Abstract
Seminal plasma (SP), the non-cellular component of semen, is a heterogeneous composite fluid built by secretions of the testis, the epididymis and the accessory sexual glands. Its composition, despite species-specific anatomical peculiarities, consistently contains inorganic ions, specific hormones, proteins and peptides, including cytokines and enzymes, cholesterol, DNA and RNA—the latter often protected within epididymis- or prostate-derived extracellular vesicles. It is beyond question that the SP participates in diverse aspects of sperm function pre-fertilization events. The SP also interacts with the various compartments of the tubular genital tract, triggering changes in gene function that prepares for an eventual successful pregnancy; thus, it ultimately modulates fertility. Despite these concepts, it is imperative to remember that SP-free spermatozoa (epididymal or washed ejaculated) are still fertile, so this review shall focus on the differences between the in vivo roles of the SP following semen deposition in the female and those regarding additions of SP on spermatozoa handled for artificial reproduction, including cryopreservation, from artificial insemination to in vitro fertilization. This review attempts, including our own results on model animal species, to critically summarize the current knowledge of the reproductive roles played by SP components, particularly in our own species, which is increasingly affected by infertility. The ultimate goal is to reconcile the delicate balance between the SP molecular concentration and their concerted effects after temporal exposure in vivo. We aim to appraise the functions of the SP components, their relevance as diagnostic biomarkers and their value as eventual additives to refine reproductive strategies, including biotechnologies, in livestock models and humans.
Keywords: epididymis, accessory sexual glands, ejaculate, seminal fluid, proteome, cytokines, antioxidants, gene expression, female genital tract, fertility, livestock, human
1. Introduction
What is fertility among species with internal fertilization? Fertility is their capacity (males and females) to produce offspring, either born from an egg or after carrying a pregnancy to term. In avian species fecundity, e.g., the potential for fertility is the most relevant in terms of gamete production and fertilization. In mammals, and particularly in eutherian species, fertility is the crucial capacity of bearing a partially hemi-allogeneic individual (the fetus) or parts of a decisive organ as the placenta. Some species are polytocous adding another level to fertility: prolificacy, imposing a grading of the capacity to fertilize and carry to term litters of various sizes.
How does semen deposition in the female influence fertility? Semen entry in the female genital tract is a pre-requisite for internal fertilization, with spermatozoa still being the most relevant factor for fecundity in terms of numbers and normality of function. However, is there any other factor than spermatozoa affecting fertility? Yes, the seminal plasma (SP), because it modulates sperm viability and function but also affects the ability of spermatozoa to interact with the lining epithelium of the female genital tract and its secretions; it even serves as a carrier of signals for the female, their immune system in particular [1,2,3,4]. However, these roles are considered not essential for fertilization, as proved by the use of epididymal or of washed ejaculated spermatozoa for in vitro fertilization (IVF), or intra-cytoplasmatic sperm injection (ICSI) and artificial reproductive techniques (ARTs) of increasing application to alleviate the increasing infertility seen in humans, thought to be related to the detrimental impacts of the environment and certain habits on male health [5,6,7]. Under the procedures of IVF and/or sperm cryopreservation, the SP has even been considered detrimental and for decades customarily removed [8,9]. However, SP seems to warrant functions beyond fertilization, and is therefore related—in many species—with fertility [5,10,11,12,13]. Noteworthy, human SP has not been explored for eventual accompanying deterioration as what spermatozoa had been [6]. In practice, and often as a consequence of empirical experiments, a minimal proportion of SP has been left present when semen is processed to produce a semen dose for artificial insemination (AI), in order to maintain a fertility similar to what is seen after natural mating [14,15,16,17,18,19,20,21,22,23]. However, the intrinsic reasons for what is present in those SP proportions remains unknown.
Seminal plasma: what is it, and what roles does it play? The SP is a composite fluid built by the concerted mixture of secretions from the testis, epididymis and the accessory sexual glands, with distinct species differences that, together with emitted aliquots of the epidydimal sperm reserves, compose the ejaculate [24]. The SP plays several roles, including modulation of sperm function, of their ability to generate energy using substrates present in the SP, to interact with the epithelia and the secretions of the female genital tract and also as a carrier of signals for the female, for their immune system in particular. The latter implies the female combats the entry of pathogens and of cells and proteins/peptides immunologically foreign to the female by eliciting a transient genital inflammation [25]. However, considering spermatozoa are also foreign cells, yet essential for fertilization, such transient inflammation is followed by the establishment of a longer-lasting state of immunological tolerance towards those spermatozoa that fortuitously colonize the oviductal sperm reservoirs [11,26,27].
Is it just the SP proteome? The SP proteome appears to participate in this double signaling, triggering a transient inflammation and the changes in the expression of genes related to immune processes at the female internal genitalia [28,29,30]. The latter transcriptomic events ultimately modulate sperm rejection or tolerance, perhaps even disclosing the relative intrinsic fertility of the male and/or female. The proteome and peptidome of semen, including proteins and peptides derived from spermatozoa and the various fractions of the SP the gametes bathe in, has been intensely studied over the past decade, with particular attention to the structure, temporal distribution and function of the major proteins [31,32,33,34,35,36]. As well, transcriptomic studies have signaled that many genes encoding the SP proteome display signs of a high rate of adaptative evolution [3,31,37]. Other components, including exosomes (epididymosomes, prostasomes and vesiculosomes) [38,39,40] and small-size proteins/peptides, such as cytokines [41] and enzymes with anti-oxidant capacity [42], are yet to be fully understood regarding sperm selection, function and fertility signaling to the female [43], as well as regarding the long-term effects on the health of the offspring, regardless of the species considered [44].
This review attempts, by including our own results on various animal classes and model species, to critically summarize the current knowledge of the roles played by the SP components on ruling fertility and prolificacy in animal models and humans. The SP’s importance is hereby particularly discussed in relation to sperm survival, function and signaling to the female immune system towards fertility modulation, in vivo findings with major relevance for the development of diagnostic/prognostic biomarkers and the refinement of reproductive strategies. The review is comprehensive yet intending to avoid reiteration of the large volume of available literature elsewhere; nonetheless, it is written as comparative as we could.
2. The Composition of the Seminal Plasma: Comparative Aspects
2.1. The Building of an Ejaculate Defines the Diversity of Its Composition
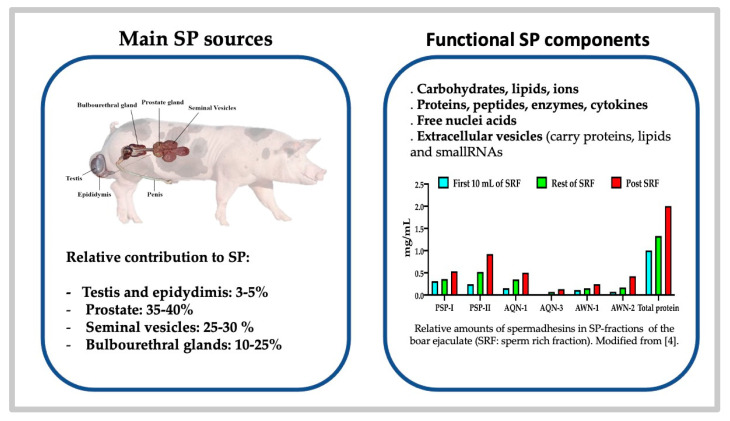
Semen, e.g., the ejaculate, is a complex suspension of spermatozoa bathing in a heterogeneous composite fluid, the so-called SP, built by contributions of the testis, the epididymis and/or the accessory sex glands; the latter conspicuously lead—in direct relation to variations in presence and gland size among animal classes and species—to differences in the volume of the ejaculate and SP composition [24,45]. In some species (as in pig), the SP builds up most of the total ejaculate volume while the cell components (spermatozoa, other classically named round cells (lining cells of the excurrent ducts, epididymis or accessory glands, migrating leukocytes and spermatogenic cells), as well as extracellular vesicles (EVs), represent barely 5% (including a minor volume of epididymal cauda fluid) [4]. Ejaculation, a basic physiological phenomenon, differs among species in timing and ejaculate volume and type [24]. Of comparative interest, differences in ejaculate volume among species seem to relate to the type of mating and the site of semen deposition. Why? Basically because female anatomy rules, with ejaculation encompassing characteristics of the female genital anatomy that, in turn, defines the mating behavior [46] and site of deposition of the semen, i.e., cloacal or vaginal deposition for low-volume ejaculates with highly concentrated sperm suspensions. Some animal classes, such as poultry, lack accessory glands [47] and thus their ejaculate is very small, yet highly concentrated in a fluid built by secretions from the testis, the rudimentary ductus epididymis and the ductus deferens [47,48]. Ruminants, also having an un-fractionated type of sperm-dense ejaculate, and builds their SP by the concerted emission of cauda epididymal contents and of the secretions of a complete series of sexual glands (prostate, seminal vesicles and bulbourethral glands) [24]. They deliver an ejaculate produced during a very quick mating (seconds) by one-time emission of spermatozoa and sexual glands secretions. In primates and some species of ungulates, semen deposition is done deep in the vagina, in front of the cervical opening or in the vaginal fornix, while in others, sperm deposition occurs intra-cervically (pigs) or even intrauterine (equine) [24]. The latter species have voluminous ejaculates with less concentrated sperm suspensions. These differences are more intricate; these ejaculates are formed by cycles of sperm emission from the cauda epididymis combined with a sequential secretion and excretion by the sexual accessory glands [24]. Moreover, dogs, horses, pigs and men have fractionated ejaculates either because there is a dominant sexual gland (prostate in dogs) or an uneven blend of the emitted spermatozoa (still contained in the fluid of the cauda epididymis) with secretions of the accessory glands, verted in sequential spurts to the urethra [24]. In pigs, to cite the animal model most intensively studied by us, the SP is sequentially built by epididymal caudal fluid and the concerted secretion of the accessory sex glands: the prostate, seminal vesicles and bulbourethral glands [24,49] (Figure 1).
Figure 1.
The sources, general functional components and variations in levels of the dominating protein, spermadhesins, along fractions of the ejaculated seminal plasma (SP) of pig, widely used by the authors as a biomedical research animal species.
Common to boar, stallion and to some extent dog or man, the ejaculate is sequentially expelled in fractions usually classed as pre-sperm, sperm-rich (SRF) and post sperm-rich, clearly defined by the amounts of spermatozoa present and the volume and type of secretion building the SP of each fraction [4,11,50]. In general, the first secretion (pre-ejaculate) present in the urethra derives from the urethral and/or bulbourethral glands (Littré and Cowper for human), containing mainly mucin, sialic acid, galactose and salts in a slightly viscous, clearly aqueous fluid). The emission of spermatozoa from the cauda epididymis to the urethra, accompanied by secretion from the prostate, initiates ejaculation (e.g., expulsion of semen into the female or into a collection vial) in a series of clearly distinct spurts. In humans, the initial spurts are usually called the SRF of the ejaculate [51], since they hold the most spermatozoa, with a blend of the acidic cauda epididymis and ampullar fluids mixed with the slightly acidic citrate and zinc-rich prostate fluid, which also contains specific peptides and proteins, such as acid phosphatase and kallikrein 3—a prostatic specific antigen (PSA) in humans) [52]. In the following spurts, building the so-called post-SRF, there is a gradual dominance of secretion from the seminal vesicles (rich in fructose, peptides, proteins, prostaglandins (PGs), etc., which is clearly basic in nature) as well as gradual diminution of sperm numbers [52]. Presence of prostaglandins or steroid hormones, such as estrogens, in the post-SRF (vesicular-derived) has been well documented, with large species variation [24]. In pigs, fractions are also classically pre-SRF (with a clear sperm-free seminal fluid that contains mainly secretion of the urethral and bulbourethral glands, as well as the prostate), the SRF (composed by the emission of portions of the cauda epididymal contents, extended in vesicular but mainly prostate gland secretions) and, finally, the post-SRF (where the fewer emitted spermatozoa are largely extended in secretions of the vesicular glands, the prostate and, by the end of the prolonged ejaculation, of the gel-rich, coagulating bulbourethral gland secretion (Figure 1)) [11].
Equines follow a similar pattern of ejaculation [53] as pigs. In either species, during the gel-rich secretion, noticeable during collection with an artificial vagina, the gloved-hand technique can virtually coagulate the entire ejaculate if placed together. In vivo, the gel fraction in these species enters the cervical canal by the end of ejaculation, a process also seen in rodents to hinder other males from deposing their semen on the already mated female. In humans, at or immediately after ejaculation, a sample of semen collected in a single vial also coagulates to form a gelatinous mass that immobilizes the spermatozoa [24,54]. If an ejaculate is collected using a split procedure (i.e., several vessels for collection of different fractions), as it presumably occurs during ejaculation in vivo, the first spurts (prostate-dominated) do not coagulate, while the last ones (vesicular-dominated) do [54]. Such coagulum is rapidly (in vivo, within minutes) or more lengthy (15–30 min in vitro) liquefied by prostatic-derived proteolytic enzymes [55]. In livestock, the gel-fraction is routinely discarded during the ejaculate collection. Noticeably, an initial sperm-peak portion is present in the first 10 mL of the pig SRF, where a vanguard sperm sub-population of about 25% of the total sperm numbers [4] seems to contain, in vivo, the first and main colonizers of the sperm reservoir in the oviduct [26]. The phenomenon seems conserved across species [56], since most human spermatozoa are, as described, present in the first (non-coagulating) fractions, so a certain proportion of them can rapidly enter the cervical canal, as extrapolated from studies that recorded sperm present in the Fallopian tubes as early as a few minutes after coitus [57].
Differences in ejaculate building among species reflects on the SP composition. The SP is basically formed by secretions of epithelial cells and thus contains electrolytes, protein and steroid hormones, sugars and proteins/peptides, including enzymes [24]. The relative proportions of these components certainly varies among species, the year [43,58] and the type of ejaculate in question. Classical examples of the latter are when either all components are ejaculated at once (e.g., poultry and ruminants) or in relative different proportions (e.g., humans, stallion, boar, etc.). For instance, the porcine pre-SRF-SP is rich in Na and Cl; the SRF-SP contains proteins, steroid hormones, glycerophosphorylcholine, fructose, glucose, inositol, citrate, bicarbonate and zinc; while the post-SRF-SP has the highest amounts of proteins, bicarbonate, zinc, Na, Cl and sialic acid [4,24,49,59]. Although different ions of the SP seem to play important roles in maintaining sperm survival and function [43], with bicarbonate modulating sperm motility or destabilizing the plasmalemma [60], or zinc as modulator of chromatin stability [61], most other roles, including those related to fertility, are connected to its large protein contents (human 25–55 g/L; poultry 7.5–9.0 g/L; boar 30–60 g/L).
2.2. The SP Proteome, What Does It Contain and What Does It Impact?
The SP of most species contains protein compounds similar to those present in blood plasma, such as pre-albumin; albumin; α-, β- and γ-globulins; transferrin; enzymes and some immunoglobulins; complement factor; as well as differential amounts of cytokines and chemokines [11]. Comprehensive sperm protein databases have been established for a plethora of species, including insects, avian, ruminants, porcine, equine, or human [11,32,34,62,63,64,65]. The sperm proteome covers the expected spectrum of function (from energy production to cell recognition), but few proteins are accurately linked to (in)fertility [66]. The seminal fluid proteome, even in species lacking all accessory sexual glands as in avian species [64,65], depicts clear phylogenetic relevance for fertility for its direct relation to sperm function, mechanisms of energy production/consumption, proteolysis and oxidoreduction.
In mammals, SP proteome studies have dramatically expanded over the past decade, allowing for the identification of large and small proteins and peptides, which now account for the thousands available [35,67,68,69,70]. The main SP proteins belong to one of three groups: proteins carrying fibronectin type II (Fn-2) modules, spermadhesins or cysteine-rich secretory proteins (CRISPs) [71]. However, differences in type and source of proteins are present among species, owing to the already named differences in glands and/or the sequence they are emptied, or the type of ejaculate they have. Comparative studies [63] have shown particular roles played by SP proteins in ruminants amply used for AI, holding single ejaculates [66,69,72,73], but also in species—including humans—with fractionated ejaculates, where the proteome type, concentration and temporal exposition are relevant [11].
The human SP has thousands of unique proteins, ~25% secretory from the accessory glands [74], and either free or present in epididymosomes and prostasomes [75,76,77]. The prostate secretion, in humans representing 20–30% of the total SP volume, and even more in other species where the gland is solely present (e.g., dogs), is the first SP portion to confront the cervical canal, being in immediate direct contact with the major numbers of emitted spermatozoa. In humans, three major proteins, all under hormone regulation, have been identified: the kallikrein PSA (mainly released by the prostate but also produced by the Littré glands), the prostatic acid phosphatase and the cysteine-rich prostate-specific protein-94 (PSP-94, β-inhibin-β-microseminoprotein) [68,74]. The primary function of PSA is the liquefaction of the coagulum by hydrolyzing the majoritarian semenogelins I and II, involved in the gelification of the latter spurts of the ejaculate (coagulum), while prostatic acid phosphatase and the PSP-94 have enzymatic growth factor action. The liquified coagulum contains products with clear biological functions, such as inhibition of sperm motility, antibacterial activity, etc., alongside with other seminal vesicle proteins that include lactoferrin, fibronectin and protein C-inhibitor [68,74]. The Cowper’s gland (which is difficult to sample as isolate) contains an extremely abundant protein: mucin [24].
