Abstract
Organic solvents used for electrolytes of dye-sensitized solar cells (DSSCs) are generally not only toxic and explosive but also prone to leakage due to volatility and low surface tension. The representative dyes of DSSCs are ruthenium-complex molecules, which are expensive and require a complicated synthesis process. In this paper, the eco-friendly DSSCs were presented based on water-based electrolytes and a commercially available organic dye. The effect of aging time after the device fabrication and the electrolyte composition on the photovoltaic performance of the eco-friendly DSSCs were investigated. Plasma treatment of TiO2 was adopted to improve the dye adsorption as well as the wettability of the water-based electrolytes on TiO2. It turned out that the plasma treatment was an effective way of improving the photovoltaic performance of the eco-friendly DSSCs by increasing the efficiency by 3.4 times. For more eco-friendly DSSCs, the organic-synthetic dye was replaced by chlorophyll extracted from spinach. With the plasma treatment, the efficiency of the eco-friendly DSSCs based on water-electrolytes and chlorophyll was comparable to those of the previously reported chlorophyll-based DSSCs with non-aqueous electrolytes.
Keywords: dye-sensitized solar cells, aqueous electrolyte, chlorophyll, plasma treatment, eco-friendly devices
1. Introduction
Due to its advantages, such as a simple fabrication process, reasonable power conversion efficiency, and relatively low production cost [1,2], dye-sensitized solar cells (DSSC) have been actively researched for the past three decades. Even though the power conversion efficiency of the DSSCs reported is relatively low compared to the counterparts, e.g., perovskite and silicon solar cells [3,4,5], continuous efforts have still been made to improve the efficiency and replace the expensive noble metal catalysts with low-cost alternatives [6,7,8]. Furthermore, the enhanced photoconversion efficiency of DSSCs under ambient light was also achieved, suggesting the potential of DSSCs for indoor applications [9].
DSSCs adopted dye molecules to effectively absorb visible sunlight and generate electricity. Electrons in the dye were excited upon light absorption and then were injected into the high-surface-area-porous TiO2 film, thereby leaving holes in the dye. The oxidized dye molecules were regenerated by a redox reaction in electrolytes. DSSCs typically employed liquid electrolytes based on organic solvents, such as acetonitrile, methoxyacetonitrile, and 3-methoxypropionitrile. Even though these solvents enabled better performance, they were likely to be highly toxic, hazardous, or explosive and had an environmental impact [2,10]. Low viscosity, low surface tension, and high volatility of the organic solvents could also cause the leakage of the electrolytes [1,11]. Additionally, the representative dyes for DSSCs were ruthenium-complex molecules, which are costly and less eco-friendly. To overcome the disadvantages in the use of the organic solvents in electrolytes and the rare-transition metal-based dyes, research to apply water-based electrolytes [10,12] and organic synthetic or naturally-derived dyes [13,14] to DSSCs was conducted. However, the efficiency of such environmentally benign DSSCs was quite low and needs to be improved.
In this paper, we developed eco-friendly DSSCs based on aqueous electrolytes and organic synthetic dyes. The organic synthetic dye that is commercially available, was used as is without further modification to reduce the production cost and develop a more versatile procedure. The pristine dye was dissolved in the water-electrolyte, including iodine (I2) and potassium iodide (KI), to be kinetically adsorbed onto the TiO2 surface. The effects of aging time and plasma treatment of the TiO2 surface on the photovoltaic performance were investigated. For the fabrication of more eco-friendly DSSCs, the synthetic dye was replaced with naturally-derived chlorophyll dye extracted from spinach. The resulting efficiency of the DSSCs with chlorophyll was compared to that with the synthetic dye and the possible ways to improve the efficiency were discussed.
2. Materials and Methods
2.1. Materials
TiO2 paste (SC-HT040, particle size: 15–20 nm) was purchased from Sharechem Co. (Hwaseong, Korea). Chloroplatinic acid hexahydrate (>37.5%, Pt base), and eosin Y disodium salt (>85%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). I2, KI, 2-propanol (IPA, 99.8%), and ethanol (95.0%) were obtained from Samchun Chemicals (Seoul, Korea). A poly(dimethylsiloxane), PDMS, elastomer kit (Sylgard 184 silicone elastomer base and curing agent) was purchased from Dow Corning Corp (Midland, TX, USA). PDMS was made by mixing the base and curing agent in a ratio of 10:1 by weight, degassing, and curing in an oven at 60 °C.
2.2. Fabrication of DSSCs with the Organic Synthetic Dye
Fluorine-doped tin oxide (FTO) glass substrates (Hanalin Tech, Seongnam, Korea) were washed by sonication in ethanol for 20 min. For a photoanode, the TiO2 paste was doctor-bladed on the FTO substrate with a thickness of 50 μm and then heated at 500 °C for 1 h in a furnace for sintering. For the Pt counter electrode, a solution of 0.005 M chloroplatinic acid hexahydrate in IPA was cast on an FTO glass with a size of 25 mm × 13 mm and was heated at 400 °C for 1 h in a furnace. The 100 μm-thick PDMS spacer was sandwiched between the anode and cathode for electrolyte filling. For the aqueous dye-electrolyte solution, 0.4 M KI, 0.02 M I2, and 0.005 M eosin Y salt were dissolved in deionized (DI) water. The plasma treatment (100 W, 50 kHz) of the TiO2 photoanodes was performed for 5 min to 60 min with a plasma system (CUTE, Femto Science Inc., Gyeonggi-do, Korea) before filling the electrolyte.
