Highlights
-
•
Ultrasonic/mechanical mixing soil washing for the removal of heavy metals was investigated.
-
•
With the aid of ultrasound, higher removal efficiency was obtained.
-
•
Ultrasound was more effective in the less extreme washing conditions.
-
•
A new novel method was suggested to understand the ultrasonic desorption mechanism.
Keywords: Cavitation, Soil washing, Heavy metals, Ultrasonic desorption, HCl, EDTA
Abstract
Ultrasound-assisted soil washing processes were investigated for the removal of heavy metals (Cu, Pb, and Zn) in real contaminated soils using HCl and EDTA. The ultrasound-assisted soil washing (US/Mixing) process was compared with the conventional soil washing (Mixing) process based on the mechanical mixing. High removal efficiency (44.8% for HCl and 43.2% for EDTA) for the metals was obtained for the most extreme conditions (HCl 1.0 M or EDTA 0.1 M and L:S = 10:1) in the Mixing process. With the aide of ultrasound, higher removal efficiency (57.9% for HCl and 50.0% for EDTA) was obtained in the same extreme conditions and similar or higher removal efficiency (e.g., 54.7% for HCl 0.5 M and L:S = 10:1 and 50.5% for EDTA 0.05 M and L:S = 5:1) was achieved even in less extreme conditions (lower HCl or EDTA concentration and L:S ratio). Therefore, it was revealed that the US/Mixing was advantageous over the conventional Mixing processes in terms of metal removal efficiency, consumption of chemicals, amount of generated washing leachate, and volume/size of washing reactor. In addition, the heavy metals removal was enhanced for the smaller soil particles in the US/Mixing process. It was due to more violent movement of smaller particles in slurry phase and more violent sonophysical effects. In order to understand the mechanism of ultrasonic desorption, the desorption test was conducted using the paint-coated beads with three sizes (1, 2, and 4 mm) for the free and attached conditions. It was found that no significant desorption/removal of paint from the beads was observed without the movement of beads in the water including floatation, collision, and scrubbing. Thus, it was suggested that the simultaneous application of the ultrasound and mechanical mixing could enhance the physical movement of the particles significantly and the very high removal/desorption could be attained.
1. Introduction
Ultrasound technology has been researched and applied in environmental engineering processes for decades. Most previous research focused on sonochemical effects including radical oxidation and pyrolysis as one of the advanced oxidation processes (AOPs) for the treatment of drinking water and wastewater [1], [2], [3], [4], [5], [6], [7], [8], [9]. Remarkable synergistic effects have been reported when it is combined with other AOPs including ultraviolet (UV) [10], [11], [12], ozone [13], [14], catalysts [15], [16], [17], and persulfate [18], [19]. Some researchers investigated the use of ultrasound in soil washing processes for the remediation of contaminated soils. Mechanical mixing of contaminated soils and washing liquid is the key step in soil washing processes and it was reported that the hybridization of the conventional mechanical mixing and novel ultrasound technology enhanced the removal of contaminants from the soils significantly [20], [21], [22], [23], [24], [25], [26]. Even though ultrasound could induce satisfactory removal with no mechanical mixing in small-scale horn-type sonicator systems, we believe that double-bath ultrasonic systems with mechanical mixing are more adequate for scale-up and industrial use.
Relatively few studies on the ultrasound-aided soil washing for the remediation of heavy metals contaminated soils compared to that of organic chemicals including petroleum hydrocarbons and persistent organic pollutants (POPs) [20], [21], [27]. The removal of metals from the soils can be more complicated by the consideration of the speciation and fractionation of heavy metals. Thus, strong acids such as HCl, HNO3, and H2SO4 are used to increase the extractability of heavy metal species in very acidic conditions. Chelating agents such as EDTA (Ethylenediaminetetraacetic acid) can be useful for the removal of heavy metals in soils that have high buffering capacities, as they can form stable metal complexes over a wide pH range. Heavy metal contaminated soils also can be remediated using the in-situ solidification/stabilization (S/S) method, which refers to the on site immobilization of heavy metals in contaminated soils using binding reagents. However, it requires long-term monitoring due to the potential for re-release of harmful contaminants [28].
It was reported that the additional use of ultrasound in the soil washing processes enhanced the removal/desorption of heavy metals from the soils due to microscale mixing and sonophysical effects including microjet and shockwave. Hwang et al. investigated the effects of ultrasound power and irradiation time on the metals removal in the sonicator system using citrate and EDTA [21]. Park and Son tested the double-bath ultrasonic system for the operation of full-scale soil washing processes [20]. Son et al. investigated the cavitational activity in heterogeneous system and the removal of metals considering the fractionation of metals before and after soil washing processes [27]. However, very little research interest has been devoted to understand the ultrasonic desorption mechanism for large-scale industrial use.
