Introduction
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, no antiviral medication to date has been proven to be beneficial except for remdesivir. With remdesivir being widely utilized in the treatment of COVID-19, many studies vouch for its overall safety profile.1 We report the first case of remdesivir-induced hemodynamically significant sinus node dysfunction that was resolved following completion of remdesivir therapy. The patient received remdesivir as an initial dose of 200 mg intravenously (IV) followed by maintenance dose of 100 mg IV for 4 days. On day 2 the patient developed cardiogenic shock secondary to marked sinus bradycardia and required dopamine infusion. Hemodynamics resolved upon completion of remdesivir therapy. Remdesivir has become standard of care for COVID-19 pneumonia and there is a paucity of data regarding its cardiac effects.
Case report
A 78-year-old white woman with a medical history significant for hypertension, prediabetes, dyslipidemia, and former tobacco use presented to the hospital with dyspnea, myalgias, fatigue, and cough for approximately 1 week. On initial presentation she was in acute hypoxic respiratory failure with an oxygen saturation of 89% on room air, with all other vital signs within normal limits. She was in respiratory distress with tachypnea, but the rest of the physical examination was unremarkable. Laboratory testing resulted in several significant findings as follows: COVID-19 nucleic acid amplification test positive, polycythemia with a hemoglobin of 16 g/dL; hyponatremia 132 mEq; reduced glomerular filtration rate of 40.33 mL/min/m2; elevated aspartate aminotransferase 84 units/L; INR 1.0; D-dimer 0.5 mg/L fibrinogen equivalent units; ferritin 467 ng/mL; C-reactive protein 5.7 mg/dL; lactate dehydrogenase 213 units/L.
On admission the patient was started on standard COVID-19 treatment protocol including antiviral therapy with remdesivir (200 mg on day 1 followed by 4 days of 100 mg IV daily) and a 5-day course of ceftriaxone and azithromycin. A 10-day course of dexamethasone, zinc, vitamin C, and vitamin D was also administered. She was admitted to the intensive care unit owing to worsening dyspnea and hypoxia. Despite noninvasive ventilation by bilevel positive airway pressure, she was subsequently intubated and placed on mechanical ventilation.
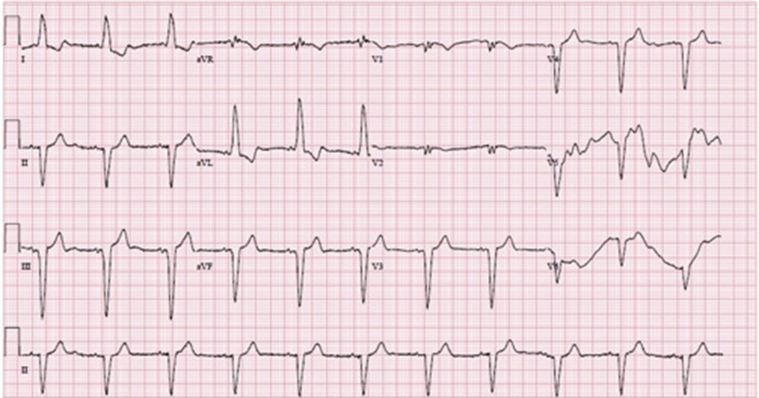
Approximately 20 hours after administration of the first dose of remdesivir the patient was found to have significant symptomatic bradycardia, with vitals revealing a blood pressure of 73/50 mm Hg and a heart rate as low as 38 beats per minute (bpm). An electrocardiogram (ECG) taken at that time (Figure 1) revealed marked sinus bradycardia with underlying left bundle branch block, compared to the initial ECG taken on admission (Figure 2) with a ventricular rate of 65 at baseline, also with existing left bundle branch block. The series of vitals taken from the time of admission to this finding of symptomatic bradycardia showed a heart rate range of 65–75 bpm and a systolic blood pressure ranging from 120 to 180 mm Hg. The patient had hemodynamic instability refractory to atropine and was started on a dopamine drip. During her 5-day course of remdesivir treatment, she remained on 5 mcg/min of dopamine and maintained normal sinus rhythm on telemetry with a heart rate of 60–65 bpm. Approximately 18 hours after the final dose of remdesivir, the dopamine was titrated off and the patient was hemodynamically stable, with ECG findings showing normal sinus rhythm with a heart rate of approximately 65 bpm (Figure 3). She was shortly extubated on day 6 of hospitalization to supplemental oxygen and was no longer found to have any evidence of sinus node dysfunction for the rest of the hospitalization. By day 11 of hospitalization, the patient unfortunately was unable to make a meaningful respiratory recovery and wished to continue her care under hospice services.
Figure 1.
Electrocardiogram during symptomatic bradycardia.
Figure 2.
Electrocardiogram on admission prior to remdesivir treatment.
Figure 3.
Telemetry strip obtained over 24 hours after final dose of remdesivir.
Discussion
Remdesivir is an internationally approved broad-spectrum antiviral that has shown promising results in the treatment of COVID-19-related lower respiratory infections by shortening time to recovery compared to placebo.2 The safety profile of remdesivir was recently evaluated since its emergence; however, there is limited peer-reviewed literature that describe cardiovascular adverse effects such as hemodynamic and electrocardiogram changes related to remdesivir administration. These adverse effects reported in recent available safety data publications include only hypotension (7.5%, 4 out of 53 patients) and atrial fibrillation (5.6%, 3 out of 53 patients). In further discussion of safety, the most commonly reported adverse events were increased liver enzymes, diarrhea, rash, and renal impairment.1,3 To the best of our knowledge, there have not been any reported cases of remdesivir-induced hemodynamically unstable sinus bradycardia.
