Abstract
For the spectroscopic assessment of brain disorders that require large-volume coverage, the requirements of RF performance and field homogeneity are high. For epilepsy, this is also challenging given the inter-patient variation in location, severity and subtlety of anatomical identification and its tendency to involve the temporal region. We apply a targeted method to examine the utility of large-volume MR spectroscopic imaging (MRSI) in surgical epilepsy patients, implementing a two-step acquisition, comprised of a 3D acquisition to cover the fronto-parietal regions, and a contiguous parallel two-slice Hadamard-encoded acquisition to cover the temporal-occipital region, both with TR/TE = 2000/40 ms and matched acquisition times. With restricted (static, first/second-order) B0 shimming in their respective regions, the Cramér-Rao lower bounds for creatine from the temporal lobe two-slice Hadamard and frontal-parietal 3D acquisition are 8.1 ± 2.2% and 6.3 ± 1.9% respectively. The datasets are combined to provide a total 60 mm axial coverage over the frontal, parietal and superior temporal to middle temporal-occipital regions. We applied these acquisitions at a nominal 400 mm3 voxel resolution in n = 27 pre-surgical epilepsy patients and n = 20 controls. In controls, 86.6 ± 3.2% voxels with at least 50% tissue (white + gray matter, excluding CSF) survived spectral quality inclusion criteria. Since all patients were clinically followed for at least 1 year after surgery, seizure frequency outcome was available for all. The MRSI measurements of the total fractional metabolic dysfunction (characterized by the Cr/NAA metric) in FreeSurfer MRI gray matter segmented regions, in the patients compared with the controls, exhibited a significant Spearman correlation with post-surgical outcome. This finding suggests that a larger burden of metabolic dysfunction is seen in patients with poorer post-surgical seizure control.
Keywords: epilepsy, Hadamard, rosette, spectroscopic imaging
1 |. INTRODUCTION
The use of proton MR spectroscopic imaging (1H-MRSI) to assess brain disorders that require large volume coverage is demanding due to the multiple requirements of RF performance, B0 field homogeneity, speed, unwanted signal suppression and signal to noise ratio (SNR). A well established approach is to select a thick transverse slab (~13 cm) and encode over it using fast trajectories for in-plane (axial slices) localization and conventional phase encoding in the slice direction.1,2 However, with the available hardware (eg with transmission B1+ of ≲17 μT), the selection of thick slabs can exacerbate chemical shift dispersion errors and slice profile imperfections at the slab edges. Also, as the available SNR at 3 T favors thicker slices and smaller encoding slice numbers, this results in increased point spread function (PSF) contamination.3–6 These factors present a challenge for 1H-MRSI applications for epilepsy given the variability between patients in anatomical location, the subtlety of visual identification, a variable level of severity or volume of abnormality and a tendency to involve temporal and/or fronto-temporal regions. Epilepsy patients also commonly exhibit multiple regions of dysfunction, not all of which are anatomically contiguous and, as suggested by Shih,7 may be based on distorted brain networks.7–9
In the past we used an in-plane fast 2D rosette trajectory at 3 T with conventional phase encoding in the slice direction,10 finding a significant relationship between SNR and linewidth with spectral quality, assessed by the Cramér-Rao lower bound (CRLB, %) for N-acetylaspartate (NAA), creatine (Cr) and choline (Cho). However, the lower brain regions extending into the middle temporal lobe are known to be more challenging for B0 shimming, which will in turn reduce spectral quality for slice based phase-encoded studies. To improve these issues, we used a two-step method that combines phase encoding in the frontal-parietal regions with multi-slice Hadamard encoding,11–13 with each acquisition performed with volume-matched B0 shimming. The first acquisition uses phase encoding and a 40 mm transverse slab covering the frontal-parietal lobes, which extends to the brain edge, and is acquired in under 10 min. The second acquisition uses Hadamard encoding to excite and encode a thinner slab to separately address the more challenging temporal region. As has been described,13 by virtue of the cascaded Hadamard RF-based multiple slice selection, the acquired slices are also free from PSF contamination and more resistant to B1+-based limitations in slice profile in comparison to a 3D phase-encoded multi-slice (slab-selected) acquisition. Due to anticipated shimming challenges in the temporal lobes, we simulate how well the medial temporal lobe (MTL) gray matter can be shimmed through use of restricted versus extended regional shimming. These acquisitions are evaluated in n = 20 healthy subjects and n = 27 surgical epilepsy patients, all of whom subsequently underwent epilepsy surgery and for all of whom at least 1 year of post-operative clinical follow-up is available.
In epilepsy, oxidative injury and mitochondrial dysfunction has been consistently found in seizure onset regions in basic and clinical research studies.14,15 For the detection of such dysfunction, MRS measurements of Cr and NAA provide a well established evaluation. Cr is a necessary component of the energy buffer phosphocreatine, and, as discussed by Connett,16 provides a measure of the energetically active cellular mass. NAA has been closely linked with neuronal mitochondrial oxygen consumption and energy demand.17–19 Thus the ratio Cr/NAA (or NAA/Cr) provides an intrinsic parameter for metabolic function, evaluating the supported cellular mass in comparison with its mitochondrion content; with neuronal metabolic dysfunction, Cr/NAA rises. MRS studies at 1.5 T and 3 T have found that the range of abnormality for Cr/NAA in epilepsy is about 20 to 50% greater than in controls,20,21 with neither of their concentrations being especially small. While Cho can be thought to be pertinent as well, its function is more based in membranes and membrane turnover, and thus changes in Cho may occur that are less related to energetic function. In our analysis, we focused on Cr/NAA to assess the performance of the acquisitions and the role of spectral filtering in regression statistics with fraction gray matter, CRLB (%) and SNR values. Control data were used to provide regression statistics to characterize the “normal” mean and standard deviation, against which we identified metabolic abnormalities in the patients. Our data indicate that the total extent of metabolic dysfunction correlates with post-surgical outcome.
2 |. METHODS
2.1 |. Subject recruitment
All studies were performed with institutional review board approval. The n = 27 medically refractory patients were recruited consecutively from the University of Pittsburgh Comprehensive Epilepsy Center, based on having complete MRSI datasets (single session, integrated 3D and Hadamard acquisitions) pre-surgery with at least 12 months of post-operative follow up. Classification of seizure outcome was performed according to the International League Against Epilepsy (ILAE) Task Force on Classification and Terminology Guidelines*.22 As applied by others,23,24 outcome analysis was also performed using a binary classification, good (ILAE I or II, largely seizure free) versus poor (ILAE III-VI, recurrent seizures). An additional n = 20 healthy controls were recruited from the university community. From 10 consecutive controls, the acquisitions were analyzed for shimming performance.
2.2 |. MR acquisitions
All imaging was performed at 3 T with a Siemens Trio system using its OEM 32-channel head coil (Siemens, Erlangen, Germany). The MRSI covered a transverse (inferior-superior, normal to the AC-PC line) 60 mm slab, comprised of a 40 mm 3D volume of interest (VOI) and a parallel contiguous two-slice 18 mm (8 mm thick slices with 2 mm gap to minimize crosstalk) VOI defined by cascaded Hadamard-encoded spectroscopic imaging (c-HSI), as shown in Figure 1. Static B0 shimming for each VOI was performed with the previously described B0 loop-encoded readout (BOLERO).25,26 This yielded two shim-current sets that were applied separately as appropriate to each VOI for that acquisitions. The 3D sequence used an in-plane 20 × 20 circular rosette trajectory for fast spectral and two axial dimensions of encoding. The slice direction was localized with conventional phase encoding yielding 20 × 20 × 12 resolution over a 200 × 200 × 48 mm3 VOI. To try to match SNR for both acquisitions, the two-slice c-HSI (200 × 200 × 18 mm3) was acquired at 28 × 28 rosette resolution with two averages. Acquisitions for both sequences were done at 1250 Hz spectral bandwidth, 10 μs sampling dwell time for a total readout duration of 320 ms. With the applied spatial filtering, the 3D and the c-HSI had effective sampling volumes of about 1300 mm3. With gradient amplitude and slew rates less than 6.5 mT/m and 55 mT/m/ms, both sequences generate minimal eddy current artifacts (no post-acquisition k-space trajectory or line-shape corrections needed) and are very quiet, operating at about 23% of maximum slew rate. Both acquisitions use TR/TE = 2000/40 ms with water suppression achieved by adiabatic inversion recovery (TI = 950 ms) and numerically optimized binomial refocusing pulse, as shown in Figure 1A. Extracranial lipid suppression was performed with a global inversion recovery, TI = 240 ms. In total, MRSI acquisitions (both sequences) required 18.5 min.