2.3. Fabrication of Chlorophyll Based DSSCs
Spinach leaves were washed with cold water, crushed, and ground in a mortar. 100 mL of ethanol was added to 10 g of spinach and then was stored in an oven at 60 °C for 30 min for chlorophyll extraction. The dark-green-colored solution obtained was filtered and centrifuged at 3000 rpm for 10 min. The resulting supernatant was used as the chlorophyll extract. The extract and DI water were mixed in a ratio of 2:1 by the volume for dyeing solution. A TiO2 photoanode was immersed in the solution for 24 h for chlorophyll adsorption. After washing with DI water, the photoanode was assembled with the Pt counter electrode and the PDMS spacer. Finally, the aqueous electrolytes with 0.4 M KI and 0.02 M I2 were injected. The plasma treatment (100 W, 50 kHz) of the TiO2 photoanodes was performed for 60 min before the chlorophyll adsorption process.
2.4. Photovoltaic Measurement
The current density–voltage (J–V) curves of the DSSCs were measured using a Keithley 2400 source meter under the illumination of the simulated solar light (100 mW/cm2, AM 1.5 G, Sol3A, Newport, Irvine, CA USA). The solar simulator was calibrated with an encapsulated reference silicon solar cell certified by the Newport Cop. PV Lab (California, USA). The active area was ~0.25 cm2.
3. Results and Discussion
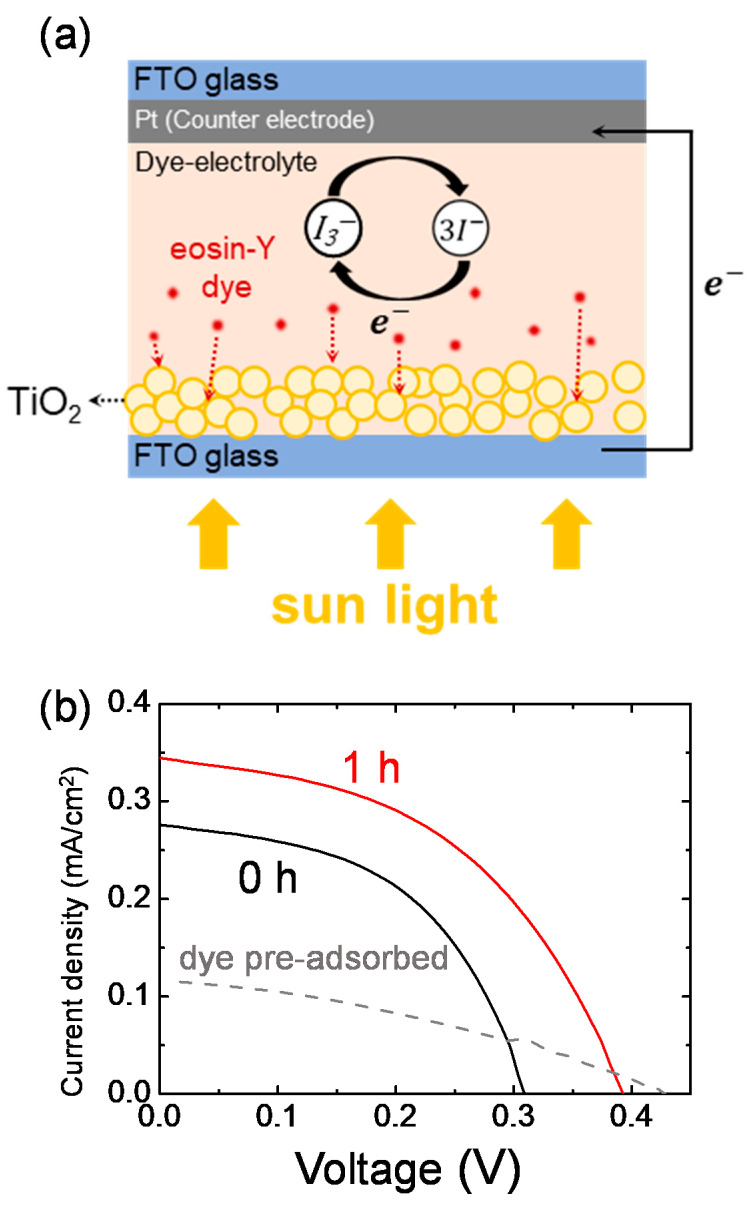
Figure 1a shows the structure of the eco-friendly DSSC. The commercial eosin Y dye itself was not effectively adsorbed onto TiO2 and was easily desorbed during washing after the dyeing step. Instead of the typical DSSCs fabrication process, we introduced the dye into the electrolyte, i.e., dye-electrolyte, so that the dye molecules were adsorbed onto TiO2 via adsorption equilibrium. Figure 1b and Table 1 compare the photovoltaic characteristics of the eco-friendly DSSCs with the dye-electrolyte to that of the DSSCs with the pre-adsorbed dye. As fabricated, the eco-friendly DSSCs showed 0.044% efficiency, which was much higher than that of the DSSCs with the pre-adsorbed dye. After 1 h of aging at room temperature, the efficiency of the eco-friendly DSSCs increased by 45%, from 0.044% to 0.064%. This was possible because it took time for the aqueous electrolytes to fully wet the TiO2 surface or for the adsorption of dye molecules onto the TiO2 to be equilibrated. As the dye-electrolyte permeated onto the porous TiO2, the contact area between dye, electrolyte, and TiO2 increased, and the series resistance in DSSCs decreased, resulting in an increase in efficiency [15]. It was found that, for the eco-friendly DSSCs, it could be more efficient to include dyes in the aqueous electrolytes instead of the pre-adsorption of dyes onto TiO2, and an appropriate aging process would be required for higher efficiency.
