Abstract
Background
The COVID-19 pandemic has highlighted the importance of respiratory protective equipment for clinicians performing airway management.
Aim
To evaluate the impact of powered air-purifying respirators, full-face air-purifying respirators and filtering facepieces on specially trained anaesthesiologists performing difficult airway procedures.
Methods
All our COVID-19 intubation team members carried out various difficult intubation drills: unprotected, wearing a full-face respirator, a filtering facepiece or a powered respirator. Airway management times and wearer comfort were evaluated and analysed.
Results
Total mean (SD) intubation times did not show significant differences between the control, the powered, the full-face respirator and the filtering facepiece groups: Airtraq 6.1 (4.4) vs. 5.4 (3.1) vs. 6.1 (5.6) vs. 7.7 (7.6) s; videolaryngoscopy 11.4 (9.0) vs. 7.7 (4.3) vs. 9.8 (8.4) vs. 12.7 (9.8) s; fibreoptic intubation 16.6 (7.8) vs.13.8 (6.7) vs. 13.6 (8.1) vs. 16.9 (9.2) s; and standard endotracheal intubation by direct laryngoscopy 8.1 (3.5) vs. 6.5 (5.6) vs. 6.2 (4.2) vs. 8.0 (4.4) s, respectively. Use of the Airtraq achieved the shortest intubation times. Anaesthesiologists rated temperature and vision significantly better in the powered respirator group.
Conclusions
Advanced airway management remains unaffected by the respiratory protective equipment used if performed by a specially trained, designated team. We conclude that when advanced airway skills are performed by a designated, specially trained team, airway management times remain unaffected by the respiratory protective equipment used.
Keywords: MERIT, COVID-19, SARS-CoV-2, PPE, RPE, Airway management
Glossary of terms
- AGPs
Aerosol-generating procedures.
- CBRN
Chemical, Biological and Radio-Nuclear.
- COVID-19
Coronavirus disease 2019.
- FFP
Filtering Facepiece.
- HCID
High consequence infectious diseases.
- HCW
Healthcare worker.
- MERIT
Mobile endotracheal rapid intubation team.
- PAPR
Powered air-purifying respirator.
- PPE
Personal protective equipment.
- RPE
Respiratory protective equipment.
- SAR
Severe acute respiratory syndrome.
1. Introduction
With more than 144 million cases of coronavirus disease 2019 (COVID-19) globally and the death toll now exceeding 3 million, the COVID-19 pandemic represents one of the greatest public health challenges in over a century [1]. COVID-19 predominantly causes a viral pneumonia and almost a fifth of hospitalized patients require critical care admission [2]. Up to 88% of these critical care patients require tracheal intubation and mechanical ventilation [3,4].
The airway management of COVID-19 patients requires healthcare professionals to wear personal protective equipment (PPE) [[5], [6], [7], [8], [9]]. The bioaerosol infection risk to tracheal intubation teams may arise from general patient contact but is especially high during airway management. Intubation, extubation, airway suctioning, non-invasive mechanical ventilation and surgical airway insertion are aerosol-generating procedures (AGPs) [[5], [6], [7]]. There is now a growing body of evidence that healthcare workers are being infected with SARS-CoV-2 following tracheal intubation of COVID-19 patients [10,11].
During the Severe Acute Respiratory Distress pandemic in 2003, nearly a quarter of those infected were healthcare workers (HCWs) [12]. In Canada, this was reported to be as high as 43%, with anaesthesiologists amongst the highest risk group to contract the disease [[12], [13], [14]]. Guidance on infection prevention, control and transmission-based precautions for at risk medical staff when managing patients with suspected or confirmed COVID-19 has been published by the World Health Organisation and by Public Health England [6,7]. These guidelines are based on evidence from previous pandemics with regular updates being made as new evidence emerges.
When treating patients with highly infectious diseases, HCWs must wear adequate respiratory, cutaneous and eye protection [14,15]. Moreover, aerosol-generating procedures (AGPs) during airway management require specific infection prevention strategies [[16], [17], [18]]. Urgent airway management can be difficult even without respiratory protective equipment (RPE). However, it is well established that this can be even more challenging in chemical, biological, radiological or nuclear environments due to limitations posed on mobility, vision and speech intelligibility, as well as the generation of noise and heat when PPE is worn [[19], [20], [21], [22]].
