Abstract
Background
A chronic imbalance of energy intake and energy expenditure results in excess fat storage. The obesity often caused by this overweight is detrimental to the health of millions of people. Understanding both sides of the energy balance equation and their counter-regulatory mechanisms is critical to the development of effective therapies to treat this epidemic.
Scope of review
Behaviors surrounding ingestion have been reviewed extensively. This review focuses more specifically on energy expenditure regarding bodyweight control, with a particular emphasis on the organs and attractive metabolic processes known to reduce bodyweight. Moreover, previous and current attempts at anti-obesity strategies focusing on energy expenditure are highlighted. Precise measurements of energy expenditure, which consist of cellular, animal, and human models, as well as measurements of their translatability, are required to provide the most effective therapies.
Major conclusions
A precise understanding of the components surrounding energy expenditure, including tailored approaches based on genetic, biomarker, or physical characteristics, must be integrated into future anti-obesity treatments. Further comprehensive investigations are required to define suitable treatments, especially because the complex nature of the human perspective remains poorly understood.
Keywords: Obesity, Energy homeostasis, Energy expenditure, Methodology, Clinical translatability
Graphical abstract
Maintenance of energy homeostasis and body weight requires a balance between energy intake, loss, and expenditure. An imbalance in this energy homeostasis leads to obesity. Attempts to treat obesity, may either focus on decreasing energy intake or increasing energy expenditure/loss; ideally combining several aspects. The major organs involved are shown in this illustration.
Abbreviations
- EI
energy intake
- EE
energy expenditure
- TEE
total EE
- RMR
resting metabolic rate
- TEF
thermic effect of food
- AEE
activity related EE
- BMI
body mass index
- REE
resting energy expenditure
- BMR
basal metabolic rate
- NAFLD
non-alcoholic fatty liver disease
- T2D
type 2 diabetes
- NEAT
non-exercise activity thermogenesis
- FFM
fat-free mass
- GLP-1
glucagon-like peptide 1
- PYY
peptide tyrosine tyrosine
- GLP-1RA
glucagon-like peptide 1 receptor agonists
- BAT
brown adipose tissue
- NST
non-shivering thermogenesis
- OCR
oxygen consumption rate
- DLW
doubly labelled water
- dERO,H
differential elimination rates of oxygen and hydrogen
- rCO2
rate of CO2
- RQ
respiratory quotient
- RER
respiratory exchange ratio
- Ucp1
uncoupling protein 1
- echoMRI
magnetic resonance imaging
- PET
positron emission tomography
- FDG
fluorodeoxyglucose
- PET/CT
PET combined with computed tomography
- miRNA
microRNA
- LC-MS/MS
liquid chromatography with tandem mass spectrometry
- 12,13-diHOME
12,13-dihydroxy-9Z-octadecenoic acid
- CYP450
cytochrome P450
- 12-LOX
12-lipoxygenase
- 12(S)-HEPE
12(S)-hydroxyeicosapentaenoic acid
- GWAS
genome-wide-association studies
- DiRECT
diabetes remission clinical trial
1. Prologue
Recidivistic weight gain is one critical complication in the treatment of obesity. Potent counter-regulatory mechanisms where changes in energy intake (EI) or energy expenditure (EE) act reciprocally lead to the failure of dieting and exercise as a treatment option for overweight and obesity. Understanding the mechanisms of these complex regulations to ensure a balanced energy homeostasis is therefore critical in developing more effective, persistent, and safe therapeutic interventions. Thus far, therapeutic approaches aiming at increasing EE have either failed to demonstrate efficacy or have been associated with severe side effects, including altered heart function or muscle wasting [1,2]. Therefore, the safe increase of EE remains critical for the development of future therapies. In this perspective review, we highlight key strategies and challenges that emerge when interfering with EE as a therapeutic goal.
In greater detail, we review the current knowledge about the regulation of energy homeostasis and possible attempts for modulation. Further highlighted are the major organs involved in this process, which are the brain, muscle, adipose tissue, and liver [3], as well as metabolic processes that are targeted to achieve meaningful changes in bodyweight and/or body fat. The pathophysiology of obesity is the result of an imbalance between EI and EE where a decrease in EE, one of the contributors to a positive energy balance, promotes this process. We further discuss past and present attempts at therapeutic strategies [1,2,4] for the treatment of obesity as well as possible compensatory mechanisms that might contribute to the occurrence of bodyweight regain [[5], [6], [7]]. In addition, the technical challenges preventing accurate and precise measurements of EE in cells, disease-related animal models (typically rodents), and humans are discussed. Furthermore, approaches toward monitoring organ-specific activity using specific biomarkers can serve as an important basis for developing tissue-based therapies. Finally, it is imperative to investigate all mechanisms affecting EE and to figure out how interventions targeting EE can be translated from cellular systems to animal models and humans in order to ensure that they can be applied as therapeutics. Because of the extensive diversity of genetic, environmental, and lifestyle factors among obesity patients [8,9], all of these factors must ultimately play a role in the development of tailored approaches for the respective subpopulations.
2. Energy homeostasis and body weight control
2.1. Regulation of energy homeostasis and control of EE
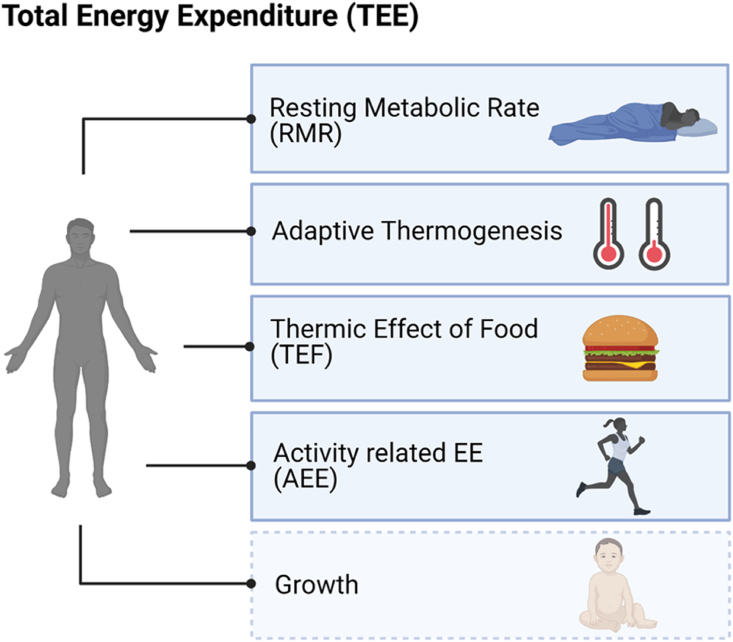
Maintenance of energy homeostasis (balance) and bodyweight requires a balance of EI and EE. Total EE (TEE) is made up of the sum of calories that an organism burns per day, and is composed of five major components: 1) resting metabolic rate (RMR), 2) thermic effect of food (TEF), 3) activity related EE (AEE), 4) adaptive thermogenesis, and 5) growth [[10], [11], [12]] (Figure 1). While EI varies greatly between different meals, changes in RMR are rather long-term. Increases in body mass cause an increase in RMR (due to increased tissue mass), TEF (due to greater food intake), and AEE (due to the increased cost of moving a larger body). Decreases in body mass result in opposite changes. In addition to these “passive” modifications of EE, there are “active” or “regulatory” components of EE changes, called adaptive thermogenesis, that occur due to feedback from the altered body mass. It comprises changes in resting and non-RMR, amounts to 10–15% of TEE, and poses a substantial limitation on changes in bodyweight [13]. TEF has been proposed to buffer the effect of abnormally large EI [14]. However, this appears counterintuitive from an evolutionary point of view [15], as evolution preserves traits ensuring reproductive success. A long-term substantial increase in AEE decreases RMR [16] and is associated with an increase in muscle efficiency (lower increase in AEE over time) and food intake without a major net change in TEE [17].
Figure 1.
Components of total energy expenditure. Total EE (TEE) is composed of five major components: 1) resting metabolic rate (RMR); 2) adaptive thermogenesis; 3) thermic effect of food (TEF); 4) activity related EE (AEE); and 5) growth.
The composition of dietary macronutrients in the form of carbohydrates, fat, and protein further affects EE as well as energy homeostasis with a constant dietary energy content [18]. The synthesis and breakdown of proteins and carbohydrates are associated with changes in levels of water and extracellular fluids. Carbohydrates also affect extracellular fluid by influencing renal sodium excretion. Protein is built up from amino acids and is not “stored” like carbohydrates or triglycerides in higher-order structures that serve as a macronutrient, which requires more energy than the synthesis of glycogen or triglycerides. Low-carbohydrate diets can therefore increase TEE [19,20], but the initially beneficial effects on the body's weight and composition appear to be relatively minor due to the long-term weight regain caused by rapid physiological adaptations [21,22]. Diet-induced changes in EE depend on the processing of the ingested nutrients and their partitioning into tissues and/or their mobilization [23].
A regulatory feedback loop between central and peripheral regions controls EE and EI. The brain receives long-term feedback signals from adipose tissue and lean body mass as well as information about short-term nutrient availability in relation to individual meals [24,25]. This also involves neuroendocrine signaling [26]. The integration of these signals operates to maintain the body's weight and composition by modulating energy homeostasis [27,28].
The fat mass increases and lean mass decreases often associated with aging are observed even under conditions when no change in bodyweight occurs. Changes in RMR and macronutrient oxidation are potential causes for the observed changes in body composition [29]. In a large study, RMR varied between 1344.7 and 1927.49 kcal/day in males and between 1277.83 and 1542.94 kcal/day in females [30]. In addition to age, gender, and body size/composition, hormonal status and physical activity explain a large part of these differences in RMR among individuals. Of course, there is also a substantial genetic component [31] that is associated with the variations in individual RMR (at similar bodyweight and composition), TEF, and AEE.
2.2. Dissecting thermogenic contributions of key organs
To understand energy homeostasis on a body-wide level, it is important to dissect the thermogenic effects of different tissues as well as their degree of contribution. Therefore, in addition to body mass index (BMI), bodyweight, and height, it is also important to investigate the thermogenic activity and capacity of individual organs and tissues when estimating resting energy expenditure (REE). Values are available for specific organ-tissue oxygen consumption or energy flux rates, which is important to establish the basis of observed differences in energy requirements between individuals as well as between groups such as young, old, lean, and obese [3,32,33].
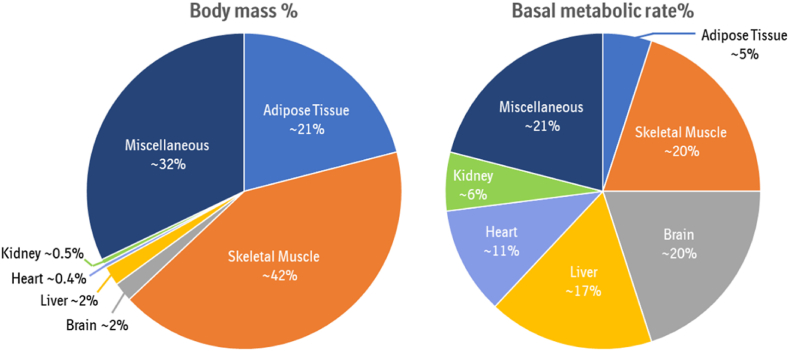
Specific organs, such as the adipose tissue (∼5% EE), skeletal muscle (∼20% EE), brain (∼20% EE), liver (∼17% EE), heart (∼11% EE), and kidneys (∼6% EE), are the greatest contributors to the basal metabolic rate (BMR) [3,[33], [34], [35]]. Skeletal muscle and adipose tissue, despite their lower metabolic rate per gram of organ weight compared to the liver, kidney, heart, or brain, are relevant to the whole-body EE due to their larger organ mass (Figure 2), with their contribution being even further elevated upon exposure to exercise and cold [2,36,37]. Therefore, it is important to consider the biochemical pathways that determine the metabolic rate of individual tissues with a larger size. Advances in organ-tissue prediction models to better assess human EE have been reviewed by Heymsfield et al. [38].
