Abstract
Background
Severe insulin resistance is an uncommon finding in patients with type 2 diabetes but is often associated with difficult to managing blood glucose. While severe insulin resistance is most frequently seen in the setting of medication side effects or rare genetic conditions, this report of two cases highlights the presence of severe insulin resistance in the setting of severe COVID-19 and explores how this may contribute to the poor prognosis of patients with diabetes who become infected with SARS-CoV-2.
Case presentation
Here we present the cases of two African-American women with pre-existing type 2 diabetes who developed severe COVID-19 requiring mechanical ventilation and concurrent severe insulin resistance with total daily insulin dose requirements of greater than 5 unit/kg. Both patients received aggressive insulin infusion and subcutaneous insulin therapy to obtain adequate glucose management. As their COVID-19 clinical course improved, their severe insulin resistance improved as well.
Conclusions
The association between critical illness and hyperglycemia is well documented in the literature, however severe insulin resistance is not commonly identified and may represent a unique clinical feature of the interaction between SARS-CoV-2 infection and type 2 diabetes.
Keywords: Diabetes, COVID-19, Severe Insulin Resistance
Introduction
SARS-CoV-2 infection is associated with severe hyperglycemia, which has been associated with poor patient outcomes [1–4]. While hyperglycemia is a feature of many severe infections, severe insulin resistance is less frequently documented. More commonly, severe insulin resistance is seen in the setting of medications or rare genetic disorders [5]. Here we present two cases of patients with severe Coronavirus Disease (COVID-19) who presented with insulin requirements greater than 5 units/kg. We use these cases as a starting point to explore the possible interactions between glucose homeostasis, SARS-CoV-2 infection and insulin treatment.
Case report 1
A 41-year old African-American woman with a history of type 2 diabetes for at least 2 years, obesity (body mass index (BMI), 44 kg/m2), hypertension, hyperlipidemia and sleep apnea presented to the emergency department at an outside hospital with acute worsening of shortness of breath which had begun one week prior to presentation. On presentation she was hypoxic with associated confusion and was emergently intubated and mechanically ventilated. Her diabetes history was notable for a worsening of glucose control over the past two years, with her hemoglobin A1c increasing from 8.4 % to 2018 to 12.8 % in August of 2019 in the setting of medication non-adherence and ongoing depression. At the time of admission, her hemoglobin A1c was 11.6 %. Chart review indicated that she was prescribed Lantus 60 units daily, metformin 1000 mg twice daily, glimepiride 4 mg twice daily with meals and 1.5 mg dulaglutide weekly as an outpatient. Physical exam was notable for acanthosis nigricans.
On admission, laboratory testing was notable for a sodium of 130 mEq/L, potassium 5.0 mEq/L, chloride 93 mmol/L, bicarbonate 16 mmol/L (anion gap 21), blood glucose 760 mg/dL, creatinine 1.65 mg/dL (eGFR 44), lactic acid of 8.33 mmol/L and serum β-hydroxybutyrate of 2.69 mmol/L. Arterial blood gas showed a pH of 7.36, pCO2 of 31 mmHg and pO2 of 55 mmHg, consistent with a mixed anion gap metabolic acidosis and respiratory alkalosis. SARS-CoV-2 PCR testing was positive. See Table 1 for additional laboratory values, including inflammatory markers, which were initially elevated on admission.
Table 1.
