Abstract
The ability to escape predation modulates predator–prey interactions and represents a crucial aspect of organismal life history, influencing feeding, mating success, and survival. Thanatosis, also known as death feigning or tonic immobility (TI), is taxonomically widespread, but understudied in blood-feeding vectors. Hematophagous arthropods, such as ticks, are unique among animals as their predators (birds, mice, lizards, frogs, and other invertebrates) may also be their source of food. Therefore, the trade-off between predator avoidance and host-seeking may shift as the time since the last bloodmeal increases. Because ticks are slow-moving and unable to fly, or otherwise escape, we predicted that they may use TI to avoid predation, but that TI would be influenced by time since the last bloodmeal (starvation). We therefore aimed to quantify this relationship, examining the effect of starvation, body mass, and ontogeny on TI for two tick species: Dermacentor variabilis (Say) (Acari: Ixodidae) and Rhipicephalus sanguineus (Latreille) (Acari: Ixodidae). As we predicted, the duration and use of TI decreased with time since feeding and emergence across species and life stages. Therefore, ticks may become more aggressive in their search for a bloodmeal as they continue to starve, opting to treat potential predators as hosts, rather than avoiding predation by feigning death. Antipredator behaviors such as TI may influence the intensity and amount of time ticks spend searching for hosts, driving patterns of tick-borne pathogen transmission. This identification and quantification of a novel antipredation strategy add a new component to our understanding of tick life history.
Keywords: Thanatosis, death feigning, stress, antipredator strategy
Predation is a major selective pressure in evolution, driving diverse adaptations in morphological, physiological, and behavioral defenses among prey (Ruxton et al. 2019). One well-documented behavioral defense is tonic immobility (TI), also known as death feigning or thanatosis (Fabre 1900, Humphreys and Ruxton 2018). TI typically occurs when prey are physically restrained or in close proximity with a potential predator and adopt a motionless posture, which can last from seconds to hours (Ruxton et al. 2019). Studies of animal behavior have suggested a wide variety of mechanisms through which TI may facilitate prey survival including causing visually guided predators to lose interest, signaling to predators that the prey possesses defense chemicals, assuming a posture that makes consumption more difficult, or simply escaping from perceived hazards by dropping (Humphreys and Ruxton 2018, 2019). Perhaps the simplest antipredatory tactic is dropping, which is often used in conjunction with TI by first dropping to the ground and then feigning death (Francke et al. 2008, Castellanos and Barbosa 2011, Gillespie and Acheampong 2012, Gish et al. 2012, Ma and Ma 2012). Generally, TI is thought to reduce the severity and length of predator attacks and has been described in diverse taxa, including mammals, birds, fish, reptiles, crustaceans, and insects (Humphreys and Ruxton 2018).
Although immobility is a hallmark of TI, several physiological indicators may co-occur including reductions in breathing, heart rate, and body temperature, as well as secretions of bodily fluids or defensive chemicals (Rogers and Simpson 2014). Evidence suggests that the duration and frequency of TI are heritable, as strains of red flour beetles divergently selected for short and long durations of TI exhibit numerous biochemical, physiological, and behavioral differences (Nakayama and Miyatake 2010, Nishi et al. 2010, Miyatake et al. 2019, Uchiyama et al. 2019). Increased frequency and duration of TI corresponds to shorter leg lengths, lower dopamine production, and increased stress resistance to cold, heat, and vibrations (Miyatake et al. 2008, 2019; Matsumura and Miyatake 2019; Uchiyama et al. 2019). Importantly, long durations of TI have been directly linked to decreased overall activity and lower mating success (Nakayama and Miyatake 2010). Shifts in these traits may have significant implications for arthropod vectors as they may be adaptive (decreasing the instances of predation) but also set evolutionarily constraints on behavior. The link between TI and overall activity has significant implications for disease systems, as vector activity levels, particularly the amount of time vectors spend seeking a host, are correlated with transmission rates. (Elliott 1972, Rund et al. 2016, Murdock et al. 2017, Gandon 2018). For hematophagous arthropods, hosts may also become predators either through defensive behaviors or by consuming the vector (Samish and Rehacek 1999, Lazzari 2009). Surprisingly, there is little information about the cues vectors use for locating a host and avoiding defensive or predatory behavior (Wynne et al. 2020). Therefore, quantifying the propensity and duration of TI in ticks offers a unique opportunity to examine trade-offs associated with host-seeking and predator defense behaviors.
Several studies have directly shown that ticks experience predation across all life stages by diverse predators, including arthropods, amphibians, reptiles, and small mammals (Mwangi et al. 1991a, Samish and Rehacek 1999, Samish and Alekseev 2001). Most commonly, ticks are predated by ants, which consume engorged and unengorged ticks of all life stages (Hoogstraal 1956, Jenkins 1964, Harris and Burns 1972, Whitford and Ettershank 1975, Fleetwood et al. 1984, Barré et al. 1991, Verissimo 1995, Yoder and Domingus 2003). In temperate forests, wood ants regulate populations of ixodid ticks, attacking both unfed and engorged nymphs and adult ticks (Zingg et al. 2018). Spiders are also known predators of ticks (Krivolutsky 1963, Wilkinson 1970a, Ault and Elliott 1979, Clifford et al. 1980, Spielman 1988, Carroll 1995). In lab studies, wolf spiders killed twice as many unfed Ixodes scapularis as Amblyomma variegatum, possibly because the latter produces defensive excretions (Carroll 1995). Lizards, frogs, and toads also consume ticks, with toads preferring ticks to other prey items (Duffy 1983, Garcia et al. 1985). Rats, gerbils, hamsters, shrews, and mice have also been observed to feed on ticks, even digging up ticks buried in leaf litter of experimental plots (Milne 1950, Wilkinson 1970b, Short and Norval 1982, Mwangi et al. 1991b). Several studies have documented tick predation by birds; oxpeckers feed almost exclusively on ticks (Bezuidenhout and Stutterheim 1980). Domestic birds such as chickens and guineas reduce the severity of tick infestations in livestock pens (Milne 1950, Barré et al. 1991, Hassan et al. 1991, Dantas-Torres 2007) and in the lab, magpies preferred unfed, adult ticks to other forms of prey (Samuel 1991). Because ticks experience predation by diverse taxa and are unable to flee, TI may be a suitable antipredatory strategy and influenced by the trade-off between predator avoidance and blood feeding.
Feeding is inherently risky as it increases the chance of detection by predators (Bernays 1997). Many animals trade-off the risk of predation against the risk of starvation and therefore reduced feeding is a common reaction to the presence of predators as well as phenotypically plastic shifts in development, behavior, and physiology (Pond and Mattacks 1985, Houston et al. 1993, Walters et al. 2017). The physiological state of the individual is critical because nutritional reserves buffer the animal against starvation if food intake is insufficient to meet metabolic demands (Lima 1986, Witter et al. 1994). The relationship between body mass and predator avoidance has led to the ‘mass-dependent starvation-predation risk trade-off hypothesis’, which has been widely tested among populations and species (Le Maho et al. 1981, Gagliano et al. 2007, Pelletier et al. 2008, Bonter et al. 2013). This hypothesis suggests that body mass influences predation risk through two mechanisms: first, increased body mass reduces the urgency of feeding and therefore the risk of predation, and second, increased body mass could hinder escape from predators, if prey is slowed by excess mass. For ticks, the link between body mass and predator avoidance may be driven by the need to feed increasing as body mass decreases and ticks continue to starve. Because ticks feed on several of the same animals that are predators, the trade-off between starvation and predator avoidance may be particularly pronounced and shift with physiological conditions such as body mass and pathogen infection status.
Ticks transmit a plethora of pathogens, which impact human and animal health and are consequently one of the most economically costly and important arthropod vectors (Randolph 2004, Brites-Neto et al. 2015). TI has been previously described in mites but has never been directly measured in ticks, although some studies mention ticks ‘playing dead’ (Ebermann 1991, Sonenshine 2004, Yoder et al. 2009). Here we examine the propensity of TI in two species of hard ticks, Dermacentor variabilis (Say) (Acari: Ixodidae) and Rhipicephalus sanguineus (Latreille) (Acari: Ixodidae). Both D. variabilis and R. sanguineus are prolific vectors of numerous human and wildlife diseases and including Rocky Mountain spotted fever and tularemia (Oliver 1989). Several studies describe the physiological and molecular characteristics of these two important ticks but much less is known about their ecology and behavior, which probably drive population dynamics, host interactions, and the prevalence of these vectors and the diseases they carry (Burg 2001, Steiert and Gilfoy 2002, Yoder et al. 2006, Rosendale et al. 2016, Minigan et al. 2018).
