Abstract
Background:
There is little information concerning the biocompatibility of mouthwashes containing metal nanoparticles. This study was conducted to assess the biocompatibility of colloidal solutions containing zinc oxide (ZnO), copper oxide (CuO), titanium dioxide (TiO2), and silver (Ag) nanoparticles compared with chlorhexidine (CHX) in a culture of human gingival fibroblasts (HGFs).
Materials and Methods:
This was an in vitro, experimental study. Nanoparticles, including ZnO, CuO, TiO2, and Ag, were purchased and added to a water-based solution to produce mouthwashes. The colloidal solutions and CHX were prepared at the minimum inhibitory concentration (MIC) against Streptococcus mutans and Streptococcus sanguis. Cytotoxicity was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay on HGFs at the concentrations of MIC, 0.1 MIC, and 0.01 MIC. To determine apoptosis, DNA fragmentation was assessed as “sub-G1” peak on DNA content histogram. The data were analyzed using repeated measures analysis at P < 0.05.
Results:
At all concentrations, the highest and lowest mean of cell viability was related to TiO2 and ZnO groups, respectively. At MIC, the mean cell viability was significantly greater in the TiO2 group than the other groups (except the Ag group) (P < 0.05). At the concentration of 0.01 MIC, the mean cell viability in the colloidal solution containing ZnO nanoparticles was significantly lower than the other solutions (P < 0.05). The CHX and CuO-containing solution displayed the highest rate of apoptosis among the groups.
Conclusion:
The TiO2-containing solution can be suggested as a suitable alternative to CHX to provide antiseptic effects with minimal toxicity.
Key Words: Apoptosis, biocompatibility, cytotoxicity, mouthwashes, nanoparticles
INTRODUCTION
Dental caries and periodontal problems are the most prevalent oral diseases worldwide.[1] So far, great attempts have been made to produce materials that have effective antibacterial properties against oral pathogens. One of the most common approaches to attack oral microbial biofilm is the use of mouthwashes as adjuncts to mechanical methods of oral hygiene. The mouthwashes are especially useful in individuals who cannot maintain effective oral healthcare such as traumatized or disabled patients with inappropriate personal skills.[2] The patients undergoing fixed orthodontic therapy are also at great risk of exposure to acidogenic bacteria over the course of treatment, as orthodontic appliances cause food accumulation and interfere with mechanical methods of plaque removal such as tooth brushing and dental flossing.[3,4] However, the administration of commercially chemical agents is associated with numerous drawbacks. For example, the use of chlorhexidine (CHX) mouthwash leads to an unpleasant taste sensation, mucosal irritation, and staining of teeth and tooth-colored restorative materials.[5] In patients with fixed orthodontic appliances, the use of fluoride-containing mouthwashes may lead to corrosion, increased friction in sliding mechanics, and decreased tensile strength and bending strength of wires.[6,7,8] Therefore, searching for an ideal mouthwash to attack microbial plaque without any side effects is still continuing.
Nanotechnology is one of the most important developments in the field of medicine and dentistry. Nanotechnology is the knowledge of producing materials and structures in the range of 0.1–100 nm in size by different physical and chemical methods. The decrease in particle size (<100 nm in diameter) leads to greater surface area, and thus, the interaction with organic and inorganic molecules enhances.[9] Today, the importance of nanosized bacteriostatic materials is more important than ever due to the increase in new microbial strains resistant to antibiotics. It has been demonstrated that metal nanoparticles present excellent bactericidal and bacteriostatic properties.[9,10] In a previous study, the anti-bacterial properties of new colloidal solutions containing nanoparticles of copper oxide (CuO), zinc oxide (ZnO), titanium dioxide (TiO2) and silver (Ag) were investigated, and the minimum inhibitory concentration (MIC) of each solution was determined against Streptococcus mutans and Streptococcus sanguis.[11] However, when planning to produce new mouthwashes, the biocompatibility should be paid due attention. Concerning their artificial production, exposing humans to nanoparticles can pose potential health risks. The cytotoxicity of mouthwashes is important as these materials are in contact with oral mucosa over prolonged periods and can cause irritation and damage to oral and periodontal tissues.[12]
There are several reports on the toxicity of metal nanoparticles and some evidence regarding the toxic effects of CHX on oral cells and animal studies.[13,14,15,16] According to the authors' knowledge, there is no information regarding the cytotoxic effects of colloidal solutions containing metal nanoparticles when applied as mouthwashes. The present study was conducted to evaluate the biocompatibility of colloidal solutions containing ZnO, CuO, TiO2, and Ag nanoparticles compared with CHX mouthwash in a culture medium of human gingival fibroblasts (HGFs).