Figure 1.
(a) The structure of eco-friendly DSSCs. (b) Density–voltage (J–V) graphs of the eco-friendly DSSCs at aging time of 0 h and 1 h. The concentration of the dye-electrolyte was 5 mM eosin Y dye, 0.4 M KI, and 0.02 M I2. The dotted line is the J–V graph of the DSSCs where the eosin Y dye was pre-adsorbed onto TiO2 photoanode.
Table 1.
Photovoltaic performances of water-based dye-sensitized solar cells (DSSCs) at different aging times, compared with the DSSCs with pre-adsorbed dye.
| State of Dye | Aging Time (h) | VOC (V) | JSC (mA/cm2) | Fill Factor (%) | Efficiency (%) |
|---|---|---|---|---|---|
| Dye in electrolyte (dye-electrolyte) |
0 | 0.31 | 0.28 | 50.3 | 0.044 |
| 1 | 0.39 | 0.35 | 47.1 | 0.064 | |
| Dye pre-adsorbed | - | 0.43 | 0.12 | 34.2 | 0.017 |
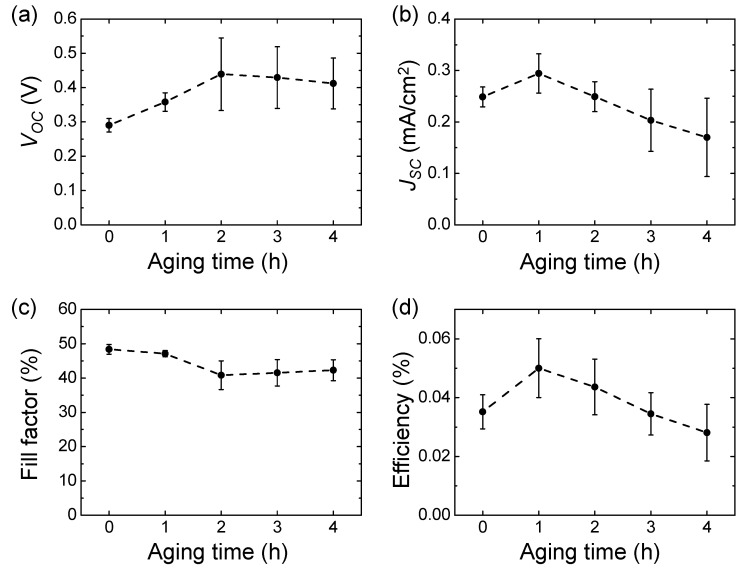
Figure 2 shows the changes in VOC, JSC, fill factor, and efficiency of eco-friendly DSSCs depending on aging time. The VOC and fill factor did not change significantly over time, while JSC and the resulting efficiency were highest at 1 h of aging time and then gradually decreased. The possible reasons for the decrease in JSC are the following: (1) dye degradation [16,17,18], (2) dye detachment from the TiO2 surface into the electrolyte by strong adsorption of water molecules onto the TiO2 [19,20], and (3) recombination by contact of the dye-free TiO2 surface with aqueous electrolytes [15]. A further experiment is now underway to improve the stability of the eco-friendly DSSCs by selecting more durable, commercially available organic dyes.
Figure 2.
Photovoltaic characteristics of the eco-friendly DSSCs as a function of aging time: (a) VOC, (b) JSC, (c) fill factor, and (d) efficiency. The concentration of the dye-electrolyte was 5 mM eosin Y dye, 0.4 M KI, and 0.02 M I2.
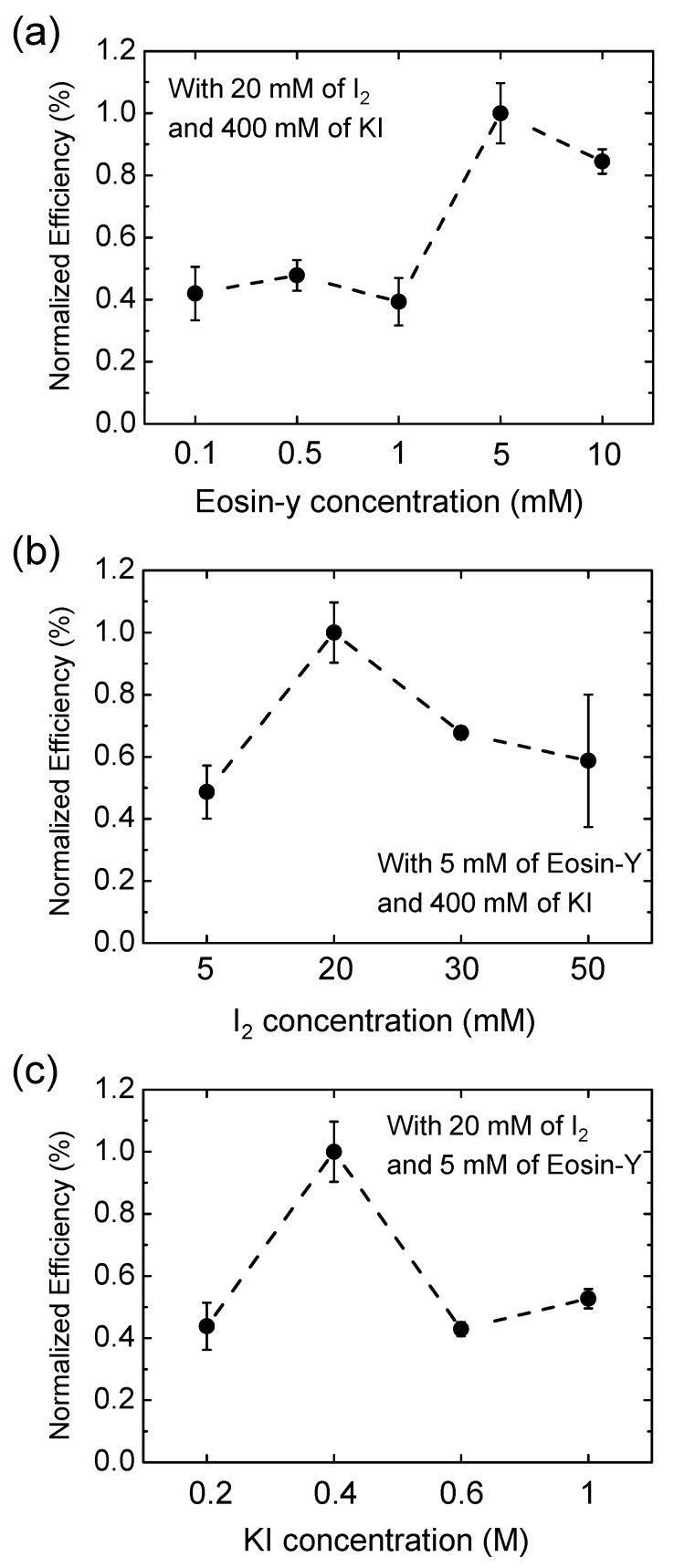
