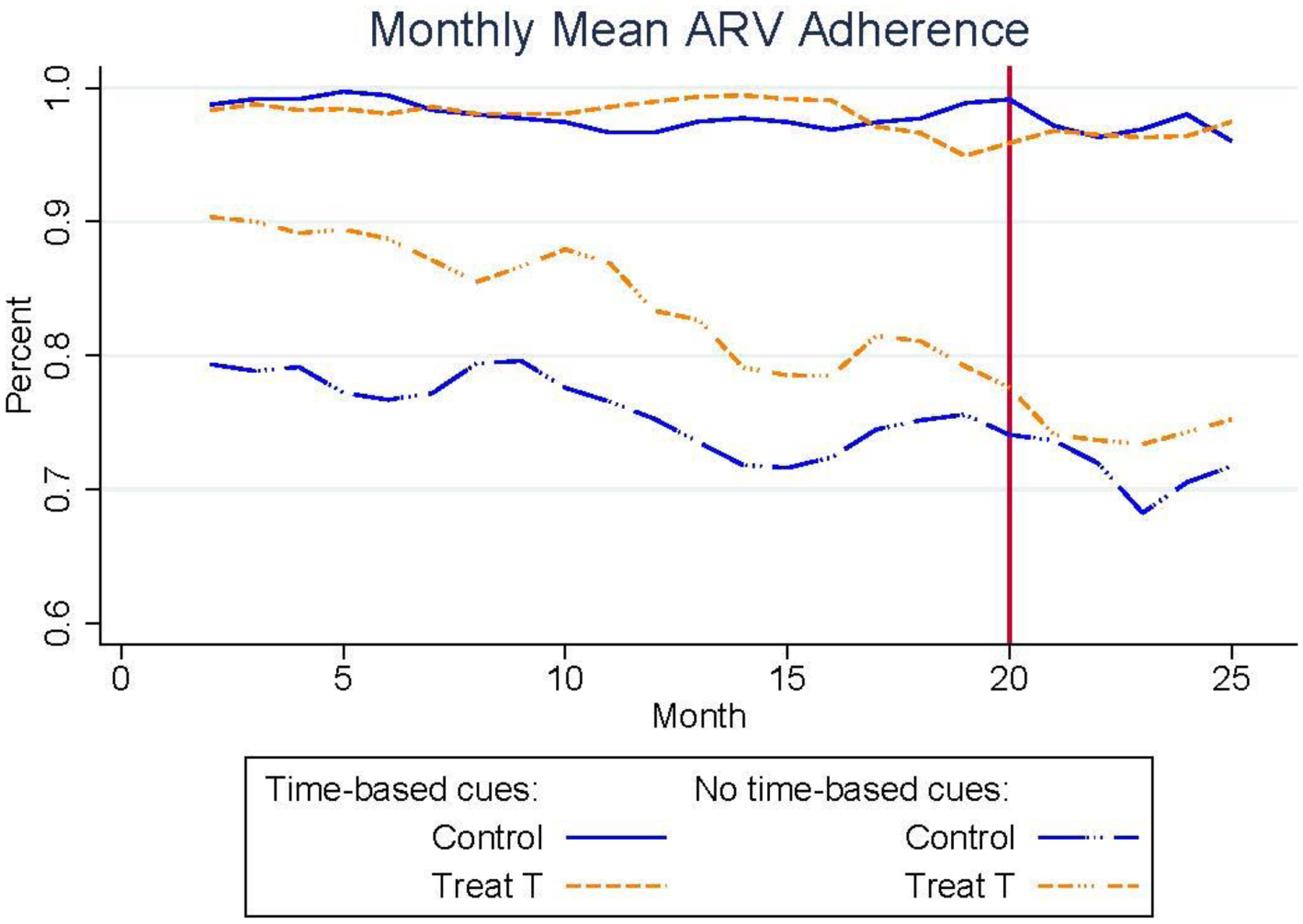
These two figures show that both treatment groups experienced a small but consistent increase in mean ARV adherence during the 20-month intervention period relative to the control group participants who only received the standard HIV care and adherence counseling. The second treatment group (“Treat T2”) that was eligible for prizes conditional on maintaining mean adherence of at least 90% showed the largest increases in adherence rates; the monthly difference in mean adherence between treatment group 2 and the control group averaged 6.6 p.p. (SD 1.2 p.p.) over the 20-month intervention and ranged from 8.3 p.p. (SD 1.4 p.p.) in month 16 to 2.4 p.p. (SD 1.2 p.p.) in month 18. The first treatment group (“Treat T1”) that was eligible for prizes conditional on timely clinic appointment attendance displayed smaller increases in adherence rates relative to the control with an average increase of 2.7 p.p. (SD 0.9 p.p.) over the 20-month intervention that ranged from a maximum monthly increase of 5.5 p.p. (SD 1.6 p.p.) in month 17 to a minimum increase of 0.5 p.p. (SD 0.4 p.p.) in month 19. This weaker effect experienced by the first treatment group is consistent with the literature that suggests incentives are less effective for infrequently performed behaviors that are not direct inputs into participants’ health, such as preventative health screenings (Goldzahl, Hollard, and Jusot 2018; Mehta et al. 2019; Gupta et al. 2016). Still, the monthly mean adherence between the pooled treatment groups (“Treat T”) was consistently higher than the control group and the cumulative mean adherence in the pooled treatment group over months 2 through 20 was 5.6 p.p. (SD 3.1 p.p.) higher than the control group. The statistical significance of these differences is tested formally in the regression analyses below.
While the average mean ARV adherence among the control group at baseline was 79.8% (SD 17.9%), ARV medication protocols require mean adherence rates of 90% or higher in order to achieve the full treatment benefits (Bangsberg et al. 2001; de Olalla Garcia et al. 2002). This high threshold for attaining the full treatment benefits underscores the importance of developing adherence support tools, and motivated our design of the second treatment group that directly incentivized participants to attain mean adherence rates above 90%. To assess the impact of the intervention on participants’ ability to meet this threshold, Figure 2 displays the percent of participants in each study group with cumulative mean ARV adherence greater than or equal to 90%, along with the percent of participants in the pooled treatment group with at least 90% cumulative mean ARV adherence. The percent of participants in the control group who reached cumulative mean adherence rates of 90% or more starts at 57.4% in month 2 and falls continuously to 30.6% in month 20. Importantly, both treatment groups displayed higher likelihoods of meeting the 90% mean adherence threshold. On average, the percent of participants meeting the 90% threshold in the first treatment group was 5.4 p.p. higher than the control group in the final month of the intervention, while the second treatment group was 16.3 p.p. more likely to reach at least 90% mean adherence in the final month of the intervention (month 20).
Figure 2.
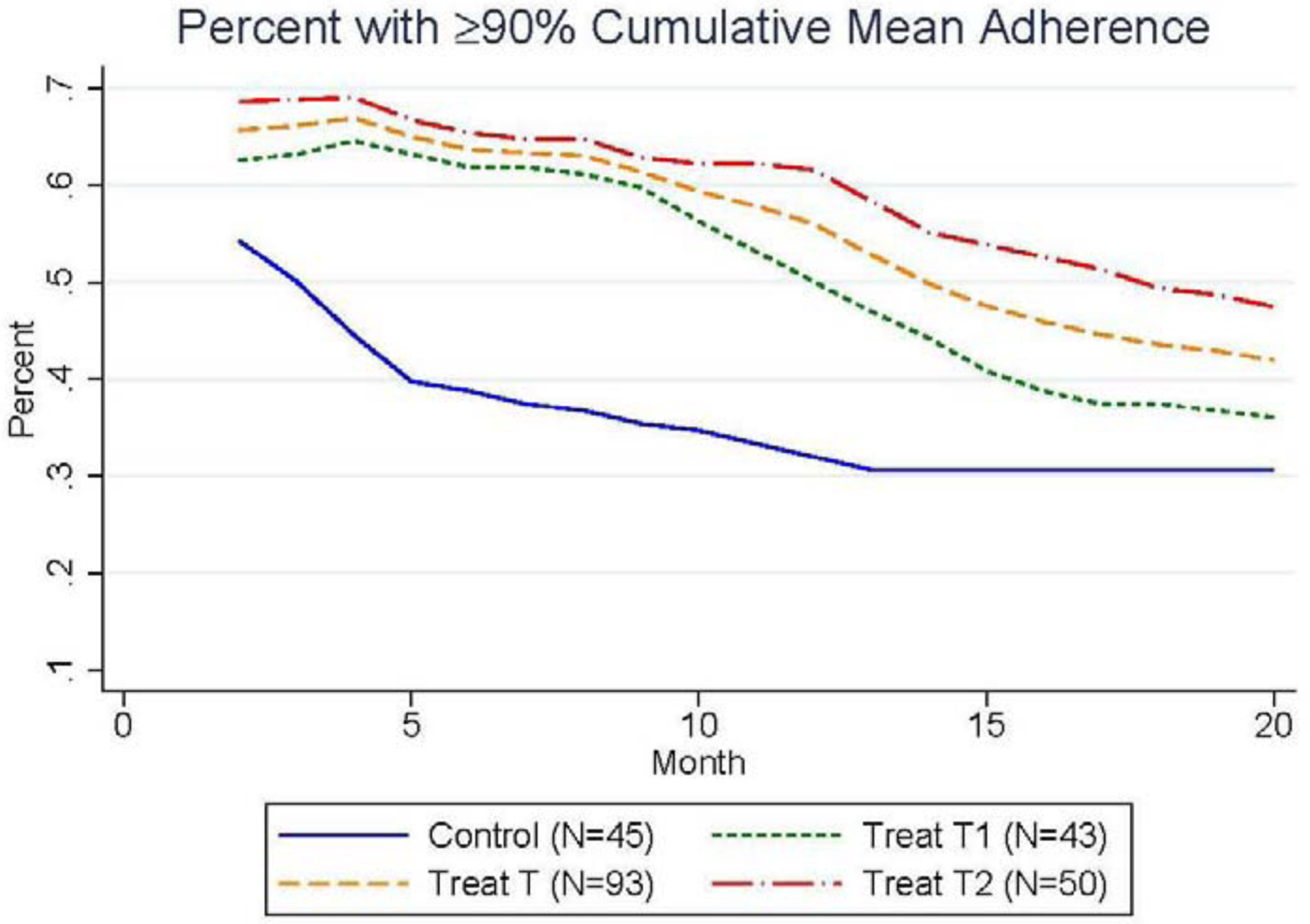
Positive Impact of Incentives on the Percent of Participants with at Least 90% Cumulative Mean Adherence Over the 20-Month Intervention
In Table 4, we present regression estimates to test the significance of the average treatment effects displayed in Figure 1. For these regressions, we run models of the form:
where yi is the cumulative mean ARV adherence, are indicator variables equal to 1 for individuals in the indicated treatment group and 0 for those in the control group, and Xi captures the remaining observable socioeconomic and health differences between participants presented in Tables 1–3. Specifically, Xi includes participants’ age in years, an indicator for being female, having at least a primary education, an indicator for being married, a categorical household asset index measured by ownership of 10 common household items, an indicator for home ownership, the logarithm of both monthly income and the travel costs to the clinic, reporting that health limits any one of six physical activities of daily living, a categorical mental health index measured by an 11-item module identifying the presence of mental illness symptoms, and the intrinsic motivation and present-biased preference measures discussed earlier. Since the randomization procedures successfully balanced the study groups across these observable dimensions, as seen in Table 1, the following regression results were largely unchanged with the inclusion of these additional controls. This model was estimated using both OLS and a censored Tobit regression to account for the bounded nature of the cumulative mean ARV adherence measure, which was recorded as the proportion of pills taken as prescribed. The results are both quantitatively and qualitatively similar between these two estimation methods, and the model parameters from both estimation methods using heteroscedasticity-consistent standard errors are displayed in Table 4.
