Highlights:
-
•
Addition of ionic liquid has impacts acoustic cavitation by increasing its efficiency.
-
•
Ionic liquid alkyl chain length effects the viscosity & surface tension of water.
-
•
Presence of ionic liquid may extend the life of radicals formed from sonolysis effect.
Keywords: Ionic Liquid, Ultrasound, Ultrasound-assisted extraction, Microalgae, Lipids
Abstract
This study investigates the potential of using small amounts of ionic liquids (IL) to enhance ultrasound-assisted extraction of lipids content from green microalgae. Three imidazolium-based ILs (butyl, octyl and dodecyl), each of them with two anions (bromide and acetate) were tested as additives. Viscosity and surface tension of the ILs aqueous mixtures were analyzed to determine the influence of ILs’ anions and alkyl chain length, whereas KI dosimetry experiments were used as an indicator of radicals formation. A key finding suggests that the small addition of ILs improves the ultrasonication either by enhancing the viscosity and reducing the water surface tension, leading to a more powerful acoustic cavitation process or by increasing HO° production likely to oxidize the microalgae cells membranes, and consequently disrupting them on a more efficient manner. KI dosimetry also revealed that long ILs alkyl chain is detrimental. This experimental observation is confirmed thus strengthened as the yield of extracted lipids from green microalgae has shown an incremental trend when the IL concentration also increased. These hypotheses are currently under investigation to spot detailed impact of ILs on cavitation process.
1. Introduction
In recent years, low frequency ultrasound-assisted extraction of lipids has emerged as an alternative technique associated with other conventional mechanical disruption methods. This recent technology appeared to be an affordable and powerful tool able to enhance many types of extraction processes [1], [2], [3], [4]. Low frequency ultrasound is found to be an economical and eco-friendly method with simple and easy set-up conditions while shortening processing time, with high reproducibility and high purity products. Furthermore, its ability to work preferentially at low temperature makes ultrasound an ideal technique of lipid extraction [5].
Within this framework, the use of ultrasound has proven to be invaluable in the field of microalgae bioprospecting, specifically in lipid extraction from microalgae. This has been echoed in several articles reporting on the efficiency of using ultrasound for lipid extraction [6], [7], [8], [9]. The common agreement among these studies is that the solvents used with ultrasound play an important role towards induced mechanical effects. In regard to this, two key factors are surface tension and viscosity of the solvent. Water has proven to be an appropriate solvent for cell disruption as it displays a lower vapour pressure and higher surface tension than most of organic solvents. Even if the energetic threshold to initiate cavitation is higher to reach in water as compared in organic solvents, bubbles formation will collapse in a much more energetic way with stronger shockwaves and shear forces. Lipids however display a hydrophobic nature preventing effective diffusion of the lipids into pure water. Used alone, low viscosity liquids exhibit very high vapour pressure and low surface tension as compared to water and acoustic cavitation in this single medium lead to a less energetic phenomenon [10], [11], [12]. In order to display amphiphilic properties, to increase viscosity and to decrease vapour pressure, using such compounds as additives would be appropriate to improve the efficiency of lipid extraction in water-mediated solution.
ILs are molten salts that are liquids at room temperature. They can be regarded as green chemicals with an outstanding design potential due to their components, cationic and anionic molecular core structures with customizable side chains [13]. Their key features, relying on their cation–anion combination, are thermally stable, liquid at wide temperature range, with low vapour pressure and non-flammability. They also have the property to exhibit dual characteristics of being polar and non-polar depending on the combination of cation and anion. Thus, these properties make them as an ideal candidate for extracting total lipids from any biomass [14], [15], [16]
As mentioned, most studies have focused either ultrasound-mediated or full ILs-mediated extractions to enhance lipid production from microalgae. To the best of our knowledge, previous studies have never focused on combination of both ultrasonication and catalytic presence of hydrophilic ILs in water-mediated extraction. Therefore, the present work aims to investigate the effect of ILs incremental addition towards enhanced cellular disruption ability of sonication. Different alkyl chain lengths of cation (C4, C8 and C12) and different anions (bromide and acetate) have been investigated for a better understanding on ILs properties effect towards low frequency ultrasound efficiency on lipid extraction.
2. Materials and methods
2.1. Chemicals
All chemicals and solvents were used as received without prior purification: 1-methylimidazole (purity ≥ 99%, Sigma-Aldrich), 1-bromoctane (for synthesis, Merck), 1-bromobutane (for synthesis, Merck), 1-bromododecane (purity ≥ 97%), silver nitrate (AgNO3, Merck), acetonitrile (for analysis, Merck), ethyl acetate (for analysis, Merck), diethyl ether (for analysis, Merck), acetic acid (glacial, 100% Merck), chloroform (for analysis, Merck) methanol (for analysis, Merck) and hexane (for analysis, Merck). Dried Chlorella vulgaris (C. vulgaris) powder was obtained from Centre for Biofuel and Biochemical Research, Universiti Teknologi PETRONAS. Structure confirmation of all synthesized ILs was performed by means of proton NMR spectroscopy (Bruker Avance 500, USA).