The dye-electrolyte concentration in the eco-friendly DSSCs was optimized by comparing the efficiency depending on the concentration of the eosin Y dye, I2, and KI (Figure 3). When the concentration of eosin Y was less than 1 mM, there was no significant change in the efficiency. The efficiency was highest at 5 mM of eosin Y and slightly decreased at 10 mM. Similarly, the efficiency was highest at 20 mM of I2 and 0.4 M of KI. Thus, the photovoltaic efficiency of the eco-friendly DSSCs was strongly affected by the concentration of the dye-electrolyte, and the resulting optimal composition was 5 mM of eosin Y dye, 20 mM of I2, and 0.4 M of KI.
Figure 3.
Effect of dye-electrolyte concentration on efficiency according to (a) concentration of eosin-Y dye, (b) concentration of I2, and (c) concentration of KI. The efficiency values were normalized based on that with the dye-electrolyte including 5 mM eosin Y dye, 0.4 M KI, and 0.02 M I2.
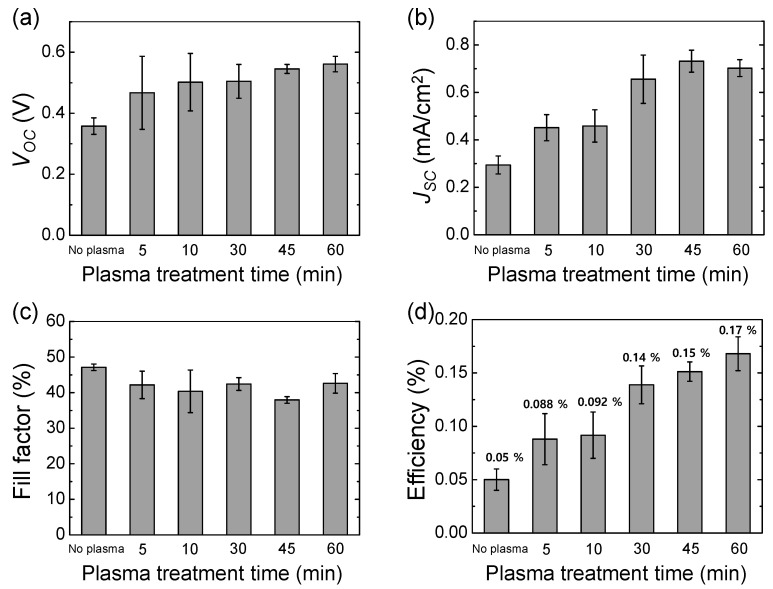
It was reported that the plasma treatment of TiO2 could enhance hydrophilicity [21,22], surface roughness and reactivity [23,24], and the reduction of oxygen vacancies [22,25,26,27]. We hypothesized that these factors would improve the dye adsorption onto TiO2 and the affinity of an interface of TiO2 and the water-based electrolyte. Figure 4 and Table 2 show the effects of the plasma treatment of TiO2 on the photovoltaic performance of the eco-friendly DSSCs. As the plasma treatment time increased, the VOC and JSC were significantly improved until 45 min of plasma treatment, while the fill factor hardly changed between 40–50%. As a result, the average efficiency increased by ~3.4 times from 0.05 to 0.17% via 1 h of plasma treatment of TiO2. Thus, it turned out that the plasma treatment of TiO2 was an effective way of improving the photovoltaic performance of the eco-friendly DSSCs.
Figure 4.
Effect of the plasma treatment of TiO2 on photovoltaic characteristics of the eco-friendly DSSCs: (a) VOC, (b) JSC, (c) fill factor and (d) efficiency. The atmospheric air plasma treatment was used. The concentration of the dye-electrolyte was 5 mM eosin Y dye, 0.4 M KI, and 0.02 M I2.
Table 2.