Respiratory protection devices are designed either as half or full-face masks. A half mask respirator is a facepiece which covers the nose, mouth and chin of the wearer and should be worn with a visor for eye protection [23]. They are assigned lower protection factors than full-face masks [24]. When full-face masks are being used as part of a filtering device, air is either actively drawn into the mask via the filter by the wearer's inspiratory lung-power or from a power assisted filtering device [23]. Powered air-purifying respirators (PAPR) consist of a battery-operated turbo unit, a filter and a loose-fitting headtop [23]. There is no need for fit-testing these devices and their design eliminates the issues of heat build-up, dead-space ventilation and airflow resistance often seen with the other fit-tested face masks [23,24].
The current study was designed to measure the impact of PPE on the performance of airway specialists in a simulated airway emergency.
We recruited all anaesthesiologists who were part of the mobile endotracheal rapid intubation team (MERIT), a specialist tracheal intubation team established specifically to manage COVID-19 patients requiring tracheal intubation [25]. This study evaluates and compares the impact of different powered and standard respirators on simulated difficult airway procedures in members of a specially trained and experienced tracheal intubation team.
The primary outcome measure of this study was defined as the difference in intubation times for various airway management procedures, wearing different RPE and performed by experienced clinicians who had previously performed a number of real-patient tracheal intubations.
Wearer comfort was a secondary outcome measure of the study.
2. Methods
The study received Guy's and St Thomas' NHS Foundation COVID-19 emergency Trust Research and Development approval and was designed as an amendment of the United Kingdom Clinical Research Network (UKCRN) portfolio ID 204282. The study did not require review by a REC within the UK Health Department as the research only involved staff as participants.
2.1. Recruitment
Members of MERIT underwent a multidisciplinary team (MDT) training programme for three weeks leading up to the start date of the service [25]. This involved a multimodal approach, where team members were shown videos on eFONA techniques followed by practising on manikins, asked to read action cards on MERIT intubating techniques and then underwent MDT simulation drills where they performed in-situ intubation and failed intubation drills in full PPE. These were all scored according to the action cards and anyone who missed more than two key steps in the process would be asked to repeat the simulation drills until they successfully completed every step. All simulation training was followed by debriefing sessions to discuss the drills and answer any questions. Experienced anaesthetic nurses made up the rest of the team. Each MERIT team consisted of two anaesthesiologists and two anaesthesiology nurses.
A protocolized, standardised approach was adopted for each intubation where the same action cards were followed; this allowed for the same technique and equipment to be used for every intubation, all of which were practised in the simulation drills.
All fourteen consultant anaesthesiologists gave written informed consent after having been given a detailed explanation of the treatment protocol and formal training in PPE use. All volunteers were instructed that they could withdraw from the study at any time. The study applied all classical contraindications for research in respiratory protection (including asthma, claustrophobia or panic disorders). In addition to that, none of the anaesthesiologists who volunteered to participate in the MERIT team at the beginning of the first wave of the COVID-19 pandemic were allowed to present any of these contraindications or other co-morbidities making them vulnerable to contracting the disease.
2.2. Study design
This observational crossover study evaluates and compares the impact of different powered and standard respirators on simulated difficult airway procedures in members of a specialist tracheal intubation team. The primary outcome measure was defined as time to successful intubation for each combination of airway management technique and PPE. Wearer comfort of each RPE device was a secondary outcome measure.
The objective of this single centre study was to determine the time taken for tracheal intubation in a simulated difficult airway model amongst recruited MERIT anaesthesiologists using four airway management techniques and when wearing four types of RPE, resulting in a total of sixteen endotracheal intubations. This was based at St Thomas’ Hospital, London, which is one of the four UK high consequence infectious diseases (HCID) centers.
2.3. Personal protective equipment
Each participant performed the airway management techniques in four different types of PPE: no RPE (control group), a disposable half-mask FFP3 device, a full-face FFP3 device and a Powered air-purifying respirator (PAPR).