Remdesivir is a prodrug that is subsequently metabolized to nucleoside triphosphate once it is transported intracellularly via enzymatic reactions. The mechanism of its metabolite is to inhibit the SARS-CoV-2 RNA-dependent RN polymerase, an enzyme used for viral replication.4 One of the leading theories of how remdesivir can induce sinus node dysfunction is based on its active metabolized triphosphate form and how it closely resembles adenosine 5′-triphosphate (ATP) structurally.5 ATP and its metabolized form of adenosine is known to exert negative chronotropic and dromotropic effects on sinus node automaticity and AV nodal conduction. This effect is mediated by ATP’s ability to suppress the sinus and AV nodes by transiently enhancing vagal tone in the heart.6, 7, 8 Therefore, it can be postulated that the remdesivir metabolite may exert the same deleterious effects on the conduction system.
In a review of related cases, we identified 3 case reports that reported remdesivir as a cause for acute normotensive marked sinus bradycardia. This was observed only after the initiation of remdesivir treatment, with all cases having resolution of bradycardia when remdesivir was discontinued.4,8,9 No cases thus far have described the development of sinus node dysfunction or cardiogenic shock associated with remdesivir treatment.
In our case report, the patient lacked any prior cardiac history and had normal telemetry monitor and ECG findings prior to remdesivir administration. Furthermore, the patient did not receive any other medications that could have caused her bradycardia. Dexamethasone and remdesivir were started simultaneously as a 10-day and 5-day course, respectively. Although dexamethasone is known to cause bradycardia, it was not considered the cause because the bradycardia resolved upon finishing the remdesivir course while dexamethasone was given for a full 10-day course. To further evaluate remdesivir’s likelihood of precipitating the observed clinical event, the Naranjo scoring system, or adverse drug reaction probability scale, was utilized. This scoring system is used to assess the potential causal relationship between clinical events and the drug in question.10,11 Our patient scored a 7 out of 10 total points, implying that the use of remdesivir in this patient’s case was the probable culprit of her hemodynamic changes and marked sinus bradycardia, given its abrupt onset with administration and rapid complete resolution on discontinuation.
Conclusion
Remdesivir is currently at the forefront of treatment in COVID-19 disease, given its overall results in mortality benefit and favorable safety data. There are currently no case reports that have linked remdesivir with hemodynamically significant sinus node dysfunction. With the proliferation of remdesivir as a mainstay therapy for COVID-19, further investigations are needed to elucidate its effect on the cardiac conduction system.
Key Teaching Points.
-
•
Remdesivir is currently used on a global scale in the treatment against COVID-19 disease.
-
•
Prompt recognition of remdesivir-induced hemodynamically unstable sinus bradycardia is crucial during treatment course of COVID-19.
-
•
Remdesivir-induced hemodynamically unstable sinus bradycardia can be supported with the use of dopamine infusion to complete its treatment course if necessary.
Acknowledgments
The patient provided informed consent. This research was supported in part by HCA Healthcare and/or an HCA Healthcare–affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
Footnotes
Funding Sources: The authors have no funding sources to disclose. Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Fan Q., Zhang B., Ma J., Zhang S. 2020). Safety profile of the antiviral drug remdesivir: an update. Biomed Pharmacother. 2020;130:110532. doi: 10.1016/j.biopha.2020.110532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beigel J., Tomashek K., Dodd L. (2020, November 05). Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grein J., Ohmagari N., Shin D. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eastman R.T., Roth J.S., Brimacombe K.R. (2020, May 27). Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. PubMed Central (PMC) ACS Cent Sci. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Touafchia A., Bagheri H., Carrie D. Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns. Clin Microbiol Infect. 2021;27 doi: 10.1016/j.cmi.2021.02.013. 791.e5–791.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubitosa J.C., Kakar P., Gerula C. Marked sinus bradycardia associated with remdesivir in COVID-19: a case and literature review. JACC Case Rep. 2020;2:2260–2264. doi: 10.1016/j.jaccas.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelleg A., Hurt C.M., Katchanov G. Anatomic-functional correlates of adenosine-5’-triphosphate triggered vagal depressor reflex. In: Belardinelli L., Pelleg A., editors. Adenosine and Adenine Nucleotides: From Molecular Biology to Integrative Physiology. Springer; Boston, MA: 1995. [Google Scholar]
- 8.Gupta A.K., Parker B.M., Priyadarshi V., Parker J. Cardiac adverse events with remdesivir in COVID-19 infection. Cureus. 2020;12(10):e11132. doi: 10.7759/cureus.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Codez M.I., Rodriguez-Gonzalez M., Gutierrez-Rosa I. Severe sinus bradycardia associated with Remdesivir in a child with severe SARS-CoV-2 infection. Eur J Pediatr. 2021;180:1627. doi: 10.1007/s00431-021-03940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murayama H., Sakuma M., Takahashi Y., Morimoto T. (2018, January 03). Improving the assessment of adverse drug reactions using the Naranjo Algorithm in daily practice: the Japan Adverse Drug Events Study. Bps publications. Retrieved February 18, 2021, from. Pharmacol Res Perspect. 2018;6 doi: 10.1002/prp2.373. https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1002/prp2.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Adverse Drug Reaction Probability Scale (Naranjo) in Drug Induced Liver Injury. https://www.ncbi.nlm.nih.gov/books/NBK548069/ [Updated 2019 May 4]. Available from: [PubMed]