Abstract
Mediterranean spotted fever (MSF) is an emerging zoonosis caused by Rickettsia conorii. The MSF typically presents with a triad of fever, generalized cutaneous rash and inoculation eschar, but its clinical spectrum may range from a mild febrile illness to a potentially life-threatening condition, being central nervous system involvement highly rare. We report the clinical case of a 63-year-old male patient with MSF complicated by acute encephalitis and multi-organic failure.
Keywords: Ricketsia connori, Mediterranean spotted fever, Encephalitis
Introduction
Mediterranean spotted fever (MSF) is a zoonosis caused by Rickettsia conorii, usually characterized by fever, generalized cutaneous rash and inoculation eschar. It is usually a self-limited disease, but it may potentially be fatal, especially in patients with risk factors. The standard treatment is doxycycline 200 mg/day for 5–7 days, but there is limited evidence of its efficacy in severe cases, including encephalitis, which may need a more prolonged or alternative scheme.
Case report
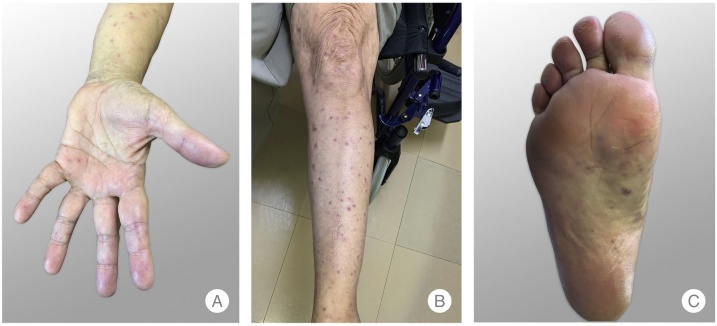
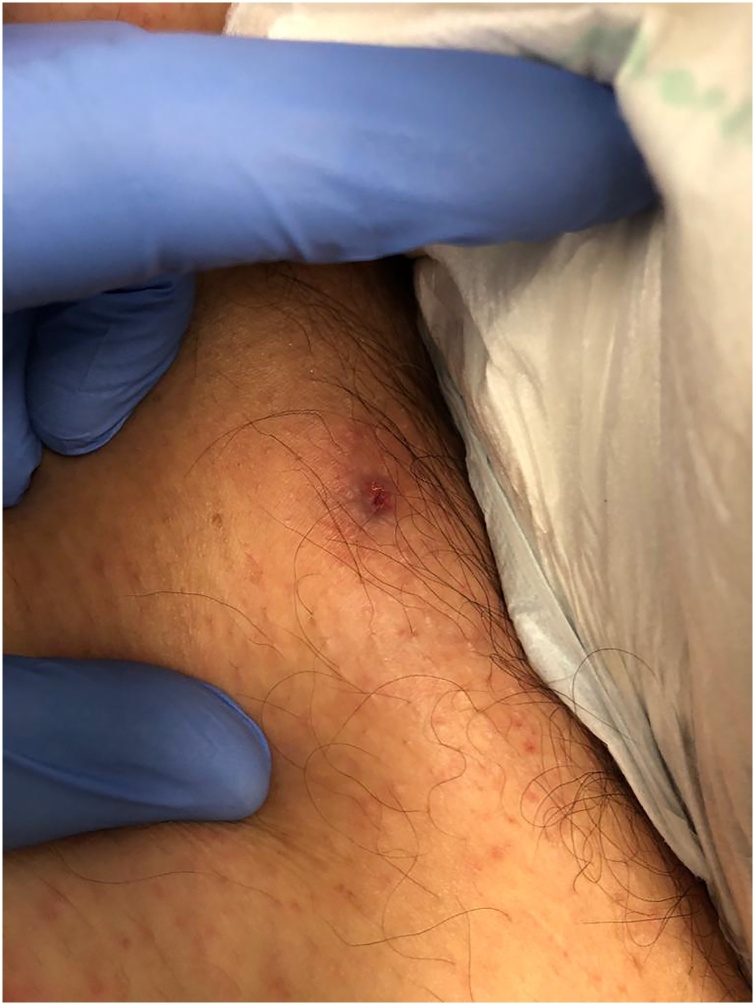
A 63-year-old Caucasian man presented to the Emergency Room (ER) with a severe holocranial headache, fever and myalgia for five days. The patient was a rural worker and had noticed a possible insect bite on the right arm two days before. A maculopapular rash on the trunk and an eschar on the right arm were found and he was discharged with doxycycline 100 mg twice a day. Two days later, he was readmitted to the ER for worsening symptoms. His past medical history was unremarkable. The physical examination revealed fever (39.4 °C), tachycardia (130 bpm), a maculopapular rash with palm and plantar involvement (Fig. 1A–C) and a right arm eschar (Fig. 2). The initial complete blood count revealed leukocytosis (13,600/μL with neutrophilia) and thrombocytopenia (platelets 97,000/μL). The remainder blood chemistries showed an acute kidney injury (creatinine 1.76 mg/dL), elevated liver enzymes (AST 287 U/L, ALT 217 U/L, AP 361 U/L, GGT 317 U/L, total bilirubin 3.2 mg/dL and direct bilirubin 2.2 mg/dL), LDH 758 U/L and C-reactive protein 16.2 mg/dL. Urinalysis, chest radiography and abdominal ultrasound were unremarkable. Fluids were started, doxycycline route was changed to intravenous, and the patient was admitted to the Internal Medicine department.
