Abstract
Background
Angiotensin converting enzyme 2 (ACE2) has recently been identified as the functional receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent response for novel coronavirus disease 2019 (COVID-19). This study aimed to explore the roles of ACE2, apelin and sodium-glucose cotransporter 2 (SGLT2) in SARS-CoV-2-mediated cardiorenal damage.
Methods and Results
The published RNA-sequencing datasets of cardiomyocytes infected with SARS-CoV-2 and COVID-19 patients were used. String, UMAP plots and single cell RNA sequencing data were analyzed to show the close relationship and distinct cardiorenal distribution patterns of ACE2, apelin and SGLT2. Intriguingly, there were decreases in ACE2 and apelin expression as well as marked increases in SGLT2 and endothelin-1 levels in SARS-CoV-2-infected cardiomyocytes, animal models with diabetes, acute kidney injury, heart failure and COVID-19 patients. These changes were linked with downregulated levels of interleukin (IL)-10, superoxide dismutase 2 and catalase as well as upregulated expression of profibrotic genes and pro-inflammatory cytokines/chemokines. Genetic ACE2 deletion resulted in upregulation of pro-inflammatory cytokines containing IL-1β, IL-6, IL-17 and tumor necrosis factor α. More importantly, dapagliflozin strikingly alleviated cardiorenal fibrosis in diabetic db/db mice by suppressing SGLT2 levels and potentiating the apelin-ACE2 signaling.
Conclusion
Downregulation of apelin and ACE2 and upregulation of SGLT2, endothelin-1 and pro-inflammatory cytokines contribute to SARS-CoV-2-mediated cardiorenal injury, indicating that the apelin-ACE2 signaling and SGLT2 inhibitors are potential therapeutic targets for COVID-19 patients.
Keywords: Angiotensin converting enzyme 2, Apelin, Severe acute respiratory syndrome coronavirus 2, COVID-19, SGLT2 inhibitor, Inflammation
Graphical abstract
1. Introduction
Coronavirus disease 2019 (COVID-19) which was induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a worldwide devastating pandemic, with 137,571,000 cases as well as 2,962,000 deaths as of April 14th, 2021. Patients with pre-existing hypertension, diabetes mellitus (DM), heart failure (HF) were prone to be infected with SARS-CoV-2 and more likely to develop severe symptoms [1]. As SARS-CoV-2 had remarkable tropism for the heart and kidney, cardiorenal injury were evidenced as common complications in COVID-19 with poor prognosis [2,3]. According to an observational, retrospective study of patients with confirmed COVID-19 infection, the mortality of patients developed acute heart failure was 46.8%, which was significantly higher than 19.7% of the others [4]. In addition, the in-hospital mortality in COVID-19 patients with acute kidney injury (AKI) was 45% in comparison with the mortality of 7% in those without AKI [5]. Currently, the pathophysiological mechanisms of cardiorenal injury involved in COVID-19 remain largely unknown. Therefore, it's of great significance to find potential therapeutic targets for COVID-19.
Recently, angiotensin converting enzyme 2 (ACE2) has garnered great attention as the functional receptor for SARS-CoV-2 [6]. Binding of SARS-CoV-2 to ACE2 receptor leads to the downregulation of ACE2, which will shift the renin-angiotensin system (RAS) peptide balance from the angiotensin (Ang)-(1–7)-Mas pathway to the Ang II-AT1R pathway, thereby enhancing the detrimental effects of SARS-CoV-2 and burst of inflammatory cytokine release among COVID-19 patients [[6], [7], [8]]. Notably, protective effects of recombinant human ACE2 (rhACE2) were shown in acute lung injury, post-myocardial infarction (MI)-induced HF and hypertensive kidney injury [6,[8], [9], [10]]. Besides, apelin, a positive regulator of ACE2, has been demonstrated as a negative regulator of the Ang II-AT1R pathway during hypertension and HF [6,8,11]. Thus, targeting the apelin-ACE2 signaling may be promising therapeutic targets for SARS-CoV-2-mediated cardiorenal dysfunction. However, the exact effects of the apelin-ACE2 signaling in COVID-19 remain to be further elucidated. Sodium-glucose cotransporter 2 (SGLT2) inhibitors have been shown to reduce body mass and systolic blood pressure levels and improve endothelial dysfunction beyond glycemic control [12]. Moreover, dapagliflozin, a selective SGLT2 inhibitor, has been demonstrated its therapeutic effects on various cardiovascular and renal diseases [13]. This study aimed to evaluate the effects of SARS-CoV-2 infection and dapagliflozin therapy on the apelin, ACE2 and SGLT2 signaling as well as cardiorenal fibrotic injury during diabetes, HF and COVID-19 status. We also explored the published RNA-sequencing (RNA-seq) datasets of cardiomyocytes (CMs) infected with SARS-CoV-2, COVID-19 patients and animals with different disease conditions containing MI-HF, AKI and diabetes, providing potential therapeutic targets for SARS-CoV-2-mediated cardiorenal injury.
