Highlights
-
•
The use of HP-UAE as a strategy to intensify extraction is still incipient.
-
•
The latest trends and state of the art of HP-UAE extraction processes are detailed.
-
•
The influence of physical and medium parameters on the HP-UAE processes was discussed.
-
•
The main applications of HP-UAE, mostly in the last ten years, were presented.
-
•
The scale of application and the main mathematical models were discussed.
Keywords: Ultrasound, Ultrasound-assisted extraction, Supercritical fluid extraction, Pressurized liquid extraction, Natural products, Bioactive compounds
Abstract
Natural products are a source of a wide range of chemical compounds, from pigments to bioactive compounds, which can be extracted and used in different applications. Due to consumer awareness, the interest in natural compounds significantly increased in the last decades, prompting the search for more efficient and environmentally friendly extraction techniques and methods. Pressurized liquids and fluids (sub and supercritical) are being explored to extract natural compounds within the green process concept. The combination of these techniques with ultrasound has emerged as an alternative to intensify the extraction process efficiently. In this context, this work presents a comprehensive review and current insights into the use of high-pressure systems, specifically supercritical fluid extraction and pressurized liquid extraction assisted by ultrasound, as emerging technologies for extracting bioactive compounds from natural products. The extraction mechanisms, applications, and the influence of operational parameters in the process are addressed, in addition to an analysis of the main challenges to be overcome for widespread application.
1. Introduction
Natural products have become an attractive market for food, pharmaceutical, and cosmetic industries due to consumer awareness and changing living habits associated with increased knowledge about the negative impact of artificial substances on health. Furthermore, the diversity of chemical compounds in nature with a broad range of known and unknown biological and technological properties have an enormous potential to be explored. The global market for natural ingredients is projected to grow annually at a substantial rate of 7.1% from 2019 to 2024, reaching a market value of USD 43 billion by the end of 2024 [1].
However, to fully explore their potential in different applications, it is necessary to extract these compounds from the raw material matrix. Conventional extraction methods, such as solvent extraction, maceration, cold pressing, and hydro-distillation, are commonly used to isolate several chemical compounds from natural matrices. Due to the increasing energy costs, the drive to reduce environmental impacts and greenhouse gas emissions, actors are being challenged to develop innovative technologies to achieve legal requirements (emissions, product safety, and control) and optimization of the industrial process (cost reduction, increase in quality and functionality, etc.) [2]. These extraction processes have been classified as novel, emerging, innovative, or non-conventional technologies and include a wide range of techniques [3], [4].
Ultrasound-assisted extraction, supercritical fluid extraction, microwave-assisted extraction, high-pressure processing, pulsed electric fields, high voltage electrical discharges are some of these techniques that have aroused interest in recent years. The main advantages of emerging techniques over conventional extraction methods are that, in general, they meet the requirements of the green process concept [5]. This concept aims to avoid/minimize the use of toxic organic solvents, reduce extraction time, process temperature, and energy consumption, intensify the mass transfer and extraction yields, and preserve phytocomplex integrity, especially in the presence of thermo-sensitive components [3], [6]. Besides, in many cases, these technologies are an energy-efficient alternative since they enable maximal yields in reduced extraction time [7].
High-pressure extraction techniques, including sub and supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), and gas-expanded liquids extraction (GXL), among others, are increasingly being used for the extraction of natural compounds from complex matrices [8]. Besides of being highly efficient and selective, these techniques are also scalable and can be coupled to other processes within an intensification or biorefinery strategy [9].
A very efficient, and still little explored, way of intensifying high-pressure extraction processes is propagating ultrasound waves in the extraction medium. Because of the numerous advantages that ultrasound-assisted extraction offers, innovative techniques such as ultrasound-assisted Soxhlet extraction, ultrasound-assisted Clevenger distillation, continuous ultrasound-assisted extraction, and a combination of ultrasound with other processes such as microwave, extrusion, and supercritical fluid extraction have been proposed in the past two decades [2].
A quick search on the Web of Science database reveals that publications regarding extraction assisted by ultrasound (UAE) increased exponentially in the last decade, as shown in Fig. 1. Ultrasound has also been used to intensify high-pressure extraction techniques, although on a much smaller scale for obvious reasons. In this article, the acronym HP-UAE is used to refer to high-pressure techniques intensified using ultrasound in a general way. There are successful reports for the extraction of a series of bioactive compounds, including caffeine [10], capsaicinoids [11], [12], apigenin [13], phenolics [14], [15], piceatannol [16], among others. The use of HP-UAE is relatively recent but has shown great potential to enhance natural product extraction with applications in many different fields.
Fig. 1.
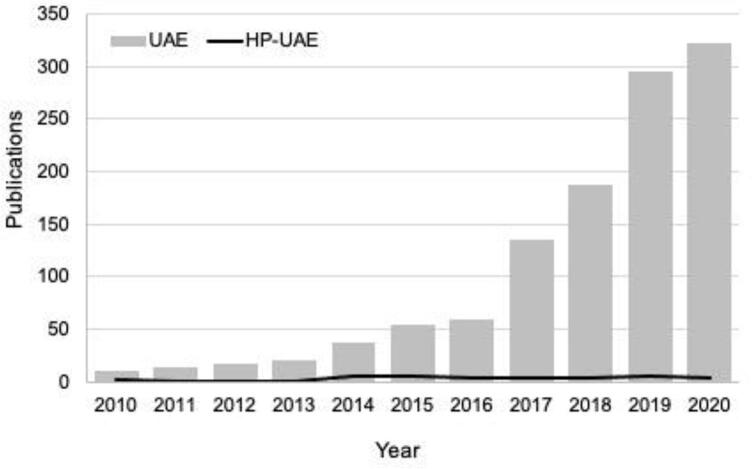
The number of studies published about ultrasound-assisted extraction (UAE) and high-pressure ultrasound-assisted extraction (HP-UAE) from 2010 to 2020 (Web of Science database, November 2020). The search terms used for UAE publications were ultrasound, bioactive, and extraction.
Thus, this study tries to provide a comprehensive review and current insights into the use of high-pressure systems, namely SFE and PLE assisted by ultrasound, as emerging technologies for extracting bioactive compounds from plant and animal matrices. The coupling of high-pressure extraction techniques with the ultrasound system can be performed in different ways [17]. Still, in this article, it is important to note that the focus will be given exclusively to ultrasound-assisted processes made in situ, which means ultrasound and SFE/PLE are used simultaneously in the same reactor. For these cases, the term UASFE has been suggested for ultrasound-assisted supercritical extraction and UAPLE for ultrasound-assisted pressurized liquid extraction. Thus, the other coupling strategies, such as the in-line combination, for instance, US and SFE/PLE application afterward or vice versa, will not be addressed in this review. The extraction mechanisms, applications (mainly in the last decade), and the main operational parameters' influence are addressed in this review. Additionally, we also provide a critical analysis of the challenges to overcome the problems towards its industrial-scale application of HP-UAE.
2. Extraction mechanisms assisted by ultrasound
2.1. Ultrasound-assisted extraction coupled with high-pressure systems (HP-UAE)
Ultrasound are sound waves with a frequency higher than the audible range for humans (16–18 kHz). The higher frequency limit is 5 MHz for propagation in gas and 500 MHz for liquid and solids [18]. The fundamental mechanism of ultrasound is based on the transformation of electric to mechanical energy through transducers, promoting a mechanical vibration in high frequency (>20 kHz). The application of ultrasound in liquids may cause acoustic streaming and, if the liquid medium is composed of gas nuclei, they can be submitted to compression/rarefaction cycles and collapse. The microbubbles' collapse is defined as “acoustic cavitation” and can take place either symmetrically or asymmetrically. When the collapse is symmetric, shock waves promote agitation and energy transfer to the medium, facilitating the disruption of the intermolecular interactions of the target compounds with the matrix (e.g., carbohydrates, lipids, proteins). In contrast, the asymmetrical collapse may create microjets, capable of damaging or rupturing cell walls [19]. Additionally, cavitation can be “stable” or “transient”. For stable cavitation, the bubbles are formed at higher ultrasound frequencies (>hundreds kHz) without creating a substantial collapse; meanwhile, the transient cavitation takes place at lower frequencies (<hundreds kHz), promoting a violent bubble collapse [20].
Cavitational effects induced by ultrasound are the main causes for the increment of heat and mass transfer in liquids; meanwhile, vibration and acoustic streaming are the main causes for that increment in a gaseous environment [21], [22]. The power of ultrasound waves may promote the dissipation of the mechanical energy causing a “heating effect” of the ultrasonic system, consequently increasing the diffusivity effects in solid medium. Another effect caused by ultrasound waves is the “sponge effect”, which may appear due to the alternative compressions/expansions of the tissue matrix. In this case, the liquid located at the inner part of the particle is released to the solid surface generating a surface tension strong enough to keep the water molecules inside the material's capillarities, creating microscopic channels that facilitate the mass transfer [23].
An additional consequence of the acoustic cavitation is related to the cell and tissue disruption, which may enhance the mass transfer phenomena due to the release of the particles from the vegetable matrices' cell wall, increasing the solute–solvent contact. Moreover, the formation of cavities and microchannels have also been attributed as the main reasons for the rise of the mass transfer phenomena in food processing assisted by ultrasound [24], [25], [26].
Furthermore, the use of power ultrasound represents an effective manner of enhancing mass transfer processes due to the production of small-scale agitation in SFE processes. High-intensity ultrasound is probably the only manner to generate agitation in SFE because of the restriction in using mechanical stirrers in such a system [27].
When passing through the medium, mechanical energy can be dissipated through absorption, scattering, and reflection. The absorption of ultrasonic waves by the material converts mechanical energy into heat by the particles' friction. The ultrasonic energy may decrease due to the particles' oscillation (scattering); On the other hand, the dissipation of energy may also occur because of the interference between incident and reflected waves (reflection) [20].
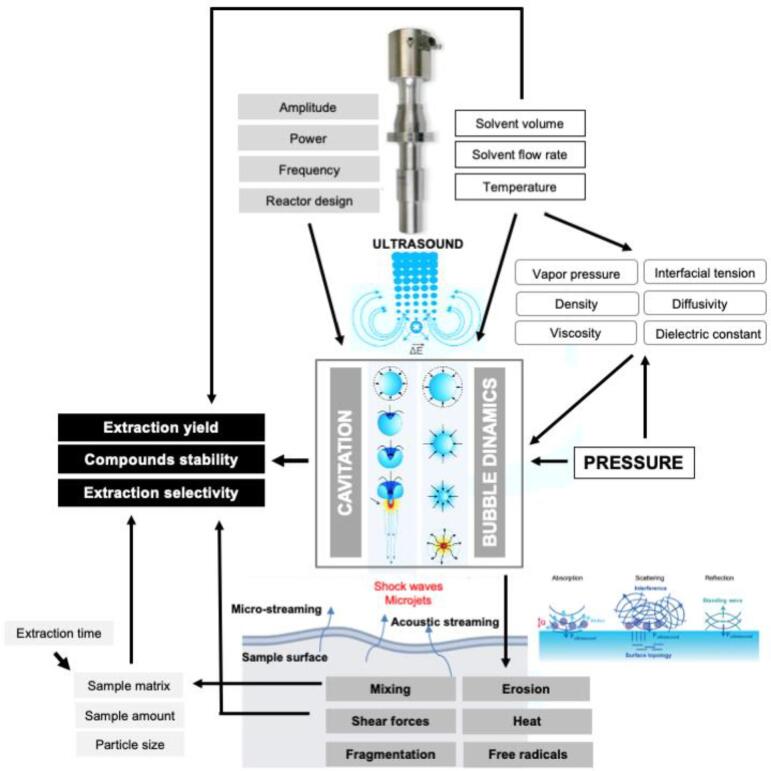
The physical effects on the matrix caused by the application of ultrasound in the extraction medium will depend on several factors. Some studies proposed evaluating the cavitational effects on the vegetable matrices using field emission scanning microscopy (FESEM). Illustratively, cell wall disruption was observed by Balachandran et al. [28] when using FESEM to evaluate the vegetable matrix after supercritical fluid extraction assisted by ultrasound (UASFE) of gingerols from ginger, as shown in Fig. 2.
Fig. 2.
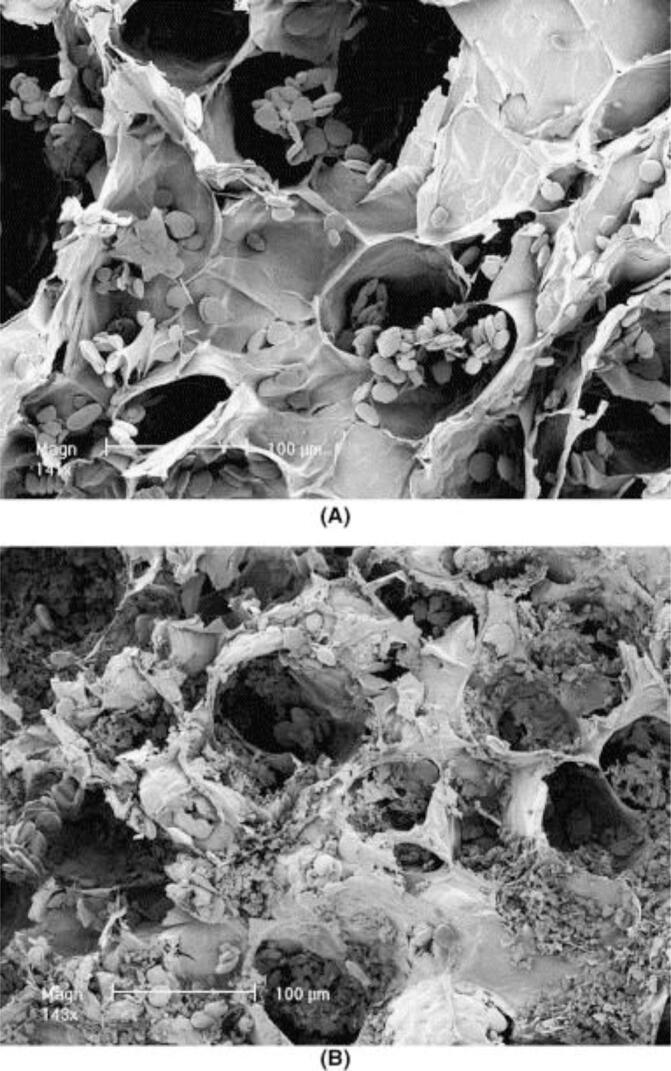
FESEM pictures of ginger particles. (A) Experiments without the influence of ultrasound, (B) experiments with ultrasound [28]. Reproduced with permission from Elsevier.
In this case, the formation of air pockets trapped inside the pores of the ginger particles could expand, and therefore damaging the cell walls. Also, Barrales et al. [29] observed through FESEM that the mechanical vibration caused by the ultrasound was capable of releasing a significant amount of material from inside the seed particles of Passiflora edulis sp. to the vegetable surface. However, in some reports, the use of ultrasound did not produce any surface crack or damage on the matrix, despite the higher extraction yields [11], [30].
The use of ultrasound for longer extraction times can naturally increase the internal temperature of the extraction vessel. Consequently, the solutes’ solubility may increase, reducing the viscosity and increasing the solvent's diffusivity. Nevertheless, the effects of the main operational parameters (e.g., pressure, temperature, particle size, ultrasound power, time, among others) need to be carefully studied when a high-pressure-ultrasound system is being used. Moreover, the complexity of the bioactive compounds present in natural products must be considered when evaluating a coupled process. Fig. 3 presents a schematic diagram of the interaction between ultrasound with the main physical and medium parameters and the cavitational effects on the sample matrix's surface. In the following section, the techniques UASFE and UAPLE for the recovery of bioactive from plant matrices will be presented and the effects of the main physical and environmental parameters will be discussed.
