Abstract
Background
The pathological changes in Alzheimer's Disease (AD) and other neurodegenerative disorders begin decades prior to their clinical expression. However, the clinical diagnosis of neurodegenerative dementias is not straightforward. Lactoferrin is an iron-binding, antimicrobial glycoprotein with a plethora of functions, including acting as an important immune modulator and by having a bacteriocidic effect. Two previous studies indicated that salivary lactoferrin could differentiate between neurodegenerative dementias.
Methods
A total of 222 cerebrospinal fluid (CSF) and saliva samples from a consecutive, mixed memory clinic population were analysed for lactoferrin. In addition, the association between lactoferrin in CSF and saliva and the concentration of tau, phosphorylated tau (p-tau) and amyloid 1–42 (Aβ42) in CSF were addressed.
Findings
CSF lactoferrin was assessed for the first time in a cohort of patients with neurodegenerative dementias. No significant differences were found in the levels of CSF or saliva lactoferrin between the diagnostic groups. In addition, no significant relationships were found between lactoferrin levels and tau, p-tau and Aβ42, respectively.
Interpretation
Neither CSF nor saliva lactoferrin could differentiate between neurodegenerative dementias in this study.
Keywords: Alzheimer's disease, Dementia, CSF, Saliva, Lactoferrin, Biomarkers
Research in Context.
Evidence before this study
The pathological changes of Alzheimer's Disease (AD) and other neurodegenerative dementias begin decades prior to their clinical expression, and for that reason it is essential to find new biomarkers to detect these diseases at an early stage. Prior to this study, a systematic review; “Biomarkers for Alzheimer's Disease in Saliva: A Systematic Review,” Gleerup et al., was conducted to investigate salivary biomarkers for the diagnosis of Alzheimer's Disease (AD). The systematic review found that beta amyloid 1–42 (Aβ42), tau, lactoferrin, and selected metabolites could be candidates for future salivary biomarkers for AD. However, more studies should be carried out in larger sample sizes and in representative, clinical cohorts. One other research group has investigated salivary lactoferrin in AD and found that salivary lactoferrin could differentiate patients diagnoses with AD from healthy controls more accurately than Aβ42 and tau in cerebrospinal fluid (CSF).
Added value of this study
To the best of our knowledge, this consecutive study is the first to evaluate the levels of CSF lactoferrin. However, lactoferrin in the CSF does not seem to have diagnostic potential for AD or other neurodegenerative dementias. Furthermore, salivary lactoferrin was investigated, but although two other studies found salivary lactoferrin to be excellent in differentiating AD from other dementias using the same methods as in this study, this consecutive study could not reproduce these results in a consecutive, mixed, memory clinic cohort.
Implications of all the available evidence
This study is a true representation of the clinical reality in a mixed memory clinic cohort, but unfortunately lactoferrin in neither CSF nor saliva, seemed to be a valid diagnostic biomarker for AD or other dementia-related diseases. However, the need for new biomarkers and the easy accessibility of saliva, warrants more studies of biomarkers in saliva for the early diagnosis of AD and other neurodegenerative dementias.
Alt-text: Unlabelled box
1. Introduction
Dementia-related diseases are an increasing health concern, and it is estimated that around 46.8 million people worldwide are suffering from dementia, with an increasing prevalence in the older age groups [1]. Alzheimer's Disease (AD) is the leading cause of progressive dementia and accounts for approximately 60–80% of all dementia-related cases [2]. Currently, the diagnosis of AD and many other neurodegenerative diseases rely primarily on clinical assessment, neuroimaging and, to some extent, analysis of biomarkers in the cerebrospinal fluid (CSF). However, neuroimaging is expensive, exposes to radiation and some methods lack molecular specificity. Biomarkers obtained from the CSF, most commonly tau, phosphorylated tau (p-tau), and beta amyloid 1–42 (Aβ42), are well-established biomarkers with a good diagnostic precision used to detect the pathology of AD [3]. However, a lumbar puncture is an invasive procedure, which can potentially lead to complications and adverse effects [4]. Blood biomarkers are a highly developing field that encompasses a great diagnostic potential for differentiating between neurodegenerative and non-neurodegenerative diseases. Blood Aβ42 and p-tau have shown to be very specific to the pathology of AD and their diagnostic accuracy is comparable to a CSF sample [5], [6], [7], [8]. Several studies have also investigated blood neurofilament light chain (NfL), and have found that blood NfL is increased in both AD and other neurodegenerative dementias [9], [10], [11], [12]. Although blood biomarkers are easier to obtain than a CSF sample, it is nonetheless an invasive procedure. Quantitative computerized analysis of electroencephalography (qEEG), which is non-invasive, quick and inexpensive, has recently been highlighted as a potential beneficial diagnostic and prognostic biomarker in dementia in larger multicentre studies [13]. However, the relevance and validity of this method in dementia evaluation is presently under further investigations. The diagnosis of neurodegenerative dementias and the implementation in clinical practice of both neuroimaging and analysis of CSF biomarkers is not straightforward. There are no disease-modifying treatments available for the most common dementia diagnoses, but currently 132 agents are being investigated in on-going trials for treatment for AD [14], especially in preclinical and early stages of the disease. For the reasons above, it is essential to find new methods to detect AD and other neurodegenerative diseases at an early stage to enable disease-modifying trials to be initiated in the preclinical or prodromal phase, and maybe in the future serve as treatment monitoring. Furthermore, these methods must be easy to perform, inexpensive, potentially non-invasive and complications and adverse effects should be limited. Saliva could be sampled in primary care, in situations with limited access to more advanced diagnostic procedures, and furthermore serve as screening of large numbers of participants in clinical trials.
