Abstract
Simple Summary
The optimal treatment and management of patients with brain cancer depend on the molecular characteristics of their tumour. Since the tumour changes with time, it is, therefore, essential to characterise the tumour of each patient at the exact time of selecting the most suitable therapeutic strategy. However, obtaining a tumour biopsy for its characterisation is a risky and invasive procedure and, sometimes, not even feasible, leading to a lack of information about the tumour. These challenges can be overcome by using a liquid biopsy of cerebrospinal fluid. Brain cancer cells release DNA into the cerebrospinal fluid, and the analysis of the cell-free circulating tumour DNA can reveal the genetic profile of brain cancer in a relatively noninvasive manner. In this review, we revise the recent results in this field that show how circulating tumour DNA in cerebrospinal fluid can provide diagnostic and prognostic information, identify potential therapeutic targets, monitor the tumour response or resistance to treatment, and help to identify tumour relapse.
Abstract
The correct characterisation of central nervous system (CNS) malignancies is crucial for accurate diagnosis and prognosis and also the identification of actionable genomic alterations that can guide the therapeutic strategy. Surgical biopsies are performed to characterise the tumour; however, these procedures are invasive and are not always feasible for all patients. Moreover, they only provide a static snapshot and can miss tumour heterogeneity. Currently, monitoring of CNS cancer is performed by conventional imaging techniques and, in some cases, cytology analysis of the cerebrospinal fluid (CSF); however, these techniques have limited sensitivity. To overcome these limitations, a liquid biopsy of the CSF can be used to obtain information about the tumour in a less invasive manner. The CSF is a source of cell-free circulating tumour DNA (ctDNA), and the analysis of this biomarker can characterise and monitor brain cancer. Recent studies have shown that ctDNA is more abundant in the CSF than plasma for CNS malignancies and that it can be sequenced to reveal tumour heterogeneity and provide diagnostic and prognostic information. Furthermore, analysis of longitudinal samples can aid patient monitoring by detecting residual disease or even tracking tumour evolution at relapse and, therefore, tailoring the therapeutic strategy. In this review, we provide an overview of the potential clinical applications of the analysis of CSF ctDNA and the challenges that need to be overcome in order to translate research findings into a tool for clinical practice.
Keywords: central nervous system malignancies, brain cancer, circulating tumour DNA, cerebrospinal fluid, liquid biopsies
1. Introduction
Central nervous system (CNS) malignancies affect both children and adults worldwide and are responsible for substantial morbidity and mortality. An epidemiological study of CNS cancer between 1990 and 2016 revealed that the age-standardised incidence rate has increased by 17.3% globally, with 330,000 incident cases and 227,000 deaths globally in 2016 [1].
CNS cancer consists of primary tumours and intracranial metastases. Most CNS tumours (>90%) occur in the brain, with the remaining located in the meninges, spinal cord and nerves. Depending on the anatomical region and the tumour type, the neurological signs and symptoms will vary and may include headaches, seizures, loss of vision, paralysis, speech disturbance, and motor deficits [2].
CNS tumours are diagnosed using neuroimaging techniques such as magnetic resonance imaging (MRI) or computed tomography; however, to obtain pathological information and molecular diagnosis, tumour biopsies are required. The treatment strategy for primary CNS tumours consists of either obtaining a biopsy or performing a surgical resection, combined, when appropriate, with postoperative radiotherapy and chemotherapy [3].
CNS tumour prognosis is diverse since there are distinct entities with different histopathological characteristics and molecular profiles. Therefore, characterising the tumour specimen is essential for accurate diagnosis and prognosis, as well as to identify potential therapeutic targets. To improve disease control, primary brain tumours or single nodule brain metastases are resected. However, obtaining tumour biopsies is not always possible due to their location, particularly when CNS tumours occur in vital regions such as the basal ganglia or the brain stem. In addition, patients with disseminated disease may not be eligible for such procedures [4,5,6].
In cases where tumour resection or obtaining a biopsy is possible, the sample obtained may not be representative of tumour heterogeneity [7,8,9], and, therefore, multiple sampling may be required to confirm the pathological diagnosis in some cases. Moreover, the analysis of the sample obtained only provides a static snapshot from the time of resection. It is important to monitor the patient’s response to treatment during the course of the disease, particularly to distinguish true disease progression from a pseudoprogression induced by treatment. Sometimes a new or enlarging area of contrast enhancement is observed, but it is not easy to assess whether it is the result of tumour growth or an inflammatory response [10]. This can be challenging when using conventional imaging techniques.
Tumours evolve over time, particularly under the selective pressure of therapy, which can result in the expansion of pre-existing resistant clones or the acquisition of de novo resistant alterations [7]. Thus, the genomic characteristics at relapse may differ from the genomic landscape at first occurrence. In some cases, treatment decisions at relapse are just based on the characteristics of the primary tissue obtained [11,12]. The known evolution of tumours and the absence of longitudinal tumour sampling may, therefore, lead to imprecise diagnosis and clinical management.
For these reasons, there is an urgent need to develop less invasive methods to identify and validate tumour biomarkers that provide real-time information to aid in diagnosing and monitoring CNS malignancies. Overall, this will help to adjust the therapeutic strategy and guide treatment decisions based on the current tumour profile and its burden.
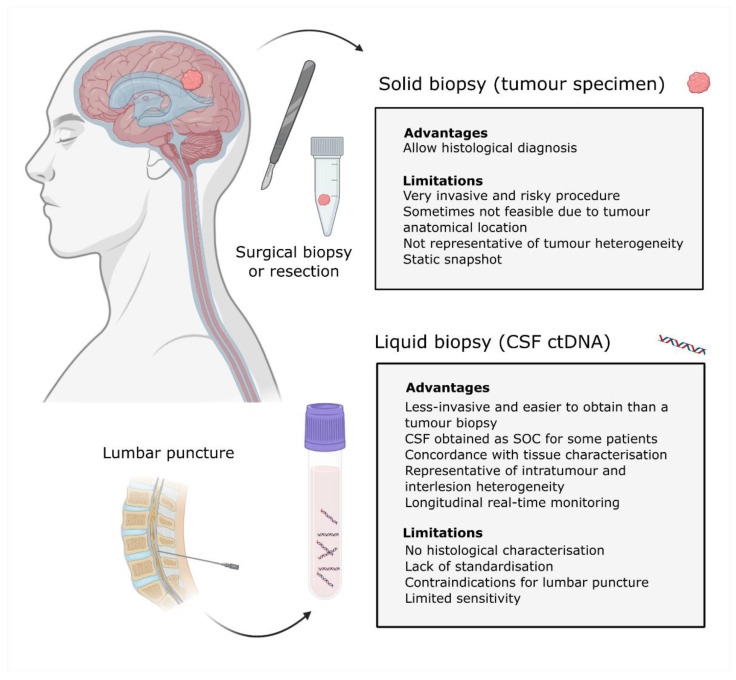
An alternative to a tumour biopsy is a liquid biopsy (Figure 1). Liquid biopsies are emerging as noninvasive tools that can provide longitudinal information about the tumour genomic landscape and facilitate patient monitoring. It consists of the analysis of biomarkers, including circulating tumour cells, exosomes and circulating tumour nucleic acids that are present in bodily fluids such as blood, cerebrospinal fluid (CSF), urine and saliva [13,14].
Figure 1.
Solid vs. liquid biopsies. Schematic representation of the tumour biopsy and CSF samples obtained from a patient with a CNS malignancy. Advantages and limitations for each methodology are indicated. Definition of acronyms: standard of care (SOC), cerebrospinal fluid (CSF), circulating tumour DNA (cfDNA) and central nervous system (CNS).
In this review, we will discuss the potential applications of circulating tumour DNA (ctDNA) in CSF as a biomarker for CNS malignancies, the challenges that we need to overcome, and future perspectives for its implementation in the clinical setting.
2. Circulating Cell-Free DNA and Circulating Tumour DNA
Cells release DNA that then circulate in bodily fluids. The fraction of cell-free DNA (cfDNA) that is shed by cancer cells, presumably undergoing apoptosis or necrosis, is known as ctDNA and carries genomic alterations that can be detected using PCR-based or next-generation sequencing (NGS)-based methods [14,15,16,17].
