Abstract
Three new (1–3) and 25 known compounds were isolated from the crude extract of Cassia abbreviata. The chemical structures of new compounds were established by extensive spectroscopic analyses including 1D and 2D NMR and HRESIMS. Cassiabrevone (1) is the first heterodimer of guibourtinidol and planchol A. Compound 2 was a new chalcane, while 3 was a new naphthalene. Cassiabrevone (1), guibourtinidol-(4α→8)-epiafzelechin (4), taxifolin (8), oleanolic acid (17), piceatannol (22), and palmitic acid (28), exhibited potent anti-HIV-1 activity with IC50 values of 11.89 µM, 15.39 µM, 49.04 µM, 7.95 µM, 3.58 µM, and 15.97 µM, respectively.
Keywords: Cassiaabbreviata, Fabaceae, anti-HIV, heterodimer, flavonoid
1. Introduction
Cassia abbreviata is a small-to-medium-sized branched tree of the Fabaceae. It is widely spread in the tropics, especially in southeast Africa, with a long history in traditional medicine for the treatment of numerous conditions [1], such as headaches, diarrhea, constipation, some skin diseases, malaria, syphilis, pneumonia, stomach troubles, uterine pains, and gonorrhea [2,3]. Pharmacological studies indicated that C. abbreviata showed a broad spectrum of biological activities, including CNS depression [4], hypoglycemia [5], anti-AIDS [6], hepatoprotection [7], antioxidant [8], antibacterial [9], etc. Although some fatty acid compositions were analyzed from its seed oil by gas chromatography (GC) [10], while several dimeric and trimeric flavonoids were proposed on the basis of the UPLC–MS spectroscopic data [11], the chemical component investigation on C. abbreviata was seldom reported. Up to now, only a new flavan [12] and two novel trimeric proanthocyanidins [9] were isolated.
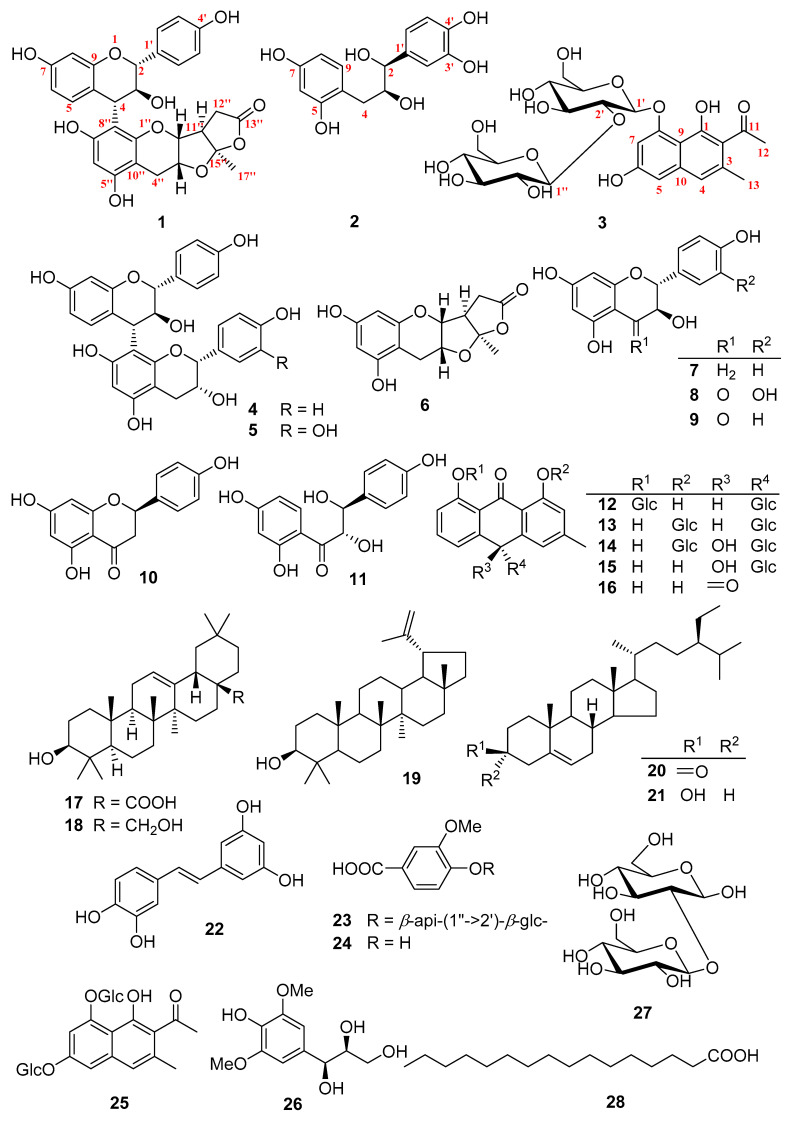
Recently, we screened several crude extracts from different plants of Cassia species and found that C. abbreviata showed potent anti-HIV-1 activity. Therefore, a systematic phytochemical investigation was carried out, which led to the isolation of three new (1–3) and 25 known (4–28) compounds (Figure 1). Herein, we report the isolation, structure, and anti-HIV-1 activity of these compounds.