MATERIALS AND METHODS
This was an in vitro, experimental study. Nanoparticles including zinc oxide (nanoZnO), copper oxide (nanoCuO), titanium dioxide (nanoTiO2), all at a concentration of 1000 ppm, and silver (nanoAg), at a concentration of 4000 ppm, were purchased from PlasmaChem GmbH (Berlin, Germany). As reported by the supplier, nanoparticles demonstrated >98% purity after ignition. The nanoparticles were then added to a water-based rinse to produce colloidal solutions, which could serve as mouthwashes. The distribution and size of nanoparticles in the colloidal solutions were examined using a particle size analyzer. The colloidal solutions were prepared at MIC against S. mutans and S. sanguis, as determined in our previous investigation [Table 1].[11] The solutions were sterilized in a gravity autoclave before cell toxicity tests. The study groups were as follows:
Table 1.
The MIC (μg/ml) of different solutions containing nanoparticles and CHX against Streptococcus mutans and Streptococcus sanguis
| Group | Definition | MIC(μg/ml) |
|---|---|---|
| 1 | ZnO | 0.390 |
| 2 | CuO | 12.5 |
| 3 | TiO2 | 0.097 |
| 4 | Ag | 25 |
| 5 | CHX | 62.5 |
MIC: Minimum inhibitory concentration; ZnO: Zinc oxide; CuO: Copper oxide, TiO2: Titanium dioxide; Ag: Silver; CHX: Chlorhexidine
Group 1: The colloidal solution containing zinc oxide nanoparticles (ZnO)
Group 2: The colloidal solution containing copper oxide nanoparticles (CuO)
Group 3: The colloidal solution containing titanium dioxide nanoparticles (TiO2)
Group 4: The colloidal solution containing silver nanoparticles (Ag)
Group 5: The CHX mouthwash (positive control).
Cell line and cell culture
HGFs were purchased from Pasteur Institute, Tehran, Iran. The experiments were carried out at the BuAli Research Institute, Mashhad University of Medical Sciences, Mashhad, Iran. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum, 4 mMol of L-gluthamine, 100 μ/mL of streptomycin, and 100 units/mL of penicillin.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
To measure cytotoxicity of different solutions, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay was used. The toxicity assessment was contemplated at MIC and at 0.1 MIC and 0.01 MIC dilutions. Five experiments were performed for each concentration of different colloidal solutions. Normal cells (fibroblasts) were seeded in 5000 cells per well in a 96-well plate. The cells were incubated at 37°C for 24 h in a humidified atmosphere with 5% CO2 and 95% air to allow fibroblasts to be attached to the bottom of the wells.
Afterward, the culture medium over cells was discarded, and equal volumes of mouthwashes were added to the wells to stimulate the fibroblasts for 1 h. Five wells containing only culture medium were used as controls. The mouthwashes were then replaced with DMEM growth medium and the plates were grown at 37°C, 5% CO2 and 95% air for 24 h.
After that, the supernatant was removed and 20 μl of MTT solution (at the concentration of 5 mg/mL) was added to the medium in each well. The plates were then incubated for 4 h under standard conditions. Following incubation, the medium was discarded from the wells and 100 μL of dimethyl sulfoxide (DMSO) was added per well to dissolve the formazan crystals. The optical density (OD) of the plates was measured at 570 nm in an ELIZA plate reader, and the reference OD was 630 nm.
Cell viability was then calculated in percentage of the control group according to the following formula:
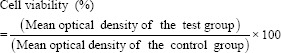
Cell viability was then classified as follows:[17]
Non-cytotoxic : >90% viability
Slightly cytotoxic: 60%–90% viability
Moderately cytotoxic: 30%–59% viability
Severely cytotoxic: <30% viability.
DNA fragmentation
To detect apoptotic cells, DNA fragmentation was assessed as “sub-G1” peak on DNA content histogram. For sub-G1 analysis, normal cells (fibroblasts) were seeded at a density of 2 × 105 cells per well in a 24-well plate. The cells were incubated for 24 h at 37°C, 5% CO2, and 95% air to allow fibroblasts to adhere to the bottom of the wells. Subsequently, the growth medium was replaced with equal volumes of colloidal solutions or CHX to expose fibroblasts for 1 h, followed by replacement with regular growth medium. The cells were incubated under standard conditions.