Increased concentrations of cfDNA have been detected in pathological conditions like trauma, infection and cancer, or even other physiological conditions like exercise [18].
For patients with an extracranial disease, plasma ctDNA has been detected across different cancer types. However, blood may not be a suitable source from patients with CNS malignancies since ctDNA levels were infrequently detected in plasma [19,20]. ctDNA was detectable in the plasma of >75% of patients with advanced cancers, such as bladder, colorectal, gastroesophageal, ovarian, pancreatic, breast, melanoma, hepatocellular, and head and neck cancers, in contrast with <10% of glioma patients (2/27) [19].
The proportion of ctDNA in the blood is small and varies depending on tumour characteristics, including type, grade and burden [16,19]. In contrast, the total amount of ctDNA in CSF is increased, making it an ideal biofluid to characterise and monitor CNS cancer [20,21]. Interestingly, the levels of ctDNA in CSF may be influenced by tumour burden, tumour progression and anatomical location of the tumour, with regard to the proximity to CSF reservoirs [21,22,23].
3. Cerebrospinal Fluid as a Source of ctDNA
CSF is a clear bodily fluid secreted by the choroid plexus that is present in the subarachnoid space of the brain, the spinal cord and the central canal [24].
CSF is in direct contact with the brain parenchyma, and several studies have shown that CSF is a reliable source of cell-free ctDNA, providing advantages over plasma or serum for the analysis of CNS tumours [20,21].
Several studies have reported the ability to detect ctDNA in CSF of patients with CNS malignancies. Gene mutations and molecular alterations have been detected in the CSF DNA of CNS cancer patients [25,26,27,28,29,30], followed by genomic landscape characterisation of CSF ctDNA with the development of high throughput sequencing technologies [20,21,22,23,31,32,33,34,35,36].
CSF samples can be accessed through a lumbar puncture or obtained from the ventricles under certain circumstances. Patients with posterior fossa tumours tend to present with hydrocephalus, a condition in which the CSF accumulates within the cerebral ventricles and/or subarachnoid spaces [37,38,39]. A lumbar puncture is contraindicated in these patients, given the risk of brain herniation; therefore, CSF is obtained from the ventricles during procedures that are performed to alleviate intracranial pressure and drain the excess of CSF [38,40,41,42].
As part of the CNS staging criteria for certain brain tumours, a diagnostic lumbar puncture is routinely performed as standard of care for CSF cytology evaluation of CNS lymphoma, CNS metastasis, and medulloblastoma [43,44,45,46]. In these cases, the CSF samples collected as standard of care can be further used to characterise the ctDNA and provide information about the tumour [20,34,47].
4. Clinical Applications of the CSF ctDNA for CNS Malignancies
CNS tumours are a heterogeneous group of malignancies [48]. In most cases, tumour resection is required to reduce tumour burden and mass effect [49]. However, liquid biopsies can be used to complement histopathological diagnosis and are essential for those patients with inoperable tumours.
The monitoring of CNS malignancies is currently performed by imaging techniques; however, these are not sensitive for microscopic disease [50]. A complementary liquid biopsy of CSF could, therefore, be performed to aid clinical assessment by determining the response to treatment, differentiating pseudoprogression from true progression and tracking levels of residual disease. In addition, the genomic characterisation of CSF ctDNA can facilitate the identification of actionable genomic alterations that confer sensitivity or resistance to clinically available drugs and the detection of mechanisms of resistance at relapse.
The distinct clinical applications of the analysis of CSF ctDNA are discussed below for patients with distinct types of CNS cancer.
4.1. Diffuse Gliomas
4.1.1. A Diagnostic and Prognostic Tool
Among diffuse gliomas, glioblastoma (GBM) is the most common malignant brain tumour in adults, with a 2-year survival of 18% and 5-year overall survival (OS) of 4% [51]. Providing an accurate molecular profile for diagnosis and prognosis is essential and can be achieved with a CSF liquid biopsy. The analysis of the mutational status of IDH1, IDH2, ATRX, TP53, TERT, H3F3A and HIST1H3B in CSF ctDNA facilitates the molecular diagnosis of diffuse gliomas and provides prognostic information in a relatively noninvasive manner [22]. In addition, TERT promoter mutations have been detected in the CSF ctDNA of GBM patients, and shorter OS of patients with high variant allele frequency (VAF) has been observed. The results from this pilot study suggested that VAF levels of the TERT promoter mutation could be a predictor of poor survival [52]. In more recent studies, CSF was obtained from lumbar punctures in glioma patients, and ctDNA was detected and was associated with disease burden, tumour progression, and adverse outcomes [20,23]. Moreover, most patients with detectable ctDNA had a negative cytopathologic analysis, and ctDNA was not detected in plasma [20,23].
Diffuse midline glioma (DMG) is a tumour entity characterised by a K27M mutation in either H3F3A or HIST1H3B/C; it is usually located in the brain stem, thalamus and spinal cord [48]. Within H3 K27M-mutant DMG, diffuse intrinsic pontine glioma (DIPG) is a rapidly growing tumour in the brain stem that typically arises in young children and is associated with poor survival [53]. The anatomical location of these tumours, the brainstem, makes them difficult and dangerous to biopsy. Importantly, H3 K27M mutations can be detected in the CSF ctDNA, facilitating diagnosis and opening the possibility of avoiding diagnostic surgical biopsies [22,54,55].
The molecular characterisation and understanding of DIPG biology have been improved from specimens obtained from rare diagnostic biopsies and postmortem tissue donations [56,57,58,59]. Indeed, the lack of surgical specimens can be overcome by the analysis of CSF ctDNA to aid in the management of patients with DIPG and contribute to the molecular study of this disease to accelerate research. An NGS panel of 68 genes commonly mutated in brainstem tumours was used to study a cohort of 57 patients with brainstem tumours, including 23 patients with DIPG. Mutations were detected in the CSF ctDNA of 82.5% of patients, and the presence of H3F3A/HIST1H3B mutations was correlated with poor OS while the IDH1 mutation predicted better OS [54]. Moreover, longitudinal analysis of CSF samples offers the possibility of monitoring and allows the tumour evolution of this dismal disease to be studied.
4.1.2. Monitoring and Therapeutic Strategies
The number of actionable genomic alterations for patients with primary brain tumours is limited. The most relevant biomarker for glioma is MGMT promoter methylation status. MGMT promoter methylation causes the loss of MGMT expression, and since it is involved in DNA repair by reversing DNA alkylation, MGMT promoter methylation renders cells more susceptible to temozolomide and is associated with longer survival [60,61,62,63]. MGMT promoter methylation was detected using methylation-specific PCR from genomic DNA extracted from the CSF of glioma patients, with higher sensitivity than from serum [64].
A potential biomarker for GBM is epidermal growth factor receptor (EGFR). EGFR amplification and EGFRvIII mutation have been detected in RNA within extracellular vesicles circulating in CSF [65]. This could be of high interest as a biomarker to predict response to future EGFRvIII-targeted therapies in GBMs.
Longitudinal analysis of CSF ctDNA from glioma patients showed the evolution of the cancer genome through the mutational changes detected [23].
4.2. Brain and Leptomeningeal Metastases
4.2.1. CSF ctDNA Facilitates Diagnosis and Allows Tumour Genomic Characterisation
About 20–40% of patients with advanced-stage cancers of the lung, breast and melanoma develop brain metastasis, and approximately 5–8% of these patients are diagnosed with leptomeningeal metastasis. These are devastating diseases that carry a poor prognosis and are often resistant to treatment [66,67,68].
In addition to brain metastasis present in the brain parenchyma, malignant cells can seed the leptomeninges, causing leptomeningeal metastasis [67,69,70]. The diagnosis of leptomeningeal metastasis is based on clinical symptoms, MRI scans, and cytology analysis of CSF [71]. However, up to 20% of patients with positive clinical and radiographic signs presented false-negative CSF cytology [72,73]. Several studies have shown that ctDNA can be detected in the CSF of patients with negative cytology analysis [20,27,32,74]. Cytology has limited sensitivity, and the analysis of ctDNA can complement the diagnosis of leptomeningeal metastases.