2.2. Methods
2.2.1. Synthesis of 1st generation bromide-based IL
Bromide-based ILs (1-butyl-3-methylimidazolium bromide [Bmim][Br]) was synthesized using preexisting protocol [17]. 1-methylimidazole was reacted with 1-bromobutane in a 3-neck round bottom flask. For acetate-based ILs, similarly to [Bmim][Br], pre-existing protocol was referred to synthesize [Bmim][OAc].
[Omim][Br] was reacted with glacial acetic acid in a 3-neck round bottom flask. The 3-neck round bottom flask was then attached to a reflux condenser and refluxed for 48 h at 40 °C in neat conditions. The resulting mixture was then washed with diethyl ether and ethyl acetate. The residual solvent was removed with a rotary evaporator (Buchi Rotavapor R-300, Switzerland). NMR analysis was conducted to determine the purity of the IL. Similar synthesis route was used for the synthesis of 1-octyl-3-methylimidazolium bromide [Omim][Br], 1-dodecyl-3-methylimidazlium bromide [Domim][Br], 1- octyl-3-methylimidazolium acetate [Omim][OAc] and 1-dodecyl-3-methylimidazlium acetate [Domim][OAc]. Given the long alkyl chain of [Domim], the synthesis time was doubled from 48 h to 96 h to ensure complete reaction of starting chemicals.
2.2.2. Surface tension of IL in water
The synthesized IL were mixed with water at differing ratio (from 0.1% to 20% w/v). Surface tension of the mixtures was measured via the pendant drop method using OCA Contact Angle System (DataPhysics Instrument, Germany). 1 ml of sample was taken with a syringe to be analyzed. All experiments were conducted in triplicates.
2.2.3. Viscosity of IL in water
Water and IL mixtures were subjected to viscosity and density measurement using SVM 3000 Viscometer (Anton Paar, Austria). Approximately 3 or 4 ml of the sample were used for each measure. The instrument was set to M5-Single temperature mode and the measurement was carried out at 25 °C. All experiments were conducted in triplicates.
2.2.4. Chemical dosimetry
In order to determine the impact of the viscosity and surface tension towards the acoustic cavitation, chemical dosimetry using potassium iodide was performed as a simple and robust method [18]. The chemical dosimetry to monitor triiodide formation may be adequate to quantitatively prove if the small addition of ILs into water either disturbs or enhances the water sonolysis producing HO° radicals. The full chemical equation is as shown below[19]:
0.1 mol of potassium iodide was dissolved in 1L of deionized water. 50 ml of solution was subjected to ultrasonication for 30 min with using a 20 kHz ultrasonic probe at 50% amplitude (Sonics VCX 750, USA). The calorimetric power released by the probe was measured to be at 30 W. The spectrophotometer absorbance at 355 nm was measured every 5 min using Biochrome Libra S60 UV-Spectrophotometer [18].
2.2.5. Ultrasound mediated extraction
IL mediated ultrasonication of the microalgae were conducted based on an optimized protocol. Each IL was sampled at a volume / volume (v/v) concentration of 0.5%, 1%, 1.5%, 2%, 2.5%, 5%, 10% and 20%, and completed with 50 ml distilled water containing 0.2 g of microalgae. The mixtures were then subjected to a 20 kHz irradiation with a 13 mm ultrasonic probe at 50% amplitude for 20 min at 2 s:1s pulse mode. A circulating water bath (Julabo SW22, Germany) was attached to the double-jacket ultrasonic vessel to ensure a constant temperature of 15 °C, to avoid any temperature changes due to the sonication effect. A first control experiment without the presence of IL was also conducted.
2.2.6. Lipid extraction and purification
The well-established Bligh and Dyer [20] method was used as extraction method. However, due to the addition of IL into the system, the original method has been slightly modified to accommodate the addition of the IL as follow: 50 ml of a methanol/chloroform (2:1) mixture was added into the algae mixture. The resulting solution was then centrifuged (Hettich Rotofix 32A, Germany) at 4000 rpm for 15 min. The separated bottom layer was transferred into a new flask and dried at 55 °C. The dried sample was then mixed with a hexane/water (1:1) solution to remove any residual IL eventually dissolved in the chloroform mixture. The hexane layer containing lipid was then transferred into a new glass vial and dried. Then, the lipids contents were weighed and expressed in terms of algae dry weight percentage (% DW).
