Abstract
Crystal-storing histiocytosis (CSH) is a rare event in disorders associated with monoclonal gammopathy and is mostly associated with the accumulation of immunoglobulins (Igs) in the cytoplasm of histiocytes. In this article, we present a case of a 75-year-old female with IgG kappa monoclonal gammopathy of undetermined significance (MGUS) and signs of a non-crystallized version of immunoglobulin-storing histiocytosis (IgSH) in a vertebra corpus. Furthermore, we performed a literature review based on all cases of storing histiocytosis identified by literature search between 1987 and 2020 and identified 140 cases in total. The median age at diagnosis was 60 years (range 18–91), with an equal sex distribution (51% men). The majority of the patients had an underlying neoplastic B-cell disorder, most often multiple myeloma (MM), MGUS, or lymphoplasmacytic lymphoma (LPL). The main affected organ systems or tissue sites were bone (n = 52), followed by head and neck (n = 31), kidney (n = 23), lung (n = 20), and gastrointestinal (GI)-tract (n = 18). IgG was the main immunoglobulin class involved, and most cases were associated with kappa light chain expression. We conclude that IgSH is a rare disease entity but should be considered with unusual findings in several organ systems associated with monoclonal gammopathy, especially with kappa light chain expression.
Keywords: storing histiocytosis, crystal-storing histiocytosis, immunoglobulin, MGUS 5, B-cell neoplasia
1. Introduction
Crystal-storing histiocytosis (CSH) is a rare disorder characterized by the accumulation of crystallized deposits in the cytoplasm of histiocytes. The entity has been associated with underlying lymphoproliferative or plasma cell disorders, such as monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma (MM), or lymphoplasmacytic lymphoma (LPL) [1,2,3]. The histiocytes in CSH contain crystallized material, although there have been reported cases of immunoglobulin-storing histiocytosis (IgSH) without a crystallized pattern of the deposited immunoglobulins [4,5]. IgSH can present in both a localized and a generalized form and may include a wide range of tissue sites and organs [1,2]. Herein, we present a patient with increasing back pain after a minor injury. She was diagnosed with a localized form of non-crystallized IgSH. We discuss the clinical findings, diagnostic workup, and therapeutic options. Furthermore, we performed a systematic review of the literature regarding IgSH and summarized the findings in this rare disease entity.
2. Case Report
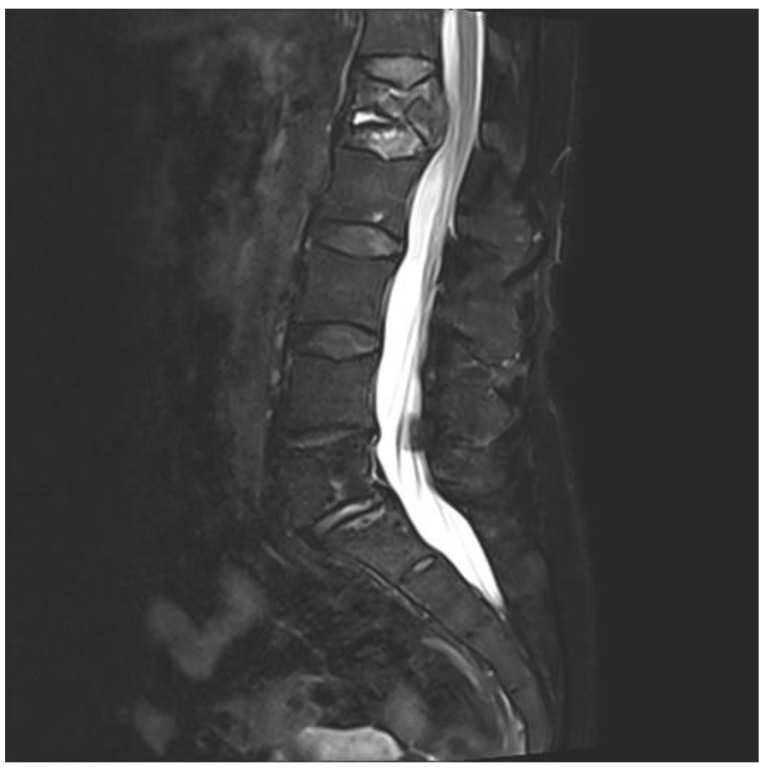
A 75-year-old woman presented with acute pain in the lower back after a trivial incident. Her past medical history included hypertension, hypercholesterolemia, type 2 diabetes mellitus, hypothyroidism, migraine, and arrhythmia. Initial radiological examinations demonstrated no fracture. However, over the next few weeks, her back pain increased. By magnetic resonance imaging (MRI), she was diagnosed with a compression fracture in L1, although with no spinal stenosis (Figure 1). The fracture was initially managed conservatively; however, she had persistent opioid-dependent lower back pain and was admitted to the orthopedic department for further clinical and laboratory diagnostic workup to exclude a pathologic etiology for her fracture.
Figure 1.
Magnetic resonance imaging (MRI) of lumbar column. The figure demonstrating collapse and fracture of vertebra corpus L1, with edema and compression against the spinal cord.
When blood tests revealed anemia, elevated sedimentation rate (SR), and a monoclonal (M)-protein by serum electrophoresis (Table 1), she was transferred to the hematology unit, as MM or other plasma cell dyscrasia was suspected. A bone marrow aspirate was performed and demonstrated a slightly hypocellular bone marrow with megakaryocytes present. There was normal maturation in the erythrocytopoiesis and the granulocytopoiesis, without expansion of lymphoid cells. The plasma cells accounted for 4% of nucleated marrow cells. Furthermore, whole-body low dose computed tomography (CT) for assessment of osteolytic lesion as part of MM was performed without detecting other lesions. Hence, the patient did not fulfill the diagnostic criteria for MM. However, based on the findings of monoclonal gammopathy by serum electrophoresis and the absence of findings supporting MM, she was diagnosed with MGUS. For further diagnostic workup, a CT-guided biopsy of the lesion in L1 was performed.
Table 1.
Diagnostic blood test from the patients.
| Analysis | Values | References |
|---|---|---|
| Hemoglobin (g/dL) | 10.1 | 11.7–15.3 |
| EVF | 0.33 | 0.35–0.46 |
| CRP (mg/L) | 18 | <5 |
| SR (mm/t) | 69 | 1–30 |
| Creatinine (µmol/t) | 78 | 45–90 |
| Protein (g/L) | 69 | 62–78 |
| IgG (g/L) | 16.1 | 6.0–15.3 |
| IgA (g/L) | 0.76 | 0.8–4.0 |
| IgM (g/L) | 0.51 | 0.3–2.30 |
| Kappa free light chains (mg/L) | 54.0 | 6.7–22.4 |
| Lambda free light chains (mg/L) | 25.0 | 8.3–27.0 |
| Ratio kappa/lambda free light chains | 2.16 | 0.31–1.56 |
| S-protein electrophoresis | Monoclonal band | |
| S-immunofixation | Monoclonal band type IgG kappa. | |
| M-protein (mg/L) | 7.1 | 0 |
The table demonstrates different analyses performed, the obtained value, and the given reference area. Abbreviations: EVF, erythrocyte volume fraction; CRP, C-reactive protein; SR, sedimentation rate; Ig, immunoglobulin.
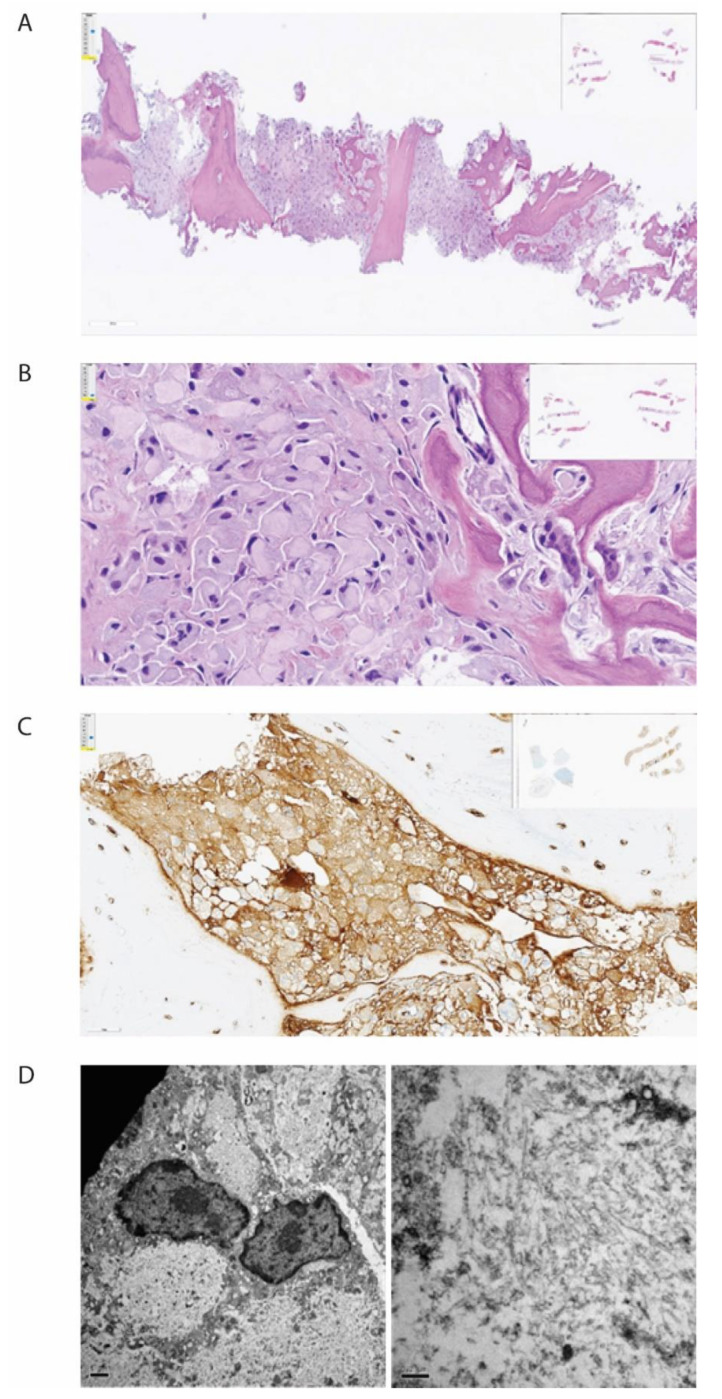
The bone biopsy showed only sparse monoclonal plasma cells, but vast amounts of deposits intracellularly stored in histiocytes, inconsistent with amyloid (Figure 2). In immunohistochemical staining for light chains, the deposits were somewhat stronger positive for kappa than for lambda. In additional stains, IgG was colocalized with kappa and CD68, making it probable that the deposits consisted of whole immunoglobulins. By electron microscopic examination, there was abundant bright, predominantly amorphous material, but also some tubular structures with a diameter between 25–30 nm. No relation to cell organs was seen. Additionally, small amounts of electron-dense amorphous material were found. Attempts with mass spectrometry gave no conclusive results.
