Abstract
Objective
Patients with hypothyroidism due to Hashimoto’s disease (HD) may experience persisting symptoms despite normal serum thyroid hormone (TH) levels. Several hypotheses have been postulated to explain these persisting symptoms. We hypothesized that thyroid autoimmunity may play a role.
Design
A systematic literature review.
Methods
A PubMed search was performed to find studies investigating the relation between the presence of thyroid autoimmunity and (persisting) symptoms. Included studies were critically appraised by the Newcastle – Ottawa Scale (NOS) and then subdivided into (A) disease-based studies, comparing biochemically euthyroid patients with HD, and euthyroid patients with non-autoimmune hypothyroidism or euthyroid benign goitre, and (B) (general) population-based studies. Due to different outcome measures among all studies, meta-analysis of data could not be performed.
Results
Thirty out of 1259 articles found in the PubMed search were included in this systematic review. Five out of seven disease-based studies found an association between thyroid autoimmunity and symptoms or lower quality of life (QoL). Sixteen of 23 population-based studies found a comparable positive association. In total, the majority of included studies reported an association between thyroid autoimmunity and persisting symptoms or lower QoL in biochemically euthyroid patients.
Conclusion
(Thyroid) autoimmunity seems to be associated with persisting symptoms or lower QoL in biochemically euthyroid HD patients. As outcome measures differed among the included studies, we propose the use of similar outcome measures in future studies. To prove causality, a necessary next step is to design and conduct intervention studies, for example immunomodulation vs. placebo preferably in the form of a randomized controlled trial, with symptoms and QoL as main outcomes.
Keywords: Hashimoto’s disease, Persisting symptoms, Quality of life, Thyroid auto-immunity, Hypothyroidism
Highlights
-
•
This review reports on a relation between thyroid autoimmunity and symptoms.
-
•
Persisting symptoms in euthyroid HD patients may be associated with autoimmunity.
-
•
Thyroid autoimmunity and symptoms were associated in population-based studies.
1. Introduction
Hypothyroidism is defined as lower than optimal thyroid hormone (TH) production by the thyroid gland, resulting in too low or suboptimal plasma TH concentrations [1]. The most frequent cause of hypothyroidism is thyroid dysfunction, also known as primary hypothyroidism [2]. This type of hypothyroidism is characterized by a low or (low-)normal serum free thyroxine (FT4) concentration in combination with a (very) high or elevated thyrotropin (TSH) concentration, and can be a congenital or acquired problem. Worldwide, the most frequent causes of acquired primary hypothyroidism are iodine deficiency and (chronic) autoimmune thyroiditis [3,4].
Autoimmune thyroiditis or Hashimoto’s disease (HD) is an autoimmune disorder, in which T- and B cells (slowly) destruct the thyroid gland. A key role in this process seems to be reserved for cytotoxic T cells that are activated by excessively stimulated CD4 positive T cells. Serological markers are anti-thyroid antibodies: anti-thyroid peroxidase and anti-thyroglobulin antibodies (TPO- and Tg-abs, respectively), produced by B cells [5,6].
In case of clinical suspicion of hypothyroidism, thyroid function assessment consists of TSH and FT4 measurement [6]. HD is diagnosed by the presence of TPO- or Tg-abs, plus or minus characteristic thyroid ultrasound abnormalities, such as reduced echogenicity [3,6,7]. Common, but also quite unspecific complaints in (primary) hypothyroidism are fatigue, weight gain with poor appetite, constipation, concentration problems and depression [[8], [9], [10]]. Treatment of hypothyroidism consists of daily administration of levothyroxine (LT4) [6,8,11]. LT4 is preferable to triiodothyronine (T3) because of its longer serum half-life [6,11]. Furthermore, the thyroid gland mainly produces thyroxine (T4), which is converted into its active metabolite T3 in peripheral tissues [6,8].
Despite normalized TSH and FT4 levels by LT4 treatment, approximately five to ten percent of HD patients experience persisting symptoms [[12], [13], [14]]. With respect to these persisting symptoms, several hypotheses have been postulated and discussed: 1) TSH is not a perfect marker; consequently, there standard LT4 treatment may not result in a truly biochemically euthyroid state [4]; therefore, some experts suggest to treat with a supraphysiological LT4 dose, which would result in a suppressed TSH, but fewer complaints; however, not all studies show the same results, and higher LT4 doses may increase the risk of cardiovascular disease; 2) a healthy thyroid gland produces approximately 80–90% T4 and 10–20% T3 [8,11,15]; since not all administered LT4 will be converted into active T3, combination therapy of LT4 and LT3 may result in less persisting symptoms; however, until now, it has not been shown that adding LT3 is better than LT4 alone [8]; 3) since deiodinase type 2 (DIO2) facilitates peripheral deiodination of T4 into active T3, patients with DIO2 gene polymorphisms may have variable peripheral T3 availability; in such cases LT4 treatment alone may not be enough [16,17]; with the Thr92Ala DIO2 polymorphism being present in 12–36% of the population [18], this might explain persisting symptoms in a considerable part of affected patients. Yet, none of these three hypotheses about the cause of persisting symptoms in treated patients with HD has been definitely proven. Therefore, according to the American Thyroid Association guideline from 2014, currently LT4-monotherapy is the best choice of treatment in hypothyroidism [8].
In the past years results of several studies have suggested that persisting symptoms in HD patients may be related to autoimmunity [[19], [20], [21]]; for example, in a systematic review Siegmann et al. reported a possible correlation between depression and anxiety disorders, and thyroid autoimmunity [22]. While hypothyroidism in HD patients is treated with TH, the autoimmune process affecting the thyroid gland is left untreated. Although, it has been shown that serum TPO-Ab levels decline in most patients with HD who are taking LT4 after a mean of 50 months, TPO-Ab levels became negative in only 16% of the studied patients, illustrating that the majority of patients have persisting elevated TPO-Ab levels [23]. We therefore hypothesized that persisting symptoms in treated patients with HD may be related to autoimmunity. Already in the 1960s [24], it has been recognized that, regardless of thyroid function, thyroid autoimmunity may cause neurological or psychiatric symptoms; in the absence of another obvious cause this clinical picture was called Hashimoto’s encephalopathy. The idea that thyroid autoimmunity causes the encephalitis has been abandoned, and is replaced by the hypothesis that these patients suffer from autoimmunity that not only affects the thyroid, but also the brain. Hence the name “Steroid-Responsive Encephalopathy with Autoimmune Thyroiditis” (SREAT). With this in mind, we hypothesized that persisting symptoms encountered in TH treated HD patients also results from autoimmunity affecting the brain. Besides thyroid autoimmunity other latent autoimmune diseases could hypothetically play a role in persisting symptoms in treated HD patients. A recent meta-analysis showed that (latent) poly-autoimmunity is common in patients with an autoimmune thyroid disorder. However, its effect on the course of the persisting symptoms is still unclear [25].
The main objective of this systematic review was to find out whether or not the presence of thyroid autoimmunity is associated with persisting symptoms in HD patients. We performed a literature search in PubMed for original studies investigating the relation between the presence of thyroid autoimmunity and symptoms performed in (LT4 treated) euthyroid patients with hypothyroidism due to HD compared with euthyroid patients with non-autoimmune hypothyroidism or euthyroid benign goitre screened for persisting symptoms, or in general or specific non-HD populations (persons positive or negative for anti-thyroid antibodies, screened for symptoms with specific questionnaires). The “general populations” consisted of either healthy persons, or of patients prone for autoimmune thyroid disease because of already existing other autoimmune disease.
2. Methods
This systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 guidelines [26].
2.1. Information sources and literature search
For this systematic review the PubMed database was searched for relevant articles. The search was conducted with Mesh and TIAB key terms, using the components Population and Outcome of the PICO-strategy by Glasziou et al.: ‘autoimmune hypothyroidism’ and ‘persisting symptoms’, respectively [27]. The following equivalents of these key terms were used: 1) autoantibodies, autoimmunity, autoantigens, autoantibody, antibody, 2) hypothyroidism, 3) thyroglobulin, iodide peroxidase, thyrotropin receptors, TSH receptor, TPO, peroxidase, 4) Hashimoto disease, autoimmune thyroiditis, Hashimoto, Hashimoto’s thyroiditis, 5) brain diseases, behavioural symptoms, mental disorders, signs and symptoms, quality of life, brain, fatigue, depression. The equivalent terms were combined as follow: ((1 AND (2 OR 3)) OR 4) AND 5.
2.2. Study selection and quality assessment
Title and abstract screening were performed independently by two of the authors (KLG and ASPvT). Full text screening was performed together. Inclusion criteria were original studies investigating the relation between the presence of thyroid autoimmunity and symptoms performed in (LT4 treated) euthyroid patients with hypothyroidism due to HD compared with euthyroid patients with non-autoimmune hypothyroidism or euthyroid benign goitre who were screened for persisting symptoms, or in well-defined (general) populations. Exclusion criteria were: review articles, case reports or series, articles in other languages than English, articles about thyroid antibodies without any relation to thyroid disorders, and articles about HD in relation to other diseases than persisting symptoms or quality of life. Part of the full text screening was a critical appraisal to evaluate the quality of each study following the Newcastle-Ottawa Scale (NOS), a quality assessment form for cohort studies. In this eight-item checklist, we considered thyroid autoimmunity as the ‘exposure’, and - as explained earlier - the various persisting symptoms as the ‘outcome’. Due to the cross-sectional design of many of the included studies, these could not be fully scored on all aspects (e.g., follow-up) of the outcome domain. The original thresholds for good or fair studies were therefore not always applicable. Nevertheless, the NOS scale gave a good indication of the quality of the included studies.
As already mentioned in the introduction, included articles were categorized in either 1) disease-based studies: groups of (LT4 treated) euthyroid patients with hypothyroidism due to HD compared with euthyroid non-autoimmune hypothyroidism patients or euthyroid patients with benign goitre, or 2) (general) population-based studies. In the population-based studies the results of different well-being questionnaires were analysed in relation to the presence or absence of thyroid auto-antibodies in well-defined groups. These studies were subsequently subdivided into 2A) studies in healthy persons, and 2B) studies performed in patients prone for thyroid autoimmunity, and thus prone for poly-autoimmunity, because of already existing other autoimmune disease, e.g., rheumatoid arthritis or celiac disease. The main reasons for making this subdivision were that patients suffering from other autoimmune disease might have had knowledge of having a higher chance of also developing autoimmune thyroid disease, and that symptoms of “other” autoimmune diseases may resemble those of autoimmune thyroid disease/HD. This makes results from these studies related to the research question of this systematic review somewhat more difficult to interpret. Nonetheless, these studies were included if there was independent evaluation of a possible relation between thyroid autoimmunity and (persisting) symptoms or QoL.
2.3. Data presentation
Disease-based studies are presented according to the way patients were assessed or to used outcome measure, in the following order: 1) well-being (including all neuropsychological tests, quality of life, fatigue and other mood-parameters) and 2) brain-function (including functional imaging of the brain). Results of the population-based studies are presented in the following order: 1) (truly) healthy persons, 2) individuals recruited from primary care facilities, 3) postpartum women, 4) pregnant women, 5) perimenopausal women, 6) individuals with an already existing other autoimmune disease.
Data are presented as four evidence tables, two for the population-based studies, and two for the disease-based studies: study and patient characteristics (Table 1, Table 3), and study results (Table 2, Table 4).
Table 1.