FIGURE 1.
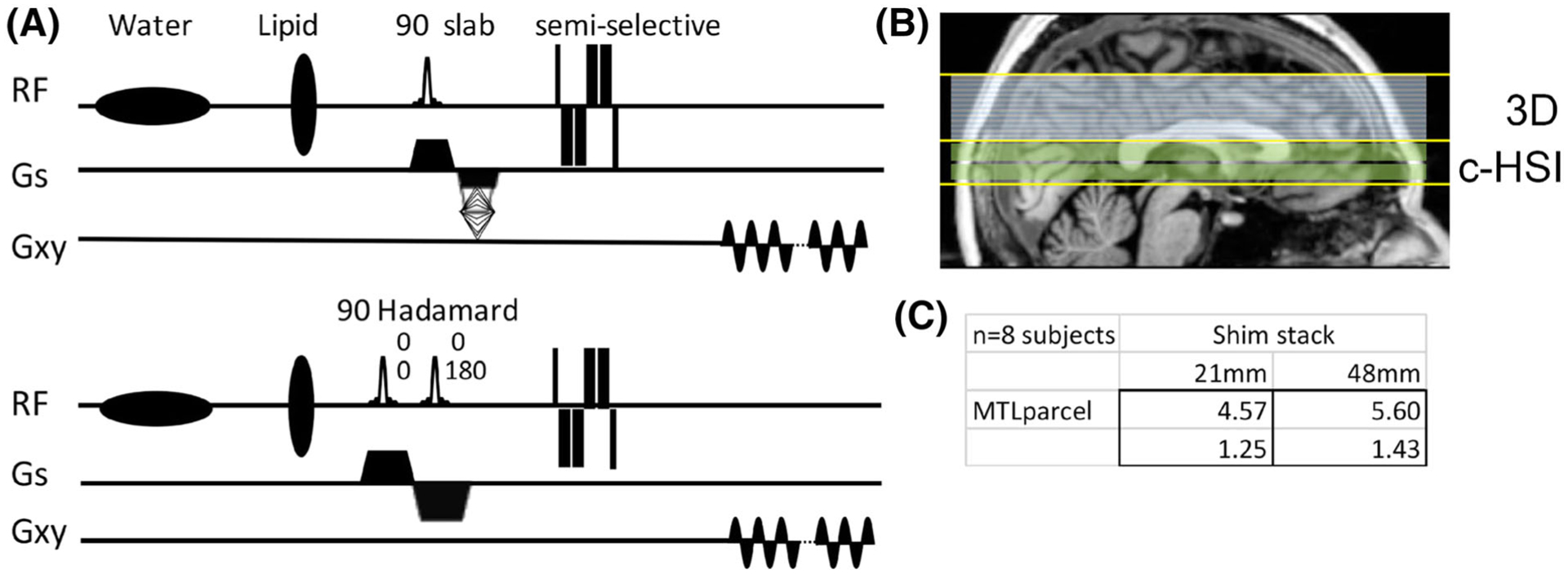
A, Pulse sequences showing the phase-encoded (top) and Hadamard slice-encoded (bottom) acquisitions. Because the Hadamard encoding is performed with cascaded slice excitations with RF phase encoding (two slices at 0°, 0° and 0°, 180°), the echo times for each slice are slightly different, 40 ms and 45 ms. B, The typical longitudinal coverage of the 3D and Hadamard acquisitions is shown. C, The effect of targeted shimming is shown in the table (mean and standard deviation): using a restricted (21 mm) or large (48 mm) shim region, with a significant improvement in MTL field homogeneity (σB0) with the smaller shim region
Structural imaging was performed using either MPRAGE or MP2RAGE acquisitions (1 mm3 isotropic spatial resolution) and T2 FLAIR (fluid-attenuated inversion recovery; 0.7 × 0.7 × 2 mm3) acquisitions.
2.3 |. Data analysis
Reconstruction of the spectroscopic images was performed as described previously,25 with phasing and coil combination coefficients determined from spatially matched non-water-suppressed rosette acquisitions (15° flip angle, 0.38 s TR).
The effect of the restricted B0 shimming in the medial temporal region was numerically examined in the controls’ data (n = 10, consecutive subjects). This was performed by calculating the predicted homogeneity after shimming using either a 21 mm (seven slices) stack that covers the middle temporal lobe or a parallel 48 mm stack that adds superiorly to the inferior 21 mm for a total of 16 slices (Figure 1B). For these simulations, the accuracy of our previously described BOLERO26 B0 field maps is within 0.005 ppm for its predicted and achieved standard deviation.27 Using the FreeSurfer parcellated images of the MTL gray matter as a mask, a 16% better B0 homogeneity was found for the restricted versus large volume shim (Figure 1C).
For VOI positioning and tissue segmentation, the 1 mm isotropic T1-weighted images were used. Segmentation into gray matter, white matter, cerebrospinal fluid (CSF) and parcellation was done with the FreeSurfer package (http://surfer.nmr.mgh.harvard.edu/). Co-registration of the MPRAGE MRI with the gradient echo scout images defining the MRSI and shimming regions(s) was done with SPM12 (http://www.fil.ion.ac.uk/spm/). The fraction of gray matter was calculated as the ratio of gray matter volume normalized to the sum of white and gray matter with exclusion of the brain stem.
Patient (n = 27) and control (n = 20; 10 young, <45 years, and 10 old, >45 years) group spectral data were analyzed with LCModel (http://www.s-provencher.com) using 12 metabolites’ basis spectra (NAA, N-acetylaspartylglutamate, Cr, phosphocholine, glycerophosphorylcholine, myo-inositol, glutamate, glutamine, gamma-amino butyric acid, lactate, glutathione, aspartate). These were calculated with GAMMA28 using the applied moderate echo pulse sequence with default macromolecule and lipid simulated components. To assess the filtering criteria applied for spectral quality in the 3D and HSI data, the number of retained voxels and size of standard error of regression were considered. The number of voxels falling outside of the regression-calculated prediction interval was also assessed. The base filtering requirements were taken with total brain (TB) content of more than 50%. Additional criteria of linewidth, CRLB (%) and macromolecule contamination at 2.0 ppm were used. The spectral parameters of CRLB (%), linewidth and SNR were compared between the 3D and c-HSI acquisitions in all controls. Regression statistics (slope, intercept and standard error of regression, determined for neocortical and limbic parcels) for the detection of abnormal voxels were determined from controls.
2.4 |. Patient data analysis
Three approaches (A, B, C) were used to assess the relationship between the MRSI data and patient outcomes. For A and B, a two-parameter correlational approach was taken, linking a single measure of overall metabolic dysfunction with outcome.
In A, the total fractional amount of brain Cr/NAA abnormality (number of abnormal voxels/total number of available voxels) was determined.
In B, a definition of the distributed regional metabolic dysfunction (RMD) was used as follows. Based on FreeSurfer classification, voxels were grouped into 4 × 2 regional parcels: anterior quadrant (frontal excepting cingulate cortex), posterior quadrant (parietal and occipital), lateral temporal (lateral temporal and central anterior cingulate cortex) and medial temporal (medial temporal, insular and rostral anterior cingulate cortex). These RMD regions were chosen based on their importance in the typical distribution of localization-related epilepsy cases. The fractional number of abnormal voxels (over total available voxels) within each control subject was identified and a mean and standard deviation of the fractional number of average voxels per regional parcel (including the effect of the acquisition 3D or HSI) calculated in that cohort. The regression analysis determined from the control subjects was then applied to the patient data and the fractional number of “abnormal” (±2 standard deviations or more compared with the age-matched controls) voxels for each regional parcel determined. The number of abnormal parcels (out of the eight studied) is the RMD score for each patient; ie, the highest possible abnormality is 8 (metabolic dysfunction in all eight parcels) and the lowest is 0. This RMD approach specifically enables dependence on the spatial distribution of dysfunction, and by virtue of the grouping of voxels effectively applies a clustering threshold to increase accuracy.