The stallion displays not only a similar fractionated ejaculate as humans [78], but shows equivalent main SP proteins, such as HSPs (horse seminal proteins 1–8), Fn-2 and CRISPs [79]. Most HSPs are of low molecular weight (14–30 kDa), form multi-protein aggregates, and all but HSP-4- are capable of attaching to the sperm surface. The prevalent HSP 1–2 short Fn-2 type heparin-binding proteins (70–80% of the total protein) modulates capacitation, changing the sperm membrane structure [80]. Studies of isolated ejaculate fractions showed HSP-1 was the major protein present in all, while the first fractions contained acrosine inhibitor, prostate-specific antigen (PSA) and PSA and other kallikrein-family proteins (HSP-6 and HSP-8), and the other HSPs were present in the rest of the fractions [53]. The equine CRISP-3 (HSP-3) is associated with fertility [81], probably in relation to the tolerance of spermatozoa to preservation [82], as it was shown it occurs in a dose-dependent manner [83].
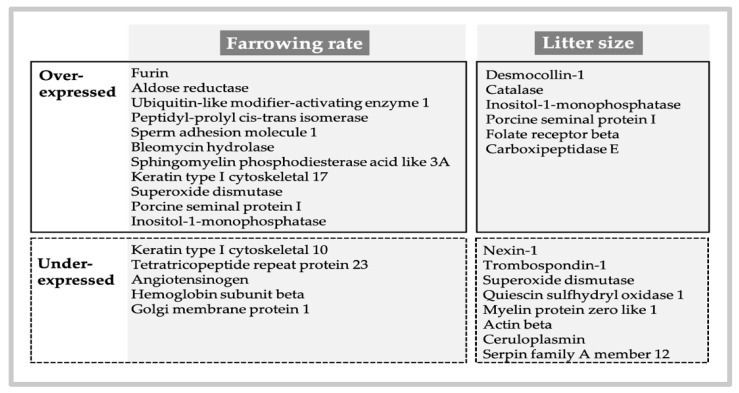
Another species with a fractionated ejaculate, whose delivery sequence resembles humans, is the pig, our most commonly used animal biomedical model [84]. Of interest, likewise to humans, the porcine SRF has a sperm-peak portion where 25% of all the ejaculated spermatozoa are present within a volume of 10 mL, i.e., 1/20 of the total ejaculate [4]. This sperm-peak portion appears as the vanguard sperm sub-population, which firstly negotiates female barriers and colonize the oviductal sperm reservoir [26]. This sperm-peak portion does not coagulate (alike human) and it is rather protein-poor, yet containing epididymal lipocalins and an inhibitor of acrosin/trypsin [4]. In contrast, the latter ejaculated fractions contain increasing amounts of SP proteins, verted by the vesicular glands (Figure 1). Between 75 and 90% of these SP proteins belong to the multi-functional spermadhesin lectin family of 12–16 kDa glycoproteins (the heparin-binding HBPs Alanine–Glutamine–Asparagine proteins AQN-1&3, the Alanine–Tryptophan–Asparagine proteins (AWNs) and the non-heparin binding porcine seminal plasma proteins I and II (PSP-I and PSP-II) [4,11,22,32,85]. These spermadhesins, the PSPs in particular, attach sequentially to the sperm plasma membrane from the testis to the ejaculate [86], promoting sperm survival through membrane stabilization [87], and fertilization capability [87,88] by modulating capacitation and sperm-oviduct/oocyte interactions [85,86,89,90,91]. Moreover, they appear to regulate the timing of ovulation [92] and further showing immunostimulatory activities in vitro and in vivo, presumably in relation to specific cytokines [4,11,25,93]. Besides spermadhesins, a plethora (several hundred specific to Sus scrofa taxonomy) of other less abundant pig-specific SP proteins are present [32,34], yet only a few influence the reproductive processes. Noteworthy, their presence and degree of expression relate to specific ejaculate fractions, associating quantitative variation to the effects depicted by the SP of these fractions, particularly the SRF vs. the post-SRF [2,32,94,95]. Up to 16 SP proteins represented in the Sus scrofa taxonomy were differentially expressed between the SRF and post-SRF. Eight of these, overexpressed in SRF, had been previously related to sperm membrane function, capacitation, the acrosome reaction and zona pellucida binding [32,33]. A follow-up study, using a pre-fractionation step by solid phase extraction, detected further numbers of less abundant SP proteins in boars with different farrowing rates and litter sizes after AI of 10,526 sows. Here, there was an overexpression of SP UBA1, SPAM-1, AKR1B1 and furin in high-fertile boars and of CAT and DSC-1 among boars registering large litter sizes, clearly confirming the relevance of the SP proteome for boar fertility [34] (Figure 2).
Figure 2.
Over- and underexpression of less abundant specific seminal plasma proteins in the animal model pig (Sus scrofa) analyzed using iTRAQ-based quantitative proteomics and their relation to fertility, as farrowing and prolificacy (litter size) (modified from [33,96,97].
It is thus evident that the proteins of the SP exert multiple functions, starting with their general influence as a vehicle aiding sperm survival when semen is highly extended [43,87] or processed [22,98,99], to specific events while adsorbed to the sperm plasma membrane, reinforcing its stability during uterine sperm transport [100]. Besides, specific SP-proteins have pro-inflammatory capacities, contributing as a trigger for the well-known post-mating primary, transient inflammation whose role is to cleanse the intra-uterine lumen from foreign cells, proteins and eventual pathogens, as well as from excessive spermatozoa, e.g., those not involved neither in colonization of the tubal reservoirs or fertilization. Moreover, SP-signaling is, as we shall later see, also involved in long-lasting signaling to the female, towards a more immunotolerant environment in preparation for the descending embryo and the triggering of a long-lasting immune modulation towards tolerance of the hemi-allogeneic embryos/placenta bearing paternal antigens [11,101,102].
Interestingly, the roles of the seminal fluid proteins mentioned for these mammals seem to be highly conserved across animal classes. The transfer of seminal proteins induces particular post-mating changes in female insects, inducing changes in behavior regarding feeding and decreased receptivity for re-mating, as well as of gene expression of the mating-dependent genes [103]. Such changes in gene expression can modify metabolism and enhance egg production, modulate sperm storage and competition, or trigger expression of antimicrobial peptides for immune defense [104]. Our own studies in phylogenetically distant species, such as chicken and pigs, has confirmed that exposure to SP proteins in vivo induces gene expression changes, affecting both the innate and adaptative immunological responses of the female, in direct relation to sperm survival and fertility [31,105].
2.3. Cytokines in the Seminal Plasma: The Effectors of Such Signaling?
The SP of most species contains differential amounts of a 5–20 kDa peptidomes with pro- and anti(or tolerance-related)-cytokines and chemokines [11]. Both in human and boar, cytokine amounts of both inflammatory (tumor necrosis factor (TNF)-α, interferon-γ (IFN-γ), interleukins (IL) −6, −8, granulocyte-macrophage colony-stimulating factor (GMCSF)), and anti-inflammatory cytokines (thymus- and activation-regulated chemokine (TARC), macrophage colony-stimulating factor (M-CSF) as well as transforming growth factor-β (TGF-β)1–3) were higher in the vesicular-dominated fractions [11,41,106]. This relation between cytokine concentrations and SP-fraction was consistent among the TGF-β1–2, IFN-γ and IL-6, IL-10 and GM-CSF [41,106,107,108,109]. In the human and pig, cytokines relate to sperm viability, semen function and hence fertility [41,70,95,110]. As well, cytokines—probably in concert with other SP proteins [11,111]—play a role in modulating the uterine immune-cytokine network [6,88,112,113,114,115,116,117], to facilitate the transitions in immune responses by the female mentioned earlier and what appears central for successful fertility [93,102,118,119,120]. In this context, it is also worth noting the function of the adipokines secreted by white adipose tissue. Adipokines have, from being considered endocrine regulators of energy metabolism and secreted by white adipose tissue, evolved as involved in fertility regulation, perhaps acting as metabolic sensors [121]. Leptin and adiponectin are now accompanied by the novel adipokines resistin, chemerin, apelin and visfatin, which, alongside cognate receptors, are now evidently expressed in the reproductive tissues of human and animals, including the testis [121], and affect various reproductive events [122]; their disarray or absence can also cause infertility [123]. Being produced by the testis or incoming into the male genital tract, they have been identified in SP in humans and animals, including the pig [124,125], and they attach via specific receptors to the sperm plasmalemma [126]. Their impact appears related to sperm morphology, function as well as being involved in inhibiting capacitation, or in inflammatory processes in infertile individuals [127]. Noteworthy, levels of some adipokines in SP, such as leptin, are 10-fold higher in the boar than in humans [125].
2.4. Enzymes of the Seminal Plasma: Do They Play a Role in Fertility or Are They Simple Markers of Sperm Function?
In general, about 3% of proteins in SP are enzymes [70], with some related to sperm quality, such as lipases, matrix metalloproteinases (MMPs) and glycosidases (β-glucuronidase (BG), α-glucosidase, β-glucosidase, α-galactosidase, β-galactosidase and β-N-acetylglucosaminidase (NAG), etc.) [24]. The pig SP contains also several antioxidative enzymes, such as superoxide dismutase (SOD), catalase (CAT), gamma-glutamyl transferase (GGT), glutathione peroxidase (GPx)/phospholipid hydroperoxide glutathione peroxidase, glutathione reductase/S-transferase and paraoxonase type 1 (PON-1), displaying the highest levels in the fractions of the ejaculate containing most spermatozoa [43]. Most of these enzymes alongside antioxidants such as glutathione, ascorbate, pyruvate, taurin and vitamin E (α-tocopherol) in SP protect spermatozoa against excessive levels of reactive oxygen species (ROS), which could lead to lipid membrane peroxidation and consequently in sperm damage and death [128]. This protective action against ROS damage is reflected in the fertility of the semen, particularly after processing [129]. Particular enzymes appear significantly associated with the fertility data of males, including the epididymal lipocalin-type PGD2 synthase in equines [130,131] and pigs [94], the PON-1 [132]; or the lactate dehydrogenase isoenzyme LDH-C4 [133] also in the pig.
3. The Particulate Seminal Plasma: The Most Relevant Component of Seminal Plasma?
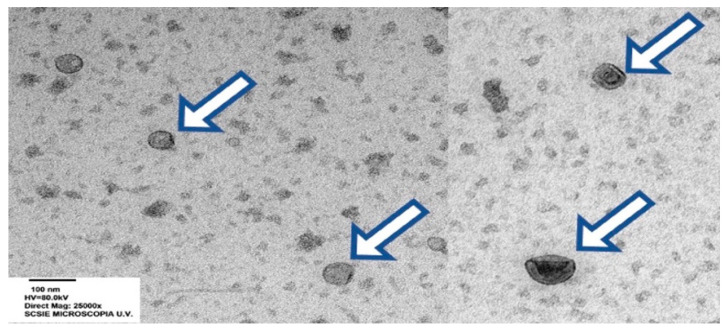
Having reached this section in this review essay, it appears confirmed that the SP is evidently far from being a simple fluid, as it contains diverse molecules. Analysis of a tube containing a collected ejaculate that has been spun at relatively low speed/time to form a pellet of the suspended spermatozoa and eventual somatic cells revealed—using electron microscopy, for instance [134]—a fluid with a large number of membrane vesicles of various dimensions, aspect and, particularly, origin (Figure 3).
Figure 3.
Transmission electron micrographs of extracellular vesicles (EVs, arrows) in porcine seminal plasma (bar: 100 nm), courtesy of Barranco et al. [39].
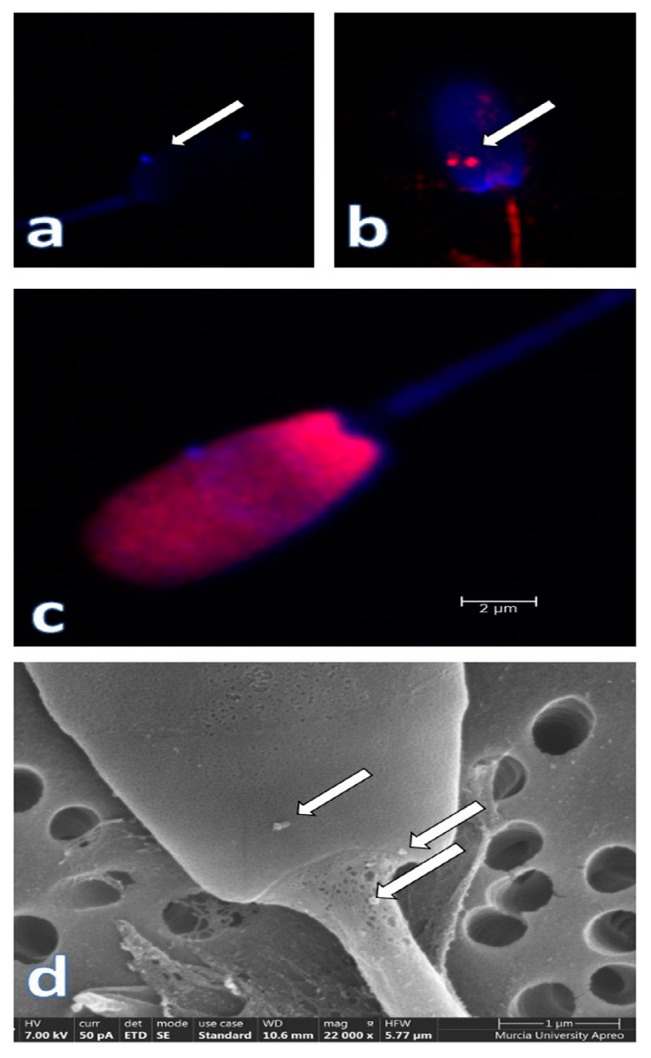
These EVs are shed by the different organs that contribute their secretion to the seminal plasma, namely, the testes, ducti epididymis and the accessory sexual glands, explaining why they display diverse cell-derived membrane structures. These EVs sequentially interact with the spermatozoa (Figure 4) in their journey towards ejaculation and, when ultimately within the female genital tract, interact with its epithelial lining.
Figure 4.
Seminal plasma extracellular vesicles (EVs) attached to the plasmalemma of ejaculated pig spermatozoa (arrows). Confocal microscopy microphotographs show the CD63 (a) (blue, Alexia fluor 405) and CD9 (b) (red, phycoerythrin, arrows) immunostained EVs. In (c), a composite image of a spermatozoon stained with Hoechst 33,342 and propidium iodide, while a scanning electron microscopy (SEM) micrograph showing some EVs attached to the membrane in the neck and head sperm domains is shown in (d). Courtesy of our graduates L Padilla and I Barranco.
Such interactions with the female are well reviewed in reference [135], and the large number of references therein. EVs are conserved structures along humans [136] and animals [39,40,75,137,138,139,140], and thus they are prompt to explain how influential they might be for crucial events in reproduction. Just consider the fact that the most relevant of these lipid bilayer nanovesicles, either being exosomes (processed multivesicular bodies released by exocytosis, of 30–100 nm diameter) or the 100–1000 nm size out-budded plasma membrane microvesicles), encapsulate a rather complex load of lipids, signaling proteins, small non-coding and regulatory RNAs [134,140], which basically define the SP EVs as information carriers from the producing organs to specific targets, these being spermatozoa or the female internal genital tract. Their role is to modify the functions related to sperm motility [141], capacitation [142,143,144] and perhaps fertilizing capacity, or even the immune responsiveness of the female against paternally derived antigens [102]. These active biomolecules are better protected from degradation and loss-of-function within EVs than the free SP-molecules are, an aspect that we should seriously consider if we aim to find relevant biomarkers for (dys)function [145] or use as signaling components, or even as particulate therapeutic additives [146].