Table 4:
Treatments on Cumulative Mean ARV Adherence
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
|---|---|---|---|---|---|---|---|
| (OLS) | (OLS) | (Tobit) | (Tobit) | Mean Adherence | Mean Adherence | Mean Adherence | |
| Mean Adherence | Mean Adherence | Mean Adherence | Mean Adherence | 25th Quantile | 50th Quantile | 75th Quantile | |
| T1 group | 0.0448 (0.033) | 0.0260 (0.035) | 0.0468 (0.034) | 0.0280 (0.033) | −0.0062 (0.058) | 0.0267 (0.047) | 0.0308 (0.031) |
| T2 group | 0.0633* (0.036) | 0.0539* (0.033) | 0.0633* (0.036) | 0.0539* (0.031) | 0.1092 (0.080) | 0.1055*** (0.036) | 0.0401* (0.023) |
| Controls included | No | Yes | No | Yes | Yes | Yes | Yes |
| Observations | 138 | 138 | 138 | 138 | 138 | 138 | 138 |
| Mean of dep var | 0.835 | 0.835 | 0.835 | 0.835 | 0.752 | 0.876 | 0.967 |
Standard errors in parentheses
p < 0.10,
p < 0.05,
p < 0.01
Notes: This table presents the coefficient estimates from both OLS and Tobit (censored) regression models of cumulative mean ARV adherence over the 20-month intervention on indicator variables for the two treatment groups, estimated using heteroscedasticity-consistent standard errors. Columns 1 and 3 (2 and 4) show the coefficients from the models estimated without (and with) all of the demographic controls presented in Tables 1–3, including participants’ age, gender, education, marital status, a measure of household assets, home ownership status, monthly income, travel costs to the clinic, household size, measures of physical and mental health, intrinsic motivation, and present-biased preferences. Columns 5 – 7 display coefficients from the same model estimated with all of the demographic controls as a quantile regression at the 25th, 50th, and 75th percentiles of cumulative mean ARV adherence over the 20-month intervention. The average cumulative mean ARV adherence was 83.5% among the analytic sample (N=138).
The Tobit regression coefficient estimates in columns 3 and 4 of Table 4 show the impact of each treatment on cumulative mean ARV adherence relative to the control, without and with the inclusion of control variables, respectively. From the full regression model, we find that the first treatment group displayed a 2.8 p.p. higher cumulative mean adherence over the 20-month intervention, but this increase was not statistically significant. The second treatment group improved cumulative mean adherence by 5.4 p.p., which is significant at the 10% level (p = 0.07). Since the average cumulative mean ARV adherence among all participants was 83.5%, this treatment effect represents a meaningful increase in adherence towards the targeted 90% threshold. To better understand which participants experienced the greatest benefits from the intervention, the results in columns 5 – 7 of Table 4 show quantile regression coefficients for the same linear regression model estimated at the 25th, 50th, and 75th percentiles of the observed cumulative mean ARV adherence distribution. These estimates show that the second treatment was most effective for participants who were just below the 90% threshold. Specifically, for the bottom 25th percentile, the second treatment increased adherence by 10.9 p.p. from an average of 75.2%, although this effect was not significant. Instead, the second treatment significantly improved adherence at the median by 10.6 p.p. (p = 0.01) from an average of 87.6%, which indicates that this treatment was successfully designed for the participants who stood to benefit the most from our intervention by successfully reaching the 90% mean adherence threshold.
To directly assess the effect of each treatment on the likelihood of reaching the 90% mean ARV adherence threshold, the following logistic regression model was estimated:
where yi is an indicator for reaching a cumulative mean ARV adherence of at least 90% over the 20-month intervention period and . The variables are indicator variables equal to 1 for individuals in the indicated treatment group and 0 for those in the control group, and Xi contains the same set of socioeconomic and health controls utilized above and presented in Tables 1 – 3. The odds ratios from this logistic model estimated through maximum likelihood procedures with heteroscedasticity-consistent standard errors without (and with) the inclusion of control variables are presented in column 1 (and 2) of Table 5. The results show that the first treatment had an insignificant impact on participants’ odds of reaching the 90% mean adherence threshold, while the second treatment increased participants’ odds by 2.37 (p = 0.06).
Table 5:
Treatments on Percent with ≥90% Cumulative Mean Adherence
| (1) | (2) | |
|---|---|---|
| Pct. ≥ 90% Mean Adhere | Pct. ≥ 90% Mean Adhere | |
| Tl group | 1.1851 (0.524) | 1.0141 (0.442) |
| T2 group | 2.3068** (0.977) | 2.3731* (1.086) |
| Controls included | No | Yes |
| Observations | 138 | 138 |
| Mean of dep var | 0.442 | 0.442 |
| SD of dep var | 0.498 | 0.498 |
Odds ratios (i.e. exponentiated coefficients); Standard errors in parentheses
p < 0.10,
p < 0.05,
p < 0.01
Notes: This table presents the estimated odds ratios (exponentiated coefficients) from a logistic regression model of whether participants reached at least 90% cumulative mean ARV adherence over the 20-month intervention on indicator variables for the two treatment groups, estimated using heteroscedasticity-consistent standard errors. Columns 1 (and 2) show the coefficients from the model estimated without (and with) demographic controls that include participants’ age, gender, education, marital status, a measure of household assets, home ownership status, monthly income, travel costs to the clinic, household size, measures of physical and mental health, intrinsic motivation, and present-biased preferences. Roughly 44.2% percent of participants reached the 90% cumulative mean ARV adherence threshold over the 20-month intervention period.
Persistence of Treatment Effects:
The short-term use of incentives in this study was motivated by the economic theory of habit formation which contends that habits are formed with the daily repetition of a behavior. To assess the degree of habit formation among the participants in this study, the following analyses will describe participants’ post-intervention behavior during the 6 months following the withdraw of incentives. As previously mentioned, a significant degree of dropout randomly occurred after month 20, so the analyses of post-incentives behavior were performed among the 60 participants with complete observations through month 26. The group-level monthly and cumulative mean adherence rates for this sample of complete observations are presented for all 26 months of the study in Figure 3, where the end of the intervention in month 20 is indicated with a vertical line.
Figure 3.
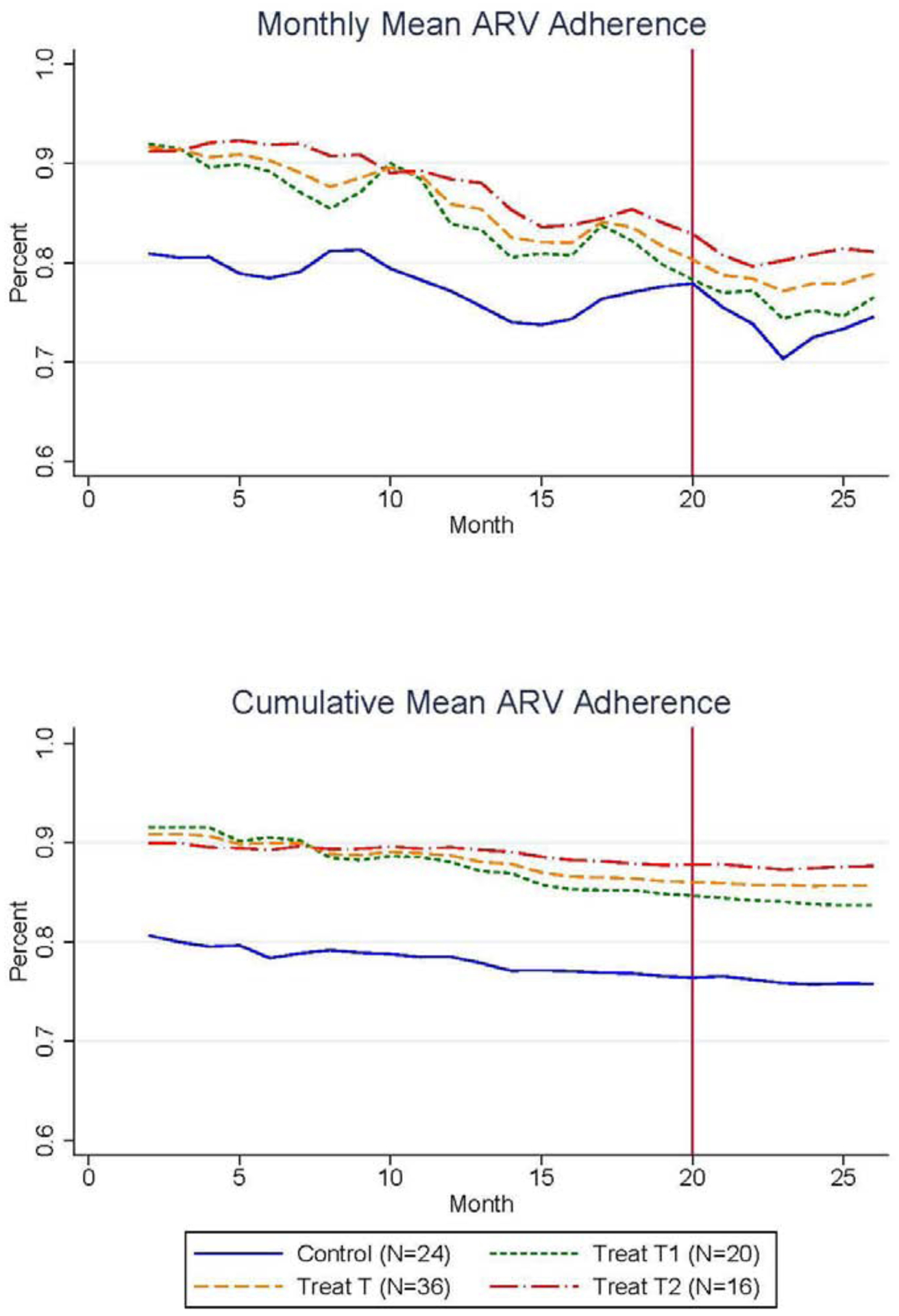
Impact of Incentives on Monthly and Cumulative Mean Adherence Over the 20-Month Intervention and 6 Months Post-Intervention
Two important trends are clearly visible in Figure 3a: 1.) on average, all incentivized participants experienced a steady decline in mean adherence once incentives were withdrawn, and 2.) the control group experienced a similar decline in mean adherence, so the level difference in mean adherence between treatment and control groups persisted through month 26. Evidence of treatment effect persistence is magnified in Figure 3b, which presents the cumulative mean ARV adherence (the pre-specified main outcome) over all 26 months for the three study groups. This graph shows that the difference in cumulative adherence between treatment and control groups established through the first 20 months was maintained during the 6 months after incentives were withdrawn.