Because ticks are small, slow, and conspicuous while questing, we predicted that they would use TI as an antipredatory tactic. We also predicted that both the instances and durations of TI would decrease as ticks starve and opt to treat predators as potential food sources. Lastly, we predicted that as body mass decreases, which is likely indicative of reduced nutritional resources, both the duration and instances of TI would decrease. Our studies show that TI is a readily employed antipredator strategy for two species of ticks. We also show that TI varies with life stage, body mass, and feeding status, with ticks exhibiting increased activity and decreased TI as time since their last bloodmeal increases. Comparative RNA-seq between beetles selected for long and short TI to D. variaiblis during starvation reveals putative molecular factors associated with this process in ticks. Our results suggest that ticks, similar to many organisms, may take greater behavioral risks to feed as nutritional resources deplete.
Materials and Methods
Study Animals
Engorged larvae and nymphs of both R. sanguineus and D. variabilis were obtained from Ecto Services Inc. (Henderson, NC). These colonies are maintained in controlled conditions and supplemented regularly with field-collected individuals. All ticks were kept under 93% relative humidity (RH), 25 ± 1°C, and 12:12 light:dark (L:D) h, and larvae were fed on rabbits (Oryctolagus cuniculus), whereas sheep (Ovis aries) were used to feed nymphs. For this study, engorged ticks at each life stage were received by our lab a few days following feeding. Two weeks after molting from engorged nymphs to adults, adult female ticks were separated and added to experimental treatment groups and kept in environmental chambers at 93% RH, 24°C, and 12 (L:D) h for the duration of the study. Newly emerged nymphs were also kept in the same conditions. Ticks were tested for TI within 1–2 mo (unstarved) or 5 mo (partially starved) or 6–9 mo (starved), following feeding and emerging into nymphs or adults. These thresholds were based on previous work in D. variabilis, which shows that major shifts in tick physiology, behavior, and transcriptome occur after approximately 12, 24, and 36 wk following emergence and without feeding (Rosendale et al. 2019). A subset of R. sanguineus was tested at 2 mo and again at 6 mo after feeding and emergence to determine whether time since feeding and emergence alone shift the duration and frequency of TI in the same individuals.
Measurement of TI
Ticks were weighed to the nearest milligram (Acculab ALC 210.4, Sartorius, NY) at the beginning of each trial. To measure the duration and number of disturbances required to elicit TI, we placed each tick on the benchtop within a 30 × 40 cm arena and gave them 10 min to adjust to their surroundings. Typically, individuals began to move around the arena within 1–2 min. Because microhabitat has been known to influence the prevalence of TI, we measured all animals in the same environment, described above. After transferring a test animal to the arena, TI was induced by using blunt featherweight forceps (BioQuip, Rancho Dominguez, CA) to simulate a bird or ant attack. The forceps were used to softly grasp and release the ticks, typically eliciting TI within one to three encounters. TI was defined as the point when the tick adducted its legs beneath its body and remained immobile for at least 1 s (Quadros et al. 2012). The end of TI was defined as the point at which ticks extended their legs, which was typically followed by immediate locomotion. The number of encounters required to elicit TI was recorded with a tally counter, and the behavioral duration was timed with a stopwatch. If individuals did not exhibit TI after 10 simulated predator encounters, they were counted as unresponsive. This threshold was previously used to categorize individuals that do not exhibit TI (Quadros et al. 2012).
Statistical Analysis
The relationship between body mass and response to a simulated predator and the duration of TI was tested with logistic regression. The differences in the duration of TI among species, life stages, and time since adult emergence were tested with analysis of variance (ANOVA) and post hoc Tukey’s tests. Also, for each species, the relationship between duration and body mass was tested with analysis of covariance. We used linear regression to determine whether there was relationship between the encounters to elicit TI and duration of response. All tests were conducted using the R Software v. 3.6.1 (R Core Team 2019).
Comparative RNA-Seq Analysis for Genes Underlying TI
To examine specific gene expression changes putatively associated with TI, we compared genes expressed during tick starvation with those expressed by beetles selected for long and short TI (Rosendale et al. 2016, 2019; Uchiyama et al. 2019). This was accomplished by BLAST comparison of genes with increased transcript levels under long and short TI to those from a de novo transcriptome for D. variabilis (GGQS00000000.1). These matches were compared with those expressed 4 wk (before starvation) and 36 wk (during starvation) following adult emergence. RNA-seq data sets are available under the NCBI Bioproject PRJNA437454. Significance was based on the analysis in Rosendale et al. (2019), where transcripts were differentially expressed with a false detection rate of P ≤ 0.05 and fold change ≥ |2|. Along with a direct comparison, we also examined differential expression of genes associated with insulin and tyrosine metabolism and dopamine signaling as these specific categories were associated with long and short TI in beetles (Uchiyama et al. 2019).
Results
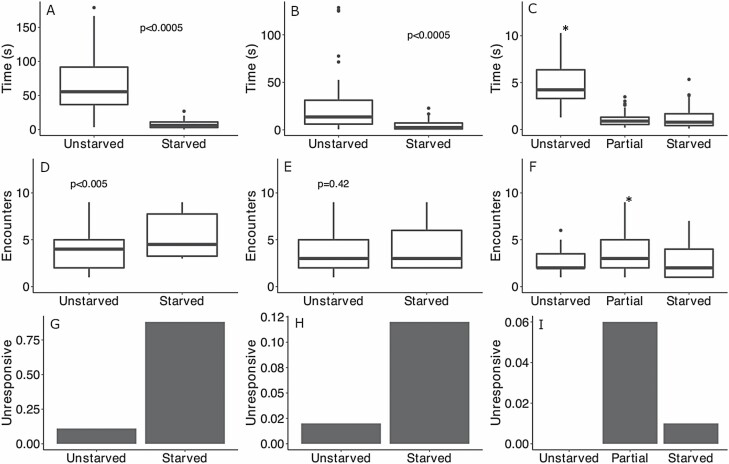
We found significant effects of time because adult emergence, life stage, body mass, and species on both the number of encounters required to elicit TI and the duration of TI. Furthermore, there was a strong relationship between the duration of TI and the encounters required to elicit TI in adults (F1,196 = 14.88, P < 0.0005) and nymphs (F1,212 = 4.88, P = 0.028) with ticks that more readily exhibited TI spending the longest amount of time in TI. This significant relationship between the duration of TI and number of disturbances required to elicit TI was consistent within life stages, feeding status, and species. In both starved and unstarved adult ticks, D. variabilis had longer durations of TI (F1,268 = 22.55, P < 0.0005) and required more encounters to elicit TI (F1,268 = 38.64, P < 0.0005) compared with R. sanguineus. On average, D. variabilis required six predator encounters before exhibiting TI and were immobile for approximately 60 s, relative to R. sanguineus that remained in TI for approximately 25 s and exhibited TI after approximately three encounters (Table 1).
Table 1.
Variation in the duration of TI (time) and number of disturbances (encounters) required to elicit TI among life stages and feeding status for two species of ticks, Dermacentor variabilis and Rhipicephalus sanguineus
| Species | Months since feeding and emergence | Status | Life stage | Encounters, mean ± SD | Time (s), mean ± SD | Unresponsive (n) | Responsive (n) | Total (n) | Proportion of unresponsive individuals |
|---|---|---|---|---|---|---|---|---|---|
| Dermacentor variabilis | 1 | Unstarved | Adult | 4 ± 3 | 67.5 ± 38.4 | 10 | 78 | 88 | 0.11 |
| Dermacentor variabilis | 6 | Starved | Adult | 9 ± 4 | 4.4 ± 3.7 | 77 | 11 | 88 | 0.88 |
| Dermacentor variabilis | 2 | Unstarved | Nymph | 3 ± 1 | 4.9 ± 2.4 | 0 | 80 | 80 | 0.00 |
| Dermacentor variabilis | 5 | Partial | Nymph | 4 ± 2 | 1.1 ± 0.7 | 3 | 56 | 59 | 0.06 |
| Dermacentor variabilis | 9 | Starved | Nymph | 3 ± 2 | 1.2 ± 1.0 | 1 | 79 | 80 | 0.01 |
| Rhipicephalus sanguineus | 1 | Unstarved | Adult | 4 ± 2 | 25.6 ± 31.9 | 1 | 49 | 50 | 0.02 |
| Rhipicephalus sanguineus | 6 | Starved | Adult | 5 ± 3 | 5.1 ± 3.1 | 6 | 44 | 50 | 0.12 |
Number of unresponsive individuals indicates the number of individuals that did not exhibit TI after 10 simulated predator encounters and are therefore excluded from the analysis of encounters and time. Values are means ± SD. See text and Fig. 1 for statistical details.
