Abstract
Some authors have been proposing the use of cavity disinfectants in order to reduce, or even eliminate, the effect of the microorganisms present in a dental cavity before a restoration is placed. The aim of this study was to evaluate the effect of different cavity disinfectants on bond strength and clinical success of composite and glass ionomer restorations on primary teeth. The research was conducted using Cochrane Library, PubMed/MEDLINE, SCOPUS, and Web of Science for articles published up to February 2021. The search was performed according to the PICO strategy. The evaluation of the methodological quality of each in vitro study was assessed using the CONSORT checklist for reporting in vitro studies on dental materials. Sixteen in vitro studies and one in situ study fulfilled the inclusion criteria and were analyzed. Chlorhexidine was the most studied cavity disinfectant, and its use does not compromise dentin bonding. Sodium hypochlorite is a promising alternative, but more research on its use is required to clearly state that it can safely be used as a cavity disinfectant for primary teeth. Although other disinfectants were studied, there is a low-level evidence attesting their effects on adhesion, therefore their use should be avoided.
Keywords: cavity disinfectants, primary teeth, adhesion, bond strength
1. Introduction
Dental caries has a high prevalence worldwide, affecting more than 2.4 thousand million adults and 620 thousand children with primary teeth [1]. It can be defined as a multifactorial pathology arising from the interaction between dental structure and microbial biofilm, due to an imbalance between remineralization and demineralization, with the last one prevailing [2,3].
Although complete removal of the decayed and necrotic tissue is directly related to restorations’ clinical success, cariogenic bacteria can be pushed deep inside the dentinal tubules while removing the carious tissue and remain viable for a long time. In fact, the remaining of cariogenic bacteria in the cavity can be associated with the development of secondary caries [4,5].
According to Dalkilic et al. [6], fermenting microorganisms can remain viable for 139 days in a restored cavity. Moreover, bacteria present in the smear layer may remain viable and proliferate, allowing their metabolism products to reach and to cause inflammatory changes in the dental pulp. Bacteria penetration through restoration and teeth interface (microinfiltration) can also explain restorations’ failure [7,8,9].
As so, some authors have been proposing the use of cavity disinfectants in order to reduce, or even eliminate, the effect of the microorganisms present in a dental cavity before a restoration is placed [8,9,10].
Among the available disinfectants, chlorhexidine, sodium hypochlorite, and fluoridated solutions are the most used. Despite their benefits, their effect on adhesion to dentin, especially that of primary teeth, is still unknown [7,11,12].
Among the pediatric population, dental caries treatment is the most common procedure to be performed in a dental appointment [12]. Restorations’ success rate is associated with dentist’s experience and patient’s collaboration. However, one of the most common causes of failure is the development of secondary caries [13,14,15].
Thereby, the aim of this systematic review was to evaluate the effect of the application of different cavity disinfectants on bond strength and clinical success of composite and glass ionomer restorations on primary teeth.
2. Results
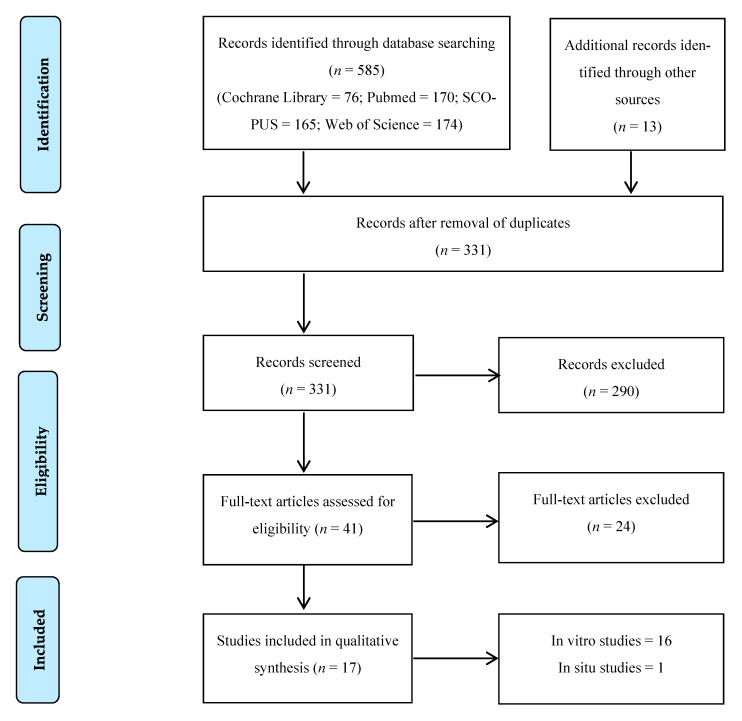
Initial research on electronic databases resulted in 585 articles. After evaluating titles and abstracts, 41 articles were selected for full text analysis, and of those, 17 studies fulfilled the inclusion and exclusion criteria. The flowchart of the data selection process is detailed in Figure 1.
Figure 1.
Flowchart of the data selection process.
Sixteen in vitro studies [12,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30] were included in this systematic review.
The earliest study was published in 2003 [12], and the most recent one in 2020 [29].
Most authors used primary molars [12,16,18,19,20,21,22,23,24,25,26,27,28,30], but Monghini et al. [17] evaluated canines, and Mohammadi et al. [29] used anterior teeth. Sample size varied from 2 [25] to 20 [28,31] teeth per group.
Even though all authors studied healthy dentin, Ersin et al. [18] additionally evaluated carious dentin, and Lenzi et al. [22,23] also evaluated demineralized dentin (artificially induced lesions).
After extraction, teeth were stored in thymol [12,18,28], chloramine [16,22,23,27,30], distilled water [16,20,22,23,24,25,27,30,32], saline solution [17,26], or sodium azide [17,21]. Ricci et al. [19] and Mohammadi [29] did not report data on the storage medium used after teeth extraction.
All authors used water to store the specimens after adhesive experiments and before bond strength evaluation.
All authors reported results on adhesion to composite resin. Only Ersin et al. [18] also reported results on adhesion to glass ionomer materials.
Most of the authors reported the use of 2% chlorhexidine [12,18,19,20,22,23,25,28,29] as a cavity disinfectant. A few studies reported results on the application of sodium hypochlorite [16,24,27], Er:YAG laser [17,21,26], KTP laser [25], ozone [25], doxycycline [29], ethylenediaminetetraacetic acid (EDTA) [29], propolis [25], and Aqua-prep™ (Bisco, Schaumburg, IL, USA) [26].
Except for Vieira et al. [12], all of the authors studying the effect of 2% chlorhexidine as a cavity disinfectant [18,19,20,22,23,25,28,29] reported positive results, allowing for maintenance or a statistically significant increase in bond strength values. These values ranged from 7.58 ± 3.18 MPa [25] to 66.45 ± 8.3 MPa [28] in resin specimens and from 7.1 ± 5.2 MPa to 14.4 ± 6.6 MPa [18] in caries-affected dentin in glass ionomer specimens. Vieira et al. [12] were the only authors applying chlorhexidine before etching the specimens with phosphoric acid.
The authors evaluating the effect of the application of sodium hypochlorite tested different concentrations, ranging from 2.5% [27] to 10% [16]. Regardless of the concentration, all authors [16,24,27] reported positive results, allowing for maintenance or even a statistically significant increase in bond strength values. These values ranged from 9.9 ± 0.2 MPa [16] to 18.45 ± 2.30 MPa [24].
The Er:YAG laser was evaluated by three studies [17,21,26]. Monghini et al. [17] reported statistically significant negative results when testing the laser with three different working parameters. However, Scatena et al. [21] did not find statistically significant differences regarding bond strength results for different focal distances (mm), and Yildiz et al. [26] even reported a statistically significant increase in bond strength values. The bond strength values of these studies ranged from 5.07 ± 2.62 MPa [21] to 20.57 ± 9.02 MPa [26].
Oznurhan et al. [25] assessed the use of a KTP laser as a cavity disinfectant and found no statistically significant differences when comparing its results to the ones of the control group (9.58 ± 2.92 and 6.38 ± 2.47 MPa, respectively).