The selected PAPR system was the 3M Scott-Duraflow (Scott Safety Ltd, West Pimbo, Skelmersdale, UK), (Fig. 1 ). This features a loose fitting flow-hood connected via a corrugated hose to a turbofan-unit containing a battery and filters. Weighing 1.4 kg, it provides an airflow of 160L/min, has a battery operating time of 8 h, generates less than 70 dB during use and provides an assigned respiratory protection factor of 40.
Fig. 1.

Powered air-purifying respirator (PAPR) with hood.
The full-face air-purifying respirator (APR) used consisted of the Promask (Scott Safety Ltd, West Pimbo, Skelmersdale, UK) (Fig. 2 ). This respirator is approved to the regulatory standards of EN136 Class 3 for use in a chemical, biological, radiological or nuclear environment. The Promask weighs 530g, has a panoramic visor, maintains re-breathed CO2 levels significantly below 1% and has an assigned protection factor of 40.
Fig. 2.

Full-face air-purifying respirator (APR).
Both reusable respiratory protective devices used the 3M™ Scott™ PF10 P3 Filter, with its EN148-1 compliant 40 mm thread, designed for use with defence and public safety respirators. The filter is suitable for military and first responder applications, protecting against solid and liquid particles, radioactive and toxic particles, and microorganisms, e.g. bacteria and viruses.
The disposable FFP3 mask was the 3M 8833 (Fig. 3 ). This valved half mask respirator is CE approved to the regulatory standards of EN149:2001 and A:2009, weighs 20g and has an assigned protection factor of 20. In addition to the half mask, a Guardian Visor face shield was used for eye protection (Guardian, Girvan, UK).
Fig. 3.
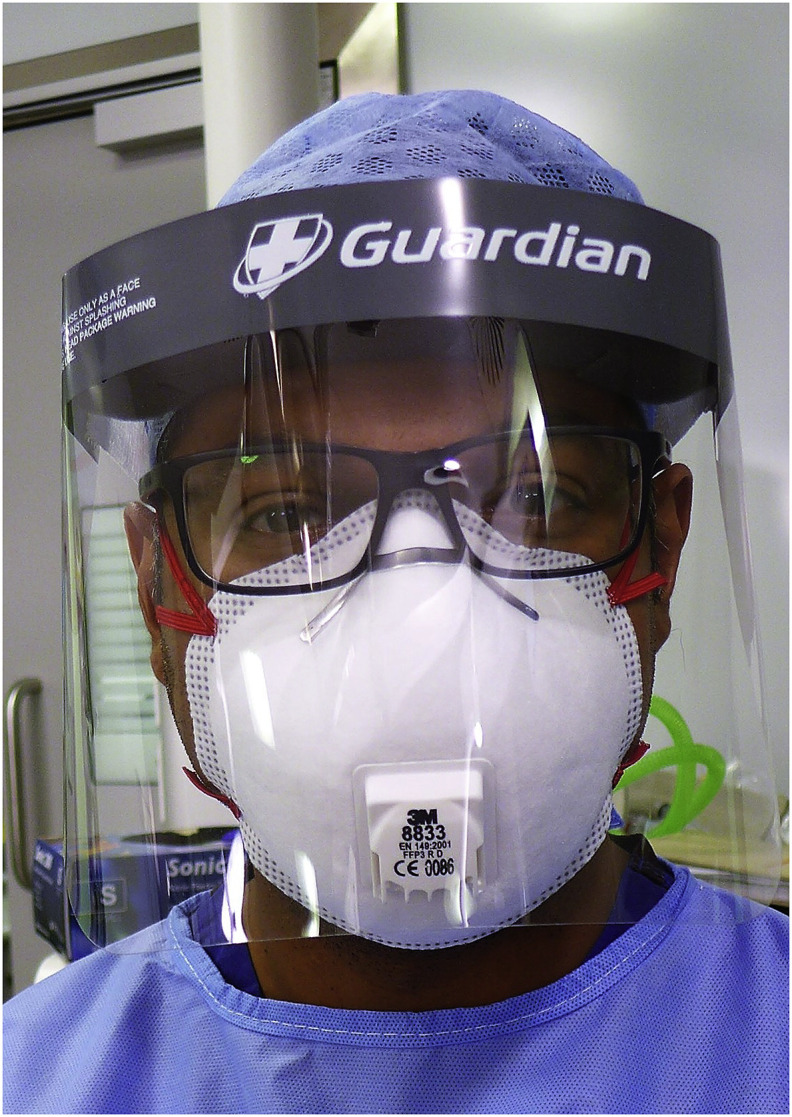
FFP3 mask in combination with face shield.