The purpose of this study was to understand various operational parameters including the liquid:solid ratio, the washing agent (HCl and EDTA) concentration, the soil particle size on the removal of heavy metals (Cu, Pb, and Zn) in the ultrasound-assisted washing processes as one of the basic steps for the optimal design of industrial-scale processes. In addition, ultrasonic desorption tests using red paint coated beads were conducted to evaluate the role of ultrasound in the washing processes.
2. Experimental methods
2.1. Sonoreactor
Fig. 1 shows a schematic of the double-bath-type ultrasonic system in this study. The system consisted of a rectangular stainless-steel sonoreactor (L × W × H: 20 cm × 20 cm × 20 cm) with a 28 kHz ultrasonic transducer module (Mirae Ultrasonic Tech., KOR) and a rectangular stainless-steel washing vessel (L × W × H: 15 cm × 15 cm × 15 cm), which was submerged in the sonoreactor. The sonoreactor was filled with 2 L of water and the temperature was maintained at 20–25 ℃ using a cooling system. The electrical working power was 170 ± 10 W, measured using a power meter (HPM-300A, AD Power, KOR). An overhead stirrer was applied in the vessel at the rate of 200 rpm and the washing time was 20 min.
Fig. 1.
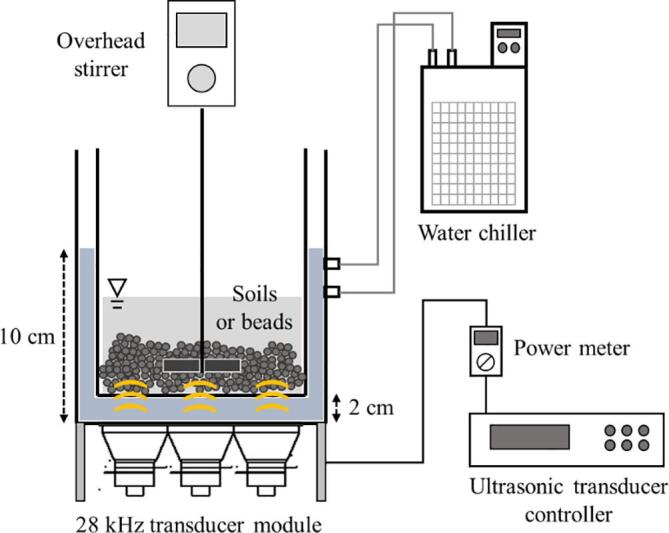
Schematic of the double-bath-type ultrasonic system used in this study.
2.2. Soil washing tests
Two kinds of soil samples contaminated with heavy metals were obtained from a closed railway depot in Korea and used for mechanical soil washing (Mixing) and ultrasonic/mechanical soil washing (US/Mixing) tests. The first soil sample was contaminated with Cu, Pb, and Zn and prepared using a #4 (4.75 mm) sieve for HCl (Hydrochloric acid) or EDTA (Ethylenediaminetetraacetic acid) soil washing processes. The initial concentrations of the sieved soil sample for Cu, Pb, and Zn were 485 ± 20, 990 ± 65, and 576 ± 22 mg/kg, respectively. Each initial concentration was higher than the regulation level in Korea according to the Soil Environment Conservation Act. The concentrations of washing liquid were 0.1, 0.4, and 1.0 M and 0.01, 0.05, and 0.10 M for HCl and EDTA, respectively. The applied liquid:soil (L:S) ratio was 3:1, 5:1, and 10:1 using a 100 g of soils. The second soil sample was contaminated with Cu and Zn and used to investigated the effect of soil particle size in HCl soil washing processes. The sample was classified into four groups using #4 (4.75 mm), #10 (2.00 mm), and #50 (0.30 mm) sieves: ~ 4.75 mm (Cu: 157 ± 7 mg/kg, Zn: 410 ± 38 mg/kg); 2.00 ~ 4.75 mm (Cu: 141 ± 21 mg/kg, Zn: 311 ± 69 mg/kg); 0.30 ~ 2.00 mm (Cu: 137 ± 5 mg/kg, Zn: 418 ± 15 mg/kg); ~ 0.30 mm (Cu: 175 ± 4 mg/kg, Zn: 516 ± 20 mg/kg) as shown in Fig. S1. A 0.5 M HCl solution was used and the L:S ratio was 3:1 using a 200 g of soils.
The concentrations of heavy metals were quantified using a trace metal digestion system (SMA20A, Gerhardt, DEU) and an ICP-OES (inductively coupled plasma-optical emission spectrometry) (720-ES, Varian, USA) according to the aqua-regia method (3 g of soil in 7 mL of HNO3 + 1 mL of HCl) in the Korean standard method for soil pollution [20], [27].
