Abstract
Xanthohumol (XH) is an important prenylated flavonoid that is found within the inflorescence of Humulus lupulus L. (Hop plant). XH is an important ingredient in beer and is considered a significant bioactive agent due to its diverse medicinal applications, which include anti-inflammatory, antimicrobial, antioxidant, immunomodulatory, antiviral, antifungal, antigenotoxic, antiangiogenic, and antimalarial effects as well as strong anticancer activity towards various types of cancer cells. XH acts as a wide ranging chemopreventive and anticancer agent, and its isomer, 8-prenylnaringenin, is a phytoestrogen with strong estrogenic activity. The present review focuses on the bioactivity of XH on various types of cancers and its pharmacokinetics. In this paper, we first highlight, in brief, the history and use of hops and then the chemistry and structure–activity relationship of XH. Lastly, we focus on its prominent effects and mechanisms of action on various cancers and its possible use in cancer prevention and treatment. Considering the limited number of available reviews on this subject, our goal is to provide a complete and detailed understanding of the anticancer effects of XH against different cancers.
Keywords: xanthohumol, Humulus lupulus L., cancer, proliferation, apoptosis, therapy, prevention, molecular targets
1. Introduction
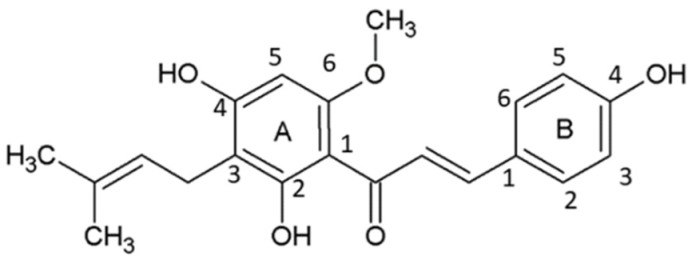
Tremendous interest has emerged toward bioactive natural compounds owing to their pharmacological activities in various chronic diseases, such as neoplasms, neurological diseases, viral and bacterial infections, and many more. Among different naturally-occurring compounds, hops, which are obtained from the inflorescence (female) of Humulus lupulus L. (family Cannabaceae), have gained attention due to their potential bioactive effects; for instance, anti-inflammatory, antimicrobial [1], antioxidant [2], immunomodulatory [3], antiviral, antifungal [4], antigenotoxic [5], antiangiogenic [6], antimalarial [7], diacylglycerol acyl transferase inhibition [8], and anticancer effects [9]. A diverse array of bioactive phenolic compounds, which are prenylated or prenylated flavonoids in nature, are present in hops. The chief prenylated chalcone available in hops is xanthohumol (XH, Figure 1) which is an ingredient used by the beer industry to enhance its aroma and bitter taste [10]. Chemically, XH is written as 3′-[3, 3-dimethyl allyl]-2′,4′,4-trihydroxy-6′-methoxychalcone [11].
Figure 1.
Chemical structure of xanthohumol depicting A and B rings.
The prenylated flavonoids, such as isoxanthohumol, 6-prenylnaringenin, and 8-prenylnaringenin (8-PN), are isomers of XH, which have potent anti-inflammatory, antineoplastic, antidiabetic, estrogenic, antiviral, and antibacterial activities [12]. XH’s structure was identified first in 1957 by Verzele et al. [13], but its advantageous pharmacological properties were not treasured until the 1990s. Prenylflavonoids which are obtained from hops have various biological activities against many ailments, including neoplasms, osteoporosis, postmenopausal hot flashes, digestive issues, neuralgia, toothaches, tension headaches, and earaches [14,15]. In 2007, the Committee on Herbal Medicinal Products of European Medicines Agency reported XH use in conventional medicine for the gentle treatment of symptoms of insomnia and mental stress. Further, the treatment for sleep disturbances, anxiety, and some other diseases by hops has been approved by Commission E of the Germany and European Scientific Cooperative on Phytotherapy [16].
The antiatherogenic activity of XH was investigated by Hirata et al. [17] using an in vivo hamster model. Their findings revealed the anti-atherosclerosis effect of XH is mediated by improved reverse cholesterol transport through efflux of cholesterol from macrophages and excretion to feces. The same study also explored the hepatic transcript analysis of XH, which concluded that XH enhanced the mRNA expression of cyp7a1 and abcg8. The findings of Hirata et al. [17] also concluded that XH decreases apolipoprotein B secretion, prevents oxidation of low-density lipoprotein, and inhibits the synthesis of triglycerides.
Stevens and Page [14] reported the various health applications of XH, including anticancer agents, dietary supplements, antioxidant activities, and estrogenic effects. XH is a wide spectrum chemopreventive phytochemical constituent which shows its activity by inhibiting early-stage tumor growth and metabolic activation of procarcinogens. These procarcinogens, such as 2-amino-3-methylimidazo[4,5-f] quinoline, are present in meats, and their activation is prevented by the inhibition of cytochrome P450 enzyme. Stevens and Page [14] confirmed that the dietetic intake of the XH through regular beer is not enough for achieving a chemopreventive effect. However, Magalhaes et al. [4] reviewed the production of XH-enriched beer and its antioxidant, cardioprotective, anti-inflammatory, antiproliferative, cancer chemopreventive, and broad spectrum anti-infective effects. XH mixed with phenethyl isothiocyanate activated Nrf2 and inhibited nuclear factor-κB (NF-κB) in pancreatic cells [18]. Another study by Lin et al. [19] performed various in vitro and in vivo experiments using XH in the context of several chronic diseases. Being a food supplement, XH has a vast array of biological effects and is emerging as a molecule of interest in treating numerous diseases. A recent publication of Iniguez et al. [20] reviewed the effects of XH on nutrition-related noncommunicable diseases. XH has promising effects against cancer by inhibiting angiogenesis, metastasis, and biotransformation of carcinogens as well as influencing programmed cell death and cell cycle arrest. So far, the work published on the health benefits of the XH has provided evidence of various biological effects; however, there is a need to evaluate its toxicity in humans and to develop bioavailability assays for prescribing effective doses. It is also important to study the nutritional content of XH.
Gerhäuser [21] reported the cancer chemoprotective activity of XH with diverse inhibitory mechanisms at various carcinogenesis stages, such as initiation, promotion, and progression. DNA synthesis inhibition, arresting of cell cycle at S phase and activity of enzymes was modulated by XH. Preneoplastic lesions were prevented by XH in organ culture of mouse mammary gland. Jiang et al. [11] reviewed the anticancer activity of XH against various types of cancers and found it was mediated through inhibition of metastasis and carcinogenesis as well as the modulation of key signaling pathways such as suppressing ERK1/2 and inducing reactive oxygen species (ROS). Additionally, it was found that XH can be used as a radio- and chemosensitizer.
Jiang et al. [11] also reviewed the limitations of XH in clinical applications. The major concern is its low bioavailability, which was reported by Nookandeh et al. [22] based on studies conducted on female Sprague Dawley rats, in which the orally administered XH was excreted within 48 h through feces and urine [22]. Pang et al. [23] concluded that the reason behind XH’s poor bioavailability was its localization in the cytosol and ability to bind to cellular proteins. Therefore, attempts must be made to improve the bioavailability of XH by changing the structure and optimizing its physical and chemical properties. It is also reported that interaction of XH with the phosphatidylcholine membrane causes biophysical change in its bilayers.
Ventureli et al. [24] reviewed bioavailability studies of XH. The estimated bioavailability of XH in rats was found to be 33.1% when administered orally and the range of low and high doses were 1.86 and 16.9 mg/kg body weight, respectively. The low solubility of XH is the limiting factor for gastrointestinal absorption. There is limited information available about the bioavailability and bioactivity of XH in humans, but preclinical studies on XH suggests that it has a cancer-preventive effect.
This review will provide complete and up-to-date information on the plausible therapeutic applications of XH against different cancer types. We have also discussed, in detail, the biopharmaceutical limitations of XH that must be resolved, its various important pharmacokinetic aspects, and its novel molecular targets. In this review, we will first highlight, in brief, the history and use of hops as well as the chemistry and structure–activity relationship (SAR) of XH. Then, we analyze the prominent activities of XH on different types of cancers, its mechanism of action, and its possible use to treat and prevent numerous oncologic diseases.
2. Description and Utilization of Hops
2.1. History of Hops
The joy of drinking beer and art of brewing is over 5500 years old. In 1935, a voyage of archaeologists from the Pennsylvania University Museum and the American Schools of Oriental Science in Mesopotamia discovered a seal baked into pottery that depicted the contents of a brewery vat which was stirred with long poles by two brewery workers. This instance was dated back to 3500–3100 BC [13].
Historical findings concluded that the origin of hops was in Asia, precisely in the lowlands of the Caucasus, southern Syria, and fertile Mesopotamia. From a botanical point of view, China is considered the place of origin as it is the solitary nation globally native to all hop species (Humulus lupulus L., H. scandens (Lour.) Merr., and H. yunnanensis Hu) [25,26,27]. The Slavic tribes were the first to cultivate and use hops for preserving and seasoning beer, beginning in 1500 to 1000 BC. Starting in the 13th century, other countries began to use hops for brewing purposes as well [28]. Pliny (23–79 AD), the great Roman naturalist, referred to hops as “wolf of the willow” because of twinning growth of hops among the willows, proved as “destructive as a wolf to a flock of sheep”. Hops have been cultivated in Germany in the Hallertau region since 736 AD, and in 860 AD, a hop garden was also planted in Nandlstadt [29]. Many monasteries were popular for their hopped beers during the Middle Ages (500 to 1400–1500 CE). The trade of beer has grown drastically due to the expansion of the towns and it was the main drink along with food at that time. In Germany from 1320–30 AD, hopped beer was usually preferred. Later in the 14th century, Belgium’s hop crop grew in importance, and northern German towns benefited from the sale of their hopped beer [30]. Due to the commercialization of hops from the 1990s onwards, the production of hops has been drastically increased.
Promptly, the medicinal possessions of hops were discovered and have made their route into traditional medicine. Conferring to the old herbarium for medicinal treatment, hops were used for the treatment of many conditions, like liver disease, foot odor, leprosy, disturbances in sleep, constipation, and for catharsis of blood. Hops alcohol extracts have been utilized in Chinese medicine for pulmonary tuberculosis and acute bacterial dysentery treatment as well as in Ayurvedic therapies in India [28].
2.2. Hop Botany
Hops belong to the Humulus genus which is made up of perennial, dioecious, and climbing vines. It is part of the Cannabaceae family which includes the Cannabis genus, also known as hemp, marijuana, or hashish [31,32]. There was a belief for years that the representation of genus Humulus was mainly by two species, i.e., Humulus lupulus L., the common hop, and H. japonicas Zieb et Zucc, the Japanese hop [33]. However, H. yunnanensis Hu was first described in 1936, and was thought to have originated from the Yunnan province of southern China at high elevations. H. yunnanensis was later identified as a third species of its own in 1978 [34]. The popular hop species H. japonicas has been an annual plant in Japan, China, and other neighboring islands and is mostly used for gardening purposes [35].
