Abstract
Non-alcoholic fatty liver disease (NAFLD) has become the most common liver pathology worldwide due to the rising prevalence of obesity. This term includes changes from simple steatosis to steatohepatitis and fibrosis. It was previously thought to be a hepatic manifestation of metabolic syndrome, but recent literature describes this relation as much more complex and bi-directional. Development of NAFLD is associated with other metabolic syndrome components but it can also exacerbate insulin resistance and increase cardiovascular risk. Recently a lot of attention is brought to the role of lipids and lipotoxicity in pathogenesis and progression of non-alcoholic fatty disease. It seems that some lipid classes can be protective against liver injury while others are harmful in excessive amounts. This study presents an overview of the main lipids involved in the pathogenesis of non-alcoholic fatty liver disease and summarizes their association with lipotoxicity, insulin resistance, oxidative stress and other processes responsible for its progression.
Keywords: non-alcoholic fatty liver disease, lipotoxicity, non-alcoholic steatohepatitis
Introduction
Following the global epidemic of obesity, the prevalence of non-alcoholic fatty liver disease (NAFLD) has risen as well, making it a major cause of liver disease worldwide [1]. It is estimated to affect 30% of adults and up to 10% of children [2]. The term NAFLD encompasses a broad spectrum of conditions from simple fat accumulation to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis and hepatocellular carcinoma (HCC) [3, 4]. NAFLD can be defined as steatosis in > 5% of hepatocytes in histological examination or proton fat fraction > 5.6% assessed by proton magnetic resonance spectroscopy (1HMRS) with the exclusion of secondary causes of steatosis and excessive alcohol consumption (≥ 30 g for men and ≥ 20 g for women). However, it has been proven that NAFLD can coexist with other liver pathologies, resulting in more severe liver injury [5]. In children diagnostic criteria include steatosis in ultrasonography and abnormal liver tests [6]. Liver biopsy remains the gold-standard method for definitive NAFLD diagnosis, but due to its invasiveness and high price it is usually used for subjects who are at increased risk of having NASH and/or advanced fibrosis or their diagnosis is uncertain [7].
Non-alcoholic fatty liver disease and metabolic syndrome
The link between NAFLD and metabolic syndrome (MetS) is under debate. According to the International Diabetes Federation the definition of MetS includes central obesity (diagnosed based on increased waist circumference) and two of the following: increased triglyceride concentration [≥ 1.7 mmol/l (150 mg/dl), reduced high-density lipoprotein (HDL) cholesterol concentration (< 1.03 mmol/l (40 mg/dl) in males and < 1.29 mmol/l (50 mg/dl) in females) or specific treatment for these lipid abnormalities, increased blood pressure (systolic: ≥ 130 mmHg or diastolic: ≥ 85 mmHg) or treatment of previously diagnosed hypertension and fasting plasma glucose ≥ 5.6 mmol/l (100 mg/dl) or previously diagnosed type 2 diabetes [8]. In children all the values should be assessed according to percentile charts [9]. There is a strong interlink between NAFLD and MetS components – abdominal obesity [10], dyslipidemia [11, 12], hypertension [13] and impaired glucose metabolism [14]. The prevalence of MetS among NAFLD patients is estimated between 18% in nonobese NAFLD and 67% in obese NAFLD patients [3, 15]. Therefore it was primarily thought that NAFLD is the hepatic manifestation and a consequence of metabolic syndrome [16]. However, it seems that the relationship between NAFLD and MetS is complex and bi-directional – on one hand development of NAFLD is linked to MetS component, while on the other NAFLD can promote type 2 diabetes and hypertension or increase the risk of cardiovascular events [17-19]. In the literature, a new term has been mentioned – metabolic dysfunction-associated fatty liver disease. It is used for hepatic steatosis accompanying overweight/obesity, diabetes mellitus or presence of metabolic abnormalities (e.g. hypertension, insulin resistance, dyslipidemia) [20].
Dyslipidemia
Dyslipidemia affects almost 70% of patients with NAFLD [3]. It is characterized by lipid triad – increased concentration of serum triglycerides, increased small, dense, low-density lipoprotein (sdLDL), and low HDL cholesterol [21]. It is suggested that all these disturbances are linked and probably initiated by very-low-density lipoprotein (VLDL) overproduction and impaired lipoprotein catabolism [22].