However, let us go back to the fact that the SP is built by several secretions and, moreover, the fact that spermatozoa are exposed to EVs during one of the most crucial periods once shed from the seminiferous epithelium: sperm maturation in the epididymis [147]. During this journey, lasting days, the spermatocrit is concentrated, and the spermatozoa sequentially gain capacity to move forward and to fertilize [148,149], to be finally stored, quiescent, in the epididymal cauda [147,150]. During this processing, the spermatozoa are exposed to electrolyte changes, to an hyperosmotic and increasingly acidic milieu [150], but, noteworthy, also to the bombardment of 20–250 size EVs (named epididymosomes, [75,151] that are shed by the lining principal cells and that modify their cargo of protein [152,153], which includes antioxidative enzymes [42,129,132,154,155] as well as their phospholipid and cholesterol content [156], conveying an extensive lipid remodeling [157,158] that would stabilize the plasmalemma but also prepare for the destabilization changes that occur during capacitation. The EVs also contain a very particular load of ncRNA (particularly miRNAs, [140,159,160], a matter we shall discuss later. Epididymosomes are tethered to receptors on the sperm plasma membrane via proteins as tetraspanins [161] or cell-surface glycoproteins involved in cell-to-cell interaction, such as CD44 [40]. These proteins are up to now mostly used as markers [39,40] despite their roles in the biogenesis, cargo selection and cell targeting of the EVs, or in cell contact and uptake [161]. Considering the latter, CD44, suggested as an eventual marker for epididymosomes [40], is the main receptor for hyaluronan, the most conspicuous glycosaminoglycan present in the oviductal sperm reservoir and the expanded cumulus cells [105], and recognized as interplay between the spermatozoa and its surroundings in crucial events such as colonization of the tubal sperm reservoir or capacitation, as well as sperm location of the oocyte [105]. In sum, it appears evident that epididymosomes convey fundamental mediation during sperm maturation [162] (Cornwall 2009), promoting acquisition of sperm motility, oxidation–reduction, metabolism, capacitation, acrosome reaction and fertilization [137,152,153,163,164,165]. Alongside, there seems that the epididymosome load of ubiquitin [166] or ELSPBP1 (epididymal sperm binding protein 1) [167] aid tagging defective or dead spermatozoa that are to be eliminated along the journey or after ejaculation [152,166,167,168]. Epididymosomes are also contained in the caudal fluid that accompanies the spermatozoa emitted during ejaculation [40,169]. In species with fractionated ejaculates, the first portion/s is/are often called the sperm-peak portion [4], being the one primarily extended with prostatic fluid and the first to enter the female genital tract and whose fortuitously contained spermatozoa are even overrepresented in the oviductal sperm reservoir [26]. Obviously, epididymosomes can modify both ejaculated spermatozoa [40,75] and/or the influence the female genital tract [27,40,75,102]. In chickens, where a rudimentary epididymis is prevalent, we have not been successful in detecting relevant amounts of epididymosomes in seminal fluid, at least not using the same tetraspanin/CD44 markers we used in pigs [170]. In turn, a very recent study, using ultracentrifugation, the WB of the protein markers and EM has shown the presence of small EVs (possibly exosomes), in the seminal fluid of roosters with different fertility with capacity to putatively incorporate into spermatozoa [171]. Our own studies using pig as model animal have shown that specific fractions of the ejaculate or even their sperm-free SP elicit dramatic modifications to the expression of genes related to sperm survival and function as well as to the immune status of the female’s various compartments of the internal genital tract—pre-ovulation [29,172] but even during early embryo development [173]. We have seen a similar set of results in chicken in response to mating, despite the uncertainty of the presence of similar types/amounts of EVs in roosters [170], implying the response might well be a conserved mechanism in animals with internal fertilization [3]. Since there is a mix of EVs shed by the prostate or the seminal vesicles (or even perhaps the bulbourethral glands) [76,169,174,175], pig SP EVs show heterogeneity in surface markers [39,40,140], probably because they also have a unique high level of cholesterol and sphingomyelin [143] and of lipoproteins (LDL/HDL) [40], which, by stabilizing the plasma membrane, could later aid in regulating sperm membrane fluidity before and during sperm capacitation [56]. As mentioned above, the SP EVs have demonstrated capacity to bind to and even fuse with the sperm plasma membrane to deliver their cargo [76,138,176,177], influencing sperm function [142,143,144], but also via proteins such as CD59, to protect spermatozoa from responses by the immune system [135]. SP EVs have also proven, as did other EVs [178], to influence the local immunity of the female internal tract [179], and to induce inflammatory and immune responses by pig uterine cells [4,38,180]. Whether the changes in immune-related genes we have detected in peri-ovulatory sows following mating or AI, with selected ejaculate fractions (particularly the sperm-peak portion) or their sperm-free SP [29], or even in sows having pre-implantation embryos [173], are conveyed mainly by free molecules, by SP-EVs or it is the effect of a concerted signaling, remains to be experimentally tested. Such exploration of the action of the epididymosomes, prostasomes and vesiculosomes is possible using a proper experimental design, in pigs and in other species with fractionated ejaculation, since the various fractions containing major subpopulations of EVs can be manually collected. Their separate harvesting and characterization, if possible, could make possible the design of experiments for their separate roles on spermatozoa and in females, in vitro or in vivo. The prospect of using EVs as biomarkers for fertility or additives for improvement is exciting, considering that the use of crude SP or even isolated proteins/enzymes suffers from a short functional lifespan often caused by nearby proteases.
There is increasing evidence that the load of non-coding, smallRNA molecules in semen differentially contributes to reproduction, and that the dysregulation of their expression can explain many dysfunctions associated with infertility [181]. Small RNAs are present within spermatozoa [27] as well as in the SP-EVs [140] and most likely free in the SP, or bound to membrane structures (of the spermatozoa, other cells or cell debris). These RNA molecules are part of the set of paternal RNAs whose transcriptional origin differs in between. Some of these were originally transcribed in the testes and retained selectively in spermatozoa entering the epididymis; another set were RNAs of epididymal or accessory gland origin that were added to the spermatozoa via EVs. Finally, we have paternal RNAs that, enclosed in EVs or free in the SP, further directly interact with the female internal genital tract.
Processing of the collected semen is of utmost importance since it can surely mix up the different allocations of the small RNAs being detected. Many small RNAs have been explored and detected in the male genital tract and semen, including microRNAs (miRNAs), PIWI-interacting RNAs (piRNAs), YRNA and transfer RNA (tRNA)-derived small RNAs (tsRNAs) [136,182,183,184]. The most commonly explored small RNAs are the single-stranded 22–24 nucleotides miRNAs, considered important regulators of post-transcriptional gene expression [182,185] and detected in the testicles, spermatozoa, free in the SP and within SP EVs [181]. The secondly most found are the piRNAs, built by 24–31 nucleotides, which are able to bind to the PIWI proteins to modulate spermatogenesis [186]. Moreover, there is evidence that circular RNAs (circRNAs) are also present in spermatozoa [187], including the pig [188]. The small RNAs present in boar semen are particularly detected within spermatozoa, accounting for about 1% of the sperm total RNAs, and include miRNAs, tRNA fragments, piwiRNAs, miRNAs and YRNAs [182], but also the above mentioned circRNA. The latter appears to be an in vivo long-lived (24 h) RNA molecule owing to its closed-loop structure, which can both act as a sponge for miRNAs, protecting them from degradation, but even be related to sperm phenotypic variables, such as sperm motility [188].
Differential analyses of the pig sperm transcriptome have shown the relevant associations with sperm quality [189] and fertility [27,190], confirming data in other species [182,191,192,193,194]. The SP EVs also contain diverse smallRNAs, among which the majority are messenger RNA fragments (mRNA, about 25% of the total reads) followed by piRNAs (15%, almost 20,000), miRNAs (9%, almost 300) and very small amounts of tsRNA (0.01%) [140]. The most abundant, likely functional miRNA was ssc-miR-21-5p, which is related to the inhibition of the VCL gene that, by constraining sperm capacitation, is considered to negatively affect fertility [195]. The other most common miRNAs were ssc-miR-148a-3p, ssc-miR-10a-5p as well as ssc-miR-200b and ssc-miR-10b [140], the latter present within spermatozoa and in SP EVs [189]. Such double location reinforces the need of considering the role of EVs as adding material to the spermatozoa yet reminding us of the relevance of proper isolation of the components of semen, so that they can sequenced separately but compared at the same time. Unfortunately, sample sizes are uneven, and semen from boars with well-studied and established fertility must be primarily studied [190], a shortage nowadays. However, how are these miRNAs affecting fertility? Directly, by affecting sperm quality, of course [196]. However, for some species, such as bovine or particularly porcine, breeding males are selected for fertility and discarded when their AI-results are compromised, even when their sperm quality surpasses the established thresholds for normality [197]. So, what could these miRNAs rule that impacts fertility? As already mentioned, SP has the capability of eliciting inflammatory and long-lasting immune signaling by the female genital tract [27,29,117,173], being even able to cause release of miRNAs from the lining epithelia [180]. Responses from the female can be issued by spermatozoa alone [198] or even by the act of copulation [199], but the evidence is that these changes are rather modest, compared to what the SP can elicit, either alone or as semen (i.e., including the suspended spermatozoa). Many of the top miRNAs detected in pig spermatozoa [27] and SP EVs [140] can impact TLR4 signaling [200], and promote the production of cytokines that modulates immune responses [27,180,201] to the extent of modulating the fertility of the males considered [27,190].
4. What Proof Do We Really Have That Seminal Plasma Really Affects Fertility?
We have assumed that the fertility of a sire is not only ruled by the spermatozoa but also by the SP. However, can we separate these two intrinsic components of semen? One could state that this is what has been proven when IVF was established, putting most weight on spermatozoa, which included the removal of the native SP. IVF and intracytoplasmic sperm injection (ICSI) of ejaculated or epididymal spermatozoa, or even elongated spermatids that have never gone through sperm maturation, has resulted in fertilization, embryo development, pregnancies and births in humans [202] and animals [203], implying that the SP is not a mandatory component. However, human fertility (as baby births) after embryo transfer of IVF/ICSI “fertilized” oocytes has been steady around 30% since 1994, in general terms [204]. Such a steady low efficiency has, together with experimental evidence of the effects of seminal plasma on the female, called for the application of SP during or after human embryo transfer [205,206,207]. Growing evidence has established SP playing major roles in embryo development and birth rates [102], and although the matter remains unsolved for humans, the experimental evidence from various animal models has quite nicely defined these effects on the female [12,93,120] (Figure 5).
Figure 5.
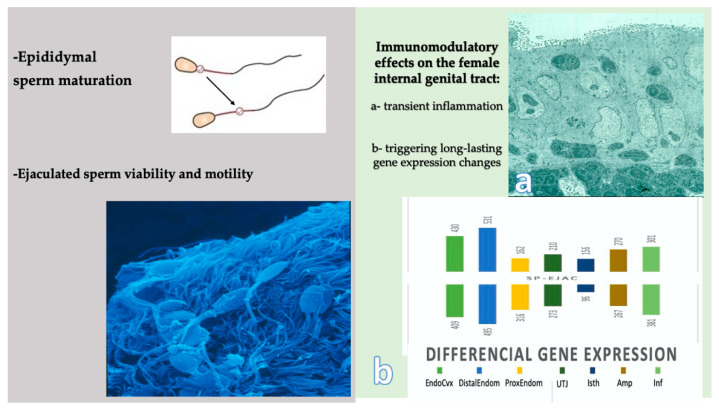
Major recognized functional effects of seminal plasma (SP) in vivo. In the male (left panel), the cytoplasmic sperm cytoplasmic droplet is lost at ejaculation, and SP promotes forward motility of the viable ejaculated spermatozoa along the female genital tract. In the female (right panel), SP elicits immunomodulatory effects in the genital tract, firstly through a migration of leukocytes from the lamina propria through the epithelium during an initial transient inflammation (a), concomitant with a triggering of long-lasting differential gene expression changes in the female (b): EndoCvx: endocervix; Distal-Prox Endom: distal/proximal endometrium; UTJ: utero-tubal junction; Isth: isthmus; Amp: ampullas; Inf: infundibulum.
The composition of SP and the differences among the ejaculate fractions has help define the possible influence on the fertility of the male [88,96] via interactions with the female genital tract [27,29,208,209]. Fractions seem to vary in their capacity to elicit inflammation, i.e., the porcine post-SRF fraction, by containing higher amounts of SP-proteins, particularly of the PSP-I/PSP-II, is capable to induce entry of polymorphonuclear leukocytes to the uterine lumen within minutes of exposure [25] (Figure 5). Thus, the post-SRF is considered as less permissive for fertility, compared to the SRF that is “less” protein-rich, and contains many of the proteins and peptides present in the sperm-friendly caudal epididymal fluid [4,11,210]. However, one should not forget that the situation in vivo—particularly for species with fractionated ejaculation and semen deposition intra-cervix or utero—is far from the analysis of an ejaculate in a test tube.
Do we have evidence that the SP per se is, by inducing the changes of gene expression in the internal genitalia of the female we have already pointed out, able to mark differences in fertility when added separately from spermatozoa? The answer seems to be yes, as rates in early embryo survival [88,93,102,120,173,208], implantation [101,102,115], placental development [116], farrowing, litter size and even offspring development and health [44] could be improved by additional inseminations with homologous/pools of SP.
Many of the above studies, and of many others being rather empirical in nature, however, show simple associations between the presence of SP and effects, with some of these complex in nature. The evidence gathered via association studies, at least regarding livestock fertility, show for proteins [11,33,35,36,43] or cytokines [41,70] either positive, negative or ambiguous statistical relations. This variation is not surprising, considering inter- or intra-male variations in composition and the relative amounts of these molecules and even the effects of handling [43,58,109]. Such variations, together with specific differences in experimental layout, are present among the plethora of contradictory reports [36,94,131,211,212]. However, this should not be a definitive hurdle, and finding a direct relation between a particular protein and fertility, despite remaining elusive, should be the purpose of future research to either secure suitable biomarkers for diagnostic/prognosis purposes or even the isolation of specific molecules to be used as additives. Associations are not definitory and, particularly, do not reveal mechanistic elements, calling for deeper experimental studies. We have attempted to recently disclose the role that native SP infusions during estrus (i.e., in relation to AI) could have on the modification of certain cytokines produced by the endometrium and released to the lumen (rather than focusing on the SP-cytokines) during the pre-implantation period. Such an estrus SP infusion has been tested earlier [213], revealing that several cytokines, such as GMCSF (relevant to early embryo development and present in pig genitalia) [108], were related to evident changes in pre-implantational embryo development, a matter we have confirmed; infusions of SP modifies the transcriptional pattern of the endometrium and advances embryo development in pigs [208]. Noteworthy, the uterine environment during the pre-implantational period in pigs seems to have a dominant anti-inflammatory cytokine profile [93] throughout, and the SP infusions definitely induce overexpression of genes associated with embryo development and implantation in Day 6 porcine blastocysts [173] (Figure 6).
Figure 6.
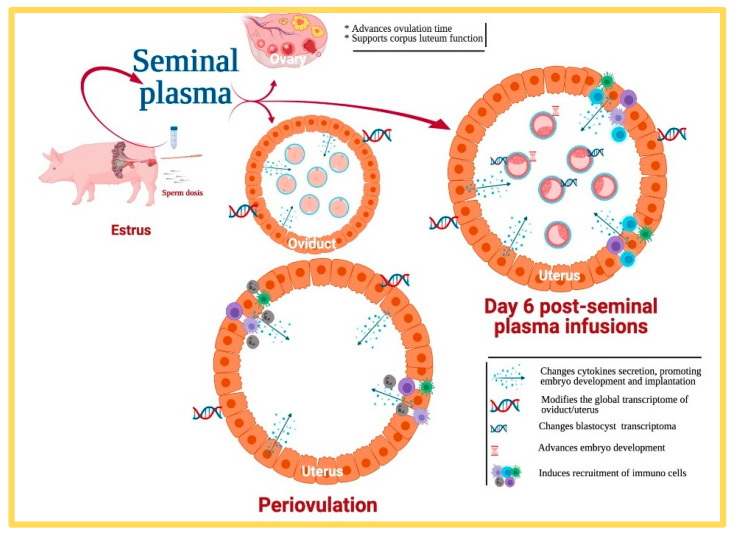
Infusions of additional sperm-free pig seminal plasma during estrus causes a plethora of functional effects in the female, from advancing ovulation, supporting corpora lutea development, to a significant number of events six days later, at the oviduct and uterus.
Among the relevant genes overexpressed, SP-treated sows showed an overactivation of the transforming growth factor-ß (TGF-ß) signaling pathway, known as the pathways supporting the proliferation of Treg cells by regulating dendritic cell function [214] and thus increasing the development of Treg cells and helping control the immune response of the female in response to the presence of hemi-allogeneic embryos as early as Day 6 of pregnancy [215]. Since TGF-ß is recognized as present in boar SP [106,107,213] and its action has been tested in relation to litter size [216], the question arose as to whether its infusion at estrus would be responsible for the above changes seen. The experiment we performed compared intrauterine infusion of TGF-ß1 with native SP prior to insemination for their effects on both endometrial cytokines and on the development of pre-implantation embryos. It was rather evident that both SP and TGF-ß1 were able to influence endometrial cytokines, but only SP impacted pre-implantation pig embryo development, thus implying SP has a concerted action of various factors influencing embryo development by way of a modified gene expression by the female endometrium [120]. Finding out which particular factors are mechanistically most relevant seems appropriate and experimentally possible, albeit complicated. However, the prospect of finding a group of relevant biomarkers, and eventually additives, raises the bid.
5. What Else Can Be Done with Our Current Knowledge of SP?
5.1. Can We Improve Andrology Diagnosis by Using Specific SP Biomarkers?
Most likely; this is indicated by the many SP proteins that have been associated with decreased motility and viability, and further to fertility [32,34,63,94,217], since these would be very valuable for diagnosing which sires can be prevented or removed from breeding. With some components of SP being associated with (in)fertility, identification of the males bearing presence or most-likely higher amounts of some can aid their removal as breeding sires, paving the way for the use of more fertile ones, and thus via diagnostics improve fertility in the long run. Molecules showing association with (in)fertility are innumerable, but association between variables is not definitory of function in a general population. Markers for sperm dysfunction [218] and (in)fertility have been detected in spermatozoa [27,190,219,220] and in the SP [221,222] of many species, including humans [218,222,223,224,225] and livestock [70,226]. They vary from gene presence [227], markers of Sertoli cell function [228], proteins [36,229,230,231], anti-oxidative enzymes [223,232,233] and metabolites [234,235,236] to exosome-associated molecules [237,238] and microRNAs [181,239,240,241].
On the down side, the methodologies to identify these markers are rather complicated and still only available at specialized laboratories [97], but we have seen major developments to mark the presence or even levels of specific proteins using sensors, so that these methods will probably evolve for wider use. In addition, seminal plasma handling protocols would have to be standardized to achieve comparable results between laboratories [109].
