Abstract
Chemokines are a large subfamily of cytokines known for their ability to facilitate cell migration, most notably leukocytes, throughout the body. Chemokines are necessary for a functioning immune system in both health and disease and have received considerable attention for their roles in orchestrating temporal-spatial regulation of immune cell populations in cancer. Gliomas comprise a group of common central nervous system (CNS) primary tumors that are extremely challenging to treat. Immunotherapy approaches for highly malignant brain tumors offer an exciting new avenue for therapeutic intervention but so far, have seen limited successful clinical outcomes. Herein we focus on important chemokine/chemokine receptor systems in the regulation of pro- and anti-tumor mechanisms, highlighting potential therapeutic advantages of modulating these systems in malignant gliomas and other cancers.
Keywords: Glioblastoma, Chemokine, Tumor microenvironment, MDSC, Microglia, Immunotherapy
1. Introduction
1.1. Overview of chemokines and chemokine receptors
Chemokines are small, secreted chemotactic cytokines known for their role in directing the movement of immune cells throughout the body (Charo & Ransohoff, 2006; Griffith, Sokol, & Luster, 2014; Nagarsheth, Wicha, & Zou, 2017). This large cytokine subfamily consists of approximately 50 endogenous ligands in humans and mice (Griffith et al., 2014; Sokol & Luster, 2015). Chemokines are divided into four families based on the relative position of cysteine (C) residues near the amino-terminus: CC, CXC, CX3C, and XC (Hughes & Nibbs, 2018; Nagarsheth et al., 2017; Sokol & Luster, 2015). These conserved cysteines form disulfide bridges which provide the structural prerequisite for chemokine receptor binding (Miller & Mayo, 2017). Chemokine receptors are a diverse family of G protein coupled receptors (GPCRs). Structurally, they belong to the class A rhodopsin-like gene family typified by their seven transmembrane spanning domains, extracellular N-terminus that contributes to ligand binding, and the presence of conserved intracellular motifs that comprise structural components of G-protein and arrestin docking sites (Fraser, Wang, Robinson, Gocayne, & Venter, 1989; Monteclaro & Charo, 1996; Monteclaro & Charo, 1997). Chemokine receptors are expressed throughout the body, most commonly on populations of immune cells (Yamasaki et al., 2012). Expression on immune cells can be driven by inflammation, with little to no constitutive expression noted (Connor et al., 2004; Mack et al., 2005; van Helden, Zaiss, & Sijts, 2012). Additionally, under inflammatory conditions, expression of chemokine receptors can been detected on non-immune cells, e.g. endothelial cells (Weber, Nelson, Grone, & Weber, 1999).
When bound to their cognate receptors, chemokines exert a range of functions within the tissues they occupy. Homeostatic chemokines such as CCL17, CXCL14, and CXCL15 (Stewart & Smyth, 2009) are constitutively produced and secreted during normal physiological states, and are essential for immune surveillance as they are necessary for proper regulation of leukocyte levels in tissues and organs (Chen et al., 2018). However, homeostatic chemokine functions extend beyond immune cell trafficking, for instance having roles in organogenesis (Cardona, Garcia, & Cardona, 2013; Fernandez & Lolis, 2002; Zlotnik, Burkhardt, & Homey, 2011). Mounting evidence points toward a role for homeostatic chemokines in development, as these genes are strongly conserved throughout evolution (Zlotnik, Yoshie, & Nomiyama, 2006). Inflammatory chemokines are produced during times of infection or other pro-inflammatory stimulus (Stewart & Smyth, 2009). The chief function of this large subset of chemokines is rapid induction of leukocyte mobilization to an infected or injured site (Fernandez & Lolis, 2002). However, it is also been shown that inflammatory chemokines are implicated in other physiological processes such as angiogenesis (Gerber, Hippe, Buhren, Muller, & Homey, 2009).
Chemokine receptors display a range of functionality which includes regulation of chemotaxis, control of extracellular ligand levels via ligand internalization (Cardona et al., 2008), and roles in proliferation of non-malignant cells (Binder et al., 2009; Meng et al., 2013; Xia, Entman, & Wang, 2013). Responding to pathogen infection, tissue damage and/or the presence of malignant cells, their role in a healthy inflammatory immune response is well characterized (Raman, Sobolik-Delmaire, & Richmond, 2011). The varied functions of the chemokines and chemokine receptors highlight the wide impacts these systems have on the progression, and potentially treatment, of many diseases. Herein, we focus on the role of chemokine/chemokine receptor systems on cancer progression, emphasizing critical activities in controlling the presence of immune cells within the tumor microenvironment. In addition, we highlight pharmacologic approaches under consideration that target chemokine receptors in order to slow cancer progression, specifically focusing on opportunities in malignant brain tumors.
1.2. Chemokine/chemokine receptors in cancer progression and treatment resistance
Chemokines can impact tumor progression through a variety of mechanisms that include direct effects on cancer cell proliferation and indirectly via regulation of angiogenesis and recruitment of immune cells that facilitate tumor growth and metastasis (Caronni et al., 2016; Chow & Luster, 2014; Mantovani et al., 2010). Binding and subsequent activation of chemokine receptors expressed on tumor cells can directly promote proliferation through activation of downstream signaling pathways including PI3K/AKT/NF-kB and the MAPK/ERK pathway (Balkwill, 2004; Liang et al., 2018; Teicher & Fricker, 2010). Furthermore, chemokine stimulation of these receptors has been shown to prevent apoptosis in tumor cells via regulation of the balance of anti-apoptotic Bcl-2 and pro-apoptotic Bax and caspase-3 (Xu et al., 2012) thereby promoting tumor cell survival. A role for autocrine chemokine signaling in tumor progression has also been shown. In glioblastoma stem-like cells, CCL5/CCR5 and CXCL12/CXCR4 autocrine signaling increases cell survival and potentiates self-renewal activity (Gatti et al., 2013; Novak et al., 2020; Pan, Smithson, Ma, Hambardzumyan, & Gutmann, 2017). Other chemokine receptor axes help drive the cancer stem-like state (Kundu et al., 2019; Zou & Wicha, 2015), suggesting the importance of direct chemokine/chemokine receptor regulation in cancer development. Chemokines of the CC and CXC family play critical roles in promoting angiogenesis (Ridiandries, Tan, & Bursill, 2016; Santoni et al., 2014). Conversely, chemokines including CCL21, CXCL4, CXCL9, CXCL10, and CXCL11 have been shown to inhibit angiogenesis (Strieter, Burdick, Gomperts, Belperio, & Keane, 2005), further complicating our understanding of the precise function of chemokine systems in regulating angiogenesis. A role for the chemokines in promoting metastasis is not surprising as chemotaxis is a consequence of chemokine receptor signaling pathways. A common chemokine dependent pro-metastatic pathway relies on the expression of CXCR4 on tumor cells that responds to the CXCR4 ligand, CXCL12. CXCL12 is highly expressed in various organs, allowing for invasion of CXCR4+ tumor cells into these distant tissues from their site of origin (Chow & Luster, 2014; Darash-Yahana et al., 2004). Other chemokine receptor systems are known to play roles in the metastatic process. For instance, CCR7 expression on cancer cells mediates migration of tumor cells into lymph nodes in various cancer subtypes, principally through interaction with its ligands, CCL19 and CCL21, which are highly expressed in lymphatic tissue (Takanami, 2003; Zlotnik et al., 2011). As a result, metastasis of CCR7+ cancer cells into the lymphatic system is common, and can negatively impact treatment of these cancers. Taken together, use of the chemokine systems by cancer cells allows for tumor progression via increased angiogenesis, cancer cell proliferation and survival, and metastasis.
1.3. Chemokines and chemokine receptors regulate immune cell trafficking: focus on glioma
The accumulation and positioning of leukocytes is of critical immunological importance in both health and disease. Chemokines modulate immune surveillance mechanisms through the trafficking of leukocytes out of the bone marrow and into distant tissues (Hughes & Nibbs, 2018), as well as the recruitment of immune cells to tumors. By altering immune cell infiltration (Sciume, Santoni, & Bernardini, 2010), including recruitment of leukocyte subsets into immune-privileged environments such as the CNS, chemokine expression may influence tumor growth (Brown et al., 2007; Kitai et al., 2007; Leung, Wong, Chung, Chan, & Yuen, 1997; Nishimura et al., 2006). In glioma, the immune-suppressive microenvironment is largely driven by molecules expressed by malignant cells as well as immune cells which have gained access to the tumor. In glioma, the chief immune-suppressive cell types include tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and T regulatory (Treg) cells.
TAMs and MDSCs share many similar properties. The mechanism by which these immune suppressive cells traffic into tumors is not completely defined, although data support a role for chemokine receptors in this process (Chang et al., 2016). Indeed, many reports indicate Ly6Chi inflammatory monocytes and CD11b+,Ly6Chi, Ly6G− M-MDSCs express CCR2 (Feng et al., 2015). Furthermore, it has been established that the CCL2/CCR2 signaling axis plays a role in development and progression of various cancers, including highly refractory types such as glioblastoma (GBM) and pancreatic cancer (Monti et al., 2003). Further contributing to its part in cancer development, a role for CCR2 in mobilization of leukocytes from the bone marrow has been reported (Engel et al., 2008; Fujimura et al., 2015; Serbina & Pamer, 2006; Tsou et al., 2007) and the mechanism likely involves interactions with another chemokine receptor, CXCR4 (Jung, Mithal, Park, & Miller, 2015), though CCR2 mediated egress of cells from the bone marrow and influx into the tumors may be mediated by any of the ligands for CCR2. The two primary ligands for this receptor, CCL2 and CCL7 (Charo et al., 1994), have been shown to be expressed directly by gliomas as well as by surrounding tissues influenced by the tumor cells (Chang et al., 2016). As such, expression of CCR2 and its ligands play an integral role in development and progression of GBM by shaping the immunosuppressive TME via recruitment of suppressive cells from the periphery. In addition to CCL2, CCL7 has been shown to be integral in migration of CCR2+ monocytes out of the bone marrow (Tsou et al., 2007). Given the redundancy in ligands for CCR2, approaches targeting either CCL2 (MCP-1) or CCL7 (MCP-3) individually may not be fruitful (Lim, Yuzhalin, Gordon-Weeks, & Muschel, 2016), warranting direct targeting of CCR2. Results from our lab (Flores-Toro et al., 2020) and others (Chen et al., 2017) provide evidence showing myeloid-like cells gain access to the glioma microenvironment and can be distinguished by their expression of CCR2 as well as CX3CR1. Elevated CCL2/CCR2 expression in GBM patients indicates a more negative prognosis (Chang et al., 2016), with patients exhibiting low levels of CCL2 surviving longer than individuals expressing high levels of this chemokine. Extending from this, the most commonly recruited immune suppressive cell, CCR2+ MDSCs, are elevated in the periphery of these patients (Alban et al., 2018). CCR2 expression is also noted on numerous other immune cells, including monocytes, T-cells, immature B cells, NK cells, basophils, and dendritic cells (Yamasaki et al., 2012), with inflammation as a common driving factor (Connor et al., 2004; Mack et al., 2005; van Helden et al., 2012). These alternative CCR2-expressing cells are less studied in general and their roles in glioma progression are not clear.