Lipotoxicity
The pathogenesis of NAFLD has not been fully established yet. The two-hit hypothesis was based on the assumption that sedentary lifestyle, high fat diet and obesity resulting in hepatic lipid accumulation acts as a first hit, making the liver prone to other factors acting as a second hit [23]. This theory has now been replaced by more complex, multiple-hit hypothesis. It states that not only diet and lifestyle but also genetic factors lead to dyslipidemia, insulin resistance, and adipocyte dysfunction, resulting in endoplasmic reticulum stress, oxidative stress, release of proinflammatory cytokines and mitochondrial dysfunction, thus promoting inflammation and fibrosis [24]. Lipotoxicity is one of the most investigated mechanisms in the pathogenesis of NAFLD. The term was originally used to describe a process of lipid-induced cell injury observed in B-cells in type 2 diabetes and muscles in MetS [25]. As both mentioned diseases are strongly linked to NAFLD, lipotoxicity turned out to have a significant role in pathogenesis of liver steatosis, inflammation and fibrosis. It transpired that it is not only the quantity of accumulated lipids but also the type of lipid molecule is of importance in the process of liver cell injury [26]. The role of certain lipid species in the pathogenesis of NAFLD is described below.
Triglycerides
Triglycerides (TG) represent a major form of intrahepatic lipids accumulated in NAFLD. Increased intake of dietary TG and transport of fatty acids (FA) released from insulin-resistant adipose tissue as well as intensive de novo lipogenesis in the liver are important pathways leading to intrahepatic triglyceride accumulation. Dietary TG are hydrolyzed into monoacylglycerols and FA by pancreatic lipase in the duodenum. Products of this process are then emulsified by bile salts and transported to enterocytes to form triglycerides. TG combine with cholesterol, phospholipids and proteins to form chylomicrons that are transported to muscles and adipose tissue as a source of energy. Remaining TG are transported to the liver to release non-esterified FA in a process of lipolysis. In the fed state the main source of energy in the liver is glucose rather than lipids. Therefore excessive FA instead of being B-oxidized are incorporated into TG that are stored as lipid droplets or secreted in VLDL [27]. Although TG are responsible for liver steatosis, it seems that they play a protective role against liver damage in the process of lipotoxicity. A study by Yamaguchi et al. demonstrated that inhibiting diacylglycerol acyltransferase 2 (an enzyme catalyzing the final step of triglyceride synthesis in the liver) in obese mice promotes conversion of simple steatosis into NASH and fibrosis. Therefore it seems that TG accumulation helps protect hepatocytes by incorporation of hepatotoxic FA [28]. Similar observations were made by Liu et al. – obese mice fed with high fat diet (HFD) together with an inhibitor of triglyceride lipase had significantly lower levels of FA and less severe histological changes in the liver than mice fed with HFD without addition of this drug. It would confirm the hypothesis about the protective role of TG accumulation against lipotoxicity [29, 30].
