To the editor:
As mass vaccinations for coronavirus disease 2019 (COVID-19) are being administered worldwide, rare reports of adverse events are emerging. We report a case of minimal change disease presenting with nephrotic syndrome 1 week after a first injection of the COVID-19 vaccine (Pfizer-BioNTech).
A 77-year-old white male with a 15-year history of type 2 diabetes mellitus without retinopathy received a first dose of the Pfizer-BioNTech vaccine on March 17, 2021. Medical history included obesity, prior smoking, and coronary artery disease. Baseline serum creatinine ranged from 1.0 to 1.3 mg/dl, with no proteinuria over the previous year. Outpatient medications included atorvastatin, aspirin, dulaglutide, empagliflozin, glipizide, losartan, metformin, and metoprolol. There was no history of nonsteroidal anti-inflammatory drug use. Seven days after vaccination, he presented to his local physician complaining of abrupt onset of lower-extremity edema. Laboratory testing revealed 4+ proteinuria by dipstick and serum albumin of 2.5 g/dl. Nephrology consultation 12 days after vaccination found anasarca with 13.6-kg weight gain due to edema, elevated blood pressure (152/81 mm Hg), and 4+ proteinuria on urinalysis with inactive urine sediment, prompting hospital admission. Laboratory evaluation by 14 days after vaccination showed 24-hour urine protein of 23.2 g/d, serum creatinine of 2.33 mg/dl, and serum albumin of 3.0 g/dl. Complete blood cell count was normal, and hemoglobin A1c was 7.5%. Serologies included elevated C3 and C4 and negative hepatitis B surface antigen and hepatitis C antibody.
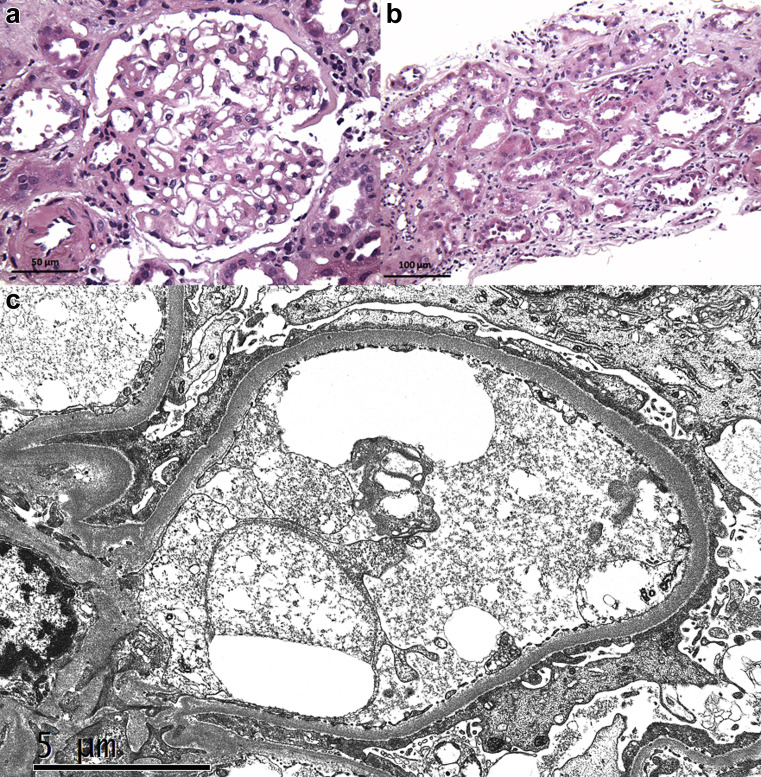
A kidney biopsy was performed 16 days after vaccination (Figure 1 ). Among 7 glomeruli sampled for light microscopy, 4 were globally sclerotic and 3 were histologically unremarkable. There was 25% tubular atrophy and interstitial fibrosis with moderate arteriosclerosis. Cortical tubules displayed diffuse acute epithelial injury. No immune deposits were identified by immunofluorescence (2 glomeruli) or electron microscopy (2 glomeruli). Electron microscopy revealed 100% podocyte foot process effacement, leading to a diagnosis of minimal change disease with acute tubular injury. The ultrastructural findings of minimal segmental mesangial sclerosis and glomerular basement membrane thickening (mean, 460 nm) suggested underlying mild diabetic changes.