Migration of Tregs to the tumor microenvironment is, in part, a result of expression of CCL17 and CCL22 acting on CCR4 (Gobert et al., 2009). Tregs have been shown in multiple models to express CCR4 (Curiel et al., 2004; Jacobs et al., 2010; Miller et al., 2006). This is relevant in GBM, where approximately 74% of Tregs isolated from GBM patient peripheral blood express CCR4, which is significantly higher than the 43% of these cells from healthy individuals that express this receptor (Jordan et al., 2008). Additionally, recruitment of CCR4+ Tregs has been shown to promote tumor growth and proliferation (Gobert et al., 2009). Taken together, the CCR4, CCL22, and CCL17 chemokine axis in glioma may offer a novel therapeutic target in GBM. However, determining the physiological significance of inhibiting this axis, particularly in pre-clinical models, has been limited.
Given the role chemokines play in GBM, it is no surprise there is great interest in understanding the composition of the GBM tumor micro environment (TME), and how the chemokine/chemokine receptor axis may be harnessed in new therapeutic approaches for GBM patients.
2. Glioma immune microenvironment
Considered an immune-privileged compartment, infiltration of peripheral immune cells into brain parenchyma is tightly regulated by the blood/brain barrier (BBB). Under non-pathological conditions, microglia (resident macrophage-like cells arising from embryonic progenitor cells) represent the major functional immune cell present within this tissue (Graeber, Scheithauer, & Kreutzberg, 2002). However, tumor development increases permeability of the BBB resulting in tumor infiltration of macrophages and, to a lesser extent, lymphocytes. Recruitment and activation of immune-suppressive cells is owed to the enhanced secretion of pro-inflammatory cytokines noted in GBM patients, including interleukin-10 (IL-10) (Huettner, Czub, Kerkau, Roggendorf, & Tonn, 1997), transforming growth factor β (TGFβ) (Maxwell, Galanopoulos, Neville-Golden, & Antoniades, 1992), and CCL2 (Chang et al., 2016). These recruited peripheral immune cells have substantial impact on tumor progression and response to antitumor therapy that may limit the efficacy of established immune based therapies in glioma patients.
2.1. Glioma infiltrating immune cells: Suppressive and effector cells
Chemokines have both pro- and anti-tumor effects within the glioma microenvironment (Fig. 1). Infiltrating CD8+ T cells, interferon-γ (IFNγ)-expressing T helper 1 (TH1) cells, natural killer (NK) cells, and B cells have anti-tumor immune properties (Nagarsheth et al., 2017; Vilgelm & Richmond, 2019). These infiltrating immune cells are recruited to the TME through a variety of chemokine/chemokine receptor interactions. CD8+ T cells drive anti-tumor immunity through secretion of cytotoxic molecules, namely granzyme B and perforin, and induce apoptosis mediated cell death in tumor cells (van der Leun, Thommen, & Schumacher, 2020). CD8+ T cells, IFNγ-expressing TH1 cells, and NK cells are recruited by CXC subfamily chemokines including CXCL9, CXCL10, CXCL11 that activate CXCR3 (Nagarsheth et al., 2017). While tumor-infiltrating B cells are less well studied and do not have clear anti-tumor effects, high levels of tumor-infiltrating B cells are associated with survival advantages in other forms of cancer (Milne et al., 2009; Nedergaard, Ladekarl, Nyengaard, & Nielsen, 2008; Schmidt et al., 2008). B cells are classically recruited to the TME through the CXCR5/CXCL13 chemokine axis (Henneken, Dorner, Burmester, & Berek, 2005), and are found in low numbers in glioma (Gieryng, Pszczolkowska, Walentynowicz, Rajan, & Kaminska, 2017). Natural killer (NK) cells are important effectors of anti-tumor responses. These cells are able to regulate adaptive immune effects through cytokine and chemokine release. NK cells are classically defined by the expression of CD56 and the lack of CD3-TCR complex. Additional NK cell subpopulations are elucidated by their receptor repertoire and functionality (Harmon et al., 2016; Lugthart et al., 2016; Stegmann et al., 2016). Describing precise NK subsets in glioma is limited, but CD56highperforinlow NK subsets have been identified in some solids tumors. Accumulation of these perturbed cytotoxic NK cells were attributed to neoplastic tissue downregulating CXCL12, which attracts CD56low NK cells, and upregulation of CXCL9, CXCL10 – attracting CD56high NK cells (Carrega et al., 2008; Carrega et al., 2014). Furthermore, CX3CL1 produced by neurons mediate CX3CR1+, IFN-gamma expressing NK cell recruitment in glioma and is associated with a favorable prognosis (Ren et al., 2019).
Fig. 1.
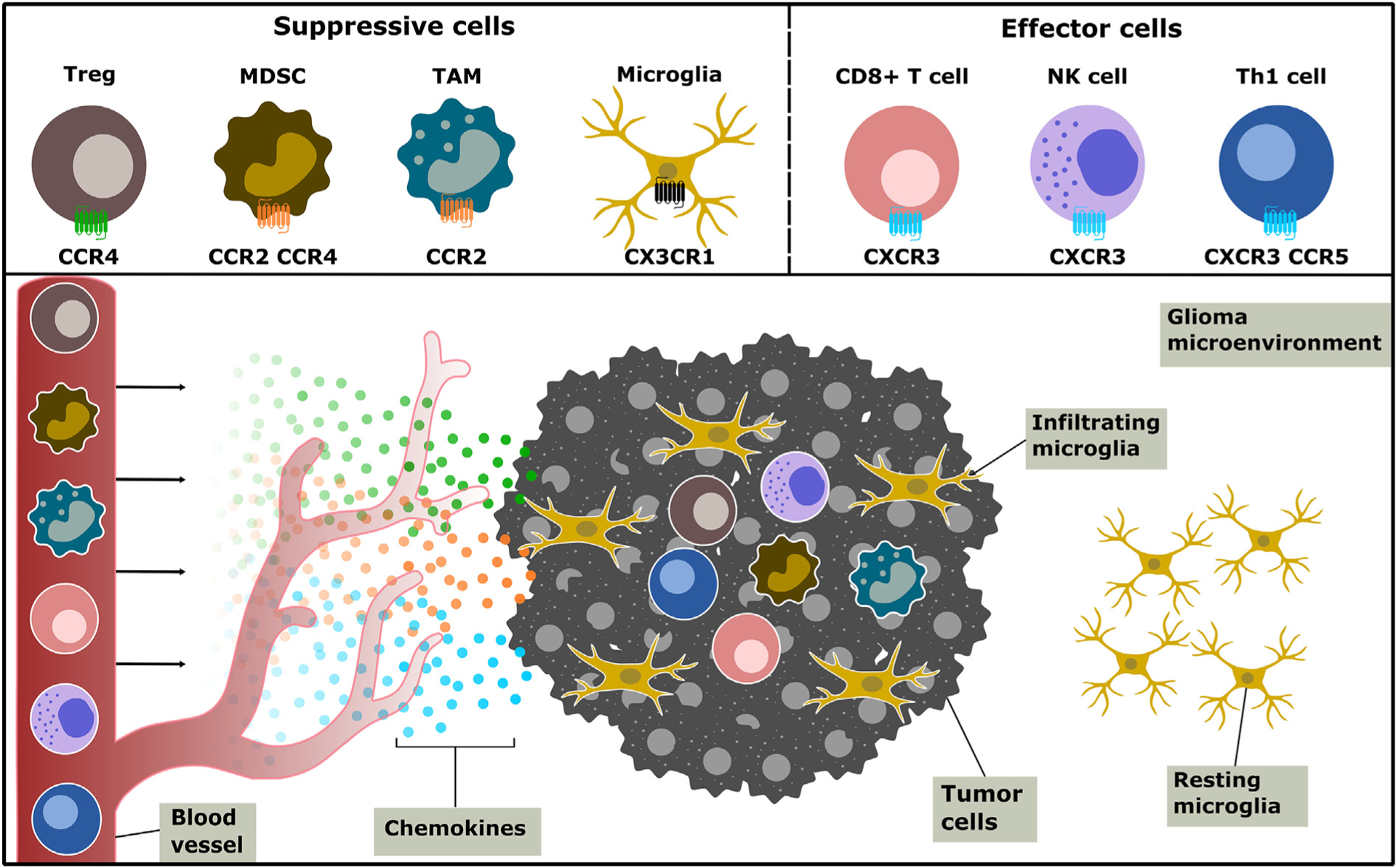
Chemokines shape the glioma immune landscape by recruiting chemokine receptor-expressing immune cells to the glioma microenvironment. Pro-tumor immune-suppressive cells Tregs, MDSCs, and TAMs are honed from the periphery to the site of the tumor through a variety of chemokine receptors, such as CCR2 and CCR4. Anti-tumor immune cells, including CD8+ and Th1 T cells and NK cells, infiltrate the tumor microenvironment and illicit tumor-killing mechanisms. These effector cells express the chemokine receptor CXCR3. CX3CR1-expressing microglia, a CNS resident macrophage, make up the bulk of the glioma infiltrating immune cells and may exhibit both pro- and anti-tumor effects.