Gaseous ozone and ozonated water [25] were also tested as cavity disinfectants. The authors reported a maintenance of the bond strength values when using gaseous ozone (5.84 ± 2.62 MPa vs. 6.38 ± 2.47 MPa for the control group) and a statistically significant increase of the bond strength values when using ozonated water (11.12 ± 2.41 MPa vs. 6.38 ± 2.47 MPa for the control group).
Aqua-prep™ [26], an aqueous solution of fluoride and hydroxyethyl methacrylate (HEMA), 2% Doxycycline [29], 17% EDTA [29], and 30% propolis [25] were all evaluated in only one study each, and no statistically significant differences were found between test and control groups.
Relevant information on each in vitro study is summarized in Table 1.
Table 1.
Characteristics of the in vitro studies included in the systematic review.
| Authors, Year | Groups (n) | Teeth | Storage | Materials | Results (MPa) |
|---|---|---|---|---|---|
| Vieira et al., 2003 [12] | G1—37% phosphoric acid + adhesive (10) + resin G2—2% CHX + 37% phosphoric acid + adhesive (10) + resin |
Molars | 0.1% Thymol | Adhesive: 3M Single Bond (3M, USA) Resin: FiltekTM Z250 (3M, USA) |
G1: 19.88 ± 1.04 G2: 17.99 ± 1.15 G1*/G2 |
| Correr et al., 2004 [16] | G1—35% phosphoric acid + adhesive 1 (15) G2—35% phosphoric acid + 10% NaOCl + adhesive 1 (15) G3—37% phosphoric acid + adhesive 2 (15) G4—37% phosphoric acid + 10% NaOCl + adhesive 2 (15) G5–Adhesive 3 (15) G6—10% NaOCl + adhesive 3 (15) + resin |
Molars | 0.5% Chloramine | Adhesive: 1–3M Single Bond; 2–Prime & Bond 2.1® (Dentsply, Brazil); 3–ClearfillTM SE Bond (Kuraray, Houston, TX, USA) Resin: FiltekTM Z250 (3M, USA) |
G1: 15.8 ± 1.9 G2: 14.6 ± 1.3 G3: 10.2 ± 0.7 G4: 9.9 ± 0.2 G5: 13.3 ± 1.2 G6: 10.7 ± 1.0 G1*/G3 |
| Monghini et al., 2004 [17] | G1—None (12) G2—Laser Er;YAG 60 mJ/2 Hz (12) G3—Laser Er;YAG 80 mJ/2 Hz (12) G4—Laser Er;YAG 100 mJ/2 Hz (12) + 35% phosphoric acid + adhesive + resin |
Canines | 0.9% Saline solution with 0.4% sodium azide | Adhesive: 3M Single Bond Laser: Kavo Key Laser 2 (Kavo Dental, Germany) Resin: FiltekTM Z250 |
G1:17.89 ± 4.75 G2:12.34 ± 4.85 G3:10.30 ± 3.67 G4:10.41 ± 4.20 G1*/G2;G3;G4 |
| Ersin et al., 2009 [18] | G1—25% polyacrlylic acid + 2% CHX + GIC 1 (sound dentin) (3) G2—25% polyacrlylic acid + 2% CHX + GIC 1 (carious dentin) (3) G3—25% polyacrlylic acid + GIC 1 (sound dentin) (3) G4—25% polyacrlylic acid + GIC 1 (carious dentin) (3) G5—2% CHX + GIC 2 (sound dentin) (3) G6—2% CHX + GIC 2 (carious dentin) (3) G7—GIC 2 (sound dentin) (3) G8—GIC 2 (carious dentin) (3) G9—37% phosphoric acid + 2% CHX + adhesive + resin (sound dentin) (3) G10–37% phosphoric acid + 2% CHX + adhesive + resin (carious dentin) (3) G11—37% phosphoric acid + adhesive + resin (sound dentin) (3) G12—37% phosphoric acid + adhesive + resin (carious dentin) (3) |
Molars | 0.1% Thymol | Adhesive: Prime & Bond®; GIC: 1–KetacTM Molar (3M, Germany); 2–VitremerTM (3M, USA) Resin–SurefilTM (Dentsply, USA) |
G1: 8.7 ± 4.3 G2: 7.1 ± 5.2 G3: 9.2 ± 5.2 G4: 10.3 ± 6.6 G5: 12.4 ± 5.7 G6: 14.4 ± 6.6 G7: 11.2 ± 4.8 G8: 13.8 ± 4.9 G9: 22.9 ± 6.9 G10: 23.2 ± 6.2 G11: 20.2 ± 5.8 G12: 22.1 ± 6.2 G9*/G1;G2;G3;G4;G5;G6;G7;G8 G10*/G1;G2;G3;G4;G5;G6;G7;G8 G11*/G1;G2;G3;G4;G5;G6;G7;G8 G12*/G1;G2;G3;G4;G5;G6;G7;G8 |
| Ricci et al., 2010 [19] | 35% phosphoric acid + G1—2% CHX + adhesive 1 (4) G2—deionized water + adhesive 1 (4) G3—2% CHX + adhesive 2 (4) G4—deionized water + adhesive 2 (4) G5—2% CHX + adhesive 3 (4) G6—deionized water + adhesive 3 (4) + resin |
Molars | NA | Adhesive: 1–AdperTM Single Bond (3M, USA); 2–Prime & Bond NT® (Dentsply, USA); 3–Excite® DSC (Ivoclar, Liechtenstein) Resin: FiltekTM Z250 |
G1: 47.4 ± 9.5 G2: 41.4 ± 11.9 G3: 48.0 ± 9.8 G4: 40.8 ± 13.4 G5: 45.2 ± 9.2 G6: 43.4 ± 12.0 G1*/G2; G3*/G4 |
| Leitune et al., 2011 [20] | 37% phosphoric acid + G1—Adhesive (24 h) (10) G2—Adhesive (6 months) (10) G3—2% CHX + Adhesive (24 h) (10) G4—2% CHX + Adhesive (6 months) (10) |
Molars | Distilled water | Adhesive: AdperTM ScotchbondTM Multi Purpose (3M, USA) Resin: FiltekTM Z250 |
G1: 22.37 ± 3.69 G2: 19.93 ± 2.05 G3: 22.30 ± 3.66 G4: 24.48 ± 2.24 G2*/G4 |
| Scatena et al., 2011 [21] | G1–None (10) G2—Laser Er:YAG (80 mJ, 11 mm) (10) G3—Laser Er:YAG (80 mJ, 12 mm) (10) G4—Laser Er:YAG (80 mJ, 16 mm) (10) G5—Laser Er:YAG (80 mJ, 17 mm) (10) G6—Laser Er:YAG (80 mJ, 20 mm) (10) + 37% phosphoric acid + adhesive + resin |
Molars | 0.4% Sodium azide | Laser: Kavo Key Laser 2 Adhesive: 3M Single Bond Resin: FiltekTM Z250 |
G1: 7.32 ± 3.83 G2: 5.07 ± 2.62 G3: 6.49 ± 1.64 G4: 7.71 ± 0.66 G5: 7.33 ± 0.02 G6: 9.65 ± 2.41 G2*/G4;G6 |
| Manfro et al., 2012 [30] | 37% phosphoric acid + G1—water + adhesive (7) G2—water + adhesive (12 months) (7) G3—0.5% CHX + adhesive (7) G4—0.5% CHX + adhesive (12 months) (7) G5—2% CHX + adhesive (7) G6—2% CHX + adhesive (12 months) (7) + resin |
Molars | 0.5% Chloramine | Adhesive: 3M Single Bond Resin: FiltekTM Z250 |
G1: 50.8 ± 12.8 G2: 20.4 ± 3.7 G3: 49.3 ± 2.6 G4: 32.3 ± 7.9 G5: 44.0 ± 8.7 G6: 34.6 ± 5.1 G1*/G2; G2*/G4;G6; G3*/G4; G5*/G6 |
| Lenzi et al., 2012 [22] | 35% phosphoric acid + G1—distilled water + adhesive (sound dentin) (5) G2—2% CHX + adhesive (sound dentin) (5) G3—distilled water + adhesive (artificial caries) (5) G4—2% CHX + adhesive (artificial caries) (5) |
Molars | 0.5% Chloramine | Adhesive: AdperTM Single Bond 2 Resin: FiltekTM Z250 |
G1: 30.8 ± 2.2 G2: 32.8 ± 3.8 G3: 24.5 ± 3.8 G4: 25.6 ± 3.6 G1*/G3;G4; G2*/G3;G4 |
| Aras et al., 2013 [24] | G1—37% phosphoric acid (10) G2—37% phosphoric acid + 5% NaOCl (10) G3—5% NaOCl + 37% phosphoric acid (10) + adhesive + resin |
Molars | Distilled water | Adhesive: Gluma® Confort Bond (Herause-Kulzer, Germany) Resin: Charisma® (Herause-Kulzer, Germany) |
G1: 14.51 ± 2.89 G2: 18.45 ± 2.30 G3: 17.06 ± 2.99 G1*/G2 |
| Lenzi et al., 2014 [23] | 35% phosphoric acid + G1—distilled water + adhesive (sound dentin) (5) G2—distilled water + adhesive (sound dentin) (6 months) (5) G3—2% CHX (without rinsing) + adhesive (sound dentin) (5) G4—2% CHX (without rinsing) + adhesive (sound dentin) (6 months) (5) G5—distilled water + adhesive (artificial lesion) (5) G6—distilled water + adhesive (artificial lesion) (6 months) (5) G7—2% CHX (without rinsing) + adhesive (artificial lesion) (5) G8—2% CHX (without rinsing) + adhesive (artificial lesion) (6 months) (5) |