Saliva is a slightly acidic body fluid produced by the submandibular gland, the sublingual gland, the parotid gland and other minor glands, and consists mainly of water, glucose and a plethora of different proteins and ions. Saliva is an easily obtained source of potential biomarkers and saliva diagnostics have already been suggested in several areas, among these endocrinological, autoimmune and metabolic diseases [15], [16], [17], [18], cardiovascular diseases [19], cancer [20], HIV [21,22], and CNS disorders [23]. The composition of saliva can mirror the hormonal, emotional, metabolic, nutritional and immunological state of an individual [24]. Studies have reported that many of the proteins found in saliva, can also to be found in blood, which further emphasizes the value of a diagnostic salivary biomarker [25]. Several studies have investigated biomarkers in saliva for the diagnosis of AD and other neurodegenerative diseases, and biomarkers such as Aβ42, tau, lactoferrin, and selected metabolites have been suggested to be potential salivary biomarkers for the diagnosis of AD, but with conflicting results [26], [27], [28], [29], [30], [31], [32], [33], [34]. The mechanisms by which these biomarkers are excreted from the CNS into saliva is still not fully understood, however different theories have been proposed. Studies have reported that proteins can be transported from the blood into the saliva through either microfiltration or active or passive transport by passing through cells within the different salivary glands and the gingival sulcus [24,35]. Furthermore, it has been suggested that selected biomarkers for neurodegenerative diseases, such as Aβ42, are either expressed or produced by the salivary glands and other organs [32,34]. The salivary glands secrete saliva in response to parasympathetic innervation from the glossopharyngeal and the facial cranial nerves. Another theory is based on the finding that neurodegeneration also occurs in nerve terminals in the parasympathetic nervous system (PNS), which could result in an altered composition and production of saliva, thereby mirroring the AD pathological changes in the CNS [36]. Studies have even suggested, that biomarkers are excreted directly into saliva from the degenerated axons of the glossopharyngeal and the facial cranial nerves [36].
Lactoferrin is an important antimicrobial peptide in saliva, and has been proposed to be a potential biomarker for the diagnosis of AD. The iron-binding glycoprotein is produced by many exocrine glands of the body but is mainly found in the oral cavity. Lactoferrin is an important immune modulator, it transports iron and acts as the first line of defence of the body against bacteria, virus, fungi, free radicals etc [37]. In humans, one study found that decreased salivary lactoferrin could differentiate patients diagnosed with AD from healthy controls more accurately than Aβ42 and total tau in CSF [38]. Furthermore, it was shown, by same research group, that salivary lactoferrin could distinguish between prodromal AD, AD and frontotemporal dementia (FTD) [39]. The role of lactoferrin as a biomarker in a mixed memory clinic population is unknown. In the present consecutive study, lactoferrin was measured in CSF and saliva from patients with various neurodegenerative diseases was investigated for its potential in the diagnosis of AD and other neurodegenerative disorders.
2. Material and methods
2.1. Ethics
This study was conducted between 20 March 2019 and 20 December 2019, at either the Copenhagen Memory Clinic, Copenhagen University Hospital, Rigshospitalet, or at the Regional Dementia Research Center, Zealand University Hospital, Roskilde. The project was approved by the Ethical Comitee of the Capital Region of Denmark (H-19000651) and the Danish Data Protection Agency (VD-2019-105), and all patients gave informed consent to participation.