During late tumor progression, chemokines often attract tumor-promoting leukocytes such as macrophages, MDSCs, and Tregs (Vilgelm & Richmond, 2019). Additionally, numbers of TAMs are associated with poor patient prognosis in some cancers (Pollard, 2004). CCL2 is a key determinant in the recruitment of TAMs in multiple malignancies including gliomas (Arenberg et al., 2000; Leung et al., 1997; Ueno et al., 2000). MDSCs represent a heterogenous population of myeloid cells that confer immunosuppressive properties in the TME (Veglia, Perego, & Gabrilovich, 2018). MDSCs of the granulocytic lineage are recruited by CXCL8 (Alfaro et al., 2016), while monocytic derived MDSCs are recruited by CCL2, CXCL5, and CXCL12 (Kitamura et al., 2015; Yang et al., 2008). CCL2 is largely responsible for glioma associated monocytic MDSC recruitment. Tregs are also responsible for promoting tumorigenesis, and are recruited to the tumor via expression of CCL22 by macrophages and tumor cells (Curiel et al., 2004). Although chemokines are mainly responsible for recruiting leukocytes to the tumor, pro-tumor effects of chemokines are extensive and include inhibition of antitumor immune responses, promotion of cancer stemness, and increased angiogenesis (Nagarsheth et al., 2017). As described in this section, multiple immune cell types comprise the TME of gliomas and contribute to the immunosuppressive nature of this environment by equally diverse means.
2.2. Astrocytes secrete glioma modulating growth factors
The highly immune-suppressed TME classically observed in gliomas substantially limits the efficacy of established immunotherapy approaches, as is reflected in clinical trial outcomes. Numerous factors contribute to the immune suppressed TME, primarily driven by infiltrating cells and various environmental factors combining, often synergistically, to produce the characteristic GBM TME. Among the variety of cells impacting the glioma microenvironment, astrocytes are typically present and capable of manipulating GBM behavior. Tumor-associated astrocytes often potentiate glioblastoma growth, invasion, and proliferation through the secretion of growth factors, cytokines, chemokines, and other soluble factors (Mega et al., 2020; Wang, Pan, Che, Cui, & Li, 2010; Zhang, Zhou, Cui, Liu, & Shen, 2020). Notably, astrocyte-derived chemokine CCL20 stimulates the CCR6-NF-kB signaling pathway and mediates HIF-1α expression to further enhance GBM proliferation (Jin et al., 2018).
2.3. Role of tumor-associated myeloid cells: Macrophages, MDSCs, microglia
TAMs make up the bulk of the tumor immune cell infiltrate and are a hallmark of high grade gliomas (Badie & Schartner, 2000; Roggendorf, Strupp, & Paulus, 1996). Infiltration of these cells is known to influence disease progression, with both resident microglia and bone marrow-derived infiltrating monocytes potentially exerting tumor growth promoting activity (Chang et al., 2016; Chen et al., 2017; Feng et al., 2015; Hutter et al., 2019; Liu, Luo, Streit, & Harrison, 2008; Raychaudhuri et al., 2015; Zhang et al., 2012). Approaches that specifically target TAM and MDSC populations have been employed in various tumor types and shown to enhance immunogenicity, even reversing resistance to checkpoint blockade in some instances (Flores-Toro et al., 2020; Holmgaard, Zamarin, Lesokhin, Merghoub, & Wolchok, 2016; Srivastava et al., 2012; Stromnes et al., 2014; Vincent et al., 2010; Yu et al., 2013).
MDSCs have potential for wide-ranging impacts on T-cell activation and proliferation (Gabrilovich & Nagaraj, 2009). These effects are exerted via an array of mechanisms including arginase 1 (ARG1) and inducible nitric oxide synthase (iNOS) expression (Kusmartsev, Li, & Chen, 2000; Mills, Shearer, Evans, & Caldwell, 1992), reactive oxygen species (ROS) production (Corzo et al., 2009; Huang et al., 2013), and recruitment of Tregs (Huang et al., 2006). As a hallmark of MDSC suppressive function, ARG1-dependent depletion of L-arginine has a profound negative effect on cytotoxic lymphocyte (CTL) function (Bronte et al., 2003). Reduced tryptophan levels, as well as presence of kynurenine catabolites, impede CTL function and promote T-cell anergy (Fallarino et al., 2002; Fallarino et al., 2003). Glioma cell expression of indoleamine-2,3-dioxygenase (IDO) further shapes the nutrient depleted TME as tryptophan catabolism drives production of kynurenine (Miyazaki et al., 2009). Tryptophan catabolism in tumor-infiltrating cells also contributes to the overall suppressive phenotypes in cancer. Upregulation of the rate-limiting enzyme, IDO, in tryptophan catabolism has been observed in tumor associated MDSCs which further impairs cytotoxic T cell responses and survival (Parker, Beury, & Ostrand-Rosenberg, 2015; Schafer et al., 2016; Yu et al., 2013). A study using a mixed brain cell culture model also confirmed astrocytes, microglia, and neurons express IDO (Guillemin, Smythe, Takikawa, & Brew, 2005). Additionally, the biologically active kynurenine metabolite, quinolinic acid (QA) (Wirthgen, Hoeflich, Rebl, & Gunther, 2017), accumulates in glioma cells (Opitz et al., 2011), which are unable to generate QA from kynurenine. However, microglia are able to produce detectable amounts of QA (Guillemin et al., 2005), further supporting the notion that infiltrating immune cells contribute to tryptophan mediated immunosuppression.
Production of nitric oxide due to enhanced expression of iNOS (Mazzoni et al., 2002) alters the oxidative state of the TME, further impairing T-cell function. In addition to increased ROS production, the hypoxic TME described above induces expression of PD-L1 on TAMs (Noman et al., 2014), directly impeding proper CTL function upon binding to its cognate receptor, PD-1. Furthermore, studies have suggested that infiltration of MDSCs into the GBM microenvironment is associated with a reduction in infiltrating lymphocytes, specifically preventing the entry of CD8+ T-cells into the tumor (Molon et al., 2011; Raychaudhuri et al., 2015), and thus acting to affect the TIL population as well as function. Extending from these findings, attempts to therapeutically target the TAM population in cancer are ongoing, with promising results observed in certain cancer subtypes. The absence of TAM populations appears to markedly decrease breast and endometrial carcinoma growth rates (Lewis & Pollard, 2006; Lin, Nguyen, Russell, & Pollard, 2001) and infiltration of TAMs appears to increase in the response of prostate cancer to treatments such as radiation (Escamilla et al., 2015), chemotherapy (Xu et al., 2013), and immunotherapies in GBM (Stafford et al., 2016). Taken together, MDSCs are known to potentiate immune-suppression in GBM and, therefore, may contribute to the failure of immune based therapies in GBM (Chae et al., 2015; Chang et al., 2016; Gielen et al., 2015; Kohanbash et al., 2013; Prosniak et al., 2013; Raychaudhuri et al., 2011; Raychaudhuri et al., 2015).
CX3CR1 is a distinct chemokine receptor with a single ligand, CX3CL1 (also known as fractalkine). In healthy brain tissue, CX3CR1+ microglia are the most prominent immune cell. CX3CR1+ cells are abundant within murine GL261 gliomas, and previous results have determined CX3CR1 deficiency has no impact on the presence of these cells within tumors; a slight reduction in median survival time was seen in GL261 tumor bearing CX3CR1-deficent mice (Liu et al., 2008). Deletion of CX3CR1 leads to reduced survival in a PDGFB-driven proneural glioma model (Feng et al., 2015). In addition, CX3CR1 depletion led to increased numbers of Ly6Chi inflammatory monocytes in these tumors, a myeloid population known to express CCR2 (Feng et al., 2015). These results suggest that myeloid-like cells, which are present within gliomas, have distinct and potentially opposing roles in tumor progression, and therefore may present intriguing candidates for novel diagnostic assays.
Expression profile analysis shows glioma-associated microglia/macrophages polarize toward a phenotype partially overlapping with the M1 or M2a, M2b, and M2c phenotypes, but display a unique repertoire of chemokine/receptor pairs: CX3CL1/CX3CR1, CXCL16/CXCR6 and CXCL12/CXCR4/CXCR7 (Hattermann et al., 2014; Szulzewsky et al., 2015). This data is notable as these chemokine/chemokine receptor pairs are frequently involved in tumor progression (Hattermann, Held-Feindt, Ludwig, & Mentlein, 2013; Hattermann, Ludwig, Gieselmann, Held-Feindt, & Mentlein, 2008; Hattermann & Mentlein, 2013; Held-Feindt et al., 2010). For instance, glioma-associated macrophages are a chief source of pro-tumorigenic proteins such as GPNMB and SPP1 (Szulzewsky et al., 2015).
3. Chemokines and chemokine receptors as biomarkers
3.1. Peripheral monitoring of chemokines as a prognostic marker
Normal human monocytes exposed to glioma cells have been shown to adopt an immunosuppressive phenotype (Rodrigues et al., 2010). As mentioned above, macrophages, dendritic cells and T cell immunity are linked through the macrophage-derived CCL22 signaling axis (Mantovani, Gray, Van, & Sozzani, 2000). CCL22 is a CC subfamily chemokine that is a potent chemoattractant for CD4 and CD8 T cells and dendritic cells expressing the CCL22 receptor CCR4. Peripheral levels of CCL22 have been identified as a marker for macrophages and dendritic cells of immunosuppression, glioma risk, and survival duration. In a recent study, Zhou et al. assessed 1208 glioma cases and 976 controls for peripheral levels of CCL22. CCL22 levels were significantly lower among glioma cases compared to controls while higher CCL22 levels were associated with longer survival in all cases, including GBM. These data suggest peripheral levels of CCL22 may be used as a marker of immune status with potential prognostic value (Zhou et al., 2015). This notion is bolstered as data suggest relationships between peripheral chemokine levels and prognosis extend beyond gliomas (Chen et al., 2012; Panse et al., 2008; Thomas, Mir, Kapur, Bae, & Singh, 2019; Tsukinaga et al., 2015), and may be predictive of immunotherapy success across cancer types (Koguchi et al., 2015; Sanmamed et al., 2017).