Brain metastasis can present different genomic alterations compared to their primary extracranial tumour [12]. Several studies have shown that the analysis of CSF ctDNA enables the characterisation of the genomic complexity of CNS metastases, including intratumour heterogeneity, revealed with the identification of trunk and private genomic alterations. Moreover, the genomic landscape of CNS metastasis, including the brain lesion’s private alterations, was better represented from the ctDNA in the CSF than plasma [20,32,75]. Analysis of CSF ctDNA from a cohort of 26 patients with leptomeningeal metastases from non-small cell lung cancer (NSCLC) revealed their unique genetic profiles, including mutations in several driver genes, copy number variations (CNVs) in MET, ERBB2, KRAS, ALK, and MYC, and loss of heterozygosity in TP53 [76].
4.2.2. Patient Monitoring and Identification of Therapeutic Targets
There are several targeted therapies for brain metastases [77,78,79,80,81,82,83,84,85,86]. For EGFR-mutated brain metastases from NSCLC, first-, second- and third- generation EGFR tyrosine kinase inhibitors (TKIs) are available [87,88,89,90]. In addition, for NSCLC with ALK gene rearrangement, CNS penetration and therapeutic potential were exhibited by second-generation ALK inhibitors [84,85,86]. There are also targeted therapies for patients diagnosed with HER2+ breast cancer and melanoma patients, including BRAF and MEK inhibitors [82,83,91,92]. For the treatment of leptomeningeal metastases, targeted therapies for the aforementioned actionable genomic alterations may also be effective [93].
The availability of targeted therapies highlights the importance of the identification of actionable genomic alterations or resistance mutations in genes, including EGFR, ALK, BRAF and HER2, which have been detected from CSF ctDNA in several studies [20,21,31,32,94,95,96]. For example, EGFR–TKI resistance mutation EGFR T790M has been detected in the CSF ctDNA of lung cancer patients [94,97].
A study of 21 patients with brain metastasis from NSCLC compared the NGS results obtained from different samples to reveal the mutation pattern of driver genes for each patient. Mutations were detected in 95.2%, 66.7% and 39% of patients from CSF ctDNA, plasma ctDNA, and plasma circulating tumour cells, respectively. The most mutated gene was EGFR, followed by KIT, PIK3CA, TP53, SMAD4, ATM, SMARCB1, PTEN and FLT3 (all >15%). For EGFR mutations, the detection rate was 57.1% (12/21) from CSF ctDNA, which, interestingly, was higher for patients with leptomeningeal (81.8%; 9/11) compared with brain parenchymal (30%; 3/10) metastases [98].
The analysis of CSF ctDNA can also contribute to monitoring response to treatment. Metastasis in the CNS developed in a patient with HER2+ breast cancer. Analysis of baseline CSF ctDNA revealed mutations in TP53 and PIK3CA and amplification in ERBB2 and cMYC. Following treatment with T-DM1, extracranial disease control was achieved, and marker levels in plasma decreased. However, the levels increased in CSF ctDNA, consistent with poor treatment benefit to the CNS [99].
Altogether, these results indicate that CSF is a more suitable fluid than plasma to reveal the mutational profile of CNS metastases and can aid in diagnosis, tailored treatment selection and monitoring.
4.3. CNS Lymphoma
Malignant B-cells can infiltrate the CNS and are associated with poor prognosis, particularly at relapse [100]. Primary CNS lymphoma is defined by the absence of systemic disease in contrast to secondary CNS lymphoma that presents infiltration into the CNS with previous or concomitant systemic lymphoma [101,102].
ctDNA has only been detected in the plasma of a minority of patients with restricted CNS lymphoma [103,104]. In contrast, several studies of patients with CNS lymphomas detected ctDNA in CSF [47,105,106,107,108,109].
Diagnosis and monitoring of CNS lymphoma are challenging, given the difficulties of tumour biopsies and the lack of sensitivity of CSF standard analysis (cytology and flow cytometry) and neuroimaging. The detection of the MYD88 L265P mutation strongly suggests the diagnosis of primary CNS lymphoma, and this mutation has been detected in the CSF ctDNA of patients with CNS lymphoma [47,106,108,109], showing that the analysis of CSF ctDNA could complement the diagnosis.
An NGS-based analysis of the CSF cfDNA of 8 patients with CNS lymphoma, at recurrence, detected tumour-derived genetic alterations and showed that the clearance of ctDNA from CSF was associated with sustained tumour responses [105].
The comparison of CSF ctDNA with plasma ctDNA and CSF standard analysis (cytology and flow cytometry) revealed that the analysis of CSF ctDNA better detected CNS disease in patients with B-cell lymphoma [47]. Moreover, longitudinal analysis of CSF ctDNA levels allowed the monitoring of response to treatment, the detection of residual disease and predicted relapse. The dynamic changes observed in CSF ctDNA recapitulated the evolution of the disease for patients with CNS lymphoma [47].
4.4. Medulloblastoma
CNS tumours are the leading cause of cancer-related mortality in children and adolescents due to the aggressiveness of certain subtypes, including medulloblastoma and high-grade gliomas such as DMG [110,111].
Medulloblastoma (MB), an embryonal tumour of the CNS, is the most aggressive brain tumour in childhood that can also occur in adults, although this is less common [112]. MB is a complex and evolving heterogeneous disease that can be divided into four molecular consensus subgroups (WNT, SHH, Group 3 and Group 4), with further subtypes identified [113,114,115,116,117]. The lack of sufficient sample, intratumour heterogeneity or the presence of disseminated disease make diagnosis and monitoring difficult [8,118,119,120,121]. However, hydrocephalus is common amongst these patients, and CSF samples can be obtained prior to tumour surgical resection or biopsy [40,42]. In addition, CSF samples are routinely collected through a lumbar puncture for cytology analysis to assess metastatic dissemination according to Chang’s M-staging system, in combination with brain and spinal MRIs [45].
The study of paediatric patients with MB showed that ctDNA was more abundant in CSF (76.9%, 10/13 patients) than plasma (1/13 patients) for patients with negative CSF cytology results. Moreover, exome sequencing of CSF ctDNA recapitulated the tumour mutational burden and the genomic alterations, including MB common mutations (PTCH1, TP53), CNVs (MYCN and GLI2 amplification) and arm-level chromosomal aberrations (chromosome 17p loss), providing diagnostic and prognostic information [34]. Longitudinal CSF samples were also collected, and ctDNA analysis detected residual disease, identified intratumour and interlesion heterogeneity, and revealed a genomic transformation of the tumour at relapse [34]. More recently, another study reported the detection of ctDNA in CSF; however, shared genetic mutations between CSF and the tumour specimen were only identified in 22% (2/9) of patients. The authors suggested that this could be explained by the time-interval differences between tumour and CSF collection [122].
MB also presents abnormal DNA methylation changes, with distinct epigenetic signatures identified across MB subtypes that can be altered during tumour progression and treatment [114,123]. The epigenetic analysis of CSF ctDNA from 4 MB patients (3 with matching tumour samples) was attained. A positive correlation of tumour and CSF samples was identified, suggesting that CSF ctDNA could be used to monitor changes in MB tumour DNA methylomes and hydroxymethylomes. In addition, DNA methylation markers of diagnostic and prognostic value could be detected in the CSF ctDNA [124]. In summary, CSF ctDNA analysis could facilitate the clinical management of paediatric patients with MB.
5. Challenges and Limitations
Obtaining CSF samples is less invasive than surgery; however, in some cases, a lumbar puncture is not feasible. Contraindications to performing a lumbar puncture include risk for cerebral herniation, abnormal intracranial pressure and coagulation abnormalities [41].
The analysis of ctDNA could be used as a biomarker of residual disease. However, it will be important to establish the dates of sample collection, particularly postsurgery, since the abundance of trauma-induced cfDNA, up to 4 weeks from the surgical procedure, could dilute the fraction of ctDNA and influence the results [125].