3. Results and discussion
3.1. Viscosity and surface tension analysis of water with IL as additive
One of the major factors affecting the intensity of acoustic cavitation is the viscosity of the liquid medium. All agreed that as the viscosity of the liquid increases, the formation of microbubble would be more difficult and slower due to its lower oscillating effect. However this condition may also lead to a stronger bubbles’ collapse and to superior sheer forces [21]. As such, for a better microalgae cellular disruption, a slight increase in viscosity could be beneficial.
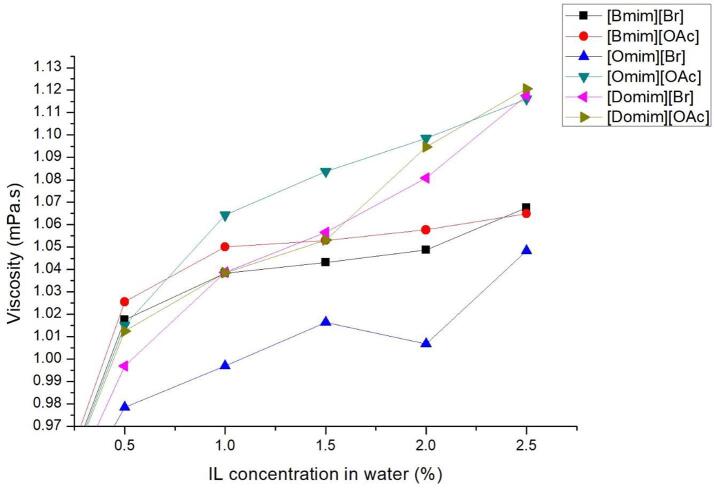
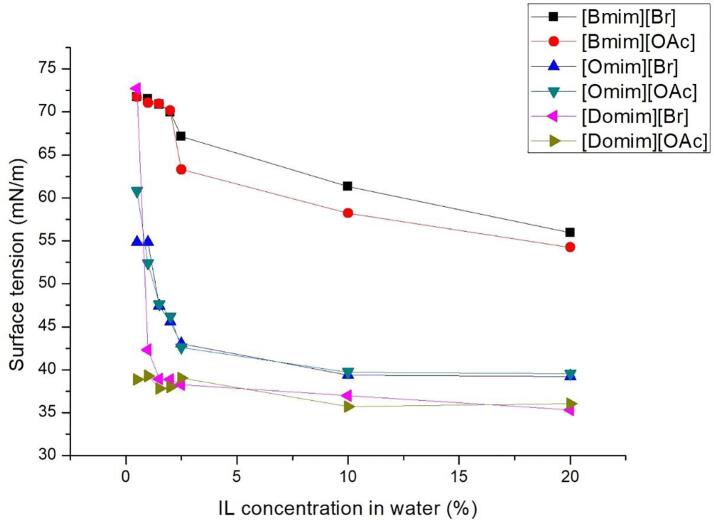
Another major factor that effects the principles of acoustic cavitation is the surface tension. Again, it is generally agreed that as the surface tension of a liquid decreases, it inherently helps to a more important formation of active microbubbles. However, there are two downsides of this condition: firstly, the numerous formed bubbles may deactivate ultrasonic irradiation by suppressing cavitation phenomenon (cushioning effect) and secondly the implosion of the microbubble would lead to less powerful micro-jet formation, thus weaker sheer forces. As such, the objective is to find a compromise between high viscosity and low surface tension thanks to the addition of IL in water as an additive. Both viscosity (Fig. 1) and surface tension (Fig. 2) are measured with different mass percentages of added ILs.
Fig. 1.
Effect of IL addition on IL-water mixtures viscosity.
Fig. 2.
Effect of IL addition on IL-water mixtures surface tension.
As expected, the addition of IL increases the viscosity of water as shown in Fig. 1. Indeed, the addition of IL into the system increased the viscosity of water from 0.89 mPa.s to more than 1.05 mPa.s at 2.5% of the ILs of different cations. The difference of the anion however does not appear to be influencing the viscosity as the difference between [Br] and [OAc] anion was found to be within the limit of 0.04 mPa.s – 0.08 mPa.s.
Fig. 2 emphasized that the addition of small amounts of IL helped in the reduction of water surface tension. As the concentration of the IL increases, the surface tension of the liquid decreases accordingly. For all IL, above 2.5%, the surface tension has almost reached its maximum reduction. Subsequently, at 10% and 20% of ILs concentration, the surface tension slightly decreased for butyl-based ILs but remained roughly constant for octyl and dodecyl-based ILs. When water becomes saturated with IL, the later begin to form micelles when reaching the critical micellar concentration (CMC) [22].
