Abstract
Simple Summary
Cutaneous Melanoma (CM), arising from pigment-producing melanocytes in the skin, is an aggressive cancer with high metastatic potential. While cutaneous melanoma represents only a fraction of all skin cancers (<5%), it accounts for most skin-cancer-related deaths worldwide. Immune checkpoint inhibition has been the first therapeutic approach to significantly benefit patient survival after treatment. Nevertheless, the immunosuppressive tumor microenvironment and the intrinsic and acquired treatment resistance of melanoma remain crucial challenges. Combining local and systemic treatment offers the potential to augment therapeutic response and overcome resistance, although, complex drug combinations can harbor an increased risk of immune-related adverse events. The aim of this review is to give current insight into studies combining systemic and local therapeutic approaches to overcome drug resistance, prime melanoma cells for therapy, and improve overall treatment response in CM patients.
Abstract
To date, the skin remains the most common cancer site among Caucasians in the western world. The complex, layered structure of human skin harbors a heterogenous population of specialized cells. Each cell type residing in the skin potentially gives rise to a variety of cancers, including non-melanoma skin cancer, sarcoma, and cutaneous melanoma. Cutaneous melanoma is known to exacerbate and metastasize if not detected at an early stage, with mutant melanomas tending to acquire treatment resistance over time. The intricacy of melanoma thus necessitates diverse and patient-centered targeted treatment options. In addition to classical treatment through surgical intervention and radio- or chemotherapy, several systemic and intratumoral immunomodulators, pharmacological agents (e.g., targeted therapies), and oncolytic viruses are trialed or have been recently approved. Moreover, utilizing combinations of immune checkpoint blockade with targeted, oncolytic, or anti-angiogenic approaches for patients with advanced disease progression are promising approaches currently under pre-clinical and clinical investigation. In this review, we summarize the current ‘state-of-the-art’ as well as discuss emerging agents and regimens in cutaneous melanoma treatment.
Keywords: skin cancer, melanoma, cancer therapy, immunotherapy, targeted therapy, intratumoral therapy, combination therapy
1. Background
Cancer of the skin is considered a growing epidemic among Caucasians in the western world, with an alarming increase of non-melanoma skin cancer and cutaneous melanoma (CM) incidence rates of up to 44% over the last decade [1,2,3]. Moreover, increased accumulated exposure to ultraviolet (UV) radiation of the sun over lifespan due to growing life expectancy and the global climate crisis are considered major catalysts of increased incidence and mortality of skin cancer. Whilst CM is still most common in the male population aged 50–70 years, rates of CM in young adults, specifically in young women, are constantly increasing over the last years. Although recently approved novel targeted and immunotherapeutic approaches for CM treatment have been able to contain mortality rates of melanoma patients, it is predicted that nearly half a million people will be diagnosed with CM by 2040 with an increase in incidence of 62% and an increase of mortality of up to 74% [4,5,6]. In contrast to non-melanoma skin cancer (0.69) the age-standardized annual mortality rate for CM is about 1.5 (per 100,000 population) in the United States. These alarming numbers make the development of novel targeted therapeutic options and constant adjustments of the current state-of-the-art regimens essential in successful CM treatment. Several combinations involving immune checkpoint inhibition (ICI), targeted therapy through mutant-BRAF inhibition, intratumoral application of immunomodulators, oncolytic viruses, and anti-angiogenic approaches are being trialed to prime the anti-tumor response, enhance the sensitivity of CM cells to therapeutic interventions, and overcome therapy resistance of mutant cancer cells.
Here, we describe current intervention strategies in CM and give pre-clinical insight into research avenues of future combination therapies in CM (Figure 1a–e).
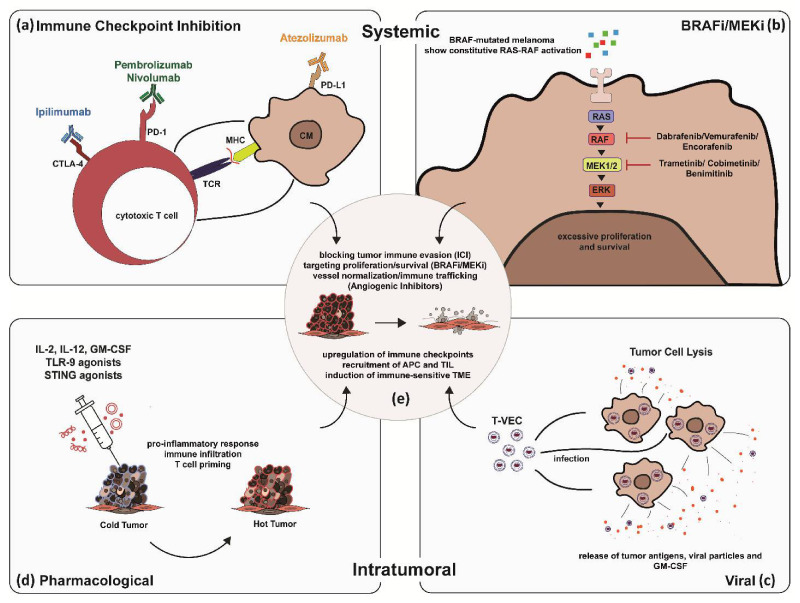
Figure 1.
Synergistic effects of combined systemic and intratumoral therapy in skin cancer treatment exemplified by CM. Systemic immune checkpoint inhibition (a–e): Blocking of immune checkpoints on cytotoxic T Lymphocytes (CTL) and CM cells disrupting tumor immune evasion (a). Systemic BRAFi/MEKi: Blocking overactivation of RAS-RAF signaling pathway in BRAF-mutated melanoma by BRAF and MEK inhibitors (b). Intratumoral application of genetically engineered oncolytic viral particles to express checkpoint inhibitors on CM cells, force production and release of tumor antigens and GM-CSF to prime T cell immune response and induce tumor cell lysis (c). Intratumoral pharmacological application of IL-2, IL12, GM-CSF, TLR-9 and STING agonists: Priming locally advanced CM to become immunogenic (d).
2. Immune Checkpoint Inhibition
Understanding the process of immune-surveillance, or more specifically the capability of the immune system to identify and target neoplastic cells for destruction, revolutionized the treatment of a range of solid tumors [7,8]. Tumor cells are capable of hijacking the patients’ T cell immune checkpoints, such as cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1), which act as negative regulators of immune response by surface expression of members of the B7 family of immune-regulatory ligands and B7 homologs such as programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 1 (PD-L2) [9,10,11] (Figure 1a). Tumor cells thus use a mechanism, which inherently is meant to protect the immune system from aberrant auto-immune responses. T cell receptor (TCR)-dependent T cell activation is mediated by several co-stimulatory signals, most notably the CD28 surface receptor, which is highly expressed at basal levels. By contrast, CTLA-4 is only induced following antigen presentation and directly opposes CD28 stimulation [12,13,14]. Similarly, PD1 is only induced after TCR stimulation, and whilst CTLA-4 mostly acts within lymphoid organs, PD1 functions predominantly within peripheral tissues [12,15]. Other immune checkpoints include T cell immunoglobulin mucin-3 (TIM-3), which regulates T cell tolerance by inhibiting expansion and promoting apoptosis of Th1 and Th17 cells, thus leading to CD8+ T cell depletion. Furthermore, lymphocyte activated gene-3 (LAG-3), T cell immunoglobulin, and immunoreceptor tyrosine-based inhibitory motif (TIGIT), Glucocorticoid-induced TNFR family-related gene (GITR) and V-domain Ig suppressor of T cell activation (VISTA) contribute to activation and inhibitory function of regulatory T cells [16,17,18]. Whilst targeted therapy is generally associated with high but short-term treatment response rates in skin cancer patients, immune checkpoint blockade has mostly shown lower but more durable responses [19,20,21].
Remarkably, recent treatment of skin cancer with immune checkpoint inhibitors (ICI) was particularly successful with high and long-term overall response rates (ORR) (40–60%). Approved and currently in-use ICI for skin cancer treatment include cytokines that target the IL-2 and IFNAR1/2 pathways such as aldesleukin and interferon/peginterferon alfa-2b, PD-1 inhibitors such as pembrolizumab and nivolumab, PD-L1 inhibitors such as atezolizumab, and the first clinically approved monoclonal antibody for CM, ipilimumab, that targets CTLA-4 [22]. Ipilumab, although having a relatively low ORR of <20%, was the first therapeutic option to improve overall survival in metastatic melanoma patients significantly and is thus predominantly used in melanoma treatment [23,24]. Treatment of melanoma was the first to gravely benefit from the approval of immune checkpoint blockade, with ICI being the first therapy to significantly improve overall survival (OS) in patients with advanced disease states. The early success of ipilimumab in 2011 propelled ICI into clinical use and shifted the standard-of-care of advanced melanoma management. The anti-CTLA-4 monoclonal antibody directed against the inhibitory receptor exempts the inhibitory effect of CTLA-4 activation, resulting in activation of T lymphocytes and thus the destruction of tumor cells. Even though ORRs achieved with ipilimumab are below 20%, some patients experience long-term survival using this anti-CTLA-4 regimen. In pretreated CM patients with advanced disease, for example, ipilimumab significantly increased median OS when compared to the peptide vaccine gp100 (10.6 versus 6.4 months). After three years, the OS rate was about 20% followed by a plateau of the survival curve for up to 10 years. Recently, a randomized placebo-controlled study in stage III patients with CM demonstrated that adjuvant ipilimumab increases relapse-free survival (RFS) and OS. Nevertheless, more than half of patients developed severe side effects (grade 3 or 4) with ipilimumab, and five patients (1.1%) even had a grade 5 outcome [23,24,25,26,27]. As a result of ipilimumab-induced T cell activation, a variety of immune-mediated adverse effects (irAEs) have been observed, particularly including colitis, skin rashes, hepatitis, and less frequently hypophysitis.