3.2. Tumor-associated chemokine receptor expression predicts prognosis
Expression levels of chemokine receptors at the site of the tumor may have prognostic value in gliomas. CXCR7 is highly transcribed in glioma cell lines and mediates anti-apoptotic effects upon activation (Hattermann et al., 2010). Further studies show high expression of CXCR7 is correlated with poor overall survival in GBM patients independent of factors such as age, Karnofsky performance scores, chemotherapy, IDH1 mutation, MGMT methylation status, and tumor size (Deng et al., 2017).
The chemokine receptor CXCR3 has three endogenous ligands, CXCL9, CXCL10, and CXCL11, and has been detected in many malignancies, although the functional role of this system in cancer is unclear. In gliomas, CXCR3 can promote growth and migration of malignant cells (Honeth, Staflin, Kalliomaki, Lindvall, & Kjellman, 2006; Liu et al., 2011). Additionally, CXCR3 and its ligands are highly expressed in glioma samples and primary patient-derived cell lines. CXCR3 has also been shown to be a prognostic marker for primary GBM. A study demonstrated high expression of CXCR3 confers poor overall survival in GBM patients (Pu et al., 2015).
Extending from the potential use of chemokines/chemokine receptors as diagnostic tools, the tight association of many of these ligand/receptor pairs with cancer progression, and their highly druggable nature, make them attractive targets for development of novel monotherapies, or as part of a combinatorial approach.
4. Therapeutic approaches targeting the chemokine/chemokine receptor axis
4.1. Direct targeting of the chemokine/chemokine receptor axis
Monotherapies directed at blocking the chemokine/chemokine receptor axis have been attempted but are extremely limited in the treatment of gliomas. The use of monoclonal antibodies to block CCR4 signaling has shown promise in renal cancer. Affi 5, a CCR4 blocking mAb, reduced tumor growth and increased the infiltration of NK cells in a preclinical model of renal cancer (Berlato et al., 2017). Further use of CCR4 mAb have also been applied in adult T-cell lymphoma, with the recent approval of a fully humanized, defucosylated IgG1 antibody (Mogamulizumab) in Japan (Ollila, Sahin, & Olszewski, 2019). However, there has been growing concerns over safety of using mAbs to target the chemokine/chemokine receptor axis in patients that have undergone allogenic bone marrow transplant. Mogamulizumab was shown to deplete regulatory T cells, which may increase the risk of graft vs host disease (Fuji & Shindo, 2016).
Small-molecule antagonists have also been developed to block the chemokine/chemokine receptor axis. In a murine model of multiple myeloma, CCX721 (a CCR1 antagonist) reduced tumor growth and osteolysis, targeting osteoclasts and their precursors (Dairaghi et al., 2012; Vallet et al., 2007). These results were replicated through the blocking of the CCR1 ligand, CCL3 (Oyajobi et al., 2003). AMD3465, a highly selective CXCR4 antagonist has been shown to reduce the growth of GBM and medulloblastoma cell line xenografts. This anti-tumor activity is associated with the blocking of cAMP suppression (Yang et al., 2007). Furthermore, a new class of CXCR4-antagonist cyclic peptide (Peptide R) impaired the metabolic activity, proliferation, and migration of U87MG cells in vitro and reduced tumor volume in an orthotopic U87MG glioma mouse model (Mercurio et al., 2016). Targeting CXCR4 is not limited to monotherapy approaches (Gravina et al., 2017; Liu & Yang, 2020; Redjal, Chan, Segal, & Kung, 2006).
CCR7 mediates cancer cell dissemination, migration, and metastasis, making this chemokine receptor axis a promising target (Legler, Uetz-von, & Hauser, 2014). CCR7 inhibition, using siRNA technology, decreased the number of metastasis in a colon carcinoma xenograft model (Yu et al., 2008). Another study showed that silencing CCR7 resulted in a decrease of cell proliferation, migration, and invasion of prostate cancer in vitro and in vivo (Chi, Du, Fu, Zhang, & Wang, 2015).
4.2. Immune based approaches in combination with chemokine/chemokine receptor axis targeting
Much progress has been made over the last decade in the development and effective application of various immune-based treatment modalities for cancer. However, despite the known contribution of an immune suppressive TME to the progression of many cancers, much of which may rely on the chemokine/chemokine receptor axis, use of single treatment immune therapy has failed to show efficacy for all patients. While the exact cause of this discrepancy is unknown, a common thread among the cancer subtypes least benefitted by immune based approaches is low mutational burden (Alexandrov et al., 2013; Lawrence et al., 2013). These cancers poorly respond to monotherapies, though coupling of existing immunotherapies with novel antagonists directed against chemokine receptors may be a potential method to circumvent immune suppression and gain efficacy in otherwise resistant cancers.
A recent study examined such an approach in pre-clinical models of GBM, utilizing checkpoint inhibition via monoclonal antibody targeting of PD-1 in combination with a small molecule antagonist targeting CCR2 (Flores-Toro et al., 2020). An anti-PD-1 survival benefit was unmasked in KR158 glioma-bearing CCR2-deficient mice. MDSCs within these established gliomas decreased with a concomitant increase in overall CCR2+ cells and CD11b+/Ly6Chi MDSCs within bone marrow of CCR2-deficient mice. The CCR2 antagonist, CCX872, increased median survival as a monotherapy in KR158 glioma-bearing animals, and further increased median and overall survival when combined with anti-PD-1. However, combination of CCX872 and anti-PD-1 demonstrated the most promising survival advantage, prolonging median survival time in 005 GSC GBM-bearing mice which had not shown a monotherapy response with either CCX872 or anti-PD-1. In both models, CCX872 decreased tumor associated MDSCs and increased these cells within the bone marrow. Examination of tumor-infiltrating lymphocytes (TILs) revealed both an increase in population, as well as decreased expression of exhaustion markers in CD4+ and CD8+ T-cells following combination treatment. A survival benefit of combining anti-CXCR4 and anti-PD-1 immunotherapy in the GL261 glioma model has also been reported (Wu et al., 2019). Animals that received the combination therapy showed decrease populations of tumor-infiltrating immunosuppressive cells and improved CD4+/CD8+ ratios. These data establish that combining chemokine receptor blockers and PD-1 blockade can extend survival in clinically relevant murine glioma models and serves as a proof of concept for this type of treatment approach in other immune therapy resistant cancer subtypes.
4.3. Targeting of the chemokine/chemokine receptor axis in other malignancies
While the previous result shows a positive proof-of-concept, the use of chemokine/chemokine receptor targeting drugs in combination with immunotherapies has been attempted in various other cancers. The small molecule antagonist CCX9588, which targets CCR1, was tested for efficacy in a murine model of breast cancer in combination with anti-PD-L1. In this study, a synergistic effect of the combination therapy was demonstrated, with an associated reduction in infiltrating myeloid cells (Jung et al., 2015). Preclinical results investigating the CCR2 inhibitor, CCX872, in combination with anti-PD-1 treatment in pancreatic cancer were reported at the 2016 AACR meeting (Abstract A107, Jung et al.) and showed reduced myeloid cell infiltration in murine models of pancreatic cancer as a consequence to CCX872 treatment. In similar models of pancreatic cancer, targeting of CXCR2 via genetic ablation was shown to improve response to anti-PD-1 administration, potentially through inhibition of metastasis (Steele et al., 2016).
Use of CCR2 targeting molecules in mouse models of ovarian cancer showed CCR2 blockade was able to augment the efficacy of peptide vaccines, and remodel the immune suppressive TME noted in these tumors (Binder et al., 2017). As peptide vaccines have not shown clinical efficacy, use of CCR2 inhibition in combination may be a viable alternative in ovarian cancer patients. Additionally, in models of ovarian cancer, inhibition of CXCR2 using SB225002 was shown to improve efficacy of Sorafenib (Devapatla, Sharma, & Woo, 2015), while another CXCR2 antagonist, Navarixin, was able to show benefit in similar models by synergizing with MAPK signaling inhibition (Young et al., 2017).
5. Targeting of the chemokine/chemokine receptor axis in clinical trials
Limited use of chemokine/chemokine receptor antagonists as a monotherapy, or in combination with immunotherapy, has been attempted in clinical trials of cancer patients, with such approaches being particularly rare in GBM patients. While preclinical data suggest these approaches may be beneficial in GBM patients, to date these trials have primarily focused on other cancer types. Below is a discussion of these trials, highlighting those including GBM patients, as well as trials in other cancer subtypes where the chemokine/chemokine receptor antagonists have yet to be investigated in glioma patients.
5.1. Clinical trials of monotherapy approaches
To date, monotherapy based clinical trials directed at the chemokine/chemokine receptor axis are limited (cite https://clinicaltrials.gov/accessed10/21/2020). However, a Phase 2 clinical trial in GBM patients (NCT03746080) is currently recruiting to evaluate the efficacy of Plerixafor (CXCR4 antagonist) after maximal safe surgical resection and during standard of care. An ongoing trial, NCT02765165, is recruiting to evaluate a CXCR4 inhibitor, USL311, in subjects with advanced solid tumors (Phase 1) and relapsed/recurrent GBM (Phase 2). The study aims to explore the safety, tolerability, pharmacokinetics, and efficacy of USL311 as a single-agent and in combination with Lomustine chemotherapy. Furthermore, a Phase 3 clinical trial studying Mogamulizumab, a blocking CCR4 mAb, for the treatment of relapsed/refractory cutaneous T-cell lymphoma is still active. This 372-participant randomized trial aims to compare progression free survival of subjects who receive Mogamulizumab versus those who receive Vorinostat (NCT01728805).
