Abstract
The effects of exposure to atmospheric pollution on the incidence and mortality due to COVID-19 have been studied but not for sulfur dioxide (SO2) in most studies. However, most studies failed to consider important cofounding factors in the estimation of health effects of air pollution. The objective of the study was to assess the short- and long-term effects of air pollution on the COVID-19 risk and fatality in Lombardy and Veneto. Air pollutants were studied based on monitoring station information in Lombardy and Veneto from January 2013 to May 2020. The daily number of cases and deaths of COVID-19 were collected from the reports of the Italian Ministry of Health in Italy. A generalized linear model with the generalized estimating equation method was used to evaluate the effects of short- and long-term exposure to air pollution on the COVID-19 outbreak in Lombardy and Veneto. After adjusting for other covariates, we found that short-term exposure to PM2.5 and PM10 had a tendency to increase the incidence and mortality of COVID-19 than long-term exposure, while for other air pollutants, including SO2 and NO2, long-term exposure was more significant than short-term exposure. Both short- and long-term exposure of SO2 resulted in increased health effects on COVID-19 pandemic. Our findings suggest that exposure to atmospheric pollution has a significant impact on COVID-19 pandemic and call for further researches to deeply investigate this topic.
Keywords: Atmospheric pollution, Generalized estimating equation, COVID-19 risk
1. Introduction
In December 2019, cases of atypical pneumonia caused by infection with severe acute respiratory syndrome coronavirus 2 were suddenly observed in the metropolitan city of Wuhan (China) (Li et al., 2020; Wu et al., 2020). The World Health Organization (WHO) gave coronavirus disease 2019 the acronym COVID-19. Patients with COVID-19 often experience pulmonary edema, acute respiratory stress syndrome and respiratory failure, or complications with other serious disorders, including organ failure, septic shock, and cardiovascular dysfunction, which are fatal in many cases and require intensive care unit (ICU) support in some cases (Chen et al., 2020).
The first country in Europe to be affected by the COVID-19 pandemic was Italy, followed by other European countries and United States, with outbreaks even larger than that originally observed in China (Fanelli and Piazza, 2020; Remuzzi and Remuzzi, 2020). The WHO announced the COVID-19 pandemic, reporting 4.8 million cases and more than 318,527 victims worldwide (as of May 19, 2020). Notably, the fatality rate of COVID-19-positive cases is the highest in the world (up to 14.2%). Although national-level Italian data were published, compared with regional-level data, such data enabled a weaker interpretation of the Italian COVID-19 outbreak.
The Lombardy region is home to a sixth of the Italian population (10.08 million inhabitants) and accounts for 37.1% of cases and 49.2% of deaths in the country as of May 19, 2020. The COVID-19 outbreak started in Italy with two cases: one in Codogno, Lombardy (Grasselli et al., 2020) and one in Vo Euganeo, Veneto. On February 25, 2020, Lombardy and Veneto had 240 and 43 confirmed cases, respectively, which increased to 80,089 and 18,373, respectively, on May 19. The difference was more than 61,676 cases between these two regions and how this progressive difference increase relates to the two regions was unclear. Among multiple factors, pandemic control strategies implemented by governors were provided to explain the geographical differences (Odone et al., 2020). However, variation in the occurrence of infection and associated fatality cannot be fully explained by strict containment actions, stay-at-home orders, or social distancing.
Lombardy and Veneto are two neighboring regions in northern Italy (Fig. 1 ), which is the first part of Italy to become industrialized and is the most developed and productive area of the country, with one of the highest gross domestic products per capita in Europe. Because of high industrialization and a lack of wind due from being located between mountain ranges, air pollution remains a severe problem in Northern Italy. Even if smog levels have decreased drastically since the 1970s and 1980s, Northern Italy is one of the most polluted regions of the world, possibly the worst in Europe in terms of smog and air pollution due to extensive human activities and its terrain, leading to the stagnation of pollutants (Fattore et al., 2011).
Fig. 1.
Locations of lombardy and veneto in Italy.
The WHO has clearly defined air pollution as a major public health risk factor, resulting in a 7% increase in overall mortality per 10 μg/m3 increase in annual average of PM₂.₅ level since 2015 (World Health Organization Media Centre, 2014). It has also been emphasized that the health authorities need to conduct medical reading of environmental monitoring data at the national, regional or local level (Iriti et al., 2020). An association between short-term exposure to air pollution and the COVID-19 outbreak in China was described (Zhu et al., 2020). The contribution of long-term exposure to atmospheric pollution to the increased incidence of and mortality due to COVID-19 was suggested for the recent outbreak in 71 Italy provinces (Fattorini and Regoli, 2020). However, researchers of these studies failed to consider important cofounding factors that are different in these provinces.
In the current study, we analyzed the contribution of different air pollutants, namely nitrogen dioxide (NO2), particulate matter (PM2.5 and PM10), ozone (O3), and sulfur dioxide (SO2), to the incidence, mortality, and fatality rates of COVID-19 in the Lombardy and Veneto regions, both short- and long-term, for following up to 8 years.