Figure 2.
Average contribution of organs to body mass and basal metabolic rate. There is a discrepancy between organ contribution to body mass compared to basal metabolic rate. Skeletal muscle and adipose tissue for example have a lower metabolic rate per gram organ weight compared to liver, kidney, heart, or brain, but due to their larger organ mass are more relevant for whole body energy expenditure (modified from [3,35]).
Clear differences are observed during cold-induced thermogenesis when measuring adipose tissue metabolic activity in brown, beige, and white adipocytes, indicating the importance of tissue localization [3]. Increasing the amount of thermogenic brown or beige adipocytes in humans is associated with a substantial contribution to whole-body EE and an improvement in metabolic parameters [[39], [40], [41], [42], [43], [44]]. In addition, skeletal muscle is an important organ for thermogenesis due to its ability to induce energy-expending futile cycles [45,46]. During mild cold exposure, the relative contribution of heat-generating organs such as skeletal muscles can increase to account for at least 40% of EE [47]. A more detailed review on the molecular pathways of both thermogenic organs was published by Betz and Enerbäck [48]. Finally, a recent review highlights the importance of liver energy metabolism, especially in the context of metabolic associated fatty liver disease. Based on preclinical studies performed with different small molecule mitochondrial uncouplers, the authors propose increasing EE via mitochondrial uncoupling specifically in the liver as an attractive therapeutic approach to treat metabolic associated fatty liver disease, non-alcoholic steatohepatitis, and type 2 diabetes (T2D) [49].
Knowing the relative thermogenic contribution of these organs and the scope for which their contribution can be modified is important for the identification of key organs or metabolic processes that target meaningful and safe changes in whole-body EE.
2.3. Obesity and EE
A positive energy balance leads to the development of obesity, with low RMR and low non-exercise activity thermogenesis (NEAT) representing two of the risk factors [50]. The lowering of bodyweight results from a negative energy balance and leads to a curve-linear ending in a new steady state as opposed to a continuous one. Thus, a new equilibrium between EI and EE is reached [13,51]. Individual responses to weight loss are thereby variable and appear to be related to the degree of adaptive thermogenesis. The different adaptive thermogenesis responses characterize the so-called thrifty [52] (favoring weight gain) and spendthrifty [13] (favoring weight loss) phenotypes, respectively. Interestingly, recent studies indicate that thriftier individuals have less capacity for cold-induced thermogenesis and that BAT activation seems to be indicative of those individuals’ predisposition to weight gain [53,54].
Adaptive thermogenesis occurs with short- and long-term weight reduction. A maintenance of bodyweight reduction ≥10% is accompanied by an ∼20–25% reduction in TEE [13]. This decrease corresponds with a 300–400 kcal reduction of daily caloric intake to maintain the same bodyweight and physical activity of an individual that did not undergo a weight loss intervention [13]. The difficulties experienced by individuals who reduce their bodyweight and aim to maintain said reduction reflect the potent counter-regulatory mechanisms. Those include adaptations in EI and adaptive thermogenesis, which are both controlled by redundant metabolic, neuroendocrine, and autonomic systems [55].
The adaptive changes in EE and EI associated with weight loss are highly persistent [56] and have been described in various models [57]. The traditional “set-point model” is influenced by control theory thinking and suggests a negative-feedback loop that defends bodyweight at a “pre-defined” level. It is still the prevailing model of bodyweight regulation depicted in textbooks, although it has been disputed for decades and is inconsistent with many experimental findings [5,58]. An alternative model compares bodyweight regulation to water in a lake. In this non-regulated system, the level of the lake (fat stores) passively “settles” at an equilibrium that is determined by the inflow (EI) matching the outflow (EE) [59].
Presumably, bodyweight regulation does not follow either of these models. Bodyweight is actively regulated, but a clear-cut defended bodyweight set point could not be established. Moreover, active compensatory responses in humans mainly occur after reductions in bodyweight and appear to be negligible after increases in bodyweight. The defense mechanism against potentially harmful weight loss is suggested to be more evolutionarily beneficial than the development of a resistance to increases in bodyweight. This theory led to the hypothesis that active compensatory mechanisms would only be activated at a lower and an upper intervention point, with no active regulation in between [59]. Again, the available evidence argues against such a model. For excellent in-depth discussions about these issues, see Geary [5] and Ravussin et al. [6].
Since Kennedy's lipostatic hypothesis [60], models of bodyweight regulation have mainly focused on fat mass as the main targeted parameter, with the discovery of leptin as a major hormonal signal defending bodyweight loss promoting this “adipocentric” concept of bodyweight regulation. However, there is solid evidence that supports the existence of an independent active regulation of fat-free mass (FFM) in addition to fat mass [[61], [62], [63], [64]]. This is practically relevant because the feedback loop that drives EI to restore FFM may lead to collateral fattening [64].
Most importantly, physiological adaptations that favor weight re-gain are triggered simultaneously with an initial weight loss, which can be achieved by a number of different therapeutic approaches, as mentioned below.
2.4. Anti-obesity strategies and EE
2.4.1. Lifestyle
Lifestyle changes made to reduce bodyweight through dietary restrictions and/or exercise have historically applied the so-called 3,500 kcals rule, i.e., the idea that eating 500 fewer kcals/day yields one pound of fat loss/week. This rule, however, does not account for dynamic changes in energy balance and hence underestimates the change in intake or expenditure required to achieve this loss [65].
Dieting reduces bodyweight more efficiently than exercise, but is accompanied by endocrine changes [66], reductions in EE, and an increased eating drive [65,67], all of which favor weight regain.
According to the constrained TEE model proposed by Pontzer et al. [16], the total level of daily EE increases linearly alongside progressive increases in physical activity but eventually plateaus with higher rates of physical activity (>230 activity counts per minute per day). This might be explained by compensatory mechanisms like decreases in REE and increases in muscle efficiency [7], both of which limit the bodyweight lowering efficacy of exercise. Despite improving general health, particularly by reducing cardiovascular risk factors and increasing insulin sensitivity, exercise alone only moderately reduces bodyweight [68,69].
The combination of both dietary restrictions and exercise demonstrated a greater potential for weight loss compared to either of the two interventions alone, achieving and maintaining a weight loss ≥10% after 1 year [70] and ≥5% after 8 years [71]. However, long-term dietary restrictions in combination with exercise are also associated with metabolic adaptations, including a persistent reduction (about 500 kcal/d from 30 weeks to 6 years) in RMR despite substantial weight regain [72].
2.4.2. Bariatric surgery
Bariatric surgery achieves a long-term weight loss of ≥25% after 5 years [73,74], with minor increases of weight after up to 20 years post-surgery [[75], [76], [77]]. Gastric bypass patients showed a larger reduction in FFM and smaller reductions in RMR, plasma leptin, and thyroid hormones than patients who underwent lifestyle intervention. There is a transient decrease in RMR/FFM for 1–2 years after bariatric surgery, as the larger mass of organs with a high–metabolic rate leads to most of the significant weight loss during that time [78]. This indicates that the surgery somehow enhances eating inhibitory signals. The lower small intestinal gut peptides glucagon-like peptide-1 (GLP-1) and peptide tyrosine tyrosine (PYY) are likely candidates in this context, as has been discussed extensively by Holst and colleagues [79]. They are established satiation signals [80], and their release is massively stimulated in response to meals after bariatric surgery. Several lines of evidence [79] indicate that GLP-1 and PYY play a role in the beneficial effects of bariatric surgery on humans, although experiments with pertinent receptor knockout mice appear to indicate that endogenous GLP-1 is not necessary for the reduction in bodyweight that occurs after bariatric surgery in mice [81]. The mechanisms that cause the weight loss after bariatric surgery may therefore differ between humans and rodents. In any case, the weight loss observed following bariatric surgery is a complex phenomenon to which different factors contribute [81].
2.4.3. Pharmacotherapy
Pharmacotherapies directly manipulating EE to lower weight were already attracting attention in the 19th century. A variety of previous attempts at treating obesity have been reviewed in detail by Müller et al. [1] and Chen et al. [2]. Many of them were initially approved for their ability to decrease bodyweight by increasing EE but were withdrawn as safety concerns started to outweigh the pharmacological benefits [82,83]. The mitochondrial uncoupler 2,4,-dinitrophenol has been suspended by the Food and Drug Administration, based on substantial side effects such as rashes and cataracts [1,2]. In particular, adverse cardiovascular events observed with the serotonin and noradrenaline reuptake inhibitor Sibutramine outweighed the benefits of weight loss and led to the withdrawal of the drug in 2010 [84]. Similarly, the beta-3 receptor agonist mirabegron leads to an increase in heart rate and blood pressure depending on the dose, despite its effects on increased EE [85].
Currently approved pharmacological interventions for obesity treatment rather support weight loss by decreasing EI through the reduction of food intake or energy absorption. Among those are the lipase inhibitor orlistat [86] or the GLP-1RA semaglutide [87], with GLP-1RA being recognized as the most effective and safe treatment for obesity to date. The EI-lowering principle of GLP-1RA showed clinical benefits not only for weight loss but also for cardiovascular risk reduction, despite an increase in heart rate [[87], [88], [89]], although the discrepancy between an increased heart rate and undesirable cardiovascular effects needs to be evaluated further. These evaluations may alter the popular view of such changes when developing new EE increasing approaches.
In addition, the simultaneous targeting of different biological mechanisms has garnered interest over the last few years. These combination approaches include different EE assets, as well as combining EI and EE to achieve a greater weight loss through either multitarget drugs or the combination of multiple independent therapies. Either way, these mixtures simultaneously target different mechanisms and thereby deliver synergistic or complementary pharmacology to aim for efficacies that are currently only achieved through bariatric surgeries. There are more detailed reviews about the history and benefits of polyagonists targeting the receptors for GLP-1, GIP, and glucagon [1,4,90].
2.5. Considerations on compensatory mechanisms
Regardless of the therapeutic intervention, it is important to identify potential compensatory responses by simultaneously quantifying the major contributing thermogenic processes and organs. For example, daily cold exposure in humans resulted in a nearly two-fold increase in brown adipose tissue (BAT) thermogenesis without eliciting any changes in shivering activity or whole-body heat production [91,92]. This was made possible by shifting the contribution of non-shivering thermogenesis (NST) from skeletal muscle (via proton leak) to BAT [91]. Similarly, several types of metabolically futile cycles may be recruited simultaneously but adapt differentially under varying metabolic conditions.
Our current understanding regarding these and other compensatory mechanisms in humans remain limited. However, the measurement of as many metabolic processes and components of total daily EE as possible remains critical in identifying energy compensations in humans as well as its therapeutic potential to treat or prevent the development of metabolic diseases.