2. Materials and methods
Detailed methods are available in the online supplemental section.
2.1. Obtaining published data and string analysis
The raw gene counts data of adult human CMs and human embryonic stem cell (hESC)-derived CMs infected with SARS-CoV-2 and mock was downloaded from the GEO repository database under the series number GSE151879 [14]. The normalized counts data for RNA-seq of isolated monocytes from peripheral blood of 6 COVID-19 patients and 3 healthy controls were available with accession number GSE160351 [15]. The normalized reads counts data for RNA-seq of buffy coat samples from COVID19+ and COVID19− critically ill patients were accessed from GSE154998 [16]. GEO Datasets SOFT files were available from https://www.ncbi.nlm.nih.gov/gds. The heart data of post-MI HF rats was from GDS4907, and the kidney data of AKI mice was from GDS4864. In addition, we used the protein and RNA expression data of ACE2, SGLT2 and apelin in different human tissues, and related single cell RNA-seq (scRNA-seq) gene expression data in kidney and heart through the Human Protein Atlas (HPA) (http://www.proteinatlas.org/). Furthermore, the protein-protein interaction (PPI) networks among ACE2, apelin and SGLT2 were obtained from the String database (https://string-db.org/).
2.2. Animals protocols
12-week-old male ACE2 knockout (KO) and wild type (WT) littermate, C57BL/6 db/db and littermate db/m mice were purchased from Vital River Inc.(Beijing, China). The C57BL/6 db/db and littermate db/m mice were randomly divided into three groups: db/m control group (n = 9), db/db group (DM) (n = 7) and dapagliflozin-treated db/db group (DM + DAPA) (n = 7). 1 mg/kg dapagliflozin (AstraZeneca, USA) or saline was administrated for db/db mice by gavage once daily for 4 weeks. All animals were anesthetized and sacrificed to collect blood and tissues for the subsequent experiments and the detailed schematic illustration of the animal experimental design was shown in Fig. S1. The plasma of ACE2KO (n = 3) and WT mice (n = 3) were used for the subsequent cytokine protein arrays. All experiments were approved by Institutional Animal Care and Use Committee at Beijing Chaoyang Hospital affiliated to Capital Medical University and Shanghai Jiao Tong University School of Medicine in China.
3. Results
3.1. Downregulation of ACE2 and upregulation of ET-1, pro-inflammatory cytokines, chemokines in COVID-19 patients and SARS-CoV-2-infected CMs
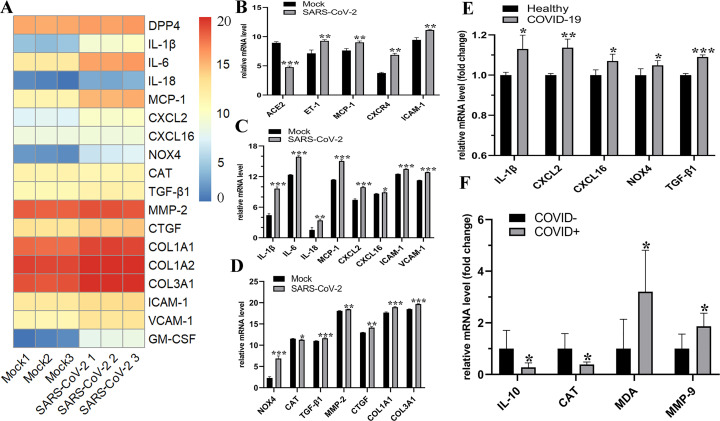
We analyzed the RNA-seq datasets to explore the changes of ACE2, endothelin-1 (ET-1), pro-inflammatory factors involved in SARS-CoV-2 infection and COVID-19 patients. Heatmap analysis revealed the differentially expressed genes containing pro-inflammatory cytokines, chemokines, oxidative stress- and fibrosis-related genes in adult human CMs infected with SARS-CoV-2 (Fig. 1A). Intriguingly, compared with mock, infection with SARS-CoV-2 resulted in a significant reduction in ACE2 expression and a marked increase in ET-1 level. These changes were associated with elevated levels of adhesion