Fig. 3.
Schematic diagram of physical and medium parameters interaction during the high-pressure extraction process assisted by ultrasound. Adapted from Radziuk and Möhwald [20], Seah and Teo [31], and Kim et al. [32].* *Reproduced with permission from Royal Society of Chemistry. Chan Kim Y, Min H, Yu J, et al. Forced infiltration of silica beads into densely-packed glass fiber beds for thin composite laminates. RSC Adv. 2016; 6(94):91341–91348.17.
2.2. Extraction with supercritical fluids and pressurized liquids assisted by ultrasound
Supercritical fluid extraction is emerging as one of the most exciting alternatives for extracting natural products due to its efficiency and green appeal. A supercritical fluid is any substance maintained above its critical pressure and temperature, where it has a mix of properties between liquid and gas. Carbon dioxide is the most used supercritical fluid due to its advantageous properties (low critical points, toxicity, cost, among others.). By adjusting temperature and pressure, it is possible to manipulate the fluid's density and viscosity, determining the solvent power of supercritical CO2. SFE using CO2 is relatively widespread, but UASFE has been increasingly used to extract natural products.
However, SFE alone is a complex and challenging technique. When the US is combined with a supercritical fluid, it further increases the extraction process's complexity since several interactions exist between operational variables [17], [33]. The study of the main parameters influencing the UASFE processes is essential to maximizing the extraction at reduced solvent consumption and energy costs. There are several applications of UASFE for the extraction of bioactive compounds in the literature. A compilation of the available reports and conditions used is presented in Table 1.
Table 1.
Reports regarding the application of supercritical fluids for extraction assisted by ultrasound (UASFE) of bioactive compounds.
| Scale | Matrix | Extract | Device | Condition | Ref. |
|---|---|---|---|---|---|
| Pilot plant | Almonds (Prunus amygdalus) | Oil fraction | US probe | US power: 100 W Frequency: 20 kHz Solvent: CO2 Sample mass: 1500 g CO2 flow rate: 20 kg/h Pressure: 28 MPa Temperature: 55 °C Time: 510 min |
[22] |
| Bench system | Ginger (Zingiber officinale) | Gingerols | US water bath | US power: 300 W Frequency: 20 kHz Solvent: CO2 Pressure: 16 MPa Temperature: 40 °C Time: ~ 380 min |
[18] |
| Pilot plant | Adlay seed (Coix lachrymal-jobi L. var. Adlay) | Oil and coixenolide | US probe | US power: 0, 50, 91, 97 W and 110 W Frequency: 20 kHz Solvent: CO2 Sample mass: 100 g CO2 flow rate: 1.5–4 L/h Pressure: 10–30 MPa Temperature: 30–55 °C Time: up to 4.5 h |
[23] |
| Pilot plant | Marigold (Tagetes erecta L.) | Lutein esters | US probe | US power: 100 to 400 W Frequency: 25 to 33 kHz US irradiation time: 3 to 9 s (9 s interval) Solvent: CO2 Sample mass:100 g CO2 flow rate: 5–10 kg/h Particle size: 0.198–0.245 to 0.350–0.833 mm Pressure: 17.5–32.5 MPa Temperature: 35–55 °C |
[24] |
| Pilot plant | Green tea | Caffeine | US probe | US power: 0–150 W Frequency: 20 kHz Solvent: CO2 Sample mass: 40 g CO2 flow rate: 5 L/h Moisture: 0–40% Pressure: 15–32 MPa Temperature: 45–65 °C Time: 30–600 min |
[7] |
| Pilot plant | Almonds and cocoa cake | Oil fraction | US probe | US power: 85 WFrequency: 19 kHzSolvent: CO2CO2 flow rate: 10–15 kg/h (almond) Sample mass: 1500 g Pressure: 20–32 MPa (almond) Temperature: 45–60 °C (almond) Time: 3.5–4 h |
[25] |
| Bench system | Scutellaria barbata D. Don | Oleanolic acid (OA) and ursolic acid (UA) | US water bath | Two-steps extraction 1 step (15 min fixed): US-assisted static extraction Frequency: 40 kHz US power: 185 W 2 step: sc-CO2 extraction Solvent: CO2 + modifier (ethanol and water) Water content in ethanol modifier: 60–100% % modifier: 0–16.7% Particle size: ≤0.21 to 0.925 mm CO2 flow rate: 0.7 to 2.5 mL/min Sample mass: 5 g Temperature: 35–75 °C Time: 10–100 min |
][26] |
| Pilot plant | Raw cocoa beans | Cocoa butter | US probe | US power: 58 W Solvent: CO2 CO2 flow rate: 0.39 L/h Sample mass: 30 g Frequency: 30 kHz Pressure: 40 and 55 MPa Temperature: 40 °C Time: 30–360 min |
[27] |
| Pilot plant | Blackberry (Rubus sp.) industrial residues | Anthocyanins, and phenolic compounds | US probe | US power: 0, 200 and 400 W Solvent: CO2 and CO2 + modifier (ethanol and water) Sample mass: 5 g Pressure: 15, 20 and 25 MPa Temperature: 40, 50 and 60 °C Time: 120 min (SFE without modifier) and 54–57 min (SFE with modifier) S/F: 400 kg solvent/kg feed |
[17] |
| Bench system | Syzygium aromaticum flower bud (clove) | Eugenol, beta-caryophyllene, eugenyl acetate and alfa-humulene | US water bath | Two-steps extraction 1 step (35 min fixed): US-assisted static extraction Frequency: 40 kHz US power: 185 W 2 step: sc-CO2 extraction Solvent: CO2 Particle size: ≤0.21 to 0.925 mm CO2 flow rate: 0.4 to 1.8 g/min Sample mass: 5 g emperature: 30, 40 and 50 °C Pressure: 10, 15 and 20 MPa Time: 5–30 min |
[28] |
| Bench system | Hedyotis corymbosa | Oleanolic acid (OA) and ursolic acid (UA) | US water bath | Two-steps extraction 1 step (15 min fixed): US-assisted static extraction Frequency: 40 kHz US power: 185 W 2 step: sc-CO2 extraction Solvent: CO2 + ethanol/water (80/20 v/v) % modifier:12.5 (v/v)CO2 flow rate: 2.2 mL/min Sample mass: 5 g Pressure: 11–32 MPa Temperature: 33–68 °CTime: up to 155 min |
[10] |
| Bench system | Hedyotis diffusa and Hedyotis corymbosa | Oleanolic acid (OA) and ursolic acid (UA) | US water bath | Two-steps extraction 1 step (15 min fixed): US-assisted static extraction Frequency: 40 kHz US power: 185 W 2 step: sc-CO2 extraction Solvent: CO2 + ethanol/water (70–100%) % modifier: 0–15% (v/v) CO2 flow rate: 0.6–2.5 mL/min Sample mass: 5 g Pressure: 10.4–30 MPa Temperature: 30–70 °C Time: 10–145 min |
[29] |
| Pilot plant | Capsicum frutescens L. | Capsaicinoids | US probe | US power: 200–360 W Frequency: 20 kHz Solvent: CO2 S/F: 600 kg CO2/kg feed Pressure: 15 MPa Temperature: 40 °C Time: 60–240 min S/F: 600 ± 2 kg CO2/kg feed |
[9] |
| Pilot plant | Passiflora edulis sp by-products | Fatty acids, tocopherol and tocotrienol | US probe | US power: 0–640 W Frequency: 20 kHz Solvent: CO2Sample mass: 5 g S/F: 210 CO2/kg feed Pressure: 16 and 26 MPa Temperature: 40 °C Time: 20 min (static) and 100 min (dynamic) |
[19] |
| Bench system | Hedyotis corymbosa | Oleanolic acid (OA) and ursolic acid (UA) | US water bath | Two-steps extraction 1 step: Static extraction Time: 15 min (US assisted) and 30 min (without US) 2 step: Dynamic extraction Frequency: 40 kHz US power: 185 W Solvent: CO2 + ethanol/water (70–90%) O2 flow rate: 0.162 L/h Sample mass: 5 g % modifier: 0–15 (v/v) Pressure: 10–31.5 MPa Temperature: 32–64 °C Time: 10 to 150 min |
[30] |
| Pilot plant | Dedo de moça pepper (Capsicum baccatum L. var. pendulum) | Phenolics, antioxidants and capsaicinoids | US probe | US power: 200–600 W Frequency: 20 kHz Solvent: CO2 Sample mass: 5 g Pressure: 15–25 MPa Temperature: 40 °C Extraction time: 120 min US Time: 40–80 min CO2 flow rate: 1.77 × 10-4 kg/s S/F: 484 ± 20 kg CO2/kg feed |
[31] |
| Semi-batch | Clove buds (Syzygium aromaticum (L.) Merr. & Perry) | Clove oil and α-humulene | US water bath | US power: 185 W Frequency: 40 kHz Solvent: CO2 Sample mass: 20 g Pressure: 9–25 MPa Temperature: 32–50 °C US Time: 115 min (75% duty cycle) CO2 flow rate: 0.084 kg/h |
[32] |
| Pilot plant | Cumbaru oil (Dipteryx alata Vogel) | Fatty acids methyl esters (FAME) | US probe | US power: 360 W Frequency: 20 kHz Solvent: CO2 Sample mass: 3 g Pressure: 15–35 Pa Temperature: 40–60 °C US Time: 360 min CO2 flow rate: 0.6876 kg/h S/F: 456 ± 11 kg CO2/kg feed |
[33] |
| Pilot plant | Curcumin from turmeric (Curcuma longa L.) | Curcumin | US water bath | US power: 600 W Frequency: 45 kHz Solvents: CO2 and ethanol (10–20% v/v)Sample mass: 1 g Pressure: 15–25 MPa Temperature: 40–60 °C US Time: 30–120 minCO2 flow rate: 1.2 – 2.4 L/h |
[34] |
| Pilot plant | Hedyotis corymbosa | Oleanolic acid (OA) and ursolic acid (UA) | US water bath | US power: 185 W Frequency: 40 kHz Solvents: CO2 and ethanol% (modifier 11.0% (v/v) (UASFE) and 14.5% (v/v) (SFE) ethanol–water mixture (80%, v/v)) Sample mass: 5 g Pressure: 10–31.5 MPa Temperature: 32–53 °C Static time: 15 min (UASFE) and 30 min (SFE) Dynamic time: 80 min (UASFE) and 150 min (SFE) CO2 + aqueous ethanol flow rate: 2.1 mL/min (UASFE) and 2.7 mL/min (SFE) |
[35] |
| Pilot plant | Oleaginous yeast (Candida sp. LEB-M3) | Fatty acids methyl esters (FAME) | US probe | US power: 800 W Frequency: 20 kHz Solvent: CO2 Sample mass: 3 g Pressure: 15–25 MPa Temperature: 40–50 °C Extraction time: 180 min US Time: 60 min CO2 flow rate: 2.51 × 10−4 kg/s S/F: 820–904 kg/kg |
[36] |
| Pilot plant | Oregano | Phenolics and antioxidants | Transducer 1: short rod-shaped head mass; Transducer 2: long rod-shaped head mass; Transducer 3: stepped circular head mass; Transducer 4: multiplate circular head mass | US power: 60 W Frequency: 30 kHz Solvents: CO2 and ethanol Sample mass: 5.5 ± 0.5 g Pressure: 35 MPa Temperature: 35 °C Extraction time: 15–60 min US Time: 15–60 min CO2 flow rate: 1.0 ± 0.1 kg/h Ethanol flow rate: 0.5 mL/min |
[37] |
| Pilot plant | Rice bran oil (Oryza sativa L.) | Antioxidants | US probe | US power: 160–320 W Frequency: 20 kHz Solvent: CO2 Sample mass: 20 g Pressure: 25 MPa Temperature: 40 °C Extraction time: 120 min US Time: 40–120 min CO2 flow rate: 14.82 g CO2/min |
[38] |
| Batch system | Scutellaria barbata D. Don | Apigenin | US water bath | US power: 185 W Frequency: 40 kHz Solvents: CO2 and ethanol Sample mass: 20 gPressure: 12.5–30 MPa Temperature: 32–64 °C Extraction time: 15 min (static) + 5–280 min (dynamic) US Time: 15 min CO2 flow rate: 0.17–0.83 g/min Cosolvent: 11.6% (v/v) aqueous ethanol (80%, v/v) 75% ultrasonic duty cycle |
[39] |
| Pilot plant | Agave (A. salmiana) bagasse | Antioxidants and saponins | Cylindrical head-mass transducer (TA) and multiplate head-mass transducer (TB) | US power: 60 W Frequency: 30 kHz Solvents: CO2 and ethanol Sample mass: 5 and 10 g Ethanol: 5.0–10.0% (v/v) Pressure: 15–45 MPa Temperature: 40–60 °C Extraction time: 60 min US Time: 60 min CO2 flow rate: 1.0 ± 0.1 kg/h Mass load: 0.043 and 0.086 g/cm3 |
[40] |
| Semibatch system | Scutellaria barbata D. Don | Flavonoids | US water bath | US power: 185 W Frequency: 40 kHz Solvents: CO2, ethanol, water Sample mass: 20 g Pressure: 10.5–31.5 MPa Temperature: 33–62 °C Extraction time: 15–30 min (static) + 5–240 min (dynamic) US Time: 90 min O2 flow rate: 0.27–0.85 g/min Cosolvent: ethanol/water = 75/ 25, v/v (0–14.5%) 75% ultrasonic duty cycle |
[41] |
| Pilot plant | Cucurbitacin E (CuE) from Iberisamara seeds | CuE oil | US water bath | US power: 50–250 WFrequency: 40 kHz Solvents: CO2 and ethanol (3–1v%) Sample mass: 4 g Pressure: 10–30 MPa Temperature: 40–60 °C Extraction time: 15 min (static) + 20–100 min (dynamic) CO2 flow rate: 1–3 mL/min |
[42] |
The application of ultrasound in extraction processes with pressurized liquids has been relatively little explored. Although there are some apparent similarities between PLE and SFE, many questions regarding the influence of process parameters such as ultrasound power and frequency, pressure, temperature, particle size, type of solvent, experimental apparatus, among others, have not yet been thoroughly elucidated.
However, it should be noted that the extraction process with pressurized liquids assisted by ultrasound is complex from a phenomenological point of view, in which different mechanisms of mass and energy transfer co-occur and interact with each other. Additionally, the ultrasound application affects the sample matrix by various means, such as erosion, sonoporation, shear forces, fragmentation, capillary effect, and detexturation, possibly sequentially [2], further increasing the complexity of the extraction process. Thus, understanding the individual and combined impact of process variables is still a great challenge for scientists and engineers, as we will see below. Table 2 shows the studies regarding applying pressurized liquids solvents for extraction assisted by ultrasound of bioactive compounds published in the last decades.
Table 2.