3. Measuring EE
Assessing EE, including the contributions of single organs, is important to better understand the role of EE in the context of obesity as well as from a drug discovery perspective. Therefore, the use of in vitro systems and preclinical models serves two main purposes: to generate a general mechanistic understanding of physiological and pathophysiological processes, and to determine whether this knowledge can ultimately be translated to influence human biology. The proper regulation of metabolic activity is crucial for cell survival and the healthy function of a tissue, whilst deregulation has been linked to the onset and progression of multiple diseases, such as obesity and metabolic syndrome. As described above, influencing muscle, fat, and/or liver to modulate EE remains an attractive option. Therefore, it is important to correctly assess EE in vitro, ex vivo, or in vivo.
3.1. Preclinical methodologies
3.1.1. Reliable methods to study EE in vitro
To elucidate tissue-specific biology and the pathways that lead to an increased level of EE, the ability to accurately measure increases in oxidative capacity, as well as the activity of the cells themselves, is important. Several methods are currently being used to define oxygen partial pressure, substrate utilization, and thermodynamics.
Direct measures: Cellular heat production directly reflects metabolic changes using thermodynamic approaches. Infrared thermography [93,94], microcalorimetry [95,96], and fluorescent thermosensors [[97], [98], [99], [100], [101], [102]]all offer appropriate physiological read-outs, as they measure real-time fluctuations in thermogenesis. However, these techniques are not frequently used in research as they are time-consuming, often require high cell counts, and do not inform about the substrates utilized. Furthermore, the heat signal is often used to monitor biological processes, but rarely allows for quantitative interpretation [103].
Indirect measures: Indirect measures are typically employed to quantitate the heat production-related phenomenon in cells using surrogate readouts, which are mainly respirometry-based. The XF Flux Analyzer (Seahorse Bioscience) has proven to be a workhorse in determining both aerobic and anaerobic metabolism of cultured cells/tissue through real-time measurements of oxygen consumption rate (OCR, key readout of oxidative phosphorylation) as well as extracellular acidification rate (indicator of glycolysis) [104]. To further investigate specific substrate preferences, one has to dissect between glycolysis and the oxidation of major fuel substrates glucose, glutamine, and fatty acids to identify the primary substrate utilized [105]. The complexity is further increased as the data interpretation depends not only on the substrates in the buffer but also on additional sinks, such as albumin. Although respiration can also be measured via the Clark oxygen electrode with the option of manual titration of several compounds, it does, however, require a large sample quantity and is a low-throughput platform [[106], [107], [108]]. Another way to determine molecular oxygen is the usage of luminescence-based probes. The big advantage of this technology is the simplicity of the measurement procedure as well as the high-sample throughput, with the drawbacks being a lower sensitivity and the inability to measure several compounds [[108], [109], [110], [111], [112]]. The electron paramagnetic resonance spectroscopy is another technology where cells and oxygen sensors are distributed homogenously throughout the samples, enabling the precise detection of low-oxygen concentrations in solution, but it requires a closed and sealed set-up and cannot be used with several compounds [108,113]. Additional considerations, such as cellular ATP demand, signaling events, substrate availability, and utilization, as well as effects on mitochondrial membrane potential, mitochondrial content, and dynamics, must be considered [[114], [115], [116], [117], [118]]. Given the variety of possible methodologies, it is important to choose the most suitable method for assessing EE in vitro. One must consider the necessity of a high throughput for drug discovery in combination with a sensitive and reliable detection, as well as the identification of mechanistic insights. Therefore, the combination of multiple technologies is recommended.
In addition to the right technology, it is also important to choose the correct cellular system for mechanistic studies. To achieve this, discrepancies between cell lines and primary cells must be considered in addition to translatability issues between animal models and humans. Immortalized cell lines offer a suitable model to generate valuable data [119]. Nevertheless, some molecules and mechanisms can only be investigated within human primary cells, e.g., certain microRNA (miRNA) and locked nucleic acids. However, humans are outbred, and strong clonal effects may occur with primary human cells. In addition, based on donor variability, it would be difficult to reach the standardization required for reproducibility. Therefore, cell lines provide a more stable model. To increase translation and relevance to the human physiology, primary cells, especially from humans, should still be considered [120]. Testing organoid cultures and ex vivo tissue biopsies for the improvement of the physiological relevance should also be taken into consideration when looking for the most appropriate technology to assess EE in vitro.
Key findings in vitro should be verified for their physiological relevance. Therefore, measuring EE in vivo is invaluable to biological interpretation and is vital for the physiological extrapolation of both health and disease progression. This includes characterization of the factors that influence EE and the dysregulation of energy balance that leads to obesity. Hereby, it is important to note that the organism behaves as an open thermodynamic system and thus the net energy balance is dictated by the EI and EE. In the following paragraph, we want to provide theoretical, practical, and analytical considerations one should be aware of when measuring EE and whole energy homeostasis.
3.1.2. EI (food) and metabolized energy
Foremost, it is important to keep in mind that not all food is metabolized. To calculate EI in absolute terms, one must calculate the metabolized energy [121,122]. The total energy of the ingested food and the total unabsorbed energy present in feces is experimentally measured using bomb calorimetry. Metabolized energy, as well as the energy equivalence of individual components, can be calculated using the derivatives of Atwater's system [122,123].
However, several limitations should be considered for these calculations: they correspond to short-term measurements and assume that the bodyweight remains constant during the experimental period. Should there be a change in bodyweight, it must be accounted for as total energy lost.
In certain pathologies (e.g., diabetes and other kidney diseases), the urinary wastage of the energy could account for a larger proportion.
Because the number of cells in the body and gut microbes are approximately equal [124] (with most of them being anaerobes), we must also contemplate the effect of inter-group interventions (such as diets or antibiotics) that could alter the gut microbiome to confound the data.
There is no information available on the significance of micronutrient availability and mobilization in these calculations. This should be considered especially when the environment/genetic intervention is expected to alter the biology of micronutrients.
As mentioned above, TEE is based on five components. In preclinical studies, a segregation of the TEE's key components is used to generate insights into predominant metabolic pathways, therapeutic prospects, and physiological correlations of specific experimental interventions.
There are several ways to experimentally measure TEE and derive its individual components using direct and indirect calorimetry. Besides these validated methodologies, there are additional methods for estimating TEE [[125], [126], [127]] that can be used to cross-validate the findings from indirect calorimetry.
3.1.3. Methodologies to measure EE in vivo
Direct calorimetry: All components of TEE (except growth) culminate in heat loss through radiation, convection, evaporation, or conduction [128]. Direct calorimetry gives the most accurate quantitation of heat loss by an organism. However, because of (a) the very high cost, (b) the violable assumption that the entire EI is reflected in the form of heat loss during the measurement period, (c) the lack of information on the type of fuel being oxidized, and (d) the lower segregation capacity of an individual component of TEE, direct calorimetry has largely been replaced by respirometry-based measurements. The direct calorimetry methodologies and instrumentation have evolved considerably overtime since Rubner's first calorimetric measurement was made in 1894 [126]. A detailed description of the variants of the direct calorimeter and its principle are reviewed in detail by Kenny et al. [129], Kaiyala and Ramsay [128], and Webb [130]. Although recent decades have experienced a very limited use of direct calorimetry in preclinical studies, it still remains a gold standard [128].
Doubly labelled water (DLW): There is an isotopic equilibrium of oxygen between water and respiratory CO2 when oxygen is re-cycled as H2O or CO2. Hydrogen, in contrast, leaves the body mainly as water. The double-labeled water technique utilizes these differential elimination rates of oxygen and hydrogen (dERO,H) by using heavy isotopes of hydrogen (2H) and oxygen (18O). The dERO,H is based on the principle of indirect calorimetry and is applied to estimate the rate of CO2 production (rCO2) [12,131]. The rCO2 can be used to estimate the metabolic rate [132]. Over the years, the DLW method has been adapted for both preclinical and clinical studies [133,134]. The main advantage of this method is that it can be used within the social context of freely moving animals. A combination of indirect calorimetry and DLW can be used to consolidate the findings in cases where respirometry data is ambiguous.
Indirect calorimetry: The philosophical concept of the fire of life and the important role of oxygen has been around for centuries [135]. Originating in the 18th century, this concept was discovered by Lavoisier and Laplace, who were the first to use an ice calorimeter to measure animal heat [136]. In 1849, Regnault and Reiset performed oxygen consumption measurements on a closed-circuit system and on carbon dioxide absorbers [137]. They concluded that the ratio of CO2 production to oxygen consumption during respiration is a function of the composition of ingested food. Later, Zuntz & Schumburg [138], as well as Lusk [139], established that one mole of molecular oxygen is utilized to oxidize one mole of carbohydrate and that this process liberates one mole of CO2. The ratio of the volume of liberated CO2 to utilized oxygen (VCO2/VO2) was derived and referred to as the respiratory quotient (RQ). The RQ for carbohydrate oxidation was found to be ∼1, while the RQ for fat was ∼0.7. In modern terminology, RQ is reserved for the direct measurement of moles of oxygen consumption and CO2 released at tissue level. In contrast, the respiratory exchange ratio (RER) is calculated from the gas content of breath. Therefore, the favored term for organismal studies is RER, which is a reliable indicator of the fuel type being used and has been widely utilized in metabolic and nutritional research [140]. These findings helped found the starting point for the development of the modern indirect calorimeter, which is presently the most commonly used method for calculating EE. EE and RER are normally calculated from the volume of utilized oxygen and liberated CO2. The estimation of EE is made using Weir's formulae [141].
3.1.4. Technological advancements in indirect calorimetry
In recent years, there have been several technical advancements in the instrumentation and analysis capacity of commercial indirect calorimeters. One of the most significant developments was the addition of a carbon isotope sensor in-line with regular respiratory analysis. This enables the study of the oxidation of various metabolites with unprecedented convenience and precision. It is now possible to label the specific carbon atoms (13C) of metabolites and analyze the rate of 13CO2 production with a unique speed and accuracy. This leads to excellent in vivo mechanistic studies on the metabolism of exogenous versus endogenous substrates and provides novel details via the tracing of specific labelled metabolites and substrates [142].
The second advancement is the extension of the commercial analyzers to integrate the sensor for other gases (e.g. CH4 and H2) along with oxygen and CO2 sensors [142]. This provides a unique opportunity to dissect the effect of dietary intervention in preclinical models and has a huge potential in the field of microbiome and its crosstalk with systemic metabolism.
The third advancement in this field is the size-reduction and sensitivity of telemetry-based implantable sensors. With the availability of implantable telemetric sensors for electrocardiogram, electroencephalogram, electromyogram, tissue temperature, etc., a combination of indirect calorimetry and 13CO2 sensors with tissue-implanted temperature sensors can yield deep insight into the organ-specific thermogenesis along with RER, REE, and the contribution of a specific metabolic pathway through the use of the isotope sensor data.
Measuring thermogenesis: There are several ways to infer the magnitude of thermogenic response (reviewed by Speakman, 2013) [11]. The defense of core body temperature and OCR have been used as surrogates for thermogenic response upon cold exposure. Nonetheless, it is difficult to distinguish the NST from shivering thermogenesis. To measure NST, it is common to acclimatize the animal at thermoneutrality and inject noradrenaline to activate brown adipose NST through a direct stimulation of β-adrenergic receptors. In ideal cases, however, the NST component should be validated in uncoupling protein 1 (Ucp1) knockout animals by demonstrating the lack of the intervention's effect on Ucp1 knockout animals [143]. It is also important to consider the history of the animal's cold exposure, as it alters the NST quite drastically (reviewed by Speakman, 2013) [11].