Figure 2.
Histopathological examination of biopsy from corpus vertebrae. The figure demonstrates the biopsy from the patient’s corpus vertebrae L1. (A) Hematoxylin-eosin stain in low magnification demonstrating bone repair and bone marrow with the absence of organized hematopoiesis and maximal infiltration by histiocytes. (B) Hematoxylin-eosin stain in higher magnification demonstrating histiocytes with intracellular amorphous material. (C) Immunohistochemical staining for kappa light chain, demonstrating positivity in the histiocytes (brown color). (D) Electron microscopy, low magnification, showing bright intracellular material (left) and high magnification, showing no fibrils but spread tiny tubules (right).
After clinical, radiological, histopathological, and biochemical examination, it was concluded that the patient had MGUS with secondary IgSH, without any other underlying malignant disease. The patient had no sure sign of CRAB-criteria associated with MM: hypercalcemia, renal failure, anemia with Hb < 10 g/dL, or osteolytic bone disease. The patient, on the other hand, had persistent pain and difficulty moving, and she was, therefore, accepted for surgical intervention with vertebroplasty. She was operated on with the insertion of pedicle screws in vertebra corpora Th12 and L2, followed by repositioning of the fracture in L1 and the insertion of cement in the corpora with access via the pedicles bilaterally. The peri- and postoperative courses were without complications. The patient had striking relief of pain after surgery; she was no longer dependent on analgesics and could resume normal daily life activities. The current follow-up by the back surgeon and hematologist for her MGUS does not show signs of disease progression.
3. Literature Review
3.1. Methods and Classification
We performed a systematic PubMed search with the term “Storing Histiocytosis” in the title and identified 107 relevant published articles from 1987 to July 2020. The term “Storing Histiocytosis” was used to include both CSH and IgSH. Only cases with sufficient information written in English were included, and six articles were thereby rejected. From 101 articles, we identified 140 cases of assumed IgSH (Table 2). Other data collected were the type of material deposited within histiocytes, age, gender, involved sites, type of immunoglobulin if available, and the presence or not of an underlying lymphoproliferative disease or plasma cell dyscrasia (Table 2).
Table 2.
Overview of literature search.
| Case nr. | Organs/Tissue Sites | Age | Sex | Type | LP–PCD | Material within Histiocytes (ISH) | Serum Immunofixation | Ref. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Bonemarrow | Kidney | GI-Tract | Liver | Abdomen unsp. | Heart | Thymus | Breast | Lung | Spleen | Head an Neck | Lymph Node | ||||||||
| 1 | 71 | M | L | MGUS | NA | IgG kappa | [6] | |||||||||||||
| 2 | 57 | M | G | LPL | IgM/IgG/IgD kappa/lambda | IgG kappa | [7] | |||||||||||||
| 3 | 56 | F | L | MM | Kappa | IgG kappa | [8] | |||||||||||||
| 4 | 56 | M | L | MALT lymphoma | Kappa | NA | [9] | |||||||||||||
| 5 | 75 | F | G | MM | Kappa | IgG kappa | [10] | |||||||||||||
| 6 | 66 | F | L | MM | Kappa | IgA kappa | [11] | |||||||||||||
| 7 | 86 | M | L | MZL | Negative | IgM | ||||||||||||||
| 8 | 68 | F | L | NA | Kappa | Kappa | [12] | |||||||||||||
| 9 | 49 | M | G | MM | NA | NA | ||||||||||||||
| 10 | 74 | F | G | DLBCL | IgM kappa | IgM kappa | [13] | |||||||||||||
| 11 | 83 | F | L | MM | Kappa | NA | [14] | |||||||||||||
| 12 | 73 | F | G | MGUS | NA | IgG kappa | [15] | |||||||||||||
| 13 | 79 | M | L | MM | Negative | NA | [16] | |||||||||||||
| 14 | 71 | M | L | LPL | IgM Kappa | IgM kappa | [17] | |||||||||||||
| 15 | 67 | M | L | MGUS | IgG kappa | IgG kappa | ||||||||||||||
| 16 | 74 | M | L | MM | IgG kappa | IgG kappa | ||||||||||||||
| 17 | 65 | M | G | None | Negative | IgG kappa | [18] | |||||||||||||
| 18 | 72 | F | L | MM | Kappa | Kappa | [19] | |||||||||||||
| 19 | 36 | M | L | None | Kappa | Negative | [1] | |||||||||||||
| 20 | 63 | M | L | B-cell lymphoma | NA | IgM Kappa | [2] | |||||||||||||
| 21 | 40 | M | L | MM | Kappa | Kappa | ||||||||||||||
| 22 | 71 | M | G | MM | Kappa | IgG kappa | ||||||||||||||
| 23 | 65 | F | L | MM | Kappa | IgA Kappa | ||||||||||||||
| 24 | 70 | M | G | MM | NA | IgG Kappa | ||||||||||||||
| 25 | 73 | M | L | MGUS | NA | IgG Kappa | ||||||||||||||
| 26 | 56 | M | L | MM | NA | IgG Kappa | ||||||||||||||
| 27 | 60 | M | G | MM | Kappa | IgG kappa | [20] | |||||||||||||
| 28 | 48 | M | L | MM | Kappa | Kappa | [21] | |||||||||||||
| 29 | 70 | M | L | MM | NA | IgG kappa | [22] | |||||||||||||
| 30 | 36 | F | L | MALT lymphoma | Kappa | IgG | [23] | |||||||||||||
| 31 | 27 | F | L | EMZL | Lambda | Lambda | [24] | |||||||||||||
| 32 | 67 | M | L | MGRS | Kappa | IgG kappa | [25] | |||||||||||||
| 33 | 51 | M | G | MM | IgG/lambda/kappa | IgG lambda | [26] | |||||||||||||
| 34 | 75 | M | L | MM | IgG kappa | IgG kappa | ||||||||||||||
| 35 | 46 | F | L | MM | NA | IgA kappa | ||||||||||||||
| 36 | 74 | M | L | LPL | IgM | IgM | ||||||||||||||
| 37 | 63 | M | L | LPL | Lambda | IgM lambda | ||||||||||||||
| 38 | 79 | M | L | LPL | Kappa | IgM kappa | ||||||||||||||
| 39 | 43 | M | G | EMZL | IgA kappa | Negative | ||||||||||||||
| 40 | 63 | F | L | EMZL | IgM lambda | NA | ||||||||||||||
| 41 | 50 | F | L | EMZL | IgM lambda | NA | ||||||||||||||
| 42 | 33 | F | L | EMZL | Kappa | NA | ||||||||||||||
| 43 | 73 | F | L | EMZL | NA | NA | ||||||||||||||
| 44 | 58 | M | L | EMZL | NA | NA | ||||||||||||||
| 45 | 68 | F | L | SMZL | Lambda | Negative | ||||||||||||||
| 46 | 56 | M | L | None | NA | Negative | [27] | |||||||||||||
| 47 | 58 | M | L | EMZL | Kappa | NA | [28] | |||||||||||||
| 48 | 46 | M | L | MM | Lambda | NA | [29] | |||||||||||||
| 49 | 62 | F | L | EMZL | IgM | NA | [30] | |||||||||||||
| 50 | 80 | F | L | LPL | NA | IgM kappa | [31] | |||||||||||||
| 51 | 52 | M | L | MM | Negative | IgG kappa | ||||||||||||||
| 52 | 57 | F | L | MM | Kappa | IgA kappa | [32] | |||||||||||||
| 53 | 53 | F | G | LPL | NA | IgM lambda | [33] | |||||||||||||
| 54 | 69 | M | G | MGUS | NA | Kappa | [34] | |||||||||||||
| 55 | NA | M | L | None | NA | NA | [35] | |||||||||||||
| 56 | 71 | F | L | MALT lymphoma | IgG kappa | Negative | [36] | |||||||||||||
| 57 | 43 | F | L | MGUS | Neg | IgD kappa | [37] | |||||||||||||
| 58 | 91 | F | G | EMZL | IgM/IgG/kappa | IgM kappa | [38] | |||||||||||||
| 59 | 77 | M | L | MZL | Kappa | Negative | [39] | |||||||||||||
| 60 | 53 | M | L | MALT lymphoma | NA | NA | [40] | |||||||||||||
| 61 | 38 | F | L | MM | IgA kappa | IgA kappa | [41] | |||||||||||||
| 62 | 30 | F | L | None | NA | Negative | [42] | |||||||||||||
| 63 | 54 | F | L | MALT lymphoma | Kappa | NA | [43] | |||||||||||||
| 64 | 72 | F | L | BPDCN | NA | Negative | [44] | |||||||||||||
| 65 | 32 | M | L | EMZL | NA | NA | [45] | |||||||||||||
| 66 | 55 | F | L | None | IgA/IgG/IgM/kappa/lambda | Negative | [46] | |||||||||||||
| 67 | 54 | F | L | MGUS | Kappa | NA | [47,48] | |||||||||||||
| 68 | 89 | F | L | MZL | Kappa | NA | ||||||||||||||
| 69 | 50 | F | L | MZL | Kappa | NA | ||||||||||||||
| 70 | 63 | M | L | MM | Kappa | NA | ||||||||||||||
| 71 | 68 | M | L | MGUS | IgG kappa | IgG kappa | ||||||||||||||
| 72 | 78 | F | L | MGUS | NA | NA | [49] | |||||||||||||
| 73 | 80 | M | L | None | NA | Negative | [50] | |||||||||||||
| 74 | 20 | F | L | None | Lambda | NA | [51] | |||||||||||||
| 75 | 62 | F | G | MGUS | NA | IgG kappa | [52] | |||||||||||||
| 76 | 64 | M | L | EMZL | NA | NA | [53] | |||||||||||||
