Abstract
Patients recovering from coronavirus disease 2019 (COVID-19) may not return to a pre-COVID functional status and baseline levels of healthcare needs after discharge from acute care hospitals. Since the long-term outcomes of COVID-19 can be more severe in patients with underlying cardiorespiratory diseases, we aimed at verifying the impact of a preexisting cardiorespiratory comorbidity on multidisciplinary rehabilitation in post-COVID-19 patients. We enrolled 95 consecutive patients referring to the Pulmonary Rehabilitation Unit of Istituti Clinici Scientifici Maugeri Spa SB, IRCCS of Telese Terme, Benevento, Italy after being discharged from the COVID-19 acute care ward and after recovering from acute COVID-19 pneumonia. Forty-nine of them were not suffering from underlying comorbidities, while 46 had a preexisting cardiorespiratory disease. Rehabilitation induced statistically significant improvements in respiratory function, blood gases and the ability to exercise both in patients without any preexisting comorbidities and in those with an underlying cardiorespiratory disease. Response to the rehabilitation cycle tended to be greater in those without preexisting comorbidities, but DLco%-predicted was the only parameter that showed a significant greater improvement when compared to the response in the group of patients with underlying cardiorespiratory comorbidity. This study suggests that multidisciplinary rehabilitation may be useful in post-COVID-19 patients regardless of the presence of preexisting cardiorespiratory comorbidities.
Keywords: COVID-19, Multidisciplinary rehabilitation, Cardiorespiratory diseases
1. Introduction
An Italian study showed that 87.4% of patients recovering from COVID-19 reported persistence of symptoms, particularly fatigue and dyspnea, even two months after being discharged [1]. Furthermore, many patients after hospitalization, which is usually prolonged, have decreased respiratory movement, pain and weakness in lower limbs, skin lesions, decreased metabolism, joint stiffness, and venous stasis. In keeping with this, some manifestations of systemic dysfunction (e.g. respiratory impairment, muscle weakness, neuropathy, and psychological disorders) may still occur [2]. This delayed recovery of symptoms has been termed ‘post-COVID-19 syndrome’ or ‘long COVID’ [3].
An early bedside rehabilitation program is strongly recommended for these patients [4]. Unfortunately, much of the information we have on the effects of respiratory rehabilitation relates to the management of critically ill patients in acute care settings, while the rehabilitation of the post-acute patient referring to a long-term care facility is very poor [5].
Taking into account these information gaps, the International Multiprofessional Steering Committee of Cochrane Rehabilitation REH-COVER (Rehabilitation for COVID-19: an Evidence-Based Response) action has recently highlighted that data on the efficacy of specific rehabilitation approaches in the acute and also post-acute phase are needed [6].
Since the long-term outcomes of COVID-19 can be more severe in patients with underlying cardiorespiratory diseases [7,8], we aimed at verifying the impact of preexisting cardiorespiratory comorbidities on multidisciplinary rehabilitation program in post-acute COVID-19 patients admitted to a Pulmonary Rehabilitation Unit. To this end, we compared the effects of rehabilitation in two groups of patients, one free of preexisting comorbidities and the other with an underlying cardiorespiratory disease.
2. Patients and methods
2.1. Patients
In this study, we recruited 95 patients consecutively referring to the Pulmonary Rehabilitation Unit of the Istituti Clinici Scientifici Maugeri Spa SB, IRCCS of Telese Terme, Benevento, Italy after being discharged from the COVID-19 acute care ward and after recovering from acute COVID-19 pneumonia (Table 1 ). Forty-nine of them were not suffering from underlying comorbidities, while 46 had a preexisting cardiorespiratory disease. The average age of those without any chronic disease, of which 41 were male and 8 female, was 61.5 (95% CI: 58.4–64.6) years, while that of subjects with an underlying cardiorespiratory disease, of which 39 were male and 7 female, was 65.3 (95% CI: 62.8–67-8) years. According to a recent meta-analysis, male patients with COVID-19 have almost three times the risk of requiring admission at intensive treatment unit (OR: 2.84; 95% CI: 2.06–3.92) as well as a higher risk of death (OR: 1.39; 95% CI: 1.31–1.47) compared to females [9]. This finding accounts for the high prevalence of male patients experiencing a more severe disease, thus requiring post-acute pulmonary rehabilitation. The most common preexisting illnesses were hypertension (27 patients), ischemic heart disease (11 patients) and chronic obstructive pulmonary disease (COPD) (9 patients).
Table 1.