Another limitation is that ctDNA is not detected in all patients with CNS malignancies. Detection of ctDNA may be influenced by tumour burden, tumour progression and anatomical location [21,22,23]. Therefore, further research to determine the biological factors involved and improve technological sensitivity will be required.
For patient monitoring through the analysis of specific mutations by sensitive techniques such as droplet digital PCR (ddPCR), prior knowledge of the tumour genetic profile is required, and new mutations (not present in the primary sample) can appear in the relapse setting. In contrast, whole-exome sequencing (WES), shallow whole-genome sequencing (WGS) or specific gene panels might provide more information to aid with tumour characterisation and monitor residual disease. However, it is important to consider sensitivity, turnaround time and cost-effectiveness.
In addition, imaging techniques may sometimes reflect either inflammatory processes from treatment or neoplastic progression [10]. Further research is needed to investigate whether the analysis of CSF ctDNA can help distinguish between true progression from pseudoprogression.
To determine the impact of the results and translate them into a tool for clinical practise, standardisation of protocols and larger studies with more patients will be required. The implementation of well-designed and controlled clinical trials will be essential to validate the use of CSF ctDNA as a liquid biopsy for the clinical management of patients.
6. Conclusions and Future Insights
CNS cancer is a dismal disease. It has elevated mortality and disabling effects on patients and is a massive burden on global health care systems. However, early detection and treatment can result in improved outcomes [1].
Several studies of the CSF ctDNA of patients with different types of primary and metastatic CNS tumours have been performed and show promising results, highlighting the potential of CSF ctDNA as a biomarker.
The challenges will be to translate these findings into clinically validated assays to improve patient healthcare. Standardisation of protocols and further studies and clinical trials will be necessary to translate the current results into a feasible tool for its implementation in the clinical setting.
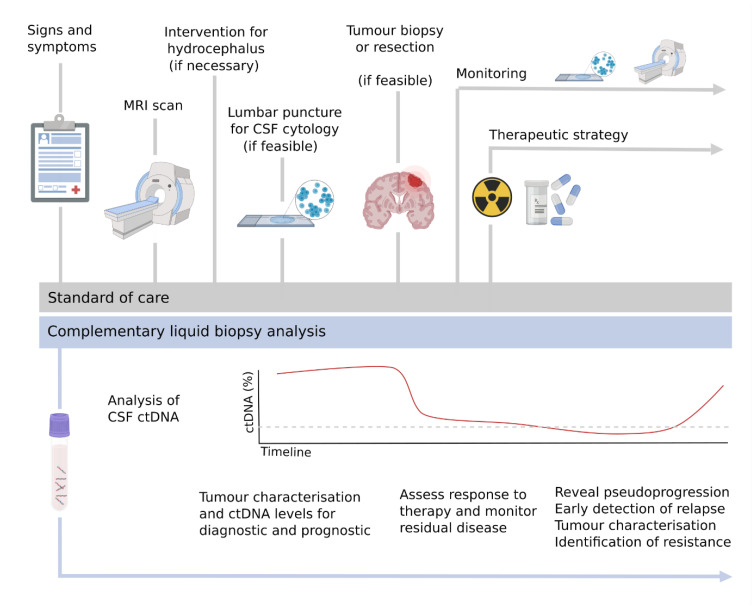
A liquid biopsy of CSF can characterise the tumour for diagnosis and provide prognostic information, also complementing the information obtained from the tumour sample if a biopsy or resection is feasible (Figure 1). During patient follow-up, it could be used to monitor the response to treatment through the levels of ctDNA and the detection of minimal residual disease. Importantly, it can facilitate early detection of relapse and identify therapeutic targets or mechanisms of resistance in order to adjust the therapeutic strategy at relapse (Figure 2).
Figure 2.
Liquid biopsies in the clinical setting. Schematic representation of the standard of care for patients with CNS malignancies and the complementation with a longitudinal analysis of CSF ctDNA.
Altogether, the analysis of CSF ctDNA remains a promising strategy to improve the clinical management of patients with CNS malignancies, and further studies are required to make liquid biopsies a standard clinical tool.
Acknowledgments
The authors would like to thank the patients and their families who took part in the research studies. Parts of the figures were created with BioRender.com (accessed on 14 March 2021).
Author Contributions
Writing—original draft preparation, L.E.; writing—review and editing, F.M.-R. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Fundación Asociación Española contra el Cáncer (AECC), FERO, the Ramón Areces Foundation, the Cellex Foundation, BBVA (CAIMI), the ISCIII, FIS (PI19/00318) and the Juan de la Cierva fellowship (L.E.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
J.S. is a cofounder of Mosaic Biomedicals and has ownership interests from Mosaic Biomedicals and Northern Biologics. J.S. has received grant/research support from Mosaic Biomedicals, Northern Biologics, Roche/Glycart and Hoffmann la Roche. The remaining authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patel A.P., Fisher J.L., Nichols E., Abd-Allah F., Abdela J., Abdelalim A., Fitzmaurice C. Global, regional, and national burden of brain and other CNS cancer, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:376–393. doi: 10.1016/S1474-4422(18)30468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alentorn A., Hoang-Xuan K., Mikkelsen T. Presenting signs and symptoms in brain tumors. Handb. Clin. Neurol. 2016;134:19–26. doi: 10.1016/b978-0-12-802997-8.00002-5. [DOI] [PubMed] [Google Scholar]
- 3.Preusser M., Marosi C. Neuro-oncology in 2016: Advances in brain tumour classification and therapy. Nat. Rev. Neurol. 2017;13:71–72. doi: 10.1038/nrneurol.2017.3. [DOI] [PubMed] [Google Scholar]
- 4.Owen S., Souhami L. The management of brain metastases in non-small cell lung cancer. Front. Oncol. 2014;4:248. doi: 10.3389/fonc.2014.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson S.D., Wagner K.M., Prabhu S.S., McAleer M.F., McCutcheon I.E., Sawaya R. Neurosurgical management of brain metastases. Clin. Exp. Metastasis. 2017;34:377–389. doi: 10.1007/s10585-017-9860-z. [DOI] [PubMed] [Google Scholar]
- 6.Wijdicks E.F.M. Historical awareness of the brainstem: From a subsidiary structure to a vital center. Neurology. 2020;95:484–488. doi: 10.1212/WNL.0000000000010504. [DOI] [PubMed] [Google Scholar]
- 7.Dagogo-Jack I., Shaw A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018;15:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 8.Morrissy A.S., Cavalli F.M.G., Remke M., Ramaswamy V., Shih D.J.H., Holgado B.L., Farooq H., Donovan L.K., Garzia L., Agnihotri S., et al. Spatial heterogeneity in medulloblastoma. Nat. Genet. 2017;49:780–788. doi: 10.1038/ng.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sottoriva A., Spiteri I., Piccirillo S.G., Touloumis A., Collins V.P., Marioni J.C., Curtis C., Watts C., Tavaré S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA. 2013;110:4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thust S.C., van den Bent M.J., Smits M. Pseudoprogression of brain tumors. J. Magn. Reason. Imaging. 2018;48:571–589. doi: 10.1002/jmri.26171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson B.E., Mazor T., Hong C., Barnes M., Aihara K., McLean C.Y., Fouse S.D., Yamamoto S., Ueda H., Tatsuno K., et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343:189–193. doi: 10.1126/science.1239947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brastianos P.K., Carter S.L., Santagata S., Cahill D.P., Taylor-Weiner A., Jones R.T., Van Allen E.M., Lawrence M.S., Horowitz P.M., Cibulskis K., et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015;5:1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz L.A., Jr., Bardelli A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siravegna G., Mussolin B., Venesio T., Marsoni S., Seoane J., Dive C., Papadopoulos N., Kopetz S., Corcoran R.B., Siu L.L., et al. How liquid biopsies can change clinical practice in oncology. Ann. Oncol. 2019;30:1580–1590. doi: 10.1093/annonc/mdz227. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzenbach H., Hoon D.S., Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 16.Wan J.C.M., Massie C., Garcia-Corbacho J., Mouliere F., Brenton J.D., Caldas C., Pacey S., Baird R., Rosenfeld N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer. 2017;17:223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 17.Heitzer E., Haque I.S., Roberts C.E.S., Speicher M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019;20:71–88. doi: 10.1038/s41576-018-0071-5. [DOI] [PubMed] [Google Scholar]