Analysis C used a multi-variate approach to consider whether these data could provide some estimation of what regions of brain are most critical for this evaluation. This applies a logistic regression to the dysfunctional fractional volume from the eight parcels, linked to a binary outcome class, grouping ILAE classes I-II as “good” and III-VI as “poor”. This type of classification is pertinent, given the common difficulty in quantifying seizure occurrence in patient groups.23,24
3 |. RESULTS
3.1 |. Cascaded Hadamard (c-HSI) and 3D phase-encoded performance
Figure 2 shows data from the two-slice c-HSI and 3D spectroscopic images from a control. With typical voxel sizes, the extent of metabolic dysfunction in epilepsy is generally elevated by about 20 to 50% of the mean value of Cr/NAA at 1.5 T and 3 T.20,21 Thus, with relatively high concentration and amplitudes for Cr and NAA, we do not anticipate that thresholds based on the CRLB (%) will exclude voxels of interest.29 To systematically assess the spectral performance of the 3D and c-HSI acquisitions, filtering parameters based on CRLB (%), SNR, linewidth and macromolecule/lipid contamination were evaluated. In this analysis, we determined the abnormality of any given voxel from its anticipated Cr/NAA value as a function of fraction gray matter (separately determined from the T1-weighted anatomical images).10,30 The performance of the acquisitions is assessed by the size of the standard error of the regression (ie the variation of the Cr/NAA value beyond that due to the voxel’s tissue fraction gray matter) and voxel retention. Starting with a base parameter of 50% TB (gray + white matter) inclusion, Table 1 shows that increasing TB inclusion to 60 and 75% improves the regression standard error although at the cost of substantial voxel loss. The addition of linewidth (<0.15 ppm), macromolecule exclusion (MM2.0/Cr < 3.0) and less than 20% CRLB are incrementally advantageous for the standard error. As anticipated, the improvement in standard error decreased the number of voxels that fall outside of the 99% prediction interval using the regression statistics as defined by (solely) the 50% TB inclusion. In this analysis, to minimize intervoxel PSF effects, we included every other (non-contiguous) in-plane voxel. For consistency, the same spectral filtering parameters were applied to all subjects, young and old.
FIGURE 2.
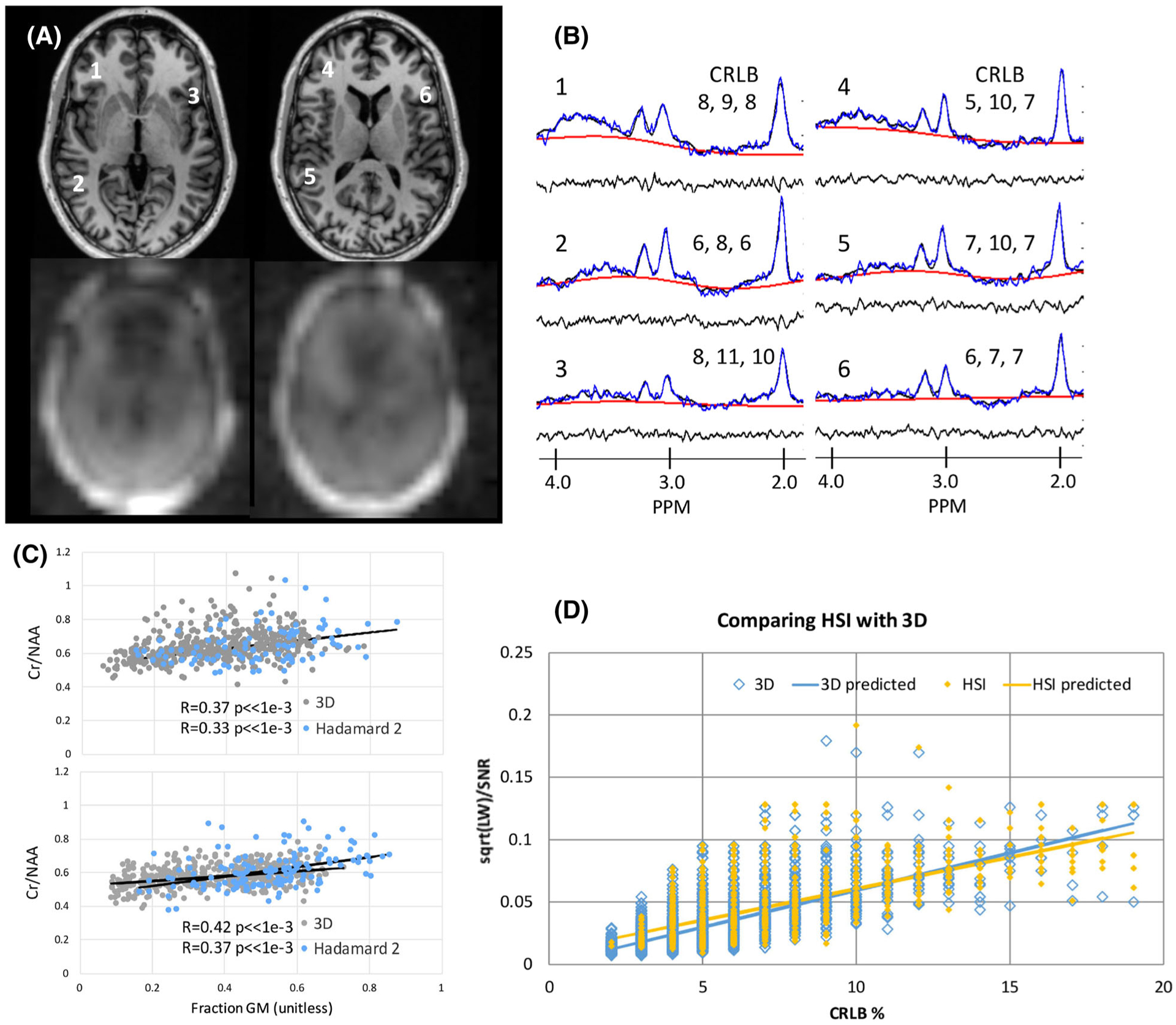
Two-slice c-HSI in controls. A, Scout MRI and magnitude NAA images. B, Sample spectra (positions indicated in A) showing the CRLB (%) for NAA, Cr and Cho. The raw with baseline, residual and fitted spectra are shown. C, For two subjects, regressions of Cr/NAA with voxel gray matter fraction are shown from the c-HSI and the adjacent 3D acquisitions, finding non-significantly different slopes and intercepts. See text for description of filtering. D, A plot of the CRLB (%) versus calculated √(LW)/SNR shows similar correlations for the 3D and c-HSI acquisitions, both significant (p < 0.001 for both), with R = 0.67 and R = 0.64 respectively
TABLE 1.
Filtering performance with 3D and c-HSI acquisitions, old and young controls, in neocortical gray matter
| Filte | 3D | HIS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TB | LW < 0.15 ppm | MM2.0/Cr < 3 | CRLB (%) < 20% | % vox retain (incr) | % regress SE (decr) | % vox beyond 99% PI (decr)a | % vox retain (incr) | % regress SE (decr) | % vox beyond 99% PI (decr)a | |
| Old | 50 | + | + | + | 91.10% | 56.90% | 3.60% | 71.50% | 27.30% | 0.00% |
| 50 | + | 0 | + | 96.50% | 60.40% | 13.30% | 81.50% | 29.00% | 0.00% | |
| 50 | + | + | 0 | 89.90% | 74.10% | 19.30% | 72.40% | 64.80% | 12.50% | |
| 50 | + | 0 | 0 | 98.10% | 96.00% | 79.50% | 90.50% | 84.10% | 62.50% | |
| 50 | 0 | 0 | 0 | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | |
| 60 | 0 | 0 | 0 | 81.80% | 64.90% | 25.30% | 87.20% | 85.90% | 62.50% | |
| 75 | 0 | 0 | 0 | 41.10% | 52.60% | 2.40% | 51.40% | 76.00% | 25.00% | |
| Young | 50 | + | + | + | 91.20% | 53.20% | 6.10% | 78.00% | 29.00% | 0.00% |
| 50 | + | 0 | + | 97.00% | 58.00% | 22.40% | 86.30% | 29.70% | 0.00% | |
| 50 | + | + | 0 | 91.80% | 55.70% | 14.30% | 81.40% | 36.60% | 11.10% | |
| 50 | + | 0 | 0 | 98.60% | 85.90% | 89.80% | 91.80% | 69.20% | 50.00% | |
| 50 | 0 | 0 | 0 | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | 100.00% | |
| 60 | 0 | 0 | 0 | 82.40% | 53.20% | 16.30% | 88.90% | 96.50% | 83.30% | |
| 75 | 0 | 0 | 0 | 41.70% | 45.00% | 6.10% | 54.50% | 108.40% | 66.70% | |
For old and young controls, each row has defined filtering criteria, with all values compared with 50% TB (bold italics). The +/0 indicates whether filtering is used: LW, reject voxels at >0.15 ppm; MM, reject voxels with MM2.0ppm/Cr of >3.0; CRLB (%), reject voxels with CRLB > 20%. TB defined here as GM + WM. Filtering with 60% and 75% TB are also shown.