Reports dealing with pressurized liquid solvents assisted by ultrasound for the extraction of bioactive compounds.
| Technique | Scale | Matrix | Extract | Device | Condition | Ref. |
|---|---|---|---|---|---|---|
| MP-UAE1 | Bench system | Dry tomato pomace | Carotenoids | US probe | Frequency: 20 kHz Amplitude: 58 to 94 µm US power: ~ 60–125 W Particle diameter: 4 ± 1 mm Pressure: 0–100 kPa Solvent: hexane and etanol (1:1) S/F: 33.33 (mL solvent/g sample) Temperature: 25, 35 e 45 °C Extraction time: up to 10 min |
[49] |
| PHP-UAE2 | Bench system | Mate leaves (Ilex paraguariensi) | Solid extracted material | Cylinder-piston system immersed in a US water bath | Frequency: 47 kHz Amplitude: not specified US power: not specified Particle diameter: 1.13x10-3mPressure: 135.3, 202.9, 270.5 and 338.2 kPa Solvent: water S/F: 20 (mL solvent/ g sample) Temperature: 16.5 °C Extraction time: 7 h Time step: 20 min (pulsed pressure) and 60 min (constant pressure) |
[50] |
| HP-UAE3 | Bench system | Ligusticum chuanxiong rhizome | Ferulic acid, senkyunolide I, senkyunolide H, senkyunolide A, ligustilide and levistolide A | US water bath | Frequency: 40 kHz Amplitude: not specified US power: 75–250 W Particle diameter: 20 mesh (0.841 mm) to 100 mesh (0.149 mm) Pressure: 0.05–10 MPa Solvent: ethanol and methanol in water Temperature: 20–70 °C Extraction time: 30–90 min S/F: 20–200 (mL solvent/g sample) |
[51] |
| HP-UAE3 | Bench system | Ligusticum chuanxiong | Polysaccharides | US water bath | Frequency: not specified Amplitude: not specified US power: 175–200 W Particle diameter: not specified Pressure: 10 MPa Solvent: water S/F: 100 (mL solvent/ g sample) Temperature: 70–90 °C Extraction time: 20–30 min S/F: 200 mL/g |
[52] |
| UAPLE4 | Bench system | Pomegranate (Punica granatum L.) peels. | Phenolics | US probe | Frequency: 19 kHz Amplitude: not specified US power: 0–800 W (generator) or 0–38.5 W (tip of the probe) Particle size: 0.68 and 1.05 mm Pressure: 10 MPa Solvent: water, ethanol + water (30, 50, 70% (v/v)) S/F: 20 (mL solvent/g sample) Temperature: 50–100 °C Extraction time: 10 min Number of cycles: 1–5 |
[11] |
| UAPLE-expanded gás | Pilot plant | Pomegranate peel (Punica granatum L.) | Phenolics | US probe | Frequency: 19 kHz Amplitude: not specified US power: 0–600 W (generator) or 0–28.9 W (tip of the probe) Particle size: 0.5–1.0 mm and > 1.0 mm N2 initial pressure: 0 to 1.5 MPa System pressure: 0 to 3.0 MPa Solvent: water S/F: 20 (mL solvent/g sample) Temperature: 40 °C Extraction time: 20 min Number of cycles: 1–3 and 1–5 |
[12] |
| HP-UAE3 | Bench system | Hovenia dulcis polyssacharides (HDPS) | Antioxidants, sugar content, uronic acid and protein content | US probe | Frequency: 40 kHz Amplitude: not specified US power: 120–420 W Particle size: 0.250 mm Pressure: 5 MPa Solvent: water and nitrogen S/F: 10–35 (mL solvent/g sample) Temperature: 30–80 °C Extraction time: 5–50 min |
[53] |
| HP-UAE3 | Bench system | Mentha haplocalyx polysaccharides (MHP) | AntioxidantsSugar content, uronic acid and protein content | US probe | US power: 250–350 W Frequency: 40 kHz Solvent: water and nitrogen S/F: 20 – 30 (mL solvent/g sample)Pressure: 10 MPa Temperature: 60–80 °C Extraction time: 20–40 min US Time: 20–40 min |
[54] |
| UAPLE4 | Pilot plant | Defatted passion fruit bagasse (DPFB) | Phenolics and piceatannolAntioxidants and sugar | US probe | Frequency: 20 kHz Amplitude: not specified US power: 240, 440 and 640 W Particle size: 0.086 ± 0.01 mm Pressure: 10.0 ± 0.5 MPa Solvents: ethanol and water at 75% (w/w) S/F: 300 (g solvent/g sample) Temperature: 65 °C (without US) and 71–77 °C (with US) Extraction time: 300 min Solvent flow rate: 5.0 g/min |
[13] |
| MTS5 | Bench system | Pomegranate peels | Phenolics | US probe | Frequency: 20 kHz Amplitude: 100% Pressure: 100, 300, and 500 kPa Solvent: 30% Ethanol S/F: 50 (ml solvent/g sample) Temperature: 25, 35, and 50 °C Extraction time: 15 s (0.25 min) to 300 s (5 min) |
[55], [34] |
MP-UAE1: manosonication, PHP-UAE2: pulsed hydrostatic pressure ultrasound assisted extraction, HP-UAE3: high-pressure ultrasonic-assisted extraction, UAPLE4: pressurized liquid extraction assisted by ultrasound, MTS5: manothermosonication.
2.2.1. Physical parameters
In the UASFE and UAPLE processes, physical parameters are related to the variables that directly influence the cavitational bubbles’ formation, including the ultrasound power, shape, and dimensions of the ultrasonic vibrating element (probe or bath).
2.2.1.1. Experimental apparatus
According to Table 1, the most commonly used ultrasound types of equipment for UASFE are the ultrasonic water bath (US bath) and the ultrasonic probe (US probe). Among the main advantages of US bath are its low cost, easy maintenance, and the use of many samples to be extracted simultaneously [2]. However, the ultrasonic waves' distribution is less homogeneous and disperse in a bath apparatus, resulting in a lower ultrasonic intensity (0.1–1 W/cm2) than that of the US probe, which concentrates the energy on a small area [35].
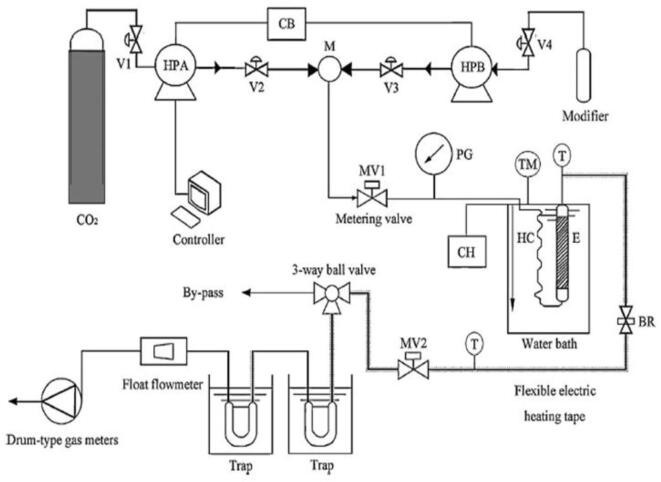
Balachandran et al. [28] extracted gingerols from ginger using a 150 mL stainless steel extractor coupled to a 20 kHz ultrasonic transducer and an ultrasound power of 300 W. The extractor was immersed in a US bath and connected to an online UV–vis spectrophotometer; meanwhile, the system was pressurized with liquid CO2 through a syringe pump. Yang et al. [36] used a semi-continuous flow high-pressure system to extract oleanolic and ursolic acids from Scutellaria barbata D. Don. The system was composed of a 43 mL stainless steel tubular extractor immersed in a US bath working at a frequency of 40 kHz and at ultrasound power of 185 W. A specific amount of glass wool was added at both ends of the extractor to avoid blocking the pipelines. Pressurized CO2 and ethanol were mixed before entering the extractor through syringe pumps. The schematic diagram is shown in Fig. 4. The same apparatus was employed to recover oleanoic and ursolic acids from Hedyotis corymbose [13], Hedyotis diffusa and Hedyotis corymbosa [13], [37], [38], apigenin [39], and flavonoids from Scutellaria barbata D. Don [40]. A similar system was used by Liu et al. [41] to obtain extracts of Cucurbitacin E (CuE) oil from Iberis amara seeds. The authors used a 30 mL stainless steel tubular extractor immersed in a US bath, operating at a frequency of 300 kHz and ultrasound power from 50 to 250 W.
Fig. 4.
Schematic diagram of supercritical CO2 extraction apparatus. V1, V2, V3, V4: stopping valve (on–off valve); HPA, HPB: syringe pump; M: mixer; CB: circulation bath; CH: circulating heater; MV1, MV2: micro-metering valve; HC: heating coil; E: extraction vessel; PG: pressure gauge; BR: backpressure regulator; T: thermocouple; TM: mercury-in-glass thermometer. Reproduced from Yang et al. [36] with permission from Elsevier.
There are also reports of direct systems, where the ultrasound probe is in contact with the sample. It must have a minimum space between the probe and the vessel to avoid any damage to the vessel's wall and minimize the loss of ultrasonic intensity. The system can be divided into three different parts: i) the transducer; ii) the amplifier, and; iii) the probe [42]. The piezoelectric transducer is the most used for the extraction of bioactive compounds from natural products. This material converts electrical to sound energy, producing ultrasound power. Among the main advantages, we can mention its lower cost and smaller size [43]. Titanium is the most common material used in US probes because of its resistance to corrosion and higher temperatures. However, the probe's tip's possible erosion is a shortcoming to be considered [44].
A system composed of a piezoelectric transducer coupled to a 13 mm diameter titanium probe was applied to extract capsaicinoids from malagueta (Capsicum frutescens L.) [12] and dedo de moça (Capsicum baccatum L. var. pendulum) [11] peppers, anthocyanins from Blackberry (Rubus sp.) industrial residues [30], tocopherol and tocotrienols from Passiflora edulis sp by-products [29], and antioxidants from rice bran oil (Oryza sativa L.) [45]. The frequency used in these reports was 20 kHz using a 300 mL cylindrical stainless steel extractor cell in which the US probe was in direct contact with the samples. The extraction bed was formed by a glass wool layer and a filter in both extremities of the cell to avoid blocking of the extraction line, and two layers of glass beads to complete the volume of the cell, as shown in Fig. 5.
Fig. 5.
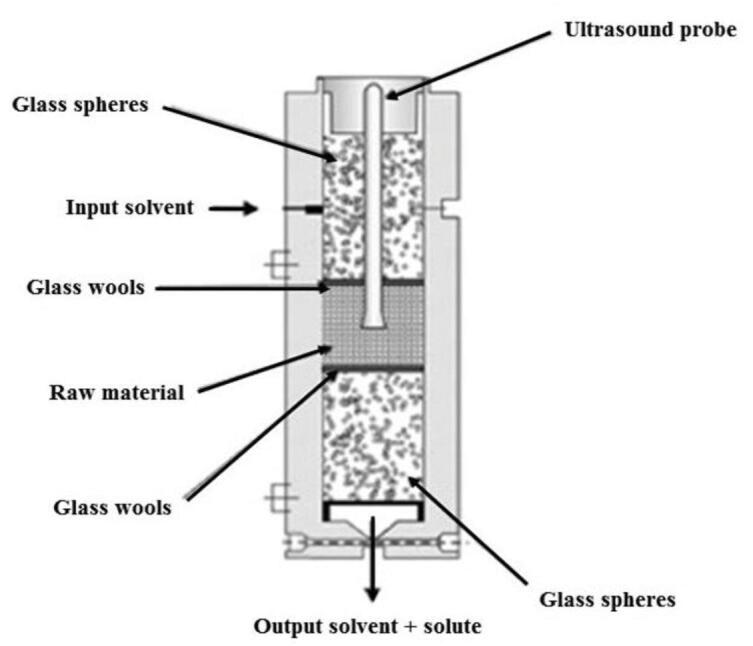
Diagram of the extraction bed used in UASFE composed by two layers of glass wool, two layers of glass spheres and a layer of the raw material. Adapted from Santos et al. [12]. Reproduced with permission from Elsevier.
The use of extractor vessels of different sizes was also employed to extract bioactive compounds through an US probe using the piezoelectric transducer. Riera et al. [46] used a 5 L capacity vessel to recover oil fraction from almonds (Prunus amygdalus), Hu et al. [47] and Gao et al. [48] worked with a 1 L capacity vessel to extract coixenolide from adlay seed (Coix lachrymal-jobi L. var. Adlay) and lutein esters from marigold (Tagetes erecta L.), respectively. When choosing the extraction cell dimensions, one may consider the possible losses of ultrasonic intensities with the size of the extractor, which means a less homogeneous ultrasonic wave distribution.
Santos-Zea et al. [49] evaluated the effects of four different transducers, namely T1 (short rod-shaped head mass), T2 (long rod-shaped head mass), T3 (stepped circular head mass), and T4 (multiplate circular head mass) in the UASFE of bioactive compounds from oregano. The authors observed that transducer T4 provided the highest recovery of phenolics and antioxidants due to its higher surface area design, promoting the solvent's turbulence and therefore enhancing the solute–solvent contact. Santos-Zea et al. [50] applied two different transducers namely TA (cylindrical head-mass transducer) and TB (multiplate head-mass transducer), to extract antioxidants and saponins from agave (A. salmiana) bagasse. In this study, TB was a better transducer to remove the target compounds due to higher energy transmitted and a more effective distribution of the acoustic field to the samples.
In general, the greatest challenge of any ultrasound-assisted system is the extraction cell design, and the associated loss of ultrasonic power. In the bath’s case, the thick walls of the extraction cell needs to withstand the pressure attenuate the ultrasonic waves from the transducers reducing the power effectively reaching the extraction medium. Moreover, for ultrasonic baths, the vibration has to cross the thick walls of the pressurized vessel. In contrast, the ultrasonic probe can be introduced in the pressurized vessel being in direct contact with the sample. Besides, for ultrasonic probes, the ultrasound energy is generated from the inside. However, the probe design and how the extraction cell is connected to the probe can cause loss, or dissipation of power, in the transmission process between the transducer and the probe's tip. Additionally, all the energy is concentrated at the tip, and as the depth of the sample in the extraction cell increases, less energy reaches the sample, imposing limits to the extraction cell dimensions.
It is worth mentioning that ultrasound at low-frequencies (20–100 kHz) and high acoustic power has been widely applied in food processing technologies [51], [52], [53]. However, the design of the ultrasound vessels is still a challenge when the scale-up of the process is aimed, mainly due to the complexity of the cavitational system and the understanding of its relation to other physical phenomena. The frequency of the ultrasonic waves is of significant importance affecting the number and size of the produced bubbles. Lower frequencies may induce the formation of fewer and larger bubbles, gathering more energy and forming micro-jets after their implosion. On the contrary, more and smaller bubbles are formed when higher frequencies are applied, gathering less energy [54], [55].
Moreover, the ultrasound efficiency is depended on other parameters, such as pressure, temperature, generator capacity, shape, size, and material of the transducer. In most cases, the amount of electrical energy supplied to the equipment is the only information provided regarding the ultrasound equipment's operation. Thus, the acoustic fields' characterization is not an easy task when assembling a new ultrasound vessel [49].
Santos-Zea et al. [49] reported the importance of an adequate choice of the transducer geometry to maximize the amount of energy transmitted into the medium when extracting antioxidants from oregano. Entezari, Pétrier, and Devidal [56] studied the phenol degradation, reporting that a cylindrical stainless-steel tube reactor was more effective than other conventional reactors due to the higher surface area of the sonicator, the reactor’s geometry, and the uniformity of the acoustic field. In a similar trend, Faid et al. [57] evaluated the mass transfer coefficients of radial and axial profiles for horn, cup horn, and a tube emitter. The authors observed that for horn and cup horn, the maximum mass transfer coefficient was reached on the axis of the ultrasonic beam but decreased abruptly on each side. On the contrary, the tube emitter showed a good radial homogeneity for both the tube wall and along the axis.