5.2. Can We Enhance Sperm Function and Cryosurvival Using Seminal Plasma?
A parallel approach, complementing diagnostics for (in)fertility, is to identify molecules that enhance fertility in the semen of individuals already fertile. Yet, we need to remember that SP has been historically described as friend and foe to sperm survival. Being evolutionarily present, its roles for sperm function depend on what we consider. Spermatozoa are highly specialized cells, programmed for destabilization during their journey to the site of fertilization. They are covered by SP proteins whose adsorption to the plasma membrane prevent its destabilization and disruption, once gathered under the title of “decapacitation factors” [210], relevant for in vitro processing and in vivo survival [100,242]. However, this friendly role can soon change. For instance, if maintained in its native SP on a lab bench or even in a very simple medium, ejaculated spermatozoa will sequentially perish, mostly due to temperature changes, a lack of energy substrates and due to the action of the proteolytic enzymes in the SP and the dead cells, which will moreover change the osmolarity and ionic composition in the suspension. However, before this happens, many spermatozoa will interestingly capacitate [243], simply because many SP proteins shall aggregate and separate from the sperm plasma membrane and because phospholipid-binding SP proteins will induce continuous cholesterol efflux [244,245,246]. Consequently, for some species (pig and horse [247]), the SP is customarily removed during semen processing, but maintained for others, i.e., ruminants, where the problem seems minor, nonexistent, or its presence is considered vital [248,249]. The species differences accompany particularities in ejaculation and semen deposition in vivo; the first species having fractionated, intra-utero deposition, while the latter are vaginal depositors of a bulk, highly concentrated ejaculate.
Semen being processed, for any species, seems to benefit from the presence of SP for sperm survival and function, for instance when high-extension rates are applied [248,250,251]. In species with fractionated ejaculation of less concentrated semen, such as pigs and horses, SP is relevant when the semen is cooled [250,251,252,253,254], and particularly, when cryopreserved (Figure 7) [2,22,43,95,255], to the extent of affecting embryo development in vitro [256]. The impact of SP on freezability enabled not only the selection of males as good or bad freezers [70,257,258] but proved that the addition of SP from good-freezer boars could improve the cryosurvival of the spermatozoa of other males [259], and even increase fertility in equines [17]. Several conclusions could be made from these experiments, namely, that spermatozoa require the presence of a certain time of exposure to SP [2,260], or even a certain proportion of SP all along (often around an empirical proportion rate of 10–20% [16,22,261], in order to maintain sperm survival and fertility post AI. The moment of SP addition varied, in most instances appearing most efficient when added post-thaw [18,22,261,262,263,264].
Figure 7.
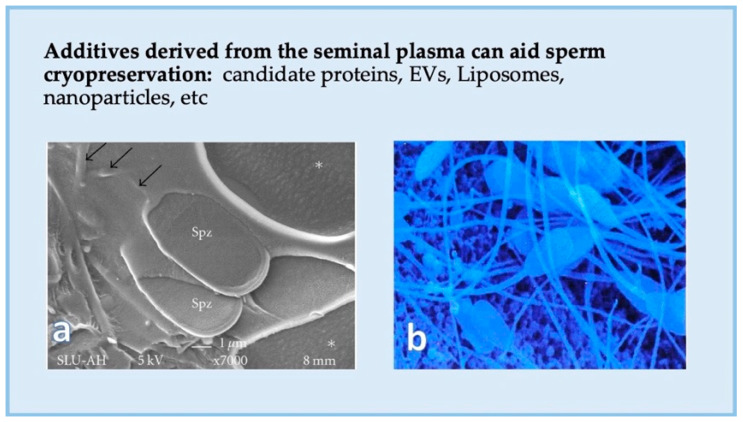
Additives derived from the seminal plasma can aid the cryopreservation of pig spermatozoa. Scanning electron microscopy (SEM) micrographs of (a) frozen (arrows show the frozen extender, embedding spermatozoa (spz and small arrows) as well as areas of frozen free water (*), Cryo-SEM), and (b) frozen–thawed boar spermatozoa.
6. The Future
In the end, would it be at all possible to use exogenous SP components to improve fertility after AI with extended, cooled, or frozen–thawed semen? Probably, the pertaining experimentation provides us with irrefutable proof that the associations seen between diverse molecules in SP and the fertility of the males go beyond that: associations. The reason for these words of caution resides in the fact that, despite many molecules, particularly proteins or antioxidative enzymes were presented as directly related to male or semen fertility, and their effect on semen has been elusive, sometimes arguing that the relation-to-effect seen was probably concerted, multifactorial and not simply related to one particular molecule. Moreover, the administration of such molecules, and particularly its resistance to degradation and its capacity to act onto living spermatozoa, are considered relevant hurdles to be explored. Hope is now focused on the growing knowledge on EVs and their functional cargo, mostly because they would be protected by the membrane-built EVs and because they would probably be the best delivered to living spermatozoa [175]. There are several strategies, such as isolation and preservation of the EVs derived from the SP of recognized and well-documented highly fertile males, to be used as additives for improvement of fertility [265]. The rationale follows proven experiments where the SP of good freezers could improve the cryosurvival in less-good freezers [259]. However, one matter is cryosurvival and another fertility as such, although they are highly related. Another approach could be the design of “synthetic EVs”, mimicking liposomes [266], which have been proven as relevant for protecting spermatozoa during cooling and post-AI. An intermediate approach is to combine the isolation of native SP EVs with techniques to upload specific molecules, relevant for fertility. All these approaches are technically possible today, particularly when intending to improve in vitro embryo production (IPV) (Figure 8).
Figure 8.
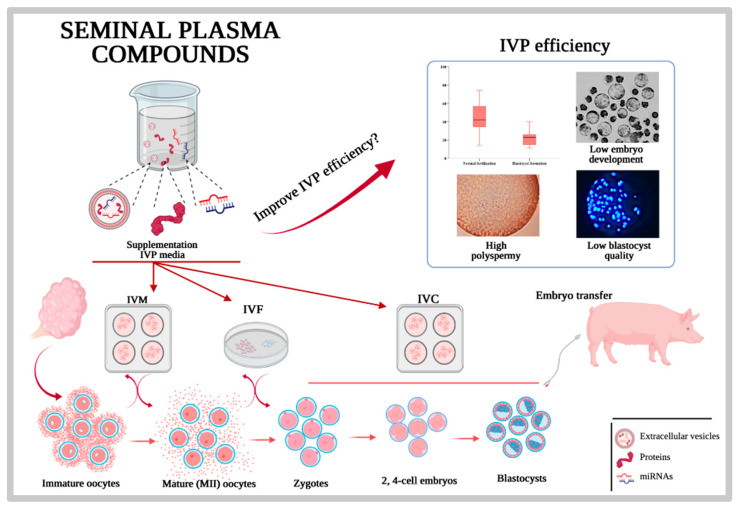
Seminal plasma can via specific proteins, native or synthetic extracellular vesicles and miRNas improve the nowadays sub-optimal in vitro porcine embryo production (IVP).
However, we still need to determine the mechanisms ruling fertility for many of the molecules present in these SP-EVs, and the role they play on the immunoregulation of the female genital tract, modulating the capacity of the female to allow the presence and development of pregnancy to term, and for some species of interest, particularly for their value as livestock but also as suitable animal models for human reproduction.
7. Conclusions
This review has critically summarized the current knowledge of the roles the SP and its particular components apparently at play in ruling fertility and prolificacy. Its focus has been to provide a comparative view, including humans, and considering suitable experimental animal models and livestock as well. The SP’s importance was particularly discussed in relation to sperm survival, function and signaling to the female immune system towards fertility modulation. Considering many in vivo findings with major relevance for the development of diagnostic/prognostic biomarkers and the refinement of reproductive strategies, particular interest has been put on the immunoregulation of the female genitalia, since it appears determinant for fertility in all species and even animal classes explored so far. Based on the available knowledge, it is expected that novel experimental research on SP EVs shall be of utmost relevance to advance not only our knowledge but our capacity to manipulate fertility.
Acknowledgments
To all hereby cited past and current graduate students and post-docs.
Author Contributions
Conceptualization, H.R.-M.; writing—original draft preparation, H.R.-M.; writing—review and editing, H.R.-M., E.A.M., J.J.C., F.J.P.V. and J.R.; project administration, H.R.-M., E.A.M., J.J.C., F.J.P.V. and J.R.; funding acquisition, H.R.-M., E.A.M., J.J.C., F.J.P.V. and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
The research of the authors was funded by the Research Council FORMAS, Stockholm (Project 2017-00946 and Project 2019-00288), the Swedish Research Council (Vetenskapsrådet, VR; project 2015-05919) Stockholm, Sweden and the MICINN (Spain) and FEDER EU-funds (AGL2015-69735-R, AGL2015-69738-R, PID2020-113493RB-100, RTI2018-093525-B-I00), Madrid, Spain.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design, writing or in the decision to publish the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rodríguez-Martínez H., Saravia F., Wallgren M., Roca J., Peña F.J. Influence of seminal plasma on the kinematics of boar spermatozoa during freezing. Theriogenology. 2008;70:1242–1250. doi: 10.1016/j.theriogenology.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Saravia F., Wallgren M., Johannisson A., Calvete J.J., Sanz L., Peña F.J., Roca J., Rodríguez-Martínez H. Exposure to the seminal plasma of different portions of the boar ejaculate modulates the survival of spermatozoa cryopreserved in MiniFlatPacks. Theriogenology. 2009;71:662–675. doi: 10.1016/j.theriogenology.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 3.Atikuzzaman M., Alvarez-Rodriguez M., Vicente-Carrillo A., Johnsson M., Wright D., Rodriguez-Martinez H. Conserved gene expression in sperm reservoirs between birds and mammals in response to mating. BMC Genomics. 2017;18:98. doi: 10.1186/s12864-017-3488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Martinez H., Kvist U., Saravia F., Wallgren M., Johannisson A., Sanz L., Peña F., Martinez E., Roca J., Vazquez J., et al. The physiological roles of the boar ejaculate. Soc. Reprod. Fertil. Suppl. 2009;66:1–21. doi: 10.1530/biosciprocs.18.0001. [DOI] [PubMed] [Google Scholar]
- 5.McGraw L.A., Suarez S.S., Wolfner M.F. On a matter of seminal importance. Bioessays. 2015;37:142–147. doi: 10.1002/bies.201400117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromfield J.J. Seminal fluid and reproduction: Much more than previously thought. J. Assist. Reprod. Genet. 2014;31:627–636. doi: 10.1007/s10815-014-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Martinez H. Assisted reproductive techniques for cattle breeding in developing countries: A critical appraisal of their value and limitations. Reprod. Domest. Anim. 2012;47:21–26. doi: 10.1111/j.1439-0531.2011.01961.x. [DOI] [PubMed] [Google Scholar]
- 8.CHANG M.C. A detrimental effect of seminal plasma on the fertilizing capacity of sperm. Nature. 1957;179:258–259. doi: 10.1038/179258a0. [DOI] [PubMed] [Google Scholar]
- 9.Pavaneli A.P.P., da Silva Passarelli M., de Freitas F.V., Ravagnani G.M., Torres M.A., Martins S.M.M.K., Yeste M., de Andrade A.F.C. Removal of seminal plasma prior to liquid storage of boar spermatozoa: A practice that can improve their fertilizing ability. Theriogenology. 2019;125:79–86. doi: 10.1016/j.theriogenology.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Mogielnicka-Brzozowska M., Kordan W. Characteristics of selected seminal plasma proteins and their application in the improvement of the reproductive processes in mammals. Pol. J. Vet. Sci. 2011;14:489–499. doi: 10.2478/v10181-011-0074-z. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Martinez H., Kvist U., Ernerudh J., Sanz L., Calvete J.J. Seminal plasma proteins: What role do they play? Am. J. Reprod. Immunol. 2011;66:11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 12.Funahashi H. Methods for Improving In Vitro and In Vivo Boar Sperm Fertility. Reprod. Domest. Anim. 2015;50(Suppl. 2):40–47. doi: 10.1111/rda.12568. [DOI] [PubMed] [Google Scholar]
- 13.Robertson S.A., Sharkey D.J. Seminal fluid and fertility in women. Fertil. Steril. 2016;106:511–519. doi: 10.1016/j.fertnstert.2016.07.1101. [DOI] [PubMed] [Google Scholar]
- 14.Rozeboom K.J., Troedsson M.H., Hodson H.H., Shurson G.C., Crabo B.G. The importance of seminal plasma on the fertility of subsequent artificial inseminations in swine. J. Anim. Sci. 2000;78:443–448. doi: 10.2527/2000.782443x. [DOI] [PubMed] [Google Scholar]
- 15.Abad M., Sprecher D.J., Ross P., Friendship R.M., Kirkwood R.N. Effect of sperm cryopreservation and supplementing semen doses with seminal plasma on the establishment of a sperm reservoir in gilts. Reprod. Domest. Anim. 2007;42:149–152. doi: 10.1111/j.1439-0531.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 16.Abad M., Garcia J.C., Sprecher D.J., Cassar G., Friendship R.M., Buhr M.M., Kirkwood R.N. Effect of insemination-ovulation interval and addition of seminal plasma on sow fertility to insemination of cryopreserved sperm. Reprod. Domest. Anim. 2007;42:418–422. doi: 10.1111/j.1439-0531.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 17.Heise A., Kähn W., Volkmann D.H., Thompson P.N., Gerber D. Influence of seminal plasma on fertility of fresh and frozen-thawed stallion epididymal spermatozoa. Anim. Reprod. Sci. 2010;118:48–53. doi: 10.1016/j.anireprosci.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 18.García J., Domínguez J.C., Peña F., Alegre B., Gonzalez R., Castro M.J., Habing G., Kirkwood R. Thawing boar semen in the presence of seminal plasma: Effects on sperm quality and fertility. Anim. Reprod. Sci. 2009;119:160–165. doi: 10.1016/j.anireprosci.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 19.McPherson F.J., Nielsen S.G., Chenoweth P.J. Semen effects on insemination outcomes in sows. Anim. Reprod. Sci. 2014;151:28–33. doi: 10.1016/j.anireprosci.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Chutia T., Biswas R.K., Tamuli M.K., Deka B.C., Sinha S., Goswami J., Banik S., Kayastha R.B. Effect of holding of semen and washing of seminal plasma on quality and fertility of Hampshire boar semen preserved at liquid state. Anim. Reprod. Sci. 2014;145:141–149. doi: 10.1016/j.anireprosci.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Tsikis G., Reynaud K., Ferchaud S., Druart X. Seminal plasma differentially alters the resistance of dog, ram and boar spermatozoa to hypotonic stress. Anim. Reprod. Sci. 2018;193:1–8. doi: 10.1016/j.anireprosci.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Recuero S., Fernandez-Fuertes B., Bonet S., Barranco I., Yeste M. Potential of seminal plasma to improve the fertility of frozen-thawed boar spermatozoa. Theriogenology. 2019;137:36–42. doi: 10.1016/j.theriogenology.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz W.G., Rizo J.A., Carvalheira L.R., Ahmed B.M.S., Estrada-Cortes E., Harstine B.R., Bromfield J.J., Hansen P.J. Effects of intrauterine infusion of seminal plasma at artificial insemination on fertility of lactating Holstein cows. J. Dairy Sci. 2019;102:6587–6594. doi: 10.3168/jds.2019-16251. [DOI] [PubMed] [Google Scholar]
- 24.Mann T., Lutwak-Mann C. In: Male Reproductive Function and Semen. Mann T., Lutwak-Mann C., editors. Springer; London, UK: 1981. [Google Scholar]
- 25.Rodriguez-Martinez H., Saravia F., Wallgren M., Martinez E.A., Sanz L., Roca J., Vazquez J.M., Calvete J.J. Spermadhesin PSP-I/PSP-II heterodimer induces migration of polymorphonuclear neutrophils into the uterine cavity of the sow. J. Reprod. Immunol. 2010;84:57–65. doi: 10.1016/j.jri.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Wallgren M., Saravia F., Rodriguez-Martinez H. The vanguard sperm cohort of the boar ejaculate is overrepresented in the tubal sperm reservoir in vivo. J. Reprod. Dev. 2010;56:68–72. doi: 10.1262/jrd.09-125K. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Rodriguez M., Martinez C., Wright D., Barranco I., Roca J., Rodriguez-Martinez H. The Transcriptome of Pig Spermatozoa, and Its Role in Fertility. Int. J. Mol. Sci. 2020;21:1572. doi: 10.3390/ijms21051572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atikuzzaman M., Mehta Bhai R., Fogelholm J., Wright D., Rodriguez-Martinez H. Mating induces the expression of immune- and pH-regulatory genes in the utero-vaginal junction containing mucosal sperm-storage tubuli of hens. Reproduction. 2015;150:473–483. doi: 10.1530/REP-15-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez-Rodriguez M., Atikuzzaman M., Venhoranta H., Wright D., Rodriguez-Martinez H. Expression of immune regulatory genes in the porcine internal genital tract is differentially triggered by spermatozoa and seminal plasma. Int. J. Mol. Sci. 2019;20:513. doi: 10.3390/ijms20030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mateo-Otero Y., Sánchez J.M., Recuero S., Bagés-Arnal S., McDonald M., Kenny D.A., Yeste M., Lonergan P., Fernandez-Fuertes B. Effect of Exposure to Seminal Plasma Through Natural Mating in Cattle on Conceptus Length and Gene Expression. Front. Cell Dev. Biol. 2020;8:341. doi: 10.3389/fcell.2020.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atikuzzaman M., Sanz L., Pla D., Alvarez-Rodriguez M., Rubér M., Wright D., Calvete J.J., Rodriguez-Martinez H. Selection for higher fertility reflects in the seminal fluid proteome of modern domestic chicken. Comp. Biochem. Physiol. Part D Genomics Proteomics. 2017;21:27–40. doi: 10.1016/j.cbd.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Perez-Patiño C., Barranco I., Parrilla I., Valero M.L., Martinez E.A., Rodriguez-Martinez H., Roca J. Characterization of the porcine seminal plasma proteome comparing ejaculate portions. J. Proteomics. 2016;142:15–23. doi: 10.1016/j.jprot.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Patiño C., Parrilla I., Barranco I., Vergara-Barberán M., Simó-Alfonso E.F., Herrero-Martínez J.M., Rodriguez-Martínez H., Martínez E.A., Roca J. New In-Depth Analytical Approach of the Porcine Seminal Plasma Proteome Reveals Potential Fertility Biomarkers. J. Proteome Res. 2018;17:1065–1076. doi: 10.1021/acs.jproteome.7b00728. [DOI] [PubMed] [Google Scholar]