Whatever the anion is, the same surface tension reduction is observed for each cation, indicating that the cation has a great influence on the surface tension of the mixture. It is obvious that the alkyl chain of the IL plays a major role towards the reduction of the water surface tension with CMC value limit. Surface tension does not undergo drastic variation beyond CMC value. Furthermore, between butyl-based and octyl-based IL, we observed a large drop of surface tension: octyl- and dodecyl-based IL (46% and 51% respectively) shows a higher reduction of surface tension against the butyl-based IL (23%). This is due to longer alkyl chain length that increases the hydrophobicity of the IL and reduces the surface tension of water caused by the increasing rate of CMC formation. Higher CMC values may reduce the intermolecular forces between water molecules, reducing the surface tension of water. This also explains that octyl-based IL seem to reach CMC much earlier compared to its butyl counterpart. This point is further shown on the IL -water surface tension of [Domim]. The CMC concentration is achieved much earlier at 0.5% IL in water undoubtedly explained by the longest side alkyl chains length (C12) of the three tested cationic core moieties.
3.2. Chemical dosimetry
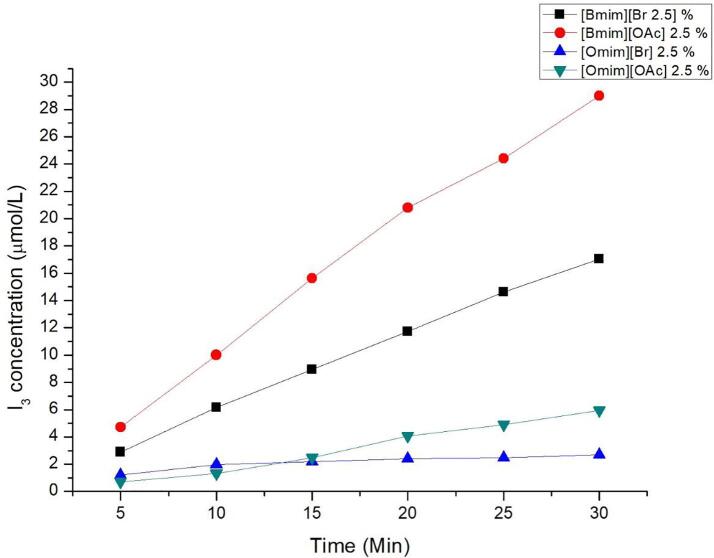
Fig. 3 depicts the iodometry results using [Bmim][Br], [Bmim][OAc], [Omim][Br] and [Omim][OAc] at 2.5% of IL. The results depicts the concentration of iodide in the presence of IL after removing the control. The mechanism of dosimetry is given in 2.2.4.
Fig. 3.
Chemical dosimetry of IL in water.
The results indicate that the addition of IL onto water does not decrease or disturb the radical production but on the contrary does increase the formation rate of triiodide. This is more evident with [Bmim] based IL. Both [Bmim][Br] and [Bmim][OAc] shows a steady increment of triiodide concentration. It is, to the best of our knowledge, the first time a research group shows that the addition of an IL in an aqueous solution submitted to ultrasound provides a higher rate of detected triiodide. This effect cannot be simply ascribed to the surfactant behavior of ILs. We have indeed attempted to perform several experiments with classical surfactants. 0.05–2% of Sodium Lauryl Sulfate (SLS) and N-Dodecyl Pyridinium Chloride (PDC) under low frequency ultrasound. However, our attempts have proven unsuccessful. Heavy foams in water immediately formed preventing a full extraction efficiency [23], [24]. Similar effect was observed when using high amount of IL (at concentration higher than 2.5%). This could be due to the CMC effect as explained earlier. In this regard, addition of small amounts of ILs into water may help not only to allow easier diffusion of lipid into water but also simultaneously to increase vapour pressure and decrease surface tension of the bulk medium leading to harsher cavitation activity. Attempt at using [Domim] based ILs have proven to be unsuccessful due to the high viscosity of the mixture. The effects obtained appeared to be similar with the classical surfactants.
Free radicals are highly transitory species that react quickly with other present molecules or by recombining through complex mechanistic pathways [25]. In a normal biological system, the occurrence of these free radicals is generally not favored due to their ability to cause severe damage to cell membrane. The free electrons on these radicals attack macromolecules causing cell damage and homeostatic disruption [26], [27]. The presence of these radicals causes oxidative damage towards cellular membrane, specifically membrane lipids. These process are known as lipid peroxidation where the radicals causes a chain reaction that damages unsaturated fatty acids commonly found in cell membrane, causing a destabilization of the cell membrane [28], [29]. Although they may seem harmful for biological organism, the formation of these radicals could benefit in the field of cellular disruption. The higher concentration of these radicals in a liquid sample, the stronger the cellular disruption effect will be expected when coupled with further shear forces of the acoustic cavitation.