molecules ICAM-1, VCAM-1 and pro-inflammatory cytokines/chemokines including interleukin (IL)-1β, IL-6, IL-18, MCP-1 and CXCL2 in hESC-derived CMs and adult human CMs (Fig. 1B and C). Moreover, decreased expression of catalase and increased levels of NOX4 were exhibited in both SARS-CoV-2 infected adult human CMs and COVID-19 patients (Fig. 1D−F). Notably, there were marked upregulations of pro-fibrotic factors TGF-β1, MMP-2, CTGF, collagen type I α 1 chain (COL1A1) and COL3A1 in SARS-CoV-2 infected adult human CMs (Fig. 1D). Consistent with the in vitro experiment of SARS-CoV-2 infection, patients with COVID-19 exhibited downregulated levels of anti-inflammatory factor IL-10 and remarkable higher levels of pro-inflammatory factors IL-1β and CXCL2 (Fig. 1E and F). Thus, SARS-CoV-2-infection-meidated ACE2 reduction was partially responsible for severe inflammation, fibrosis and oxidative injury in CMs.
Fig. 1.
Levels of ACE2, ET-1, pro-inflammatory cytokines and fibrosis-related genes in COVID-19 patients and SARS-CoV-2 infected CMs. (A−D) Heatmap analysis and the relative expression of ACE2, inflammatory factors, chemokines, oxidative stress- and fibrosis-related genes in adult human CMs (A, C and D) and hESC-derived CMs (B) infected with SARS-CoV-2 virus. n = 3. Levels of pro-inflammatory factors, chemokines expression of peripheral monocytes from COVID-19 patients and healthy controls (E). n = 3–6. The relative expression levels of anti-inflammatory and oxidative stress-related factors between leucocyte RNA samples derived from critically ill COVID+ versus COVID− samples (F). n = 7. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001 versus mock or healthy or COVID− group.
3.2. Decreased apelin-ACE2 signaling and increased levels of ET1, pro-inflammatory factors, and fibrosis-related genes in post-MI HF rats, AKI and ACE2KO mice
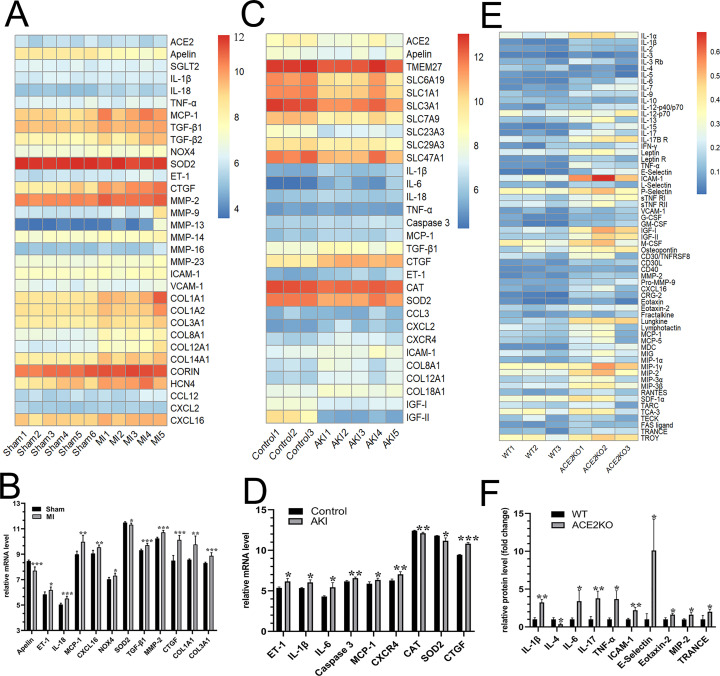
We next evaluated the expression of ACE2 and apelin in the context of cardiorenal injury. In the hearts of post-MI HF rats, there were a significant decrease in apelin and marked elevations in ET-1, NOX4 and pro-inflammatory factors containing IL-18, MCP-1, CXCL16 (Fig. 2A and B). Moreover, levels of fibrosis-related genes including TGF-β1, MMP-2, CTGF, COL1A1 and COL3A1 were obviously increased in the hearts of post-MI HF rats (Fig. 2B). We further inferred that both ACE2 and apelin exhibited a downregulated tendency in the kidneys of AKI mice by RNA-seq datasets analysis (Fig. 2C). Importantly, elevated expression of ET-1, IL-1β, IL-6, caspase 3, MCP-1, CXCR4, and CTGF were shown in AKI kidneys linked with decreased catalase and SOD2 (Fig. 2D). Cytokine protein arrays demonstrated that genetic ACE2 deficiency resulted in downregulation of anti-inflammatory cytokine IL-4 and upregulation of pro-inflammatory cytokines/chemokines in kidneys including IL-1β, IL-6, IL-17, TNF-α, MIP-2, RANTES and ICAM-1 (Fig. 2E and F). Collectively, inactivation of the apelin-ACE2 signaling resulted in exacerbated endothelial injury, inflammation, cardiorenal fibrosis and oxidative injury.