- 34.Pérez-Patiño C., Parrilla I., Li J., Barranco I., Martínez E.A., Rodriguez-Martínez H., Roca J. The Proteome of Pig Spermatozoa Is Remodeled During Ejaculation. Mol. Cell. Proteomics. 2019;18:41–50. doi: 10.1074/mcp.RA118.000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Druart X., Rickard J.P., Tsikis G., de Graaf S.P. Seminal plasma proteins as markers of sperm fertility. Theriogenology. 2019;137:30–35. doi: 10.1016/j.theriogenology.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 36.Kang S., Pang W.-K., Ryu D.-Y., Song W.-H., Rahman M.S., Park Y.-J., Pang M.-G. Porcine seminal protein-I and II mRNA expression in boar spermatozoa is significantly correlated with fertility. Theriogenology. 2019;138:31–38. doi: 10.1016/j.theriogenology.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 37.Ramm S.A., Oliver P.L., Ponting C.P., Stockley P., Emes R.D. Sexual selection and the adaptive evolution of mammalian ejaculate proteins. Mol. Biol. Evol. 2008;25:207–219. doi: 10.1093/molbev/msm242. [DOI] [PubMed] [Google Scholar]
- 38.Bai R., Latifi Z., Kusama K., Nakamura K., Shimada M., Imakawa K. Induction of immune-related gene expression by seminal exosomes in the porcine endometrium. Biochem. Biophys. Res. Commun. 2018;495:1094–1101. doi: 10.1016/j.bbrc.2017.11.100. [DOI] [PubMed] [Google Scholar]
- 39.Barranco I., Padilla L., Parrilla I., Álvarez-Barrientos A., Pérez-Patiño C., Peña F.J., Martínez E.A., Rodriguez-Martínez H., Roca J. Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles. Sci. Rep. 2019;9:11584. doi: 10.1038/s41598-019-48095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez-Rodriguez M., Ljunggren S.A., Karlsson H., Rodriguez-Martinez H. Exosomes in specific fractions of the boar ejaculate contain CD44: A marker for epididymosomes? Theriogenology. 2019;140:143–152. doi: 10.1016/j.theriogenology.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Barranco I., Padilla L., Perez-Patino C., Vazquez J.M., Martinez E.A., Rodriguez-Martinez H., Roca J., Parrilla I. Seminal Plasma Cytokines Are Predictive of the Outcome of Boar Sperm Preservation. Front. Vet. Sci. 2019;6:436. doi: 10.3389/fvets.2019.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barranco I., Roca J., Tvarijonaviciute A., Rubér M., Vicente-Carrillo A., Atikuzzaman M., Ceron J.J., Martinez E.A., Rodriguez-Martinez H. Measurement of activity and concentration of paraoxonase 1 (PON-1) in seminal plasma and identification of PON-2 in the sperm of boar ejaculates. Mol. Reprod. Dev. 2015;82:58–65. doi: 10.1002/mrd.22444. [DOI] [PubMed] [Google Scholar]
- 43.Parrilla I., Martinez E.A., Gil M.A., Cuello C., Roca J., Rodriguez-Martinez H., Martinez C.A. Boar seminal plasma: Current insights on its potential role for assisted reproductive technologies in swine. Anim. Reprod. 2020;17:e20200022. doi: 10.1590/1984-3143-ar2020-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan H.L., Watkins A.J. The influence of seminal plasma on offspring development and health. Semin. Cell Dev. Biol. 2020;97:131–137. doi: 10.1016/j.semcdb.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Beyler S., Zaneveld L. The Male Accessory Sex Glands. In: Zaneveld L., Chatterton R., editors. Biochemistry of Mammalian Reproduction. John Wiley & Sons; Hoboken, NJ, USA: 1982. pp. 65–88. [Google Scholar]
- 46.Lombardi J. Comparative Vertebrate Reproduction. Kluwer Academic Publishers; Boston, MA, USA: 1998. [Google Scholar]
- 47.LAKE P.E. The male reproductive tract of the fowl. J. Anat. 1957;91:116–129. [PMC free article] [PubMed] [Google Scholar]
- 48.Etches R.J. The Male. In: Etches R., editor. Reproduction in Poultry. CAB International; Wallingford, Oxon: 1996. pp. 208–233. ISBN 0851987389 9780851987385. [Google Scholar]
- 49.Lavon U., Boursnell J.C. The split ejaculate of the boar: Contributions of the epididymides and seminal vesicles. J. Reprod. Fertil. 1975;42:541–552. doi: 10.1530/jrf.0.0420541. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Martinez H. Sperm function in cattle and pigs: Morphological and functional aspects. Arch. Tierzucht. 2001;44:102–113. [Google Scholar]
- 51.Purvis K., Magnus O., Mørkås L., Abyholm T., Rui H. Ejaculate composition after masturbation and coitus in the human male. Int. J. Androl. 1986;9:401–406. doi: 10.1111/j.1365-2605.1986.tb00902.x. [DOI] [PubMed] [Google Scholar]
- 52.Owen D.H., Katz D.F. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 2005;26:459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 53.Kareskoski A.M., del Alamo M.M.R., Güvenc K., Reilas T., Calvete J.J., Rodriguez-Martinez H., Andersson M., Katila T. Protein composition of seminal plasma in fractionated stallion ejaculates. Reprod. Domest. Anim. 2011;46:e79–e84. doi: 10.1111/j.1439-0531.2010.01641.x. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida K., Kawano N., Yoshiike M., Yoshida M., Iwamoto T., Morisawa M. Physiological roles of semenogelin I and zinc in sperm motility and semen coagulation on ejaculation in humans. Mol. Hum. Reprod. 2008;14:151–156. doi: 10.1093/molehr/gan003. [DOI] [PubMed] [Google Scholar]
- 55.Jonsson M., Linse S., Frohm B., Lundwall A., Malm J. Semenogelins I and II bind zinc and regulate the activity of prostate-specific antigen. Biochem. J. 2005;387:447–453. doi: 10.1042/BJ20041424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodríguez-Martínez H. State of the art in farm animal sperm evaluation. Reprod. Fertil. Dev. 2007;19:91–101. doi: 10.1071/RD06104. [DOI] [PubMed] [Google Scholar]
- 57.Sobrero A.J., Macleod J. The immediate postcoital test. Fertil. Steril. 1962;13:184–189. doi: 10.1016/S0015-0282(16)34447-8. [DOI] [PubMed] [Google Scholar]
- 58.Padilla L., Lucas X., Parrilla I., Perez-Patiño C., Rodriguez-Martinez H., Roca J., Barranco I. Period of Boar Ejaculate Collection Contributes to the Yearly Intra-Male Variability of Seminal Plasma Cytokines. Biology. 2020;9:105. doi: 10.3390/biology9050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Claus R. Physiological role of seminal components in the reproductive tract of the female pig. J. Reprod. Fertil. Suppl. 1990;40:117–131. [PubMed] [Google Scholar]
- 60.Rodríguez-Martínez H., Saravia F., Wallgren M., Tienthai P., Johannisson A., Vázquez J.M., Martínez E., Roca J., Sanz L., Calvete J.J. Boar spermatozoa in the oviduct. Theriogenology. 2005;63:514–535. doi: 10.1016/j.theriogenology.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 61.Björndahl L., Kvist U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2010;16:23–29. doi: 10.1093/molehr/gap099. [DOI] [PubMed] [Google Scholar]
- 62.Duncan M.W., Thompson H.S. Proteomics of semen and its constituents. Proteomics. Clin. Appl. 2007;1:861–875. doi: 10.1002/prca.200700228. [DOI] [PubMed] [Google Scholar]
- 63.Druart X., Rickard J.P., Mactier S., Kohnke P.L., Kershaw-Young C.M., Bathgate R., Gibb Z., Crossett B., Tsikis G., Labas V., et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J. Proteomics. 2013;91:13–22. doi: 10.1016/j.jprot.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 64.Marzoni M., Castillo A., Sagona S., Citti L., Rocchiccioli S., Romboli I., Felicioli A. A proteomic approach to identify seminal plasma proteins in roosters (Gallus gallus domesticus) Anim. Reprod. Sci. 2013;140:216–223. doi: 10.1016/j.anireprosci.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Labas V., Grasseau I., Cahier K., Gargaros A., Harichaux G., Teixeira-Gomes A.-P., Alves S., Bourin M., Gérard N., Blesbois E. Qualitative and quantitative peptidomic and proteomic approaches to phenotyping chicken semen. J. Proteomics. 2015;112:313–335. doi: 10.1016/j.jprot.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 66.de Azevedo Viana A.G., Ribeiro I.M., Carvalho R.P.R., Memili E., Moura A.A., Machado-Neves M. Functional attributes of seminal proteins in bull fertility: A systematic review. Reproduction. 2021;161:459–475. doi: 10.1530/REP-20-0392. [DOI] [PubMed] [Google Scholar]
- 67.Westfalewicz B., Dietrich M.A., Mostek A., Partyka A., Bielas W., Niżański W., Ciereszko A. Analysis of bull (Bos taurus) seminal vesicle fluid proteome in relation to seminal plasma proteome. J. Dairy Sci. 2017;100:2282–2298. doi: 10.3168/jds.2016-11866. [DOI] [PubMed] [Google Scholar]
- 68.Samanta L., Parida R., Dias T.R., Agarwal A. The enigmatic seminal plasma: A proteomics insight from ejaculation to fertilization. Reprod. Biol. Endocrinol. 2018;16:41. doi: 10.1186/s12958-018-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leahy T., Rickard J.P., Bernecic N.C., Druart X., de Graaf S.P. Ram seminal plasma and its functional proteomic assessment. Reproduction. 2019;157:R243–R256. doi: 10.1530/REP-18-0627. [DOI] [PubMed] [Google Scholar]
- 70.Roca J., Perez-Patiño C., Barranco I., Padilla L.C., Martínez E.A., Rodriguez-Martinez H., Parrilla I. Proteomics in fresh and preserved pig semen: Recent achievements and future challenges. Theriogenology. 2020;150:41–47. doi: 10.1016/j.theriogenology.2020.01.066. [DOI] [PubMed] [Google Scholar]
- 71.Kelly V., Kuy S., Palmer D., Xu Z., Davis S., Cooper G. Characterization of bovine seminal plasma by proteomics. Proteomics. 2006;6:5826–5833. doi: 10.1002/pmic.200500830. [DOI] [PubMed] [Google Scholar]
- 72.Viana A.G.A., Martins A.M.A., Pontes A.H., Fontes W., Castro M.S., Ricart C.A.O., Sousa M.V., Kaya A., Topper E., Memili E., et al. Proteomic landscape of seminal plasma associated with dairy bull fertility. Sci. Rep. 2018;8:16323. doi: 10.1038/s41598-018-34152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu Q., Pan L., Huang D., Wang Z., Hou Z., Zhang M. Proteomic profiles of buffalo spermatozoa and seminal plasma. Theriogenology. 2019;134:74–82. doi: 10.1016/j.theriogenology.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 74.Camargo M., Intasqui P., Bertolla R.P. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin. Androl. 2018;28:6. doi: 10.1186/s12610-018-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sullivan R., Saez F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction. 2013;146:R21–R35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- 76.Aalberts M., Stout T.A.E., Stoorvogel W. Prostasomes: Extracellular vesicles from the prostate. Reproduction. 2014;147:R1–R14. doi: 10.1530/REP-13-0358. [DOI] [PubMed] [Google Scholar]
- 77.Machtinger R., Laurent L.C., Baccarelli A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update. 2016;22:182–193. doi: 10.1093/humupd/dmv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de la Torre J., Sánchez-Martín P., Gosálvez J., Crespo F. Equivalent seminal characteristics in human and stallion at first and second ejaculated fractions. Andrologia. 2017;49 doi: 10.1111/and.12708. [DOI] [PubMed] [Google Scholar]
- 79.Graham J.K. Analysis of stallion semen and its relation to fertility. Vet. Clin. North Am. Equine Pract. 1996;12:119–130. doi: 10.1016/S0749-0739(17)30299-7. [DOI] [PubMed] [Google Scholar]
- 80.Ekhlasi-Hundrieser M., Schäfer B., Kirchhoff C., Hess O., Bellair S., Müller P., Töpfer-Petersen E. Structural and molecular characterization of equine sperm-binding fibronectin-II module proteins. Mol. Reprod. Dev. 2005;70:45–57. doi: 10.1002/mrd.20187. [DOI] [PubMed] [Google Scholar]
- 81.Hamann H., Jude R., Sieme H., Mertens U., Töpfer-Petersen E., Distl O., Leeb T. A polymorphism within the equine CRISP3 gene is associated with stallion fertility in Hanoverian warmblood horses. Anim. Genet. 2007;38:259–264. doi: 10.1111/j.1365-2052.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 82.Kareskoski A.M., Palviainen M., Johannisson A., Katila T. Upregulation of CRISP-3 and kallikrein in stallion seminal plasma is associated with poor tolerance of cooled storage. Reprod. Domest. Anim. 2020;55:496–502. doi: 10.1111/rda.13643. [DOI] [PubMed] [Google Scholar]
- 83.Bubenickova F., Postlerova P., Simonik O., Sirohi J., Sichtar J. Effect of Seminal Plasma Protein Fractions on Stallion Sperm Cryopreservation. Int. J. Mol. Sci. 2020;21:6415. doi: 10.3390/ijms21176415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walters E.M., Agca Y., Ganjam V., Evans T. Animal models got you puzzled?: Think pig. Ann. N. Y. Acad. Sci. 2011;1245:63–64. doi: 10.1111/j.1749-6632.2011.06345.x. [DOI] [PubMed] [Google Scholar]
- 85.Töpfer-Petersen E., Romero A., Varela P.F., Ekhlasi-Hundrieser M., Dostàlovà Z., Sanz L., Calvete J.J. Spermadhesins: A new protein family. Facts, hypotheses and perspectives. Andrologia. 1998;30:217–224. doi: 10.1111/j.1439-0272.1998.tb01163.x. [DOI] [PubMed] [Google Scholar]
- 86.Caballero I., Vázquez J.M., García E.M., Roca J., Martínez E.A., Calvete J.J., Sanz L., Ekwall H., Rodríguez-Martínez H. Immunolocalization and possible functional role of PSP-I/PSP-II heterodimer in highly extended boar spermatozoa. J. Androl. 2006;27:766–773. doi: 10.2164/jandrol.106.000539. [DOI] [PubMed] [Google Scholar]
- 87.Caballero I., Parrilla I., Almiñana C., del Olmo D., Roca J., Martínez E.A., Vázquez J.M. Seminal plasma proteins as modulators of the sperm function and their application in sperm biotechnologies. Reprod. Domest. Anim. 2012;47(Suppl. 3):12–21. doi: 10.1111/j.1439-0531.2012.02028.x. [DOI] [PubMed] [Google Scholar]
- 88.Bromfield J.J. A role for seminal plasma in modulating pregnancy outcomes in domestic species. Reproduction. 2016;152:R223–R232. doi: 10.1530/REP-16-0313. [DOI] [PubMed] [Google Scholar]
- 89.Calvete J.J., Ensslin M., Mburu J., Iborra A., Martínez P., Adermann K., Waberski D., Sanz L., Töpfer-Petersen E., Weitze K.F., et al. Monoclonal antibodies against boar sperm zona pellucida-binding protein AWN-1. Characterization of a continuous antigenic determinant and immunolocalization of AWN epitopes in inseminated sows. Biol. Reprod. 1997;57:735–742. doi: 10.1095/biolreprod57.4.735. [DOI] [PubMed] [Google Scholar]
- 90.Rodríguez-Martinez H., Iborra A., Martínez P., Calvete J.J. Immunoelectronmicroscopic imaging of spermadhesin AWN epitopes on boar spermatozoa bound in vivo to the zona pellucida. Reprod. Fertil. Dev. 1998;10:491–497. doi: 10.1071/RD98111. [DOI] [PubMed] [Google Scholar]
- 91.Calvete J., Sanz L., Garcia E.M., Caballero I., Parrilla I., Martinez E., Roca J., Vazquez J., Saravia F., Wallgren M., et al. On the biological function of boar spermadhesin PSP-I/PSP-II. Reprod Domest Anim. 2005;40:331. [Google Scholar]
- 92.Waberski D., Claassen R., Hahn T., Jungblut P.W., Parvizi N., Kallweit E., Weitze K.F. LH profile and advancement of ovulation after transcervical infusion of seminal plasma at different stages of oestrus in gilts. J. Reprod. Fertil. 1997;109:29–34. doi: 10.1530/jrf.0.1090029. [DOI] [PubMed] [Google Scholar]