In addition, none of the previous study had mentioned that the presence of classical surfactants could enhance the production rate of HO° with any chosen chemical dosimetry. This unusual result may be explained by two hypotheses. First, it has been recently shown that IL may be able to extend lifetime of radicals in radical polymerization reactions [30], [31]. So it can be assumed that ejected HO° from cavitation bubble collapse may be more stabilized by the presence of ILs, consequently increasing their lifetime to react with encountered iodide ions prior to recombination. The second hypothesis refers to the increased viscosity and reduced surface tension of the aqueous solutions, increasing the Blake threshold and substantially increasing bubble collapses intensity which leads to a higher production of HO°. Finally, both hypotheses may also occur simultaneously. Fig. 3 also highlights the exceptional chemical inertness of these ILs compounds as one would expect that the production of iodine would have dramatically decreased as compared to the control experiment without IL since HO° should have oxidized them.
3.3. Ionic liquid mediated lipid extraction
Table 1 depicts the lipid yield obtained from the control experiments.
Table 1.
Total lipid extracted from C. vulgaris with respective cellular disruption process.
| Cell disruption method | Yield (%) |
|---|---|
| Soxhlet | 34.4 ± 1.3 |
| Ultrasonic | 13.3 ± 0.1 |
Maximum lipid recovered from C. vulgaris under ultrasonic irradiation (after optimization of usual parameters such as time, power and temperature) without addition of ILs was at 13%. This result seems to be in accordance with various studies that have obtained similar yield from the same C. vulgaris species. It is generally agreed upon that the obtainable lipid yield from C. vulgaris usually range from 12% to 20% dry weight with conventional cellular disruption and extraction method [32], [33], [34]. This amount could however be increased depending upon the changes made during the cellular disruption process or modifying the algae culture nutrients as observed in the study conducted by Choi et al. They were able to almost double the lipid yield obtained by modifying the solvent. Their lipid extraction with hexane and hexane-methanol mixture both yielded 18.4% and 18.5% lipid. However, extraction performed with chloroform–methanol mixture reached a surprising 37.8%, double the lipid yield from the other two methods. This inherently proves that there is more lipid content within C. vulgaris than 20% and the base yield could be increased with an optimized extraction procedure. This is supported by our result obtained with Soxhlet procedure. The total obtainable lipid extracted with Soxhlet was as high as 34.4%, mirroring the results obtained by Choi et al. To obtain this amount, the Soxhlet extraction was done three times, where the biomass was repeatedly grinded and extracted until no weight changes obtained.
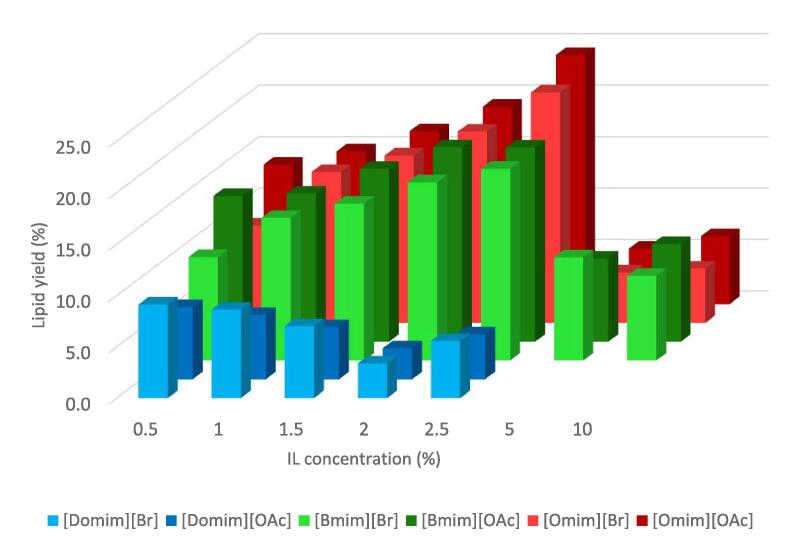
To quantify the effect of IL concentration on 20 kHz-assisted extraction, experiments were carried out with the following conditions: ILs were added incrementally in water and subjected to ultrasonication at 30 W of calorimetric power for a duration of 20 min. The sonication was done at 2 s:1s rest run interval (2 s of sonication followed by 1 s without ultrasound). Results are given in Fig. 4.
Fig. 4.
Percentage of lipids obtained from C. vulgaris by different concentration IL (frequency: 20 kHz; power: 30 W; duration: 20 min; volume: 50 ml; temperature: 15 °C).
For butyl-based ILs, the extracted lipid yield obtained have shown an increase with incremental addition of IL, from 0.1 to 2.5%. For [Bmim][Br], the lipid yield obtained at 0.5% IL was 10.1% DW and this steadily increases with each IL concentration, where at 2.5% concentration, lipid yield observed was at 18.7% DW, almost 9% yield increment from the lowest concentration with an increment rate of 85%. The same trend may also be observed with lipid extraction using [Bmim][OAc] as additive. At 0.5% IL, the lipid yield observed was at 14.2% DW and at 2.5% IL, an increment rate of 34% was observed.