After the anti-CTLA-4 proof of concept showing that checkpoint blockade is in fact a beneficial approach to combat CM, pembrolizumab, and nivolumab were studied in this condition. These two monoclonal antibodies against PD-1 were approved by the FDA in 2014, becoming the first-line treatment option in metastatic melanoma. Large, randomized investigations have demonstrated that mono-nivolumab or -pembrolizumab were superior to mono-ipilimumab. Mono-pembrolizumab in the management of naïve as well as pretreated patients resulted in sustained ORR of 30 to 40% [27,28,29]. In previously untreated CM patients, pembrolizumab revealed OS rates of 51% and 41% after three and five years, respectively. Studies of mono-nivolumab demonstrated ORR of 32% in treatment naïve patients and 40% in pretreated CM. The three-year OS rate for mono-nivolumab in therapy-naive patients was 42%, while the five-year OS in pretreated CM patients with this monotherapy was 35%. Cross-trial comparisons of homogeneous groups of CM patients with mono-pembrolizumab or -nivolumab treatment revealed similar results with respect to the clinical endpoints and side effects [27,28,29,30]. Furthermore, adjuvant monotherapy using nivolumab or pembrolizumab is now the preferred treatment option in patients with resected stage III disease, in particular, stage IIIB to IIID. Compared to ipilimumab, nivolumab has improved RFS with lower rates of adverse events. In this clinical setting, pembrolizumab therapy revealed significantly longer RFS than placebo [31]. Moreover, neoadjuvant melanoma trials, also including immunotherapies, currently investigate agents with promising clinical and biomarker results [32]. As previously mentioned, however, ICI can produce a wide range of irAEs affecting a multitude of organs such as skin, gastrointestinal tract, endocrine system, heart, lung, kidneys, and the nervous system. The most frequent irAEs induced by anti-PD-1 agents are hypo- or hyperthyroidism, pneumonitis, cutaneous reactions (Figure 2), including severe conditions (Stevens-Johnson syndrome, etc.), and hepatitis. irAEs may develop at any time as indicated by a wide range of first occurrence for different organs (e.g., from few days after initiation up to 15 months for skin or up to 12 months for the gastrointestinal tract) [33,34]. In general, oral and intravenous corticosteroids are the mainstay of irAEs management. Depending on the irAEs, their severity and non-responsiveness to corticosteroids, other immunomodulatory, and immunosuppressive drugs may be indicated.
Figure 2.
Showing a 59-year old male patient with metastatic melanoma and multiple benign melanocytic nevi on the back (a). After 4 cycles (3 months) of anti-PD-1 plus anti-CTLA-4 combination therapy, he developed marked nevi lightening (b, highlighted by arrowheads).
With the attempt to further increase the number of patients who benefit from immunotherapy, combination therapies using anti-CTLA-4 and anti-PD-1 antibodies have been studied in large trials. Two studies demonstrated that the combination of nivolumab plus ipilimumab (ORR: 56.7%) resulted in higher clinical benefit when compared to -nivolumab (ORR: 43.7%) or mono-ipilimumab (ORR: 19%). Five-year OS rates were 52% in the combined treatment arm, 44% in the mono-nivolumab arm, and 26% in the mono-ipilimumab arm [26,27,28,29]. In 2015, the combination of nivolumab plus ipilimumab was approved based on positive ORR and PFS data. In case of primary or secondary resistance to anti-PD-1, monotherapy combination or mono-ipilimumab is a potential therapeutic approach. Today, the respective benefits of combination ICI versus sequential ICI are still unclear. Combination therapies are associated with much higher rates of irAEs, which are justified by long-term disease response. However, the subgroup of patients who might benefit from the combination is not known prior to therapy, potentially exposing patients to unnecessary toxicity [25,26,27,28,29,30,31]. Brain metastases are a common cause of disabling neurologic complications and poor prognosis in CM patients. In a phase 2 trial, patients with small, untreated, and asymptomatic brain metastasis were enrolled—it was demonstrated that ipilimumab plus nivolumab have clinically meaningful intracranial efficacy (56% of intracranial response). The safety profile was similar to those reported for the combination in patients without brain metastasis [35]. Another phase 2 clinical trial compared the combination of nivolumab plus ipilimumab versus nivolumab alone. Despite the small sample size, ICIs combination was superior to nivolumab monotherapy, with a higher proportion of patients achieving intracranial response [35]. An exemplary case shows almost complete remission after ICI combination treatment is depicted in Figure 3. However, the combination of anti-PD-1 and anti-CTLA-4 agents is associated with even higher irAEs and drop-out rates when compared to monotherapy regimens. Hence, ICI can exhibit a variety of irAEs, which range in severity but can have detrimental effects on a patient’s quality of life and well-being, limiting subsequent treatment options [36]. Combinations of two or more therapies (e.g., BRAF/MEKi, T-VEC, or anti-angiogenic modulators) with ICI, although potentially leading to improved efficacy, can increase the incidence and severity of irAEs. Overcoming irAEs thus presents a major challenge in immunotherapeutic and combination approaches to target CM.
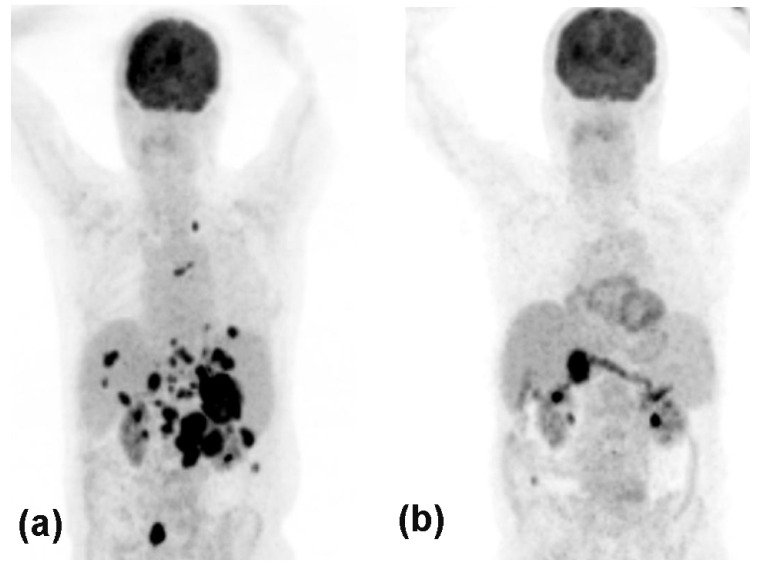
Figure 3.
PET-CT of an 80-year old male showing massive melanoma metastases in the entire abdomen (a), which almost completely resolved after two cycles of anti-PD-1 plus an-ti-CTLA-4 combination therapy (b).
Despite recent advances in ICI therapy in CM, a subgroup of patients does not respond to ICI treatment, with a significant portion of patients relapsing within 2 years. ICI resistance can be caused by a variety of factors, including an immunosuppressive tumor microenvironment, levels of tissue-specific neoantigens, altered tumor-infiltrating lymphocyte function, and specific oncogenic alterations in the heterogenous tumor entities [37,38,39]. Therefore, regimens are being studied, including new immunomodulators and combination treatments using ICI with targeted therapy, such atezolizumab, vemurafenib, and cobimetinib. Currently, in-use ICIs and BRAF/MEK inhibitors are summarized in Table 1. Furthermore, evidence is accumulating on the use of new immunomodulatory treatments, for example, addressing LAG3, TIM3, and GITR [16,40].
Table 1.
Currently in-use ICIs and BRAF/MEK inhibitors for CM.
| Biological Classification | Drug Name | Mechanism | Main Target | Study |
|---|---|---|---|---|
| Immune Checkpoint Inhibition (ICI) | ||||
| Monoclonal Antibody | Ipilimumab | priming of anti-tumor immune response by checkpoint receptor inhibiton on immune and melanoma cells | CTLA4 | NCT00094653 |
| Pembrolizumab | PD1 | KEYNOTE-001 KEYNOTE-002 KEYNOTE-006 |
||
| Nivolumab | PD1 | NCT01844505 | ||
| Atezolizumab | PDL1 | IMspire150 NCT02908672 |
||
| Targeted Therapy | ||||
| Kinase Inhibitor | Dabrafenib | inhibition of MAPK/ERK overactivation in BRAF-mutant melanoma | BRAF mutant | BREAK-2 BREAK-3 |
| Vermurafenib | NCT01689519 | |||
| Encorafenib | NCT01909453 | |||
| Trametinib | MEK | METRIC MEK113583 |
||
| Cobimetinib | NCT01689519 | |||
| Binimetinib | NCT01909453 | |||
3. Targeted Therapy
Pharmacological approaches to target skin cancer have been widely studied in the last two decades. Targeting cancer by focusing on cancer type-specific genetic alterations and the most consequential signaling cascade abnormalities in tumor initiation and progression are direct and profound applications to rehabilitate cellular homeostasis. In NMSC, several critical pharmacological modulators are known to exhibit favorable effects. Targets of systemic therapies include COX2, Toll-like-receptors (TLR), growth factor receptors such as EGFR and the sonic hedgehog, and m-TOR signaling pathways [41,42,43,44,45,46].