All anaesthesiologists wore a long-sleeved surgical gown and gloves during each procedure.
2.4. Patient simulator
The airway management skills were carried out on a Deluxe Laerdal Airway Management Trainer™ (Laerdal Medical Ltd, Orpington, UK), which was configured to simulate a direct laryngoscopy Cormack and Lehane grade 2b view.
The manikin was placed on a standard stretcher and the airway management scenarios were carried out with all our genuine anaesthetic equipment in one of our operating rooms.
2.5. Study protocol and data collection
The study consisted of one cohort that undertook the tasks in all four protection levels.
The treatment tasks remained identical in each group, that is, intubation using one of the four intubation devices stated below, but we randomised the order in which the three types of RPE and standard operating department attire was worn to counter any learning effects. The order of PPE/standard operating attire use was determined by sealed envelopes before starting the simulation. The investigator was blinded to the contents of the envelope. All volunteers were briefed on the scenario and the sequence of the tasks; as aforementioned, they additionally received formal training in the RPE used.
A conventional laryngoscope with a Mac 3 blade was used for direct laryngoscopy, whilst a standard Airtraq, size two, using the eyepiece, was the device utilised for indirect tracheal intubation (Airtraq, 48930 Getxo, Spain). For the videolaryngoscopy group, the Storz C-Mac (Storz, Tuttlingen, Germany) was used with a standard Macintosh blade. The Ambu aScope 4 regular bronchoscope was used for the fibreoptic intubation group (Ambu A/S, 2750 Ballerup, Denmark).
The investigator measured the times from picking up the airway device of each airway management procedure until visually confirming the correct tube placement inside the mannikin. Following the simulation scenarios, anaesthesiologists completed a wearer comfort questionnaire evaluating mobility, noise, heat, vision, and speech intelligibility of each respiratory protection system used.
2.6. Statistical analysis
The required sample size had been established by our previous investigations using the same model [15,[19], [20], [21], [22]] and the power calculation was based on a study that measured a standardised airway management scenario using powered respirator hoods [21]. The statistical analysis was performed using Statistical Package for the Social Sciences Statistics v26.0 (IBM, Armonk, New York, NY, USA). Continuous data were normality tested using the Shapiro–Wilk test; this was followed up by multiple comparisons of the time periods by Analysis of Variance (ANOVA). The data of the five-point scale wearer comfort evaluation form was analysed with the Mann-Whitney test. A p value of <0.05 was considered statistically significant.
3. Results
All fourteen MERIT anaesthesiologists volunteered to participate in the study and were able to successfully accomplish all treatment objectives. All airway interventions ended with a successful endotracheal tube placement. The treatment times are displayed in Table 1 .
Table 1.
Treatment times of the individual airway management tasks. Values are mean (±SD) n:14, p values are for comparisons between the two three respiratory protective equipment groups [VL (videolaryngoscopy), FOI (fibreoptic intubation)].
| Control seconds |
Powered respirator seconds |
Full Face respirator seconds |
NR FFP3 Mask seconds |
p-value | |
|---|---|---|---|---|---|
| Airtraq intubation | 6.1 (4.4) | 5.4 (3.1) | 6.1 (5.6) | 7.7 (7.6) | 0.243 |
| VL intubation | 11.4 (9.0) | 7.7 (4.3) | 9.8 (8.4) | 12.7 (9.8) | 0.036 |
| FOI intubation | 16.6 (7.8) | 13.8 (6.7) | 13.6 (8.1) | 16.9 (9.2) | 0.105 |
| Mac intubation | 8.1 (3.5) | 6.5 (5.6) | 6.2 (4.2) | 8.0 (4.4) | 0.090 |
| Donning | (0) | 33.8 (8.3) | 33.4 (9.5) | 30.7 (10.4) | 0.250 |
| Number of failures | 0 | 0 | 0 | 0 |
Intubation times in all study arms were unaffected by the respiratory protective device used, i.e. airway management times of the Airtraq, the videolaryngoscopy, the fibreoptic intubation and the direct laryngoscopy group were independent of the type of respiratory protection used.