Photovoltaic performance parameters of the eco-friendly DSSCs with and without plasma treatment at 1 h of aging time.
| Plasma Treatment |
VOC (V) | JSC (mA/cm2) | Fill Factor (%) | Efficiency (%) |
|---|---|---|---|---|
| Without plasma |
0.35 | 0.29 | 47.1 | 0.05 |
| With plasma for 60 min |
0.56 | 0.70 | 42.6 | 0.17 |
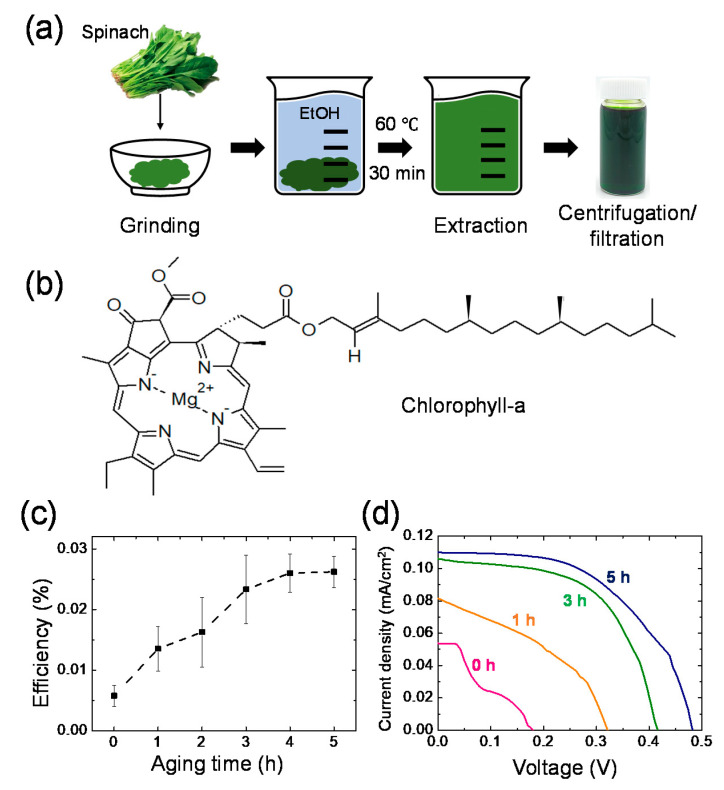
As the next step toward further eco-friendly DSSCs, the organic synthetic dye was replaced with chlorophyll derived from natural spinach leaves. The extraction process to obtain the chlorophyll stock solution is shown in Figure 5a. Due to its long hydrocarbon chain, as shown in Figure 5b, chlorophyll was barely soluble in aqueous electrolytes. Instead, TiO2 was stained for 24 h in the solution where the chlorophyll stock solution and DI water were mixed at the ratio of 2:1 (v/v) [28,29]. The TiO2 photoanode stained by the spinach chlorophyll was assembled with the spacer and the Pt counter electrode, followed by adding the aqueous KI/I2 electrolytes. Figure 5c,d shows the photovoltaic characteristics of the eco-friendly DSSCs with chlorophyll as a function of aging time. The efficiency of the DSSCs gradually increased with time and stabilized after 3 h of aging time. The resulting efficiency after the aging step was ~0.026%, which was higher than that of the DSSCs with pre-adsorbed eosin Y, as discussed in Table 1.
Figure 5.
(a) Process of chlorophyll extraction from spinach. (b) Molecular structure of chlorophyll-a. (c) Change in the efficiency of the eco-friendly DSSCs with chlorophyll depending on the aging time and (d) corresponding J–V graphs.
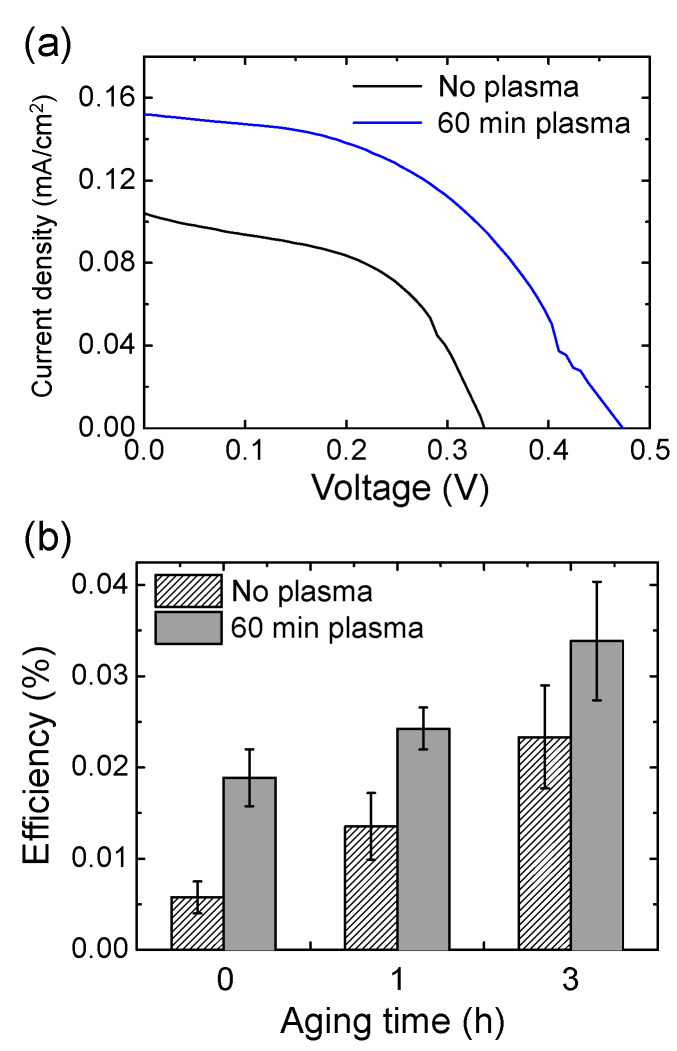
We investigated the effect of the plasma treatment of the TiO2 photoanode on the photovoltaic performance of the eco-friendly DSSCs based on chlorophyll. It was also reported that chlorophyll was better adsorbed onto the plasma-treated TiO2 surface due to the reduction of the oxygen vacancies [25,30]. The TiO2 photoanode of the eco-friendly DSSCs was treated with air plasma for 60 min before chlorophyll staining. Figure 6 and Table 3 compare the photovoltaic characteristics of the chlorophyll-based DSSCs with and without the plasma treatment. Both VOC and JSC increased after the plasma treatment for 60 min. As a result, when compared at 3 h of aging time, the plasma treatment improved the efficiency by ~50%. Notably, the resulting efficiency was comparable to those of the previously reported chlorophyll-based DSSCs with non-aqueous electrolytes [31,32,33]. The efficiency was still low, even compared to the eosin-Y-based DSSCs in Figure 4d. This was largely due to the low current density and needed to be significantly improved for practical use. According to the literature [34], 10 g of spinach contains 6.91 mg of chlorophyll. In this study, if we assume that all of the chlorophyll molecules in spinach were completely extracted, the maximum molar concentration of the chlorophyll staining solution was ~0.052 mM. Therefore, the actual concentration should be relatively low compared to the typical concentration for dye staining of DSSCs [35,36,37]. The efficiency of the eco-friendly DSSCs could be further improved by optimizing the conditions of the chlorophyll staining and the plasma treatment.
Figure 6.