In advanced melanoma, the most common druggable mutations are found in the MAPK/ERK pathway [47,48] (Figure 1b). Up to half of the patients diagnosed with CM carry activating mutations in the serine-threonine kinase of the BRAF gene (BRAF-V600), with almost a quarter of patients (15–25%) carrying mutations in the RAS gene (Q61R, Q61K), which downstream activate RAF, MEK, and ERK. [49,50,51]. Other mutations associated with poor outcomes include CDKN2A and TP53 in ~13% and ~15% of melanoma patients, respectively [52]. MAPK/ERK pathway mutations enhance proliferation, survival, and spread of melanoma cells, and thus, patients carrying the mutation are eligible for treatment with BRAF and MEK inhibitors. In BRAF-mutant metastatic melanoma, BRAF and MEK inhibitors have proven to improve survival, although half of the patients develop resistance within a year [53,54]. Moreover, treatment with BRAF and MEK inhibitors is associated with toxicity, as shown in a case of widespread acneiform rash developing 2 months after the initiation of anti-BRAF plus anti-MEK combination therapy (Figure 4). Several adverse events (AE), including hyperkeratosis, rash, alopecia, skin papilloma, palmar-plantar hyperkeratosis, and arthralgia, as well as rare adverse events such as cutaneous SCC and pyrexia were observed. Most used BRAF/MEK inhibitor combinations include Dabrafenib/Trametinib, Vermurafenib/Cobimetinib, and Encorafenib/Binimetinib. In addition, BRAFi was associated with increased antigen expression, lymphocyte homing, and a decrease in immunosuppressive cytokine release in melanoma cell lines and patients’ biopsies, providing a rationale for ICI-BRAF/MEKi combinations [55,56,57,58]. This was further explored in a first-in-human clinical trial of dabrafenib, trametinib, and pembrolizumab in which 11/15 patients (73%) showed an objective response and 6/15 continued with a response at a median follow-up of 27 months. Triple therapy had higher PFS (16 months) compared to dabrafenib and trametinib double therapy (10.3 months) with a median duration of 18.7 months. [58,59]. Recently, a randomized, double-blind phase 3 trial on 514 patients suffering from stage III-IV BRAFV600-positive melanoma evaluated the use of atezolizumab in combination with vermurafenib and cobimetinib. Progression-free survival was significantly prolonged from 10.6 months in the control group to 15.1 months in the atezolizumab group with similar ORR (65% vs. 66%) [60]. While triple therapy combinations were prone to a higher incidence of AEs, these studies were the first to indicate that BRAFi/MEKi/anti-PD1/PDL1 combinations have the potential to increase the frequency of long-lasting antitumor responses in BRAF v600-mutant melanoma patients [58,60].
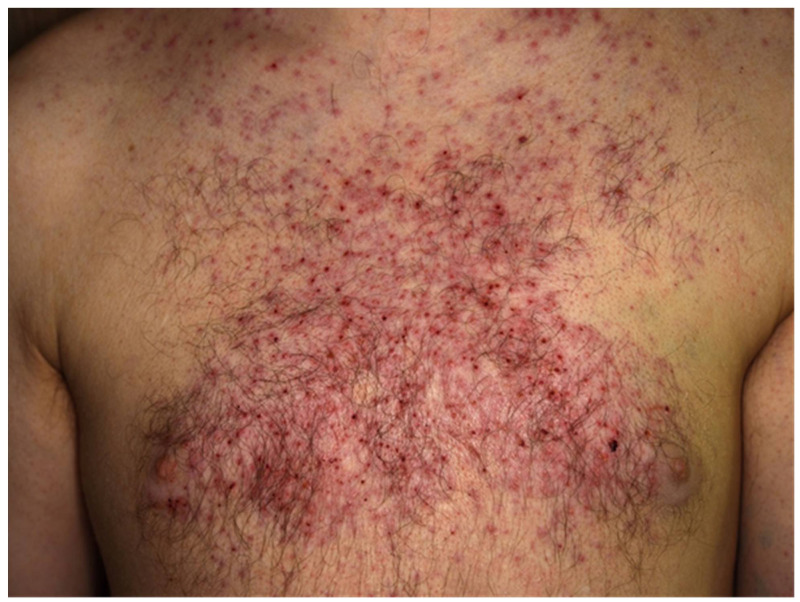
Figure 4.
Widespread acneiform rash developing 2 months after the initiation of anti-BRAF plus anti-MEK combination therapy in a 33-year-old male with metastatic melanoma.
As with ICI, targeted therapies are also associated with AEs occurring of any grade in almost all patients treated with the combination therapy. In patients treated with BRAFi/MEKi, grade 3 to 4 AEs have been observed in about 50%. The discontinuation rate due to AEs of BRAFi/MEKi is about 15% and thus much lower as compared to combinations of anti-PD-1 and anti-CTLA-4. The most frequently reported AEs of BRAFi/MEKi include cutaneous toxicities (Figure 4; e.g., acneiform rashes, photosensitivity, palmoplantar hyperkeratosis), diarrhea, pyrexia, hepatic toxicities, arthralgia, cardiovascular toxicities (e.g., hypertension, QT-prolongation), ocular AEs (Figure 5; retinal detachment, uveitis), and rarely pneumonitis.
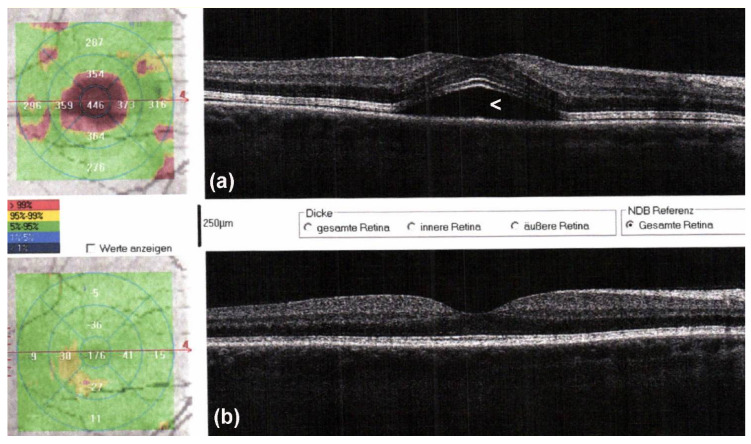
Figure 5.
Optical coherence tomography (OCT) scans of a male patient with metastatic melanoma started targeted therapy with encorafenib and binimetinib. Two days after therapy initiation, he noticed blurry vision. On OCT, serous retinal detachment with subfoveolar fluid (a, arrowhead) is observed, which spontaneously resolved after withholding targeted therapy for 5 days (b).
4. Intratumoral Tumor Therapy and Combinations
Oncolytic Viral Therapy (OVT) utilizes replication-competent native or genetically engineered herpes and adenoviruses to selectively target, infect, and lyse tumor cells. Moreover, oncolytic viruses have the potential to be used as vehicles for transduction of immunomodulatory transgenes in a tumor-promoter-driven system to enhance anti-tumor immunity and assist in immune checkpoint blockade (Figure 1c). The first oncolytic virus approved in 2015 for melanoma treatment was the engineered herpes-simplex virus 1 (HSV-1) Talimogene laherparepvec (T-VEC). Deletion of RL1 and US12, encoding for ICP34.5 and ICP47 in T-VEC blocks the ability of the virus to hijack the replication machinery of normal cells by making it susceptible to the anti-viral cell response through protein kinase R (PKR) activation. Tumor cells, due to a disrupted PKR and IFN pathway, thus become main targets for infection and lysis by T-VEC. Moreover, it was found that insertion of US11 and GM-CSF improved oncolytic effect and augmented immune response leading to systemic activation of CD8+ TIL [61,62,63,64,65]. Several reports have shown intratumoral injection of T-VEC to be effective in the treatment of recurrent and locally advanced Merkel cell carcinoma (MCC) [66,67]. A recent report of T-VEC and concurrent pembrolizumab treatment led to a complete response in 51-year old patients with refractory MCC [68]. Two cases showed a complete and partial response in two 69-year old and 76-year-old Caucasian males with pre-treated anti-PD-1 refractory MCC after concurrent PD1/PDL1 inhibition and T-VEC treatment [69]. Nevertheless, T-VEC/ICI combinations in MCC treatment remain largely under-studied. In CM, several clinical studies were published showing good efficacy and tolerability, with few reported severe AEs of T-VEC treatment in patients with stage III-IV melanoma [70,71,72,73,74]. Recently, final analysis of the OPTiM phase III trials comparing T-VEC versus GM-CSF treatment in 436 patients with unresectable stage III-IV melanoma, T-VEC was shown to improve long-term efficacy and was well tolerated with median OS for T-VEC treatment of 23.3 months and ORR of 31.5% [72]. Combining T-VEC and ICI or BRAF/MEK inhibition in CM treatment are being widely studied [61,63]. T-VEC can prime the anti-tumor response and turn immunologically ‘cold tumors’ to become ‘hot’ by induction of IFN signaling overcoming immunosuppression of the TME. Moreover, T-VEC can induce PD-1 expression in tumor cells, making them susceptible for ICI and viral GM-CSF, and chemokine release can lead to attraction and maturation of APCs that can cross prime CD8+ TIL in anti-tumor response. First, phase I/II studies on T-VEC/ipilimumab or pembrolizumab combinations show higher effectiveness of T-VEC/ICI combinational therapy versus T-VEC monotherapy in stage I/II melanoma [74]. Patients receiving ipilimumab/T-VEC combinations experienced a higher incidence of pseudo-progression, and combinational therapy was associated with higher ORR (39%) [75]. Similarly, pembrolizumab/T-VEC combinations were well tolerated with an ORR of 62% [61]. Another oncolytic adenovirus, ONCOS-102, that was engineered to express GM-CSF, in combination with pembrolizumab, showed promising results in melanoma mouse models and is currently undergoing a pilot study in advanced melanoma patients after anti-PD1 treatment [76] (NCT03003676).