Mean fibreoptic intubation times were significantly longer compared to Airtraq, videolaryngoscopy and direct laryngoscopy times. Donning times were not affected by the different PPE used.
Anaesthesiologists rated their personal sensation of heat build-up and perceived vision significantly higher in the powered respirator group (Table 2 ). Conversely, noise levels and mobility scored significantly lower in this group compared to the standard respirator and FFP3 mask group. Speech intelligibility scored significantly better in the full-face respirator as well as in the FFP3 respirator group.
Table 2.
Wearer comfort evaluation form, scale: 0 poor to 5 excellent. Values are median (IQR [ range]) n:14, p values are for comparisons between the three respiratory protective equipment groups.
| Control | Powered respirator |
full-face respirator |
FFP3 Mask |
p-value PAPR-APR/ PAPR-FFP/ APR-FFP |
|
|---|---|---|---|---|---|
| Mobility | 5 (0 [0]) | 3 (2–4 [[1], [2], [3], [4]]) | 4 (2–4 [[1], [2], [3], [4], [5]]) | 4 (2–4 [[1], [2], [3], [4], [5]]) | 0.001 0.000 0.528 |
| Noise | 5 (0 [0]) | 3 (1–3 [[1], [2], [3], [4]]) | 4 (2–4 [[1], [2], [3], [4], [5]]) | 5 (2–4 [[1], [2], [3], [4], [5]]) | 0.000 0.000 0.264 |
| Heat | 5 (0 [0]) | 4.5 (3–4 [[2], [3], [4], [5]]) | 3 (1–2 [0–4]) | 3 (1–2 [0–4]) | 0.000 0.000 0.979 |
| Vision | 5 (0 [0]) | 5 (2–4 [[1], [2], [3], [4]]) | 4 (1–3 [[1], [2], [3], [4], [5]]) | 3.5 (1–3 [[1], [2], [3], [4], [5]]) | 0.003 0.004 0.227 |
| Speech intelligibility | 5 (0 [0]) | 4 (1–4 [0–5]) | 4 (2–4 [[1], [2], [3], [4], [5]]) | 4 (2–4 [[1], [2], [3], [4], [5]]) | 0.015 0.024 0.570 |
We also found that the specially trained MERIT anaesthesiologists required half the time to complete standard laryngoscopy, Airtraq and fibreoptic intubation using the more challenging Deluxe Laerdal Airway Management Trainer (inflated tongue) compared to our previous pre-COVID-19 study where senior anaesthesiology residents were recruited to intubate the standard Laerdal Airway Management Trainer [28].
4. Discussion
Emergency airway management in simulated hazardous Chemical, Biological and Radio-Nuclear (CBRN) environments had come into focus following several high-profile terrorist attacks but also pandemics such as SARS in 2003.
Most studies comparing the effect of different types of respiratory protection reported a significant impact of PPE on various airway management skills in anaesthesiologists, intensivists or paramedics [[19], [20], [21], [22],[26], [27], [28]].
This study has found that wearing standard and powered respirators does not significantly prolong simulated advanced intubation procedures when performed by an experienced and skilled team. This important and novel finding strongly supports the strategy of having designated intubating teams during healthcare crises like the COVID-19 surge. PPE familiarity, experience and confidence seem to have a major influence on airway management in hazardous environments.
These findings contrast with previous publications mainly focussing on CBRN scenarios [[19], [20], [21], [22],[26], [27], [28]] where intubation times were prolonged. However, these studies lacked a specially trained and experienced intubation team of anaesthesiology consultants. In a retrospective observational case series of 202 COVID-19 intubations, Yao and colleagues demonstrated that a highly skilled intubation team who used tools that they were most comfortable with resulted in a first attempt success rate of 89%, with all patients successfully intubated without the need for rescue front of neck access techniques [29]. This study demonstrates the value of using a well-trained and designated intubation team to perform these challenging intubations. In sight of our results, we are in full agreement with one of the key messages of Bainbridge's editorial [30] that training for anaesthesiologists regarding infectious disease control and prevention should start in residency: it needs to include in-depth knowledge concerning AGPs and requirements for RPE, as well as training of technical skills like donning, doffing and airway management when using PPE.