Fatty acids
Non-esterified fatty acids are among most common molecules suspected for lipotoxicity. Hepatic FAs derive from the plasma pool (adipose tissue lipolysis), are synthetized in the liver (de novo lipogenesis), or are released from lysosomes after autophagy. Their plasma concentration depends on the feeding state – it rises during fasting as they serve as the main substrate for various tissues’ metabolism. Postprandial levels of FA decrease as a result of insulin release and its anti-lipolytic action. However, it is known that NAFLD is strongly linked to insulin resistance (IR) [31]. In this state, circulating FA levels are high despite increased insulin concentration due to tissue resistance to its action [32]. Therefore their hepatic uptake is increased. Hepatic FAs are transformed into acyl-CoA molecules and can either undergo B-oxidation or be incorporated into triglycerides or other lipids. Accumulation of FA as complex lipids promote steatosis but it seems that it plays a protective role against liver injury. Excess FAs that are not incorporated into more complex forms are lipotoxic to hepatocytes – they lead to endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress followed by reactive oxygen species (ROS) formation. These processes activate proinflammatory and profibrotic pathways, promoting progression of the disease towards NASH [24, 27, 33]. A lot of attention has been brought to the influence of FAs on metabolic syndrome according to their saturation and length of the chain. Saturated fatty acids (SFA) are thought to increase production of proinflammatory M2 macrophages and inflammatory cytokines, thus promoting insulin resistance. Monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) have a positive influence on glucose metabolism, decreasing insulin resistance; they seem to reduce hepatic steatosis and promote anti-inflammatory cytokines. Therefore it seems that unsaturated FA play a protective role against liver injury [34]. Moreover, data suggest that a low SFA diet decreases liver fat content and improves insulin sensitivity. Similar observations were made with a diet rich in PUFA and MUFA – they improve liver steatosis and are beneficial for insulin sensitivity [35]. Among MUFAs, oleic acid is the best known for its positive role – it was observed that it promotes SFA incorporation into triglyceride, therefore protecting cells from SFA-mediated lipotoxicity [36]. The biological effect of PUFA seems to depend on the localization of the double bond. There are two main classes of PUFA – n-3 PUFA (including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) and n-6 PUFA (including arachidonic acid (AA)). A lot of research has been done on the beneficial role of n-3 PUFA in liver function, modulation of oxidative stress, endothelial function, and their anti-inflammatory properties [37]. Their positive role has been described in atherosclerosis, cardiovascular disease, diabetes, metabolic syndrome and many other diseases [38-40]. Importantly, anti-inflammatory and anti-oxidative properties of n-3 PUFA were observed to ameliorate liver damage in NAFLD, making them a potential therapeutic target in this disease [41-43]. On the other hand, n-6 PUFA are considered to be proinflammatory and prothrombotic [37]. However, accumulating evidence suggests that the n-6/n-3 PUFA ratio is more important considering inflammation than concentrations of n-6 and n-3 PUFA. It was observed that an increased n-6/n-3 ratio is associated with an increased risk of obesity and NAFLD [44-46]. Therefore it would justify dietary supplementation of n-3 PUFA as potential treatment of NAFLD [42, 47, 48].
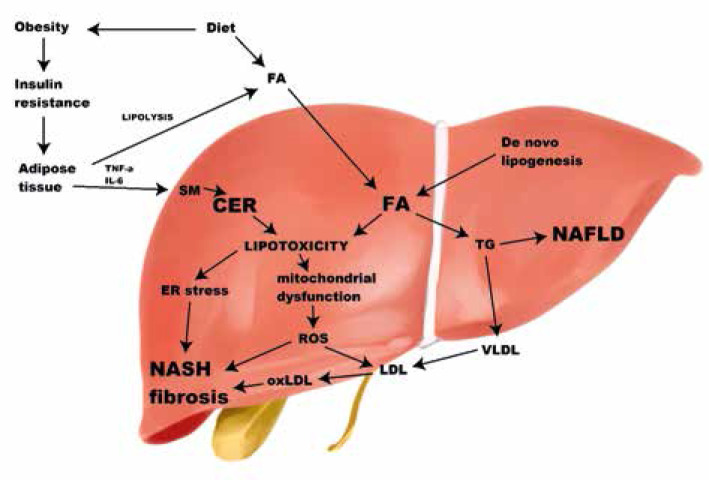
Fig. 1.