Figure 4 plots the likelihood of reaching the 90% cumulative mean adherence threshold among all three study groups and the pooled treatment group among the 60 participants with complete observations through 26 months. Again, a persistent level difference existed between the treatment and control groups for this secondary outcome over the full 26-month period. Tables 6 and 7 provide regression results that describe the significance of the level differences between study groups observed in Figures 3 and 4, respectively. Specifically, Table 6 shows that the second treatment group had 10.6 p.p. (p = 0.031) higher cumulative mean ARV adherence over the 20-month intervention and 6-month post-intervention period relative to the control group (months 2 – 26). Similarly, the second treatment group had 4.75 (p = 0.03) greater odds of reaching the 90% cumulative mean adherence threshold over this 26-months period. To examine the level differences in adherence behavior between study groups just over the post-incentivization period, Tables 8 and 9 present regression results for the cumulative mean adherence during the 6 months post-incentives and the odds of reaching the 90% cumulative mean adherence threshold during the 6 months post-incentives, respectively. Similar to the results over months 2 – 26, the second treatment group had 11.2 p.p. (p=0.03) higher cumulative mean ARV adherence and a 2.82 (p=0.02) greater odds of reaching the 90% cumulative mean adherence threshold over months 21 – 26.
Figure 4.
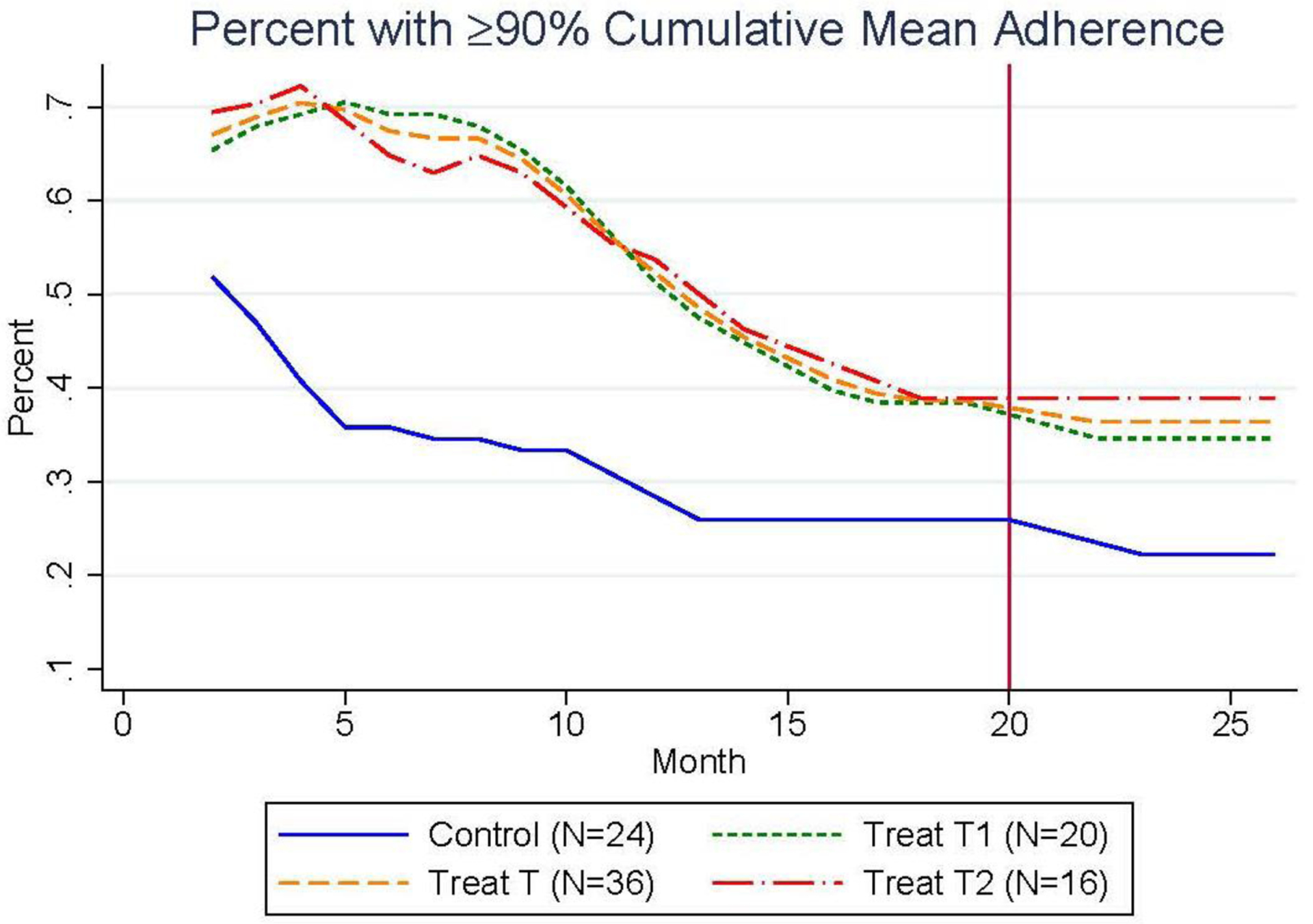
Impact of Incentives on the Percent of Participants with at Least 90% Cumulative Mean Adherence Over the 20-Month Intervention and 6 Months Post-Intervention
Table 6:
Treatments on Cumulative Mean Adherence Including Post-Incentives (Months 2 – 26)
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
|---|---|---|---|---|---|---|---|
| (OLS) | (OLS) | (Tobit) | (Tobit) | Mean Adherence | Mean Adherence | Mean Adherence | |
| Mean Adherence | Mean Adherence | Mean Adherence | Mean Adherence | 25th Quantile | 50th Quantile | 75th Quantile | |
| Tl group | 0.0490 (0.049) | 0.0682 (0.049) | 0.0365 (0.046) | 0.0532 (0.045) | 0.0368 (0.103) | 0.0286 (0.059) | 0.0302 (0.031) |
| T2 group | 0.1246*** (0.046) | 0.1263** (0.048) | 0.0921** (0.042) | 0.1063** (0.047) | 0.1507 (0.106) | 0.0591 (0.049) | 0.0559** (0.024) |
| Controls included | No | Yes | No | Yes | Yes | Yes | Yes |
| Observations | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Mean (Qntl.) of dep var | 0.833 | 0.833 | 0.833 | 0.833 | 0.723 | 0.875 | 0.938 |
Standard errors in parentheses
p < 0.10,
p < 0.05,
p < 0.01
Notes: This table presents the coefficient estimates from both OLS and Tobit (censored) regression models of cumulative mean ARV adherence over the 20-month intervention and 6 months post-incentives on indicator variables for the two treatment groups, estimated using heteroscedasticity-consistent standard errors. Columns 1 and 3 (2 and 4) show the coefficients from the models estimated without (and with) demographic controls that include participants’ age, gender, education, marital status, a measure of household assets, home ownership status, monthly income, travel costs to the clinic, household size, measures of physical and mental health, intrinsic motivation, and present-biased preferences. Columns 5 – 7 display coefficients from the same model estimated with all of the demographic controls as a quantile regression at the 25th, 50th, and 75th percentiles of cumulative ARV adherence over months 2 – 26. The average cumulative ARV adherence over months 2 –26 was 83.3% among the full sample (N=60).
Table 7:
Treatments on Percent ≥90% Cumulative Adherence Including Post-Incentives
| (1) | (2) | |
|---|---|---|
| Pct. ≥ 90% Mean Adhere | Pct. ≥ 90% Mean Adhere | |
| T1 group | 1.9870 (1.273) | 2.1231 (1.676) |
| T2 group | 4.0476** (2.193) | 4.7451** (2.673) |
| Controls included | No | Yes |
| Observations | 60 | 60 |
| Mean of dep var | 0.433 | 0.433 |
Odds ratios (i.e. exponentiated coefficients); Standard errors in parentheses
p < 0.10,
p < 0.05,
p< 0.01
Notes: This table presents the estimated odds ratios (exponentiated coefficients) from a logistic regression model of whether participants reached at least 90% cumulative mean ARV adherence over the 20-month intervention and the 6 months post-incentives on indicator variables for the two treatment groups, estimated using heteroscedasticity-consistent standard errors. Columns 1 (and 2) show the coefficients from the model estimated without (and with) demographic controls that include participants’ age, gender, education, marital status, a measure of household assets, home ownership status, monthly income, travel costs to the clinic, household size, measures of physical and mental health, intrinsic motivation, and present-biased preferences. Roughly 43.3% percent of participants reached the 90% cumulative mean ARV adherence threshold over the 20-month intervention and the 6 months post-incentives (months 2 – 26).
Table 8:
Treatments on Cumulative Mean Adherence Over Post-Incentives (Months 21 – 26)
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
|---|---|---|---|---|---|---|---|
| (OLS) | (OLS) | (Tobit) | (Tobit) | Mean Adherence | Mean Adherence | Mean Adherence | |
| Mean Adherence | Mean Adherence | Mean Adherence | Mean Adherence | 25th Quantile | 50th Quantile | 75th Quantile | |
| T1 group | 0.0712 (0.059) | 0.0813 (0.061) | 0.0707 (0.058) | 0.0843 (0.062) | 0.0686 (0.061) | 0.0578 (0.059) | 0.0780 (0.046) |
| T2 group | 0.1205** (0.061) | 0.1211** (0.063) | 0.1104** (0.052) | 0.1121** (0.053) | 0.1026 (0.065) | 0.1073** (0.053) | 0.0741* (0.036) |
| Controls included | No | Yes | No | Yes | Yes | Yes | Yes |
| Observations | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Mean (Qntl.) of dep var | 0.794 | 0.794 | 0.794 | 0.794 | 0.721 | 0.816 | 0.928 |
Standard errors in parentheses
p < 0.10,
p < 0.05,
p < 0.01
Notes: This table presents the coefficient estimates from both OLS and Tobit (censored) regression models of cumulative mean ARV adherence over the 6 months post-incentives (months 21 – 26) on indicator variables for the two treatment groups, estimated using heteroscedasticity-consistent standard errors. Columns 1 and 3 (2 and 4) show the coefficients from the models estimated without (and with) demographic controls that include participants’ age, gender, education, marital status, a measure of household assets, home ownership status, monthly income, travel costs to the clinic, household size, measures of physical and mental health, intrinsic motivation, and present-biased preferences. Columns 5 – 7 display coefficients from the same model estimated with all of the demographic controls as a quantile regression at the 25th, 50th, and 75th percentiles of cumulative ARV adherence over the post-incentives period (months 21 – 26). The average cumulative ARV adherence over months 21 –26 is 79.4% among the full sample (N=60).