Patients’ clinical characteristics.
| Patients without comorbidities (n = 49) | Patients with comorbidities (n = 46) | |
|---|---|---|
| Age (years) | 61.5 ± 1.6 | 65.3 ± 1.2 |
| Sex | ||
|
41 | 39 |
|
8 | 7 |
| Smokers | 3 | 7 |
| Ex-smokers | 20 | 21 |
| FVC % predicted | 76.7 ± 3.2† | 72.6 ± 2.9 § |
| FEV1% predicted | 79.9 ± 3,1† | 73.2 ± 3.3 § |
| 6MWT (meters) | 242.5 ± 14.6 | 200.5 ± 16.6 |
| Comorbidities | ||
|
27 | |
|
7 | |
|
9 | |
|
2 | |
|
11 | |
|
8 | |
|
9 | |
|
3 | |
|
2 | |
| Number of comorbidities | ||
|
11 | |
|
28 | |
|
5 | |
|
2 | |
† spirometry was not available in 11 patients; § spirometry was not available in 21 patients.
Values are expressed as mean ± ES.
3. Methods
The study was approved by the Institutional Review Board of Istituto Nazionale Tumori, Fondazione Pascale, Naples, Italy with reference number ICS 11/20, and all patients provided written informed consent to use their de-identified data for future research.
Upon entering the ward, pulmonary function test (PFT) and evaluation of carbon monoxide diffusion capacity (DLCO) were performed whenever possible. Furthermore, always when the patient's clinical conditions allowed it, a 6-min walking test (6MWT) was performed.
PFTs were performed using an automated equipment (Vyasis, Milan, Italy) according to American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines [10]. Forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and the FEV1/FVC ratio were measured. Moreover, measurement of DLco using the single-breath method was performed according to ATS/ERS guidelines [11].
The 6MWT was also performed in accordance with the ATS/ERS guidelines [12]. The 6-min walking distance (6MWD) was measured in metres and the other outcomes that have been reported, such as symptoms of dyspnoea and fatigue, were measured at the beginning and at the end of the test. Each patient was asked to walk at his/her own pace to cover maximum distance possible in the allotted time.
Immediately afterwards, all patients began a 30-day respiratory rehabilitation program, in addition to the appropriate pharmacological treatment.
At the end of the rehabilitation program, patients underwent again PFT, DLco measurement and 6MWT.
In all patients, arterial blood gas levels were measured while breathing room air before and at the end of the rehabilitation cycle.
3.1. Pulmonary rehabilitation
All included patients underwent a 5-week pulmonary rehabilitation program with daily sessions (6 sessions/week). The program consisted of 30 sessions following the official ATS/ERS statement on pulmonary rehabilitation [13], including physical exercise training, dietary counselling, and psychosocial counselling. Physical exercise training was the cornerstone of the program, consisting of exercises to strengthen groups of muscles in the upper and lower extremities, treadmill walking and stationary cycling. All exercises were performed at moderate to high intensity to obtain an overload stimulus, and the training intensity was increased during the rehabilitation period, based on dyspnea and fatigue symptom scores. All patients underwent flexibility exercises, general physical exercise for lower and upper extremities, and daily supervised 30-min outdoor walks.
3.2. Statistical analysis
Statistical analysis was performed using Prism 8 software package (GraphPad Software Inc, USA). Data were expressed as mean and 95% confidence interval (CI) (standard errors have been reported in figures). Analysis of spirometric, 6MWT and blood gases changes collected before and after rehabilitation was performed using the Student's t-test for paired variables. Comparison between subjects with no previous pathology and those with preexisting cardiopulmonary comorbidity was made with unpaired t-test and Welch's correction. All results were expressed as 2-tailed values, P values < 0.05 being statistically significant.