Molars | Distilled water | Adhesive: AdperTM Single Bond Resin: FiltekTM Z250 |
G1: 30.7 ± 2.2 G2: 25.9 ± 5.7 G3: 32.8 ± 3.8 G4: 31.3 ± 2.6 G5: 26.2 ± 5.4 G6: 20.0 ± 3.9 G7: 28.3 ± 3.4 G8: 26.9 ± 5.9 G1*/G5;G7; G2*/G6;G8 G3*/G5;G7 G4*/G6;G8 |
| Oznurhan et al., 2015 [25] | G1—2% CHX (2) G2—30% propolis (2) G3—Gaseous ozone (2) G4—Ozonated water (2) G5—Laser KTP (2) G6—None (2) + adhesive + resin |
Molars | Distilled water | Adhesive: AdperTM Prime & Bond NT® Resin: Tetric® N-Ceram (Ivoclar Vivadent, Liechenstein) Laser: Smartlite D (Deka, Italy) |
G1: 7.58 ± 3.18 G2: 7.42 ± 2.28 G3: 5.84 ± 2.62 G4: 11.12 ± 2.41 G5: 9.58 ± 2.92 G6: 6.38 ± 2.47 G3*/G5; G4*/G1/G2/G3/G6 |
| Yildiz et al., 2015 [26] | G1—37% phosphoric acid (3) G2—37% phosphoric acid + Aqua-Prep™ (without rinsing) (3) G3—Laser Er:YAG (10 Hz, 8 mm) (3) + adhesive + resin |
Molars | Saline solution | Adhesive: AdperTM Single Bond 2 Resin: FiltekTM Z250 Laser: Fidelis Plus III (Fotona, Slovenia) Aqua-PrepTM (Bisco, USA) |
G1: 14.28 ± 5.22 G2: 18.35 ± 7.94 G3: 20.57 ± 9.02 G1*/G3 |
| Bahrololoomi et al., 2017 [27] | 35% phosphoric acid + G1–none (14) G2–2.5% NaOCl (14) G3–5.25% NaOCl (14) + adhesive + resin |
Molars | 0.5% Chloramine | Adhesive: One-Step® Plus (Bisco, USA) Resin: AELITE (Bisco, USA) |
G1: 13.56 ± 3.36 G2: 13.53 ± 3.64 G3: 14.36 ± 3.64 |
| Ebrahimi et al., 2018 [28] | G1—37% phosphoric acid + adhesive 1 (20) G2—37% phosphoric acid + adhesive 1 (3 months) (20) G3—37% phosphoric acid + adhesive 1 + 2% CHX (without rinsing) (20) G4—37% phosphoric acid + adhesive 1 + 2% CHX (without rinsing) (3 months) (20) G5—Adhesive 2 (20) G6—Adhesive 2 (3 months) (20) G7—Adhesive 2 (Primer) + 2% CHX (without rinsing) + adhesive 2 (bond) (20) G8—Adhesive 2 (primer) + 2% CHX (without rinsing) + adhesive 2 (bond) (3months) (20) |
Molars | 0.1% Thymol + water | Adhesive: 1–AdperTM Single Bond 2–ClearfilTM SE Bond Resin: FiltekTM Z250 |
G1: 25.43 ± 12.94 G2: 39.96 ± 21.75 G3: 66.45 ± 8.3 G4: 39.02 ± 23.29 G5: 47.83 ± 19.83 G6: 53.36 ± 18.05 G7: 46.25 ± 9.34 G8: 56.4 ± 22.18 G1*/G3 |
| Mohammadi et al., 2020 [29] | 37% phosphoric acid + G1–PBS (15) G2—2% CHX (without rinsing) (15) G3—2% Doxycycline (without rinsing) (15) G4—17% EDTA (15) + adhesive |
Anterior teeth | - | Adhesive: AdperTM Single Bond 2 Resin: FiltekTM Z250 |
G1: 6.20 ± 2.11 G2: 5.60 ± 2.69 G3: 8.82 ± 3.29 G4: 7.50 ± 3.94 G2*/G3 |
CHX–Chlorhexidine; EDTA–Ethylenediaminetetraacetic Acid; GIC–Glass Ionomer Cement; NaOCl–Sodium hypochlorite; *–Statistically significant difference (p < 0.05).
No clinical studies were identified, and only one in situ study regarding the use of a cavity disinfectant in primary teeth was evaluated. Ricci et al. [31] developed a split-mouth experimental protocol that included children aged between 8 and 11 years with at least two contralateral primary molars with small carious lesions. Chlorhexidine was used as a cavity disinfectant after enamel and dentin were etched with 35% phosphoric acid. The solution was removed with absorbent papers, and the cavities were restored with Prime & Bond NT® (Dentsply, York, PA, USA) and Filtek™ Z250 (3M, Saint Paul, MN, USA). All the procedures were done under rubber dam, and the teeth were collected later, after exfoliation. The teeth were grouped according to the time of oral function after restoration: up to 30 days, 1 to 5 months, 10 to 12 months, and 18 to 20 months. A progressive decrease in bond strength values was reported for control and experimental groups as the time in oral function increased. However, a statistically significant decrease was reported sooner for the control group (it started after 1–5 months, while for the experimental group it started after 10–12 months). Also, significantly higher bond strength values were reported for the experimental group after 1–5 and 18–20 months.
Quality Assessment
Methodological quality assessment outcomes are presented in Table 2. All studies presented accurate information regarding each item from 1 to 10. However, none of them provided results with confidence intervals. In addition, only two studies [26,29] reported study limitations and sources of potential bias (item 12).
Table 2.
Modified CONSORT checklist for reporting in vitro studies of dental materials.
| Studies | Item | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Abstract |
2a Introduction (Background) |
2b Introduction (Objectives) |
3 Methods (Intervention) |
4 Methods (Outcomes) |
10 Methods (Statistical Methods) |
11 Results (Outcomes and Estimation) |
12 Discussion (Limitations) |
13 Other Information (Funding) |
14 Other Information (Protocol) |
|
| Vieira et al., 2003 [12] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | No | No |
| Correr et al., 2004 [16] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | No | No |
| Monghini et al., 2004 [17] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | No | No |
| Ersin et al., 2009 [18] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | No | No |
| Ricci et al., 2010 [19] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | Yes | No |
| Leitune et al., 2011 [20] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | No | No |
| Scatena et al., 2011 [21] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | No | No |
| Manfro et al., 2012 [30] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | No | No |
| Lenzi et al., 2012 [22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | Yes | No |
| Aras et al., 2013 [24] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | Yes | No |
| Lenzi et al., 2014 [23] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | Yes | No |
| Oznurhan et al., 2015 [25] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | Yes | No |
| Yildiz et al., 2015 [26] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | Yes | Yes | No |
| Bahrololoomi et al., 2017 [27] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | Yes | No |
| Ebrahimi et al., 2018 [28] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | No | Yes | No |
| Mohammadi et al., 2020 [29] | Yes | Yes | Yes | Yes | Yes | Yes | Yes a | Yes | Yes | No |
a No confidence interval.