- 18.Crigna A.T., Samec M., Koklesova L., Liskova A., Giordano F.A., Kubatka P., Golubnitschaja O. Cell-free nucleic acid patterns in disease prediction and monitoring-hype or hope? EPMA J. 2020;11:1–25. doi: 10.1007/s13167-020-00226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bettegowda C., Sausen M., Leary R.J., Kinde I., Wang Y., Agrawal N., Bartlett B.R., Wang H., Luber B., Alani R.M., et al. Detection of circulating tumor DNA in early-and late-stage human malignancies. Sci. Transl. Med. 2014;6:224ra224. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Mattos-Arruda L., Mayor R., Ng C.K.Y., Weigelt B., Martínez-Ricarte F., Torrejon D., Oliveira M., Arias A., Raventos C., Tang J., et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 2015;6:8839. doi: 10.1038/ncomms9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Springer S., Zhang M., McMahon K.W., Kinde I., Dobbyn L., Ptak J., Brem H., Chaichana K., Gallia G.L., et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc. Natl. Acad. Sci. USA. 2015;112:9704–9709. doi: 10.1073/pnas.1511694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-Ricarte F., Mayor R., Martínez-Sáez E., Rubio-Pérez C., Pineda E., Cordero E., Cicuéndez M., Poca M.A., López-Bigas N., Ramon Y.C.S., et al. Molecular Diagnosis of Diffuse Gliomas through Sequencing of Cell-Free Circulating Tumor DNA from Cerebrospinal Fluid. Clin. Cancer Res. 2018;24:2812–2819. doi: 10.1158/1078-0432.CCR-17-3800. [DOI] [PubMed] [Google Scholar]
- 23.Miller A.M., Shah R.H., Pentsova E.I., Pourmaleki M., Briggs S., Distefano N., Zheng Y., Skakodub A., Mehta S.A., Campos C., et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565:654–658. doi: 10.1038/s41586-019-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghersi-Egea J.F., Strazielle N., Catala M., Silva-Vargas V., Doetsch F., Engelhardt B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018;135:337–361. doi: 10.1007/s00401-018-1807-1. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt-Gräff A., Hummel M., Anagnostopoulos I., Stoltenburg G., Stein H. Primary brain lymphoma in acquired immunodeficiency syndrome. Immunophenotype and molecular pathologic characterization in stereotactic biopsy, autopsy and cerebrospinal fluid cytology. Pathologe. 1995;16:75–80. doi: 10.1007/s002920050079. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes C.H., Honsinger C., Sorenson G.D. PCR-detection of tumor-derived p53 DNA in cerebrospinal fluid. Am. J. Clin. Pathol. 1995;103:404–408. doi: 10.1093/ajcp/103.4.404. [DOI] [PubMed] [Google Scholar]
- 27.Swinkels D.W., de Kok J.B., Hanselaar A., Lamers K., Boerman R.H. Early detection of leptomeningeal metastasis by PCR examination of tumor-derived K-ras DNA in cerebrospinal fluid. Clin. Chem. 2000;46:132–133. doi: 10.1093/clinchem/46.1.132. [DOI] [PubMed] [Google Scholar]
- 28.Shi W., Lv C., Qi J., Zhao W., Wu X., Jing R., Wu X., Ju S., Chen J. Prognostic value of free DNA quantification in serum and cerebrospinal fluid in glioma patients. J. Mol. Neurosci. 2012;46:470–475. doi: 10.1007/s12031-011-9617-0. [DOI] [PubMed] [Google Scholar]
- 29.Yang H., Cai L., Zhang Y., Tan H., Deng Q., Zhao M., Xu X. Sensitive detection of EGFR mutations in cerebrospinal fluid from lung adenocarcinoma patients with brain metastases. J. Mol. Diagn. 2014;16:558–563. doi: 10.1016/j.jmoldx.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Touat M., Duran-Peña A., Alentorn A., Lacroix L., Massard C., Idbaih A. Emerging circulating biomarkers in glioblastoma: Promises and challenges. Expert Rev. Mol. Diagn. 2015;15:1311–1323. doi: 10.1586/14737159.2015.1087315. [DOI] [PubMed] [Google Scholar]
- 31.Pan W., Gu W., Nagpal S., Gephart M.H., Quake S.R. Brain tumor mutations detected in cerebral spinal fluid. Clin. Chem. 2015;61:514–522. doi: 10.1373/clinchem.2014.235457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pentsova E.I., Shah R.H., Tang J., Boire A., You D., Briggs S., Omuro A., Lin X., Fleisher M., Grommes C., et al. Evaluating Cancer of the Central Nervous System Through Next-Generation Sequencing of Cerebrospinal Fluid. J. Clin. Oncol. 2016;34:2404–2415. doi: 10.1200/JCO.2016.66.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouliere F., Mair R., Chandrananda D., Marass F., Smith C.G., Su J., Morris J., Watts C., Brindle K.M., Rosenfeld N. Detection of cell-free DNA fragmentation and copy number alterations in cerebrospinal fluid from glioma patients. EMBO Mol. Med. 2018;10 doi: 10.15252/emmm.201809323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escudero L., Llort A., Arias A., Diaz-Navarro A., Martínez-Ricarte F., Rubio-Perez C., Mayor R., Caratù G., Martínez-Sáez E., Vázquez-Méndez É., et al. Circulating tumour DNA from the cerebrospinal fluid allows the characterisation and monitoring of medulloblastoma. Nat. Commun. 2020;11:5376. doi: 10.1038/s41467-020-19175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seoane J., De Mattos-Arruda L., Le Rhun E., Bardelli A., Weller M. Cerebrospinal fluid cell-free tumour DNA as a liquid biopsy for primary brain tumours and central nervous system metastases. Ann. Oncol. 2019;30:211–218. doi: 10.1093/annonc/mdy544. [DOI] [PubMed] [Google Scholar]
- 36.Le Rhun E., Seoane J., Salzet M., Soffietti R., Weller M. Liquid biopsies for diagnosing and monitoring primary tumors of the central nervous system. Cancer Lett. 2020;480:24–28. doi: 10.1016/j.canlet.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen T.T., Smith M.V., Rodziewicz G.S., Lemke S.M. Hydrocephalus caused by metastatic brain lesions: Treatment by third ventriculostomy. J. Neurol. Neurosurg. Psychiatry. 1999;67:552–553. doi: 10.1136/jnnp.67.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marx S., Reinfelder M., Matthes M., Schroeder H.W.S., Baldauf J. Frequency and treatment of hydrocephalus prior to and after posterior fossa tumor surgery in adult patients. Acta Neurochir. 2018;160:1063–1071. doi: 10.1007/s00701-018-3496-x. [DOI] [PubMed] [Google Scholar]
- 39.Won S.Y., Dubinski D., Behmanesh B., Bernstock J.D., Seifert V., Konczalla J., Tritt S., Senft C., Gessler F. Management of hydrocephalus after resection of posterior fossa lesions in pediatric and adult patients-predictors for development of hydrocephalus. Neurosurg. Rev. 2020;43:1143–1150. doi: 10.1007/s10143-019-01139-8. [DOI] [PubMed] [Google Scholar]
- 40.Sainte-Rose C., Cinalli G., Roux F.E., Maixner R., Chumas P.D., Mansour M., Carpentier A., Bourgeois M., Zerah M., Pierre-Kahn A., et al. Management of hydrocephalus in pediatric patients with posterior fossa tumors: The role of endoscopic third ventriculostomy. J. Neurosurg. 2001;95:791–797. doi: 10.3171/jns.2001.95.5.0791. [DOI] [PubMed] [Google Scholar]
- 41.Engelborghs S., Niemantsverdriet E., Struyfs H., Blennow K., Brouns R., Comabella M., Dujmovic I., van der Flier W., Frölich L., Galimberti D., et al. Consensus guidelines for lumbar puncture in patients with neurological diseases. Alzheimer Dement. 2017;8:111–126. doi: 10.1016/j.dadm.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee M., Wisoff J.H., Abbott R., Freed D., Epstein F.J. Management of hydrocephalus in children with medulloblastoma: Prognostic factors for shunting. Pediatr. Neurosurg. 1994;20:240–247. doi: 10.1159/000120797. [DOI] [PubMed] [Google Scholar]