Study and patient characteristics of disease-based studies.
| Article |
Research question |
Study design |
Patients |
Controls |
||||
|---|---|---|---|---|---|---|---|---|
| Sample size (N) | Gender F (N) | Mean Age in yrs (±SD, range) | Sample size (N) | Gender F (N) | Mean Age in yrs (±SD, range) | |||
| STUDIES WITH OUTCOME MEASUREMENT: WELL-BEING | ||||||||
| ZIVALJEVIC 201526 | What is the QoL of HT patients compared to patients with BG? And does thyroid surgery improve the health of this patients even with normal hormonal status on LT4 treatment? | Cohort study, prospective | 27 euthyroid HT (LT4 treatment) | 26 (96%) | 52.2 (±10.9, median: 52.0) | 116 euthyroid BG (LT4 treatment) | 99 (85%) | 52.6 (±12.9, median: 55.0) |
| GIYNAS AYHAN 201427 | What is the current prevalence of major depression and anxiety disorders in patients with euthyroid HT and euthyroid goiter? And does HT increases the risk of depressive or anxiety disorders compared with endemic/non-endemic goiter or controls? | Cohort study, retrospective | 51 euthyroid HT (no treatment) | 49 (96.1%) | 35.1 (±7.75, 20-45) |
45 euthyroid endemic / non-endemic goiter (no treatment) | 41 (91.1%) | 35.47 (±6.74) |
| LOUWERENS 201228 | What is the impact of the cause of hypothyroidism on fatigue and fatigue-related symptoms in patients treated for hypothyroidism of different origin (AIH vs. DTC)? | Cross-sectional study | 138 euthyroid AIT (LT4 treatment) | 119 (86.2%) | 48.3 (±9.8) | 140 euthyroid DTC (LT4 treatment) | 114 (81.4%) | 49.3 (±13.3) |
| BAZZICHI 201229 | Is there a predisposition for the development of FM in patients with HT with or without SCH compared with SCH alone? | Cross-sectional study | 21 SCH + HT | - | - | 13 SCH without HT | 12 (92.3%) | 38.54 (±15.33) |
| OTT 201119 | Are higher anti-TPO levels associated with an increased symptom load and a decreased QoL in a female euthyroid patient cohort? (with/without treatment) | Cohort study, prospective | 47 Anti-TPO >121.0 IU/mL | 47 (100%) | 52.3 (±12.7) | 379 Anti-TPO ≤121.0 IU/mL | 379 (100%) | 54.6 (±12.0) |
| STUDIES WITH OUTCOME MEASUREMENT: BRAIN FUNCTION | ||||||||
| LEYHE 201321 | Is there an association between the performance in d2 attention testing and GM density of the LIFG on MRI in euthyroid HT patients compared to euthyroid patients undergoing hormonal substitution for goiter or after thyroid surgery? | Cohort study, retrospective | 13 euthyroid HT (treatment) | 11 (84.6%) | 43.0 (±12) | 12 euthyroid goiter/post-surgery (treatment) | 9 (75.0%) | 47.0 (±13) |
| LEYHE 200820 | Is there a neuropsychological impairment in a subgroup of HT patients indicating a subtle brain dysfunction independent of thyroid dysfunction? | Cohort study, prospective | 26 euthyroid HT (treatment) | 23 (88.5%) | 46.0 (±1.9) | 25 euthyroid goiter/post-surgery (treatment) | 19 (82.6%) | 49.8 (±1.9) |
AIH = autoimmune hypothyroidism, AIT = autoimmune thyroiditis, anti-TPO = anti thyroid peroxidase, BG = benign goiter, DTC = differentiated thyroid carcinoma, FM = fibromyalgia, GM = grey matter, HT = Hashimoto’s thyroiditis, LIFG = left inferior frontal gyrus, LT4 = Levothyroxine 4, MRI = magnetic resonance imaging, QoL = quality of life, SCH = subclinical hypothyroidism.
Table 3.
Study and patients characteristics of population-based studies.
| ARTICLE |
RESEARCH QUESTION |
STUDY DESIGN |
PATIENTS CHARACTERISTICS |
|||
|---|---|---|---|---|---|---|
| Population | Sample size (N) | Mean Age in yrs (±SD, range) or range | Gender F (N) | |||
| PATIENTS FROM THE GENERAL POPULATION | ||||||
| KRYSIAK 201632 | Is the association between hypothyroidism and sexuality a consequence of a hypometabolic state or thyroid autoimmunity, and is sexual dysfunction associated with mood disturbances? | Cross-sectional | General | 68 | 30 | 68 (100%) |
| DELITALA 201630 | Is there an association between depressive symptoms and thyroid autoimmunity, determined by the presence of TPO-abs? | Cross-sectional | General population | 3138 | 36.3-64.7 | 1763 (56%) |
| FJAELLEGAARD 2015 31 | What is the significance of elevated anti-TPO as a marker of poor well-being and depression in euthyroid individuals and individuals with SCH? | Cross-sectional | General population | 7634 | Median: 53.0 (43-63) | 3938 (52%) |
| ISEME 2015 34 | What is the association between the presence of autoantibodies at baseline and change in depressive symptom score over 5 years follow-up? | Cohort study, retrospective | General population | 1207 out of 2049 | Median: 65.69 (±12.65, 55-85) (2049 participants) |
965 (47%) |
| ITTERMANN 2015 35 | What is the association between TPO-abs and depression and anxiety? | Cross-sectional | General population | 1644 | Median: 50 (39-61) | 776 (47.2%) |
| VAN DE VEN 2012 36 | Is there a relationship between the presence of TPO-abs and fatigue in euthyroid subjects? | Cross-sectional | General population | 5439 out of 5897 | 55.6 (±17.9, 18-98) (5897 participants) | 3101 (53%) (out of 5897 participants) |
| VAN DE VEN 2012 37 | What is the association between the presence of TPO-abs and the prevalence and severity of depression? | Cross-sectional | General population | 1125 | 56.8 (±5.7) | 546 (49%) |
| GRIGOROVA 2012 38 | What is the relationship between Tg-abs and performance on neuropsychological tests in healthy, euthyroid women? | Cross-sectional | General population | 122 | 51 (±15.2, 25-75) | 122 (100%) |
| ENGUM 2005 39 | What is the relationship between thyroid autoimmunity and depression or anxiety in a population-based sample? | Cross-sectional | General population | 30175 (anti-TPO measured in 2445) | 40-84 | 1737 (71%) (out of 2445 anti-TPO measured participants) |
| GRABE 2005 40 | Is autoimmune thyroiditis associated with mental and physical complaints in the general population? | Cross-sectional | General population | 1006 | >20 | 1006 (100%) |
| STRIEDER 2005 41 | Is there an association between TPO-abs, an early marker for AITD, and self-reported stress? | Cross-sectional | General population | 759 | 18-65 | 759 (100%) |
| CARTA 2004 33 | What is the relationship between mood and anxiety disorders and thyroid autoimmunity? | Cross-sectional | General population | 222 | >18 | 127 (57.2%) |
| PATIENTS FROM A PRIMARY CARE FACILITY | ||||||
| BUNEVICIUS 2007 50 | What is the impact of thyroid immunity, evident by hypo-echoic thyroid ultrasound pattern, on prevalence of depression and anxiety symptoms in a primary care setting? | Cross-sectional | Primary care | 474 | 52.0 (18-89) | 348 (73%) |
| KIRIM 2012 51 | Is the frequency of depression elevated in patients with chronic autoimmune thyroiditis and normal thyroid function? | Cross-sectional | Endocrinology Outpatient Clinic |
201 | 38.0 (±11; 18-65) | 197 (98%) |
| BAZZICHI 2007 52 | What are the characteristics of thyroid autoimmunity in patients affected by FM and what are the relationships between clinical data and symptoms? | Cross-sectional | Fibromyalgia | 120 | 50.64 (±12.42, 18-75) | 115 (96%) |
| POSTPARTUM WOMEN | ||||||
| GROER 2013 43 | What is the relationship between TPO status, development of PPT and dysphoric moods across pregnancy and postpartum? | Cohort study, prospective | Post-partum women | 135 | ≥18 and ≤45 | 135 (100%) |
| MCCOY 2008 45 | What is the relationship between quantified mood and thyroid measures? | Cohort study, prospective | Post-partum women | 51 | ≥18 | 51 (100%) |
| HARRIS 1989 46 | What is the relationship between PPTD and thyroid antibodies, and mood disorders? | Cohort study, prospective | Post-partum women | 147 | 17-40 | 147 (100%) |
| PREGNANT WOMEN | ||||||
| WESSELOO 201847 | What is the association between a positive TPO-Ab status during early gestation and first-onset postpartum depression? | Cohort study, prospective | Pregnant women and post-partum | 1075 | 30.4 (±3.5) | 1075 (100%) |
| POP 2006 48 | What is the relation between thyroid parameters (TSH, FT4 and TPO-abs) and an episode of major depression at different trimesters during pregnancy? | Cohort study, prospective | Pregnant women | 1017 | 29.0 (±0.5) | 1017 (100%) |
| PERIMENOPAUSAL WOMEN | ||||||
| POP 1998 42 | What is the relationship between autoimmune thyroid dysfunction and depression in perimenopausal women? | Cross-sectional | Perimenopausal women | 583 | 49.9 (±2.2) | 583 (100%) |
| PATIENTS WITH ANOTHER AUTOIMMUNE DISEASE | ||||||
| AHMAD 2015 44 | How does AIT affect the clinical presentation of established RA with particular reference to FM and CWP? | Cohort study, retrospective | Patients with RA | 204 | 58.23 (±13.06) | 188 (92%) |
| CARTA 2002 49 | What is the relationship between celiac disease and psychiatric disorders and what is the relevance of associated thyroid disease in the development of psychiatric illnesses in celiac patients? | Case-control study, retrospective | Patients with coeliac disease | 36 | 41.1 (±15.3, 18-64) | 27 (75%) |
AIT = autoimmune thyroiditis, AITD(s) = autoimmune thyroid disease(s), Anti-TPO = anti thyroid peroxidase, CWP = chronic widespread pain, F = female, FM = fibromyalgia, FT4 = free thyroxine 4, N = number, PPT = post-partum thyroiditis, PPTD = post-partum thyroid disease, RA = rheumatoid arthritis, SCH = subclinical hypothyroidism, SD = standard deviation, Tg-ab(s) = thyroglobulin antibody(-ies), TPO = thyroid peroxidase, TPO-abs(s) = thyroid peroxidase antibody(-ies), TSH = thyroid stimulating hormone.
Table 2.
Results of disease-based studies.