Laboratory values measured on admission
| Laboratory Test | Patient 1 | Patient 2 |
|---|---|---|
| WBC (cells x103/uL) | 19.9 | 18.34 |
| Neutrophils (cells x103/uL) | 17.3 | 14.12 |
| Lymphocytes (cells x103/uL) | 0.8 | 2.57 |
| Monocytes (cells x103/uL) | 1.9 | 1.28 |
| Hemoglobin (g/dL) | 13.7 | 11.6 |
| Serum Chemistries | ||
| Sodium (mEq/L) | 130 | 141 |
| Potassium (mEq/L) | 5 | 4.6 |
| Chloride (mEq/L) | 93 | 100 |
| Bicarbonate (mEq/L) | 16 | 27 |
| BUN (mg/dL) | 19 | 74 |
| Creatinine (mg/dL) | 1.65 | 2.01 |
| Glucose (mg/dL) | 760 | 272 |
| Anion Gap | 21 | 14 |
| Calcium (mg/dL) | 8.7 | 9.2 |
| Albumin (g/dL) | 3.9 | 3.8 |
| Alkaline Phosphatase (IU/L) | 105 | 98 |
| AST (units/L) | 81 | 44 |
| ALT (units/L) | 44 | 69 |
| Total Bilirubin (mg/dL) | 1.1 | <0.2 |
| Arterial Blood Gas | ||
| Arterial pH | 7.36 | 7.4 |
| Arterial CO2 (mmHg) | 31 | 56 |
| Arterial O2 (mmHg) | 55 | 68 |
| Inflammatory Markers | ||
| ESR (mm/hr) | 85 | 120 |
| CRP (mg/L) | 128.5 | 121.7 |
| CK (units/L) | - | 82 |
| Fibrinogen (mg/dL) | 669 | 295 (transfer) |
| LDH (units/L) | ||
| Admission | 810 | 885 |
| Transfer | - | 871 |
| Procalcitonin (ng/mL) | ||
| Admission | - | 0.72 |
| Transfer | 0.17 | 0.21 |
| D-Dimer (ng/mL) | ||
| Admission | 1547 | 969 |
| Transfer | 12.19 | > 35 |
| Ferritin (ng/mL) | ||
| Admission | 98 | 744 |
| Transfer | - | 595.3 |
| Triglycerides (mg/dL) | ||
| Admission | 352 | 166 |
| Transfer | 347 | 295 |
| Hemoglobin A1c | 11.60% | 7.30% |
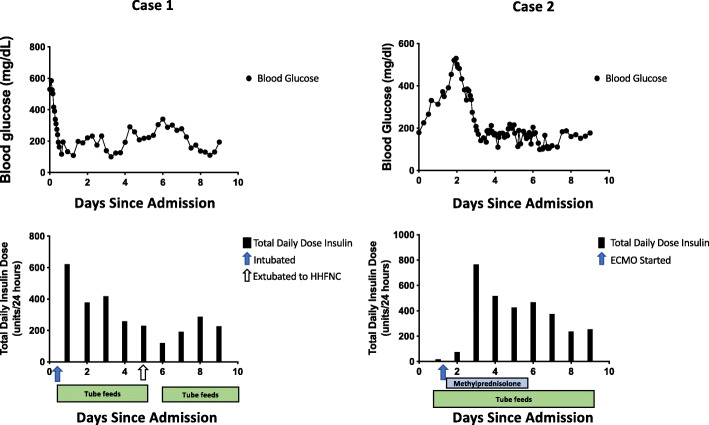
Due to the elevated serum ketones and anion-gap metabolic acidosis, she was treated for diabetic ketoacidosis (DKA) with a continuous insulin infusion at 7 units/hr (0.05 units/kg/hr) along with a normal saline infusion. Her blood glucose levels remained elevated in the 400–500 mg/dL range while her anion gap decreased to 15 but did not resolve. Over the next 36 h her insulin infusion rate peaked at 34.5 units/hr (0.26 units/kg/hr) and she was started on 50 units of insulin glargine and 50 units of regular insulin every 6 h in addition to the insulin infusion to facilitate weaning off of the insulin drip. During the first 36 h following transfer to our hospital, she had an average insulin requirement of 5 units/kg/day. Her insulin infusion was weaned off within 48 h and her subcutaneous insulin requirements continued to be elevated at 3.24 units/kg/day. Following extubation on day 6, her insulin requirements dropped to 211 units per day (1.64 units/kg) and she continued to require over 200 units of subcutaneous insulin daily for the next 13 days (Fig. 1). Her insulin requirements improved as she was weaned off of heated high flow nasal cannula and transitioned from tube feeds to a diabetic diet. Prior to discharge to a subacute rehabilitation center, she was transitioned to her home regimen of 60 units Lantus, metformin, glimepiride and dulaglutide. While in the hospital she had well-controlled blood sugars on this regimen. Two months after discharge, while still at the subacute rehabilitation center, her hemoglobin A1c was 6.0 %. Her kidney function had returned to her baseline (eGFR > 90).