Figure 1.
Compounds 1–28 from Cassia abbreviata.
2. Results and Discussion
Compound 1 showed a molecular ion peak at m/z 535.1590 [M + H]+ in its positive HRESIMS (Figure S18), corresponding to the molecular formula of C29H26O10. Its 1H and 13C NMR spectroscopic data in DMSO-d6 (Table 1) exhibited 29 carbon signals, consisting of one ABX [δH 6.12 (1H, d, J = 2.4 Hz, H-8); 6.15 (1H, dd, J = 8.4, 2.4 Hz, H-6); 6.40 (1H, d, J = 8.4 Hz, H-5); δC 102.0 (d, C-8), 108.3 (d, C-6), 118.6 (s, C-10), 129.5 (d, C-5), 155.3 (s, C-9), 155.8 (s, C-7)], one 1,4-disubsituted [δH 6.76 (2H, d, J = 8.5 Hz, H-3′,5′); 7.24 (2H, d, J = 8.6 Hz, H-2′,6′); δC 114.8 (d × 2, C-3′,5′), 129.3 (d × 2, C-2′,6′), 130.3 (s, C-1′), 157.1 (s, C-4′)] and one penta-substituted [δH 6.09 (1H, s, H-6″); δC 95.2 (d, C-6″), 97.4 (s, C-10″), 107.7 (s, C-8″), 151.7 (s, C-9″), 154.0 (s, C-5″), 155.4 (s, C-7″)] benzoic moieties, besides to one methyl [δH 0.97 (3H, s, Me-17″); δC 23.8 (q, C-17″)], two sp3 methylenes [δH 2.57 (dd, J = 17.7, 5.3 Hz, H-4″β), 2.67 (d, J = 17.7 Hz, H-4″α); 2.62 (dd, J = 19.0, 4.8 Hz, H-12″α), 2.98 (dd, J = 19.0, 11.6 Hz, H-12″β); δC 20.2 (t, C-4″), 31.1 (t, C-12″)], four oxygenated sp3 methines [δH 4.19 (d, J = 1.6 Hz, H-2″); 4.26 (br. t, J = 9.1 Hz, H-3); 4.39 (t, J = 2.5 Hz, H-3″); 4.48 (d, J = 9.5 Hz, H-2); δC 69.5 (d, C-3), 72.8 (d, C-3″), 78.7 (d, C-2″), 82.9 (d, C-2)], two sp3 methines [δH 2.36 (dd, J = 11.7, 4.6 Hz, H-11″); 4.40 (d, J = 9.1 Hz, H-4); δC 40.4 (d, C-4), 50.2 (d, C-11″)], one carbonyl (δC 174.5 s, C-13″), and one acetalic quaternary carbon (δC 115.6 s, C-15″).
Table 1.
1H (500 Hz) and 13C (125 Hz) NMR spectroscopic data of 1–3 (δ in ppm, J in Hz within parentheses).
| No. | 1 a | 1 b | 2 b | 3 b | ||||
|---|---|---|---|---|---|---|---|---|
| δ C | δ H | δ C | δ H | δ C | δ H | δ C | δ H | |
| 1 | 153.7 C | |||||||
| 2 | 82.9 CH | 4.48 (d, 9.5) | 84.3 CH | 4.56 (d, 9.6) | 80.1 CH | 4.90 overlap | 123.3 C | |
| 3 | 69.5 CH | 4.26 (dd, 9.5, 9.1) | 72.3 CH | 4.40 (dd, 9.6, 9.1) | 67.8 CH | 4.19 (br. t, 4.0) | 135.1 C | |
| 4 | 40.4 CH | 4.40 (d, 9.1) | 41.8 CH | 4.58 (d, 9.1) | 34.1 CH2 | 3.14 (dd, 16.2, 4.2) | 119.6 CH | 6.86 s |
| 2.75 (dd, 16.2, 3.0) | ||||||||
| 5 | 129.5 CH | 6.40 (d, 8.4) | 130.2 CH | 6.60 (d, 8.6) | 156.7 C | 104.8 CH | 6.64 (d, 2.0) | |
| 6 | 108.3 CH | 6.15 (dd, 8.4, 2.4) | 109.9 CH | 6.24 (dd, 8.6, 2.4) | 104.0 CH | (d, 2.4) | 157.9 C | |
| 7 | 155.8 C | 157.0 C | 157.8 C | 102.7 d | 6.74 (d, 2.0) | |||
| 8 | 102.0 CH | 6.12 (d, 2.4) | 103.3 CH | 6.24 (d, 2.4) | 109.6 CH | (dd, 8.2, 2.4) | 156.5 C | |
| 9 | 155.3 C | 156.9 C | 131.7 CH | 6.90 (d, 8.2) | 109.3 C | |||
| 10 | 118.6 C | 120.6 C | 111.9 C | 139.5 C | ||||