Lipid classes and their role in development of non-alcoholic fatty liver disease and lipotoxicity. Obesity leads to insulin resistance, which is strongly linked to non-alcoholic fatty liver disease (NAFLD). In the insulin resistant state, circulating fatty acids are high, because of impaired anti-lipolytic action of insulin. Hepatic fatty acids (FA) derive from plasma, de novo lipogenesis or are released from lysosomes. An excessive amount of FA can be either incorporated into triglycerides or take part in a process of lipotoxicity. It seems that despite being responsible for liver steatosis, triglycerides play a protective role against liver injury. Ceramides can derive from FA, converted from sphingomyelin or sphingoid bases in lysosomes. Conversion from sphingomyelin is stimulated by inflammatory cytokines (TNF-α, IL-6). Both ceramides and FA are lipotoxic – they cause endoplasmic reticulum stress, mitochondrial dysfunction, and oxidative stress, and lead to increased synthesis of reactive oxygen species that take part in oxLDL synthesis. These processes increase inflammation, leading to non-alcoholic steatohepatitis, and promote fibrosis. FA – fatty acids, TNF-α – tumor necrosis factor α, IL-6 – interleukin 6, SM – sphingomyelin, NAFLD – non-alcoholic fatty liver disease, ROS – reactive oxygen species, TG – triglycerides, VLDL – very-low-density lipoprotein, LDL – low-density lipoprotein, oxLDL – oxidized low-density lipoprotein, NASH – non-alcoholic steatohepatitis
Ceramides
Ceramides are sphingolipid (SPL) metabolites that seem to be associated with hepatic injury in a mechanism of lipotoxicity as well. There are three main sources of ceramides – de novo synthesis in endoplasmic reticulum from circulating FAs, conversion from another SPL, sphingomyelin (SM), or conversion from long-chain sphingoid bases in endosomes and lysosomes. As mentioned above, in a state of insulin resistance, adipose tissue lipolysis is not inhibited by insulin, and therefore levels of circulating FA are high. Synthesis of ceramide depend mostly on availability of saturated FA – the excessive amount that cannot be incorporated into more complex lipids (TG) is used as a substrate in ceramide production [49]. It is commonly known that obesity is a state of chronic, low grade inflammation, associated with increased release of inflammatory cytokines from adipose tissue – tumor necrosis factor α (TNF-α), interleukin 6 (IL-6) and others [50]. These inflammatory signals were reported to trigger sphingomyelinases that are responsible for the other way of ceramide generation – conversion from SM [51]. Increased levels of ceramides were associated with obesity, insulin resistance and other metabolic disturbances [52, 53]. Moreover, a growing body of literature has described their role in pathogenesis of non-alcoholic liver disease and progression to NASH in both adult and pediatric populations [54-56]. It seems that ceramides containing long side chains – palmitic or stearic – are more lipotoxic than other ceramides by promoting weight gain and worsening insulin sensitivity [53, 57]. A high concentration of ceramides was observed to increase hepatic insulin resistance, activate inflammatory pathways and cytokine release and promote mitochondrial ROS production [58, 59]. All of these processes contribute to increased hepatocyte apoptosis and fibrosis [60]. Inhibiting ceramide synthesis was found to ameliorate liver steatosis and fibrosis in rats; therefore it may represent another potential therapeutic strategy [61].
Oxidized low-density lipoprotein
Oxidized low-density lipoprotein (oxLDL) is another component suspected for hepatic lipotoxicity. Production of this lipid peroxide is a result of an imbalance between ROS formation and the capacity of the antioxidative system. In a state of FA overload, excessive FA are B-oxidized in adipose tissue mitochondria and peroxisomes and contribute to ROS formation [62]. OxLDL is well known for its key pathogenic role in formation and progression of atherosclerotic plaque by promoting infiltration of macrophages followed by their activation and inflammatory cytokine release [63]. Moreover, oxLDL was found to be linked to cardiovascular disease [64], obesity [65], diabetes mellitus [66] and metabolic syndrome [67]. In the pediatric population oxLDL was found to correlate with waist-hip ratio (WHR) and its concentration was significantly higher in obese/overweight children in comparison to lean patients [68]. Similarly, Matusik et al. reported significantly higher levels of oxLDL in obese children when compared to lean, sport trained children [69]. Kelly et al. performed a study on levels of oxLDL in obese children and observed its positive correlation with BMI and waist circumference and significant association with insulin resistance [70]. It seems that oxidative stress plays a crucial role in NAFLD progression towards NASH [62, 71]. Nobili et al. reported high prevalence of oxidative stress in pediatric NAFLD and its correlation with steatohepatitis severity [72]. OxLDL was found to promote development of NASH and fibrosis. This process can be mediated by various mechanisms in different hepatic cells. The most important one is oxLDL incorporation into Kupffer cells, their activation and promotion of the inflammatory pathway. Moreover, oxLDL may be internalized by hepatocytes, activating the inflammasome and promoting their apoptosis. OxLDL may also activate hepatic stellate cells, promoting the pro-fibrotic pathway, and liver sinusoidal endothelial cells, causing endothelial damage [73, 74]. Interestingly Ho et al. reported that oxLDL contributes to atherosclerosis in the portal vein, causing vascular damage, portal venous inflammation and fibrosis [75]. These observations suggest an important role of oxidative stress in pathogenesis of MS components as well as development and progression of NAFLD.