| ARTICLE | MAIN OUTCOME MEASURE | RESULTS | CONCLUSION | DO RESULTS SUPPORT THE HYPOTHESIS? | ||
|---|---|---|---|---|---|---|
| STUDIES WITH OUTCOME MEASUREMENT: WELL-BEING | ||||||
| ZIVALJEVIC 201526 | Histologically confirmed HT or BG. |
HT N=27 |
Other BG N=116 |
P-value | Preoperatively, hypothyroid symptoms were significantly more expressed; sex life was significantly worse in HT patients than in BG patients (p=0.025 and p=0.007, respectively). Pre-operative differences in other domains were not significant. | No, except for a significantly worse QoL score on the domain “sex life” |
| QoL pre-operative in ThyPRO domains: | Postoperatively, goiter symptoms were significantly worse in the HT patients than in the BG patients (=0.042). This result is probably due to the fact that an inflammatory process develops around the thyroid gland in HT patients, but not in BG patients. | |||||
| - Goiter symptoms | 21.4 (±11.2) | 20.9 (±14.0) | 0.505 | |||
| - Hyperthyroid symptoms | 16.3 (±11.7) | 20.3 (±14.7) | 0.159 | |||
| - Hypothyroid symptoms | 24.8 (±22.1) | 15.0 (±15.9) | 0.025 | |||
| - Eye symptoms | 4.9 (±7.2) | 6.2 (±11.0) | 0.579 | |||
| - Tiredness | 33.6 (±20.2) | 35.7 (±23.7) | 0.957 | |||
| - Cognitive impairment | 13.4 (±20.0) | 13.6 (±17.0) | 0.926 | |||
| - Anxiety | 21.8 (±20.8) | 23.1 (±18.9) | 0.564 | |||
| - Depression | 27.6 (±16.3) | 25.4 (±18.1) | 0.377 | |||
| - Emotional susceptibility | 19.5 (±18.9) | 22.5 (±17.7) | 0.330 | |||
| - Social life | 9.0 (±18.9) | 7.9 (±13.1) | 0.579 | |||
| - Daily life | 16.3 (±14.2) | 15.3 (±17.0) | 0.289 | |||
| - Sex life | 20.0 (±24.7) | 14.4 (±27.7) | 0.007 | |||
| - Cosmetic complaints | 11.6 (±14.0) | 10.9 (±13.7) | 0.777 | |||
| - Overall QoL | 36.1 (±28.0) | 33.0 (±28.8) | 0.473 | |||
| QoL post-operative in ThyPRO domains: | ||||||
| - Goiter symptoms | 2.9 (±5.1) | 1.8 (±6.6) | 0.042 | |||
| - Hyperthyroid symptoms | 9.1 (±10.4) | 8.3 (±7.4) | 0.853 | |||
| - Hypothyroid symptoms | 15.0 (±15.9) | 10.6 (±11.7) | 0.176 | |||
| - Eye symptoms | 4.5 (±7.6) | 2.1 (±4.5) | 0.110 | |||
| - Tiredness | 20.5 (±14.1) | 17.5 (±14.4) | 0.125 | |||
| - Cognitive impairment | 8.9 (±11.4) | 9.9 (±13.9) | 0.948 | |||
| - Anxiety | 10.8 (±14.8) | 10.6 (±16.8) | 0.891 | |||
| - Depression | 15.7 (±13.1) | 15.0 (±12.4) | 0.834 | |||
| - Emotional susceptibility | 10.2 (±9.6) | 9.5 (±9.0) | 0.717 | |||
| - Social life | 5.3 (±10.9) | 3.4 (±6.7) | 0.537 | |||
| - Daily life | 6.2 (±8.9) | 7.6 (±12.1) | 0.803 | |||
| - Sex life | 4.2 (±11.5) | 3.5 (±12.3) | 0.556 | |||
| - Cosmetic complaints | 2.9 (±5.5) | 1.5 (±4.2) | 0.078 | |||
| - Overall QoL | 2.8 (±8.0) | 3.4 (±11.8) | 0.845 | |||
| GIYNAS AYHAN 201427 | Prevalence of psychiatric diagnoses according DSM-IV: | HT N =51 | Goiter N=45 | P-value | A higher percentage of major depression, OCD, PD, GAD, an anxiety disorder, any depressive disorder and any psychiatric disorder was found in the HT group compared with the goiter group. However, differences were not statistically significant. | No |
| - Major depression | 15 (29.4%) | 10 (22.2%) | 0.489 | |||
| - Dysthymic disorder | 2 (3.9%) | 4 (8.9%) | 0.414 | |||
| - OCD | 8 (15.7%) | 3 (6.7%) | 0.209 | |||
| - PD | 6 (11.8%) | 3 (6.7%) | 0.495 | |||
| - GAD | 3 (5.9%) | 1 (2.2%) | 0.620 | |||
| - Phobic disorder | 3 (3.9%) | 3 (6.7%) | 0.663 | |||
| - An anxiety disorder | 19 (37.3%) | 11 (24.4%) | 0.194 | |||
| - Any depressive disorder | 17 (33.3%) | 11 (24.4%) | 0.375 | |||
| - Any psychiatric disorder | 27 (52.9%) | 17 (37.8%) | 0.155 | |||
| LOUWERENS 201228 | Scores on five MFI-20 subscales between AIH and DTC: | AIH N=138 |
DTC N=140 |
P-value | Patients with AIH were significantly more fatigued in contrast to patients with hypothyroidism after total thyroidectomy, which could not be attributed to thyroid or clinical parameters. Therefore these findings probably represent a disease-specific decrease in QoL | Yes |
| - General fatigue | 15.1 ± 4.3 | 11.0 ± 4.8 | <0.001 | |||
| - Physical fatigue | 13.0 ± 4.1 | 9.9 ± 4.9 | <0.001 | |||
| - Reduction in activity | 11.6 ±4.6 | 8.8 ± 4.1 | <0.001 | |||
| - Reduction in motivation | 11.0 ± 4.4 | 8.6 ± 3.8 | <0.001 | |||
| - Mental fatigue | 12.7 ± 4.9 | 9.5 ± 4.8 | <0.001 | |||
| BAZZICHI 201229 | FM comorbidity in HT patients with SCH compared to SCH alone. Scores of FIQ and VAS for fatigue and pain in the different studied group of patients. |
HT + SCH N=21 28.5% |
SCH alone N=13 0.0% |
P-value | HT patients with FM comorbidity had a significantly higher mean duration of disease with respect to the all other thyroid patients (8.50 ±6.20 vs. 3.67 ±2.75 years, P=0.0022). | Yes |
| N=39 | N=13 | - | HT patients (SCH+/-) had a higher incidence of clinical symptoms and significantly higher values of FIQ, VAS pain and VAS fatigue scores compared to patients affected by SCH alone. | |||
| - FIQ (mean, SD) | 43.13 (24.97) | 17.39 (14.48) | 0.001 | |||
| - VAS fatigue (mean, SD) | 4.51 (3.36) | 1.54 (2.54) | 0.006 | |||
| - VAS pain (mean, SD) | 3.03 (3.19) | 0.38 (0.77) | 0.009 | |||
| OTT 201119 | Thyroid histology based calculation of anti-TPO concentration cut-off, predictive of lymphocytic infiltration of the thyroid gland. |
Anti-TPO >121IU/mL N=47 |
Anti-TPO ≤121 IU/mL N=379 |
P-value | Histologically confirmed HT showed significantly higher anti-TPO levels than those without histological signs of HT. | Yes |
| Preoperative general symptom questionnaire | Increased anti-TPO levels were found to be associated with a lower quality of life and various general symptoms (chronic fatigue, dry hair, getting easily fatigued, dysphagia, chronic irritability, chronic nervousness). | |||||
| - Chronic fatigue | 31 (66.0%) | 185 (48.8%) | 0.027 | |||
| - Dry skin | 24 (51.1%) | 168 (44.3%) | 0.381 | |||
| - Dry hair | 18 (38.3%) | 77 (20.3%) | 0.005 | |||
| - Vaginal dryness | 8 (17%) | 58 (15.3%) | 0.759 | |||
| - Chronic sensation of cold | 14 (29.8%) | 82 (21.6%) | 0.207 | |||
| - Frequent sweating | 23 (48.9%) | 165 (43.5%) | 0.482 | |||
| - Becoming easily fatigued | 21 (44.7%) | 111 (29.3%) | 0.031 | |||
| - Chronic weakness | 7 (14.9%) | 39 (10.3%) | 0.034 | |||
| - Dysphagia | 15 (31.9%) | 63 (16.6%) | 0.011 | |||
| - Chronic weeping | 13 (27.7%) | 86 (22.7%) | 0.447 | |||
| - Chronic irritability | 21 (44.7%) | 95 (25.1%) | 0.004 | |||
| - Chronic lack of concentration | 15 (31.9%) | 71 (18.7%) | 0.033 | |||
| - Chronic nervousness | 36 (67.6%) | 149 (39.3%) | <0.001 | |||
| - Frequent mood swings | 17 (36.2%) | 110 (29.0%) | 0.312 | |||
| Anti-TPO >121 IU/mL | Anti-TPO ≤121 IU/mL | |||||
| QoL by SF-36 Questionnaire: | N=78 | N=346 | ||||
| - General health | 61.3±22.6 | 68.2±17.6 | 0.015 | |||
| - Physical functioning | 75.9±22.5 | 82.8±20.8 | 0.062 | |||
| - Role physical | 68.2±37.1 | 80.3±31.6 | 0.011 | |||
| - Bodily pain | 74.7±22.6 | 80.3±23.8 | 0.137 | |||
| - Vitality | 50.5±17.3 | 57.0±18.5 | 0.025 | |||
| - Social functioning | 74.4±21.6 | 82.3±20.8 | 0.020 | |||
| - Role emotional | 78.8±31.4 | 80.2±33.5 | 0.798 | |||
| - Mental health | 61.7±20.3 | 66.8±18.0 | 0.050 | |||
| Correlation between histological signs of HT with anti-TPO levels: |
HT 367.4±134.7 IU/ml |
Non-HT 28.2±65.7 IU/ml |
<0.001, r2 = 0.46 |
|||
| STUDIES WITH OUTCOME MEASUREMENT: BRAIN FUNCTION | ||||||
| LEYHE 201321 | Neurocognitive function assessed by the d2 attention test. | No significant differences in the total score of the d2 attention test were detected between groups (P=0.9). | Performance in attention testing is associated with GM density LIFG in patients with HT, but not in patients with other thyroid diseases. Particularly low achievement was associated with reduced GM density of this brain region suggesting an influence of autoimmune processes on the frontal cortex in this disease. This could be due to not yet known antibodies affecting brain morphology or an influence of thyroid antibodies themselves. | Yes | ||
| GM density on MRI was correlated with d2 test scores. | A significant correlation between GM density and d2 test total score could be shown for the opercular part of the LIFG (MNI coordinates: x=038, y=25, Z score=3.89, k=153 voxels, P<0.05) in patients with HT (r=0.88, P<0.001, Cohen’s d=1.89), but not in the control group (r=-0.06, P=0.94, Cohen’s d=-0.06). | |||||
| A negative relationship between GM density and level of TPO-Abs was found in the HT patients which, however, scarcely failed to reach significance (r=-0.44, P=0.06, Cohen’s d=0.49). | ||||||
| LEYHE 200820 | Neurocognitive function assessed by the d2 attention test. |
Control group N=25 |
Hashimoto’s thyroiditis N=26 |
P-value | No significant differences between groups were detected comparing the main values of the performances in the neuropsychological tests. However, significantly more patients with HT than in controls were found with z-scores below the normal range (less than -1.5) in de d2 attention test regarding total scores. | Yes |
| Number of patients below the normal range (z-scores). | These results point to subtle brain dysfunction in a group of patients with HT who were euthyroid and without diagnosed neuropsychiatric disease. | |||||
| - D2 total score I: total number of items processed minus errors. | 3 | 10 | 0.0302 | |||
| - D2 total score II: number of correctly processed items minus errors. | 1 | 11 | 0.0013 | |||
AIH = autoimmune hypothyroidism, anti-TPO = anti-TPO = anti thyroid peroxidase, BG = benign goiter, DSM-IV = diagnostic and statistical manual of mental disorders IV, DTC = differentiated thyroid carcinoma, FM = fibromyalgia, FIQ = fibromyalgia impact questionnaire, GAD = generalized anxiety disorder, GM = grey matter, HT = Hashimoto’s thyroiditis, LIFG = left inferior frontal gyrus, MFI-20 = multidimensional fatigue inventory, MNI = Montreal neurological institute, OCD = obsessive compulsive disorder, PD = panic disorder, QoL = quality of life, SCH = subclinical hypothyroidism, SD = standard deviation, SF-36 = short form 36 questionnaire, Thy-PRO = thyroid-specific patient reported outcome, VAS = visual analogue scale.
Table 4.