| 1′ | 130.3 C | 131.7 C | 132.2 C | 104.8 CH | 4.75 (d, 7.7) | |||
| 2′ | 129.3 CH | 7.24 (d, 8.6) | 130.3 CH | 7.31 (d, 8.5) | 115.4 CH | 6.99 (d, 1.7) | 79.8 CH | 3.89 m |
| 3′ | 114.8 CH | 6.76 (d, 8.5) | 116.0 CH | 6.81 (d, 8.5) | 145.9 C | 78.0 CH | 3.34 m | |
| 4′ | 157.1 C | 158.5 C | 146.0 C | 78.2 CH | 3.76 m | |||
| 5′ | 114.8 CH | 6.76 (d, 8.5) | 116.0 CH | 6.81 (d, 8.5) | 116.0 CH | 6.78 (d, 8.2) | 71.0 CH | 3.50 m |
| 6′ | 129.3 CH | 7.24 (d, 8.6) | 130.3 CH | 7.31 (d, 8.5) | 119.4 CH | 6.82 (dd, 8.2, 1.7) | 66.9 CH2 | 3.23 m; 3.92 m |
| 1″ | 100.4 CH | 5.23 (d, 7.9) | ||||||
| 2″ | 78.7 CH | 4.19 (d, 1.6) | 81.0 CH | 4.22 (d, 2.4) | 78.4 CH | 3.54 m | ||
| 3″ | 72.8 CH | 4.39 (dd, 5.3, 1.6) | 75.0 CH | 4.45 (ddd, 5.1, 2.4, 1.5) | 78.0 CH | 3.34 m | ||
| 4″ | 20.2 CH2 | 2.67 (d, 17.7) | 21.3 CH2 | 2.89(d, 17.9) | 75.4 CH | 3.25 m | ||
| 2.57 (dd, 17.7, 5.3) | 2.64 (dd, 17.9, 5.1) | |||||||
| 5″ | 154.0 C | 155.6 C | 71.0 CH | 3.50 m | ||||
| 6″ | 95.2 CH | 6.09 s | 96.3 CH | 6.09 s | 62.3 CH2 | 3.74 m; 3.93 m | ||
| 7″ | 155.4 C | 157.1 C | ||||||
| 8″ | 107.7 C | 109.4 C | ||||||
| 9″ | 151.7 C | 153.0 C | ||||||
| 10″ | 97.4 C | 99.6 C | ||||||
| 11″(11) | 50.2 CH | 2.36 (dd, 11.7, 4.7) | 52.1 CH | 2.39 (dd, 11.6, 4.5) | 208.3 C | |||
| 12″(12) | 31.1 CH2 | 2.98 (dd, 19.0, 11.7) | 32.8 CH2 | 2.93 (dd, 19.2, 11.6) | 32.7 CH3 | 2.58 s | ||
| 2.62 (dd, 19.0, 4.7) | 2.60 (dd, 19.2, 4.5) | |||||||
| 13″(13) | 174.8 C | 177.2 C | 20.2 CH3 | 2.23 s | ||||
| 15″ | 115.9 C | 118.4 C | ||||||
| 17″ | 23.8 CH3 | 0.97 s | 24.4 CH3 | 1.10 s | ||||
a Recorded in DMSO-d6. b Recorded in CD3OD.
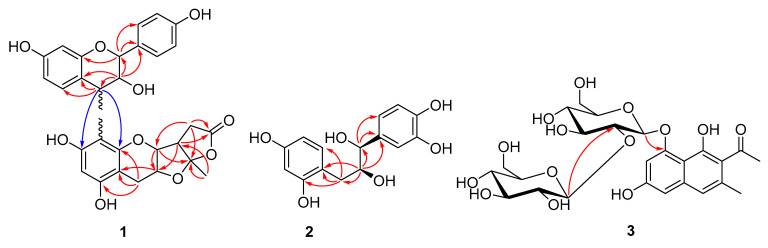
In the heteronuclear multiple bond connectivity (HMBC) spectrum, a diagnostic ketal-γ-lactone moiety could easily be deduced according to correlations of H-2″ to C-3″/C-15″, H-3″ to C-10″, H-11″ to C-3″/C-13″, H2-12″ to C-13″/C-15″, and H3-17″ to C-11″/C-15″, which by further cross peaks of H2-4″ to C-5″/C-9″/C-10″ constructed the fragment of planchol A (6) [13]. Moreover, HMBC correlations of H-2 to C-1′/C-2′/C-9, H-3 to C-1′/C-2/C-4/C-10, and H-4 to C-5/C-9/C-10 and the large coupling constant of H-2/H-3 (3JH2-H3 = 9.5 Hz) could be used to establish another fragment of guibourtinidol [14]. These two fragments could be connected via C-4 and C-8″ by the key HMBC correlations of H-4 to C-7″/C-9″ (Figure 2). According to the large coupling constant between H-3 and H-4 (3JH3-H4 = 9.1 Hz), the stereochemistry of C-4 was assumed to be α-orientation [15]. On the basis of the above evidence and from the perspective of the biosynthetic pathway, the structure of 1 was then determined to be guibourtinidol (4α→8) planchol A, and named cassiabrevone.