In a pre-clinical study, MAPK inhibition was shown to enhance T-VEC replication in murine and human melanoma cell lines. BRAFi leads to enhanced T-VEC oncolysis in BRAF-mutated melanoma lines, while MEKi increased T-VEC effectiveness in both BRAF-mutated and BRAF-wildtype cell lines [63]. Thus, combining T-VEC and BRAF/MEK inhibition might represent a potentially promising avenue to enhance T-VEC efficacy in BRAF-mutated and BRAF-wildtype melanoma and requires further pre-clinical and clinical validation.
Cytokines such as interleukin 2 (IL-2) and granulocyte-macrophage colony-stimulating factor (GM-CSF) were among the first intratumoral regimens assessed in melanoma [77,78] (Figure 1d). In the USA, systemically administered IL-2 is approved for the treatment of metastatic CM. Even though the anti-tumor efficacy of intratumoral IL-2 appears to be durable, it is limited to the injected lesions suggesting that intratumoral IL-2 does not have strong systemic effects. Unlike systemic IL-2, however, intratumoral IL-2 is generally well tolerated [79,80]. In a phase 2 study, tavokinogene telseplasmid—a synthetic plasmid encoding the cytokine IL-12—demonstrated the induction of an anti-tumor immune response and a high control rate in patients with CM. In 2017, this drug was given an orphan drug status in the USA for the management of unresectable metastatic melanoma [81]. Another type of intratumoral approach in development comprises the pattern recognition receptor (PRR) agonists, including Toll-like receptor (TLR) agonists and stimulator of interferon genes (STING) agonists. Three TLR-9 agonists, such as SD-101, IMO-2125, and CMP-001, are under investigation in combination treatments [82]. Moreover, it has been detected that cyclic dinucleotides may represent immune adjuvants by activating STING, in turn stimulating a pro-inflammatory immune response. Hence, phase 1 studies on 2 intratumoral STING agonists have been initiated [79,83]. A synergy between intratumoral agents and ICI may be expected. In fact, treatment regimens combining therapies that have different modes of action without enhancing toxicity are probable to feature in future investigations [79]. There is increasing data indicating that the combination of intratumoral agents and systemic regimens can even achieve responses in anti-PD1-refractory cancers, thereby overcoming resistance. Importantly, intratumoral strategies may be considered for use in any cancer that is injectable [79].
5. Potential of Anti-Angiogenic Immunotherapy in CM Treatment
The role of tumor angiogenesis in cancer has been known for nearly 50 years [84,85]. Under homeostatic conditions, blood vessels are considered highly organized, specialized systems with organ-specific functions [86]. Disruption of pro- and anti-angiogenic balance in tumor angiogenesis can lead to unorganized and permeable vessels lacking proper barrier function. This, in turn, can increase the spread and risk of metastasis formation, which has detrimental effects on patient survival outcomes. Abnormal angiogenesis is at the core of melanoma growth and metastasis formation, with melanoma carcinogenesis being highly dependent on the recruitment of blood vessels from the periphery by promoting angiogenesis through the release of vascular endothelial growth factor (VEGF) [87,88].
In an advanced setting, melanoma cells are capable of hijacking angiogenic programs, which can promote metastatic dissemination through lymphatic and hematogenous routes [89,90,91]. Still, anti-angiogenic monotherapy, although improving disease-free interval (DFI), did not prove to have durable and substantial anti-tumor activity in CM [92]. Additionally, VEGF decreases during aging leading to a poorer response in older compared to young patients treated with anti-VEGF inhibitors such as bevacizumab [93]. VEGF is reported to contribute to ICI resistance in mice by reducing CTL trafficking into the TME whilst favoring Treg infiltration through the permeable endothelium [94]. Furthermore, VEGF levels are found to be higher in ICI non-responders than responders indicating an immuno-suppressive function. Moreover, it is reported that high VEGF concentrations induce inhibitory receptor expression forcing CTL exhaustion, decreasing ICI effectiveness [95,96]. Although the immunomodulatory effect of angiogenic molecules such as VEGF and angiopoietins, which can control immune trafficking through regulation of adhesion molecules, was known for the last decade, the emergence of ICI and the challenge of therapeutic resistance in a subset of patients made angiogenic inhibitors potential targets of interest to support ICI and overcome treatment resistance [97,98,99]. Moreover, the dysfunctional tumor vasculature can play a crucial role in immune evasion by cancer cells using an angiogenesis-induced endothelial immune cell barrier hampering antitumor immunity. It was thus suggested that exposure to pro-angiogenic factors leads to endothelial cell anergy, reduced upregulation of endothelial adhesion molecules, and thus decreased leukocyte adhesion, extravasation, and immune infiltration [100,101,102]. Hence, vessel normalization and immune modulation by angiogenic inhibitors exhibit synergistic effects that can potentiate cancer immunity and the anti-tumor response of ICI [103,104,105].
In recent years, the use of ICI in combination with anti-angiogenic modulators such as bevacizumab was widely studied in several solid tumors, including non-small-cell lung cancer [106], renal carcinoma [107,108], hepatocellular carcinoma [109,110], and endometrial cancer [33]. Moreover, recently a landmark phase III randomized clinical study in which patients with unresectable hepatocellular carcinoma were treated with a PD-L1 inhibitor (atezolizumab) and a VEGF inhibitor (bevacizumab) combination found a significantly improved overall and progression-free survival when compared to a group treated with the standard of care protein kinase inhibitor, sorafenib [34]. Combinations of ICI with systemic and local treatments for HCC are already under evaluation in large-scale clinical trials, and atezolizumab/bevacizumab combinations are predicted to soon become the standard of care as first-line therapy in HCC [111].
In CM, pre-clinical studies have previously suggested that angiopoietin-2 and VEGFA inhibition by a bispecific antibody can elicit antitumor immunity and enhance PD-1 blockade in a tumor transplant mouse model of melanoma [112]. Remarkably, in BRAF-mutated melanoma, VEGF blockade was even suggested to benefit long-lasting tumor responses, delay onset of BRAFi resistance, and induce macrophage infiltration [113]. Although adjuvant bevacizumab treatment was demonstrated to not significantly affect 5-year disease-free survival in resected melanoma with a high-risk of recurrence, several studies are currently evaluating the safety and efficacy of ICI/bevacizumab combinations [92]. A phase I trial in advanced melanoma patients has shown VEGF-A blockade with ipilimumab administration to be safe with increased CD8 T cell and macrophage infiltration in tumor biopsies as well as improved survival with a median of 25.1 months [113,114]. Two phase II studies evaluating ipilimumab monotherapy vs. ipilimumab/bevacizumab in unresectable stage III/IV melanoma and atezolizumab/bevacizumab in patients with locally advanced and metastatic cutaneous and mucosal melanoma are currently ongoing and recruiting (NCT01950390/NCT04091217). In addition, two phase I trials evaluating pembrolizumab plus angiopoietin-1/-2-neutralizing peptibody, AMG386, as well as tremelimumab plus anti-angiopoietin-2 antibody, MEDI3617, are recruiting and ongoing (NCT03239145/NCT02141542). Overall anti-angiogenic inhibitors have the potential to support ICI immunotherapy and help in overcoming treatment resistance in advanced melanoma patients. A summary of currently studied therapeutic combinations for advanced melanoma treatment is depicted in Table 2.
Table 2.