Prospective clinical research in such high stake's conditions are very difficult to achieve. To address this, we have simulated difficult airway settings in manikins, with all subjects wearing the various PPE devices. The value and relevance of manikin-based research has been questioned [31,32] but a systematic review of airway management research over a period between 2006-17 revealed that manikin studies accounted for over a fifth of these. Manikin studies therefore do play an important role in airway management studies but their benefits on clinical practice are still a matter of debate [33].
When comparing the intubation times across all four groups in our study, there was no significant difference found between the various types of respiratory protective devices used, demonstrating that even in this setting a specially trained and skilled HCW is unlikely to be affected by the device used. Given the novelty of COVID-19, there is a relative lack of specific evidence-based information regarding the level of respiratory protection required. According to the latest Cochrane database analysis, it remains unclear as to which type of PPE protects best, what is the best way to put PPE on (i.e. donning) or to remove PPE (i.e. doffing), and how to train HCWs to use PPE as instructed [34].
We used the RPE currently recommended by Public Health England [7] and the Health and Safety Executive [24]. All our MERIT anaesthesiologists had therefore undergone quantitative fit testing to ensure sufficient protection factors of the individual masks used. The reusable and non-reusable respiratory protective devices tested in our study reflect the actual range of equipment used by anaesthesiologists, nurses and ODPs in our trust during the COVID-19 pandemic.
There are some limitations of our study. The study took place in a controlled, non-hazardous environment and the participants were not required to physically exert themselves; therefore stress levels are different from real life scenarios.
Furthermore, heat build-up and the effects of standard respirators on breathing resistance over time cannot be commented on. Additionally, the manikins in our study were unable to replicate some of the real-life problems that may occur such as blood, secretions or expired water vapour clouding the indirect laryngoscopic view.
In conclusion, our study has demonstrated that wearing different types of RPE does not significantly prolong simulated intubation procedures when performed by a designated, trained and experienced team. Powered respirators seem to be significantly favourable in respect of vision and temperature, whereas full face and half mask air-purifying respirators took preference regarding their lower noise levels, better speech intelligibility and overall mobility. We found that our designated, specially trained MERIT anaesthesiologists required half the airway management times to complete standard laryngoscopy, Airtraq and fibreoptic intubation using the more challenging Deluxe Laerdal Airway Management Trainer (compared to our previous pre-COVID-19 study [28]), highlighting the importance of establishing specialist tracheal intubation teams to manage the airways of COVID-19 patients.
Author contributions
JS: This author designed the study protocol, created the simulation model, facilitated data collection and manuscript preparation.
CC: This author helped with manuscript preparation.
PG: This author helped with data analysis and manuscript preparation.
SR: This author helped with data collection and manuscript preparation.
IA: This author helped with study conception and manuscript preparation.
Declaration of competing interest
No competing interests declared.
Acknowledgements
The authors wish to thank Prof David Crouch (3M Global Subject Matter Expert - Application Engineering, Defense & Public Safety) for the provision of free samples of RPE. This work was supported by the Department of Anaesthetics, Guy's and St Thomas’ NHS Hospital Trust, London, UK and the NIHR GSTFT/KCL Biomedical Research Centre, F16 Tower Wing Guy's Hospital, London SE1 9RT.
Footnotes
This work was supported by the Anaesthetic Department of Guy's and St Thomas' NHS Foundation Trust, London.
Appendix. Wearer Comfort Evaluation Form
 PAPR - powered air purifying respirator |
 APR - Full face air-purifying respirator |
|
|---|---|---|
| Mobility | [0 1 2 3 4 5] Worst Best |
[0 1 2 3 4 5] Worst Best |
| Noise | [0 1 2 3 4 5] Worst Best |
[0 1 2 3 4 5] Worst Best |
| Heat | [0 1 2 3 4 5] Worst Best |
[0 1 2 3 4 5] Worst Best |
| Vision | [0 1 2 3 4 5] Worst Best |
[0 1 2 3 4 5] Worst Best |
| Speech Intelligibility | [0 1 2 3 4 5] Worst Best |
[0 1 2 3 4 5] Worst Best |
| Freetext | ||
 FFP3 and face shield |
Control | |
| Mobility | [0 1 2 3 4 5] Worst Best |
[0 1 2 3 4 5] Worst Best |
| Noise | [0 1 2 3 4 5] Worst Best |
[0 1 2 3 4 5] Worst Best |
| Heat | [0 1 2 3 4 5] Worst Best |
[0 1 2 3 4 5] Worst Best |
| Vision | [0 1 2 3 4 5] Worst Best |
[0 1 2 3 4 5] Worst Best |
| Speech Intelligibility | [0 1 2 3 4 5] Worst Best |
[0 1 2 3 4 5] Worst Best |
| Freetext |
References
- 1.World Health Organization Coronavirus disease (COVID-19) dashboard. https://covid19.who.int Available from URL.