4. Results
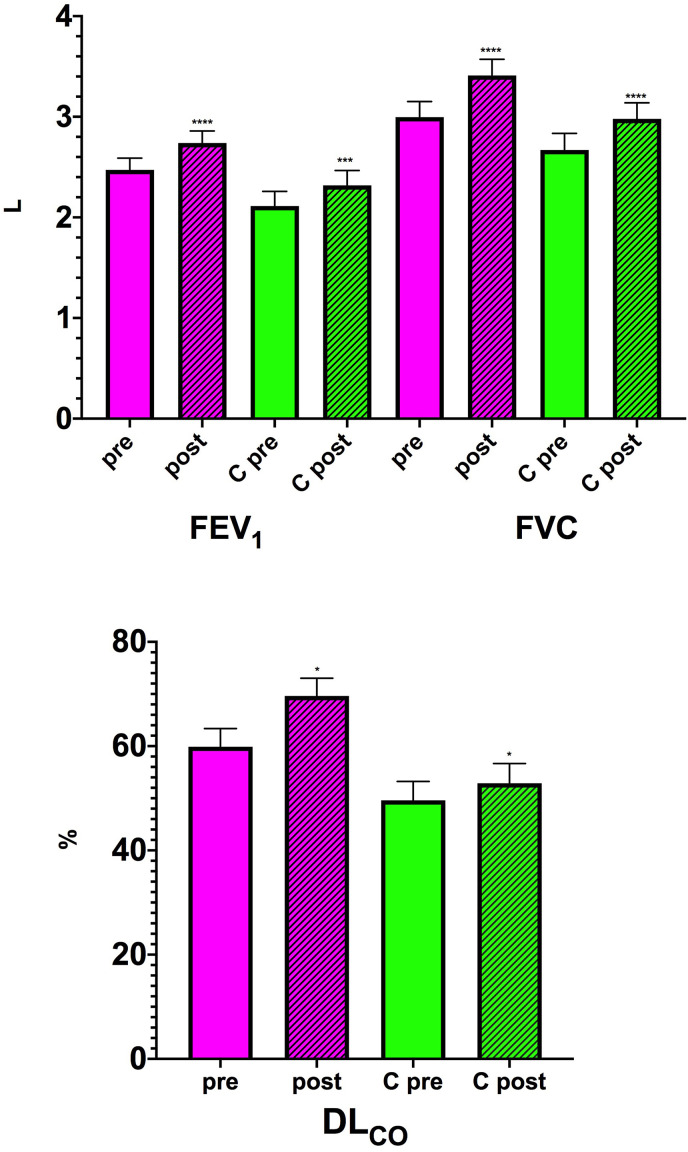
At the end of the rehabilitation program, during which no adverse events occurred, in the group of patients without underlying cardiorespiratory comorbidities and in which spirometry could also be performed at admission, there was a significant improvement in pulmonary function measurements, with FEV1 increasing by 267.1 mL (95% CI: 161.3–372.9; P < 0.0001) and a mean increase in FVC of 415.0 mL (95% CI: 278.1–551.9; P < 0.0001) (Fig. 1 ). DLCO%-predicted improved by 9.7% (95% CI: 6.0–13.5%; P = 0.016) (Fig. 1). The PFT data refer to 38 patients because the other 11 were unable to perform the PFT when they were admitted to our ward. Before starting rehabilitation, among the patients with no previous pathology, 30 had a DLCO <80% indicating the presence of a diffusion deficit [14], in 13 it was not possible to perform the test and only 6 had a value ≥ 80%.
Fig. 1.
– FEV1, FVC and DLCO %-predicted before (pre) and at the end of the rehabilitation program (post) in patients with no previous pathology (magenta) and those with preexisting cardiopulmonary comorbidity (C green). *P < 0.05, ***P < 0.001, ****P < 0.0001 post-rehabilitation vs pre-rehabilitation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
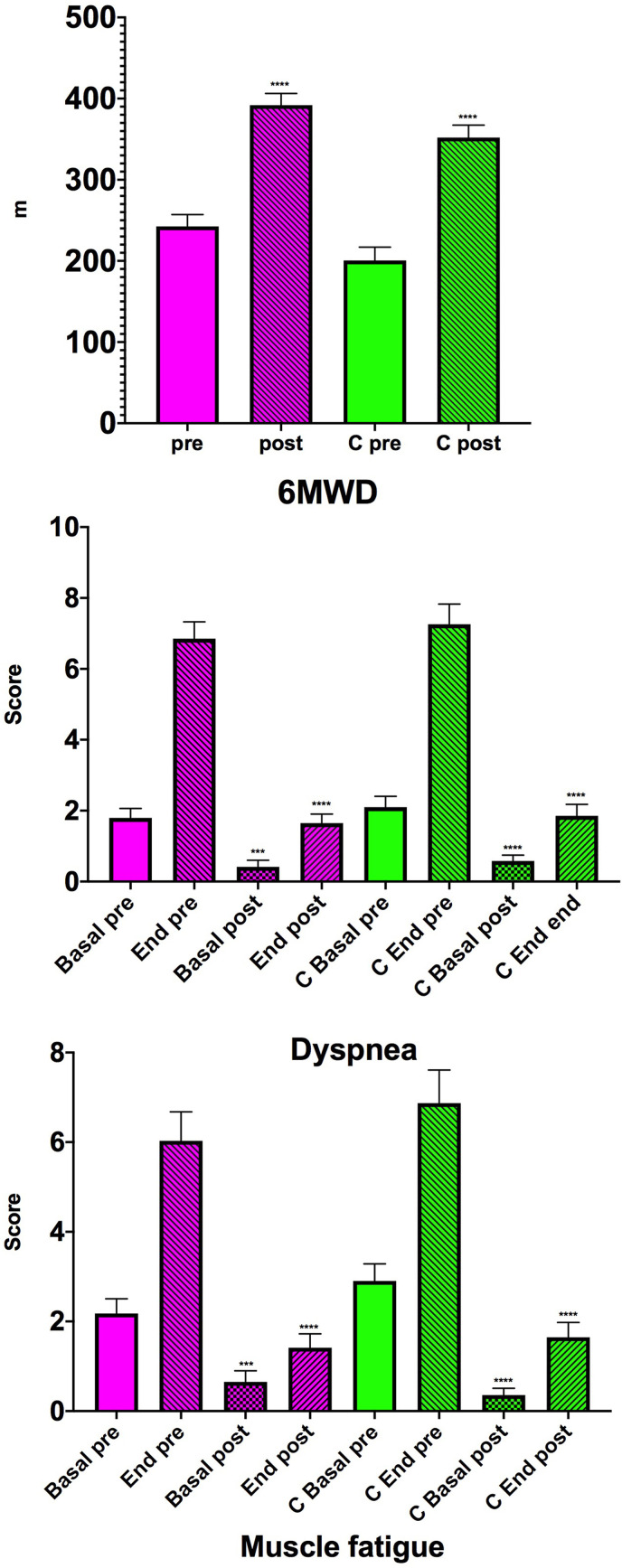