Combinational treatment strategies for CM.
| Biological Classification |
Drug Name | Mechanism | Main Target | Study |
|---|---|---|---|---|
| Combined Targeted Therapy | ||||
| Kinase Inhibitor | Dabrafenib + Trametinib | inhibition of BRAF-MEK pathway reactivation, decreased tumor cell survival in BRAF-mutants | BRAF mutant, MEK |
NCT01584648 NCT0159790 |
| Vermurafenib + Cobimetinib | NCT01689519 | |||
| Encorafenib + Binimetinib | COMBO450 | |||
| ICI + Combined Targeted Therapy | ||||
| Monoclonal Antibody + Kinase Inhibitor | Pembrolizumab + Dabrafenib + Trametinib | PD1 checkpoint blockade, decreased tumor cell survival in BRAF-mutants | PD1, RAF, MEK | NCT02130466 |
| Atezolizumab + Vermurafenib + Cobimetinib | PDL1 checkpoint blockade, decreased tumor cell survival in BRAF-mutants | PDL1, RAF, MEK |
NCT02908672 IMspire150 |
|
| Intratumoral Stimulation | ||||
| Cytokine | GM-CSF | stimulation of tumor immune response by GM-CSF | APCs, TILs | E4697 |
| IL-2 | stimulation of tumor immune response by IL2 | TILs, NK cells |
NCT01672450 NCT00204581 |
|
| Oncolytic Virus | Talimogene laherparepvec (T-VEC) | oncolysis, activation of anti-tumor immune response through IFN signalling | tumor cells, innate and adaptive Immunity | NCT00769704 |
| ONCOS-102 | oncolysis, immune activation by GM-CSF | tumor cells, innate and adaptive Immunity | NCT03003676 | |
| Plasmid | Tavokinogene telseplasmid | stimulating diffentiation, activation of the adaptive immune system | APCs, TILs | NCT01502293 |
| TLR9 agonists | SD-101; IMO-2125; CMP-001; CPG 7909 | induction of CD8 T cell response enhancing uptake, destruction of cancer cells | TLR9 in APCs, TILs |
NCT02521870 NCT02644967 NCT02680184 |
| STING agonists | ADU-S100, MK-1454 | anti-tumor response through type I IFN signalling activation | STING in tumor cells, TIL, APCs |
NCT02675439 NCT03172936 NCT03010176 |
| Immune Checkpoint Inhibition + Intratumoral Stimulation | ||||
| Oncolytic Virus + Monoclonal Antibody | T-VEC + Ipilimumab | immunological priming of TME to enhance PD-1 blockade efficacy | tumor cells, TILs, APCs | NCT01740297 |
| T-VEC + Pembrolizumab | MASTERKEY265 KEYNOTE034 |
|||
| ONCOS-102 + Pembrolizumab | NCT03003676 | |||
| Intratumoral Stimulation + Targeted Therapy | ||||
| Oncolytic Virus + Kinase Inhibitor | T-VEC + Trametinib | oncolysis, adaptive immune activation, MEK inhibition to reduce tumor cell survival | tumor cells, MEK, TILs and APCs | NCT03088176 |
| ICI + Anti-Angiogenesis | ||||
| Monoclonal Antibody | Bevacizumab | vessel normalization, immunomodulation, checkpoint inhibition | VEGF | AVAST-M |
| Ipilimumab + Bevacizumab | CTLA4, VEGF | NCT01950390 | ||
| Atezolizumab + Bevacizumab | PDL1, VEGF | NCT04091217 | ||
| Monoclonal Antibody + Neutralizing Peptibody | Pembrolizumab + AMG386 | vessel normalization, immunomodulation, checkpoint inhibition | PD1, Angpt1/2 | NCT03239145 |
| Tremelimumab + MEDI3617 | CTLA4, Angpt2 | NCT02141542 | ||
6. Conclusions and Future Perspective
The development of patient-centered approaches for targeted treatment will be one of the main challenges in CM care in the next decade. Combining therapeutic options offers the unique opportunity to tailor treatment to patient- and cancer-specific conditions utilizing the synergistic effects of existing therapeutic approaches (Figure 1e). Combinations of several systemic treatment options such as ICI, BRAFi/MEKi, and angiogenic inhibitors for metastatic melanoma, as well as intratumoral and systemic immunotherapeutic applications to prime and induce immuno-sensitivity of locally advanced CM show promising results and potential to overcome acquired and endogenous treatment resistance. Nevertheless, limitations for increasingly complex treatment combinations remain. Patients treated with ICI can exhibit a range of non-cutaneous and cutaneous irAEs, which range in severity and can have detrimental effects on a patient’s quality of life and well-being, limiting subsequent treatment options [36]. Combining two or more therapeutic approaches, although potentially leading to improved efficacy, can increase the incidence and severity of irAEs. As discussed in previous sections, there is a range of potentially life-threatening irAEs affecting a multitude of organs, but there are also less severe cutaneous irAEs substantially impacting patient’s quality of life. Cutaneous irAEs are the most common adverse events occurring in up to 50% of patients undergoing ICI resemble autoimmune disorders mostly presenting as primary dermatoses [115,116,117]. Future investigations will have to address emerging non-cutaneous and cutaneous AEs as well as potential augmentation of treatment interventions combining different modes of action whilst minimizing toxicity. In addition, we would like to emphasize that the survival of patients with CM is significantly improved when the tumor is detected at a very early stage—a time when simple surgery is fully sufficient to cure the patient’s CM. Hence, we also want to refer to emerging novel techniques under current investigation, which might significantly improve the accuracy of early CM diagnosis. A variety of innovative optical and acoustic technology-based techniques, such as confocal laser-scanning microscopy, optical coherence tomography (OCT), photoacoustic/ultrasound/OCT, multiphoton excited fluorescence imaging, and stepwise two-photon excited fluorescence, have been developed to increase the diagnostic accuracy for the non-invasive melanoma diagnosis [118,119,120,121,122,123,124]. Advancing diagnostic tools and biomarkers to identify subgroup beneficiaries of specific treatment combinations prior to therapy can thus become the next step in patient-centered personalized CM treatment.
Abbreviations
AE: Adverse events; irAE: Immune-related adverse events; Blimp-1: B lymphocyte-induced maturation protein 1; BRAFi: BRAF inhibition; CM: Cutaneous melanoma; CTLA4: Cytotoxic T-Lymphocyte Associated Protein 4; CTL: Cytotoxic T-Lymphocyte; GM-CSF: Granulocyte-macrophage colony-stimulating factor; ICI: Immune checkpoint blockade; ITIM: Immunoreceptor tyrosine-based inhibitory motif; KLRG-1: Killer cell lectin-like receptor sub family G; LAG3: Lymphocyte-activation protein 3; MAPK: Mitogen-activated protein kinase; MCC: Merkel cell carcinoma; MEKi: MEK inhibiton; NSCLC: Non-small-cell lung cancer; OVT: Oncolytic viral therapy; OS: Overall survival; ORR: Overall response rate; PD-L1: Programmed cell death ligand 1; PFS: Progression-free-survival; PKA: Protein kinase A; PRR: Pattern recognition receptor; RFS: Relapse-free survival; T-VEC: Talimogene laherparepvec; TCR: T-cell receptor; TIGIT: T cell immunoreceptor with Ig and tyrosine-based inhibitory motif (ITIM) domains; TIM3: T cell immunoglobulin domain and mucin domain-containing protein3; TLR: Toll-like receptor; TME: Tumor microenvironment; Treg: Regulatory T; UV: Ultraviolet; VEGF: Vascular endothelial growth factor.
Author Contributions
L.S. and T.G. designed the review. D.K. drafted the manuscript. M.S., T.G. and D.K. helped to modify the manuscript. L.S. and D.K. provided the figures and tables. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study conforms to the applicable local requirements regarding ethical and investigational committee review (ethics review board code #4749-13), informed consent, and other statutes or regulations regarding the protection of the rights and welfare of human subjects participating in medical research.
Informed Consent Statement
Informed consent was obtained from all subjects for publication of the figures.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.