| 77 | 57 | F | L | EMZL | NA | NA | ||||||||||||||
| 78 | 48 | F | G | MGUS | Kappa | IgG kappa | [54] | |||||||||||||
| 79 | 51 | F | L | MGUS | IgM/IgG | NA | [55] | |||||||||||||
| 80 | 38 | M | L | None | IgG/kappa/lambda | Negative | [56] | |||||||||||||
| 81 | 32 | F | L | None | Kappa | NA | [57] | |||||||||||||
| 82 | 50 | F | L | LPL | NA | IgM kappa | [58] | |||||||||||||
| 83 | 67 | M | L | MM | IgG | IgG | [59] | |||||||||||||
| 84 | 27 | F | L | None | NA | NA | [60] | |||||||||||||
| 85 | 54 | F | L | EMZL | Kappa | NA | [61] | |||||||||||||
| 86 | 75 | F | L | MGUS | NA | IgG kappa | [62] | |||||||||||||
| 87 | 63 | M | L | MM | NA | IgG kappa | [63] | |||||||||||||
| 88 | 53 | F | L | MALT lymphoma | Non-crystallized IgG kappa | IgA kappa | [5] | |||||||||||||
| 89 | 52 | M | L | MGUS | NA | IgM kappa | [64] | |||||||||||||
| 90 | 70 | M | L | DLBCL | NA | IgM kappa | ||||||||||||||
| 91 | 65 | M | L | MGUS | NA | IgG kappa | ||||||||||||||
| 92 | 64 | M | L | MG | Negative | IgG kappa | [65] | |||||||||||||
| 93 | 76 | F | L | MZL | IgM lambda | IgM lambda | [66] | |||||||||||||
| 94 | 66 | M | G | MM | NA | IgG kappa | [67] | |||||||||||||
| 95 | 66 | M | L | MGUS | Lambda | IgG lambda | [68] | |||||||||||||
| 96 | 65 | M | G | None | NA | Kappa | [69] | |||||||||||||
| 97 | 54 | M | L | MM | NA | IgG kappa | [70] | |||||||||||||
| 98 | 81 | F | L | MALT lymphoma | IgM kappa | Negative | [71] | |||||||||||||
| 99 | 56 | F | L | None | Kappa/lambda | Negative | [72] | |||||||||||||
| 100 | 52 | M | L | MM | Non-crystallized kappa | NA | [4] | |||||||||||||
| 101 | 41 | M | L | MM | NA | IgD kappa | [73] | |||||||||||||
| 102 | 62 | F | L | MM | Kappa | IgG kappa | [74] | |||||||||||||
| 103 | 70 | F | L | None | Kappa | Kappa | [75] | |||||||||||||
| 104 | 79 | F | L | MM | IgA kappa | IgA kappa | [76] | |||||||||||||
| 105 | 69 | F | L | MALT lymphoma | NA | Negative | [77] | |||||||||||||
| 106 | 49 | M | G | MGUS | Kappa | IgG kappa | [78] | |||||||||||||
| 107 | 72 | F | G | MM | Kappa | IgA kappa | ||||||||||||||
| 108 | 62 | F | L | MM | NA | IgG kappa | [79] | |||||||||||||
| 109 | 74 | F | L | MM | Negative | IgA lambda | [80] | |||||||||||||
| 110 | 51 | M | G | MM | IgG kappa | Kappa | [81] | |||||||||||||
| 111 | 59 | M | L | EMZL | IgG/IgM/kappa/lambda | NA | [82] | |||||||||||||
| 112 | 58 | M | L | MZL | Lambda | IgM lambda | [83] | |||||||||||||
| 113 | 73 | M | G | MM | IgA kappa | IgA kappa | [3] | |||||||||||||
| 114 | 62 | F | L | EMZL | Kappa | Negative | [84] | |||||||||||||
| 115 | 48 | M | L | MM | IgA kappa | IgA kappa | [85] | |||||||||||||
| 116 | 53 | M | L | MM | IgA kappa | Negative | ||||||||||||||
| 117 | 50 | M | G | MM | IgG kappa | Negative | ||||||||||||||
| 118 | 77 | F | L | MM | Kappa | Negative | ||||||||||||||
| 119 | 66 | M | L | MGUS | IgA kappa | IgA kappa | ||||||||||||||
| 120 | 66 | M | G | LPL | IgM kappa | Negative | ||||||||||||||
| 121 | 68 | F | L | LPL | IgM kappa | IgM/IgG/kappa | ||||||||||||||
| 122 | 53 | F | L | LPL | IgM kappa | IgM kappa | ||||||||||||||
| 123 | 70 | M | G | LPL | IgM kappa | NA | ||||||||||||||
| 124 | 70 | M | G | LPL | IgM lambda | NA | ||||||||||||||
| 125 | 35 | F | L | LPL | IgA kappa | IgG lambda | ||||||||||||||
| 126 | 54 | F | L | None | IgG lambda | NA | ||||||||||||||
| 127 | 72 | F | L | LPL | IgM kappa | NA | [86] | |||||||||||||
| 128 | 44 | M | G | LPL | IgG kappa | IgG kappa | [87] | |||||||||||||
| 129 | 73 | F | L | None | IgG/kappa/lambda | IgG | [88] | |||||||||||||
| 130 | 81 | F | L | MALT lymphoma | IgM lambda | NA | [89] | |||||||||||||
| 131 | 61 | F | L | LPL | IgM/IgG/kappa/lambda | IgM kappa | [90] | |||||||||||||
| 132 | 54 | F | L | None | IgA/IgM/IgG/kappa/lambda | NA | [91] | |||||||||||||
| 133 | 46 | M | L | LPL | Negative | IgG/IgM/lambda | [92] | |||||||||||||
| 134 | 49 | M | L | B-cell lymphoma | NA | NA | [93] | |||||||||||||
| 135 | 78 | F | G | LPL | Negative | IgM kappa | [94] | |||||||||||||
| 136 | 77 | F | G | LPL | IgM kappa | NA | ||||||||||||||
| 137 | 18 | F | L | LPL | Negative | NA | ||||||||||||||
| 138 | 75 | M | G | MM | IgG kappa | NA | [95] | |||||||||||||
| 139 | 60 | M | G | MM | IgA kappa | NA | [96] | |||||||||||||
| 140 | 75 | F | L | MGUS | Non-crystallized kappa | IgG kappa | Pres | |||||||||||||
The table demonstrates the cases of storing histiocytosis we identified by literature review. The cases are numbered, and the different organ systems affected are identified by different colors in the left columns. To the right, we present patient age, sex, associated disorders, material identified, and results of immunofixation. Abbreviations: LP–PCD—Lymphoproliferative–Plasma cell disorder; M—Male; F—Female; L—Localized; G—Generalized; ISH—In-situ hybridization; MGUS—Monoclonal gammopathy of undetermined significance; MGRS—Monoclonal gammopathy of renal significance; MM—Multiple myeloma; LPL—Lymphoplasmacytic lymphoma; DLBCL—Diffuse large B-cell lymphoma; BPDCN—Blastic plasmacytoid dendritic cell neoplasia; MALT lymphoma—Mucosa-associated lymphoid tissue lymphoma; EMZL—Extranodal marginal zone lymphoma; SMZL—Splenic marginal zone lymphoma; MZL—Marginal zone lymphoma; NA—Not available.
3.2. Classification and Etiology
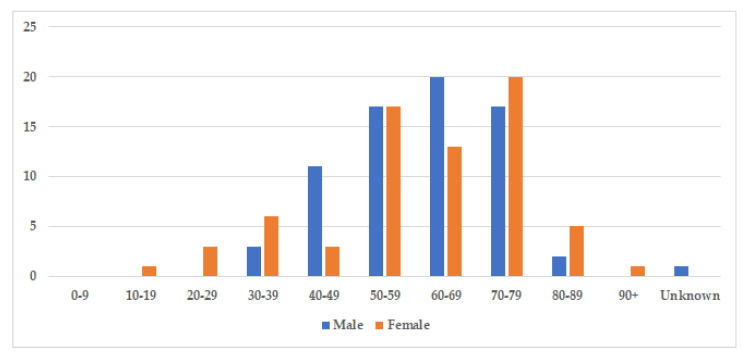
The median age at diagnosis was 60.5 years for both sexes (age-range women 18–91, age-range men 32–86), and there was a nearly equal sex distribution with 71 men (51%) and 69 women (49%) (Table 3). The age and gender distribution are presented in Figure 3. Cases were divided into two subgroups: localized and generalized, as proposed by Dogan et al. in 2012 [55]. Cases with only one involved site were characterized as localized (77%), while cases with two or more involved sites were classified as generalized (23%) (Table 3).
Table 3.
Demographic and pathological data from identified cases.
| Number (n) | Percentage (%) | |
|---|---|---|
| Sex | ||
| Men | 71 | 51 |
| Women | 69 | 49 |
| Etiology | ||
| Localized | 108 | 77 |
| Generalized | 32 | 23 |
| 1. SH with underlying LP–PCD | 122 | 87 |
| MGUS/MGRS | 21 | 15 |
| MM | 43 | 31 |
| LPL | 21 | 15 |
| DLBCL | 2 | 1 |
| BPDCN | 1 | 1 |
| MALT lymphoma | 10 | 7 |
| EMZL/SMZL | 23 | 16 |
| B-cell lymphoma not specified | 2 | 1 |
| 2. SH without underlying LP–PCD | 17 | 12 |
| 3. SH with unknown history | 1 | 1 |
The table demonstrates the distribution of cases of storing histiocytosis regarding age, gender, localized versus generalized disease and the presence or not of an underlying lymphoproliferative or plasma cell disorder. Abbreviations: LP–PCD—lymphoproliferative–Plasma cell disorder; MGUS—Monoclonal gammopathy of undetermined significance; MGRS—Monoclonal gammopathy of renal significance; MM—Multiple myeloma; LPL—Lymphoplasmacytic lymphoma; DLBCL—Diffuse Large B-cell lymphoma; BPDCN—Blastic plasmacytoid dendritic cell neoplasia; MALT lymphoma—Mucosa-associated lymphoid tissue lymphoma; EMZL—Extra nodal marginal zone lymphoma; SMZL—Splenic marginal zone lymphoma.
Figure 3.
Age and gender distribution. The figure displays the incidence of storing histiocytosis in different decades. The median age at diagnosis was 60.5 years for both sexes (range 18–91, and slightly more cases were identified amongst men with 71 men (51%), compared to 69 women (49%).
3.3. Organ Affection
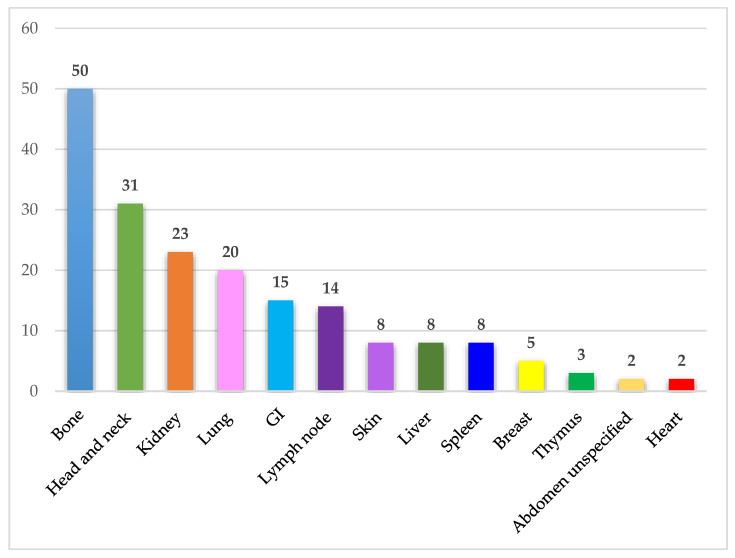
IgSH can be found in a wide range of organs/tissues. The most frequently involved organ systems and tissue sites included bone, head and neck, kidney, lung, gastrointestinal mucosa, and lymph node. The organ and tissue site involvement in the identified cases is presented in Figure 4.
Figure 4.