As a result of the rehabilitation program, the 6MWD increased by 149.2 m (95% CI: 128.3– 170.2; P < 0.0001) (Fig. 2 ). In the 6MWT performed before starting rehabilitation, the dyspnea score was 1.8 (95% CI: 1.2–2.3) at resting (time 0) and 6.9 (95% CI: 5.9–7.8) the end of the test. Rehabilitation induced a significant reduction in this score. In fact, in the test performed at the end of the rehabilitation cycle the dyspnea score was reduced to 0.4 (95% CI: 0.03–0.8; P = 0.0003 vs pre-rehabilitation) at resting and to 1.6 (95% CI: 1.1–2.1; P < 0.0001 vs pre-rehabilitation) at the end of the test (Fig. 2). Muscle fatigue scores also dropped significantly thanks to rehabilitation, going from 2.2 (95% CI: 1.5–2.8) to 6.0 (95% CI: 4.7–7.3) on the test performed on admission and from 0.6 (95% CI: 0.1–1.1; P = 0.0004 vs pre-rehabilitation) to 1.4 (95% CI: 0.8–2.0; P < 0.0001 vs pre-rehabilitation) on the test performed before discharge (Fig. 2). The 6MWT was performed before and after rehabilitation in 34 patients. It was, however, possible to perform a 6MWT at the end of the rehabilitation program in 14 of the other 15 patients.
Fig. 2.
– 6MWD, dyspnea and muscle fatigue scores before (pre) and at the end of the rehabilitation program (post) in patients with no previous pathology (magenta) and those with preexisting cardiopulmonary comorbidity (C green). ***P < 0.001, ****P < 0.0001 post-rehabilitation vs pre-rehabilitation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
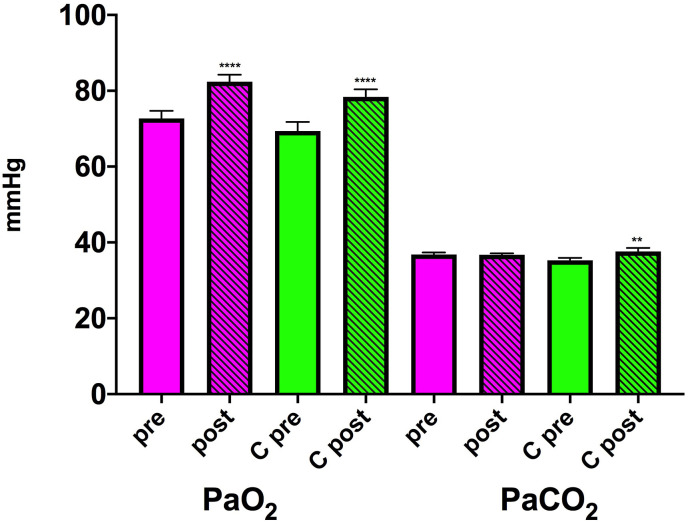
The mean changes in PaO2 and PaCO2 measured on ambient air are shown in Fig. 3 .
Fig. 3.