Tissue level resolution into thermogenesis: It is difficult to precisely assign the expended energy to a certain tissue within the animal's body. Nonetheless, by using the tissue-implanted telemetric temperature sensors, it is now possible to monitor slight changes in tissue temperature upon cold exposure without systemic interference. The real-time data monitoring advancement is gaining popularity over conventional methods not only due to the precision and resolution but also due to the non-ambiguity of the data.
3.1.5. General considerations for reliable indirect calorimetry analysis
Several excellent reviews have outlined the best practices for the design and execution of calorimetric experiments [[144], [145], [146]]. Most of these guidelines are subjective and vary according to the objective of a particular study. However, certain aspects that must be considered in any preclinical calorimetric experiment are discussed below.
Acclimation, Food intake, EE, and thermogenesis: As rodents are social animals, individual housing constitutes a stressful situation and impacts many important aspects, such as behavior, and often leads to reduced food intake and body weight [147,148]. Therefore, before experimental analysis, animals should be appropriately acclimatized to experimental conditions. It should be noted that changes in the housing conditions may also affect the results of EE measurements in mice. Further detailed studies comparing single-housed with group-housed mice using physiological paradigms will be required to address this point. Besides being caused by stress, changes in food intake may also be the consequence of experimental intervention (genetic, pharmacological, or nutritional). Therefore, one should carefully measure both the EI and EE to accurately attribute the change of increased EE or reduced EI. Another conceivable but often ignored aspect is the contribution of thermogenesis. Most calorimetric studies are performed at an ambient temperature of 22–25 °C [149,150]. These temperatures are below thermoneutral conditions for mice. Therefore, the mice actively regulate body temperature through NST. However, the intervention, even if it does not affect the thermogenesis directly, might alter the rate of thermogenesis. For example, effects on fur and skin might increase heat loss and thereby further increase the metabolic rate [151,152]. In such cases, it is important to carefully delineate the thermogenic contribution. In another scenario, an intervention might affect the BMR, but the calorimetry may not be able to discern the difference due to a compensatory increase in energy consumption for thermogenesis. Therefore, one should thoroughly investigate the origin of difference in EE or the lack thereof.
Body composition and normalization of data: To study a novel genetic model or intervention, it is prudent to first establish baseline data for food intake, bodyweight, and body composition during the anticipated study period. In such a pilot study, it is sensible to first employ a non-invasive body composition analysis (such as magnetic resonance imaging (MRI) based measurement, e.g., echoMRI) and perform endpoint analysis to determine tissue composition. Even if these background data are unavailable, it is recommended to assess the body composition of every experimental animal using non-invasive methods before and after the calorimetric measurement. To normalize the EE data, it is recommended that lean body mass be used. To circumvent the issue that division by lean mass overcompensates for the mass effect, one should consider data analysis using relevant covariates in an ANCOVA, as it includes the mass of each individual organ separately [144].
Intervention-related alteration in absorption efficiency or gut microbiome: When the intervention is expected to cause considerable change to absorption of macronutrients (e.g., lipase inhibitors treatment) or the intervention causes alterations in the gut microbiome, it is necessary to calculate the metabolized energy of every experimental animal group. The lack of this might cause an artefact in data or could mask the actual effect of intervention. We therefore recommend running a pilot experiment, whenever appropriate, to first estimate the metabolizable energy before performing an indirect calorimeter analysis.
3.2. Clinical methodologies
3.2.1. Measuring whole-body EE in humans
For translational studies in humans, indirect calorimetry enables the non-invasive measurement of EE at a relatively low cost [140]. Current research calorimeters usually use the so-called canopy technique, which measures the amount of CO2 and oxygen in a constant flow of air. This technique is highly precise, with a variation coefficient of less than 2% [153].
Indirect calorimetry is generally confined to measuring EE during limited time periods, i.e., in the range of hours. Whole body calorimeters, which are basically small rooms equipped with exact measurements of airflow and devices to quantify oxygen and CO2 in the exhausted air, can theoretically be used to measure EE over longer periods of time; however, individuals cannot be studied in their usual environment. Mobile indirect calorimeters have also been developed but generally lack the precision of conventional devices and are uncomfortable to wear, thus precluding long-term measurements. A promising alternative to assess free-living EE is the use of DLW [134,154,155]. The levels of 18O and 2H in blood, urine, and exhaled air are measured with isotope ratio mass spectrometry. This method is used to quantify total daily EE, and therefore must be combined with indirect calorimetry and activity trackers to quantify the components that make up this expenditure, including the RMR, TEF exercise activity thermogenesis, and NEAT.
Activity trackers and mobile devices offer the possibility of counting steps and assessing physical activity by means of other sensors, such as GPS tracking. The devices often calculate estimates of EE which has been tested against calorimetry in some instances [156,157]. It should, however, be noted that these algorithms do not offer the accuracy and reliability necessary in the research setting, with R2 being in the range of 0.5–0.6. This is also the case with equations used to derive EE from anthropometric parameters such as the Harris Benedict [158] or Mifflin Formula [159].
3.2.2. Organ-specific metabolism and its contribution to whole-body EE
An important consideration when investigating whole-body EE in humans is understanding the specific metabolic rates of various organs and thermogenic processes. Some attempts have been made to estimate organ metabolic rates in humans and animals using tissue preparations [3,32,33]. However, these tissue preparations often lack the electrical activity, metabolites, or substrates required for their optimal function, thereby limiting their extrapolation to in vivo physiological conditions. More commonly, organ-specific oxygen consumption in vivo has been determined using an iteration of the Fick principle, which combines measurements of regional or organ blood flow with differences in arterial and venous concentrations of oxygen. While this historically required the catheterization of arteries and/or veins across regions encompassing the organ of interest [34,160], advancements in molecular imaging over the past decades have allowed for the metabolic rate and mass of specific organs to be directly quantified non-invasively in vivo [34].
The gold standard for the measurement of organ-specific oxygen consumption requires the use of quantitative positron emission tomography (PET) with 15O-labelled carbon monoxide ([15O]CO), radiolabeled water ([15O]H2O), and inhaled oxygen gas ([15O]O2). This allows for the absolute quantification of organ blood volume, blood flow, and metabolic rate of oxygen, respectively. Other indirect measures of organ metabolic rate have been applied using PET with [11C]acetate, which can quantify tricarboxylic acid cycle turnover (or [11C]CO2 production) [161,162], or 31phosphorous-magnetic resonance spectroscopy (31P-MRS) to examine the relative changes in ATP, phosphocreatine, and inorganic phosphate [163,164]. From these direct in vivo measurements combined with measurements of organ weight, the relative contribution to whole-body EE of the most metabolically active tissues can be calculated.
3.3. Brown/beige adipose tissue biomarkers to estimate EE
The standard method for measuring BAT activity is 18F-fluorodeoxyglucose (FDG) uptake using PET combined with computed tomography (PET/CT). This technique was also the basis for the (re) discovery of BAT in adult humans by several groups around the world between 2007 and 2009 [[165], [166], [167], [168], [169]]. However, FDG-PET/CT exposes the patients to ionizing radiation from the 18F-labeled FDG glucose tracer and the PET scan radiation dose. Moreover, it requires exposure to cold in order to activate the BAT thermogenesis necessary to induce uptake of glucose [170]. Cold exposure induces norepinephrine release by the sympathetic nerves not only in BAT, but also in the cardiovascular system, and can therefore be problematic in patients with obesity and concomitant cardiovascular diseases. Finally, PET is generally a high-cost imaging tool and FDG-PET/CT is overall an expensive diagnostic tool requiring dedicated PET/CT scanners [171]. Taken together, new diagnostic tools that allow for the safe (non-radioactive, non-invasive) and affordable assessment of thermogenic adipose tissue activity in humans are required.
Beyond its well-recognized function in energy dissipation, BAT has been recognized as an endocrine organ that synthesizes and secretes different molecules, such as peptides, lipids, metabolites, and microRNA (miRNA), to regulate systemic metabolism [[172], [173], [174]]. Several groups have identified lipid species, referred to as lipokines, that exert signaling effects and act as metabolic messengers in regulating nutrient utilization, thermogenesis, and insulin sensitivity [175]. These lipid species can be detected in serum and tissue by liquid chromatography with tandem mass spectrometry (LC-MS/MS). In both mice and humans, there are marked changes in lipid compositions of adipose tissues and circulation upon a cold challenge. 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME) is one of the cold-induced lipokines found to be produced by BAT [176]. 12,13-diHOME is a metabolite derived from linoleic acid by the sequential enzymatic reactions of cytochrome P450 (CYP450) monooxygenase and soluble epoxide hydrolase. Circulating levels of 12,13-diHOME are significantly higher in human subjects acutely exposed to cold [176] or exercise [177]. 12,13-diHOME acts in an autocrine/paracrine manner to facilitate fatty acid utilization in BAT and reduce plasma triglyceride concentration in obese mice [176]. Besides 12,13-diHOME, cold exposure enhances the enzymatic activity of 12-lipoxygenase (12-LOX) in BAT, leading to elevated circulating levels of 12-LOX metabolites [178]. The administration of one of these 12-LOX-derived lipids, 12(S)-hydroxyeicosapentaenoic acid (12(S)-HEPE), into obese mice significantly improved their glucose tolerance and insulin sensitivity. In humans, circulating levels of 12(S)-HEPE are raised in response to the specific β3-adrenergic agonist mirabegron. Since the plasma levels of 12,13-diHOME and 12-HEPE are positively correlated with BAT activity, as measured by FDG uptake [176,178,179], it is suggested that these lipids could serve as surrogate biomarkers for BAT activities in humans. Recently, Kulterer et al. used [18F]-FDG PET/CT to define the presence or absence of detectable BAT activity and found that the presence of active BAT is associated with cold-induced EE. Their studies also revealed that circulating levels of 12,13-diHOME and 12-HEPE are higher in human subjects with detectable BAT activity compared to those with undetectable BAT activity [179]. The levels of both lipokines are elevated in subjects with active BAT in response to cold exposure, reinforcing the notion that 12,13-diHOME and 12-HEPE are cold-induced, BAT-derived lipokines. Notably, the levels of 12,13-diHOMe and 12(S)-HEPE in human plasma correlate negatively with bodyweight and insulin resistance [176,178,180], indicating a potential role of these lipid mediators in modulating human obesity and diabetes.
miRNAs, small non-coding RNAs of approximately 25 nucleotide size, mediate the specific post-transcriptional gene silencing of their target mRNAs by binding to an untranslated region (prominently at 3′) and marking them for degradation [181]. Specific miRNAs could potentially serve as biomarkers. miRNA are involved in many biological processes, including the regulation of differentiation of brown and beige/brite adipocytes [182], with an important example being miR155, which regulates the transcription factor CAAT enhancer binding protein β [183]. Moreover, miRNAs are actively secreted/released by cells and are involved in inter-organ and –cellular communication. Thus, miRNAs can be found in the extracellular space and bodily fluids, including blood [184] and urine. These extracellular miRNAs are either packaged in extracellular vesicles or form protein-miRNA complexes [185]. Extracellular miRNAs have great diagnostic potential for several diseases including cancer [186,187], T2D, and obesity [185]. Adipose tissues have been shown to contribute to body-wide extracellular vesicle secretion [188,189]. Interestingly, brown adipocytes not only release extracellular vesicles but also significantly increase the extracellular vesicle release after stimulation with cAMP, the second messenger that mediates adrenergic- and cold-induced BAT activation [188]. Moreover, cold exposure in mice induces a nine-fold increase in extracellular vesicle release from BAT [188]. BAT-derived extracellular vesicles contain several hundred miRNAs, and the profiling of extracellular vesicles isolated from the serum of cold-exposed or CL-316,243-treated mice revealed that several miRNAs are either up- or downregulated after BAT activation [188]. The levels of one of these exosomal miRNAs, miR92a, in the serum reflected brown fat activity in mice [188]. Importantly, analysis of extracellular vesicles isolated from cold-exposed humans that were either BAT-positive or –negative – as measured by FDG PET/CT – revealed that miR92a levels in human serum inversely correlate with human BAT activity. Thus, exosomal miR-92a represents a potential first serum biomarker for BAT activity in mice and humans. Further studies are required to elucidate whether miR92a or other miRNAs can be used as diagnostic biomarkers for BAT activity in larger cohorts, including patients with obesity or diabetes, as well as in patients with weight loss. The usage of biomarkers could be helpful tools in predicting the success of EE approaches to treat obesity.