2.3. Hop Cultivation
Around 97% of the total cultivated hops worldwide are destined for the brewing industry, and H. lupulus L. is the most commonly grown hop for brewing. The production of hops globally is dominated by the USA and Germany, which produce around 70–80% of the world’s supply [36]. Hops are grown throughout most moderate climate regions and its cultivation requires ample sunlight, warm temperatures, high annual rainfall, and fertile soil. The major regions associated with Hop cultivation are given in Table 1.
Table 1.
Leading hop producing countries in the world.
| Country | Region |
|---|---|
| Germany | Hallertau region |
| USA | Washington, Oregon, and Idaho |
| Other countries | Poland, Czech Republic, South Africa, England, Slovenia, Ukraine, China, Australia, and New Zealand |
2.4. XH and Brewing
The basic brewing process includes mashing, boiling, fermentation, maturation, filtration, and bottling. The entire brewing process starts with the creation of a mash from malted barley combined with hot water. Malted starch is converted to sugars during this stage. The wort which contains elevated levels of sugar in water is then drawn from the base of mash and poured into a brewing kettle to boil [37,38,39]. During the wort boiling process, bitter taste and palatability to beer, hops are added at different times to build on aroma. The boiled wort is allowed to cool and is aerated. Yeast is then added to the wort for fermentation and generation of byproducts like alcohol and carbon dioxide take place. After fermentation, the green beer undergoes maturation and is then filtered. After filtration, it is carbonated and moved to the holding tank for bottling [4].
Wunderlich et al. [40] developed a technology known as XAN technology which is used during the brewing process to create XH-enriched beer. For unfiltered larger beers, an XH content of around 1–3 mg/L is obtained using this technique, and for filtered dark beers, an XH content of up to 10 mg/L can be achieved. In another study, an increase in XH yield and inhibition of XH isomerization during wort boiling was achieved by using special malts and cereals and the substances generated from the roasting process [30].
Although thought of as a modern invention, hop extracts have been created and used since the 19th century. In 1863, a steam method of hops extraction was patented by Richard Morland. In 1867, another method for extraction of hops using steam was patented by Theophile Breihaupt. Additionally, a method of hop extraction using carbon disulfide alone or a mixture of carbon disulfide and alcohol, ether, or chloroform was patented by John Johnson from Pennsylvania in 1869 [29].
3. Chemistry and SAR of XH
The structure of the XH molecule is made up of open-chain flavonoids with trans-configured A and B aromatic rings joined through a three-carbon, α,β-unsaturated carbonyl system substituted by hydroxyl groups at the 4th, 2nd, and 4th positions, methoxy group at the 6th position, and a prenyl unit at the 3rd position (Figure 1).
The biological action of the prenylated chalcones is due to the existence of the α,β-unsaturated keto group. The molecule’s lipophilicity is improved by replacing the A-ring with a prenyl unit and –OCH3 group, which gives it a robust affinity for biological membranes [41].
The SAR of XH was studied by Nuti et al. [42] to evaluate the antiangiogenic property by replacing the phenolic group on the B-ring of XH with various substituents provided with different steric and electronic properties like halogens, nitro groups, or methoxy groups. The phenyl group present on ring A was kept unchanged as it was found to be important for antiangiogenic activity. The analogues of the XH were synthesized by substituting various substituents that have shown potent antiangiogenic property, except for the compounds with chlorine and methoxymethyl groups because of poor solubility [42]. The antioxidant activity of desmethylxanthohumol was studied by synthesizing and evaluating its analogues. The SAR study revealed that lower toxicity was shown by the closed ring (cyclization of prenyl group) analogue than desmethylxanthohumol on human umbilical vein endothelial cells (HUVECs) and pheochromocytoma of the rat adrenal medulla (PC12) cells. Better antioxidant property was shown by compounds with 2,3,4-trihydroxyl phenyl and 2,3-dihydroxyl phenyl groups than by their counterparts with 2,4-dihydroxyl phenyl and 4-hydroxyl phenyl groups. Likewise, better antioxidant activity was shown by dimers than by their corresponding monomers. The chalcone with 2,4-dihydroxyl phenyl and 4-hydroxyl phenyl groups, 2,3,4-trihydroxyl phenyl, and the 2,3-dihydroxyl phenyl groups are easily oxidized to ortho-quinone in the H2O2-induced oxidation procedure and have more potent antioxidant properties than other tested compounds [43].
4. Anticancer Potential of XH Based on Preclinical Evidence
Several studies evaluating the potential of XH in cancer treatment have found that it is effective against numerous different cancer models both in vitro and in vivo. Various researchers have examined the anticancer effect of XH in different cancer types which are summarized in Table 2 and Table 3 and discussed in the following sections.
Table 2.
Antineoplastic effects and underlying mechanisms of action of XH based on in vitro experiments.
| Cancer Type | Cell Line | Effects | Mechanisms | Conc. | Reference |
|---|---|---|---|---|---|
| Breast cancer | Hs57BT and MDA-MB-231 | Decreased cell viability, cell invasion and proliferation | None | 4.78–6.7 µM | [57] |
| MDA-MB-231 | Decreased cell viability | ↑Caspase-3; ↑caspase-9; ↓Bax | 10 and 20 µM | [56] | |
| MCF-7 | Decreased proliferation | ↓ALP isoenzymes | 10 µM | [50] | |
| Adriamycin-resistant MCF-7 | Decreased cell viability, stemness, and increased radio- and chemosensitivity | ↑Apoptosis; ↑γ-H2AX; ↓STAT3; ↓MDR1; ↓EGFR | 10 µM | [59,68] | |
| Cervical cancer | Ca Ski | Decreased proliferation | ↑Apoptosis; ↑caspase-3; ↑caspase-8; ↑caspase-9; ↑cell cycle arrest; ↑p53; ↓XIAP | 59.96 µM | [61] |
| Choliangiocarcinoma | KKU-M139 and KKU-M214 | Decreased cell growth | ↓STAT3 | 20 and 50 µM | [63] |
| Colon cancer | 40-16 colon cancer | Decreased proliferation | None | 4.1, 3.6 and 2.6 µM | [65] |
| HT-29 and CDD-18Co | Decreased cell viability | ↑Apoptosis; ↑caspase-3; ↑caspase-9; ↓cyclin B1; ↓MEK/ERK; ↓G2/M phase of cell cycle | 10 and 100 µM | [69] | |
| HT-29 | Decreased cell viability | None | 48 and 72 µM | [70] | |
| HCT115 | Decreased proliferation | ↓ABCC 1,2,3; ↓ABCB1 | 10.2 µM | [66] | |
| Colorectal cancer | FHC, CCD841, CoN, HT29, SW480, LOVO, HCT116 and SW620 | Decreased cell proliferation, cell viability, and colony formation | ↑Apoptosis; ↓HK2; ↓glycolysis; ↓EGFR-Akt |
25 µM | [71] |
| Esophageal cancer | KYSE30, KYSE70, KYSE410, and KYSE450 | Suppressed proliferation, foci formation, and anchorage-independent colony growth | ↓Apoptosis; ↑cell cycle arrest (G1 phase); ↓Bax; ↓cyclin D1; ↓cyt. c; ↓cleaved-PARP; ↓Bcl-2; ↓cyclin D3; ↓KRT18 | 0.3, 0.6, 1.25, and 2.5 µM | [72] |
| Glioblastoma | U87 glioblastoma | Decreased cell viability | ↑Apoptosis; ↓IGFBP2/Akt/Bcl‑2; ↑mIR-204-3p; ↑ERK/c-Fos | 25 µM | [73] |
| T98G | Decreased cell viability | ↑Apoptosis; ↑ROS; ↑p-p38; ↓p‑ERK1/2; ↑cleavage of PARP ↓caspase-3; ↓caspase-9 | 20 µM | [74] | |
| LN229, T98G and U87-MG | Inhibited proliferation, viability, and colony formation | ↓Akt-GSK3β-FBW7-c-Myc protein, ↓HK2 protein | 2, 5, and 10 µM | [75] | |
| Hematological cancers | Acute lymphoblastic leukemia L1210 and adriamycin-resistant L1210 | Decreased cell viability, invasion and migration | ↑Apoptosis; ↓Akt; ↓FAK; ↓NF-κB | 2.5, 5, and 10 µM | [76] |
| Chronic myeloid leukemia KBM-5 | Suppressed invasion | ↑Apoptosis; ↓IKK activity; ↓p65 nuclear translocation; ↓IκBα degradation and phosphorylation; ↓TRAF-2; ↓cIAP-1; ↓cIAP2; ↓survivin; ↓XIAP; ↓Bcl-xL | 50 µM | [77] | |
| Bcr-Abl+ myeloid leukemia cells K562 | Decreased adhesion to endothelial cells, cell viability, and invasion | ↑Apoptosis; ↓MMP-2; ↓Bcr-Abl; ↑p21; ↑p53 | 2.5, 5, and 10 µM | [78] | |
| Laryngeal cancer | RK33 and RK45 | Decreased cell viability | ↑Apoptosis; ↑caspase-3; ↑caspase-8; ↑caspase-9; ↑p53; ↑p21; ↓cyclin D1; ↓ERK1/2 | 12.3 and 22.5 µM | [79] |
| SCC4 | Decreased proliferation | ↑Apoptosis; ↑PARP; ↑p53; ↑AIF; ↓Bcl-2; ↓Mcl-1 | 20, 30, and 40 µM | [80] | |
| Liver cancer | HepG2 | None | None | 10 µM | [81] |
| Huh7, Hep3B, SK-Hep1, and HepG2 | Decreased colony forming, cell viability and confluency ability | ↓HES1; ↓Notch1 pathway | 5 µM | [82] | |
| Hep3B and HA22T/VGH | None | None | 108 and 166 µM | [83] | |
| Melanoma | B16 | Decreased IBMX-induced melanogenesis | ↓Tyrosine enzyme activity | 0.5, 1.5, and 10 µM | [84] |
| SK-MEL-2 | Decreased proliferation | ↓DNA topoisomerase 1 | 14.4 µM | [66] | |
| Ovarian cancer | A-2780 | Decreased proliferation | None | 0.52 and 5.2 µM | [70] |
| OVCAR3 and SKOV3 | Decreased proliferation | ↓Notch1 pathway; ↑p21; ↑cell cycle arrest | 10, 20, and 30 µM | [85] | |
| Oral squamous cell carcinoma | OSCC | Decreased cell viability and reversed radioresistance | ↓Survivin; ↑mitochondrial apoptotic signaling; ↓Akt-Wee1-CDK1 | 1–5 µM | [86] |
| Pancreatic cancer | PANC1 and BxPC3 | Decreased proliferation, viability, and colony formation | ↑Apoptosis; ↓p-STAT3 | 5–100 µM | [87] |
| BxPC3, MXPaCa2, and AsPC1 | Inhibited cell proliferation | ↓NF-κB; ↓VGEF ↓IL-8; ↓mRNA | 0.5–25 µmol/L | [88] | |
| Prostate cancer | Hormone-refractory AR−PC3 | Decreased cell viability | ↑Apoptosis; ↓activation of NF-κB | 2.5–20 µM | [89] |
| Hormone-sensitive AR+, hormone-refractory AR− PC3, LNCaP and DU145 | Decreased cell viability | ↑Apoptosis; ↓NF-κB; ↓p65; ↓p-Akt; ↓p-mTOR; ↓survivin; ↓Bcl-2 | 24 and 40 µM | [90] | |
| Hormone-refractory AR− PC3, DU145 PC3, DU145 | Decreased proliferation, invasion, and migration | ↓p-FAK; ↓p-Akt | 2.5, 5, and 10 µM | [91] | |
| Thyroid cancer | MTC (medullary thyroid cancer cells) | Decreased proliferation and malignant phenotype | ↑ERK1/2 phosphorylation | 10, 20, and 30 µM | [92] |
Table 3.