(a) J–V curve graphs of the eco-friendly DSSCs with chlorophyll with and without plasma treatment for 60 min at 3 h of aging time. (b) Changes in the efficiency of DSSC with and without plasma treatment for 60 min according to aging time.
Table 3.
Photovoltaic performance parameters of the chlorophyll-based DSSCs with and without plasma treatment at 3 h of aging time.
| Plasma Treatment |
VOC (V) | JSC (mA/cm2) | Fill Factor (%) | Efficiency (%) |
|---|---|---|---|---|
| Without plasma |
0.46 | 0.089 | 56.4 | 0.023 |
| With plasma for 60 min |
0.46 | 0.14 | 52.4 | 0.033 |
4. Conclusions
In conclusion, the eco-friendly DSSCs were fabricated by employing the water-based electrolyte and the commercial organic dye of eosin Y. The commercial dye without any further modification was introduced into the water-electrolytes to deal with the issue of poor adsorption of eosin Y onto TiO2. The effects of the aging time and the composition of the dye-electrolyte on the photovoltaic performance of the eco-friendly DSSCs were investigated. To improve the dye adsorption and wettability of the water-based electrolyte, the surface of the TiO2 photoanode was treated by air plasma. It turns out that the plasma treatment was highly effective. The photovoltaic efficiency of the eco-friendly DSSCs increased by ~3.4 times after the plasma treatment, compared to that without the plasma treatment. For more eco-friendly DSSCs, the organic synthetic dye was replaced by the naturally-derived chlorophyll. Finally, the eco-friendly DSSCs based on the chlorophyll photosensitizer were fabricated. The resulting efficiency with the plasma treatment was comparable to those of the chlorophyll-based DSSCs with non-aqueous electrolytes. Even though many issues still need to be solved, such as low photovoltaic efficiency, expensive Pt catalyst, etc., we believe that such an eco-friendly DSSCs based on aqueous electrolytes and natural photosensitizers could be the suitable energy device structure with a minimum environmental footprint in the future.
Author Contributions
Conceptualization, H.-J.K.; Formal analysis, J.-H.K.; Investigation, J.-H.K., S.-Y.P., D.-H.L. and S.-Y.L.; Methodology, J.-H.K.; Resources, J.C.; Supervision, H.-J.K.; Writing—original draft, J.-H.K., S.-Y.P., D.-H.L. and S.-Y.L.; Writing—review & editing, J.C. and H.-J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Research Program funded by SeoulTech (Seoul National University of Science and Technology).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Iftikhar H., Sonai G.G., Hashmi S.G., Nogueira A.F., Lund P.D. Progress on Electrolytes Development in Dye-Sensitized Solar Cells. Materials. 2019;12:1998. doi: 10.3390/ma12121998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bella F., Gerbaldi C., Barolo C., Grätzel M. Aqueous dye-sensitized solar cells. Chem. Soc. Rev. 2015;44:3431–3473. doi: 10.1039/C4CS00456F. [DOI] [PubMed] [Google Scholar]
- 3.Green M.A., Dunlop E.D., Hohl-Ebinger J., Yoshita M., Kopidakis N., Ho-Baillie A.W. Solar cell efficiency tables (Version 55) Prog. Photovolt. Res. Appl. 2019;28:3–15. doi: 10.1002/pip.3228. [DOI] [Google Scholar]
- 4.NREL N. Research Cell Efficiency Records. [(accessed on 1 April 2021)]; Available online: https://www.nrel.gov/pv/cell-efficiency.html.