Conflicts of Interest
L.S. has received speakers and/or advisory board honoraria from BMS, Sun-Pharma, MSD, and Novartis. T.G. has received speakers and/or advisory board honoraria from BMS, Sanofi-Genzyme, MSD, Novartis Pharma, Roche, Abbvie, Almirall, Janssen, Lilly, Pfizer, Pierre Fabre, Merck-Serono, outside the submitted work. D.K. and M.S. have no conflict of interest to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paulson K.G., Gupta D., Kim T.S., Veatch J.R., Byrd D.R., Bhatia S., Wojcik K., Chapuis A.G., Thompson J.A., Madeleine M.M., et al. Age-Specific Incidence of Melanoma in the United States. JAMA Dermatol. 2020;156:57–64. doi: 10.1001/jamadermatol.2019.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apalla Z., Lallas A., Sotiriou E., Lazaridou E., Ioannides D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017;7:1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guy G.P., Thomas C.C., Thompson T., Watson M., Massetti G.M., Richardson L.C. Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. MMWR Morb. Mortal. Wkly. Rep. 2015;64:591. [PMC free article] [PubMed] [Google Scholar]
- 4.Skin Cancer Foundation Skin Cancer Facts and Statistics. Skin Cancer Information. Last Updated: January 13, 2021. [(accessed on 25 April 2021)]; Available online: https://www.skincancer.org/skin-cancer-information/skin-cancer-facts/
- 5.Seite S., Del Marmol V., Moyal D., Friedman A. Public primary and secondary skin cancer prevention, perceptions and knowledge: An international cross-sectional survey. J. Eur. Acad. Dermatol. Venereol. 2017;31:815–820. doi: 10.1111/jdv.14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Pineros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 7.Dunn G.P., Old L.J., Schreiber R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 9.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei S.C., Duffy C.R., Allison J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 11.Sun C., Mezzadra R., Schumacher T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchbinder E.I., Desai A. CTLA-4 and PD-1 Pathways. Am. J. Clin. Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esensten J.H., Helou Y.A., Chopra G., Weiss A., Bluestone J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity. 2016;44:973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beyersdorf N., Kerkau T., Hünig T. CD28 co-stimulation in T-cell homeostasis: A recent perspective Introduction: T-cell responses and the role of CD28 co-stimulation. Immuno Targets Ther. 2015;4:111–122. doi: 10.2147/ITT.S61647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fife B.T., Bluestone J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 16.Qin S., Xu L., Yi M., Yu S., Wu K., Luo S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer. 2019;18:155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tundo G.R., Sbardella D., Lacal P.M., Graziani G., Marini S. On the Horizon: Targeting Next-Generation Immune Checkpoints for Cancer Treatment. Chemotherapy. 2019;64:62–80. doi: 10.1159/000500902. [DOI] [PubMed] [Google Scholar]
- 18.De Sousa Linhares A., Leitner J., Grabmeier-Pfistershammer K., Steinberger P. Not All Immune Checkpoints Are Created Equal. Front. Immunol. 2018;9:1909. doi: 10.3389/fimmu.2018.01909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozar I., Margue C., Rothengatter S., Haan C., Kreis S. Many ways to resistance: How melanoma cells evade targeted therapies. Biochim. Biophys. Acta Rev. Cancer. 2019;1871:313–322. doi: 10.1016/j.bbcan.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Kim T., Amaria R.N., Spencer C., Reuben A., Cooper Z.A., Wargo J.A. Combining targeted therapy and immune checkpoint in-hibitors in the treatment of metastatic melanoma. Cancer Biol. Med. 2014;11:237. doi: 10.7497/j.issn.2095-3941.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prieto P.A., Reuben A., Cooper Z.A., Wargo J.A. Targeted Therapies Combined with Immune Checkpoint Therapy. Cancer J. 2016;22:138–146. doi: 10.1097/PPO.0000000000000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulson K.G., Lahman M.C., Chapuis A.G., Brownell I. Immunotherapy for skin cancer. Int. Immunol. 2019;31:465–475. doi: 10.1093/intimm/dxz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodi F.S., O’Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., Gonzalez R., Robert C., Schadendorf D., Hassel J.C., et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott D., Haanen J., Chen T.-T., Lorigan P., O’Day S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20) Ann. Oncol. 2013;24:2694–2698. doi: 10.1093/annonc/mdt291. [DOI] [PubMed] [Google Scholar]
- 25.Naing A., Hajjar J. Immunotherapy. Springer; Cham, Switzerland: 2018. [Google Scholar]
- 26.Ralli M., Botticelli A., Visconti I.C., Angeletti D., Fiore M., Marchetti P., Lambiase A., De Vincentiis M., Greco A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020;2020:1–12. doi: 10.1155/2020/9235638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuryk L., Bertinato L., Staniszewska M., Pancer K., Wieczorek M., Salmaso S., Caliceti P., Garofalo M. From Conventional Therapies to Immuno-therapy: Melanoma Treatment in Review. Cancers. 2020;12:3057. doi: 10.3390/cancers12103057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barrios D.M., Do M.H., Phillips G.S., Postow M.A., Akaike T., Nghiem P., Lacouture M.E. Immune checkpoint inhibitors to treat cutaneous malignancies. J. Am. Acad. Dermatol. 2020;83:1239–1253. doi: 10.1016/j.jaad.2020.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonardi G.C., Candido S., Falzone L., Spandidos D.A., Libra M. Cutaneous melanoma and the immunotherapy revolution (Review) Int. J. Oncol. 2020;57:609–618. doi: 10.3892/ijo.2020.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaremba A., Zimmer L., Griewank K.G., Ugurel S., Roesch A., Schadendorf D., Livingstone E. Immunotherapy for malignant melanoma. Internist. 2020;61:669–675. doi: 10.1007/s00108-020-00812-1. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J.V., Buchbinder E.I. The Evolution of Adjuvant Therapy for Melanoma. Curr. Oncol. Rep. 2019;21:1–7. doi: 10.1007/s11912-019-0858-3. [DOI] [PubMed] [Google Scholar]
- 32.Kelly Z.R., Gorantla V.C., Davar D. The Role of Neoadjuvant Therapy in Melanoma. Curr. Oncol. Rep. 2020;22:1–11. doi: 10.1007/s11912-020-00944-5. [DOI] [PubMed] [Google Scholar]
- 33.Makker V., Rasco D., Vogelzang N., Brose M., Cohn A., Mier J., DiSimone C., Hyman D., Stepan D., Dutcus C., et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: Final analysis of a multicentre, open-label, single-arm, phase 2 trial. Gynecol. Oncol. 2020;159:6–7. doi: 10.1016/j.ygyno.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.-Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocel-lular Carcinoma. N. Engl. J. Med. 2020;380:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 35.Gutzmer R., Vordermark D., Hassel J.C., Krex D., Wendl C., Schadendorf D., Sickmann T., Rieken S., Pukrop T., Höller C., et al. Melanoma brain metastases—Interdisciplinary management recommendations 2020. Cancer Treat. Rev. 2020;89:102083. doi: 10.1016/j.ctrv.2020.102083. [DOI] [PubMed] [Google Scholar]
- 36.Michot J., Bigenwald C., Champiat S., Collins M., Carbonnel F., Postel-Vinay S., Berdelou A., Varga A., Bahleda R., Hollebecque A., et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Imbert C., Montfort A., Fraisse M., Marcheteau E., Gilhodes J., Martin E., Bertrand F., Marcellin M., Burlet-Schiltz O., Peredo A.G., et al. Resistance of melanoma to immune checkpoint inhibitors is overcome by targeting the sphingosine kinase-1. Nat. Commun. 2020;11:437. doi: 10.1038/s41467-019-14218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Q., Lei Y., Li X., Guo F., Liu M. A highlight of the Mechanisms of Immune Checkpoint Blocker Resistance. Front. Cell Dev. Biol. 2020;8:580140. doi: 10.3389/fcell.2020.580140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lecocq Q., Keyaerts M., Devoogdt N., Breckpot K. The Next-Generation Immune Checkpoint LAG-3 and Its Therapeutic Potential in Oncology: Third Time’s a Charm. Int. J. Mol. Sci. 2020;22:75. doi: 10.3390/ijms22010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villani A., Fabbrocini G., Costa C., Annunziata M.C., Scalvenzi M. Merkel Cell Carcinoma: Therapeutic Update and Emerging Therapies. Dermatol. Ther. 2019;9:209–222. doi: 10.1007/s13555-019-0288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowey C.L. Targeted Therapy for Advanced Basal-Cell Carcinoma: Vismodegib and Beyond. Dermatol. Ther. 2013;3:17–31. doi: 10.1007/s13555-013-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogata D., Namikawa K., Otsuka M., Asai J., Kato H., Yasuda M., Maekawa T., Fujimura T., Kato J., Takenouchi T., et al. Systemic treatment of patients with advanced cutaneous squamous cell carcinoma: Response rates and outcomes of the regimes used. Eur. J. Cancer. 2020;127:108–117. doi: 10.1016/j.ejca.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Stein J.E., Brothers P., Applebaum K., Gaskin A., Wang H., Taube J.M., Lipson E.J. A phase 2 study of nivolumab (NIVO) alone or plus ipilimumab (IPI) for patients with locally advanced unresectable (laBCC) or metastatic basal cell carcinoma (mBCC) J. Clin. Oncol. 2019;37:TPS9595. doi: 10.1200/JCO.2019.37.15_suppl.TPS9595. [DOI] [Google Scholar]
- 45.Epstein E.H. Basal cell carcinomas: Attack of the hedgehog. Nat. Rev. Cancer. 2008;8:743–754. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mason R., Au L., Garces A.I., Larkin J. Current and emerging systemic therapies for cutaneous metastatic melanoma. Expert Opin. Pharmacother. 2019;20:1135–1152. doi: 10.1080/14656566.2019.1601700. [DOI] [PubMed] [Google Scholar]