5.2. Clinical trials of combination approaches
A phase Ib/II trail (NCT02732938) investigating the use of an orally delivered CCR2 antagonist, PF-04136309 (Cullis et al., 2017), in combination with Abraxane (a nanoparticle bound form of Paclitaxel) and Gemcitabine for treatment of pancreatic cancer began recruiting 2017. Unfortunately, this study found a concerning safety profile and clinical data were unable to show a benefit of the combination therapy in pancreatic cancer patients (Noel et al., 2020). As this approach modality has recently gained traction, numerous studies are currently recruiting. Among those are trials for breast cancer (NCT03599453: chemokine modulation combined with Pembrolizumab), colorectal cancer (NCT03403634: chemokine modulation combined with Celecoxib), and B cell lymphoma (NCT03929107: IL-7 and CCL19 expressing chimeric antigen receptor T-cell (CAR-T) cell therapy).
While use of chemokine receptor antagonists in combination with immune-based approaches has been limited, coupling these agents with chemotherapies has been more common. In pancreatic cancer, a Phase I clinical trial was conducted to investigate the efficacy of dual treatment with PF-04136309 and standard FOLFIRINOX (Fluorouracil, Leucovorin Calcium, Irinotecan Hydrochloride, and Oxaliplatin) treatment (NCT01413022). Final results concluded the combination therapy was safe and tolerable by patients, with minimal impact on primary endpoints (Nywening et al., 2016). Approaches using chemotherapeutics and chemokine receptor antagonism has been tested in clinical trials for the treatment of breast cancer as well. A phase I trial (NCT01837095) using combination of Eribulin and Balixafortide (CXCR4 antagonist) was conducted in HER2-negative metastatic breast cancer patients (Pernas et al., 2018) and concluded with a positive safety profile. Further studies in breast cancer include a Phase Ib study (NCT02001974) determining the safety of combination Paclitaxel with Reparixin (CXCR1 and CXCR2 inhibitor). This study concluded that combination therapy was safe for use in patients, warranting further investigation (Schott et al., 2017).
6. Conclusion
Decades of research has yielded a wealth of data in support of the role of the chemokine signaling axis in the development and progression of various cancers. While the mechanism(s) by which these effects are exerted on the tumor are most likely related to modulation of immune responses, growing evidence, such as those discussed above, point to additional contributions, including promotion of cancer cell stemness and angiogenesis. Given the varied roles of chemokines in the progression of cancer, these cytokines present as attractive candidates for targeted therapies, with great potential of such therapies having wide application. Taken together, it is reasonable to continue investigation of chemokines and chemokine receptors as novel therapeutic targets, particularly in otherwise refractory cancers such as GBM.
Acknowledgements
J.K.H: National Institutes of Health/NINDS R01: NS108781 Florida Center for Brain Tumor Research (FCBTR).
Abbreviations:
- ARG1
Arginase 1
- BBB
Blood-brain barrier
- C
Cysteine
- CAR-T
Chimeric antigen receptor T-cell
- CCL2/MCP1
Chemokine ligand 2
- CCL7/MCP3
Chemokine ligand 7
- CCR2
Chemokine receptor 2
- CNS
Central nervous system
- CTL
Cytotoxic lymphocytes
- CTLA-4
Cytotoxic T-lymphocyte-associated protein-4
- CXCL8
Interleukin-8
- DC
Dendritic cells
- GBM
Glioblastoma
- GPCR
G protein coupled receptor
- HIF-1α
Hypoxia inducible factor-1α
- IDH
Isocitrate dehydrogenase
- IDO
Indoleamine-2,3-dioxygenase
- IFN-γ
Interferon-γ
- IL-10
Interleukin-10
- iNOS
Induced nitric oxide synthase
- MDSC
Myeloid derived suppressor cell
- MGMT
O6-methylguanine-DNA methyltransferase
- NK
Natural killer
- PD-1
Programmed death receptor 1
- PD-L1
Programmed death ligand 1
- ROS
Reactive oxygen species
- TAM
Tumor associated macrophage
- TGFβ
Transforming growth factor β
- TH1
T helper 1
- TILs
Tumor infiltrating lymphocytes
- TME
Tumor microenvironment
- Treg
T regulatory cell
Footnotes
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Reference
- Alban TJ, Alvarado AG, Sorensen MD, Bayik D, Volovetz J, Serbinowski E, … Lathia JD (2018). Global immune fingerprinting in glioblastoma patient peripheral blood reveals immune-suppression signatures associated with prognosis. JCI. Insight 3, e122264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, … Stratton MR (2013). Signatures of mutational processes in human cancer. Nature 500, 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP, … Melero I (2016). Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clinical Cancer Research 22, 3924–3936. [DOI] [PubMed] [Google Scholar]
- Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Strom SR, Burdick MD, … Strieter RM (2000). Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunology, Immunotherapy 49, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badie B, & Schartner JM (2000). Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery 46, 957–961. [DOI] [PubMed] [Google Scholar]
- Balkwill F (2004). Cancer and the chemokine network. Nature Reviews. Cancer 4, 540–550. [DOI] [PubMed] [Google Scholar]
- Berlato C, Khan MN, Schioppa T, Thompson R, Maniati E, Montfort A, … Balkwill FR (2017). A CCR4 antagonist reverses the tumor-promoting microenvironment of renal cancer. The Journal of Clinical Investigation 127, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder NB, Niederreiter B, Hoffmann O, Stange R, Pap T, Stulnig TM, … Redlich K (2009). Estrogen-dependent and C-C chemokine receptor-2-dependent pathways determine osteoclast behavior in osteoporosis. Nature Medicine 15, 417–424. [DOI] [PubMed] [Google Scholar]
- Binder PS, Cullinan D, Nywening T, Wilkinson-Ryan I, Belt B, Goedegebuure P, … Hawkins W (2017). CCR2 blockade alters the tumor microenvironment immune infiltrate and enhances anti-tumor activity in ovarian cancer. Gynecologic Oncology 145, 36. [Google Scholar]
- Bronte V, Serafini P, De SC, Marigo I, Tosello V, Mazzoni A, … Zanovello P (2003). IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. Journal of Immunology 170, 270–278. [DOI] [PubMed] [Google Scholar]
- Brown CE, Vishwanath RP, Aguilar B, Starr R, Najbauer J, Aboody KS, & Jensen MC (2007). Tumor-derived chemokine. MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. Journal of Immunology 179, 3332–3341. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, … Ransohoff RM (2008). Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood 112, 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona SM, Garcia JA, & Cardona AE (2013). The fine balance of chemokines during disease: trafficking, inflammation, and homeostasis. Methods Biol 1013, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caronni N, Savino B, Recordati C, Villa A, Locati M, & Bonecchi R (2016). Cancer and Chemokines. Methods Biol 1393, 87–96. [DOI] [PubMed] [Google Scholar]
- Carrega P, Bonaccorsi I, Di Carlo E, Morandi B, Paul P, Rizzello V, … Ferlazzo G (2014). CD56(bright)perforin(low) noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. Journal of Immunology 192, 3805–3815. [DOI] [PubMed] [Google Scholar]
- Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, … Ferlazzo G (2008). Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer 112, 863–875. [DOI] [PubMed] [Google Scholar]
- Chae M, Peterson TE, Balgeman A, Chen S, Zhang L, Renner DN, … Parney IF (2015). Increasing glioma-associated monocytes leads to increased intratumoral and systemic myeloid-derived suppressor cells in a murine model. Neuro-Oncology 17, 978–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, … Lesniak MS (2016). CCL2 Produced by the Glioma Microenvironment Is Essential for the Recruitment of Regulatory T Cells and Myeloid-Derived Suppressor Cells. Cancer Research 76, 5671–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, & Coughlin SR (1994). Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proceedings of the National Academy of Sciences of the United States of America 91, 2752–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, & Ransohoff RM (2006). The many roles of chemokines and chemokine receptors in inflammation. The New England Journal of Medicine 354, 610–621. [DOI] [PubMed] [Google Scholar]
- Chen K, Bao Z, Tang P, Gong W, Yoshimura T, & Wang JM (2018). Chemokines in homeostasis and diseases. Cellular & Molecular Immunology 15, 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Shi M, Yu GZ, Qin XR, Jin G, Chen P, & Zhu MH (2012). Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World Journal of Gastroenterology 18, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Feng X, Herting CJ, Garcia VA, Nie K, Pong WW, … Hambardzumyan D (2017). Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Research 77, 2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BJ, Du CL, Fu YF, Zhang YN, & Wang RW (2015). Silencing of CCR7 inhibits the growth, invasion and migration of prostate cancer cells induced by VEGFC. International Journal of Clinical and Experimental Pathology 8, 12533–12540. [PMC free article] [PubMed] [Google Scholar]
- Chow MT, & Luster AD (2014). Chemokines in cancer. Cancer Immunology Research 2, 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor SJ, Paraskevopoulos N, Newman R, Cuan N, Hampartzoumian T, Lloyd AR, & Grimm MC (2004). CCR2 expressing CD4+ T lymphocytes are preferentially recruited to the ileum in Crohn’s disease. Gut 53, 1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, … Gabrilovich DI (2009). Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. Journal of Immunology 182, 5693–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis J, Siolas D, Avanzi A, Barui S, Maitra A, & Bar-Sagi D (2017). Macropinocytosis of Nab-paclitaxel Drives Macrophage Activation in Pancreatic Cancer. Cancer Immunology Research 5, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, … Zou W (2004). Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine 10, 942–949. [DOI] [PubMed] [Google Scholar]
- Dairaghi DJ, Oyajobi BO, Gupta A, McCluskey B, Miao S, Powers JP, … Jaen JC (2012). CCR1 blockade reduces tumor burden and osteolysis in vivo in a mouse model of myeloma bone disease. Blood 120, 1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, … Peled A (2004). Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. The FASEB Journal 18, 1240–1242. [DOI] [PubMed] [Google Scholar]