The plates were then prepared for flow cytometry analysis. The cells were exposed to the hypotonic solution (50 μg/ml propidium iodide in 0.1% sodium citrate + 0.1% Triton X-100) for 1 day at 4°C. Cell cycle analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, USA), and the distribution of cells that were in the Sub-G1 peak was calculated. The test was performed in duplicate at the concentration of MIC for different solutions, and the mean value was reported.
Statistical analysis
The normality of the data was assessed with the Shapiro–Wilk test. The repeated measures analysis was run to compare the cytotoxicity of colloidal solutions containing metal nanoparticles at various concentrations (MIC, 0.1 MIC and 0.01 MIC). The statistical analysis was performed through SPSS (Statistical Package for the Social Sciences, version 16, Chicago, IL, USA), and P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
There was a borderline interaction between the group and the concentration (P = 0.054). Therefore, the two factors were analyzed separately.
Comparison of cell viability between different groups
Table 2 presents the mean and standard deviations regarding cell viability in three concentrations (MIC, 0.1 MIC, and 0.01 MIC) of different mouthwashes. All colloidal solutions and CHX induced some degree of growth inhibition in HGFs. At all the concentrations studied in this study, the highest and lowest mean of cell viability was related to TiO2 and ZnO groups, respectively [Table 2].
Table 2.
The descriptive statistics and the results of statistical analysis to compare the cell viability of fibroblasts after exposure to colloidal solutions and chlorhexidine at different dilutions of minimum inhibitory concentration
| Group | MIC* | 0.1 MIC | 0.01 MIC* | Statistical analysis(P) |
|---|---|---|---|---|
| ZnO | 22.0±4.9a | 39.0±7.0 | 51.2±8.5a | 0.001 |
| CuO | 22.2±8.7a | 54.0±4.1 | 65.6±7.9b | <0.001 |
| TiO2 | 36.9±12.8b | 47.2±12.2 | 76.0±2.1b | 0.001 |
| Ag | 33.2±7.1a,b | 45.2±11.9 | 64.8±10.4b | 0.002 |
| CHX | 22.3±1.3a | 43.5±4.2 | 67.1±12.6b | 0.001 |
| One-way ANOVA(P) | 0.014 | 0.133 | 0.007 |
*The groups that have been defined with different letters show statistically significant difference at P<0.05, whereas those with the same letter are statistically comparable. MIC: Minimum inhibitory concentration; ZnO: Zinc oxide; CuO: Copper oxide, TiO2: Titanium dioxide; Ag: Silver; CHX: Chlorhexidine
One way analysis of variance (ANOVA) revealed a significant difference in cell viability between the five groups at MIC, and 0.01 MIC [P < 0.05; Table 2], but the difference between groups at 0.1 MIC was not significant (P = 0.133). Pairwise comparisons by Duncan test demonstrated that at MIC, the mean cell viability was significantly greater in the TiO2 group than all other groups (except the Ag group) (P < 0.05), whereas other comparisons were not statistically significant [P > 0.05; Table 2]. At the concentration of 0.01 MIC, the mean cell viability in the colloidal solution containing ZnO nanoparticles was significantly lower than the other solutions (P < 0.05), whereas other groups showed comparable cell viability to each other [P > 0.05; Table 2].
Comparison of cell viability between different dilutions of minimum inhibitory concentration (MIC) per group
At MIC, all solutions (except TiO2 and Ag groups) were severely toxic to HGFs. The cell toxicity reduced after diluting the solutions, and hence all groups showed moderate inhibition of growth at the 0.1 MIC dilution. At 0.01 MIC, the ZnO-containing solution was moderately toxic to fibroblasts, whereas other groups induced slight growth inhibition.
Table 2 shows that in all groups, the difference in cell viability between various concentrations (MIC, 0.1 MIC and 0.01 MIC) was statistically significant (P < 0.05). A post hoc least significant difference (LSD) test revealed that in the ZnO group, the mean cell viability at MIC was significantly lower than that of the 0.1 and 0.01 MICs (P < 0.05), but there was no significant difference in cell viability between the 0.1 and 0.01 MIC dilutions (P = 0.122). In the CuO and CHX groups, the mean cellular viability at all three concentrations (MIC, 0.1 MIC and 0.01 MIC) were significantly different from each other (P < 0.05). In the colloidal solutions containing Ag or TiO2 nanoparticles, the mean cell viability was significantly greater at 0.01 MIC compared to 0.1 MIC and MIC (P < 0.05), and there was no significant difference in toxicity between the 0.1 MIC and MIC dilutions (P > 0.05).