- 43.Balhuizen J.C., Bots G.T., Schaberg A., Bosman F.T. Value of cerebrospinal fluid cytology for the diagnosis of malignancies in the central nervous system. J. Neurosurg. 1978;48:747–753. doi: 10.3171/jns.1978.48.5.0747. [DOI] [PubMed] [Google Scholar]
- 44.Cohen N.R., Phipps K., Harding B., Jacques T.S. Is CSF cytology a useful diagnostic procedure in staging paediatric CNS tumours? Cytopathology. 2009;20:256–260. doi: 10.1111/j.1365-2303.2009.00676.x. [DOI] [PubMed] [Google Scholar]
- 45.Chang C.H., Housepian E.M., Herbert C., Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–1359. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 46.Rahimi J., Woehrer A. Overview of cerebrospinal fluid cytology. Handb. Clin. Neurol. 2017;145:563–571. doi: 10.1016/b978-0-12-802395-2.00035-3. [DOI] [PubMed] [Google Scholar]
- 47.Bobillo S., Crespo M., Escudero L., Mayor R., Raheja P., Carpio C., Rubio-Perez C., Tazón-Vega B., Palacio C., Carabia J., et al. Cell free circulating tumor DNA in cerebrospinal fluid detects and monitors central nervous system involvement of B-cell lymphomas. Haematologica. 2021;106:513–521. doi: 10.3324/haematol.2019.241208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 49.Rees J.H. Diagnosis and treatment in neuro-oncology: An oncological perspective. Br. J. Radiol. 2011;84:S82–S89. doi: 10.1259/bjr/18061999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aquino D., Gioppo A., Finocchiaro G., Bruzzone M.G., Cuccarini V. MRI in Glioma Immunotherapy: Evidence, Pitfalls, and Perspectives. J. Immunol. Res. 2017;2017:5813951. doi: 10.1155/2017/5813951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poon M.T.C., Sudlow C.L.M., Figueroa J.D., Brennan P.M. Longer-term (≥ 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: A systematic review and meta-analysis. Sci. Rep. 2020;10:11622. doi: 10.1038/s41598-020-68011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juratli T.A., Stasik S., Zolal A., Schuster C., Richter S., Daubner D., Juratli M.A., Thowe R., Hennig S., Makina M., et al. TERT Promoter Mutation Detection in Cell-Free Tumor-Derived DNA in Patients with IDH Wild-Type Glioblastomas: A Pilot Prospective Study. Clin. Cancer Res. 2018;24:5282–5291. doi: 10.1158/1078-0432.CCR-17-3717. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman L.M., Veldhuijzen van Zanten S.E.M., Colditz N., Baugh J., Chaney B., Hoffmann M., Lane A., Fuller C., Miles L., Hawkins C., et al. Clinical, Radiologic, Pathologic, and Molecular Characteristics of Long-Term Survivors of Diffuse Intrinsic Pontine Glioma (DIPG): A Collaborative Report from the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018;36:1963–1972. doi: 10.1200/JCO.2017.75.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan C., Diplas B.H., Chen X., Wu Y., Xiao X., Jiang L., Geng Y., Xu C., Sun Y., Zhang P., et al. Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol. 2019;137:297–306. doi: 10.1007/s00401-018-1936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azad T.D., Jin M.C., Bernhardt L.J., Bettegowda C. Liquid biopsy for pediatric diffuse midline glioma: A review of circulating tumor DNA and cerebrospinal fluid tumor DNA. Neurosurg. Focus. 2020;48:E9. doi: 10.3171/2019.9.FOCUS19699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kambhampati M., Perez J.P., Yadavilli S., Saratsis A.M., Hill A.D., Ho C.Y., Panditharatna E., Markel M., Packer R.J., Nazarian J. A standardized autopsy procurement allows for the comprehensive study of DIPG biology. Oncotarget. 2015;6:12740–12747. doi: 10.18632/oncotarget.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kambhampati M., Panditharatna E., Yadavilli S., Saoud K., Lee S., Eze A., Almira-Suarez M.I., Hancock L., Bonner E.R., Gittens J., et al. Harmonization of postmortem donations for pediatric brain tumors and molecular characterization of diffuse midline gliomas. Sci. Rep. 2020;10:10954. doi: 10.1038/s41598-020-67764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baugh J., Bartels U., Leach J., Jones B., Chaney B., Warren K.E., Kirkendall J., Doughman R., Hawkins C., Miles L., et al. The international diffuse intrinsic pontine glioma registry: An infrastructure to accelerate collaborative research for an orphan disease. J. Neurooncol. 2017;132:323–331. doi: 10.1007/s11060-017-2372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veldhuijzen van Zanten S.E.M., Lane A., Heymans M.W., Baugh J., Chaney B., Hoffman L.M., Doughman R., Jansen M.H.A., Sanchez E., Vandertop W.P., et al. External validation of the diffuse intrinsic pontine glioma survival prediction model: A collaborative report from the International DIPG Registry and the SIOPE DIPG Registry. J. Neurooncol. 2017;134:231–240. doi: 10.1007/s11060-017-2514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hegi M.E., Diserens A.C., Gorlia T., Hamou M.F., de Tribolet N., Weller M., Kros J.M., Hainfellner J.A., Mason W., Mariani L., et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 61.Hegi M.E., Liu L., Herman J.G., Stupp R., Wick W., Weller M., Mehta M.P., Gilbert M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 62.Szopa W., Burley T.A., Kramer-Marek G., Kaspera W. Diagnostic and Therapeutic Biomarkers in Glioblastoma: Current Status and Future Perspectives. Biomed. Res. Int. 2017;2017:8013575. doi: 10.1155/2017/8013575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weller M., van den Bent M., Tonn J.C., Stupp R., Preusser M., Cohen-Jonathan-Moyal E., Henriksson R., Le Rhun E., Balana C., Chinot O., et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18:e315–e329. doi: 10.1016/S1470-2045(17)30194-8. [DOI] [PubMed] [Google Scholar]
- 64.Wang Z., Jiang W., Wang Y., Guo Y., Cong Z., Du F., Song B. MGMT promoter methylation in serum and cerebrospinal fluid as a tumor-specific biomarker of glioma. Biomed. Rep. 2015;3:543–548. doi: 10.3892/br.2015.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Figueroa J.M., Skog J., Akers J., Li H., Komotar R., Jensen R., Ringel F., Yang I., Kalkanis S., Thompson R., et al. Detection of wild-type EGFR amplification and EGFRvIII mutation in CSF-derived extracellular vesicles of glioblastoma patients. Neuro Oncol. 2017;19:1494–1502. doi: 10.1093/neuonc/nox085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tabouret E., Chinot O., Metellus P., Tallet A., Viens P., Gonçalves A. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 67.Le Rhun E., Weller M., Brandsma D., Van den Bent M., de Azambuja E., Henriksson R., Boulanger T., Peters S., Watts C., Wick W., et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann. Oncol. 2017;28:iv84–iv99. doi: 10.1093/annonc/mdx221. [DOI] [PubMed] [Google Scholar]
- 68.Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol. 2010;11:871–879. doi: 10.1016/S1470-2045(10)70034-6. [DOI] [PubMed] [Google Scholar]