4. Clinical perspective
A substantial proportion of individuals in today's environment gain significant excess bodyweight during their lifetime. Tailored approaches are necessary to provide precision medicine catered to individual needs. Interactions between genetic, environmental, and lifestyle factors contribute to obesity and must be taken into account during the development of therapies [8,9].
4.1. Patient subpopulations
Genetic factors such as heritability and genetic variants, including epigenetics, play a role in the pathogenesis of obesity [190,191]. While a few causes of monogenetic obesity have been identified, only a small amount of the apparent heritability of obesity has been explained until today (reviewed by Xia and Grant) [192]. Because the BMI distribution is very wide, indicating differences in individual responses to a common obesogenic environment, large genome-wide-association studies (GWAS) were performed (recent GWAS obesity reviews [193,194]) to investigate the genetic mechanisms underlying the development of extreme cases of obesity and to improve the understanding of the factors responsible for excessive weight gain in certain populations. A number of genetic loci were identified to be associated with BMI and adiposity traits, with prominent genes near loci enriched for central nervous system expression as well as for fat distribution in adipose tissue itself, confirming that these two tissues are important for bodyweight control. However, current associations explain only a minor percentage (2–3%) of the variation in adult BMI. Hence, the low level of variance in these results clearly calls into question the clinical relevance of GWAS-identified obesity genes [195]. Therefore, a re-launch of future obesity GWAS studies employing more biologically meaningful phenotypes (e.g., EE, in-depth body composition analyses, and longitudinal weight changes) could provide a better mechanistic understanding of how genetics influence bodyweight and could help to design personalized treatments. Moreover, only a few studies on the other side of the BMI range exist, investigating the physiology and genetic architecture of healthy thinness (defined as BMI < 18 in the absence of disease). In a recent publication, the heritability of thinness was found to be comparable with that of severe obesity [196]. The loci identified overlapped with those already identified in association with obesity. However, novel loci were also identified and could be studied in more detail to improve the understanding of the physiology of habitual leanness. Interestingly, a distinct molecular signature of adipose tissue indicating a potential role of adipose tissue and EE in human thinness was suggested in another recent study [197]. This demonstrates that obesity and leanness being opposite phenotypes provide interesting starting points for assessing causal gene variations related to bodyweight and composition as well as understanding differences between individuals and identifying subpopulations.
4.2. Environmental factors and lifestyle
Environmental changes such as a sedentary lifestyle may further explain the mean weight gain observed in a population [198,199]. However, despite said environmental changes as well as the physiological adaptations outlined above, about 25% of any given population can maintain a stable bodyweight overtime without adhering to caloric restriction or excessive exercise [200,201]. Conversely, various large studies show that the lower rates of occupational-related (non-exercise) physical activity observed over the past few decades are associated with an increase in obesity rates (USA [198]; UK [202]). In addition, availability of energy-dense food [199], chronic stress [203], and disrupted sleep [204] add to the dysregulation of energy balance.
One explanation of the changes in energy balance is centered in the central nervous system. As mentioned above, there is a constant crosstalk between the brain and adipose tissue that controls body fat mass and energy homeostasis [27,28]. Furthermore, changes in hormone levels throughout life also favor bodyweight gain [205]. Drug-induced weight gain is a common side effect of medications such as beta-blockers, sulfonylureas, or centrally active substances [206] and needs to be taken into account during weight lowering approaches in these individuals.
These contributors to bodyweight gain already imply that the pathophysiology of the disease varies among patients and that it is important to capture these factors and provide treatments that match each individual patient's needs.
Taken together, the basis for non-response to certain treatments is complex and poorly understood. Therefore, there is an urgent need for a chronically effective pharmacotherapy that increases EE and/or decreases EI to manage the disease long-term.
4.3. Outlook
In the clinical context, different phenotypes of patients with obesity require tailored therapeutic approaches. In some patients with a high degree of physical activity, e.g., relatively young males who work physically, a reduction in EI quickly leads to considerable weight loss. Thus, an approach using a calorie-reduced diet or a GLP-1 agonist which reduces appetite and EI may be a highly effective strategy. Conversely, another common phenotype in obesity clinics presents relatively low amounts of RMR. As reviewed earlier in chapter 2, this phenomenon is mostly present following very low calorie diets [72] or bariatric surgery [207]. The further reduction of EI proves difficult in these patients and is often unsuccessful. While physical exercise can increase the TEE, its efficacy is often minimal because patients are either limited in the time they can spend exercising or in their exercise capacity. Disease conditions such as hyperthyroidism and pheochromocytoma demonstrate that an increase of RMR can lead to significant weight loss [[208], [209], [210]]. Prior experience with the energy-expending drug 2,4,-dinitrophenol also underscores that the therapeutic targeting of EE is essentially feasible [211]. This indicates that future obesity therapies should target RMR, an important component of the calorie balance. Furthermore, patients in clinical and observational trials are not phenotyped to an extent that allows for the definition of the different subtypes. Differences in treatment duration, with some studies investigating effects after several weeks and others after a year, a huge BMI range, and a rather small sample size make it difficult to compare results.
Moreover, patients that experienced weight regain and weight cycling driven by a reduction in RMR would benefit from new approaches to increase EE [72] the most. However, current data on personalized obesity treatments that also address EE is extremely limited. Most weight loss trials used low-calorie diets (e.g., diabetes remission clinical trial (DiRECT)). In addition, weight loss programs with a significant amount of exercise are associated with decreases in RMR [212] that make it mostly impossible to maintain a stable bodyweight. Therefore, it might be speculated that, in patients with low EE due to a sedentary lifestyle and/or decreases in RMR due to weight loss attempts, increasing EE by activating BAT or other uncoupling mechanisms could not only promote weight loss but, more importantly, weight maintenance.
Together, the challenge for all weight loss attempts seems to be the physiological response to a negative energy balance as metabolic efficiency is increased, which results in a decrease of RMR, at least in the short-term. Therefore, attempts to increase RMR to complement reduced EI are urgently needed, especially in obesity patients who struggle with weight maintenance and weight cycling.
5. Epilogue
Energy mobilization and expenditure are subjective but decisive factors for chronic bodyweight homeostasis. Through this perspective, we put forth a concise discussion on key considerations during the experimental data analysis from preclinical in vitro, in vivo, and clinical setups. We project that, in the future, patient-centric care must include not only BMI, but also the evaluation of disease history (weight cycling history, concomitant conditions, medication, eating behavior, environment, physical functioning, and chronic pain that enables dedicated chronic therapy in patients with obesity) as well as individuals' energy homeostatic and metabolic features. Clinical measurement of EE might also help to decide if it is better for a given patient to target EI or to focus on intervention of EE. Through precision medicine, an optimized therapeutic benefit is given by targeting an individual patient's needs based on genetic, biomarker, phenotypic, or psychosocial characteristics.
Acknowledgments
We thank Dr. Thomas Klein, Dr. Alexander Pfeifer, Dr. Tamara Baader-Pagler, Dr. Claus Thamer, and Dr. Jürgen Schymeinsky for their assistance in the preparation of this manuscript. The graphical abstract, as well as Figure 1 have been made with biorender.com.
Conflict of interest
None declared.