Time Since Emergence
Time since feeding and subsequent emergence significantly influenced both the duration and propensity for TI across all ticks. Starvation decreased the duration of TI in adult D. variabilis by over 16-fold (F1,169 = 174.1, P < 0.0005) and doubled number of encounters required to elicit TI (Fig. 1A–D; F1,99 = 20.09, P < 0.0005). Similarly, unstarved adult R. sanguineus spent nearly five times longer in TI than starved conspecifics (Fig. 1B; F1,98 = 20.48, P < 0.0005) and exhibited TI after approximately three simulated predator encounters (Figs. 1E; F1,96 = 4.08, P = 0.046). The number of encounters required to elicit TI decreased with the time since the emergence in all nymphs (ANOVA, F1,211 = 4.88, P < 0.008), but was highest in the partially starved group (Fig. 1F; Tukey’s HSD, P = 0.006) and was not significantly different between the starved and unstarved nymphs (Tukey’s HSD, P = 0.38, and P = 0.14, respectively). The duration of TI decreased significantly with time since feeding and emergence among all nymphs (Fig. 1C; ANOVA, F1,211 = 137.3, P < 0.00005) but was not significantly different between the partially starved (5 mo since feeding and emergence) and starved (9 mo since feeding and emergence) groups (Fig. 1F; Tukey’s HSD, P = 0.93). The number of unresponsive individuals was defined as ticks, which did not exhibit TI after 10 simulated predator encounters. Numbers of unresponsive individuals increased 11-fold in starved D. variabilis and 6-fold in starved R. sanguineus adults (Fig. 1G and H; Table 1). Furthermore, all unstarved nymphs exhibited TI before 10 predator encounters and 3 partially starved and 1 starved nymph remained unresponsive (Fig. 1I; Table 1).
Fig. 1.
The duration of TI in seconds was measured in unstarved and starved adult Dermacentor variabilis and Rhipicephalus sanguineus (A and B, respectively), and unstarved, partially starved, and starved D. variabilis nymphs (C). The number of encounters required to elicit TI was measured in unstarved and starved adult D. variabilis, adult R. sanguineus (D and E, respectively), and unstarved, partially starved, and starved D. variabilis nymphs (F). (G–I) The proportion of individuals that were unresponsive to encounters with predators (not responding after 10 encounters) in unstarved and starved adult D. variabilis, adult R. sanguineus, and D. variabilis nymphs, respectively. Symbols (*) and P values indicate statistical significance.
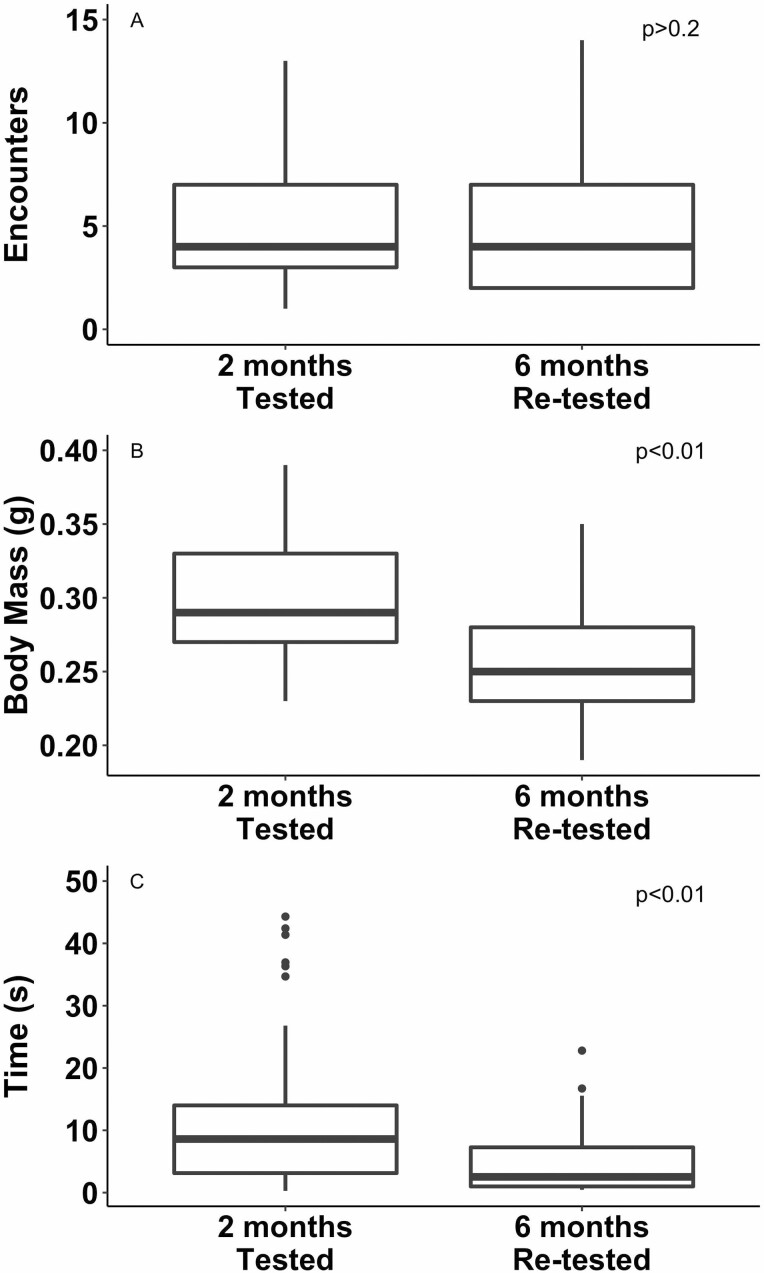
When measured at 2-mo postfeeding and after emergence and again at 6-mo postfeeding and after emergence, R. sanguineus showed no difference in the number of encounters required to elicit TI (Fig. 2A; F1,96 = 4.08, P = 0.046) and lost nearly 15% body mass between 2- and 6-mo postfeeding and after emergence (Fig. 2B). Additionally, ticks spent significantly less time in TI after 6 mo without feeding (Fig 2C; F1,96 = 4.08, P = 0.046). Overall, the duration of TI decreased by ~55% in 4 mo without feeding, with individuals measured at 2-mo postfeeding and after emergence spending approximately 9 s in TI but only 2 s after 6 mo of starvation.
Fig. 2.
Rhipicephalus sanguineus (n = 50) were measured twice at 2- and 6-mo postadult emergence. The number of encounters with a simulated predator required to elicit TI (A), body mass (B), and duration of TI (C) at 2-mo postfeeding and after emergence (unstarved) and again at 6-mo postfeeding and after emergence (starved) are indicated by boxplots. P values indicate statistical significance.
Life Stage
Both starved and unstarved nymphs required fewer encounters to elicit TI than starved and unstarved adult conspecifics (F1,160 = 112.3, P < 0.0001), but nymphs remained immobile for shorter durations (F1,160 = 92.8, P < 0.0001). In general, adult ticks maintained TI about 4 s longer than nymphs in both starved and unstarved groups (Table 1). Unstarved nymphs typically exhibited TI after approximately three encounters, whereas unstarved adults encountered a simulated predator five times before using TI.
Unstarved adults had required approximately two more encounters with simulated predators before exhibiting TI compared with unstarved nymphs (Table 1; F1,161 = 24.64, P < 0.0005), but feigned death for significantly longer periods (nearly 60 s longer) than unstarved nymphs (Table 1; F1,161 = 173.2, P < 0.0005). We observed a similar pattern in starved adults, which require more disturbances than starved nymphs to exhibit TI (F1,161 = 113.8, P < 0.0005), but also spend significantly greater amounts of time in TI than nymphs (F1,161 = 91.3, P < 0.0005).
Mass Effects
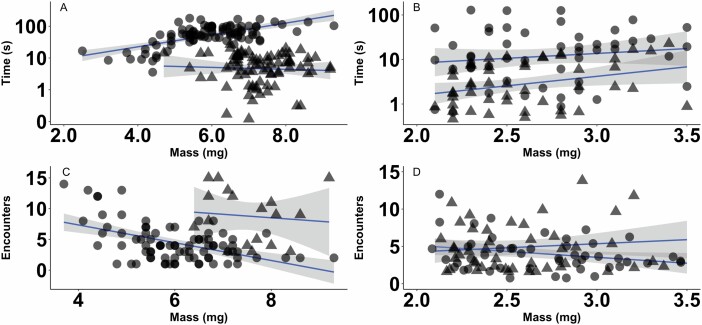
Overall, the duration of TI increased significantly with body mass (F1,268 = 65.08, P < 0.0005). This pattern held true in starved, adult D. variabilis (Fig. 3A; F1,86 = 35.05, P < 0.0005) and R. sanguineus (Fig. 3B; F1,48 = 12.78, P = 0.0008), but not unstarved conspecifics (F1,81 = 1.413, P = 0.238; F1,48 = 0.022, P = 0.882). Larger, unstarved nymphs also spent more time in TI than smaller counterparts (F1,73 = 4.31, P = 0.041).