- 47.Savoia P., Fava P., Casoni F., Cremona O. Targeting the ERK Signaling Pathway in Melanoma. Int. J. Mol. Sci. 2019;20:1483. doi: 10.3390/ijms20061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S., Rauch J., Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020;21:1102. doi: 10.3390/ijms21031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayward N.K., Wilmott J.S., Waddell N., Johansson P.A., Field M.A., Nones K., Patch A.M., Kakavand H., Alexandrov L.B., Burke H., et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–180. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 50.Menzies A.M., Haydu L.E., Visintin L., Carlino M.S., Howle J.R., Thompson J.F., Kefford R.F., Scolyer R.A., Long G.V. Distinguishing Clinicopathologic Features of Patients with V600E and V600K BRAF-Mutant Metastatic Melanoma. Clin. Cancer Res. 2012;18:3242–3249. doi: 10.1158/1078-0432.CCR-12-0052. [DOI] [PubMed] [Google Scholar]
- 51.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 52.Zhang T., Dutton-Regester K., Brown K.M., Hayward N.K. The genomic landscape of cutaneous melanoma. Pigment. Cell Melanoma Res. 2016;29:266–283. doi: 10.1111/pcmr.12459. [DOI] [PubMed] [Google Scholar]
- 53.Hauschild A., Grob J.J., Demidov L.V., Jouary T., Gutzmer R., Millward M., Rutkowski P., Blank C.U., Miller W.H., Jr., Kaempgen E., et al. Dabrafenib in BRAF-mutated metastatic mela-noma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 54.Trunzer K., Pavlick A.C., Schuchter L., Gonzalez R., McArthur G.A., Hutson T.E., Moschos S.J., Flaherty K.T., Kim K.B., Weber J.S., et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J. Clin. Oncol. 2013;31:1767–1774. doi: 10.1200/JCO.2012.44.7888. [DOI] [PubMed] [Google Scholar]
- 55.Bradley S.D., Chen Z., Melendez B., Talukder A., Khalili J.S., Rodriguez-Cruz T., Liu S., Whittington M., Deng W., Li F., et al. BRAFV600E Co-opts a Conserved MHC Class I Internalization Pathway to Diminish Antigen Presentation and CD8+ T-cell Recognition of Melanoma. Cancer Immunol. Res. 2015;3:602–609. doi: 10.1158/2326-6066.CIR-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mandalà M., De Logu F., Merelli B., Nassini R., Massi D. Immunomodulating property of MAPK inhibitors: From translational knowledge to clinical implementation. Lab. Investig. 2016;97:166–175. doi: 10.1038/labinvest.2016.132. [DOI] [PubMed] [Google Scholar]
- 57.Frederick D.T., Piris A., Cogdill A.P., Cooper Z.A., Lezcano C., Ferrone C.R., Mitra D., Boni A., Newton L.P., Liu C., et al. BRAF Inhibition Is Associated with Enhanced Melanoma Antigen Expression and a More Favorable Tumor Microenvironment in Patients with Metastatic Melanoma. Clin. Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ribas A., Lawrence D., Atkinson V., Agarwal S., Miller W.H., Carlino M.S., Fisher R., Long G.V., Hodi F.S., Tsoi J., et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat. Med. 2019;25:936–940. doi: 10.1038/s41591-019-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ascierto P.A., Ferrucci P.F., Fisher R., Del Vecchio M., Atkinson V., Schmidt H., Schachter J., Queirolo P., Long G.V., Di Giacomo A.M., et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat. Med. 2019;25:941–946. doi: 10.1038/s41591-019-0448-9. [DOI] [PubMed] [Google Scholar]
- 60.Gutzmer R., Stroyakovskiy D., Gogas H., Robert C., Lewis K., Protsenko S., Pereira R.P., Eigentler T., Rutkowski P., Demidov L., et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835–1844. doi: 10.1016/S0140-6736(20)30934-X. [DOI] [PubMed] [Google Scholar]
- 61.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E., et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017;170:1109–1119. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russell S.J., Peng K.-W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bommareddy P.K., Aspromonte S., Zloza A., Rabkin S.D., Kaufman H.L. MEK inhibition enhances oncolytic virus immunother-apy through increased tumor cell killing and T cell activation. Sci. Transl. Med. 2018;10:eaau0417. doi: 10.1126/scitranslmed.aau0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bommareddy P.K., Patel A., Hossain S., Kaufman H.L. Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma. Am. J. Clin. Dermatol. 2017;18:1–15. doi: 10.1007/s40257-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kohlhapp F.J., Kaufman H.L. Molecular Pathways: Mechanism of Action for Talimogene Laherparepvec, a New Oncolytic Virus Immunotherapy. Clin. Cancer Res. 2016;22:1048–1054. doi: 10.1158/1078-0432.CCR-15-2667. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen M.H., Leong S.P., Abendroth R., Kashani-Sabet M., Kim K.B. Complete clinical response to intralesional talimogene laherparepvec injection in a patient with recurrent, regionally advanced Merkel cell carcinoma. JAAD Case Rep. 2019;5:849–851. doi: 10.1016/j.jdcr.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westbrook B.C., Norwood T.G., Terry N.L.J., McKee S.B., Conry R.M. Talimogene laherparepvec induces durable response of re-gionally advanced Merkel cell carcinoma in 4 consecutive patients. JAAD Case Rep. 2019;5:782–786. doi: 10.1016/j.jdcr.2019.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lara K.M., In G.K., Matcuk G.R., Mehta A., Hu J.S. Talimogene laherparepvec in combination with pembrolizumab leads to a complete response in a patient with refractory Merkel cell carcinoma. JAAD Case Rep. 2018;4:1004–1006. doi: 10.1016/j.jdcr.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knackstedt R., Sussman T., McCahon L., Song J.-M., Funchain P., Gastman B. Pre-treated anti-PD-1 refractory Merkel cell carcinoma successfully treated with the combination of PD-1/PD-L1 axis inhibitors and TVEC: A report of two cases. Ann. Oncol. 2019;30:1399–1400. doi: 10.1093/annonc/mdz187. [DOI] [PubMed] [Google Scholar]
- 70.Perez M.C., Zager J.S., Amatruda T., Conry R., Ariyan C., Desai A., Kirkwood J.M., Treichel S., Cohan D., Raskin L. Observational study of talimogene laherparepvec use for melanoma in clinical practice in the United States (COSMUS-1) Melanoma Manag. 2019;6:MMT19. doi: 10.2217/mmt-2019-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mohr P., Haferkamp S., Pinter A., Weishaupt C., Huber M.A., Downey G., Öhrling K., Loquai C., Louie K.S. Real-World Use of Talimogene Laherparepvec in German Patients with Stage IIIB to IVM1a Melanoma: A Retrospective Chart Review and Physician Survey. Adv. Ther. 2019;36:101–117. doi: 10.1007/s12325-018-0850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andtbacka R.H.I., Collichio F., Harrington K.J., Middleton M.R., Downey G., Öhrling K., Kaufman H.L. Final analyses of OPTiM: A ran-domized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresec-table stage III-IV melanoma. J. Immunother. Cancer. 2019;7:1–11. doi: 10.1186/s40425-019-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Louie R.J., Perez M.C., Jajja M.R., Sun J., Collichio F., Delman K.A., Lowe M., Sarnaik A.A., Zager J.S., Ollila D.W. Real-World Outcomes of Talimogene Laherparepvec Therapy: A Multi-Institutional Experience. J. Am. Coll. Surg. 2019;228:644–649. doi: 10.1016/j.jamcollsurg.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 74.LaRocca C.A., Leboeuf N.R., Silk A.W., Kaufman H.L. An Update on the Role of Talimogene Laherparepvec (T-VEC) in the Treatment of Melanoma: Best Practices and Future Directions. Am. J. Clin. Dermatol. 2020;21:821–832. doi: 10.1007/s40257-020-00554-8. [DOI] [PubMed] [Google Scholar]
- 75.Chesney J., Puzanov I., Collichio F., Milhem M.M., Hauschild A., Chen L., Sharma A., Garbe C., Singh P., Mehnert J.M. Patterns of response with talimogene laherparepvec in combination with ipilimumab or ipilimumab alone in metastatic unresectable melanoma. Br. J. Cancer. 2019;121:417–420. doi: 10.1038/s41416-019-0530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuryk L., Møller A.S.W., Jaderberg M. Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pem-brolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. Oncoimmunology. 2019;8:e1532763. doi: 10.1080/2162402X.2018.1532763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Byers B.A., Temple-Oberle C.F., Hurdle V., McKinnon J.G. Treatment of in-transit melanoma with intra-lesional interleukin-2: A systematic review. J. Surg. Oncol. 2014;110:770–775. doi: 10.1002/jso.23702. [DOI] [PubMed] [Google Scholar]
- 78.Boyd K.U., Wehrli B.M., Temple C.L. Intra-lesional interleukin-2 for the treatment of in-transit melanoma. J. Surg. Oncol. 2011;104:711–717. doi: 10.1002/jso.21968. [DOI] [PubMed] [Google Scholar]
- 79.Middleton M.R., Hoeller C., Michielin O., Robert C., Caramella C., Öhrling K., Hauschild A. Intratumoural immunotherapies for unresectable and metastatic melanoma: Current status and future perspectives. Br. J. Cancer. 2020;123:885–897. doi: 10.1038/s41416-020-0994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weide B., Derhovanessian E., Pflugfelder A., Eigentler T.K., Radny P., Zelba H., Pföhler C., Pawelec G., Garbe C. High response rate after intratumoral treatment with interleukin-2: Results from a phase 2 study in 51 patients with metastasized melanoma. Cancer. 2010;116:4139–4146. doi: 10.1002/cncr.25156. [DOI] [PubMed] [Google Scholar]
- 81.Canton D.A., Shirley S., Wright J., Connolly R., Burkart C., Mukhopadhyay A., Twitty C., Qattan K.E., Campbell J.S., Le M.H., et al. Melanoma treatment with intratumoral electroporation of tavokinogene telseplasmid (pIL-12, tavokinogene telseplasmid) Immunotherapy. 2017;9:1309–1321. doi: 10.2217/imt-2017-0096. [DOI] [PubMed] [Google Scholar]
- 82.Melisi D., Frizziero M., Tamburrino A., Zanotto M., Carbone C., Piro G., Tortora G. Toll-Like Receptor 9 Agonists for Cancer Therapy. Biomedicines. 2014;2:211–228. doi: 10.3390/biomedicines2030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fu J., Kanne D.B., Leong M., Glickman L.H., McWhirter S.M., Lemmens E., Mechette K., Leong J.J., Lauer P., Liu W., et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 2015;7:283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sherwood L.M., Parris E.E., Folkman J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 85.Folkman J. What Is the Evidence That Tumors Are Angiogenesis Dependent? J. Natl. Cancer Inst. 1990;82:4–7. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 86.Augustin H.G., Koh G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science. 2017;357:eaal2379. doi: 10.1126/science.aal2379. [DOI] [PubMed] [Google Scholar]