Fig. 1.
Glucose levels and total daily insulin dose during the first 10 days following admission. Glucose levels (black circles, upper panel) and total daily dose of insulin (black bars, lower panel) by days following admission. Respiratory interventions indicated by arrows. Blue bars represent duration of 1 mg/kg methylprednisolone treatment for ARDS. Green bars represent duration of tube feeds
Case 2
A 47-year old African-American woman with a history of pre-diabetes on metformin, obesity (BMI 39 kg/m2), hypertension and hyperlipidemia presented with cough, dyspnea, hypoxia and fever and was emergently intubated. She was found to be SARS-CoV-2 positive. She was initially treated with hydroxychloroquine, azithromycin, 40 mg methylprednisolone twice daily and high dose vitamin C with blood glucoses recorded in the 300 s. Her hemoglobin A1c was 7.3 % on admission.
Regarding her diabetes history, she was diagnosed with pre-diabetes in 2016 with a hemoglobin A1c of 5.9 % and started on metformin therapy. Her hemoglobin A1c ranged between 5.5 and 6.2 % until her admission for COVID-19.
Following admission, her course was complicated by significant difficulty with oxygenation, pneumomediastinum due to barotrauma and acute kidney injury. She was ultimately transferred to our institution for extra-corporeal membrane oxygenation (ECMO) from an outside hospital. On arrival at our hospital, she received 1 dose of sarilumab (human monoclonal antibody against interleukin-6 (IL-6)) and was started on IV methylprednisolone. She rapidly developed worsening hyperglycemia with glucose levels above 400 mg/dL in the setting of methylprednisolone treatment and was started on an insulin infusion. Subcutaneous insulin was initiated simultaneously to assist in weaning off the insulin infusion as rapidly as possible. This protocol has recently been published in Gianchandani et al. [6]. Over the next 24 h she required insulin drip rates up to 45 units/hr in addition to 20 units insulin glargine twice daily and 16 units regular insulin every 6 h (see Fig. 1). The subcutaneous insulin was titrated up over 4 days until she was receiving 30 units of insulin glargine twice a day and 56 units of regular insulin every 6 h with drip rates between 2 and 10 units/hr. Her 24-hour insulin requirements peaked at 6.45 units/kg/day. After discontinuation of methylprednisolone her insulin requirements decreased significantly, her insulin infusion was titrated off and subcutaneous insulin was decreased to Lantus 25 units twice daily and regular insulin 48 units every 6 h. Despite this improvement, she did continue to require over 200 units of insulin daily via subcutaneous injection even after discontinuation of the methylprednisolone. Six days after methylprednisolone discontinuation, her insulin requirements dropped precipitously, which coincided with ECMO decannulation. Insulin was discontinued completely 12 days later. She continued to require mechanical ventilation for over 2 months and was discharged to a long-term care facility for further ventilator weaning. Six months after discharge from the hospital, her creatinine had not yet returned to baseline, but had improved to 1.12 (eGFR 58). Her hemoglobin A1c at that time was 5.9 % without any diabetes medications. She had successfully been liberated from the ventilator and returned home.
Discussion
Over the last year, a picture has emerged that illustrates the increased risk of severe infection and death in patients with diabetes who develop COVID-19. Almost 30 % of patients with diabetes who present to the hospital with COVID-19 require intubation and over 10 % will die within a week [1]. Patients with diabetes are more likely to require hospitalization and ICU admission and have a higher risk of death than those without diabetes [2–4]. Here we present the cases of two patients with a history of diabetes that developed COVID-19, difficult to control hyperglycemia and severe insulin resistance, prompting us to consider the mechanisms underlying the poor prognosis for patients with diabetes and how glucose control and insulin treatment might interact with this infection.