Results of population-based studies.
| Article | Main outcome measure | Results | Authors’ conclusion | Do results support the hypothesis? | ||
|---|---|---|---|---|---|---|
| PATIENTS FROM THE GENERAL POPULATION | ||||||
| KRYSIAK 2016 32 | Autoimmune SCH vs non-autoimmune SCH compared on multiple variables |
Non-autoimmune SCH N = 17 |
Autoimmune SCH N = 17 |
P-value | Both autoimmune thyroiditis and subclinical hypothyroidism are associated with a lower total FSFI score, lower scores in selected FSFI domains and higher BDI-II score. These disturbances are particularly pronounced in women whose secondary hypothyroidism results from autoimmune thyroiditis. The obtained results suggest that both thyroid autoimmunity and thyroid hypofunction disturb female sexual function and that their deteriorating effect on women's sexuality are additive. | Yes |
| BDI-II score (mean, SD) | 11.3 (3.9) | 15.6 (3.4) | < 0.05 | |||
| Depressive symptoms (n, %) | 6 (35) | 10 (59) | < 0.05 | |||
| Mild symptoms (n, %) | 6 (35) | 9 (53) | < 0.05 | |||
| Moderate symptoms (n, %) | 0 (0) | 1 (6) | – | |||
| Severe symptoms (n, %) | 0 (0) | 0 (0) | – | |||
| FSFI score (mean, SD) | 27.87 (3.62) | 23.74 (4.00) | – | |||
| Sexual desire (mean, SD) | 4.30 (0.48) | 3.38 (0.51) | < 0.01 | |||
| Sexual arousal (mean, SD) | 4.75 (0.67) | 4.25 (0.46) | – | |||
| Lubrication (mean, SD) | 4.70 (0.51) | 4.10 (0.48) | – | |||
| Orgasm (mean, SD) | 4.38 (0.60) | 3.95 (0.50) | – | |||
| Sexual satisfaction (mean, SD) | 4.92 (0.68) | 3.94 (0.47) | < 0.01 | |||
| Dyspareunia | 4.82 (0.65) | 4.12 (0.60) | – | |||
| DELITALA 2016 30 | Relation of TPO-abs and CES-D. Result of multiple regression analysis and logistic regression analysis, adjusted for age, sex, obesity (BMI≥30), smoking, and education. | CES-D Continuous: | Relation of TPO-abs + vs. TPO-abs – | β (se) −0.304 (0.394) P = 0.440 |
No support was found for an association between thyroid autoimmunity (TPO-abs) and depressive symptoms in a community-based cohort. | No |
| Relation of TPO-abs titer | 0.001 (0.001) P = 0.626 |
|||||
| CES-D > 16: | Relation of TPO-abs + vs. TPO-abs – | OR (95% CI) 1.20 (0.75–2.60) P = 0.126 |
||||
| Relation of TPO-abs titer | 1.00 (0.66–3.95) P = 0.300 |
|||||
| FJAELLEGAARD 2015 31 | Percentage of all euthyroid subjects with: | Anti-TPO – | Anti-TPO + | P-value | No significant differences were found in well-being or depression between euthyroid TPO-abs positive and TPO-abs negative individuals. | No |
| N = 7015 | N = 619 | |||||
| 1. Depression assessed with MDI questionnaire on depression categories: |
||||||
| - 0–3% “No” | 39 | 39 | – | |||
| - 4–19% “Low” | 56 | 56 | – | |||
| - 20–25% “Medium” | 3 | 2 | 0.8 | |||
| - >25% “High” | 2 | 3 | – | |||
| - DSM-IV MDD | 2 | 2 | 0.8 | |||
| 2. Well-being raw score ≥50% | 85 | 86 | 0.4 | |||
| ISEME 2015 34 | Change in CES-D from baseline to follow-up (5yr) |
TPO-abs – | TPO-abs + | Interaction term coefficient (r); 95% CI (P-value) | No significant association was found between change in CES-D score over time and TPO-abs. | No |
| Baseline | 4.47 | 3.95 | – | |||
| 5-year follow-up | 6.85 | 6.56 | – | |||
| Change | 2.38 | 2.61 | 0.23; −1.28-1.75 (0.76) | |||
| Adjusted for variables (gender, cholesterol, hypertension, medication, smoking, BMI). | ||||||
| Baseline | 4.16 | 3.06 | – | |||
| 5-year follow-up | 6.41 | 5.42 | – | |||
| Change | 2.25 | 2.36 | 0.11; −2.23-2.45 (0.93) | |||
| ITTERMANN 2015 35 | MDD, multivariable poisson regression models: |
Anti-TPO-increased (M ≥ 60, F ≥ 100 IU/mL) N = 115 (7.0%) |
P-value | This study detected significant associations between positive TPO-Abs and lifetime depression when excluding individuals with thyroid medication. Furthermore, no significant association was found between TPO-abs and recent depression in this study. A significant positive association between increased TPO-abs and MDD 12 months, but that finding was neither confirmed for positive TPO-abs nor for a BDI-II≥12. |
Yes | |
| RR (95% Confidence Interval) | ||||||
| - Global | 1.29 (0.78–2.11) | – | ||||
| - Recurrent | 1.18 (0.60–2.34) | – | ||||
| - Last 12 months | 2.88 (1.47–5.65) | – | ||||
| - Global lifetime | – | – | ||||
| - Global recurrent | – | – | ||||
| - BDI-II ≥12 | 0.93 (0.52–1.65) | – | ||||
| - Anxiety excl. specific phobias | 1.80 (0.96–3.38) | |||||
| MDD, multivariable poisson regression models: |
Anti-TPO positive (≥200 IU/mL) N = 54 (3.3%) RR (95% Confidence Interval) |
|||||
| - Global | 1.24 (0.54–2.84) | – | ||||
| - Recurrent | 1.47 (0.55–3.92) | – | ||||
| - Last 12 months | 1.78 (0.56–5.74) | – | ||||
| - Global lifetime | 2.14 (1.13–4.06) | 0.020 | ||||
| - Global recurrent | 3.30 (1.21–9.01) | 0.020 | ||||
| - BDI-II ≥12 | 0.42 (0.13–1.34) | – | ||||
| - Anxiety excl. specific phobias | 1.89 (0.75–4.81) | – | ||||
| VAN DE VEN 2012 36 | Self-reported fatigue and scores of RAND-36 vitality subscale and SFQ in euthyroid subjects free of known thyroid disorder (N = 5439): |
Euthyroid anti-TPO – N = 4870 |
Euthyroid anti-TPO + N = 569 |
RR or RC and [CI] | No association between the level of TPO-abs and fatigue was found. | No |
| - Self reported fatigue | 33.9% | 34.6% | RR 1.0 [0.8–1.1] | |||
| - RAND-36 vitality subscale | 66.3 | 66.3 | RC 0.7 [-0.9-2.2] | |||
| - SFQ | 11.1 | 11.4 | RC 0.1 [-0.5-0.7] | |||
| VAN DE VEN 2012 37 | Percentage of subjects with (number in brackets after % = number of patients that completed the specific questionnaire): | TPO-ab ≤12 kIU/l | TPO-ab >12 kIU/l | RR (95%CI), P-value | The presence of TPO-abs is associated with trait characteristics factors like neuroticism and lifetime diagnosis of depression, whereas thyroid function is not. | Yes |
| - Current depression | 15.3% (N = 791) | 19.1% (N = 115) | 1.2 (0.8–1.9) | The presence of TPO-abs may be a vulnerability marker for depression. | ||
| - Lifetime depression | 16.7% (N = 882) | 24.2% (N = 124) | 1.4 (1.0–2.1), < 0.05 | |||
| Scores: |
Difference from reference group (95% CI), P-value |
No significant relationship between the presence of TPO-abs and state markers of depression was found in the general population. | ||||
| - BDI | 5.1 (N = 791) | 6.0 (N = 115) | 0.74 (−0.2–1.7) | |||
| - EPQ-RSS neuroticism subscale | 3.2 (N = 879) | 4.1 (N = 121) | 0.7 (0.1–1.3), < 0.05 | |||
| GRIGOROVA 2012 38 | Significant correlations between scores on the executive function tests and thyroid hormone levels: Design fluency perseverative errors (corrected) Design fluency (total errors) Word fluency (total errors) Trails B errors. |
Higher Tg-ab levels were positively correlated with more errors on: - Trail Making Test Part B (r = 0.470; P = 0.000) - Word Fluency Test (r = 0.284; P = 0.023) - Design Fluency (r = 0.28; P = 0.045) test. The demographic, mood, and neuropsychological test data of all participants with Tg-Ab levels lower than 20mU/L were merged into a low (<20mU/L) Tg-ab group (N = 96) and compared to that of the women whose Tg-ab levels were >20mU/L (high Tg-ab group; N = 29). There were no significant differences between the groups on any of the demographic or mood scores. However, the women in the high Tg-ab group made significantly more perseverative errors on the Design Fluency test (P = 0.003) compared to women in the log Tg-ab group. |
The hypothesis that higher levels of Tg-ab would be associated with worse performance on all of the neuropsychological tests was partially supported. Only on the Trails Making Test-Part B, the Design Fluency and the Word Fluency tests, higher levels of Tg-abs were associated with more errors. These findings suggest that higher levels of Tg-abs antibodies are related to poorer performance on tasks of executive functions. | Yes | ||
| ENGUM 2005 39 | Prevalence (%) in TPO-abs positive subjects (cut-off 200U/mL) compared to general population: |
TPO-abs – (reference category): N = 29,180 (General population) |
TPO-abs + N = 995 |
P-value | The presence of TPO-abs was not associated with depression or anxiety. | No |
| - HADS-D (≥8) | 13.2% | 11.6% | 0.125 | |||
| - HADS-A (≥8) | 16.7% | 16.3% | 0.709 | |||
| Logistic regression analysis of depression or anxiety as dependent variables in relation to thyroid antibodies, when controlling for age, gender and thyroid function | OR adj. (95%) | |||||
| 1 | 0.92 (0.69–1.22) P = 0.557 | HADS-D ≥8 | ||||
| 1 | 0.76 (0.45–1.26) P = 0.285 | HADS-D ≥11 | ||||
| 1 | 0.93 (0.72–1.20) P = 0.584 | HADS-A ≥8 | ||||
| 1 | 1.18 (0.77–1.81) P = 0.447 | HADS-A ≥11 | ||||
| GRABE 2005 40 | Explorative comparison of symptoms between women, MANOVA (adjusted mean [SE]): |
Euthyroid without goiter N = 961 |
Euthyroid Autoimmune thyroiditis N = 30 |
MANOVA | There is some preliminary evidence, that AIT, even without pathologic changes in thyroid hormones, could alter mental well-being at least in females. Therefore, AIT could be associated with negative well-being independently from the current thyroid function. | Yes |
| - Tachycardia | 1.6 [0.02] | F = 4.8; df = 1990; P = 0.03 | ||||
| - Anxiety | 1.5 [0.02] | F = 7.1; df = 1990; P = 0.008 | ||||
| - Globus sensation | 1.3 [0.02] | F = 1.7; df = 1990; P = 0.19 | ||||
| - Nausea |
1.3 [0.02] | F = 2.5; df = 1, 990; P = 0.11 | ||||
| Adjusted for age, gender, education and marital status | ||||||
| STRIEDER 2005 41 | Experienced stress in TPO-abs positive and TPO-abs negative euthyroid subjects. (Mean [SD]) |
TPO-abs – N = 576 |
TPO-abs + N = 183 |
P-value, observed [corrected for age] | No association between recently experienced stressful life events, daily hassles or mood and the presence or absence of TPO antibodies was found in euthyroid women. | No |
| Recent life events - Total life events |
11.2 [6.2] |
10.3 [6.1] |
0.09 [0.97] |
|||
| - Unpleasant events | 4.7 [3.4] | 4.6 [3.5] | 0.68 [0.68] | |||
| - Pleasant events | 5.2 [3.5] | 4.5 [3.5] | 0.02 [0.66] | |||
| - Total unpleasantness | 16.7 [12.4] | 15.1 [11.0] | 0.13 [0.68] | |||
| - Total pleasantness | 18.9 [12.6] | 15.9 [11.4] | 0.01 [0.38] | |||
| Daily Hassles - Total number |
25.2 [14.1] |
23.8 [13.6] |
0.24 [0.83] |
|||
| - Intensity per hassle | 1.3 [0.4] | 1.3 [0.4] | 0.52 [0.38] | |||
| - Total intensity of all | 35.4 [25.5] | 32.2 [22.9] | 0.15 [0.57] | |||
| Positive and Negative affect schedule scale - Report negative feelings |
22.2 [7.3] |
22.1 [7.4] |
