Abstract
Background:
Necrotizing pancreatitis survivors develop complications beyond infected necrosis that often require invasive intervention. Remarkably few data have cataloged these late complications after acute necrotizing pancreatitis resolution. We sought to identify the types and incidence of complications after necrotizing pancreatitis.
Design:
An observational study was performed evaluating 647 patients with necrotizing pancreatitis captured in a single-institution database between 2005 and 2017 at a tertiary care hospital. Retrospective review and analysis of newly diagnosed conditions attributable to necrotizing pancreatitis was performed. Exclusion criteria included the following: death before disease resolution (n = 57, 9%) and patients lost to follow-up (n = 12, 2%).
Results:
A total of 578 patients were followed for a median of 46 months (range, 8 months to 15 y) after necrotizing pancreatitis. In 489 (85%) patients 1 or more complications developed and included symptomatic disconnected pancreatic duct syndrome (285 of 578, 49%), splanchnic vein thrombosis (257 of 572, 45%), new endocrine insufficiency (195 of 549, 35%), new exocrine insufficiency (108 of 571, 19%), symptomatic chronic pancreatitis (93 of 571, 16%), incisional hernia (89 of 420, 21%), biliary stricture (90 of 576, 16%), chronic pain (44 of 575, 8%), gastrointestinal fistula (44 of 578, 8%), pancreatic duct stricture (30 of 578, 5%), and duodenal stricture (28 of 578, 5%). During the follow-up period, a total of 340 (59%) patients required an invasive intervention after necrotizing pancreatitis resolution. Invasive pancreatobiliary intervention was required in 230 (40%) patients.
Conclusion:
Late complications are common in necrotizing pancreatitis survivors. A broad variety of problems manifest themselves after resolution of the acute disease process and often require invasive intervention. Necrotizing pancreatitis patients should be followed lifelong by experienced clinicians.
Introduction
Acute pancreatitis (AP) accounts for nearly 300,000 hospital admissions annually in the United States, resulting in more than US$2.2 billion in acute health care costs each year.1,2 Worldwide, an increasing incidence of AP has been observed.3,4 After resolution of AP, patients may experience permanent endocrine and exocrine insufficiency or failure.5,6 Additional consequences include recurrent AP, chronic pancreatitis, and impaired quality of life.7–9 The likelihood of developing these complications is directly associated with the severity of the earlier episode of AP.6,7,9,10 Most patients with AP experience mild disease; however, 10% to 20% of patients will progress to necrotizing pancreatitis (NP).11–13
Necrotizing pancreatitis is an acute process that associates with a prolonged disease course, the need for intensive care, frequent readmission, high rates of infection, and the need for invasive interventions.12,14,15 Studies cataloging and critically evaluating the complications in survivors of NP are lacking. Available series are often small in sample size, have limited follow-up, or are restricted to discrete complications such as pancreatic insufficiency, chronic pancreatitis, quality of life, and hernia.16–29 In our robust clinical experience managing NP patients, we have observed a diverse spectrum of complications in survivors. The aim of this study was to evaluate the types and incidence of these complications after an episode of NP.
We hypothesized that complications are common in survivors of necrotizing pancreatitis and include a number of extrapancreatic pathologic conditions in addition to the previously described pancreatic complications.
Methods
Patient population
This observational study included all consecutive patients treated at Indiana University Health University Hospital ([IU-UH] Indianapolis, IN) with a diagnosis of NP between 2005 and 2017 regardless of age, etiology, or treatment strategy. Our institutional practice throughout the duration of this report was that all NP patients are evaluated by a multidisciplinary team including consultant gastroenterologist and pancreatic surgeons. An institutional database was retrospectively reviewed to evaluate demographic and clinical information related to the acute NP disease process and all NP-related complications during the follow-up period. Only patients treated for NP during the acute disease process are included in this database. Patients treated for NP elsewhere and referred for the treatment of complications after disease resolution are not included. It is the practice of IU-UH physicians to follow all NP patients long-term after acute disease resolution. Inperson follow-up and a minimum follow-up period of 6 months were required to be included in the study. This study received exempt status from the institutional review board. All data were compiled and reported in compliance with the Health Insurance Portability and Accountability Act.
Parameters assessed
At the time of hospital admission, the following data were collected: pancreatitis etiology, age, sex, method of admission, and comorbidities. Parameters recorded during the course of acute NP included the following: computed tomography severity index (CTSI), extent of pancreatic necrosis, persistent organ failure (>48 hours), infected necrosis, intervention type, and disease duration. Complications evaluated included the following: pancreatic endocrine insufficiency, pancreatic exocrine insufficiency, symptomatic chronic pancreatitis (CP), symptomatic disconnected pancreatic duct syndrome (DPDS), splanchnic vein thrombosis (SVT), presinusoidal portal hypertension, pancreatic duct stricture, bile duct stricture, duodenal stricture, chronic pain syndrome, gastrointestinal (GI) fistula, incisional hernia, and any interventions after NP disease resolution. Analysis of complications was performed only on patients surviving acute NP and excluded those patients who died before disease resolution or those patients lost to follow-up.
Definitions
Acute pancreatitis was defined according to the revised Atlanta classification as two or more of the following: typical abdominal pain (acute onset, severe epigastric abdominal pain radiating to the back), serum lipase or amylase a minimum of three times the upper limit of laboratory normal, and characteristic findings of acute pancreatitis on cross-sectional imaging.30 Necrotizing pancreatitis was defined as the lack of pancreatic parenchymal enhancement or findings of peripancreatic necrosis such as an acute necrotic collection or walled off necrosis.30 CTSI was calculated in each patient (Table 1).31
Table 1.
Computed tomography severity index
| Prognostic indicator | Points |
|---|---|
| Pancreatic inflammation | |
| Normal pancreas | 0 |
| Focal or diffuse enlargement of the pancreas | 1 |
| Intrinsic pancreatic abnormalities with inflammatory changes in peripancreatic fat | 2 |
| Single, ill-defined fluid collection or phlegmon | 3 |
| Two or more poorly defined collections or presence of gas in or adjacent to the pancreas | 4 |
| Pancreatic necrosis | |
| None | 0 |
| <30% | 2 |
| 30%–50% | 4 |
| >50% | 6 |
Organ failure was defined according to the modified Marshall scoring system for organ dysfunction.30 Infected necrosis was suggested by the presence of gas within the collection on cross-sectional imaging and confirmed by microbiology culture of aseptically obtained specimens.30 Disease resolution was defined as the absence of clinical symptoms attributable to pancreatic necrosis and radiographic resolution of pancreatic necrosis. Mortality before disease resolution was recorded and included any patient death, regardless of etiology. Acute NP disease resolution was defined as clinical and radiographic resolution of pancreatic necrosis. Follow-up was defined as an in-person visit with their pancreatic specialist.