3. Discussion
A cavity disinfectant must not only have a strong antimicrobial effect but also not compromise the adhesion of the restorative material to the dental substrates [7,32]. The majority of the studies on this topic reports results on permanent teeth, but the structural and mechanical properties of the primary teeth make it necessary to carry out experimental protocols testing this type of teeth [33,34]. Compared to permanent teeth, primary teeth have thinner enamel and dentin, are less mineralized due to their lower concentration of calcium and potassium ions, have a hybrid layer more prone to be degraded [35], and their dentin has a lower tubule density [18,36,37]. This may explain why bond strength values of composite materials in primary teeth are lower than those of permanent teeth [38].
Dental adhesion may be affected not only by the cavity disinfectant used but also by the dental substrate. In order to minimize its effect, it is recommended to perform adhesion tests in the superficial dentin of healthy teeth, ideally without restorations [39]. Deep dentin is mainly composed of dentinal tubules and a small percentage of intertubular dentin. Superficial dentin has a higher percentage of organic components (collagen) and of intertubular dentin and a lower number of dentinal tubules [40,41,42].
The differences between healthy and caries-affected dentin should also be underlined. The caries-affected dentin is more porous and softer due to its partial demineralization, which leads to a less effective adhesion [43,44,45]. In fact, some of the articles included in this systematic review evaluated the effect of a cavity disinfectant in healthy and affected dentin [18,22,23], and Lenzi et al. [22,23] reported significant lower bond strength values for the affected-dentin groups.
Besides dentin’s quality (superficial/deep dentin, permanent/primary teeth, healthy/carious dentin, amount of collagen and number, diameter, orientation, and size of dentinal tubules), moisture, contaminants, adhesive systems, solvents, and phosphoric acid/acidic primers are all factors affecting bond strength to dentin [46,47,48,49]. As so, the inclusion of at least one control group per study was mandatory for a study to be included in this systematic review.
All of the studies reported the use of a storage medium before the samples were submitted to the experimental protocol. The ISO/TS 11405/2015 (Dentistry–Testing of adhesion to tooth structure) [39] provides guidance for testing adhesion between dental substrates and restorative materials. This ISO/TS recommends the use of a 0.5% chloramine solution or of distilled water as a storage medium for the extracted teeth. If chloramine is chosen, it should be replaced by distilled water after one week. Despite these recommendations, some authors used other solutions, such as thymol [12,18,28]. The use of other solutions is not recommended by the ISO/TS 11405/2015, since it may affect dentin’s mechanical properties. In fact, Santana et al. [50] reported that the use of thymol as a storage medium led to impaired adhesion.
After the restorations were made, all authors stated that the samples were kept in water, which is exactly the recommendation of the ISO/TS 11405/2015 (ISO 3696:1987, grade 3) [51].
Almost all authors reported results on adhesion to molars, which is also in line with the recommendations of the ISO/TS 11405/201545. However, Monghini et al. [17] and Mohammadi et al. [29] used anterior teeth.
Most authors [12,18,19,20,22,23,25,28,29] evaluated the effect of chlorhexidine as a cavity disinfectant. Chlorhexidine has been widely used in dentistry, mainly because of its antimicrobial properties, including against Streptococcus mutans, and of its antiplaque effect [52,53,54,55]. Chlorhexidine is also well known for its ability to inhibit matrix metalloproteinases due to its strong collagenolytic activity, reducing the degradation of the hybrid layer [56,57], which may justify the positive results reported by almost all authors. Although only Ersin et al. [18] evaluated the effect of chlorhexidine on the adhesion to a glass ionomer material, the authors also reported positive results.
Similar results were previously reported for permanent teeth [58], which makes chlorhexidine the most consensual cavity disinfectant to be used in clinical practice. Not only adhesion to dentin is not only adequate after its use but, as stated by some authors [59,60], it can even be enhanced. As so, chlorhexidine presents as a safe and effective product to be used as a cavity disinfectant.
Sodium hypochlorite is commonly used as a cavity disinfectant due to its favorable properties: antibacterial action against aerobic bacteria, such as S. mutans, wettability, and deproteinization [61,62,63,64,65]. Although all authors studying the effect of the use of sodium hypochlorite as a cavity disinfectant in primary teeth reported positive results, only three articles [16,24,27] were identified. Since there are just a few studies reporting results on primary teeth and that the use of sodium hypochlorite as a cavity disinfectant in permanent teeth is still a matter of discussion [58], caution is required when choosing this product as a cavity disinfectant.
Initially presented as an alternative to the use of burs for cavity preparation, the Erbium:Ytrium (Er:YAG) laser was first introduced in 1989 by Hibst and Keller [66]. From then on, lasers have been used in numerous dentistry fields such as oral surgery, periodontics, endodontics, and prosthodontics [67]. However, similarly to what was reported for permanent teeth [58], there is no consensus regarding the use of lasers as cavity disinfectants, with some authors reporting an impairment of adhesion [17], and others reporting maintenance or even an enhancement of the bond strength values [21,26]. Moreover, even though some authors did not report secondary side effects [66,68,69], lasers may lead to overheating of the dental structures, which may induce pulp injuries, hydroxyapatite changes, and excessive dentin dehydration [17,70,71,72,73,74,75,76]. Given the results, the use of lasers as a cavity disinfection method should be avoided.
Both gaseous ozone and ozonated water have been recently introduced as alternatives to cavity disinfection due to their known antimicrobial and strong antioxidant properties. Polydorou et al. [77] reported that gaseous ozone eliminated 99.9% of the microorganisms in carious lesions in 20 s. In addition to its great antimicrobial activity (including against S. mutans), ozone also has antifungal and antiviral properties [78]. Authors analyzing the effect of either ozonated water or gaseous ozone on adhesion reported positive results [25], which may be justified by the opening of the dentinal tubules caused by oxygen [79,80,81,82,83]. Although there is limited information about the use of ozone as a cavity disinfectant in primary teeth, it looks like a promising alternative.
EDTA is an organic compound responsible for chelating calcium and potassium ions and for selective removal of hydroxyapatite crystals, which allows for the maintenance of the collagen matrix [84,85]. It is widely used in endodontics to improve shaping of the entire root canal system and to dissolve the inorganic components of the smear layer [86]. Although the reported results were positive (no differences on bond strength values after using it as a cavity disinfectant), only one study [29] evaluated it. A few articles on permanent teeth [58] also showed that EDTA presents as a promising alternative, but there is a clear need for further research.
Aqua-prepTM [26], 2% doxycycline [29], and 30% propolis [25] were all evaluated by studies included in this review, and the reported results were positive, but only one article was included for each product. Given the limited scientific evidence associated with these products (even in permanent teeth [58]), their use as cavity disinfectants should be avoided.
The limitations of this systematic review mainly reflect the shortcomings of the included articles. No clinical studies on the topic were identified, and such studies are essential to analyze the effects of the different cavity disinfectants when applied in the oral cavity. In addition, there is no information on the best application time and on the durability of bond interfaces over time. Also, there are several studies reporting results on different adhesive systems (total etch, self-etch, universal) but given the different methods applied, it is impossible to draw conclusions regarding this matter.
Further studies with standardized protocols should be developed to allow solid conclusions and recommendations concerning this issue. The effect of the incorporation of cavity disinfectants into adhesive systems must also be evaluated, since it may reduce clinical steps, which is of great importance in pediatric dentistry.
4. Materials and Methods
The present systematic review was registered on the International Prospective Register of Systematic Reviews (PROSPERO) platform (ID CRD42020199614) and followed the PRISMA protocol (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols) [87].
The research questions were developed according to the PICO (Population, Intervention, Comparison, Outcome) methodology, as described in Table 3.
Table 3.