Figure 2.
The key HMBC correlations of 1–3.
Although dimers or trimers of flavonoids were commonly found in nature, cassiabrevone is the first example of a heterodimer formed by flavanol and planchol A. It might be biosynthesized from a natural chalconol by dehydration, oxidation, and final endo-attacking via neighboring group participation induced Friedel–Crafts reaction (Figure 3).
Figure 3.
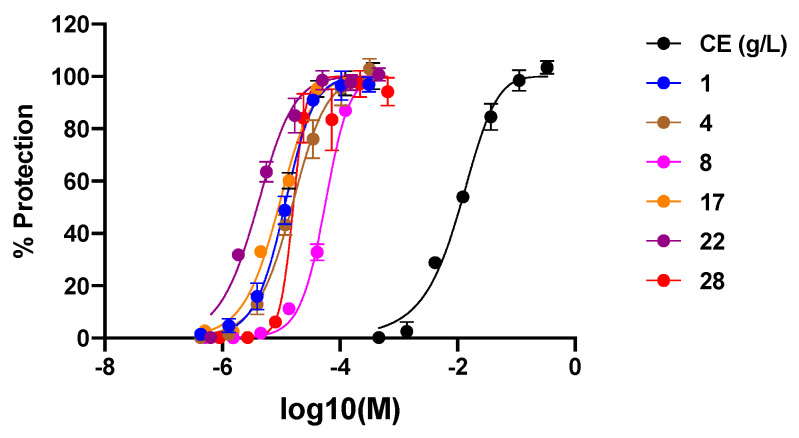
Protective effects of the crude extract (CE) along with compounds 1, 4, 8, 17, 22, and 28 of Cassia abbreviata against HIV-1 infection (n = 3).
Compound 2 was assigned the molecular formula C15H16O6 from its positive HRESIMS at m/z 293.0947 [M + H]+. The 1H and 13C NMR spectroscopic data of 2 were very similar to those of epifiliferol [16,17], except that an ABX aromatic ring instead of an AX benzoic moiety was found in 2. The assumption was confirmed by the HMBC correlation of H2-4 (δH 2.75, 1H, dd, J = 16.2, 3.0 Hz, H-4a; 3.14,1H, dd, J = 16.2, 4.0 Hz, H-4b) to C-5 (δC 156.7 s)/C-9 (δC 131.7 d)/C-10 (δC 111.9 s). The small coupling constant between H-2 and H-3 (3JH2/H3 = 4.0 Hz) further confirmed the erythro-configuration of 2. Accordingly, the structure of 2 was assigned as 9-dehydroxyepifiliferol. Interestingly, it might be originated by the same biosynthetic precursor as 1, via a nucleophilic displacement reaction, followed by the oxidation reaction.
The molecular formula of compound 3 was assigned to be C24H30O13 according to its sodium adduct ion peak at m/z 557.1792 [M + H]+, suggesting ten degrees of unsaturation. The 1H and 13C NMR spectra (Table 1) of 3 were almost the same as those of cassiaglycoside II (17) [18], except that the β-d-glucopyranosyl moiety at the C-6 position was shifted to the C-2′ position. This was evidenced by the downfield shift from 74.9 to 79.8 of the C-2′ position. Further confirmation could be observed by the HMBC correlations of H-1″ (δH 5.23, 1H, d, J = 7.9 Hz) to C-2′ (δC 79.8 d) and H-1′ (δH 1H, 4.75, d, J = 7.7 Hz) to C-8 (δC 156.5 s). By detailed analysis of its HSQC, COSY, and HMBC NMR spectroscopic data, compound 3 was then elucidated as 6-deglucopyranosyl-2′-glucopyransoyl cassiaglycoside II, and named cassiaglycoside V.