0.89 [0.88] |
|||
| - Report positive feelings | 38.3 [5.3] | 38.2 [5.1] | 0.91 [0.91] | |||
| CARTA 2004 33 | Association between anti-TPO+, mood and anxiety disorders: | OR anti-TPO + vs anti-TPO – | P-value (95% CI) | Anti-TPO positivity is associated with a higher lifetime risk of a diagnosis of one mood or anxiety disorder. | Yes | |
| - One anxiety diagnosis (GAD + PD + SP + ADNOS) | 4.2 | 0.001 (1.9–38.8) | ||||
| - One mood diagnosis (MDE + DD + DDNOS) |
2.9 | 0.011 (1.4–6.6) | ||||
| - GAD | 2.7 | 0.058 (0.97–7.5) | ||||
| - PD | 5.4 | 0.096 (0.7–37.3) | ||||
| - SP | 3.6 | 0.111 (0.7–7.6) | ||||
| - ADNOS | 4.0 | 0.045 (1.1–15.5) | ||||
| - MDE | 2.7 | 0.033 (1.1–6.7) | ||||
| - DD | 5.2 | 0.250 (0.3–16.8) | ||||
| - DDNOS | 4.4 | 0.049 (1–19.3) | ||||
| PATIENTS FROM A PRIMARY CARE FACILITY | ||||||
| BUNEVICIUS 2007 50 | Number of pre-menopausal women (N = 153) with: |
Normo-echoic thyroid N = 137 |
Hypo-echoic thyroid (AITD) N = 16 | P-value | Thyroid autoimmunity, evaluated by a relatively simple, cost effective but reliable technique, ultrasonographic imaging of the thyroid gland, is associated with mood symptoms in primary health care patients, especially in pre-menopausal women. | Yes |
| - HADS depression >10 | 4 (3%) | 3 (19%) | 0.02 | |||
| - HADS anxiety >10 | 27 (20%) | 6 (38%) | 0.2 | |||
| - MINI diagnoses major depression | 21 (15%) | 3 (19%) | 0.7 | |||
| - MINI diagnoses AD: | ||||||
| Panic disorder | 6 (4%) | 2 (13%) | 0.2 | |||
| Social phobia | 8 (6%) | 2 (13%) | 0.3 | |||
| Generalized anxiety | 30 (33%) | 5 (31%) | 0.4 | |||
| - Depression or anxiety disorder | 40 (29%) | 8 (50%) | 0.09 | |||
| KIRIM 2012 51 | Number of subjects HRDS levels positive for thyroid autoantibodies vs. negative. |
Thyroid auto- antibodies – N = 107 |
Thyroid auto- antibodies + N = 94 |
P value | Patients with euthyroid chronic autoimmune thyroiditis showed an elevated frequency of depression and a higher rate of severe depression. HDRS scores were correlated to age only in the control group and not in patients with euthyroid chronic AIT, suggesting a possible link between depression and euthyroid Hashimoto's Disease. | Yes |
| - Normal (0–7) | 94 (87.9%) | 8 (8.5%) | – | |||
| - Mild-medium (8-23) | 13 (12.1%) | 51 (54.3%) | – | |||
| - Severe-very severe (19–53) | 0 (0%) | 35 (37.2%) | – | |||
| Average HDRS value: | 3.65 (±3.17) | 16.05 (±6.05) | <0.001 | |||
| BAZZICHI 2007 52 | Percentage of FM patients with clinical characteristics: |
Anti-TPO – N = 70 |
Anti-TPO + N = 50 |
P-value | The results suggest a relationship between thyroid autoimmunity and FM, and highlight the association between thyroid autoimmunity and some typical symptoms such as: dysuria, allodynia, sore throat, blurred vision and dry eyes. Thyroid autoimmunity is a marker of the severity of FM, especially if patients are in post-menopausal status. |
Yes |
| - Dry eyes | 36.5% | 56.0% | <0.05 | |||
| - Burning/pain with urination | 10.0% | 36.0% | <0.01 | |||
| - Allodynia | 32.4% | 73.5% | <0.01 | |||
| - Blurred vision | 22.5% | 48.9% | <0.01 | |||
| - Sore throat | 16.9% | 43.7% | <0.01 | |||
| POSTPARTUM WOMEN | ||||||
| GROER 2013 43 | POMS-D and POMS-A at the time of pregnancy measurement. |
Anti-TPO – N = 72 |
Anti-TPO + N = 63 (pregnant) N = 47 (post-partum) |
The 63 TPO-positive pregnant women had statistically significantly higher scores on the POMS depression-dejection (POMS-D) subscale (8.5) compared to TPO-negative women (5.9) at the time of pregnancy measurement (P = 0.028). Depression symptom reports were higher postpartum for TPO-positive than PPT-negative mothers, F(1.129) = 9.1, P = 0.003. POMS-A subscale scores, F(1.131) = 6.4, P = 0.013, and total mood disturbance scores, F(1.130) = 5.3, P = 0.023, were also higher in the TPO-positive group than in the TPO-negative group. |
In pregnant women, more clinical depression and higher depressive symptom scores were found when TPO positive, and the same pattern continued postpartum. The findings support a relationship between dysphoric moods and TPO antibody status across the peripartum period. |
Yes |
| MCCOY 2008 45 | Score on 10-item EPDS score 4 weeks postpartum. |
TPO-abs – N = 44 |
TPO-abs + N = 7 Seven subjects had positive antibody tests at 4 weeks postpartum. |
P-value The 7 participants with positive antibody tests were more likely than their counterparts to have higher EPDS scores. P = 0.0428 |
TPO-abs + women tended to have higher scores on the EPDS at 4 weeks post-partum than TPO-abs – women, even when PPD was not present. | Yes |
| HARRIS 1989 46 | Psychiatric assessment according to DSM-III criteria for depressed mood by a psychiatrist on three questionnaires: The Rasking 3-area scale for depression, The MADS and The Edinburgh Postnatal depression scale. |
Anti-TPO – N = 82 (56%) |
Anti-TPO + N = 65 (44%) The frequency of cases of postnatal depression at the time of assessment was not significantly different in Ab + compared with Ab- women (x2 test). This was true for both microsomal and thyroglobulin antibody status. |
P-value Not mentioned. |
The presence of autoantibodies showed little association with depressed mood. | No |
| PREGNANT WOMEN | ||||||
| WESSELOO 2018 47 | Risk of self-reported first-onset postpartum depression: |
Anti-TPO + 121 (11.3%) |
Anti-TPO – 954 |
Adjusted OR, P-value (95% CI) | Women with a positive TPO-ab status during early gestation are at increased risk for self-reported first-onset depression at four months postpartum, but not at other time points. This period coincides with the typical postpartum rebound phenomena of the maternal immune system, which suggest an overlap in the etiology of first-onset postpartum depression and auto-immune thyroid dysfunction. | Yes |
| - 6 weeks | 1.7% | 2.1% | – | |||
| - 4 months | 5.8% (7/121) | 2.1% | 3.8, 0.017 (1.3–11.6)∗ |
|||
| - 8 months | 1.7% | 3.0% | – | |||
| - 12 months | 0.8% | 2.2% | – | |||
| POP 2006 48 | Assessment of depression by CIDI: Multiple logistic regression analysis in 1017 women at two different assessments during gestation |
OR Increased TPO-abs titers (>35) N = 1017 |
95% CI | At 12 and 24 weeks gestation, an elevated titre of TPO-abs was significantly related to depression as well as other confounders. | Yes | |
| - 12 weeks gestation | 2.1 | 1.1–5.8 | ||||
| - 24 weeks gestation | 2.8 | 1.9–7.1 | ||||
| PERIMENOPAUSAL WOMAN | ||||||
| POP 1998 42 | Multiple logistic regression analysis with depression (score ≥12 on the Edinburgh Depression Scale) as dependent variable. |
Low TPO-ab levels (<100 U/mL) N = 525 (90%) |
High TPO-ab levels (≥100 U/mL) N = 58 (10%) |
95% CI |
Women with a high concentration of TPO-abs are at risk for depression, a relationship that still exists after adjustment for other (psycho-social) determinants of depression. | Yes |
| OR | 1 | 3.0 | 1.3–6.8 | |||
| The occurrence of financial problems, caring for parents, a previous episode of depression in the woman's life, the occurrence of a major life event, and an elevated concentration of TPO-Ab (≥100 U/mL) were all significantly and independently related to depression. | ||||||
| PATIENTS WITH AUTOIMMUNE DISEASE | ||||||
| AHMAD 2015 44 |
No Autoimmune Thyroid Disease N = 130 |
Autoimmune Thyroid Disease N = 74 |
P-value |
This study shows a positive association between AIT and the presence of TPO-abs, and FM or CWP in patients with established RA. | Yes | |
| FM or CWP | 17% | 40% | <0.01 | |||
| OR | OR (95%CI) | |||||
| TPO-abs adjusted OR for FM (adjusted for age, sex, DM, BMI and spinal Degenerative Disc Disorder) |
1 | 4.458 (1.950–10.191) | < 0.001 | |||
| CARTA 2002 49 |
Anti-TPO – N = 25 |
Anti TPO+ N = 11 |
P-value |
Celiac patients with positive anti-TPO were more frequently affected by lifetime MDD than celiac patients without anti-TPO. A similar significant association between PD and TPO + among celiac patients was found. | Yes | |
| Number of celiac patients effected with lifetime MDD | 6 (24%) | 9 (81.8%) | <0.01 | |||
| Number of celiac patients effected with PD | 1 (4%) | 4 (36.4%) | <0.01 | |||
ab = antibody, Adj. = adjusted, ADNOS = anxiety disorder not otherwise specified, AIT = autoimmune thyroiditis, AITD(s) = autoimmune thyroid disease(s), anti-TPO = anti thyroid peroxidase, anti-TPO+/- = anti-TPO positive/negative, BDI = Beck depression inventory, BMI = body mass index, CES-D = centre for epidemiological studies-depression scale, CI = confidence interval, CIDI = the composite international diagnostic interview, CWP = chronic widespread pain, DD = dysthymic disorder, DDNOS = depressive disorder not otherwise specified, df = degrees of freedom, DM = Diabetes Mellitus, DSM = diagnostic and statistical manual of mental disorders, EPDS = Edinburgh postnatal depression subscale, EPQ-RSS = Eysenck personality questionnaire revised short scale, F = female, FSFI = female sexual function index, GAD = generalized anxiety disorder, HADS = hospital anxiety and depression scale, HADS-A = HADS-anxiety, HADS-D = HADS-depression, HDRS = Hamilton depression rating scale, M = male, MADS = Montgomery-Asberg depression rating scale, MANOVA = multivariate analysis of variance, MDE = major depressive episode, MDI = WHO major (ICD-10) depression inventory, MDD = major depression diagnosis, MINI = mini international neuropsychiatric interview, N = number, OR = odds ratio, PD = panic disorder, POMS-A = profile of mood states checklists for anxiety, POMS-D = profile of mood states checklists for depression, PPD = post-partum depression, PPT = post-partum thyroiditis, RA = rheumatoid arthritis, RAND-36 = research and development-36, RC = regression coefficient, RR = relative risk, SCH = subclinical hypothyroidism, SD = standard deviation, SE = standard error, SFQ = shortened fatigue questionnaire, SP = social phobia, Tg-ab(s) = thyroglobulin antibody(-ies), TPO = thyroid peroxidase, TPO-ab(s) = thyroid peroxidase antibody(-ies), TPO-ab+/- = TPO-ab positive/negative, yr = year.
Different sample: anti-TPO + (n = 119), anti-TPO–(n = 934). Self-reported first-onset depression rates 5.0% and 1.5% resp.
2.4. Statistical analysis
Due to different outcome measures and presentation of results in all studies, data could not be aggregated. Therefore, meta-analysis was not performed. Instead, a qualitative synthesis of the included data was performed.