Fig. 3.
The duration of TI in seconds and number of encounters required to elicit TI in relation to body mass (mg) in starved (triangles) and unstarved (circles) adult D. variabilis (A and C, respectively), and adult Rhipicephalus sanguineus (B and D, respectively). Trend lines indicate significant (P < 0.05) relationships and 95% confidence interval for unstarved D. variabilis adults (A, P < 0.0005), starved adult R. sanguineus (B, P = 0.0008), and unstarved D. variabilis adults (C, P < 0.0005). Adult body mass did not influence the number of encounters required to elicit a response in adult R. sanguineus (C).
For all adult ticks, the relationship between the number of encounters required to elicit TI and body mass was insignificant (F1,195 = 0.87, P = 0.35). This was the case for both starved adult D. variabilis (Fig. 3C) and adult R. sanguineus (Fig. 3D). Although there was a highly significant influence of mass on adult unstarved D. variabilis, with smaller adults requiring more simulated predator encounters than their larger counterparts (Fig. 3C; F1,81 = 26.99, P < 0.0005). Although this trend was insignificant within starved D. variabilis (F1,81 = 0.67, P = 0.413) and R. sanguineus (F1,48 = 0.06, P = 0.812), both groups trended toward the same pattern, suggesting that individuals of greater mass exhibit TI with fewer predator encounters (Fig. 3C and D). For nymphs, the number of encounters required to elicit TI increased with body mass (F1,212 = 12.59, P = 0.0005), where larger individuals endured more disturbances before entering TI than their smaller counterparts. This pattern was primarily driven by larger, starved, and partially starved nymphs, which required the most encounters to elicit TI (F1,66 = 4.11, P = 0.047), whereas body mass of unstarved nymphs did not influence the number of disturbances required to elicit TI (F1,73 = 0.50, P = 0.483).
Transcript Differences Underlying Short and Long TI That Are Affected by Starvation
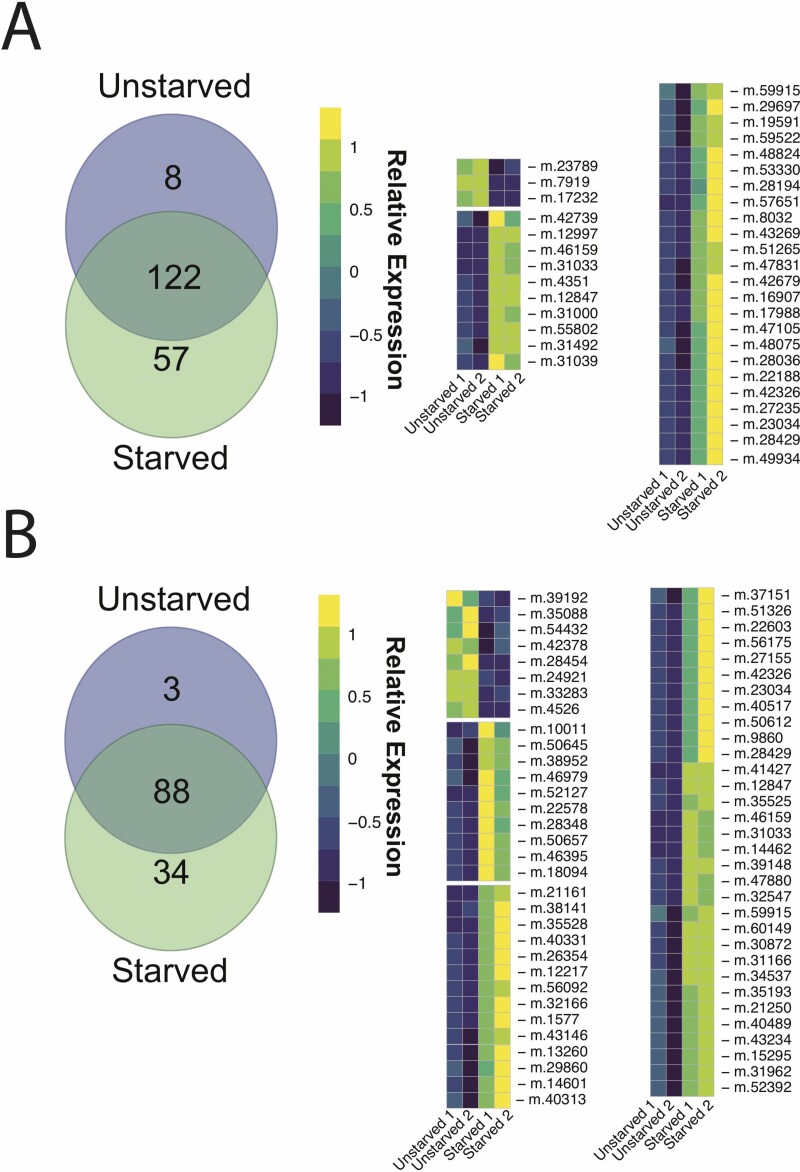
Our comparative analyses revealed 312 gene matches between a transcriptome for D. variabilis and genes differentially expressed in T. castaneum selected for short and long durations of TI (Fig. 4). Three differentially expressed genes were shared between beetles with long TI durations and unstarved ticks, which also exhibited long durations of TI. These three genes are putatively involved in maintenance of membrane ion balance, degradation of gangliosides, and mitochondrial translation. Additionally, 57 differentially expressed genes were shared between beetles bred for short durations of TI and starved ticks, which also exhibited short durations of TI. Within these 57 genes were stress proteins, including HSP-70, HSP-90, and proteins, which modulate immunity, including cytochrome P450, which is responsible for metabolic detoxification, and tryptase (Uchiyama et al. 2019). Of interest was shared differential expression in tyrosine-protein kinase, which is associated with dopamine biosynthesis, and several longevity-related genes, including Z9 acyl-CoA desaturases and fatty acyl-CoA reductases between the short TI duration beetles and starved ticks. Using the Database for Annotation, Visualization and Integrated Discovery (DAVID), the 57 differentially expressed genes that were shared between short duration beetles and starved ticks were associated with GO terms for oxidoreductase activity (The Gene Ontology Consortium 2019).
Fig. 4.
Venn diagrams of differentially expressed genes (left) in unstarved and starved ticks compared with artificially selected beetles for short durations (A) and long durations (B) of TI and corresponding heatmaps (right) for transcript levels of TI-related genes among starved and unstarved ticks. Colors indicate relative levels of expression from high (lightest) low (darkest; see Supp Tables [online only] for data).
Discussion
TI may increase the chance of surviving a predatory attack through several potential mechanisms, including reducing the interest of visually guided predators, exposing attackers to defensive chemicals, or deterring predators that will not consume dead prey (Rogers and Simpson 2014). Although death-feigning behavior has been known for over a century, substantial gaps remain in the quantification of its prevalence across taxonomic levels and the underlying factors that drive its occurrence, particularly with regard to arthropod vectors (Humphreys and Ruxton 2018). Here we examine TI and factors that influence TI, including starvation, body mass, and ontogeny in two species of hard ticks. We show that both the duration of TI and the number of simulated predator encounters required to elicit TI shift with time since feeding and emergence as well as body mass. TI also varies between species, life stages, and body sizes.
We used two response variables, the duration of TI and the encounters required to elicit TI, as metrics of TI propensity in ticks. The frequency of TI was strongly correlated with the duration of TI in both species and it is likely that both of these metrics provide robust estimates of behavioral differences in ticks. Ticks are unique among animals previously measured for TI because as hematophagous arthropods, their predators may also act as hosts, leading to a direct trade-off between death feigning and host-seeking. Given this obvious trade-off, we believe the number of simulated predator encounters may be a particularly relevant metric for understanding TI in vectors, which must balance predator avoidance with host-seeking. Our data suggest that ticks switch from predator avoidance to aggressive host-seeking, as starvation progresses, increasing the number of predator encounters required to elicit TI and shortening the duration of TI (or loss of the behavior altogether). This trade-off between predator avoidance and feeding is only one example of several associated trade-offs with TI that have been previously described.