By comparison of the NMR and MS data with those published in the literature, 25 known compounds were determined to be guibourtinidol-(4α→8)-epiafzelechin (4) [15], guibourtinidol-(4α→8)-epicatechin (5) [15], planchol A (6) [13], (+)-afzelechin (7) [19], taxifolin (8) [20], dihydrokaempferol (9) [20,21], naringenin (10) [22], rhusopolyphenol E (11) [23], cascaroside D (12) [24], 1″-deoxyaloin B-1-O-β-d-glucopyranoside (13) [24], 10-hydroxycascaroside C (14) [24], cassialoin (15) [24], chrysophanol (16) [25], oleanolic acid (17) [26], erythrodiol (18) [26], lupeol (19) [27], β-sitosterone (20) [28], β-sitosterol (21) [29], piceatannol (22) [30], markhamioside F (23) [31], vanillic acid (24) [32], cassiaglycoside II (25) [18], (7S, 8S)-syringoylglycerol (26) [33], β-d-glucopyranosyl (1→2)-β-d-glucopyranoside (27) [34], and palmitic aicd (28) [35].
The crude extract of Cassia abbreviata and all isolated compounds were assessed for their anti-HIV activity in MT4 cells infected by the reference strain HIV-1 IIIB (Figure 3). Cassiabrevone (1), guibourtinidol-(4α→8)-epiafzelechin (4), taxifolin (8), oleanolic acid (17), piceatannol (22), and palmitic acid (28) inhibited HIV-1 infection at noncytotoxic concentration and showed IC50 values ranging from 3.58 to 49.04 µM (Table 2). Enfuvirtide and Plerixafor are entry inhibitors for positive controls.
Table 2.
The IC50 values of compounds 1, 4, 8, 17, 22, and 28 harboring an anti-HIV-1 activity.
| Compounds | IC50 (µM) | |
|---|---|---|
| HIV-1 Infection (µM) | Cytotoxicity | |
| CE a | 9.98 ± 3.88 (µg/mL) | >1000 (μg/mL) |
| Cassiabrevone (1) | 11.89 ± 2.14 | >333 |
| Guibourtinidol-(4α→8)-epiafzelechin (4) | 15.39 ± 9.09 | >333 |
| Taxifolin (8) | 49.04 ± 5.02 | >333 |
| Oleanolic acid (17) | 7.95 ± 2.57 | >333 |
| Piceatannol (22) | 3.58 ± 0.27 | >333 |
| Palmitic acid (28) | 15.97 ± 3.04 | >333 |
| Enfuvirtide (T20) b | 0.0096 ± 0.001 | >1 |
| Plerixafor (AMD3100) b | 0.075 ± 0.009 | >1 |
a CE: crude extract of Cassia abbreviate. b Enfuvirtide and Plerixafor: positive controls. Each experiment was conducted three times and data were expressed as means ± SD.
3. Materials and Methods
3.1. General Experimental Procedures
NMR spectra were recorded on Bruker 500 MHz spectrometer using TMS as an internal standard. The HRESIMS spectra were measured on a Waters Xevo G2 Q-TOF mass spectrometer. Optical rotations were measured with an Anton Paar MCP100 polarimeter. Chemical shifts were recorded in δ values using solvent signals DMSO-d6 (δH 2.50/δC 39.5) and CD3OD (δH 3.0/δC 49.0) as references. Column chromatography (CC) was performed on silica gel, Sephadex LH-20, and ODS (octadecyl silane).
3.2. Plant Material
The mature shrubs of the plant (3 kg) were collected in Makueni County, Kenya, and the identity of Cassia abbreviata was confirmed by DNA barcoding.
3.3. Extraction and Isolation
Barks and roots of Cassia abbreviata were pulverized and extracted with 95% EtOH four times at room temperature. The extracts were combined and concentrated to provide a crude extract (CE, ca 420 g). Then, it was suspended in deionized water and partitioned successively with CHCl3, EtOAc, and n-BuOH, to provide an EtOAc-soluble extract (306.7 g) and an n-BuOH-soluble extract (103.5 g). The EtOAc extract was subjected to CC over silica gel eluting with a gradient CHCl3-MeOH (0→100%) to obtain eight fractions (Fr.1–Fr.8). Fr.2 was further separated by ODS and Sephadex LH-20 chromatography, and finally obtained 16 (58.1 mg) and 19 (16.8 mg) by preparative thin-layer chromatography (prep. TLC). Compounds 20 (32.7 mg) and 21 (68.5 mg) were purified from Fr.3 by repeated ODS CC, Sephadex LH-20 chromatography, and prep. TLC. Fr.5 was subjected to ODS CC, Sephadex LH-20 chromatography, and prep. TLC to give 9 (9.3 mg), 10 (11.5 mg), 11 (5.1 mg), 17 (0.8 mg), 18 (3.5 mg), 24 (11.5 mg), and 28 (6.9 mg). Purification of Fr.6 by ODS and Sephadex LH-20 chromatography, followed by prep. TLC led to the isolation of 1 (2.4 mg), 2 (3.4 mg), 4 (7.6 mg), 5 (6.5 mg), 6 (17.8 mg), 7 (40.9 mg), 8 (17.1 mg), 15 (140.3 mg), 22 (75.9 mg), and 26 (11.4 mg). The n-BuOH-soluble part was separated via ODS CC and Sephadex LH-20 chromatography, respectively. Final purification by prep. TLC obtained 3 (14.8 mg), 12 (20.0 mg), 13 (24.0 mg), 14 (22.0 mg), 23 (11.0 mg), 25 (21.0 mg), and 27 (28.7 mg).