- Deng L, Zheng W, Dong X, Liu J, Zhu C, Lu D, … Deng D (2017). Chemokine receptor CXCR7 is an independent prognostic biomarker in glioblastoma. Cancer Biomarkers 20, 1–6. [DOI] [PubMed] [Google Scholar]
- Devapatla B, Sharma A, & Woo S (2015). CXCR2 Inhibition Combined with Sorafenib Improved Antitumor and Antiangiogenic Response in Preclinical Models of Ovarian Cancer. PLoS One 10, e0139237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel DR, Maurer J, Tittel AP, Weisheit C, Cavlar T, Schumak B, … Kurts C (2008). CCR2 mediates homeostatic and inflammatory release of Gr1(high) monocytes from the bone marrow, but is dispensable for bladder infiltration in bacterial urinary tract infection. Journal of Immunology 181, 5579–5586. [DOI] [PubMed] [Google Scholar]
- Escamilla J, Schokrpur S, Liu C, Priceman SJ, Moughon D, Jiang Z, … Wu L (2015). CSF1 receptor targeting in prostate cancer reverses macrophage-mediated resistance to androgen blockade therapy. Cancer Research 75, 950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, … Puccetti P (2002). T cell apoptosis by tryptophan catabolism. Cell Death and Differentiation 9, 1069–1077. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Vacca C, Orabona C, Spreca A, Fioretti MC, & Puccetti P(2003). T cell apoptosis by kynurenines. Adv. Exp Med. Biol 527, 183–190. [DOI] [PubMed] [Google Scholar]
- Feng X, Szulzewsky F, Yerevanian A, Chen Z, Heinzmann D, Rasmussen RD, … Hambardzumyan D (2015). Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget 6, 15077–15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez EJ, & Lolis E (2002). Structure, function, and inhibition of chemokines. Annual Review of Pharmacology and Toxicology 42, 469–499. [DOI] [PubMed] [Google Scholar]
- Flores-Toro JA, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Charo IF, … Harrison JK (2020). CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proceedings of the National Academy of Sciences of the United States of America 117, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Wang CD, Robinson DA, Gocayne JD, & Venter JC (1989). Sitedirected mutagenesis of m1 muscarinic acetylcholine receptors: conserved aspartic acids play important roles in receptor function. Molecular Pharmacology 36, 840–847. [PubMed] [Google Scholar]
- Fuji S, & Shindo T (2016). Friend or foe? Mogamulizumab in allogeneic hematopoietic stem cell transplantation for adult T-cell leukemia/lymphoma. Stem Cell Investig 3, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura N, Xu B, Dalman J, Deng H, Aoyama K, & Dalman RL (2015). CCR2 inhibition sequesters multiple subsets of leukocytes in the bone marrow. Scientific Reports 5, 11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, & Nagaraj S (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews. Immunology 9, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti M, Pattarozzi A, Bajetto A, Wurth R, Daga A, Fiaschi P, … Barbieri F (2013). Inhibition of CXCL12/CXCR4 autocrine/paracrine loop reduces viability of human glioblastoma stem-like cells affecting self-renewal activity. Toxicology 314, 209–220. [DOI] [PubMed] [Google Scholar]
- Gerber PA, Hippe A, Buhren BA, Muller A, & Homey B (2009). Chemokines in tumor-associated angiogenesis. Biological Chemistry 390, 1213–1223. [DOI] [PubMed] [Google Scholar]
- Gielen PR, Schulte BM, Kers-Rebel ED, Verrijp K, Petersen-Baltussen HM, Ter LM, … Adema GJ (2015). Increase in both CD14-positive and CD15-positive myeloid-derived suppressor cell subpopulations in the blood of patients with glioma but pre-dominance of CD15-positive myeloid-derived suppressor cells in glioma tissue. Journal of Neuropathology and Experimental Neurology 74, 390–400. [DOI] [PubMed] [Google Scholar]
- Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, & Kaminska B (2017). Immune microenvironment of gliomas. Laboratory Investigation 97, 498–518. [DOI] [PubMed] [Google Scholar]
- Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, … Menetrier-Caux C (2009). Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Research 69, 2000–2009. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Scheithauer BW, & Kreutzberg GW (2002). Microglia in brain tumors. Glia 40, 252–259. [DOI] [PubMed] [Google Scholar]
- Gravina GL, Mancini A, Marampon F, Colapietro A, Delle Monache S, Sferra R, … Festuccia C (2017). The brain-penetrating CXCR4 antagonist, PRX177561, increases the antitumor effects of bevacizumab and sunitinib in preclinical models of human glioblastoma. Journal of Hematology & Oncology 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JW, Sokol CL, & Luster AD (2014). Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annual Review of Immunology 32, 659–702. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, & Brew BJ (2005). Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 49, 15–23. [DOI] [PubMed] [Google Scholar]
- Harmon C, Robinson MW, Fahey R, Whelan S, Houlihan DD, Geoghegan J, & O’Farrelly C (2016). Tissue-resident Eomes(hi) T-bet(lo) CD56(bright) NK cells with reduced proinflammatory potential are enriched in the adult human liver. European Journal of Immunology 46, 2111–2120. [DOI] [PubMed] [Google Scholar]
- Hattermann K, Held-Feindt J, Lucius R, Muerkoster SS, Penfold ME, Schall TJ, & Mentlein R (2010). The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Research 70, 3299–3308. [DOI] [PubMed] [Google Scholar]
- Hattermann K, Held-Feindt J, Ludwig A, & Mentlein R (2013). The CXCL16-CXCR6 chemokine axis in glial tumors. Journal of Neuroimmunology 260, 47–54. [DOI] [PubMed] [Google Scholar]
- Hattermann K, Ludwig A, Gieselmann V, Held-Feindt J, & Mentlein R (2008). The chemokine CXCL16 induces migration and invasion of glial precursor cells via its receptor CXCR6. Molecular and Cellular Neurosciences 39, 133–141. [DOI] [PubMed] [Google Scholar]
- Hattermann K, & Mentlein R (2013). An infernal trio: the chemokine CXCL12 and its receptors CXCR4 and CXCR7 in tumor biology. Annals of Anatomy 195, 103–110. [DOI] [PubMed] [Google Scholar]
- Hattermann K, Sebens S, Helm O, Schmitt AD, Mentlein R, Mehdorn HM, & Held-Feindt J (2014). Chemokine expression profile of freshly isolated human glioblastoma-associated macrophages/microglia. Oncology Reports 32, 270–276. [DOI] [PubMed] [Google Scholar]
- van Helden MJ, Zaiss DM, & Sijts AJ (2012). CCR2 defines a distinct population of NK cells and mediates their migration during influenza virus infection in mice. PLoS One 7, e52027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held-Feindt J, Hattermann K, Muerkoster SS, Wedderkopp H, Knerlich-Lukoschus F, Ungefroren H, … Mentlein R (2010). CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs). Experimental Cell Research 316, 1553–1566. [DOI] [PubMed] [Google Scholar]
- Henneken M, Dorner T, Burmester GR, & Berek C (2005). Differential expression of chemokine receptors on peripheral blood B cells from patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Research & Therapy 7, R1001–R1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgaard RB, Zamarin D, Lesokhin A, Merghoub T, & Wolchok JD (2016). Targeting myeloid-derived suppressor cells with colony stimulating factor-1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3-dioxygenase-expressing tumors. EBioMedicine 6, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeth G, Staflin K, Kalliomaki S, Lindvall M, & Kjellman C (2006). Chemokine-directed migration of tumor-inhibitory neural progenitor cells towards an intracranially growing glioma. Experimental Cell Research 312, 1265–1276. [DOI] [PubMed] [Google Scholar]
- Huang A, Zhang B, Wang B, Zhang F, Fan KX, & Guo YJ (2013). Increased CD14(+) HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunology, Immunotherapy 62, 1439–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, … Chen SH (2006). Gr-1 +CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Research 66, 1123–1131. [DOI] [PubMed] [Google Scholar]
- Huettner C, Czub S, Kerkau S, Roggendorf W, & Tonn JC (1997). Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Research 17, 3217–3224. [PubMed] [Google Scholar]
- Hughes CE, & Nibbs RJB (2018). A guide to chemokines and their receptors. The FEBS Journal 285, 2944–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter G, Theruvath J, Graef CM, Zhang M, Schoen MK, Manz EM, … Cheshier SH (2019). Microglia are effector cells of CD47-SIRPalpha antiphagocytic axis disruption against glioblastoma. Proc. Natl. Acad. Sci. U. S. A 116, 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, & Adema GJ (2010). Prognostic significance and mechanism of Treg infiltration in human brain tumors. Journal of Neuroimmunology 225, 195–199. [DOI] [PubMed] [Google Scholar]
- Jin P, Shin SH, Chun YS, Shin HW, Shin YJ, Lee Y, … Park JW (2018). Astrocyte-derived CCL20 reinforces HIF-1-mediated hypoxic responses in glioblastoma by stimulating the CCR6-NF-kappaB signaling pathway. Oncogene 37, 3070–3087. [DOI] [PubMed] [Google Scholar]
- Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, & Heimberger AB (2008). Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunology, Immunotherapy 57, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Bischof A, Ebsworth K, Ertl L, Schall TJ, & Charo IF (2015). Combination therapy of chemokine receptor inhibition plus PDL-1 blockade potentiates antitumor effects in a murine model of breast cancer. Journal for Immunotherapy of Cancer 3, P227. [Google Scholar]
- Jung H, Mithal DS, Park JE, & Miller RJ (2015). Localized CCR2 Activation in the Bone Marrow Niche Mobilizes Monocytes by Desensitizing CXCR4. PLoS One 10, e0128387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai R, Ishisaka K, Sato K, Sakuma T, Yamauchi T, Imamura Y, … Kubota T (2007). Primary central nervous system lymphoma secretes monocyte chemoattractant protein 1. Medical Molecular Morphology 40, 18–22. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, … Pollard JW (2015). CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. The Journal of Experimental Medicine 212, 1043–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koguchi Y, Hoen HM, Bambina SA, Rynning MD, Fuerstenberg RK, Curti BD, … Bahjat KS (2015). Serum Immunoregulatory Proteins as Predictors of Overall Survival of Metastatic Melanoma Patients Treated with Ipilimumab. Cancer Research 75, 5084–5092. [DOI] [PubMed] [Google Scholar]