Fig. 1.
Maculopapular rash with palm and plantar involvement.
Fig. 2.
Inoculation eschar in the right arm.
On the next day he exhibited a worsening of symptoms, with persistent fever (39 °C) and diffuse neurologic symptoms with decreased level of consciousness (Glasgow Coma Scale of 10), lethargy and acute confusion. Furthermore, neurological examination showed conjugate eye deviation and right homonymous hemianopsia, without meningeal signs or motor dysfunction. The head CT scan was normal and the electroencephalogram revealed diffuse slowing of electrical brain impulses without epileptic activity. A lumbar puncture was performed and cerebrospinal fluid analysis revealed elevated protein (158 mg/dL) and leukocytes (48/μL with polymorphonuclear predominance). Doxycycline was stopped and ceftriaxone (2 g twice a day), ampicillin (1 g four times a day) and acyclovir (1 g three times a day) were started. Serologic tests for Rickettsia conorii, Coxiella burnetii, Borrelia burgdorferi, Brucella spp, Listeria monocytogenes, syphilis, HIV 1 and 2, Herpes 1 and 2, Cytomegalovirus, Epstein-Barr virus and Hepatitis A, B and C viruses were negative, as were blood, urine and CSF cultures. CSF meningitis panel by PCR for common viruses, bacteria, and fungi associated with meningitis and encephalitis was also negative. Two days later a new head CT scan and a MRI were performed, which showed no abnormalities, with a GCS of 5, tachypnea (32 cpm), tachycardia (140 bpm), hypotension (70/50 mmHg) and no response to fluids, he was transferred to the Intensive Care Unit for hemodynamic and ventilator support. Serology for Rickettsia conorii was repeated two weeks later and showed elevated IgM and IgG titers (>1:1280 and 1:5120 respectively). Since his clinical condition improved, antimicrobials were de-escalated to doxycycline 100 mg twice daily and the patient was discharged with full clinical and laboratory recovery.
Discussion
MSF is a zoonosis caused by Rickettsia conorii, an obligate intracellular gram negative bacterium, whose only vector identified in Portugal is Rhipicephalus sanguineus (brown dog tick) [1,2]. It is widely distributed throughout southern Europe, Africa, and the Middle East, with most cases occurring during the summer [1]. Although MSF is usually a benign self-limited exanthematous febrile illness [3], its clinical spectrum may range from a mild febrile illness to a potentially life-threatening condition [4]. MSF typically presents with a triad of fever, generalized cutaneous rash and inoculation eschar, but other manifestations may occur [2,4]. Diagnosis is suggested by clinical presentation and confirmed by serological tests, namely immunofluorescense detection of IgM and IgG, which is positive after 7–15 days of the onset of the disease [4]. When high clinical suspicion for MSF is present and initial serological tests are negative, as in our case, tests should be repeated based on the high rate of false negatives in the first days.
The most severe complications may include acute kidney failure, thrombocytopenia, myocarditis, pneumonitis and shock [3]. Although higher rates have been observed, the current fatality rate is 3–7 %, and the main risk factors for severe disease are advanced age, alcoholism, immunocompromised status, and diabetes [1]. There are few reports in the literature describing central nervous system involvement in MSF [3] and only five cases of adults with encephalitis related to Rickettsia conorii infection have been mentioned. Three of them had pleocytosis and elevated protein levels in CSF [2]. Encephalitis criteria diagnosis includes the presence of encephalopathy and at least two of the following: fever, seizures or focal neurological findings, CSF pleocytosis, and electroencephalogram or neuroimaging findings suggestive of encephalitis [5]. MSF treatment usually includes doxycycline 200 mg daily for 5–7 days, but in severe cases a more prolonged treatment may be needed [1]. From the reported cases of MSF with neurological involvement, the majority of patients recovered with long-term sequelae but our patient experienced full recovery [2].
References
- 1.Duque V., Ventura C., Seixas D. Mediterranean spotted fever and encephalitis: a case report and review of the literature. J Infect Chemother. 2012;18(1):105–108. doi: 10.1007/s10156-011-0295-1. [DOI] [PubMed] [Google Scholar]
- 2.Sousa Almeida R., Pego P.M., Pinto M.J., Matos Costa J. A rare case of Mediterranean Spotted Fever and encephalitis. Case Rep Infect Dis. 2016;2016:1–3. doi: 10.1155/2016/2421540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aliaga L., Sánchez-Blázquez P., Rodríguez-Granger J., Sampedro A., Orozco M., Pastor J. Mediterranean spotted fever with encephalitis. J Med Microbiol. 2009;58(4):521–525. doi: 10.1099/jmm.0.004465-0. [DOI] [PubMed] [Google Scholar]
- 4.Sekeyová Z., Danchenko M., Filipčík P., Fournier P.E. Rickettsial infections of the central nervous system. PLoS Negl Trop Dis. 2019;13(8):1–18. doi: 10.1371/journal.pntd.0007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatesan A. Diagnosis and management of acute encephalitis. Neurol Clin Pract. 2018;18(2):206–215. doi: 10.1212/CPJ.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]