- 69.Remon J., Le Rhun E., Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat. Rev. 2017;53:128–137. doi: 10.1016/j.ctrv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Dudani S., Mazzarello S., Hilton J., Hutton B., Vandermeer L., Fernandes R., Ibrahim M.F., Smith S., Majeed H., Al-Baimani K., et al. Optimal Management of Leptomeningeal Carcinomatosis in Breast Cancer Patients-A Systematic Review. Clin. Breast Cancer. 2016;16:456–470. doi: 10.1016/j.clbc.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 71.Pan Z., Yang G., He H., Yuan T., Wang Y., Li Y., Shi W., Gao P., Dong L., Zhao G. Leptomeningeal metastasis from solid tumors: Clinical features and its diagnostic implication. Sci. Rep. 2018;8:10445. doi: 10.1038/s41598-018-28662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glantz M.J., Cole B.F., Glantz L.K., Cobb J., Mills P., Lekos A., Walters B.C., Recht L.D. Cerebrospinal fluid cytology in patients with cancer: Minimizing false-negative results. Cancer. 1998;82:733–739. doi: 10.1002/(SICI)1097-0142(19980215)82:4<733::AID-CNCR17>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 73.Clarke J.L., Perez H.R., Jacks L.M., Panageas K.S., Deangelis L.M. Leptomeningeal metastases in the MRI era. Neurology. 2010;74:1449–1454. doi: 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y., He J.Y., Zou Y.L., Guo X.S., Cui J.Z., Guo L., Bu H. Evaluating the cerebrospinal fluid ctDNA detection by next-generation sequencing in the diagnosis of meningeal Carcinomatosis. BMC Neurol. 2019;19:331. doi: 10.1186/s12883-019-1554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ge M., Zhan Q., Zhang Z., Ji X., Zhou X., Huang R., Liang X. Different next-generation sequencing pipelines based detection of tumor DNA in cerebrospinal fluid of lung adenocarcinoma cancer patients with leptomeningeal metastases. BMC Cancer. 2019;19:143. doi: 10.1186/s12885-019-5348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y.S., Jiang B.Y., Yang J.J., Zhang X.C., Zhang Z., Ye J.Y., Zhong W.Z., Tu H.Y., Chen H.J., Wang Z., et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: A new medium of liquid biopsy. Ann. Oncol. 2018;29:945–952. doi: 10.1093/annonc/mdy009. [DOI] [PubMed] [Google Scholar]
- 77.Rochet N.M., Kottschade L.A., Markovic S.N. Vemurafenib for melanoma metastases to the brain. N. Engl. J. Med. 2011;365:2439–2441. doi: 10.1056/NEJMc1111672. [DOI] [PubMed] [Google Scholar]
- 78.Welsh J.W., Komaki R., Amini A., Munsell M.F., Unger W., Allen P.K., Chang J.Y., Wefel J.S., McGovern S.L., Garland L.L., et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J. Clin. Oncol. 2013;31:895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seoane J., De Mattos-Arruda L. Brain metastasis: New opportunities to tackle therapeutic resistance. Mol. Oncol. 2014;8:1120–1131. doi: 10.1016/j.molonc.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Long G.V., Trefzer U., Davies M.A., Kefford R.F., Ascierto P.A., Chapman P.B., Puzanov I., Hauschild A., Robert C., Algazi A., et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 81.Margolin K., Ernstoff M.S., Hamid O., Lawrence D., McDermott D., Puzanov I., Wolchok J.D., Clark J.I., Sznol M., Logan T.F., et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 82.Lin N.U., Diéras V., Paul D., Lossignol D., Christodoulou C., Stemmler H.J., Roché H., Liu M.C., Greil R., Ciruelos E., et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 83.Bachelot T., Romieu G., Campone M., Diéras V., Cropet C., Dalenc F., Jimenez M., Le Rhun E., Pierga J.Y., Gonçalves A., et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 84.Gadgeel S.M., Gandhi L., Riely G.J., Chiappori A.A., West H.L., Azada M.C., Morcos P.N., Lee R.M., Garcia L., Yu L., et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): Results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–1128. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 85.Costa D.B., Shaw A.T., Ou S.H., Solomon B.J., Riely G.J., Ahn M.J., Zhou C., Shreeve S.M., Selaru P., Polli A., et al. Clinical Experience With Crizotinib in Patients with Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J. Clin. Oncol. 2015;33:1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crinò L., Ahn M.J., De Marinis F., Groen H.J., Wakelee H., Hida T., Mok T., Spigel D., Felip E., Nishio M., et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity with Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J. Clin. Oncol. 2016;34:2866–2873. doi: 10.1200/JCO.2015.65.5936. [DOI] [PubMed] [Google Scholar]
- 87.Iuchi T., Shingyoji M., Sakaida T., Hatano K., Nagano O., Itakura M., Kageyama H., Yokoi S., Hasegawa Y., Kawasaki K., et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;82:282–287. doi: 10.1016/j.lungcan.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 88.Porta R., Sánchez-Torres J.M., Paz-Ares L., Massutí B., Reguart N., Mayo C., Lianes P., Queralt C., Guillem V., Salinas P., et al. Brain metastases from lung cancer responding to erlotinib: The importance of EGFR mutation. Eur. Respir. J. 2011;37:624–631. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- 89.Schuler M., Wu Y.L., Hirsh V., O’Byrne K., Yamamoto N., Mok T., Popat S., Sequist L.V., Massey D., Zazulina V., et al. First-Line Afatinib versus Chemotherapy in Patients with Non-Small Cell Lung Cancer and Common Epidermal Growth Factor Receptor Gene Mutations and Brain Metastases. J. Thorac. Oncol. 2016;11:380–390. doi: 10.1016/j.jtho.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 90.Remon J., Steuer C.E., Ramalingam S.S., Felip E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann. Oncol. 2018;29:i20–i27. doi: 10.1093/annonc/mdx704. [DOI] [PubMed] [Google Scholar]
- 91.Swain S.M., Baselga J., Miles D., Im Y.H., Quah C., Lee L.F., Cortés J. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: Results from the randomized phase III study CLEOPATRA. Ann. Oncol. 2014;25:1116–1121. doi: 10.1093/annonc/mdu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartsch R., Berghoff A.S., Preusser M. Breast cancer brain metastases responding to primary systemic therapy with T-DM1. J. Neurooncol. 2014;116:205–206. doi: 10.1007/s11060-013-1257-5. [DOI] [PubMed] [Google Scholar]
- 93.Taillibert S., Chamberlain M.C. Leptomeningeal metastasis. Handb. Clin. Neurol. 2018;149:169–204. doi: 10.1016/b978-0-12-811161-1.00013-x. [DOI] [PubMed] [Google Scholar]
- 94.Jiang B.-Y., LI Y., Chuai S., Zhang Z., Yang J.-J., Zhong W., Zhou Q., Wu Y.-L. NGS to reveal heterogeneity between cerebrospinal fluid and plasma ctDNA among non-small cell lung cancer patients with leptomeningeal carcinomatosis. J. Clin. Oncol. 2017;35:9022. doi: 10.1200/JCO.2017.35.15_suppl.9022. [DOI] [Google Scholar]
- 95.Zhao Y., He J.Y., Cui J.Z., Meng Z.Q., Zou Y.L., Guo X.S., Chen X., Wang X., Yan L.T., Han W.X., et al. Detection of genes mutations in cerebrospinal fluid circulating tumor DNA from neoplastic meningitis patients using next generation sequencing. BMC Cancer. 2020;20:690. doi: 10.1186/s12885-020-07172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma C., Zhang J., Tang D., Ye X., Li J., Mu N., Li Z., Liu R., Xiang L., Huang C., et al. Tyrosine Kinase Inhibitors Could Be Effective Against Non-small Cell Lung Cancer Brain Metastases Harboring Uncommon EGFR Mutations. Front. Oncol. 2020;10:224. doi: 10.3389/fonc.2020.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kawahara A., Abe H., Murata K., Ishii H., Azuma K., Takase Y., Hattori S., Naito Y., Akiba J. Screening system for epidermal growth factor receptor mutation detection in cytology cell-free DNA of cerebrospinal fluid based on assured sample quality. Cytopathology. 2019;30:144–149. doi: 10.1111/cyt.12660. [DOI] [PubMed] [Google Scholar]