2.2. Subjects
Saliva samples were collected from patients referred for cognitive assessment. A total of 222 patients were included in the study, and CSF and saliva samples were collected from healthy controls (HC) (n = 20), MCI (n = 56), AD (n = 71) and non-AD patients (n = 75) were obtained. The group of non-AD patients included individuals with vascular dementia (VaD) (n = 11), mixed dementia (n = 8), FTD (n = 15), dementia with Lewy bodies (DLB) (n = 7), normal pressure hydrocephalus (NPH) (n = 16), Progressive supranuclear palsy (PSP) (n = 1), Parkinson's disease with dementia (PDD) (n = 1), alcoholic dementia (n = 6), and other either dementias of unknown aetiology (n = 6) or dementia types due to a non-neurodegenerative disease (n = 4). All patients were diagnosed at a consensus conference after an extensive evaluation, including clinical and neuropsychological examination, structural (Magnetic Resonance imaging or in a few cases Computerized Tomography), and in most subjects supplemented with 18F-fluordeoxyglucose positron emission tomography (18F-FDG-PET). CSF biomarkers for AD (tau, p-tau, and Aβ42) were used for diagnostic purposes with a cut-off for Aβ42 of 875 pg/mL [40]. The patients diagnosed with AD fulfilled the NIA-AA criteria [41], and the patients with MCI fulfilled the criteria suggested by the International Working Group in Mild Cognitive Impairment, where MCI can be caused by several etiologies [42]. The patients diagnosed with VaD fulfilled Society for Vascular Behavior and Cognitive Disorders (VASCOG) criteria [43], and the patients with mixed dementia fulfilled both the NIA-AA criteria and the VASCOG criteria [41,43]. The diagnosis of DLB was established according to the fourth report of the DLB consortium [44], while the patients diagnosed with FTD fulfilled the criteria for the behavioural variant [45] or the criteria for non-fluent aphasia or the semantic variant of FTD [46]. The diagnosis of NPH was established according to the international guideline criteria for iNPH [47]. PSP and PDD fulfilled the criteria suggested by the International Parkinson and Movement Disorder Society [48,49], while alcoholic dementia was diagnosed according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) [50]. The HC were recruited for research purposes and did not fulfil any of the criteria for dementia or MCI. All HC were amyloid negative in CSF, although absolute values were only available for nine out of 20 HC.
2.3. Sample
2.3.1. Saliva collection
To control for any diurnal variation of lactoferrin, all saliva samples from the Copenhagen Memory Clinic, Copenhagen University Hospital, Rigshospitalet, were collected around noon, while saliva samples from the Regional Dementia Research Centre, Zealand University Hospital were collected between 9:15 AM and 10:15 AM. All saliva samples were collected immediately after lumbar puncture. All subjects were asked to refrain from drinking, eating, smoking etc. for at least 30 min prior to the saliva sampling, which is equivalent to the time frame of the lumbar puncture. In addition, patients were requested to rinse their mouth with water, before providing a 1–3 mL whole, unstimulated saliva sample in a 15 mL polypropylene falcon tube.
2.3.2. Cerebrospinal fluid collection
CSF samples were obtained immediately prior to saliva sampling by lumbar puncture [51] and collected in polypropylene tubes [52].
2.3.3. Sample processing
Following saliva and CSF sampling, the falcon tubes were placed on ice until they were centrifuged at 2000 rpm for 10 min at 4°C. The saliva and CSF samples were redistributed in 250 µL aliquots and stored at −80 °C until further analysis.
2.3.4. Biomarker assays
All saliva and CSF samples were analysed for lactoferrin using the Lactoferrin (HLF2) Human ELISA Kit (ab108882) according to the instructions provided by the manufacturer [53]. Saliva samples were diluted 1:1000, however a few samples were outside the sensitivity range of the assay and were diluted 1:2000. CSF samples were run undiluted unless they were outside the sensitivity range of the assay. Then 1:10 diluted samples were used. CSF Αβ42 levels were determined using sandwich ELISA (INNOTEST® β-AMYLOID(1-42) Fujirebio). CSF levels of total tau and p-tau were determined using sandwich ELISA INNOTEST hTau Ag, and INNOTEST PHOSPHO-TAU(181P), respectively. The estimation of total protein in saliva was analysed using the PierceTM BCA Protein Assay Kit (ThermoFisher, Scientific), while the concentration of total protein in CSF was analysed using a turbidimetric method on a COBAS instrument at the department of Clinical Biochemistry at Copenhagen University Hospital, Rigshospitalet.
2.4. Statistical analyses
All statistics were performed in GraphPad Prism. An Anderson-Darling test was performed on all data on lactoferrin in saliva and CSF and on total protein in saliva and CSF to test for normal distribution. All lactoferrin data followed a non-normal distribution and were logarithmic transformed. The logarithmic transformed data were again tested for normal distribution with an Anderson-Darling test, but the data did still not follow a normal distribution, and for that reason the data was analysed with a nonparametric test. A Kruskal–Wallis test analysis was performed on lactoferrin in CSF and saliva and on the total protein concentration in CSF and saliva. Due to variations in total protein concentration, lactoferrin in CSF and saliva was normalized to the concentration of total protein, and Kruskal–Wallis test for normalized lactoferrin in CSF and saliva were performed. As a sub analysis, the levels of lactoferrin, total protein and normalized lactoferrin in CSF and saliva were assessed by a Kruskal–Wallis test. This was done in diagnostic groups contained in the non-AD group. In addition, simple linear regressions with 95% confidence intervals were performed on the relationship between normalized lactoferrin in CSF and saliva between the groups. To assess the association of CSF Aβ42, p-tau, and tau with normalized lactoferrin in CSF and saliva, simple linear regressions were performed. The level for statistical significance was set at with a P < 0·05.
2.5. Funding source
The study was supported by Lundbeck Foundation, Grosserer L. F. Foghts Foundation, Augustinus Foundation, Frimodt-Heineke Foundation, and the Foundation for Neurological Research. The Danish Dementia Biobank was supported by the Absalon Foundation of May 1st 1978 and Simon Spies Foundation. The funders had no role in the conceptualization, study design, data collection, analysis, interpretation of data, in writing the paper or in the decision to submit the paper for publication.