2.3. Glass beads tests
Three kinds of beads with mean diameters of 1.0, 2.0, and 4.0 mm were tested to investigate the mechanism of ultrasonic washing processes. For desorption tests, the beads were coated with a red paint using an oil-based spray paint and exposed to ultrasound irradiation under the water : beads ratios of 3:1, 5:1, and 10:1. The turbidity induced by the detachment of the paint was measured using a UV–vis spectrophotometer (Vibra S60, Biochrom Ltd., UK). For the visualization of cavitational active zone, uncoated/transparent beads were used in the luminol solution (0.1 g/L luminol and 1 g/L NaOH). SCL (Sonochemiluminescence) images were obtained using an exposure-controlled digital camera (α58, Sony Corp., JPN) in a completely dark room [29], [30]. The aluminum foil tests were conducted to visualize the sonophysical effects. The thickness of aluminum foil was 15 μm [25].
3. Results and discussion
3.1. Soil washing processes
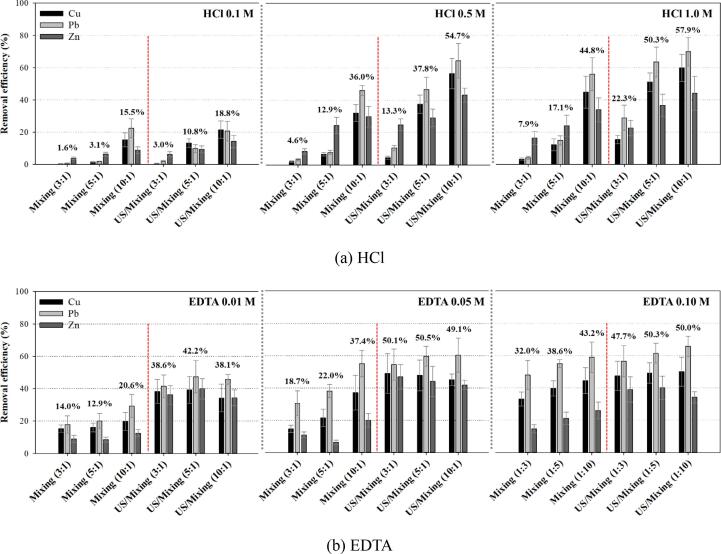
Mechanical washing (Mixing) processes and ultrasonic/mechanical washing (US/Mixing) processes were investigated for the heavy metal removal from soils using the HCl solution (0.1, 0.5, and 1.0 M) and EDTA solution (0.01, 0.05, and 0.10 M) under various liquid (washing liquid):solid (dried contaminated soil) ratio conditions (3:1, 5:1, and 10:1) as shown in Fig. 2. HCl was selected due to its high effectiveness among organic and inorganic acids and EDTA was considered because it is one of the most applicable chelating agents in previous research. No significant removal was observed in the only application of ultrasound (ultrasonic washing (US) processes) as previously reported in the bath-type systems [20], [27].
Fig. 2.
Removal efficiency in the mechanical (Mixing) and combined ultrasonic and mechanical (US/Mixing) washing processes using HCl and EDTA (The number above each of the bar group represents the average removal efficiency of the group of Cu, Pb, and Zn.).
For the washing processes using strong acid, it is known that extremely acidic condition in slurry phase (washing liquid + soil) is required for the significant removal of heavy metal species from soil particles via ion exchange and dissolution [27], [28]. In both Mixing and US/Mixing processes, it was found that lower removal efficiency (1.6 ~ 18.8%) was obtained for lower HCl concentration (0.1 M) where the acidity of the HCl solution did not overwhelm the buffering capacity of the soil, induced by the presence of organic matters and calcareous components, in this study. The pH in slurry with 0.1 M HCl solution ranged from 1.7 to 2.6 (The theoretical pH in 0.1 M HCl solution is 1.0.). For 0.5 and 1.0 M HCl condition (The theoretical pH for 0.5 and 1.0 M HCl solution was 0.3 and 0.0, respectively.), relatively low removal efficiency (4.6 ~ 22.3%) was attained for low L:S ratio of 3:1 and 5:1 or no ultrasound conditions in spite of very acidic condition (pH −0.2 ~ 0.1). It might be because some spots such as soil particle pores were not well exposed to the washing liquid and less acidic condition, unfavorable for the metal removal, could be formed. It was reported that the wettability of soil particles could be determined by the characteristics of particle surface [31]. As the L:S ratio increased, the density of the slurry decreased and the effect of mechanical mixing for the contact of metal species in soil particle pores could be enhanced significantly under the same mixing rate. The degree of particle-to-particle collision and scrubbing could also increase for higher S:L ratio. In addition, the additional application of ultrasound could induce cavitation events in particle pores due the presence of dissolved gas molecules entrapped in pores and enhance micro-scale contact of the washing liquid in the interior of the soil particles [32], [33]. Sonophysical effects including microjet and shockwave from the interior and the exterior of particles could also result in the fragmentation of particles into smaller particles with larger surface area [24].