Endocrine insufficiency was defined as a serum hemoglobin A1c of >6.5% and the requirement of exogenous insulin supplementation. Exocrine insufficiency was diagnosed clinically and defined as pancreatic enzyme replacement therapy with improvement of symptoms typical of exocrine insufficiency. Formal exocrine insufficiency testing was not routinely performed. Symptomatic CP was diagnosed on cross-sectional or endoscopic ultrasound in the setting of symptoms characteristic of CP.32 Disconnected pancreatic duct syndrome was diagnosed when parenchymal necrosis involved ≥2 cm of pancreas resulting in viable upstream pancreatic tissue and extravasation of contrast or total cutoff of the main pancreatic duct on endoscopic retrograde pancreatography (ERP) or magnetic resonance pancreatography (MRP).33 SVT was diagnosed on contrast enhanced cross-sectional imaging in the presence of a filling defect within the lumen of the portal vein, superior mesenteric vein, and/or splenic vein.34 Radiographic evidence of presinusoidal portal hypertension in patients with SVT included esophageal or gastric varices with collateral vessel development. Pancreatic duct stricture was diagnosed on ERP or MRP as a localized narrowing of the pancreatic duct measuring (ISP)5 mm in length.35 Bile duct stricture was diagnosed on endoscopic retrograde or magnetic resonance cholangiography and defined as a narrowing of the extrahepatic bile duct <75% of the diameter of the unaffected duct in the setting of 1 or more elevated liver function tests.36 Duodenal stricture was diagnosed based on endoscopic or radiographic findings of duodenal narrowing and symptoms of gastric outlet obstruction. A chronic pain syndrome was diagnosed in patients requiring treatment by a physician specialized in chronic pain management under a signed pain contract and without an identifiable correctable structural pathology. During the course of the study, a variety of chronic pain assessments were used to aide diagnosis. Because of this, to meet the definition of a chronic pain syndrome in this study, the diagnosis must have been independently established by the treating pain specialist’s expert clinical opinion.
Resolution of pancreatic necrosis was defined as radiographic resolution of pancreatic necrosis in the absence of symptoms contributable to pancreatic necrosis and no further pancreatic necrosis directed intervention during follow-up. Any intervention to treat complications of NP after this point was considered as repeat intervention after disease resolution as long as pancreatic necrosis was not further addressed.
Necrotizing pancreatitis treatment strategy
The period included in this study spans an era of significant change in the management of NP. Early in the study period, 2005–2009, operative pancreatic debridement was frequently the first step in treating pancreatic necrosis. In 2008, a transition to percutaneous drainage as the first step in intervention began and was widely accepted by 2010. At this time, endoscopic debridement was introduced as an initial option to treat pancreatic necrosis and applied more widely beginning in 2014. The current treatment strategy at IU-UH reflects the evidence-based guidelines published in 2013 by the International Association of Pancreatology and the American Pancreatic Association and the American Gastroenterological Association update recently published in 2020.37,38 It is noteworthy that, as a tertiary referral center, 75% to 80% of NP patients are referred to our institution as transfers. These cases often reflect patients who have failed less-invasive necrosis intervention at facilities other than IU-UH, and therefore a higher proportion of IU-UH NP patients undergo operative intervention.
During the convalescent period of acute NP, patients are routinely seen in clinic at 2- to 4-week intervals with cross-sectional imaging to evaluate the evolution and determine resolution of pancreatic necrosis. After acute NP has resolved, follow-up includes an annual clinic evaluation by the responsible pancreatologist (pancreatic surgeon or gastroenterologist) to screen for symptoms that may be attributable to an earlier episode of NP. Routine imaging is not performed in this setting. Diagnostic evaluation is considered in these visits only when clinically indicated to evaluate new or persistent symptoms.
Statistical analysis
Data were recorded using Microsoft Excel 2005-2018 (Microsoft, Inc, Redmond, WA) and analyzed with IBM SPSS Statistics v 25.0 (IBM, Inc, Armonk, NY). Categorical data were described as numbers with percentages and compared using the Pearson χ2 statistic. Continuous data were described as median values with range or mean values with standard error of the mean and compared using the Mann-Whitney U test or Student’s t test. Associations between each individual complication and potential risk factors were evaluated, including age, sex, pancreatitis etiology, organ failure, infected necrosis, degree of gland necrosis, CTSI, location of gland necrosis, duration of disease, and type of necrosis intervention required. The Supplementary Table outlines these results in their entirety; however, among each individual complication only the statistically significant risk factors are reported in the body of the text. Analyzed were 13 potential risk factors for each complication. To control for multiple comparisons, the Bonferroni correction was used and P values <.00385 were accepted as statistically significant.39 Only patients at risk for each complication were analyzed. Thus, patients were censored from individual complication analysis in the event that complication was present before the onset of NP. Therefore, when describing each complication, denominators are listed with the number and percentage.
Results
A total of 647 NP patients were treated between 2005 and 2017. The most common pancreatitis etiology was biliary (n = 317, 49%) followed by alcohol (n = 133, 21%), post-ERCP (n = 39, 6%), and hypertriglyceridemia (n = 39, 6%). No specific etiology of pancreatitis was identified in 15% of patients (n = 99). Median age at diagnosis was 52 years (range, 13–96 years); 66% of patients were male (n = 424). Reflecting the regional referral pattern, patients were more commonly transferred to IU-UH (n = 501, 77%) than admitted primarily (n = 146, 23%).
The mean CTSI was 6.7 ± 0.08 and 36% (n = 236) of patients developed organ failure. The most common organ failure was respiratory (n = 210, 32%), followed by renal (n = 148, 23%) and cardiovascular (n = 94, 15%); multiple organ failure developed in 143 (22%) patients. The mean index hospital admission length of stay was 27.1 ± 1.1 days. The management strategy of all patients with NP is presented in Fig 1. In patients requiring intervention for pancreatic necrosis a median of 2 procedures (range, 1–14 procedures) were required (Fig 2). Infected necrosis was documented in 355 (55%) patients based on positive culture obtained from specimens obtained under sterile conditions during surgery (59%), percutaneous drain placement (34%), or endoscopic debridement (7%). The mean number of total inpatient hospital days before disease resolution was 48.4 ± 3.0 days.
Fig. 1.