Antineoplastic effects and underlying mechanisms of action of XH based on in vivo experiments.
| Cancer Type | Animal Model | Effects | Mechanisms | Dose (Route) | Duration | Reference |
|---|---|---|---|---|---|---|
| Breast cancer | BALB/c mouse tumor model by using 4T1 cell lines | Suppressed tumor growth; decreased tumor weight and size | ↓Survivin; ↑caspase cleavage, ↓Notch-1; ↓Ki-67 |
100 and 200 mg/kg | 14 days | [51] |
| Colorectal cancer | Xenograft mouse model by using FHC, SW620, LOVO, CCD841, SW480, CoN, HT29, and HCT116 | Inhibited tumor cell proliferation | ↑Apoptosis; ↑cyt. c release | 10 mg/kg (i.p.) | Every two days | [71] |
| Male Sprague Dawley rats by using SW480 CRC cells | Inhibited tumor cell proliferation | ↑Apoptosis; ↓wnt/β-catenin signaling ↓Bax; ↓ Bcl-2; ↓caspase-3; ↓iNOS; ↓COX-2 | 5 mg/kg for alternate days | 8 weeks | [93] | |
| Esophageal cancer | Patient-derived xenograft mouse model by using KYSE30 cell lines | Decreased tumor volume and weight | ↑Apoptosis; Ki-67; ↓KRT18 | 40, 80, and 160 mg/kg (p.o.) | 64 days | [72] |
| Glioblastoma | Xenograft mouse model by using LN229, U87MG, and T98G cell lines | Reduced tumor weight | ↓Akt-GSK3β-FBW7-c-Myc protein | 10 mg/kg for every three days (i.p.) | 32 days | [75] |
| Lung cancer | Xenograft mouse model by using HCC827 cells | Suppressed tumor growth | ↓Cyclin D1; ↓ERK1/2-fra1 signaling pathway | 10mg/kg (i.p.) | 32 days | [94] |
| Pancreatic cancer | Subcutaneous xenograft mouse model by using BXPC-3 cells | Inhibited tumor growth and angiogenesis | ↓NF-κB activation ↓tube formation; ↓VGEF; ↓IL8 | 10 mg/kg/week | 5 weeks | [88] |
4.1. Breast Cancer
The most prevalent form of female cancer worldwide is breast cancer [44,45]. The Notch signaling pathway has significant importance in the normal development of breast cancer cells by deciding cell fate and self-renewal in stem cells [46]. Notch also acts as an oncogene in the growth and progression of breast cancer, and maladaptive amplification of this pathway is associated with increased frequency of breast cancer [47,48]. Thus, inhibition of Notch1 expression can lead to growth suppression and programmed cell death in breast cancer cells [49,50]. The targeting of XH to Notch1 pathway was assessed by two positive controls such as dual antiplatelet therapy (γ-secretase inhibitor) and Notch1 functional assay. The activity of luciferase was determined using a luciferase reporter assay for when Notch1 was bound to the CBF1 transgene. XH reduced the expression of Notch 1, survivin, and Ki-67 and enhanced the expression of caspase-3. The experiment conducted by Sun et al. [51] showed that XH can suppress the growth of breast tumors and promote programmed cell death. Both in vivo and in vitro studies of XH were performed by Sun et al. on breast cancer. The in vivo study used tumorigenicity assays to determine that XH was effective in suppressing tumor growth on a mouse model of breast tumors. The in vitro studies revealed that there is a decrease in notch signaling pathway, and apoptotic regulators such as Bcl-2, caspase-3, and Bcl-extra-large, which was confirmed by MTT assay, Western blot analysis, and flow cytometry [52].
XH orally administered to nude mice which were previously inoculated with breast cancer cells (MCF-7), resulting in central tumor necrosis, a reduced number of inflammatory cells and, an area of focal proliferation, an increased percentage of apoptotic cells, and lower microvessel density. The antiangiogenic effects of XH have also been confirmed and compared with controls via factor VIII expression immunoblotting in XH-treated tumors [53].
Breast cancer is graded based on the expression of progesterone and, estrogen receptors (PR&ER), and human epidermal growth factor receptor 2 (HER2), such as (1) ER+, a positive estrogen receptor; (2) HER2+, a HER2 overexpressor which may be ER+ or ER−; or (3) TNBCs, a triple-negative subtype that does not express any of these receptors. Until now, successful therapies have not been identified for TNBCs [54]. Hs578 T and MDA-MB-231 breast cancer cells are widely used in in vitro studies as cell models for TNBCs [55]. XH was found to inhibit MDA-MB-231 cell proliferation by apoptosis induction through a mitochondria- and caspase- dependent pathway [56]. XH treatment for 24 h repressed the development of MDA-MB-231 and Hs578 T cells with IC50s of 6.7 and 4.78 μM, respectively. XH inhibited the invasive phenotype of Hs578 T and MDA-MB-231 cells as well [57]. The uncharacteristic expression of cancerous ALP isoenzymes, which is a biomarker for prognosis of different cancers, has been observed in many malignant tissues [58]. According to the findings of Guerreiro and team [50], the intestinal ALP (IALP) isoenzyme of ALP, which was overexpressed in malignant tissues, was inhibited by XH in MCF-7 cells. However, XH had no significant effect on other types of ALP isoenzyme, i.e., TNS-ALP. Additionally, chemo- and radiosensitizing experiments on MCF-7/ADR cells revealed that XH was able to attenuate MCF-7/ADR cell sensitivity to adriamycin therapies and radiation that inhibited the expression of epidermal growth factor receptor (EGFR), signal transducer and activator of transcription 3 (STAT3), and multidrug resistance mutation 1 (MDR1), also known as ATP binding cassette subfamily B member 1 (ABCB1). These findings indicate that XH could be a powerful chemosensitizer and radiosensitizer, and warrant comprehensive clinical investigation for the potential therapy of breast cancer [59].
4.2. Cervical Cancer
Cervical cancer is a prominent initiator of cancer anguish and death in women all over the globe. Nearly two-thirds of examined women are found to have locally advanced cervical cancer, which has poor prognosis [60]. Hence, there is an immediate need for novel, efficient treatments. Yong et al. [61] reported that XH induced apoptosis in the Ca Ski cervical cancer cell line. XH also trigger S phase cell cycle arrest and amplified the activity of caspase-3, caspase-8, and caspase-9. Additionally, the expression of cleaved poly-ADP-ribose polymerase (PARP), apoptosis-inducing factor (AIF), and p53 increased, while concentration-dependent expression of Bcl-2 and X-chromosome linked inhibitor of apoptosis protein (XIAP) was decreased. These findings suggest that apoptosis induced by XH could involve intrinsic and extrinsic apoptotic pathways.
4.3. Cholangiocarcinoma
Cholangiocarcinoma is the most common hepatic and biliary neoplasm with 5-year survival rate less than 10%. The anticancer activity of XH was studied on various cell lines of human cholangiocarcinoma (CC-SW-1, SG-231, and CCLP1). XH strongly decreased colony formation, confluency of cells, and cell proliferation. XH showed anti-cholangiocarcinogenic activity, by enhancing the proapoptotic markers, reducing cell cycle regulatory proteins, and suppressing the antiapoptotic markers, enhanced apoptosis and cell cycle arrest. At molecular level, XH reduced the growth of cholangiocarcinoma by inhibiting the Notch1/Akt pathway [62]. There is a crucial role played by STAT3 in the formation of cholangiocarcinoma. XH has the capability to target STAT3 via the Akt-NF-κB signaling pathway, which leads to the inhibition of the proliferation of cells. Therefore, reduction in the activity of STAT3 with 50 µM XH prominently decreased cell growth and enhanced apoptosis when given orally by mixing XH in drinking water and did not exhibit toxicity. Nonetheless, the major limitation is the poor solubility of XH in water [63]. In another study [64], XH exhibited an antiproliferative effect against cholangiocarcinoma by reducing BCL-2 and increasing BAX expression. XH also suppressed BECLIN-1-dependent autophagy, which restricted its toxicity.
4.4. Colon Cancer
Presently, colon cancer is one of the leading causes of death in men and women globally. Its incidence is expected to have a significant increase, rising to 1.1 million death by 2030. XH showed antiproliferative effect on various colon cancer cell lines with IC50 values of 2.6, 3.6, and 4.1 µM for 24, 48, and 72 h, respectively [65]. A strong cytotoxicity was exerted by XH against HCT-15 colon cancer cell line after a 24 h treatment with an IC50 value of 3.6 µM. The studies of Lee et al. [66] recommended XH to be used with other chemopreventive agents to decreased drug resistance by inhibiting the efflux drug transporter MDR1, multidrug resistance protein (MRP) 1 (drug efflux gene ABCC1), MRP2 (drug efflux gene ABCC2), and MRP3 (drug efflux gene ABCC3) to decrease the drug resistance [11]. XH showed anticancer activity in colorectal cell lines SW480 by activating the ataxia telangiectasia mutated pathway and also by enhancing the DNA damage response [67]. XH also demonstrated antitumor activity in the 40–16 colon cancer cell line derived from HCT116 by downregulating Bcl-2 and by activating caspase-3 and caspase-7 [65].
4.5. Esophageal Cancer
Esophageal squamous cell carcinoma is the leading cause of deaths globally. The anticancer activity of XH was studied on KYSE30 cell lines in which XH exhibited an antiproliferative effect and foci formation. Keratin 18 (KRT18) was the major target of XH for exhibiting anticancer activity in KYSE30 cell lines. Apoptosis and cell cycle arrest at G1 phase was also major activities of XH and were associated with the markers, such as Bax, PARP (cleaved), cyclin D1 and D3, and cytochrome c [72]. XH also targeted Akt1/2 to suppress esophageal cancer. This targeting mainly involved the downregulation of GSK3β, S6K, and mammalian target of rapamycin (mTOR). XH also decreased the volume and weight of tumors in PDXs which largely express Akt [95].
4.6. Glioblastoma
Glioblastoma is a very aggressive brain cancer related with an extremely poor prognosis in adults. There is a great need to develop new treatments due to the developing drug resistance against the presently available chemotherapeutic agents [96]. XH was studied for its potency towards glioblastoma by using T98G cells. It was found that it reduced cell viability and enhanced the apoptosis which involved cleavage of PARP and activation of caspase-3 and caspase-9 in a concentration- and time-dependent manner. Intracellular ROS was also enhanced by XH [97]. XH decreased the viability of U87 cell lines as well. A recent study revealed that XH suppressed the glycolysis through HK2 inhibition, which led to suppression of glioblastoma [75].