- 2.International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) COVID-19 Report: 27th April 2020. https://isaric.tghn.org/covid-19-clinical-research-resources/ Available from URL.
- 3.Grasselli G., Zangrillo A A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. J. Am. Med. Assoc. 2020;323 doi: 10.1001/jama.2020.5394. 1574-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Intensive Care National Audit & Research Centre . 2020. ICNARC Report on COVID-19 in Critical Care.https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports Available from URL. [Google Scholar]
- 5.Wei H., Jiang B., Behringer E., et al. Controversies in airway management of COVID-19 patients: updated information and international expert consensus recommendations. Br. J. Anaesth. 2021;126:361–366. doi: 10.1016/j.bja.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Public Health England Guidance on Infection Prevention and Control for COVID-19. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control Available from URL.
- 7.World Health Organization Coronavirus disease (COVID-19) technical guidance: infection prevention and control. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-and-control Available from URL.
- 8.Miller L., Luković E., Wagener G. Guiding airway management and personal protective equipment for COVID-19 intubation teams. Br. J. Anaesth. 2020;125:e288–e290. doi: 10.1016/j.bja.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z., Wu Z., Zhao H., Zuo M. Personal protective equipment during tracheal intubation in patients with COVID-19 in China: a cross-sectional survey. Br. J. Anaesth. 2020;125:e420–e422. doi: 10.1016/j.bja.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J., Sun M., Li N., Suo X., Xia Z., Zuo M., Liu R. Acquired infection after intubating patients with COVID-19: a retrospective pilot study. J. Clin. Anesth. 2020;67:110006. doi: 10.1016/j.jclinane.2020.110006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Boghdadly K., Wong D.J.N., Owen R., et al. Risks to healthcare workers following tracheal intubation of patients with COVID-19: a prospective international multicentre cohort study. Anaesthesia. 2020;75:1437–1447. doi: 10.1111/anae.15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Summary W.H.O. 2004. Of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003.https://www.who.int/csr/sars/country/table2004_04_21/en/ Available from URL. [Google Scholar]
- 13.Kamming D., Gardam M., Chung F. Anaesthesia and SARS. Br. J. Anaesth. 2003;90:715–718. doi: 10.1093/bja/aeg173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orser B. Recommendations for endotracheal intubation of COVID-19 patients. Anesth. Analg. 2020;130:1109–1110. doi: 10.1213/ANE.0000000000004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher J. In: Handbook of Respiratory Protection. first ed. Racz L., Yamamoto D., Eninger R., editors. Taylor and Francis; Boca Raton, Fl: 2018. Respiratory protection for medical first responders and receivers; pp. 72–91. [Google Scholar]
- 16.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the difficult airway society, the association of anesthesiologists the intensive care society, the faculty of intensive care medicine and the royal college of anaesthetists. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Zundert T.C.R.V., Barach P., J Van Zundert A.A. Revisiting safe airway management and patient care by anaesthetists during the COVID-19 pandemic. Br. J. Anaesth. 2020;125:863–867. doi: 10.1016/j.bja.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saracoglu K.T., Saracoglu A., Demirhan R. Airway management strategies for the Covid 19 patients: a brief narrative review. J. Clin. Anesth. 2020;66:109954. doi: 10.1016/j.jclinane.2020.109954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumacher J., Arlidge J., Garnham F., Ahmad I. A randomised crossover simulation study comparing the impact of chemical, biological, radiological or nuclear substance personal protection equipment on the performance of advanced life support interventions. Anaesthesia. 2017;72:592–597. doi: 10.1111/anae.13842. [DOI] [PubMed] [Google Scholar]