Increased durations of TI are strongly correlated with other behavioral traits, and physiological and morphological characteristics, including overall activity levels, exploratory behavior, dopamine production, leg length, and even mating success (Humphreys and Ruxton 2018, Matsumura and Miyatake 2019, Uchiyama et al. 2019). These correlative patterns may lead to significant life-history trade-offs with risk averse (greater propensity for and longer durations of TI) individuals being generally less-active and subsequently having lower mating success (Nakayama and Miyatake 2010). Beetles selectively bred for long and short durations of TI showed significant transcriptional differences in the enzymatic precursors of dopamine (Uchiyama et al. 2019). Several genes involved in dopaminergic pathways were expressed at higher levels in individuals with long durations of TI, while insulin signaling-related genes were more highly expressed in individuals with shorter durations of TI (Uchiyama et al. 2019). Although these differences were observed in selectively bred beetle strains for long and short durations of TI, we tested whether these transcriptional differences would also be prevalent in unstarved and starved ticks with respectively long and short durations of TI.
Interestingly, progressive transcriptional shifts throughout starvation mirrored some of the transcriptional changes observed in long and short TI duration beetles (Rosendale et al. 2016, Uchiyama et al. 2019). Several stress-related genes and tyrosine protein kinase, which is involved in dopamine biosynthesis, overlapped between starved ticks and artificially selected beetles, both of which exhibit short durations of TI. Dopamine and other neurochemicals can affect motivational state and have previously been shown to decrease TI in several species of birds and mammals (Ettinger and Thompson 1978, Jones and Faure 1981, Meyer et al. 1984). Injections of dopamine or octopamine reduced the duration of TI in T. castaneum beetles, suggesting that these neurochemicals may be important regulators of predator responses among insects (Nishi et al. 2010). Notably, both starved ticks and selected beetles with low propensity for TI had lower relative expression of stress proteins, including HSP-70 and HSP-90 than conspecifics with longer durations of TI (Uchiyama et al. 2019). These parallels in stress protein expression could suggest that behavioral differences in TI could be induced by both selection and acute stressful physiological conditions. Other studies have also shown acute physiological stress, including extreme temperatures (Miyatake et al. 2008, Quadros et al. 2012), overcrowding (Tojo 1991), and starvation (Miyatake 2001, Li et al. 2019, Segovia et al. 2019), to influence the prevalence of TI in populations. Starvation influences TI in several species, generally leading to decreased durations of TI as starvation persists (Miyatake 2001, Bilde et al. 2006, Li et al. 2019). Although neither harvestman nor wasps (King and Leaich 2006, Segovia et al. 2019) exhibit changes in TI duration due to prolonged starvation. Body mass may also act as a proxy for nutritional status, with smaller individuals becoming more starved with time since feeding than their larger counterparts (Hochachka and Somero 2002). This could explain why body mass influences the frequency and duration of TI in our study.
Our results match other studies showing that individuals with greater mass have longer durations of TI (Hozumi and Miyatake 2005, Quadros et al. 2012, Neves and Pie 2018). This relationship could suggest that larger individuals are more risk averse or potentially more attractive to predators (Farkas 2016). Larger individuals may also have more available resources (e.g., lipid reserves), given their greater body mass. If true, the effect of body mass may be an extension of the starvation effect, where smaller, more resource-limited individuals are in greater need of a bloodmeal and therefore more prone to host-seeking than predator avoidance. Thus, individual variability in body mass may be tightly linked to nutritional status and drive the observed differences in TI, suggesting an even stronger role of resource-limitation in modulating TI. Further support of this hypothesis is the decreased durations in TI and decreased body mass in R. sanguineus measured at 2- and 6-mo postfeeding and after emergence. This is particularly relevant because TI duration decreased with body mass in the same individuals, compared with the other experiments, which measured separate treatment groups and individuals. This finding directly points to behavior flexibility in individuals, which shift from long durations of TI to short durations of TI, rather than just correlative differences between larger and smaller ticks or those with varying levels of nutritional resources. Another, nonexclusive possibility is that larger ticks may be more conspicuous to prey, leading to longer durations of TI and increased anti-predatory behavior (Mänd et al. 2007). This is further supported by the fact that adults typically sustain TI for longer periods than nymphs with comparable levels of starvation. Predators have been shown to have stronger aversions to aposematic prey with larger body sizes (Mänd et al. 2007). Previous studies have also shown that warning signals, including chemical secretions, offset the cost of large, conspicuous body size (Ruxton et al. 2019). In frogs, smaller individuals are selectively disadvantaged because predators can discern that they have relatively low levels of chemical defense compared with larger frogs (Flores et al. 2015). This could also apply to ticks, which secrete protective squalene when confronted by ants (Yoder and Domingus 2003).
Repellent substances, including chemical defenses, putrescence, or other secretions, may increase survival during a predator attack, particularly when paired with TI (Gabrielsen and Smith 1985). Death displays with chemical defenses are common and thought to increase the chance that a visually guided predator will shift focus from or be repelled by chemical defenses, rather than spend time overpowering its prey (Rogers and Simpson 2014). When confronted by a potential predator, American opossums and hog nosed snakes not only assume a contorted, thantotic posture but also urinate, defecate, and secrete a foul-smelling fluid from anal glands (Gehlbach 1970, Gabrielsen and Smith 1985). Red tree frogs release an ammonia-like substance to deter potential predators (Escobar-Lasso and González-Duran 2012). Ticks have been previously shown to secrete defensive allomones predominantly composed of squalene in conjunction with other unknown chemicals, from large pores on their dorsolateral surface (Yoder et al. 1999). These pores, previously termed sagittiform sensilla because of presumed sensory functions, are widespread on metastriate ticks, which produce a waxy substrate often protecting them from predation (Yoder et al. 1993a, 2009). The amount of secretion can be quite substantial, averaging around 2% of a tick’s body mass (Yoder et al. 1993b, Sonenshine 2004). When attacked by fire ants, unfed adult D. variabilis were observed to secrete defensive squalene, which significantly reduced instances of predation (Yoder and Domingus 2003). Chemical secretions are likely a critical component during tick-ant interactions and although tick allomones do not have a repellant odor, they do temporarily neutralize predator aggression (Zingg et al. 2018, Showler et al. 2019). Tick allomones are comparable to the majority of allomones in found insects and may be utilized as an anti-predatory strategy in tandem with TI, similarly to other species (Blum 1985, Yoder et al. 1993a). Interestingly, ticks replete of squalene and other associated chemicals (either by repeated predator attacks or by dehydration) were more vulnerable to attack (Yoder et al. 1993b). The relationship between starvation and allomone secretion in ticks is unknown, but long bouts of starvation are likely to reduce potential production or reserves of allomones and subsequently could be linked to the behavioral differences we see in ticks of variable body mass and nutritional status.
Several caveats to our results should be considered. Although lab-reared ticks may not perfectly represent wild ticks, many previous studies have found clear parallels in behavior, physiology, and morphology between lab-reared and wild-collected ticks. Furthermore, many of the patterns we have observed, including effects of ontogeny, starvation, and body mass, have also been documented in other species (Jones and Faure 1981, Miyatake 2001, Hozumi and Miyatake 2005, King and Leaich 2006, Farkas 2016), suggesting that our findings are broadly applicable and likely representative of other tick species. During our field collections of American dog ticks, we have consistently observed TI-like behaviors when handling nymphs and adults, further supporting that TI is an ecologically important behavior exhibited by wild ticks. We therefore have little reason to assume that our results are not indicative of ticks in the wild.
A potential difference between our experimental trails and tick predator encounters in the wild is the fact that our trials used a horizontal testing surface, but wild ticks often encounter predators while questing vertically on vegetation. Consequently, the behavior we observed in the lab when ticks adducted their legs and remained immobile could result in ‘dropping’ in the wild. Dropping is an antipredator tactic that has been recorded in a variety of taxa and temporality removes an individual from a perceived hazard. It is often paired with other prey defense strategies such as prolonged death feigning or chemical excretions (Humphreys and Ruxton 2019). In our study, we observed that ticks may continue to feign death long after an initial predator encounter (quantified by the duration of TI) and other work has shown that ticks secrete chemical squalene in response to ants or other predators (Yoder et al. 1993b). Therefore, in the wild, ticks likely use a combination of these strategies—dropping, continual death feigning, and chemical secretions—to reduce predation. Further experiments that directly quantify dropping propensities in ticks, using vertical rather than horizontal testing spaces, would help clarify the complex interplay between these strategies in wild ticks. Last, because age, body mass, and starvation all covary within our study, it is difficult to attribute the behavioral changes in tick TI to a single factor. However, it is worth noting that body mass influenced the duration of TI in the unstarved individuals but not within starved individuals. This effect was consistent in both species and suggests that starvation may drive the duration of TI to a greater extent than body mass. Similarly, although age and starvation covary in our study, we believe that the patterns we observed are driven by differences in starvation rather than age because we see both within- and among-life stage variability in TI. Even if TI is affected by age alone, starvation, and stress tolerance have been directly linked to physiological age in ticks, where periods of stress exposure yield ‘older’ ticks even though the time since emergence is shorter (Uspensky 1995, McCue et al. 2017, Pool et al. 2017, Rosendale et al. 2017). This effect of physiological aging could be critical to feeding and pathogen transmission as questing could be more aggressive and TI reduced following repeated stress bouts.