- 5.Yoshikawa K., Kawasaki H., Yoshida W., Irie T., Konishi K., Nakano K., Uto T., Adachi D., Kanematsu M., Uzu H., et al. Silicon heterojunction solar cell with interdigitated back contacts for a photoconversion efficiency over 26% Nat. Energy. 2017;2:1–8. doi: 10.1038/nenergy.2017.32. [DOI] [Google Scholar]
- 6.Kakiage K., Aoyama Y., Yano T., Oya K., Fujisawa J.-I., Hanaya M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015;51:15894–15897. doi: 10.1039/C5CC06759F. [DOI] [PubMed] [Google Scholar]
- 7.Nemala S.S., Kartikay P., Agrawal R.K., Bhargava P., Mallick S., Bohm S. Few layers graphene based conductive composite inks for Pt free stainless steel counter electrodes for DSSC. Sol. Energy. 2018;169:67–74. doi: 10.1016/j.solener.2018.02.061. [DOI] [Google Scholar]
- 8.Ju M.J., Jeon I.-Y., Kim H.M., Choi J.I., Jung S.-M., Seo J.-M., Choi I.T., Kang S.H., Kim H.S., Noh M.J., et al. Edge-selenated graphene nanoplatelets as durable metal-free catalysts for iodine reduction reaction in dye-sensitized solar cells. Sci. Adv. 2016;2:e1501459. doi: 10.1126/sciadv.1501459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y., Liu Y., Zakeeruddin S.M., Hagfeldt A., Grätzel M. Direct Contact of Selective Charge Extraction Layers Enables High-Efficiency Molecular Photovoltaics. Joule. 2018;2:1108–1117. doi: 10.1016/j.joule.2018.03.017. [DOI] [Google Scholar]
- 10.Zhang H., Qiu L., Xu D., Zhang W., Yan F. Performance enhancement for water based dye-sensitized solar cells via addition of ionic surfactants. J. Mater. Chem. A. 2013;2:2221–2226. doi: 10.1039/C3TA14571A. [DOI] [Google Scholar]
- 11.Galliano S., Bella F., Bonomo M., Viscardi G., Gerbaldi C., Boschloo G., Barolo C. Hydrogel Electrolytes Based on Xanthan Gum: Green Route Towards Stable Dye-Sensitized Solar Cells. Nanomaterials. 2020;10:1585. doi: 10.3390/nano10081585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Law C., Pathirana S.C., Li X., Anderson A.Y., Barnes P.R.F., Listorti A., Ghaddar T.H., O′regan B.C. Water-Based Electrolytes for Dye-Sensitized Solar Cells. Adv. Mater. 2010;22:4505–4509. doi: 10.1002/adma.201001703. [DOI] [PubMed] [Google Scholar]
- 13.Syafinar R., Gomesh N., Irwanto M., Fareq M., Irwan Y. Chlorophyll Pigments as Nature Based Dye for Dye-Sensitized Solar Cell (DSSC) Energy Procedia. 2015;79:896–902. doi: 10.1016/j.egypro.2015.11.584. [DOI] [Google Scholar]
- 14.Hassan H.C., Abidin Z.H.Z., Chowdhury F.I., Arof A.K. A High Efficiency Chlorophyll Sensitized Solar Cell with Quasi Solid PVA Based Electrolyte. Int. J. Photoenergy. 2016;2016:1–9. doi: 10.1155/2016/3685210. [DOI] [Google Scholar]
- 15.Yang R.-Y., Chen H.-Y., Lai F.-D. Performance Degradation of Dye-Sensitized Solar Cells Induced by Electrolytes. Adv. Mater. Sci. Eng. 2012;2012:1–4. doi: 10.1155/2012/902146. [DOI] [Google Scholar]
- 16.Agrell H.G., Lindgren J., Hagfeldt A. Degradation mechanisms in a dye-sensitized solar cell studied by UV–VIS and IR spectroscopy. Sol. Energy. 2003;75:169–180. doi: 10.1016/S0038-092X(03)00248-2. [DOI] [Google Scholar]
- 17.Hinsch A., Kroon J.M., Kern R., Uhlendorf I., Holzbock J., Meyer A., Ferber J. Long-term stability of dye-sensitised solar cells. Prog. Photovolt. Res. Appl. 2001;9:425–438. doi: 10.1002/pip.397. [DOI] [Google Scholar]
- 18.Koo H.-J., Velev O.D. Regenerable Photovoltaic Devices with a Hydrogel-Embedded Microvascular Network. Sci. Rep. 2013;3:srep02357. doi: 10.1038/srep02357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Hagfeldt A., Xiao X.-R., Lindquist S.-E. Investigation of influence of redox species on the interfacial energetics of a dye-sensitized nanoporous TiO2 solar cell. Sol. Energy Mater. Sol. Cells. 1998;55:267–281. doi: 10.1016/S0927-0248(98)00111-1. [DOI] [Google Scholar]
- 20.Jung Y.-S., Yoo B., Lim M.K., Lee S.Y., Kim K.-J. Effect of Triton X-100 in water-added electrolytes on the performance of dye-sensitized solar cells. Electrochim. ACTA. 2009;54:6286–6291. doi: 10.1016/j.electacta.2009.06.006. [DOI] [Google Scholar]
- 21.Han J.-B., Wang X., Wang N., Wei Z.-H., Yu G.-P., Zhou Z.-G., Wang Q.-Q. Effect of plasma treatment on hydrophilic properties of TiO2 thin films. Surf. Coat. Technol. 2006;200:4876–4878. doi: 10.1016/j.surfcoat.2005.04.036. [DOI] [Google Scholar]