The ultrasound-assisted extraction process using pressurized solvents is extraordinarily versatile and can be conducted in various ways. The literature reports different equipment, which gives particular characteristics for each extraction process. Among the different configurations used, it is worth mentioning the systems similar to those used in UASFE in concept, using baths and probes to propagate ultrasound waves in the extraction medium.
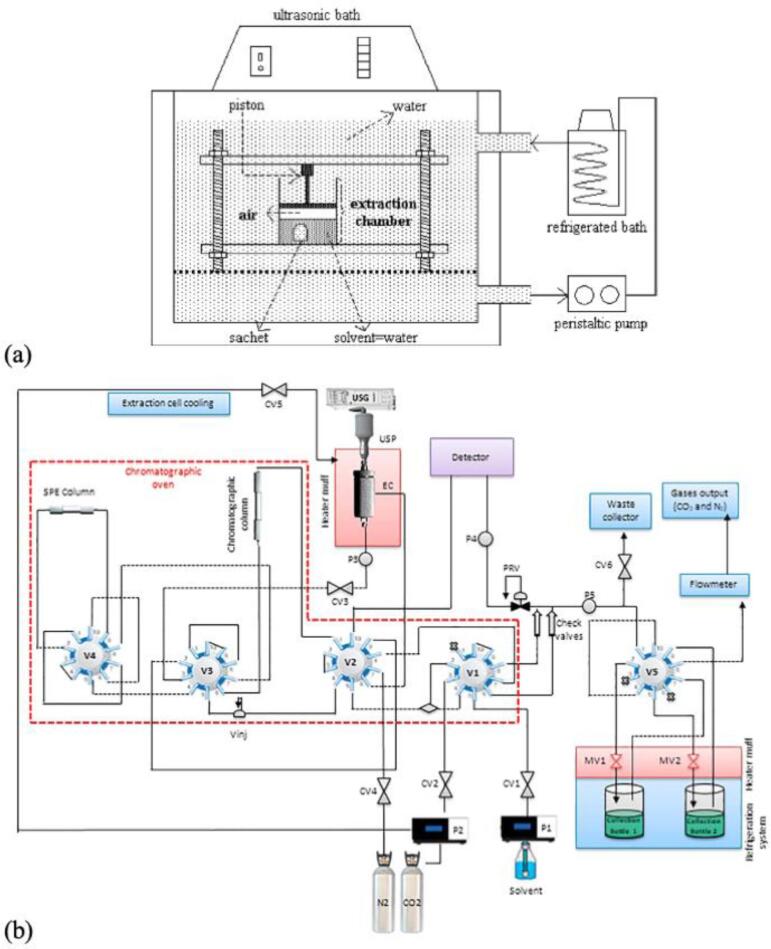
A few examples are using ultrasound bath-based systems. Kotovicz et al. [58] studied the pulsed hydrostatic pressure and ultrasound-assisted extraction of soluble matter from mate leaves. The equipment consisted of an extraction chamber containing a sample wrapped in filter paper and the extraction solvent. The vessel was sealed at the upper end with an internal piston mechanically moved up to a fixed position. The extraction vessel fed with the solid–solvent mixture was accommodated in an ultrasonic bath operated at a frequency of 47 kHz. This configuration, presented in Fig. 6a, allowed the authors to perform ultrasonic extraction experiments assisted and non-assisted by pulsed hydrostatic pressure at different pressures and fixed temperature. Liu et al. [59], [60] used high-pressure extraction vessels containing the sample and solvent to perform a high-pressure ultrasonic-assisted extraction. The system was pressurized and partially immersed in the ultrasonic bath to proceed with the extraction process. This configuration allowed the authors to evaluate the effect of ultrasound power and temperature on the extraction of bioactive compounds of interest.
Fig. 6.
Experimental apparatus used for extraction assisted by ultrasound using liquids at high pressure: (a) ultrasound bath system [58] and (b) ultrasound probe system - Integrated EXTRACT-US diagram system. P1: Liquid pump; P2: CO2 pump; PRV: Pressure Regulating Valve; EC: Extraction Cell; USG: Ultrasound Generator; SPE: Solid Phase Extraction; USP: Ultrasound Probe; P1-5: Pressure transducer; V1-5: Automatic Valve 2-position/10-port; CV1-6: Check Valve; Vinj: Manual injection valve; MV1-2: Micrometric valve. *Dotted section represents the chromatographic oven [14]. All images were reproduced with permission from Elsevier.
On the other hand, ultrasound probe-based systems showed great versatility and, consequently, more possibilities to evaluate the high-pressure extraction process assisted by ultrasound. Luengo et al. [61] performed the extraction of carotenoids from tomato waste in a pressurized nitrogen extraction chamber equipped with a sonication horn connected to an ultrasonic generator. The sample and the extraction solvent were inserted into the extraction chamber; the mixture was then pressurized with nitrogen until constant pressure and later submitted to ultrasound waves. This process was called manisonication.
Sumere et al. [14] and Santos et al. [15] performed extractions combining pressurized liquids with ultrasound in a device called EXTRACT-US. The system consists of an ultrasound probe coupled to an extraction cell, and HPLC pumps to pressurize the solvents. It is also equipped with a chromatographic column and a UV–VIS detector to analyze the extracts. A schematic diagram of the Integrated EXTRACT-US system is shown in Fig. 6b. With this system, it was possible to evaluate the influence of parameters such as pressure, temperature, ultrasound power, and the number of extraction cycles. Additionally, in the experiments carried out by Santos et al. [15], a differential was the use of expansion gas in high-pressure water to maximize the cavitation caused by the application of ultrasound to improve the extraction.
Yang et al. [62] and Chen et al. [63] employed a high-pressure ultrasonic extraction system composed basically of a high-pressure nitrogen gas bottle, an ultrasonic generator, and a high-pressure extraction reaction kettle coupled to an ultrasonic horn. With this system, the authors evaluated the influence of process parameters such as ultrasound power, solvent composition, temperature, and extraction time.
Viganó et al. [16] used the dynamic PLE method to study process variables’ effect on extracting bioactive compounds from passion fruit bagasse (Passiflora edulis sp.). The experiments were carried out in an extraction unit composed of an extraction cell coupled to an ultrasound probe and an HPLC pump that pumped a pressurized solvent with the continuous flow throughout the extraction. The authors evaluated the influence of temperature and ultrasound power on extraction yields.
2.2.1.2. Ultrasound power
Balachandran et al. [28] reported that ultrasound in SFE processes might induce cracks or disruption of the vegetable matrices’ cell walls, favoring the release of compounds the medium. The rupture can enhance solvation power due to the rise in the solute–solvent interaction. Soares et al. [45] evaluated the effects of ultrasound power (160–320 W) for different ultrasound times (40–120 min) in the extraction of antioxidants from rice bran (Oryza sativa L.) oil. The authors noticed that at fixed extraction time (40 min), the highest extraction yield was obtained at 160 W. In contrast, the lowest yield was achieved at 320 W. Barrales et al. [29] studied the effects of different ultrasound power (0–640 W) on the recovery of fatty acids, tocopherols, and tocotrienols from Passiflora edulis sp. The authors observed that higher ultrasound power reduced the extraction of the target compounds. In a similar trend, Dias et al. [11] noticed that an increment in the ultrasound power from 200 to 600 W at the highest pressure tested (25 MPa) reduced the extraction yield at the beginning of the extraction kinetic (CER step). The authors reported that higher ultrasound powers and pressures might have caused the compression of the extraction bed, obstructing the solvent’s flow or reducing the sample-solvent contact. In contrast, Reátegui et al. [30] observed that the highest ultrasound power (400 W) and pressure (25 MPa) resulted in the highest global yield, having the effects of the solvation power more significant in the extraction results.
Santos et al. [12] studied the effects of ultrasound power (0–360 W) and ultrasound time (60–240 min) on the recovery of capsaicinoids from malagueta peppers (Capsicum frutescens L.), keeping pressure and temperature fixed at 15 MPa and 40 °C, respectively. The authors noticed an increment in the global yield when the ultrasound power raised from 200 to 360 W for 60 min. Hu et al. [47] tested different ultrasound powers (0–110 W) for the extraction of oil and coixenolide (Coix lachrymal-jobi L. var. Adlay) from adlay seeds. In this study, sonication effects were more pronounced at an ultrasound range from 90 to 110 W. Moreover, the authors noticed a direct relation between the ultrasound power and the vibration at the solid-solvent interface, showing that the sonication results were more significant in overcoming the static pressure on the ultrasound probe's tip. However, for ultrasound powers from 0 to 90 W, the vibration effects of the transducers on the solid-solvent interface might not be significant on the recovery of the aimed compounds.
Regarding the UAPLE, the amount of energy that enters the extraction medium directly affects the cavitation phenomenon. It is related to bubble number and lifetime, in addition to the collapse pressure of bubbles [64]. As the amount of energy is related to ultrasound power, it is essential to evaluate this variable's effect on the extraction process. Some authors measured the actual applied acoustic power in the extraction process with pressurized liquid assisted by ultrasound [14], [15], [16], [61]. The actual applied acoustic power is a crucial parameter to assess the influence of power on the extraction process since it indicates the amount of energy that effectively enters the liquid. It can be obtained by direct or indirect methods, which estimate the energy transferred by chemical or physical changes in the extraction medium. Among the physical methods, the most used are hydrophones or optical microscopes to measure sound pressure, the aluminum foil method, and the calorimetric method. For chemical methods, indirect measurements of OH• radicals formed by sonoluminescence or chemical dosimeters can be used [2].
Most of the evaluated studies report that, at constant pressure, a higher ultrasound power promotes significant changes in the matrix by inducing greater shear forces. A usual approach is to study the effect of power on the target compound's extraction yield to obtain the best results with the minimum energy consumption [2], [14], [15], [16], [59], [60], [61], [62], [63].
Luengo et al. [61] reported a positive effect of ultrasound power on the extraction yield of carotenoids from tomato waste by manosonication. They observed that the maximum extraction yield of carotenoids was linear related to the ultrasound power (power delivered to the extraction medium) regardless of the external pressure or ultrasonic amplitude. A similar observation was made by Liu et al. [60] when evaluating the process of extracting bioactive compounds at high pressure assisted by ultrasound from the rhizome of Ligusticum chuanxiong. The authors reported that extraction yield increased when the applied ultrasound power varied from 75 to 175 W.
Sumere et al. [14] evaluated the combined process of ultrasound-assisted extraction and pressurized liquid extraction (UAPLE) to improve the efficiency, selectivity, and yield of the extraction of phenolic compounds from pomegranate peel. The influence of several variables of the extraction yield process, including ultrasound power (0–800 W at the generator) and particle size (1.05 and 0.68 mm), were analyzed according to the yield of 20 different phenolic compounds. A significant main effect on phenolic compounds' content was observed varying the ultrasound power and particle size, besides a positive interaction between these two variables, mostly observed for large particles (1.05 nm) at 480 and 640 W. The authors reported that ultrasound power did not affect the extraction using small particle samples. However, when using samples with larger particle sizes, ultrasound power had a significant impact. Extractions conducted under US power of 480 and 640 W resulted in the highest recovery of phenolics. According to the authors, the increase in total phenolic yield is mainly due to cavitation, and the mechanical effect resulted from the application of ultrasound. Combining these two factors increased the surface area of contact between the solid and liquid phases, improved the solvent's penetration in the matrix, and accelerated the analytes' mass transfer to the extraction solvent [65].
Another significant effect that the application of ultrasound can promote at ambient pressure and high pressures is the degradation of compounds. The application of ultrasound, especially in aqueous solutions, can generate free radicals from water molecules' dissociation. These free radicals can trigger the oxidation of compounds and breakage of bonds, resulting in the modification of macromolecular structures and the reduction of molecular weight [66], [67].
2.2.2. Medium parameters
The extraction medium parameters present intrinsic properties that significantly affect the yield of UAPLE and UASFE processes.
2.2.2.1. Pressure, temperature, and solvent
The combined effects of pressure and temperature on the UASFE of natural matters are predominant in understanding the behavior of the mass transfer mechanisms involved in the processes. Moreover, these parameters are essential to predict the influence of the CO2 density and the vapor pressure on the target compounds. The search for the optimum pressure and temperature conditions is critical to maximizing the extraction yields at reduced operational time and costs. Wei and Yang [68] evaluated the effects of different pressure (10.4–30 MPa) and temperature (30–70 °C) on the recovery of oleanoic and ursolic acids from Hedyotis diffusa and Hedyotis corymbosa at 185 W. The authors observed that higher pressures (up to 24.5 MPa) and temperatures (up to 55 °C) diminished the extraction yield due to reduced diffusivity. Moreover, the lower yields obtained at higher temperatures and pressures may be related to the small formation of cavitational bubbles. Reduced diffusivities and solubilities of the aimed compounds were also observed by Hu et al. [47], Wei et al. [69], and Santos et al. [70] when applying higher pressures (up to 35 MPa) and temperatures (up to 60 °C) through UASFE.
Higher pressure and ultrasound power can diminish the void fraction and space between the molecules, reducing the convective effects caused by ultrasound. Hu et al. [47] reported that the ultrasound power must overcome the static pressure against the probe to promote vibrational effects. Moreover, the internal temperature of the system may rise when applying ultrasound. At lower pressures and higher temperatures, the internal temperature effects may reduce the CO2 density and its solvation power. Bubble formation may also be avoided using higher pressures, which may decrease the cavitation effects [29].
On the contrary, Wei et al. [38] applied UASFE to recover oleanoic and ursolic acids from Hedyotis corymbosa noticing that the solubility of the aimed compounds and the diffusional effects were favored at higher pressures (up to 31.5 MPa) and temperatures (up to 53 °C). A similar trend was found by Yang et al. [40] when evaluated the flavonoids extraction from Scutellaria barbata D. Don. Combining ultrasound with higher pressure (up to 28 MPa) and temperature (up to 52 °C) increased the flavonoids content up to 19%. Liu et al. [41] extracted cucurbitacin oil from Iberis amara seeds observing that at 25 MPa and 55 °C, the global yield for UASFE raised by 21.6% compared to SFE. Besides, the authors reported that the convective effects were more predominant than the diffusional ones. Tang et al. [10] noticed a higher extraction yield of caffeine from green tea when using higher pressures (up to 32 MPa), temperatures (up to 65 °C), and ultrasound powers (up to 150 W). A possible explanation for this behavior is the hydrogen bond’s rupture between water, caffeine, and supercritical carbon dioxide (SC-CO2).
Generally, UASFE experiments improve the recoveries of the aimed compounds in comparison to the SFE methods. Temperature and pressure will determine the fluid density (and thus, target compounds' solubility) and affect mass transfer between the sample matrix and the fluid. These variables will also affect the propagation of ultrasonic waves in the fluid, the bubble dynamics, and the cavitation phenomena. Therefore, the observed extraction yields result from overlapping effects of the temperature and pressure on the fluid's properties, ultrasound propagation, and bubble dynamics. However, the evidence provided by the studies mentioned above suggests that lower pressures are more favorable for higher ultrasound powers since the internal temperature can be accurately controlled. Higher pressures are encouraged at later stages of the extraction, especially when diffusional mechanisms are more prominent.
The system pressure has a direct influence on the cavitation phenomenon in the UAPLE processes. If the external pressure increases, higher acoustic pressure will be required to induce cavitation [2]. However, once the cavitation threshold is reached under external pressure, the cavitation bubble collapse's intensity will be higher than without pressure. As a consequence, there will be an enhancement in sonochemical effects [71], [72].