Ticks are notorious vectors of numerous pathogens, and although much of their physiology has been previously studied and described, little is known about their behavior outside of factors related to host questing (Loye and Lane 1988, Perret et al. 2000, Tomkins et al. 2014, Arsnoe et al. 2015, Kjeldgaard et al. 2019) and how differences in tick behavior may influence disease dynamics. Our study is the first to quantitatively describe the frequency and duration of TI in ticks. The duration of TI decreased among all ticks and life stages as time since feeding increased, suggesting an interesting behavioral progression from risk averse, unstarved ticks to aggressive starving individuals. Although we did not observe strong differences in the number of encounters required to induce TI with regard to starvation in R. sanguineus, we did see major differences between starved and unstarved D. variabilis. Therefore, this may be a useful metric for estimating behavioral differences among hematophagous arthropods, which are unique as their predators may also become hosts. Along with previous work on tick chemical defenses, our results provide a more complete view of antipredatory strategies in ticks. To our knowledge, TI has never previously been reported in an arthropod vector and potentially represents an important aspect of predator–prey interactions in disease systems. These behavioral adaptations may be particularly advantageous for ambush/sit-and-wait vectors such as ticks, which probably rely on the energetic savings of quiescence during long periods of starvation between hosts. Therefore, TI, rather than fleeing predation, may be particularly attractive for energetically limited organisms, such as ticks. Understanding the complex dynamics of tick antipredator behavior may lead to a more holistic view of the ecological processes driving tick-borne diseases.
Supplementary Material
Acknowledgments
We thank Samuel Baily for assistance with analysis and figure development for the comparative genetic component of this study. We also thank Thomas Arya for assistance with the care and maintenance of ticks throughout the course of this study and two anonymous reviewers whose comments improved the quality of our manuscript. This work was funded by the David H. Smith Conservation Research Fellowship to K.J.O. and the National Science Foundation (DEB-1654417 to J.B.B.), United States Department of Agriculture National Institute of Food and Agriculture (2018-67013-28495 to J.B.B.), and National Institutes of Health (1R01AI148551-01A1 to J.B.B.).
References Cited
- Arsnoe, I. M., Hickling G. J., Ginsberg H. S., McElreath R., and Tsao J. I.. . 2015. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human lyme disease risk. PLoS One 10: e0127450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault, S. K., and Elliott K. D.. . 1979. Spider predation (Araneae: Salticidae) on Ornithodoros coriaceus (Acarina: Argasidae), with a survey of the predators of the genus Ornithodoros. J. Med. Entomol. 15: 570–571. [Google Scholar]
- Barré, N., Mauléon H., Garris G. I., and Kermarrec A.. . 1991. Predators of the tick Amblyomma variegatum (Acari: Ixodidae) in Guadeloupe, French West Indies. Exp. Appl. Acarol. 12: 163–170. [DOI] [PubMed] [Google Scholar]
- Bernays, E. A. 1997. Feeding by lepidopteran larvae is dangerous. Ecol. Entomol. 22: 121–123. [Google Scholar]
- Bezuidenhout, J. D., and Stutterheim C. J.. . 1980. A critical evaluation of the role played by the red-billed oxpecker Buphagus erythrorhynchus in the biological control of ticks. Une Evaluation Critique du role Joue par l’oisea u, Buphagus er Ythrorhynchus, dans le Controle Biologique des Tiques. (https://repository.up.ac.za/handle/2263/53998).
- Bilde, T., Tuni C., Elsayed R., Pekár S., and Toft S.. . 2006. Death feigning in the face of sexual cannibalism. Biol. Lett. 2: 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum, M. S. 1985. Fundamentals of insect physiology. John Wiley & Sons, New York. [Google Scholar]
- Bonter, D. N., Zuckerberg B., Sedgwick C. W., and Hochachka W. M.. . 2013. Daily foraging patterns in free-living birds: exploring the predation–starvation trade-off. Proc. R. Soc. B 280: 20123087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brites-Neto, J., Duarte K. M., and Martins T. F.. . 2015. Tick-borne infections in human and animal population worldwide. Vet. World 8: 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg, J. G. 2001. Seasonal activity and spatial distribution of host-seeking adults of the tick Dermacentor variabilis. Med. Vet. Entomol. 15: 413–421. [DOI] [PubMed] [Google Scholar]
- Carroll, J. F. 1995. Laboratory evaluation of predatory capabilities of a common wolf spider (Araneae: Lycosidae) against two species of ticks (Acari: Ixodidae). Proc. Entomol. Soc. Wash. 97: 746–749. [Google Scholar]
- Castellanos, I., and Barbosa P.. . 2011. Dropping from host plants in response to predators by a polyphagous caterpillar. J. Lepi Soc. 65: 270–272. [Google Scholar]
- Clifford, C. M., Hoogstraal H., Radovsky F. J., Stiller D., and Keirans J. E.. . 1980. Ornithodoros (Alectorobius) amblus (Acarina: Ixodoidea: Argasidae): identity, marine bird and human hosts, virus infections, and distribution in Peru. J. Parasitol. 66: 312–323. [PubMed] [Google Scholar]
- Dantas-Torres, F. 2007. Rocky Mountain spotted fever. Lancet Infect. Dis. 7: 724–732. [DOI] [PubMed] [Google Scholar]
- Duffy, D. C. 1983. The ecology of tick parasitism on densely nesting Peruvian seabirds. Ecology 64: 110–119. [Google Scholar]
- Ebermann, E. 1991. Thanatosis or feigning death in mites of the family Scutacaridae. The Acari: reproduction, development and life-history strategies. Springer, Dordrecht, The Netherlands. p. 399–401. [Google Scholar]
- Elliott, R. 1972. The influence of vector behavior on malaria transmission. Am. J. Trop. Med. Hyg. 21: 755–763. [DOI] [PubMed] [Google Scholar]
- Escobar-Lasso, S., and González-Duran G. A.. . 2012. Strategies employed by three Neotropical frogs (Amphibia: Anura) to avoid predation. Herpetol Notes 5: 79–84. [Google Scholar]
- Ettinger, R. H., and Thompson R. W.. . 1978. The role of dopaminergic systems in the mediation of tonic immobility (animal hypnosis) in chickens. Bull. Psychon Soc. 12: 301–302. [Google Scholar]
- Fabre, J. H. 1900. Souvenirs entomologiques Livre VII-Étude sur l’instinct et les moeurs des insectes: Libraire Delagrave, Paris, France. [Google Scholar]
- Farkas, T. E. 2016. Body size, not maladaptive gene flow, explains death-feigning behaviour in Timema cristinae stick insects. Evol. Ecol. 30: 623–634. [Google Scholar]
- Fleetwood, S. C., Teel P. D., and Thompson G.. . 1984. Impact of imported fire ant on lone star tick mortality in open and canopied pasture habitats of east central Texas [Amblyomma americanum, Solenopsis invicta]. Southwest. Entomol. 9: 158–163. (http://agris.fao.org/agris-search/search.do?recordID=US8532630).