- 22.Park K.-H., Dhayal M. High efficiency solar cell based on dye sensitized plasma treated nano-structured TiO2 films. Electrochem. Commun. 2009;11:75–79. doi: 10.1016/j.elecom.2008.10.020. [DOI] [Google Scholar]
- 23.Wang W., Chen J., Luo J., Zhang Y., Gao L., Liu Y., Sun J. Effects of low pressure plasma treatments on DSSCs based on rutile TiO2 array photoanodes. Appl. Surf. Sci. 2015;324:143–151. doi: 10.1016/j.apsusc.2014.10.099. [DOI] [Google Scholar]
- 24.Kim H.J., Kim J., Hong B. Effect of hydrogen plasma treatment on nano-structured TiO2 films for the enhanced performance of dye-sensitized solar cell. Appl. Surf. Sci. 2013;274:171–175. doi: 10.1016/j.apsusc.2013.03.006. [DOI] [Google Scholar]
- 25.Chang H., Yang Y.-J., Li H.-C., Hsu C.-C., Cheng I.-C., Chen J.Z., Hsu J.C.-C. Preparation of nanoporous TiO2 films for DSSC application by a rapid atmospheric pressure plasma jet sintering process. J. Power Sources. 2013;234:16–22. doi: 10.1016/j.jpowsour.2013.01.113. [DOI] [Google Scholar]
- 26.Parvez K., Yoo G.M., Kim J.H., Ko M.J., Kim S.R. Comparative study of plasma and ion-beam treatment to reduce the oxygen vacancies in TiO2 and recombination reactions in dye-sensitized solar cells. Chem. Phys. Lett. 2010;495:69–72. doi: 10.1016/j.cplett.2010.06.038. [DOI] [Google Scholar]
- 27.Weerasinghe J., Sen S., Kumari J., Dissanayake M., Senadeera G., Thotawatthage C., Ekanayake M., Zhou R., Cullen P.J., Sonar P., et al. Efficiency enhancement of low-cost metal free dye sensitized solar cells via non-thermal atmospheric pressure plasma surface treatment. Sol. Energy. 2021;215:367–374. doi: 10.1016/j.solener.2020.12.044. [DOI] [Google Scholar]
- 28.Arof A.K., Ping T.L. Chlorophyll. BoD–Books on Demand; Norderstedt, Germany: 2017. Chlorophyll as Photosensitizer in Dye-Sensitized Solar Cells; pp. 105–121. [DOI] [Google Scholar]
- 29.Al-Alwani M.A., Mohamad A.B., Kadhum A.A.H., Ludin N.A. Effect of solvents on the extraction of natural pigments and adsorption onto TiO2 for dye-sensitized solar cell applications. Spectrochim. ACTA Part. A Mol. Biomol. Spectrosc. 2015;138:130–137. doi: 10.1016/j.saa.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Kim J.T., Kim S.H. Surface modification of TiO2 electrode by various over-layer coatings and O2 plasma treatment for dye sensitized solar cells. Sol. Energy Mater. Sol. Cells. 2011;95:336–339. doi: 10.1016/j.solmat.2010.04.045. [DOI] [Google Scholar]
- 31.Amao Y., Komori T. Bio-photovoltaic conversion device using chlorine-e6 derived from chlorophyll from Spirulina adsorbed on a nanocrystalline TiO2 film electrode. Biosens. Bioelectron. 2004;19:843–847. doi: 10.1016/j.bios.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 32.Lim A., Manaf N.H., Tennakoon K., Chandrakanthi R.L.N., Lim L.B.L., Bandara J.M.R.S., Ekanayake P. Higher Performance of DSSC with Dyes from Cladophora sp. as Mixed Cosensitizer through Synergistic Effect. J. Biophys. 2015;2015:1–8. doi: 10.1155/2015/510467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arifin Z., Soeparman S., Widhiyanuriyawan D., Sutanto B., Suyitno S. Performance enhancement of dye-sensitized solar cells (DSSCs) using a natural sensitizer. International Conference on Engineering, Science and Nanotechnology 2016 (ICESNANO 2016) Int. J. Photoenergy. 2017:2704864. doi: 10.1063/1.4968376. [DOI] [Google Scholar]
- 34.Bohn T., Walczyk T. Determination of chlorophyll in plant samples by liquid chromatography using zinc–phthalocyanine as an internal standard. J. Chromatogr. A. 2004;1024:123–128. doi: 10.1016/j.chroma.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 35.Rahman M., Umar A.A., Taslim R., Salleh M.M. Effect of organic dye, the concentration and dipping time of the organic dye N719 on the photovoltaic performance of dye-sensitized ZnO solar cell prepared by ammonia-assisted hydrolysis technique. Electrochim. ACTA. 2013;88:639–643. doi: 10.1016/j.electacta.2012.10.146. [DOI] [Google Scholar]
- 36.Chiba Y., Islam A., Watanabe Y., Komiya R., Koide N., Han L. Dye-Sensitized Solar Cells with Conversion Efficiency of 11.1% Jpn. J. Appl. Phys. 2006;45:L638–L640. doi: 10.1143/JJAP.45.L638. [DOI] [Google Scholar]
- 37.Freitag M., Teuscher J., Saygili Y., Zhang X., Giordano F., Liska P., Hua J., Zakeeruddin S.M., Moser J.-E., Grätzel M., et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photon. 2017;11:372–378. doi: 10.1038/nphoton.2017.60. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.