Fig. 2.
Abnormal apelin-ACE2 signaling and levels of ET-1, pro-inflammatory and fibrosis-related factors in post-MI HF rats, AKI and ACE2KO mice. (A−D) Heatmap analysis and the relative mRNA levels of ACE2, apelin, pro-inflammatory factors, oxidative stress- and fibrosis-related genes in the hearts between post-MI HF rats and sham group (A and B); and the kidneys between AKI mice and control group (C and D). n = 3–6. Heatmap analysis and the relative protein levels of renal pro-inflammatory cytokines and chemokines between ACE2KO and WT mice (E and F). n = 3. ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001 versus sham or control or WT group.
3.3. String and sc-RNA seq data exhibited the PPI network, cardiorenal distribution patterns and UMAP plots of ACE2, SGLT2 and apelin
To predict the functional protein associating network among ACE2, apelin and SGLT2, we constructed the PPI network using String analysis. As demonstrated in Fig. S2, ACE2 exhibited close associations with apelin, SGLT2 and classical pro-inflammatory factors, oxidative stress- and fibrosis-related factors. We further explored the different distribution patterns of ACE2, SGLT2 and apelin in normal human hearts and kidneys and their expressional condition in each cell type cluster from the HPA. ACE2 was shown to be highly expressed in smooth muscle cells, fibroblasts and endothelial cells in heart while apelin mainly in endothelial cells, fibroblasts and CMs. In addition, the expression of SGLT2 was relatively higher in CMs, endothelial cells and fibroblasts (Fig. S3A−I). Interestingly, ACE2, apelin and SGLT2 exhibited the highest levels in proximal tubular cells among all cell clusters in kidneys (Fig. S4A−I). Thus, abnormal apelin-ACE2 and SGLT2 signaling may play key roles in cardiac fibrosis remodeling, endothelial dysfunction and kidney function regulation during COVID-19 progression.
3.4. Distinct RNA and protein levels and cardiorenal distribution of ACE2, SGLT2 and apelin in normal human tissues from the HPA
We further investigated the HPA and obtained detailed information for the mRNA and protein expression levels of ACE2, SGLT2 and apelin in different human tissues. ACE2 is widely expressed in human tissues, mainly in kidney and heart. The protein expression of ACE2 in kidney is prominently higher than that in other tissues (Fig. S5A−D). Furthermore, SGLT2 is typically expressed in the kidney and testis, and exhibited the highest level in kidney (Fig. S5B−E). Apelin is widely expressed in heart and kidney tissues (Fig. S5C). Representative immunohistochemistry staining demonstrated that ACE2 and SGLT2 protein were prominent in kidney, specifically expressed in renal proximal tubular cells (Fig. S5F,G).