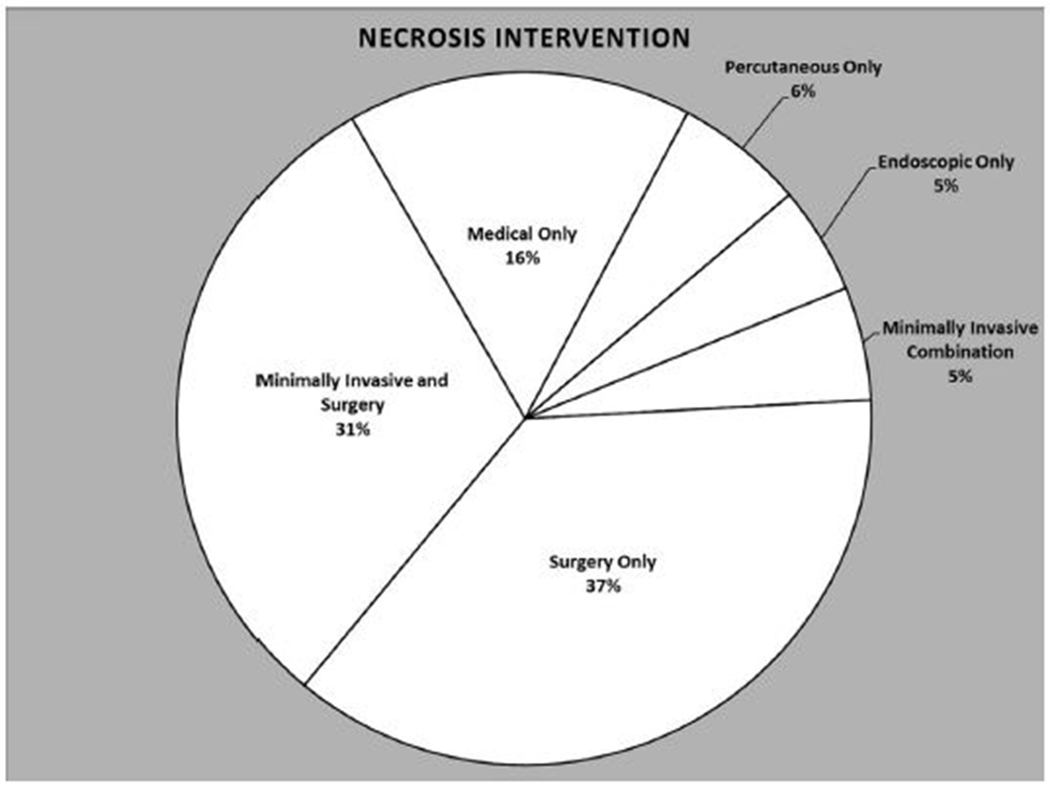
Definitive necrosis intervention of the overall cohort.
Fig. 2.
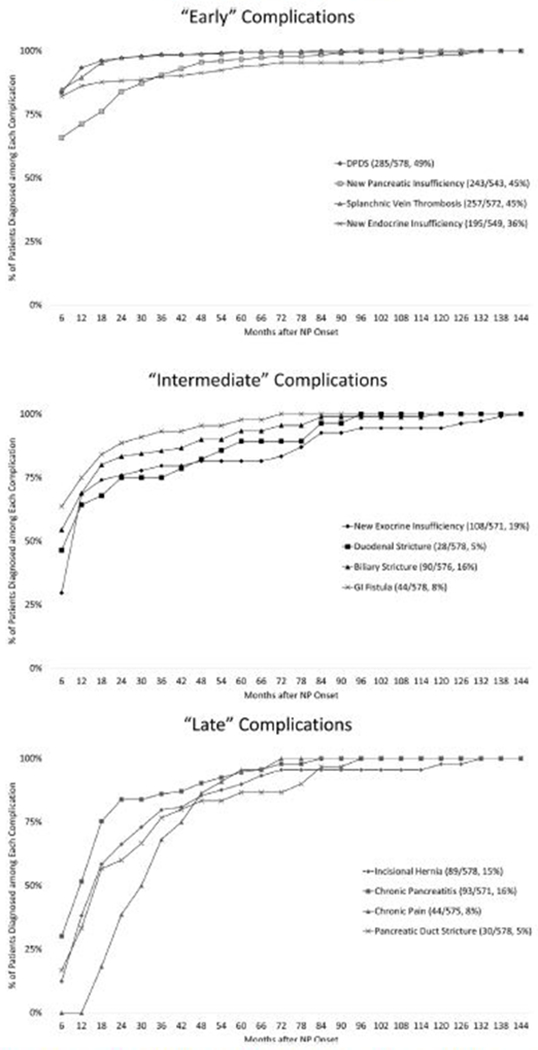
Overview of the incidence of each complication of necrotizing pancreatitis and the timing of diagnosis relative to necrotizing pancreatitis onset.
Overall mortality was 9% (n = 57), and 12 patients (2%) were lost to long-term follow-up. These 69 patients were censored from further analysis of complications after NP resolution and therefore the remaining analysis includes a total of 578 patients. The median time to disease resolution was 6.4 months (range, 1.2–30.7 months). The median duration of follow-up after NP onset was 46 months (range, 8 months–15 years). During the follow-up period, 85% of patients (n = 489) developed 1 or more complications of NP. A total of 340 (59%) patients required an invasive intervention after NP resolution. Invasive pancreatobiliary intervention was required in 230 (40%) patients. An overview of the complications is presented in Fig 3.
Fig. 3.