Figure 1 compares the cell viability of different mouthwashes at the concentrations of MIC, 0.1 MIC, and 0.01 MIC.
Figure 1.
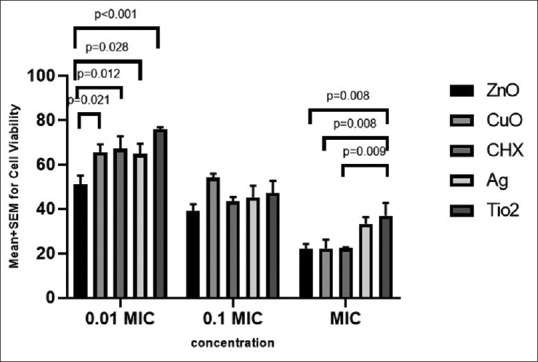
Comparison of cell viability in different groups and various dilutions of minimum inhibitory concentration (MIC).
Sub-G1 analysis
Figure 2 delineates the rate of apoptosis in different colloidal solutions and CHX at the concentration of MIC. The CHX group (96.1%) and CuO-containing solution (93.6%) displayed the highest rate of apoptosis, followed by the colloidal solutions containing Ag, TiO2, or ZnO nanoparticles. Almost no apoptosis was observed in the unstimulated control group.
Figure 2.
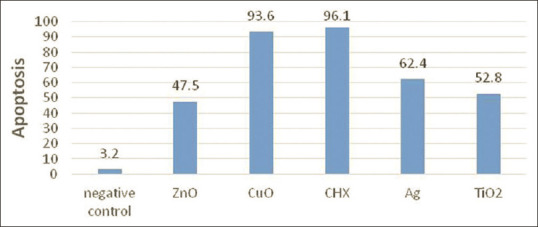
The apoptosis rate in different groups.
DISCUSSION
The present study examined the biocompatibility of several colloidal solutions containing CuO, ZnO, TiO2, and Ag nanoparticles when applied as mouthwashes in HGFs. The colloidal solutions and CHX were tested for toxicity at MIC and at 0.1 and 0.01 MIC dilutions. MICs were obtained according to the outcomes of a previous study,[11] which was performed to investigate the antimicrobial properties of the colloidal solutions containing metal nanoparticles. The gingival fibroblasts were evaluated in this experiment, as they are predominant cells in the soft connective tissue of the mouth.
In the present study, the cells were exposed to mouthwashes for 1 h to detect the cytotoxicity and genotoxicity. Previous studies used different durations of cell exposure ranging from 5 min to 24 h to detect the biocompatibility of mouthwashes.[6,18,19] Although patients routinely keep the mouthwash for 1 min or less in the oral cavity, it should be noted that the components of mouth rinses are adsorbed to the tooth surfaces and soft tissues and released over extended times. During this period, the oral tissues are in contact with progressively lower concentrations of mouth rinses.
Exposure of cells to toxic compounds may lead to necrosis or apoptosis. The necrosis occurs as a result of a traumatic and acute injury. In this process, cell membrane integrity disappears and cell death occurs. The MTT colorimetric method is a frequently-used technique to assess cell viability.[20] The test is based on the cleavage of MTT (a tetrazolium dye) by the mitochondrial succinate dehydrogenase enzyme in living cells, which leads to the formation of insoluble formazan. The formazan is then solved in DMSO and produces a purple color. The main advantages of MTT assay are its rapidity and precision and the ability to quantify the percentage of living cells, according to the absorption of the purple light.[21] In the present study, the highest and lowest mean of cell viability at MIC, 0.1 MIC, and 0.01 MIC belonged to TiO2 and ZnO groups, respectively. At the concentration of MIC, the mean cell viability in the colloidal solution containing TiO2 nanoparticles was significantly higher than that of the other groups (except the Ag group). It was also found that at 0.01 MIC, the average viability in the colloidal solution containing ZnO nanoparticles was significantly lower compared to other colloidal solutions and CHX.