The percentage of voxels falling outside of the 99% prediction interval (PI) as defined from 50% TB filtered regression. The arrows in the header row indicate the preferred direction of the parameter. SE, standard error of regression.
Over the control volunteers, these filtering steps resulted in an overall voxel retention of 86.6 ± 3.2%: 73.9 ± 7.1% and 91.1 ± 2.8% for the c-HSI and 3D respectively. Patients were similar, although they had greater variability, at 85.0 ± 8.5% overall: 75.7 ± 12.8% and 90.2 ± 7.5% for the c-HSI and 3D respectively. In the controls, the number of voxels falling outside of the 50% TB prediction interval was 6.1% or less for both the 3D and HSI acquisitions. The mean CRLBs (%) (NAA, Cr and Cho) from the c-HSI and 3D acquisitions were 7.3 ± 3.3, 8.1 ± 2.2, 8.6 ± 2.1 and 4.5 ± 1.9, 6.3 ± 1.9, 6.7 ± 2.1 respectively. The RMS SNRNAA as reported by LCM for these data were 7.2 ± 2.7 and 12.5 ± 4.0; linewidths were 0.083 ± 0.029 and 0.061 ± 0.022 ppm respectively. Figure 2A–C shows representative spectra and regression analysis for the Cr/NAA value with fractional gray matter content for two subjects. Finally, we have previously shown that the CRLB (%) can be estimated from ,10 given that the spectra are well behaved and modeled by non-overlapping singlet resonances. Thus Figure 2D shows the adequacy of the singlet model to describe both the 3D and c-HSI acquisitions, comparing the CRLBest against the measured CRLB. The regression lines for the 3D and HSI are both separately significant (p < 0.001) but not different from each other. Thus, to apply a consistent statistical model to all the c-HSI and 3D data, matched regression parameters (intercept, slope and standard error) were used in the analysis, taking into account age (old, >45, versus young, <45 years old), acquisition (3D or c-HSI) and tissue type (neocortical versus limbic). A Bonferroni correction was applied to minimize bias due to the multiple methods of analysis and comparisons in addition to the inclusion of all voxels (rather than a reduced number); p < 0.005 was used for significance testing. To facilitate rapid presentation of the large number of spectroscopic data, color-coded p-map overlays were created on the structural images (see Figures 3–5 for examples).
FIGURE 3.
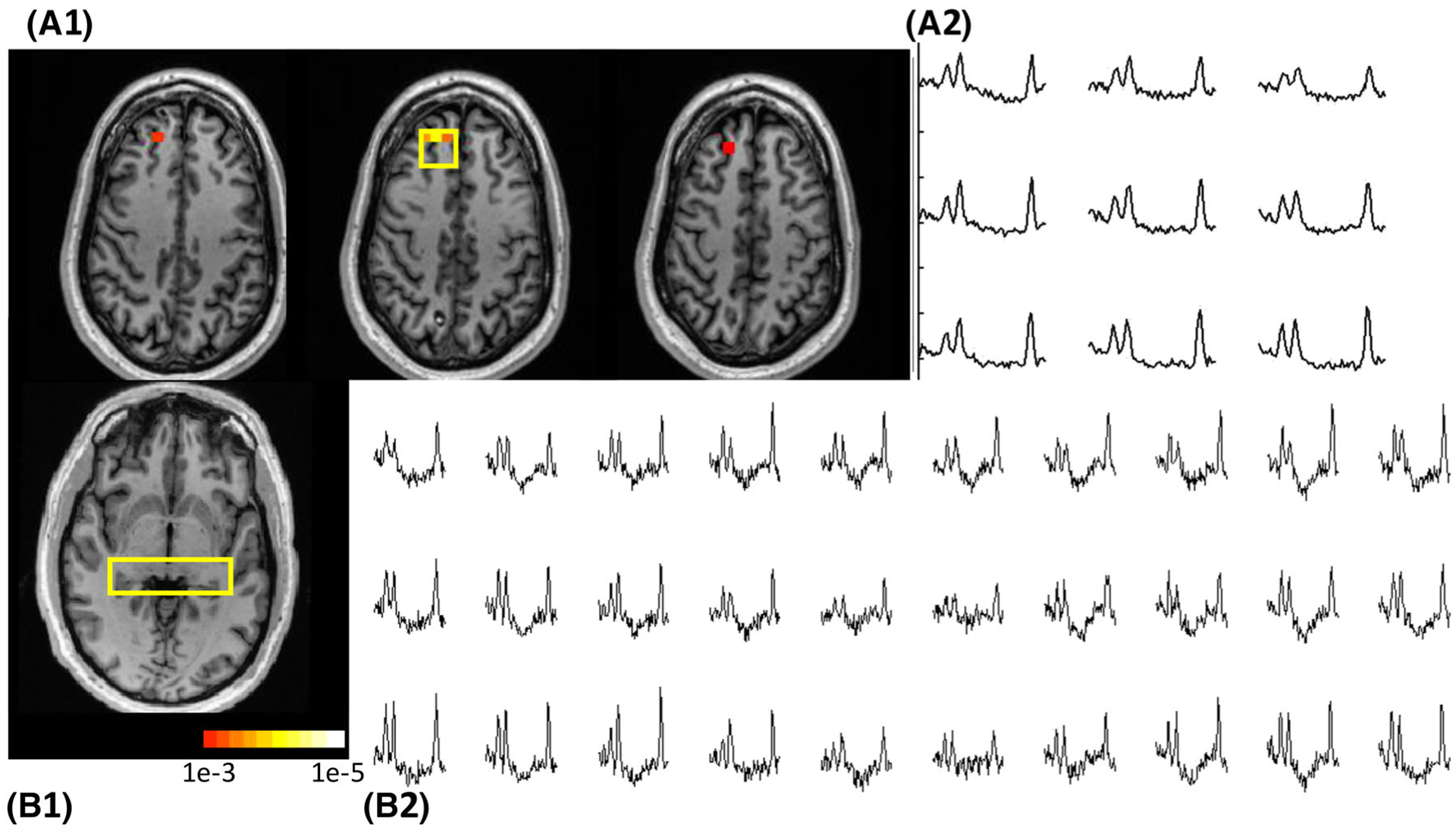
Data from Patient 4. A1, Three consecutive slices are shown from the 3D acquisition. A2, The spectra shown correspond to the box in the middle slice, and were identified as abnormal in comparison with the control. B1, B2, The c-HSI slice is shown, with corresponding bihippocampal spectra. This patient underwent right frontal lobe resection with an ILAE outcome of I. The color bar indicates p-value, and also applies to Figures 4 and 5
FIGURE 5.
Data from Patient 15, with RMD score 2. This patient had a vascular abnormality with tissue loss in the left occipital lobe causing large susceptibility changes in the region. With left hippocampal ablation this patient had an ILAE outcome of IV. Spectra from the bihippocampal and occipital regions are shown. Evident disturbance in the baseline is seen through the occipital spectra
Table 2 shows the mean Cr/NAA values of the young and old controls and patients separated by outcome. In the controls, there was no significant difference between the anterior and posterior quadrant measurements. There were significant differences between young and old subjects: from the combined left and right anterior quadrant, posterior quadrant, temporal and limbic parcels. The Cr/NAA values from the young subjects are significantly lower than those from the older subjects, eg anterior quadrant (0.56 ± 0.02 young versus 0.61 ± 0.04 old, p < 0.001) and limbic (0.64 ± 0.04 young, 0.70 ± 0.06, p < 0.01), consistent with literature.31,32 The total volume recovered in patients for the 3D sequence was 85 582 ± 13 160 mm3 and that for the c-HSI was 31 769 ± 8523 mm3. In controls the 3D sequence and c-HSI volumes were 91 563 ± 7863 mm3 and 32 053 ± 4340 mm3 respectively. Although the volume for inclusion in the patient group was commonly smaller than that for the control group, consistent with the well known volume loss seen in many epilepsy patients,33 they were not statistically different.
TABLE 2.