Cassiabrevone (1): pale yellow amorphous powder; −12.7 (c 0.1, MeOH); 1H and 13C NMR data, see Table 1; HRESIMS m/z 535.1590 [M + H]+ (calcd for C29H27O10, 535.1599).
9-Dehydroxyepifiliferol (2): white amorphous powder; −12.0 (c 0.1, MeOH); 1H and 13C NMR data, see Table 1; HRESIMS m/z 293.1009 [M + H]+ (calcd for C15H17O6, 293.1020).
Cassiaglycoside V (3): pale yellow amorphous powder; −90.0 (c 0.1, MeOH); 1H and 13C NMR data, see Table 1; HRESIMS m/z 557.1851 [M + H]+ (calcd for C25H33O14, 557.1865).
3.4. Anti-HIV-1 Infection Bioassay
MT4 cells were obtained through the NIH AIDS Reagent Program and cultured in RPMI 1640 (Lonza, Wijchen, the Netherlands) supplemented with 10% heat-inactivated fetal bovine serum (Lonza, the Netherlands) and 2mM l-glutamine (Invitrogen, Gosselies, Belgium). MT-4 cells were incubated with the crude extract of Cassia abbreviatta or the tested compounds alone to assess cytotoxicity, or HIV-1 IIIB alone or a mixture of the tested extract and compounds and HIV-1 IIIB viruses to assess protection against HIV-1 infection. After five days, protection from viral infection and the cytotoxicity were evaluated in parallel using (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, Liège, Belgium) by measuring OD540 and OD690 using a POLARstar Omega Plate Reader (BMG Labtech, Ortenberg, Germany). Data were normalized to cells without treatment. Values of OD540−OD690 were calculated to determine IC50 values in Prism. The entry inhibitors, enfuvirtide, and AMD3100 (Sigma Aldrich, Liège, Belgium), were used as positive controls.
4. Conclusions
From Cassia abbreviata, three new compounds, cassiabrevone, 9-dehydroxyfiliferol, and cassiaglycoside V, were isolated along with 25 known ones. Noteworthily, cassiabrevone is the first heterodimer by flavanol guibourtinidol and tetracyclic phenolic planchol A. Moreover, six compounds showed inhibition against HIV-1 infection with IC50 values ranging from 3 to 50 µM.
Acknowledgments
Authors thank Jean-Yves Servais and Morgane Lemaire for their excellent technical assistance.
Supplementary Materials
The following are available online, Figures S1–S17: The 1D and 2D NMR spectra of 1–3.
Author Contributions
X.Y. isolated all compounds. X.Y., Z.H., and N.W. analyzed the chemical data and wrote the manuscript. Y.Z. performed the bioactive experiments and the barcoding analysis. M.M. provided the plant material and performed the in silico ligand-based studies. J.-C.S. designed the study with A.S. and C.S.-D. C.S.-D. analyzed the data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Fonds National de la Recherche” of Luxembourg [PHD AFR grant 1189522] and the Luxembourg Institute of Health (MESR grant 20150415).