Apoptosis in ancient Greece means “falling off” of leaves from a tree. It is a form of programmed and controlled cell death, which occurs as a result of biochemical events and has advantages during the life cycle of an organism. In addition to its importance as a biological phenomenon, defective apoptotic processes have been implicated in a wide variety of diseases such as cancers. Examination of apoptosis, along with cell viability is essential for assessing the toxicity of materials. Several morphological changes occur in cells during apoptosis such as blebbing, shrinkage, chromatin condensation (pyknosis), nuclear or chromosomal fragmentation, and mRNA decay.
In this study, apoptotic cells were identified by propidium iodide staining and determining the percentage of sub-G1 fraction in HGFs. The Sub-G1 analysis is a method to detect the cells that have lost some DNA as a result of apoptosis. This test is based on the principle that the fragmented DNA leaks out of the cells during cell staining, resulting in a population of cells with reduced DNA content (a hallmark of apoptosis). Therefore, the application of a DNA dye like propidium iodide will create a DNA content histogram with apoptotic cells being represented by a sub-G0/G1 population at the left of the G0/G1 peak. The sub-G1 assay is a fast, reliable, reproducible, easy, and widely used technique to measure apoptosis. However, some authors suggested that this technique should not be used as the sole marker of apoptotic cells.[22] According to the outcomes of the present study, the rate of apoptosis was highest in CHX and CuO groups. After that, Ag-, TiO2- and ZnO-containing solutions showed higher apoptosis, respectively.
TiO2 nanoparticles showed excellent biocompatibility at MIC compared to other nanoparticles-containing solutions (except Ag) and CHX. Since MIC is the most effective concentration to affect microbial agents, this superiority is of crucial importance for clinical applications. The outcomes of this study are consistent with the results of several investigations that showed TiO2 nanoparticles are more biocompatible than other nano-scale metal counterparts.[23,24,25] Heravi et al.[24] revealed that the addition of 1 wt% TiO2 nanoparticles to an orthodontic adhesive did not significantly affect the viability of HGFs and mouse L929 fibroblasts. In contrast, Wang et al.[26] investigated the cytotoxicity and genotoxicity of ultrafine TiO2 (UF-TiO2; <100 nm in diameter) by different methods and demonstrated that UF-TiO2 can cause cytotoxicity and genotoxicity in human lymphoblastoid cells. Jia et al.[27] evaluated the toxicity potential of TiO2 nanoparticles and found that high doses of TiO2 significantly impaired the function of multiple organs in mice such as the liver, kidneys, and brain. It should be noted that the latter studies[26,27] did not compare the toxicity of TiO2 nanoparticles with other metal nano-scale counterparts and also employed higher doses of TiO2 nanoparticles compared to that used in the current investigation.
In the present study, the colloidal solution containing ZnO nanoparticles showed the lowest cell viability at MIC and at 0.1 and 0.01 MIC dilutions. The difference in cell viability between ZnO-containing solution and other groups was significant at the concentration of 0.01 MIC. This means that even low doses of ZnO should be considered toxic to HGFs. Previous studies also reported a high degree of toxicity for ZnO nanoparticles.[23,25,28] Kim et al.[25] compared the toxicity of several oxide nanoparticles (Al2O3, CeO2, TiO2, and ZnO) on two cell lines. They found that ZnO exhibited the highest cytotoxicity in terms of cell proliferation and viability, membrane integrity, and colony formation among all the nanoparticles tested. Kononenko et al.[28] treated canine kidney cells with ZnO nanoparticles, ZnO macroparticles, and ZnCl2 as a source of free Zn ions. They indicated that all zinc compounds caused similar cytotoxicity depending on the dose, but only ZnO nanoparticles significantly increased the damage to DNA and chromosomes. The authors assumed that the genotoxicity of ZnO nanoparticles was related to the particle size rather than the Zn ion release in the cell culture medium.