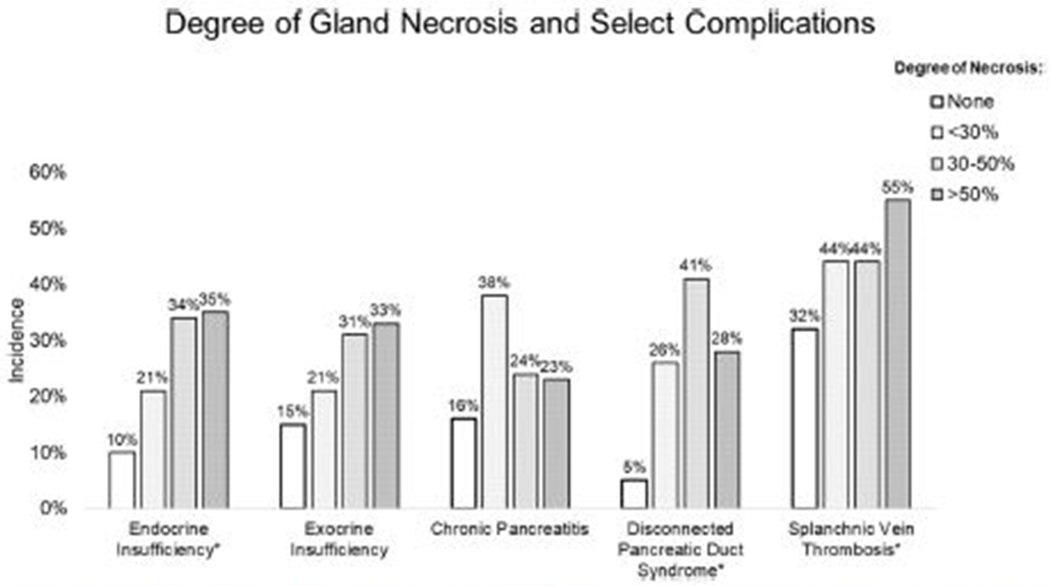
Degree of pancreatic necrosis and incidence of endocrine insufficiency, exocrine insufficiency, chronic pancreatitis, disconnected pancreatic duct syndrome, and splanchnic vein thrombosis. *Statistical significance. Complete analysis is included in the Supplemental Table.
Pancreatic insufficiency
New pancreatic endocrine insufficiency developed in 36% of patients (195 of 549 patients) either alone (n = 141) or in combination with exocrine insufficiency (n = 54). New endocrine insufficiency developed at NP onset in 77% of patients (150 of 195). In the remaining 45 patients, endocrine insufficiency was diagnosed a median of 26 months after NP onset (range, 1 month–13 years). New pancreatic exocrine insufficiency developed in 19% of patients (108 of 571 patients) either alone (n = 54) or in combination with endocrine insufficiency (n = 54). New exocrine insufficiency was diagnosed at a median of 8 months (range, 1 month–15 years). Thus, 243 of 543 (45%) patients developed new pancreatic endocrine or exocrine insufficiency. No patient diagnosed with pancreatic insufficiency regained sufficiency over time.
During the acute NP disease process, patients developing new endocrine insufficiency more frequently had organ failure (endocrine insufficiency, 41% versus no endocrine insufficiency, 29%; P = .004), infected necrosis (64% vs 49%; P = .0005), a higher mean CTSI (7.2 ± 0.1 vs 6.3 ± 0.1; P = .000002), and an increasing degree of pancreatic necrosis (Fig 4). Patients developing new onset exocrine insufficiency were observed to have higher rates of renal failure (exocrine insufficiency, 31% versus no exocrine insufficiency, 17%; P = .001) and cardiovascular failure (19% vs 9%; P = .003) during acute NP. Patients who required necrosis intervention had a higher incidence of endocrine (38%) and exocrine (21%) insufficiency compared with patients who recovered with medical therapy alone (25% and 9%, respectively); however, these differences did not reach the study’s predefined level of statistical significance. The Supplemental Table outlines the type of necrosis intervention to achieve NP resolution for patients with and without pancreatic insufficiency. No risk factors were identified in patients developing both endocrine and exocrine insufficiency.
Fig. 4.
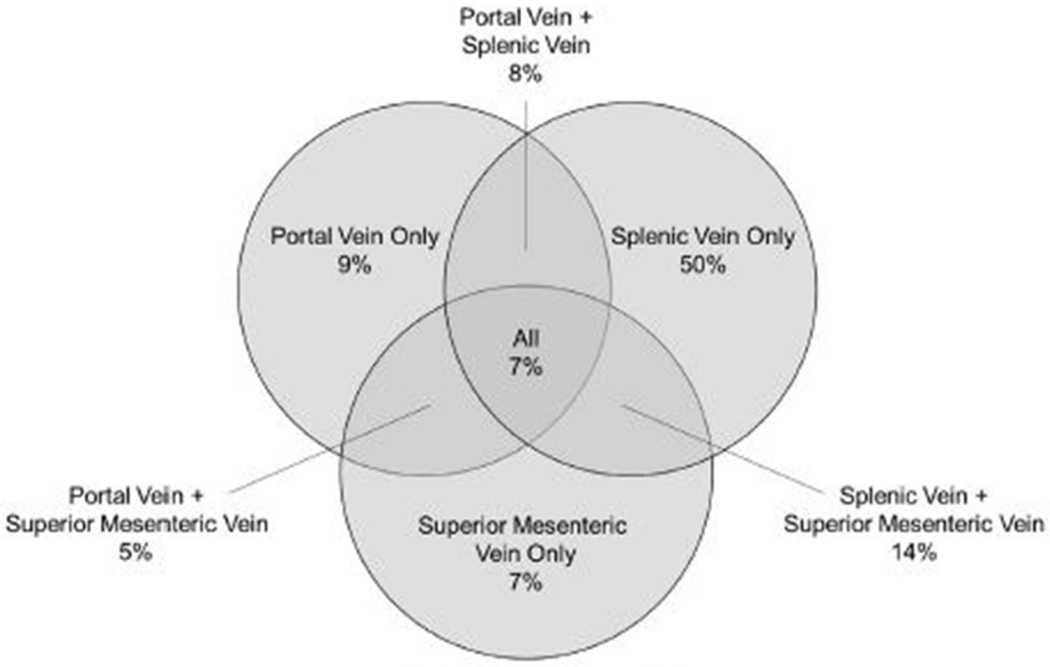
Distribution of splanchnic vein thrombosis.
Symptomatic chronic pancreatitis
Symptomatic CP was diagnosed in 16% of patients (93 of 571 patients) and manifested a median of 13 months (range, 1 month to 9 years) after the onset of NP. The most common presentation of symptomatic CP was abdominal pain (n = 69, 74%) followed by recurrent pancreatic pseudocyst (n = 15, 26%). Chronic pancreatitis was diagnosed in the remaining patients as an incidental finding on imaging (n = 3, 3%) or during the evaluation of steatorrhea (n = 3, 3%) or pancreatic head mass (n = 3, 3%). Diagnosis of CP was by ERP in 48 (52%) patients, endoscopic ultrasound in 27 (29%) patients, CT in 12 (13%) patients, and MRP in 6 (13%%) patients. Compared with patients who did not develop CP, alcohol etiology was more frequently observed in patients developing CP (CP, 31% versus no CP, 19%; P = .001). Patients who developed CP on average were younger (48 years vs 52 years), but this did not reach statistical significance (P = .018).
Patients who developed CP required necrosis intervention at rates similar to patients who did not develop CP (84% each intervention rate). The type of necrosis intervention and the NP clinical course was similar between these 2 groups (Supplemental Table).