Mean Cr/NAA values, and for patients % fraction of abnormality
| Controls | Patients | ||||
|---|---|---|---|---|---|
| Parcel | L/R | Young (n= 10) | Old (n= 10) | Good O/C | Poor O/C |
| Total | 0.58±0.02 | 0.63±0.03 | 0.63±0.06 | 0.66±0.08 | |
| 1.01±0.06 | 1.00±0.06 | ||||
| 0.07±0.07% | 0.14±0.11% | 1.38±4.75% | 3.07±6.56% | ||
| Ant Quad | L | 0.56±0.02 | 0.61±0.04 | 0.61±0.06 | 0.64±0.08 |
| 1.28±0.38 | 1.04±0.09 | ||||
| 0.38±0.66% | 1.09±2.58% | ||||
| R | 0.06±0.23% | 0.08±0.18% | 0.62±0.07 | 0.67±0.10 | |
| 0.99±0.04 | 1.02±0.07 | ||||
| 0.85±1.93% | 2.91±4.24% | ||||
| Post Quad | L | 0.58±0.03 | 0.62±0.04 | 0.60±0.05 | 0.65±0.07 |
| 0.99±0.01 | 1.03±0.09 | ||||
| 0.08±0.23% | 1.84±4.45% | ||||
| R | 0.05±0.07% | 0.04±0.14% | 0.62±0.06 | 0.69±0.12 | |
| 1.16±0.32 | 1.05±0.04 | ||||
| 0.37±1.11% | 3.78±7.03% | ||||
| Temporal | L | 0.61±0.03 | 0.65±0.05 | 0.66±0.08 | 0.69±0.10 |
| 1.18±0.08 | 1.06±0.09 | ||||
| 2.58±7.18% | 1.11±2.82% | ||||
| R | 0.00% | 0.08±0.23% | 0.70±0.10 | 0.74±0.11 | |
| 1.18±0.15 | 1.09±0.07 | ||||
| 3.51±9.99% | 5.80±10.24% | ||||
| Limbic | L | 0.64±0.04 | 0.70±0.06 | 0.69±0.07 | 0.72±0.10 |
| 1.11±0.12 | 1.12±0.10 | ||||
| 1.37±2.25% | 2.59±4.38% | ||||
| R | 0.04±0.16% | 0.73±1.02% | 0.70±0.11 | 0.75±0.10 | |
| 1.09±0.10 | 1.19±0.17 | ||||
| 1.87±4.84% | 5.34±10.58% | ||||
In the control columns, all parcels (combined left, right) from the young group are significantly different from the old group, all below p < 0.005. The fraction of limbic voxels that are abnormal is significantly larger in the old versus young group, 0.73 ± 1.02 versus 0.04 ± 0.16, p < 0.02. In the controls, each field has two entries (mean ± standard deviation): top, mean Cr/NAA over entire parcel; bottom, percentage of voxels that are scored as abnormal. In the patient columns, each field has three entries (mean and standard deviation): top, mean Cr/NAA over entire parcel; middle, mean Cr/NAA over the abnormal voxels only; bottom, percentage of abnormal voxels. The patient data are separated according to outcome: good, ILAE I, II; poor, ILAE III-VI.
3.2 |. Epilepsy patients
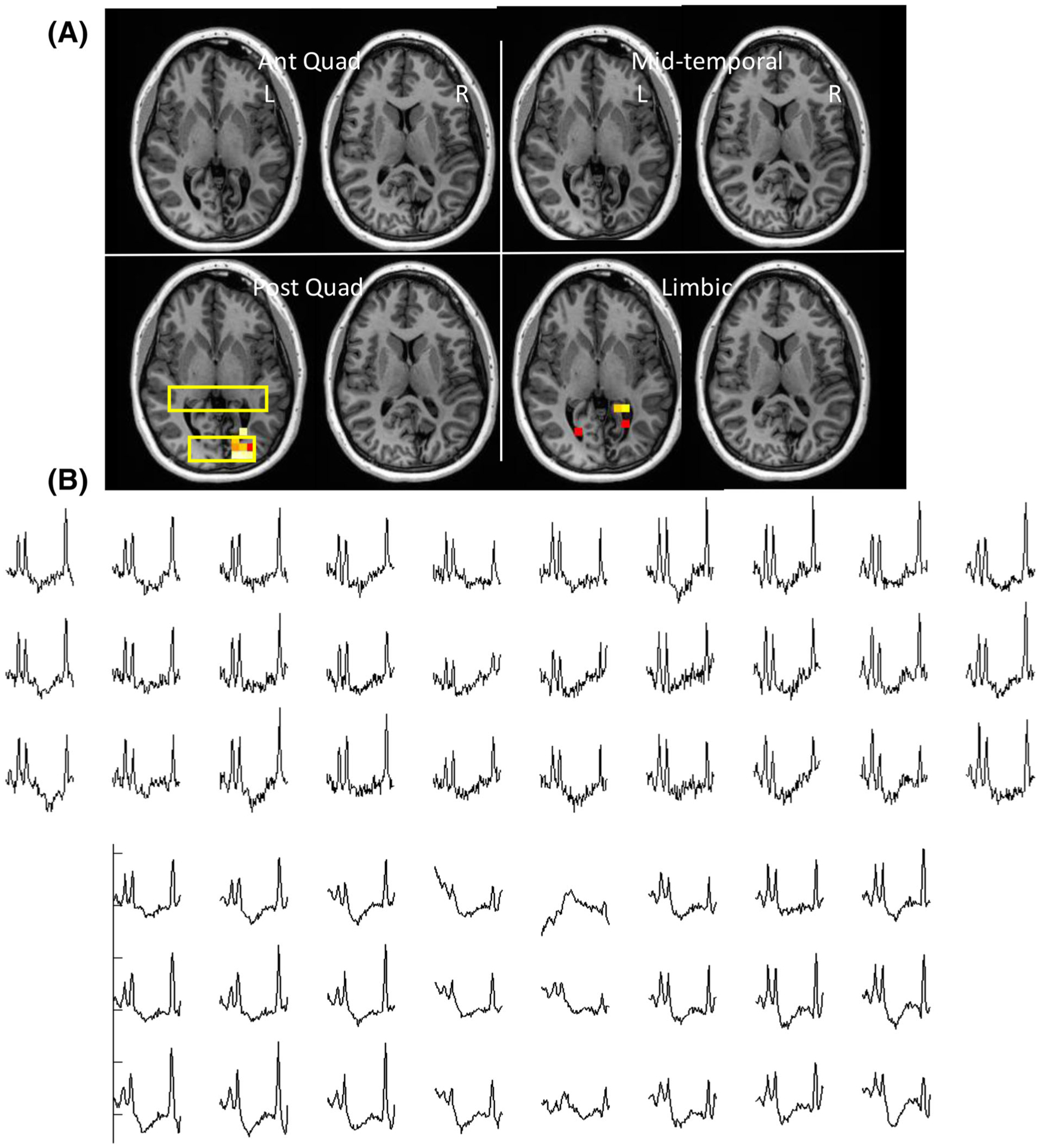
Table 3 shows the basic clinical indices for the n = 27 epilepsy patients. Figure 3 shows the p-map (overlaid with significantly abnormal Cr/NAA regions determined by regression analysis) data from Patient 4. Spectra from this 46-year-old patient are shown from the abnormal region in the right frontal lobe and the bihippocampal region, and identify the abnormally increased Cr/NAA from the right frontal region. After right frontal lobe resection of the lesion, this patient became seizure free, resulting in an ILAE Class I outcome. Figure 4 shows data from Patient 26. This 47-year-old patient had a RMD of 5, including the right temporal, posterior quadrant as well as bilateral anterior quadrant parcels. He underwent right hippocampal ablation and had right parietal-occipital RNS placement, resulting in a ILAE Class IV outcome. Sample spectra are shown identifying the region of abnormality. Figure 5 shows data from 24-year-old Patient 15, with RMD = 2 including the left posterior quadrant and left limbic parcel. This patient had a vascular abnormality (Sturge-Weber) with tissue loss in the left occipital lobe, causing large susceptibility changes in the region, manifest as a disturbance in the baseline seen through the occipital spectra. With left hippocampal ablation, this patient was seizure free for 3 months but then experienced recurrence 5 months after surgery, resulting in an ILAE Class IV outcome at 1 year.
TABLE 3.