Conclusions
Together these results provide important insights into the role of various lipid classes in development and progression of NAFLD and NASH. An important role of insulin resistance, oxidative stress, and activation of inflammatory pathways resulting in cytokine release has been underlined, therefore identifying potential therapeutic targets. It would seem that future work should be concerned more about changing the lipid profile of the liver than reducing the total amount of lipids.
Disclosure
The authors declare no conflict of interest.
References
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013; 10: 686-690. [DOI] [PubMed] [Google Scholar]
- 2.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr 2013; 162: 496-500.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73-84. [DOI] [PubMed] [Google Scholar]
- 4.Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 2010; 53: 372-384. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388-1402. [DOI] [PubMed] [Google Scholar]
- 6.Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr 2012; 54: 700-713. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67: 328-357. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006; 23: 469-480. [DOI] [PubMed] [Google Scholar]
- 9.Zimmet P, Alberti KGM, Kaufman F, et al. The metabolic syndrome in children and adolescents–an IDF consensus report. Pediatr Diabetes 2007; 8: 299-306. [DOI] [PubMed] [Google Scholar]
- 10.Finelli C, Tarantino G. Is visceral fat reduction necessary to favour metabolic changes in the liver? J Gastrointestin Liver Dis 2012; 21: 205-208. [PubMed] [Google Scholar]
- 11.Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 2016; 65: 1109-1123. [DOI] [PubMed] [Google Scholar]
- 12.Du T, Sun X, Yuan G, et al. Lipid phenotypes in patients with nonalcoholic fatty liver disease. Metabolism 2016; 65: 1391-1398. [DOI] [PubMed] [Google Scholar]
- 13.Ryoo JH, Suh YJ, Shin HC, et al. Clinical association between non-alcoholic fatty liver disease and the development of hypertension. J Gastroenterol Hepatol 2014; 29: 1926-1931. [DOI] [PubMed] [Google Scholar]
- 14.Tomic D, Kemp WW, Roberts SK. Nonalcoholic fatty liver disease: current concepts, epidemiology and management strategies. Eur J Gastroenterol Hepatol 2018; 30: 1103-1115. [DOI] [PubMed] [Google Scholar]
- 15.Zdanowicz K, Białokoz-Kalinowska I, Lebensztejn DM. Non-alcoholic fatty liver disease in non-obese children. Hong Kong Med J 2020; 26: 459-462. [DOI] [PubMed] [Google Scholar]
- 16.de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol 2008; 48 Suppl 1: S104-112. [DOI] [PubMed] [Google Scholar]
- 17.Lonardo A, Nascimbeni F, Mantovani A, et al. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J Hepatol 2018; 68: 335-352. [DOI] [PubMed] [Google Scholar]
- 18.Kim D, Touros A, Kim WR. Nonalcoholic fatty liver disease and metabolic syndrome. Clin Liver Dis 2018; 22: 133-140. [DOI] [PubMed] [Google Scholar]
- 19.Bobrus-Chociej A, Wasilewska N, Flisiak-Jackiewicz M, et al. Cardiovascular risk in children with nonalcoholic fatty liver disease (NAFLD). Curr Pediatr Rev 2020; 16: 294-297. [DOI] [PubMed] [Google Scholar]
- 20.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol 2020; 73: 202-209. [DOI] [PubMed] [Google Scholar]
- 21.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology 2010; 51: 1979-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartley A, Santos Ferreira DL, Anderson EL, et al. Metabolic profiling of adolescent non-alcoholic fatty liver disease. Wellcome Open Res 2018; 3: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci 2014; 15: 8591-8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016; 65: 1038-1048. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y, Hirose H, Ohneda M, et al. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A 1994; 91: 10878-10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrell GC, Haczeyni F, Chitturi S. Pathogenesis of NASH: how metabolic complications of overnutrition favour lipotoxicity and pro-inflammatory fatty liver disease. Adv Exp Med Biol 2018; 1061: 19-44. [DOI] [PubMed] [Google Scholar]