Symptomatic disconnected pancreatic duct syndrome
The diagnosis of symptomatic DPDS was established in 49% of patients (285 of 578 patients). Diagnosis was made a median of 2 months (range, 1 day to 6 years) after the onset of NP. Compared with patients without DPDS, patients developing DPDS had a higher incidence of necrosis involving the neck (DPDS, 63% versus no DPDS, 44%; P = .000004) and body (74% vs 54%; P < .000001) of the pancreas. An increasing degree of gland necrosis (Fig 4) and CTSI (7.1 ± 0.1 vs 6.3 ± 0.1; P < .000001) were associated with the development of DPDS. Patients with DPDS had a prolonged NP disease duration (7.0 ± 0.4 months vs 6.0 ± 0.3 months; P < .000001) and were more likely to require operative management of their necrosis either alone (42% vs 36%) or in combination with minimally invasive necrosis intervention (34% vs 24%), P < .000001.
Surgical intervention was performed in 227 (80%) patients a median of 4 months (range, 20 days to 7 years) after NP onset. Operative intervention consisted of internal drainage of the disconnected pancreatic tail (n = 107), distal pancreatectomy (n = 89), and open pancreatic debridement with spontaneous closure of pancreaticocutaneous fistula (n = 31). Detailed analysis of the operative management of symptomatic DPDS and outcomes has been reported elsewhere.40
Splanchnic vein thrombosis
Splanchnic vein thrombosis developed in 45% of patients (257 of 570 patients). The diagnosis of SVT was secured at a median of 48 days (range, 0 days to 10 years) after NP onset. The incidence of SVT was associated with an increasing degree of gland necrosis (Fig 4) and a higher CTSI (SVT, 7.0 ± 0.1 versus no SVT, 6.4 ± 0.1; P = .0001). The duration of acute NP was longer in patients developing SVT (7.4 ± 0.4 months) compared with patients without SVT (5.7 ± 0.2 months), P = .001. The pattern of SVT is presented in Fig 4. One patient with acute portal vein thrombosis developed fatal fulminant liver failure and another patient with acute superior mesenteric vein thrombosis developed intestinal ischemia requiring total enterectomy. Radiographic evidence of presinusoidal portal hypertension developed in 152 of the 257 patients (59%) with SVT. During long-term follow-up, 5 (3%) patients developed upper GI bleed attributed to presinusoidal portal hypertension. The impact of venous thromboembolism, including SVT, deep vein thrombosis, and pulmonary embolism on outcomes during acute NP have been reported elsewhere.34
Pancreaticobiliary and duodenal stricture
Pancreatic duct stricture developed in 5% of patients (30 of 578 patients) and was diagnosed at a median of 18 months (range, 2 months to 6 years) after the onset of NP. Presentation of pancreatic duct stricture included symptomatic CP (n = 14, 47%), recurrent acute pancreatitis (n = 10, 33%), or recurrent pseudocyst (n = 6, 20%). Intervention was only performed in symptomatic patients and was indicated in 28 patients (93%). A mean number of 3.5 ± 0.4 procedures was required to achieve stricture resolution a median of 6 months (range, 6 days to 6 years) after diagnosis. A total of 6 patients have ongoing pancreatic duct stricture. All patients requiring intervention underwent a combination of endoscopic and surgical therapy. No risk factors for the development of pancreatic duct stricture were identified.
Biliary stricture developed in 16% of patients (90 of 576 patients), presenting a median of 12 months (range, 14 days to 10 years) after the onset of NP. At presentation, the mean alkaline phosphatase was 557 ± 65 U/L and the mean total bilirubin was 4.3 ± 0.5 mg/dL. Intervention was required in 88 patients (98%). A mean number of 3.9 ± 0.3 procedures was required to achieve stricture resolution at a median of 6 months (range, 3 days to 8 years) after the diagnosis of biliary stricture. Intervention included endoscopic therapy only (n = 60, 68%), a combination of endoscopic, percutaneous, and surgical intervention (n = 24, 27%), percutaneous only (n = 2, 2%), and surgical only (n = 2, 2%). No risk factors for the development of biliary stricture were identified.
Duodenal stricture developed in 5% of patients (28 of 578 patients), presenting at a median of 8 months (range, 1 month to 7 years) after NP onset. Intervention was required in 17 patients (61%) to achieve resolution of stricture at a median of 17 days (range, 3 days to 7 months) after diagnosis. Intervention included surgical intervention (n = 11, 39%), a combination of endoscopic and surgical intervention (n = 5, 18%), and endoscopic intervention only (n = 1, 4%). Endoscopic intervention included the placement of a gastrojejunostomy feeding tube in 4 patients, endoscopic dilation in 1 patient, and both in 1 patient. The remaining 11 patients either died before intervention (n = 1, 4%) or had symptomatic improvement with diet modification (n = 10, 36%). No risk factors for the development of duodenal stricture were identified.
Chronic pain
A chronic pain syndrome was diagnosed in 8% of patients (44 of 575 patients) at a median of 28 months (range, 12 months to 8 years) after NP onset. An invasive procedure for symptom control was performed in 18 patients (41%). These invasive interventions included celiac plexus block/neurolysis (n = 12), intrathecal pain pump (n = 3), spinal cord stimulator (n = 2), or a combination (n = 1). All 44 patients diagnosed with a chronic pain syndrome required opioid medications. The mean age in patients developing chronic pain (43 ± 2 years) was younger than patients who did not develop chronic pain (52 ± 1 years), P = .00009.
Gastrointestinal fistula
Non-pancreatic GI fistula developed in 8% of patients (44 of 578 patients) at a median of 5 months (range, 15 days to 6 years) after onset of NP. Gastrointestinal fistula involved the colon in 16 patients, jejunum or ileum in 14 patients, duodenum in 6 patients, and stomach in 11 patients (in 2 patients the fistula involved both the duodenum and jejunum/ileum and in 1 patient the fistula involved both the jejunum/ileum and colon). Gastrointestinal fistula was associated with higher rates of organ failure, infected necrosis, and operative intervention (Supplementary Table). In addition, patients with GI fistula had a longer NP disease duration (11.9 ± 8.4 months) compared with patients without GI fistula (6.0 ± 0.2 months), P < .000001.
Intervention was required in 36 patients (86%) with GI fistula to achieve resolution at a median of 3 months (range, 5 days to 6 years) after diagnosis; 7 patients experienced spontaneous fistula closure and 1 patient died before intervention for GI fistula. A mean number of 3.5 ± 0.4 was required to achieve resolution and included surgical intervention only (n = 19); a combination of surgical, percutaneous, and/or endoscopic intervention (n = 11); and percutaneous drain alone (n = 6).