- Flores, E. E., Stevens M., Moore A. J., Rowland H. M., and Blount J. D.. . 2015. Body size but not warning signal luminance influences predation risk in recently metamorphosed poison frogs. Ecol. Evol. 5: 4603–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke, D. L., Harmon J. P., Harvey C. T., and Ives A. R.. . 2008. Pea aphid dropping behavior diminishes foraging efficiency of a predatory ladybeetle. Entomol. Exp. Appl. 127: 118–124. [Google Scholar]
- Gabrielsen, G. W., and Smith E. N.. . 1985. Physiological responses associated with feigned death in the American opossum. Acta Physiol. Scand. 123: 393–398. [DOI] [PubMed] [Google Scholar]
- Gagliano, M., McCormick M. I., and Meekan M. G.. . 2007. Survival against the odds: ontogenetic changes in selective pressure mediate growth-mortality trade-offs in a marine fish. Proc. Biol. Sci. 274: 1575–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandon, S. 2018. Evolution and manipulation of vector host choice. Am. Nat. 192: 23–34. [DOI] [PubMed] [Google Scholar]
- Garcia, M., Ferrari O., Rocha U., Verissimo C., and Homem E.. . 1985. Relative preferences of the toad Fugo paracnemis for ticks, muscoid larvae, and slugs as food. Vet. Zootec. 1: 95–99. [Google Scholar]
- Gehlbach, F. R. 1970. Death-feigning and erratic behavior in leptotyphlopid, colubrid, and elapid snakes. Herpetologica 26: 24–34. [Google Scholar]
- Gillespie, D. R., and Acheampong S.. . 2012. Dropping behaviour in Aulacorthum solani (Hemiptera: Aphididae) following attack by Aphidus ervi (Hymenoptera: Braconidae): are sticky stem bands a useful integrated pest management method? Can. Entomol. 144: 589–598. [Google Scholar]
- Gish, M., Dafni A., and Inbar M.. . 2012. Young aphids avoid erroneous dropping when evading mammalian herbivores by combining input from two sensory modalities. PLoS One 7: e32706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, W. G., and Burns E. C.. . 1972. Predation on the lone star tick by the imported fire ant. Environ. Entomol. 1: 362–365. [Google Scholar]
- Hassan, S. M., Dipeolu O. O., Amoo A. O., and Odhiambo T. R.. . 1991. Predation on livestock ticks by chickens. Vet. Parasitol. 38: 199–204. [DOI] [PubMed] [Google Scholar]
- Hochachka, P. W., and Somero G. N.. . 2002. Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Hoogstraal, H. 1956. Ticks of the Sudan (with special reference to Equatoria Province and with preliminary reviews of the genera Boophilus, Margaropus, and Hyalomma. Afr. Ixodoidea 1: 150–153. [Google Scholar]
- Houston, A. I., McNamara J. M., and Hutchinson J. M. C.. . 1993. General results concerning the trade-off between gaining energy and avoiding predation. Philos. Trans. R. Soc. 341: 375–397. [Google Scholar]
- Hozumi, N., and Miyatake T.. . 2005. Body-size dependent difference in death-feigning behavior of adult Callosobruchus chinensis. J. Insect Behav. 18: 557–566. [Google Scholar]
- Humphreys, R. K., and Ruxton G. D.. . 2018. A review of thanatosis (death feigning) as an anti-predator behaviour. Behav. Ecol. Sociobiol. 72: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys, R. K., and Ruxton G. D.. . 2019. Dropping to escape: a review of an under-appreciated antipredator defense. Biol. Rev. 94: 575–589. [DOI] [PubMed] [Google Scholar]
- Jenkins, D. W. 1964. Pathogens, parasites and predators of medically important arthropods. annotated list and bibliography. Bull. World Health Organ. 30(Suppl): 1–150. [PMC free article] [PubMed] [Google Scholar]
- Jones, R. B., and Faure J. M.. . 1981. Sex and strain comparisons of tonic immobility (“righting time”) in the domestic fowl and the effects of various methods of induction. Behav. Process. 6: 47–55. [DOI] [PubMed] [Google Scholar]
- King, B. H., and Leaich H. R.. . 2006. Variation in propensity to exhibit thanatosis in Nasonia vitripennis (Hymenoptera: Pteromalidae). J. Insect Behav. 19: 241. [Google Scholar]
- Kjeldgaard, M. K., Takano O. M., Bockoven A. A., Teel P. D., Light J. E., Hamer S. A., Hamer G. L., and Eubanks M. D.. . 2019. Red imported fire ant (Solenopsis invicta) aggression influences the behavior of three hard tick species. Exp. Appl. Acarol. 79: 87–97. [DOI] [PubMed] [Google Scholar]
- Krivolutsky, D. 1963. Eradication of larvae and nymphs of the tick Ixodes persulcatus by predators. Proc Sci Conf. 38: 187–188. [Google Scholar]
- Lazzari, C. R. 2009. Chapter 1: Orientation towards hosts in haematophagous insects: an integrative perspective. InAdvances in insect physiology, vol. 37. Academic Press, Elsevier, Amsterdam, Netherlands. p. 1–58. [Google Scholar]
- Le Maho, Y., Vu Van Kha H., Koubi H., Dewasmes G., Girard J., Ferré P., and Cagnard M.. . 1981. Body composition, energy expenditure, and plasma metabolites in long-term fasting geese. Am. J. Physiol. 241: E342–E354. [DOI] [PubMed] [Google Scholar]
- Li, H., Zhang G., Ji Y., and Wen J.. . 2019. Effects of starvation on death-feigning in adult Eucryptorrhynchus brandti (Coleoptera: Curculionidae). Ethology 125: 645–651. [Google Scholar]
- Lima, S. L. 1986. Predation risk and unpredictable feeding conditions: determinants of body mass in birds. Ecology 67: 377–385. [Google Scholar]
- Loye, J. E., and Lane R. S.. . 1988. Questing behavior of Ixodes pacificus (Acari: Ixodidae) in relation to meteorological and seasonal factors. J. Med. Entomol. 25: 391–398. [DOI] [PubMed] [Google Scholar]
- Ma, G., and Ma C. S.. . 2012. Climate warming may increase aphids’ dropping probabilities in response to high temperatures. J. Insect Physiol. 58: 1456–1462. [DOI] [PubMed] [Google Scholar]
- Mänd, T., Tammaru T., and Mappes J.. . 2007. Size dependent predation risk in cryptic and conspicuous insects. Evol. Ecol. 21: 485. [Google Scholar]
- Matsumura, K., and Miyatake T.. . 2019. Lines selected for different durations of tonic immobility have different leg lengths in the red flour beetle Tribolium castaneum. Behaviour 1: 1–15. [Google Scholar]
- McCue, M. D., Terblanche J. S., and Benoit J. B.. . 2017. Learning to starve: impacts of food limitation beyond the stress period. J. Exp. Biol. 220: 4330–4338. [DOI] [PubMed] [Google Scholar]
- Meyer, M. E., Smith R. L., and Van Hartesveldt C.. . 1984. Haloperidol differentially potentiates tonic immobility, the dorsal immobility response, and catalepsy in the developing rat. Dev. Psychobiol. 17: 383–389. [DOI] [PubMed] [Google Scholar]
- Milne, A. 1950. The ecology of the sheep tick, Ixodes ricinus; microhabitat economy of the adult tick. Parasitology 40: 14–34. [DOI] [PubMed] [Google Scholar]
- Minigan, J. N., Hager H. A., Peregrine A. S., and Newman J. A.. . 2018. Current and potential future distribution of the American dog tick (Dermacentor variabilis) in North America. Tick Tick-Borne Dis. 9: 354–362. [DOI] [PubMed] [Google Scholar]
- Miyatake, T. 2001. Effects of starvation on death-feigning in adults of Cylas formicarius (Coleoptera: Brentidae). Ann. Entomol. Soc. Am. 94: 612–616. [Google Scholar]
- Miyatake, T., Okada K., and Harano T.. . 2008. Negative relationship between ambient temperature and death-feigning intensity in adult Callosobruchus maculatus and Callosobruchus chinensis. Phys Ent. 33: 83–88. [Google Scholar]
- Miyatake, T., Matsumura K., Kitayama R., Otsuki K., Yuhao J., Fujisawa R., and Nagaya N.. . 2019. Arousal from tonic immobility by vibration stimulus. Behav. Genet. 49: 478–483. [DOI] [PubMed] [Google Scholar]
- Murdock, C. C., Luckhart S., and Cator L. J.. . 2017. Immunity, host physiology, and behaviour in infected vectors. Curr. Opin. Insect Sci. 20: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwangi, E. N., Dipeolu O. O., Newson R. M., Kaaya G. P., and Hassan S. M.. . 1991a. Predators, parasitoids and pathogens of ticks: a review. Biocontrol Sci. Technol. 1: 147–156. [Google Scholar]
- Mwangi, E. N., Newson R. M., and Kaaya G. P.. . 1991b. Predation of free-living engorged female Rhipicephalus appendiculatus. Exp. Appl. Acarol. 12: 153–162. [DOI] [PubMed] [Google Scholar]
- Nakayama, S., and Miyatake T.. . 2010. Genetic trade-off between abilities to avoid attack and to mate: a cost of tonic immobility. Biol. Lett. 6: 18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves, F. M., and Pie M. R.. . 2018. On the adult behavioral repertoire of the sawfly Perreyia flavipes Konow, 1899 (Hymenoptera: Pergidae): movement, mating, and thanatosis. Neotrop. Entomol. 47: 46–52. [DOI] [PubMed] [Google Scholar]