Luengo et al. [61] observed higher extraction yields of carotenoids from tomato waste by increasing the medium's hydrostatic pressure in the manosonication process. According to the authors, this effect is associated with the influence of static pressure on the dynamic of growth and collapse of bubbles. According to the authors, it is possible to increase the physicochemical effects of ultrasound in two ways. The first is to increase the number of cavitation bubbles, and the second is to increase the power of bubbles implosion. When the external pressure is increased, the cavitation process is impaired; however, if the ultrasound power is sufficient to cause the cavitation phenomenon, the intensity of the collapse of the bubbles increases.
In UAPLE processes, the temperature has a notable influence on the properties of the solvent. For example, the increase in temperature induces vapor pressure increase and a decrease in the solvent's viscosity and surface tension [2], affecting the extraction process's performance directly.
The influence of temperature on the manosication was studied by Luengo et al. [61], which reported that temperature was the most significant parameter in the extraction yield of carotenoids. Results indicated that the effect of temperature on extraction yield was characteristic of solid–liquid extraction processes of intracellular compounds from plant matrices. At higher temperatures, the material's solubility and diffusivity increase, which results in higher extraction yields [73]. It is known that the temperature rise reduces the intensity of cavitation when ultrasound is applied at ambient pressure due to the increased pressure of the vapor contained within the bubble by increasing temperature cushion bubble implosion. However, in manosonication, ultrasound used under pressure allows maintaining the cavitation intensity at higher temperatures [74]. Thus, it is possible to manipulate cavitation and diffusion phenomena through the matrix cell walls with the temperature. By increasing the temperature, the surface tension and the liquid medium’s viscosity are reduced, the liquid's vapor pressure increases, enhancing the formation of cavitation bubbles in the liquid medium and producing ultrasonic cavitation at low intensity [75].
Sumere et al. [14] observed that temperature, solvent, and their interaction, significantly influenced the extraction of phenolic compounds from pomegranate peel. As the ethanol percentage of the solvent increased, the extraction yield of the polar compounds decreased. Such results were expected since as ethanol percentage in the solvent increases, solvent polarity and solubility of hydrophilic compounds in the solvent reduces. The effect of the different temperatures evaluated (50, 60, 70, 80, 90, and 100 °C) varied significantly depending on the solvent's composition. The authors stated that the observed behavior is likely to be associated with a combination of various effects, such as temperature, cavitation phenomena, viscosity, dielectric constant, analytes' solubility, mass transfer, and degradation of target compounds. For instance, increasing the temperature reduces the solvent's viscosity, affecting the ability to penetrate the matrix's pores. The increase in temperature also reduces the solvents' dielectric constant, changing their chemical characteristics and their ability to extract target compounds from the matrix. Besides, high temperatures can also positively affect the solubility of the analytes in the solvent; on the other hand, it can trigger degradation processes in thermolabile compounds during the extraction process. The extraction temperature can be increased up to a limit, where the target compounds’ degradation surpasses the increased mass transfer promoted by higher temperatures in the process [14].
Besides affecting the extraction process and the interaction between the temperature and solvent, the solvent's boiling point is crucial for the cavitation phenomena. The cavitation intensity is a function of solvent temperature; when the solvent temperature is close to its boiling point or is much lower, the relative cavitation will be less intense than using intermediate temperatures [14]. For instance, the highest relative cavitation is observed between 50 and 60 °C for water, which can be two or three-fold more elevated than at 100 °C [33], [76]. Considering that solvent mixtures have different boiling points, differences in the relative cavitation achieved with these solvents at different temperatures will be observed.
2.2.2.2. Particle size
For UASFE procedures, particle size reduction implies in higher surface area to volume ratio, which means that more surface area is available to be extracted. Moreover, smaller particle sizes reduce the solvent's diffusional resistance toward the solute, which may raise the final extraction yields [36].
Riera et al. [46] applied UASFE to extract oil fraction from almonds (Prunus amygdalus), and they observed an increment in the extraction yield about 20% when the particle size reduced from 9 to 10 mm to 3–4 mm. Balachandran et al. [28] tested different particle sizes (4–8 mm) to recover gingerols from ginger (Zingiber officinale) through UASFE. The authors noticed that for 4 mm, the use of ultrasound increased the yields up to 30%. Gao et al. [48] also observed an optimum mean particle size interval between 0.245 and 0.350 mm to recover lutein esters from marigold (Tagetes erect L.). The study conducted by Santos et al. [12] evaluated the effects of different particle sizes (1.43 and 0.23 mm) on the recovery of capsaicinoids from malagueta peppers (Capsicum frutescens L.). The authors found that the global yield was 6.2% and 10% higher for large and small particles, respectively. The use of ultrasound was favorable for larger particle sizes (1.43 mm) during the whole extraction. For smaller particle sizes (0.23 mm), ultrasound was more advantageous after 5 h, in which the diffusivity mechanisms were predominant. On the other hand, Yang and Wei [37] studied the effects of different mean particle sizes (0.089–0.925 mm) on the extraction of oleanoic and ursolic acids from Hedyotis corymbose. The authors noticed an increase in the mass transfer properties due to the particle size reduction from 0.925 to 0.355 mm, but further diminishes (0.355–0.089 mm) did not increase the extraction yields.
For UASFE experiments, it is crucial to determine an optimum particle size interval to improve the extraction yields. The use of too small particles may compromise the efficiency of the process since the particles can aggregate during the extraction forming the so-called “channeling” or “short-circuiting” phenomena, where the solvent passes through the channels inside the matrix instead of the inner parts of the samples [37], [48]. It also may promote clogging of filters and valves.
Regarding the influence of particle size on the UAPLE process, Sumere et al. (2018) noted that the smallest particle diameter studied (0.68 mm) resulted in an average yield of phenolic compounds about 22% higher than that obtained for the largest particles (1.05 mm). As expected, the smallest particles' larger relative contact area favors the mass transfer of soluble compounds from the sample matrix to the solvent. On the other hand, the relative contact area between the sample matrix and the solvent is smaller in larger particles, decreasing the mass transfer rate [77]. Even though higher extraction yields were obtained using the sample with a smaller particle size, the authors reported frequent clogging of the extraction system lines, resulting in constant interruption of the extraction process. Thus, from a practical point of view, the use of small size particles was not recommended, resulting in serious reliability problems.
2.2.2.3. Co-solvent
The non-polar nature of the SC-CO2 may restrain its use as an extraction solvent to recover polar compounds from natural matter. The addition of polar co-solvents is recommended to overcome this limitation. Generally, ethanol and water are the most used co-solvents in supercritical extractions. However, the difficulty in operating these solvents at their critical pressures and temperatures makes them found in their sub-critical state. After being adsorbed by the vegetable matrices, co-solvent induces the bed's swelling, enhancing the diffusion of SC-CO2 to the matrix's inner parts, then the solute's availability is extracted [36]. On the other hand, a higher co-solvent concentration can promote the bed's channeling, reducing the final extraction yield [41].
The use of ethanol–water mixtures as co-solvent increased the recovery of oleanoic and ursolic acids from Scutellaria barbata D. Don [36] and Hedyotis corymbose [13], [37], [68] due to the enhanced solubility and diffusivity effects of the SC-CO2 into the solutes. Reátegui et al. [30] noticed that the application of co-solvents (ethanol–water mixtures) in the UASFE experiments raised the recovery of polar compounds, such as anthocyanins and phenolics from blackberry (Rubus sp.) industrial residues. A similar trend was found by Chhouk et al. [78] when an aqueous ethanol solution was used to enhance the recovery of curcumin from turmeric (Curcuma longa L.). Yang et al. [40] observed that the addition of small amounts of aqueous ethanol as co-solvent (10–14.5%) favored the extraction of flavonoids from Scutellaria barbata D. Don due to the driven polar forces of the extraction system. Liu et al. [41] reported that 12% of co-solvent raised cucurbitacin E (CuE) extraction from Iberis amara seeds.
The use of an optimum co-solvent concentration is necessary when ultrasound is applied in a supercritical system. Yang and Wei [39] noticed that the highest yield obtained for UASFE required a lower aqueous ethanol concentration (11.6%) in comparison to the highest yield for SFE (14.9%). Thus, the ultrasound application may induce a reduced operational cost since the amount of co-solvent can be diminished. However, the addition of a co-solvent to supercritical CO2 will also affect the fluid’s density and vapor pressure. Consequently, it will influence how ultrasonic waves are propagated, directly impacting the bubble dynamics and cavitation.
2.2.2.4. CO2 flow rate
The CO2 flow rate interval is another crucial parameter to be studied in UASFE experiments, which may directly impact the extraction performance and the economic aspects. The application of lower CO2 flow rates may result in longer residence times inside the extraction vessel, which will allow the SC-CO2 to diffuse inside the vegetable matrices, improving the extraction yield [13]. Generally, when the CO2 flow rate increases, the intermolecular interaction between solute and solvent rises, allowing the dissolution of the analytes by the solvent [36]. Moreover, the CO2 flow rate increment may reduce the molecules' external mass transfer resistance [68]. However, a further rise in the CO2 flow rate will induce the solvent to flow at high velocities around the channels instead of diffusing through the vegetable matrix. The solute–solvent interaction will occur in a shorter contact time, which may diminish the extraction yield.
Hu et al. [47] did not observe a significant effect of the CO2 flow rate on the SFE and UASFE of oil and coixenolide from adlay (Coix lachrymal-jobi L. var. Adlay) seeds. The authors reported an increased yield at a CO2 flow rate from 1.5 to 3.0 L/h for both SFE and UASFE, but a further increment from 3.5 to 4.0 L/h showed a plateau behavior of the extraction curves. The UASFE obtained a higher extraction yield than the SFE, demonstrating that lower CO2 flow rates were sufficient to obtain higher extraction yields. Similarly, Yang et al. [79] observed that UASFE raised the extraction yield of bioactive compounds from Syzygium aromaticum flower bud (clove) in comparison to SFE experiments. The authors obtained the highest extraction recovery at a CO2 flow rate of 1.4 g/mL for UASFE, while for SFE, a CO2 flow rate of 1.8 g/mL was necessary. Chhouk et al. [78] reported that at CO2 flow rate interval from 2.0 to 3.0 mL/min, curcumin extraction increased but further raises (from 3.0 to 4.0 mL/min) declined the yield. Besides, Liu et al. [41] observed that the CO2 flow rate from 1.0 to 2.5 mL/min significantly improved the supercritical extraction of CuE oil from Iberis amara seeds, but decreased extraction yields were noted from 2.5 to 3.0 mL/min.
Wei and Yang [68] inferred that the higher extraction recovery of oleanoic and ursolic acids from Hedyotis diffusa and Hedyotis corymbose, respectively, using UASFE was due to the micro-stirring and cavitational effects of ultrasound on the cell walls of the vegetable matrices. In this work, a CO2 flow rate of 2.1 mL/min was used to obtain the highest yield for UASFE, while 2.5 mL/min was the flow rate necessary for the highest result in SFE experiments.
2.2.2.5. Ultrasound irradiation time
The effects of ultrasound irradiation time on the extraction yield in SFE processes can be described through overall extraction curves (OECs), based on quantifying the mass of the extraction or extraction yield as a function of time. According to Sovová [80], the OECs are divided into three steps: i) constant extraction rate (CER), in which the readily solute is extracted from the external surface of the particles and convection is predominant; ii) falling extraction rate (FER) where both convection and diffusion play significant roles and iii) diffusion-controlled (DC) where the extraction is controlled by the diffusion of the solvent for the inner parts of the particles. Balachandran et al. [28] observed that applying ultrasound for 10 min favored the convectional effects. The CER period was longer, while diffusional effects were more pronounced when ultrasound was used after 200 min of extraction.
Dias et al. [11] evaluated the effects of different ultrasound irradiation times (40–80 min) on the recovery of bioactive compounds from Dedo de moça pepper (Capsicum baccatum L. var. pendulum). Still, the authors did not find a significant effect of this parameter at the level of 5%. However, the authors observed that at the lowest pressure and temperature (15 MPa and 40 °C), the CER time was shorter (10.52 min) with ultrasound application (200 W for 40 min) in comparison to the condition without ultrasound (18.62 min). Similar behavior was found by Reátegui et al. [30] for the extraction of anthocyanins and phenolics from blackberry (Rubus sp.) industrial residues. In this case, the CER time was lower (36.21 min) with ultrasound than without (42.48 min) at 15 MPa and 40 °C. Wei and Yang [68] extracted oleanolic and ursolic acids from Hedyotis diffusa and Hedyotis corymbosa, respectively, varying the ultrasound time from 10 to 145 min. In this study, the highest extraction yield was achieved for shorter times (10–80 min). A similar trend was noticed by Santos et al. [12], in which the authors concluded that the lowest ultrasound irradiation time (60 min) resulted in the highest global yield.
3. Scale of application
The described extraction methods in Sections 2.2. and 2.3. can have different objectives: i) analytical extraction for quantitative purposes; or ii) production of extract and/or purification. Although the same techniques and methods can be used, they have fundamental differences when considering ultrasound to intensify the process.
Analytical extraction methods are designed for the quantitative recovery of the target compounds through an exhaustive process. Therefore, it is necessary to extend the kinetic extraction curves until the end of the last step, controlled by diffusion (DC). Generally, the extraction process is used as sample preparation for the instrumental method of analysis (e.g., high-performance liquid chromatography - HPLC, gas chromatography – GC, mass spectrometry – MS, among others). In the analytical extraction, higher solvent to feed (S/F) are ratios usually required, meaning smaller sample sizes and higher solvent amounts. Due to the lower sample quantities, methods are relatively short, even when extending the kinetic curve until the last stage. The use of green solvents is strongly recommended and sought, but smaller amounts of organic solvents (e.g., hexane, methanol) can be tolerated.
Analytical applications can fully explore the potential of ultrasound because samples are small (a few grams), the duration of methods is relatively short. Furthermore, since small samples are used, most of the sample is in contact with the probe, minimizing energy dissipation. The costs related to the application of ultrasound for analytical chemistry are much lower due to smaller ultrasound generators and the shorter ultrasound time, which is not a critical issue. Moreover, by using ultrasound, it is possible to shorten the extraction time, mainly that related to the DC step to achieve quantitative recoveries. The DC phase is the slowest of the extraction process and can significantly benefit from the application of ultrasound, as observed by Dias et al. [11] in which the SFE (15 MPa and 40 °C) could not achieve the DC period after 120 min; meanwhile, for UASFE (15 MPa, 40 °C, and 200 W) the DC step was identified for the same extraction time.
In contrast, the production of extracts aims for higher extraction yields at reduced operational costs. The objective is to obtain a concentrated extract. Usually, quantitative recoveries may not be necessary (unless target compounds have a high value), and the extraction is interrupted in the FER stage of the kinetic curve. Overextending the extraction will only dilute the extract and increase expenses.
Using ultrasound at large scales is a real challenge, and several factors should be considered. Operational cost is a critical issue. Since lower S/F ratios are employed, a higher amount of raw material may raise the expenses due to the longer ultrasound irradiation time and/or ultrasound power requirements to scale up, keeping proportions constant. When using more sample attenuation and ultrasonic power loss may also become a problem. Additionally, the ultrasound generator size has limits, and the required power may not be achievable or too expensive to implement.
Among the possible solutions to reduce the costs are pulses, the redesign of the reactors, new types of probes, parallel operation of smaller reactors, and continuous processes. For continuous operations, the samples would be pumped together to the solvent passing through the reactor at controlled temperature and ultrasound power. In this case, the limitation is the amount of solids high-pressure pumps can operate with, implying a high S/F ratio and a more diluted extract. Additional filtration steps also are required in comparison with semi-continuous systems. Another possibility is applying ultrasound only in some specific stages of the process, the initial part or the end of the extraction.