3. Results
3.1. Demographics
A total of 222 matched saliva and CSF samples were analysed. Table 1 and Table 2 gives an overview of the obtained results. Among the groups of included patients, significant differences were observed between the sex distribution, age, mini mental state examination score (MMSE), and levels of AD biomarkers as expected. In general, the HC were younger than the other groups, had a better MMSE score, higher levels Aβ42, and lower levels of p-tau and tau.
Table 1.
Characteristics of the study cohort.
| HC (n = 20) | MCI (n = 56) | AD (n = 71) | Non-AD (n = 75) | p-value | |
|---|---|---|---|---|---|
| Sex F/M | 8/12 | 27/29 | 41/30 | 29/47 | 0·014** |
| Age, years † | 65·7 ± 10·1 | 70·4 ± 8·2 | 72·1 ± 7·3 | 73·5 ± 8·6 | 0·002 |
| MMSE score † | 28·8 ± 0·9 | 26·4 ± 3·3 | 22·6 ± 4·4 | 22·4 ± 4·0 | <0·0001 |
| CSF Aβ42 (pg/mL) † | 990·5 ± 168·5* | 907·9 ± 288·9 | 615·9 ± 186·2 | 883·0 ± 311·3 | <0·0001 |
| CSF p-tau (pg/mL) † | 48·7 ± 22·1* | 52·0 ± 27·3 | 97·7 ± 123·5 | 58·2 ± 59·0 | 0·005 |
| CSF total tau (pg/mL) † | 236·1 ± 140·2* | 338·1 ± 203·1 | 545·8 ± 275·1 | 315·2 ± 191·7 | <0·0001 |
n, number; F, female; M, male; MMSE, mini mental state examination; CSF, cerebrospinal fluid; Aβ42, amyloid 1-42; p-tau, phosphorylated tau; HC, healthy controls; MCI, mild cognitive impairment; AD, Alzheimer´s Disease.
† expressed as mean ± standard deviation (SD). *Missing data from 11 out of 20 HC, but all HC were amyloid negative.
All p-values were calculated by a one-way ANOVA, except **, which was calculated by a Chi-squared test.
Table 2.
Mean levels of the analysed biomarkers in CSF and saliva
| HC (n = 20) | MCI (n = 56) | AD (n = 71) | Non-AD (n = 75) | p-value* | |
|---|---|---|---|---|---|
| CSF lactoferrin (ng/mL) † | 15.8 ± 10.6 | 18.1 ± 10.3 | 19.5 ± 11.2 | 20.8 ± 10.7 | 0·38 |
| Saliva lactoferrin (ug/mL) † | 16·4 ± 6·6 | 26·3 ± 23·4 | 26·9 ± 26·3 | 24·4 ± 24·4 | 0·31 |
| CSF total protein (ug/mL) † | 447·9 ± 151·9 | 503·2 ± 161·1 | 481·0 ± 155·7 | 558·9 ± 313·2 | 0·12 |
| Saliva total protein (ug/mL) † | 958·3 ± 381·4 | 996·7 ± 494·6 | 1079·4 ± 604·2 | 957·0 ± 418·6 | 0·85 |
| CSF lactoferrin / CSF total protein† | 40·6 ± 27·6 | 36·1 ± 22·4 | 45·0 ± 31·0 | 44·2 ± 29·4 | 0·59 |
| Saliva lactoferrin / saliva total protein † | 24·6 ± 32·9 | 31·8 ± 26·5 | 32·2 ± 41·5 | 30·9 ± 37·1 | 0·19 |
CSF, cerebrospinal fluid; HC, healthy controls; MCI, mild cognitive impairment; AD, Alzheimer's Disease.
† expressed as mean ± standard deviation (SD). *analysed by Kruskal-Wallis test.
No correlation was found between normalized lactoferrin in CSF and Aβ42 (P = 1·0 [simple linear regression]), p-tau (P = 0·76 [simple linear regression]), or tau (P = 0·35 [simple linear regression]) in CSF. In addition, no statistically significant difference was found between normalized salivary lactoferrin and Aβ42 (P = 0·86 [simple linear regression]), p-tau (P = 0·34 [simple linear regression]) or tau (P = 0·75 [simple linear regression]) in CSF.