Prior to the COVID-19 pandemic, there was already a wealth of data demonstrating that hyperglycemia and insulin resistance during critical illness is associated with worse outcomes [7–10]. One of the remarkable features of the patients presented here is the degree of insulin resistance, with both patients requiring more than 5 units/kg/day shortly after admission. This meets the criteria for extreme insulin resistance, defined as more than 3 units/kg/day. Extreme insulin resistance is most often seen in the setting of rare genetic disorders such as lipodystrophy and severe insulin resistance syndrome, use of medications such as glucocorticoids or endocrinopathies such as Cushings [5].
As dexamethasone is one of the few treatments that decreases mortality in severe COVID-19 infection [11, 12], this is likely a significant contributor to the severe hyperglycemia in many patients with COVID-19. However, it is not the only cause, as the first patient described in this report developed severe hyperglycemia and insulin resistance despite never receiving steroids. Similarly, the second patient continued to have severe insulin resistance for 6 days following discontinuation of steroids.
Acute infections also lead to increased insulin resistance through enhanced secretion of the counter-regulatory hormones cortisol, glucagon and growth hormone [13]. In addition to the hormonal response to stress, cytokine expression may independently contribute to increasing insulin resistance during infection [14]. Thus, the robust inflammatory response in patients with SARS-CoV-2 could be a significant contributor to their severe insulin resistance [6], as both of our patients had elevated inflammatory markers that coincided with increasing insulin demands (Table 1).
The interaction between insulin resistance, severe hyperglycemia and inflammation is likely to be particularly important in the setting of SARS-CoV-2 infection, as the robust inflammatory response has been linked to poor outcomes [15]. In patients with diabetes who present with COVID-19, the inflammatory marker C-reactive protein (CRP) has been identified as one of the strongest risk factors for mortality [2], while patients with prolonged hyperglycemia have higher IL-6 and D-dimer levels and a higher risk of progressing to severe disease [16]. While inflammation leads to hyperglycemia and insulin resistance, hyperglycemia and insulin resistance also exacerbate inflammation, impair immune cell function and promote epithelial cell dysfunction, even in otherwise healthy volunteers [17–21].
Treatment of hyperglycemia in patients with COVID-19 has been particularly difficult as many centers have attempted to limit insulin infusions to preserve PPE and minimize health care provider exposure, as treatment with insulin infusion require hourly finger stick blood glucose checks. Given the extraordinary doses of insulin required in the patients presented here, it is valuable to consider the potential effects of insulin beyond its hypoglycemic effects, and in particular, its role in inflammation.
Insulin’s role in inflammation is likely to be context dependent. When evaluated in the setting of obesity-related insulin resistance, high doses of insulin may be pro-inflammatory. In both obese mice and humans, hyperinsulinemia results in elevated adipose tissue cytokine levels [22–25], while this does not occur in healthy, non-obese and non-insulin resistant patients [26]. In contrast, many studies have demonstrated that insulin treatment in the setting of sepsis is anti-inflammatory. In multiple animal models of infection, insulin treatment decreases systemic cytokines independent of glucose levels [27–29]. This finding holds true in human studies as well. In healthy patients with both type 1 and type 2 diabetes, insulin infusion (with dextrose to maintain euglycemia) leads to decreased reactive oxygen species, systemic cytokine gene expression and serum CRP levels [30–32].
Conclusions
Based on our review of the literature and exemplified by the cases presented here, it is likely that hyperglycemia in patients with COVID-19 infection worsens the dramatic inflammation and cytokine storm that accompanies severe infection. Insulin treatment reduces blood glucose levels and may have an anti-inflammatory action in the setting of COVID-19 sepsis.
Acknowledgements
Not applicable.
Abbreviations
- COVID-19
Coronavirus Disease 2019
- DKA
Diabetic ketoacidosis
- ECMO
Extra-corporeal membrane oxygenation
- CRP
C-reactive protein
- IL-6
Interleukin-6
Authors’ contributions
AHA drafted the manuscript. AHA and RYG were involved in the care of at least one of the patients described; all authors reviewed and edited the manuscript; and have approved the final version of the article.