Problem, Intervention, Comparison, Outcome (PICO) strategy.
| Parameter | In Vitro Studies | Clinical/In Situ Studies |
|---|---|---|
| P (Population) | Primary teeth / dentin discs | Children in need of a restoration |
| I (Intervention) | Restoration with prior application of a cavity disinfectant | |
| C (Comparison) | Conventional restoration | |
| O (Outcome) | Effect of cavity disinfection on dentin bond strength | Effect of cavity disinfection on clinical success |
The inclusion and exclusion criteria are presented in Table 4.
Table 4.
Inclusion and Exclusion Criteria.
| Inclusion Criteria | Primary teeth evaluation |
| Bond strength/clinical success evaluation | |
| Existence of a control group | |
| Evaluation of commercially available adhesive systems and composite resins or glass ionomer | |
| Application of only one cavity disinfectant per experimental group | |
| Report of results as mean and standard deviation | |
| Exclusion Criteria | Permanent teeth evaluation |
| Evaluation of teeth with endodontic treatment | |
| Evaluation of adhesion of cements, posts, sealants, or brackets | |
| Use of experimental adhesive systems or of mixtures of adhesives with disinfectants | |
| Revisions, animal or cell studies, letters, abstracts, comments, and clinical cases |
An electronic research was conducted in Cochrane Library (www.cochranelibrary.com), PubMed/MEDLINE (pubmed.ncbi.nlm.nih.gov), SCOPUS (www.scopus.com), and Web of Science (webofknowledge.com). The research keys used in each database can be found in Table 5.
Table 5.
Search keys used in the different databases.
| Database | Search keys |
|---|---|
| Cochrane Library | #1 MeSH descriptor: [Dentin] explode all trees |
| #2 dentin | |
| #3 cavity | |
| #4 MeSH descriptor: [Disinfection] explode all trees | |
| #5 disinfect* | |
| #6 antibacteria* | |
| #7 MeSH descriptor: [Anti-Bacterial Agents] explode all trees | |
| #8 chlorhexidine | |
| #9 MeSH descriptor: [Chlorhexidine] explode all trees | |
| #10 “sodium hypochlorite” | |
| #11 MeSH descriptor: [Sodium Hypochlorite] explode all trees | |
| #12 laser | |
| #13 MeSH descriptor: [Lasers] explode all trees | |
| #14 ozone | |
| #15 MeSH descriptor: [Ozone] explode all trees | |
| #16 “aloe vera” | |
| #17 MeSH descriptor: [Aloe] explode all trees | |
| #18 ethanol | |
| #19 MeSH descriptor: [Ethanol] explode all trees | |
| #20 EDTA | |
| #21 MeSH descriptor: [Edetic Acid] explode all trees | |
| #22 “green tea” | |
| #23 EGCG | |
| #24 “bond strength” | |
| #25 adhesion | |
| #26 adhesive | |
| #27 MeSH descriptor: [Dental Cements] explode all trees | |
| #28 primary | |
| #29 deciduous | |
| #30 MeSH descriptor: [Tooth, Deciduous] explode all trees | |
| #31 temporary | |
| #32 #1 OR #2 OR #3 | |
| #33 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 | |
| #34 #24 OR #25 OR #26 OR #27 | |
| #35 #28 OR #29 OR #30 OR #31 | |
| #36 #32 AND #33 AND #34 AND #35 | |
| PubMed | (dentin[MeSH Terms] OR dentin OR cavity) AND (disinfection[MeSH Terms] OR disinfect* OR antibacteria* OR agents, antibacterial[MeSH Terms] OR chlorhexidine[MeSH Terms] OR chlorhexidine OR “sodium hypochlorite” OR sodium hypochlorite[MeSH Terms] OR laser OR lasers[MeSH Terms] OR ozone OR ozone[MeSH Terms] OR “aloe vera” OR aloe[MeSH Terms] OR ethanol OR ethanol[MeSH Terms] OR EDTA OR Edetic acid[MeSH Terms] OR “green tea” OR EGCG) AND (“bond strength” OR adhesion OR adhesive OR adhesives[MeSH Terms]) AND (deciduous tooth[MeSH Terms] OR deciduous OR primary OR temporary) |
| SCOPUS | TITLE-ABS-KEY (dentin OR cavity) AND TITLE-ABS-KEY (disinfect* OR antibacterial* OR chlorhexidine OR “sodium hypochlorite” OR laser OR ozone OR “aloe vera” OR ethanol OR EDTA OR “green tea” OR EGCG) AND TITLE-ABS-KEY (“bond strength” OR adhesion OR adhesive) AND TITLE-ABS-KEY (primary OR deciduous OR temporary) |
| Web of Science | TS= ((dentin[MeSH Terms] OR dentin OR cavity) AND (disinfect* OR antibacteria* OR chlorhexidine OR “sodium hypochlorite” OR laser OR ozone OR “aloe vera” OR ethanol OR EDTA OR “green tea” OR EGCG) AND (“bond strength” OR adhesion or adhesive) AND (primary OR deciduous OR temporary)) |
The search was limited to articles published until 14 February 2021, with no restrictions on region, language, or year of publication. A manual search for other references in reviews and in the included articles was performed.
Duplicate articles were removed with Endnote 20 (Clarivate™, Boston, MA, USA). Two independent reviewers analyzed titles, abstracts, and full texts, and a third one’s opinion was obtained when necessary.
Selected articles were read by the same two independent authors, who collected the following data on the in vitro studies: authors and year of publication, number of elements per group (n), materials used (cavity disinfectant, type of adhesive system, and restorative material), storage, and bond strength results.
Regarding the clinical/in situ studies, the following data were acquired: authors and year of publication, type of teeth, number and ages of children per group (n), materials used (cavity disinfectant, type of adhesive system, and restorative material), and results (pigmentation, marginal gaps, or existence of carious lesions).
Quality Assessment
The evaluation of the methodological quality of each in vitro study was assessed using the modified Consolidated Standards of Reporting Trials (CONSORT) checklist [88] for reporting in vitro studies on dental materials. When applying this checklist, items 5 to 9 could not be evaluated, since these are designed to evaluate sample standardization. Two authors assessed the risk of bias independently, and any disagreement was solved by consensus.
5. Conclusions
Chlorhexidine is the most studied cavity disinfectant, and according to the results, its use does not compromise adhesion to primary dentin. Sodium hypochlorite is a promising alternative, but more research on its effects on adhesion is required to clearly state that it can be safely used as a cavity disinfectant for primary teeth. Although other disinfectants were studied, there is a low-level evidence attesting their effects on adhesion; therefore, their use should be avoided.
There is a clear need for researchers to conduct well-designed in vitro and clinical studies so more options can be identified, and the long-term effect on adhesion can be evaluated.