- 27.Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol 2017; 8: 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007; 45: 1366-1374. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Han L, Zhu L, et al. Free fatty acids, not triglycerides, are associated with non-alcoholic liver injury progression in high fat diet induced obese rats. Lipids Health Dis 2016; 15: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 2003; 100: 3077-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitade H, Chen G, Ni Y, et al. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients 2017; 9: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bechmann LP, Hannivoort RA, Gerken G, et al. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 2012; 56: 952-964. [DOI] [PubMed] [Google Scholar]
- 33.Mann JP, Feldstein AE, Nobili V. Update on lipid species and paediatric nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care 2017; 20: 110-116. [DOI] [PubMed] [Google Scholar]
- 34.Silva Figueiredo P, Carla Inada A, Marcelino G, et al. Fatty acids consumption: the role metabolic aspects involved in obesity and its associated disorders. Nutrients 2017; 9: 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yki-Järvinen H. Nutritional modulation of non-alcoholic fatty liver disease and insulin resistance. Nutrients 2015; 7: 9127-9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Li L, Liu X, et al. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci 2018; 203: 291-304. [DOI] [PubMed] [Google Scholar]
- 37.Monteiro J, Leslie M, Moghadasian MH, et al. The role of n-6 and n-3 polyunsaturated fatty acids in the manifestation of the metabolic syndrome in cardiovascular disease and non-alcoholic fatty liver disease. Food Funct 2014; 5: 426-435. [DOI] [PubMed] [Google Scholar]
- 38.Bird JK, Calder PC, Eggersdorfer M. The role of n-3 long chain polyunsaturated fatty acids in cardiovascular disease prevention and interactions with statins. Nutrients 2018; 10: 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpentier YA, Portois L, Malaisse WJ. n-3 fatty acids and the metabolic syndrome. Am J Clin Nutr 2006; 83 (6 Suppl): 1499S-1504S. [DOI] [PubMed] [Google Scholar]
- 40.Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol 2018; 9: 345-381. [DOI] [PubMed] [Google Scholar]
- 41.Scorletti E, Byrne CD. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol Aspects Med 2018; 64: 135-146. [DOI] [PubMed] [Google Scholar]
- 42.Nobili V, Alisi A, Musso G, et al. Omega-3 fatty acids: Mechanisms of benefit and therapeutic effects in pediatric and adult NAFLD. Crit Rev Clin Lab Sci 2016; 53: 106-120. [DOI] [PubMed] [Google Scholar]
- 43.Corte CD, Iasevoli S, Strologo AD, et al. Omega-3 fatty acids and fatty liver disease in children. Adv Food Nutr Res 2018; 85: 59-77. [DOI] [PubMed] [Google Scholar]
- 44.Patterson E, Wall R, Fitzgerald GF, et al. Health implications of high dietary omega-6 polyunsaturated fatty acids. J Nutr Metab 2012; 2012: 539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simopoulos AP. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016; 8: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004; 106: 635-643. [DOI] [PubMed] [Google Scholar]
- 47.Spooner MH, Jump DB. Omega-3 fatty acids and nonalcoholic fatty liver disease in adults and children: where do we stand? Curr Opin Clin Nutr Metab Care 2019; 22: 103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janczyk W, Socha P, Lebensztejn D, et al. Omega-3 fatty acids for treatment of non-alcoholic fatty liver disease: design and rationale of randomized controlled trial. BMC Pediatr 2013; 13: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martínez L, Torres S, Baulies A, et al. Myristic acid potentiates palmitic acid-induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaning de novo ceramide synthesis. Oncotarget 2015; 6: 41479-41496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engin A. The pathogenesis of obesity-associated adipose tissue inflammation. Adv Exp Med Biol 2017; 960: 221-245. [DOI] [PubMed] [Google Scholar]
- 51.Fucho R, Casals N, Serra D, et al. Ceramides and mitochondrial fatty acid oxidation in obesity. FASEB J 2017; 31: 1263-1272. [DOI] [PubMed] [Google Scholar]
- 52.Choromańska B, Myśliwiec P, Hady HR, et al. Metabolic syndrome is associated with ceramide accumulation in visceral adipose tissue of women with morbid obesity. Obesity (Silver Spring) 2019; 27: 444-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luukkonen PK, Zhou Y, Sädevirta S, et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1167-1175. [DOI] [PubMed] [Google Scholar]