4.7. Hematological Cancers
Apoptosis and growth arrest in B-acute lymphocytic leukemia (ALL) cells was induced by XH. Moreover, equal cytotoxicity was found when XH was used against adriamycin resistant ALL (L1210) cells. Additionally, prolonged XH exposure to ALL cells improved their sensitivity towards chemotherapeutic medications [76]. An increase in animal lifespan was observed by administration of 50 µg/mouse/day XH in 200 µL PBS in ALL-like xenograft mouse model, and it substantially deferred the insurgence of neurological disorders. XH produced anticarcinogenic activity on various cell lines of cancer like ALL cells and chronic myeloid leukemia (KBM-5) cells by decreasing the activation of NF-κB through the modification of IKK and p65 [77]. There was no drug resistance to XH, though drug adaptation, categorized by the downregulation of Akt, FAK, and NF-κB activities, rendered cells less invasive and more vulnerable to cytotoxic drugs [11,76]. The proliferation, resistance to apoptosis, and transformation of leukemic cells is mainly due to activation of the PI3K/Akt and NF-κB signaling pathways via oncogenic Bcr-Abl tyrosine kinase in Bcr-Abl(+) myeloid leukemia cells, and this Bcr-Abl expression in the Bcr-Abl(+) myeloid leukemia cell line and K562 cells was inhibited strongly by XH [78].
4.8. Laryngeal Cancer
Laryngeal squamous cell cancer is one of the most common cancers of head and neck in the US population and has low survival rate [98]. XH has the potential to decrease the viability of laryngeal squamous cells RK33 and RK45 with less side effects [79]. In SCC4 cells, XH exerted strong cytotoxic effect by inhibiting the expression of Bcl-2 and Mcl-1, and also by activating AIF, p53, and PARP [80].
4.9. Liver Cancer
XH was studied for its effects and mechanisms of action on hepatocellular carcinoma cellular cell lines (Hep3B, HepG2, and SK-Hep-1) by evaluating cell viability, colony-forming ability, and cell proliferation [99]. At a concentration of 5 µM and higher, XH expressively reduced the cell viability and colony-formation ability in the hepatocellular cell lines. Hepatocellular cancers were treated by inhibiting the Notch signaling pathway which was supported by reduced Notch1 and HES-1 protein expression [82]. The hepatoprotective activity of XH was found to be concentration-dependent. The expression of the proinflammatory factors like MCP-1 and type I collagen a profibrogenic gene was reduced by XH in hepatic stellate cells (HSC) [99].
The cytotoxic and antiproliferative activity of XH and its non-estrogenic derivatives were investigated by Logan et al. [100] on cell lines of hepatocellular carcinoma. Although XH has potent anticancer activity, its use is limited because of its metabolism by gut microbiota and the host’s hepatic cytochrome P450 enzyme, which transforms it to phytoestrogen 8-PN, the most potent phytoestrogen. The same study also compared the antiproliferative activity of XH with its derivatives like dihydroxanthohumol and tetrahydroxanthohumol, which are not metabolized into 8-PN. These derivatives showed more antiproliferative activity than XH in hepatocellular carcinoma (Huh7 and HepG2) cells. XH was also studied on hepatocellular carcinoma cells and it was reported that XH was effective at a concentration of 25 µM against two hepatocellular cancer cell lines, HepG2 and Huh7. XH also suppressed the proliferation, migration, IL-8 expression, and TNF-induced NF-κB activity in both the cell lines even at lower concentrations [101].
XH not only possesses a hepatoprotective effect but also showed potential effects against obesity and hepatic steatosis [99]. The involvement of Nrf2 pathway activation followed p53 induction and was probably due to the chemopreventive activity of XH in hepatocytes [102]. Additionally, XH also exhibited antimutagenic effects against different procarcinogens which are activated through cytochrome P450 enzymes [81,103]. When evaluated against hepatocellular carcinoma cells, XH and its non-estrogenic derivatives dihydroxanthohumol and tetrahydroxanthohumol showed potential anticancer effects by the induction of cell cycle arrest at the G0/G1 phase [100]. Another study showed that XH was able to exhibit anticancer effects by inducing apoptosis and growth inhibition via NF-κB/p53-apoptosis signaling pathway in human liver cancer cells [104].
4.10. Lung Cancer
Presently, lung cancer is one of the most common causes global cancer mortality. Out of 1.6 million affected people, 1.4 million people die annually with only 15% survival rate. Multidrug resistance is one of the most important reasons for the poor response of the most lung cancer patients to the standard drug regimens of cancer therapy. The major molecular target of the human lung carcinomas is ERK1/2 kinase cascade. XH was demonstrated as a potential suppressor of p90RSK and ERK1/2 kinases, activator of cellular repressor of E1A-stimulated genes 1 (CREG) protein, and inhibitor of phosphorylation of CREG in A549 lung adenocarcinoma cells. XH was also found to be a strong chemotherapeutic agent against lung carcinoma by arresting the cell cycle at G1 phase, inducing apoptosis, increasing caspase-3 activity, upregulating p53 and p21, and downregulating cyclin D1 [105].
4.11. Melanoma
Melanocytes and dendritic cells are present in the dermal–epidermal boundary of the skin and mainly synthesize melanin, a biopolymer pigment. Melanin possesses diverse functions that include chemical and toxic drug absorption, neurodevelopment in embryogenesis, determination of the appearance of an organism, aural processing, and protective coloration [84]. Several extracellular stimuli, such as isobutyl-methylxanthine, are responsible for development of melanogenesis. XH at a dose range 0.5–10 µM inhibited melanogenesis induced by isobutyl-methylxanthine in B16 melanoma cells. Tyrosinase genes (tyrosinase, TRP-1 and TRP-2) also significantly influence the melanogenesis by producing melanin pigment and activity of these genes was reduced significantly by XH. Strong cytotoxic activity of XH was observed in SK-MEL-2 melanoma cells, in which XH suppressed the activity of the DNA topo-I enzyme (topoisomerase) that was involved in adjustment of topological structure of DNA. The study revealed that XH may evolve as a novel inhibitor of topo-I. The strong inhibition of DNA topo-I enzyme led to apoptosis and antiproliferative action. Topo-I is the main target for many cancer treatments and XH is also regarded as broad-spectrum chemopreventive agent because of its topo-I inhibition [11].
A concentration-dependent cytotoxic effect of XH was observed on human melanoma cell lines in subtoxic doses. XH suppressed migratory activity, proliferation, and formation of colonies. XH also decreased the liver metastasis of murine B16 melanoma cells in C57/BL6 mice and has evolved as an important chemotherapeutic agent for hepatic metastasis of melanoma treatment [106]. The anti-melanogenesis effect of XH was also studied on human keratinocytes (HacaT) for melanosome degradation, and in normal human melanocytes and MNT-1 human melanoma cells for its anti-melanogenesis effect. In all the cell lines, XH showed prominent inhibition of melanin synthesis and melanosome export, showing its potential as an inhibitor of pigmentation in humans [107].
4.12. Oral Cancer
Approximately 2% of all cancers are oral cancer cases, and 90% of them are diagnosed as oral squamous cell carcinoma (OSCC). This is because OSCC emerged from the oral cavities of epithelial mucosa. The survivin protein is highly expressed in the tissues derived from the patients of OSCC and cell lines. XH was found to exert antitumor activity via inhibition of the overexpression of survivin and activating mitochondrial apoptotic signaling both in vivo and in vitro. XH inhibits Akt-Wee1-CDK1 signaling and causes reduction of survivin phosphorylation on Thr34, survivin ubiquitination, and degradation mediated by facilitated E3 ligase Fbxl7. Hence, XH has been emerged as a strong chemotherapeutic agent for oral cancer [86].
4.13. Ovarian Cancer
Ovarian cancer is a deadly gynecologic threat in the United States, and the fifth most common cancer. Because of the challenge in early identification, most instances of ovarian cancer are discovered at stage III or IV and have only a 15–20% survival rate [108]. When A-2780 ovarian cancer cells were treated with XH for 2 and 4 days, it was found that XH instigated exceptional cytotoxicity with IC50 estimations of 0.52 and 5.2 μM, respectively [70]. After XH therapy, significant growth inhibition and downregulation of protein expression and Notch1 transcription were discovered in SKOV3 and OVCAR3 ovarian cancer cells [85].
4.14. Pancreatic Cancer
Pancreatic carcinoma with pancreatic ductal adenocarcinoma is the major cause of cancer related deaths in the United States because of their dismal survival rate [109]. Therefore, there is an urgency to develop new treatment approaches with minimal toxicities. XH was found to be effective for the treatment of pancreatic cancer with minimal side effects in many pancreatic cell lines, such as BxPC-3 and PANC-1. XH acted by inhibiting phosphorylation of STAT3 and downregulating the expression of target genes (Bcl-xL, cyclin D1, and survivin). XH also increased the apoptosis of pancreatic cancer cells by inhibition of the Notch1 signaling pathway. The anticancer activity has been demonstrated in many cancers with activated Notch1 and STAT3 signaling [11,110]. XH in combination with phenethyl isothiocyanate reduced the proliferation of PANC-1 cells. XH and phenethyl isothiocyanate, in combination, reduced NF-κB activity and enhanced Nrf2 expression and expression of Nrf2-related genes (SOD, NQO1, and GSTP) in pancreatic cancer cells [18].
4.15. Prostate Cancer
Prostate cancer (PC) is the third most prevalent cause of death in men worldwide and is the most frequently diagnosed cancer, with a peak incidence in men over 70. PC treatment choices are determined by a number of variables, including the patient’s living standards and cancer characteristics, such as prostate-specific antigen (PSA) level, tumor stage, and tumor aggressive behavior. The majority of high-risk PC patients are tackled with a combination of radiotherapy and hormone therapy, resulting in a high chance of survival [111]. Androgen steroids influence the development and progression of PC and, accordingly, androgen ablation therapy has been used to treat various degrees of disease. Advanced therapy of PC is mainly based on the interference with androgen deprivation therapy (ADT) and AR signaling. The development of the resistance in PC towards ADT and next-generation ADT is mainly due to molecular AR- modifications. AR-V7 (AR splice variant) is the most common AR-modification that leads to resistance, and mutations in the AR gene are the second most common [112]. Due to this, there is an immediate need for the development of anti-adenocarcinogenic agents that do not produce resistance in PC cells.
Numerous studies have found XH to be effective against PC through several different mechanisms. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in PC cells was increased by XH [113]. In PC cells, XH also causes cell death that is not dependent on caspases [114]. Additionally, XH induced cell apoptosis by binding with annexin V-FITC, cleaving PARP-1, activating procaspase-3, procaspase-8 and procaspase-9, depolarizing mitochondria, and releasing cytochrome c from mitochondria. The inhibition of prosurvival Akt, NF-κB, p-mTOR, and NF-κB-regulated antiapoptotic Bcl-2 was linked to XH-induced apoptosis [90].
The anticancer activity of XH, such as induction of apoptosis and inhibition of growth, were tested on hormone-refractory and hormone-sensitive prostate cancer cell lines. The high sensitivity of prostate cancer cells towards XH (20–40 µM) was identified using cell growth or viability assays. The tumor cell destruction was primarily accomplished by apoptosis which was shown by binding of annexin V-FITC to phosphatidylserine of PC3 and PC4 cells, PARP-1 cleavage, procaspase activation, the release of cytochrome c, and depolarization of mitochondria. XH also showed a concentration-dependent suppression of cell viability in BPH-1 and PC3 cells, and decreased activation of NF-κB [89]. The above studies give a basis for the evaluation of XH clinically in treating metastatic hormone-refractory prostate cancer [90].