- 93.Parrilla I., Martinez C.A., Cambra J.M., Lucas X., Ferreira-Dias G., Rodriguez-Martinez H., Cuello C., Gil M.A., Martinez E.A. Blastocyst-Bearing Sows Display a Dominant Anti-Inflammatory Cytokine Profile Compared to Cyclic Sows at Day 6 of the Cycle. Animals. 2020;10:2028. doi: 10.3390/ani10112028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Novak S., Ruiz-Sánchez A., Dixon W.T., Foxcroft G.R., Dyck M.K. Seminal plasma proteins as potential markers of relative fertility in boars. J. Androl. 2010;31:188–200. doi: 10.2164/jandrol.109.007583. [DOI] [PubMed] [Google Scholar]
- 95.Alkmin D.V., Perez-Patiño C., Barranco I., Parrilla I., Vazquez J.M., Martinez E.A., Rodriguez-Martinez H., Roca J. Boar sperm cryosurvival is better after exposure to seminal plasma from selected fractions than to those from entire ejaculate. Cryobiology. 2014;69:203–210. doi: 10.1016/j.cryobiol.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 96.Mills K.M., Aryal U.K., Sobreira T., Minton A.M., Casey T., Stewart K.R. Shotgun proteome analysis of seminal plasma differentiate boars by reproductive performance. Theriogenology. 2020;157:130–139. doi: 10.1016/j.theriogenology.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 97.Zeng F., Chen Y., Guo C., Li C., Wei H., Li L., Meng L., Zhang S. Analysis of differentially abundant proteins related to boar fertility in seminal plasma using iTRAQ-based quantitative proteomics. J. Proteomics. 2021;236:104120. doi: 10.1016/j.jprot.2021.104120. [DOI] [PubMed] [Google Scholar]
- 98.De Lazari F.L., Sontag E.R., Schneider A., Araripe Moura A.A., Vasconcelos F.R., Nagano C.S., Dalberto P.F., Bizarro C.V., Mattos R.C., Mascarenhas Jobim M.I., et al. Proteomic identification of boar seminal plasma proteins related to sperm resistance to cooling at 17 °C. Theriogenology. 2020;147:135–145. doi: 10.1016/j.theriogenology.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 99.Höfner L., Luther A.-M., Palladini A., Fröhlich T., Waberski D. Tolerance of Stored Boar Spermatozoa to Autologous Seminal Plasma: A Proteomic and Lipidomic Approach. Int. J. Mol. Sci. 2020;21:6474. doi: 10.3390/ijms21186474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luongo C., Abril-Sánchez S., Hernández J.G., García-Vázquez F.A. Seminal plasma mitigates the adverse effect of uterine fluid on boar spermatozoa. Theriogenology. 2019;136:28–35. doi: 10.1016/j.theriogenology.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 101.Schjenken J.E., Robertson S.A. Seminal fluid and immune adaptation for pregnancy—Comparative biology in mammalian species. Reprod. Domest. Anim. 2014;49:27–36. doi: 10.1111/rda.12383. [DOI] [PubMed] [Google Scholar]
- 102.Schjenken J.E., Robertson S.A. The Female Response to Seminal Fluid. Physiol. Rev. 2020;100:1077–1117. doi: 10.1152/physrev.00013.2018. [DOI] [PubMed] [Google Scholar]
- 103.Avila F.W., Sirot L.K., LaFlamme B.A., Rubinstein C.D., Wolfner M.F. Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ben Chehida Y., Denis B., Claisse G., Joly D. [What the study of seminal fluid proteins in Drosophila tells us about the evolution of reproduction] Med. Sci. (Paris) 2014;30:651–657. doi: 10.1051/medsci/20143006015. [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez-Martinez H., Tienthai P., Atikuzzaman M., Vicente-Carrillo A., Rubér M., Alvarez-Rodriguez M. The ubiquitous hyaluronan: Functionally implicated in the oviduct? Theriogenology. 2016;86:182–186. doi: 10.1016/j.theriogenology.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 106.Barranco I., Ruber M., Perez-Patino C., Atikuzzaman M., Martinez E.A., Roca J., Rodriguez-Martinez H. The Seminal Plasma of the Boar is Rich in Cytokines, with Significant Individual and Intra-Ejaculate Variation. Am. J. Reprod. Immunol. 2015;74:523–532. doi: 10.1111/aji.12432. [DOI] [PubMed] [Google Scholar]
- 107.O’Leary S., Armstrong D.T., Robertson S.A. Transforming growth factor-β (TGFβ) in porcine seminal plasma. Reprod. Fertil. Dev. 2011;23:748–758. doi: 10.1071/RD11001. [DOI] [PubMed] [Google Scholar]
- 108.Padilla L., Martínez-Hernández J., Barranco I., Lucas X., Pastor L.M., Rodriguez-Martínez H., Roca J., Parrilla I. Granulocyte-macrophage colony stimulating factor (GM-CSF) is fully expressed in the genital tract, seminal plasma and spermatozoa of male pigs. Sci. Rep. 2020;10:13360. doi: 10.1038/s41598-020-70302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Padilla L., Barranco I., Parrilla I., Lucas X., Rodriguez-Martinez H., Roca J. Measurable Cytokine Concentrations in Pig Seminal Plasma Are Modified by Semen Handling and Storage. Biology. 2020;9:276. doi: 10.3390/biology9090276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fraczek M., Kurpisz M. Cytokines in the male reproductive tract and their role in infertility disorders. J. Reprod. Immunol. 2015;108:98–104. doi: 10.1016/j.jri.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 111.Sharkey D.J., Macpherson A.M., Tremellen K.P., Robertson S.A. Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol. Hum. Reprod. 2007;13:491–501. doi: 10.1093/molehr/gam028. [DOI] [PubMed] [Google Scholar]
- 112.Robertson S.A., Ingman W.V., O’Leary S., Sharkey D.J., Tremellen K.P. Transforming growth factor beta--a mediator of immune deviation in seminal plasma. J. Reprod. Immunol. 2002;57:109–128. doi: 10.1016/S0165-0378(02)00015-3. [DOI] [PubMed] [Google Scholar]
- 113.Robertson S.A. Seminal fluid signaling in the female reproductive tract: Lessons from rodents and pigs. J. Anim. Sci. 2007;85:36–44. doi: 10.2527/jas.2006-578. [DOI] [PubMed] [Google Scholar]
- 114.Jalali B.M., Kitewska A., Wasielak M., Bodek G., Bogacki M. Effects of seminal plasma and the presence of a conceptus on regulation of lymphocyte-cytokine network in porcine endometrium. Mol. Reprod. Dev. 2014;81:270–281. doi: 10.1002/mrd.22297. [DOI] [PubMed] [Google Scholar]
- 115.Waberski D., Schäfer J., Bölling A., Scheld M., Henning H., Hambruch N., Schuberth H.J., Pfarrer C., Wrenzycki C., Hunter R.H.F. Seminal plasma modulates the immune-cytokine network in the porcine uterine tissue and pre-ovulatory follicles. PLoS ONE. 2018;13:1–21. doi: 10.1371/journal.pone.0202654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martinez C.A., Ruber M., Rodriguez-Martinez H., Alvarez-Rodriguez M. Pig Pregnancies after Transfer of Allogeneic Embryos Show a Dysregulated Endometrial/Placental Cytokine Balance: A Novel Clue for Embryo Death? Biomolecules. 2020;10:554. doi: 10.3390/biom10040554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nongbua T., Guo Y., Ntallaris T., Rubèr M., Rodriguez-Martinez H., Humblot P., Morrell J. Bull seminal plasma stimulates in vitro production of TGF-β, IL-6 and IL-8 from bovine endometrial epithelial cells, depending on dose and bull fertility. J. Reprod. Immunol. 2020;142:103179. doi: 10.1016/j.jri.2020.103179. [DOI] [PubMed] [Google Scholar]
- 118.Robertson S.A., Sharkey D.J. The role of semen in induction of maternal immune tolerance to pregnancy. Semin. Immunol. 2001;13:243–254. doi: 10.1006/smim.2000.0320. [DOI] [PubMed] [Google Scholar]
- 119.Robertson S.A. Immune regulation of conception and embryo implantation-all about quality control? J. Reprod. Immunol. 2010;85:51–57. doi: 10.1016/j.jri.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 120.Martinez C.A., Cambra J.M., Lucas X., Ferreira-Dias G., Rodriguez-Martinez H., Gil M.A., Martinez E.A., Cuello C., Parrilla I. Intrauterine Infusion of TGF-β1 Prior to Insemination, Alike Seminal Plasma, Influences Endometrial Cytokine Responses but Does Not Impact the Timing of the Progression of Pre-Implantation Pig Embryo Development. Biology. 2021;10:159. doi: 10.3390/biology10020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Estienne A., Bongrani A., Reverchon M., Ramé C., Ducluzeau P.-H., Froment P., Dupont J. Involvement of Novel Adipokines, Chemerin, Visfatin, Resistin and Apelin in Reproductive Functions in Normal and Pathological Conditions in Humans and Animal Models. Int. J. Mol. Sci. 2019;20:4431. doi: 10.3390/ijms20184431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Estienne A., Brossaud A., Reverchon M., Ramé C., Froment P., Dupont J. Adipokines Expression and Effects in Oocyte Maturation, Fertilization and Early Embryo Development: Lessons from Mammals and Birds. Int. J. Mol. Sci. 2020;21:3581. doi: 10.3390/ijms21103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elfassy Y., Bastard J.-P., McAvoy C., Fellahi S., Dupont J., Levy R. Adipokines in Semen: Physiopathology and Effects on Spermatozoas. Int. J. Endocrinol. 2018;2018:3906490. doi: 10.1155/2018/3906490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomas S., Kratzsch D., Schaab M., Scholz M., Grunewald S., Thiery J., Paasch U., Kratzsch J. Seminal plasma adipokine levels are correlated with functional characteristics of spermatozoa. Fertil. Steril. 2013;99:1256–1263. doi: 10.1016/j.fertnstert.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 125.Lackey B.R., Gray S.L., Henricks D.M. Measurement of leptin and insulin-like growth factor-I in seminal plasma from different species. Physiol. Res. 2002;51:309–311. [PubMed] [Google Scholar]
- 126.Jope T., Lammert A., Kratzsch J., Paasch U., Glander H.-J. Leptin and leptin receptor in human seminal plasma and in human spermatozoa. Int. J. Androl. 2003;26:335–341. doi: 10.1111/j.1365-2605.2003.00434.x. [DOI] [PubMed] [Google Scholar]
- 127.Bongrani A., Elfassy Y., Brun J.S., Ramé C., Mellouk N., Fellahi S., Bastard J.P., Levy R., Vasseur C., Froment P., et al. Expression of adipokines in seminal fluid of men of normal weight. Asian J. Androl. 2019;21:528–530. doi: 10.4103/aja.aja_25_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li J., Barranco I., Tvarijonaviciute A., Molina M.F., Martinez E.A., Rodriguez-Martinez H., Parrilla I., Roca J. Seminal plasma antioxidants are directly involved in boar sperm cryotolerance. Theriogenology. 2018;107:27–35. doi: 10.1016/j.theriogenology.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 129.Barranco I., Tvarijonaviciute A., Perez-Patiño C., Parrilla I., Ceron J.J., Martinez E.A., Rodriguez-Martinez H., Roca J. High total antioxidant capacity of the porcine seminal plasma (SP-TAC) relates to sperm survival and fertility. Sci. Rep. 2015;5:18538. doi: 10.1038/srep18538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barrier-Battut I., Dacheux J.L., Gatti J.L., Rouviere P., Stanciu C., Dacheux F., Vidament M. Seminal plasma proteins and semen characteristics in relation with fertility in the stallion. Anim. Reprod. Sci. 2005;89:255–258. [PubMed] [Google Scholar]
- 131.Novak S., Smith T.A., Paradis F., Burwash L., Dyck M.K., Foxcroft G.R., Dixon W.T. Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology. 2010;74:956–967. doi: 10.1016/j.theriogenology.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 132.Barranco I., Tvarijonaviciute A., Perez-Patiño C., Alkmin D.V., Ceron J.J., Martinez E.A., Rodriguez-Martinez H., Roca J. The activity of paraoxonase type 1 (PON-1) in boar seminal plasma and its relationship with sperm quality, functionality, and in vivo fertility. Andrology. 2015;3:315–320. doi: 10.1111/andr.309. [DOI] [PubMed] [Google Scholar]
- 133.Sopková D., Andrejcakova Z., Vlckova R., Danišová O., Supuka P., Ondrasovicova S., Petrilla V. Lactate dehydrogenase as a possible indicator of reproductive capacity of boars. Indian J. Anim. Sci. 2015;85:143–147. [Google Scholar]
- 134.Jeppesen D.K., Fenix A.M., Franklin J.L., Higginbotham J.N., Zhang Q., Zimmerman L.J., Liebler D.C., Ping J., Liu Q., Evans R., et al. Reassessment of Exosome Composition. Cell. 2019;177:428–445. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tamessar C.T., Trigg N.A., Nixon B., Skerrett-Byrne D.A., Sharkey D.J., Robertson S.A., Bromfield E.G., Schjenken J.E. Roles of male reproductive tract extracellular vesicles in reproduction. Am. J. Reprod. Immunol. 2021;85:e13338. doi: 10.1111/aji.13338. [DOI] [PubMed] [Google Scholar]
- 136.Vojtech L., Woo S., Hughes S., Levy C., Ballweber L., Sauteraud R.P., Strobl J., Westerberg K., Gottardo R., Tewari M., et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014;42:7290–7304. doi: 10.1093/nar/gku347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Belleannée C., Calvo É., Caballero J., Sullivan R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol. Reprod. 2013;89:30. doi: 10.1095/biolreprod.113.110486. [DOI] [PubMed] [Google Scholar]
- 138.Du J., Shen J., Wang Y., Pan C., Pang W., Diao H., Dong W. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget. 2016;7:58832–58847. doi: 10.18632/oncotarget.11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Skalnikova H.K., Bohuslavova B., Turnovcova K., Juhasova J., Juhas S., Rodinova M., Vodicka P. Isolation and Characterization of Small Extracellular Vesicles from Porcine Blood Plasma, Cerebrospinal Fluid, and Seminal Plasma. Proteomes. 2019;7:17. doi: 10.3390/proteomes7020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu Z., Xie Y., Zhou C., Hu Q., Gu T., Yang J., Zheng E., Huang S., Xu Z., Cai G., et al. Expression Pattern of Seminal Plasma Extracellular Vesicle Small RNAs in Boar Semen. Front. Vet. Sci. 2020;7:585276. doi: 10.3389/fvets.2020.585276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Murdica V., Giacomini E., Alteri A., Bartolacci A., Cermisoni G.C., Zarovni N., Papaleo E., Montorsi F., Salonia A., Viganò P., et al. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 2019;111:897–908. doi: 10.1016/j.fertnstert.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 142.Piehl L.L., Fischman M.L., Hellman U., Cisale H., Miranda P.V. Boar seminal plasma exosomes: Effect on sperm function and protein identification by sequencing. Theriogenology. 2013;79:1071–1082. doi: 10.1016/j.theriogenology.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 143.Piehl L.L., Cisale H., Torres N., Capani F., Sterin-Speziale N., Hager A. Biochemical characterization and membrane fluidity of membranous vesicles isolated from boar seminal plasma. Anim. Reprod. Sci. 2006;92:401–410. doi: 10.1016/j.anireprosci.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 144.Siciliano L., Marcianò V., Carpino A. Prostasome-like vesicles stimulate acrosome reaction of pig spermatozoa. Reprod. Biol. Endocrinol. 2008;6:5. doi: 10.1186/1477-7827-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu Y., Shen Q., Zhang L., Xiang W. Extracellular Vesicles: Recent Developments in Aging and Reproductive Diseases. Front. Cell Dev. Biol. 2020;8:577084. doi: 10.3389/fcell.2020.577084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dang X.T.T., Kavishka J.M., Zhang D.X., Pirisinu M., Le M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells. 2020;9:2191. doi: 10.3390/cells9102191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rodriguez-Martinez H. Aspects of the electrolytic composition of boar epididymal fluid with reference to sperm maturation and storage. Reprod. Domest. Anim. 1991:13–27. [Google Scholar]
- 148.Amann R.P., Hammerstedt R.H., Veeramachaneni D.N. The epididymis and sperm maturation: A perspective. Reprod. Fertil. Dev. 1993;5:361–381. doi: 10.1071/RD9930361. [DOI] [PubMed] [Google Scholar]
- 149.Zhou W., De Iuliis G.N., Dun M.D., Nixon B. Characteristics of the Epididymal Luminal Environment Responsible for Sperm Maturation and Storage. Front. Endocrinol. (Lausanne) 2018;9:59. doi: 10.3389/fendo.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rodriguez-Martinez H., Ekstedt E., Einarsson S. Acidification of epididymal fluid in the boar. Int. J. Androl. 1990;13:238–243. doi: 10.1111/j.1365-2605.1990.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 151.Saez F., Frenette G., Sullivan R. Epididymosomes and prostasomes: Their roles in posttesticular maturation of the sperm cells. J. Androl. 2003;24:149–154. doi: 10.1002/j.1939-4640.2003.tb02653.x. [DOI] [PubMed] [Google Scholar]