Author Contributions
Conceptualization, A.C., I.A., and E.C.; methodology, A.C., I.A., A.P., C.M.M.; software, A.C., I.A.; validation, A.C., I.A., A.P., C.M.M., M.M.F., E.C.; data extraction and analysis, A.C., I.A., A.A., J.S., E.C.; writing-original draft preparation, A.C., I.A., A.A., J.S.; writing-review and editing, A.C., I.A., A.P., C.M.M., M.M.F., E.C.; supervision, A.C., I.A., E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kassebaum N.J., Bernabé E., Dahiya M., Bhandari B., Murray C.J.L., Marcenes W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015;94:650–658. doi: 10.1177/0022034515573272. [DOI] [PubMed] [Google Scholar]
- 2.Pitts N.B., Zero D.T., Marsh P.D., Ekstrand K., Weintraub J.A., Ramos-Gomez F., Tagami J., Twetman S., Tsakos G., Ismail A. Dental caries. Nat. Rev. Dis. Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 3.Askar H., Krois J., Göstemeyer G., Bottenberg P., Zero D., Banerjee A., Schwendicke F. Secondary caries: What is it, and how it can be controlled, detected, and managed? Clin. Oral Investig. 2020;24:1869–1876. doi: 10.1007/s00784-020-03268-7. [DOI] [PubMed] [Google Scholar]
- 4.Singhal D.K., Acharya S., Thakur A.S. Microbiological analysis after complete or partial removal of carious dentin using two different techniques in primary teeth: A randomized clinical trial. Dent. Res. J. 2016;13:30–37. doi: 10.4103/1735-3327.174695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson M.H., Loesche W.J., Charbeneau G.T. Bacteriologic study of a basic fuchsin caries-disclosing dye. J. Prosthet. Dent. 1985;54:51–55. doi: 10.1016/S0022-3913(85)80069-X. [DOI] [PubMed] [Google Scholar]
- 6.Dalkilic E.E., Arisu H.D., Kivanc B.H., Uctasli M.B., Omurlu H. Effect of different disinfectant methods on the initial microtensile bond strength of a self-etch adhesive to dentin. Lasers Med. Sci. 2012;27:819–825. doi: 10.1007/s10103-011-0987-x. [DOI] [PubMed] [Google Scholar]
- 7.Elkassas D.W., Fawzi E.M., Zohairy A. The effect of cavity disinfectants on the micro-shear bond strength of dentin adhesives. Eur. J. Dent. 2014;8:184–190. doi: 10.4103/1305-7456.130596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Say E.C., Koray F., Tarim B., Soyman M., Gülmez T. In vitro effect of cavity disinfectants on the bond strength of dentin bonding systems. Quintessence Int. 2004;35:56–60. [PubMed] [Google Scholar]
- 9.Hiraishi N., Yiu C.K.Y., King N.M., Tay F.R. Effect of 2% chlorhexidine on dentin microtensile bond strengths and nanoleakage of luting cements. J. Dent. 2009;37:440–448. doi: 10.1016/j.jdent.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Colares V., Franca C., Filho H.A.A. O tratamento restaurador atraumático nas dentições decídua e permanente. Rev. Port. Estomatol. Med. Dentária Cir. Maxilofac. 2009;50:35–41. doi: 10.1016/S1646-2890(09)70014-7. [DOI] [Google Scholar]
- 11.Suma N.K., Shashibhushan K.K. Effect of Dentin Disinfection with 2% Chlorhexidine Gluconate and 0.3% Iodine on Dentin Bond Strength: An in vitro Study. Int. J. Clin. Pediatric Dent. 2017;10:223–228. doi: 10.5005/jp-journals-10005-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieira R.S., Silva I.A. Bond strength to primary tooth dentin following disinfection with a chlorhexidine solution: An in vitro study. Pediatric Dent. 2003;25:49–52. [PubMed] [Google Scholar]
- 13.Franzon R., Opdam N.J., Guimarães L.F., Demarco F.F., Casagrande L., Haas A.N., Araújo F.B. Randomized controlled clinical trial of the 24-months survival of composite resin restorations after one-step incomplete and complete excavation on primary teeth. J. Dent. 2015;43:235–1241. doi: 10.1016/j.jdent.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Demarco F.F., Corrêa M.B., Cenci M.S., Moraes R.R., Opdam N.J.M. Longevity of posterior composite restorations: Not only a matter of materials. Dent. Mater. 2012;28:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Sande F.H., Collares K., Correa M.B., Cenci M.S., Demarco F.F., Opdam N. Restoration Survival: Revisiting Patients’ Risk Factors through a Systematic Literature Review. Oper. Dent. 2016;41:S7–S26. doi: 10.2341/15-120-LIT. [DOI] [PubMed] [Google Scholar]
- 16.Correr G.M., Puppin-Rontani R.M., Correr-Sobrinho L., Sinhoret M.A.C., Consani S. Effect of sodium hypochlorite on dentin bonding in primary teeth. J. Adhes. Dent. 2004;6:307–312. [PubMed] [Google Scholar]
- 17.Monghini E.M., Wanderley R.L., Pécora J.D., Dibb P.R.G., Corona S.A.M., Borsatto M.C. Bond Strength to Dentin of Primary Teeth Irradiated with Varying Er: YAG Laser Energies and SEM Examination of the Surface Morphology. Lasers Surg. Med. 2004;34:254–259. doi: 10.1002/lsm.20023. [DOI] [PubMed] [Google Scholar]
- 18.Ersin N.K., Candan U., Aykut A., Eronat C., Belli S. No Adverse Effect to Bonding Following Caries. J. Dent. Child. 2009;76:20–27. [PubMed] [Google Scholar]
- 19.Ricci H.A., Sanabe M.E., Costa C.A.S., Hebling J. Effect of chlorhexidine on bond strength of two-step etch-and-rinse adhesive systems to dentin of primary and permanent teeth. Am. J. Dent. 2010;23:128–132. [PubMed] [Google Scholar]
- 20.Leitune V.C.B., Portella F.F., Bohn P.V., Collares F.M., Samuel S.M.W. Influence of chlorhexidine application on longitudinal adhesive bond strength in deciduous teeth. Braz. Oral Res. 2011;25:388–392. doi: 10.1590/S1806-83242011000500003. [DOI] [PubMed] [Google Scholar]
- 21.Scatena C., Torres C.P., Gomes-Silva J.M., Contente M., Pécora J.D., Palma-Dibb R.G., Borsatto M.C. Shear strength of the bond to primary dentin: Influence of Er: YAG laser irradiation distance. Lasers Med. Sci. 2011;26:293–297. doi: 10.1007/s10103-010-0776-y. [DOI] [PubMed] [Google Scholar]
- 22.Lenzi T.L., Tedesco T.K., Soares F.Z.M., Loguercio A.D., Rocha R.O. Chlorhexidine does not increase immediate bond strength of etch-and-rinse adhesive to caries-affected dentin of primary and permanent teeth. Braz. Dent. J. 2012;23:438–442. doi: 10.1590/S0103-64402012000400022. [DOI] [PubMed] [Google Scholar]
- 23.Lenzi T.L., Tedesco T.K., Soares F.Z.M., Loguercio A.D., Rocha R.O. Chlorhexidine application for bond strength preservation in artificially-created caries-affected primary dentin. Int. J. Adhes. Adhes. 2014;54:51–56. doi: 10.1016/j.ijadhadh.2014.04.007. [DOI] [Google Scholar]
- 24.Aras S., Küçükeçmen H.C., Öaroǧlu S.I. Deproteinization treatment on bond strengths of primary, mature and immature permanent tooth enamel. J. Clin. Pediatric Dent. 2013;37:275–280. doi: 10.17796/jcpd.37.3.252n428508q2w204. [DOI] [PubMed] [Google Scholar]
- 25.Oznurhan F., Ozturk C., Ekci E.S. Effects of different cavity-disinfectants and potassium titanyl phosphate laser on microtensile bond strength to primary dentin. Niger. J. Clin. Pract. 2015;18:400–404. doi: 10.4103/1119-3077.151774. [DOI] [PubMed] [Google Scholar]
- 26.Yildiz E., Karaarslan E.S., Simsek M., Cebe F., Ozsevik A.S., Ozturk B. Effect of a re-wetting agent on bond strength of an adhesive to primary and permanent teeth dentin after different etching techniques. Niger. J. Clin. Pract. 2015;18:364–370. doi: 10.4103/1119-3077.151786. [DOI] [PubMed] [Google Scholar]
- 27.Bahrololoomi Z., Dadkhah A., Alemrajabi M. The Effect of Er: YAG laser irradiation and different concentrations of sodium hypochlorite on shear bond strength of composite to primary teeth’s dentin. J. Lasers Med. Sci. 2017;8:29–35. doi: 10.15171/jlms.2017.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebrahimi M., Naseh A., Abdollahi M., Shirazi A.S. Can chlorhexidine enhance the bond strength of self-etch and etch-and-rinse systems to primary teeth dentin? J. Contemp. Dent. Pract. 2018;19:404–408. [PubMed] [Google Scholar]