– PaO2 and PaCO2 before (pre) and at the end of the rehabilitation program (post) in patients with no previous pathology (magenta) and those with preexisting cardiopulmonary comorbidity (C green). ****P < 0.0001 post-rehabilitation vs pre-rehabilitation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In the group of patients with underlying cardiorespiratory diseases, FEV1, FVC and DLCO%-predicted (Fig. 1) improved by 205.5 mL (95% CI: 106.3–304.9; P = 0.0003), 310.4 mL (95% CI: 176.0–444.8; P < 0.0001) and 3.3% (95% CI: 0.7–5.9; P = 0.016), respectively. In this group, the PFT data refer to 25 patients because the other 21 were unable to perform the PFT upon entering the ward, while only 2 had a DLCO ≥ 80% and in other 19 patients clinical conditions did not allow performing this test.
The rehabilitation program significantly (P < 0.0001) increased the walking distance as assessed by the 6MWT (151.5 m, 95% CI: 121.9–181.1) (Fig. 2). The dyspnea score in the 6MWT performed at entry went from 2.1 (95% CI: 1.5–2.7) at time 0 to 7.3 (95% CI: 6.1–8.4) at the end of the test, while that of muscle fatigue went from 2.9 (95% CI: 2.1–3.7) to 6.9 (95% CI: 5.4–8.4) (Fig. 2). The dyspnea scores measured before (0.6, 95% CI: 0.2–0.9) and after (1.9, 95% CI: 1.2–2.5) the 6MWT at the end of the rehabilitation program were significantly lower (both P < 0.0001) than those measured before and after the test performed on admission to the ward. Muscle fatigue scores showed trends similar to those of dyspnea being 0.3 (95% CI: 0.04–0.7) before and 1.6 (95% CI: 1.0–2.3) after the 6MWT performed at the end of the rehabilitation program (Fig. 2). The scores measured before and after the test carried out at the end of the rehabilitation program were significantly lower (both P < 0.0001) than those measured before rehabilitation. The 6MWT was performed before and after rehabilitation in 31 patients, while in 11 other subjects it was possible to perform the 6MWT at the end of the planned hospitalization. Three patients were unable to perform any 6MWT, and 1 patient who had performed the test at admission was unable to perform it at the end of rehabilitation due to a worsening of his clinical status.
It should be noted that patients without a previous comorbidity started the rehabilitation program with better functional parameters (Table 2 ). The mean difference in FEV1 between those without a previous pathology and those suffering from an underlying cardiopulmonary pathology was 359.4 mL (95% CI: -15,2–734.1; P = 0.06), whereas that in FVC was 326.3 mL (95% CI: -130.6–783.3; P = 0.158) and difference in DLCO%-predicted was 10.3% (95% CI: 1.4–20.4; P = 0.037). Furthermore, they also suffered from less dyspnea (1.8, 95% CI: 1.2–2,3 vs. 2.1, 95% CI: 1.5–2.7), although the difference between the two groups was not statistically significant (P = 0.463). Also the 6MWD was greater in those without a previous pathology with a difference of 42.0 m (95% CI: -1.9–85.8), although even in this case the difference between the two groups was not statistically significant (P = 0.061), nor was the difference in 6MWT-induced dyspnea significant (0.4, 95% CI: -1.1–1.9; P = 0.587).
Table 2.
Differences in changes of examined parameters between patients with no underlying disease and those with preexisting cardiopulmonary comorbidity before and after rehabilitation.
| Pre-rehabilitation | P value | Post-rehabilitation | P value | |
|---|---|---|---|---|
| FEV1 | 359.4 mL (95% CI: -15,2–734.1) | 0.06 | 420.9 mL (95% CI: 36.7–805.2) | 0.0324 |
| FVC | 326.3 mL (95% CI: -130.6–783.3) | 0.158 | 430.9 mL (95% CI: -20.6–882.5) | 0.06 |
| DLCO | 10.3% (95% CI: 1.4–20.4) | 0.037 | 16.7% (95% CI: 6.5–27.0) | 0.0019 |
| 6MWD | 42.0 m (95% CI: -1.9–85.8) | 0.06 | 39.7 m (95% CI: -2.5–81) | 0.06 |
| PaO2 | 3.3 mmHg (95% CI: -2.8–9.5) | 0,284 | 4.0 mmHg (95% CI: -1.4–9-4) | 0.145 |
| PaCO2 | 1.5 mmHg (95% CI: -0.1–3.2) | 0.07 | −0.9 mmHg (95% CI: -2.9–1.2) | 0.400 |
DLCO carbon monoxide diffusion capacity %-predicted; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; partial pressure of carbon dioxide (PaCO2); partial pressure of oxygen (PaO2); 6MWD, 6-min walking distance.