2. Materials and methods
2.1. Air pollution exposure
We collected the hourly or daily monitoring data of ambient air pollutants (i.e., NO2, O3, PM2.5, PM10, and SO2) in Lombardy and Veneto in Italy from January 2013 to May 2020 from the air quality database of the European Environment Agency (EEA) (https://discomap.eea.europa.eu/map/fme/AirQualityExport.htm), as reported by member states. In this study, we calculated the short- and long-term exposure to air pollutants (i.e., STAP and LTAP) in Lombardy and Veneto to estimate the acute and chronic contributions of air pollution to the COVID-19 pandemic. The daily average concentration of air pollutants in different regions in an individual day was calculated as STAP in the corresponding location and day. For LTAP, it was defined as the mean of air pollution measurements in the individual region in the coming 8 years. The monitoring data were validated, and the measurements below detection limits were replaced using 0.5 times the detection limit.
2.2. Daily information on the COVID-19 pandemic
The daily number of cases of and deaths due to COVID-19 in Lombardy and Veneto from February 21 to May 19 in 2020 were collected from the daily reports of the Italian Ministry of Health (http://www.salute.gov.it/). We used the fatality rate (i.e., the number of deaths divided by the number of cases in the same day) to determine the severity of the COVID-19 pandemic. Furthermore, the government of Italy announced a decree implementing the quarantine of residents in two provinces (Lodi and Padua in Lombardy and Veneto, respectively) of northern Italy from February 22, 2020. Lockdown implementation was extended to the entire country on March 9, 2020, and it may have influenced the course of the COVID-19 outbreak in Italy. Therefore, we further adjusted for the effect of lockdown in models used in this study.
2.3. Statistical analysis
The generalized estimating equation (GEE) method has been widely used for longitudinal data analysis in clinical trials or epidemiological studies (Bailey et al., 2002; Neilan et al., 2017; Stawinska-Witoszynska et al., 2016; Yan et al., 2019). In the study, we used a generalized linear model with the GEE method for the count data of COVID-19 with log link function to evaluate the effects of short- and long-term exposure to air pollution on the course of the COVID-19 outbreak in Lombardy and Veneto. In the models, the dependent variable of interest was the daily cases or deaths due to COVID-19 in Lombardy and Veneto. The independent variables included LTAP and STAP data and lockdown information (LOCK).
In addition, to further evaluate the robust effects of air pollution exposure on the COVID-19 pandemic, we conducted a sensitivity analysis through adjusting exposure to the rest of the air pollutants (i.e., adjustment based on a single pollutant having the highest correlation with the air pollutant of interest and multiple pollutants). The models are expressed as follows:
| (1) |
| (2) |
| (3) |
where is the daily case or death number in the th region on theth day. and are the short- and long-term air pollution exposure, respectively. and are the single pollutants correlated largely with the pollutant of interest and all of the remaining air pollutants, respectively. is a dummy variable for lockdown implementation (equal to 1). , , , , and are the estimated regression parameters. is the residual error. In our models, we assumed that the case and death numbers were monitored daily and highly correlated based on the process of infectious diseases. Therefore, we chose the autoregressive matrix (AR(1)) as the working correlation matrix in models. The aforementioned analysis was implemented using the PROC GENMOD procedure in SAS 9.4 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Summary statistics of the COVID-19 pandemic
The descriptive statistics related to COVID-19 pandemic in Lombardy and Veneto are shown in Table 1 . The population of Lombardy (10.06 million persons) is approximately 2.05 times that of Veneto (4.91 million persons). Furthermore, the population density of Lombardy (421.93 persons per km2) is 1.58 times that of Veneto (267.42 persons per km2). In terms of the COVID-19 pandemic, the total numbers of COVID-19 cases and deaths in Lombardy (85,800 and 15,700 persons, respectively) were 4.51 and 8.55 times those in Veneto (19,000 and 1800 persons, respectively).
Table 1.
Summary statistics of the COVID-19 pandemic and air pollution in Lombardy and Veneto.
| Itemsa | Regions |
|
|---|---|---|
| Lombardy | Veneto | |
| Population | 10,060,574 | 4,905,854 |
| Population density | 421.93 | 267.42 |
| Total number of COVID-19 cases | 85,775 | 19,030 |
| Total number of COVID-19 deaths | 15,662 | 1,832 |
| STAP | ||
| NO2 | 24.89 (11.13) | 20.16 (10.05) |
| O3 | 59.80 (19.45) | 60.23 (19.70) |
| PM2.5 | 18.44 (9.16) | 18.14 (14.55) |
| PM10 | 25.92 (13.62) | 29.47 (22.44) |
| SO2 | 2.23 (0.41) | 0.70 (0.32) |
| LTAP | ||
| NO2 | 41.06(0.21) | 32.88 (0.14) |
| O3 | 48.30 (0.19) | 45.89 (0.15) |
| PM2.5 | 23.23 (0.07) | 24.26 (0.04) |
| PM10 | 32.03 (0.06) | 33.83 (0.05) |
| SO2 | 2.82 (0.01) | 1.63 (0.01) |
| LOCKDOWNb | ||
| Yes | 1 (1.12) | 1 (1.12) |
| No | 88 (98.88) | 88 (98.88) |
The units of population, population density, total number of COVID-19 cases and deaths, short-term exposure to air pollution (STAP), long-term exposure to air pollution (LTAP), and lockdown implementation (LOCKDOWN) are expressed as persons, persons/km per, persons, persons, μg/m3, μg/m3, and day, respectively. For STAP and LTAP, the average concentration and standard deviation (in brackets) are presented; for LOCKDOWN, the means of days and standard deviations (in brackets) are shown.