Affected organ systems. The figure demonstrates the number of identified cases in different organ systems. Abdomen unspecified includes one case of ascites and one case of a retroperitoneal mass. Abbreviations: GI—Gastrointestinal tract.
3.4. Immunoglobulin Classes and Immunoglobulin Restriction
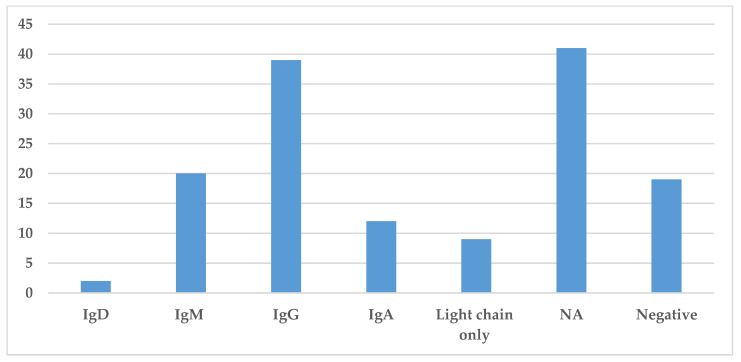
Immunoglobulin classes were collected in this literature review and are presented in Figure 5. The information regarding the specific immunoglobulin and light chain present in the serum was not always available or specified. The most common immunoglobulin found by serum protein electrophoresis and immunofixation was IgG identified in 39 cases, while IgM and IgA were identified in 20 and 12 cases, respectively. The presence of light chains only was identified in nine cases (Figure 5). Hence, in most cases, the monoclonal spike was made of both heavy and light chains.
Figure 5.
Prevalence of different immunoglobulins in Storing Histiocytosis. This figure demonstrates the distribution of immunoglobulin found in the serum by protein electrophoresis or immunofixation. Abbreviation: NA—not available.
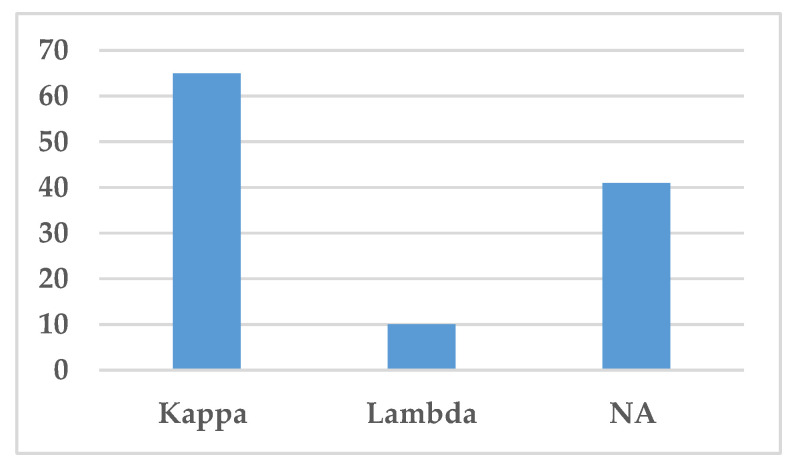
The type of light chain, i.e., either kappa or lambda light chains, is presented in Figure 6. Kappa light chain was the most common light chain associated with IgSH (65 cases), either as part of complete Ig or as free light chain alone (Figure 6). Lambda light chain was only found in 10 cases.
Figure 6.
Distribution of light chains. This figure demonstrates the distribution of light chains found in the serum by protein electrophoresis or immunofixation. Abbreviation: NA—Not available.
3.5. Etiology of Material Deposited
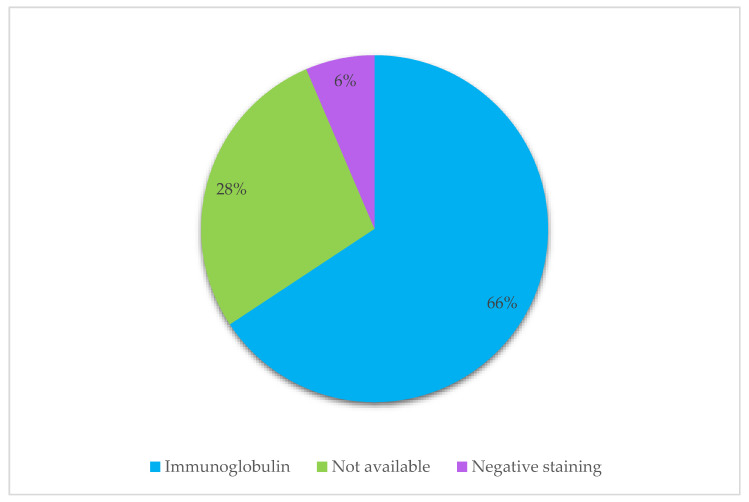
To include both non-crystallized and crystallized material within histiocytes, the term Storing Histiocytosis was used in the literature search. Three cases, including our present case, were described as “non-crystallizing” by histopathological review. On immunohistochemical analysis, the crystals are normally monoclonal, with eight exceptions in this review (Table 2). In many articles, information regarding the etiology of the crystals was insufficient and thereby classified as not available (NA). 6% had negative immunohistochemical staining for immunoglobulin, but many had an associated neoplastic B-cell disorder and had positive staining of plasma cells and lymphocytes in the surrounding area of the histiocytes and were described as an immunoglobulin-type of CSH.
4. Discussion
CSH is a very rare disease ethnicity. Therefore, very few studies related to this condition exist, and the knowledge in the field is largely based on case reporters or smaller patient series. Guidelines for diagnosis and treatment are almost non-existent. Increased knowledge and understanding about this condition are therefore welcome to the medical community in order to increase insight into this condition.
In the present paper, we describe a case of non-crystallized IgSH with, as far as we know, only two other cases previously presented in the literature [4,5]. IgSH has been linked to CSH, which is a rare entity characterized by the accumulation of crystallized material within histiocytes [1,55]. CSH can be classified according to crystal deposited, or according to etiology and associated disease, often underlying B-cell neoplasia or rarely allergic-autoimmune, drugs, metabolic or inflammatory-reactive [55] (Table 4). Immunoglobulins are the most common material deposited in CSH, with kappa being the dominant light chain [11], as also found in our study (Figure 7). Other rare types of crystallized material have been reported, including clofazimine crystals, Charcot–Leyden crystals, cystinosis, and crystallization due to exposure to silica [97,98,99,100]. Due to the finding of non-crystallized IgSH, we propose to alter the classification as shown in Table 5.
Table 4.
CSH classification according to crystal and etiology.
| According to Crystal | According to Etiology |
|---|---|
|
Associated with LP–PCD |
|
|
|
|
|
|
|
Autoimmune |
|
|
|
|
| Drugs | |
|
|
|
|
| Reactive-inflammatory | |
|
|
| Metabolic | |
|
The table demonstrates classification systems according to crystal stored and etiology. Abbreviations: LP–PCD—lymphoproliferative–Plasma cell disorder; MGUS—Monoclonal gammopathy of undetermined significance; MM—Multiple myeloma; LPL—Lymphoplasmacytic lymphoma.
Figure 7.
Distribution of the deposited material within histiocytes. The figure presents the distribution of material within histiocytes in identified cases, as described by immunohistochemical stains, to determine the origin of the deposited material.
Table 5.
Proposal of new classification.
| New Classification |
|---|
| Immunoglobulin Storing Histiocytosis |
|
|
| Non-immunoglobulin Crystallized Storing Histiocytosis |
|
|
|
|
The table demonstrates a new proposed classification system according to immunoglobulin relation.
The term “Storing Histiocytosis” was used to include non-crystallized versions of IgSH in this literature review. A total of 140 cases were identified through a PubMed Search dating from 1987 to July 2020. The crystallized material was made up of clofazimine crystals, Charcot–Leyden crystals or amyloid material in six CSH cases found in our literature search. These cases were thereby not included in the review due to their non-immunoglobulin material. We included 5 cases in our literature search that were “triple-negative,” i.e., the articles did not specify if a lymphoproliferative–plasma cell disorder was present, the type of immunoglobulin in serum, or the type of immunoglobulin within histiocytes. These were included since we considered it likely that the cases were immunoglobulin-related [27,35,42,50,60]. Woeherer et al. presented a 55-year-old male patient with a CNS tumor, which is spoken about as immunoglobulin-related CSH, although the material is not specifically characterized as immunoglobulin [27]. Vaid et al. presented a patient with a gastrointestinal CSH, where a lymphoproliferative disease was suspected and is thereby included [35]. Another case was presented, where there was an association with lymphoplasmacytic infiltrate, though not sufficient to diagnose an LP–PCD [42]. Kawano et al. described their material as crystallized immunoglobulin without specifying the immunoglobulin restriction within the histiocytes [50]. Kamimsky et al. presented a case of CNS CSH, in which the etiology behind the crystallized material was unknown. This case was included due to the possibility of it being immunoglobulin-related; while further examination was not performed, other possible materials were thought of and ruled out [60]. Crystallized immunoglobulin was found in 90 cases, while non-crystallized immunoglobulin was found in three cases [4,5]. Of the cases reviewed, 87% had an underlying neoplastic B-cell disorder, with MM being the most frequent (43 cases, 31%). IgG was the main immunoglobulin class involved, and most of the cases were associated with kappa light chain expression (Figure 6).
IgSH can be found in a wide range of organs/tissue sites and is divided into generalized and localized forms based on the number of affected organs/tissue sites. We found 32 cases (23%) of generalized disease and 108 cases (77%) of localized disease. Bone involvement was the most frequently involved organ/tissue site in our review. Dogan et al. performed an extensive review of CSH in 2012 and found that head and neck were the most frequently affected sites in the localized form and bone marrow in the generalized form [55]. The mentioned review classified 58% of the cases as localized disease, which is lower than in the present review [55]. This difference may be due to increased awareness of this entity in recent years, leading to diagnosis at an earlier time in the disease development.
CSH has been associated with underlying B-cell neoplasia in as many as 90% of the cases in earlier reviews and 87% of the IgSH cases in this review [55] (Table 2). Earlier detection of IgSH in recent years may also explain the lower percentage of LP–PCD in this literature review. Lack of complete staging in newer case reports or loss of patients to follow-up may also explain the difference in frequency. The B-cell neoplasia’s associated include the main secretory B-cell malignancies; MGUS, MM, and lymphoplasmacytic lymphoma (LPL) [1,2,3,101].
Clofazimine, a drug used to treat leprosy, has been linked to the development of CSH in two cases [102,103]. CSH has also been reported in cases of benign plasma cell proliferation such as hypergammaglobulinemia and plasma cell granuloma, which may indicate that CSH is a result of high values of abnormal immunoglobulins rather than being a result of lymphoproliferative disease [85,88].