Patient data
| No | Age | Handedness, gender | Years of epilepsy | Surgery | RMD | % of brain abnormal | ILAE |
|---|---|---|---|---|---|---|---|
| 1 | 53 | R, F | 30 | L AMTL | 1 | 0.228 | II |
| 2 | 22 | R, M | 2 | L AMTL | 0 | 0.000 | I |
| 3 | 30 | R, M | 1 | L AMTL | 2 | 0.172 | I |
| 4* | 42 | R, M | 11 | R x2 fro cav mal, resection | 1 | 0.273 | I |
| 5 | 23 | R, F | 4 | R MTL ablation | 0 | 0.000 | I |
| 6 | 36 | L, F | 14 | L MTL ablation | 4 | 0.688 | VI |
| 7 | 21 | R, M | 7 | RNS temp-par | 4 | 2.493 | IV |
| 8 | 63 | R, M | 43 | L AMTL | 3 | 0.419 | I |
| 9 | 26 | R, F | 7 | R MTL ablation | 1 | 0.137 | I |
| 10 | 50 | R, F | 49 | R MTL ablation | 8 | 4.675 | II |
| 11 | 18 | R, F | 10 | L AMTL | 0 | 0.000 | I |
| 12 | 46 | R, F | 1 | R parietal resection | 6 | 6.248 | V |
| 13 | 42 | R, F | 10 | R MTL ablation | 5 | 1.444 | V |
| 14 | 46 | R, F | 4 | L MTL ablation | 1 | 0.129 | V |
| 15* | 24 | L, F | 23 | L MTL ablation | 3 | 0.600 | IV |
| 16 | 28 | R, M | 13 | RNS bilateral MTL | 3 | 0.221 | IV |
| 17 | 19 | Ambi, M | 3 | L inferior fro ablation | 1 | 0.151 | III |
| 18 | 33 | R, M | 10 | R AMTL | 4 | 0.615 | IV |
| 19 | 64 | R, M | 62 | R fro resection | 8 | 10.941 | V |
| 20 | 26 | R, M | 6 | L MTL ablation | 3 | 0.490 | III |
| 21 | 26 | R, M | 19 | L mesial fro ablation | 0 | 0.050 | III |
| 22 | 27 | R, M | 20 | R MTL ablation | 7 | 11.221 | V |
| 23 | 36 | R, F | 6 | RNS L posterior MTL, R posterior lateral temp | 0 | 0.022 | V |
| 24 | 37 | R, F | 5 | R AMTL | 1 | 0.102 | I |
| 25 | 57 | R, M | 38 | R AMTL | 4 | 0.655 | I |
| 26* | 48 | L, M | 33 | R MTL ablation; RNS R par-occ | 5 | 4.805 | V |
| 27 | 36 | R, F | 5 | R posterior basal temp ablation | 1 | 0.078 | V |
FIGURE 4.
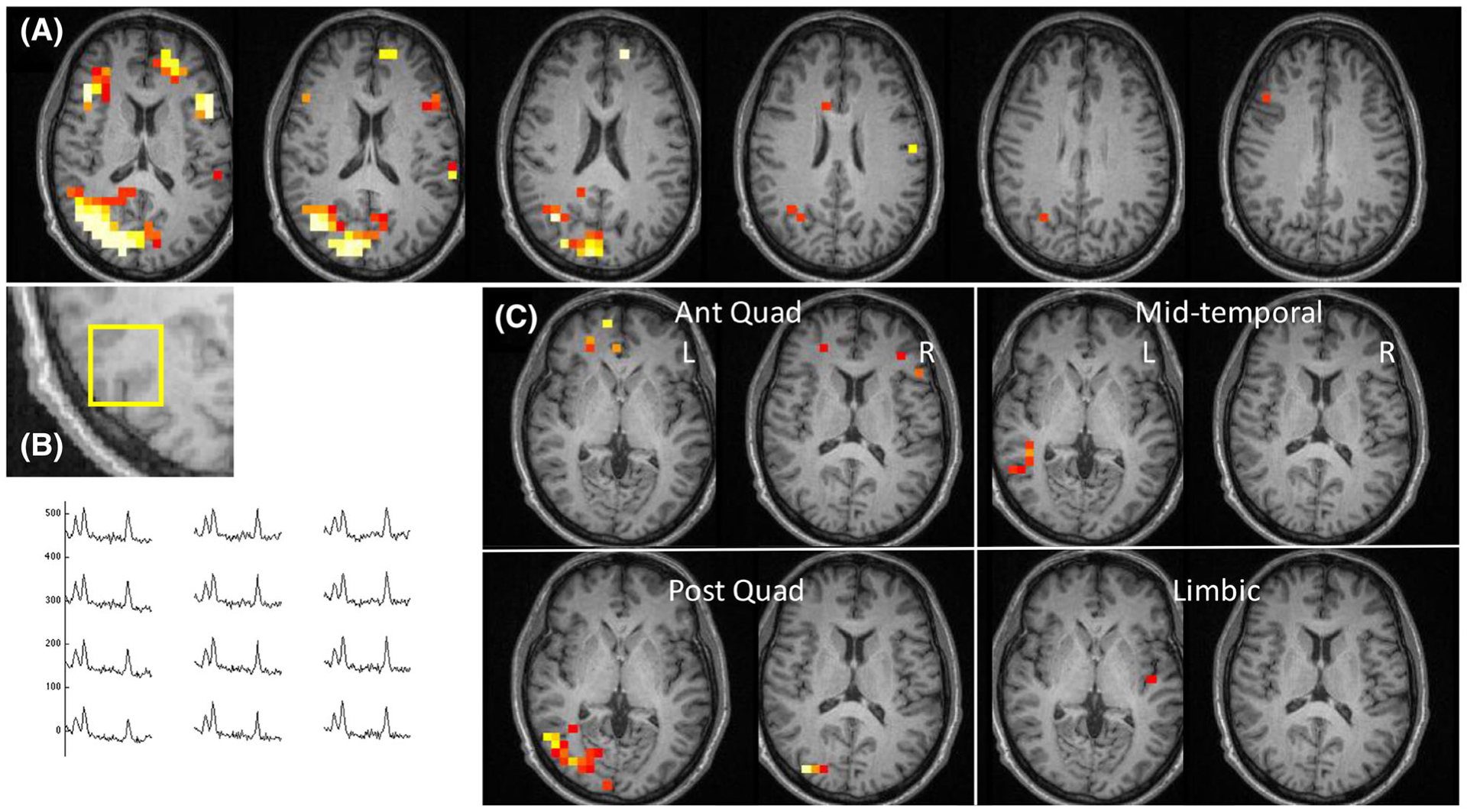
Data from Patient 26, with RMD score 5. This patient underwent right hippocampal ablation and repetitive neurostimulator placement with an ILAE outcome of IV. Spectra from the right temporal-parietal region are shown
The Cr/NA values from the good versus poor outcome patients are shown with control data in Table 2. With the patients, for each parcel, the mean and standard deviation for Cr/NAA values (top), values for the abnormal (middle) voxels and fraction abnormal (bottom) are shown. Overall, the patient groups with poor outcome exhibited a mildly worse (larger value) mean Cr/NAA than patients with good outcomes; however, there is a large amount of data scatter, reflecting the variation between patients and relatively limited extent of injury seen in many. With the detected abnormalities, the amplitude of Cr/NAA change was similar in all parcels considered. What is notable is that the fractional volume of metabolic dysfunction seen in the patients with poor outcomes appeared to be larger, particularly in the right hemisphere.
In the evaluations for outcome, with epilepsy being a primarily gray matter disorder, we also evaluated fractional gray matter (GM/GM + WM + CSF or GM/GM + WM) in relation to outcome, but found no significant rank or linear correlation. However, we did find relationships between whole brain metabolic measurements and clinical outcome (Figure 6). Analysis Method A, relating to total fraction of abnormality, found that a Spearman correlation was significant at R = 0.47, p < 0.015. Taking into account the parcel distribution through the use of the RMD score, Analysis Method B was also significant, at R = 0.39, p < 0.05. Finally, to ascertain whether these data could provide some estimate of the contribution of each of the parcels to outcome when evaluating a binary outcome (good versus poor seizure control), the results of the logistic regression (Analysis Method C) are shown in Figure 6C. The temporal (limbic and mid-temporal parcels) appear to have greater effect for classification, with the right limbic component significant at p < 0.025.
FIGURE 6.