3.2. Lactoferrin levels
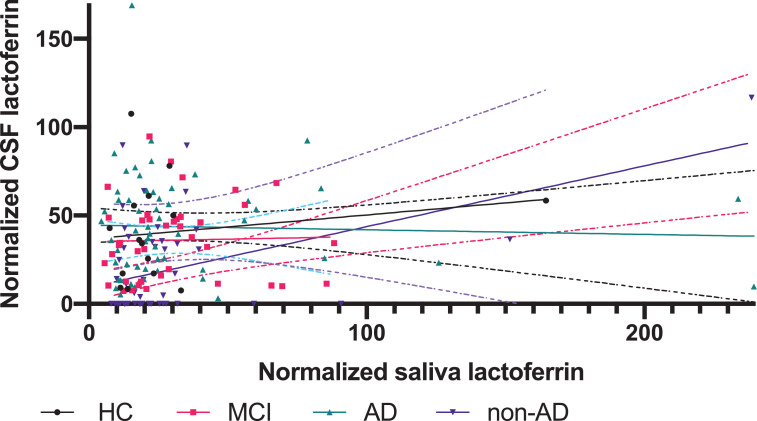
No statistically significant differences were found for lactoferrin in CSF (P=0·38 [Kruskal-Wallis test]; Fig. 1a) or for salivary lactoferrin between the diagnostic groups (P = 0·31[Kruskal–Wallis test]; Fig. 1b). In addition, the concentrations of total protein in CSF and saliva were not significantly different (P = 0·12 [Kruskal–Wallis test] and P = 0·85 [Kruskal–Wallis test]). Furthermore, no statistically significant differences between the groups were found when lactoferrin in CSF and saliva was normalized to the concentration of total protein in CSF and saliva, respectively (P = 0·59 [Kruskal–Wallis test]; Fig. 1c and P = 0·19 [Kruskal–Wallis test]; Fig. 1d). When investigating normalized lactoferrin in CSF (P = 0·49 [Kruskal–Wallis test]) and saliva (P = 0·42 [Kruskal–Wallis test]) for the diagnoses within the non-AD group, no significant differences were found. In addition, no statistically significant difference was found in the relationship between normalized lactoferrin in CSF and saliva between in HC, MCI or AD (Fig. 2). A significant correlation was found between normalized lactoferrin in CSF and saliva in the non-AD group (slope=0·34 [simple linear regression]), but caution must be taken due to the heterogenous nature of the non-AD group (Fig. 2).
Fig. 1.
a, 1b, 1c and 1d. Scatter plots of CSF and saliva lactoferrin and normalized CSF and saliva lactoferrin
Fig. 1: a): Lactoferrin levels in CSF in HC (n = 20), MCI (n = 56), AD (n = 71) and non-AD (n = 75). The scatter plot shows the median and interquartile range for each of the four groups. b): Lactoferrin levels in saliva in HC (n = 20), MCI (n = 56), AD (n = 71) and non-AD (n = 75). The scatterplot shows the median and interquartile range for each of the four groups. c): Normalized lactoferrin levels in CSF in HC (n = 20), MCI (n = 56), AD (n = 71) and non-AD (n = 75). The scatterplot shows the median and interquartile range of each of the four groups. d) Normalized salivary lactoferrin levels in HC (n = 20), MCI (n = 56), AD (n = 71) and non-AD (n = 75). The scatterplot shows the median and interquartile range of each of the four groups.
Abbreviations: HC, Healthy controls; MCI, mild cognitive impairment; AD, Alzheimer's Disease.
Fig. 2.
Relationship between normalized lactoferrin in CSF and saliva
Fig. 2: The graphs show the relationship between normalized levels of lactoferrin in CSF and saliva when comparing the four groups (HC (n = 20), MCI (n = 56), AD (n = 71) and non-AD (n = 75)). Furthermore, the graphs show the 95% confidence intervals.
Abbreviations: HC, Healthy controls; MCI, mild cognitive impairment; AD, Alzheimer's Disease.
Supplementary figure 1 and 2 give an overview of the obtained results if a cut-off for Aβ42 in CSF of 550 pg/mL was used in the AD group. With this cut-off value still no statistically significant differences were found for lactoferrin in CSF (P = 0·39 [Kruskal-Wallis test]; supplementary fig. 1a), salivary lactoferrin (P = 0·35 [Kruskal–Wallis test]; supplementary fig. 1b), normalized lactoferrin in CSF (P = 0·79 [Kruskal–Wallis test]; supplementary fig. 1c) and normalized lactoferrin in saliva P = 0·13 [Kruskal-Wallis test]; supplementary fig. 1d) between the diagnostic groups. Furthermore, no significant correlation was found between normalized lactoferrin in CSF and saliva in the AD group (slope=0.21 [simple linear regression]; supplementary figure 2).
4. Discussion
In this study, lactoferrin in CSF and saliva was investigated as a diagnostic biomarker for AD and other neurodegenerative dementias in a consecutive, mixed memory clinic cohort. To the best of our knowledge, this consecutive study is the first to investigate lactoferrin in CSF. Lactoferrin was detected in low concentrations in CSF, but no statistical significance was found between HC, MCI, AD, or non-AD.
One other research group has investigated salivary lactoferrin in AD. Their first study found that decreased lactoferrin was able to differentiate AD from HC more accurately than Aβ42 and total tau in CSF [38]. In addition, the study found a correlation between salivary lactoferrin and Aβ42 in CSF. Their second study found that decreased salivary lactoferrin could distinguish prodromal AD, AD from FTD by being specific to AD [39]. Although, salivary lactoferrin was proposed as a candidate for a new diagnostic biomarker for AD in early disease detection, the present study was not able to reproduce any of the results, neither on the HC, MCI, AD, or non-AD group. Furthermore, our study could not identify any association between salivary lactoferrin and any of the well-established dementia biomarkers (Aβ42, p-tau and tau).