- 152.Nixon B., De Iuliis G.N., Hart H.M., Zhou W., Mathe A., Bernstein I.R., Anderson A.L., Stanger S.J., Skerrett-Byrne D.A., Jamaluddin M.F.B., et al. Proteomic Profiling of Mouse Epididymosomes Reveals their Contributions to Post-testicular Sperm Maturation. Mol. Cell. Proteomics. 2019;18:S91–S108. doi: 10.1074/mcp.RA118.000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Légaré C., Akintayo A., Blondin P., Calvo E., Sullivan R. Impact of male fertility status on the transcriptome of the bovine epididymis. Mol. Hum. Reprod. 2017;23:355–369. doi: 10.1093/molehr/gax019. [DOI] [PubMed] [Google Scholar]
- 154.Barranco I., Tvarijonaviciute A., Perez-Patiño C., Vicente-Carrillo A., Parrilla I., Ceron J.J., Martinez E.A., Rodriguez-Martinez H., Roca J. Glutathione Peroxidase 5 Is Expressed by the Entire Pig Male Genital Tract and Once in the Seminal Plasma Contributes to Sperm Survival and In Vivo Fertility. PLoS ONE. 2016;11:e0162958. doi: 10.1371/journal.pone.0162958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barranco I., Perez-Patiño C., Tvarijonaviciute A., Parrilla I., Vicente-Carrillo A., Alvarez-Rodriguez M., Ceron J.J., Martinez E.A., Rodriguez-Martinez H., Roca J. Active paraoxonase 1 is synthesised throughout the internal boar genital organs. Reproduction. 2017;154:237–243. doi: 10.1530/REP-17-0300. [DOI] [PubMed] [Google Scholar]
- 156.Gervasi M.G., Visconti P.E. Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology. 2017;5:204–218. doi: 10.1111/andr.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ramos Angrimani D.S., Nichi M., Losano J.D.A., Lucio C.F., Lima Veiga G.A., Franco M.V.M.J., Vannucchi C.I. Fatty acid content in epididymal fluid and spermatozoa during sperm maturation in dogs. J. Anim. Sci. Biotechnol. 2017;8:18. doi: 10.1186/s40104-017-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou W., Stanger S.J., Anderson A.L., Bernstein I.R., De Iuliis G.N., McCluskey A., McLaughlin E.A., Dun M.D., Nixon B. Mechanisms of tethering and cargo transfer during epididymosome-sperm interactions. BMC Biol. 2019;17:35. doi: 10.1186/s12915-019-0653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sharma U., Conine C.C., Shea J.M., Boskovic A., Derr A.G., Bing X.Y., Belleannee C., Kucukural A., Serra R.W., Sun F., et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391–396. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sharma U., Sun F., Conine C.C., Reichholf B., Kukreja S., Herzog V.A., Ameres S.L., Rando O.J. Small RNAs Are Trafficked from the Epididymis to Developing Mammalian Sperm. Dev. Cell. 2018;46:481–494. doi: 10.1016/j.devcel.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jankovičová J., Sečová P., Michalková K., Antalíková J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020;21:7568. doi: 10.3390/ijms21207568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cornwall G.A. New insights into epididymal biology and function. Hum. Reprod. Update. 2009;15:213–227. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Martin-DeLeon P.A. Epididymosomes: Transfer of fertility-modulating proteins to the sperm surface. Asian J. Androl. 2015;17:720–725. doi: 10.4103/1008-682X.155538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Saewu A., Kadunganattil S., Raghupathy R., Kongmanas K., Diaz-Astudillo P., Hermo L., Tanphaichitr N. Clusterin in the mouse epididymis: Possible roles in sperm maturation and capacitation. Reproduction. 2017;154:867–880. doi: 10.1530/REP-17-0518. [DOI] [PubMed] [Google Scholar]
- 165.Gaikwad A.S., Hu J., Chapple D.G., O’Bryan M.K. The functions of CAP superfamily proteins in mammalian fertility and disease. Hum. Reprod. Update. 2020;26:689–723. doi: 10.1093/humupd/dmaa016. [DOI] [PubMed] [Google Scholar]
- 166.Sutovsky P., Moreno R., Ramalho-Santos J., Dominko T., Thompson W.E., Schatten G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J. Cell Sci. 2001;114:1665–1675. doi: 10.1242/jcs.114.9.1665. [DOI] [PubMed] [Google Scholar]
- 167.D’Amours O., Frenette G., Bordeleau L.-J., Allard N., Leclerc P., Blondin P., Sullivan R. Epididymosomes Transfer Epididymal Sperm Binding Protein 1 (ELSPBP1) to Dead Spermatozoa During Epididymal Transit in Bovine1. Biol. Reprod. 2012;87 doi: 10.1095/biolreprod.112.100990. [DOI] [PubMed] [Google Scholar]
- 168.Girouard J., Frenette G., Sullivan R. Comparative proteome and lipid profiles of bovine epididymosomes collected in the intraluminal compartment of the caput and cauda epididymidis. Int. J. Androl. 2011;34:e475–e486. doi: 10.1111/j.1365-2605.2011.01203.x. [DOI] [PubMed] [Google Scholar]
- 169.Höög J.L., Lötvall J. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J. Extracell. Vesicles. 2015;4:28680. doi: 10.3402/jev.v4.28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Alvarez-Rodriguez M., Ntzouni M., Wright D., Khan K.I., López-Béjar M., Martinez C.A., Rodriguez-Martinez H. Chicken seminal fluid lacks CD9- and CD44-bearing extracellular vesicles. Reprod. Domest. Anim. 2020;55:293–300. doi: 10.1111/rda.13617. [DOI] [PubMed] [Google Scholar]
- 171.Cordeiro L., Lin H.-L.H., Vitorino Carvalho A., Grasseau I., Uzbekov R., Besbois E. First insights on seminal extracellular vesicles in chickens of contrasted fertility. Reproduction. 2021 doi: 10.1530/REP-20-0462. [DOI] [PubMed] [Google Scholar]
- 172.Álvarez-Rodríguez M., Martinez C.A., Wright D., Rodríguez-Martinez H. The role of semen and seminal plasma in inducing large-scale genomic changes in the female porcine peri-ovulatory tract. Sci. Rep. 2020;10:5061. doi: 10.1038/s41598-020-60810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Martinez C.A., Cambra J.M., Gil M.A., Parrilla I., Alvarez-Rodriguez M., Rodriguez-Martinez H., Cuello C., Martinez E.A. Seminal Plasma Induces Overexpression of Genes Associated with Embryo Development and Implantation in Day-6 Porcine Blastocysts. Int. J. Mol. Sci. 2020;21:3662. doi: 10.3390/ijms21103662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gabrielsen J.S., Lipshultz L.I. Rapid progression in our understanding of extracellular vesicles and male infertility. Fertil. Steril. 2019;111:881–882. doi: 10.1016/j.fertnstert.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 175.Leahy T., Rickard J.P., Pini T., Gadella B.M., de Graaf S.P. Quantitative Proteomic Analysis of Seminal Plasma, Sperm Membrane Proteins, and Seminal Extracellular Vesicles Suggests Vesicular Mechanisms Aid in the Removal and Addition of Proteins to the Ram Sperm Membrane. Proteomics. 2020;20:e1900289. doi: 10.1002/pmic.201900289. [DOI] [PubMed] [Google Scholar]
- 176.Aalberts M., Sostaric E., Wubbolts R., Wauben M.W.M., Nolte-’t Hoen E.N.M., Gadella B.M., Stout T.A.E., Stoorvogel W. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim. Biophys. Acta. 2013;1834:2326–2335. doi: 10.1016/j.bbapap.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 177.Vilanova-Perez T., Jones C., Balint S., Dragovic R., L Dustin M., Yeste M., Coward K. Exosomes derived from HEK293T cells interact in an efficient and noninvasive manner with mammalian sperm in vitro. Nanomedicine (Lond) 2020;15:1965–1980. doi: 10.2217/nnm-2020-0056. [DOI] [PubMed] [Google Scholar]
- 178.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sharkey D.J., Tremellen K.P., Jasper M.J., Gemzell-Danielsson K., Robertson S.A. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J. Immunol. 2012;188:2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 180.Barranco I., Padilla L., Martinez C.A., Alvarez-Rodriguez M., Parrilla I., Lucas X., Ferreira-Dias G., Yeste M., Rodriguez-Martinez H., Roca J. Seminal Plasma Modulates miRNA Expression by Sow Genital Tract Lining Explants. Biomolecules. 2020;10:933. doi: 10.3390/biom10060933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Salas-Huetos A., James E.R., Aston K.I., Carrell D.T., Jenkins T.G., Yeste M. The role of miRNAs in male human reproduction: A systematic review. Andrology. 2020;8:7–26. doi: 10.1111/andr.12714. [DOI] [PubMed] [Google Scholar]
- 182.Jodar M. Sperm and seminal plasma RNAs: What roles do they play beyond fertilization? Reproduction. 2019;158:R113–R123. doi: 10.1530/REP-18-0639. [DOI] [PubMed] [Google Scholar]
- 183.Chen X., Zheng Y., Lei A., Zhang H., Niu H., Li X., Zhang P., Liao M., Lv Y., Zhu Z., et al. Early cleavage of preimplantation embryos is regulated by tRNA(Gln-TTG)-derived small RNAs present in mature spermatozoa. J. Biol. Chem. 2020;295:10885–10900. doi: 10.1074/jbc.RA120.013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen X., Sun Q., Zheng Y., Liu Z., Meng X., Zeng W., Lu H. Human sperm tsRNA as potential biomarker and therapy target for male fertility. Reproduction. 2021;161:111–122. doi: 10.1530/REP-20-0415. [DOI] [PubMed] [Google Scholar]
- 185.Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat. Rev. Genet. 2011;12:846–860. doi: 10.1038/nrg3079. [DOI] [PubMed] [Google Scholar]
- 186.Yang Q., Hua J., Wang L., Xu B., Zhang H., Ye N., Zhang Z., Yu D., Cooke H.J., Zhang Y., et al. MicroRNA and piRNA profiles in normal human testis detected by next generation sequencing. PLoS ONE. 2013;8:e66809. doi: 10.1371/journal.pone.0066809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chioccarelli T., Manfrevola F., Ferraro B., Sellitto C., Cobellis G., Migliaccio M., Fasano S., Pierantoni R., Chianese R. Expression Patterns of Circular RNAs in High Quality and Poor Quality Human Spermatozoa. Front. Endocrinol. (Lausanne) 2019;10:435. doi: 10.3389/fendo.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gòdia M., Castelló A., Rocco M., Cabrera B., Rodríguez-Gil J.E., Balasch S., Lewis C., Sánchez A., Clop A. Identification of circular RNAs in porcine sperm and evaluation of their relation to sperm motility. Sci. Rep. 2020;10:7985. doi: 10.1038/s41598-020-64711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gòdia M., Estill M., Castelló A., Balasch S., Rodríguez-Gil J.E., Krawetz S.A., Sánchez A., Clop A. A RNA-Seq Analysis to Describe the Boar Sperm Transcriptome and Its Seasonal Changes. Front. Genet. 2019;10:299. doi: 10.3389/fgene.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pértille F., Alvarez-Rodriguez M., da Silva A.N., Barranco I., Roca J., Guerrero-Bosagna C., Rodriguez-Martinez H. Sperm Methylome Profiling Can Discern Fertility Levels in the Porcine Biomedical Model. Int. J. Mol. Sci. 2021;22:2679. doi: 10.3390/ijms22052679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Krausz C., Escamilla A.R., Chianese C. Genetics of male infertility: From research to clinic. Reproduction. 2015;150:R159–R174. doi: 10.1530/REP-15-0261. [DOI] [PubMed] [Google Scholar]
- 192.Fagerlind M., Stålhammar H., Olsson B., Klinga-Levan K. Expression of miRNAs in Bull Spermatozoa Correlates with Fertility Rates. Reprod. Domest. Anim. 2015;50:587–594. doi: 10.1111/rda.12531. [DOI] [PubMed] [Google Scholar]
- 193.Gòdia M., Swanson G., Krawetz S.A. A history of why fathers’ RNA matters. Biol. Reprod. 2018;99:147–159. doi: 10.1093/biolre/ioy007. [DOI] [PubMed] [Google Scholar]
- 194.Ortiz-Rodriguez J.M., Ortega-Ferrusola C., Gil M.C., Martín-Cano F.E., Gaitskell-Phillips G., Rodríguez-Martínez H., Hinrichs K., Álvarez-Barrientos A., Román Á., Peña F.J. Transcriptome analysis reveals that fertilization with cryopreserved sperm downregulates genes relevant for early embryo development in the horse. PLoS ONE. 2019;14:e0213420. doi: 10.1371/journal.pone.0213420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Roa-Espitia A.L., Hernández-Rendón E.R., Baltiérrez-Hoyos R., Muñoz-Gotera R.J., Cote-Vélez A., Jiménez I., González-Márquez H., Hernández-González E.O. Focal adhesion kinase is required for actin polymerization and remodeling of the cytoskeleton during sperm capacitation. Biol. Open. 2016;5:1189–1199. doi: 10.1242/bio.017558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gòdia M., Reverter A., González-Prendes R., Ramayo-Caldas Y., Castelló A., Rodríguez-Gil J.-E., Sánchez A., Clop A. A systems biology framework integrating GWAS and RNA-seq to shed light on the molecular basis of sperm quality in swine. Genet. Sel. Evol. 2020;52:72. doi: 10.1186/s12711-020-00592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Roca J., Broekhuijse M.L.W.J., Parrilla I., Rodriguez-Martinez H., Martinez E.A., Bolarin A. Boar Differences In Artificial Insemination Outcomes: Can They Be Minimized? Reprod. Domest. Anim. 2015;50(Suppl. 2):48–55. doi: 10.1111/rda.12530. [DOI] [PubMed] [Google Scholar]
- 198.Recuero S., Sánchez J.M., Mateo-Otero Y., Bagés-Arnal S., McDonald M., Behura S.K., Spencer T.E., Kenny D.A., Yeste M., Lonergan P., et al. Mating to Intact, but Not Vasectomized, Males Elicits Changes in the Endometrial Transcriptome: Insights From the Bovine Model. Front. Cell Dev. Biol. 2020;8:547. doi: 10.3389/fcell.2020.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Alvarez-Rodriguez M., Martinez C.A., Wright D., Rodriguez-Martinez H. Does the Act of Copulation per se, without Considering Seminal Deposition, Change the Expression of Genes in the Porcine Female Genital Tract? Int. J. Mol. Sci. 2020;21:5477. doi: 10.3390/ijms21155477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sheedy F.J., Palsson-McDermott E., Hennessy E.J., Martin C., O’Leary J.J., Ruan Q., Johnson D.S., Chen Y., O’Neill L.A.J. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol. 2010;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 201.Cui B., Liu W., Wang X., Chen Y., Du Q., Zhao X., Zhang H., Liu S.-L., Tong D., Huang Y. Brucella Omp25 Upregulates miR-155, miR-21-5p, and miR-23b to Inhibit Interleukin-12 Production via Modulation of Programmed Death-1 Signaling in Human Monocyte/Macrophages. Front. Immunol. 2017;8:708. doi: 10.3389/fimmu.2017.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Marzano G., Chiriacò M.S., Primiceri E., Dell’Aquila M.E., Ramalho-Santos J., Zara V., Ferramosca A., Maruccio G. Sperm selection in assisted reproduction: A review of established methods and cutting-edge possibilities. Biotechnol. Adv. 2020;40:107498. doi: 10.1016/j.biotechadv.2019.107498. [DOI] [PubMed] [Google Scholar]
- 203.Morgan H., Eid N., Khoshkerdar A., Watkins A. Defining the male contribution to embryo quality and offspring health in assisted reproduction in farm animals. Anim. Reprod. 2020;17 doi: 10.1590/1984-3143-ar2020-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.De Geyter C., Calhaz-Jorge C., Kupka M.S., Wyns C., Mocanu E., Motrenko T., Scaravelli G., Smeenk J., Vidakovic S., Goossens V. ART in Europe, 2015: Results generated from European registries by ESHRE. Hum. Reprod. Open. 2020;2020:hoz038. doi: 10.1093/hropen/hoz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Crawford G., Ray A., Gudi A., Shah A., Homburg R. The role of seminal plasma for improved outcomes during in vitro fertilization treatment: Review of the literature and meta-analysis. Hum. Reprod. Update. 2015;21:275–284. doi: 10.1093/humupd/dmu052. [DOI] [PubMed] [Google Scholar]
- 206.Ata B., Abou-Setta A.M., Seyhan A., Buckett W. Application of seminal plasma to female genital tract prior to embryo transfer in assisted reproductive technology cycles (IVF, ICSI and frozen embryo transfer) Cochrane database Syst. Rev. 2018;2:CD011809. doi: 10.1002/14651858.CD011809.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kanannejad Z., Namavar Jahromi B., Gharesi-Fard B. T Cell Subsets Profiling in Unexplained Infertile Women with Successful and Unsuccessful in Vitro Fertilization Outcome: Focus on the Effect of Seminal Plasma. Iran. J. Allergy. Asthma. Immunol. 2019;18:163–172. doi: 10.18502/ijaai.v18i2.919. [DOI] [PubMed] [Google Scholar]