CHX is considered as the gold standard against a broad spectrum of oral microorganisms. Previous studies reported that CHX at doses similar to or below those routinely prescribed in dental patients can cause potent toxic effects on human and mammalian cells.[18,29] Some studies reported that CHX inhibited cell growth, proliferation, and collagen synthesis in a dose-dependent and time-dependent manner.[18,29] Others displayed that CHX induced a negative impact on the adhesion capacity of periodontal cells and thus interfered with the regeneration of periodontal tissues.[19,30] Other side effects of CHX include calculus formation, discoloration of teeth, restorations or tongue, and alteration in the taste perception.[5]
In the present study, CHX induced the highest apoptosis rate among all the study groups. The outcomes of this study are consistent with several studies that demonstrated the toxic effects of CHX even at low concentrations and short exposure times.[19,29] Rajabalian et al.[29] reported that CHX at a concentration of 0.001% exerted toxic effects on gingival fibroblasts and at concentrations higher than 0.001% lead to cell death in macrophage, epithelial, and osteoblast cells. Wyganowska-Swiatkowska et al.[19] exhibited that CHX at concentrations higher than 0.002% affected cell proliferation and morphology and also altered cell cycle in time-dependent and dose-dependent manners. Since the toxicity of CHX for oral cells can affect the early wound healing phase and interfere with regenerative periodontal therapy, its application for antimicrobial effects in the postsurgical dental treatments should be limited.[19] The present study verified the need to find alternative mouthwashes with less toxicity and similar antibacterial properties.
The CuO-containing solution exhibited the highest apoptosis among all metal nanoparticles-containing solutions in the present investigation. Midander et al.[31] examined copper release and the toxic aspects of nano- and micrometer-sized particles of metallic copper and copper oxide in lung cells. They exhibited a size-dependent effect with respect to both the copper release and toxicity. The nanometer-sized particles released greater amounts of copper per quantity of particles compared to the micrometer-sized particles and also induced a higher degree of DNA damage and a higher percentage of cell death compared to that caused by micrometer-sized particles.
In the present study, the solution containing Ag nanoparticles showed comparable cell viability to both TiO2-containing solution and other mouth rinses at MIC. The apoptosis rate of the Ag group was also moderate among the study groups. Therefore, Ag nanoparticles may also be considered as a viable option to be applied in oral antiseptic agents.
The cytotoxicity of metal nanoparticles may be related to the penetration of particles into the cell membrane or generation of reactive oxygen species (ROS) and subsequent genotoxicity, and cytotoxicity. Several ways have been proposed to reduce the health hazards of nanoparticles. For example, Oleszczuk et al.[32] proposed the use of surfactants to reduce the toxicity of nanoparticles, possibly thorough the formation of nanoparticle aggregates that inhibit the availability of nanoparticles to enter the cells. Sonane et al.[33] showed that antioxidants such as curcumin and Vitamin C decreased nanoparticles induced ROS and lethality and thus effectively evading the toxicity of ZnO and TiO2 nanoparticles. Further studies are warranted to elucidate the effects of adding antioxidants to metal nanoparticles-containing solutions on enhancing cell viability.
The excellent anti-microbial effects of metal nanoparticles make them a viable option to be used in oral mouthwashes and antiseptic agents. According to the outcomes of a recent study, the solution containing TiO2 nanoparticles showed effective antimicrobial properties against S. mutans and S. sanguis.[11] Considering the highest cell survival and relatively low apoptosis rate in the TiO2 containing solution, it can be suggested as a suitable alternative to CHX or incorporated into the composition of oral antimicrobial agents to provide more potent effects against microorganisms. Since the thorough simulation of the oral environment is not possible during in vitro experiments, further studies are warranted to elucidate the biocompatibility and other properties of mouthwashes containing TiO2 or Ag nanoparticles in the clinical conditions.
CONCLUSION
At MIC, 0.1 MIC, and 0.01 MIC, the highest and lowest mean of cell viability belonged to TiO2 and ZnO groups, respectively. The CHX group and CuO-containing solution displayed the highest rate of apoptosis among the study groups. The TiO2-containing solution can be suggested as a suitable alternative to CHX to provide antiseptic effects with minimal toxicity.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
Acknowledgments
The authors would like to thank the vice-chancellor for research of Mashhad University of Medical Sciences for the financial support of this project (grant number 900069). The results presented in this paper have been taken from a DDS student thesis (thesis number 2872).