With octyl-based ILs, similar to the extraction with butyl-based ILs, extraction gave an increasing trend as the concentration increases. Extraction with [Omim][Br] gave as indicated a staggering increment rate of 136% of lipid between extraction at 0.5% IL and 2.5% IL. Extraction with [Omim][OAc] observed an increment rate of 80% DW.
These results undoubtedly show a positive impact of ILs towards the lipid extraction efficiency in terms of enhancing the extractive capacity of ultrasonic method. This is especially true for extraction with [Bmim] and [Omim] based IL. We also observed that [Omim] based extraction has a slight advantage against [Bmim] based extraction. Extraction with [Omim][Br] and [Omim][OAc] has yielded 22.4% and 24.2% lipid respectively. This has shown to be 68.8% and 81.81% increment from the control.
Based on the observation, we hypothesize that due to the enhanced sonicating effect influenced by the increased viscosity of the medium contributed to stronger sheer force of the cells, hence allowing to a better interaction of the ILs to the lipids. This creates an opportunity for the ILs to interact with lipids more freely enhancing its ability to be extracted. This is further corroborated by the findings of our previous study where a detailed account on the positive interaction of IL with lipid have been given [35]. In an article by Zu et. al, they suggested that increasing ultrasonic power increases the destruction of cell walls, enabling better contact of intracellular materials with the solvent [36]. This has been echoed by several literature [37], [38], [39]. However, the downside of these studies is that they needed high acoustic power or longer sonication time in order to achieve an efficient extraction. With lower acoustic power and time, we were able to reach comparable (and even slightly higher) lipid yield compared to these methods[32], [40], [41], thanks to small addition of IL.
Incidentally, we also observed that extraction with 5% ILs for both [Bmim] and [Omim] was significantly low, whatever the anion is. We would like to put forth two hypotheses for this phenomenon. First hypothesis is the effect of high amount of IL within the water increases the viscosity of water tremendously to cause improper acoustic cavitation. This correlates with direct principle of ultrasonication that is high viscosity products are not preferred due to their inability to provide proper acoustic cavitation. Second hypothesis is the relation of surface tension towards IL-water mixture. We have assumed that large amount of IL in water may contribute to the formation of CMC. This may hamper the effect of acoustic cavitation within the water. A study conducted by Alegria et al., have shown a possible relation between the acoustic cavitation of aqueous solution and the CMC. In this study, the authors have theorized that the radicals formed during sonication are trapped near the corresponding CMC region [42]. This could attest as a reason of the decreased yield obtained during extraction from 5% and 10% IL.
Despite the overwhelming positive result of [Bmim] and [Omim], the opposite effect was achieved while using [Domim] based IL, as depicted in Fig. 4. Increasing the amount of IL into the system seems to give decreasing lipid yield. At 0.5% IL, the lipid yield obtained were only 9.1% for [Domim][Br] and 7.1% for [Domim][OAc]. They gradually decrease as the IL concentration increases. Finaly at 2.5%, both ILs gave a lipid yield lower than 6%. This result further reflects the previous hypothesis that [Domim] ILs form CMC, even at the lowest concentration.
4. Conclusion
Ultrasound-mediated lipid extraction has lately been gaining wide interest as a powerful and low-cost cellular disruption process in biomass downstream processing. In this study, we used [Bmim], [Omim] and [Domim] based IL with 2 sets of anions ([Br] and [OAc]). We have shown that small addition of IL into the ultrasound-mediated extraction system greatly enhances the lipid yield. The benefit can be linked to the properties of IL which increase the viscosity and reduce the surface tension of water, benefiting the acoustic cavitation. Increasing the alkyl chain length of the ILs also appears to benefit the extraction efficiency. Despite that, longer chain length IL was not favorable as the viscosity of liquid decreases the acoustic cavitation of ultrasound. As such, this study has shown that addition of small amount gave promising results in lipid extraction procedure.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Fundamental Research Grant Scheme (FRGS/1/2015/TK10/UTP/02/5) provided by Ministry of Higher Education (MOHE) Malaysia. We would also like to acknowledge the Centre for Biochemical and Biofuel Research, University Teknologi PETRONAS and Centre for Research in Ionic Liquids for their continued support throughout the duration of this project.
Footnotes
Given his role as Executive Editor of this journal, Jean-Marc Leveque had no involvement in the peer-review of articles for which he was an author and had no access to information regarding their peer-review. Full responsibility for the peer-review process for this article was delegated to another Editor.
Contributor Information
Sooridarsan Krishnan, Email: suridarsan@gmail.com.
Noraini Abd. Ghani, Email: noraini.ghani@utp.edu.my.