According to the binding strength of metals species to soil particles, five-step fractionation was suggested as follows in order of weak binding: (F1) exchangeable; (F2) bound to carbonates; (F3) bound to Fe-Mn oxides; (F4) bound to organic matter and sulfides; and (F5) residual [28], [34], [35]. It is well known that metal species belonging to F4 and F5 fractions are not well removed even in extremely acidic condition. Recently, Son et al. reported that more F4 and F5 metals were extracted and much higher removal efficiency was achieved in US/Mixing processes compared to Mixing processes via various cavitational reactions. In this study, low removal efficiency of 17.1% was obtained in the Mixing process (HCl 1.0 M and L:S = 5:1) with negative pH condition (-0.2) [27]. On the other hand, much higher removal efficiency of 50.3% was achieved in the US/Mixing process (HCl 1.0 M and L:S = 5:1) and it was because more F4 and F5 fractions were removed for all three metals. The initial F5 fraction of the contaminated soil was 35.2%, 18.0%, and 47.6% for Cu, Pb, and Zn, respectively. The difference in the removal efficiencies of three metals under the same condition seemed to be related with the initial F5 fraction of the soil. The highest removal efficiency was observed for Pb and followed by Cu and Zn for most conditions of higher HCl concentration and L:S ratio.
Moreover, ultrasound was more effective in the less extreme conditions such as lower HCl concentrations and L:S ratios and relatively higher removal efficiency could be yielded using less amount of strong acid and washing liquid. Similar or even higher removal was observed in the US/Mixing processes with less acid dose and less amount of washing liquid. Therefore, it was found that the additional use of ultrasound in soil washing processes was superior to the conventional mechanical soil washing processes in terms of metal removal efficiency, consumption of chemicals, amount of generated washing leachate, and volume/size of washing reactor [25], [27]. In addition, the use of less acidic solution can be one of the most crucial conditions in long-term operation of washing processes because strong acid solutions are highly corrosive to reactor materials such as stainless steel.
Fig. 2(b) also shows the effectiveness of ultrasound in the soil washing processes using EDTA. Mixing of chelating agent solution and metal-contaminated soils induces the formation of highly stable water-soluble metal complexes and the complexation reactions are generally pH independent [28]. However, an excess dose of chelating agents is required for achieving satisfactory removal due to the presence of plenty of cations such as Ca2+, Mg2+, Fe2+, and Al3+ in soils like the excess requirement of acids to maintain extremely acidic condition [28], [36]. For the lowest EDTA concentration (0.01 M), relatively low removal efficiency was obtained and ultrasound could enhance the metal removal substantially by activating the soil particles surface and pores. Only a little variation for the removal efficiencies for the L:S ratios in the US/Mixing processes (The efficiency range of 38.1 ~ 42.2%, 49.1 ~ 50.5%, and 47.7 ~ 50.3% was obtained for 0.01, 0.05, and 0.10 M of EDTA, respectively.). With no ultrasound, strong acids such as HCl can increase the reactive surface and pores by acidic dissolution of soil components and some metal species, initially unexposed to the washing liquid, can be desorbed while chelating agents such as EDTA can react with only exposed metal species. As a result of this, it seemed ultrasound played more important role than the application of higher EDTA concentration or higher L:S ratio. In this study, the optimal washing condition using EDTA was suggested as the US/Mixing processes with L:S = 1:3 and 0.05 M EDTA. Therefore, it was revealed again that US/Mixing processes had various advantages mentioned above over the conventional washing processes.
3.2. Soil particle size
Most of the previous researches focused on the remediation of sand-sized (~2 mm) soils in US or US/Mixing processes [20], [21], [22], [25], [26]. Recently we reported that ultrasound was very effective for the treatment of fine soil particles (~0.075 mm: silt and clay) in US/Mixing processes [27]. Herein, in order to investigate the effect of the soil particle size in the washing processes, the contaminated soils were sieved into four size ranges (~4.75 mm; 2.00 ~ 4.75 mm; 0.30 ~ 2.00 mm; ~ 0.30 mm) and Mixing and US/Mixing processes (0.5 M HCl and L:S = 1:3) were operated using each of the sieved soil samples as shown in Fig. 3. No significant removal was observed in the US processes as reported above. The size distribution of the original field soil sample was as follows (wt%): > 4.75 mm: 8.1%; 2.00 ~ 4.75 mm: 24.9%; 0.30 ~ 2.00 mm: 53.0%; < 0.30 mm: 14.0%. The soil sample of ~ 4.75 mm was considered as the representation of the original field soil sample because it included 91.9% of total size composition of the original field soil in this study. Higher initial concentration of heavy metal was measured for smaller soil particle samples due to the larger surface area of smaller particles.
Fig. 3.