REFERENCES
- 1.Ahrari F, Shahabi M, Fekrazad R, Eslami N, Mazhari F, Ghazvini K, et al. Antimicrobial photodynamic therapy of Lactobacillus acidophilus by indocyanine green and 810-nm diode laser. Photodiagnosis Photodyn Ther. 2018;24:145–9. doi: 10.1016/j.pdpdt.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Eslami N, Ahrari F, Rajabi O, Zamani R. The staining effect of different mouthwashes containing nanoparticles on dental enamel. J Clin Exp Dent. 2015;7:e457–61. doi: 10.4317/jced.52199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heravi F, Ahrari F, Tanbakuchi B. Effectiveness of MI Paste Plus and Remin Pro on remineralization and color improvement of postorthodontic white spot lesions. Dent Res J (Isfahan) 2018;15:95–103. [PMC free article] [PubMed] [Google Scholar]
- 4.Poosti M, Ahrari F, Moosavi H, Najjaran H. The effect of fractional CO2 laser irradiation on remineralization of enamel white spot lesions. Lasers Med Sci. 2014;29:1349–55. doi: 10.1007/s10103-013-1290-9. [DOI] [PubMed] [Google Scholar]
- 5.al-Tannir MA, Goodman HS. A review of chlorhexidine and its use in special populations. Spec Care Dentist. 1994;14:116–22. doi: 10.1111/j.1754-4505.1994.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 6.Ahrari F, Ramazanzadeh BA, Sabzevari B, Ahrari A. The effect of fluoride exposure on the load-deflection properties of superelastic nickel-titanium-based orthodontic archwires. Aust Orthod J. 2012;28:72–9. [PubMed] [Google Scholar]
- 7.Kao CT, Ding SJ, Wang CK, He H, Chou MY, Huang TH. Comparison of frictional resistance after immersion of metal brackets and orthodontic wires in a fluoride-containing prophylactic agent. Am J Orthod Dentofac Orthop. 2006;130:568e1–9. doi: 10.1016/j.ajodo.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Schiff N, Boinet M, Morgon L, Lissac M, Dalard F, Grosgogeat B. Galvanic corrosion between orthodontic wires and brackets in fluoride mouthwashes. Eur J Orthod. 2006;28:298–304. doi: 10.1093/ejo/cji102. [DOI] [PubMed] [Google Scholar]
- 9.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 10.Phan TN, Buckner T, Sheng J, Baldeck JD, Marquis RE. Physiologic actions of zinc related to inhibition of acid and alkali production by oral streptococci in suspensions and biofilms. Oral Microbiol Immunol. 2004;19:31–8. doi: 10.1046/j.0902-0055.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahrari F, Eslami N, Rajabi O, Ghazvini K, Barati S. The antimicrobial sensitivity of Streptococcus mutans and Streptococcus sangius to colloidal solutions of different nanoparticles applied as mouthwashes. Dent Res J (Isfahan) 2015;12:44–9. doi: 10.4103/1735-3327.150330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agnihotri R, Gaur S, Albin S. Nanometals in dentistry: Applications and toxicological implications-a systematic review. Biol Trace Elem Res. 2020;197:70–88. doi: 10.1007/s12011-019-01986-y. [DOI] [PubMed] [Google Scholar]
- 13.Chakrabarti S, Goyary D, Karmakar S, Chattopadhyay P. Exploration of cytotoxic and genotoxic endpoints following sub-chronic oral exposure to titanium dioxide nanoparticles. Toxicol Ind Health. 2019;35:577–92. doi: 10.1177/0748233719879611. [DOI] [PubMed] [Google Scholar]
- 14.Nazir S, Rabbani A, Mehmood K, Maqbool F, Shah GM, Khan MF, et al. Antileishmanial activity and cytotoxicity of ZnO-based nano-formulations. Int J Nanomedicine. 2019;14:7809–22. doi: 10.2147/IJN.S203351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A, Gorey B, Casey A. In vitro comparative cytotoxicity study of aminated polystyrene, zinc oxide and silver nanoparticles on a cervical cancer cell line. Drug Chem Toxicol. 2019;42:9–23. doi: 10.1080/01480545.2018.1424181. [DOI] [PubMed] [Google Scholar]
- 16.Pavičić I, Milić M, Pongrac IM, Brkić Ahmed L, Matijević Glavan T, Ilić K, et al. Neurotoxicity of silver nanoparticles stabilized with different coating agents: In vitro response of neuronal precursor cells. Food Chem Toxicol. 2020;136:110935. doi: 10.1016/j.fct.2019.110935. [DOI] [PubMed] [Google Scholar]