Table 9:
Treatments on Percent ≥90% Cumulative Adherence Over Post-Incentives
| (1) | (2) | |
|---|---|---|
| Pct. ≥ 90% Mean Adhere | Pct. ≥ 90% Mean Adhere | |
| T1 group | 1.8608 (1.143) | 1.8264 (1.554) |
| T2 group | 2.7843** (1.408) | 2.8238** (1.316) |
| Controls included | No | Yes |
| Observations | 60 | 60 |
| Mean of dep var | 0.421 | 0.421 |
Odds ratios (i.e. exponentiated coefficients; Standard errors in parentheses
p < 0.10,
p < 0.05,
p < 0.01
Notes: This table presents the estimated odds ratios (exponentiated coefficients) from a logistic regression model of whether participants reach at least 90% cumulative mean ARV adherence over the 6 months post-incentives on indicator variables for the two treatment groups, estimated using heteroscedasticity-consistent standard errors. Columns 1 (and 2) show the coefficients from the model estimated without (and with) demographic controls that include participants’ age, gender, education, marital status, a measure of household assets, home ownership status, monthly income, travel costs to the clinic, household size, measures of physical and mental health, intrinsic motivation, and present-biased preferences. Roughly 42.1% percent of participants reach the 90% cumulative mean ARV adherence threshold over the 6 months post-incentives (months 21 – 26).
A clear weakness in this evidence for the successful formation of proper medication adherence habits is that persistent level differences between the treatment and control groups occurs because adherence similarly declines for all study participants after incentives were withdrawn. This universal decline in mean adherence is clearly observed in Figure 5, which plots the change in monthly mean ARV adherence starting from the end of incentives in month 20. While the control group initially experienced a significantly larger drop in monthly mean adherence, −2.67 p.p. (p = 0.01) in month 23, the difference between study groups was not statistically significant in month 26, when all three groups had a monthly mean adherence that was approximately 2.1 p.p. below their cumulative mean adherence level in month 20.
Figure 5.
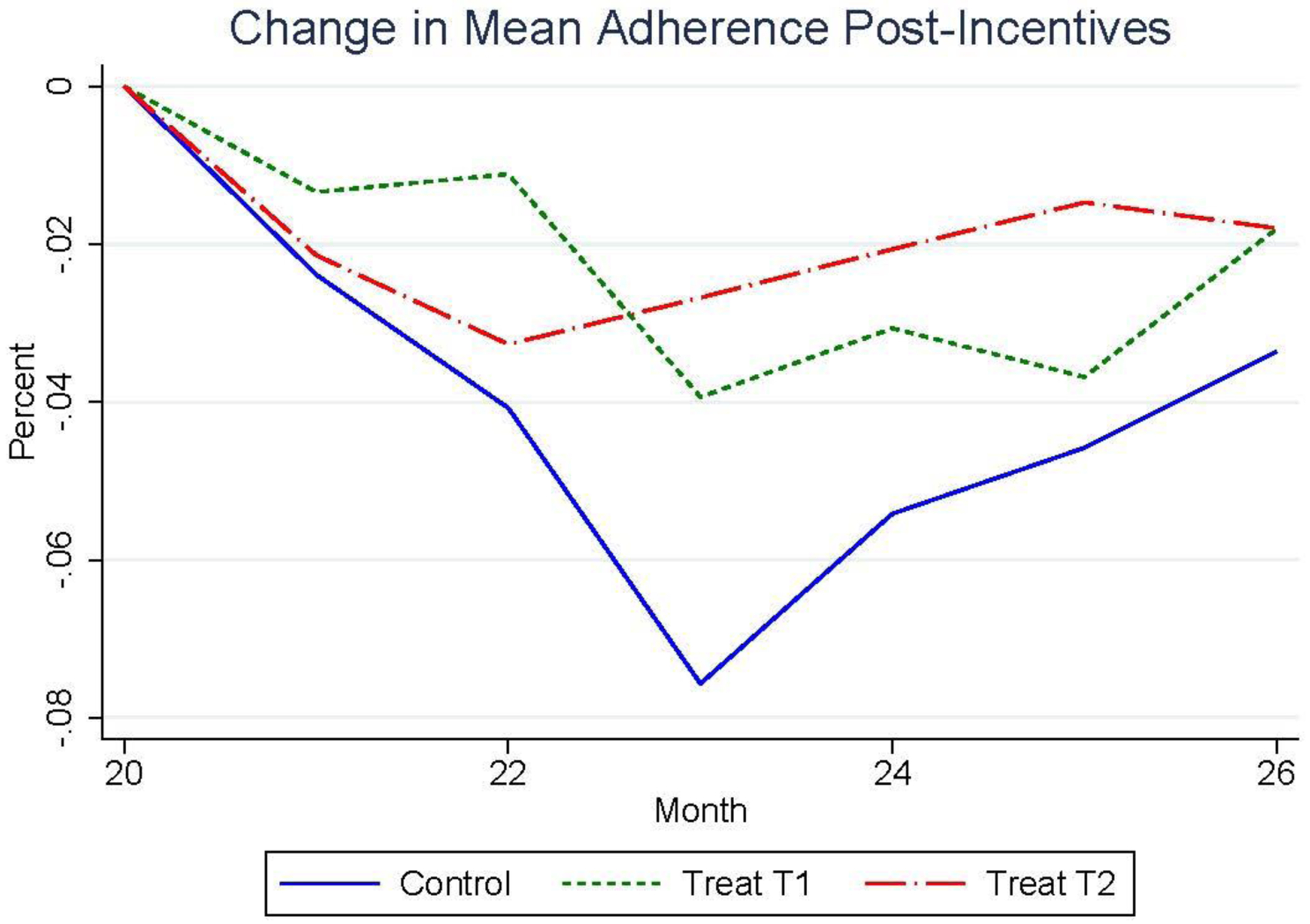
Percentage Point Change in Mean Monthly Adherence During the 6 Months Post-Intervention
To more carefully study the persistence of participants’ adherence behavior, the following regression analyses examine the monthly change in mean ARV adherence between participants’ cumulative ARV adherence through the 20-month intervention and their cumulative adherence over the 6-month post-intervention period. Specifically, Table 10 shows the coefficients from the following regression model:
where yi is the monthly change in cumulative mean ARV adherence between the 20-month intervention period and the 6-month post-intervention period, are indicator variables equal to 1 for participants in the indicated treatment group and 0 for those in the control group, and Ci is an indicator variable equal to 1 if participants are identified as using time-based pill-taking cues. Xi includes measures of participants’ age, gender, education, marital status, household assets, home ownership status, monthly income, travel costs to the clinic, physical and mental health, intrinsic motivation for ARV adherence, and an indicator of present-biased time preferences. On average, all participants saw a 3.4 p.p. drop (p = 0.03) in their cumulative mean ARV adherence after incentives were withdrawn. As displayed in column 1 of Table 8, both treatment groups experienced slightly smaller reductions in their adherence post-intervention relative to the control group, but these differences were not statistically significant. The standard economic theory of habit formation would predict that higher ARV adherence among the treatment groups during the 20-month incentivization period would lead to a larger habit stock of adherence behavior and thus a higher marginal utility for continued adherence, but these insignificant differences in behavioral persistence across study groups questions the explanatory power of this theory for habitual behavior in our setting.
Table 10:
Time-based Cues on Change in Cumulative Mean Adherence During versus Post-Incentives
| (1) | (2) | (3) | (4) | |
|---|---|---|---|---|
| Tl group | 0.0107 (0.058) | −0.0204 (0.043) | −0.0203 (0.040) | −0.0169 (0.043) |
| T2 group | 0.0277 (0.046) | −0.0441 (0.030) | −0.0435 (0.029) | −0.0506 (0.031) |
| Time-based cues | 0.0331** (0.015) | 0.0313** (0.014) | 0.0334** (0.012) | |
| Intrinsic motivation | −0.0639 (0.106) | −0.0395 (0.118) | ||
| Present-biased | - | −0.0092 (0.028) | −0.0208 (0.033) | |
| Demograpic controls included | No | No | No | Yes |
| Observations | 60 | 60 | 60 | 60 |
| Mean of dep var | −0.034 | −0.034 | −0.034 | −0.034 |
Standard errors in parentheses
p < 0.10,
p < 0.05,
p < 0.01
Notes: This table presents the coefficient estimates from a Tobit (censored) regression model of the change in cumulative mean ARV adherence between the 20-month intervention and 6 months post-incentives on indicator variables for the two treatment groups, estimated using heteroscedasticity-consistent standard errors. Columns 1 (and 2) show the coefficients from the model estimated without (and with) an indicator of whether participants used time-based adherence cues. Column 3 additionally includes measures of intrinsic motivation for ARV adherence and present-biased time preferences, and Column 4 includes include participants’ age, gender, education, marital status, a measure of household assets, home ownership status, monthly income, travel costs to the clinic, household size, measures of physical and mental health, intrinsic motivation, and present-biased preferences as demographic controls. On average, participants cumulative mean ARV adherence dropped by 3.4 percentage points between the 20-month intervention and 6 months post-incentives periods.
The use of time-based ARV adherence cues is associated with successfully maintaining adherence after incentives were withdrawn. Specifically, column 2 of Table 10 shows that the drop in cumulative mean adherence post-incentives was 3.3 p.p. (p = 0.02) smaller among participants with cued pill-taking routines. Columns 3 and 4 of Table 10 show the relatively minimal and statistically insignificant impact of participants’ intrinsic motivation and present-biased time preferences on their change in cumulative ARV adherence post-incentives, without and with demographic controls, respectively. These results run contrary to both Self-Determination Theory and the behavioral economic model of intertemporal choice. Even when including these additional psychological measures and controlling for participants’ socioeconomic and health characteristics, the only significant predictor of ARV adherence persistence is the use of time-based pill-taking cues. This finding is visualized in Figure 6, which shows the average monthly mean ARV adherence among the control and pooled treatment groups where each group is further split between those that were identified as using time-based pill-taking cues and those without a cued ARV routine. This figure shows that adherence fell by 4.7 p.p. among those without time-based pill-taking cues and increased by 1.5 p.p. among those with time-based cues during the 6 months post-incentives.