3.5. Treatment with dapagliflozin prevented cardiorenal fibrosis in diabetic mice by improving the apelin-ACE2 and SGLT2 signaling
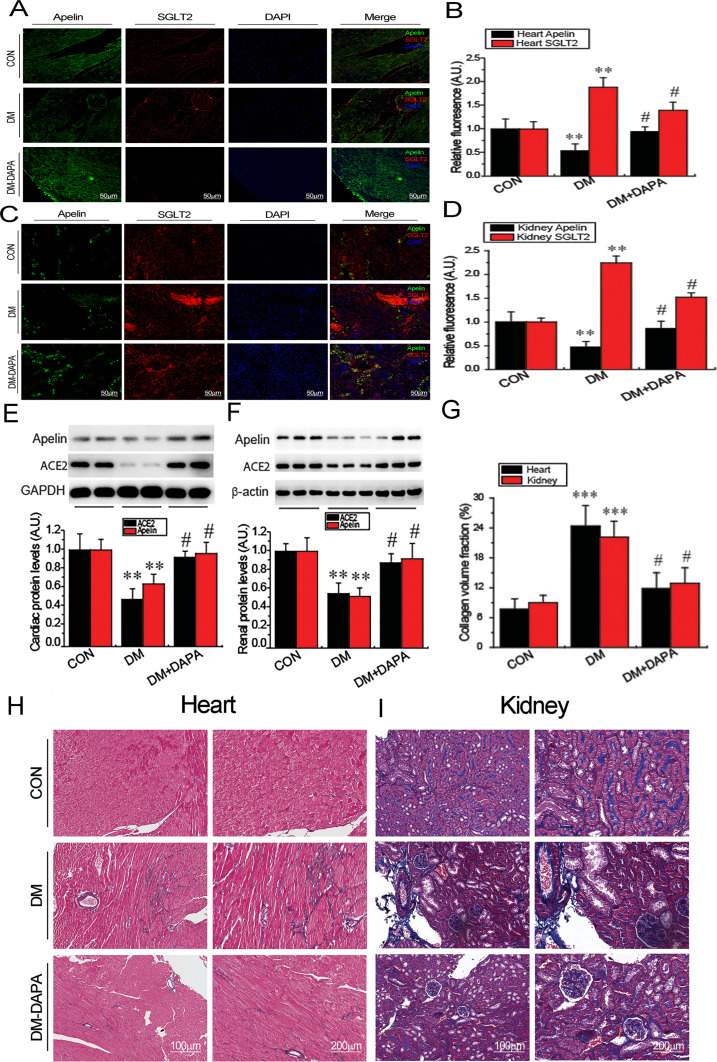
Immunofluorescence staining analysis revealed downregulated apelin and upregulated SGLT2 expression in hearts (Fig. 3A and B) and kidneys (Fig. 3C and D) of diabetic db/db mice. Western blotting array further confirmed that both ACE2 and apelin protein were reduced in heart and kidney in diabetic db/db mice (Fig. 3E and F), thereby contributing to exacerbated cardiorenal fibrosis during diabetes state (Fig. 4G−I). More importantly, administration of dapagliflozin remarkably attenuated cardiorenal fibrosis in diabetic mice by promoting cardiorenal apelin and ACE2 levels (Fig. 3). Taken together, our results demonstrated that dapagliflozin exerts cardiorenal protective effects by activating the apelin-ACE2 signaling and suppressing the SGLT2 pathway, implying potential therapeutic targets of SGLT2 inhibitors for COVID-19-mediated cardiorenal injury (Fig. 4).
Fig. 3.
Treatment with dapagliflozin (DAPA) reversed the abnormal apelin, ACE2 and SGLT2 in heart and kidney tissues and attenuated cardiorenal fibrosis of diabetic mice. Immunofluorescence revealed the expression of apelin and SGLT2 in hearts (A and B) and kidneys (C and D) of diabetic mice with or without treatment by dapagliflozin. n = 4. Western blotting showed expression of ACE2 and apelin in hearts and kidneys from diabetic mice after treatment of dapagliflozin (E and F). n = 5. Masson's trichrome staining showed enhanced cardiac and renal fibrosis in diabetic mice, which were alleviated by dapagliflozin (G−H). n = 5. ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001 versus control; #P < 0.05 versus DM group. A.U. indicates arbitrary units.
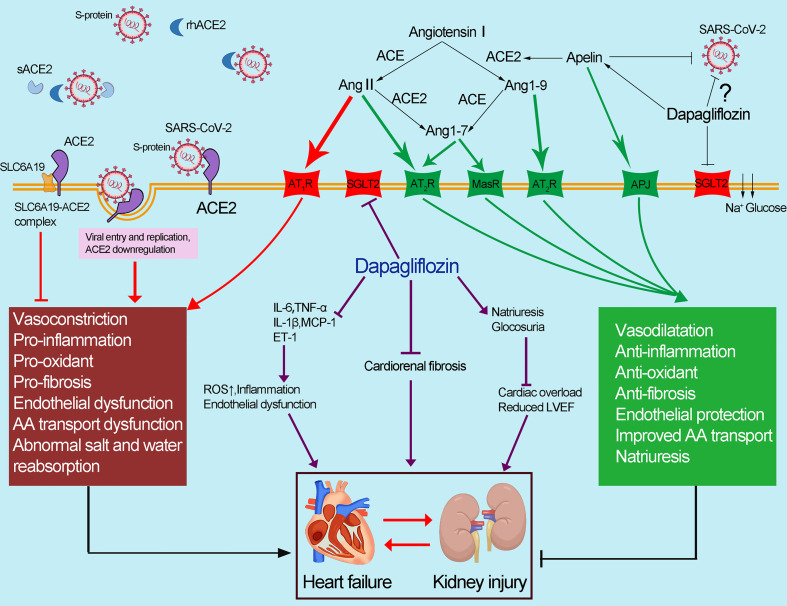
Fig. 4.