Incisional hernia
A total of 420 patients required an operation for NP or NP-related complications. Incisional hernia developed in 89 of these patients (21%) and operative repair was performed in 77 patients. Outcomes in NP patients undergoing incisional hernia repair have been reported elsewhere.23
Uncommon complications
In addition to the relatively common post-NP complications documented earlier in this report, we have treated patients manifesting a large number of less common, but not less important complications. These conditions occurred too infrequently to allow for subgroup analysis to identify risk factors. Infrequent complications associated with NP or its treatment included tracheal stenosis (n = 19), ureteral stricture (n = 7), inflammatory colon stricture (n = 7), progressive renal failure (n = 5), pancreatic ductal carcinoma (n = 5),41 irreversible gastrointestinal failure requiring multivisceral transplant (n = 4), male impotence (n = 1), metabolic peripheral polyneuropathy (n = 1), deafness (n = 1), and blindness (n = 1).
Discussion
NP is often studied as an acute process, with minimal attention paid to the complications that develop in survivors. Therefore, the overall goal of this study was to provide a broad report describing the spectrum of complications in NP. This large-volume, single-center series with very long-term follow-up allows for a greater appreciation of both the common and less frequent pancreatic and extrapancreatic complications associated with NP–85% of survivors developed a minimum of 1 complication. Most reports focus on endocrine and exocrine insufficiency and chronic pancreatitis. In this series, these complications were extremely common and had developed in 16% to 45% of NP patients with long-term follow-up. Unique to this study is the report of less frequently described pancreatic and extrapancreatic complications of NP including DPDS, SVT, presinusoidal portal hypertension, pancreaticobiliary and duodenal stricture, chronic pain, and GI fistula. Although infrequently reported, these complications are quite common with incidences ranging 5% to 49%. Awareness of these problems and their presentation is imperative in the long-term follow-up of patients surviving NP as 59% of patients in this study required invasive intervention after NP resolution. Understanding the common complications from acute NP that may be treated with invasive intervention is important in optimizing daily symptoms, quality of life, and return to baseline functional status after disease resolution. An understanding of the spectrum of complications that develop in these patients is necessary. The ideal follow-up strategy in patients who survived NP remains unknown but should consider the frequency and timing of the complications reported in this series. In most cases, annual clinic evaluation should be performed and more frequent visits considered on a case-by-case basis.
Pancreatic insufficiency after NP is common and has been associated with increasing severity of acute pancreatitis as reported elsewhere.5,6,10 Our series is consistent with these earlier reports. Any new pancreatic insufficiency developed in nearly half of patients, with new endocrine insufficiency occurring in 36% of patients and new exocrine insufficiency occurring in 19% of patients. Compared with pancreatitis patients with any severity, these complications develop at a much higher rate after NP. Thus, survivors of NP are at the highest risk of all pancreatitis patients. It follows that monitoring glucose control through serum hemoglobin A1c after resolution of NP is critical in the long-term management of these patients. Within the subset of NP patients, increasing CTSI and degree of pancreatic necrosis and the development of organ failure or infected necrosis appear to be directly associated with an increased risk for endocrine insufficiency. Increasing CTSI has been identified as a risk factor for new onset endocrine insufficiency after acute pancreatitis42; however, this study identified increasing CTSI as a risk factor within NP patients, highlighting the prognostic utility of CTSI even among the most severe cases of AP. In light of these data, NP survivors should be routinely screened for symptoms attributable to endocrine insufficiency, exocrine insufficiency, and chronic pancreatitis and undergo regular laboratory evaluation. Diagnostic imaging to evaluate for chronic pancreatitis should be considered on an individual basis. Identification and prompt treatment of pancreatic insufficiency may significantly improve quality of life and long-term health.
The deeper focus of this study was to evaluate the less frequently examined, albeit still common, complications of NP. Splanchnic vein thrombosis developed in nearly half of patients and predominantly involved the splenic vein; however, the superior mesenteric or portal vein was involved in 50% of patients with SVT. The incidence of SVT in NP in this study was much higher than the rates of 1% to 24% reported elsewhere.43 Pancreatic necrosis has been identified elsewhere44 as a risk factor for SVT. In this study, the extent of pancreatic necrosis, increasing CTSI, and prolonged NP disease duration were risk factors for SVT among NP patients. In addition, tail necrosis was identified as a risk factor for SVT, which is not surprising given the incidence of splenic vein thrombosis in NP patients and the proximity of the splenic vein to the pancreas. The ideal treatment strategy for SVT in NP remains unknown. The risks of systemic anticoagulation must be weighed against the risks of untreated SVT on an individual basis. Furthermore, over half of patients with SVT developed evidence of presinusoidal portal hypertension. This is most often left-sided or “sinistral” portal hypertension that results in gastric varices and associated gastric variceal hemorrhage. Bleeding complications of sinistral portal hypertension developed infrequently (3%) and no routine treatment for sinistral portal hypertension is necessary.45 However, it is noteworthy that SVT resulted in catastrophic complications in 2cpatients (fatal liver failure, total bowel ischemia). Furthermore, the presence of presinusoidal portal hypertension increases the complexity and risk of any operation in the left upper quadrant and this is particularly significant given the high incidence of repeat invasive intervention during long-term follow-up of NP survivors.
Invasive interventions were required in 59% of patients during long-term follow-up after NP resolution and were most commonly performed for the treatment of DPDS, pancreatic duct stricture, bile duct stricture, duodenal stricture, hernia, and GI fistula. Several of these complications have been reported in small case series; however, a significant strength of the current study is the large number of consecutive NP patients treated at a single institution, which allows for an accurate estimate of how frequent these complications are in NP. Although these complications develop with varying incidences (5%−49%), each significantly impacts quality of life. These problems may manifest late, often years after resolution of acute disease. This observation underscores the importance of educated long-term follow-up for this patient population. Treatment frequently requires multiple interventions performed over months or years to achieve symptom resolution. Recurrence of symptoms is common and typically requires repeat intervention. Few studies have reported intervention rates after NP resolution of 8% to 41%18,26–28,46; however, these are small-volume studies with short-duration follow-up and likely do not reflect the true incidence of repeat intervention after NP disease resolution.