Figure 6.
Monthly Mean Adherence Over the 20-Month Intervention and 6 Months post-Intervention Between Participants With and Without Time-Based Cues
These results on post-incentives adherence behavior suggest that time-based cues are associated with more persistent adherence, but another important question is how incentives differentially impacted participants who did and did not use time-based cues during the intervention. To provide estimates of these heterogenous treatment effects, Table 11 shows the coefficients from the following linear regression model:
where yi is the cumulative mean ARV adherence during the 20-month intervention, are indicator variables equal to 1 for participants in the indicated treatment group and 0 for those in the control group, Ci is an indicator variable equal to 1 if participants are identified as using time-based pill-taking cues, is the interaction of the treatment group and time-based cues indicator variables, and Xi contains the full set of demographic controls used in the previous models. As demonstrated by Figure 6, the average cumulative adherence was roughly 19.5 p.p. (p<0.01) higher among those who use time-based cues over the 20-month intervention. While the sample size was not statistically powered for these heterogeneous treatment effect analyses, the attenuating effect of time-based cues on the treatment suggests that this intervention was primarily effective only for those who did not use time-based pill-taking cues. Specifically, participants who did not use time-based cues had a 7.3 p.p. (p = 0.17) greater increase in cumulative mean ARV adherence from the second treatment (i.e. incentives for high ARV adherence). However, our earlier findings show these are the same participants for whom adherence declined by 3.4 p.p. in the 6 months after incentives were withdrawn. Taken together, these results show that those who used time-based cues maintained a high level of ARV adherence during both the 20-month intervention and 6 months post-intervention regardless of their study group assignment. Since one of the eligibility criteria for participation in this study was either documented disengagement from treatment or self-reported adherence problems, it is unlikely that participants successfully used time-based cues prior to the intervention. Additionally, the use of contextual cues for ARV adherence was not the focus of this intervention, so it is unclear how this intervention might have promoted the use of time-based cues for a subset of participants.
Table 11:
Treatments × Time-based Cues on Cumulative Mean Adherence During 20-Month Intervention
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | |
|---|---|---|---|---|---|---|---|
| (OLS) | (OLS) | (Tobit) | (Tobit) | Mean Adherence | Mean Adherence | Mean Adherence | |
| Mean Adherence | Mean Adherence | Mean Adherence | Mean Adherence | 25th Quantile | 50th Quantile | 75th Quantile | |
| Tl group | 0.0586 (0.0.36) | 0.0439 (0.037) | 0.0565 (0.036) | 0.0463 (0.035) | 0.0675 (0.045) | 0.0351 (0.038) | 0.0332 (0.033) |
| T2 group | 0.0684* (0.041) | 0.0628 (0.039) | 0.0684* (0.040) | 0.0631* (0.037) | 0.0070 (0.049) | 0.0915* (0.046) | 0.0435* (0.025) |
| Timo-hasod cues | 0.2206*** (0.029) | 0.1937*** (0.043) | 0.2206*** (0.029) | 0.1953*** (0.040) | 0.1743*** (0.059) | 0.1748** (0.069) | 0.0902 (0.057) |
| Tl group × Time-based cues | −0.0545 (0.037) | −0.0438 (0.048) | −0.0564 (0.037) | −0.0465 (0.046) | −0.0233 (0.088) | −0.0281 (0.074) | −0.0371 (0.074) |
| T2 group × Time-based cues | −0.0655 (0.041) | −0.0719 (0.057) | −0.0655 (0.041) | −0.0733 (0.053) | 0.0029 (0.090) | −0.0933 (0.082) | −0.0469 (0.067) |
| Controls included | No | Yes | No | Yes | Yes | Yes | Yes |
| Observations | 138 | 138 | 138 | 138 | 138 | 138 | 138 |
| Mean of dep var | 0.835 | 0.835 | 0.835 | 0.835 | 0.752 | 0.876 | 0.967 |
Standard errors in parentheses
p < 0.10,
p < 0.05,
p < 0.01
Notes: This table presents the coefficient estimates from both OLS and Tobit (censored) regression models of cumulative mean ARV adherence over the 20-month intervention on indicator variables for the two treatment groups, an indicator of whether participants use time-based pill-taking cues, and the interaction between each treatment and whether participants are using time-based cues, estimated using heteroscedasticity-consistent standard errors. Columns 1 and 3 (2 and 4) show the coefficients from the models estimated without (and with) demographic controls that include participants’ age, gender, education, marital status, a measure of household assets, home ownership status, monthly income, travel costs to the clinic, household size, measures of physical and mental health, intrinsic motivation, and present-biased preferences. Columns 5 – 7 display coefficients from the same model estimated with all of the demographic controls as a quantile regression at the 25th, 50th, and 75th percentiles of cumulative mean ARV adherence over the 20-month intervention. The average cumulative mean ARV adherence was 83.5% among the analytic sample (N=138).
Regardless of the motivation for using time-based pill-taking cues, one important benefit to using contextual cues that can be seen in the data is combatting forgetfulness. This cognitive barrier can be seen in Figure 7 which shows the likelihood of pill-taking interruptions of 48 hours or more among those with and without time-based pill-taking cues for the participants with complete observations for 26 months. A clear advantage of using one’s environment to cue daily ARV pill-taking is a reduced reliance on one’s memory, as shown by the significantly higher number of pill interruptions of at least 48 hours (1.1 interruptions per month) among those without cues as compared to the participants that used time-based cues (0.2 interruptions per month) (Wilcoxon rank-sum test: p = 0.01). Additionally, the likelihood of at least one ≥48-hour pill interruptions increased steadily over the course of the intervention and post-incentives period at roughly 2 p.p. per month for those without contextual cues, while the cued-ARV adherence group did not experience a change in their likelihood of forgetting pills.
Figure 7.
Monthly Number of 48 Hours or Longer Pill Interruptions Over Full 26 Months Between Participants With and Without Time-Based Cues
Additional Outcomes:
The incentives offered during this intervention were successful at increasing mean ARV adherence, as described above and demonstrated in Figures 1 and 2, but the intervention was not designed to incentivize or otherwise motivate the use of time-based adherence cues and correspondingly we do not observe differences among the study groups in their likelihood of using this behavioral strategy. Figure 8 shows the percent of participants in each study group that used time-based pill-taking cues in each month of the 20-month intervention among all participants (N=138). This percentage reflects the share of participants who reported using time-based cues on the most recent follow-up survey, administered at months 4, 14, and 20, and who also took 90% of their pills within a 2-hour window around their modal pill-taking time. While there was an initially larger likelihood of using time-based cues among the treatment groups relative to control group, that difference disappears in all months after month 6. This shows that incentives alone were not driving the use of time-based cues for ARV adherence. Instead, this behavioral strategy seems to have been organically adopted by the participants who were also the most successful at maintaining proper ARV adherence after incentives were withdrawn. As outlined in Table 3, these 26 participants (19% of the analytic sample) were financially more secure and had better physical and mental health, which highlights the need for novel intervention strategies that can establish cued ARV medication adherence routines among more resource-constrained and vulnerable populations.
Figure 8.
Percent of Participants of Using Time-Based Cues Across All Three Study Groups
One frequently cited concern with using incentives to initiate behavioral change is the potential of “crowding-out” participants’ intrinsic motivation for the targeted behavior, as hypothesized in Self-Determination Theory (Deci 1971; Frey and Jegen 2001). This phenomenon has been observed in psychological laboratory experiments using simple tasks that participants had high initial levels of intrinsic motivation to complete, such as completing puzzles and painting pictures (Lepper, Greene, and Nisbett 1973). Field experiments in the behavioral economics literature have subsequently provided evidence of crowding-out for more complex behaviors, such as volunteering (Frey and Goette 1999) and enforcing penalties for picking children up (Gneezy and Rustichini 2000), but these behaviors are characterized by interpersonal conflicts of interest where the targeted behavior involves a tradeoff between one’s own self-interest and potential benefits to others. However, most of the health-related behaviors that policy makers may wish to improve through incentives, such as diet, physical activity, and medication adherence, cannot be characterized has having either high initial motivation or conflicts of interest (Promberger and Marteau 2013). Instead, suboptimal performance of these health promoting behaviors is often attributed to problems of self-control and intertemporal decision-making biases (Frederick, Loewenstein, and O’Donoghue 2002), which may be successfully addressed by incentives that can enhance feelings of competence and might actually increase intrinsic motivation.
Existing studies that incentivize health-related behaviors find no evidence of motivational crowd-out (Charness and Gneezy 2009; Volpp et al. 2009; Cooke et al. 2011; Czaicki et al. 2018), and this study contributes to this literature by similarly not finding a negative relationship between incentives and intrinsic motivation for medication adherence. Specifically, Figure 9 shows that participants’ self-reported level of intrinsic motivation for ARV adherence steadily increased from the baseline survey to follow-up surveys in months 4, 14, and 20. From this figure, an increasing trend is observed for all study groups, where this measure of intrinsic motivation increased on average by 10.6% (p < 0.01) over the course of the 20-month intervention. Interestingly, while slightly higher levels of intrinsic motivation were initially observed among the participants that used time-based pill-taking cues (7.7% higher motivation index; p=0.11), relative motivation levels switch over the subsequent 20 months as the rest of the study participants increased motivation by 11.5% while no change was observed among the group that used time-based cues. Since time-based cues are more predictive of proper ARV adherence after incentives were withdrawn than intrinsic motivation, this finding corroborates previous studies showing the relative importance of contextually cued habits for maintaining long-term behaviors (Verhoeven et al. 2012; Neal, Wood, and Drolet 2013; Galla and Duckworth 2015).
Figure 9.