- 29.Mohammadi N., Parsaie Z., Jafarpour D., Bizolm F. Effect of different matrix metalloproteinase inhibitors on shear bond strength of composite attached to primary teeth dentin. Eur. J. Gen. Dent. 2020;9:147–151. doi: 10.4103/ejgd.ejgd_167_20. [DOI] [Google Scholar]
- 30.Manfro A.R.G., Reis A., Loguercio A.D., Imparato J.C.P., Raggio D.P. Effect of different concentrations of chlorhexidine on bond strength of primary dentin. Pediatric Dent. 2012;34:11E–15E. [PubMed] [Google Scholar]
- 31.Ricci H.A., Sanabe M.E., Costa C.A.S., Pashley D.H., Hebling J. Chlorhexidine increases the longevity of in vivo resin-dentin bonds. Eur. J. Oral Sci. 2010;118:411–416. doi: 10.1111/j.1600-0722.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- 32.Jowkar Z., Farpour N., Koohpeima F., Mokhtari M.J., Shafiei F. Effect of silver nanoparticles, zinc oxide nanoparticles and titanium dioxide nanoparticles on microshear bond strength to enamel and dentin. J. Contemp. Dent. Pract. 2018;19:1405–1412. [PubMed] [Google Scholar]
- 33.Koutsi V., Noonan R.G., Horner J.A., Simpson M.D., Matthews W.G., Pashley D.H. The effect of dentin depth on the permeability and ultrastructure of primary molars. Pediatric Dent. 1994;16:29–35. [PubMed] [Google Scholar]
- 34.Dourda A.O., Moule A.J., Young W.G. A morphometric analysis of the cross-sectional area of dentine occupied by dentinal tubules in human third molar teeth. Int. Endod. J. 1994;27:184–189. doi: 10.1111/j.1365-2591.1994.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto M., Ohno H., Endo K., Kaga M., Sano H., Oguchi H. The effect of hybrid layer thickness on bond strength: Demineralized dentin zone of the hybrid layer. Dent. Mater. 2000;16:406–411. doi: 10.1016/S0109-5641(00)00035-X. [DOI] [PubMed] [Google Scholar]
- 36.Angker L., Nockolds C., Swain M.V., Kilpatrick N. Quantitative analysis of the mineral content of sound and carious primary dentine using BSE imaging. Arch. Oral Biol. 2004;49:99–107. doi: 10.1016/j.archoralbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Nor J., Dennison J., Edwardsa C., Feigal R. Dentin bonding: SEM comparison teeth. Am. Acad. Pediatric Dent. 1997;19:246–252. [PubMed] [Google Scholar]
- 38.Sung E.C., Chenard T., Caputo A.A., Amodeo M., Chung E.M., Rizoiu I.M. Composite resin bond strength to primary dentin prepared with ER, CR: YSSG laser. J. Clin. Pediatric Dent. 2005;30:45–50. doi: 10.17796/jcpd.30.1.el385u211tnu2574. [DOI] [PubMed] [Google Scholar]
- 39.ISO/TS 11405:2015 Dental Materials—Testing of Adhesion to Tooth Structure. International Organisation for Standardization; Geneva, Switzerland: 2015. [Google Scholar]
- 40.Uceda-Gómez N., Reis A., Carrilho M.R.O., Loguercio A.D., Filho L.E.R. Effect of sodium hypochlorite on the bond strength of an adhesive system to superficial and deep dentin. J. Appl. Oral Sci. 2003;11:223–228. doi: 10.1590/S1678-77572003000300012. [DOI] [PubMed] [Google Scholar]
- 41.Ramos R.P., Chimello D.T., Chinelatti M.A., Nonaka T., Pécora J.D., Dibb R.G.P. Effect of Er: YAG laser on bond strength to dentin of a self-etching primer and two single-bottle adhesive systems. Lasers Surg. Med. 2002;31:164–170. doi: 10.1002/lsm.10106. [DOI] [PubMed] [Google Scholar]
- 42.Yu H.H., Zhang L., Yu F., Li F., Liu Z.Y., Chen J.H. Epigallocatechin-3-gallate and Epigallocatechin-3-O-(3-O-methyl)-gallate Enhance the Bonding Stability of an Etch-and-Rinse Adhesive to Dentin. Materials. 2017;10:183. doi: 10.3390/ma10020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pashley D.H., Carvalho R.M. Dentine permeability and dentine adhesion. J. Dent. 1997;25:355–372. doi: 10.1016/S0300-5712(96)00057-7. [DOI] [PubMed] [Google Scholar]
- 44.Pashley E.L., Talman R., Horner J.A., Pashley D.H. Permeability of normal versus carious dentin. Dent. Traumatol. 1991;7:207–211. doi: 10.1111/j.1600-9657.1991.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 45.Swift E.J.J. Dentin/enamel adhesives: Review of the literature. Pediatric Dent. 2002;24:456–461. [PubMed] [Google Scholar]
- 46.Powers J.M., O’Keefe K.L., Pinzon L.M. Factores affecting in vitro bond strength of bonding agents to human dentin. Odontology. 2003;91:1–6. doi: 10.1007/s10266-003-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carvalho R.M., Mendonça J.S., Santiago S.L., Silveira R.R., Garcia F.C.P., Tay F.R., Pashley D.H. Effects of HEMA/Solvent combinations on bond strength to dentin. J. Dent. Res. 2003;82:597–601. doi: 10.1177/154405910308200805. [DOI] [PubMed] [Google Scholar]
- 48.Guo J., Wang L., Zhu J., Yang J., Zhu H. Impact of dentinal tubule orientation on dentin bond strength. Curr. Med. Sci. 2018;38:721–726. doi: 10.1007/s11596-018-1936-8. [DOI] [PubMed] [Google Scholar]
- 49.Lima D.M., Candido M.S.M. Effect of dentin on the shear bond strength of different adhesive systems. Rev. Gaúcha Odontol. 2012;60:149–161. [Google Scholar]
- 50.Santana F.R., Pereira J.C., Pereira C.A., Neto F.A.J., Soares C.J. Influence of method and period of storage on the microtensile bond strength of indirect composite resin restorations to dentine. Braz. Oral Res. 2008;22:352–357. doi: 10.1590/S1806-83242008000400012. [DOI] [PubMed] [Google Scholar]
- 51.ISO 3696:1987—Water for Analytical Laboratory Use—Specification and Test Methods. International Organisation for Standardization; Geneva, Switzerland: 1987. [Google Scholar]
- 52.Kang H.J., Moon H.J., Shin D.H. Effect of different chlorhexidine application times on microtensile bond strength to dentin in Class I cavities. Restor. Dent. Endod. 2012;37:9. doi: 10.5395/rde.2012.37.1.9. [DOI] [Google Scholar]
- 53.Coelho A., Paula A., Carrilho T., Silva M.J., Botelho M.F., Carrilho E. Chlorhexidine mouthwash as an anticaries agent: A systematic review. Quintessence Int. 2017;48:585–591. doi: 10.3290/j.qi.a38353. [DOI] [PubMed] [Google Scholar]
- 54.Haydari M., Bardakci A.G., Koldsland O.C., Aass A.M., Sandvik L., Preus H.R. Comparing the effect of 0.06%, 0.12% and 0.2% Chlorhexidine on plaque, bleeding and side effects in an experimental gingivitis model: A parallel group, double masked randomized clinical trial. BMC Oral Health. 2017;17:1–8. doi: 10.1186/s12903-017-0400-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kandaswamy S.K., Sharath A., Priya P.G. Comparison of the Effectiveness of Probiotic, Chlorhexidine-based Mouthwashes, and Oil Pulling Therapy on Plaque Accumulation and Gingival Inflammation in 10- to 12-year-old Schoolchildren: A Randomized Controlled Trial. Int. J. Clin. Pediatric Dent. 2018;11:66–70. doi: 10.5005/jp-journals-10005-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hebling J., Pashley D.H., Tjäderhane L., Tay F.R. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J. Dent. Res. 2005;84:741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 57.Pashley D.H., Tay F.R., Yiu C., Hashimoto M., Breschi L., Carvalho R.M., Ito S. Collagen Degradation by Host-derived Enzymes during Aging. J. Dent. Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 58.Coelho A., Amaro I., Rascão B., Marcelino I., Paula A., Saraiva J., Spagnuolo G., Ferreira M.M., Marto C.M., Carrilho E. Effect of Cavity Disinfectants on Dentin Bond. Strength and Clinical Success of Composite Restorations—A Systematic Review of In Vitro, In Situ and Clinical Studies. Int. J. Mol. Sci. 2020;22:353. doi: 10.3390/ijms22010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pappas M., Burns D.R., Moon P.C., Coffey J.P. Influence of a 3-step tooth disinfection procedure on dentin bond strength. J. Prosthet. Dent. 2005;93:545–550. doi: 10.1016/j.prosdent.