3. Results
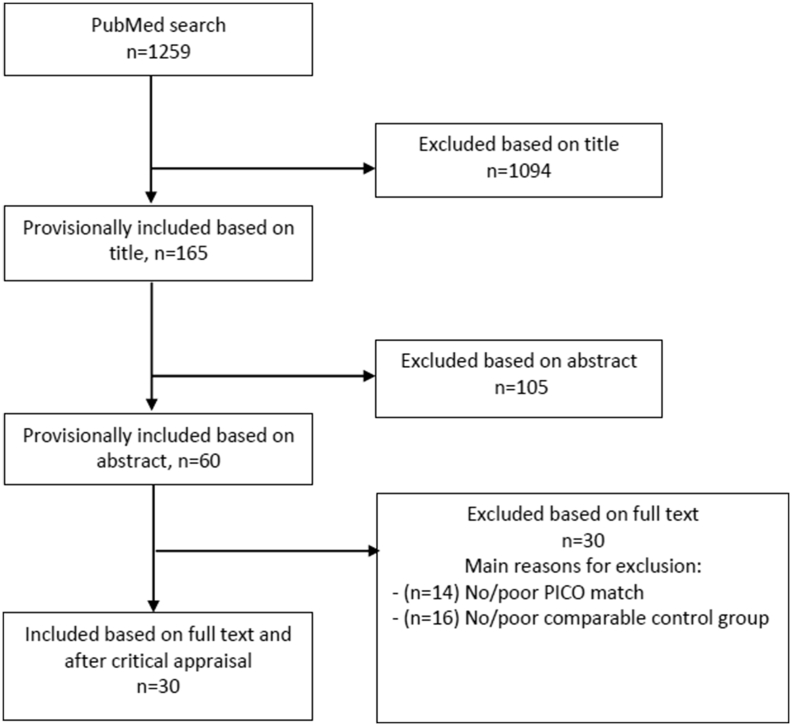
The PubMed search was performed on January 10th, 2020 in PubMed and yielded 1259 articles. We excluded 1229 articles through title, abstract or full text screening (n = 1094, n = 105, and n = 30, respectively). A total of 30 articles was included in this systematic review (Fig. 1). Seven articles could be classified as disease-based studies, twenty-three as population-based studies. The results of the critical appraisal are shown in supplementary table 1 and the used PRISMA checklist is shown in supplementary table 2.
Fig. 1.
Flowchart illustrating the results of the literature search performed in this systematic review.
3.1. Study characteristics
For study characteristics see Table 1 and Table 3. In the disease-based studies sample size ranged from n = 21 to n = 379, and mean age from 35.1 to 54.6 years. In the population-based studies sample size ranged from n = 36 to n = 7,634, and mean age from 17 to 98 years. The included studies could be classified as cross-sectional (n = 17), prospective cohort (n = 8), retrospective cohort (n = 4) or case-control (n = 1).
3.2. Disease-based studies
The results of the individual included disease-based studies are summarized in Table 2. Five disease-based studies evaluated well-being in LT4 treated patients with HD compared with patients with non-autoimmune hypothyroidism, using the following questionnaires: DSM-IV mood and anxiety disorders, FIQ, FM comorbidity, General Symptom Questionnaire, MFI-20, QoL, SCID, ThyPRO, VAS (Table 4) [19,[28], [29], [30], [31]]. Three studies reported a significant relation between persisting symptoms and thyroid autoimmunity [19,30,31]. In two studies neurocognitive function was investigated with patient and control groups as described above, using the d2 attention test. [20,21] In one of these studies the results were also related to grey matter density of the left inferior frontal gyrus determined by brain magnetic resonance imaging [21]. Both studies showed a significant association between symptoms and thyroid autoimmunity.
Thyroid function was specified in six of the seven disease-based studies. TSH, free T4 and free T3 levels were similar in HD patients and controls (non-autoimmune hypothyroidism and euthyroid benign goitre patients) in four studies [[19], [20], [21],31]. In two studies TSH values were significantly lower in the control groups [28,30], that consisted of patients who underwent thyroidectomy because of differentiated thyroid cancer and who were subsequently treated with LT4 aiming at a suppressed TSH [30]. In one study all HD patients and controls were classified as euthyroid, but thyroid function was not specified [29].
Overall, five of the seven disease-based studies found a statistically significant association between persisting symptoms and the presence of thyroid autoimmunity.
3.3. Population-based studies
The results of the individual included population-based studies are summarized in Table 4. Large and representative samples of the general population were investigated in 12 of 23 population-based studies [[32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]]. Different tests and questionnaires were used to assess the presence of persisting symptoms (BDI, CES-D, EDS, Executive Function Test, EPQ-RSS, FSFI, HADS-D/-A, MDD, MDI, RAND-36, SFQ, Symptom checklist, Life Events Checklist). Krysiak et al., Ittermann et al. and van de Ven et al. used the Beck Depression Inventory (BDI), however data were presented differently [34,37,38]. The same applies to Delitala et al. and Iseme et al. concerning the Center for Epidemiological Studies Depression scale (CES-D) [32,36]. Seven out of these 12 general population studies showed a significant relation between symptoms and thyroid autoimmunity [34,35,37,38,40,42,44]. The other eleven studies were performed in populations from a primary care facility (n = 3), postpartum women (n = 3), pregnant women (n = 2), perimenopausal women (n = 1), and patients with another autoimmune disease (n = 2) [[44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]]. In these studies, the following tests and questionnaires were used to evaluate symptoms: CIDI, Clinical Characteristics FM, EPDS, HADS-D, HADS-A, HRDS, MADS, MINI, POMS-D, POMS-A, Prevalence of FM/CWP. A significant relation between symptoms and thyroid autoimmunity was described in the three studies of individuals from a primary care facility, two studies in postpartum women, in both studies of pregnant women, in the study of perimenopausal women, and in both studies of patients with another autoimmune disease [[45], [46], [47], [48], [49], [50], [51], [52], [53], [54]].
Overall, 16 of the 23 population-based studies reported a statistically significant association between symptoms and thyroid autoimmunity. However, the total number of people studied in the seven studies showing no association between symptoms and thyroid autoimmunity was much higher (n = 20,769; study sample size range 147–7634) than the number of people in the 16 studies that did show an association (n = 8038; study sample size range 36–1644).
4. Discussion
In this systematic review we have tried to answer the question whether or not the presence of thyroid autoimmunity is associated with persisting symptoms in HD patients. Twenty-one out of 30 well-designed studies included in the review (70%) reported a probable relation between the presence of thyroid autoimmunity, and (persisting) symptoms or lower QoL. Validity of the studies was evaluated through critical appraisal following the pillars of the NOS. The included studies were divided into studies evaluating LT4 treated patients with hypothyroidism due to HD versus patients with non-autoimmune hypothyroidism (disease-based studies), and (mostly healthy general) population-based studies. An association between the presence of thyroid autoimmunity and (persisting) symptoms was found in five of the seven disease-based studies, and in 16 of the 23 population-based studies. Due to great variety in tests, questionnaires and outcome measures among the studies, data could not be combined nor could a meta-analysis be performed. Yet, to our best knowledge this is the first systematic review on this topic.
In the population-based studies, most participants with and without markers of thyroid autoimmunity - mostly TPO-abs - had a normal thyroid function. Yet, in many of these studies a number of patients had (sub-clinical) hypothyroidism or hyperthyroidism. Although some studies reported a relation between thyroid function and symptoms [32,34,37,[46], [47], [48],50], most studies that reported an association between thyroid autoimmunity and symptoms did so after correction for thyroid function. In the disease-based studies, biochemically euthyroid auto-immune hypothyroid patients reported more symptoms than biochemically euthyroid non-autoimmune hypothyroid patients or euthyroid patients with a benign goitre. In most of these studies thyroid function was similar in the HD patients and controls. Although the factor suboptimal thyroid hormone treatment cannot be ruled out, we feel that ongoing (thyroid) autoimmunity may at least play an additional role in the persisting symptoms or lower QoL of these HD patients.
Thyroid autoimmunity as cause of persisting symptoms in treated HD patients has been suggested before. Leyhe et al. reported an association between cognitive and affective disorders, and (euthyroid) autoimmune thyroid disease in their review [55]. The well-designed study by Ott et al., included in this review, showed strong evidence for a relation between thyroid auto-immunity and persisting symptoms [19]. In this study, 426 consecutive euthyroid female patients who underwent thyroid surgery for benign thyroid disease (goitre) were evaluated. Removed thyroid glands were examined for lymphocytic infiltration, and based on histology results and pre-surgery anti-TPO levels an anti-TPO concentration cut-off for “true” inflammation (121.0 IU/mL) was calculated. Subsequently, anti-TPO negative and positive patients were compared with respect to pre-operatively symptoms and QoL. A significant association between anti-TPO levels and chronic fatigue, chronic irritability, chronic nervousness, and lower QoL levels was found.
Results of several other studies are also in support of the hypothesis that there is a relation between thyroid auto-immunity and (persisting) symptoms in euthyroid HD patients. In a cross-sectional study, Watt et al. evaluated health-related QoL, using the ThyPRO, in 199 patients with auto-immune hypothyroidism with TPO-abs levels >60IU/l [56]. No association between QoL scores and thyroid function tests was seen. However, in a multivariate model the TPO-abs level was related to goitre symptoms, depression and anxiety. Half of the studied patients were euthyroid at the time of the study and only 2% were overtly hypothyroid, which may explain that no relation between thyroid function test and QoL was found. Watt el al. concluded that the health-related QoL in patients with auto-immune hypothyroidism was related to TPO-abs level, but not thyroid function. A recent Norwegian randomized trial evaluated the effect of thyroidectomy versus no thyroid surgery on persisting symptoms in adequately LT4 treated HD patients. One hundred and fifty adult euthyroid HD patients with anti-TPO concentrations greater than 1000 IU/mL and persisting symptoms were studied. SF-36 general health score, fatigue score and chronic fatigue frequency improved only in the patients who underwent thyroidectomy [57]. The median serum TPO-abs concentration decreased sharply after thyroidectomy (2232–152 IU/mL). The combination of the decrease in anti-TPO concentrations and the observed clinical improvement suggests that thyroid autoimmunity plays a role in persisting symptoms in euthyroid HD patients. Yet, an important limitation of this study is that it was not blinded by performing a sham operation instead of “no thyroid surgery”. Therefore, a placebo effect of thyroid surgery cannot be ruled out. Because these two studies lacked control groups as defined in our methods, they were not included in this review.
With respect to the population-based studies included in this systematic review, two other studies showed a relation between presence of TPO-abs, and mood and anxiety disorders [35,58]. However, these two studies were not included in this review because the investigated populations consisted of psychiatric patients which were considered to be non-representative study groups. In all included general population studies - also in the seven studies that showed no significant difference between the groups with and without thyroid autoimmunity -, a proportion of the TPO-abs positive participants experienced symptoms, while another part did not. This fits within our hypothesis that thyroid autoantibodies and thus (low-grade) thyroiditis may be present and cause symptoms, even though thyroid function is not yet compromised.
A relation between the presence of thyroid autoimmunity and neurological or psychiatric symptoms has been recognized previously in SREAT, formerly known as “Hashimoto’s encephalopathy”. In this condition, affected persons have encephalopathy that was attributed to the presence of thyroid autoimmunity [59]. For a long time, TPO-abs crossing the blood-brain barrier were held responsible, but a causative role for these abs has never been proven. It is suggested that both thyroid and brain are targets of autoimmunity, hence the name SREAT, but how is still unknown [60]. SREAT is a rare condition with an estimated prevalence of 2.1/100,000 [61]. Yet, if there is a causal relation between the (thyroid) autoimmunity in persons with TPO-abs or patients with HD on the one hand, and (persisting) symptoms on the other hand - many symptoms may be traced back to (sensing by) the brain, and might be viewed as mild brain dysfunction.