Funding
AHA is supported by grant F32 DK122660 from the National Institutes of Diabetes and Digestive and Kidney Diseases. AW has received funding from Novo Nordisk and research support from United Health Group and Eli Lilly.
Availability of data and materials
Data sharing is not applicable to this case report as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.For the CORONADO investigators. Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia [Internet]. 2020 May 29 [cited 2020 Jun 1]; Available from: http://link.springer.com/10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed]
- 2.Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care. 2020;43(7):1399-407. [DOI] [PubMed]
- 3.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):535–45. [DOI] [PMC free article] [PubMed]
- 4.Zhu L, She Z-G, Cheng X, Qin J-J, Zhang X-J, Cai J, et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31(6):S1550413120302382. [DOI] [PMC free article] [PubMed]
- 5.Ovalle F. Clinical approach to the patient with diabetes mellitus and very high insulin requirements. Diabetes Res Clin Pract. 2010;90(3):231–42. doi: 10.1016/j.diabres.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Gianchandani R, Esfandiari NH, Ang L, Iyengar J, Knotts S, Choksi P, et al. Managing Hyperglycemia in the COVID-19 Inflammatory Storm. Diabetes. 2020;69(10):dbi200022. [DOI] [PubMed]
- 7.Bonizzoli M, Zagli G, Lazzeri C, Degl’Innocenti S, Gensini G, Peris A. Early insulin resistance in severe trauma without head injury as outcome predictor? A prospective, monocentric pilot study. Scand J Trauma Resusc Emerg Med. 2012;20:69. [DOI] [PMC free article] [PubMed]
- 8.Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;4(9206):773–8. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 9.Lazzeri C, Bonizzoli M, Cianchi G, Ciapetti M, Socci F, Peris A. The prognostic role of peak glycemia and glucose variability in trauma: a single-center investigation. Acta Diabetol. 2020;57(8):931-5. [DOI] [PubMed]
- 10.Brealey D, Singer M. Hyperglycemia in critical illness: a review. J Diabetes Sci Technol. 2009;1(6):1250–60. doi: 10.1177/193229680900300604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324(13):1307. [DOI] [PMC free article] [PubMed]
- 12.The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N Engl J Med. 2020;384:NEJMoa2021436. [DOI] [PMC free article] [PubMed]
- 13.Rizza RA, Cryer PE, Gerich JE. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aljada A, Ghanim H, Assian E, Dandona P. Tumor necrosis factor-alpha inhibits insulin-induced increase in endothelial nitric oxide synthase and reduces insulin receptor content and phosphorylation in human aortic endothelial cells. Metab Clin Exp. 2002;51(4):487–91. doi: 10.1053/meta.2002.31339. [DOI] [PubMed] [Google Scholar]
- 15.Blanco-Melo D, Nilsson-Payant BE, Liu W-C, Uhl S, Hoagland D, Møller R, et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181(5):S009286742030489X. [DOI] [PMC free article] [PubMed]
- 16.Sardu C, D’Onofrio N, Balestrieri ML, Barbieri M, Rizzo MR, Messina V, et al. Outcomes in Patients With Hyperglycemia Affected by Covid-19: Can We Do More on Glycemic Control? Dia Care. 2020;43(7):dc200723. [DOI] [PMC free article] [PubMed]
- 17.Ieronymaki E, Theodorakis EM, Lyroni K, Vergadi E, Lagoudaki E, Al-Qahtani A, et al. Insulin Resistance in Macrophages Alters Their Metabolism and Promotes an M2-Like Phenotype. J Immunol. 2019;15(6):1786–97. doi: 10.4049/jimmunol.1800065. [DOI] [PubMed] [Google Scholar]
- 18.Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30(5):748–56. doi: 10.1007/s00134-004-2167-y. [DOI] [PubMed] [Google Scholar]