A patient, reported by Uthamalingam et al., presented with similar symptoms as our patient with worsening pain around the hip after a trivial fall some months earlier [22]. An MRI showed multiple compression fractures of the lumbar vertebra and a trochanteric fracture. The patient also suffered from anemia. A plasma cell dyscrasia was suspected, and serum electrophoresis demonstrated an M-component, while immunofixation identified them to be IgG kappa. A bone marrow aspirate showed both mature and immature plasma cells and also a few large histiocytes with long cytoplasmic crystals. Extensive workup led to the diagnosis of MM. Histological examination of the excised femoral head showed abundant histiocytes with intracytoplasmic crystallized material leading to the diagnosis of CSH. Since fractures in the axial skeleton are uncommon in plasma cell myeloma, the fracture of the femoral head was suspected to be caused by CSH [22]. This case has many similarities to the case presented, making common pathogenesis probable.
Regarding cases of IgSH without crystallization, very few cases have been reported. The first known case of a non-crystallized form of IgSH in the lungs was described by Chantranuwat et al. in 2007. The patient presented with dyspnea, fever, and bilateral patchy lung infiltrations whilst on chemotherapy for MM. A lung biopsy was performed demonstrating intra-alveolar accumulation of macrophages with round eosinophilic globules in the cytoplasm. These globules stained positively for kappa light chain expression. Electron microscopic examination showed no linear parallel configuration of the immunoglobulins as is seen in CSH and had several comparisons to our reported case [4]. The second case of non-crystallized IgSH was presented by Kurabayashi et al. in 2010 in a 53-year-old woman with Sjogren’s disease and MALT thymic lymphoma. When examining the biopsy of the thymoma, the accumulation of eosinophilic histiocytes was discovered, which were positive for IgG kappa [5]. All three cases showed an accumulation of kappa light chains within the histiocytes, which increases the probability of its association with CSH and common pathogenesis. Electron microscopic examination of our case showed abundant bright material within histiocytes but without a crystallized pattern with rhomboidal, needle-shaped, or parallel arrays of crystals as documented in the literature for CSH; thereby, it is an important examination to distinguish the crystallized from the non-crystallized immunoglobulins [4,13,17]. Chantranuwat, who presented the first case of IgSH, proposed to use the classification of ISH widely with subclassification of either crystallized or non-crystallized immunoglobulins [4]. We propose to use the term IgSH instead of ISH due to the widely known abbreviation of ISH to describe in situ hybridization. The histiocytes in CSH are usually strongly positive for CD68 and negative for desmin, myoglobin, muscle-specific actin, CD1a, and S-100 protein. The crystals are stained blue with phosphotungstic acid hematoxylin and positive in periodic acid-Schiff stains [18,55]. On immunohistochemical analysis, the crystals may be monoclonal, polyclonal, or not stain at all. Suboptimal tissue fixation antigen-masking resulting from the crystalline structure of the protein or altered molecular formation may lead to failure to stain [18,55]. Given the possibility of faint or negative staining on immunohistochemical analysis, the information in the clinical history regarding the presence of a serum monoclonal protein may be crucial in the identification of the deposited material as immunoglobulins [81]. The material within the reactive histiocytes is most often kappa light chains. When M-proteins are not found by regular serum immunofixation, assays for serum free-light chain identification can be used.
The pathophysiology behind the crystal formation remains unclear, with theories regarding overproduction or failure to degrade immunoglobulins intracellularly. Several studies show no or low paraprotein levels in the blood of patients diagnosed with CSH, making the theory of overproduction less likely [18]. It has also been hypothesized that DNA mutations in the sequence for immunoglobulins may result in resistance towards lysosomal degradation by macrophages [17].
Most often, the patients with CSH/IgSH present with an asymptomatic mass or swelling, although other symptoms may occur [55]. Some patients also present with symptoms such as fever or other general symptoms, revealing CSH as a more systemic inflammatory syndrome [13,26,57]. Noteworthy, this could also lead to elevated inflammatory markers, such as CRP and ferritin, and inflammatory anemia [104]. Inflammation of the serous tissues, serositis, can also occur as part of the disease. Lesesve et al. presented a woman with CSH found in ascites, while Galed-Placed et al. presented a case of CSH and MM in a pleural effusion [58,76]. In our case, the patient presented with increasing back pain, and the diagnosis was made because of her uncommon progression of a compression fracture in the L1-vertebra.
Treatment and prognosis of patients with CSH vary according to the associated underlying disease. Given the rarity of CSH, clinical studies regarding optimal treatment approaches are lacking, including the specific response of CSH following chemotherapy or simple excision. Therefore, individual therapeutic approaches based on the patient’s symptoms and disease burden are used for practical purposes. If the patient’s underlying B-cell malignancy requires treatment, such as symptomatic MM or LPL, most physicians will probably treat the disease according to the appropriate treatment algorithm. As a consequence of CSH’s association with an underlying disease, research regarding therapeutic options is limited as the symptoms following CSH decrease when the underlying cause is successfully treated [55]. However, CSH can show persistence in follow-up biopsies after chemotherapy and stem cell transplantation [85]. Local treatment with surgery or other interventions, as in our present patient, should hence be considered special for patients with localized CSH without treatment requiring systemic disease. The number of foci of CSH probably also has a prognostic impact, and patients with generalized CSH tend to have the worst prognosis than those localized CSH [3]. The symptoms of CSH can lead patients with underlying B-cell malignancies to earlier diagnostic workup CSH [3] and hence lead to diagnosis at an earlier stage of disease than would otherwise occur, leading to a better prognosis. The discovery of CSH should therefore lead to investigations to dismiss diagnoses such as MM, MGUS, LPL, or other B-cell malignancies, as many of the cases are associated with a lymphoid or plasma cell neoplasm [7,12,22]. Though the impact CSH has on the prognosis remains unclear, it seems that patients with MM and CSH have reported survival 5–15 years longer than MM patients without CSH [85,105]. Classification of CSH and disease etiology (Table 3 and Table 4) may have the potential to more uniform diagnostic algorithms and treatment decisions; therefore, clinical cases and studies are easier to compare.
Gaucher’s disease, a histiocytic storage disease, needs to be assessed as a differential diagnosis, especially when histiocytes with crystallized material are found in the bone marrow [75,85]. Gaucher’s disease is a metabolic storage disease that resembles the needle-like accumulation of immunoglobulins in the cytoplasm of histiocytes in CSH, with fine cytoplasmic striations caused by the accumulation of glucocerebroside. As opposed to the histiocytes in CSH, Gaucher cells normally stain brightly positive for iron, but when in doubt and for exact diagnosis, assays for β-glucocerebrosidase should be performed to properly differentiate [106]. There are several other diseases with a histiocytic or histiocyte-like infiltrate that need to be excluded [55].
A few cases of CSH have been reported due to clofazimine treatment in patients with leprosy, where a good clinical history and examination may be of more value in the diagnostic process than a biopsy. This finding led to the proposition to use the term clofazimine-induced CSH by Sukpanichnant et al. [102,103]. CSH associated with massive deposits of Charcot–Leyden crystals have been reported a few times, one causing colonic polyps in a 78-year-old woman and another as systemic mastocytosis in the bone marrow of a 91-year-old woman [97,98]. CSH in association with cystinosis has been described once by Gebrail et al. in a 23-year-old man with hereditary cystinosis [99]. A bone marrow biopsy was performed because of an abnormal blood count, which showed clusters of hexagonal, tubular, and rectangular cysteine crystals within macrophages. Weiss et al. described in 1978 the development of a fibrous histiocytoma in seven patients, who had been exposed to silica, most of them after injections to repair hernias [100]. Macrophages with intra- and extracellular crystals, identified as silica by x-ray diffraction, were seen in all lesions. Cho et al. presented a case of CSH, associated with MM, as a possible result of prolonged treatment with carbamazepine, an antiepileptic drug, for the management of peripheral neuropathy [19]. Cases of plasma cell neoplasm have been reported with carbamazepine exposure, but the mentioned case report addresses the first possible related CSH [19]. When serum protein electrophoresis is negative, and immunohistochemistry fails to stain the intracytoplasmic crystals, diagnostic challenges arise. Ko et al. described the diagnostic difficulties in separating immunoglobulin crystals from mycobacteria [53]. Kaminsky et al. presented a case with unknown crystal etiology and origin, debating whether their case of CSH may be due to immunoglobulin deposits or due to Pentasa-type medications, given the orange discoloration of the patient’s tissue sampling, similar to that of clofazimine use [60].
5. Conclusions
To our knowledge, we have presented the third case of non-crystallized IgSH. IgSH presents in diverse forms and locations, making the histological examination crucial in the process of identifying cases of this entity. It is likely underreported, given the diversity of the presentations and symptomatology. Chantranuwat et al. suggested in 2007 to use the term ISH widely, with a subclassification of crystallized or non-crystallized material [4]. We hereby propose to use the term IgSH to distinguish this form of Storing Histiocytosis from the abbreviation ISH, which in pathology is used for In Situ Hybridization. By our present literature review, we highlight the importance of investigating the presence of an underlying plasma cell dyscrasia or other B-cell malignancy when deposits of uncertain etiology are identified.
Acknowledgments
We are grateful for the patients and the next of kins’ willingness to publish this article.
Author Contributions
Conceptualization, H.W.-H. and H.R.; methodology H.W.-H. and H.R.; software, H.W.-H.; validation, H.W.-H.; formal analysis, H.W.-H.; investigation, H.W.-H. and H.R.; resources, H.W.-H. and H.R.; data curation, H.W.-H.; writing—original draft preparation, H.W.-H., F.L., A.L.H. and H.R.; writing—review and editing, H.W.-H., F.L., A.L.H. and H.R.; visualization, H.W.-H. and F.L.; supervision, H.R.; project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from the patient presented as part of this the study.