- 17.Ahrari F, Tavakkol Afshari J, Poosti M, Brook A. Cytotoxicity of orthodontic bonding adhesive resins on human oral fibroblasts. Eur J Orthod. 2010;32:688–92. doi: 10.1093/ejo/cjq019. [DOI] [PubMed] [Google Scholar]
- 18.Patel P, Ide M, Coward P, Di Silvio L. The effect of a commercially available chlorhexidine mouthwash product on human osteoblast cells. Eur J Prosthodont Restor Dent. 2006;14:67–72. [PubMed] [Google Scholar]
- 19.Wyganowska-Swiatkowska M, Kotwicka M, Urbaniak P, Nowak A, Skrzypczak-Jankun E, Jankun J. Clinical implications of the growth-suppressive effects of chlorhexidine at low and high concentrations on human gingival fibroblasts and changes in morphology. Int J Mol Med. 2016;37:1594–600. doi: 10.3892/ijmm.2016.2550. [DOI] [PubMed] [Google Scholar]
- 20.Jahanbin A, Shahabi M, Ahrari F, Bozorgnia Y, Shajiei A, Shafaee H, et al. Evaluation of the cytotoxicity of fiber reinforced composite bonded retainers and flexible spiral wires retainers in simulated high and low cariogenic environments. J Orthod Sci. 2015;4:13–8. doi: 10.4103/2278-0203.149610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Kajstura M, Halicka HD, Pryjma J, Darzynkiewicz Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “sub-G1” peaks on DNA content histograms. Cytometry A. 2007;71:125–31. doi: 10.1002/cyto.a.20357. [DOI] [PubMed] [Google Scholar]
- 23.Ge Y, Schimel JP, Holden PA. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ Sci Technol. 2011;45:1659–64. doi: 10.1021/es103040t. [DOI] [PubMed] [Google Scholar]
- 24.Heravi F, Ramezani M, Poosti M, Hosseini M, Shajiei A, Ahrari F. In vitro cytotoxicity assessment of an orthodontic composite containing titanium-dioxide nano-particles. J Dent Res Dent Clin Dent Prospects. 2013;7:192–8. doi: 10.5681/joddd.2013.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim IS, Baek M, Choi SJ. Comparative cytotoxicity of Al2O3, CeO2, TiO2 and ZnO nanoparticles to human lung cells. J Nanosci Nanotechnol. 2010;10:3453–8. doi: 10.1166/jnn.2010.2340. [DOI] [PubMed] [Google Scholar]
- 26.Wang JJ, Sanderson BJ, Wang H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat Res. 2007;628:99–106. doi: 10.1016/j.mrgentox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Jia X, Wang S, Zhou L, Sun L. The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res Lett. 2017;12:478. doi: 10.1186/s11671-017-2242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kononenko V, Repar N, Marušič N, Drašler B, Romih T, Hočevar S, et al. Comparative in vitro genotoxicity study of ZnO nanoparticles, ZnO macroparticles and ZnCl2 to MDCK kidney cells: Size matters. Toxicol In Vitro. 2017;40:256–63. doi: 10.1016/j.tiv.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Rajabalian S, Mohammadi M, Mozaffari B. Cytotoxicity evaluation of Persica mouthwash on cultured human and mouse cell lines in the presence and absence of fetal calf serum. Indian J Dent Res. 2009;20:169–73. doi: 10.4103/0970-9290.52894. [DOI] [PubMed] [Google Scholar]
- 30.Balloni S, Locci P, Lumare A, Marinucci L. Cytotoxicity of three commercial mouthrinses on extracellular matrix metabolism and human gingival cell behaviour. Toxicol In Vitro. 2016;34:88–96. doi: 10.1016/j.tiv.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Midander K, Cronholm P, Karlsson HL, Elihn K, Möller L, Leygraf C, et al. Surface characteristics, copper release, and toxicity of nano- and micrometer-sized copper and copper(II) oxide particles: A cross-disciplinary study. Small. 2009;5:389–99. doi: 10.1002/smll.200801220. [DOI] [PubMed] [Google Scholar]
- 32.Oleszczuk P, Jośko I, Skwarek E. Surfactants decrease the toxicity of ZnO, TiO2 and Ni nanoparticles to Daphnia magna. Ecotoxicology. 2015;24:1923–32. doi: 10.1007/s10646-015-1529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonane M, Moin N, Satish A. The role of antioxidants in attenuation of Caenorhabditis elegans lethality on exposure to TiO2 and ZnO nanoparticles. Chemosphere. 2017;187:240–7. doi: 10.1016/j.chemosphere.2017.08.080. [DOI] [PubMed] [Google Scholar]