Data Availability Statement
Not available.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maurice M.I. Handbook of African Medicinal Plants. 2nd ed. CRC Press; Boca Raton, FL, USA: 1993. [Google Scholar]
- 2.Mongalo N.I., Mafoko B.J. Cassia abbreviata Oliv, a review of its ethnomedicinal uses, toxicology, phytochemistry, possible propagation techniques and pharmacology. Afr. J. Pharm. Pharm. 2013;7:2901–2906. doi: 10.5897/AJPP12.1017. [DOI] [Google Scholar]
- 3.Ribeiro A., Romeiras M.M., Tavares J., Faria M.T. Ethnobotanical survey in Canhane village, district of Massingir, Mozambique: Medicinal plants and traditional knowledge. J. Ethnobiol. Ethnomed. 2010;6:1–15. doi: 10.1186/1746-4269-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parry O., Matambo C. Some pharmacological actions of aloe extracts and Cassia abbreviata on rats and mice. Cent. Afr. J. Med. 1992;38:409–414. [PubMed] [Google Scholar]
- 5.Bati K., Kwape T.E., Chaturvedi P. Anti-diabetic effects of an ethanol extract of Cassia abbreviata stem bark on diabetic rats and possible mechanism of its action: Anti-diabetic properties of Cassia abbreviata. J. Pharm. 2017;20:45–51. doi: 10.3831/KPI.2017.20.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leteane M.M., Ngwenya B.N., Muzila M., Namushe A., Mwinga J., Musonda R., Moyo S., Mengestu Y.B., Abegaz B.M., Andrae-Marobela K. Old plants newly discovered: Cassia sieberiana D.C. and Cassia abbreviata Oliv. Oliv. root extracts inhibit in vitro HIV-1c replication in peripheral blood mononuclear cells (PBMCs) by different modes of action. J. Ethnopharmacol. 2012;141:48–56. doi: 10.1016/j.jep.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Sobeh M., Mahmoud M.F., Abdelfattah M.A.O., Cheng H., El-Shazly A.M., Wink M. A proanthocyanidin-rich extract from Cassia abbreviata exhibits antioxidant and hepatoprotective activities in vivo. J. Ethnopharmacol. 2018;213:38–47. doi: 10.1016/j.jep.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Jobe M.C., Ncobela C.N., Kunene N.W., Opoku A.R. Effects of Cassia abbreviata extract and stocking density on growth performance, oxidative stress and liver function of indigenous chickens. Trop. Anim. Health Prod. 2019;51:2567–2574. doi: 10.1007/s11250-019-01979-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erasto P., Majinda R. Bioactive proanthocyanidins from the root bark of Cassia abbreviata. Int. J. Biol. Chem. Sci. 2011;5:2170–2179. doi: 10.4314/ijbcs.v5i5.36. [DOI] [Google Scholar]
- 10.Dangarembizi R., Chivandi E., Dawood S., Erlwanger K.H., Gundidza M., Magwa M.L., Muredzi P., Samie A. Report-The fatty acid composition and physicochemical properties of the underutilised Cassia abbreviata seed oil. Pak. J. Pharm. Sci. 2015;28:1005–1008. [PubMed] [Google Scholar]
- 11.Thomford N.E., Dzobo K., Chopera D., Wonkam A., Maroyi A., Blackhurst D., Dandara C. In vitro reversible and time-dependent CYP450 inhibition profiles of medicinal herbal plant extracts Newbouldia laevis and Cassia abbreviata: Implications for herb-drug interactions. Molecules. 2016;21:891. doi: 10.3390/molecules21070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehmlow E.V., van Ree T., Guntenhoner M. 2,4-Trans-,7 4′-dihydroxy-4-methoxyflavan from Cassia abbreviata. Phytochemistry. 1998;49:1805–1806. doi: 10.1016/S0031-9422(98)00218-0. [DOI] [PubMed] [Google Scholar]
- 13.Chang J., Case R. Cytotoxic phenolic constituents from the root of Actinidia chinensis. Planta Med. 2005;71:955–959. doi: 10.1055/s-2005-871225. [DOI] [PubMed] [Google Scholar]
- 14.Messanga B.B., Ghogomu R., Sondengam B.L., Martin M.T., Blond A., Brouard J.P., Bodo B. Calodenin C: A new guibourtinidol-(4α->8)-afzelechin from Ochna calodendron. Planta Med. 1998;64:760–761. doi: 10.1055/s-2006-957577. [DOI] [PubMed] [Google Scholar]
- 15.Malan E., Swinny E., Ferreira D., Steynberg P. The structure and synthesis of proguibourtinidins from Cassia abbreviata. Phytochemistry. 1996;41:1209–1213. doi: 10.1016/0031-9422(95)00656-7. [DOI] [Google Scholar]
- 16.Jew S., Lim D., Bae S., Kim H., Kim J., Lee J., Park H. Enantioselective synthesis of (2R,3S)-(+)-catechin. Tetrahedron. Asymmetry. 2002;13:715–720. doi: 10.1016/S0957-4166(02)00182-9. [DOI] [Google Scholar]