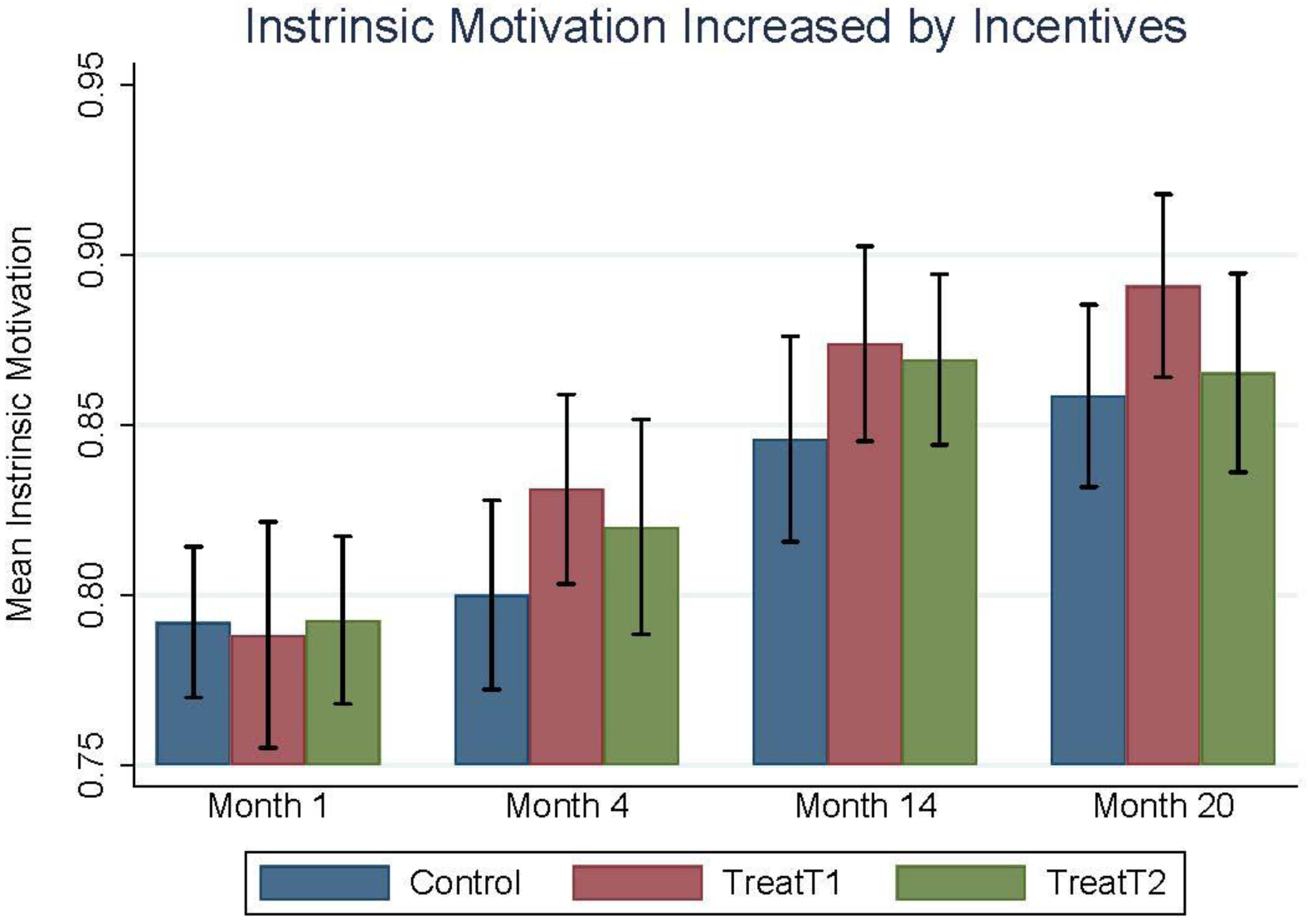
Intrinsic Motivation Measured for Each Study Group at Four Periods During the 20-Month Intervention
V. Discussion and Conclusion
This study shows that incentives can successfully improve HIV-infected patients’ ARV medication adherence, and identifies an important behavioral strategy for maintaining adherence post-incentives that warrants further theoretical and empirical research. Specifically, incentives improved participants’ cumulative ARV adherence and their likelihood of attaining at least 90% adherence during the 20-month intervention period, and the success of this intervention illustrates the importance of carefully calibrating incentive design parameters, such the frequency, magnitude, and type of rewards, for a particular behavioral setting. This study offered participants the chance of winning in-kind prizes that were selected based on extensive formative research in this clinical setting, and also tested the relative impact of incentivizing two different observable health behaviors that are necessary for ARV adherence: timely clinic attendance and cumulative ARV adherence. The results show that incentives conditional on the more frequent behavior that directly contributes to participants’ health (i.e. ARV adherence) are more successful for improving health behaviors. The significant difference in cumulative ARV adherence during the intervention between the treatment and control groups was also found to persist during the 6 months after incentives were withdrawn, which is frequently offered as evidence of habit formation in the economics literature. However, a closer inspection of the change in adherence behavior before and after incentives were withdrawn revealed that participants in all study groups experienced a similar adherence decline during the 6-month post-incentive period, except for the participants who used time-based contextual cues. Additionally, the beneficial effect of incentives appears to be largely experienced by those who did not use time-based adherence cues, as the subset of participants who used time-based cues maintained high levels of adherence throughout the study irrespective of group assignment. The importance of these contextual cues in the habit formation process has been previously described by psychological theory, and the results of this study lend further support for the use of this behavioral strategy as a mechanism for creating persistent, healthier habits.
These observed changes in ARV adherence behavior both during and after the receipt of incentives also questions the explanatory power of the standard economic model of habit formation in this setting. According to this model, the higher performance of daily pill-taking among the treatment groups led to a larger habit stock from past adherence behavior, and thus a higher marginal utility for adherence by the time incentives were withdrawn. The equal rate of decline in ARV adherence across study groups suggests that marginal utilities were not significantly increased through the accumulation of an ARV adherence habit stock; however, this intervention was carefully designed to mitigate most of the potential barriers to this theoretical habit formation process. Specifically, baseline marginal utilities were likely high for our targeted behavior, since full ARV adherence improves the health and life expectancies of people living with HIV. Additionally, pill-taking is a relatively simple behavior with little time or opportunity costs, ARV medications were provided to participants for free, and clinic access and social stigma were likely overcome by our sample of treatment-mature clients, thus the marginal cost of adherence was minimized. Finally, incentives were provided for 20 months, a period longer than the maximum time of habit formation suggested by the existing psychology literature (Lally and Gardner 2013). While these conditions did not result in ARV adherence habits for participants in the two treatment groups, a minority of participants across study groups who used time-based pill-taking cues maintained high adherence rates throughout the pre- and post-incentives periods. To reconcile this result with the classical economic habit formation model, it could be that a habit stock is only accumulated for contextually cued behaviors, that contextual cues may reduce the marginal cost of ARV adherence, or that the notion of habits as unconscious behavioral responses to cues, as suggested by Wood and Rünger (2016), may better explain ARV adherence behavior. Future research should attempt to disentangle the mechanism(s) underlying the success of contextual cues for supporting persistently high medication adherence.
Importantly, the use of time-based pill-taking cues was associated with higher household income, wealth, and better physical and mental health, which highlights the need for new intervention techniques to support successful habit formation among the less advantaged. Better financial conditions are hypothesized to facilitate persistent daily pill-taking routines because these participants can both afford to buy alarm clocks and take more leisure time, such as watching TV or participating in daily payers, all of which can serve as readily available time-based adherence cues and because higher household income and wealth can help smooth financial shocks and other potential disruptions to daily routines and work schedules. Additionally, these participants are less likely to work in the transportation or construction industries, which are common industries of employment among the less wealthy study participants, which frequently require irregular work hours and travel outside of Kampala that would disrupt context-dependent pill-taking routines. Future interventions should investigate whether stable time-based cues can still be identified and utilized among this population, and whether the lack of existing consistent routines or potential cognitive limitations among those with poor physical and mental health may undermine individuals’ ability to establish healthy habits. Since the use of contextual cues was not a focus of this intervention, it is unclear what caused a subset of participants in each study group to display this successful behavioral strategy, but future research should also investigate how study participation may focus individuals’ attention on the targeted behavior and change their performance of that behavior regardless of the specific intervention method.
In addition to identifying the importance of contextual cues for behavioral persistence, this paper uses survey measures of participants’ intrinsic motivation and intertemporal choice preferences to provide evidence that challenges alternative theories of habit formation in the psychology and economics literatures. First, participants’ self-reported intrinsic motivation for ARV adherence steadily increased during the 20-month intervention period, even among the participants receiving incentives. This contradicts the prediction that external rewards “crowd-out” intrinsic motivation, which Self-Determination Theory also posits is a necessary component for successful habit formation. Second, participants identified as having myopic (present-biased) time preferences, did not differentially form or lose ARV adherence habits as the behavioral economic model of intertemporal choice would suggest.
Overall, this paper demonstrates that context plays an important role in maintaining habitual behaviors and suggests that future research should consider using incentives to reinforce the response to contextual cues when attempting to foster persistent healthier habits. In this study, the participants who were most successful at maintaining high ARV adherence after incentives were withdrawn reported using time-based contextual cues, such as timing adherence with TV shows, daily prayers, or phone alarms. These cues served as daily reminders, but more information on participants’ full medication routine would help to better replicate this behavioral strategy in future interventions, such as how long after the cue was ARV medication taken, whether intermediate behavioral habits (e.g. filling a glass of water) were also built, and whether these participants utilize other reinforcers or social supports. Additionally, this study employed incentives over a relatively long period, 20 months, which limits the feasibility and applicability of this approach in many other behavioral settings, and future studies should investigate the ability of shorter duration incentivization interventions to successfully promote cued behavioral routines. Recent psychological studies have suggested that habits can take anywhere from 30 to 250 days to form depending on the complexity and daily frequency of the repeated behavior (Lally and Gardner 2013). In addition to better understanding the duration of this process for health behaviors of varying complexity and frequency, future research should also examine how incentives of varying frequency, magnitude, and type impact this habit formation process.
There are several limitations to this study’s findings. First, sample dropout that occurred between months 20 and 21 of the study reduced the statistical power for the analyses of ARV adherence persistence. Second, the survey measures of intertemporal choice preferences were not incentivized, and there is strong evidence that both the Multiple Price List and Intrinsic Motivation Inventory survey modules need to be significantly modified when employed across countries, cultures, and behavioral domains (Singer et al. 2016; Galizzi 2014; Carvalho, Prina, and Sydnor 2016). Third, the initial study sample size was not powered for heterogenous treatment effect analyses, so future research is needed to better understand how incentives differentially impact those who do and do not use contextual cues for the targeted behavior. Finally, this intervention did not randomize or otherwise manipulate participants’ use of contextual cues, so the documented association between the use of time-based contextual cues and persistent adherence should be considered as suggested evidence for the role of context in maintaining medication adherence. It is possible that other behavioral mechanisms were also utilized by the participants who reported using time-based cues and displayed temporal consistency in their medication behavior, which highlights the need for more research on incentivizing the use of contextual cues for building healthier habits.
Disclosure Statement
The authors declare that they have no relevant or material financial interests that relate to the research described in this paper. Research support was provided by NIMH: R34 MH096609, and Institutional Review Board (IRB) approval was acquired from the RAND Corporation: IRB # 2012-0372. The study was also approved by the HSPC Board at RAND, the IRB review board at Mildmay, and the Uganda Natinal Science Council (UNCST). The trial was registered with ClinicalTrials.gov (NCT02503072).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ryder and Heal (1973) define intertemporally dependent preferences, also known as state-dependent preferences, as u(ct, zt); where ct represents current consumption and zt is a weighted average of past consumption with exponentially declining weights, e.g. , and importantly, . That is, an increase in past consumption increases the marginal utility of current consumption.