- 20.Schumacher J., Gray S.A., Michel S., Alcock R., Brinker A. Respiratory protection during simulated emergency pediatric life support. Prehospital Disaster Med. 2013;28:33–38. doi: 10.1017/S1049023X12001525. [DOI] [PubMed] [Google Scholar]
- 21.Schumacher J., Gray S.A., Weidelt L., Prior K., Brinker A., Stratling W.M. Comparison of powered and conventional air-purifying respirators during simulated resuscitation of CBRN victims. Emerg. Med. J. 2009;26:501–505. doi: 10.1136/emj.2008.061531. [DOI] [PubMed] [Google Scholar]
- 22.Schumacher J., Runte J., Brinker A., Prior K., Heringlake M., Eichler W. Respiratory protection during high fidelity simulated resuscitation of casualties contaminated with chemical warfare agents - respiratory protection for anaesthestists during resuscitation. Anaesthesia. 2008;63:593–598. doi: 10.1111/j.1365-2044.2008.05450.x. [DOI] [PubMed] [Google Scholar]
- 23.BRITISH STANDARDS EN 529 . 2005. Respiratory Protective Devices — Recommendations for Selection, Use, Care and Maintenance — Guidance Document.https://www.breathesafety.com/files/EN5292005RespiratoryProtectiondevices.pdf Technical Committee PH/4, Respiratory protection, to Subcommittee PH/4/11. Available from URL. [Google Scholar]
- 24.Health and Safety Executive . fourth ed. 2013. Respiratory Protective Equipment at Work - A Practical Guide.https://www.hse.gov.uk/pubns/books/hsg53.htm HSG53. Available from URL. [Google Scholar]
- 25.Ahmad I., Jeyarajah J., Nair G., et al. A prospective, observational, cohort study of airway management of patients with COVID-19 by specialist tracheal intubation teams. Can. J. Anaesth. 2020;68:196–203. doi: 10.1007/s12630-020-01804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castle N., Pillay Y., Spencer N. Comparison of six different intubation aids for use while wearing CBRN-PPE: a manikin study. Resuscitation. 2011;82:1548–1552. doi: 10.1016/j.resuscitation.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Castle N., Pillay Y., Spencer N. Insertion of six different supraglottic airway devices whilst wearing chemical, biological, radiation, nuclear-personal protective equipment: a manikin study. Anaesthesia. 2011;66:983–988. doi: 10.1111/j.1365-2044.2011.06816.x. [DOI] [PubMed] [Google Scholar]
- 28.Schumacher J., Arlidge J., Dudley D., Sicinski M., Ahmad I. The impact of respiratory protective equipment on difficult airway management: a randomised, crossover, simulation study. Anaesthesia. 2020;75:1301–1306. doi: 10.1111/anae.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao W., Wang T., Jiang B., et al. Emergency tracheal intubation in 202 patients with COVD-19 in Wuhan, China: lessons learnt and international expert recommendations. Br. J. Anaesth. 2020;125 doi: 10.1016/j.bja.2020.03.026. e28–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bainbridge D. Personal protective equipment (PPE) for anesthesiologists: the need for national guidelines. Can. J. Anaesth. 2020;67:919–923. doi: 10.1007/s12630-020-01675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook T.M., Duggan L.V., Kristensen M.S. In search of consensus on ethics in airway research. Anaesthesia. 2017;72:1175–1179. doi: 10.1111/anae.13961. [DOI] [PubMed] [Google Scholar]
- 32.Irwin M.G., Ward P.A. Consensus on ethics in airway research – a reply. Anaesthesia. 2017;72:1278–1279. doi: 10.1111/anae.14021. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad I., Onwochei D.N., Muldoon S., Keane O., El-Boghdadly K. Airway management research: a systematic review. Anaesthesia. 2019;74:225–236. doi: 10.1111/anae.14471. [DOI] [PubMed] [Google Scholar]
- 34.Verbeek J.H., Rajamaki B., Ijaz S., et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst. Rev. 2020;15:CD011621. doi: 10.1002/14651858.CD011621.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]