Lockdown implementation (i.e., Yes) is defined as one or more provinces being locked down by Italy's government in Lombardy and Veneto since February 22, 2020.
Regarding lockdown implementation, the starting date for Lombardy and Veneto was the same (February 22, 2020). Italy's government first established a lockdown to restrain the spread of COVID-19 within one province each in Lombardy (i.e., Lodi) and Veneto (i.e., Padua). On March 9, 2020, the lockdown policy was extended to all the regions in Italy. In our research, we used the definition of a lockdown to adjust the effect of lockdown implementation on the COVID-19 pandemic. For example, we assumed that lockdown had effectively controlled the spread of COVID-19 infections in Lombardy and Veneto from February 22, 2020 although only in a few provinces.
3.2. Temporal variation of accumulated incidence, mortality, and fatality rates for COVID-19
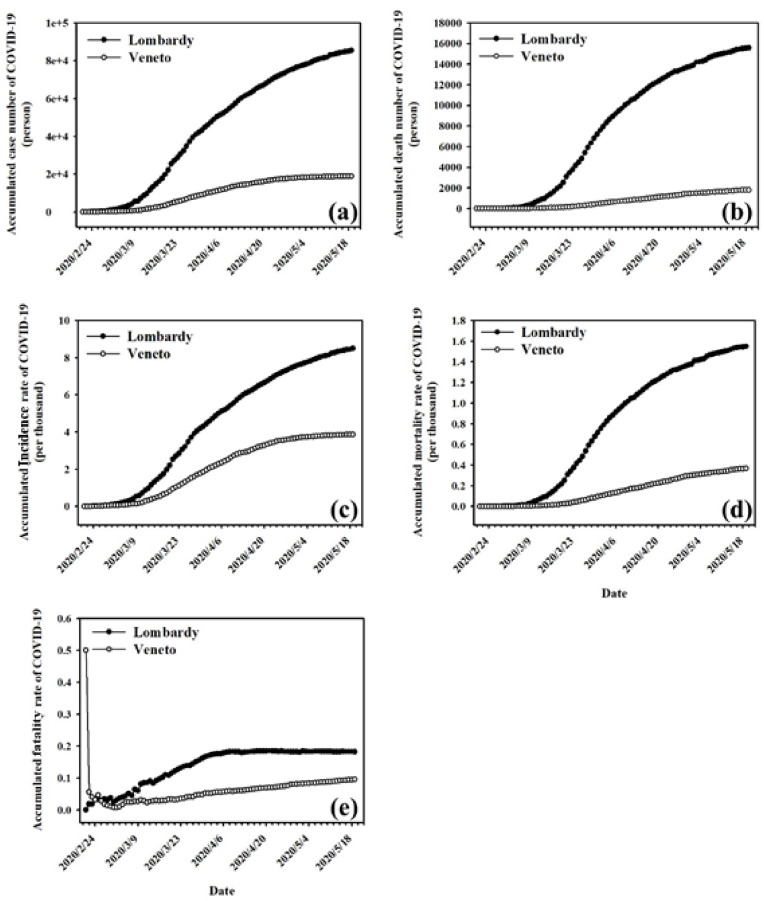
Fig. 2 shows the accumulated cases, deaths, incidence rate, mortality rate, and fatality rate related to COVID-19 in Lombardy and Veneto from February 21 to May 19, 2020. From February 22 to the day of the survey, the cumulative cases, deaths, incidence, and mortality related to COVID-19 in Lombardy increased more rapidly than in Veneto. Similarly, the cumulative COVID-19 fatality rate in Lombardy also increased faster than in Veneto, and the rate of increase declined after April 6, whereas the COVID-19 fatality rate in Veneto increased slowly at the same rate during the investigation.
Fig. 2.
Accumulated (a) cases, (b) deaths, (c) incidence rate, (d) mortality rate, and (e) fatality rate of COVID-19 in Lombardy and Veneto.