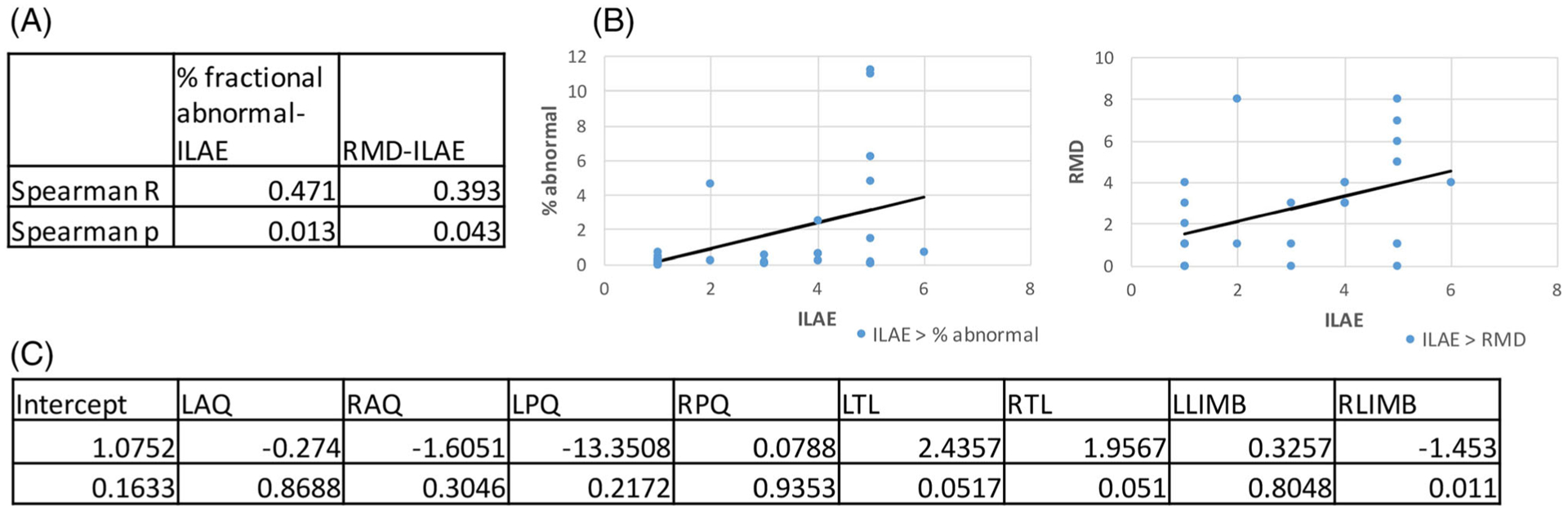
A, Table showing the rank correlation values with significance levels for volumetric MRSI abnormality with ILAE outcome. Fraction of TB and RMD count are both significant. B, Data plotted together with the Pearson (linear) correlations. C, Multivariate analysis using a binomial logistic regression analysis to consider the 4 × 2 individual parcels’ contributions to outcome classification. The limbic and temporal parcels have the greatest contribution to the regression, with the right limbic significant at p < 0.05. LAQ, RAQ, left or right anterior quadrant; LPQ, RPQ, left or right posterior quadrant; LTL, RTL, left or right temporal lobe; LLimb, RLimb, left or right limbic
4 |. DISCUSSION
With the two-part acquisition covering the large portions of frontal, parietal to mid-temporal and occipital regions, we have found that the performances of the two-slice c-HSI and 3D phase-encoded chemical shift imaging are comparable. The two-slice c-HSI approach avoids the PSF contamination that would be more of a problem for phase-encoded slices in the lower brain regions, and allows for targeted shimming of the structures of interest. In this assessment, we have used the regression analysis parameters (resulting from Cr/NAA with fraction gray matter) as a guide in determining the optimal voxel filtering. This regression analysis is based on the standard error of regression, ie the prediction interval (defined as se is the mean fraction of gray matter, Sxx is the standard deviation for the fraction of gray matter, n is the number of points) defined from the control data. In this analysis and as shown in Table 1 for controls, we calculated the number of voxels falling beyond ±2×prediction interval as a function of filtering criteria. Starting with the base criterion of 50% TB (gray + white matter), the number of voxels with values beyond the prediction interval falls quickly with the use of the linewidth threshold, macromolecule baseline and the CRLB < 20% criteria. As a result, in the controls, the retained voxel numbers were 86.6 ± 3.2% for all voxels and 73.9 ± 7.1% and 91.1 ± 2.8% for the c-HSI and 3D respectively. With these criteria, the linewidth and SNR for the c-HSI are 0.083 ± 0.029 and 7.2 ± 2.7, compared with the 3D at 0.061 ± 0.022 ppm and 12.5 ± 4.0 respectively. The SNR difference is most likely a consequence of the larger susceptibility variations seen in the inferior temporal region and the 3 T head coil, which inferiorly has been reported to exhibit decreased SNR in comparison with superior brain regions.34 Consistent with this, the CRLB (%) values are larger from the temporal c-HSI acquisitions than the 3D (for NAA, Cr, Cho these are 7.3 ± 3.3, 8.1 ± 2.2, 8.6 ± 2.1 for the HSI and 4.5 ± 1.9, 6.3 ± 1.9, 6.7 ± 2.1 for 3D), but are well behaved. As a result, the use of the regression statistics as determined by their region, acquisition (and age) from control data enables a consistent analysis over the entire study.
4.1 |. Epilepsy patients
The patient data show that there is a widespread and variable distribution of metabolic dysfunction in the epilepsy brain, with abnormal voxels exhibiting an increased Cr/NAA (irrespective of outcome, an overall value of 1.0 ± 0.12 compared with the control at 0.62 ± 0.08) that is similar between parcels. With these voxel sizes, the actual values of Cr/NAA itself do not appear to provide discriminatory value for outcome, consistent with the work of Guye et al.20 However, the amount of distributed dysfunction is pertinent, as suggested from the fractional volume of dysfunction, with nearly all parcels showing a greater amount of dysfunction in the poor outcome group. The binary logistic regression suggests that the temporal and limbic regions, and thus the HSI acquisition, are of importance for patient classification, consistent with the high incidence of temporal lobe epilepsy in this patient population.35 There may also be an effect of left versus right distribution, with the right-sided parcels showing volumetrically greater dysfunction than left. The etiology of this is unclear, but it has been identified in other contexts,36,37 possibly related to the brain’s asymmetric development and function.
We found that a fractional count of total dysfunctional brain or a distributed evaluation of metabolic dysfunction (RMD) correlates moderately and positively with worse post-surgical outcome. Current reports of surgery success range from 50% (extra-temporal lobe surgery) to 65% (temporal lobe surgery) for excellent outcome, with more recent laser ablation methods not being substantially different.35,38 Given this level of surgical success, and the understanding that the epilepsy brain exhibits complex seizure networks9,10 that likely influence the success of focal epilepsy surgery, the current data may be better understood in terms of the size and distribution of the seizure network and localization of seizure onset. With a larger volume of abnormality, the dysfunctional seizure network may be larger (including multiple potential seizure onset sites), which for focal therapy can result in more difficulty in localization of the onset site. It should also be noted that the spectroscopic Cr/NAA values can be abnormal for reasons other than seizures, and such metabolic dysfunction may make optimal response to surgery more difficult. Conversely, patients with a lower volume of abnormality may be more “focal” in nature and thus more amenable to surgical therapy.
The inferior brain region is a challenging area for robust spectroscopic imaging. While we did not cover the entire brain, this targeted approach was able to obtain comparable spectral quality between the c-HSI and 3D acquisitions. As applied in these epilepsy patients we were able to determine the fractional abnormality of the covered regions beyond that seen in equivalently studied age-matched group controls. That these results significantly correlated with surgical outcome argues that it is a relevant strategy. Additional work in B0 shimming will be needed to further extend the volume of coverage beyond the mid-temporal region, which should be pertinent to improve the prognostication accuracy of these methods.
ACKNOWLEDGEMENTS
This work is supported by NIH grants EB011639, NS090417, NS081772, NS097494 and NS112853. AT acknowledges support from the Monroy-Marks Career Development Fund and the Harold Perlman Family.
Funding information
National Institutes of Health, Grant/Award Numbers: EB011639, EB112853, NS090417, NS097494, NS112853, NS081772; Harold Perlman Family; Monroy-Marks Career Development Fund
Abbreviations:
- BOLERO
B0 loop-encoded readout
- B1+
B1 transmission
- Cho
choline
- c-HSI
cascaded Hadamard-encoded spectroscopic imaging
- Cr
creatine
- CRLB
Cramér-Rao lower bound
- CSF
cerebrospinal fluid
- GM
gray matter
- HSI
Hadamard spectroscopic imaging
- ILAE
International League Against Epilepsy
- LW
linewidth
- MRSI
MR spectroscopic imaging
- MTL
medial temporal lobe
- NAA
N-acetylaspartate
- PSF
point spread function
- RMD
regional metabolic dysfunction
- SNR
signal to noise ratio
- TB
total brain
- VOI
volume of interest
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ILAE classifications: I, seizure free, no auras; II, auras permitted, otherwise seizure free; III, 1–3 seizures/year ± auras; IV, 4 seizure days/year to 50% reduction of baseline seizures; V, less than 50% reduction of seizures from baseline seizure days up to a 100% increase in seizure frequency; VI, more than 100% increase from baseline seizure days.