At the end of the rehabilitation program, the mean difference in FEV1 between the two groups became 420.9 mL (95% CI: 36.7–805.2; P = 0.032), while that in FVC was 430.9 mL (95% CI: -20.6–882.5; P = 0.06), and DLCO%-predicted differed between those without a preexisting pathology and those suffering from an underlying cardiopulmonary comorbidity by 16.7% (95% CI: 6.5–27.0; P = 0.0019). The difference in the distance walked in the 6 min was not significant between the groups (39.7 m, 95% CI: -2.5–81; P = 0.06) and very similar to that recorded before rehabilitation. Pre-test dyspnoea was mildly (−0.2, 95% CI: -0.7–0.3) but not significantly (P = 0.505) less in the group without prior disease, difference that was substantially unchanged at the end of the test (−0.2, 95% CI: -1.0–0.6; P = 0.616).
The differences between the two groups in the changes in muscle fatigue scores were also modest and not statistically significant both before starting rehabilitation and at its end.
5. Discussion
Our study shows that a multidisciplinary rehabilitation program is extremely useful in post-COVID-19 patients, regardless of the presence of an underlying cardiorespiratory comorbidity. In particular, the significant increase in 6MWD values regardless of the health status before the onset of COVID-19 deserves a specific mention because it was indicative of a noteworthy improvement in physical function. Rehabilitation greatly reduced dyspnea and muscle fatigue in both groups. This leads us to believe that there are no substantial differences in the response to physical effort between those with preexisting cardiorespiratory comorbidities and those initially healthy, even if the latter, as expected, tended to have a better performance in terms of walking distance.
It could be argued that in order to understand whether the results of this study are specific to COVID-19 and really due to the rehabilitation program, it would have been necessary to also consider specific groups of patients without COVID-19 and post-COVID-19 patients who were not been undergoing rehabilitation. Our study focused solely on post-COVID patients because currently it is preferred not to admit patients who have not contracted SARS-CoV-2 infection to reduce risk of contracting COVID-19 and spreading the virus. In any case, our centre, as well as most of the rehabilitation centres around the world, as correctly highlighted by the Assembly on pulmonary rehabilitation of the American Thoracic Society [15], has temporarily suspended its centre-based, face-to-face programs for COVID free subjects due to concerns of infection and the need for physical distancing.
On the other hand, even following outpatient patients who do not need rehabilitation is currently extremely difficult. As also stated by the 2020 GOLD Science Committee Report on COVID-19 and COPD [16], during periods of high prevalence of COVID-19 in the community, spirometry (and we also add the other exams of our study) should be restricted to patients requiring urgent or essential tests for the diagnosis of COPD and/or to assess lung function status for interventional procedures or surgery. Adding a control group in a COVID era by entering data from groups treated in the past should be an alternative, but in our opinion it would be wrong because the protocol would be completely different.
Great importance should be given to the improvements in 6MWD, which provides an assessment of exercise capacity and can better reflect daily activity than laboratory tests [17]. The 54 m threshold for 6MWD is considered representative of a clinically significant change [18]. The majority of our patients largely exceeded this value at the end of the rehabilitation program, although it has been highlighted that a statistically significant mean increase in 6MWD in a group of study participants is often much less than a clinically significant increase in an individual patient [19]. The fact that a large number of patients who were unable to walk upon arrival in our facility and, consequently did not perform the 6MWT before rehabilitation, were able to do it at the end of the multidisciplinary rehabilitation program even in the presence of a cardiorespiratory comorbidity reinforces our view that all patients hospitalized for COVID-19 should undergo a multidisciplinary rehabilitation cycle.
We are fully aware that using a more demanding exercise capacity test, such as a cardiopulmonary exercise test, would have been more helpful in documenting that the rehabilitation cycle did not really impact differently in the two patient groups we examined, but the initial conditions of the patients discouraged the execution of this test.
Although the 6MWT can better reflect daily activity than laboratory tests, these also provide important information. As expected, the improvements in FEV1, FVC and DLCO% observed in those who were healthy prior to COVID-19 tended to be greater, but a statistically significant difference between the two groups was observed only in the FEV1 and DLCO%-predicted changes.
While the more substantial improvement in FEV1 is not surprising because this parameter is substantially reduced in subjects with cardiorespiratory pathologies [[20], [21], [22], [23]], particular attention should be paid to the differences in the DLCO values. DLCO%-predicted is the strongest independent factor associated with previous severe/critical COVID-19 [24].