In addition to SREAT or HD patients with (persisting) symptoms, brain autoimmunity may also play a role in the pathogenesis of (late) neurological symptoms in other disorders. For example, it was shown that herpes simplex virus encephalitis may promote the development of neuronal autoantibodies targeting mostly the NMDA receptor causing subsequent autoimmune encephalitis [62]. During the SARS-CoV-2 pandemic neurological symptoms in COVID-19 patients (disabling fatigue, anosmia, Guillain-Barré syndrome and encephalopathy) can persist or reemerge after clearance of SARS-CoV-2, and are hypothesized to be caused by cross-reactive antibodies generated in response to the primary viral infection [63]. These examples illustrate that a secondary brain autoimmunity may cause neurological symptoms, which is in line with our postulated hypothesis.
A strength of this systematic review is the long period from which articles are included. Although the scientific literature database was also searched for relevant articles published before 1980, none were found. The most important limitation of this systematic review is that we were not able to compare or aggregate results of studies due to different outcome measurements, and different cut-off values used for TPO-abs. For future research we therefore suggest uniformity in measurements. For example, within the field of rheumatology OMERACT (Outcome Measurements in Rheumatology) is an initiative to increase the standardization of outcomes, with successful results [64]. Since a meta-analysis could not be performed, we could also not correct for differences in sample size; the association between thyroid autoimmunity and symptoms that we found in the population-based studies may therefore be less pronounced, since the studies that showed no association were performed in considerably larger populations. Another limitation of the population-based studies is that thyroid function was not always specified, and that a possible relation between TSH or thyroid hormone levels, and symptoms was not always evaluated or corrected for. Therefore, in these studies minor differences in thyroid function may have contributed to symptoms that were attributed to thyroid auto-immunity alone. Furthermore, studies in populations with another diagnosed autoimmune disease (such as celiac disease and rheumatoid arthritis) are prone for selection bias due the chance of overt and latent poly-autoimmunity. In cases of (latent) poly-autoimmunity, it will be difficult to ascertain a possible relation between thyroid autoimmunity alone and persisting symptoms. However, the latencies for other autoantibodies may hypothetically also play a role in the clinical course of patients with HD. Therefore, it is interesting to evaluate the presence of other autoantibodies in relation to persisting symptoms of HD. Only two of the herein included studies measured other autoantibodies, but did not analyze any relationship between the autoantibodies [31,36]. Six studies reported diabetes and/or rheumatoid arthritis in their population characteristics [52], of which two studies stated that the presence of diabetes mellitus was not a confounder [19,46], and three adjusted their results for the presence of diabetes and/or rheumatoid arthritis [36,38,39]. Future studies are needed to evaluate an additional role of latencies for other autoantibodies in treated HD patients with persisting symptoms. Finally, the results reported in this review may be influenced by publication bias, although we think the chance of publication bias is small as studies both in favour and against our hypothesis were published and subsequently included in this review.
4.1. Conclusions
In summary, the majority of the included studies in this systematic review reported an association between thyroid autoimmunity and persisting symptoms or low QoL in patients with HD. Meta-analysis of data was not possible due to the wide variety of used outcome measures. Several possible causes of persisting symptoms in hypothyroid patients have been proposed previously, like LT4 not being the optimal drug for treatment of hypothyroidism and DIO2 gene polymorphisms. However, given the overall results of this systematic review (thyroid) autoimmunity per se may also play a role in persisting symptoms in a part of HD patients. This however, needs further investigation as the found association does not prove a causality. Conducting a randomized placebo-controlled trial, evaluating the effect of additional immunomodulating treatment, for example intravenous immunoglobulins versus placebo, may further elucidate the role of thyroid autoimmunity in persisting symptoms in patients with HD.
Registration and protocols
This review was not registered and no protocol was not prepared.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2021.100101.
Contributor Information
Karelina L. Groenewegen, Email: k.l.groenewegen@amsterdamumc.nl.
Christiaan F. Mooij, Email: c.mooij@amsterdamumc.nl.
A.S. Paul van Trotsenburg, Email: a.s.vantrotsenburg@amsterdamumc.nl.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chaker L., Bianco A.C., Jonklaas J., Peeters R.P. Seminar hypothyroidism. Lancet. 2017;390:1550–1562. doi: 10.1016/S0140-6736(17)30703-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiersinga W.M., Duntas L., Fadeyev V., Nygaard B., Vanderpump M.P.J. ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur. Thyroid J. 2012;1(2012):55–71. doi: 10.1159/000339444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan M.M. Clinical perspectives in the diagnosis of thyroid disease. Clin. Chem. 1999;45:1377–1383. [PubMed] [Google Scholar]
- 4.Biondi B., Wartofsky L. Treatment with thyroid hormone. Endocr. Rev. 2014;35:433–512. doi: 10.1210/er.2013-1083. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair D. Clinical and laboratory aspects of thyroid autoantibodies. Ann. Clin. Biochem. 2006;43:173–183. doi: 10.1258/000456306776865043. [DOI] [PubMed] [Google Scholar]
- 6.Caturegli P., De Remigis A., Rose N.R. Hashimoto thyroiditis: clinical and diagnostic criteria, Autoimmun. Rev. 2014;13:391–397. doi: 10.1016/j.autrev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Fisher D.A., Oddie T.H., Johnson D.E., Nelson J.C. The diagnosis of Hashimoto’s thyroiditis. J. Clin. Endocrinol. Metab. 1975;40:795–801. doi: 10.1210/jcem-40-5-795. [DOI] [PubMed] [Google Scholar]
- 8.Jonklaas J., Bianco A.C., Bauer A.J., Burman K.D., Cappola A.R., Celi F.S., Cooper D.S., Kim B.W., Peeters R.P., Rosenthal M.S., Sawka A.M. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer M., Goetz T., Glenn T., Whybrow P.C. The thyroid-brain interaction in thyroid disorders and mood disorders. J. Neuroendocrinol. 2008;20:1101–1114. doi: 10.1111/j.1365-2826.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 10.Canaris G.J., Steiner J.F., Ridgway E.C. Do traditional symptoms of hypothyroidism correlate with biochemical disease? J. Gen. Intern. Med. 1997;12:544–550. doi: 10.1046/j.1525-1497.1997.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiersinga W.M. Thyroid hormone replacement therapy. Horm. Res. 2001;56:74–81. doi: 10.1159/000048140. [DOI] [PubMed] [Google Scholar]
- 12.Saravanan P., Chau W.F., Roberts N., Vedhara K., Greenwood R., Dayan C.M. Psychological well-being in patients on “adequate” doses of L-thyroxine: results of a large, controlled community-based questionnaire study. Clin. Endocrinol. 2002;57:577–585. doi: 10.1046/j.1365-2265.2002.01654.x. [DOI] [PubMed] [Google Scholar]
- 13.Panicker V., Evans J., Bjøro T., Åsvold B.O., Dayan C.M., Bjerkeset O. A paradoxical difference in relationship between anxiety, depression and thyroid function in subjects on and not on T4: findings from the HUNT study. Clin. Endocrinol. 2009;71:574–580. doi: 10.1111/j.1365-2265.2008.03521.x. [DOI] [PubMed] [Google Scholar]
- 14.Wekking E.M., Appelhof B.C., Fliers E., Schene A.H., Huyser J., Tijssen J.G.P., Wiersinga W.M. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur. J. Endocrinol. 2005;153:747–753. doi: 10.1530/eje.1.02025. [DOI] [PubMed] [Google Scholar]
- 15.de Carvalho G.A., Bahls S.-C., Boeving A., Graf H. Effects of selective serotonin reuptake inhibitors on thyroid function in depressed patients with primary hypothyroidism or normal thyroid. Function. 2009;19:691–697. doi: 10.1089/thy.2008.0261. [DOI] [PubMed] [Google Scholar]
- 16.Bianco A.C., Casula S. Thyroid hormone replacement therapy: three “simple” questions, complex answers. Eur. Thyroid J. 2012;1:88–98. doi: 10.1159/000339447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arrojo e Drigo R., Bianco A.C. Type 2 deiodinase at the crossroads of thyroid hormone action. Int. J. Biochem. Cell Biol. 2011;43:1432–1441. doi: 10.1021/nn300902w. (Release) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dora J.M., Machado W.E., Rheinheimer J., Crispim D., Maia A.L. Association of the type 2 deiodinase Thr92Ala polymorphism with type 2 diabetes: case-control study and meta-analysis. Eur. J. Endocrinol. 2010;163:427–434. doi: 10.1530/EJE-10-0419. [DOI] [PubMed] [Google Scholar]
- 19.Ott J., Promberger R., Kober F., Neuhold N., Tea M., Huber J.C., Hermann M. Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid. 2011;21:161–167. doi: 10.1089/thy.2010.0191. [DOI] [PubMed] [Google Scholar]
- 20.Leyhe T., Müssig K., Weinert C., Laske C., Häring H.U., Saur R., Klingberg S., Gallwitz B. Increased occurrence of weaknesses in attention testing in patients with Hashimoto’s thyroiditis compared to patients with other thyroid illnesses. Psychoneuroendocrinology. 2008;33:1432–1436. doi: 10.1016/j.psyneuen.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Leyhe T., Ethofer T., Bretscher J., Künle A., Säuberlich A.L., Klein R., Gallwitz B., Häring H.U., Fallgatter A., Klingberg S., Saur R., Müssig K. Low performance in attention testing is associated with reduced grey matter density of the left inferior frontal gyrus in euthyroid patients with Hashimoto’s thyroiditis. Brain Behav. Immun. 2013;27:33–37. doi: 10.1016/j.bbi.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Siegmann E.M., Müller H.H.O., Luecke C., Philipsen A., Kornhuber J., Grömer T.W. Association of depression and anxiety disorders with autoimmune thyroiditis: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75:577–584. doi: 10.1001/jamapsychiatry.2018.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt M., Voell M., Rahlff I., Dietlein M., Kobe C., Faust M., Schicha H. Long-term follow-up of antithyroid peroxidase antibodies in patients with chronic autoimmune thyroiditis (Hashimoto’s thyroiditis) treated with levothyroxine. Thyroid. 2008;18:755–760. doi: 10.1089/thy.2008.0008. [DOI] [PubMed] [Google Scholar]
- 24.Lord Brain E.H. Jellinek K. Ball. Hashimoto’s disease and encephalopathy. Lancet. 1966;2:512–514. doi: 10.1016/s0140-6736(66)92876-5. [DOI] [PubMed] [Google Scholar]
- 25.Botello A., Herrán M., Salcedo V., Rodríguez Y., Anaya J.-M., Rojas M. Prevalence of latent and overt polyautoimmunity in autoimmune thyroid disease: a systematic review and meta-analysis. Clin. Endocrinol. 2020;93:375–389. doi: 10.1111/cen.14304. [DOI] [PubMed] [Google Scholar]