Among the studies that evaluate habits cited in this paper, the post-intervention period of observation used to measure behavioral persistence ranges from 4 weeks (e.g. Sen et al. 2014) to 6 months (e.g. Volpp et al. 2009; Thirumurthy et al. 2019), with the mean/median number of weeks equal to 15/16 weeks. This includes over 40 studies that employ incentives-based interventions to promote healthier habits, including a recent review and meta-analysis of the literature (Mantzari et al. 2015).
Mathematically this is expressed by a utility function over current and past consumption u(ct, zt); where ct represents current consumption and zt is a weighted average of past consumption with exponentially declining weights, e.g. . The influence of past consumption on current consumption is captured by the assumption that ; an increase in past consumption increases the marginal utility of current consumption.
Clinicaltrials.gov Identifier: NCT02503072
Contributor Information
Chad Stecher, Arizona State University, 500 N 3rd Street, Phoenix, AZ 85004.
Barbara Mukasa, Mildmay Uganda, Kampala, Uganda.
Sebastian Linnemayr, RAND Corporation, 1776 Main Street, Santa Monica, CA 90401.
REFERENCES
- Abegaz Tadesse Melaku, Shehab Abdulla, Gebreyohannes Eyob Alemayehu, Bhagavathula Akshaya Srikanth, and Elnour Asim Ahmed. 2017. “Nonadherence to Antihypertensive Drugs: A Systematic Review and Meta-Analysis.” Medicine 96 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams Elaine J., and Strasser Susan. 2015. “90-90-90–Charting a Steady Course to End the Paediatric HIV Epidemic.” Journal of the International AIDS Society 18: 20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland Dan, and Levy Matthew R.. 2015. “Naiveté, Projection Bias, and Habit Formation in Gym Attendance.” Management Science 61 (1): 146–60. [Google Scholar]
- Adamowicz Wiktor L., and Swait Joffre D.. 2012. “Are Food Choices Really Habitual? Integrating Habits, Variety-Seeking, and Compensatory Choice in a Utility-Maximizing Framework.” American Journal of Agricultural Economics 95 (1): 17–41. [Google Scholar]
- Ajzen Icek. 1985. “From Intentions to Actions: A Theory of Planned Behavior.” In Action Control, 11–39. Springer. [Google Scholar]
- Andersen Steffen, Harrison Glenn W., Lau Morten Igel, and Rutström E. Elisabet. 2006. “Elicitation Using Multiple Price List Formats.” Experimental Economics 9 (4): 383–405. [Google Scholar]
- Arbour Kelly P., and Martin Ginis Kathleen A.. 2009. “A Randomised Controlled Trial of the Effects of Implementation Intentions on Women’s Walking Behaviour.” Psychology and Health 24 (1): 49–65. [DOI] [PubMed] [Google Scholar]
- Armitage Christopher J. 2004. “Evidence That Implementation Intentions Reduce Dietary Fat Intake: A Randomized Trial.” Health Psychology 23 (3): 319. [DOI] [PubMed] [Google Scholar]
- Asch David A., Troxel Andrea B., Stewart Walter F., Sequist Thomas D., Jones James B., Hirsch AnneMarie G., Hoffer Karen, Zhu Jingsan, Wang Wenli, and Hodlofski Amanda. 2015. “Effect of Financial Incentives to Physicians, Patients, or Both on Lipid Levels: A Randomized Clinical Trial.” Jama 314 (18): 1926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsberg David R., Perry Sharon, Charlebois Edwin D., Clark Richard A., Roberston Marjorie, Zolopa Andrew R., and Moss Andrew. 2001. “Non-Adherence to Highly Active Antiretroviral Therapy Predicts Progression to AIDS.” Aids 15 (9): 1181–83. [DOI] [PubMed] [Google Scholar]
- Becker Gary S., and Murphy Kevin M.. 1988. “A Theory of Rational Addiction.” Journal of Political Economy 96 (4): 675–700. [Google Scholar]
- Carrera Mariana, Royer Heather, Stehr Mark, and Sydnor Justin. 2018. “Can Financial Incentives Help People Trying to Establish New Habits? Experimental Evidence with New Gym Members.” Journal of Health Economics 58: 202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho Leandro S., Prina Silvia, and Sydnor Justin. 2016. “The Effect of Saving on Risk Attitudes and Intertemporal Choices.” Journal of Development Economics 120: 41–52. [Google Scholar]
- CDC. 2016. “CDC Press Releases.” CDC. January 1, 2016. https://www.cdc.gov/media/releases/2014/p0501-preventable-deaths.html. [Google Scholar]
- Charness Gary, and Gneezy Uri. 2009. “Incentives to Exercise.” Econometrica 77 (3): 909–31. [Google Scholar]
- Cooke Lucy J., Chambers Lucy C., Añez Elizabeth V., Croker Helen A., Boniface David, Yeomans Martin R., and Wardle Jane. 2011. “Eating for Pleasure or Profit: The Effect of Incentives on Children’s Enjoyment of Vegetables.” Psychological Science 22 (2): 190–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler Rachelle Louise, Fernandez-Llimos Fernando Frommer Michael, Benrimoj Charlie, and Garcia-Cardenas Victoria. 2018. “Economic Impact of Medication Non-Adherence by Disease Groups: A Systematic Review.” BMJ Open 8 (1): e016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaicki Nancy L., Dow William H., Njau Prosper F., and McCoy Sandra I.. 2018. “Do Incentives Undermine Intrinsic Motivation? Increases in Intrinsic Motivation within an Incentive-Based Intervention for People Living with HIV in Tanzania.” Plos One 13 (6): e0196616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deci Edward L. 1971. “Effects of Externally Mediated Rewards on Intrinsic Motivation.” Journal of Personality and Social Psychology 18 (1): 105. [Google Scholar]
- Deci Edward L., Koestner Richard, and Ryan Richard M.. 1999. “A Meta-Analytic Review of Experiments Examining the Effects of Extrinsic Rewards on Intrinsic Motivation.” Psychological Bulletin 125 (6): 627. [DOI] [PubMed] [Google Scholar]
- Deci Edward L., and Ryan Richard M.. 2000. “The” What” and” Why” of Goal Pursuits: Human Needs and the Self-Determination of Behavior.” Psychological Inquiry 11 (4): 227–68. [Google Scholar]
- Deci Edward L.. 2010. “Intrinsic Motivation.” The Corsini Encyclopedia of Psychology, 1–2. [Google Scholar]
- Dilorio C, McCarty F, Resnicow K, McDonnell Holstad M, Soet J, Yeager K, Sharma SM, Morisky DE, and Lundberg B. 2008. “Using Motivational Interviewing to Promote Adherence to Antiretroviral Medications: A Randomized Controlled Study.” AIDS Care 20 (3): 273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth Angela L., Milkman Katherine L., and Laibson David. 2018. “Beyond Willpower: Strategies for Reducing Failures of Self-Control.” Psychological Science in the Public Interest 19 (3): 102–29. [DOI] [PubMed] [Google Scholar]
- Dunn Keith, Lafeuille Marie-Hélène, Jiao Xiaolong, Romdhani Hela, Emond Bruno, Woodruff Kimberly, Pesa Jacqueline, Tandon Neeta, and Lefebvre Patrick. 2018. “Risk Factors, Health Care Resource Utilization, and Costs Associated with Nonadherence to Antiretrovirals in Medicaid-Insured Patients with HIV.” Journal of Managed Care & Specialty Pharmacy 24 (10): 1040–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick Shane, Loewenstein George, and O’Donoghue Ted. 2002. “Time Discounting and Time Preference: A Critical Review.” Journal of Economic Literature 40 (2): 351–401. [Google Scholar]
- Frey Bruno S., and Goette Lorenz. 1999. “Does Pay Motivate Volunteers?” Working Paper/Institute for Empirical Research in Economics 7. [Google Scholar]
- Frey Bruno S., and Jegen Reto. 2001. “Motivation Crowding Theory.” Journal of Economic Surveys 15 (5): 589–611. [Google Scholar]
- Galizzi Matteo M. 2014. “What Is Really Behavioral in Behavioral Health Policy? And Does It Work?” Applied Economic Perspectives and Policy 36 (1): 25–60. [Google Scholar]
- Galla Brian M., and Duckworth Angela L.. 2015. “More than Resisting Temptation: Beneficial Habits Mediate the Relationship between Self-Control and Positive Life Outcomes.” Journal of Personality and Social Psychology 109 (3): 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Macarena C. 2016. “Potentially Preventable Deaths Among the Five Leading Causes of Death — United States, 2010 and 2014.” MMWR. Morbidity and Mortality Weekly Report 65. 10.15585/mmwr.mm6545a1. [DOI] [PubMed] [Google Scholar]
- Gneezy Uri, and Rustichini Aldo. 2000. “Pay Enough or Don’t Pay at All.” The Quarterly Journal of Economics 115 (3): 791–810. [Google Scholar]
- Goldzahl Léontine, Hollard Guillaume, and Jusot Florence. 2018. “Increasing Breast-Cancer Screening Uptake: A Randomized Controlled Experiment.” Journal of Health Economics 58 (March): 228–52. 10.1016/j.jhealeco.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Gollwitzer Peter M., and Brandstätter Veronika. 1997. “Implementation Intentions and Effective Goal Pursuit.” Journal of Personality and Social Psychology 73 (1): 186. [DOI] [PubMed] [Google Scholar]
- Gollwitzer Peter M., and Sheeran Paschal. 2006. “Implementation Intentions and Goal Achievement: A Meta-Analysis of Effects and Processes.” Advances in Experimental Social Psychology 38: 69–119. [Google Scholar]
- Gupta Samir, Miller Stacie, Koch Mark, Berry Emily, Anderson Paula, Pruitt Sandi L., Borton Eric, et al. 2016. “Financial Incentives for Promoting Colorectal Cancer Screening: A Randomized, Comparative Effectiveness Trial.” Official Journal of the American College of Gastroenterology | ACG 111 (11): 1630–36. 10.1038/ajg.2016.286. [DOI] [PubMed] [Google Scholar]
- Hagger Martin S., and Luszczynska Aleksandra. 2014. “Implementation Intention and Action Planning Interventions in Health Contexts: State of the Research and Proposals for the Way Forward.” Applied Psychology: Health and Well-Being 6 (1): 1–47. [DOI] [PubMed] [Google Scholar]