- 19.Nielson CP, Hindson DA. Inhibition of polymorphonuclear leukocyte respiratory burst by elevated glucose concentrations in vitro. Diabetes. 1989;38(8):1031–5. doi: 10.2337/diabetes.38.8.1031. [DOI] [PubMed] [Google Scholar]
- 20.Perner A, Nielsen SE, Rask-Madsen J. High glucose impairs superoxide production from isolated blood neutrophils. Intensive Care Med. 2003;29(4):642–5. doi: 10.1007/s00134-002-1628-4. [DOI] [PubMed] [Google Scholar]
- 21.Ghanim H, Aljada A, Daoud N, Deopurkar R, Chaudhuri A, Dandona P. Role of inflammatory mediators in the suppression of insulin receptor phosphorylation in circulating mononuclear cells of obese subjects. Diabetologia. 2007;50(2):278–85. doi: 10.1007/s00125-006-0508-9. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen DJ, Guilherme A, Danai LV, Heyda L, Matevossian A, Cohen J, et al. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Mol Metab. 2015;4(7):507–18. doi: 10.1016/j.molmet.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang AMY, Wellberg EA, Kopp JL, Johnson JD. Hyperinsulinemia in Obesity, Inflammation, and Cancer. Diabetes Metab J. 2021. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 24.Westerbacka J, Cornér A, Kannisto K, Kolak M, Makkonen J, Korsheninnikova E, et al. Acute in vivo effects of insulin on gene expression in adipose tissue in insulin-resistant and insulin-sensitive subjects. Diabetologia. 2006;49(1):132–40. doi: 10.1007/s00125-005-0075-5. [DOI] [PubMed] [Google Scholar]
- 25.Krogh-Madsen R, Plomgaard P, Keller P, Keller C, Pedersen BK. Insulin stimulates interleukin-6 and tumor necrosis factor-alpha gene expression in human subcutaneous adipose tissue. Am J Physiol Endocrinol Metab. 2004;286(2):E234–8. doi: 10.1152/ajpendo.00274.2003. [DOI] [PubMed] [Google Scholar]
- 26.Tannous A, Bradford AP, Kuhn K, Fought A, Schauer I, Santoro N. A randomised trial examining inflammatory signaling in acutely induced hyperinsulinemia and hyperlipidemia in normal weight women-the reprometabolic syndrome. PLoS One. 2021;16(3):e0247638. doi: 10.1371/journal.pone.0247638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagar JA, Edin ML, Lih FB, Thurlow LR, Koller BH, Cairns BA, et al. Lipopolysaccharide Potentiates Insulin-Driven Hypoglycemic Shock. J Immunol. 2017;15(10):3634–43. doi: 10.4049/jimmunol.1700820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holger JS, Dries DJ, Barringer KW, Peake BJ, Flottemesch TJ, Marini JJ. Cardiovascular and metabolic effects of high-dose insulin in a porcine septic shock model. Acad Emerg Med. 2010;17(4):429–35. doi: 10.1111/j.1553-2712.2010.00695.x. [DOI] [PubMed] [Google Scholar]
- 29.Brix-Christensen V, Andersen SK, Andersen R, Mengel A, Dyhr T, Andersen NT, et al. Acute hyperinsulinemia restrains endotoxin-induced systemic inflammatory response: an experimental study in a porcine model. Anesthesiology. 2004;100(4):861–70. doi: 10.1097/00000542-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Dandona P, Ghanim H, Green K, Sia CL, Abuaysheh S, Kuhadiya N, et al. Insulin infusion suppresses while glucose infusion induces Toll-like receptors and high-mobility group-B1 protein expression in mononuclear cells of type 1 diabetes patients. Am J Physiol Endocrinol Metab. 2013;304(8):E810-818. [DOI] [PubMed]
- 31.Ghanim H, Korzeniewski K, Sia CL, Abuaysheh S, Lohano T, Chaudhuri A, et al. Suppressive effect of insulin infusion on chemokines and chemokine receptors. Diabetes Care. 2010;33(5):1103–8. doi: 10.2337/dc09-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghanim H, Mohanty P, Deopurkar R, Sia CL, Korzeniewski K, Abuaysheh S, et al. Acute modulation of toll-like receptors by insulin. Diabetes Care. 2008;31(9):1827–31. doi: 10.2337/dc08-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this case report as no datasets were generated or analyzed during the current study.