3.3. Short- and long-term exposure to air pollution
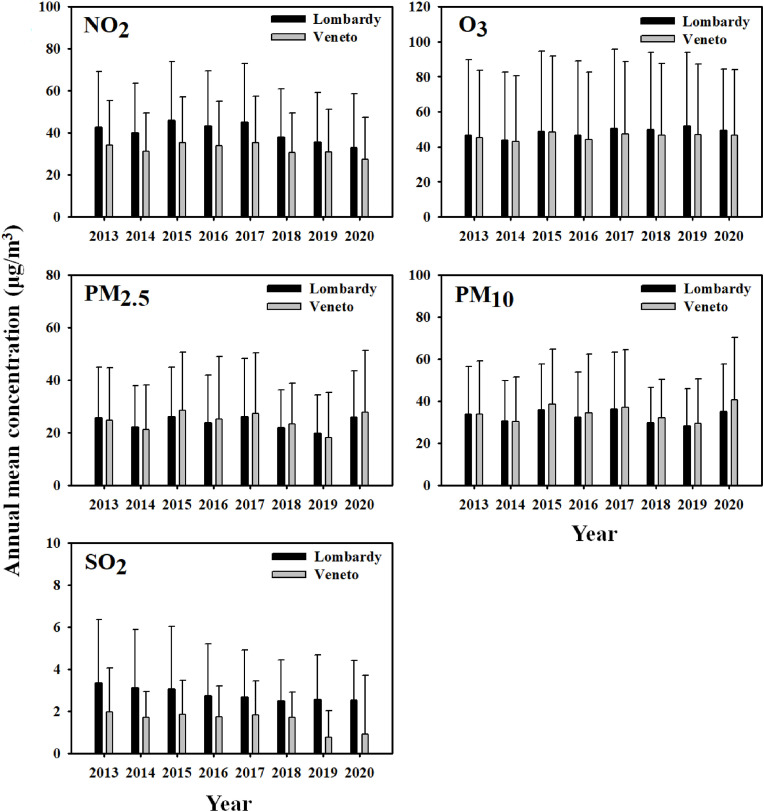
Regarding STAP, the daily mean concentrations of NO2, O3, PM2.5, PM10, and SO2 in Lombardy were 24.89, 59.80, 18.44, 25.92, and 2.23 μg/m3, respectively, whereas those in Veneto were 20.16, 60.23, 18.14, 29.47, and 0.70 μg/m3, respectively (Table 1). The mean (STAP) concentrations of NO2, PM2.5, and SO2 were higher in Lombardy than in Veneto (ratios of 1.23, 1.02, and 3.19, respectively). Regarding LTAP, the mean concentrations of NO2, O3, PM2.5, PM10, and SO2 were 41.06, 48.30, 23.23, 32.03, and 2.82 μg/m3, respectively, in Lombardy, respectively; and 32.88 μg/m3, 45.89 μg/m3, 24.26 μg/m3, 33.83 μg/m3, and 1.63 μg/m3 in Veneto, respectively (Table 1). The LTAP mean concentrations of NO2, O3 and SO2 were higher in Lombardy, and the ratios were 1.25, 1.05 and 1.73, respectively.
Fig. 3 shows the temporal variation of annual mean concentration for NO2, O3, PM2.5, PM10, and SO2 between Lombardy and Veneto regions from 2013 to 2020 (until May 19, 2020). The annual average concentrations of NO2, O3, and SO2 from 2013 to 2020 (i.e., the mean of measurements from January 1 to May 19) were all higher in Lombardy than in Veneto. In terms of particulate matter (PM2.5 and PM10), no consistent patterns were observed in the two regions.
Fig. 3.
Temporal variations in the annual mean concentrations of NO2, O3, PM2.5, PM10, and SO2in Lombardy and Veneto from 2013 to 2020.
3.4. Effect of exposure to air pollution on the COVID-19 pandemic
On the basis of the results of our model (Eq. (1)) analyzed using the GEE method, compared with other pollutants, in terms of LTAP, SO2 has the greatest effect on the incidence and mortality of COVID-19 after adjustment for the lockdown effect (Table 2 ). For example, with the increase of 1 LTAP unit in these two regions, the incidence and mortality of the COVID-19 pandemic increased by 3.16 and 5.37, respectively. Similar results were observed for the increase of 1 STAP unit, with the incidence and mortality of the COVID-19 pandemic increasing by 1.12 and 1.19, respectively. In addition, consistent results were observed for NO2 and O3. For example, the COVID-19 incidence and mortality increased by 1.20 and 1.29, respectively, per 1 unit of LTAP for NO2, whereas they increased by 1.00 each per 1 unit of STAP for NO2.
Table 2.
Regression parameters of short- and long-term exposure to air pollution using GEE method.
| Parametersa | NO2 |
O3 |
PM2.5 |
PM10 |
SO2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | |
| INTERCEPT | 2.78E-01 | 2.97E-03 | 1.34E+00 | 5.08E-10 | 1.15E-15 | 9.82E-01 | 1.57E+18 | 2.57E+22 | 1.21E-01 | 3.54E+14 | 9.55E+17 | 2.38E-02 | 1.75E+01 | 6.38E-01 | 5.04E-01 |
| STAP | 1.00E+00 | 1.00E+00 | 9.93E-01 | 1.00E+00 | 9.99E-01 | 1.00E+00 | 1.01E+00 | 1.00E+00 | 9.94E-01 | 1.00E+00 | 1.00E+00 | 9.98E-01 | 1.12E+00 | 1.19E+00 | 8.20E-01 |
| LTAP | 1.20E+00 | 1.29E+00 | 9.77E-01 | 1.78E+00 | 2.25E+00 | 9.81E-01 | 2.17E-01 | 1.33E-01 | 1.06E+00 | 4.29E-01 | 3.20E-01 | 1.09E+00 | 3.16E+00 | 5.37E+00 | 1.08E+00 |
| LOCKDOWN | |||||||||||||||
| Yes | 1.48E+00 | 1.44E+00 | 3.33E-01 | 1.44E+00 | 1.35E+00 | 3.83E-01 | 1.35E+00 | 1.28E+00 | 3.79E-01 | 1.42E+00 | 1.34E+00 | 3.87E-01 | 1.54E+00 | 1.55E+00 | 3.64E-01 |
| No | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 |
STAP, LTAP, and LOCKDOWN represent short- and long-term exposure to air pollution and lockdown implementation, respectively. Values in bold were significant at a p value of 0.05.
Conversely, PM2.5 and PM10 exhibited an opposing effect on COVID-19 incidence and mortality (Table 2). Short-term exposure to PM2.5 and PM10 has a greater effect on COVID-19 than does long-term exposure. As for the COVID-19 fatality rate (Table 2), no significant effects of LTAP and STAP of SO2 and O3 were observed. Nevertheless, in long-term exposure to NO2, PM2.5, and PM10 significantly affected COVID-19 fatality rate.