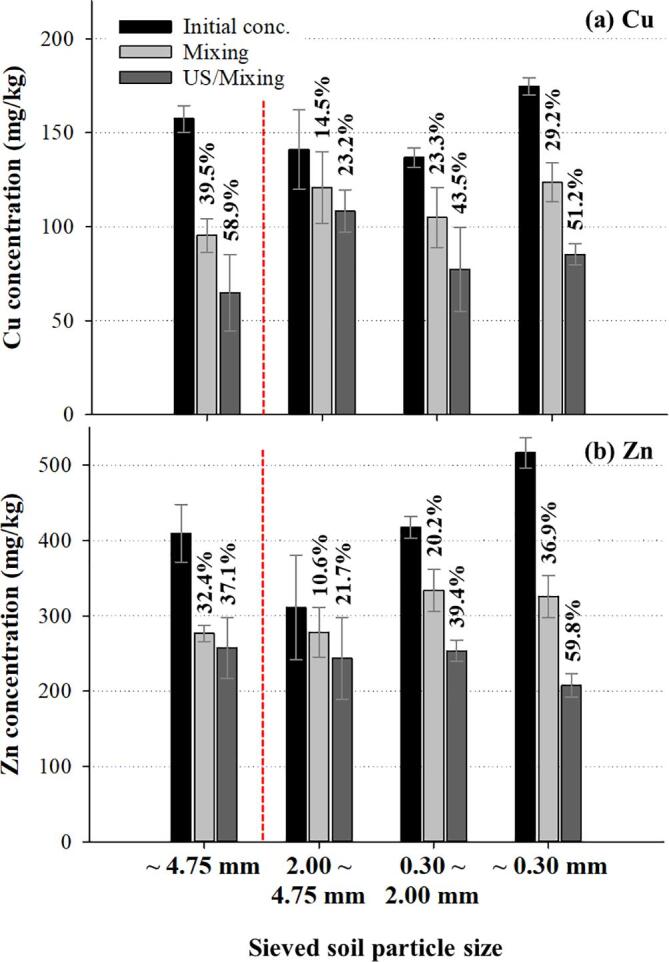
Heavy metal removal in the mechanical (Mixing) and combined ultrasonic and mechanical (US/Mixing) washing processes using HCl (The number above each of the bar represents the removal efficiency based on the initial concentration.).
As the soil particle size decreased, the removal efficiency for Cu and Zn increased in both washing processes. The movement of smaller soil particles in the washing liquid were more violent due to lower weight of each particle under the same mechanical mixing rate condition. The more violent movement induced more physical impact on the particles (particle-to-particle collision and scrubbing) and significantly enhanced the removal of heavy metals. Moreover, the increment of the removal efficiency in the US/Mixing process compared to that in the Mixing process for each particle size condition increased as the particle size decreased. It seemed that more micro-scale contact between the washing liquid and the soil particles and more active cavitation events occurred when ultrasound was applied for smaller particles. In the US processes, gentle movement of the particles in the liquid was observed for the smaller particles of 0.30 ~ 2.00 mm and ~ 0.30 mm while no movement was detected for the larger particles of 2.00 ~ 4.75 mm.
Considering the removal efficiency of each size-range soil sample, the removal efficiency of the original field sample (~4.75 mm) was not well understood. For Cu, the highest removal efficiency for both processes was obtained for the original sample. However, relatively low removal efficiency was attained in the washing process of the original sample for Zn. This might be due to more complex characteristics of the interactions between particles in slurry systems of various sized particles when mechanical mixing and ultrasound was applied. The difference in the five-step fractionation composition of heavy metal species in the different-sized contaminated soil particles might be another reason [34].
3.3. Ultrasonic desorption mechanism
In the previous chapters, we found that the only application of ultrasound resulted in no considerable removal of metals from the soils and higher effectiveness of ultrasound was observed in the US/Mixing processes for the smaller particles. In order to understand the role of ultrasound in the washing processes, ultrasonic desorption tests were conducted using the red paint coated glass beads in the US processes (No mechanical mixing was applied.). Three kinds of beads (diameter: 1, 2, and 4 mm) were applied for two cases where the beads were attached to the vessel bottom (attached beads) and not attached to it (free beads). The water : beads ratio was 3:1, 5:1, and 10:1 (The amount of the beads was 135 g.).