A specific clinical point that deserves discussion is the relationship between NP and pancreatic cancer. It is well established that pancreatic cancer can prompt acute pancreatitis; however, this diagnosis in NP is extremely difficult to make in the presence of parenchymal necrosis and distorted anatomy by pancreatic and peripancreatic collections.41 The most practical message is that clinicians should be aware of this relationship, especially in middle-aged or elderly patients with idiopathic etiology of NP, and have a low threshold for ordering pancreatic cancer tumor marker carbohydrate antigen 19-9 serum concentration to investigate this possibility.
Throughout the past decade, a drastic change in the management of NP requiring intervention has taken place.37,47,48 These innovations have translated to significant improvements in the morbidity and mortality of patients with NP. As more minimally invasive approaches are utilized, intuitively a decrease in complications such as hernia will be observed. Minimally invasive approaches have been associated with equivalent or improved rates of pancreatic insufficiency after NP47,49,50 However, the impact of evolving management strategies on complications, such as gastrointestinal fistula, pancreaticobiliary stricture, splanchnic vein thrombosis, chronic pancreatitis, and chronic pain, remain unknown. Investigation of novel management strategies should consider these complications in their evaluation.
Significant strengths of this study include its size, duration of follow-up, and few patients lost to follow-up. These strengths allow for a comprehensive inventory of the less commonly reported complications of NP; however, several complications were not evaluated in this series, including recurrent acute pancreatitis; mental illness; the impact on quality of life and functional status; and long-term renal, respiratory, and cardiovascular decline. The aim of this series was to provide an overview of the variety of complications that develop in NP. Given the definitions of individual complications used in this study, the incidence of these complications may be underestimated. On the other hand, the majority of NP patients treated in this study were referred to our institution as transfers and reflect the most complex NP cases. This may have resulted in an overestimation of complications in this study. This analysis did not include specific assessment of long-term quality-of-life metrics or psychologic wellness; however, anecdotally this represents a quite common problem that deserves further in-depth study. The retrospective nature of this study has inherent limitations in the ability to associate certain risk factors with individual complications. As an example, in patients with alcohol etiology of NP that developed CP, the ability to accurately assess alcohol cessation and volume of alcohol use after NP resolution was limited.
In conclusion, late complications are common in necrotizing pancreatitis survivors. A broad variety of problems manifest themselves months to years after resolution of the acute disease process and often require intervention after resolution of the acute disease process. Necrotizing pancreatitis patients should be followed lifelong by experienced clinicians.
Supplementary Material
Acknowledgments
Funding/Support
This study did not receive or require any funding.
Footnotes
Conflict of interest/Disclosure
The authors declare no conflicts of interest.
Supplementary data
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.surg.2020.07.004.
References
- 1.Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology. 2015;149: 1731–1741.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagenholz P, Fernández-del Castillo C, Harris N, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35: 302–307. [DOI] [PubMed] [Google Scholar]
- 3.Yadav D, Lowenfels A. Trends in the epidemiology of the first attach of acute pancreatitis: A systematic review. Pancreas. 2006;33:323–330. [DOI] [PubMed] [Google Scholar]
- 4.Yadav D, Lowenfels A. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das SL, Singh PP, Phillips AR, et al. Newly diagnosed diabetes mellitus after acute pancreatitis: A systematic review and meta-analysis. Gut. 2014;63: 818–831. [DOI] [PubMed] [Google Scholar]
- 6.Hollemans RA, Hallensleben NDL, Mager DJ, et al. Pancreatic exocrine insufficiency following acute pancreatitis: Systematic review and study level meta-analysis. Pancreatology. 2018;18:253–262. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed Ali U, Issa Y, Hagenaars JC, et al. Risk of recurrent pancreatitis and progression to chronic pancreatitis after a first episode of acute pancreatitis. Clin Gastroenterol Hepatol. 2016;14:738–746. [DOI] [PubMed] [Google Scholar]
- 8.Takeyama Y Long-term prognosis of acute pancreatitis in Japan. Clin Gastroenterol Hepatol. 2009;7(11 Suppl):S15–S17. [DOI] [PubMed] [Google Scholar]
- 9.Machicado JD, Gougol A, Stello K, et al. Acute pancreatitis has a long-term deleterious effect on physical health related quality of life. Clin Gastroenterol Hepatol. 2017;15:1435–1443.e2. [DOI] [PubMed] [Google Scholar]
- 10.Vipperla K, Papachristou GI, Slivka A, et al. Risk of new-onset diabetes is determined by severity of acute pancreatitis. Pancreas. 2016;45:e14–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavallini G, Frulloni L, Bassi C, et al. Prospective multicentre survey on acute pancreatitis in Italy (ProInf-AISP): Results on 1005 patients. Dig Liver Dis. 2004;36:205–211. [DOI] [PubMed] [Google Scholar]
- 12.Freeman M, Werner J, van Santvoort H, et al. Interventions for necrotizing pancreatitis: Summary of a multidisciplinary consensus conference. Pancreas. 2012;41:1176–1194. [DOI] [PubMed] [Google Scholar]
- 13.Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. [DOI] [PubMed] [Google Scholar]
- 14.Maatman TK, Mahajan S, Roch AM, et al. High rates of readmission in necrotizing pancreatitis: Natural history or opportunity for improvement? J Gastrointest Surg. 2019;23:1834–1839. [DOI] [PubMed] [Google Scholar]
- 15.Petrov MS, Shanbhag S, Chakraborty M, et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–820. [DOI] [PubMed] [Google Scholar]
- 16.Tzovaras G, Parks RW, Diamond T, et al. Early and long-term results of surgery for severe necrotising pancreatitis. Dig Surg. 2004;21:41–46;discussion 46–47. [DOI] [PubMed] [Google Scholar]
- 17.Umapathy C, Raina A, Saligram S, et al. Natural history after acute necrotizing pancreatitis: A large US tertiary care experience. J Gastrointest Surg. 2016;20: 1844–1853. [DOI] [PubMed] [Google Scholar]