In addition, after adjustment for other pollutants with the most relevant correlation to our outcome of interest (Eq. (2)), reliable results can be drawn to estimate the most robust effect of LTAP and STAP on the COVID-19 pandemic (Table 3 ). Table S1 shows the correlation between short-term exposure to different pollutants. Table 3 shows that LTAPs of all air pollutants significantly affected the variations of both COVID-19 incidence and mortality. For example, when for a 1-unit increase of LTAP for SO2, after adjustment for NO2 and other confounding factors, the COVID-19 incidence and mortality increased by 3.16 and 5.37, respectively. However, only NO2 and SO2 show significant short-term effect on COVID-19 incidence and mortality. Compared with long-term effects, PM2.5 and PM10 have greater short-term effects on COVID-19 incidence and mortality. In terms of fatality, we observed similar results between short- and long-term exposure (Table 2), except for PM2.5, PM10, and SO2.
Table 3.
Regression parameters of short- and long-term exposure to air pollution using GEE method adjusted for the coeffect of a single pollutant.
| Parametersa | NO2 |
O3 |
PM2.5 |
PM10 |
SO2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | |
| INTERCEPT | 2.54E-01 | 2.77E-03 | 1.35E+00 | 4.16E-10 | 7.70E-16 | 6.52E-01 | 2.59E+18 | 3.47E+22 | 1.48E-01 | 1.59E+15 | 2.12E+18 | 4.64E-02 | 1.75E+01 | 6.40E-01 | 7.29E-01 |
| STAP | 1.00E+00 | 1.00E+00 | 9.93E-01 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.01E+00 | 1.00E+00 | 9.93E-01 | 9.98E-01 | 9.99E-01 | 1.00E+00 | 1.12E+00 | 1.20E+00 | 8.66E-01 |
| LTAP | 1.20E+00 | 1.29E+00 | 9.77E-01 | 1.78E+00 | 2.26E+00 | 9.98E-01 | 2.12E-01 | 1.31E-01 | 1.05E+00 | 4.09E-01 | 3.10E-01 | 1.07E+00 | 3.16E+00 | 5.37E+00 | 1.09E+00 |
| STAP_ADJ | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.01E+00 | 9.92E-01 | 9.98E-01 | 9.99E-01 | 1.00E+00 | 1.01E+00 | 1.00E+00 | 9.93E-01 | 1.00E+00 | 1.00E+00 | 9.89E-01 |
| LOCKDOWN | |||||||||||||||
| Yes | 1.48E+00 | 1.44E+00 | 3.33E-01 | 1.46E+00 | 1.38E+00 | 3.30E-01 | 1.33E+00 | 1.27E+00 | 3.78E-01 | 1.35E+00 | 1.30E+00 | 3.79E-01 | 1.54E+00 | 1.55E+00 | 2.87E-01 |
| No | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 |
STAP, LTAP, STAP_ADJ, and LOCKDOWN represent short- and long-term exposure to air pollution, the pollutant used for adjusting the interaction with the one of our interest, and lockdown implementation, respectively. Of NO2, O3, PM2.5, PM10, and SO2, the pollutants with the highest correlations (i.e., STAP_ADJ) were O3, NO2, PM10, PM2.5, and NO2, respectively. Values in bold were significant at a p value of 0.05.
Table 4 shows the results of the full pollutant model (Eq. (3)). The effects of LTAP of overall pollutants on COVID-19 incidence and mortality were significant after adjustment for multiple pollutants and confounders. For example, a 1-unit increase of long-term SO2 exposure may lead to 3.53- and 5.64-fold increases in COVID-19 incidence and mortality, respectively. For STAP, only NO2 and SO2 contributed to COVID-19 incidence and mortality. In addition, only NO2 and O3 significantly contributed to the fatality rate. The effects of LTAP on the COVID-19 fatality rate were nonsignificant. As aforementioned, the ratio of LTAP and STAP coefficient for incidence and mortality rates were, respectively, 2.82 and 4.51 in a single pollutant model (SO2), 2.82 and 4.48 in two-pollutant models, and 3.12 and 4.66 in all-pollutant models (Table 2, Table 3, Table 4).
Table 4.
Regression parameters of short- and long-term exposure to air pollution using GEE method adjusted for the coeffects of multiple pollutants.