Comparing this consecutive study to the two other studies, the same saliva collection methods were used. In addition, the same assays were used to measure lactoferrin in saliva and to measure the concentration of total protein. A difference between the studies is that the patients in our cohort are included from a consecutive, mixed memory clinic population, and therefore may be more heterogenous and have more comorbidities than subjects enrolled in previous studies. Also, our included AD patients had a MMSE score which was around 3·4 points higher than the AD patients in the other two studies [38,39]. This difference in the cohort composition with inclusion of milder and more heterogenous cases in our study could perhaps account for some of the larger variations in lactoferrin levels observed in this study. We have no other straightforward explanation for this variation. Our sample size was comparable to previous studies, but due to larger variation in our sample, we cannot rule out that our study was underpowered. However, in our study, we saw a non-significant trend of higher lactoferrin levels in the diseased groups compared to the HC, which is opposite to the findings by the two previous studies.
With increasing dementia severity patients have a decreased ability of selfcare, and poor oral health could potentially affect the concentration of salivary lactoferrin or other salivary biomarkers. For that reason, future studies must take the salivary flow-rates, oral health and hygiene into account. Furthermore, it should be taken into account that studies have shown that antidepressants, antipsychotics and some other medications can lead to an altered saliva production [54]. The patients of our study were included prior to diagnosis, and for that reason they did not yet receive any medication for dementia, which could potentially affect their saliva production [55]. One weakness of this study is the missing data on tau, p-tau and Aβ42 from 11 out of 20 HC, even though it was known that all 20 HC were amyloid negative. Another limitation is that the sample in our study may not be representative of patients evaluated in memory clinics in general, as our center is also a tertiary referral center with more complex and atypical cases. On the other hand, a strength of the study is the external validity. The included patients are a valid representation of a clinical cohort, which furthermore eliminates selection bias.
This was the first study to investigate the diagnostic potential of CSF levels of lactoferrin in a large, consecutive cohort. Based on the results from our study, lactoferrin in CSF does not seem to be a valid diagnostic biomarker for AD. Although, two other studies found salivary lactoferrin to be an excellent diagnostic biomarker for the diagnosis of AD in early disease stages, this consecutive study could not reproduce the results in saliva in a representative, mixed memory clinic cohort. We have no clear explanation for this, and future studies of lactoferrin should explore any confounding methodological issues. The easy accessibility of saliva, however, warrants more studies of biomarkers in saliva to ascertain the clinical usefulness for an early diagnosis of AD and other neurodegenerative dementias.
Contributors
Conceptualization, Helena Sophia Gleerup, Steen Gregers Hasselbalch, and Anja Hviid Simonsen; Data curation, Helena Sophia Gleerup; Formal analysis, Helena Sophia Gleerup; Funding acquisition, Helena Sophia Gleerup, Steen Gregers Hasselbalch, and Anja Hviid Simonsen; Investigation, Helena Sophia Gleerup; Methodology, Helena Sophia Gleerup, Camilla Steen Jensen, and Peter Høgh; Project administration, Helena Sophia Gleerup; Supervision, Steen Gregers Hasselbalch and Anja Hviid Simonsen; Validation, Camilla Steen Jensen and Anja Hviid Simonsen; Visualization, Steen Gregers Hasselbalch; Writing – original draft, Helena Sophia Gleerup; Writing – review & editing, Camilla Steen Jensen, Peter Høgh, and Anja Hviid Simonsen. All authors had access to the data and accept the responsibility to submit for publication. All authors have read and approved the final version of the manuscript.
Data sharing statement
Deidentified participant data can be made available with relevant researchers after approval of a research proposal and signature of a data access agreement in accordance with Danish data protection law.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
The authors are grateful to the clinical staff at the Copenhagen Memory Clinic, Copenhagen University Hospital, Rigshospitalet and the Dementia Research Centre, Zealand University Hospital. The study was supported by Lundbeck Foundation, Grosserer L. F. Foghts Foundation, Augustinus Foundation, Frimodt-Heineke Foundation and the Foundation for Neurological Research. The Danish Dementia Biobank was supported by the Absalon Foundation of May 1st 1978 and Simon Spies Foundation.
Footnotes
Funding: The study was supported by Lundbeck Foundation, Grosserer L. F. Foghts Foundation, Augustinus Foundation, Frimodt-Heineke Foundation and the Foundation for Neurological Research. The Danish Dementia Biobank was supported by the Absalon Foundation of May 1st 1978 and Simon Spies Foundation.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103361.
Appendix]. Supplementary materials
References
- 1.Martin Prince A, Wimo A, Guerchet M, et al. World Alzheimer report 2015 the global impact of dementia: an analysis of prevalence, incidence, cost and trends. https://www.alzint.org/resource/world-alzheimer-report-2015/. Accessed November 7, 2018.
- 2.What is Alzheimer's | Alzheimer's Association. Alzheimer's association. https://www.alz.org/alzheimers-dementia/what-is-alzheimers. Accessed November 7, 2018.