Figure 1.
(a) Light microscopy shows a histologically unremarkable glomerulus (hematoxylin and eosin, original magnification ×400). (b) A low-power view shows diffuse cortical acute tubular injury with focal shedding of degenerating epithelial cells into the lumen (hematoxylin and eosin, original magnification ×200). (c) Electron microscopy demonstrates complete podocyte foot process effacement (original magnification ×8000). To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
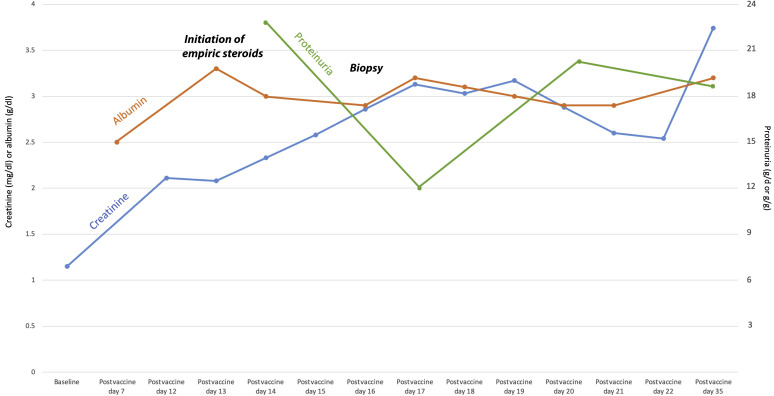
Empiric pulse methylprednisolone, 1 g daily for 3 days, was initiated on hospital admission, followed by oral prednisone, 60 mg daily, after biopsy. In the hospital, he required i.v. furosemide drip, 10 mg/h, transitioned to bumetanide, 0.25 mg/h, for 5 days for fluid overload. Creatinine peaked during the hospitalization at 3.17 mg/dl at 19 days after vaccination. The patient was discharged 3 days later with 19.8 g/g proteinuria by spot ratio, serum albumin of 2.9 g/dl, and serum creatinine of 2.54 mg/dl. At the most recent follow-up, approximately 3 weeks after initiation of corticosteroids, creatinine remained elevated at 3.74 mg/dl, with 24-hour urine protein of 18.8 g/d (Figure 2 ).
Figure 2.
Temporal trends in serum creatinine (mg/dl), serum albumin (g/dl), and proteinuria (g/d if 24-hour sample, g/g if spot sample) are graphed over the first 5 weeks after vaccination.
This is the second report of the onset of minimal change disease occurring within a week of an initial dose of the Pfizer-BioNTech vaccine. The first report was of a 50-year-old healthy man who developed lower-extremity edema 4 days after injection, followed rapidly by anasarca and acute kidney injury, with serum creatinine of 2.3 mg/dl and urine protein of 6.9 g/d on admission.1 He responded to steroid therapy with complete remission.1
The strong temporal association with vaccination in both cases suggests a rapid T cell–mediated immune response to viral mRNA as a possible trigger for podocytopathy. Acute onset of minimal change disease has also been reported in a 65-year-old woman and a 44-year-old man at 4 and 18 days, respectively, following the influenza vaccine.2 , 3 Although definitive causality is difficult to establish, greater awareness of this potential adverse effect of vaccination is needed to determine its frequency. With prompt renal biopsy and initiation of steroid therapy, complete remission of nephrotic syndrome and acute kidney injury can be achieved. It is uncertain if and when it is safe to administer a second dose of the Pfizer vaccine in these individuals.
References
- 1.Lebedev L., Sapojnikov M., Wechsler A. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:142–145. doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kielstein J.T., Termühlen L., Sohn J., Kliem V. Minimal change nephrotic syndrome in a 65-year-old patient following influenza vaccination. Clin Nephrol. 2000;54:246–248. [PubMed] [Google Scholar]
- 3.Gutiérrez S., Dotto B., Petiti J.P. Minimal change disease following influenza vaccination and acute renal failure: just a co-incidence? Nefrologia. 2012;32:414–415. doi: 10.3265/Nefrologia.pre2012.Feb.11370. [DOI] [PubMed] [Google Scholar]