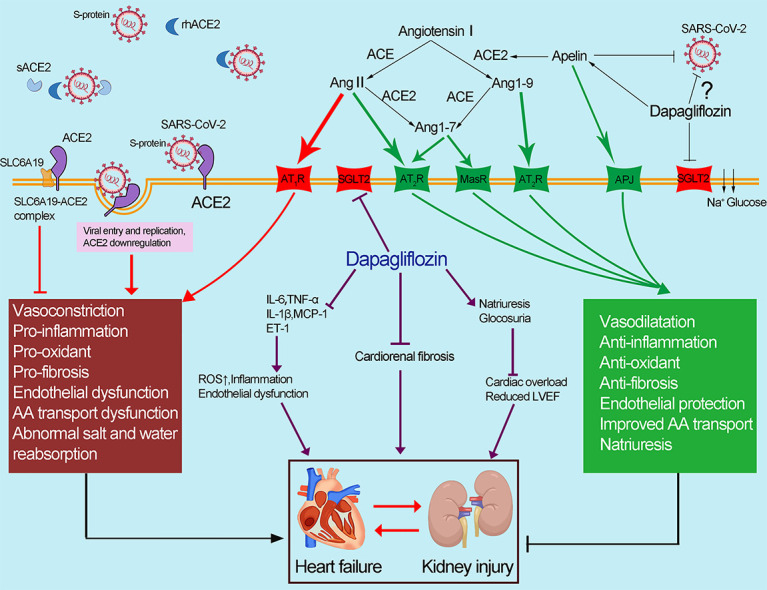
Abnormal apelin-ACE2 and SGLT2 signaling contribute to adverse cardiorenal injury in patients with COVID-19. The spike protein of SARS-CoV-2 binds to membrane-bound ACE2, resulting in the downregulation of ACE2. Downregulation of ACE2 results in the accumulation of Ang II, contributing to the deleterious effects in COVID-19 (marked in red). ACE2 could catalyze Ang II conversion to Ang-(1–7), which exerts its cardiorenal protective effects including anti-inflammation, antioxidant and antifibrosis (marked in green). Dapagliflozin has a protective effect against the cardiorenal injury through improving the abnormal apelin-ACE2 signaling, indicating that SGLT2 inhibitors, apelin and rhACE2 could be potential therapeutic approaches in COVID-19 patients with adverse cardiorenal injury. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
COVID-19 has a high prevalence among aged patients with or without comorbidities such as diabetes, HF and kidney failure [6]. ACE2 is located on the X chromosome and largely expressed in organs mainly targeted and damaged by SARS-CoV-2 [17]. Downregulation of ACE2 and the subsequent decreased Ang-(1–7)/Ang II ratio caused by SARS-CoV-2 infection play an essential role in COVID-19 associated cardiorenal dysfunction. Notably, circulating Ang II levels in SARS-CoV-2-infected patients were significantly increased and strongly associated with viral load and lung injury [18]. The vast release of cytokines in response to the viral infection resulted in a cytokine storm, subsequently leading to multiorgan dysfunction [19]. In this work, we demonstrated that there was a decrease in ACE2 expression and elevated levels of pro-inflammatory cytokines and chemokines in SARS-CoV-2-infected CMs and COVID-19 patients, suggesting that SARS-CoV-2-induced ACE2 downregulation were the primary mechanisms of organ injury during COVID-19. In subjects with higher ACE2 and lower ACE/ACE2 ratios, it appears that they are protected against COVID-19 exacerbation. Notably, sex differences in COVID-19 patients are emerging in terms of case fatality. The mortality rate among confirmed cases is lower in women than that in men, and the ACE/ACE2 activity ratio in females is lower than that in the male serum [17,20], indicating more susceptible for men to SARS-CoV-2 infection. Moreover, multiple pathological conditions, especially those of the cardiovascular system, are also characterized with a downregulation of ACE2 and elevated ACE/ACE2 ratio. Our previous published work demonstrated that Ang II-induced inflammation, oxidative stress, cardiorenal fibrotic remodeling and dysfunction were exacerbated in ACE2-deficient mice [21,22]. Besides, apelin has been demonstrated to protect against acute respiratory distress syndrome, cardiovascular injury and endothelial inflammation through activating the ACE2/Ang-(1–7)/Mas axis and blocking the Ang II-AT1R actions, indicating that apelin/ACE2 signaling could be a promising therapeutic target in ameliorating cardiorenal injuries during COVID-19 [23]. In this study, we demonstrated decreased apelin-ACE2 signaling and increased levels of pro-inflammatory factors and fibrosis-related genes in post-MI HF rats, AKI and ACE2 KO mice. The results implied that the cardiorenal injuries caused by SARS-CoV-2 could be exacerbated by inactivating of the apelin-ACE2 signaling.