- Nishi, Y., Sasaki K., and Miyatake T.. . 2010. Biogenic amines, caffeine and tonic immobility in Tribolium castaneum. J. Insect Physiol. 56: 622–628. [DOI] [PubMed] [Google Scholar]
- Oliver, J. H. 1989. Biology and systematics of ticks (Acari: Ixodida). Annu. Rev. Ecol. Evol. Syst. 20: 397–430. [Google Scholar]
- Pelletier, D., Guillemette M., Grandbois J. M., and Butler P. J.. . 2008. To fly or not to fly: high flight costs in a large sea duck do not imply an expensive lifestyle. Proc. Biol. Sci. 275: 2117–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret, J. L., Guigoz E., Rais O., and Gern L.. . 2000. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland). Parasitol. Res. 86: 554–557. [DOI] [PubMed] [Google Scholar]
- Pond, C. M., and Mattacks C. A.. . 1985. Body mass and natural diet as determinants of the number and volume of adipocytes in eutherian mammals. J. Morphol. 185: 183–193. [DOI] [PubMed] [Google Scholar]
- Pool, J. R., Petronglo J. R., Falco R. C., and Daniels T. J.. . 2017. Energy usage of known-age blacklegged ticks (Acari: Ixodidae): what is the best method for determining physiological age? J. Med. Entomol. 54: 949–956. [DOI] [PubMed] [Google Scholar]
- Quadros, A. F., Bugs P. S., and Araujo P. B.. . 2012. Tonic immobility in terrestrial isopods: intraspecific and interspecific variability. Zookeys. 176: 155–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2019. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (https://www.R-project.org). [Google Scholar]
- Randolph, S. E. 2004. Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 129(Suppl): S37–S65. [DOI] [PubMed] [Google Scholar]
- Rogers, S. M., and Simpson S. J.. . 2014. Thanatosis. Curr. Biol. 24: R1031–R1033. [DOI] [PubMed] [Google Scholar]
- Rosendale, A. J., Romick-Rosendale L. E., Watanabe M., Dunlevy M. E., and Benoit J. B.. . 2016. Mechanistic underpinnings of dehydration stress in the American dog tick revealed through RNA-Seq and metabolomics. J. Exp. Biol. 219: 1808–1819. [DOI] [PubMed] [Google Scholar]
- Rosendale, A. J., Dunlevy M. E., Fieler A. M., Farrow D. W., Davies B., and Benoit J. B.. . 2017. Dehydration and starvation yield energetic consequences that affect survival of the American dog tick. J. Insect Physiol. 101: 39–46. [DOI] [PubMed] [Google Scholar]
- Rosendale, A. J., Dunlevy M. E., McCue M. D., and Benoit J. B.. . 2019. Progressive behavioural, physiological and transcriptomic shifts over the course of prolonged starvation in ticks. Mol. Ecol. 28: 49–65. [DOI] [PubMed] [Google Scholar]
- Rund, S. S. C., O’Donnell A. J., Gentile J. E., and Reece S. E.. . 2016. Daily rhythms in mosquitoes and their consequences for malaria transmission. Insects 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruxton, G. D., Allen W. L., Sherratt T. N., Speed M. P.. . 2019. Avoiding attack: the evolutionary ecology of crypsis, aposematism, and mimicry. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Samish, M., and Alekseev E.. . 2001. Arthropods as predators of ticks (Ixodoidea). J. Med. Entomol. 38: 1–11. [DOI] [PubMed] [Google Scholar]
- Samish, M., and Rehacek J.. . 1999. Pathogens and predators of ticks and their potential in biological control. Annu. Rev. Entomol. 44: 159–182. [DOI] [PubMed] [Google Scholar]
- Samuel, W. M. 1991. Winter ticks on moose and other ungulates: factors influencing their population size. Alces 27: 169–182. [Google Scholar]
- Segovia, J. M. G., Moura R. R., and Willemart R. H.. . 2019. Starvation decreases behavioral consistency in a Neotropical harvestman. Acta Ethol. 22: 203–208. [Google Scholar]
- Short, N., and Norval R.. . 1982. Tick predation by shrews in Zimbabwe. J. Parasitol. 68: 122–127. [Google Scholar]
- Showler, A. T., Osbrink W. L. A., Dorsey B. N., and Caesar R. M.. . 2019. Metastriate ixodid life stages protected from predatory ants in Texas. Environ. Entomol. 48: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Sonenshine, D. E. 2004. Pheromones and other semiochemicals of ticks and their use in tick control. Parasitology 129(Suppl): S405–S425. [DOI] [PubMed] [Google Scholar]
- Spielman, A. 1988. Prospects for suppressing transmission of Lyme disease. Ann. N. Y. Acad. Sci. 539: 212–220. [DOI] [PubMed] [Google Scholar]
- Steiert, J. G., and Gilfoy F.. . 2002. Infection rates of Amblyomma americanum and Dermacentor variabilis by Ehrlichia chaffeensis and Ehrlichia ewingii in southwest Missouri. Vector Borne Zoonotic Dis. 2: 53–60. [DOI] [PubMed] [Google Scholar]
- The Gene Ontology Consortium . 2019. The Gene Ontology resource: 20 years and still going strong. Nucleic Acids Res. 47: D330–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo, S. 1991. Variation in phase polymorphism in the common cutworm, Spodoptera Iitura (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 26: 571–578. [Google Scholar]
- Tomkins, J. L., Aungier J., Hazel W., and Gilbert L.. . 2014. Towards an evolutionary understanding of questing behaviour in the tick Ixodes ricinus. PLoS One 9: e110028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama, H., Sasaki K., Hinosawa S., Tanaka K., Matsumura K., Yajima S., and Miyatake T.. . 2019. Transcriptomic comparison between beetle strains selected for short and long durations of death feigning. Sci. Rep. 9: 14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uspensky, I. 1995. Physiological age of ixodid ticks: aspects of its determination and application. J. Med. Entomol. 32: 751–764. [DOI] [PubMed] [Google Scholar]
- Verissimo, C. J. 1995. Natural enemies of the cattle tick. Cienc. Tecnol. 8: 35–37. [Google Scholar]
- Walters, B. T., Cheng T. N. N., Doyle J., Guglielmo C. G., Clinchy M., and Zanette L. Y.. . 2017. Too important to tamper with: predation risk affects body mass and escape behaviour but not escape ability. Funct. Ecol. 31: 1405–1417. [Google Scholar]
- Whitford, W. G., and Ettershank G.. . 1975. Factors affecting foraging activity in Chihuahuan desert harvester ants. Environ. Entomol. 4: 689–696. [Google Scholar]
- Wilkinson, P. R. 1970b. A preliminary note on predation on free-living engorged female Rocky Mountain wood ticks. J. Med. Entomol. 7: 493–496. [DOI] [PubMed] [Google Scholar]
- Wilkinson, P. R. 1970a. Factors affecting the distribution and abundance of the cattle tick in Australia: observations and hypotheses. Acarologia 12: 492–508. [PubMed] [Google Scholar]
- Witter, M. S., Cuthill I. C., and Bonser R. H. C.. . 1994. Experimental investigations of mass-dependent predation risk in the European starling, Sturnus vulgaris. Anim. Behav. 48: 201–222. [Google Scholar]
- Wynne, N. E., Lorenzo M. G., and Vinauger C.. . 2020. Mechanism and plasticity of vectors’ host-seeking behavior. Curr. Opin. Insect Sci. 40: 1–5. [DOI] [PubMed] [Google Scholar]
- Yoder, J. A., and Domingus J. L.. . 2003. Identification of hydrocarbons that protect ticks (Acari: Ixodidae) against fire ants (Hymenoptera: Formicidae), but not lizards (Squamata: Polychrotidae), in an allomonal defense secretion. Int. J. Acarol. 29: 87–91. [Google Scholar]
- Yoder, J., Pollack Richard J., and Spielman A.. . 1993a. An ant-diversionary secretion of ticks: first demonstration of an acarine allomone. J. Insect Phys. 39: 429–435. [Google Scholar]
- Yoder, J., Pollack Richard J., Spielman A., Sonenshine D. E., and Johnstons D. E.. . 1993b. Secretion of squalene by ticks. J. Insect Phys. 39: 291–296. [Google Scholar]
- Yoder, J. A., Stevens B. W., and Crouch K. C.. . 1999. Squalene: a naturally abundant mammalian skin secretion and long distance tick-attractant (Acari: Ixodidae). J. Med. Entomol. 36: 526–529. [DOI] [PubMed] [Google Scholar]
- Yoder, J. A., Benoit J. B., Rellinger E. J., and Tank J. L.. . 2006. Developmental profiles in tick water balance with a focus on the new Rocky Mountain spotted fever vector, Rhipicephalus sanguineus. Med. Vet. Entomol. 20: 365–372. [DOI] [PubMed] [Google Scholar]
- Yoder, J. A., Benoit J. B., Bundy M. R., Hedges B. Z., and Gribbins K. M.. . 2009. Functional morphology of secretion by the large wax glands (Sensilla sagittiformia) involved in tick defense. Psyche. 2009: 1–8. (https://www.hindawi.com/journals/psyche/2009/631030/abs/). [Google Scholar]
- Zingg, S., Dolle P., Voordouw M. J., and Kern M.. . 2018. The negative effect of wood ant presence on tick abundance. Parasit. Vectors 11: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.