- Harries Anthony D., Suthar Amitabh B., Takarinda Kudakwashe C., Tweya Hannock, Thu Kyaw Nang Thu, Tayler-Smith Katie, and Zachariah Rony. 2016. “Ending the HIV/AIDS Epidemic in Low-and Middle-Income Countries by 2030: Is It Possible?” F1000Research 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagee A, Remien RH, Berkman A, Hoffman S, Campos L, and Swartz L. 2011. “Structural Barriers to ART Adherence in Southern Africa: Challenges and Potential Ways Forward.” Global Public Health 6 (1): 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenica Emir. 2012. “Behavioral Economics and Psychology of Incentives.” Annu. Rev. Econ 4 (1): 427–52. [Google Scholar]
- Kruskal William H., and Wallis W. Allen. 1952. “Use of Ranks in One-Criterion Variance Analysis.” Journal of the American Statistical Association 47 (260): 583–621. [Google Scholar]
- Lally Phillippa, and Gardner Benjamin. 2013. “Promoting Habit Formation.” Health Psychology Review 7 (sup1): S137–58. [Google Scholar]
- Lepper Mark R., Greene David, and Nisbett Richard E.. 1973. “Undermining Children’s Intrinsic Interest with Extrinsic Reward: A Test of the” Overjustification” Hypothesis.” Journal of Personality and Social Psychology 28 (1): 129. [Google Scholar]
- Linnemayr Sebastian. 2017. “Behavioral Economics and HIV: A Review of Existing Studies and Potential Future Research Areas.” In Behavioral Economics and Healthy Behaviors, 141–56. Routledge. [Google Scholar]
- Linnemayr Sebastian, and Stecher Chad. 2015. “Behavioral Economics Matters for HIV Research: The Impact of Behavioral Biases on Adherence to Antiretrovirals (ARVs).” AIDS and Behavior 19 (11): 2069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemayr Sebastian, Stecher Chad, and Mukasa Barbara. 2017. “Behavioral Economic Incentives to Improve Adherence to Antiretroviral Medication.” AIDS (London, England) 31 (5): 719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein George, Price Joseph, and Volpp Kevin. 2016. “Habit Formation in Children: Evidence from Incentives for Healthy Eating.” Journal of Health Economics 45: 47–54. [DOI] [PubMed] [Google Scholar]
- Mantzari Eleni, Vogt Florian, Shemilt Ian, Wei Yinghui, Higgins Julian P. T., and Marteau Theresa M.. 2015. “Personal Financial Incentives for Changing Habitual Health-Related Behaviors: A Systematic Review and Meta-Analysis.” Preventive Medicine 75 (June): 75–85. 10.1016/j.ypmed.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta Shivan J., Pepe Rebecca S., Gabler Nicole B., Kanneganti Mounika, Reitz Catherine, Saia Chelsea, Teel Joseph, Asch David A., Volpp Kevin G., and Doubeni Chyke A.. 2019. “Effect of Financial Incentives on Patient Use of Mailed Colorectal Cancer Screening Tests: A Randomized Clinical Trial.” JAMA Network Open 2 (3): e191156–e191156. 10.1001/jamanetworkopen.2019.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier Stephan, and Sprenger Charles D.. 2015. “Temporal Stability of Time Preferences.” Review of Economics and Statistics 97 (2): 273–86. [Google Scholar]
- Milkman Katherine L., Beshears John, Choi James J., Laibson David, and Madrian Brigitte C.. 2011. “Using Implementation Intentions Prompts to Enhance Influenza Vaccination Rates.” Proceedings of the National Academy of Sciences 108 (26): 10415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkman Katherine L.. 2013. “Planning Prompts as a Means of Increasing Preventive Screening Rates.” Preventive Medicine 56 (1): 92–93. [DOI] [PubMed] [Google Scholar]
- Neal David T., Wood Wendy, and Drolet Aimee. 2013. “How Do People Adhere to Goals When Willpower Is Low? The Profits (and Pitfalls) of Strong Habits.” Journal of Personality and Social Psychology 104 (6): 959. [DOI] [PubMed] [Google Scholar]
- Oettingen Gabriele, and Gollwitzer Peter. 2010. Strategies of Setting and Implementing Goals: Mental Contrasting and Implementation Intentions.
- Olalla Garcia P. de, Knobel Hernando, Carmona Alexia, Guelar Ana, López-Colomés José L., and Caylà Joan A.. 2002. “Impact of Adherence and Highly Active Antiretroviral Therapy on Survival in HIV-Infected Patients.” Journal of Acquired Immune Deficiency Syndromes (1999) 30 (1): 105–10. [DOI] [PubMed] [Google Scholar]
- Orbell Sheina, Hodgkins Sarah, and Sheeran Paschal. 1997. “Implementation Intentions and the Theory of Planned Behavior.” Personality and Social Psychology Bulletin 23 (9): 945–54. [DOI] [PubMed] [Google Scholar]
- Pollak Robert A. 1970. “Habit Formation and Dynamic Demand Functions.” Journal of Political Economy 78 (4, Part 1): 745–63. [Google Scholar]
- Polonsky William H, and Henry Robert R. 2016. “Poor Medication Adherence in Type 2 Diabetes: Recognizing the Scope of the Problem and Its Key Contributors.” Patient Preference and Adherence 10 (July): 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promberger Marianne, and Marteau Theresa M.. 2013. “When Do Financial Incentives Reduce Intrinsic Motivation? Comparing Behaviors Studied in Psychological and Economic Literatures.” Health Psychology 32 (9): 950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamaki Lance S., Davis Terry C., Skripkauskas Silvia, Bennett Charles L., and Wolf Michael S.. 2006. “Social Stigma Concerns and HIV Medication Adherence.” AIDS Patient Care & STDs 20 (5): 359–68. [DOI] [PubMed] [Google Scholar]
- Rohde Kirsten IM, and Verbeke Willem. 2017. “We like to See You in the Gym—A Field Experiment on Financial Incentives for Short and Long Term Gym Attendance.” Journal of Economic Behavior & Organization 134: 388–407. [Google Scholar]
- Rosen Marc I., Dieckhaus Kevin, McMahon Thomas J., Valdes Barbara, Petry Nancy M., Cramer Joyce, and Rounsaville Bruce. 2007. “Improved Adherence with Contingency Management.” AIDS Patient Care and STDs 21 (1): 30–40. [DOI] [PubMed] [Google Scholar]
- Royer Heather, Stehr Mark, and Sydnor Justin. 2015. “Incentives, Commitments, and Habit Formation in Exercise: Evidence from a Field Experiment with Workers at a Fortune-500 Company.” American Economic Journal: Applied Economics 7 (3): 51–84. [Google Scholar]
- Ryan Richard M., and Deci Edward L.. 2000. “Intrinsic and Extrinsic Motivations: Classic Definitions and New Directions.” Contemporary Educational Psychology 25 (1): 54–67. [DOI] [PubMed] [Google Scholar]
- Ryan Richard M. 2008. “A Self-Determination Theory Approach to Psychotherapy: The Motivational Basis for Effective Change.” Canadian Psychology/Psychologie Canadienne 49 (3): 186. [Google Scholar]
- Ryder Harl E., and Heal Geoffrey M.. 1973. “Optimal Growth with Intertemporally Dependent Preferences.” The Review of Economic Studies 40 (1): 1–31. [Google Scholar]
- Sheeran Paschal, and Orbell Sheina. 1999. “Implementation Intentions and Repeated Behaviour: Augmenting the Predictive Validity of the Theory of Planned Behaviour.” European Journal of Social Psychology 29 (2–3): 349–69. [Google Scholar]
- Simoni Jane M., Amico K. Rivet, Pearson Cynthia R., and Malow Robert. 2008. “Strategies for Promoting Adherence to Antiretroviral Therapy: A Review of the Literature.” Current Infectious Disease Reports 10 (6): 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. Kagawa, Dressler W, George S, Baquet Claudia R., Bell Ronny A., Burhansstipanov Linda, Burke Nancy J., Dibble Suzanne, Elwood William, and Garro Linda. 2016. “Culture: The Missing Link in Health Research.” Social Science & Medicine 170: 237–46. [DOI] [PubMed] [Google Scholar]
- Sorensen James L., Haug Nancy A., Delucchi Kevin L., Gruber Valerie, Kletter Evan, Batki Steven L., Tulsky Jacqueline P., Barnett Paul, and Hall Sharon. 2007. “Voucher Reinforcement Improves Medication Adherence in HIV-Positive Methadone Patients: A Randomized Trial.” Drug and Alcohol Dependence 88 (1): 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumurthy Harsha, Asch David A., and Volpp Kevin G.. 2019. “The Uncertain Effect of Financial Incentives to Improve Health Behaviors.” JAMA. [DOI] [PubMed] [Google Scholar]
- Thirumurthy Harsha, Ndyabakira Alex, Marson Kara, Emperador Devy, Kamya Moses, Havlir Diane, Kwarisiima Dalsone, and Chamie Gabriel. 2019. “Financial Incentives for Achieving and Maintaining Viral Suppression among HIV-Positive Adults in Uganda: A Randomised Controlled Trial.” The Lancet HIV 6 (3): e155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven Aukje AC, Adriaanse Marieke A., Evers Catharine, and de Ridder Denise TD. 2012. “The Power of Habits: Unhealthy Snacking Behaviour Is Primarily Predicted by Habit Strength.” British Journal of Health Psychology 17 (4): 758–70. [DOI] [PubMed] [Google Scholar]
- Verplanken Bas, Verplanken B, and Ryan. 2018. Psychology of Habit. Springer. [Google Scholar]
- Volpp Kevin G., Troxel Andrea B., Pauly Mark V., Glick Henry A., Puig Andrea, Asch David A., Galvin Robert, Zhu Jingsan, Wan Fei, and DeGuzman Jill. 2009. “A Randomized, Controlled Trial of Financial Incentives for Smoking Cessation.” New England Journal of Medicine 360 (7): 699–709. [DOI] [PubMed] [Google Scholar]
- Vrijens Bernard, Vincze Gäbor, Kristanto Paulus, Urquhart John, and Burnier Michel. 2008. “Adherence to Prescribed Antihypertensive Drug Treatments: Longitudinal Study of Electronically Compiled Dosing Histories.” Bmj 336 (7653): 1114–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood Wendy. 2017. “Habit in Personality and Social Psychology.” Personality and Social Psychology Review 21 (4): 389–403. [DOI] [PubMed] [Google Scholar]
- Wood Wendy, and Neal David T.. 2007. “A New Look at Habits and the Habit-Goal Interface.” Psychological Review 114 (4): 843. [DOI] [PubMed] [Google Scholar]
- Wood Wendy. 2016. “Healthy through Habit: Interventions for Initiating & Maintaining Health Behavior Change.” Behavioral Science & Policy 2 (1): 71–83. [Google Scholar]
- Wood Wendy, and Rünger Dennis. 2016. “Psychology of Habit.” Annual Review of Psychology 67: 289–314. [DOI] [PubMed] [Google Scholar]