4.16. Thyroid Cancer
The incidence rate of thyroid cancer is high in both women and men, with a lower mortality rate. XH can be a potential molecule of interest because of its promotion of iodine uptake of thyrocytes (FRTL-5), which reduces the need for inadequate surgery and radiotherapy. XH decreased the proliferation and suppressed malignant phenotype (ASCL1) concentration-dependently in thyroid carcinoma. However, the rate of suppression was very low, i.e., >50%, following a treatment with 30 μM XH for 4 days [11]. Another study revealed that XH enhanced the apoptotic activity in TPC-1 human thyroid cancer cells at higher concentrations [115]. XH increased the uptake of radioiodine at nanomolar concentrations in FRTL-5 cells after 3 days of activation [116]. The Raf-1 signal was activated by XH, which led to inhibition of ASCL1, an important factor in medullary thyroid cancer development. Therefore, the activation of Raf-1 produced anticancer activity in MTC [92]. Further investigation is required for evaluating the mechanisms of anticancer activity of XH against thyroid cancer.
5. Biotransformation and Pharmacokinetics of XH
The human intestinal epithelial cell line was used to better understand the reasons for XH’s low bioavailability. Caco2, was used to study XH uptake, accumulation, and transport in the human body. After seeding the cells with XH, the studies were carried out for 18–21 days, and Vmax and Km values were determined for the accumulation of XH in Caco-2 cells [23]. It was found that XH was not absorbed by facilitated transport. Instead, absorption was mediated through the unique binding of cytosolic proteins produced in Caco-2 cells, which contributed to its poor oral bioavailability.
The biotransformation of XH was investigated using rat liver microsomes. The rats were pretreated with hepatic microsomes from isosafrole and β-naphthoflavone nonpolar metabolite. It was found that XH forms three polar metabolites which were identified by 1H NMR, mass spectrometry, and liquid chromatography analyses as (1) 5″-isopropyl-5″-hydroxydihydrofurano[2″,3″:3′,4′]-2′,4-dihydroxy-6′-methoxychalcone; (2) 5″-(2‴-hydroxyisopropyl)-dihydrofurano[2′,3″:3′,4′]-2′,4-dihydroxy-6′-methoxychalcone; and (3) a derivative of XH with an additional hydroxyl function at the B ring. A nonpolar metabolite of XH was also identified as dehydrocycloxanthohumol [117].
Microsomes of human liver were used to investigate the biotransformation of XH. It was observed that the primary path of the oxidative metabolism is the hydroxylation of a prenyl methyl group which forms hydroxylated metabolites of XH. The major possibility of XH conversion in stomach is to isoxanthohumol and to a strong phytoestrogen 8-PN. These phytoestrogens are formed from desmethylxanthohumol, a direct metabolic product of XH [118]. The excretion of XH was observed through feces when 1000 mg per kg body weight was administered [119]. In total, 22 metabolites were observed in the feces of rat most of which were flavone derivatives and modified chalcones [22]. Approximately 89% of unchanged XH remained in the intestinal tract and only 11% are its metabolites. In rats, the phase II metabolites identified revealed that they are due to demethylation, sulfation, oxidation, and hydration reactions [120]. Metabolites due to multiple biotransformations of the XH may contribute to the biological activities of XH [121].
The pharmacokinetic profile of e XH was studied by administering a single oral dose of XH to the mice at a dose level of 20, 60, or 180 mg/kg. The samples of blood were collected after different time intervals and plasma levels of XH were estimated by LC–MS/MS. The peak plasma concentration of XH has a biphasic absorption pattern, and XH was observed after 1 h and between 4–5 h after administration, and the half-life was found to be 20 h for all the respective doses [114]. The longer half-lives of XH are due to enterohepatic recirculation and slower absorption rate after administration through the oral route in humans. XH can easily assemble in intestinal cells as most XH molecules are connected to cellular proteins [122]. XH is poorly orally accessible due to the unique attachment of XH to cytosolic proteins in the intestinal epithelial cells [123]. Many examinations have been performed for investigation of the interaction of XH with the phosphatidylcholine model membranes using FT-IR, DSC, and fluorescence spectroscopy. Fast transportation through the cell membrane was shown in such experiments [124].
6. Conclusions
This review summarized recent progress on XH, that inhibits carcinogenesis and metastasis in many cancers. Traditionally hops are utilized as sedatives, antispasmodics, bitter stomachics, and antimicrobials. Current laboratory examination has shown that chalcones and flavonones from hops possess several therapeutic effects such as anti-inflammatory, chemopreventive, antioxidant, and antiproliferative properties. XH has emerged as a potential candidate for anticancer therapy. However, present studies are not sufficient to evaluate the anticancer activity of XH and more mechanistic and systematic in vivo studies must be conducted for better understanding of its efficacy for clinical development.
XH showed very promising bioactivities in both in vitro and in vivo examinations on various cancers as mentioned in Table 2 and Table 3. It was found that among all the cancers studied, XH had the most prominent therapeutic effect on the breast cancer, colon, and prostate cancer.
The development of a broad range of human cancer cell lines, such as ovarian, breast, colon, blood, and prostate cancer lines, were inhibited by XH by inducing apoptosis and by inhibiting apoptosis, and modulating various oncogenic signaling pathways (Figure 2). Several vital signaling pathways were tangled in the anticancer activity of XH, such as ROS, ERK1/2, NF-κB, and Akt. Many in vitro examinations were conducted on various cell lines for evaluating the antitumor activity of the XH on different cancers. These studies concluded that antitumor activity of XH occurs through NF-κB, IKK, and p65 inhibition in blood cancer, whereas in breast cancer, XH decreased the expression of Ki-67, survivin, and Notch 1, enhanced the expression of caspase-3, and inhibited EGFR, MDR1, and STAT3. In hematological cancer, there was downregulation of FAK, Akt, and NF-κB activity as well as strong inhibition of Bcr-Ab1 expression. In cervical cancer, apoptosis was induced by reducing PARP and p53 expression. The therapeutic activity of XH on ovarian cancer was due to downregulation of Notch1 and protein expression. Apoptosis in prostate cancer was brought by TRAIL as well as the depolarization of mitochondria by activation of procaspase-3, procaspase-8, and procaspase-9. XH regulated the MCP-1 protein and reduced the expression of Notch1 and HES-1 protein in liver cancer.
Figure 2.
Mechanistic profile of xanthohumol on various cancers. Abbreviations: APA, alternative polyadenylation; Bcl-2, B-cell lymphoma 2; c-PARP, cleaved poly (ADP-ribose) polymerase; ERK1/2, extracellular signal-regulated protein kinase; FAK, focal adhesion kinase; HES-1, split homolog 1; Hs578T, aneuploid mammary epithelium; IKK, IκB kinase; MDR1, multidrug resistance mutation 1; MRP1; multidrug resistance protein 1, MRP2, multidrug resistance protein 2, MRP3, multidrug resistance protein 3; Mcl-1, myeloid leukemia cell differentiation protein 1; NF-κB, nuclear factor-κB.
Limited in vivo examinations have been conducted to evaluate the anticancer activity of XH on different cancers (Table 3). Various xenograft mouse models were used for studying the antitumor activity of XH. Another major challenge associated with XH is its low oral bioavailability and the conversion of XH to its estrogenic derivative. To date, there are few human studies available for evaluating the therapeutic dose and toxicity of XH. Additionally, there is need to develop bioavailability assays, dose–response curves, and perform molecular modifications to the core chemical structure of XH to have a deeper understanding of its pharmacokinetic and pharmacodynamic profile. In view of the encouraging results presented here, XH seems to be a multitargeted agent and appreciable candidate for drug development for the prevention and treatment of cancer.
Author Contributions
V.H.: writing original draft and data curation; E.H., review and editing; M.Ś., review and editing; H.T., review and editing; S.J., review and editing; D.T., conceptualization, supervision, review and editing; A.B., supervision, review, critical revision, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stompor M., Żarowska B. Antimicrobial Activity of Xanthohumol and Its Selected Structural Analogues. Molecules. 2016;21:608. doi: 10.3390/molecules21050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X.-L., Zhang Y.-D., Wang T., Guo H.-Y., Liu Q.-M., Su H.-X. Evaluation on Antioxidant Effect of Xanthohumol by Different Antioxidant Capacity Analytical Methods. J. Chem. 2014;2014:249485. doi: 10.1155/2014/249485. [DOI] [Google Scholar]
- 3.Gao X., Deeb D., Liu Y., Gautam S., Dulchavsky S.A., Gautam S.C. Immunomodulatory activity of xanthohumol: Inhibition of T cell proliferation, cell-mediated cytotoxicity and Th1 cytokine production through suppression of NF-kappaB. Immunopharmacol. Immunotoxicol. 2009;31:477–484. doi: 10.1080/08923970902798132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magalhães P.J., Carvalho D.O., Cruz J.M., Guido L.F., Barros A.A. Fundamentals and health benefits of xanthohumol, a natural product derived from hops and beer. Nat. Prod. Commun. 2009;4:591–610. doi: 10.1177/1934578X0900400501. [DOI] [PubMed] [Google Scholar]
- 5.Plazar J., Filipic M., Groothuis G.M.M. Antigenotoxic effect of Xanthohumol in rat liver slices. Toxicol. In Vitro. 2008;22:318–327. doi: 10.1016/j.tiv.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Dell’Eva R., Ambrosini C., Vannini N., Piaggio G., Albini A., Ferrari N. AKT/NF-kappaB inhibitor xanthohumol targets cell growth and angiogenesis in hematologic malignancies. Cancer. 2007;110:2007–2011. doi: 10.1002/cncr.23017. [DOI] [PubMed] [Google Scholar]