References
- 1.Shirsath S.R., Sonawane S.H., Gogate P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process Intensif. 2012;53:10–23. doi: 10.1016/j.cep.2012.01.003. [DOI] [Google Scholar]
- 2.Hu Y., Kwan T.H., Daoud W.A., Lin C.S.K. Continuous ultrasonic-mediated solvent extraction of lactic acid from fermentation broths. J. Cleaner Prod. 2017;145:142–150. [Google Scholar]
- 3.Li F., Raza A., Wang Y.-W., Xu X.-Q., Chen G.-H. Optimization of surfactant-mediated, ultrasonic-assisted extraction of antioxidant polyphenols from rattan tea (Ampelopsis grossedentata) using response surface methodology. Pharmacognosy Magazine. 2017;13:446. doi: 10.4103/pm.pm_159_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L., Zhou C., Wang B., Yagoub A.-E.-G.-A., Ma H., Zhang X., Wu M. Study of ultrasonic cavitation during extraction of the peanut oil at varying frequencies. Ultrason. Sonochem. 2017;37:106–113. doi: 10.1016/j.ultsonch.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 5.Chemat F., Zill-e-Huma, Khan M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011;18(4):813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Keris-Sen U.D., Sen U., Soydemir G., Gurol M.D. An investigation of ultrasound effect on microalgal cell integrity and lipid extraction efficiency. Bioresour. Technol. 2014;152:407–413. doi: 10.1016/j.biortech.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Adam F., Abert-Vian M., Peltier G., Chemat F. “Solvent-free” ultrasound-assisted extraction of lipids from fresh microalgae cells: a green, clean and scalable process. Bioresour. Technol. 2012;114:457–465. doi: 10.1016/j.biortech.2012.02.096. [DOI] [PubMed] [Google Scholar]
- 8.Lee S.J., Yoon B.-D., Oh H.-M. Rapid method for the determination of lipid from the green alga Botryococcus braunii. Biotechnol. Tech. 1998;12:553–556. [Google Scholar]
- 9.Suali E., Sarbatly R. Conversion of microalgae to biofuel. Renew. Sustain. Energy Rev. 2012;16:4316–4342. [Google Scholar]
- 10.Ensminger D., Bond L.J. CRC Press; 2011. Ultrasonics: fundamentals, technologies, and applications. [Google Scholar]
- 11.Shah Y.T., Pandit A., Moholkar V. Springer Science & Business Media; 2012. Cavitation reaction engineering. [Google Scholar]
- 12.Ashokkumar M. The characterization of acoustic cavitation bubbles–an overview. Ultrason. Sonochem. 2011;18:864–872. doi: 10.1016/j.ultsonch.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Neumann J., Steudte S., Cho C.-W., Thöming J., Stolte S. Biodegradability of 27 pyrrolidinium, morpholinium, piperidinium, imidazolium and pyridinium ionic liquid cations under aerobic conditions. Green Chem. 2014;16:2174–2184. [Google Scholar]
- 14.Choi S.-A., Oh Y.-K., Jeong M.-J., Kim S.W., Lee J.-S., Park J.-Y. Effects of ionic liquid mixtures on lipid extraction from Chlorella vulgaris. Renewable Energy. 2014;65:169–174. [Google Scholar]
- 15.Krishnan S., Abd Ghani N., Aminuddin N.F., Quraishi K.S., Azman N.S., Cravotto G., Leveque J.-M. Microwave-assisted lipid extraction from Chlorella vulgaris in water with 0.5%–2.5% of imidazolium based ionic liquid as additive. Renewable Energy. 2020;149:244–252. [Google Scholar]
- 16.Zhou W., Wang Z., Alam M.A., Xu J., Zhu S., Yuan Z., Huo S., Guo Y., Qin L., Ma L. Repeated Utilization of Ionic Liquid to Extract Lipid from Algal Biomass. International Journal of Polymer Science. 2019;2019:1–7. doi: 10.1155/2019/9209210. [DOI] [Google Scholar]
- 17.V. Losetty, C.D. Wilfred, M.C. Shekar, Synthesis and study of ionic interactions by volumetric, transport, FT-IR and computational methods of alkyl imidazolium acetate ionic liquid with molecular solvents (DMSO, DMF & EG) at T=(293.15–363.15) K, Journal of Molecular Liquids, 224 (2016) 480-491.
- 18.Koda S., Kimura T., Kondo T., Mitome H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003;10:149–156. doi: 10.1016/S1350-4177(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 19.Pflieger R., Nikitenko S.I., Cairós C., Mettin R. Springer; 2019. Characterization of cavitation bubbles and sonoluminescence. [Google Scholar]
- 20.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 21.R.E. Apfel, 7. Acoustic cavitation, in: Methods in experimental physics, Elsevier, 1981, pp. 355-411.