More than 90% of our patients had a known or presumed diffusion deficit, although the deficit tended to be greater in those with history of cardiorespiratory comorbidities potentially affecting lung diffusing capacity. Alterations of gas transport occurs in more than half of the COVID-19 patients [25] and may be present even in subjects who had mild COVID-19 pneumonia and no or minimal persisting computed tomography (CT) abnormalities [26]. Although a reduced DLCO may reflect parenchymal lung disease, it has been suggested that a low DLCO is mainly determined by a reduced alveolar volume and not by the residual interstitial lung abnormalities or pulmonary vascular abnormalities [27]. However, COVID-19 specifically impacts the pulmonary circulation [28]. To determine whether the reduction in DLCO in patients who have recovered from COVID-19 is caused by interstitial or pulmonary vascular abnormalities, it would be desirable to use more specific measures of the alveolar-capillary membrane, such as combined DLCO and diffusion capacity of the lung for nitric oxide (DLNO) measurements or advanced imaging techniques [29]. It has been suggested that an impairment of DLNO exceeding standard DLCO may be present during the recovery from COVID-19, possibly due to loss of alveolar units with alveolar membrane damage, but relatively preserved capillary volume [26].
Having not performed this type of test, we are not able to tell if the low DLCO values we recorded were dependent on a parenchymal damage rather than a vascular involvement. However, we have noticed a remarkable improvement of this parameter that has even normalized in 7 subjects without preexisting comorbidity.
While it is relatively simple to explain the improvements in PTF and 6MWT parameters induced by rehabilitation with the positive effects elicited by the respiratory muscle training [26], it is difficult to give explanation on its effect on the diffusion deficit. It has been suggested that the severity of pulmonary inflammation may be the reason for impaired DLCO in COVID-19 patients [30]. Since there is evidence that exercise training represents a strategy to elicit an anti-inflammation effect, which may help to decrease the risk or progression of several disorders of an inflammatory nature [31], as are the parenchymal and vascular sequelae of COVID-19, we wonder if the improvement observed is linked to an anti-inflammatory effect induced by the rehabilitation cycle, an effect that in our patients was more evident in the absence of a previous cardiorespiratory damage. One could speculate that the effect should be different because of the systemic inflammation that characterises the cardiorespiratory diseases [32]. However, the impossibility of including in this study groups of patients without preexisting comorbidities or suffering from cardiorespiratory comorbidities but not been affected by COVID do not allow us to formulate solid conclusion.
Obviously, this is a hypothesis to be verified when it will be possible to compare groups with different characteristics. The study will have to use more sophisticated diagnostic tests and also consider the biomarkers of inflammation. In the meantime, as already pointed out, we strongly believe that multidisciplinary rehabilitation cycles must always be prescribed in patients who have a slow recovery from COVID-19 and this regardless if they are suffering from cardiorespiratory comorbidities.
Author contribution
M. Maniscalco: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Writing - review and editing. S. Fuschillo: Investigation. P. Ambrosino: Data curation, Investigation, Methodology, Writing - review and editing. M. Martucci: Investigation. A. Papa: Investigation. M.G. Matera: Formal analysis, Validation, Writing - review and editing. M. Cazzola: Conceptualization, Formal analysis, Validation, Writing - original draft, Writing - review and editing.
Funding
No funding.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Authors want to thank Anna Ciullo and Silvia Stufano for technical assistance.
References
- 1.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. J. Am. Med. Assoc. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prvu Bettger J., Thoumi A., Marquevich V., De Groote W., Rizzo Battistella L., Imamura M., et al. COVID-19: maintaining essential rehabilitation services across the care continuum. BMJ Glob. Health. 2020;5 doi: 10.1136/bmjgh-2020-002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenhalgh T., Knight M., A'Court C., Buxton M., Husain L. Management of post-acute COVID-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 4.Spruit M.A., Holland A.E., Singh S.J., Tonia T., Wilson K.C., Troosters T. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society and American Thoracic Society-coordinated international task force. Eur. Respir. J. 2020;56:2002197. doi: 10.1183/13993003.02197-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demeco A., Marotta N., Barletta M., Pino I., Marinaro C., Petraroli A., et al. Rehabilitation of patients post-COVID-19 infection: a literature review. J. Int. Med. Res. 2020;48 doi: 10.1177/0300060520948382. 