- 7.Frölich S., Schubert C., Bienzle U., Jenett-Siems K. In vitro antiplasmodial activity of prenylated chalcone derivatives of hops (Humulus lupulus) and their interaction with haemin. J. Antimicrob. Chemother. 2005;55:883–887. doi: 10.1093/jac/dki099. [DOI] [PubMed] [Google Scholar]
- 8.Goto K., Asai T., Hara S., Namatame I., Tomoda H., Ikemoto M., Oku N. Enhanced antitumor activity of xanthohumol, a diacylglycerol acyltransferase inhibitor, under hypoxia. Cancer Lett. 2005;219:215–222. doi: 10.1016/j.canlet.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Karabín M. Biologically Active Compounds from Hops and Prospects for Their Use. Compr. Rev. Food Sci. Food Saf. 2016;15:542–567. doi: 10.1111/1541-4337.12201. [DOI] [PubMed] [Google Scholar]
- 10.Liu M., Yin H., Liu G., Dong J., Qian Z., Miao J. Xanthohumol, a prenylated chalcone from beer hops, acts as an α-glucosidase inhibitor in vitro. J. Agric. Food Chem. 2014;62:5548–5554. doi: 10.1021/jf500426z. [DOI] [PubMed] [Google Scholar]
- 11.Jiang C.-H., Sun T.-L., Xiang D.-X., Wei S.-S., Li W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.) Front. Pharmacol. 2018;9:530. doi: 10.3389/fphar.2018.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrož M., Lněničková K., Matoušková P., Skálová L., Boušová I. Antiproliferative Effects of Hop-derived Prenylflavonoids and Their Influence on the Efficacy of Oxaliplatine, 5-fluorouracil and Irinotecan in Human ColorectalC Cells. Nutrients. 2019;11:879. doi: 10.3390/nu11040879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verzele M., Stockx J., Fontijn F., Anteunis M. Xanthohumol, a new natural chalkone. Bull. Sociétés Chim. Belg. 1957;66:452–475. doi: 10.1002/bscb.19570660137. [DOI] [Google Scholar]
- 14.Stevens J.F., Page J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry. 2004;65:1317–1330. doi: 10.1016/j.phytochem.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Zanoli P., Zavatti M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008;116:383–396. doi: 10.1016/j.jep.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Vázquez Loureiro P., Hernández Jiménez I., Sendón R., Rodriguez-Bernaldo de Quirós A., Barbosa-Pereira L. Determination of Xanthohumol in Hops, Food Supplements and Beers by HPLC. Foods. 2019;8:435. doi: 10.3390/foods8100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirata H., Uto-Kondo H., Ogura M., Ayaori M., Shiotani K., Ota A., Tsuchiya Y., Ikewaki K. Xanthohumol, a hop-derived prenylated flavonoid, promotes macrophage reverse cholesterol transport. J. Nutr. Biochem. 2017;47:29–34. doi: 10.1016/j.jnutbio.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Krajka-Kuźniak V., Cykowiak M., Szaefer H., Kleszcz R., Baer-Dubowska W. Combination of xanthohumol and phenethyl isothiocyanate inhibits NF-κB and activates Nrf2 in pancreatic cancer cells. Toxicol. In Vitro. 2020;65:104799. doi: 10.1016/j.tiv.2020.104799. [DOI] [PubMed] [Google Scholar]
- 19.Lin M., Xiang D., Chen X., Huo H. Role of Characteristic Components of Humulus lupulus in Promoting Human Health. J. Agric. Food Chem. 2019;67:8291–8302. doi: 10.1021/acs.jafc.9b03780. [DOI] [PubMed] [Google Scholar]
- 20.Iniguez A.B., Zhu M.-J. Hop bioactive compounds in prevention of nutrition-related noncommunicable diseases. Crit. Rev. Food Sci. Nutr. 2020:1–14. doi: 10.1080/10408398.2020.1767537. [DOI] [PubMed] [Google Scholar]
- 21.Gerhäuser C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer. 2005;41:1941–1954. doi: 10.1016/j.ejca.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Nookandeh A., Frank N., Steiner F., Ellinger R., Schneider B., Gerhäuser C., Becker H. Xanthohumol metabolites in faeces of rats. Phytochemistry. 2004;65:561–570. doi: 10.1016/j.phytochem.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Pang Y., Nikolic D., Zhu D., Chadwick L.R., Pauli G.F., Farnsworth N.R., van Breemen R.B. Binding of the hop (Humulus lupulus L.) chalcone xanthohumol to cytosolic proteins in Caco-2 intestinal epithelial cells. Mol. Nutr. Food Res. 2007;51:872–879. doi: 10.1002/mnfr.200600252. [DOI] [PubMed] [Google Scholar]
- 24.Venturelli S., Burkard M., Biendl M., Lauer U.M., Frank J., Busch C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition. 2016;32:1171–1178. doi: 10.1016/j.nut.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Small E. The relationships of hop cultivars and wild variants of Humulus lupulus. Can. J. Bot. 1980;58:676–686. doi: 10.1139/b80-086. [DOI] [Google Scholar]
- 26.Royle D.J. Hops. By RA Neve. London: Chapman and Hall, (1991), pp. 266, £32.50, ISBN 0-412-30330-2. Exp. Agric. 1992;28:123–124. doi: 10.1017/S0014479700023085. [DOI] [Google Scholar]
- 27.Murakami A., Darby P., Javornik B., Pais M.S.S., Seigner E., Lutz A., Svoboda P. Microsatellite DNA analysis of wild hops, Humulus lupulus L. Genet. Resour. Crop Evol. 2006;53:1553–1562. doi: 10.1007/s10722-005-7765-1. [DOI] [Google Scholar]
- 28.Olsovska J., Bostikova V., Dusek M., Jandovska V., Bogdanova K., Cermak P., Bostik P., Mikyska A., Kolar M. Humulus lupulus L.(hops)–a valuable source of compounds with bioactive effects for future therapies. Mil. Med. Sci. Lett. 2016;85:19–30. doi: 10.31482/mmsl.2016.004. [DOI] [Google Scholar]
- 29.Moir M. Hops—A millennium review. J. Am. Soc. Brew. Chem. 2000;58:131–146. doi: 10.1094/ASBCJ-58-0131. [DOI] [Google Scholar]
- 30.Edwardson J.R. Hops—Their botany, history, production and utilization. Econ. Bot. 1952;6:160–175. doi: 10.1007/BF02984875. [DOI] [Google Scholar]
- 31.The Angiosperm Phylogeny Group An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 2003;141:399–436. doi: 10.1046/j.1095-8339.2003.t01-1-00158.x. [DOI] [Google Scholar]
- 32.Stevens R. The chemistry of hop constituents. Chem. Rev. 1967;67:19–71. doi: 10.1021/cr60245a002. [DOI] [Google Scholar]
- 33.Chapman A.C. The Hop and Its Constituents: A Monograph on the Hop Plant. The Brewing Trade Review; London, UK: 1905. [Google Scholar]
- 34.Small E. A numerical and nomenclatural analysis of morpho-geographic taxa of Humulus. Syst. Bot. 1978;3:37–76. doi: 10.2307/2418532. [DOI] [Google Scholar]
- 35.Robert T.R., Wilson R.J.H. Handbook of Brewing. Taylor & Francis Group; Boca Raton, FL, USA: 2006. Hops. [Google Scholar]
- 36.Šrédl K., Prášilová M., Svoboda R., Severová L. Hop production in the Czech Republic and its international aspects. Heliyon. 2020;6:e04371. doi: 10.1016/j.heliyon.2020.e04371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brissart R., Brauninger U., Haydon S., Morand R., Palmer G.H.O., Sauvage R., Seward B. Malting Technology: Manual of Good Practice. Frachverlag Hans Carl; Wolverhampton, UK: 2000. [Google Scholar]
- 38.Goldhammer T. The Brewers Handbook: The Complete Book to Brewing Beer. Apex; Centreville, VA, USA: 2008. [Google Scholar]
- 39.De Keukeleire D. Fundamentals of beer and hop chemistry. Quim. Nova. 2000;23:108–112. doi: 10.1590/S0100-40422000000100019. [DOI] [Google Scholar]
- 40.Wunderlich S., Zürcher A., Back W. Enrichment of xanthohumol in the brewing process. Mol. Nutr. Food Res. 2005;49:874–881. doi: 10.1002/mnfr.200500051. [DOI] [PubMed] [Google Scholar]
- 41.Zamzow D.R., Elias V., Legette L.L., Choi J., Stevens J.F., Magnusson K.R. Xanthohumol improved cognitive flexibility in young mice. Behav. Brain Res. 2014;275:1–10. doi: 10.1016/j.bbr.2014.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nuti E., Bassani B., Camodeca C., Rosalia L., Cantelmo A., Gallo C., Baci D., Bruno A., Orlandini E., Nencetti S., et al. Synthesis and antiangiogenic activity study of new hop chalcone Xanthohumol analogues. Eur. J. Med. Chem. 2017;138:890–899. doi: 10.1016/j.ejmech.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Teng Y., Li X., Yang K., Li X., Zhang Z., Wang L., Deng Z., Song B., Yan Z., Zhang Y., et al. Synthesis and antioxidant evaluation of desmethylxanthohumol analogs and their dimers. Eur. J. Med. Chem. 2017;125:335–345. doi: 10.1016/j.ejmech.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 44.Guo H., Wu F., Wang Y. Overexpressed ubiquitin ligase Cullin7 in breast cancer promotes cell proliferation and invasion via down-regulating p53. Biochem. Biophys. Res. Commun. 2014;450:1370–1376. doi: 10.1016/j.bbrc.2014.06.134. [DOI] [PubMed] [Google Scholar]
- 45.Keyaerts M., Xavier C., Heemskerk J., Devoogdt N., Everaert H., Ackaert C., Vanhoeij M., Duhoux F.P., Gevaert T., Simon P. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J. Nucl. Med. 2016;57:27–33. doi: 10.2967/jnumed.115.162024. [DOI] [PubMed] [Google Scholar]
- 46.Al-Hussaini H., Subramanyam D., Reedijk M., Sridhar S.S. Notch signaling pathway as a therapeutic target in breast cancer. Mol. Cancer Ther. 2011;10:9–15. doi: 10.1158/1535-7163.MCT-10-0677. [DOI] [PubMed] [Google Scholar]
- 47.Yuan X., Zhang M., Wu H., Xu H., Han N., Chu Q., Yu S., Chen Y., Wu K. Expression of Notch1 correlates with breast cancer progression and prognosis. PLoS ONE. 2015;10:e0131689. doi: 10.1371/journal.pone.0131689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy M., Pear W.S., Aster J.C. The multifaceted role of Notch in cancer. Curr. Opin. Genet. Dev. 2007;17:52–59. doi: 10.1016/j.gde.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Robinson D.R., Kalyana-Sundaram S., Wu Y.-M., Shankar S., Cao X., Ateeq B., Asangani I.A., Iyer M., Maher C.A., Grasso C.S. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nat. Med. 2011;17:1646. doi: 10.1038/nm.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guerreiro S., Monteiro R., Martins M.J., Calhau C., Azevedo I., Soares R. Distinct modulation of alkaline phosphatase isoenzymes by 17beta-estradiol and xanthohumol in breast cancer MCF-7 cells. Clin. Biochem. 2007;40:268–273. doi: 10.1016/j.clinbiochem.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 51.Sun Z., Zhou C., Liu F., Zhang W., Chen J., Pan Y., Ma L., Liu Q., Du Y., Yang J., et al. Inhibition of breast cancer cell survival by Xanthohumol via modulation of the Notch signaling pathway in vivo and in vitro. Oncol. Lett. 2018;15:908–916. doi: 10.3892/ol.2017.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin L., Sun Z., Ren Q., Su X., Zhang D. Methyl eugenol induces potent anticancer effects in RB355 human retinoblastoma cells by inducing autophagy, cell cycle arrest and inhibition of PI3K/mTOR/Akt signalling pathway. J. BUON. 2018;23:1174–1178. [PubMed] [Google Scholar]