- 87.Arbiser J.L. Angiogenesis and the skin: A primer. J. Am. Acad. Dermatol. 1996;34:486–497. doi: 10.1016/S0190-9622(96)90444-2. [DOI] [PubMed] [Google Scholar]
- 88.Streit M., Detmar M. Angiogenesis, lymphangiogenesis, and melanoma metastasis. Oncogene. 2003;22:3172–3179. doi: 10.1038/sj.onc.1206457. [DOI] [PubMed] [Google Scholar]
- 89.Wong S.Y., Hynes R.O. Lymphatic or hematogenous dissemination: How does a metastatic tumor cell decide? Cell Cycle. 2006;5:812–817. doi: 10.4161/cc.5.8.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lacal P.M., Failla C.M., Pagani E., Odorisio T., Schietroma C., Falcinelli S., Zambruno G., D’Atri S. Human Melanoma Cells Secrete and Respond to Placenta Growth Factor and Vascular Endothelial Growth Factor. J. Investig. Dermatol. 2000;115:1000–1007. doi: 10.1046/j.1523-1747.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- 91.Cho W.C., Jour G., Aung P.P. Role of angiogenesis in melanoma progression: Update on key angiogenic mechanisms and other associated components. Semin. Cancer Biol. 2019;59:175–186. doi: 10.1016/j.semcancer.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 92.Corrie P.G., Marshall A., Nathan P.D., Lorigan P., Gore M., Tahir S., Faust G., Kelly C.G., Marples M., Danson S.J., et al. Adjuvant bevacizumab for melanoma patients at high risk of recurrence: Survival analysis of the AVAST-M trial. Ann. Oncol. 2018;29:1843–1852. doi: 10.1093/annonc/mdy229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fane M.E., Ecker B.L., Kaur A., Marino G.E., Alicea G.M., Douglass S.M., Chhabra Y., Webster M.R., Marshall A., Colling R., et al. sFRP2 Supersedes VEGF as an Age-related Driver of Angiogenesis in Melanoma, Affecting Response to Anti-VEGF Therapy in Older Patients. Clin. Cancer Res. 2020;26:5709–5719. doi: 10.1158/1078-0432.CCR-20-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Motz G.T., Santoro S.P., Wang L.P., Garrabrant T., Lastra R.R., Hagemann I.S., Lal P., Feldman M.D., Benencia F., Coukos G. Tumor endothelium FasL establishes a selec-tive immune barrier promoting tolerance in tumors. Nat. Med. 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen P.-L., Roh W., Reuben A., Cooper Z.A., Spencer C.N., Prieto P.A., Miller J.P., Bassett R.L., Gopalakrishnan V., Wani K., et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov. 2016;6:827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fares C.M., Van Allen E.M., Drake C.G., Allison J.P., Hu-Lieskovan S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book. 2019;39:147–164. doi: 10.1200/EDBK_240837. [DOI] [PubMed] [Google Scholar]
- 97.Lee W.S., Yang H., Chon H.J., Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020;52:1475–1485. doi: 10.1038/s12276-020-00500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fukumura D., Kloepper J., Amoozgar Z., Duda D.G., Jain R.K. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat. Rev. Clin. Oncol. 2018;15:325. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yi M., Jiao D., Qin S., Chu Q., Wu K., Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer. 2019;18:1–12. doi: 10.1186/s12943-019-0974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Griffioen A., Damen C., Blijham G., Groenewegen G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood. 1996;88:667–673. doi: 10.1182/blood.V88.2.667.bloodjournal882667. [DOI] [PubMed] [Google Scholar]
- 101.Griffioen A.W., Damen C.A., Martinotti S., Blijham G.H., Groenewegen G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: The role of angiogenic factors. Cancer Res. 1996;56:1111–1117. [PubMed] [Google Scholar]
- 102.Huinen Z.R., Huijbers E.J.M., van Beijnum J.R., Nowak-Sliwinska P., Griffioen A.W. Anti-angiogenic agents—overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 2021:1–14. doi: 10.1038/s41571-021-00496-y. [DOI] [PubMed] [Google Scholar]
- 103.Pober J.S., Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hendry S.A., Farnsworth R.H., Solomon B., Achen M.G., Stacker S.A., Fox S.B. The role of the tumor vasculature in the host im-mune response: Implications for therapeutic strategies targeting the tumor microenvironment. Front. Immunol. 2016;7:261. doi: 10.3389/fimmu.2016.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fares J., Fares M.Y., Khachfe H.H., Salhab H.A., Fares Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target Ther. 2020;5:1–17. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reck M., Mok T.S.K., Nishio M., Jotte R.M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N., Rodríguez-Abreu D., Moro-Sibilot D., et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019;7:387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 107.Rini B.I., Plimack E.R., Stus V., Gafanov R., Hawkins R., Nosov D., Pouliot F., Alekseev B., Soulières D., Melichar B., et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 108.Rini B.I., Powles T., Atkins M.B., Escudier B., McDermott D.F., Suarez C., Bracarda S., Stadler W.M., Donskov F., Lee J.L., et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–2415. doi: 10.1016/S0140-6736(19)30723-8. [DOI] [PubMed] [Google Scholar]
- 109.Pishvaian M., Lee M., Ryoo B.-Y., Stein S., Lee K.-H., Verret W., Spahn J., Shao H., Liu B., Iizuka K., et al. Updated safety and clinical activity results from a phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC) Ann. Oncol. 2018;29:viii718–viii719. doi: 10.1093/annonc/mdy424.028. [DOI] [Google Scholar]
- 110.Stein S., Pishvaian M.J., Lee M.S., Lee K.-H., Hernandez S., Kwan A., Liu B., Grossman W., Iizuka K., Ryoo B.-Y. Safety and clinical activity of 1L atezolizumab + bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC) J. Clin. Oncol. 2018;36:4074. doi: 10.1200/JCO.2018.36.15_suppl.4074. [DOI] [Google Scholar]
- 111.Sangro B., Sarobe P., Hervás-Stubbs S., Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021:1–19. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schmittnaegel M., Rigamonti N., Kadioglu E., Cassará A., Rmili C.W., Kiialainen A., Kienast Y., Mueller H.J., Ooi C.H., Laoui D., et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 2017;9:385. doi: 10.1126/scitranslmed.aak9670. [DOI] [PubMed] [Google Scholar]
- 113.Wu X., Giobbie-Hurder A., Liao X., Lawrence D., Mcdermott D., Zhou J., Rodig S., Hodi F.S. VEGF Neutralization Plus CTLA-4 Blockade Alters Soluble and Cellular Factors Associated with Enhancing Lymphocyte Infiltration and Humoral Recognition in Melanoma. Cancer Immunol. Res. 2016;4:858–869. doi: 10.1158/2326-6066.CIR-16-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hodi F.S., Lawrence D., Lezcano C., Wu X., Zhou J., Sasada T., Zeng W., Giobbie-Hurder A., Atkins M.B., Ibrahim N., et al. Bevacizumab plus Ipilimumab in Patients with Metastatic Melanoma. Cancer Immunol. Res. 2014;2:632–642. doi: 10.1158/2326-6066.CIR-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tattersall I.W., Leventhal J.S. Cutaneous toxicities of immune checkpoint inhibitors: The role of the dermatologist. Yale J. Biol. Med. 2020;93:123. [PMC free article] [PubMed] [Google Scholar]
- 116.Habre M.M., Habre S.B.S., Kourie H.R. Dermatologic adverse events of checkpoint inhibitors: What an oncologist should know. Immunotherapy. 2016;8:1437–1446. doi: 10.2217/imt-2016-0074. [DOI] [PubMed] [Google Scholar]
- 117.Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. Am. J. Clin. Dermatol. 2018;19:345–361. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- 118.Gambichler T., Schmid-Wendtner M.H., Plura I., Kampilafkos P., Stücker M., Berking C., Maier T. A multicentre pilot study investigating high-definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J. Eur. Acad. Dermatol. Venereol. 2015;29:537–541. doi: 10.1111/jdv.12621. [DOI] [PubMed] [Google Scholar]
- 119.Ferrante di Ruffano L., Dinnes J., Deeks J.J., Chuchu N., Bayliss S.E., Davenport C., Takwoingi Y., Godfrey K., O’Sullivan C., Matin R.N., et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018;4:CD013189. doi: 10.1002/14651858.CD013189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shahriari N., Grant-Kels J.M., Rabinovitz H., Oliviero M., Scope A. Reflectance confocal microscopy: Diagnostic criteria of common benign and malignant neoplasms, dermoscopic and histopathologic correlates of key confocal criteria, and diagnostic algorithms. J. Am. Acad. Dermatol. 2021;84:17–31. doi: 10.1016/j.jaad.2020.05.154. [DOI] [PubMed] [Google Scholar]
- 121.Turani Z., Fatemizadeh E., Blumetti T., Daveluy S., Moraes A.F., Chen W., Mehregan D., Andersen P.E., Nasiriavanaki M. Optical Radiomic Signatures Derived from Optical Coherence Tomography Images Improve Identification of Melanoma. Cancer Res. 2019;79:2021–2030. doi: 10.1158/0008-5472.CAN-18-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kratkiewicz K., Manwar R., Rajabi-Estarabadi A., Fakhoury J., Meiliute J., Daveluy S., Mehregan D., Avanaki K.M. Photoacoustic/Ultrasound/Optical Coherence Tomography Evaluation of Melanoma Lesion and Healthy Skin in a Swine Model. Sensors. 2019;24:2815. doi: 10.3390/s19122815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hai P., Qu Y., Li Y., Zhu L., Shmuylovich L., Cornelius L.A., Wang L.V. Label-free high-throughput photoacoustic tomography of suspected circulating melanoma tumor cells in patients in vivo. J. Biomed. Opt. 2020;25:1–17. doi: 10.1117/1.JBO.25.3.036002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meng X., Chen J., Zhang Z., Li K., Li J., Yu Z., Zhang Y. Non-invasive optical methods for melanoma diagnosis. Photodiagnosis Photodyn. Ther. 2021;34:102266. doi: 10.1016/j.pdpdt.2021.102266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the published article.