- 54.Wasilewska N, Bobrus-Chociej A, Harasim-Symbor E, et al. Increased serum concentration of ceramides in obese children with nonalcoholic fatty liver disease. Lipids Health Dis 2018; 17: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuel VT, Shulman GI. Nonalcoholic fatty liver disease, insulin resistance, and ceramides. N Engl J Med 2019; 381: 1866-1869. [DOI] [PubMed] [Google Scholar]
- 56.Régnier M, Polizzi A, Guillou H, et al. Sphingolipid metabolism in non-alcoholic fatty liver diseases. Biochimie 2019; 159: 9-22. [DOI] [PubMed] [Google Scholar]
- 57.Turpin SM, Nicholls HT, Willmes DM, et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 2014; 20: 678-686. [DOI] [PubMed] [Google Scholar]
- 58.Levy M, Castillo SS, Goldkorn T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem Biophys Res Commun 2006; 344: 900-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pagadala M, Kasumov T, McCullough AJ, et al. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol Metab 2012; 23: 365-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon J, Ouro A, Ala-Ibanibo L, et al. Sphingolipids in non-alcoholic fatty liver disease and hepatocellular carcinoma: ceramide turnover. Int J Mol Sci 2019; 21: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang M, Li C, Liu Q, et al. Inhibiting ceramide synthesis attenuates hepatic steatosis and fibrosis in rats with non-alcoholic fatty liver disease. Front Endocrinol (Lausanne) 2019; 10: 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quijano C, Trujillo M, Castro L, et al. Interplay between oxidant species and energy metabolism. Redox Biol 2016; 8: 28-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012; 32: 2045-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao S, Zhao D, Wang M, et al. Association between circulating oxidized LDL and atherosclerotic cardiovascular disease: a meta-analysis of observational studies. Can J Cardiol 2017; 33: 1624-1632. [DOI] [PubMed] [Google Scholar]
- 65.Park S, Yoo HJ, Jee SH, et al. Weighting approaches for a genetic risk score and an oxidative stress score for predicting the incidence of obesity. Diabetes Metab Res Rev 2020; 36: e3230. [DOI] [PubMed] [Google Scholar]
- 66.Marin MT, Dasari PS, Tryggestad JB, et al. Oxidized HDL and LDL in adolescents with type 2 diabetes compared to normal weight and obese peers. J Diabetes Complications 2015; 29: 679-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hurtado-Roca Y, Bueno H, Fernandez-Ortiz A, et al. Oxidized LDL is associated with metabolic syndrome traits independently of central obesity and insulin resistance. Diabetes 2017; 66: 474-482. [DOI] [PubMed] [Google Scholar]
- 68.Calcaterra V, De Giuseppe R, Biino G, et al. Relation between circulating oxidized-LDL and metabolic syndrome in children with obesity: the role of hypertriglyceridemic waist phenotype. J Pediatr Endocrinol Metab 2017; 30: 1257-1263. [DOI] [PubMed] [Google Scholar]
- 69.Matusik P, Prokopowicz Z, Norek B, et al. Oxidative/antioxidative status in obese and sport trained children: a comparative study. Biomed Res Int 2015; 2015: 315747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly AS, Jacobs DR Jr, Sinaiko AR, et al. Relation of circulating oxidized LDL to obesity and insulin resistance in children. Pediatr Diabetes 2010; 11: 552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spahis S, Delvin E, Borys JM, et al. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid Redox Signal 2017; 26: 519-541. [DOI] [PubMed] [Google Scholar]
- 72.Nobili V, Parola M, Alisi A, et al. Oxidative stress parameters in paediatric non-alcoholic fatty liver disease. Int J Mol Med 2010; 26: 471-476. [DOI] [PubMed] [Google Scholar]
- 73.Houben T, Brandsma E, Walenbergh SMA, et al. Oxidized LDL at the crossroads of immunity in non-alcoholic steatohepatitis. Biochim Biophys Acta Mol Cell Biol Lipids 2017; 1862: 416-429. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Q, Liu J, Liu J, et al. oxLDL induces injury and defenestration of human liver sinusoidal endothelial cells via LOX1. J Mol Endocrinol 2014; 53: 281-293. [DOI] [PubMed] [Google Scholar]
- 75.Ho CM, Ho SL, Jeng YM, et al. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. J Inflamm (Lond) 2019; 16: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]