- 53.Monteiro R., Calhau C., Silva A.O.E., Pinheiro-Silva S., Guerreiro S., Gärtner F., Azevedo I., Soares R. Xanthohumol inhibits inflammatory factor production and angiogenesis in breast cancer xenografts. J. Cell. Biochem. 2008;104:1699–1707. doi: 10.1002/jcb.21738. [DOI] [PubMed] [Google Scholar]
- 54.Jitariu A.-A., Cîmpean A.M., Ribatti D., Raica M. Triple negative breast cancer: The kiss of death. Oncotarget. 2017;8:46652. doi: 10.18632/oncotarget.16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bassey-Archibong B.I., Kwiecien J.M., Milosavljevic S.B., Hallett R.M., Rayner L.G.A., Erb M.J., Crawford-Brown C.J., Stephenson K.B., Bédard P.A., Hassell J.A. Kaiso depletion attenuates transforming growth factor-β signaling and metastatic activity of triple-negative breast cancer cells. Oncogenesis. 2016;5:e208. doi: 10.1038/oncsis.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoo Y.B., Park K.S., Kim J.B., Kang H.J., Yang J.H., Lee E.K., Kim H.Y. Xanthohumol inhibits cellular proliferation in a breast cancer cell line (MDA-MB231) through an intrinsic mitochondrial-dependent pathway. Indian J. Cancer. 2014;51:518–523. doi: 10.4103/0019-509X.175328. [DOI] [PubMed] [Google Scholar]
- 57.Kim S.Y., Lee I.-S., Moon A. 2-Hydroxychalcone and xanthohumol inhibit invasion of triple negative breast cancer cells. Chem. Biol. Interact. 2013;203:565–572. doi: 10.1016/j.cbi.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Wada N., Fujisaki M., Ishii S., Ikeda T., Kitajima M. Evaluation of bone metabolic markers in breast cancer with bone metastasis. Breast Cancer. 2001;8:131–137. doi: 10.1007/BF02967492. [DOI] [PubMed] [Google Scholar]
- 59.Kang Y., Park M.A., Heo S.-W., Park S.-Y., Kang K.W., Park P.-H., Kim J.-A. The radio-sensitizing effect of xanthohumol is mediated by STAT3 and EGFR suppression in doxorubicin-resistant MCF-7 human breast cancer cells. Biochim. Biophys. Acta. 2013;1830:2638–2648. doi: 10.1016/j.bbagen.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Marquina G., Manzano A., Casado A. Targeted agents in cervical cancer: Beyond bevacizumab. Curr. Oncol. Rep. 2018;20:40. doi: 10.1007/s11912-018-0680-3. [DOI] [PubMed] [Google Scholar]
- 61.Yong W.K., Abd Malek S.N. Xanthohumol induces growth inhibition and apoptosis in ca ski human cervical cancer cells. Evid. Based Complement. Altern. Med. 2015;2015:921306. doi: 10.1155/2015/921306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walden D., Kunnimalaiyaan S., Sokolowski K., Clark T.G., Kunnimalaiyaan M. Antiproliferative and apoptotic effects of xanthohumol in cholangiocarcinoma. Oncotarget. 2017;8:88069–88078. doi: 10.18632/oncotarget.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dokduang H., Yongvanit P., Namwat N., Pairojkul C., Sangkhamanon S., Yageta M.S., Murakami Y., Loilome W. Xanthohumol inhibits STAT3 activation pathway leading to growth suppression and apoptosis induction in human cholangiocarcinoma cells. Oncol. Rep. 2016;35:2065–2072. doi: 10.3892/or.2016.4584. [DOI] [PubMed] [Google Scholar]
- 64.Thongchot S., Thanee M., Loilome W., Techasen A., Boonmars T., Sa-Ngiamwibool P., Titapun A., Yongvanit P., Isidoro C., Namwat N. Curative effect of xanthohumol supplementation during liver fluke-associated cholangiocarcinogenesis: Potential involvement of autophagy. J. Tradit. Complement. Med. 2020;10:230–235. doi: 10.1016/j.jtcme.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pan L., Becker H., Gerhäuser C. Xanthohumol induces apoptosis in cultured 40-16 human colon cancer cells by activation of the death receptor- and mitochondrial pathway. Mol. Nutr. Food Res. 2005;49:837–843. doi: 10.1002/mnfr.200500065. [DOI] [PubMed] [Google Scholar]
- 66.Lee S.H., Kim H.J., Lee J.S., Lee I.-S., Kang B.Y. Inhibition of topoisomerase I activity and efflux drug transporters’ expression by xanthohumol. from hops. Arch. Pharm. Res. 2007;30:1435–1439. doi: 10.1007/BF02977368. [DOI] [PubMed] [Google Scholar]
- 67.Scagliarini A., Mathey A., Aires V., Delmas D. Xanthohumol, a Prenylated Flavonoid from Hops, Induces DNA Damages in Colorectal Cancer Cells and Sensitizes SW480 Cells to the SN38 Chemotherapeutic Agent. Cells. 2020;9:932. doi: 10.3390/cells9040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu M., Yin H., Qian X., Dong J., Qian Z., Miao J. Xanthohumol, a Prenylated Chalcone from Hops, Inhibits the Viability and Stemness of Doxorubicin-Resistant MCF-7/ADR Cells. Molecules. 2016;22:36. doi: 10.3390/molecules22010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X., An L.J., Li Y., Wang Y., Zhao L., Lv X., Guo J., Song A.L. Xanthohumol chalcone acts as a powerful inhibitor of carcinogenesis in drug-resistant human colon carcinoma and these effects are mediated via G2/M phase cell cycle arrest, activation of apoptotic pathways, caspase activation and targeting Ras /MEK/ERK p. J. BUON. 2019;24:2442–2447. [PubMed] [Google Scholar]
- 70.Miranda C.L., Stevens J.F., Helmrich A., Henderson M.C., Rodriguez R.J., Yang Y.H., Deinzer M.L., Barnes D.W., Buhler D.R. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humulus lupulus) in human cancer cell lines. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1999;37:271–285. doi: 10.1016/S0278-6915(99)00019-8. [DOI] [PubMed] [Google Scholar]
- 71.Liu W., Li W., Liu H., Yu X. Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int. J. Biol. Sci. 2019;15:2497–2508. doi: 10.7150/ijbs.37481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin S., Song M., Zhao R., Liu X., Kang W.K., Lee J.M., Kim Y.E., Zhang C., Shim J.-H., Liu K., et al. Xanthohumol Inhibits the Growth of Keratin 18-Overexpressed Esophageal Squamous Cell Carcinoma in vitro and in vivo. Front. Cell Dev. Biol. 2020;8:366. doi: 10.3389/fcell.2020.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen P.-H., Chang C.-K., Shih C.-M., Cheng C.-H., Lin C.-W., Lee C.-C., Liu A.-J., Ho K.-H., Chen K.-C. The miR-204-3p-targeted IGFBP2 pathway is involved in xanthohumol-induced glioma cell apoptotic death. Neuropharmacology. 2016;110:362–375. doi: 10.1016/j.neuropharm.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 74.Festa M., Capasso A., D’Acunto C.W., Masullo M., Rossi A.G., Pizza C., Piacente S. Xanthohumol induces apoptosis in human malignant glioblastoma cells by increasing reactive oxygen species and activating MAPK pathways. J. Nat. Prod. 2011;74:2505–2513. doi: 10.1021/np200390x. [DOI] [PubMed] [Google Scholar]
- 75.Yuan J., Peng G., Xiao G., Yang Z., Huang J., Liu Q., Yang Z., Liu D. Xanthohumol suppresses glioblastoma via modulation of Hexokinase 2 -mediated glycolysis. J. Cancer. 2020;11:4047–4058. doi: 10.7150/jca.33045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benelli R. The AKT/NF-κB inhibitor xanthohumol is a potent anti-lymphocytic leukemia drug overcoming chemoresistance and cell infiltration. Biochem. Pharmacol. 2012;83:1634–1642. doi: 10.1016/j.bcp.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Harikumar K.B., Kunnumakkara A.B., Ahn K.S., Anand P., Krishnan S., Guha S., Aggarwal B.B. Modification of the cysteine residues in IkappaBalpha kinase and NF-kappaB (p65) by xanthohumol leads to suppression of NF-kappaB-regulated gene products and potentiation of apoptosis in leukemia cells. Blood. 2009;113:2003–2013. doi: 10.1182/blood-2008-04-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monteghirfo S., Tosetti F., Ambrosini C., Stigliani S., Pozzi S., Frassoni F., Fassina G., Soverini S., Albini A., Ferrari N. Antileukemia effects of xanthohumol in Bcr/Abl-transformed cells involve nuclear factor-kappaB and p53 modulation. Mol. Cancer Ther. 2008;7:2692–2702. doi: 10.1158/1535-7163.MCT-08-0132. [DOI] [PubMed] [Google Scholar]
- 79.Sławińska-Brych A., Król S.K., Dmoszyńska-Graniczka M., Zdzisińska B., Stepulak A., Gagoś M. Xanthohumol inhibits cell cycle progression and proliferation of larynx cancer cells in vitro. Chem. Biol. Interact. 2015;240:110–118. doi: 10.1016/j.cbi.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 80.Li Y., Wang K., Yin S., Zheng H., Min D. Xanthohumol inhibits proliferation of laryngeal squamous cell carcinoma. Oncol. Lett. 2016;12:5289–5294. doi: 10.3892/ol.2016.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plazar J., Zegura B., Lah T.T., Filipic M. Protective effects of xanthohumol against the genotoxicity of benzo(a)pyrene (BaP), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and tert-butyl hydroperoxide (t-BOOH) in HepG2 human hepatoma cells. Mutat. Res. 2007;632:1–8. doi: 10.1016/j.mrgentox.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 82.Kunnimalaiyaan S., Sokolowski K.M., Balamurugan M., Gamblin T.C., Kunnimalaiyaan M. Xanthohumol inhibits Notch signaling and induces apoptosis in hepatocellular carcinoma. PLoS ONE. 2015;10:e0127464. doi: 10.1371/journal.pone.0127464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ho Y.-C., Liu C.-H., Chen C.-N., Duan K.-J., Lin M.-T. Inhibitory effects of xanthohumol from hops (Humulus lupulus L.) on human hepatocellular carcinoma cell lines. Phytother. Res. 2008;22:1465–1468. doi: 10.1002/ptr.2481. [DOI] [PubMed] [Google Scholar]
- 84.Koo J.-H., Kim H.T., Yoon H.-Y., Kwon K.-B., Choi I.-W., Jung S.H., Kim H.-U., Park B.-H., Park J.-W. Effect of xanthohumol on melanogenesis in B16 melanoma cells. Exp. Mol. Med. 2008;40:313–319. doi: 10.3858/emm.2008.40.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drenzek J.G., Seiler N.L., Jaskula-Sztul R., Rausch M.M., Rose S.L. Xanthohumol decreases Notch1 expression and cell growth by cell cycle arrest and induction of apoptosis in epithelial ovarian cancer cell lines. Gynecol. Oncol. 2011;122:396–401. doi: 10.1016/j.ygyno.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 86.Li M., Gao F., Yu X., Zhao Q., Zhou L., Liu W., Li W. Promotion of ubiquitination-dependent survivin destruction contributes to xanthohumol-mediated tumor suppression and overcomes radioresistance in human oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2020;39:88. doi: 10.1186/s13046-020-01593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jiang W., Zhao S., Xu L., Lu Y., Lu Z., Chen C., Ni J., Wan R., Yang L. The inhibitory effects of xanthohumol, a prenylated chalcone derived from hops, on cell growth and tumorigenesis in human pancreatic cancer. Biomed. Pharmacother. 2015;73:40–47. doi: 10.1016/j.biopha.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 88.Saito K., Matsuo Y., Imafuji H., Okubo T., Maeda Y., Sato T., Shamoto T., Tsuboi K., Morimoto M., Takahashi H., et al. Xanthohumol inhibits angiogenesis by suppressing nuclear factor-κB activation in pancreatic cancer. Cancer Sci. 2018;109:132–140. doi: 10.1111/cas.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colgate E.C., Miranda C.L., Stevens J.F., Bray T.M., Ho E. Xanthohumol, a prenylflavonoid derived from hops induces apoptosis and inhibits NF-kappaB activation in prostate epithelial cells. Cancer Lett. 2007;246:201–209. doi: 10.1016/j.canlet.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 90.Deeb D., Gao X., Jiang H., Arbab A.S., Dulchavsky S.A., Gautam S.C. Growth inhibitory and apoptosis-inducing effects of xanthohumol, a prenylated chalone present in hops, in human prostate cancer cells. Anticancer Res. 2010;30:3333–3339. [PMC free article] [PubMed] [Google Scholar]