References
- 1.Müller T., Clemmensen C., Finan B., DiMarchi R., Tschöp M. Anti-obesity therapy: from rainbow pills to polyagonists. Pharmacological Reviews. 2018;70:712–746. doi: 10.1124/pr.117.014803. [DOI] [PubMed] [Google Scholar]
- 2.Chen K.Y. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. Journal of Biological Chemistry. 2020;295:1926–1942. doi: 10.1074/jbc.REV119.007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rolfe D., Brown G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiological Reviews. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 4.Tschöp M.H. Unimolecular polypharmacy for treatment of diabetes and obesity. Cell Metabolism. 2016;24:51–62. doi: 10.1016/j.cmet.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Geary N. Control-theory models of body-weight regulation and body-weight-regulatory appetite. Appetite. 2020;144:104440. doi: 10.1016/j.appet.2019.104440. [DOI] [PubMed] [Google Scholar]
- 6.Ravussin Y., Leibel R.L., Ferrante A.W., Jr. A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metabolism. 2014;20:565–572. doi: 10.1016/j.cmet.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbaum M. Resistance training reduces skeletal muscle work efficiency in weight-reduced and non–weight-reduced subjects. Obesity. 2018;26:1576–1583. doi: 10.1002/oby.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marti A., Moreno-Aliaga M., Hebebrand J., Martinez J. Genes, lifestyles and obesity. International Journal of Obesity. 2004;28:S29–S36. doi: 10.1038/sj.ijo.0802808. [DOI] [PubMed] [Google Scholar]
- 9.Marti A., Martinez-González M.A., Martinez J.A. Interaction between genes and lifestyle factors on obesity: nutrition society silver medal lecture. Proceedings of the Nutrition Society. 2008;67:1–8. doi: 10.1017/S002966510800596X. [DOI] [PubMed] [Google Scholar]
- 10.Leibel R.L., Rosenbaum M., Hirsch J. Changes in energy expenditure resulting from altered body weight. New England Journal of Medicine. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 11.Speakman J.R. Measuring energy metabolism in the mouse–theoretical, practical, and analytical considerations. Frontiers in Physiology. 2013;4:34. doi: 10.3389/fphys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndahimana D., Kim E.-K. Measurement methods for physical activity and energy expenditure: a review. Clinical Nutrition Research. 2017;6:68–80. doi: 10.7762/cnr.2017.6.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum M., Leibel R.L. Adaptive thermogenesis in humans. International Journal of Obesity. 2010;34:S47–S55. doi: 10.1038/ijo.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachman E.S. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 15.Liao W.H., Henneberg M., Langhans W. Immunity-based evolutionary interpretation of diet-induced thermogenesis. Cell Metabolism. 2016;23:971–979. doi: 10.1016/j.cmet.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Pontzer H. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Current Biology. 2016;26:410–417. doi: 10.1016/j.cub.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King N.A. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity. 2007;15:1373–1383. doi: 10.1038/oby.2007.164. [DOI] [PubMed] [Google Scholar]
- 18.Hall K.D. Energy balance and its components: implications for body weight regulation. American Journal of Clinical Nutrition. 2012;95:989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebbeling C.B. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. Bmj. 2018;363 doi: 10.1136/bmj.k4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira M.A., Swain J., Goldfine A.B., Rifai N., Ludwig D.S. Effects of a low–glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482–2490. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 21.de Souza R.J. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. American Journal of Clinical Nutrition. 2012;95:614–625. doi: 10.3945/ajcn.111.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu T. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. American Journal of Epidemiology. 2012;176:S44–S54. doi: 10.1093/aje/kws264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall K.D. Quantification of the effect of energy imbalance on bodyweight. The Lancet. 2011;378:826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grannell A., De Vito G., Murphy J.C., le Roux C.W. The influence of skeletal muscle on appetite regulation. Expert Review of Endocrinology & Metabolism. 2019;14:267–282. doi: 10.1080/17446651.2019.1618185. [DOI] [PubMed] [Google Scholar]
- 25.Dulloo A.G. Physiology of weight regain: lessons from the classic Minnesota Starvation Experiment on human body composition regulation. Obesity Reviews. 2021 doi: 10.1111/obr.13189. [DOI] [PubMed] [Google Scholar]
- 26.Henningsen J.B., Scheele C. Brown adipose tissue: a metabolic regulator in a hypothalamic cross talk? Annual Review of Physiology. 2020;83 doi: 10.1146/annurev-physiol-032420-042950. [DOI] [PubMed] [Google Scholar]
- 27.Bartness T.J., Liu Y., Shrestha Y.B., Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Frontiers in Neuroendocrinology. 2014;35:473–493. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pénicaud L., Lorsignol A. Springer; 2013. Physiology and Physiopathology of adipose tissue; pp. 171–185. [Google Scholar]
- 29.St-Onge M.-P., Gallagher D. Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition. 2010;26:152–155. doi: 10.1016/j.nut.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller M.J. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. American Journal of Clinical Nutrition. 2004;80:1379–1390. doi: 10.1093/ajcn/80.5.1379. [DOI] [PubMed] [Google Scholar]
- 31.Bouchard C., Dériaz O., Pérusse L., Tremblay A. Genetics of energy expenditure in humans. The Genetics of Obesity. 1994;2:135–146. [Google Scholar]
- 32.Schmidt-Nielsen K., Knut S.-N. Cambridge university press; 1984. Scaling: why is animal size so important? [Google Scholar]
- 33.Kinney J.M. Raven Press; 1992. Energy metabolism: tissue determinants and cellular corollaries. [Google Scholar]
- 34.Müller M.J., Wang Z., Heymsfield S.B., Schautz B., Bosy-Westphal A. Advances in the understanding of specific metabolic rates of major organs and tissues in humans. Current Opinion in Clinical Nutrition and Metabolic Care. 2013;16:501–508. doi: 10.1097/MCO.0b013e328363bdf9. [DOI] [PubMed] [Google Scholar]
- 35.Dulloo A.G., Jacquet J., Solinas G., Montani J.-P., Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. International Journal of Obesity. 2010;34:S4–S17. doi: 10.1038/ijo.2010.234. [DOI] [PubMed] [Google Scholar]
- 36.Stanford K.I., Middelbeek R.J., Goodyear L.J. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes. 2015;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peres Valgas da Silva C., Hernández-Saavedra D., White J.D., Stanford K.I. Cold and exercise: therapeutic tools to activate brown adipose tissue and combat obesity. Biology. 2019;8:9. doi: 10.3390/biology8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heymsfield S.B. Human energy expenditure: advances in organ-tissue prediction models. Obesity Reviews. 2018;19:1177–1188. doi: 10.1111/obr.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibrahim M.M. Subcutaneous and visceral adipose tissue: structural and functional differences. Obesity Reviews. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee M.-J., Wu Y., Fried S.K. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Molecular Aspects of Medicine. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahn C.R., Wang G., Lee K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. Journal of Clinical Investigation. 2019;129:3990–4000. doi: 10.1172/JCI129187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosell M. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. American Journal of Physiology-Endocrinology and Metabolism. 2014;306:E945–E964. doi: 10.1152/ajpendo.00473.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith W.S., Broadbridge R., East J.M., Lee A.G. Sarcolipin uncouples hydrolysis of ATP from accumulation of Ca2+ by the Ca2+-ATPase of skeletal-muscle sarcoplasmic reticulum. Biochemical Journal. 2002;361:277–286. doi: 10.1042/0264-6021:3610277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Meis L. The thermogenic activity of rat brown adipose tissue and rabbit white muscle Ca2+-ATPase. IUBMB Life. 2005;57:337–345. doi: 10.1080/15216540500092534. [DOI] [PubMed] [Google Scholar]
- 47.u Din M. Human brown adipose tissue [15 O] O 2 PET imaging in the presence and absence of cold stimulus. European Journal of Nuclear Medicine and Molecular Imaging. 2016;43:1878–1886. doi: 10.1007/s00259-016-3364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Betz M.J., Enerbäck S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nature Reviews Endocrinology. 2018;14:77–87. doi: 10.1038/nrendo.2017.132. [DOI] [PubMed] [Google Scholar]
- 49.Goedeke L., Shulman G.I. Therapeutic potential of mitochondrial uncouplers for the treatment of metabolic associated fatty liver disease and NASH. Molecular Metabolism. 2021:101178. doi: 10.1016/j.molmet.2021.101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravussin E. Reduced rate of energy expenditure as a risk factor for body-weight gain. New England Journal of Medicine. 1988;318:467–472. doi: 10.1056/NEJM198802253180802. [DOI] [PubMed] [Google Scholar]
- 51.Müller M.J., Enderle J., Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Current Obesity Report. 2016;5:413–423. doi: 10.1007/s13679-016-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reinhardt M. A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes. 2015;64:2859–2867. doi: 10.2337/db14-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piaggi P. Metabolic determinants of weight gain in humans. Obesity. 2019;27:691–699. doi: 10.1002/oby.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hollstein T. Reduced brown adipose tissue activity during cold exposure is a metabolic feature of the human thrifty phenotype. Metabolism. 2021;117:154709. doi: 10.1016/j.metabol.2021.154709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polidori D., Sanghvi A., Seeley R.J., Hall K.D. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity. 2016;24:2289–2295. doi: 10.1002/oby.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hao Z. Reprogramming of defended body weight after R oux-En-Y gastric bypass surgery in diet-induced obese mice. Obesity. 2016;24:654–660. doi: 10.1002/oby.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Müller M.J., Geisler C., Heymsfield S.B., Bosy-Westphal A. Recent advances in understanding body weight homeostasis in humans. F1000Research. 2018;7 doi: 10.12688/f1000research.14151.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wirtshafter D., Davis J.D. Set points, settling points, and the control of body weight. Physiology & Behavior. 1977;19:75–78. doi: 10.1016/0031-9384(77)90162-7. [DOI] [PubMed] [Google Scholar]
- 59.Speakman J.R. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Disease models & mechanisms. 2011;4:733–745. doi: 10.1242/dmm.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kennedy G. The role of depot fat in the hypothalamic control of food intake in the rat. Proceedings of the Royal Society of London. Series B-Biological Sciences. 1953;140:578–592. doi: 10.1098/rspb.1953.0009. [DOI] [PubMed] [Google Scholar]
- 61.Lissner L. Body composition and energy intake: do overweight women overeat and underreport? American Journal of Clinical Nutrition. 1989;49:320–325. doi: 10.1093/ajcn/49.2.320. [DOI] [PubMed] [Google Scholar]
- 62.Blundell J.E. Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. British Journal of Nutrition. 2012;107:445–449. doi: 10.1017/S0007114511003138. [DOI] [PubMed] [Google Scholar]
- 63.Dulloo A.G., Jacquet J., Girardier L. Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues. American Journal of Clinical Nutrition. 1997;65:717–723. doi: 10.1093/ajcn/65.3.717. [DOI] [PubMed] [Google Scholar]
- 64.Dulloo A.G., Jacquet J., Miles-Chan J.L., Schutz Y. Passive and active roles of fat-free mass in the control of energy intake and body composition regulation. European Journal of Clinical Nutrition. 2017;71:353–357. doi: 10.1038/ejcn.2016.256. [DOI] [PubMed] [Google Scholar]
- 65.Rosenbaum M., Hirsch J., Gallagher D.A., Leibel R.L. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. American Journal of Clinical Nutrition. 2008;88:906–912. doi: 10.1093/ajcn/88.4.906. [DOI] [PubMed] [Google Scholar]