- 17.Cangelosi B., Clematis F., Monroy F., Roversi P.F., Troiano R., Curir P., Lanzotti V. Filiferol, a chalconoid analogue from Washingtonia filifera possibly involved in the defence against the Red Palm Weevil Rhynchophorus ferrugineus Olivier. Phytochemistry. 2015;115:216–221. doi: 10.1016/j.phytochem.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura S., Zhang Y., Nakashima S., Oda Y., Wang T., Yoshikawa M., Matsuda H. Structures of aromatic glycosides from the seeds of Cassia auriculata. Chem. Pharm. Bull. 2016;64:970–974. doi: 10.1248/cpb.c16-00198. [DOI] [PubMed] [Google Scholar]
- 19.Tai B.H., Trung T.N., Nhiem N.X., Ha do T., Van Men C., Duong V.B., Van Luong H., Song S., Bae K., Kim Y.H. A new flavan-3-ol and the anti-inflammatory effect of flavonoids from the fruit peels of Wisteria floribunda. J. Asian Nat. Prod. Res. 2011;13:1061–1068. doi: 10.1080/10286020.2011.603306. [DOI] [PubMed] [Google Scholar]
- 20.Herz W., Gibaja S., Bhat S.V., Srinivasan A. Dihydroflavonols and other flavonoids of Eupatorium species. Phytochemistry. 1972;11:2859–2863. doi: 10.1016/S0031-9422(00)86525-5. [DOI] [Google Scholar]
- 21.Binutu O.A., Cordell G.A. Constituents of Afzelia bella stem bark. Phytochemistry. 2001;56:827–830. doi: 10.1016/S0031-9422(01)00006-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z.H., Li Q., Huang M., Xu P.F., Yang L.P., Zhai Y.Y., Zhang Z.Z., Zhang W.K., Niu C., Wang H. Chemical constituents of Callicarpa macrophylla. Chem. Nat. Compd. 2020;56:1125–1127. doi: 10.1007/s10600-020-03243-4. [DOI] [Google Scholar]
- 23.Kim K.H., Moon E., Choi S.U., Kim S.Y., Lee K.R. Polyphenols from the bark of Rhus verniciflua and their biological evaluation on antitumor and anti-inflammatory activities. Phytochemistry. 2013;92:113–121. doi: 10.1016/j.phytochem.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Krenn L., Pradhan R., Presser A., Reznicek G., Kopp B. Anthrone C-glucosides from Rheum emodi. Chem. Pharm. Bull. 2004;52:391–393. doi: 10.1248/cpb.52.391. [DOI] [PubMed] [Google Scholar]
- 25.Prateeksha , Yusuf M.A., Singh B.N., Sudheer S., Kharwar R.N., Siddiqui S., Abdel-Azeem A.M., Fernandes Fraceto L., Dashora K., Gupta V.K. Chrysophanol: A natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules. 2019;9:68. doi: 10.3390/biom9020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shamma M., Rosenstock P. The triterpenes of Heliabravoa chende. J. Org. Chem. 1959;24:726–728. doi: 10.1021/jo01087a623. [DOI] [Google Scholar]
- 27.Imam S., Azhar I., Hasan M.M., Ali M.S., Ahmed S.W. Two triterpenes lupanone and lupeol isolated and identified from Tamarindus indica linn. Pak. J. Pharm. Sci. 2007;20:125–127. [PubMed] [Google Scholar]
- 28.Prachayasittikul S., Suphapong S., Worachartcheewan A., Lawung R., Ruchirawat S., Prachayasittikul V. Bioactive metabolites from Spilanthes acmella Murr. Molecules. 2009;14:850–867. doi: 10.3390/molecules14020850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weihrauch J.L., Gardner J.M. Sterol content of foods of plant origin. J. Am. Diet. Assoc. 1978;73:39–47. [PubMed] [Google Scholar]
- 30.Boue S.M., Shih B.Y., Burow M.E., Eggleston G., Lingle S., Pan Y.B., Daigle K., Bhatnagar D. Postharvest accumulation of resveratrol and piceatannol in sugarcane with enhanced antioxidant activity. J. Agric. Food Chem. 2013;61:8412–8419. doi: 10.1021/jf4020087. [DOI] [PubMed] [Google Scholar]
- 31.Kanchanapoom T., Kasai R., Yamasaki K. Phenolic glycosides from Markhamia stipulata. Phytochemistry. 2002;59:557–563. doi: 10.1016/S0031-9422(01)00466-6. [DOI] [PubMed] [Google Scholar]
- 32.Shataer D., Abdulla R., Ma Q.L., Liu G.Y., Aisa H.A. Chemical composition of extract of Corylus avellana Shells. Chem. Nat. Compd. 2020;56:338–340. doi: 10.1007/s10600-020-03024-z. [DOI] [Google Scholar]
- 33.Matsuura H., Miyazaki H., Asakawa C., Amano M., Yoshihara T., Mizutani J. Isolation of α-glusosidase inhibitors from hyssop (Hyssopus officinalis) Phytochemistry. 2004;65:91–97. doi: 10.1016/j.phytochem.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Li N., Li X., Hou B.L., Meng D.L. New disaccharoside from Camptosorus sibiricus Rupr. Nat. Prod. Res. 2008;22:1379–1383. doi: 10.1080/14786410701824866. [DOI] [PubMed] [Google Scholar]
- 35.Hamid A.A., Aiyelaagbe O.O., Negi A.S., Kaneez F., Luqman S., Oguntoye S.O., Kumar S.B., Zubair M. Isolation and antiproliferative activity of triterpenoids and fatty acids from the leaves and stem of Turraea vogelii Hook. f. ex benth. Nat. Prod. Res. 2019;33:296–301. doi: 10.1080/14786419.2018.1446133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not available.