- Kohanbash G, McKaveney K, Sakaki M, Ueda R, Mintz AH, Amankulor N, … Okada H (2013). GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-alpha. Cancer Research 73, 6413–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu N, Ma X, Brox R, Fan X, Kochel T, Reader J, Tschammer N and Fulton A The Chemokine Receptor CXCR3 Isoform B Drives Breast Cancer Stem Cells. Breast Cancer: Basic and Clinical Research, (2019) 13: 1178223419873628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev SA, Li Y, & Chen SH (2000). Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. Journal of Immunology 165, 779–785. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, … Getz G (2013). Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legler DF, Uetz-von AE, & Hauser MA (2014). CCR7: roles in cancer cell dissemination, migration and metastasis formation. The International Journal of Biochemistry & Cell Biology 54, 78–82. [DOI] [PubMed] [Google Scholar]
- van der Leun AM, Thommen DS, & Schumacher TN (2020). CD8(+) T cell states in human cancer: insights from single-cell analysis. Nature Reviews. Cancer 20, 218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung SY, Wong MP, Chung LP, Chan AS, & Yuen ST (1997). Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathologica 93, 518–527. [DOI] [PubMed] [Google Scholar]
- Lewis CE, & Pollard JW (2006). Distinct role of macrophages in different tumor micro-environments. Cancer Research 66, 605–612. [DOI] [PubMed] [Google Scholar]
- Liang K, Liu Y, Eer D, Liu J, Yang F, & Hu K (2018). High CXC Chemokine Ligand 16 (CXCL16) Expression Promotes Proliferation and Metastasis of Lung Cancer via Regulating the NF-kappaB Pathway. Medical Science Monitor 24, 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Yuzhalin AE, Gordon-Weeks AN, & Muschel RJ (2016). Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 7, 28697–28710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, & Pollard JW (2001). Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. The Journal of Experimental Medicine 193, 727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Luo D, Reynolds BA, Meher G, Katritzky AR, Lu B, … Harrison JK (2011). Chemokine receptor CXCR3 promotes growth of glioma. Carcinogenesis 32, 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Luo D, Streit WJ, & Harrison JK (2008). CX3CL1 and CX3CR1 in the GL261 murine model of glioma: CX3CR1 deficiency does not impact tumor growth or infiltration of microglia and lymphocytes. Journal of Neuroimmunology 198, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F and Yang B Double-Targeted Knockdown of miR-21 and CXCR4 Inhibits Malignant Glioma Progression by Suppression of the PI3K/AKT and Raf/MEK/ERK Pathways. Biomed Res Int (2020) 2020: 7930160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugthart G, Melsen JE, Vervat C, Ostaijen-Ten Dam MM, Corver WE, Roelen DL, … Schilham MW (2016). Human Lymphoid Tissues Harbor a Distinct CD69+CXCR6+ NK Cell Population. Journal of Immunology 197, 78–84. [DOI] [PubMed] [Google Scholar]
- Mack M, Schneider MA, Moll C, Cihak J, Bruhl H, Ellwart JW, … Schlondorff D (2005). Identification of antigen-capturing cells as basophils. Journal of Immunology 174, 735–741. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Gray PA, Van DJ, & Sozzani S (2000). Macrophage-derived chemokine (MDC). Journal of Leukocyte Biology 68, 400–404. [PubMed] [Google Scholar]
- Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, & Bonecchi R (2010). The chemokine system in cancer biology and therapy. Cytokine & Growth Factor Reviews 21, 27–39. [DOI] [PubMed] [Google Scholar]
- Maxwell M, Galanopoulos T, Neville-Golden J, & Antoniades HN (1992). Effect of the expression of transforming growth factor-beta 2 in primary human glioblastomas on immunosuppression and loss of immune surveillance. Journal of Neurosurgery 76, 799–804. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, … Segal DM (2002). Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. Journal of Immunology 168, 689–695. [DOI] [PubMed] [Google Scholar]
- Mega A, Hartmark Nilsen M, Leiss LW, Tobin NP, Miletic H, Sleire L, … Ostman A (2020). Astrocytes enhance glioblastoma growth. Glia 68, 316–327. [DOI] [PubMed] [Google Scholar]
- Meng YH, Li H, Chen X, Liu LB, Shao J, Chang KK, … Li DJ (2013). RANKL promotes the growth of decidual stromal cells in an autocrine manner via CCL2/CCR2 interaction in human early pregnancy. Placenta 34, 663–671. [DOI] [PubMed] [Google Scholar]
- Mercurio L, Ajmone-Cat MA, Cecchetti S, Ricci A, Bozzuto G, Molinari A, … Minghetti L (2016). Targeting CXCR4 by a selective peptide antagonist modulates tumor microenvironment and microglia reactivity in a human glioblastoma model. Journal of Experimental & Clinical Cancer Research 35, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, & Pisa P (2006). CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. Journal of Immunology 177, 7398–7405. [DOI] [PubMed] [Google Scholar]
- Miller MC, & Mayo KH (2017). Chemokines from a Structural Perspective. International Journal of Molecular Sciences 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Shearer J, Evans R, & Caldwell MD (1992). Macrophage arginine metabolism and the inhibition or stimulation of cancer. Journal of Immunology 149, 2709–2714. [PubMed] [Google Scholar]
- Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, … Nelson BH (2009). Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 4, e6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Moritake K, Yamada K, Hara N, Osago H, Shibata T, … Tsuchiya M (2009). Indoleamine 2,3-dioxygenase as a new target for malignant glioma therapy. Laboratory investigation. Journal of Neurosurgery 111, 230–237. [DOI] [PubMed] [Google Scholar]
- Molon B, Ugel S, Del PF, Soldani C, Zilio S, Avella D, … Viola A (2011). Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. The Journal of Experimental Medicine 208, 1949–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteclaro FS, & Charo IF (1996). The amino-terminal extracellular domain of the MCP-1 receptor, but not the RANTES/MIP-1alpha receptor, confers chemokine selectivity. Evidence for a two-step mechanism for MCP-1 receptor activation. The Journal of Biological Chemistry 271, 19084–19092. [DOI] [PubMed] [Google Scholar]
- Monteclaro FS, & Charo IF (1997). The amino-terminal domain of CCR2 is both necessary and sufficient for high affinity binding of monocyte chemoattractant protein 1. Receptor activation by a pseudo-tethered ligand. The Journal of Biological Chemistry 272, 23186–23190. [DOI] [PubMed] [Google Scholar]
- Monti P, Leone B, Marchesi F, Balzano G, Zerbi A, Scaltrini F, … Piemonti L (2003). The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Research 63, 7451–7461. [PubMed] [Google Scholar]
- Nagarsheth N, Wicha MS, & Zou W (2017). Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nature Reviews. Immunology 17, 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard BS, Ladekarl M, Nyengaard JR, & Nielsen K (2008). A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecologic Oncology 108, 106–111. [DOI] [PubMed] [Google Scholar]
- Nishimura F, Dusak JE, Eguchi J, Zhu X, Gambotto A, Storkus WJ, & Okada H (2006). Adoptive transfer of type 1 CTL mediates effective anti-central nervous system tumor response: critical roles of IFN-inducible protein-10. Cancer Research 66, 4478–4487. [DOI] [PubMed] [Google Scholar]
- Noel M, O’Reilly EM, Wolpin BM, Ryan DP, Bullock AJ, Britten CD, … Lowery MA (2020). Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Investigational New Drugs 38, 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, … Chouaib S (2014). PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. The Journal of Experimental Medicine 211, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M, Koprivnikar Krajnc M, Hrastar B, Breznik B, Majc B, Mlinar M, … Lah Turnsek T (2020). CCR5-Mediated Signaling Is Involved in Invasion of Glioblastoma Cells in Its Microenvironment. International Journal of Molecular Sciences 21, 4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, … Linehan DC (2016). Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. The Lancet Oncology 17, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollila TA, Sahin I, & Olszewski AJ (2019). Mogamulizumab: a new tool for management of cutaneous T-cell lymphoma. Oncotargets and Therapy 12, 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, … Platten M (2011). An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203. [DOI] [PubMed] [Google Scholar]
- Oyajobi BO, Franchin G, Williams PJ, Pulkrabek D, Gupta A, Munoz S, … Mundy GR (2003). Dual effects of macrophage inflammatory protein-1alpha on osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. Blood 102, 311–319. [DOI] [PubMed] [Google Scholar]
- Pan Y, Smithson LJ, Ma Y, Hambardzumyan D, & Gutmann DH (2017). Ccl5 establishes an autocrine high-grade glioma growth regulatory circuit critical for mesenchymal glioblastoma survival. Oncotarget 8, 32977–32989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panse J, Friedrichs K, Marx A, Hildebrandt Y, Luetkens T, Barrels K, … Atanackovic D (2008). Chemokine CXCL13 is overexpressed in the tumour tissue and in the peripheral blood of breast cancer patients. British Journal of Cancer 99, 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KH, Beury DW, & Ostrand-Rosenberg S (2015). Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv Cancer Res 128, 95–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas S, Martin M, Kaufman PA, Gil-Martin M, Gomez PP, Lopez-Tarruella S, … Cortes J (2018). Balixafortide plus eribulin in HER2-negative metastatic breast cancer: a phase 1, single-arm, dose-escalation trial. The Lancet Oncology 19, 812–824. [DOI] [PubMed] [Google Scholar]
- Pollard JW (2004). Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews. Cancer 4, 71–78. [DOI] [PubMed] [Google Scholar]
- Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, Apanasovich TV, … Hooper DC (2013). Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clinical Cancer Research 19, 3776–3786. [DOI] [PubMed] [Google Scholar]
- Pu Y, Li S, Zhang C, Bao Z, Yang Z, & Sun L (2015). High expression of CXCR3 is an independent prognostic factor in glioblastoma patients that promotes an invasive phenotype. Journal of Neuro-Oncology 122, 43–51. [DOI] [PubMed] [Google Scholar]