Data Availability Statement
The data presented in this study are available in Table 2 in the present article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flanagan M.E., Keene C.D., Louis D.N., Juric-Sekhar G. Localized crystal-storing histiocytosis of the posterior fossa. Neuropathology. 2018;38:529–534. doi: 10.1111/neup.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang H., Chiu A., Reichard K.K. Crystal-Storing Histiocytosis in Bone Marrow: A Clinicopathologic Study of Eight Cases and Review of the Literature. Am. J. Clin. Pathol. 2018;149:148–163. doi: 10.1093/ajcp/aqx150. [DOI] [PubMed] [Google Scholar]
- 3.Lebeau A., Zeindl-Eberhart E., Muller E.C., Muller-Hocker J., Jungblut P.R., Emmerich B., Lohrs U. Generalized crystal-storing histiocytosis associated with monoclonal gammopathy: Molecular analysis of a disorder with rapid clinical course and review of the literature. Blood. 2002;100:1817–1827. doi: 10.1182/blood.V100.5.1817.h81702001817_1817_1827. [DOI] [PubMed] [Google Scholar]
- 4.Chantranuwat C. Noncrystallized form of immunoglobulin-storing histiocytosis as a cause of chronic lung infiltration in multiple myeloma. Ann. Diagn. Pathol. 2007;11:220–222. doi: 10.1016/j.anndiagpath.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Kurabayashi A., Iguchi M., Matsumoto M., Hiroi M., Kume M., Furihata M. Thymic mucosa-associated lymphoid tissue lymphoma with immunoglobulin-storing histiocytosis in Sjogren’s syndrome. Pathol. Int. 2010;60:125–130. doi: 10.1111/j.1440-1827.2009.02486.x. [DOI] [PubMed] [Google Scholar]
- 6.Zizzo M., De Marco L., Zanelli M., Annessi V., Manenti A., Ascani S., Pedrazzoli C. Localized Crystal-Storing Histiocytosis Involving Lower Rectum. Int. J. Surg. Pathol. 2020;28:415–416. doi: 10.1177/1066896919874407. [DOI] [PubMed] [Google Scholar]
- 7.Reeders J., Arnold C., Chen J., Kirwan P., Lynnhtun K. Crystal-storing histiocytosis leading to the identification of IgG-kappa secreting lymphoplasmacytic lymphoma with crystalline nephropathy. Pathology. 2020;52:283–286. doi: 10.1016/j.pathol.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Kilic I., Picken M.M., Velankar M.M., Pambuccian S.E. Bone marrow imprints of crystal-storing histiocytosis. Diagn. Cytopathol. 2020;48:244–252. doi: 10.1002/dc.24363. [DOI] [PubMed] [Google Scholar]
- 9.Joo M., Kim N.H. Gastric crystal-storing histiocytosis with concomitant mucosa-associated lymphoid tissue lymphoma. J. Pathol. Transl. Med. 2020;54:332–335. doi: 10.4132/jptm.2020.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contejean A., Larousserie F., Bouscary D., Dohan A., Deau-Fischer B., Szwebel T.A., Dhooge M., Terris B., Vignon M. A colonic mass revealing a disseminated crystal storing histiocytosis secondary to indolent multiple myeloma: A case report with literature review. BMC Gastroenterol. 2020;20:239. doi: 10.1186/s12876-020-01364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel A.N., Casey J., Kaur J., Uppal G. Two Cases of Crystal-storing Histiocytosis Diagnosed by Morphology, Immunohistochemistry, and Ultrastructural Examination. Appl. Immunohistochem. Mol. Morphol. 2019;29:e1–e4. doi: 10.1097/PAI.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 12.Tomsula J., Meis J.M., Koy R.D., Monheit J., Zieske A., Ro J., Ayala A. Crystal storing histiocytosis: Unusual clinical presentations in two patients. Ann. Diagn. Pathol. 2019;40:13–17. doi: 10.1016/j.anndiagpath.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Tao Q., Zhang W., Chen Z., Gao L., Yan J., Wang M., Xiang C., Liu W. Generalized crystal-storing histiocytosis with diffuse large B-cell lymphoma and monoclonal gammopathy in a Chinese elderly woman: A case report. BMC Cancer. 2019;19:514. doi: 10.1186/s12885-019-5734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebecchini C., Trimeche M., Rosselet A., de Leval L. Crystal-Storing Histiocytosis. Int. J. Surg. Pathol. 2019;27:399–400. doi: 10.1177/1066896918796949. [DOI] [PubMed] [Google Scholar]
- 15.Michon A., Cohen Aubart F., Haroche J., Charlotte F., Maksud P., Amoura Z. Long-bones involvement in generalized crystal-storing histiocytosis. Jt. Bone Spine. 2019;86:652–653. doi: 10.1016/j.jbspin.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Jaitly V., Hu Z., Ayala G., Wahed M.A., Nguyen N.D., Brown R.E. M2 Macrophages in Crystal Storing Histiocytosis Associated with Plasma Cell Myeloma. Ann. Clin. Lab. Sci. 2019;49:666–670. [PubMed] [Google Scholar]
- 17.Gupta R.K., Rosenberg A.Z., Bagnasco S.M., Arend L.J. Renal crystal-storing histiocytosis involving glomeruli—A comprehensive clinicopathologic analysis. Ann. Diagn. Pathol. 2019;43:151403. doi: 10.1016/j.anndiagpath.2019.151403. [DOI] [PubMed] [Google Scholar]
- 18.Galeano-Valle F., Diaz-Crespo F.J., Melero-Martin R., Apaza-Chavez J.E., Del-Toro-Cervera J., Demelo-Rodriguez P. Massive generalized crystal-storing histiocytosis associated with extracellular crystalline nephropathy: Clinical, immunohistochemical, and ultrastructural studies of a unique disorder and review of the literature. CEN Case Rep. 2019;8:166–172. doi: 10.1007/s13730-019-00385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho W.C., Hafeez S., Shen P. Crystal-Storing Histiocytosis with Plasma Cell Neoplasm in the Setting of Chronic Carbamazepine Exposure. J. Pathol. Transl. Med. 2019;53:142–144. doi: 10.4132/jptm.2018.05.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boudhabhay I., Titah C., Talbot A., Harel S., Verine J., Touchard G., Kaaki S., Gabison E., Vasseur V., Mauget-Faysse M., et al. Multiple myeloma with crystal-storing histiocytosis, crystalline podocytopathy, and light chain proximal tubulopathy, revealed by retinal abnormalities: A case report. Medicine. 2018;97:e13638. doi: 10.1097/MD.0000000000013638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C.K., Yang A.H., Lai H.C., Lin B.S. Combined proximal tubulopathy, crystal-storing histiocytosis, and cast nephropathy in a patient with light chain multiple myeloma. BMC Nephrol. 2017;18:170. doi: 10.1186/s12882-017-0584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uthamalingam P., Mehta S. Crystal-Storing Histiocytosis: Report of a Rare Case Presenting with Pathological Fracture of Femur. Is There More to the Entity? Int. J. Surg. Pathol. 2017;25:458–461. doi: 10.1177/1066896917696746. [DOI] [PubMed] [Google Scholar]
- 23.Kokuho N., Terasaki Y., Kunugi S., Onda N., Urushiyama H., Terasaki M., Hino M., Gemma A., Hatori T., Shimizu A. Localized pulmonary crystal-storing histiocytosis complicating pulmonary mucosa-associated lymphoid tissue lymphoma presenting with multiple mass lesions. Hum. Pathol. 2017;65:180–186. doi: 10.1016/j.humpath.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Balakrishna J.P., Jaffe E.S. Crystal-storing histiocytosis associated with thymic extranodal marginal zone lymphoma. Blood. 2017;130:1683. doi: 10.1182/blood-2017-07-794230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah S., Sethi S., Arend L., Geetha D. Crystal-storing histiocytosis. Kidney Int. 2016;89:507. doi: 10.1016/j.kint.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Kanagal-Shamanna R., Xu-Monette Z.Y., Miranda R.N., Dogan A., Zou D., Luthra R., Weber D.M., O’Malley D.P., Jorgensen J.L., Khoury J.D., et al. Crystal-storing histiocytosis: A clinicopathological study of 13 cases. Histopathology. 2016;68:482–491. doi: 10.1111/his.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woehrer A., Kovacs G.G. Clinical Neuropathology image 1-2015: Crystal-storing histiocytosis of the central nervous system. Clin. Neuropathol. 2015;34:4–5. doi: 10.5414/NP300847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal R., Damato B., Coupland S.E. Conjunctival extranodal marginal zone B-cell lymphoma with crystal-storing histiocytosis. Acta Ophthalmol. 2015;93:e602–e603. doi: 10.1111/aos.12682. [DOI] [PubMed] [Google Scholar]
- 29.Lv Y., Liu Y., Li X., Yan Q., Wang Z. Plasmacytoma with crystal-storing histiocytosis exhibiting FGFR3 and IgH translocation. Pathology. 2015;47:82–85. doi: 10.1097/PAT.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 30.Loghavi S., Khoury J.D. Unusual breast mass: Lymphoma with crystal-storing histiocytosis. Blood. 2015;125:2445. doi: 10.1182/blood-2015-01-623082. [DOI] [PubMed] [Google Scholar]
- 31.Li J.J., Henderson C. Cutaneous crystal storing histiocytosis: A report of two cases. J. Cutan. Pathol. 2015;42:136–143. doi: 10.1111/cup.12413. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.S., Im K., Park S.N., Park H.S., Kim J.A., Choi Q., Kim S.Y., Cha C.H., Oh H.S., Kim I.H., et al. A challenging diagnosis: Crystal-storing histiocytosis in plasma cell myeloma. Am. J. Clin. Pathol. 2015;143:300–304. doi: 10.1309/AJCPC7RWYPYG8UGY. [DOI] [PubMed] [Google Scholar]
- 33.Baird S.M., Kenealy M.K., Hoy R. Complete remission of Waldenstrom’s associated generalized crystal-storing histiocytosis of IgM lambda subtype with bortezomib-based combination chemotherapy. Leuk. Lymphoma. 2015;56:3233–3235. doi: 10.3109/10428194.2015.1036261. [DOI] [PubMed] [Google Scholar]
- 34.Aline-Fardin A., Bender S., Fabiani B., Buob D., Brahimi S., Verpont M.C., Mothy M., Ronco P., Boffa J.J., Aucouturier P., et al. Pseudo-Peritoneal Carcinomatosis Presentation of a Crystal-Storing Histiocytosis With an Unmutated Monoclonal kappa Light Chain. Medicine. 2015;94:e1247. doi: 10.1097/MD.0000000000001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaid A., Caradine K.D., Lai K.K., Rego R. Isolated gastric crystal-storing histiocytosis: A rare marker of occult lymphoproliferative disorders. J. Clin. Pathol. 2014;67:740–741. doi: 10.1136/jclinpath-2014-202247. [DOI] [PubMed] [Google Scholar]