The production of extracts has a promising field for higher aggregated value products, such as pure substances or high-priced raw materials. In these cases, ultrasound may enhance the extraction yield and improve the compound's recovery in the DC step. However, concerns are related to the charges, environmental impact, and further usage of the extracts when a higher solvent amount is applied.
The scale-up of a high-pressure system assisted by ultrasound is still challenging for the companies that aim to expand their production from lab- to industrial scale. At a lab-scale, the combination of high-pressure systems and low-frequency ultrasound has significantly enhanced the extraction yield of the target compounds. At the industrial scale, the nature and amount of the matrix will determine the type of device (US-bath or US-probe) used. Generally, US-probe is recommended for a continuous or semi-continuous system. A larger sample is irradiated in a smaller reactor volume, thus enhancing the ultrasonic density (ultrasound power per volume of the reactor). On the other hand, US-bath requires that the vessel has a larger surface area and agitation system. Nowadays, it is possible to find some companies that produce reactors from the laboratory (up to 50 L) to industrial-scale (up to 1000 L) in a high-pressure process [2].
The main operational parameter that affects the costs of a high-pressure system is the extraction time. For scale-up purposes, the optimum extraction time is desired to maximize the extraction yield and reduce the costs. Del Valle [81] reported that the effects of the extraction time were more pronounced in the CER step and the effects of ultrasound were more significant at times used for cost analysis. This trend demonstrated the efficiency of ultrasound to reduce the costs of the system. Besides, for scale-up purposes, ultrasound has a lower implementation price than other non-thermal technologies, such as high hydrostatic pressure and pulsed electrical fields, which turns ultrasound a feasible method to be used at the industrial scale [82].
4. Mathematical modeling
The use of mathematical modeling to predict the behavior of SC-CO2 extractions is crucial to understand the main effects of the operational parameters on the mass transfer mechanisms of the process. The models used for SC-CO2 extractions are divided into kinetic-based and solubility-based models. According to Dassoff and Li [83], the kinetic-based models can be classified in the linear driving forces (LDF) model [84], the broken and intact cell (BIC) model [80], and the combined LDF and BIC model [85], [86]. On the contrary, the solubility-based models evaluate the effects of density and/or solubility through state equations [87].
The broken and intact cell (BIC) model developed by Sovová [80] considers that at the inlet portion of the cell, the solvent is free of the solute, having one part in contact with the solvent and another maintained intact inside the extractor. The BIC model makes some assumptions, such as the constants’ solid particle size and the solute distribution inside the extractor. Moreover, this model assumes that the mass transfer resistance may be lower to the broken cells. Still, ultrasound can influence the initial considerations due to the micro-and macro-mixing effects [83]. The BIC model predicts the adjustment of three parameters ( – the intact solid ratio; - the mass transfer coefficient in the fluid; and - the mass transfer coefficient in the solid phase). Recently, the behavior of UASFE experiments has been predicted through the BIC model. Barrales et al. [29] applied the BIC model, observing an increase of the CER time when the ultrasound was applied. Moreover, diminished when pressure raised from 16 to 26 MPa, minimizing the effects of ultrasound at higher pressures. The increase of with ultrasound can be explained due to the more prominent effects of ultrasonic waves to release the inner particles from the matrix during the CER period [12], [45]. However, Dias et al. [11] observed a diminish of the when ultrasound was used at higher pressures, which led to higher average absolute relative deviation (AARD) percentages for UASFE (13.01–19.42%) in comparison to SFE experiments (11.32–11.36%).
Another kinetic-based model is the BET-based developed by Pardo et al. [88], supported by the Brunauer-Emmett-Teller (BET) theory of adsorption. This model is described by one equation that relates the extraction yield as a function of time with three adjusted parameters (3P) ( – the solute mass fraction in the first monolayer; - the solubility of the solute; and K – the ratio between the adsorption equilibrium constants). However, when it is assumed equality of the equilibrium constants (K ≈ 1) the model is reduced to a simpler form with two adjustable parameters (2P) ( and ). Dias et al. [11] proposed applying the BIC and the BET-based models to predict the extraction kinetics' behavior and the effects of the operational parameters, mainly those related to the ultrasound use. The authors noticed that ultrasound usage at 15 MPa promoted a lower AARD for 3P (10.54%) in comparison to 2P (14.26%), but a slight difference was observed at 25 MPa (8.34% for 3P and 8.03% for 2P).
Generally, the use of ultrasound in a supercritical process may alter a mathematical model's efficiency since the initial considerations may be compromised, and then its final validation. From the published works, the BET-based model showed to be more accurate in predicting the experimental data for UASFE than the BIC’s model. The use of fewer parameters to be adjusted make the BET-based model feasible to be studied for scale-up proposes at reduced operational costs.
Regarding the extraction with pressurized liquids assisted by ultrasound, only a few studies were concerned with the process’s theoretical description [16], [58]. Two different models were suggested to reproduce the kinetic results of solute mass fractions during the ultrasonic extraction assisted and non-assisted by pulsed hydrostatic pressure by Kotovicz et al. [58]. For the extraction at constant pressure, the solute-mass transfer was assumed to be governed exclusively by diffusion. In contrast, for the extraction subjected to hydrostatic pressure pulses, a combination of internal convection and diffusion in the sample matrix was adopted to explain the removal of the solute from the leaves. The modeling of the ultrasound-assisted extractions at constant pressure demonstrated an exponential increase of the diffusion coefficient and an asymptotic rise of equilibrium solute mass fraction in the liquid phase with pressure. The two-dimensional diffusive model was able to check the progress of extraction, besides reproducing the positive influence of pressure on the kinetics and extraction yields at equilibrium.
Viganó et al. [16] studied the extraction of bioactive compounds from defatted passion fruit bagasse with pressurized liquids assisted by ultrasound. The authors used the two and three-line spline models to fit the experimental extraction curves described by Rodrigues et al. [89] and Meireles [90]. According to this strategy, an overall extraction curve can be described by a straight-line family, usually comprising 2 to 3 lines, depending on the process. The fitted lines can be attributed to three different mass transfer mechanisms based on classic descriptions of the extraction periods: CER, FER, and DC. The slope of the first line represents the mass-transfer rate of the CER period, called MCER. tCER is the time corresponding to the interception of the two lines, which can be related to the minimum time an extraction cycle should last. YCER is the mass ratio of solute in the liquid phase at the bed outlet, and it can be obtained by dividing MCER by the mean solvent flow rate for the CER period. The yield relative to the CER period is RCER or minimum yield expected from the extraction process at a given temperature, pressure, solvent flow rate, and solid matrix properties [90]. The two- and three-lines spline models were fitted to the experimental data obtained by Viganó et al. [16], but the three-line model presented the best fit for all responses. CER, FER, and DC periods were identified for all the kinetics evaluated. The authors observed a notable variation in the parameter b1, or the MCER, with the extraction condition. The application of ultrasound during the extraction resulted in a higher MCER than the PLE without ultrasound application at 65 °C for both cases.
5. Concluding remarks and future perspectives
High-pressure systems assisted by ultrasound to extract bioactive compounds from natural products are a promising field to be explored in some industrial segments, such as food, chemical, and pharmaceutical. Ultrasonic waves can enhance the extraction yield due to the bubbles’ cavitational effects on the vegetable matrices’ cell walls, improving the mass transfer phenomena between solute and solvent. High-pressure systems have shown to be feasible in recovering the target compounds through GRAS solvents, reducing waste and environmental impacts.
However, there is a lack in the literature dealing with the cost analysis and scale-up of high-pressure systems assisted by ultrasound. The industrial-scale process is still challenging for many companies, mainly due to the high expenses with facilities, extraction time, and ultrasound power. Moreover, the lack of specialist laborers, weak competition between supplier companies, and fewer start-ups inserted in the market can also be highlighted as barriers to the scale-up of these kinds of techniques.
Due to the generally lower power density and reactor size limitations, the use of a reactor vessel design that allows enough penetration of the ultrasound probe inside the extraction bed can be an option to enhance the final yields. For this point, the evaluation of different transducer geometries and positions inside the vessel may be helpful (Fig. 7).
Fig. 7.
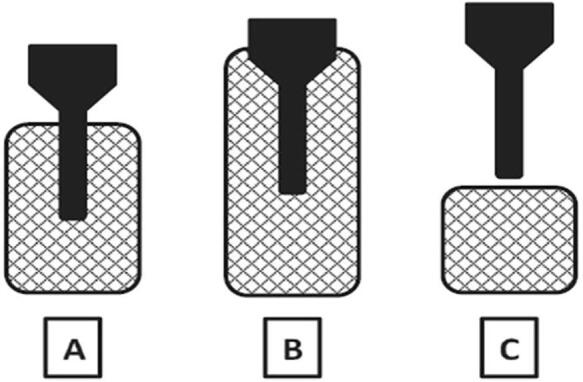
Different transducer positions for ultrasonic-assisted extraction: A – the probe is inside, and the piezoelectric transducer is outside the extraction vessel; B – both the probe and the piezoelectric transducer are inside the extraction vessel; C – both the probe and the piezoelectric transducer are outside the extraction vessel. Adapted from Santos-Zea et al. [49]. Reproduced with permission from Elsevier.
The energy dissipation rate is an important parameter that needs to be monitored because it is related to the cavitational effects affecting the number, size, and distribution of the bubbles. Moreover, the control of the increment of temperature in the bulk of the fluid is necessary since it can alter the gas solubility, vapor pressure, and then the generation of cavitational bubbles. The irradiatiońs frequency, vessel geometry, surface area, and position of the transducers are some parameters that need to be established to optimize the power dissipation. Once the power dissipation is optimized, the power density (W/m3) may be calculated, providing the energy requirement per volume for physicochemical reactions [64].
Maintaining the uniform distribution of the cavitational activity is critical when the optimization of the ultrasound system is aimed. When assembling an ultrasound vessel, one may consider the influence of some physical parameters, such as temperature, amplitude, shape, and size of the ultrasound vessel, material, and diameter of the probe [49]. Besides, the transducer’s position and the fluid height are of major importance when the distribution of the cavitational effects is evaluated. The cavitational activity increases with the increment of the ratio between the transducer’s diameter to the vessel’s diameter, affecting the level of turbulent dissipation of energy and the acoustic streaming’s intensity. Furthermore, the use of multiple transducers is encouraged to obtain a uniform and maximized cavitational activity [64]. The choice of adequate transducers and power generators is also a key factor to avoid foaming and corrosion of the equipment. Although some probe materials like glass and ceramic are available, their fragility can restrict the cavitational effects. However, titanium is usually the most used probe material because of its resistance to heat and corrosion [91].
An efficient acoustic coupling and excitation mode requires understanding the wave propagation nature and radiation from ultrasound vessels. Moreover, important recommendations may be followed to optimize an ultrasound system's efficiency, such as selecting the irradiatiońs frequency, the maintenance of an optimum power dissipation level (W/m3), and the use of a larger irradiation surface of the transducers. Besides, low viscosity, low vapor pressure, and high surface tension fluids are necessary to enhance the cavitational effects. Understanding the fluid's flow behavior is crucial to select the vesselś dimensions and transducer’s location [64].
Some works have applied mathematical models to describe the cavitation formation [92], [93]. However, only a small number of cavitational bubbles is modeled due to the complexity of the phenomena. The mapping of the pressure distribution and its correlation to achieve the pressure threshold are necessary to produce cavitation [94]. Moreover, the use of finite element modeling (FEM) has been adopted to design and optimize ultrasonic transducers [93].
Studies of the effects’ evaluation of other operational parameters, such as amplitude, pulse, and frequency of ultrasound waves, are also encouraged. Larger amplitudes may cause compression/shearing cycles in the solvent molecules, which may change their density, a crucial parameter to be monitored in a high-pressure system. Pulse duration and/or pulse interval optimization are necessary to reduce the costs with time and energy; meanwhile, studies of the effects of high (100–1000 kHz) and low (20–100 kHz) frequencies are essential to a better understanding of the formation of the cavitational bubbles.
The combination of ultrasound with ionic liquids (ILs) has also been extensively investigated in the extraction area. A recent review showed that around 40% of the published works of combined ultrasound/ILs focus on the extraction and micro-extraction processes [95]. This synergic effect can be attributed to the high solvation power and chemical stability of the ILs and the ease of operation, lower solvent consumption, and the facilitated mass transport effects. Moreover, ILs can improve the sonochemistry effects in the extraction through the formation of microjets, erosion, and shockwaves [95]. High-pressure systems coupled to the combined ultrasound/ILs system may improve the extraction yield, reduce operational time and energy consumption, and increase the final compounds’ purity. However, the possible formation of free radicals is a drawback that needs to be monitored during the process.
Studies of the synergic effects of ultrasound and deep eutectic solvents (DESs) on the extraction of bioactive compounds are still incipient in the literature. Biodegradability, low toxicity, and low cost of DESs are some advantages, but their higher viscosity and lower diffusivity may diminish the mass transfer mechanisms [96]. In this case, the application of high-pressure solvents can overcome this limitation by reducing the DESs viscosity. Moreover, SC-CO2 can replace water as solvent facilitating the solubilization of the non-polar components.
The use of expanded gases in high-pressure processes to recover bioactive compounds is also another promising field to be explored. Expanded gases can produce new cavitational bubbles enhancing the ultrasound extraction efficiency [15]. Besides, studies of the effects of the main operational parameters (e.g., pressure, ultrasound power, solvent amount) can be suggested.
Instantaneous Controlled Pressure Drop process or DIC is another extraction technology that can be applied together with the US. In this thermo-mechanical process, the sample is subjected to a fast transition from high steam pressure to vacuum, promoting sample swelling and texture changing. As a result, there is an increase in porosity and specific surface and decreases the sample matrix's diffusion resistance. The combination of US and DIC can improve the extraction kinetics and has been successfully applied to extraction with yield enhancements and reduced treatment time [97]. More information on this topic can be obtained in the review by Chemat et al. [2].
The combination of ultrasound during physical and chemical treatments can also be highlighted. In the context of the extraction of bioactive compounds from vegetable matrices, it is noticed that ultrasound can act either by independent or combined mechanisms. Among the main mechanisms, we can mention fragmentation [98], [99], erosion [100], sonocapillary [101], [102], sonoporation [103], local shear stress [104], and detexturation [105]. For all these mechanisms, the collapse of the cavitational bubbles within the fluid is predominant in incrementing the mass transfer phenomena raising the extraction yields. However, in some cases, ultrasound impacts the vegetable matrices not by a single mechanism but following a chain of mechanisms as observed by Khadhraoui et al. [106]. The authors observed that a chain detexturation mechanism in the following order (erosion, shear forces, sonoporation, fragmentation, capillary effect, and detexturation) led to the total detexturation of rosemary (Rosmarinus officinalis L.) leaves.
To sum up, when choosing a high-pressure system assisted by ultrasound, one may consider an appropriate set of operational parameters to reduce costs and optimize the extraction yield. The use of greener technologies (e.g., UASFE, UAPLE, HP-UAE, among others) has shown to be viable to be applied in the lab-scale, reducing the extraction time and increasing the quality of the final products. However, the process's scale-up is still a challenge to overcome, and many studies are necessary to shed more light on the critical points and find solutions to specific applications.