- 22.Fendler J., Fendler E. Academic; New York: 1975. Catalysis in micellar and macromolecular chemistry. [Google Scholar]
- 23.Zhang C., Wang Q., Xia H., Qiu G. Ultrasonically induced microemulsion polymerization of styrene. Eur. Polym. J. 2002;38:1769–1776. [Google Scholar]
- 24.J. O'sullivan, B. Murray, C. Flynn, I. Norton, The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins, Food Hydrocolloids, 53 (2016) 141-154.
- 25.Suslick K.S. Sonochemistry. Science. 1990;247:1439–1446. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- 26.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Rreviews. 2010;4:118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Djordjevic V.B. Free radicals in cell biology. Int. Rev. Cytol. 2004;237:57–91. doi: 10.1016/S0074-7696(04)37002-6. [DOI] [PubMed] [Google Scholar]
- 28.Jancic S.A., Stosic B.Z. Cadmium effects on the thyroid gland. Vitam. Horm. 2014;94:391–425. doi: 10.1016/B978-0-12-800095-3.00014-6. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs P., Perez-Pinzon M.A., Dave K.R. Cerebral ischemia in diabetics and oxidative stress. Diabetes: Oxidative Stress and Dietary Antioxidants. 2014:15–23. [Google Scholar]
- 30.Harrisson S., Mackenzie S.R., Haddleton D.M. Pulsed laser polymerization in an ionic liquid: strong solvent effects on propagation and termination of methyl methacrylate. Macromolecules. 2003;36:5072–5075. [Google Scholar]
- 31.L. Cheng, Y. Zhang, T. Zhao, H. Wang, Free radical polymerization of acrylonitrile in green ionic liquids, in: Macromolecular Symposia, Wiley Online Library, 2004, pp. 9-16.
- 32.dos Santos R.R., Moreira D.M., Kunigami C.N., Aranda D.A.G., Teixeira C.M.L.L. Comparison between several methods of total lipid extraction from Chlorella vulgaris biomass. Ultrason. Sonochem. 2015;22:95–99. doi: 10.1016/j.ultsonch.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Guckert J.B., Cooksey K.E., Jackson L.L. Lipid sovent systems are not equivalent for analysis of lipid classes in the microeukaryotic green alga. Chlorella, Journal of Microbiological Methods. 1988;8:139–149. [Google Scholar]
- 34.Griffiths M.J., Harrison S.T. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009;21:493–507. [Google Scholar]
- 35.Krishnan S., Ghani N.A., Aminuddin N.F., Quraishi K.S., Azman N.S., Cravotto G., Leveque J.-M. Microwave-assisted lipid extraction from Chlorella vulgaris in water with 0.5%–2.5% of imidazolium based ionic liquid as additive. Renewable Energy. 2020;149:244–252. [Google Scholar]
- 36.Zu G., Zhang R., Yang L., Ma C., Zu Y., Wang W., Zhao C. Ultrasound-assisted extraction of carnosic acid and rosmarinic acid using ionic liquid solution from Rosmarinus officinalis. Int. J. Mol. Sci. 2012;13:11027–11043. doi: 10.3390/ijms130911027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L., Wang H., Zu Y.-G., Zhao C., Zhang L., Chen X., Zhang Z. Ultrasound-assisted extraction of the three terpenoid indole alkaloids vindoline, catharanthine and vinblastine from Catharanthus roseus using ionic liquid aqueous solutions. Chem. Eng. J. 2011;172:705–712. [Google Scholar]
- 38.Cao X., Ye X., Lu Y., Yu Y., Mo W. Ionic liquid-based ultrasonic-assisted extraction of piperine from white pepper. Anal. Chim. Acta. 2009;640:47–51. doi: 10.1016/j.aca.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 39.Han D., Zhu T., Row K.H. Ultrasonic extraction of phenolic compounds from Laminaria japonica Aresch using ionic liquid as extraction solvent. Bull. Korean Chem. Soc. 2011;32:2213. [Google Scholar]
- 40.Araujo G.S., Matos L.J., Fernandes J.O., Cartaxo S.J., Gonçalves L.R., Fernandes F.A., Farias W.R. Extraction of lipids from microalgae by ultrasound application: prospection of the optimal extraction method. Ultrason. Sonochem. 2013;20:95–98. doi: 10.1016/j.ultsonch.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y., Hong A., Zhang D., Li L. Comparison of cell rupturing by ozonation and ultrasonication for algal lipid extraction from Chlorella vulgaris. Environ. Technol. 2014;35:931–937. doi: 10.1080/09593330.2013.856954. [DOI] [PubMed] [Google Scholar]
- 42.Alegria A.E., Lion Y., Kondo T., Riesz P. Sonolysis of aqueous surfactant solutions: probing the interfacial region of cavitation bubbles by spin trapping. The Journal of Physical Chemistry. 1989;93:4908–4913. [Google Scholar]