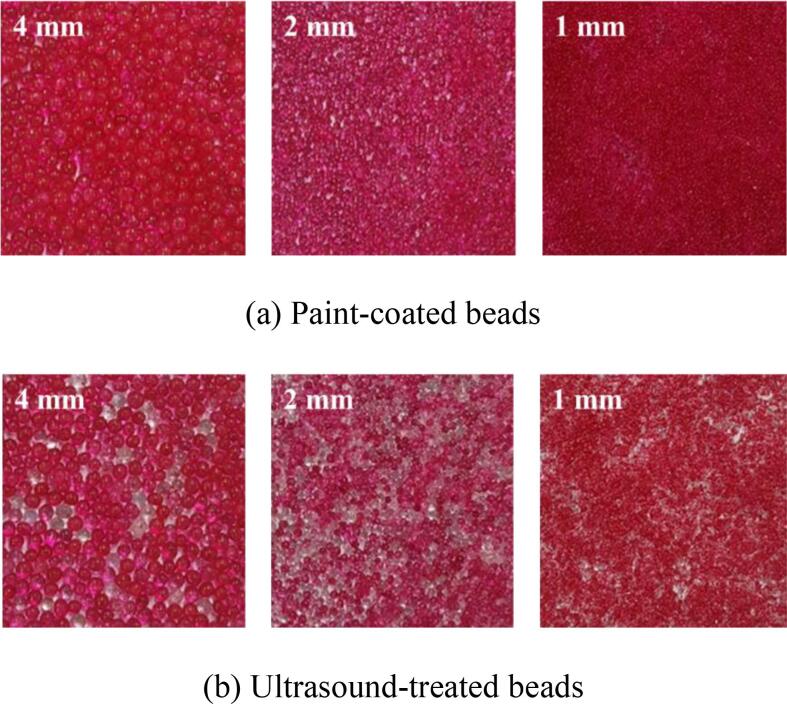
As shown in Fig. 4, much higher removal of the paint was observed for the smaller beads in the free beads condition and no significant removal was obtained in the attached beads condition. The difference in the removal of the paint from the beads could be understood by looking at the movement of beads under ultrasound irradiation. For the free condition, the 4 mm beads vibrated and rotated gently with no meaningful deviation from the original position in the vessel. Gentle scrubbing occurred between the beads and a small amount of the paint was released from the beads. On the other hand, the 1 and 2 mm beads were floated and more violent collision and scrubbing between beads were induced by the ultrasound force. As a result of this, a large amount of the paint was released for the small beads. For the attached condition, no movement of the beads was detected and no removal was induced. Interestingly, the removal of the paint occurred randomly. As shown in Fig. 5, complete removal was observed in some beads while other painted beads were flawless for all cases of the free beads condition.
Fig. 4.
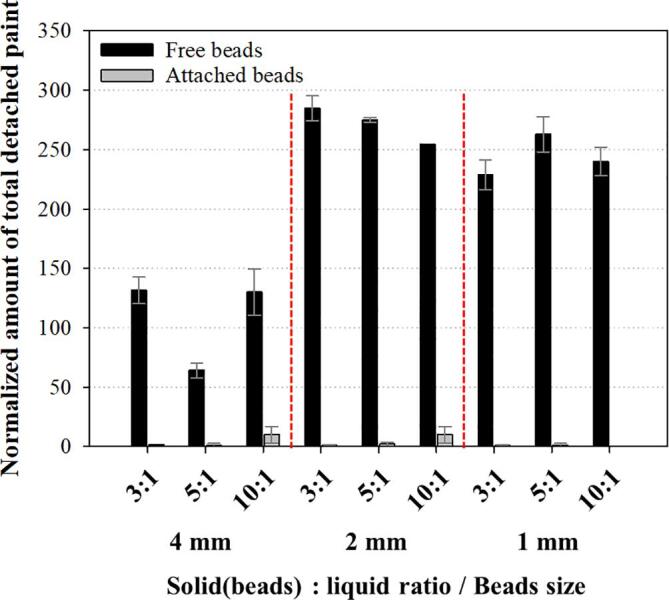
Ultrasonic paint removal from the glass beads for under various conditions (Free beads: the glass beads were not attached to the vessel bottom; Attached beads: the beads were attached to it).
Fig. 5.
The paint-coated beads and ultrasound-treated beads for various bead sizes.
From the results shown in Fig. 4, Fig. 5, we suggest the ultrasonic desorption mechanism in the beads tests as follows: the cavitational activity including microjet and shock wave for the desorption was not considerable when the particles were fixed; the desorption was mainly induced by the ultrasound-force-induced violent physical movement including floating, collision and attrition scrubbing of the beads; the ultrasonic desorption occurred in the limited active zone and no meaningful effect was activated outside the active zone; the additional mechanical mixing could enhance the physical movement of the beads significantly and the very high removal/desorption could be achieved. It should be noted that noticeable cavitational activity including sonophysical effects (Aluminum foil tests: Fig. S2) and sonochemical effects (Sonochemiluminescence (SCL) tests: Fig. S3) was observed in the presence of the beads. However, it seemed that the cavitational activity was not large enough to induce observable removal of the paint from the beads in this study.
4. Conclusion
For the remediation of heavy metals contaminated soils, the ultrasonic and mechanical mixing soil washing processes was investigated under various operational conditions including HCl concentration (0.1, 0.5, and 1.0 M), EDTA concentration (0.01, 0.05, 0.10 M), liquid:soil ratio (3:1, 5:1, and 10:1), and soil particles size (~4.75 mm, 2.00 ~ 4.75 mm, 0.30 ~ 2.00 mm, and ~ 0.30 mm). It was found that the hybridization of ultrasound technology and the conventional mechanical mixing resulted in significant enhancement of the metals removal and the effectiveness of ultrasound was markedly improved in the less extreme washing conditions (lower HCl or EDTA concentration, lower liquid:soil ratio, and smaller soil particle size). The glass beads test was conducted to understand the ultrasonic desorption mechanism using the paint-coated beads with various sizes (1, 2, and 4 mm). It was revealed that the particle movement and particle-to-particle collision and scrubbing were essential for the satisfactory desorption and removal induced by ultrasound.