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Carrilho M.R.O., Carvalho R.M., Goes M.F., Hipólito V., Geraldeli S., Tay F.R., Pashley D.H., Tjäderhane L. Chlorhexidine preserves dentin bond in vitro. J. Dent. Res. 2007;86:90–94. doi: 10.1177/154405910708600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salles M.M., Badaró M.M., Arruda C.N.F., Leite V., Silva C., Watanabe E., Oliveira V., Paranhos H. Antimicrobial activity of complete denture cleanser solutions based on sodium hypochlorite and Ricinus communis—A randomized clinical study. J. Appl. Oral Sci. 2015;23:637–6342. doi: 10.1590/1678-775720150204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Estrela C., Estrela C.R.A., Decurcio D.A., Hollanda A.C.B., Silva J.A. Antimicrobial efficacy of ozonated water, gaseous ozone, sodium hypochlorite and chlorhexidine in infected human root canals. Int. Endod. J. 2007;40:85–93. doi: 10.1111/j.1365-2591.2006.01185.x. [DOI] [PubMed] [Google Scholar]
- 63.Arslan S., Ozbilge H., Kaya E.G., Er O. In vitro antimicrobial activity of propolis, BioPure MTAD, sodium hypochlorite, and chlorhexidine on Enterococcus faecalis and Candida albicans. Saudi Med. J. 2011;32:479–483. [PubMed] [Google Scholar]
- 64.Cha H.S., Shin D.H. Antibacterial capacity of cavity disinfectants against Streptococcus mutans and their effects on shear bond strength of a self-etch adhesive. Dent. Mater. J. 2016;35:147–152. doi: 10.4012/dmj.2015-175. [DOI] [PubMed] [Google Scholar]
- 65.Ahuja B., Yeluri R., Baliga S., Munshi A.K. Enamel deproteinization before acid etching--a scanning electron microscopic observation. J. Clin. Pediatric Dent. 2010;35:169–172. doi: 10.17796/jcpd.35.2.9gw7147381836380. [DOI] [PubMed] [Google Scholar]
- 66.Hibst R., Keller U. Experimental studies of the application of the Er: YAG laser on dental hard substances: I. Measurement of the ablation rate. Lasers Surg. Med. 1989;9:338–344. doi: 10.1002/lsm.1900090405. [DOI] [PubMed] [Google Scholar]
- 67.Franke M., Taylor A.W., Lago A., Fredel M.C. Influence of Nd: YAG laser irradiation on an adhesive restorative procedure. Oper. Dent. 2006;31:604–609. doi: 10.2341/05-110. [DOI] [PubMed] [Google Scholar]
- 68.Keller U., Hibst R. Experimental studies of the application of the Er: YAG laser on dental hard substances: II. Light microscopic and SEM investigations. Lasers Surg. Med. 1989;9:345–351. doi: 10.1002/lsm.1900090406. [DOI] [PubMed] [Google Scholar]
- 69.Tokonabe H., Kouji R., Watanabe H., Nakamura Y., Matsumoto K. Morphological changes of human teeth with Er: YAG laser irradiation. J. Clin. Laser Med. Surg. 1999;17:7–12. doi: 10.1089/clm.1999.17.7. [DOI] [PubMed] [Google Scholar]
- 70.Nelson D.G., Jongebloed W.L., Featherstone J.D. Laser irradiation of human dental enamel and dentine. N. Z. Dent. J. 1986;82:74–77. [PubMed] [Google Scholar]
- 71.Hossain M., Nakamura Y., Yamada Y., Kimura Y., Nakamura G., Matsumoto K. Ablation depths and morphological changes in human enamel and dentin after Er: YAG laser irradiation with or without water mist. J. Clin. Laser Med. Surg. 1999;17:105–109. doi: 10.1089/clm.1999.17.105. [DOI] [PubMed] [Google Scholar]
- 72.Armengol V., Jean A., Rohanizadeh R., Hamel H. Scanning electron microscopic analysis of diseased and healthy dental hard tissues after Er: YAG laser irradiation: In vitro study. J. Endod. 1999;25:543–546. doi: 10.1016/S0099-2399(99)80376-8. [DOI] [PubMed] [Google Scholar]
- 73.Gonçalves M., Corona S.A.M., Palma-Dibb R.G., Pécora J.D. Influence of pulse repetition rate of Er: YAG laser and dentin depth on tensile bond strength of dentin-resin interface. J. Biomed. Mater. Res. 2008;86:477–482. doi: 10.1002/jbm.a.31636. [DOI] [PubMed] [Google Scholar]
- 74.Ferreira L.S., Apel C., Francci C., Simoes A., Eduardo C.P., Gutknecht N. Influence of etching time on bond strength in dentin irradiated with erbium lasers. Lasers Med. Sci. 2010;25:849–854. doi: 10.1007/s10103-009-0715-y. [DOI] [PubMed] [Google Scholar]
- 75.Martínez-Insua A., Dominguez L.S., Rivera F.G., Santana-Penín U.A. Differences in bonding to acid-etched or Er: YAG-laser-treated enamel and dentin surfaces. J. Prosthet. Dent. 2000;84:280–288. doi: 10.1067/mpr.2000.108600. [DOI] [PubMed] [Google Scholar]
- 76.Munck J., Meerbeek B., Yudhira R., Lambrechts P., Vanherle G. Micro-tensile bond strength of two adhesives to Erbium:YAG-lased vs. bur-cut enamel and dentin. Eur. J. Oral Sci. 2002;110:322–329. doi: 10.1034/j.1600-0722.2002.21281.x. [DOI] [PubMed] [Google Scholar]
- 77.Polydorou O., Pelz K., Hahn P. Antibacterial effect of an ozone device and its comparison with two dentin-bonding systems. Eur. J. Oral Sci. 2006;114:349–353. doi: 10.1111/j.1600-0722.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- 78.Bocci V.A. Scientific and medical aspects of ozone therapy. State of the art. Arch. Med. Res. 2006;37:425–435. doi: 10.1016/j.arcmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Nagayoshi M., Kitamura C., Fukuizumi T., Nishihara T., Terashita M. Antimicrobial effect of ozonated water on bacteria invading dentinal tubules. J. Endod. 2004;30:778–781. doi: 10.1097/00004770-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 80.Baysan A., Lynch E. Effect of ozone on the oral microbiota and clinical severity of primary root caries. Am. J. Dent. 2004;17:56–60. [PubMed] [Google Scholar]
- 81.Baysan A., Whiley R.A., Lynch E. Antimicrobial effect of a novel ozone-generating device on micro-organisms associated with primary root carious lesions in vitro. Caries Res. 2000;34:498–501. doi: 10.1159/000016630. [DOI] [PubMed] [Google Scholar]
- 82.Castillo A., Galindo-Moreno P., Avila G., Valderrama M., Liébana J., Baca P. In vitro reduction of mutans streptococci by means of ozone gas application. Quintessence Int. 2008;39:827–831. [PubMed] [Google Scholar]
- 83.Fagrell T.G., Dietz W., Lingström P., Steiniger F., Norén J.G. Effect of ozone treatment on different cariogenic microorganisms in vitro. Swed. Dent. J. 2008;32:139–147. [PubMed] [Google Scholar]
- 84.Wang J., Song W., Zhu L., Wei X. A comparative study of the microtensile bond strength and microstructural differences between sclerotic and Normal dentine after surface pretreatment. BMC Oral Health. 2019;19:1–10. doi: 10.1186/s12903-019-0899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thompson J.M., Agee K., Sidow S., McNally K., Lindsey K., Borke J., Elsalanty M., Tay F.R. Inhibition of endogenous dentin matrix metalloproteinases by ethylenediaminetetraacetic acid. J. Endod. 2012;38:62–65. doi: 10.1016/j.joen.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Youm S.H., Jung K.H., Son S.A., Kwon Y.H., Park J.K. Effect of dentin pretreatment and curing mode on the microtensile bond strength of self-adhesive resin cements. J. Adv. Prosthodont. 2015;7:317–322. doi: 10.4047/jap.2015.7.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;62:1–34. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Faggion C.M. Guidelines for reporting pre-clinical in vitro studies on dental materials. J. Evid. Based Dent. Pract. 2012;12:182–189. doi: 10.1016/j.jebdp.2012.10.001. [DOI] [PubMed] [Google Scholar]