A high-glucose diet contributed to the augmentation of ACE/ACE2 ratio in the heart [17], the subsequent imbalance of the Ang-(1–7)/Ang II was assumed to be the major contributor to progressively worsened systemic manifestations of COVID-19. Consistently, we revealed, for the first time, that both apelin and ACE2 were downregulated in heart and kidney of diabetic db/db mice, leading to exacerbated cardiorenal fibrosis. SGLT2 inhibitors, although originally developed as antidiabetic drugs, have exhibited dramatically effects of reducing cardiovascular mortality and hospitalization for HF and decelerating progression of chronic kidney disease event in absence of diabetes [24]. Beyond improving glycemic control, early natriuresis with a reduction in plasma volume, mitigating endothelial damage, improving mitochondrial function and myocardial efficiency, a reduction in systolic blood pressure levels and changes in tissue sodium handling are all likely to be the benefits of SGLT2 inhibitors [13], all of which have been proposed as plausible mechanisms of cardiorenal protection in COVID-19 patients.
More importantly, we found that dapagliflozin treatment potentiated the expression of apelin and ACE2, contributing to the alleviation of cardiorenal fibrosis and injury. Intriguingly, it was demonstrated that recombinant human soluble ACE2 (rhsACE2) could inhibit SARS-CoV-2 infection of Vero-E6 cells and human capillary and kidney organoids in a dose dependent manner [25]. Thus, it was postulated that exogenous supplement of rhsACE2 may be a potential therapeutic approach for the administration of COVID-19. Given its cardiorenal protective effects, SGLT2 inhibitors are thought to be of potential significance for ameliorating cardiorenal dysfunction in COVID-19. Further studies are needed to explore whether SGLT2 inhibitors could be safely applied in patients with COVID-19.
5. Conclusion
Downregulation of ACE2 mediated by SARS-CoV-2 infection and the subsequent exacerbated inflammation, oxidative stress, endothelial damage and fibrosis are responsible for adverse cardiorenal injury of COVID-19 patients. In the present study, we found marked decreases in apelin and ACE2 expression and significant increases in SGLT2, ET-1, pro-inflammatory factors in post-MI HF rats, AKI and diabetic mice. More importantly, we determined, for the first time, that dapagliflozin had a protective effect against the cardiorenal injury through improving the abnormal apelin-ACE2 signaling, indicating that the apelin-ACE2 signaling and SGLT2 inhibitors could be potential therapeutic targets for COVID-19. Further pre-clinical and clinical studies aimed to explore the potential therapeutic effects of SGLT2 inhibitors in COVID-19 need to be conducted.
Statement of authorship
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Declaration of Competing Interest
All authors declare that they have no conflicts of interest.
Acknowledgements
This study was supported by the General Program and the National Major Research Plan Training Program of the National Natural Science Foundation of China (No. 91849111; 81770253; 81370362; 91339108; 81500038; 81300044), and Shanghai Sailing Program (20YF1444100).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2021.05.029.