CRediT authorship contribution statement
Jongbok Choi: Validation, Writing - original draft. Dukyoung Lee: Methodology, Investigation. Younggyu Son: Conceptualization, Methodology, Writing - review & editing, Visualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Research Foundation of Korea [NRF-2021R1A2C1005470] and by the Korea Ministry of Environment (MOE) as “SEM (Subsurface Environment Management)” Program [project No. 2021002470001].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105574.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mahamuni N.N., Adewuyi Y.G. Advanced oxidation processes (AOPs) involving ultrasound for waste water treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010;17(6):990–1003. doi: 10.1016/j.ultsonch.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Son Y., Lim M., Khim J., Kim L.H., Ashokkumar M. Comparison of calorimetric energy and cavitation energy for the removal of bisphenol-A: The effects of frequency and liquid height. Chem. Eng. J. 2012;183:39–45. [Google Scholar]
- 3.Neppolian B., Doronila A., Grieser F., Ashokkumar M. Simple and Efficient Sonochemical Method for the Oxidation of Arsenic(III) to Arsenic(V) Environ. Sci. Technol. 2009;43(17):6793–6798. doi: 10.1021/es900878g. [DOI] [PubMed] [Google Scholar]
- 4.Torres R.A., Pétrier C., Combet E., Carrier M., Pulgarin C. Ultrasonic cavitation applied to the treatment of bisphenol A. Effect of sonochemical parameters and analysis of BPA by-products. Ultrason. Sonochem. 2008;15(4):605–611. doi: 10.1016/j.ultsonch.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Pétrier C., Combet E., Mason T. Oxygen-induced concurrent ultrasonic degradation of volatile and non-volatile aromatic compounds. Ultrason. Sonochem. 2007;14(2):117–121. doi: 10.1016/j.ultsonch.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y., Petrier C., Waite T.D. Sonolysis of 4-chlorophenol in aqueous solution: Effects of substrate concentration, aqueous temperature and ultrasonic frequency. Ultrason. Sonochem. 2006;13:415–422. doi: 10.1016/j.ultsonch.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Daisuke K., Chiemi H., Hideyuki M., Tomoki T., Yuichiro S., Chiaki K., Katsuto O., Atsushi S. Effects of ultrasonic frequency and initial concentration on degradation of methylene blue. Jpn. J. Appl. Phys. 2014;53:07KE03. [Google Scholar]
- 8.Kobayashi D., Sano K., Takeuchi Y., Terasaka K. Effect of irradiation distance on degradation of phenol using indirect ultrasonic irradiation method. Ultrason. Sonochem. 2011;18(5):1205–1210. doi: 10.1016/j.ultsonch.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Gogate P.R., Sivakumar M., Pandit A.B. Destruction of Rhodamine B using novel sonochemical reactor with capacity of 7.5 l. Sep. Purif. Technol. 2004;34(1-3):13–24. [Google Scholar]
- 10.Park B., Cho E., Son Y., Khim J. Distribution of electrical energy consumption for the efficient degradation control of THMs mixture in sonophotolytic process. Ultrason. Sonochem. 2014;21(6):1982–1987. doi: 10.1016/j.ultsonch.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 11.Son Y., Lim M., Khim J., Ashokkumar M. Attenuation of UV Light in Large-Scale Sonophotocatalytic Reactors: The Effects of Ultrasound Irradiation and TiO2 Concentration. Ind. Eng. Chem. Res. 2012;51:232–239. [Google Scholar]
- 12.Joseph C.G., Li Puma G., Bono A., Krishnaiah D. Sonophotocatalysis in advanced oxidation process: A short review. Ultrason. Sonochem. 2009;16(5):583–589. doi: 10.1016/j.ultsonch.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z., Abramova A., Nikonov R., Cravotto G. Sonozonation (sonication/ozonation) for the degradation of organic contaminants – A review. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105195. [DOI] [PubMed] [Google Scholar]
- 14.Alfonso-Muniozguren P., Hazzwan Bohari M., Sicilia A., Avignone-Rossa C., Bussemaker M., Saroj D., Lee J. Tertiary treatment of real abattoir wastewater using combined acoustic cavitation and ozonation. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104986. [DOI] [PubMed] [Google Scholar]