300060520948382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Sire A., Andrenelli E., Negrini F., Patrini M., Lazzarini S.G., Ceravolo M.G. Rehabilitation and COVID-19: a rapid living systematic review by Cochrane Rehabilitation Field updated as of December 31st, 2020 and synthesis of the scientific literature of 2020. Eur. J. Phys. Rehabil. Med. 2021 doi: 10.23736/S1973-9087.21.06870-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Ramirez D.C., Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: a systematic review and meta-analysis. Respir. Med. 2020;171:106096. doi: 10.1016/j.rmed.2020.106096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peckham H., de Gruijter N.M., Raine C., Radziszewska A., Ciurtin C., Wedderburn L.R., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax. 2006;61:744–746. doi: 10.1136/thx.2006.061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham B.L., Brusasco V., Burgos F., Cooper B.G., Jensen R., Kendrick A., et al. Executive Summary: 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017;49:16E0016. doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 12.Holland A.E., Spruit M.A., Troosters T., Puhan M.A., Pepin V., Saey D., et al. An official European Respiratory Society/American Thoracic Society technical standard: field-walking tests in chronic respiratory disease. Eur. Respir. J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 13.Rochester C.L., Vogiatzis I., Holland A.E., Lareau S.C., Marciniuk D.D., Puhan M.A., et al. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2015;192:1373–1386. doi: 10.1164/rccm.201510-1966ST. [DOI] [PubMed] [Google Scholar]
- 14.Johnson D.C. DLCO: adjust for lung volume, standardised reporting and interpretation. Eur. Respir. J. 2017;50:1700940. doi: 10.1183/13993003.00940-2017. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society . American Thoracic Society; 2020. Assembly on Pulmonary Rehabilitation: Guidance for Re-opening Pulmonary Rehabilitation Programs.https://www.thoracic.org/members/assemblies/assemblies/pr/resources/ats-pr-assembly-re-opening-pr-document-final.pdf accessed 2021 March 28]. Available from: [Google Scholar]
- 16.Halpin D.M.G., Criner G.J., Papi A., Singh D., Anzueto A., Martinez F.J., et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee Report on COVID-19 and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2021;203(1):24–36. doi: 10.1164/rccm.202009-3533SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasekaba T., Lee A.L., Naughton M.T., Williams T.J., Holland A.E. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern. Med. J. 2009;39:495–501. doi: 10.1111/j.1445-5994.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 18.Redelmeier D.A., Bayoumi A.M., Goldstein R.S., Guyatt G.H. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am. J. Respir. Crit. Care Med. 1997;155:1278–1282. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 19.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Hawthorne V.M., Watt G.C., Hart C.L., Hole D.J., Smith G.D., Gillis C.R. Cardiorespiratory disease in men and women in urban Scotland: baseline characteristics of the Renfrew/Paisley (midspan) study population. Scot. Med. J. 1995;40:102–107. doi: 10.1177/003693309504000402. [DOI] [PubMed] [Google Scholar]
- 21.Doherty D.E. A review of the role of FEV1 in the COPD paradigm. COPD. 2008;5:310–318. doi: 10.1080/15412550802363386. [DOI] [PubMed] [Google Scholar]
- 22.Engström G., Melander O., Hedblad B. Population-based study of lung function and incidence of heart failure hospitalisations. Thorax. 2010;65:633–638. doi: 10.1136/thx.2010.135392. [DOI] [PubMed] [Google Scholar]
- 23.Wang B., Zhou Y., Xiao L., Guo Y., Ma J., Zhou M., et al. Association of lung function with cardiovascular risk: a cohort study. Respir. Res. 2018;19:214. doi: 10.1186/s12931-018-0920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y., Tan C., Wu J., Chen M., Wang Z., Luo L., et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir. Res. 2020;21:163. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nusair S. Abnormal carbon monoxide diffusion capacity in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020;56:2001832. doi: 10.1183/13993003.01832-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barisione G., Brusasco V. Lung diffusing capacity for nitric oxide and carbon monoxide following mild-to-severe COVID-19. Phys. Rep. 2021;9 doi: 10.14814/phy2.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potus F., Mai V., Lebret M., Malenfant S., Breton-Gagnon E., Lajoie A.C., et al. Novel insights on the pulmonary vascular consequences of COVID-19. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;319:L277–L288. doi: 10.1152/ajplung.00195.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman D.G., Badal T., King G.G., Thamrin C. Caution in interpretation of abnormal carbon monoxide diffusion capacity in COVID-19 patients. Eur. Respir. J. 2021;57:2003263. doi: 10.1183/13993003.03263-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gosselink R., De Vos J., van den Heuvel S.P., Segers J., Decramer M., Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur. Respir. J. 2011;37:416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 30.Qin W., Chen S., Zhang Y., Dong F., Zhang Z., Hu B., et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at three-month follow-up. Eur. Respir. J. 2021 doi: 10.1183/13993003.03677-2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bay M.L., Pedersen B.K. Muscle-organ crosstalk: focus on immunometabolism. Front. Physiol. 2020;11:567881. doi: 10.3389/fphys.2020.567881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramalho S.H.R., Shah A.M. Lung function and cardiovascular disease: a link. Trends Cardiovasc. Med. 2021;31(2):93–98. doi: 10.1016/j.tcm.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]