- 26.Page M., McKenzie J., Bossuyt P., Boutron I., Hoffmann T., Mulrow C., Shamseer L., Tetzlaff J., Akl E., Brennan S., Chou R., Glanville J., Grimshaw J., Hróbjartsson A., Lalu M., Li T., Loder E., Mayo-Wilson E., McDonald S., McGuinnes L., Stewart L., Tjomas J., Tricco A., Welch V., Whiting P., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. MetaArXic Preprints. 2020 doi: 10.31222/osf.io/v7gm2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaziou P., del Mar C., Salisbury J. second ed. Blackwell Publishing Ltd; Oxford: 2007. Evidence-based Practice Workbook; pp. 21–38. [Google Scholar]
- 28.Zivaljevic V.R., Bukvic Bacotic B.R., Sipetic S.B., Stanisavljevic D.M., Maksimovic J.M., Diklic A.D., Paunovic I.R. Quality of life improvement in patients with Hashimoto thyroiditis and other goiters after surgery: a prospective cohort study. Int. J. Surg. 2015;21:150–155. doi: 10.1016/j.ijsu.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Giynas Ayhan M., Uguz F., Askin R., Gonen M.S. The prevalence of depression and anxiety disorders in patients with euthyroid Hashimoto’s thyroiditis: a comparative study. Gen. Hosp. Psychiatr. 2014;36:95–98. doi: 10.1016/j.genhosppsych.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Louwerens M., Appelhof B.C., Verloop H., Medici M., Peeters R.P., Visser T.J., Boelen A., Fliers E., Smit J.W.A., Dekkers O.M. Fatigue and fatigue-related symptoms in patients treated for different causes of hypothyroidism. Eur. J. Endocrinol. 2012;167:809–815. doi: 10.1530/EJE-12-0501. [DOI] [PubMed] [Google Scholar]
- 31.Bazzichi L., Rossi A., Zirafa C., Monzani F., Tognini S., Dardano A., Santini F., Tonacchera M., De Servi M., Giacomelli C., De Feo F., Doveri M., Massimetti G., Bombardieri S. Thyroid autoimmunity may represent a predisposition for the development of fibromyalgia? Rheumatol. Int. 2012;32:335–341. doi: 10.1007/s00296-010-1620-1. [DOI] [PubMed] [Google Scholar]
- 32.Delitala A.P., Terracciano A., Fiorillo E., Orrù V., Schlessinger D., Cucca F. Depressive symptoms, thyroid hormone and autoimmunity in a population-based cohort from Sardinia. J. Affect. Disord. 2016;191:82–87. doi: 10.1016/j.jad.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fjaellegaard K., Kvetny J., Allerup P.N., Bech P., Ellervik C. Well-being and depression in individuals with subclinical hypothyroidism and thyroid autoimmunity—a general population study, Nord. J. Psychiatry. 2015;69:73–78. doi: 10.3109/08039488.2014.929741. [DOI] [PubMed] [Google Scholar]
- 34.Krysiak R., Drosdzol-Cop A., Skrzypulec-Plinta V., Okopien B. Sexual function and depressive symptoms in young women with thyroid autoimmunity and subclinical hypothyroidism. Clin. Endocrinol. 2016;84:925–931. doi: 10.1111/cen.12956. [DOI] [PubMed] [Google Scholar]
- 35.Carta M.G., Loviselli A., Hardoy M.C., Massa S., Cadeddu M., Sardu C., Carpiniello B., Dell’Osso L., Mariotti S. The link between thyroid autoimmunity (antithyroid peroxidase autoantibodies) with anxiety and mood disorders in the community: a field of interest for public health in the future. BMC Psychiatr. 2004;4:25. doi: 10.1186/1471-244X-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iseme R.A., McEvoy M., Kelly B., Agnew L., Attia J., Walker F.R., Oldmeadow C., Boyle M. Autoantibodies are not predictive markers for the development of depressive symptoms in a population-based cohort of older adults. Eur. Psychiatr. 2015;30:694–700. doi: 10.1016/j.eurpsy.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Ittermann T., Völzke H., Baumeister S.E., Appel K., Grabe H.J. Diagnosed thyroid disorders are associated with depression and anxiety. Soc. Psychiatr. Psychiatr. Epidemiol. 2015;50:1417–1425. doi: 10.1007/s00127-015-1043-0. [DOI] [PubMed] [Google Scholar]
- 38.van de Ven A.C., Netea-Maier R.T., de Vegt F., Ross H.A., Sweep F.C.G.J., Kiemeney L.a., Hermus A.R., den Heijer M. Is there a relationship between fatigue perception and the serum levels of thyrotropin and free thyroxine in euthyroid subjects? Thyroid. 2012;22:1236–1243. doi: 10.1089/thy.2011.0200. [DOI] [PubMed] [Google Scholar]
- 39.Van de Ven A.C., Muntjewerff J.W., Netea-Maier R.T., de Vegt F., Ross H.A., Sweep F.C.G.J., Kiemeney L.A., Vos P.E., Buitelaar J.K., Hermus A.R.M.M., den Heijer M., Janzing J.G.E. Association between thyroid function, thyroid autoimmunity, and state and trait factors of depression. Acta Psychiatr. Scand. 2012;126:377–384. doi: 10.1111/j.1600-0447.2012.01870.x. [DOI] [PubMed] [Google Scholar]
- 40.Grigorova M., Sherwin B.B. Thyroid hormones and cognitive functioning in healthy, euthyroid women: a correlational study. Horm. Behav. 2012;61:617–622. doi: 10.1016/j.yhbeh.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engum A., Bjoro T., Mykletun A., Dahl A.A. Thyroid autoimmunity, depression and anxiety; are there any connections? An epidemiological study of a large population. J. Psychosom. Res. 2005;59:263–268. doi: 10.1016/j.jpsychores.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Grabe H.J., Völzke H., Lüdemann J., Wolff B., Schwahn C., John U., Meng W., Freyberger H.J. Mental and physical complaints in thyroid disorders in the general population. Acta Psychiatr. Scand. 2005;112:286–293. doi: 10.1111/j.1600-0447.2005.00586.x. [DOI] [PubMed] [Google Scholar]
- 43.Strieder T.G.A., Prummel M.F., Tijssen J.G.P., Brosschot J.F., Wiersinga W.M. Stress is not associated with thyroid peroxidase autoantibodies in euthyroid women. Brain Behav. Immun. 2005;19:203–206. doi: 10.1016/j.bbi.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Pop V.J., Maartens L.H., Leusink G., van Son M.J., Knottnerus A.A., Ward A.M., Metcalfe R., Weetman A.P. Are autoimmune thyroid dysfunction and depression related? J. Clin. Endocrinol. Metab. 1998;83:3194–3197. doi: 10.1210/jcem.83.9.5131. [DOI] [PubMed] [Google Scholar]
- 45.Groer M.W., Vaughan J.H. Positive thyroid peroxidase antibody titer is associated with dysphoric moods during pregnancy and postpartum. J. Obstet. Gynecol. Neonatal Nurs. 2013;42:E26–E32. doi: 10.1111/j.1552-6909.2012.01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmad J., Blumen H., Tagoe C.E. Association of antithyroid peroxidase antibody with fibromyalgia in rheumatoid arthritis. Rheumatol. Int. 2015;35:1415–1421. doi: 10.1007/s00296-015-3278-1. [DOI] [PubMed] [Google Scholar]
- 47.McCoy S.J.B., Beal J.M., Payton M.E., Stewart A.L., DeMers A.M., Watson G.H. Postpartum thyroid measures and depressive symptomology: a pilot study. J. Am. Osteopath. Assoc. 2008;108:503–507. [PubMed] [Google Scholar]
- 48.Harris B., Fung H., Johns S., Kologlu M., Bhatti R., McGregor A.M., Richards C.J., Hall R. Transient post-partum thyroid dysfunction and postnatal depression. J. Affect. Disord. 1989;17:243–249. doi: 10.1016/0165-0327(89)90006-2. [DOI] [PubMed] [Google Scholar]
- 49.Wesseloo R., Kamperman A.M., Bergink V., Pop V.J.M. Thyroid peroxidase antibodies during early gestation and the subsequent risk of first-onset postpartum depression: a prospective cohort study. J. Affect. Disord. 2018;225:399–403. doi: 10.1016/j.jad.2017.08.058. [DOI] [PubMed] [Google Scholar]
- 50.Pop V.J., a Wijnen H., Lapkienne L., Bunivicius R., Vader H.L., Essed G.G. The relation between gestational thyroid parameters and depression: a reflection of the downregulation of the immune system during pregnancy? Thyroid. 2006;16:485–492. doi: 10.1089/thy.2006.16.485. [DOI] [PubMed] [Google Scholar]
- 51.Carta M.G., Hardoy M.C., Boi M.F., Mariotti S., Carpiniello B., Usai P. Association between panic disorder, major depressive disorder and celiac disease: a possible role of thyroid autoimmunity. J. Psychosom. Res. 2002;53:789–793. doi: 10.1016/S0022-3999(02)00328-8. [DOI] [PubMed] [Google Scholar]
- 52.Bunevicius R., Peceliuniene J., Mickuviene N., Bunevicius A., Pop V.J., Girdler S.S. Mood and thyroid immunity assessed by ultrasonographic imaging in a primary health care. J. Affect. Disord. 2007;97:85–90. doi: 10.1016/j.jad.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 53.Kirim S., Keskek S.O., Koksal F., Haydardedeoglu F.E., Bozkirli E., Toledano Y. Depression in patients with euthyroid chronic autoimmune thyroiditis. Endocr. J. 2012;59:705–708. doi: 10.1507/endocrj.EJ12-0035. [DOI] [PubMed] [Google Scholar]
- 54.Bazzichi L., Rossi A., Giuliano T., Feo F., Giacomelli C., Consensi A., Ciapparelli A., Consoli G., Dell’Osso L., Bombardieri S. Association between thyroid autoimmunity and fibromyalgic disease severity. Clin. Rheumatol. 2007;26:2115–2120. doi: 10.1007/s10067-007-0636-8. [DOI] [PubMed] [Google Scholar]
- 55.Leyhe T., Müssig K. Cognitive and affective dysfunctions in autoimmune thyroiditis. Brain Behav. Immun. 2014;41:261–266. doi: 10.1016/j.bbi.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Watt T., Watt T., Hegedüs L., Hegedüs L., Bjorner J.B., Bjorner J.B., Groenvold M., Groenvold M., Bonnema S.J., Bonnema S.J., Rasmussen Å.K., Rasmussen Å.K., Feldt-Rasmussen U., Feldt-Rasmussen U. Is thyroid autoimmunity per se a determinant of quality of life in patients with autoimmune hypothyroidism? Eur. Thyroid J. 2012;1:186–192. doi: 10.1159/000342623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guldvog I., Reitsma L.C., Johnsen L., Lauzike A., Gibbs C., Carlsen E., Lende T.H., Narvestad J.K., Omdal R., Kvaløy J.T., Hoff G., Bernklev T., Søiland H. Thyroidectomy versus medical management for euthyroid patients with hashimoto disease and persisting symptoms: a randomized trial. Ann. Intern. Med. 2019;170:453–464. doi: 10.7326/M18-0284. [DOI] [PubMed] [Google Scholar]
- 58.Le Donne M., Settineri S., Benvenga S. Early pospartum alexithymia and risk for depression: relationship with serum thyrotropin, free thyroid hormones and thyroid autoantibodies. Psychoneuroendocrinology. 2012;37:519–533. doi: 10.1016/j.psyneuen.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Laurent C., Capron J., Quilleroi B., Thomad G., Alamowitch S., Fain O., Mekinian A. Steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT): characteristics, treatment and outcome in 251 cases from the literature. Autoimmun. Rev. 2016;15:1129–1133. doi: 10.1016/j.autrev.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Churilov L.P., Sobolevskaia P.A., Stroev Y.I. Thyroid gland and brain: enigma of Hashimoto’s encephalopathy. Best Pract. Res. Clin. Endocrinol. Metabol. 2019;33:101364. doi: 10.1016/j.beem.2019.101364. [DOI] [PubMed] [Google Scholar]
- 61.Ferracci F., Bertiato G., Moretto G. Hashimoto’s encephalopathy: epidemiologic data and pathogenetic considerations. J. Neurol. Sci. 2004;217:165–168. doi: 10.1016/j.jns.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Armangue T., Spatola M., Vlagea A., Mattozzi S., Cárceles-Cordon M., Martinez-Heras E., Llufriu S., Muchart J., Erro M.E., Abraira L., Moris G., Monros-Giménez L., Corral-Corral Í., Montejo C., Toledo M., Bataller L., Secondi G., Ariño H., Martínez-Hernández E., Juan M., Marcos M.A., Alsina L., Saiz A., Rosenfeld M.R., Graus F., Dalmau J. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kreye J., Reincke S.M., Prüss H. Do cross-reactive antibodies cause neuropathology in COVID-19? Nat. Rev. Immunol. 2020:1–2. doi: 10.1038/s41577-020-00458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tugwell P., Boers M., Brooks P., Simon L., Strand V., Idzerda L. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials. 2007;8:1–6. doi: 10.1186/1745-6215-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.