- 15.Neppolian B., Ciceri L., Bianchi C.L., Grieser F., Ashokkumar M. Sonophotocatalytic degradation of 4-chlorophenol using Bi2O3/TiZrO4 as a visible light responsive photocatalyst. Ultrason. Sonochem. 2011;18(1):135–139. doi: 10.1016/j.ultsonch.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Harada H. Sonophotocatalytic decomposition of water using TiO2 photocatalyst. Ultrason. Sonochem. 2001;8(1):55–58. doi: 10.1016/s1350-4177(99)00050-4. [DOI] [PubMed] [Google Scholar]
- 17.Torres R.A., Nieto J.I., Combet E., Pétrier C., Pulgarin C. Influence of TiO2 concentration on the synergistic effect between photocatalysis and high-frequency ultrasound for organic pollutant mineralization in water. Appl. Catal. B-Environ. 2008;80(1-2):168–175. [Google Scholar]
- 18.Ferkous H., Merouani S., Hamdaoui O., Pétrier C. Persulfate-enhanced sonochemical degradation of naphthol blue black in water: Evidence of sulfate radical formation. Ultrason. Sonochem. 2017;34:580–587. doi: 10.1016/j.ultsonch.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Darsinou B., Frontistis Z., Antonopoulou M., Konstantinou I., Mantzavinos D. Sono-activated persulfate oxidation of bisphenol A: Kinetics, pathways and the controversial role of temperature. Chem. Eng. J. 2015;280:623–633. [Google Scholar]
- 20.B. Park, Y. Son, Ultrasonic and mechanical soil washing processes for the removal of heavy metals from soils, Ultrason. Sonochem. 35, Part B (2017) 640-645. [DOI] [PubMed]
- 21.Hwang S., Park J., Namkoong W. Ultrasonic-Assisted Extraction to Release Heavy Metals from Contaminated Soil. J. Ind. Eng. Chem. 2007;13:650–656. [Google Scholar]
- 22.Feng D., Aldrich C. Sonochemical treatment of simulated soil contaminated with diesel. Adv. Environ. Res. 2000;4(2):103–112. [Google Scholar]
- 23.Shrestha R.A., Pham T.D., Sillanpää M. Effect of ultrasound on removal of persistent organic pollutants (POPs) from different types of soils. J. Hazard. Mater. 2009;170(2-3):871–875. doi: 10.1016/j.jhazmat.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 24.Son Y., Nam S., Ashokkumar M., Khim J. Comparison of energy consumptions between ultrasonic, mechanical, and combined soil washing processes. Ultrason. Sonochem. 2012;19(3):395–398. doi: 10.1016/j.ultsonch.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Son Y., Cha J., Lim M., Ashokkumar M., Khim J. Comparison of Ultrasonic and Conventional Mechanical Soil-Washing Processes for Diesel-Contaminated Sand. Ind. Eng. Chem. Res. 2011;50(4):2400–2407. [Google Scholar]
- 26.Feng D., Lorenzen L., Aldrich C., Maré P.W. Ex situ diesel contaminated soil washing with mechanical methods. Miner. Eng. 2001;14(9):1093–1100. [Google Scholar]
- 27.Son Y., Lee D., Lee W., Park J., Hyoung Lee W., Ashokkumar M. Cavitational activity in heterogeneous systems containing fine particles. Ultrason. Sonochem. 2019;58 doi: 10.1016/j.ultsonch.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Dermont G., Bergeron M., Mercier G., Richer-Laflèche M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008;152:1–31. doi: 10.1016/j.jhazmat.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Choi J., Lee H., Son Y. Effects of gas sparging and mechanical mixing on sonochemical oxidation activity. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Son Y., No Y., Kim J. Geometric and operational optimization of 20-kHz probe-type sonoreactor for enhancing sonochemical activity. Ultrason. Sonochem. 2020;65 doi: 10.1016/j.ultsonch.2020.105065. [DOI] [PubMed] [Google Scholar]
- 31.Woche S.K., Goebel M.O., Mikutta R., Schurig C., Kaestner M., Guggenberger G., Bachmann J. Soil wettability can be explained by the chemical composition of particle interfaces - An XPS study. Sci. Rep. 2017;7:42877. doi: 10.1038/srep42877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leighton T.G. Academic Press; London: 1994. The Acoustic Bubble. [Google Scholar]
- 33.Young F.R. Imperial College Press; London: 1999. Cavitation. [Google Scholar]
- 34.Bacon J.R., Davidson C.M. Is there a future for sequential chemical extraction? Anlst. 2008;133:25–46. doi: 10.1039/b711896a. [DOI] [PubMed] [Google Scholar]
- 35.Tessier A., Campbell P.G.C., Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979;51(7):844–851. [Google Scholar]
- 36.Zou Z., Qiu R., Zhang W., Dong H., Zhao Z., Zhang T., Wei X., Cai X. The study of operating variables in soil washing with EDTA. Environ. Pollut. 2009;157(1):229–236. doi: 10.1016/j.envpol.2008.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.