- 91.Venè R., Benelli R., Minghelli S., Astigiano S., Tosetti F., Ferrari N. Xanthohumol impairs human prostate cancer cell growth and invasion and diminishes the incidence and progression of advanced tumors in TRAMP mice. Mol. Med. 2012;18:1292–1302. doi: 10.2119/molmed.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cook M.R., Luo J., Ndiaye M., Chen H., Kunnimalaiyaan M. Xanthohumol inhibits the neuroendocrine transcription factor achaete-scute complex-like 1, suppresses proliferation, and induces phosphorylated ERK1/2 in medullary thyroid cancer. Am. J. Surg. 2010;199 doi: 10.1016/j.amjsurg.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu H., Zhang L., Li G., Gao Z. Xanthohumol protects against Azoxymethane-induced colorectal cancer in Sprague-Dawley rats. Environ. Toxicol. 2020;35:136–144. doi: 10.1002/tox.22849. [DOI] [PubMed] [Google Scholar]
- 94.Gao F., Li M., Zhou L., Liu W., Zuo H., Li W. Xanthohumol targets the ERK1/2‑Fra1 signaling axis to reduce cyclin D1 expression and inhibit non‑small cell lung cancer. Oncol. Rep. 2020;44:1365–1374. doi: 10.3892/or.2020.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu X., Song M., Wang P., Zhao R., Chen H., Zhang M., Shi Y., Liu K., Liu F., Yang R., et al. Targeted therapy of the AKT kinase inhibits esophageal squamous cell carcinoma growth in vitro and in vivo. Int. J. Cancer. 2019;145:1007–1019. doi: 10.1002/ijc.32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reitman Z.J., Winkler F., Elia A.E.H. New Directions in the Treatment of Glioblastoma. Semin. Neurol. 2018;38:50–61. doi: 10.1055/s-0038-1623534. [DOI] [PubMed] [Google Scholar]
- 97.Festa M., Caputo M., Cipolla C., D’Acunto C., Rossi A., Tecce M., Capasso A. The involvement of xanthohumol in the expression of annexin in human malignant glioblastoma cells. Open Biochem. J. 2013;7:1–10. doi: 10.2174/1874091X01307010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cosetti M., Yu G.-P., Schantz S.P. Five-year survival rates and time trends of laryngeal cancer in the US population. Arch. Otolaryngol. Head Neck Surg. 2008;134:370–379. doi: 10.1001/archotol.134.4.370. [DOI] [PubMed] [Google Scholar]
- 99.Weiskirchen R., Mahli A., Weiskirchen S., Hellerbrand C. The hop constituent xanthohumol exhibits hepatoprotective effects and inhibits the activation of hepatic stellate cells at different levels. Front. Physiol. 2015;6:140. doi: 10.3389/fphys.2015.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Logan I.E., Miranda C.L., Lowry M.B., Maier C.S., Stevens J.F., Gombart A.F. Antiproliferative and Cytotoxic Activity of Xanthohumol and Its Non-Estrogenic Derivatives in Colon and Hepatocellular Carcinoma Cell Lines. Int. J. Mol. Sci. 2019;20:1203. doi: 10.3390/ijms20051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dorn C., Weiss T.S., Heilmann J., Hellerbrand C. Xanthohumol, a prenylated chalcone derived from hops, inhibits proliferation, migration and interleukin-8 expression of hepatocellular carcinoma cells. Int. J. Oncol. 2010;36:435–441. doi: 10.1055/s-0029-1246396. [DOI] [PubMed] [Google Scholar]
- 102.Krajka-Kuźniak V., Paluszczak J., Baer-Dubowska W. Xanthohumol induces phase II enzymes via Nrf2 in human hepatocytes in vitro. Toxicol. In Vitro. 2013;27:149–156. doi: 10.1016/j.tiv.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 103.Ferk F., Huber W.W., Filipic M., Bichler J., Haslinger E., Misík M., Nersesyan A., Grasl-Kraupp B., Zegura B., Knasmüller S. Xanthohumol, a prenylated flavonoid contained in beer, prevents the induction of preneoplastic lesions and DNA damage in liver and colon induced by the heterocyclic aromatic amine amino-3-methyl-imidazo[4,5-f]quinoline (IQ) Mutat. Res. 2010;691:17–22. doi: 10.1016/j.mrfmmm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 104.Zhao X., Jiang K., Liang B., Huang X. Anticancer effect of xanthohumol induces growth inhibition and apoptosis of human liver cancer through NF-κB/p53-apoptosis signaling pathway. Oncol. Rep. 2016;35:669–675. doi: 10.3892/or.2015.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sławińska-Brych A., Zdzisińska B., Dmoszyńska-Graniczka M., Jeleniewicz W., Kurzepa J., Gagoś M., Stepulak A. Xanthohumol inhibits the extracellular signal regulated kinase (ERK) signalling pathway and suppresses cell growth of lung adenocarcinoma cells. Toxicology. 2016;357–358:65–73. doi: 10.1016/j.tox.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 106.Seitz T., Hackl C., Freese K., Dietrich P., Mahli A., Thasler R.M., Thasler W.E., Lang S.A., Bosserhoff A.K., Hellerbrand C. Xanthohumol, a Prenylated Chalcone Derived from Hops, Inhibits Growth and Metastasis of Melanoma Cells. Cancers. 2021;13:511. doi: 10.3390/cancers13030511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goenka S., Simon S.R. Depigmenting effect of Xanthohumol from hop extract in MNT-1 human melanoma cells and normal human melanocytes. Biochem. Biophys. Rep. 2021;26:100955. doi: 10.1016/j.bbrep.2021.100955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Musella A., Bardhi E., Marchetti C., Vertechy L., Santangelo G., Sassu C., Tomao F., Rech F., D’Amelio R., Monti M. Rucaparib: An emerging parp inhibitor for treatment of recurrent ovarian cancer. Cancer Treat. Rev. 2018;66:7–14. doi: 10.1016/j.ctrv.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 109.Siegel R.L., Miller K.D., Jemal A. Cancer Statistics, 2017. CA. Cancer J. Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 110.LeComte M.D., Spees J.L. Notch1-STAT3-ETBR signaling in brain injury and cancer. Cytokine. 2016;80:64–65. doi: 10.1016/j.cyto.2015.08.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dinesh T.A., Nair P., Abhijath V., Jha V., Aarthy K. Economics of cancer care: A community-based cross-sectional study in Kerala, India. South Asian J. Cancer. 2020;9:7. doi: 10.4103/sajc.sajc_382_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steinestel J., Luedeke M., Arndt A., Schnoeller T.J., Lennerz J.K., Wurm C., Maier C., Cronauer M.V., Steinestel K., Schrader A.J. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget. 2019;10:4213. doi: 10.18632/oncotarget.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kłósek M., Mertas A., Król W., Jaworska D., Szymszal J., Szliszka E. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis in Prostate Cancer Cells after Treatment with Xanthohumol-A Natural Compound Present in Humulus lupulus L. Int. J. Mol. Sci. 2016;17:837. doi: 10.3390/ijms17060837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Legette L., Karnpracha C., Reed R.L., Choi J., Bobe G., Christensen J.M., Rodriguez-Proteau R., Purnell J.Q., Stevens J.F. Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol. Nutr. Food Res. 2014;58:248–255. doi: 10.1002/mnfr.201300333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Carvalho D.O., Freitas J., Nogueira P., Henriques S.N., Carmo A.M., Castro M.A., Guido L.F. Xanthohumol inhibits cell proliferation and induces apoptosis in human thyroid cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018;121:450–457. doi: 10.1016/j.fct.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 116.Radović B., Schmutzler C., Köhrle J. Xanthohumol stimulates iodide uptake in rat thyroid-derived FRTL-5 cells. Mol. Nutr. Food Res. 2005;49:832–836. doi: 10.1002/mnfr.200500053. [DOI] [PubMed] [Google Scholar]
- 117.Yilmazer M., Stevens J.F., Deinzer M.L., Buhler D.R. In vitro biotransformation of xanthohumol, a flavonoid from hops (Humulus lupulus), by rat liver microsomes. Drug Metab. Dispos. 2001;29:223–231. [PubMed] [Google Scholar]
- 118.Possemiers S., Heyerick A., Robbens V., De Keukeleire D., Verstraete W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J. Agric. Food Chem. 2005;53:6281–6288. doi: 10.1021/jf0509714. [DOI] [PubMed] [Google Scholar]
- 119.Hanske L., Loh G., Sczesny S., Blaut M., Braune A. Recovery and metabolism of xanthohumol in germ-free and human microbiota-associated rats. Mol. Nutr. Food Res. 2010;54:1405–1413. doi: 10.1002/mnfr.200900517. [DOI] [PubMed] [Google Scholar]
- 120.Jirásko R., Holcapek M., Vrublová E., Ulrichová J., Simánek V. Identification of new phase II metabolites of xanthohumol in rat in vivo biotransformation of hop extracts using high-performance liquid chromatography electrospray ionization tandem mass spectrometry. J. Chromatogr. A. 2010;1217:4100–4108. doi: 10.1016/j.chroma.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 121.Bartmanska A., Tronina T., Poplonski J., Huszcza E. Biotransformations of prenylated hop flavonoids for drug discovery and production. Curr. Drug Metab. 2013;14:1083–1097. doi: 10.2174/1389200214666131211151855. [DOI] [PubMed] [Google Scholar]
- 122.Van Breemen R.B., Yuan Y., Banuvar S., Shulman L.P., Qiu X., Alvarenga R.F.R., Chen S.-N., Dietz B.M., Bolton J.L., Pauli G.F., et al. Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol. Nutr. Food Res. 2014;58:1962–1969. doi: 10.1002/mnfr.201400245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wolff H., Motyl M., Hellerbrand C., Heilmann J., Kraus B. Xanthohumol uptake and intracellular kinetics in hepatocytes, hepatic stellate cells, and intestinal cells. J. Agric. Food Chem. 2011;59:12893–12901. doi: 10.1021/jf203689z. [DOI] [PubMed] [Google Scholar]
- 124.Wesołowska O., Gąsiorowska J., Petrus J., Czarnik-Matusewicz B., Michalak K. Interaction of prenylated chalcones and flavanones from common hop with phosphatidylcholine model membranes. Biochim. Biophys. Acta. 2014;1838:173–184. doi: 10.1016/j.bbamem.2013.09.009. [DOI] [PubMed] [Google Scholar]