- 36.Tsuji T., Yamasaki H., Hirano T., Toyozumi Y., Arima N., Tsuda H. Crystal-storing histiocytosis complicating marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Int. J. Hematol. 2014;100:519–520. doi: 10.1007/s12185-014-1669-9. [DOI] [PubMed] [Google Scholar]
- 37.Thakral B., Courville E. Crystal-storing histiocytosis with IgD kappa-associated plasma cell neoplasm. Blood. 2014;123:3540. doi: 10.1182/blood-2014-03-565788. [DOI] [PubMed] [Google Scholar]
- 38.Tahara K., Miyajima K., Ono M., Sugio Y., Yamamoto I., Tamiya S. Crystal-storing histiocytosis associated with marginal-zone lymphoma. Jpn. J. Radiol. 2014;32:296–301. doi: 10.1007/s11604-014-0302-4. [DOI] [PubMed] [Google Scholar]
- 39.Saluja K., Thakral B., Eldibany M., Goldschmidt R.A. Crystal storing histiocytosis associated with marginal zone B-cell lymphoma: A rare initial clinical presentation diagnosed by fine-needle aspiration. CytoJournal. 2014;11:17. doi: 10.4103/1742-6413.134439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radhakrishnan S., Maneksha V., Adulkar N. Crystal-storing histiocytosis masquerading ocular adnexal lymphoma: A case report and review of literature. Ophthalmic Plast. Reconstr. Surg. 2014;30:e67–e69. doi: 10.1097/IOP.0b013e31829c41f7. [DOI] [PubMed] [Google Scholar]
- 41.Orr B.A., Gallia G.L., Dogan A., Rodriguez F.J. IgA/kappa-restricted crystal storing histiocytosis involving the central nervous system characterized by proteomic analysis. Clin. Neuropathol. 2014;33:23–28. doi: 10.5414/NP300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaudhary S., Navarro M., Laser J., Berman E., Bhuiya T. Localized crystal-storing histiocytosis presenting as a breast nodule: An unusual presentation of a rare entity. Breast J. 2014;20:539–542. doi: 10.1111/tbj.12307. [DOI] [PubMed] [Google Scholar]
- 43.Zhang C., Myers J.L. Crystal-storing histiocytosis complicating primary pulmonary marginal zone lymphoma of mucosa-associated lymphoid tissue. Arch. Pathol. Lab. Med. 2013;137:1199–1204. doi: 10.5858/arpa.2013-0252-CR. [DOI] [PubMed] [Google Scholar]
- 44.Zardawi I.M., Szabo F. Monoclonal plasma cell proliferation associated with crystal-storing histiocytosis on a background of plasmacytoid dendritic cell tumour in a patient with stable chronic myelomonocytic leukaemia. Histopathology. 2013;62:967–972. doi: 10.1111/his.12102. [DOI] [PubMed] [Google Scholar]
- 45.Yu S.C., Yao M., Liao S.L. Crystal-storing histiocytosis in a patient with ocular extranodal marginal zone lymphoma. Br. J. Haematol. 2013;160:419. doi: 10.1111/bjh.12149. [DOI] [PubMed] [Google Scholar]
- 46.Yano Y., Nagahama T., Matsui T., Chuman K., Takeichi M., Hirai F., Yao K., Nishimata N., Haraoka S., Iwashita A. Gastric crystal-storing histiocytosis detected with asymptomatic Sjogren’s syndrome: Report of a case and summary. Clin. J. Gastroenterol. 2013;6:237–242. doi: 10.1007/s12328-013-0388-8. [DOI] [PubMed] [Google Scholar]
- 47.Rossi G., De Rosa N., Cavazza A., Mengoli M.C., Della Casa G., Nannini N., Colby T.V. Localized pleuropulmonary crystal-storing histiocytosis: 5 cases of a rare histiocytic disorder with variable clinicoradiologic features. Am. J. Surg. Pathol. 2013;37:906–912. doi: 10.1097/PAS.0b013e31827b1618. [DOI] [PubMed] [Google Scholar]
- 48.Rossi G., Morandi U., Nannini N., Fontana G., Pifferi M., Casali C. Crystal-storing histiocytosis presenting with pleural disease. Histopathology. 2010;56:403–405. doi: 10.1111/j.1365-2559.2010.03481.x. [DOI] [PubMed] [Google Scholar]
- 49.Miura T.E., Takihi I.Y., Maekawa Y.H., Chauffaille Mde L., Rizzatti E.G., Sandes A.F. Iron staining in gammopathy-related crystal-storing histiocytosis: A misleading feature to the differential diagnosis with Gaucher’s disease. Mol. Genet. Metab. 2013;110:414–415. doi: 10.1016/j.ymgme.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Kawano N., Beppu K., Oyama M., Himeji D., Yoshida S., Kuriyama T., Ono N., Masuyama H., Yamashita K., Yamaguchi K., et al. Successful surgical treatment for pulmonary crystal-storing histiocytosis following the onset of gastric non-hodgkin lymphoma. J. Clin. Exp. Hematop. 2013;53:241–245. doi: 10.3960/jslrt.53.241. [DOI] [PubMed] [Google Scholar]
- 51.Johnson M., Mazariegos J., Lewis P.J., Pomakova D. Crystal storing histiocytosis presenting as a temporal lobe mass lesion. Surg. Neurol. Int. 2013;4:112. doi: 10.4103/2152-7806.117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duquesne A., Werbrouck A., Fabiani B., Denoyer A., Cervera P., Verpont M.C., Bender S., Piedagnel R., Brocheriou I., Ronco P., et al. Complete remission of monoclonal gammopathy with ocular and periorbital crystal storing histiocytosis and Fanconi syndrome. Hum. Pathol. 2013;44:927–933. doi: 10.1016/j.humpath.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Ko H.M., da Cunha Santos G., Boerner S.L., Bailey D.J., Geddie W.R. Negative images of crystalline immunoglobulin in crystal storing histiocytosis: A potential cytologic mimic of mycobacteria in smears. Diagn. Cytopathol. 2012;40:916–919. doi: 10.1002/dc.21677. [DOI] [PubMed] [Google Scholar]
- 54.Hu X., Liu J., Bai C., Wang J., Song X. Bortezomib combined with thalidomide and dexamethasone is effective for patient with crystal-storing histiocytosis associated with monoclonal gammopathy of undermined significance. Eur. J. Haematol. 2012;89:183–184. doi: 10.1111/j.1600-0609.2012.01800.x. [DOI] [PubMed] [Google Scholar]
- 55.Dogan S., Barnes L., Cruz-Vetrano W.P. Crystal-storing histiocytosis: Report of a case, review of the literature (80 cases) and a proposed classification. Head Neck Pathol. 2012;6:111–120. doi: 10.1007/s12105-011-0326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Da Cruz Perez D.E., Silva-Sousa Y.T., de Andrade B.A., Rizo V.H., Almeida L.Y., Leon J.E., de Almeida O.P. Crystal-storing histiocytosis: A rare lesion in periapical pathology. Ann. Diagn. Pathol. 2012;16:527–531. doi: 10.1016/j.anndiagpath.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Costanzi C., Bourdette D., Parisi J.E., Woltjer R., Rodriguez F., Steensma D., Lucchinetti C.F. Crystal-storing histiocytosis: An unusual relapsing inflammatory CNS disorder. Mult. Scler. Relat. Disord. 2012;1:95–99. doi: 10.1016/j.msard.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lesesve J.F., Bronowicki J.P., Galed-Placed I. Crystal-storing histiocytosis in ascites from a patient with IgM kappa lymphoplasmacytic lymphoma. Cytopathology. 2011;22:207–208. doi: 10.1111/j.1365-2303.2010.00823.x. [DOI] [PubMed] [Google Scholar]
- 59.Khurram S.A., McPhaden A., Hislop W.S., Hunter K.D. Crystal storing histiocytosis of the tongue as the initial presentation of multiple myeloma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011;111:494–496. doi: 10.1016/j.tripleo.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 60.Kaminsky I.A., Wang A.M., Olsen J., Schechter S., Wilson J., Olson R. Central nervous system crystal-storing histiocytosis: Neuroimaging, neuropathology, and literature review. Am. J. Neuroradiol. 2011;32:E26–E28. doi: 10.3174/ajnr.A1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao F.F., Khalbuss W.E., Austin R.M., Monaco S.E. Cytomorphology of crystal storing histiocytosis in the breast associated with lymphoma: A case report. Acta Cytol. 2011;55:302–306. doi: 10.1159/000324558. [DOI] [PubMed] [Google Scholar]
- 62.Todd W.U., Drabick J.J., Benninghoff M.G., Frauenhoffer E.E., Zander D.S. Pulmonary crystal-storing histiocytosis diagnosed by computed tomography-guided fine-needle aspiration. Diagn. Cytopathol. 2010;38:274–278. doi: 10.1002/dc.21193. [DOI] [PubMed] [Google Scholar]
- 63.Qureshi A., Kashif M. Crystal-storing histiocytosis. Blood. 2010;115:2568. doi: 10.1182/blood-2009-10-241901. [DOI] [PubMed] [Google Scholar]
- 64.El Hamel C., Thierry A., Trouillas P., Bridoux F., Carrion C., Quellard N., Goujon J.M., Aldigier J.C., Gombert J.M., Cogne M., et al. Crystal-storing histiocytosis with renal Fanconi syndrome: Pathological and molecular characteristics compared with classical myeloma-associated Fanconi syndrome. Nephrol. Dial. Transpl. 2010;25:2982–2990. doi: 10.1093/ndt/gfq129. [DOI] [PubMed] [Google Scholar]
- 65.Sailey C.J., Alexiev B.A., Gammie J.S., Pinell-Salles P., Stafford J.L., Burke A. Crystal-storing histiocytosis as a cause of symptomatic cardiac mass. Arch. Pathol. Lab. Med. 2009;133:1861–1864. doi: 10.5858/133.11.1861. [DOI] [PubMed] [Google Scholar]
- 66.Laszlo R., Degrell P., Kellermayer M., Bollmann D., Egyed M., Seress L., Pajor L. Crystal-storing histiocytosis associated with only one of two consecutive, but genetically unrelated B-cell lymphomas. Pathol. Res. Pract. 2009;205:273–278. doi: 10.1016/j.prp.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Farooq U., Bayerl M.G., Abendroth C.S., Verma N., Talamo G. Renal crystal storing histiocytosis in a patient with multiple myeloma. Ann. Hematol. 2009;88:807–809. doi: 10.1007/s00277-008-0660-z. [DOI] [PubMed] [Google Scholar]
- 68.De Alba Campomanes A.G., Rutar T., Crawford J.B., Seiff S., Goodman D., Grenert J. Crystal-storing histiocytosis and crystalline keratopathy caused by monoclonal gammopathy of undetermined significance. Cornea. 2009;28:1081–1084. doi: 10.1097/ICO.0b013e318199f73b. [DOI] [PubMed] [Google Scholar]
- 69.Keane C., Gill D. Multi-organ involvement with crystal-storing histiocytosis. Br. J. Haematol. 2008;141:750. doi: 10.1111/j.1365-2141.2008.07131.x. [DOI] [PubMed] [Google Scholar]