CRediT authorship contribution statement
Arthur Luiz Baião Dias: Conceptualization, Investigation, Writing - original draft, Project administration. Ana Carolina de Aguiar: Conceptualization, Investigation, Writing - original draft. Maurício A. Rostagno: Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors acknowledge the financial support received from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (151005/2019-2) and from the São Paulo Research Foundation (FAPESP) (2018/14582-5). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Financial code 001 and Process 88887.310558/2018-00.
References
- 1.Global Bioactive ingredients Market Research Report: Information by Ingredient Type (Probiotic, Prebiotic & Amino Acid, Omega 3 Fatty Acid, Phytoextract, Carotenoids and others), by Source (Plant, Animal and Microbial), by Application (Food & Beverages, P, Mark. Res. Futur. (2020) 90. https://www.marketresearchfuture.com/reports/bioactive-ingredients-market-5371 (accessed December 5, 2020).
- 2.Chemat F., Rombaut N., Sicaire A.G., Meullemiestre A., Fabiano-Tixier A.S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Koubaa M., Mhemdi H., Fages J. Recovery of valuable components and inactivating microorganisms in the agro-food industry with ultrasound-assisted supercritical fluid technology. J. Supercrit. Fluids. 2018;134:71–79. doi: 10.1016/j.supflu.2017.12.012. [DOI] [Google Scholar]
- 4.Chaves J.O., de Souza M.C., da Silva L.C., Lachos-Perez D., Torres-Mayanga P.C., Machado A.P.d.F., Forster-Carneiro T., Vázquez-Espinosa M., González-de-Peredo A.V., Barbero G.F., Rostagno M.A. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020;8 doi: 10.3389/fchem.2020.507887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barba F.J., Roselló-Soto E., Marszałek K., Bursać Kovačević D., Režek Jambrak A., Lorenzo J.M., Chemat F., Putnik P. Green food processing: concepts, strategies, and tools. Green Food Process. Tech. 2019:1–21. doi: 10.1016/b978-0-12-815353-6.00001-x. [DOI] [Google Scholar]
- 6.Chemat F., Abert Vian M., Fabiano-Tixier A.-S., Nutrizio M., Režek Jambrak A., Munekata P.E.S., Lorenzo J.M., Barba F.J., Binello A., Cravotto G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020;22(8):2325–2353. doi: 10.1039/C9GC03878G. [DOI] [Google Scholar]
- 7.Chemat F., Abert-Vian M., Fabiano-Tixier A.S., Strube J., Uhlenbrock L., Gunjevic V., Cravotto G. Green extraction of natural products. Origins, current status, and future challenges, TrAC. Trends Anal. Chem. 2019;118:248–263. doi: 10.1016/j.trac.2019.05.037. [DOI] [Google Scholar]
- 8.J. Martínez, A.C. de Aguiar, A.P. da F. Machado, F.M. Barrales, J. Viganó, P. dos Santos, Process Integration and Intensification, in: A.B.T.-C.F. Cifuentes (Ed.), Compr. Foodomics, Elsevier, Oxford, 2021: pp. 786–807. https://doi.org/https://doi.org/10.1016/B978-0-08-100596-5.22819-9.
- 9.Gallego R., Bueno M., Herrero M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae – An update. TrAC Trends Anal. Chem. 2019;116:198–213. doi: 10.1016/j.trac.2019.04.030. [DOI] [Google Scholar]
- 10.W.Q. Tang, D.C. Li, Y.X. Lv, J.G. Jiang, Extraction and removal of caffeine from green tea by ultrasonic-enhanced supercritical fluid, J. Food Sci. 75 (2010). https://doi.org/10.1111/j.1750-3841.2010.01604.x. [DOI] [PubMed]
- 11.Dias A.L.B., Arroio Sergio C.S., Santos P., Barbero G.F., Rezende C.A., Martínez J. Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L. var. pendulum) Ultrason. Sonochem. 2016;31:284–294. doi: 10.1016/j.ultsonch.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Santos P., Aguiar A.C., Barbero G.F., Rezende C.A., Martínez J. Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrason. Sonochem. 2015;22:78–88. doi: 10.1016/j.ultsonch.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y.-C., Wei M.-C. Ethanol solution-modified supercritical carbon dioxide extraction of triterpenic acids from Hedyotis corymbosa with ultrasound assistance and determination of their solubilities. Sep. Purif. Technol. 2015;150:204–214. doi: 10.1016/j.seppur.2015.06.037. [DOI] [Google Scholar]
- 14.Sumere B.R., de Souza M.C., dos Santos M.P., Bezerra R.M.N., da Cunha D.T., Martinez J., Rostagno M.A. Combining pressurized liquids with ultrasound to improve the extraction of phenolic compounds from pomegranate peel (Punica granatum L.) Ultrason. Sonochem. 2018;48:151–162. doi: 10.1016/j.ultsonch.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Santos M.P., Souza M.C., Sumere B.R., da Silva L.C., Cunha D.T., Bezerra R.M.N., Rostagno M.A. Extraction of bioactive compounds from pomegranate peel (Punica granatum L.) with pressurized liquids assisted by ultrasound combined with an expansion gas. Ultrason. Sonochem. 2019;54:11–17. doi: 10.1016/j.ultsonch.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Viganó J., Assis B.F.d.P., Náthia-Neves G., dos Santos P., Meireles M.A.A., Veggi P.C., Martínez J. Extraction of bioactive compounds from defatted passion fruit bagasse (Passiflora edulis sp.) applying pressurized liquids assisted by ultrasound. Ultrason. Sonochem. 2020;64:104999. doi: 10.1016/j.ultsonch.2020.104999. [DOI] [PubMed] [Google Scholar]
- 17.Rostagno M.A., D’Arrigo M., Martínez J.A., Martínez J.A. Combinatory and hyphenated sample preparation for the determination of bioactive compounds in foods. TrAC - Trends Anal. Chem. 2010;29(6):553–561. doi: 10.1016/j.trac.2010.02.015. [DOI] [Google Scholar]
- 18.Mason T.J., Povey M.J.W. 1st ed. Springer; US: 1997. Ultrasound in Food Processing. [Google Scholar]
- 19.Ashokkumar M. The characterization of acoustic cavitation bubbles - An overview. Ultrason. Sonochem. 2011;18(4):864–872. doi: 10.1016/j.ultsonch.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Radziuk D., Möhwald H. Ultrasonic mastering of filter flow and antifouling of renewable resources. ChemPhysChem. 2016;17(7):931–953. doi: 10.1002/cphc.v17.710.1002/cphc.201500960. [DOI] [PubMed] [Google Scholar]
- 21.Suslick K.S., Nyborg W.L. Ultrasound: its chemical, physical and biological effects. J. Acoust. Soc. Am. 1990;87(2):919–920. doi: 10.1121/1.398864. [DOI] [Google Scholar]
- 22.Peshkovsky A.S., Peshkovsky S.L. Nova Science Publishers; New York: 2010. Acoustic Cavitation Theory and Equipment Design Principles for Industrial Applications of High-Intensity Ultrasound. [Google Scholar]
- 23.Muralidhara, H.S., Ensminger D., Putnam A. Acoustic dewatering and drying (low and high frequency): state of the art review. Dry. Technol. 1985;3(4):529–566. doi: 10.1080/07373938508916296. [DOI] [Google Scholar]
- 24.Garcia-Noguera J., Oliveira F.I.P., Gallão M.I., Weller C.L., Rodrigues S., Fernandes F.A.N. Ultrasound-assisted osmotic dehydration of strawberries: effect of pretreatment time and ultrasonic frequency. Dry. Technol. 2010;28(2):294–303. doi: 10.1080/07373930903530402. [DOI] [Google Scholar]
- 25.Patero T., Augusto P.E.D. Ultrasound (US) enhances the hydration of sorghum (Sorghum bicolor) grains. Ultrason. Sonochem. 2015;23:11–15. doi: 10.1016/j.ultsonch.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Ozuna C., Puig A., Garcia-Perez J.V., Cárcel J.A. Ultrasonically enhanced desalting of cod (Gadus morhua). Mass transport kinetics and structural changes. LWT - Food Sci. Technol. 2014;59:130–137. doi: 10.1016/j.lwt.2014.05.062. [DOI] [Google Scholar]
- 27.Chemat F., Zill-e-Huma, Khan M.K. Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason. Sonochem. 2011;18(4):813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Balachandran S., Kentish S.E., Mawson R., Ashokkumar M. Ultrasonic enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006;13(6):471–479. doi: 10.1016/j.ultsonch.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Barrales F.M., Rezende C.A., Martínez J. Supercritical CO2 extraction of passion fruit (Passiflora edulis sp.) seed oil assisted by ultrasound. J. Supercrit. Fluids. 2015;104:183–192. doi: 10.1016/j.supflu.2015.06.006. [DOI] [Google Scholar]
- 30.Pasquel Reátegui J.L., Machado A.P.D.F., Barbero G.F., Rezende C.A., Martínez J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids. 2014;94:223–233. doi: 10.1016/j.supflu.2014.07.019. [DOI] [Google Scholar]
- 31.Seah B.C.Q., Teo B.M. Recent advances in ultrasound-based transdermal drug delivery. Int. J. Nanomed. 2018;13:7749–7763. doi: 10.2147/IJN.S174759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan Kim Y.e., Min H., Yu J., Hong S.Y., Wang M., Kim S.H., Suhr J., Lee Y.K., Kim K.J., Nam J.-D. Forced infiltration of silica beads into densely-packed glass fibre beds for thin composite laminates. RSC Adv. 2016;6(94):91341–91348. doi: 10.1039/C6RA14969C. [DOI] [Google Scholar]
- 33.J.M. Rostagno, Mauricio A. Prado, Natural Product Extraction: Principles and Applications, Royal Society of Chemistry, 2013.
- 34.Güzel N., Kahraman O., Feng H. Solid-liquid extraction by manothermosonication: recapturing the value of pomegranate peels and nanocomplexation of extracts with pea protein. ACS Sustain. Chem. Eng. 2020;8(44):16671–16679. doi: 10.1021/acssuschemeng.0c06316. [DOI] [Google Scholar]
- 35.Bermúdez-Aguirre D., Mobbs T., Barbosa-Cánovas G.V. Ultrasound Applications in Food Processing BT - Ultrasound Technologies for Food and Bioprocessing. Springer; New York, New York, NY: 2011. pp. 65–105. [DOI] [Google Scholar]
- 36.Yang Y.C., Wei M.C., Hong S.J., Huang T.C., Lee S.Z. Development/optimization of a green procedure with ultrasound-assisted improved supercritical carbon dioxide to produce extracts enriched in oleanolic acid and ursolic acid from Scutellaria barbata D. Don. Ind. Crops Prod. 2013;49:542–553. doi: 10.1016/j.indcrop.2013.05.013. [DOI] [Google Scholar]
- 37.Yang Y.C., Wei M.C. A combined procedure of ultrasound-assisted and supercritical carbon dioxide for extraction and quantitation oleanolic and ursolic acids from Hedyotis corymbosa. Ind. Crops Prod. 2016;79:7–17. doi: 10.1016/j.indcrop.2015.10.038. [DOI] [Google Scholar]
- 38.Wei M.C., Hong S.J., Yang Y.C. Isolation of triterpenic acid-rich extracts from Hedyotis corymbosa using ultrasound-assisted supercritical carbon dioxide extraction and determination of their fictitious solubilities. J. Ind. Eng. Chem. 2017;48:202–211. doi: 10.1016/j.jiec.2017.01.003. [DOI] [Google Scholar]
- 39.Yang Y.C., Wei M.C. Development and characterization of a green procedure for apigenin extraction from Scutellaria barbata D. Don, Food Chem. 2018;252:381–389. doi: 10.1016/j.foodchem.2017.12.086. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y.C., Wang C.S., Wei M.C. Kinetics and mass transfer considerations for an ultrasound-assisted supercritical CO2 procedure to produce extracts enriched in flavonoids from Scutellaria barbata. J. CO2 Util. 2019;32:219–231. doi: 10.1016/j.jcou.2019.04.008. [DOI] [Google Scholar]
- 41.Liu X.-y., Ou H., Gregersen H. Ultrasound-assisted supercritical CO2 extraction of cucurbitacin E from Iberis amara seeds. Ind. Crops Prod. 2020;145:112093. doi: 10.1016/j.indcrop.2020.112093. [DOI] [Google Scholar]
- 42.Bermúdez-aguirre D., Mobbs T., Barbosa-cánovas G.V. Ultrasound Technol. Food Bioprocess. 2011 doi: 10.1007/978-1-4419-7472-3. [DOI] [Google Scholar]
- 43.Belwal T., Chemat F., Venskutonis P.R., Cravotto G., Jaiswal D.K., Bhatt I.D., Devkota H.P., Luo Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC - Trends Anal. Chem. 2020;127:115895. doi: 10.1016/j.trac.2020.115895. [DOI] [Google Scholar]
- 44.Cravotto G., Binello A. Low-frequency, high-power ultrasound-assisted food component extraction. Elsevier. 2016 doi: 10.1016/B978-0-08-100294-0.00001-8. [DOI] [Google Scholar]
- 45.Soares J.F., Prá V.D., Barrales F.M., Dos Santos P., Kuhn R.C., Rezende C.A., Martínez J., Mazutti M.A. Extraction of rice bran oil using supercritical CO2 combined with ultrasound. Brazilian J. Chem. Eng. 2018;35:785–794. doi: 10.1590/0104-6632.20180352s20160447. [DOI] [Google Scholar]
- 46.Riera E., Golás Y., Blanco A., Gallego J.A., Blasco M., Mulet A. Mass transfer enhancement in supercritical fluids extraction by means of power ultrasound. Ultrason. Sonochem. 2004;11(3-4):241–244. doi: 10.1016/j.ultsonch.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Hu A.-J., Zhao S., Liang H., Qiu T.-Q., Chen G. Ultrasound assisted supercritical fluid extraction of oil and coixenolide from adlay seed. Ultrason. Sonochem. 2007;14(2):219–224. doi: 10.1016/j.ultsonch.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y., Nagy B., Liu X., Simándi B., Wang Q.i. Supercritical CO2 extraction of lutein esters from marigold (Tagetes erecta L.) enhanced by ultrasound. J. Supercrit. Fluids. 2009;49(3):345–350. doi: 10.1016/j.supflu.2009.02.006. [DOI] [Google Scholar]
- 49.Santos-Zea L., Antunes-Ricardo M., Gutierrez-Uribe J.A., García-Pérez J.V., Benedito J. Effect of ultrasound transducer design on the acoustically-assisted supercritical fluid extraction of antioxidants from oregano. Ultrason. Sonochem. 2018;47:47–56. doi: 10.1016/j.ultsonch.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 50.Santos-Zea L., Gutiérrez-Uribe J.A., Benedito J. Effect of ultrasound intensification on the supercritical fluid extraction of phytochemicals from Agave salmiana bagasse. J. Supercrit. Fluids. 2019;144:98–107. doi: 10.1016/j.supflu.2018.10.013. [DOI] [Google Scholar]
- 51.Bermúdez-Aguirre D., Corradini M.G., Mawson R., Barbosa-Cánovas G.V. Modeling the inactivation of Listeria innocua in raw whole milk treated under thermo-sonication. Innov. Food Sci. Emerg. Technol. 2009;10:172–178. doi: 10.1016/j.ifset.2008.11.005. [DOI] [Google Scholar]