- 18.Connor S, Alexakis N, Raraty MG, et al. Early and late complications after pancreatic necrosectomy. Surgery. 2005;137:499–505. [DOI] [PubMed] [Google Scholar]
- 19.Gupta R, Wig JD, Bhasin DK, et al. Severe acute pancreatitis: The life after. J Gastrointest Surg. 2009;13:1328–1336. [DOI] [PubMed] [Google Scholar]
- 20.Tsiotos GG, Luque-de León E, Sarr MG. Long-term outcome of necrotizing pancreatitis treated by necrosectomy. Br J Surg. 1998;85:1650–1653. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda T, Ueda T, Takeyama Y, et al. Long-term outcome of severe acute pancreatitis. J Hepatobiliary Pancreat Surg. 2008;15:397–402. [DOI] [PubMed] [Google Scholar]
- 22.Winter Gasparoto R, Racy M, De Campos T. Long-term outcomes after acute necrotizing pancreatitis: What happens to the pancreas and to the patient? JOP. 2015;16:159–166. [DOI] [PubMed] [Google Scholar]
- 23.Al-Azzawi HH, Kuhlenschmidt H, Howard TJ, et al. The burden of incisional hernia in necrotizing pancreatitis: How can we improve? Am J Surg. 2010;199: 310–314;discussion 314. [DOI] [PubMed] [Google Scholar]
- 24.Chandrasekaran P, Gupta R, Shenvi S, et al. Prospective comparison of long term outcomes in patients with severe acute pancreatitis managed by operative and non operative measures. Pancreatology. 2015;15:478–484. [DOI] [PubMed] [Google Scholar]
- 25.Uomo G, Gallucci F, Madrid E, et al. Pancreatic functional impairment following acute necrotizing pancreatitis: Long-term outcome of a non-surgically treated series. Dig Liver Dis. 2010;42:149–152. [DOI] [PubMed] [Google Scholar]
- 26.Cinquepalmi L, Boni L, Dionigi G, et al. Long-term results and quality of life of patients undergoing sequential surgical treatment for severe acute pancreatitis complicated by infected pancreatic necrosis. Surg Infect (Larchmt). 2006;7(Suppl 2):S113–S116. [DOI] [PubMed] [Google Scholar]
- 27.Ross AS, Irani S, Gan SI, et al. Dual-modality drainage of infected and symptomatic walled-off pancreatic necrosis: Long-term clinical outcomes. Gastrointest Endosc. 2014;79:929–935. [DOI] [PubMed] [Google Scholar]
- 28.Seewald S, Ang TL, Richter H, et al. Long-term results after endoscopic drainage and necrosectomy of symptomatic pancreatic fluid collections. Dig Endosc. 2012;24:36–41. [DOI] [PubMed] [Google Scholar]
- 29.Endlicher E, Volk M, Feuerbach S, et al. Long-term follow-up of patients with necrotizing pancreatitis treated by percutaneous necrosectomy. Hepatogastroenterology. 2003;50:2225–2228. [PubMed] [Google Scholar]
- 30.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. [DOI] [PubMed] [Google Scholar]
- 31.Balthazar E, Robinson D, Megibow A, et al. Acute pancreatitis: Value of CT in establishing prognosis. Radiology. 1990;174:331–336. [DOI] [PubMed] [Google Scholar]
- 32.Conwell DL, Lee LS, Yadav D, et al. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: Evidence-based report on diagnostic guidelines. Pancreas. 2014;43:1143–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandrasegaran K, Tann M, Jennings G, et al. Disconnection of the pancreatic duct: An Important but overlooked complication of severe acute pancreatitis. Radiographics. 2007;27:1389–1400. [DOI] [PubMed] [Google Scholar]
- 34.Roch AM, Maatman TK, Carr RA, et al. Venous thromboembolism in necrotizing pancreatitis: An underappreciated risk. J Gastrointest Surg. 2019;23: 2430–2438. [DOI] [PubMed] [Google Scholar]
- 35.Axon A, Classen M, Cotton P, et al. Pancreatography in chronic pancreatitis: International definitions. Gut. 1984;25:1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cote GA, Slivka A, Tarnasky P, et al. Effect of covered metallic stents compared with plastic stents on benign biliary stricture resolution: A randomized clinical trial. JAMA. 2016;315:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1–e15. [DOI] [PubMed] [Google Scholar]
- 38.Baron TH, DiMaio CJ, Wang AY, et al. American Gastroenterological Association Clinical Practice Update: Management of pancreatic necrosis. Gastroenterology. 2020;158:67–75 e1. [DOI] [PubMed] [Google Scholar]
- 39.Vickerstaff V, Omar RZ, Ambler G. Methods to adjust for multiple comparisons in the analysis and sample size calculation of randomised controlled trials with multiple primary outcomes. BMC Med Res Methodol. 2019;19:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maatman TK, Roch AM, Heimberger MA, et al. Disconnected pancreatic duct syndrome: Spectrum of operative management. J Surg Res. 2020;247:297–303. [DOI] [PubMed] [Google Scholar]
- 41.Lewellen KA, Maatman TK, Heimberger MA, et al. Pancreatic adenocarcinoma causing necrotizing pancreatitis: Not as rare as you think? J Surg Res. 2020;250: 53–58. [DOI] [PubMed] [Google Scholar]
- 42.Tu J, Yang Y, Zhang J, et al. Effect of the disease severity on the risk of developing new-onset diabetes after acute pancreatitis. Medicine (Baltimore). 2018;97:e10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nadkarni NA, Khanna S, Vege SS. Splanchnic Venous Thrombosis and Pancreatitis. Pancreas. 2013;42(6):924–931. [DOI] [PubMed] [Google Scholar]
- 44.Easler J, Muddana V, Furlan A, et al. Portosplenomesenteric venous thrombosis in patients with acute pancreatitis is associated with pancreatic necrosis and usually has a benign course. Clin Gastroenterol Hepatol. 2014;12:854–862. [DOI] [PubMed] [Google Scholar]
- 45.Butler JR, Eckert GJ, Zyromski NJ, et al. Natural history of pancreatitis-induced splenic vein thrombosis: A systematic review and meta-analysis of its incidence and rate of gastrointestinal bleeding. HPB (Oxford). 2011;13:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smoczynski M, Marek I, Dubowik M, et al. Endoscopic drainage/debridement of walled-off pancreatic necrosis—Single center experience of 112 cases. Pancreatology. 2014;14:137–142. [DOI] [PubMed] [Google Scholar]
- 47.Van Santvoort H, Besselink M, Bakker O, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491–1502. [DOI] [PubMed] [Google Scholar]
- 48.Besselink M The ‘step-up approach’ to infected necrotizing pancreatitis: Delay, drain, debride. Dig Liver Dis. 2011;43:421–422. [DOI] [PubMed] [Google Scholar]
- 49.Haney CM, Kowalewski KF, Schmidt MW, et al. Endoscopic versus surgical treatment for infected necrotizing pancreatitis: A systematic review and meta-analysis of randomized controlled trials. Surg Endosc. 2020;34:2429–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Brunschot S, Hollemans RA, Bakker OJ, et al. Minimally invasive and endoscopic versus open necrosectomy for necrotising pancreatitis: A pooled analysis of individual data for 1980 patients. Gut. 2018;67:697–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