Appendix A. Supplementary data
Supplementary material
References
- 1.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topol E.J. COVID-19 can affect the heart. Science. 2020;370:408–409. doi: 10.1126/science.abe2813. [DOI] [PubMed] [Google Scholar]
- 4.Rey J.R., Caro-Codón J., Rosillo S.O., Iniesta Á.M., Castrejón-Castrejón S., Marco-Clement I., et al. Heart failure in COVID-19 patients: prevalence, incidence and prognostic implications. Eur. J. Heart Fail. 2020;22:2205–2215. doi: 10.1002/ejhf.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkelmayer W.C., Khairallah P., Charytan D.M. Nephrology and COVID-19. JAMA. 2020;324:1137–1138. doi: 10.1001/jama.2020.16779. [DOI] [PubMed] [Google Scholar]
- 6.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amraei R., Rahimi N. COVID-19, renin-angiotensin system and endothelial dysfunction. Cells. 2020;9 doi: 10.3390/cells9071652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z.Z., Wang W., Jin H.Y., Chen X., Cheng Y.W., Xu Y.L., et al. Apelin is a negative regulator of angiotensin II-mediated adverse myocardial remodeling and dysfunction. Hypertension. 2017;70:1165–1175. doi: 10.1161/HYPERTENSIONAHA.117.10156. [DOI] [PubMed] [Google Scholar]
- 9.Li X., Liu Y., Song J., Zhong J. Increased plasma ACE2 concentration does not mean increased risk of SARS-CoV-2 infection and increased fatality rate of COVID-19. Acta Pharm. Sin. B. 2020;10:2010–2014. doi: 10.1016/j.apsb.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Z., Song J.J., Martin S., Yang X.C., Zhong J.C. The Elabela-APJ axis: a promising therapeutic target for heart failure. Heart Fail. Rev. 2020:1–10. doi: 10.1007/s10741-020-09957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W., Yan J., Pan W., Tang M. Apelin/Elabela-APJ: a novel therapeutic target in the cardiovascular system. Ann. Transl. Med. 2020;8:243. doi: 10.21037/atm.2020.02.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowie M.R., Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020;17:761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 13.Zelniker T.A., Braunwald E. Mechanisms of Cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75:422–434. doi: 10.1016/j.jacc.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Chen S., Yang L., Nilsson-Payant B., Han Y., Jaffré F., Zhu J., et al. SARS-CoV-2 infected cardiomyocytes recruit monocytes by secreting CCL2. Res. Sq. 2020 doi: 10.21203/rs.3.rs-94634/v1. rs.3.rs–94634. [DOI] [Google Scholar]
- 15.Brunetta E., Folci M., Bottazzi B. Macrophage expression and prognostic significance of the long pentraxin PTX3 in COVID-19. Nat. Immunol. 2021;22:19–24. doi: 10.1038/s41590-020-00832-x. [DOI] [PubMed] [Google Scholar]
- 16.Gill S.E., Dos Santos C.C., O'Gorman D.B., Carter D.E., Patterson E.K., Slessarev M., et al. Transcriptional profiling of leukocytes in critically ill COVID19 patients: implications for interferon response and coagulation. Intensive Care Med. Exp. 2020;8:75. doi: 10.1186/s40635-020-00361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagliaro P., Penna C. ACE/ACE2 ratio: a key also in 2019 coronavirus disease (Covid-19)? Front. Med. (Lausanne) 2020;7:335. doi: 10.3389/fmed.2020.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fara A., Mitrev Z., Rosalia R.A., Assas B.M. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10:200160. doi: 10.1098/rsob.200160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penna C., Mercurio V. Sex-related differences in COVID-19 lethality. Br. J. Pharmacol. 2020;177:4375–4385. doi: 10.1111/bph.15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong J., Basu R., Guo D., Chow F.L., Byrns S., Schuster M., et al. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. [DOI] [PubMed] [Google Scholar]
- 22.Jin H.Y., Chen L.J., Zhang Z.Z., Xu Y.L., Song B., Xu R., et al. Deletion of angiotensin-converting enzyme 2 exacerbates renal inflammation and injury in apolipoprotein E-deficient mice through modulation of the nephrin and TNF-alpha-TNFRSF1A signaling. J. Transl. Med. 2015;13:255. doi: 10.1186/s12967-015-0616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeedi Saravi S.S., Beer J.H. Apelin-potential therapy for COVID-19? J. Mol. Cell. Cardiol. 2020;145:84–87. doi: 10.1016/j.yjmcc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Packer M., Anker S.D., Butler J., Filippatos G., Pocock S.J., Carson P., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 25.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913.e907. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material