- 208.Martinez C.A., Cambra J.M., Parrilla I., Roca J., Ferreira-Dias G., Pallares F.J., Lucas X., Vazquez J.M., Martinez E.A., Gil M.A., et al. Seminal Plasma Modifies the Transcriptional Pattern of the Endometrium and Advances Embryo Development in Pigs. Front. Vet. Sci. 2019;6:1–16. doi: 10.3389/fvets.2019.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Saint-Dizier M., Mahé C., Reynaud K., Tsikis G., Mermillod P., Druart X. Sperm interactions with the female reproductive tract: A key for successful fertilization in mammals. Mol. Cell. Endocrinol. 2020;516:110956. doi: 10.1016/j.mce.2020.110956. [DOI] [PubMed] [Google Scholar]
- 210.Hernández-Silva G., Chirinos M. Proteins from male and female reproductive tracts involved in sperm function regulation. Zygote. 2019;27:5–16. doi: 10.1017/S096719941800062X. [DOI] [PubMed] [Google Scholar]
- 211.De Lazari F.L., Sontag E.R., Schneider A., Moura A.A.A., Vasconcelos F.R., Nagano C.S., Mattos R.C., Jobim M.I.M., Bustamante-Filho I.C. Seminal plasma proteins and their relationship with sperm motility and morphology in boars. Andrologia. 2019;51:e13222. doi: 10.1111/and.13222. [DOI] [PubMed] [Google Scholar]
- 212.Pavaneli A.P.P., Recuero S., Chaves B.R., Garcia-Bonavila E., Llavanera M., Pinart E., Bonet S., De Andrade A.F.C., Yeste M. The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis. Int. J. Mol. Sci. 2020;21:4520. doi: 10.3390/ijms21124520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.O’Leary S., Jasper M.J., Warnes G.M., Armstrong D.T., Robertson S.A. Seminal plasma regulates endometrial cytokine expression, leukocyte recruitment and embryo development in the pig. Reproduction. 2004;128:237–247. doi: 10.1530/rep.1.00160. [DOI] [PubMed] [Google Scholar]
- 214.Ghiringhelli F., Ménard C., Terme M., Flament C., Taieb J., Chaput N., Puig P.E., Novault S., Escudier B., Vivier E., et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Robertson S.A. GM-CSF regulation of embryo development and pregnancy. Cytokine Growth Factor Rev. 2007;18:287–298. doi: 10.1016/j.cytogfr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 216.Rhodes M., Brendemuhl J.H., Hansen P.J. Litter characteristics of gilts artificially inseminated with transforming growth factor-beta. Am. J. Reprod. Immunol. 2006;56:153–156. doi: 10.1111/j.1600-0897.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 217.Foxcroft G.R., Dyck M.K., Ruiz-Sanchez A., Novak S., Dixon W.T. Identifying useable semen. Theriogenology. 2008;70:1324–1336. doi: 10.1016/j.theriogenology.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 218.Candenas L., Chianese R. Exosome Composition and Seminal Plasma Proteome: A Promising Source of Biomarkers of Male Infertility. Int. J. Mol. Sci. 2020;21:7022. doi: 10.3390/ijms21197022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Moura A., Memili E. Functional aspects of seminal plasma and sperm proteins and their potential as molecular markers of fertility. Anim. Reprod. 2016;13:191–199. doi: 10.21451/1984-3143-AR884. [DOI] [Google Scholar]
- 220.Gross N., Peñagaricano F., Khatib H. Integration of whole-genome DNA methylation data with RNA sequencing data to identify markers for bull fertility. Anim. Genet. 2020;51:502–510. doi: 10.1111/age.12941. [DOI] [PubMed] [Google Scholar]
- 221.Dyck M.K., Foxcroft G.R., Novak S., Ruiz-Sanchez A., Patterson J., Dixon W.T. Biological markers of boar fertility. Reprod. Domest. Anim. 2011;46(Suppl. 2):55–58. doi: 10.1111/j.1439-0531.2011.01837.x. [DOI] [PubMed] [Google Scholar]
- 222.Wang F., Yang W., Ouyang S., Yuan S. The Vehicle Determines the Destination: The Significance of Seminal Plasma Factors for Male Fertility. Int. J. Mol. Sci. 2020;21:8499. doi: 10.3390/ijms21228499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Otasevic V., Kalezic A., Macanovic B., Jankovic A., Stancic A., Garalejic E., Korac A., Korac B. Evaluation of the antioxidative enzymes in the seminal plasma of infertile men: Contribution to classic semen quality analysis. Syst. Biol. Reprod. Med. 2019;65:343–349. doi: 10.1080/19396368.2019.1600171. [DOI] [PubMed] [Google Scholar]
- 224.Kumar N., Singh N.K. Emerging role of Novel Seminal Plasma Bio-markers in Male Infertility: A Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;253:170–179. doi: 10.1016/j.ejogrb.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 225.Kumar N., Singh N.K. An Insight into Novel Sperm Cell Proteins as Bio-markers for Male Infertility: A Review. Curr. Mol. Med. 2021 doi: 10.2174/1566524021666210121142612. [DOI] [PubMed] [Google Scholar]
- 226.Archana S.S., Selvaraju S., Binsila B.K., Arangasamy A., Krawetz S.A. Immune regulatory molecules as modifiers of semen and fertility: A review. Mol. Reprod. Dev. 2019;86:1485–1504. doi: 10.1002/mrd.23263. [DOI] [PubMed] [Google Scholar]
- 227.Hashemi M.-S., Mozdarani H., Ghaedi K., Nasr-Esfahani M.H. Could analysis of testis-specific genes, as biomarkers in seminal plasma, predict presence of focal spermatogenesis in non-obstructive azoospermia? Andrologia. 2020;52:e13483. doi: 10.1111/and.13483. [DOI] [PubMed] [Google Scholar]
- 228.Barranco I., Fernandez-Fuertes B., Padilla L., Delgado-Bermúdez A., Tvarijonaviciute A., Yeste M. Seminal Plasma Anti-Müllerian Hormone: A Potential AI-Boar Fertility Biomarker? Biology. 2020;9:78. doi: 10.3390/biology9040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Thelie A., Réhault-Godbert S., Poirier J.-C., Fouchécourt S., Blesbois E. The seminal acrosin-inhibitor ClTI1/SPINK2 is a fertility-associated marker in the chicken. Mol. Reprod. Dev. 2019;86 doi: 10.1002/mrd.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Janiszewska E., Kratz E.M. Could the glycosylation analysis of seminal plasma clusterin become a novel male infertility biomarker? Mol. Reprod. Dev. 2020;87:515–524. doi: 10.1002/mrd.23340. [DOI] [PubMed] [Google Scholar]
- 231.Cunha Bustamante-Filho I., Renato Menegassi S., Ribas Pereira G., Dias Salton G., Mosena Munari F., Roberto Schneider M., Costa Mattos R., Otávio Jardim Barcellos J., Pereira Laurino J., Obino Cirne-Lima E., et al. Bovine seminal plasma osteopontin: Structural modelling, recombinant expression and its relationship with semen quality. Andrologia. 2021;53:e13905. doi: 10.1111/and.13905. [DOI] [PubMed] [Google Scholar]
- 232.Liu Y., Zhang F., Dai L. Cyclooxygenase 1 (COX1) as an indicator of sperm quality in humans. Andrologia. 2020;52:e13537. doi: 10.1111/and.13537. [DOI] [PubMed] [Google Scholar]
- 233.Llavanera M., Delgado-Bermúdez A., Mateo-Otero Y., Padilla L., Romeu X., Roca J., Barranco I., Yeste M. Exploring Seminal Plasma GSTM3 as a Quality and In Vivo Fertility Biomarker in Pigs-Relationship with Sperm Morphology. Antioxidants. 2020;9:741. doi: 10.3390/antiox9080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mehrparvar B., Chashmniam S., Nobakht F., Amini M., Javidi A., Minai-Tehrani A., Arjmand B., Gilany K. Metabolic profiling of seminal plasma from teratozoospermia patients. J. Pharm. Biomed. Anal. 2020;178:112903. doi: 10.1016/j.jpba.2019.112903. [DOI] [PubMed] [Google Scholar]
- 235.Mateo-Otero Y., Fernández-López P., Gil-Caballero S., Fernandez-Fuertes B., Bonet S., Barranco I., Yeste M. (1)H Nuclear Magnetic Resonance of Pig Seminal Plasma Reveals Intra-Ejaculate Variation in Metabolites. Biomolecules. 2020;10:906. doi: 10.3390/biom10060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang Y.-T., Liu Y., Liang H.-L., Xu Q.-Q., Liu Z.-H., Weng X.-G. Metabolomic differences of seminal plasma between boars with high and low average conception rates after artificial insemination. Reprod. Domest. Anim. 2021;56:161–171. doi: 10.1111/rda.13861. [DOI] [PubMed] [Google Scholar]
- 237.Panner Selvam M.K., Agarwal A., Sharma R., Samanta L., Gupta S., Dias T.R., Martins A.D. Protein Fingerprinting of Seminal Plasma Reveals Dysregulation of Exosome-Associated Proteins in Infertile Men with Unilateral Varicocele. World J. Mens. Health. 2019 doi: 10.5534/wjmh.180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Baskaran S., Panner Selvam M.K., Agarwal A. Exosomes of male reproduction. Adv. Clin. Chem. 2020;95:149–163. doi: 10.1016/bs.acc.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 239.Corral-Vazquez C., Salas-Huetos A., Blanco J., Vidal F., Sarrate Z., Anton E. Sperm microRNA pairs: New perspectives in the search for male fertility biomarkers. Fertil. Steril. 2019;112:831–841. doi: 10.1016/j.fertnstert.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 240.Xu Y., Zhang Y., Yang Y., Liu X., Chen Y. Seminal plasma miR-210-3p is a biomarker for screening dyszoospermia caused by varicocele. Andrologia. 2019;51:e13244. doi: 10.1111/and.13244. [DOI] [PubMed] [Google Scholar]
- 241.Radtke A., Dieckmann K.-P., Grobelny F., Salzbrunn A., Oing C., Schulze W., Belge G. Expression of miRNA-371a-3p in seminal plasma and ejaculate is associated with sperm concentration. Andrology. 2019;7:469–474. doi: 10.1111/andr.12664. [DOI] [PubMed] [Google Scholar]
- 242.Oliphant G., Reynolds A.B., Thomas T.S. Sperm surface components involved in the control of the acrosome reaction. Am. J. Anat. 1985;174:269–283. doi: 10.1002/aja.1001740308. [DOI] [PubMed] [Google Scholar]
- 243.Fraser L.R. The “switching on” of mammalian spermatozoa: Molecular events involved in promotion and regulation of capacitation. Mol. Reprod. Dev. 2010;77:197–208. doi: 10.1002/mrd.21124. [DOI] [PubMed] [Google Scholar]
- 244.Way A.L., Griel L.C.J., Killian G.J. Effects of accessory sex gland fluid on viability, capacitation, and the acrosome reaction of cauda epididymal bull spermatozoa. J. Androl. 2000;21:213–219. [PubMed] [Google Scholar]
- 245.Manjunath P., Thérien I. Role of seminal plasma phospholipid-binding proteins in sperm membrane lipid modification that occurs during capacitation. J. Reprod. Immunol. 2002;53:109–119. doi: 10.1016/S0165-0378(01)00098-5. [DOI] [PubMed] [Google Scholar]
- 246.Bergeron A., Crête M.-H., Brindle Y., Manjunath P. Low-density lipoprotein fraction from hen’s egg yolk decreases the binding of the major proteins of bovine seminal plasma to sperm and prevents lipid efflux from the sperm membrane. Biol. Reprod. 2004;70:708–717. doi: 10.1095/biolreprod.103.022996. [DOI] [PubMed] [Google Scholar]
- 247.Brinsko S.P., Crockett E.C., Squires E.L. Effect of centrifugation and partial removal of seminal plasma on equine spermatozoal motility after cooling and storage. Theriogenology. 2000;54:129–136. doi: 10.1016/S0093-691X(00)00331-9. [DOI] [PubMed] [Google Scholar]
- 248.Maxwell W.M., Welch G.R., Johnson L.A. Viability and membrane integrity of spermatozoa after dilution and flow cytometric sorting in the presence or absence of seminal plasma. Reprod. Fertil. Dev. 1996;8:1165–1178. doi: 10.1071/RD9961165. [DOI] [PubMed] [Google Scholar]
- 249.Pérez-Pé R., Cebrián-Pérez J.A., Muiño-Blanco T. Semen plasma proteins prevent cold-shock membrane damage to ram spermatozoa. Theriogenology. 2001;56:425–434. doi: 10.1016/S0093-691X(01)00574-X. [DOI] [PubMed] [Google Scholar]
- 250.Caballero I., Vazquez J.M., Mayor G.M., Almiñana C., Calvete J.J., Sanz L., Roca J., Martinez E.A. PSP-I/PSP-II spermadhesin exert a decapacitation effect on highly extended boar spermatozoa. Int. J. Androl. 2009;32:505–513. doi: 10.1111/j.1365-2605.2008.00887.x. [DOI] [PubMed] [Google Scholar]
- 251.Garner D.L., Thomas C.A., Gravance C.G., Marshall C.E., DeJarnette J.M., Allen C.H. Seminal plasma addition attenuates the dilution effect in bovine sperm. Theriogenology. 2001;56:31–40. doi: 10.1016/S0093-691X(01)00540-4. [DOI] [PubMed] [Google Scholar]
- 252.Höfner L., Luther A.-M., Waberski D. The role of seminal plasma in the liquid storage of spermatozoa. Anim. Reprod. Sci. 2020;220:106290. doi: 10.1016/j.anireprosci.2020.106290. [DOI] [PubMed] [Google Scholar]
- 253.Gaitskell-Phillips G., Martín-Cano F.E., Ortiz-Rodríguez J.M., Silva-Rodríguez A., Rodríguez-Martínez H., Gil M.C., Ortega-Ferrusola C., Peña F.J. Seminal plasma AnnexinA2 protein is a relevant biomarker for stallions which require removal of seminal plasma for sperm survival upon refrigeration. Biol. Reprod. 2020;103:1275–1288. doi: 10.1093/biolre/ioaa153. [DOI] [PubMed] [Google Scholar]
- 254.Parrilla I., Vazquez J.M., Caballero I., Gil M.A., Hernandez M., Roca J., Lucas X., Martinez E.A. Optimal characteristics of spermatozoa for semen technologies in pigs. Soc. Reprod. Fertil. Suppl. 2009;66:37–50. [PubMed] [Google Scholar]
- 255.Vadnais M.L., Roberts K.P. Effects of seminal plasma on cooling-induced capacitative changes in boar sperm. J. Androl. 2007;28:416–422. doi: 10.2164/jandrol.106.001826. [DOI] [PubMed] [Google Scholar]
- 256.Suzuki K., Asano A., Eriksson B., Niwa K., Nagai T., Rodriguez-Martinez H. Capacitation status and in vitro fertility of boar spermatozoa: Effects of seminal plasma, cumulus-oocyte-complexes-conditioned medium and hyaluronan. Int. J. Androl. 2002;25:84–93. doi: 10.1046/j.1365-2605.2002.00330.x. [DOI] [PubMed] [Google Scholar]
- 257.Al-Essawe E.M., Wallgren M., Wulf M., Aurich C., Macías-García B., Sjunnesson Y., Morrell J.M. Seminal plasma influences the fertilizing potential of cryopreserved stallion sperm. Theriogenology. 2018;115:99–107. doi: 10.1016/j.theriogenology.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 258.González-Cadavid V., Martins J.A.M., Moreno F.B., Andrade T.S., Santos A.C.L., Monteiro-Moreira A.C.O., Moreira R.A., Moura A.A. Seminal plasma proteins of adult boars and correlations with sperm parameters. Theriogenology. 2014;82:697–707. doi: 10.1016/j.theriogenology.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 259.Hernández M., Roca J., Calvete J.J., Sanz L., Muiño-Blanco T., Cebrián-Pérez J.A., Vázquez J.M., Martínez E.A. Cryosurvival and in vitro fertilizing capacity postthaw is improved when boar spermatozoa are frozen in the presence of seminal plasma from good freezer boars. J. Androl. 2007;28:689–697. doi: 10.2164/jandrol.107.002725. [DOI] [PubMed] [Google Scholar]
- 260.Eriksson B.M., Vazquez J.M., Martinez E.A., Roca J., Lucas X., Rodriguez-Martinez H. Effects of holding time during cooling and of type of package on plasma membrane integrity, motility and in vitro oocyte penetration ability of frozen-thawed boar spermatozoa. Theriogenology. 2001;55:1593–1605. doi: 10.1016/S0093-691X(01)00505-2. [DOI] [PubMed] [Google Scholar]
- 261.Okazaki T., Abe S., Yoshida S., Shimada M. Seminal plasma damages sperm during cryopreservation, but its presence during thawing improves semen quality and conception rates in boars with poor post-thaw semen quality. Theriogenology. 2008;71:491–498. doi: 10.1016/j.theriogenology.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 262.Okazaki T., Shimada M. New strategies of boar sperm cryopreservation: Development of novel freezing and thawing methods with a focus on the roles of seminal plasma. Anim. Sci. J. 2012;83:623–629. doi: 10.1111/j.1740-0929.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 263.Fernández-Gago R., Álvarez-Rodríguez M., Alonso M.E., González J.R., Alegre B., Domínguez J.C., Martínez-Pastor F. Thawing boar semen in the presence of seminal plasma improves motility, modifies subpopulation patterns and reduces chromatin alterations. Reprod. Fertil. Dev. 2017;29:1576–1584. doi: 10.1071/RD15530. [DOI] [PubMed] [Google Scholar]
- 264.Kaeoket K., Chanapiwat P., Tummaruk P., Techakumphu M., Kunavongkrit A. A preliminary study on using autologous and heterologous boar sperm supernatant from freezing processes as post-thawing solution: Its effect on sperm motility. Trop. Anim. Health Prod. 2011;43:1049–1055. doi: 10.1007/s11250-011-9804-6. [DOI] [PubMed] [Google Scholar]
- 265.Gervasi M.G., Soler A.J., González-Fernández L., Alves M.G., Oliveira P.F., Martín-Hidalgo D. Extracellular Vesicles, the Road toward the Improvement of ART Outcomes. Animals. 2020;10:2171. doi: 10.3390/ani10112171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Saadeldin I.M., Khalil W.A., Alharbi M.G., Lee S.H. The Current Trends in Using Nanoparticles, Liposomes, and Exosomes for Semen Cryopreservation. Animal. 2020;10:2281. doi: 10.3390/ani10122281. [DOI] [PMC free article] [PubMed] [Google Scholar]