- 70.Kar R., Dutta S., Bhargava R., Tyagi S. Crystal storing histiocytosis: A rare presentation of plasma cell myeloma. Indian J. Hematol. Blood Transfus. 2008;24:63–66. doi: 10.1007/s12288-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kusakabe T., Watanabe K., Mori T., Iida T., Suzuki T. Crystal-storing histiocytosis associated with MALT lymphoma of the ocular adnexa: A case report with review of literature. Virchows Arch. 2007;450:103–108. doi: 10.1007/s00428-006-0323-1. [DOI] [PubMed] [Google Scholar]
- 72.Joo M., Kwak J.E., Chang S.H., Kim H., Chi J.G., Moon Y.S., Kim K.M. Localized gastric crystal-storing histiocytosis. Histopathology. 2007;51:116–119. doi: 10.1111/j.1365-2559.2007.02710.x. [DOI] [PubMed] [Google Scholar]
- 73.Stokes M.B., Aronoff B., Siegel D., D’Agati V.D. Dysproteinemia-related nephropathy associated with crystal-storing histiocytosis. Kidney Int. 2006;70:597–602. doi: 10.1038/sj.ki.5001524. [DOI] [PubMed] [Google Scholar]
- 74.Pock L., Stuchlik D., Hercogova J. Crystal storing histiocytosis of the skin associated with multiple myeloma. Int. J. Derm. 2006;45:1408–1411. doi: 10.1111/j.1365-4632.2006.02919.x. [DOI] [PubMed] [Google Scholar]
- 75.Pitman S.D., Wang J., Serros E.R., Zuppan C. A 70-year-old woman with acute renal failure. Crystal-storing histiocytosis. Arch. Pathol. Lab. Med. 2006;130:1077–1078. doi: 10.5858/2006-130-1077-AYWWAR. [DOI] [PubMed] [Google Scholar]
- 76.Galed-Placed I. Immunoglobulin crystal-storing histiocytosis in a pleural effusion from a woman with IgA kapa multiple myeloma: A case report. Acta Cytol. 2006;50:539–541. doi: 10.1159/000326010. [DOI] [PubMed] [Google Scholar]
- 77.Fairweather P.M., Williamson R., Tsikleas G. Pulmonary extranodal marginal zone lymphoma with massive crystal storing histiocytosis. Am. J. Surg. Pathol. 2006;30:262–267. doi: 10.1097/01.pas.0000178093.99889.f7. [DOI] [PubMed] [Google Scholar]
- 78.De Lastours V., Papo T., Cazals-Hatem D., Eden A., Feydy A., Belmatoug N., Chauveheid M.P., Lidove O., Fantin B. Bone involvement in generalized crystal-storing histiocytosis. J. Rheumatol. 2006;33:2354–2358. [PubMed] [Google Scholar]
- 79.Tholouli E., Krebs M., Reeve R., Houghton J.B. Crystal-storing histiocytosis in a patient with IgG kappa multiple myeloma. Br. J. Haematol. 2005;128:412. doi: 10.1111/j.1365-2141.2004.05362.x. [DOI] [PubMed] [Google Scholar]
- 80.Zioni F., Giovanardi P., Bozzoli M., Artusi T., Bonacorsi G., Sighinolfi P. Massive bone marrow crystal-storing histiocytosis in a patient with IgA-lambda multiple myeloma and extensive extramedullary disease. A case report. Tumori J. 2004;90:348–351. doi: 10.1177/030089160409000318. [DOI] [PubMed] [Google Scholar]
- 81.Papla B., Spolnik P., Rzenno E., Zdunczyk A., Rudzki Z., Okon K., Szczepanski W., Dabros W., Stachura J. Generalized crystal-storing histiocytosis as a presentation of multiple myeloma: A case with a possible pro-aggregation defect in the immunoglobulin heavy chain. Virchows Arch. 2004;445:83–89. doi: 10.1007/s00428-004-1031-3. [DOI] [PubMed] [Google Scholar]
- 82.Sun Y., Tawfiqul B., Valderrama E., Kline G., Kahn L.B. Pulmonary crystal-storing histiocytosis and extranodal marginal zone B-cell lymphoma associated with a fibroleiomyomatous hamartoma. Ann. Diagn. Pathol. 2003;7:47–53. doi: 10.1053/adpa.2003.50008. [DOI] [PubMed] [Google Scholar]
- 83.Sethi S., Cuiffo B.P., Pinkus G.S., Rennke H.G. Crystal-storing histiocytosis involving the kidney in a low-grade B-cell lymphoproliferative disorder. Am. J. Kidney Dis. 2002;39:183–188. doi: 10.1053/ajkd.2002.29914. [DOI] [PubMed] [Google Scholar]
- 84.Coupland S.E., Foss H.D., Hummel M., Stein H. Extranodal marginal zone B-cell lymphoma of the lacrimal gland associated with crystal-storing histiocytosis. Ophthalmology. 2002;109:105–110. doi: 10.1016/S0161-6420(01)00837-5. [DOI] [PubMed] [Google Scholar]
- 85.Jones D., Bhatia V.K., Krausz T., Pinkus G.S. Crystal-storing histiocytosis: A disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum. Pathol. 1999;30:1441–1448. doi: 10.1016/S0046-8177(99)90166-1. [DOI] [PubMed] [Google Scholar]
- 86.Prasad M.L., Charney D.A., Sarlin J., Keller S.M. Pulmonary immunocytoma with massive crystal storing histiocytosis: A case report with review of literature. Am. J. Surg. Pathol. 1998;22:1148–1153. doi: 10.1097/00000478-199809000-00015. [DOI] [PubMed] [Google Scholar]
- 87.Garcia J.F., Sanchez E., Lloret E., Martin J., Piris M.A. Crystal-storing histiocytosis and immunocytoma associated with multifocal fibrosclerosis. Histopathology. 1998;33:459–464. doi: 10.1046/j.1365-2559.1998.00531.x. [DOI] [PubMed] [Google Scholar]
- 88.Bosman C., Camassei F.D., Boldrini R., Piro F.R., Saponara M., Romeo R., Corsi A. Solitary crystal-storing histiocytosis of the tongue in a patient with rheumatoid arthritis and polyclonal hypergammaglobulinemia. Arch. Pathol. Lab. Med. 1998;122:920–924. [PubMed] [Google Scholar]
- 89.Llobet M., Castro P., Barcelo C., Trull J.M., Campo E., Bernado L. Massive crystal-storing histiocytosis associated with low-grade malignant B-cell lymphoma of MALT-type of the parotid gland. Diagn. Cytopathol. 1997;17:148–152. doi: 10.1002/(SICI)1097-0339(199708)17:2<148::AID-DC12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 90.Kaufmann O., Hansen A., Deicke P., Burmester G.R., Dietel M. Subcutaneous crystal-storing histiocytosis associated with lymphoplasmacytic lymphoma (immunocytoma) Pathol. Res. Pract. 1996;192:1148–1151. doi: 10.1016/S0344-0338(96)80036-7. [DOI] [PubMed] [Google Scholar]
- 91.Jones D., Renshaw A.A. Recurrent crystal-storing histiocytosis of the lung in a patient without a clonal lymphoproliferative disorder. Arch. Pathol. Lab. Med. 1996;120:978–980. [PubMed] [Google Scholar]
- 92.Harada M., Shimada M., Fukayama M., Kaneko T., Kitazume K., Weiss S.W. Crystal-storing histiocytosis associated with lymphoplasmacytic lymphoma mimicking Weber-Christian disease: Immunohistochemical, ultrastructural, and gene-rearrangement studies. Hum. Pathol. 1996;27:84–87. doi: 10.1016/S0046-8177(96)90143-4. [DOI] [PubMed] [Google Scholar]
- 93.Friedman M.T., Molho L., Valderrama E., Kahn L.B. Crystal-storing histiocytosis associated with a lymphoplasmacytic neoplasm mimicking adult rhabdomyoma: A case report and review of the literature. Arch. Pathol. Lab. Med. 1996;120:1133–1136. [PubMed] [Google Scholar]
- 94.Kapadia S.B., Enzinger F.M., Heffner D.K., Hyams V.J., Frizzera G. Crystal-storing histiocytosis associated with lymphoplasmacytic neoplasms. Report of three cases mimicking adult rhabdomyoma. Am. J. Surg. Pathol. 1993;17:461–467. doi: 10.1097/00000478-199305000-00004. [DOI] [PubMed] [Google Scholar]
- 95.Yamamoto T., Hishida A., Honda N., Ito I., Shirasawa H., Nagase M. Crystal-storing histiocytosis and crystalline tissue deposition in multiple myeloma. Arch. Pathol. Lab. Med. 1991;115:351–354. [PubMed] [Google Scholar]
- 96.Takahashi K., Naito M., Takatsuki K., Kono F., Chitose M., Ooshima S., Mori N., Sakuma H., Uchino F. Multiple myeloma, IgA kappa type, accompanying crystal-storing histiocytosis and amyloidosis. Acta Pathol. Jpn. 1987;37:141–154. doi: 10.1111/j.1440-1827.1987.tb03142.x. [DOI] [PubMed] [Google Scholar]
- 97.Alayed K.M., Alabdulaali M.K., Alkhairy K.S., Elnour S., Alhajjaj A. Aggressive systemic mastocytosis with Charcot-Leyden crystals-associated crystal storing histiocytosis in bone marrow. Pathology. 2010;42:85–87. doi: 10.3109/00313020903434652. [DOI] [PubMed] [Google Scholar]
- 98.Lewis J.T., Candelora J.N., Hogan R.B., Briggs F.R., Abraham S.C. Crystal-storing histiocytosis due to massive accumulation of charcot-leyden crystals: A unique association producing colonic polyposis in a 78-year-old woman with eosinophilic colitis. Am. J. Surg. Pathol. 2007;31:481–485. doi: 10.1097/01.pas.0000213420.46127.9c. [DOI] [PubMed] [Google Scholar]
- 99.Gebrail F., Knapp M., Perotta G., Cualing H. Crystalline histiocytosis in hereditary cysinosis. Arch. Pathol. Lab. Med. 2002;126:1135. doi: 10.5858/2002-126-1135-CHIHC. [DOI] [PubMed] [Google Scholar]
- 100.Weiss S.W., Enzinger F.M., Johnson F.B. Silica reaction simulating fibrous histiocytoma. Cancer. 1978;42:2738–2743. doi: 10.1002/1097-0142(197812)42:6<2738::AID-CNCR2820420632>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 101.Balakrishna J., Chen A., Urken M. Crystal storing histiocytosis clinically mimicking metastatic carcinoma: Report of a case and reviews of literature. Head Neck. 2016;38:E95–E98. doi: 10.1002/hed.24321. [DOI] [PubMed] [Google Scholar]
- 102.Pais A.V., Pereira S., Garg I., Stephen J., Antony M., Inchara Y.K. Intra-abdominal, crystal-storing histiocytosis due to clofazimine in a patient with lepromatous leprosy and concurrent carcinoma of the colon. Lepr. Rev. 2004;75:171–176. doi: 10.47276/lr.75.2.171. [DOI] [PubMed] [Google Scholar]
- 103.Sukpanichnant S., Hargrove N.S., Kachintorn U., Manatsathit S., Chanchairujira T., Siritanaratkul N., Akaraviputh T., Thakerngpol K. Clofazimine-induced crystal-storing histiocytosis producing chronic abdominal pain in a leprosy patient. Am. J. Surg. Pathol. 2000;24:129–135. doi: 10.1097/00000478-200001000-00016. [DOI] [PubMed] [Google Scholar]
- 104.Ganz T. Anemia of Inflammation. N. Engl. J. Med. 2019;381:1148–1157. doi: 10.1056/NEJMra1804281. [DOI] [PubMed] [Google Scholar]
- 105.Pasqualetti P., Festuccia V., Collacciani A., Casale R. The natural history of monoclonal gammopathy of undetermined significance. A 5- to 20-year follow-up of 263 cases. Acta Haematol. 1997;97:174–179. doi: 10.1159/000203676. [DOI] [PubMed] [Google Scholar]
- 106.Amir G., Ron N. Pulmonary pathology in Gaucher’s disease. Hum. Pathol. 1999;30:666–670. doi: 10.1016/S0046-8177(99)90092-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in Table 2 in the present article.