- Raman D, Sobolik-Delmaire T, & Richmond A (2011). Chemokines in health and disease. Experimental Cell Research 317, 575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri B, Rayman P, Huang P, Grabowski M, Hambardzumyan D, Finke JH, & Vogelbaum MA (2015). Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. Journal of Neuro-Oncology 122, 293–301. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, … Finke J (2011). Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro-Oncology 13, 591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redjal N, Chan JA, Segal RA, & Kung AL (2006). CXCR4 inhibition synergizes with cytotoxic chemotherapy in gliomas. Clinical Cancer Research 12, 6765–6771. [DOI] [PubMed] [Google Scholar]
- Ren F, Zhao Q, Huang L, Zheng Y, Li L, He Q, … Zhang Y (2019). The R132H mutation in IDH1 promotes the recruitment of NK cells through CX3CL1/CX3CR1 chemotaxis and is correlated with a better prognosis in gliomas. Immunology and Cell Biology 97, 457–469. [DOI] [PubMed] [Google Scholar]
- Ridiandries A, Tan JT, & Bursill CA (2016). The Role of CC-Chemokines in the Regulation of Angiogenesis. International Journal of Molecular Sciences 17, 1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, … Parney IF (2010). Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro-Oncology 12, 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggendorf W, Strupp S, & Paulus W (1996). Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathologica 92, 288–293. [DOI] [PubMed] [Google Scholar]
- Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, … Melero I (2017). Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Annals of Oncology 28, 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni M, Bracarda S, Nabissi M, Massari F, Conti A, Bria E, … Cascinu S (2014). CXC and CC chemokines as angiogenic modulators in nonhaematological tumors. BioMed Research International 2014, 768758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer CC, Wang Y, Hough KP, Sawant A, Grant SC, Thannickal VJ, … Deshane JS (2016). Indoleamine 2,3-dioxygenase regulates anti-tumor immunity in lung cancer by metabolic reprogramming of immune cells in the tumor microenvironment. Oncotarget 7, 75407–75424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Bohm D, von TC, Steiner E, Puhl A, Pilch H, … Gehrmann M (2008). The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Research 68, 5405–5413. [DOI] [PubMed] [Google Scholar]
- Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, … Wicha M (2017). Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with HER-2-Negative Metastatic Breast Cancer. Clinical Cancer Research 23, 5358–5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciume G, Santoni A, & Bernardini G (2010). Chemokines and glioma: invasion and more. Journal of Neuroimmunology 224, 8–12. [DOI] [PubMed] [Google Scholar]
- Serbina NV, & Pamer EG (2006). Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature Immunology 7, 311–317. [DOI] [PubMed] [Google Scholar]
- Sokol CL, & Luster AD (2015). The chemokine system in innate immunity. Cold Spring Harbor Perspectives in Biology 7, a016303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava MK, Zhu L, Harris-White M, Kar UK, Huang M, Johnson MF, … Sharma S (2012). Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One 7, e40677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford JH, Hirai T, Deng L, Chernikova SB, Urata K, West BL, & Brown JM (2016). Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro-Oncology 18, 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, … Morton JP (2016). CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell 29, 832–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann KA, Robertson F, Hansi N, Gill U, Pallant C, Christophides T, … Maini MK (2016). CXCR6 marks a novel subset of T-bet(lo)Eomes(hi) natural killer cells residing in human liver. Scientific Reports 6, 26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart TJ, & Smyth MJ (2009). Chemokine-chemokine receptors in cancer immunotherapy. Immunotherapy 1, 109–127. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Burdick MD, Gomperts BN, Belperio JA, & Keane MP (2005). CXC chemokines in angiogenesis. Cytokine & Growth Factor Reviews 16, 593–609. [DOI] [PubMed] [Google Scholar]
- Stromnes IM, Brockenbrough JS, Izeradjene K, Carlson MA, Cuevas C, Simmons RM, … Hingorani SR (2014). Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 63, 1769–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulzewsky F, Pelz A, Feng X, Synowitz M, Markovic D, Langmann T, … Kettenmann H (2015). Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS One 10, e0116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami I (2003). Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. International Journal of Cancer 105, 186–189. [DOI] [PubMed] [Google Scholar]
- Teicher BA, & Fricker SP (2010). CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clinical Cancer Research 16, 2927–2931. [DOI] [PubMed] [Google Scholar]
- Thomas JK, Mir H, Kapur N, Bae S, & Singh S (2019). CC chemokines are differentially expressed in Breast Cancer and are associated with disparity in overall survival. Scientific Reports 9, 4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, … Charo IF (2007). Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. The Journal of Clinical Investigation 117, 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukinaga S, Kajihara M, Takakura K, Ito Z, Kanai T, Saito K, … Koido S (2015). Prognostic significance of plasma interleukin-6/−8 in pancreatic cancer patients receiving chemoimmunotherapy. World Journal of Gastroenterology 21, 11168–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, … Matsushima K (2000). Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clinical Cancer Research 6, 3282–3289. [PubMed] [Google Scholar]
- Vallet S, Raje N, Ishitsuka K, Hideshima T, Podar K, Chhetri S, … Anderson KC(2007). MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood 110, 3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veglia F, Perego M, & Gabrilovich D (2018). Myeloid-derived suppressor cells coming of age. Nature Immunology 19, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgelm AE, & Richmond A (2019). Chemokines Modulate Immune Surveillance in Tumorigenesis, Metastasis. and Response to Immunotherapy. Front Immunol 10, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, … Ghiringhelli F (2010). 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Research 70, 3052–3061. [DOI] [PubMed] [Google Scholar]
- Wang K, Pan L, Che X, Cui D, & Li C (2010). Sonic Hedgehog/GLI(1) signaling pathway inhibition restricts cell migration and invasion in human gliomas. Neurological Research 32, 975–980. [DOI] [PubMed] [Google Scholar]
- Weber KS, Nelson PJ, Grone HJ, & Weber C (1999). Expression of CCR2 by endothelial cells : implications for MCP-1 mediated wound injury repair and In vivo inflammatory activation of endothelium. Arteriosclerosis, Thrombosis, and Vascular Biology 19, 2085–2093. [DOI] [PubMed] [Google Scholar]
- Wirthgen E, Hoeflich A, Rebl A, & Gunther J (2017). Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Frontiers in Immunology 8, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Maxwell R, Xia Y, Cardarelli P, Oyasu M, Belcaid Z, … Lim M (2019). Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. Journal of Neuro-Oncology 143, 241–249. [DOI] [PubMed] [Google Scholar]
- Xia Y, Entman ML, & Wang Y (2013). CCR2 regulates the uptake of bone marrow-derived fibroblasts in renal fibrosis. PLoS One 8, e77493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Escamilla J, Mok S, David J, Priceman S, West B, … Wu L (2013). CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Research 73, 2782–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Liu L, Qiu X, Liu Z, Li H, Li Z, … Wang E (2012). CCL21/CCR7 prevents apoptosis via the ERK pathway in human non-small cell lung cancer cells. PLoS One 7, e33262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Liu L, Lin J, & Ransohoff RM (2012). Role of CCR2 in immunobiology and neurobiology. Clinical and Experimental Neuroimmunology 3, 16–29. [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, … Moses HL (2008). Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Jackson E, Woerner BM, Perry A, Piwnica-Worms D, & Rubin JB (2007). Blocking CXCR4-mediated cyclic AMP suppression inhibits brain tumor growth in vivo. Cancer Research 67, 651–658. [DOI] [PubMed] [Google Scholar]
- Young HL, Rowling EJ, Bugatti M, Giurisato E, Luheshi N, Arozarena I, … Hurlstone A (2017). An adaptive signaling network in melanoma inflammatory niches confers tolerance to MAPK signaling inhibition. The Journal of Experimental Medicine 214, 1691–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Du W, Yan F, Wang Y, Li H, Cao S, … Ren X (2013). Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. Journal of Immunology 190, 3783–3797. [DOI] [PubMed] [Google Scholar]
- Yu S, Duan J, Zhou Z, Pang Q, Wuyang J, Liu T, … Chen Y (2008). A critical role of CCR7 in invasiveness and metastasis of SW620 colon cancer cell in vitro and in vivo. Cancer Biology & Therapy 7, 1037–1043. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou Y, Cui B, Liu Z, & Shen H (2020). Novel insights into astrocyte-mediated signaling of proliferation, invasion and tumor immune microenvironment in glioblastoma. Biomedicine & Pharmacotherapy 126, 110086. [DOI] [PubMed] [Google Scholar]
- Zhang J, Sarkar S, Cua R, Zhou Y, Hader W, & Yong VW (2012). A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis 33, 312–319. [DOI] [PubMed] [Google Scholar]
- Zhou M, Bracci PM, McCoy LS, Hsuang G, Wiemels JL, Rice T, … Wiencke JK (2015). Serum macrophage-derived chemokine/CCL22 levels are associated with glioma risk, CD4 T cell lymphopenia and survival time. International Journal of Cancer 137, 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Burkhardt AM, & Homey B (2011). Homeostatic chemokine receptors and organ-specific metastasis. Nature Reviews. Immunology 11, 597–606. [DOI] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O, & Nomiyama H (2006). The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biology 7, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W, & Wicha MS (2015). Chemokines and cellular plasticity of ovarian cancer stem cells. Oncoscience 2, 615–616. [DOI] [PMC free article] [PubMed] [Google Scholar]