| Parametersa | NO2 |
O3 |
PM2.5 |
PM10 |
SO2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | Incidence | Mortality | Fatality | |
| INTERCEPT | 2.57E-01 | 3.21E-03 | 4.12E-01 | 1.13E-10 | 1.72E-16 | 2.01E-04 | 1.93E+17 | 1.15E+22 | 3.46E+03 | 1.94E+14 | 5.24E+17 | 4.52E+01 | 1.27E+01 | 5.85E-01 | 5.16E-01 |
| STAP | 9.96E-01 | 9.98E-01 | 9.96E-01 | 1.00E+00 | 9.99E-01 | 1.00E+00 | 1.01E+00 | 1.00E+00 | 9.94E-01 | 9.98E-01 | 9.99E-01 | 1.00E+00 | 1.13E+00 | 1.21E+00 | 8.46E-01 |
| LTAP | 1.20E+00 | 1.28E+00 | 1.01E+00 | 1.82E+00 | 2.32E+00 | 1.19E+00 | 2.35E-01 | 1.35E-01 | 7.01E-01 | 4.33E-01 | 3.20E-01 | 8.81E-01 | 3.53E+00 | 5.64E+00 | 1.13E+00 |
| STAP_ADJ | |||||||||||||||
| NO2 | – | – | – | 9.97E-01 | 1.00E+00 | 9.98E-01 | 9.97E-01 | 9.99E-01 | 9.97E-01 | 9.97E-01 | 9.99E-01 | 9.97E-01 | 9.96E-01 | 9.98E-01 | 9.96E-01 |
| O3 | 1.00E+00 | 9.99E-01 | 1.00E+00 | – | – | – | 1.00E+00 | 9.99E-01 | 1.00E+00 | 1.00E+00 | 9.99E-01 | 1.00E+00 | 1.00E+00 | 9.99E-01 | 1.00E+00 |
| PM2.5 | 1.01E+00 | 1.00E+00 | 9.93E-01 | 1.01E+00 | 1.00E+00 | 9.93E-01 | – | – | – | 1.01E+00 | 1.00E+00 | 9.94E-01 | 1.01E+00 | 1.00E+00 | 9.93E-01 |
| PM10 | 9.98E-01 | 9.99E-01 | 1.00E+00 | 9.98E-01 | 9.99E-01 | 1.00E+00 | 9.98E-01 | 9.99E-01 | 1.00E+00 | – | – | – | 9.98E-01 | 9.99E-01 | 1.00E+00 |
| SO2 | 1.13E+00 | 1.21E+00 | 8.58E-01 | 1.13E+00 | 1.21E+00 | 7.58E-01 | 1.13E+00 | 1.21E+00 | 7.76E-01 | 1.13E+00 | 1.21E+00 | 8.18E-01 | – | – | – |
| LOCKDOWN | |||||||||||||||
| Yes | 1.42E+00 | 1.51E+00 | 2.92E-01 | 1.39E+00 | 1.44E+00 | 2.73E-01 | 1.38E+00 | 1.42E+00 | 2.75E-01 | 1.39E+00 | 1.45E+00 | 2.87E-01 | 1.42E+00 | 1.50E+00 | 2.92E-01 |
| No | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 | 1.00E+00 |
STAP, LTAP, STAP_ADJ, and LOCKDOWN represent short- and long-term exposure to air pollution, the pollutant used for adjusting the interaction with the one of our interest, and lockdown implementation, respectively. Values in bold were significant at a p value of 0.05.
To further evaluate the reliability of our results, the effect of meteorology on the association of short-term air pollution and COVID-19 pandemic was considered in models. The meteorological data (e.g., the daily means of temperature and relative humidity) in Lombardy and Veneto regions during the study period were collected from the NASA earth science's applied sciences program. We reanalyzed the association of short-term air pollution and COVID-19 pandemic after adjusting for the meteorological information in models. In the results (Tables S2–S4), we found the similar patterns of estimated coefficients for short-term air pollutants compared with the results of Table 2, Table 3, Table 4 It supported the reliability of our finding related to the association of air pollution and COVID-19 pandemic when considering the influence of meteorology.
4. Discussion
Among air pollutants, the current focus is mainly on NO2, PM2.5, PM10, and O3, which frequently occur at increased concentrations in large areas of the planet (Ogen, 2020). However, the roles of SO2 in COVID-19 risk and severity are few (Conticini et al., 2020), if any, emphasized, although inhaled SO2 causes pulmonary and systemic inflammation, leading to toxic effects both in the respiratory and cardiopulmonary systems (Meng et al., 2003) and causing fibrotic respiratory disease (Wigenstam et al., 2016).
In this study, the concentrations of tropospheric air pollutants including SO2, which were extracted from the air quality database of the EEA, were used to explain the spatial variation of incidences, mortalities, and fatalities in Lombardy and Veneto. The air quality data show that annual mean concentrations of SO2 were higher over Lombardy region than over Veneto region in the past years (Fig. 3). One of the possible reasons was that the high concentration of SO2 in Lombardy is accompanied by downward airflow, which causes SO2 to accumulate near the surface. The terrain of Lombardy, which is dominated by mountains and canyons, combined with reversed atmospheric conditions (e.g., temperature inversion), prevents the spread of air pollutants, which can lead to a high incidence of respiratory diseases and inflammation among local residents. Furthermore, we observed an asymmetric variation of cases and deaths due to COVID-19 in the two regions (Fig. 2). It is interesting to consider that long-term exposure may be an important reason for the high COVID-19 mortality observed in these areas. The physiopathological event leading to the ICU admissions and consequent mortality is acute respiratory distress syndrome (ARDS), which is a sever event, and its treatment is usually only supportive and requires mechanical ventilation. Regardless of the cause (Aisiku et al., 2016), excessive activation of the innate immune system is considered to play a crucial role in related events: inflammatory cytokines and chemokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, IL-17, and IL-18, and several growth factors are overexpressed in ARDS, triggering the apoptosis cascade and epithelial-mesenchymal transition (Gouda et al., 2018). In addition, their high serum and bronchoalveolar lavage levels seem to be associated with poor prognosis (Butt et al., 2016). Although these findings are not validated and thus not applicable to routine clinical practice, they further medical goals regarding suitable potential biomarkers and targets for treatment.