- 66.Sumithran P. Long-term persistence of hormonal adaptations to weight loss. New England Journal of Medicine. 2011;365:1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 67.Polidori D., Sanghvi A., Seeley R.J., Hall K.D. How strongly does appetite counter weight loss? Quantification of the feedback control of human energy intake. Obesity. 2016;24:2289–2295. doi: 10.1002/oby.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swift D.L. The effects of exercise and physical activity on weight loss and maintenance. Progress in Cardiovascular Diseases. 2018;61:206–213. doi: 10.1016/j.pcad.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 69.Catenacci V.A., Wyatt H.R. The role of physical activity in producing and maintaining weight loss. Nature Clinical Practice Endocrinology & Metabolism. 2007;3:518–529. doi: 10.1038/ncpendmet0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foster-Schubert K.E. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity. 2012;20:1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity. 2014;22:5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fothergill E. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity. 2016;24:1612–1619. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inge T.H. Five-year outcomes of gastric bypass in adolescents as compared with adults. New England Journal of Medicine. 2019;380:2136–2145. doi: 10.1056/NEJMoa1813909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heshka S. Resting energy expenditure and organ-tissue body composition 5 Years after bariatric surgery. Obesity Surgery. 2020;30:587–594. doi: 10.1007/s11695-019-04217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Adams T.D. Weight and metabolic outcomes 12 years after gastric bypass. New England Journal of Medicine. 2017;377:1143–1155. doi: 10.1056/NEJMoa1700459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carlsson L.M. Life expectancy after bariatric surgery in the Swedish obese subjects study. New England Journal of Medicine. 2020;383:1535–1543. doi: 10.1056/NEJMoa2002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mingrone G. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. The Lancet. 2021;397:293–304. doi: 10.1016/S0140-6736(20)32649-0. [DOI] [PubMed] [Google Scholar]
- 78.Lamarca F., Melendez-Araújo M.S., de Toledo I.P., Dutra E.S., de Carvalho K.M.B. Relative energy expenditure decreases during the first year after bariatric surgery: a systematic review and meta-analysis. Obesity Surgery. 2019:1–12. doi: 10.1007/s11695-019-03934-0. [DOI] [PubMed] [Google Scholar]
- 79.Holst J.J. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surgery for Obesity and Related Diseases. 2018;14:708–714. doi: 10.1016/j.soard.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Langhans W., Holst J.J. Afferent endocrine control of eating. Neuroendocrinology of appetite. 2016:24–54. [Google Scholar]
- 81.Clemmensen C. Gut-brain cross-talk in metabolic control. Cell. 2017;168:758–774. doi: 10.1016/j.cell.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adan R.A. Mechanisms underlying current and future anti-obesity drugs. Trends in Neurosciences. 2013;36:133–140. doi: 10.1016/j.tins.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Astrup A. Mass Medical Soc; 2010. [Google Scholar]
- 84.Scheen A.J. Sibutramine on cardiovascular outcome. Diabetes Care. 2011;34:S114–S119. doi: 10.2337/dc11-s205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loh R.K. Acute metabolic and cardiovascular effects of mirabegron in healthy individuals. Diabetes, Obesity and Metabolism. 2019;21:276–284. doi: 10.1111/dom.13516. [DOI] [PubMed] [Google Scholar]
- 86.Karhunen L. Effect of orlistat treatment on body composition and resting energy expenditure during a two-year weight-reduction programme in obese Finns. International Journal of Obesity. 2000;24:1567–1572. doi: 10.1038/sj.ijo.0801443. [DOI] [PubMed] [Google Scholar]
- 87.Wilding J.P. Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine. 2021 doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 88.Marso S.P. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Howell R., Wright A.M., Clements J.N. Clinical potential of liraglutide in cardiovascular risk reduction in patients with type 2 diabetes: evidence to date. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2019;12:505. doi: 10.2147/DMSO.S174568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brandt S.J., Götz A., Tschöp M.H., Müller T.D. Gut hormone polyagonists for the treatment of type 2 diabetes. Peptides. 2018;100:190–201. doi: 10.1016/j.peptides.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blondin D.P. Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue. The Journal of Physiology. 2017;595:2099–2113. doi: 10.1113/JP273395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blondin D.P. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. Journal of Clinical Endocrinology & Metabolism. 2014;99:E438–E446. doi: 10.1210/jc.2013-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paulik M.A. Development of infrared imaging to measure thermogenesis in cell culture: thermogenic effects of uncoupling protein-2, troglitazone, and β-adrenoceptor agonists. Pharmaceutical Research. 1998;15:944–949. doi: 10.1023/a:1011993019385. [DOI] [PubMed] [Google Scholar]
- 94.Law J. The use of infrared thermography in the measurement and characterization of brown adipose tissue activation. Temperature. 2018;5:147–161. doi: 10.1080/23328940.2017.1397085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clark D.G., Brinkman M., Neville S.D. Microcalorimetric measurements of heat production in brown adipocytes from control and cafeteria-fed rats. Biochemical Journal. 1986;235:337–342. doi: 10.1042/bj2350337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nedergaard J., Cannon B., Lindberg O. Microcalorimetry of isolated mammalian cells. Nature. 1977;267:518–520. doi: 10.1038/267518a0. [DOI] [PubMed] [Google Scholar]
- 97.Kriszt R. Optical visualisation of thermogenesis in stimulated single-cell brown adipocytes. Scientific Reports. 2017;7:1–14. doi: 10.1038/s41598-017-00291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie T.-R., Liu C.-F., Kang J.-S. Dye-based mito-thermometry and its application in thermogenesis of brown adipocytes. Biophysics Reports. 2017;3:85–91. doi: 10.1007/s41048-017-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hayashi T., Fukuda N., Uchiyama S., Inada N. A cell-permeable fluorescent polymeric thermometer for intracellular temperature mapping in mammalian cell lines. PloS One. 2015;10 doi: 10.1371/journal.pone.0117677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gota C., Okabe K., Funatsu T., Harada Y., Uchiyama S. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. Journal of the American Chemical Society. 2009;131:2766–2767. doi: 10.1021/ja807714j. [DOI] [PubMed] [Google Scholar]
- 101.Peng H.-S., Huang S.-H., Wolfbeis O.S. Ratiometric fluorescent nanoparticles for sensing temperature. Journal of Nanoparticle Research. 2010;12:2729–2733. [Google Scholar]
- 102.Kiyonaka S. Genetically encoded fluorescent thermosensors visualize subcellular thermoregulation in living cells. Nature Methods. 2013;10:1232–1238. doi: 10.1038/nmeth.2690. [DOI] [PubMed] [Google Scholar]
- 103.Maskow T., Paufler S. What does calorimetry and thermodynamics of living cells tell us? Methods. 2015;76:3–10. doi: 10.1016/j.ymeth.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 104.Bugge A., Dib L., Collins S. Vol. 538. Elsevier; 2014. Methods in enzymology; pp. 233–247. [Google Scholar]
- 105.Schweizer S., Oeckl J., Klingenspor M., Fromme T. Substrate fluxes in brown adipocytes upon adrenergic stimulation and uncoupling protein 1 ablation. Life Science Alliance. 2018;1 doi: 10.26508/lsa.201800136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murphy A.N., Bredesen D.E., Cortopassi G., Wang E., Fiskum G. Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proceedings of the National Academy of Sciences. 1996;93:9893–9898. doi: 10.1073/pnas.93.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clark L.C., JR., Wolf R., Granger D., Taylor Z. Continuous recording of blood oxygen tensions by polarography. Journal of Applied Physiology. 1953;6:189–193. doi: 10.1152/jappl.1953.6.3.189. [DOI] [PubMed] [Google Scholar]
- 108.Diepart C. Comparison of methods for measuring oxygen consumption in tumor cells in vitro. Analytical Biochemistry. 2010;396:250–256. doi: 10.1016/j.ab.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 109.Vanderkooi J.M., Maniara G., Green T.J., Wilson D.F. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. Journal of Biological Chemistry. 1987;262:5476–5482. [PubMed] [Google Scholar]
- 110.Papkovsky D.B. Vol. 381. Elsevier; 2004. Methods in enzymology; pp. 715–735. [Google Scholar]
- 111.Dmitriev R.I., Papkovsky D.B. Optical probes and techniques for O 2 measurement in live cells and tissue. Cellular and Molecular Life Sciences. 2012;69:2025–2039. doi: 10.1007/s00018-011-0914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hynes J. Investigation of drug-induced mitochondrial toxicity using fluorescence-based oxygen-sensitive probes. Toxicological Sciences. 2006;92:186–200. doi: 10.1093/toxsci/kfj208. [DOI] [PubMed] [Google Scholar]
- 113.James P.E., Jackson S.K., Grinberg O.Y., Swartz H.M. The effects of endotoxin on oxygen consumption of various cell types in vitro: an EPR oximetry study. Free Radical Biology and Medicine. 1995;18:641–647. doi: 10.1016/0891-5849(94)00179-n. [DOI] [PubMed] [Google Scholar]
- 114.Ma Y. Functional analysis of molecular and pharmacological modulators of mitochondrial fatty acid oxidation. Scientific Reports. 2020;10:1–13. doi: 10.1038/s41598-020-58334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berry M.N. Intracellular mitochondrial membrane potential as an indicator of hepatocyte energy metabolism: further evidence for thermodynamic control of metabolism. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1988;936:294–306. doi: 10.1016/0005-2728(88)90005-9. [DOI] [PubMed] [Google Scholar]
- 116.Hill B.G. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biological Chemistry. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochemical Journal. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huynh F.K., Green M.F., Koves T.R., Hirschey M.D. Vol. 542. Elsevier; 2014. Methods in enzymology; pp. 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaur G., Dufour J.M. 2012. Taylor & Francis. [Google Scholar]
- 120.Pan C., Kumar C., Bohl S., Klingmueller U., Mann M. Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Molecular & Cellular Proteomics. 2009;8:443–450. doi: 10.1074/mcp.M800258-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mayxard L. The Atwater system of calculating the caloric value of diets. Journal of Nutrition. 1944;28:443–452. [Google Scholar]
- 122.Sánchez-Peña M.J. Calculating the metabolizable energy of macronutrients: a critical review of Atwater's results. Nutrition Reviews. 2017;75:37–48. doi: 10.1093/nutrit/nuw044. [DOI] [PubMed] [Google Scholar]
- 123.Atwater W.O. US Dept. of Agriculture; 1910. Principles of nutrition and nutritive value of food. [Google Scholar]
- 124.Sender R., Fuchs S., Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 125.Ravussin Y., Gutman R., LeDuc C.A., Leibel R.L. Estimating energy expenditure in mice using an energy balance technique. International Journal of Obesity. 2013;37:399–403. doi: 10.1038/ijo.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Levine J.A. Measurement of energy expenditure. Public Health Nutrition. 2005;8:1123–1132. doi: 10.1079/phn2005800. [DOI] [PubMed] [Google Scholar]
- 127.Wilson R.P. Estimates for energy expenditure in free-living animals using acceleration proxies: a reappraisal. Journal of Animal Ecology. 2020;89:161–172. doi: 10.1111/1365-2656.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaiyala K.J., Ramsay D.S. Direct animal calorimetry, the underused gold standard for quantifying the fire of life. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2011;158:252–264. doi: 10.1016/j.cbpa.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kenny G.P., Notley S.R., Gagnon D. Direct calorimetry: a brief historical review of its use in the study of human metabolism and thermoregulation. European Journal of Applied Physiology. 2017;117:1765–1785. doi: 10.1007/s00421-017-3670-5. [DOI] [PubMed] [Google Scholar]
- 130.Webb P. The measurement of energy expenditure. Journal of Nutrition. 1991;121:1897–1901. doi: 10.1093/jn/121.11.1897. [DOI] [PubMed] [Google Scholar]
- 131.Lifson N., Gordon G.B., McClintock R. Measurement of total carbon dioxide production by means of D2O18. Journal of Applied Physiology. 1955;7:704–710. doi: 10.1152/jappl.1955.7.6.704. [DOI] [PubMed] [Google Scholar]
- 132.Lifson N., McClintock R. Theory of use of the turnover rates of body water for measuring energy and material balance. Journal of Theoretical Biology. 1966;12:46–74. doi: 10.1016/0022-5193(66)90185-8. [DOI] [PubMed] [Google Scholar]
- 133.Guidotti S., Meijer H.A., Dijk G.v. Validity of the doubly labeled water method for estimating CO2 production in mice under different nutritional conditions. American Journal of Physiology-Endocrinology and Metabolism. 2013;305:E317–E324. doi: 10.1152/ajpendo.00192.2013. [DOI] [PubMed] [Google Scholar]
- 134.Westerterp K.R. Doubly labelled water assessment of energy expenditure: principle, practice, and promise. European Journal of Applied Physiology. 2017;117:1277–1285. doi: 10.1007/s00421-017-3641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mtaweh H., Tuira L., Floh A.A., Parshuram C.S. Indirect calorimetry: history, technology, and application. Frontiers in Pediatrics. 2018;6:257. doi: 10.3389/fped.2018.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lavoisier A., De Laplace P. Histoire de l'Académie des Sciences. Année. 1780;355 [Google Scholar]
- 137.Regnault H.-V., Reiset J. Bachelier; 1849. Recherches chimiques sur la respiration des animaux des diverses classes. [Google Scholar]
- 138.Zuntz N., Schumburg W.A.E.F. Vol. 6. Hirschwald; 1901. Studien zu einer Physiologie des Marsches. [Google Scholar]
- 139.Lusk G. Analysis ofthe oxidation ofmixtures ofcarbohydrate and fat. Journal of Biological Chemistry. 1924;59:1–2. [Google Scholar]