REFERENCES
- 1.Lecocq A, Le Fur Y, Maudsley AA, et al. Whole-brain quantitative mapping of metabolites using short echo three-dimensional proton MRSI. J Magn Reson Imaging. 2015;42(2):280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maghsudi H, Schmitz B, Maudsley AA, et al. Regional metabolite concentrations in aging human brain: comparison of short-TE whole brain MR spectroscopic imaging and single voxel spectroscopy at 3T. Clin Neuroradiol. 2019;30(2):251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maudsley AA. Sensitivity in Fourier imaging. J Magn Reson. 1986;68:363–366. [Google Scholar]
- 4.Hetherington HP, Pan JW, Chu WJ, Mason GF, Newcomer BR. Biological and clinical MRS at ultra-high field. NMR Biomed. 1997;10(8):360–371. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Bolinger L, Subramanian VH, Leigh JS. Errors of Fourier chemical shift imaging and their corrections. J Magn Reson. 1991;92:64–72. [Google Scholar]
- 6.Goelman G, Liu S, Gonen O. Reducing voxel bleed in Hadamard-encoded MRI and MRS. Magn Reson Med. 2006;55(6):1460–1465. [DOI] [PubMed] [Google Scholar]
- 7.Shih JJ. It’s all about the networks. Epilepsy Curr. 2019;19(3):165–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller SG, Ebel A, Barakos J, et al. Widespread extrahippocampal NAA/(Cr+Cho) abnormalities in TLE with and without mesial temporal sclerosis. J Neurol. 2011;258(4):603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartolomei F, Lagarde S, Wendling F, et al. Defining epileptogenic networks: contribution of SEEG and signal analysis. Epilepsia. 2017;58(7):1131–1147. [DOI] [PubMed] [Google Scholar]
- 10.Schirda CV, Zhao T, Yushmanov VE, et al. Fast 3D rosette spectroscopic imaging of neocortical abnormalities at 3 T: assessment of spectral quality. Magn Reson Med. 2018;79(5):2470–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher DW, Haselgrove JC, Bolinger L. High-resolution imaging using Hadamard encoding. Magn Reson Imaging. 1999;17(10):1457–1468. [DOI] [PubMed] [Google Scholar]
- 12.Cohen O, Tal A, Gonen O. Three-dimensional Hadamard-encoded proton spectroscopic imaging in the human brain using time-cascaded pulses at 3 Tesla. Magn Reson Med. 2014;72(4):923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tal A, Zhao T, Schirda C, Hetherington H, Pan JW, Gonen O. Fast, regional 3D hybrid (1D-Hadamard 2D-rosette) 1H MR spectroscopic imaging in the human temporal lobes. NMR Biomed. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folbergrová J, Kunz WS. Mitochondrial dysfunction in epilepsy. Mitochondrion. 2012;12(1):35–40. [DOI] [PubMed] [Google Scholar]
- 15.Waldbaum S, Patel M. Mitochondrial dysfunction and oxidative stress: a contributing link to acquired epilepsy? J Bioenerg Biomembr. 2010;42(6):449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connett RJ. Analysis of metabolic control: new insights using scaled creatine kinase model. Am J Physiol. 1988;254(6 Pt 2):R949–R959. [DOI] [PubMed] [Google Scholar]
- 17.Bates T, Strangward M, Keelan J, Davey G, Munro P, Clark J. Inhibition of N-acetyl aspartate production: implications for 1H MRS studies in vivo. NeuroReport. 1996;7(8):1397–1400. [PubMed] [Google Scholar]
- 18.Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20(4/5):271–276. [DOI] [PubMed] [Google Scholar]
- 19.Pan JW, Takahashi K. Inter-dependence of NAA and high energy phosphates in human brain. Ann Neurol. 2005;57(1):92–97. [DOI] [PubMed] [Google Scholar]
- 20.Guye M, Le Fur Y, Confort-Gouny S, et al. Metabolic and electrophysiological alterations in subtypes of temporal lobe epilepsy: a combined proton magnetic resonance spectroscopic imaging and depth electrodes study. Epilepsia. 2002;43(10):1197–1209. [DOI] [PubMed] [Google Scholar]
- 21.Mueller SG, Laxer KD, Suhy J, Lopez RC, Flenniken DL, Weiner MW. Spectroscopic metabolic abnormalities in mTLE with and without MRI evidence for mesial temporal sclerosis using hippocampal short-TE MRSI. Epilepsia. 2003;44(7):977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Commission on Neurosurgery of the International League Against Epilepsy (ILAE) 1997–2001:Wieser H, Blume W, Fish D, et al. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42(2):282–286. [PubMed] [Google Scholar]
- 23.Mohan M, Keller S, Nicolson A, et al. The long-term outcomes of epilepsy surgery. PLoS ONE. 2018;13(5):e0196274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiebe S, Blume WT, Girvin JP, Eliasziw M, for the Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. [DOI] [PubMed] [Google Scholar]
- 25.Schirda CV, Tanase C, Boada FE. Rosette spectroscopic imaging: optimal parameters for alias-free, high sensitivity spectroscopic imaging. J Magn Reson Imaging. 2009;29(6):1375–1385. [DOI] [PubMed] [Google Scholar]
- 26.Hetherington H, Chu WJ, Gonen O, Pan JW. Robust fully automated shimming of the human brain for high field 1H spectroscopic imaging. Magn Reson Med. 2006;56(1):26–33. [DOI] [PubMed] [Google Scholar]
- 27.Pan JW, Lo KM, Hetherington HP. Role of very high order and degree B0 shimming for spectroscopic imaging of the human brain at 7 tesla. Magn Reson Med. 2012;68(4):1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith S, Levante T, Meier BH, Ernst RR. Computer simulations in magnetic resonance. An object-oriented programing approach. J Magn Reson A. 1994;106(1):75–105. [Google Scholar]
- 29.Kreis R The trouble with quality filtering based on relative Cramer Rao lower bounds. Magn Reson Med. 2016;75(1):15–18. [DOI] [PubMed] [Google Scholar]
- 30.Pan JW, Duckrow RB, Gerrard J, et al. 7T spectroscopic imaging in surgical treated epilepsy. Epilepsia. 2013;54(9):1668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sporn L, MacMillan EL, Ge R, Greenway K, Vila-Rodriguez F, Laule C. Longer repetition time proton MR spectroscopy shows increasing hippocampal and parahippocampal metabolite concentrations with aging. J Neuroimaging. 2019;29(5):592–597. [DOI] [PubMed] [Google Scholar]
- 32.Gruber S, Pinker K, Riederer F, et al. Metabolic changes in the normal ageing brain: consistent findings from short and long echo time proton spectroscopy. Eur J Radiol. 2008;68(2):320–327. [DOI] [PubMed] [Google Scholar]
- 33.Farid N, Girard HM, Kemmotsu N, et al. Temporal lobe epilepsy: quantitative MR volumetry in detection of hippocampal atrophy. Radiology. 2012; 264(2):542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockmann JP, Witzel T, Keil B, et al. A 32-channel combined RF and B0 shim array for 3T brain imaging. Magn Reson Med. 2016;75(1):441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramey WL, Martirosyan NL, Lieu CM, Hasham HA, Lemole GM Jr, Weinand ME. Current management and surgical outcomes of medically intractable epilepsy. Clin Neurol Neurosurg. 2013;115(12):2411–2418. [DOI] [PubMed] [Google Scholar]
- 36.Voets NL, Beckmann CF, Cole D, Hong SJ, Bernasconi A, Bernasconi N. Structural substrates for resting network disruption in temporal lobe epilepsy. Brain. 2012;135(8):2350–2357. [DOI] [PubMed] [Google Scholar]
- 37.Chiang S, Stern JM, Engel J, Levin H, Haneef Z. Differences in graph theory functional connectivity in left and right temporal lobe epilepsy. Epilepsy Res. 2014;108(10):1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta K, Cabaniss B, Kheder A, et al. Stereotactic MRI-guided laser interstitial thermal therapy for extratemporal lobe epilepsy. Epilepsia. 2020;61(8): 1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]