- 3.Simonsen AH, Herukka SK, Andreasen N. Recommendations for CSF AD biomarkers in the diagnostic evaluation of dementia. Alzheimer’s Dement. 2017;13(3):274–284. doi: 10.1016/j.jalz.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Costerus JM, Brouwer MC, van de Beek D. Technological advances and changing indications for lumbar puncture in neurological disorders. Lancet Neurol. 2018;17(3):268–278. doi: 10.1016/S1474-4422(18)30033-4. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura A, Kaneko N, Villemagne VL. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nat Publ Gr. 2018;554 doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- 6.Karikari TK, Benedet AL, Ashton NJ. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer's Disease Neuroimaging Initiative. Mol Psychiatry. 2020:1–14. doi: 10.1038/s41380-020-00923-z. [DOI] [PubMed] [Google Scholar]
- 7.Moscoso A, Grothe MJ, Ashton NJ. Brain. 2020. Time course of phosphorylated-tau181 in blood across the Alzheimer's disease spectrum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lantero Rodriguez J, Karikari TK, Suárez-Calvet M. Plasma p-tau181 accurately predicts Alzheimer's disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020;140(3):267–278. doi: 10.1007/s00401-020-02195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattsson N, Andreasson U, Zetterberg H. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74(5):557–566. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashton NJ, Leuzy A, Lim YM. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol Commun. 2019;7(1):5. doi: 10.1186/s40478-018-0649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson O, Janelidze S, Hall S. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88(10):930–937. doi: 10.1212/WNL.0000000000003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashton N, Janelidze S, Al Khleifat A. Research Square. 2020. Diagnostic value of plasma neurolament light: a multicentre validation study. [DOI] [Google Scholar]
- 13.Schjønning Nielsen M, Simonsen AH, Siersma V. Quantitative electroencephalography analyzed by statistical pattern recognition as a diagnostic and prognostic tool in mild cognitive impairment: results from a nordic multicenter cohort study. Dement Geriatr Cognit Dis Extra. 2018;8(3):426–438. doi: 10.1159/000490788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer's disease drug development pipeline: 2019. Alzheimer's Dement Transl Res Clin Interv. 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan A, Rao M V., Veeranna Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. 2017;9(4) doi: 10.1101/CSHPERSPECT.A018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sashikumar R, Kannan R, Chennai I. Salivary glucose levels and oral candidal carriage in type II diabetics. YMOE. 2010;109:706–711. doi: 10.1016/j.tripleo.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 17.Streckfus C., Bigler L., Navazesh IA-H M. Cytokine concentrations in stimulated whole saliva among patients with primary Sjögren's syndrome, secondary Sjögren's syndrome, and patients with primary Sjögren's syndrome receiving varying doses of interferon for symptomatic treatment of the condition. Clinical Oral Investigation. 2001;5(2):133–135. doi: 10.1007/s007840100104. [DOI] [PubMed] [Google Scholar]
- 18.Walt DR, Blichartz TM, Hayman RB. Microsensor arrays for saliva diagnostics. Ann N Y Acad Sci. 2007;1098(1):389–400. doi: 10.1196/annals.1384.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adam DJ, Milne AA, Evans SM. Serum amylase isoenzymes in patients undergoing operation for ruptured and non-ruptured abdominal aortic aneurysm. J Vasc Surg. 1999;30(2):229–235. doi: 10.1016/S0741-5214(99)70132-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Farrell JJ, Zhou H. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology. 2010;138(3):949–957. doi: 10.1053/j.gastro.2009.11.010. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emmons W. Accuracy of oral specimen testing for human immunodeficiency virus. Am J Med. 1997;102:15–20. doi: 10.1016/s0002-9343(97)00033-8. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 22.Malamud D, Rodriguez-Chavez IR. Saliva as a diagnostic fluid. Dent Clin N Am. 2011;55(1):159–178. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hùgh P, Oturai A, Schreiber K. Apoliprotein E and multiple sclerosis: impact of the epsilon-4 allele on susceptibility. Clin Type Progress Rate. 2020 doi: 10.1177/135245850000600403. www.nature.com/msAccessed November 24. [DOI] [PubMed] [Google Scholar]
- 24.Spielmann N, Wong DT. Saliva: diagnostics and therapeutic perspectives. Oral Dis. 2011;17(4):345–354. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gug IT, Tertis M, Hosu O, Cristea C. Salivary biomarkers detection: analytical and immunological methods overview. TrAC – Trends Anal Chem. 2019;113:301–316. doi: 10.1016/j.trac.2019.02.020. [DOI] [Google Scholar]