- 52.Char C.D., Mitilinaki E., Guerrero S.N., Alzamora S.M. Use of high-intensity ultrasound and UV-C light to inactivate some microorganisms in fruit juices. Food Bioprocess Technol. 2010;3(6):797–803. doi: 10.1007/s11947-009-0307-7. [DOI] [Google Scholar]
- 53.Kentish S., Wooster T.J., Ashokkumar M., Balachandran S., Mawson R., Simons L. The use of ultrasonics for nanoemulsion preparation. Innov. Food Sci. Emerg. Technol. 2008;9:170–175. doi: 10.1016/j.ifset.2007.07.005. [DOI] [Google Scholar]
- 54.Luque de Castro M.D., Priego-Capote F. Ultrasound-assisted preparation of liquid samples. Talanta. 2007;72:321–334. doi: 10.1016/j.talanta.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Machado A.P.D.F., Sumere B.R., Mekaru C., Martinez J., Bezerra R.M.N., Rostagno M.A. Extraction of polyphenols and antioxidants from pomegranate peel using ultrasound: influence of temperature, frequency and operation mode. Int. J. Food Sci. Technol. 2019;54:2792–2801. doi: 10.1111/ijfs.14194. [DOI] [Google Scholar]
- 56.Entezari M.H., Pétrier C., Devidal P. Sonochemical degradation of phenol in water: a comparison of classical equipment with a new cylindrical reactor. Ultrason. Sonochem. 2003;10:103–108. doi: 10.1016/S1350-4177(02)00136-0. [DOI] [PubMed] [Google Scholar]
- 57.Faïd F., Contamine F., Wilhelm A.M., Delmas H. Comparison of ultrasound effects in different reactors at 20kHz. Ultrason. Sonochem. 1998;5(3):119–124. doi: 10.1016/s1350-4177(98)00009-1. [DOI] [PubMed] [Google Scholar]
- 58.Kotovicz V., Wypych F., Zanoelo E.F. Pulsed hydrostatic pressure and ultrasound assisted extraction of soluble matter from mate leaves (Ilex paraguariensis): Experiments and modeling. Sep. Purif. Technol. 2014;132:1–9. doi: 10.1016/j.seppur.2014.04.036. [DOI] [Google Scholar]
- 59.Liu J.L., Zheng S.L., Fan Q.J., Yuan J.C., Yang S.M., Kong F.L. Optimisation of high-pressure ultrasonic-assisted extraction and antioxidant capacity of polysaccharides from the rhizome of Ligusticum chuanxiong. Int. J. Biol. Macromol. 2015;76:80–85. doi: 10.1016/j.ijbiomac.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 60.Liu J.L., Zheng S.L., Fan Q.J., Yuan J.C., Yang S.M., Kong F.L. Optimization of high-pressure ultrasonic-assisted simultaneous extraction of six major constituents from ligusticum chuanxiong rhizome using response surface methodology. Molecules. 2014;19:1887–1911. doi: 10.3390/molecules19021887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luengo E., Condón-Abanto S., Condón S., Álvarez I., Raso J. Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Sep. Purif. Technol. 2014;136:130–136. doi: 10.1016/j.seppur.2014.09.008. [DOI] [Google Scholar]
- 62.Yang B., Wu Q., Luo Y., Yang Q., Wei X., Kan J. High-pressure ultrasonic-assisted extraction of polysaccharides from Hovenia dulcis: extraction, structure, antioxidant activity and hypoglycemic. Int. J. Biol. Macromol. 2019;137:676–687. doi: 10.1016/j.ijbiomac.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 63.Chen G., Fang C., Chen X., Wang Z., Liu M., Kan J. High-pressure ultrasonic-assisted extraction of polysaccharides from Mentha haplocalyx: structure, functional and biological activities. Ind. Crops Prod. 2019;130:273–284. doi: 10.1016/j.indcrop.2018.12.086. [DOI] [Google Scholar]
- 64.Gogate P.R., Sutkar V.S., Pandit A.B. Sonochemical reactors: important design and scale up considerations with a special emphasis on heterogeneous systems. Chem. Eng. J. 2011;166(3):1066–1082. doi: 10.1016/j.cej.2010.11.069. [DOI] [Google Scholar]
- 65.Pan Z., Qu W., Ma H., Atungulu G.G., McHugh T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2011;18(5):1249–1257. doi: 10.1016/j.ultsonch.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Jacotet-Navarro M., Rombaut N., Deslis S., Fabiano-Tixier A.-S., Pierre F.-X., Bily A., Chemat F. Towards a “dry” bio-refinery without solvents or added water using microwaves and ultrasound for total valorization of fruit and vegetable by-products. Green Chem. 2016;18(10):3106–3115. doi: 10.1039/C5GC02542G. [DOI] [Google Scholar]
- 67.Ince N.H. Ultrasound-assisted advanced oxidation processes for water decontamination. Ultrason. Sonochem. 2018;40:97–103. doi: 10.1016/j.ultsonch.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Wei M.C., Yang Y.C. Kinetic studies for ultrasound-assisted supercritical carbon dioxide extraction of triterpenic acids from healthy tea ingredient Hedyotis diffusa and Hedyotis corymbosa. Sep. Purif. Technol. 2015;142:316–325. doi: 10.1016/j.seppur.2015.01.008. [DOI] [Google Scholar]
- 69.Wei M.C., Xiao J., Yang Y.C. Extraction of α-humulene-enriched oil from clove using ultrasound-assisted supercritical carbon dioxide extraction and studies of its fictitious solubility. Food Chem. 2016;210:172–181. doi: 10.1016/j.foodchem.2016.04.076. [DOI] [PubMed] [Google Scholar]
- 70.Dos Santos P., De Aguiar A.C., Viganó J., Boeing J.S., Visentainer J.V., Martínez J. Supercritical CO<inf>2</inf> extraction of cumbaru oil (Dipteryx alata Vogel) assisted by ultrasound: global yield, kinetics and fatty acid composition. J. Supercrit. Fluids. 2016;107 doi: 10.1016/j.supflu.2015.08.018. [DOI] [Google Scholar]
- 71.Leong T., Ashokkumar M., Kentish S. The fundamentals of power ultrasound - A review. Acoust. Aust. 2011;39:54–63. [Google Scholar]
- 72.H.M. Santos, C. Lodeiro, J.-L. Capelo-Martínez, The power of ultrasound, in: J.-L. Capelo-Martínez (Ed.), Ultrasound Chem. Anal. Appl., Wiley-VCH Verlag, 2009: pp. 1–16.
- 73.Hojnik M., Škerget M., Knez Ž. Extraction of lutein from Marigold flower petals - Experimental kinetics and modelling. LWT - Food Sci. Technol. 2008;41(10):2008–2016. doi: 10.1016/j.lwt.2007.11.017. [DOI] [Google Scholar]
- 74.Raso J., Mañas P., Pagán R., Sala F.J. Influence of different factors on the output power transferred into medium by ultrasound. Ultrason. Sonochem. 1999;5(4):157–162. doi: 10.1016/S1350-4177(98)00042-X. [DOI] [PubMed] [Google Scholar]
- 75.Zhu C.P., Zhai X.C., Li L.Q., Wu X.X., Li B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015;177:139–146. doi: 10.1016/j.foodchem.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 76.Capote F.P., Luque de Castro M.D. Elsevier Science; 2006. Analytical Applications of Ultrasound. [Google Scholar]
- 77.Qu W., Pan Z., Ma H. Extraction modeling and activities of antioxidants from pomegranate marc. J. Food Eng. 2010;99(1):16–23. doi: 10.1016/j.jfoodeng.2010.01.020. [DOI] [Google Scholar]
- 78.Chhouk K., Wahyudiono W., Kanda H., Goto M. Comparison of conventional and ultrasound assisted supercritical carbon dioxide extraction of curcumin from turmeric (Curcuma longa L.) Eng. J. 2017;21(5):53–65. doi: 10.4186/ej10.4186/ej.2017.21.510.4186/ej.2017.21.5.53. [DOI] [Google Scholar]
- 79.Yang Y.C., Wei M.C., Hong S.J. Ultrasound-assisted extraction and quantitation of oils from Syzygium aromaticum flower bud (clove) with supercritical carbon dioxide. J. Chromatogr. A. 2014;1323:18–27. doi: 10.1016/j.chroma.2013.10.098. [DOI] [PubMed] [Google Scholar]
- 80.Sovová H. Rate of the vegetable oil extraction with supercritical CO2—I. Modelling of extraction curves. Chem. Eng. Sci. 1994;49:409–414. doi: 10.1016/0009-2509(94)87012-8. [DOI] [Google Scholar]
- 81.Del Valle J.M. Extraction of natural compounds using supercritical CO2: Going from the laboratory to the industrial application. J. Supercrit. Fluids. 2015;96:180–199. doi: 10.1016/j.supflu.2014.10.001. [DOI] [Google Scholar]
- 82.L. Astráin-Redín, S. Ciudad-Hidalgo, J. Raso, S. Condón, G. Cebrián, I. Álvarez, Application of High-Power Ultrasound in the Food Industry, in: S. Karakuş (Ed.), Sonochemical React., IntechOpen, 2020.
- 83.Dassoff E.S., Li Y.O. Mechanisms and effects of ultrasound-assisted supercritical CO 2 extraction. Trends Food Sci. Technol. 2019;86:492–501. doi: 10.1016/j.tifs.2019.03.001. [DOI] [Google Scholar]
- 84.Glueckauf E., Coates J.I. The influence of incomplete equilibrium on the front boundary of chrornatogram and on the effectiveness of seputation. Theory Chromatogr. 1947:1315–1321. doi: 10.1039/jr9470001315. [DOI] [PubMed] [Google Scholar]
- 85.Fiori L., Basso D., Costa P. Supercritical extraction kinetics of seed oil: a new model bridging the “broken and intact cells” and the “shrinking-core” models. J. Supercrit. Fluids. 2009;48(2):131–138. doi: 10.1016/j.supflu.2008.09.019. [DOI] [Google Scholar]
- 86.Oliveira E.L.G., Silvestre A.J.D., Silva C.M. Review of kinetic models for supercritical fluid extraction. Chem. Eng. Res. Des. 2011;89(7):1104–1117. doi: 10.1016/j.cherd.2010.10.025. [DOI] [Google Scholar]
- 87.Saldaña M.D.A., Tomberli B., Guigard S.E., Goldman S., Gray C.G., Temelli F. Determination of vapor pressure and solubility correlation of phenolic compounds in supercritical CO2. J. Supercrit. Fluids. 2007;40(1):7–19. doi: 10.1016/j.supflu.2006.05.001. [DOI] [Google Scholar]
- 88.Pardo-Castaño C., Velásquez M., Bolaños G. Simple models for supercritical extraction of natural matter. J. Supercrit. Fluids. 2015;97:165–173. doi: 10.1016/j.supflu.2014.09.044. [DOI] [Google Scholar]
- 89.Rodrigues V.M., Sousa E.M.B.D., Monteiro A.R., Chiavone-Filho O., Marques M.O.M., Meireles M.A.A. Determination of the solubility of extracts from vegetable raw material in pressurized CO2: a pseudo-ternary mixture formed by cellulosic structure+solute+solvent. J. Supercrit. Fluids. 2002;22:21–36. doi: 10.1016/S0896-8446(01)00108-5. [DOI] [Google Scholar]
- 90.M.A.A. Meireles, Extraction of bioactive compounds from latin american plants, in: J.L. Martinez (Ed.), Supercrit. Fluid Extr. Nutraceuticals Bioact. Compd., 1st ed., CRC Press, Boca Raton, 2007: pp. 243–274. https://doi.org/https://doi.org/10.1201/9781420006513.
- 91.Wen C., Zhang J., Zhang H., Dzah C.S., Zandile M., Duan Y., Ma H., Luo X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops – A review. Ultrason. Sonochem. 2018;48:538–549. doi: 10.1016/j.ultsonch.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 92.Lais H., Lowe P.S., Gan T.-H., Wrobel L.C. Numerical modelling of acoustic pressure fields to optimize the ultrasonic cleaning technique for cylinders. Ultrason. Sonochem. 2018;45:7–16. doi: 10.1016/j.ultsonch.2018.02.045. [DOI] [PubMed] [Google Scholar]
- 93.Abboud N.N., Wojcik G.L., Vaughan D.K., Jr J.M., Powell D.J., Nikodym L. Finite element modeling for ultrasonic transducers. Proc. SPIE. 1998 doi: 10.1117/12.308015. [DOI] [Google Scholar]
- 94.Wei Z., Weavers L.K. Combining COMSOL modeling with acoustic pressure maps to design sono-reactors. Ultrason. Sonochem. 2016;31:490–498. doi: 10.1016/j.ultsonch.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 95.Chatel G., MacFarlane D.R. Ionic liquids and ultrasound in combination: Synergies and challenges. Chem. Soc. Rev. 2014;43(23):8132–8149. doi: 10.1039/C4CS00193A. [DOI] [PubMed] [Google Scholar]
- 96.Patil S.S., Pathak A., Rathod V.K. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: a greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochem. 2021;70:105267. doi: 10.1016/j.ultsonch.2020.105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allaf T., Tomao V., Ruiz K., Chemat F. Instant controlled pressure drop technology and ultrasound assisted extraction for sequential extraction of essential oil and antioxidants. Ultrason. Sonochem. 2013;20:239–246. doi: 10.1016/j.ultsonch.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 98.Kusters K.A., Pratsinis S.E., Thoma S.G., Smith D.M. Ultrasonic fragmentation of agglomerate powders. Chem. Eng. Sci. 1993;48:4119–4127. doi: 10.1016/0009-2509(93)80258-R. [DOI] [Google Scholar]
- 99.Kusters K.A., Pratsinis S.E., Thoma S.G., Smith D.M. Energy—size reduction laws for ultrasonic fragmentation. Powder Technol. 1994;80:253–263. doi: 10.1016/0032-5910(94)02852-4. [DOI] [Google Scholar]
- 100.Petigny L., Périno-Issartier S., Wajsman J., Chemat F. Batch and continuous ultrasound assisted extraction of boldo leaves (Peumus boldus Mol.) Int. J. Mol. Sci. 2013;14(3):5750–5764. doi: 10.3390/ijms14035750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mason T.J. Some neglected or rejected paths in sonochemistry – A very personal view. Ultrason. Sonochem. 2015;25:89–93. doi: 10.1016/j.ultsonch.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 102.N. V. Malykh, V.M. Petrov, G. Sankin, On sonocapillary effect, in: 5th World Congr. Ultrason., Paris, 2003: pp. 1343–1346.
- 103.Karshafian R., Bevan P.D., Williams R., Samac S., Burns P.N. Sonoporation by ultrasound-activated microbubble contrast agents: effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med. Biol. 2009;35(5):847–860. doi: 10.1016/j.ultrasmedbio.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 104.Vilkhu K., Manasseh R., Mawson R., Ashokkumar M. Ultrasonic recovery and modification of food ingredients. In: Barbosa-Canovas G., editor. Ultrasound Technol. Food Bioprocess. Springer; New York, NY: 2011. pp. 345–368. [DOI] [Google Scholar]
- 105.Chemat S., Lagha A., AitAmar H., Bartels P.V., Chemat F. Comparison of conventional and ultrasound-assisted extraction of carvone and limonene from caraway seeds. Flavour Fragr. J. 2004;19(3):188–195. [Google Scholar]
- 106.Khadhraoui B., Turk M., Fabiano-Tixier A.S., Petitcolas E., Robinet P., Imbert R., El Maâtaoui M., Chemat F. Histo-cytochemistry and scanning electron microscopy for studying spatial and temporal extraction of metabolites induced by ultrasound. Towards chain detexturation mechanism. Ultrason. Sonochem. 2018;42:482–492. doi: 10.1016/j.ultsonch.2017.11.029. [DOI] [PubMed] [Google Scholar]