- 140.Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988;37:287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- 141.Weir J.d.V. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of Physiology. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fernández-Calleja J.M. Extended indirect calorimetry with isotopic CO 2 sensors for prolonged and continuous quantification of exogenous vs. total substrate oxidation in mice. Scientific Reports. 2019;9:1–13. doi: 10.1038/s41598-019-47977-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cannon B., Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. Journal of Experimental Biology. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 144.Tschöp M.H. A guide to analysis of mouse energy metabolism. Nature Methods. 2012;9:57. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Even P.C., Nadkarni N.A. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2012;303:R459–R476. doi: 10.1152/ajpregu.00137.2012. [DOI] [PubMed] [Google Scholar]
- 146.Gupta R.D., Ramachandran R., Padmanaban Venkatesan S.A., Joseph M., Thomas N. Indirect calorimetry: from bench to bedside. Indian Journal of Endocrinology and Metabolism. 2017;21:594. doi: 10.4103/ijem.IJEM_484_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Würbel H. Ideal homes? Housing effects on rodent brain and behaviour. Trends in Neurosciences. 2001;24:207–211. doi: 10.1016/s0166-2236(00)01718-5. [DOI] [PubMed] [Google Scholar]
- 148.Voelkl B. Reproducibility of animal research in light of biological variation. Nature Reviews Neuroscience. 2020;21:384–393. doi: 10.1038/s41583-020-0313-3. [DOI] [PubMed] [Google Scholar]
- 149.Overton J.M. Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. International Journal of Obesity. 2010;34:S53–S58. doi: 10.1038/ijo.2010.240. [DOI] [PubMed] [Google Scholar]
- 150.Ravussin Y., LeDuc C.A., Watanabe K., Leibel R.L. Effects of ambient temperature on adaptive thermogenesis during maintenance of reduced body weight in mice. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2012;303:R438–R448. doi: 10.1152/ajpregu.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nedergaard J., Cannon B. The browning of white adipose tissue: some burning issues. Cell Metabolism. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 152.Kruse V., Neess D., Færgeman N.J. The significance of epidermal lipid metabolism in whole-body physiology. Trends in Endocrinology and Metabolism. 2017;28:669–683. doi: 10.1016/j.tem.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 153.Blond E. A new indirect calorimeter is accurate and reliable for measuring basal energy expenditure, thermic effect of food and substrate oxidation in obese and healthy subjects. E-SPEN, the European E-Journal of Clinical Nutrition and Metabolism. 2011;6:e7–e15. [Google Scholar]
- 154.Schoeller D.A. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1986;250:R823–R830. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- 155.Schoeller D.A., Taylor P.B., Shay K. Analytic requirements for the doubly labeled water method. Obesity Research. 1995;3:15–20. doi: 10.1002/j.1550-8528.1995.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 156.Diaz K.M. Fitbit®: an accurate and reliable device for wireless physical activity tracking. International Journal of Cardiology. 2015;185:138–140. doi: 10.1016/j.ijcard.2015.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Van Remoortel H. Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PloS One. 2012;7 doi: 10.1371/journal.pone.0039198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Harris J.A., Benedict F.G. A biometric study of human basal metabolism. Proceedings of the National Academy of Sciences of the United States of America. 1918;4:370. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mifflin M.D. A new predictive equation for resting energy expenditure in healthy individuals. American Journal of Clinical Nutrition. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 160.Holliday M., Potter D., Jarrah A., Bearg S. The relation of metabolic rate to body weight and organ size. Pediatric Research. 1967;1:185–195. doi: 10.1203/00006450-196705000-00005. [DOI] [PubMed] [Google Scholar]
- 161.Ouellet V. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. Journal of Clinical Investigation. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Labbé S.M. Normal postprandial nonesterified fatty acid uptake in muscles despite increased circulating fatty acids in type 2 diabetes. Diabetes. 2011;60:408–415. doi: 10.2337/db10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Befroy D.E., Shulman G.I. Magnetic resonance spectroscopy studies of human metabolism. Diabetes. 2011;60:1361–1369. doi: 10.2337/db09-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van de Weijer T., Schrauwen-Hinderling V.B. Application of magnetic resonance spectroscopy in metabolic research. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2019;1865:741–748. doi: 10.1016/j.bbadis.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 165.Virtanen K.A. Functional brown adipose tissue in healthy adults. New England Journal of Medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 166.Cypess A.M. Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.van Marken Lichtenbelt W.D. Cold-activated brown adipose tissue in healthy men. New England Journal of Medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 168.Saito M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nedergaard J., Bengtsson T., Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. American Journal of Physiology-Endocrinology and Metabolism. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 170.Bartelt A. Brown adipose tissue activity controls triglyceride clearance. Nature Medicine. 2011;17:200. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 171.Berger M., Gould M.K., Barnett P.G. The cost of positron emission tomography in six United States Veterans Affairs hospitals and two academic medical centers. American Journal of Roentgenology. 2003;181:359–365. doi: 10.2214/ajr.181.2.1810359. [DOI] [PubMed] [Google Scholar]
- 172.Wang G.X., Zhao X.Y., Lin J.D. The brown fat secretome: metabolic functions beyond thermogenesis. Trends in Endocrinology and Metabolism: Trends in Endocrinology and Metabolism. 2015;26:231–237. doi: 10.1016/j.tem.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Villarroya F., Cereijo R., Villarroya J., Giralt M. Brown adipose tissue as a secretory organ. Nature Reviews. Endocrinology. 2017;13:26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 174.Scheele C., Wolfrum C. Brown adipose crosstalk in tissue plasticity and human metabolism. Endocrine Reviews. 2020;41:53–65. doi: 10.1210/endrev/bnz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lynes M.D., Kodani S.D., Tseng Y.H. Lipokines and thermogenesis. Endocrinology. 2019;160:2314–2325. doi: 10.1210/en.2019-00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lynes M.D. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nature Medicine. 2017;23:631–637. doi: 10.1038/nm.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stanford K.I. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metabolism. 2018;27:1357. doi: 10.1016/j.cmet.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Leiria L.O. 12-Lipoxygenase regulates cold adaptation and glucose metabolism by producing the omega-3 lipid 12-HEPE from Brown fat. Cell Metabolism. 2019;30:768–783. doi: 10.1016/j.cmet.2019.07.001. e767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kulterer O.C. The presence of active Brown adipose tissue determines cold-induced energy expenditure and oxylipin profiles in humans. Journal of Clinical Endocrinology & Metabolism. 2020;105 doi: 10.1210/clinem/dgaa183. [DOI] [PubMed] [Google Scholar]
- 180.Vasan S.K. The proposed systemic thermogenic metabolites succinate and 12,13-diHOME are inversely associated with adiposity and related metabolic traits: evidence from a large human cross-sectional study. Diabetologia. 2019;62:2079–2087. doi: 10.1007/s00125-019-4947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 182.Chen Y., Pan R., Pfeifer A. Regulation of brown and beige fat by microRNAs. Pharmacology & Therapeutics. 2017;170:1–7. doi: 10.1016/j.pharmthera.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 183.Chen Y. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nature Communications. 2013;4:1–13. doi: 10.1038/ncomms2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nielsen S. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PloS One. 2014;9 doi: 10.1371/journal.pone.0087308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mori M.A., Ludwig R.G., Garcia-Martin R., Brandão B.B., Kahn C.R. Extracellular miRNAs: from biomarkers to mediators of physiology and disease. Cell metabolism. 2019 doi: 10.1016/j.cmet.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ogata-Kawata H. Circulating exosomal microRNAs as biomarkers of colon cancer. PloS One. 2014;9 doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang W. 57–67. Springer; 2017. Bioinformatics in MicroRNA research. [Google Scholar]
- 188.Chen Y. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nature Communications. 2016;7:1–9. doi: 10.1038/ncomms11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Thomou T. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Loos R.J., Bouchard C. Obesity–is it a genetic disorder? Journal of Internal Medicine. 2003;254:401–425. doi: 10.1046/j.1365-2796.2003.01242.x. [DOI] [PubMed] [Google Scholar]
- 191.Bell C.G., Walley A.J., Froguel P. The genetics of human obesity. Nature Reviews Genetics. 2005;6:221–234. doi: 10.1038/nrg1556. [DOI] [PubMed] [Google Scholar]
- 192.Xia Q., Grant S.F. The genetics of human obesity. Annals of the New York Academy of Sciences. 2013;1281:178. doi: 10.1111/nyas.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Young K.L., Graff M., Fernandez-Rhodes L., North K.E. Genetics of obesity in diverse populations. Current Diabetes Reports. 2018;18:145. doi: 10.1007/s11892-018-1107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Goodarzi M.O. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. The Lancet Diabetes & endocrinology. 2018;6:223–236. doi: 10.1016/s2213-8587(17)30200-0. [DOI] [PubMed] [Google Scholar]
- 195.Müller M.J. The case of GWAS of obesity: does body weight control play by the rules? International Journal of Obesity. 2005;42:1395–1405. doi: 10.1038/s41366-018-0081-6. (2018) [DOI] [PubMed] [Google Scholar]
- 196.Riveros-McKay F. Genetic architecture of human thinness compared to severe obesity. PLoS Genetics. 2019;15 doi: 10.1371/journal.pgen.1007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ling Y. Persistent low body weight in humans is associated with higher mitochondrial activity in white adipose tissue. American Journal of Clinical Nutrition. 2019;110:605–616. doi: 10.1093/ajcn/nqz144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Church T.S. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PloS One. 2011;6 doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hill J.O., Peters J.C. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 200.Kärkkäinen U., Mustelin L., Raevuori A., Kaprio J., Keski-Rahkonen A. Successful weight maintainers among young adults—a ten-year prospective population study. Eating Behaviors. 2018;29:91–98. doi: 10.1016/j.eatbeh.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 201.Truesdale K.P. Changes in risk factors for cardiovascular disease by baseline weight status in young adults who maintain or gain weight over 15 years: the CARDIA study. International Journal of Obesity. 2006;30:1397–1407. doi: 10.1038/sj.ijo.0803307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Millward D.J. Energy balance and obesity: a UK perspective on the gluttony v. sloth debate. Nutrition Research Reviews. 2013;26:89–109. doi: 10.1017/s095442241300005x. [DOI] [PubMed] [Google Scholar]
- 203.De Vriendt T., Moreno L.A., De Henauw S. Chronic stress and obesity in adolescents: scientific evidence and methodological issues for epidemiological research. Nutrition, Metabolism, and Cardiovascular Diseases. 2009;19:511–519. doi: 10.1016/j.numecd.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 204.Knutson K.L., Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Annals of the New York Academy of Sciences. 2008;1129:287. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rogge M.M., Gautam B. Biology of obesity and weight regain: implications for clinical practice. Journal of the American Association of Nurse Practitioners. 2017;29:S15–S29. doi: 10.1002/2327-6924.12504. [DOI] [PubMed] [Google Scholar]
- 206.Ness-Abramof R., Apovian C.M. Drug-induced weight gain. Drugs of Today. 2005;41:547. doi: 10.1358/dot.2005.41.8.893630. [DOI] [PubMed] [Google Scholar]
- 207.Thivel D. Surgical weight loss: impact on energy expenditure. Obesity Surgery. 2013;23:255–266. doi: 10.1007/s11695-012-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lahesmaa M. Hyperthyroidism increases brown fat metabolism in humans. Journal of Clinical Endocrinology & Metabolism. 2014;99:E28–E35. doi: 10.1210/jc.2013-2312. [DOI] [PubMed] [Google Scholar]
- 209.Petrák O. Changes in energy metabolism in pheochromocytoma. Journal of Clinical Endocrinology & Metabolism. 2013;98:1651–1658. doi: 10.1210/jc.2012-3625. [DOI] [PubMed] [Google Scholar]
- 210.Klímová J. FGF21 levels in pheochromocytoma/functional paraganglioma. Cancers. 2019;11:485. doi: 10.3390/cancers11040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tainter M., Stockton A., Cutting W. Use of dinitrophenol in obesity and related conditions: a progress report. The Journal of the American Medical Association. 1933;101:1472–1475. [Google Scholar]
- 212.Belko A.Z., Van Loan M., Barbieri T.F., Mayclin P. Diet, exercise, weight loss, and energy expenditure in moderately overweight women. International Journal of Obesity. 1987;11:93–104. [PubMed] [Google Scholar]