According to our results (Table 2, Table 3, Table 4), long-term exposure to SO2 significantly affected the COVID-19 pandemic after adjustment for other covariates, and its influence was higher than that of STAP. As for other air pollutants besides particulate matter, similar results were also observed after adjustment for multiple pollutants and lockdown. As mentioned in related studies, air pollution is among the most well-known causes of chronic inflammation, which ultimately leads to excessive activation of the innate immune system. Levels of pro-inflammatory cytokines, such as TNF-α, IFN-γ, in allergic mice (ovalbumin challenge) were increased 6- to 8-fold by exposure to PM2.5 particles before challenge compared with control filter extract (Yang et al., 2019). High systemic inflammation also impairs cardiac function, as demonstrated by another group of mice exposed to PM2.5 and PM10 (Radan et al., 2019). Moreover, these findings have been extensively confirmed in humans; even in healthy people, nonsmokers, and young people, PM2.5 and PM10 caused systemic inflammation with the upregulation of platelet-derived growth factor, vascular endothelial growth factor, TNFα, IL-1, and IL-6 (Tsai et al., 2019; Pope et al., 2016). Among other more common pollutants, SO2 also plays a crucial role in inducing systemic and respiratory inflammation both in vitro and in vivo (Knorst et al., 1996). The commonest source of SO2 exposure is from fossil fuel combustion. Italy has two sources of SO2 emission, namely volcanic emissions and biofuel combustion. Biofuel combustion is the main source of SO2 emissions in Lombardy (Stefano, 2012). Notably, due to worldwide Clean Air Act efforts, anthropogenic emissions of SO2 into the troposphere have remarkably reduced in the West; it had peaked during the year 1972 at approximately 131 megatons and decreased by approximately 48 megatons by the year 2000 (Smith et al., 2011). In our study, we observed a decreasing trend of annual mean SO2 level in two regions in northern Italy from 2013 to 2020. However, the effects of SO2 exposure are long term; a long-term study can explain why the fatality rate is high in elderly people. In our recent in vivo study on the effects of exposure to air pollution on idiopathic pulmonary fibrosis, SRM 1649b (Albinet et al., 2019), a well characterized urban dust standard reference material, was applied, which contains the main organic and inorganic substances produced in the mid-1970s, when fossil fuel combustion was at its peak in the United States (Ellerman, 2004). Macrophages activated through particulate matter uptake secreted KC (a murine IL-8 homolog) to recruit neutrophil and aggravate bleomycin-induced pulmonary fibrosis (Cheng et al., 2019). These studies have shown that exposure to SO2 or related air pollution can cause lung inflammation, and it is now necessary to determine whether the initial inflammation in the body is related to the immune system's response to COVID-19. When the body experiences chronic respiratory stress, its ability to resist infection is limited.
Notably, after adjusting for other covariates, we also observed that the short-term exposure to PM2.5 and PM10 had a tendency to increase COVID-19 incidence and mortality than long-term exposure. These findings are supported by previous studies of Setti et al., where they found a significant correlation between the daily PM10 exceedances and COVID-19 spreading in Italy (Setti et al., 2020a). In addition, we observed the new confirmed cases of COVID-19 still appeared during the lockdown period. We speculate on two reasons for the occurrence of new COVID-19 cases after the lockdown in northern Italy. First, patients who have been infected with COVID-19 before the lockdown were still in the incubation period. Second, the SARS-COV-2 virus may spread through the air by attaching to PM2.5/PM10 particles as demonstrated in previous study (Setti et al., 2020b), thereby spreading COVID-19 disease to other people.
5. Conclusions
In our study, we analyzed the temporal variability of incidence, mortality, and fatality rate related to COVID-19 as well as atmospheric pollution exposure in Lombardy and Veneto regions in Italy from February 21 to May 19, 2020. We observed that the incidence, mortality and fatality rates related to COVID-19 in Lombardy increased more rapidly than in Veneto. As for air pollution exposure, the annual average concentrations of NO2, O3, and SO2 were consistently higher in Lombardy than in Veneto from 2013 to 2020 as well as particulate matter (PM2.5 and PM10). Furthermore, we explored the effects of STAP and LTAP on COVID-19 risk and fatality. The results showed that short-term exposure to PM2.5 and PM10 had a tendency to increase the incidence and mortality of COVID-19 than long-term exposure. As for other air pollutants, both short- and long-term exposure resulted in increased health effects on COVID-19 pandemic, especially for SO2. Our findings indicated the significant role of exposure to SO2 in COVID-19 pandemic via inducing systemic and respiratory inflammation in human bodies, suggesting that further studies are warranted.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study is supported by China Medical University, Taiwan (CMU105-S-48, CMU106-S-34, and CMU107-Z-04), and China Medical University Hospital, Taiwan (DMR-110-228).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.111293.