- 98.Ma C., Yang X., Xing W., Yu H., Si T., Guo Z. Detection of circulating tumor DNA from non-small cell lung cancer brain metastasis in cerebrospinal fluid samples. Thorac. Cancer. 2020;11:588–593. doi: 10.1111/1759-7714.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Siravegna G., Geuna E., Mussolin B., Crisafulli G., Bartolini A., Galizia D., Casorzo L., Sarotto I., Scaltriti M., Sapino A., et al. Genotyping tumour DNA in cerebrospinal fluid and plasma of a HER2-positive breast cancer patient with brain metastases. ESMO Open. 2017;2:e000253. doi: 10.1136/esmoopen-2017-000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamamoto W., Tomita N., Watanabe R., Hattori Y., Nakajima Y., Hyo R., Hashimoto C., Motomura S., Ishigatsubo Y. Central nervous system involvement in diffuse large B-cell lymphoma. Eur. J. Haematol. 2010;85:6–10. doi: 10.1111/j.1600-0609.2010.01438.x. [DOI] [PubMed] [Google Scholar]
- 101.Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. IARC; Lyon, France: 2017. [Google Scholar]
- 102.El-Galaly T.C., Cheah C.Y., Bendtsen M.D., Nowakowski G.S., Kansara R., Savage K.J., Connors J.M., Sehn L.H., Goldschmidt N., Shaulov A., et al. Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur. J. Cancer. 2018;93:57–68. doi: 10.1016/j.ejca.2018.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fontanilles M., Marguet F., Bohers É., Viailly P.J., Dubois S., Bertrand P., Camus V., Mareschal S., Ruminy P., Maingonnat C., et al. Non-invasive detection of somatic mutations using next-generation sequencing in primary central nervous system lymphoma. Oncotarget. 2017;8:48157–48168. doi: 10.18632/oncotarget.18325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hattori K., Sakata-Yanagimoto M., Suehara Y., Yokoyama Y., Kato T., Kurita N., Nishikii H., Obara N., Takano S., Ishikawa E., et al. Clinical significance of disease-specific MYD88 mutations in circulating DNA in primary central nervous system lymphoma. Cancer Sci. 2018;109:225–230. doi: 10.1111/cas.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grommes C., Tang S.S., Wolfe J., Kaley T.J., Daras M., Pentsova E.I., Piotrowski A.F., Stone J., Lin A., Nolan C.P., et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood. 2019;133:436–445. doi: 10.1182/blood-2018-09-875732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hiemcke-Jiwa L.S., Minnema M.C., Radersma-van Loon J.H., Jiwa N.M., de Boer M., Leguit R.J., de Weger R.A., Huibers M.M.H. The use of droplet digital PCR in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematol. Oncol. 2018;36:429–435. doi: 10.1002/hon.2489. [DOI] [PubMed] [Google Scholar]
- 107.Hiemcke-Jiwa L.S., Leguit R.J., Snijders T.J., Bromberg J.E.C., Nierkens S., Jiwa N.M., Minnema M.C., Huibers M.M.H. MYD88 p.(L265P) detection on cell-free DNA in liquid biopsies of patients with primary central nervous system lymphoma. Br. J. Haematol. 2019;185:974–977. doi: 10.1111/bjh.15674. [DOI] [PubMed] [Google Scholar]
- 108.Hickmann A.K., Frick M., Hadaschik D., Battke F., Bittl M., Ganslandt O., Biskup S., Döcker D. Molecular tumor analysis and liquid biopsy: A feasibility investigation analyzing circulating tumor DNA in patients with central nervous system lymphomas. BMC Cancer. 2019;19:192. doi: 10.1186/s12885-019-5394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rimelen V., Ahle G., Pencreach E., Zinniger N., Debliquis A., Zalmaï L., Harzallah I., Hurstel R., Alamome I., Lamy F., et al. Tumor cell-free DNA detection in CSF for primary CNS lymphoma diagnosis. Acta Neuropathol. Commun. 2019;7:43. doi: 10.1186/s40478-019-0692-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 111.Udaka Y.T., Packer R.J. Pediatric Brain Tumors. Neurol. Clin. 2018;36:533–556. doi: 10.1016/j.ncl.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 112.Smoll N.R., Drummond K.J. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J. Clin. Neurosci. 2012;19:1541–1544. doi: 10.1016/j.jocn.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 113.Taylor M.D., Northcott P.A., Korshunov A., Remke M., Cho Y.J., Clifford S.C., Eberhart C.G., Parsons D.W., Rutkowski S., Gajjar A., et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Northcott P.A., Buchhalter I., Morrissy A.S., Hovestadt V., Weischenfeldt J., Ehrenberger T., Gröbner S., Segura-Wang M., Zichner T., Rudneva V.A., et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547:311–317. doi: 10.1038/nature22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cavalli F.M.G., Remke M., Rampasek L., Peacock J., Shih D.J.H., Luu B., Garzia L., Torchia J., Nor C., Morrissy A.S., et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell. 2017;31:737–754.e736. doi: 10.1016/j.ccell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schwalbe E.C., Lindsey J.C., Nakjang S., Crosier S., Smith A.J., Hicks D., Rafiee G., Hill R.M., Iliasova A., Stone T., et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: A cohort study. Lancet Oncol. 2017;18:958–971. doi: 10.1016/S1470-2045(17)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hovestadt V., Ayrault O., Swartling F.J., Robinson G.W., Pfister S.M., Northcott P.A. Medulloblastomics revisited: Biological and clinical insights from thousands of patients. Nat. Rev. Cancer. 2020;20:42–56. doi: 10.1038/s41568-019-0223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koschmann C., Bloom K., Upadhyaya S., Geyer J.R., Leary S.E. Survival After Relapse of Medulloblastoma. J. Pediatr. Hematol. Oncol. 2016;38:269–273. doi: 10.1097/MPH.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 119.Zapotocky M., Mata-Mbemba D., Sumerauer D., Liby P., Lassaletta A., Zamecnik J., Krskova L., Kyncl M., Stary J., Laughlin S., et al. Differential patterns of metastatic dissemination across medulloblastoma subgroups. J. Neurosurg. Pediatr. 2018;21:145–152. doi: 10.3171/2017.8.PEDS17264. [DOI] [PubMed] [Google Scholar]
- 120.Ramaswamy V., Remke M., Bouffet E., Faria C.C., Perreault S., Cho Y.J., Shih D.J., Luu B., Dubuc A.M., Northcott P.A., et al. Recurrence patterns across medulloblastoma subgroups: An integrated clinical and molecular analysis. Lancet Oncol. 2013;14:1200–1207. doi: 10.1016/S1470-2045(13)70449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hill R.M., Kuijper S., Lindsey J.C., Petrie K., Schwalbe E.C., Barker K., Boult J.K., Williamson D., Ahmad Z., Hallsworth A., et al. Combined MYC and P53 defects emerge at medulloblastoma relapse and define rapidly progressive, therapeutically targetable disease. Cancer Cell. 2015;27:72–84. doi: 10.1016/j.ccell.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sun Y., Li M., Ren S., Liu Y., Zhang J., Li S., Gao W., Gong X., Liu J., Wang Y., et al. Exploring genetic alterations in circulating tumor DNA from cerebrospinal fluid of pediatric medulloblastoma. Sci. Rep. 2021;11:5638. doi: 10.1038/s41598-021-85178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hovestadt V., Jones D.T., Picelli S., Wang W., Kool M., Northcott P.A., Sultan M., Stachurski K., Ryzhova M., Warnatz H.J., et al. Decoding the regulatory landscape of medulloblastoma using DNA methylation sequencing. Nature. 2014;510:537–541. doi: 10.1038/nature13268. [DOI] [PubMed] [Google Scholar]
- 124.Li J., Zhao S., Lee M., Yin Y., Li J., Zhou Y., Ballester L.Y., Esquenazi Y., Dashwood R.H., Davies P.J.A., et al. Reliable tumor detection by whole-genome methylation sequencing of cell-free DNA in cerebrospinal fluid of pediatric medulloblastoma. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abb5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Henriksen T.V., Reinert T., Christensen E., Sethi H., Birkenkamp-Demtröder K., Gögenur M., Gögenur I., Zimmermann B.G., Dyrskjøt L., Andersen C.L. The effect of surgical trauma on circulating free DNA levels in cancer patients-implications for studies of circulating tumor DNA. Mol. Oncol. 2020;14:1670–1679. doi: 10.1002/1878-0261.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.