- 26.Gleerup HS, Hasselbalch SG, Simonsen AH. Biomarkers for Alzheimer’s disease in saliva: a systematic review. Dis Markers. 2019 doi: 10.1155/2019/4761054. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashton NJ, Ide M, Zetterberg H, Blennow K. Salivary biomarkers for Alzheimer's disease and related disorders. Neurol Ther. 2019;8(2):83–94. doi: 10.1007/s40120-019-00168-1. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi M, Sui Y-T, Peskind ER. Salivary tau species are potential biomarkers of Alzheimer's disease. J Alzheimers Dis. 2011;27(2):299–305. doi: 10.3233/JAD-2011-110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pekeles H, Qureshi HY, Paudel HK, Schipper HM, Gornistky M, Chertkow H. Development and validation of a salivary tau biomarker in Alzheimer's disease. Alzheimer's Dement Diagnosis. Assess Dis Monit. 2018:1–8. doi: 10.1016/J.DADM.2018.03.003. (April) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashton NJ, Ide M, Schöll M. No association of salivary total tau concentration with Alzheimer's disease. Neurobiol Aging. 2018;70:125–127. doi: 10.1016/j.neurobiolaging.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Bermejo-Pareja F, Antequera D, Vargas T, Molina JA, Carro E. Saliva levels of Abeta1-42 as potential biomarker of Alzheimer's disease: a pilot study. BMC Neurol. 2010;10:108. doi: 10.1186/1471-2377-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGeer PL, Guo JP, Lee M, Kennedy K, McGeer EG. Alzheimer's disease can be spared by nonsteroidal anti-inflammatory drugs. J Alzheimers Dis. 2018;62(3):1219–1222. doi: 10.3233/JAD-170706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabbagh MN, Shi J, Lee M. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer's disease dementia from normal controls: preliminary findings. BMC Neurol. 2018;18(1):155. doi: 10.1186/s12883-018-1160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee M, Guo JP, Kennedy K, Mcgeer EG, McGeer PL. A method for diagnosing Alzheimer's disease based on salivary amyloid-β protein 42 levels. J Alzheimer's Dis. 2017;55(3):1175–1182. doi: 10.3233/JAD-160748. [DOI] [PubMed] [Google Scholar]
- 35.Jasim H, Carlsson A, Hedenberg-Magnusson B, Ghafouri B, Ernberg M. Saliva as a medium to detect and measure biomarkers related to pain. Sci Rep. 2018;8(1):3220. doi: 10.1038/s41598-018-21131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farah R, Haraty H, Salame Z, Fares Y, Ojcius DM. Said Sadier N. Salivary biomarkers for the diagnosis and monitoring of neurological diseases. Biomed J. 2018;41(2):63–87. doi: 10.1016/j.bj.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B, Timilsena YP, Blanch E, Adhikari B. Lactoferrin: Structure, function, denaturation and digestion. Crit Rev Food Sci Nutr. 2019;59(4):580–596. doi: 10.1080/10408398.2017.1381583. [DOI] [PubMed] [Google Scholar]
- 38.Carro E, Bartolomé F, Bermejo-Pareja F. Early diagnosis of mild cognitive impairment and Alzheimer’s disease based on salivary lactoferrin. Alzheimer’s Dement. 2017;8:131–138. doi: 10.1016/j.dadm.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González-Sánchez M, Bartolome F, Antequera D. EBioMedicine. 2020. Decreased salivary lactoferrin levels are specific to Alzheimer's disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tijms BM, Willemse EAJ, Zwan MD. Unbiased approach to counteract upward drift in cerebrospinal fluid amyloid-β 1–42 analysis results. Clin Chem. 2018;64(3):576–585. doi: 10.1373/clinchem.2017.281055. [DOI] [PubMed] [Google Scholar]
- 41.McKhann GM, Knopman DS, Chertkow H. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winblad B, Palmer K, Kivipelto M. Mild cognitive impairment – beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 43.Sachdev P, Kalaria R, O’Brien J. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28(3):206–218. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKeith IG, Boeve BF, DIckson DW. Diagnosis and management of dementia with Lewy bodies. Neurology. 2017;89(1):88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. A J Neurol. 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed]
- 46.Gorno-Tempini ML, Hillis AE, Weintraub S. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b01e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 10.1227/01.NEU.0000168185.29659.C5. [DOI] [PubMed]
- 48.Höglinger GU, Respondek G, Stamelou M. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32(6):853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emre M, Aarsland D, Brown R. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 50.ICD-10 Version:2010. https://icd.who.int/browse10/2010/en#. WHO. Accessed October 23, 2020.
- 51.Engelborghs S, Niemantsverdriet E, Struyfs H. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimer’s Dement Diagnosis Assess Dis Monit. 2017;8:111–126. doi: 10.1016/j.dadm.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Del Campo M, Mollenhauer B, Bertolotto A. Recommendations to standardize preanalytical confounding factors in Alzheimers and Parkinsons disease cerebrospinal fluid biomarkers: An update. Biomark Med. 2012;6(4):419–430. doi: 10.2217/bmm.12.46. [DOI] [PubMed] [Google Scholar]
- 53.Lactoferrin H. ab108882 Lactoferrin Human ELISA Kit. Abcam. 2018;(August).
- 54.Einhorn OM, Georgiou K, Tompa A. Salivary dysfunction caused by medication usage. Physiol Int. 2020;107(2):195–208. doi: 10.1556/2060.2020.00019. [DOI] [PubMed] [Google Scholar]
- 55.Miranda-Rius J, Brunet-Llobet L, Lahor-Soler E, Farré M. Salivary secretory disorders, inducing drugs, and clinical management. Int J Med Sci. 2015;12(10):811–824. doi: 10.7150/ijms.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.