Credit author statement
Chi-Chang Ho: Writing – original draft, Software, Formal analysis. Shih-Chieh Hung: Conceptualization, Methodology, Supervision. Wen-Chao Ho: Conceptualization, Methodology, Supervision.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aisiku I.P., et al. Plasma cytokines IL-6, IL-8, and IL-10 are associated with the development of acute respiratory distress syndrome in patients with severe traumatic brain injury. Crit. Care. 2016;20:288. doi: 10.1186/s13054-016-1470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albinet A., et al. Analysis and determination of secondary organic aerosol (SOA) tracers (markers) in particulate matter standard reference material (SRM 1649b, urban dust) Anal. Bioanal. Chem. 2019;411:5975–5983. doi: 10.1007/s00216-019-02015-6. [DOI] [PubMed] [Google Scholar]
- Bailey W.C., et al. Predictive model to identify positive tuberculosis skin test results during contact investigations. J. Am. Med. Assoc. 2002;287:996–1002. doi: 10.1001/jama.287.8.996. [DOI] [PubMed] [Google Scholar]
- Butt Y., et al. Acute lung injury: a clinical and molecular review. Arch. Pathol. Lab Med. 2016;140:345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- Chen N., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng I.Y., et al. Particulate matter increases the severity of bleomycin-induced pulmonary fibrosis through KC-mediated neutrophil chemotaxis. Int. J. Mol. Sci. 2019;21 doi: 10.3390/ijms21010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conticini E., et al. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-CoV-2 lethality in Northern Italy? Environ. Pollut. 2020;261:114465. doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerman A.D. The sources of emission reductions: evidence from U.S. SO2 emissions from 1985 through 2002. 2004. http://web.mit.edu/ceepr/www/publications/workingpapers.html
- Fanelli D., Piazza F. Analysis and forecast of COVID-19 spreading in China, Italy and France. Chaos, Solit. Fractals. 2020;134:109761. doi: 10.1016/j.chaos.2020.109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore E., et al. Human health risk in relation to air quality in two municipalities in an industrialized area of Northern Italy. Environ. Res. 2011;111:1321–1327. doi: 10.1016/j.envres.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Fattorini D., Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ. Pollut. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouda M.M., et al. Inflammatory and fibrinolytic system in acute respiratory distress syndrome. Lung. 2018;196:609–616. doi: 10.1007/s00408-018-0150-6. [DOI] [PubMed] [Google Scholar]
- Grasselli G., et al. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. J. Am. Med. Assoc. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- Iriti M., et al. Air pollution and health: the need for a medical reading of environmental monitoring data. Int. J. Environ. Res. Publ. Health. 2020;17 doi: 10.3390/ijerph17072174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorst M.M., et al. Effect of sulfur dioxide on cytokine production of human alveolar macrophages in vitro. Arch. Environ. Health. 1996;51:150–156. doi: 10.1080/00039896.1996.9936009. [DOI] [PubMed] [Google Scholar]
- Li Q., et al. Early transmission dynamics in wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z., et al. Oxidative damage of sulfur dioxide inhalation on lungs and hearts of mice. Environ. Res. 2003;93:285–292. doi: 10.1016/s0013-9351(03)00045-8. [DOI] [PubMed] [Google Scholar]
- Neilan A.M., et al. Association of risk of viremia, immunosuppression, serious clinical events, and mortality with increasing age in perinatally human immunodeficiency virus-infected youth. Jama Pediatr. 2017;171:450–460. doi: 10.1001/jamapediatrics.2017.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odone A., et al. COVID-19 deaths in Lombardy, Italy: data in context. The Lancet Public Health. 2020;5 doi: 10.1016/S2468-2667(20)30099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020;726:138605. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C.A., et al. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ. Res. 2016;119:1204–1214. doi: 10.1161/CIRCRESAHA.116.309279. 3rd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radan M., et al. Gallic acid protects particulate matter (PM10) triggers cardiac oxidative stress and inflammation causing heart adverse events in rats. Environ. Sci. Pollut. Res. Int. 2019;26:18200–18207. doi: 10.1007/s11356-019-05223-w. [DOI] [PubMed] [Google Scholar]
- Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., et al. Potential role of particulate matter in the spreading of COVID-19 in Northern Italy: first observational study based on initial epidemic diffusion. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti L., et al. SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: first evidence. Environ. Res. 2020;188:109754. doi: 10.1016/j.envres.2020.109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.J., et al. Anthropogenic sulfur dioxide emissions: 1850-2005. Atmos. Chem. Phys. 2011;11:1101–1116. [Google Scholar]
- Stawinska-Witoszynska B., et al. Trends in the incidence rates of chronic hepatitis B in Poland in the years 2005 - 2013. Hepat. Mon. 2016;16 doi: 10.5812/hepatmon.32692. e32692-e32692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano C. 2012. Atmospheric Emissions in Lombardy: Findings from the INEMAR Emission Inventory. Report No. 978-88-903557-2-1. [Google Scholar]
- Tsai D.H., et al. Effects of short- and long-term exposures to particulate matter on inflammatory marker levels in the general population. Environ. Sci. Pollut. Res. Int. 2019;26:19697–19704. doi: 10.1007/s11356-019-05194-y. [DOI] [PubMed] [Google Scholar]
- Wigenstam E., et al. Inhaled sulfur dioxide causes pulmonary and systemic inflammation leading to fibrotic respiratory disease in a rat model of chemical-induced lung injury. Toxicology. 2016;368–369:28–36. doi: 10.1016/j.tox.2016.08.018. [DOI] [PubMed] [Google Scholar]
- World Health Organization Media Centre https://www.who.int/mediacentre/news/releases/2014/air-pollution/en/ Available online:
- Wu F., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q.L., et al. Identifying risk factors of A(H7N9) outbreak by wavelet analysis and generalized estimating equation. Int. J. Environ. Res. Publ. Health. 2019;16 doi: 10.3390/ijerph16081311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., et al. The influence of PM2.5 on lung injury and cytokines in mice. Exp Ther Med. 2019;18:2503–2511. doi: 10.3892/etm.2019.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., et al. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.