Abstract
As the world continues to grapple with the reality of coronavirus disease, global research communities are racing to develop practical solutions to adjust to the new challenges. One such challenge is the control of indoor air quality in the COVID-19 era and beyond. Since COVID-19 became a global pandemic, the “super spread” of the virus has continued to amaze policymakers despite measures put in place by public health officials to sensitize the general public on the need for social distancing, personal hygiene, etc. In this work, we have reviewed the literature to demonstrate, by investigating the historical and present circumstances, that indoor spread of infectious diseases may be assisted by the conditions of the HVAC systems. While little consideration has been given to the possibility of indoor airborne transmission of the virus, the available reports have demonstrated that the virus, with average aerodynamic diameter up to 80–120 nm, is viable as aerosol in indoor atmosphere for more than 3 h, and its spread may be assisted by the HVAC systems. Having reviewed the vulnerability of the conventional ventilation systems, we recommend innovative air circulation concept supported by the use of UVGI in combination with nanoporous air filter to combat the spread of SARS-CoV-2 and other harmful microbes in enclosed spaces.
Keywords: Coronavirus disease, COVID-19 spread, Public health, HVAC systems, Ventilation systems, Aerosols
Nomenclature
- COVID-19 –
Coronavirus Disease 2019
- HVAC –
Heating, Ventilation and Air-conditioning
- SARS-CoV-1 –
Severe Acute Respiratory Syndrome Coronavirus 1
- SARS-CoV-2 –
Severe Acute Respiratory Syndrome Coronavirus 2
- MERS –
Middle East Respiratory Syndrome
- WHO –
World Health Organization
- H1N1 –
Hemagglutinin1 Neuraminidases1 – Protein found on the outer shell of swine flu virus
- SBS –
Sick Building Syndrome
- HEPA –
High-Efficiency Particulate Air
- ULPA –
Ultra Low Particulate Air
- RH –
Relative Humidity
- UVGI –
Ultraviolet Germicidal Irradiation
- OPC –
Optical Particle Counter
- AMAS –
Active Microbial Air Sampler
- CFU –
Community Forming Units
- AIIRs –
Airborne Infection Isolation rooms
- CDC –
Centre for Disease Control and Prevention
- ACH –
Air changes per hour
- UVC –
Ultraviolet light type C
- ASHRAE –
American Society of Heating, Refrigerating and Air-Conditioning Engineers
- VOCs –
Volatile Organic Compounds
1. Introduction
The rate and severity of SARS-CoV-2 (the pathogen that causes COVID-19 disease) spread have awakened researchers in the built environment to take further steps to improve indoor air quality. Although there is still no agreement as to whether SARS-CoV-2 is airborne, a large number of available reports reveals that transmission of the virus is possible even when there are no physical contacts with infected/contaminated persons or fomites (Tellier et al., 2019; Dietz et al., 2019). Moreover, about 239 scientists, reporting from 32 countries, have written an open letter to the World Health Organization (WHO), confirming the airborne transmission route of SARS-CoV-2 ("O acknowledges 'eviden, 2020). This shows that, to some extent, the virus can be contracted through contaminated air (Lewis, 2020; Morawska and Cao, 2020a), especially in buildings where the percentage of fresh air-makeup is small (Chen and Zhao, 2020). Moreover, there have been reported accounts of active and transmittable viruses in the circulated air in indoor environments. In 2005, after its outbreak between the years 2002 and 2004, Booth et al. (Boothet al., 2005) reported an active and viable SARS-CoV-1 (a known human coronavirus closely related to SARS-CoV-2), and in 2020, Lednicky et al. (Lednickyet al., 2020) reported an active SARS-CoV-2, both in the air samples taken from the hospitals where coronavirus patients were being treated. Furthermore, in a recent work carried out to evaluate the stability of SARS-CoV-2 in the air and on surfaces, van Doremalen et al. (Van Doremalenet al., 2020) reported the virus to be viable as aerosols in the air for more than 3 h, which is similar in aerosol stability to its sister virus, SARS-CoV-1. The stronger argument, following ASHRAE report (Schoenet al., 2009) in their document that described the aerobiology of infectious diseases, is that SARS-CoV-2, to some extent, is indoor airborne due to the nature of the virus. The phrase “to some extent” is used to describe aerosol-classification of SARS-CoV-2 because the spread of the virus follows an opportunistic transmission route— infections that are traditionally transmitted via other routes (e.g. physical touching and fomite), but can equally be contracted by using aerosols as a means to propagate in favorable atmospheres. Since the outbreak of SARS-CoV-2, in order to prevent further spread of the virus, public health officials and government bodies have focused on sensitizing the general public on the need for social distancing (Chen et al., 2020), personal hygiene, and the use of face masks (Repiciet al., 2020), little attention has been given to the possibility of airborne transmission. Although it is understandable why public health officials have paid little attention to the possibility of airborne transmission of the ongoing outbreak of SARS-CoV-2, it is difficult to measure or detect the virus directly in the air (Chiaet al., 2020; Faridiet al., 2020). However, historical accounts and the present reported cases have revealed airborne transmission route as a possible contagion pathway of many known infectious diseases, such as H1N1 influenza virus (Klontz et al., 1989), adenovirus, rhinovirus (Wat, 2004) and coronavirus (Leunget al., 2020; Morawska and Cao, 2020b). Furthermore, in a recent comprehensive work carried out to ascertain the indoor airborne nature of SARS-CoV-2, Hadei et al. (Hadeiet al., 2021) obtained air samples from public transportation facilities (buses, airport and subways), banks, post office, shopping centers and government offices, in which 64% of the samples collected were returned positive. The question that most experts and researchers continue to ask is whether the viral loads of SARS-CoV-2 detected in the air samples are enough to constitute COVID-19 (Lednickyet al., 2020; Liuet al., 2020; Nissenet al., 2020). The answer to the question has been mostly inconclusive (Lewis, 2020; Al Huraimel et al., 2020), however, there is a general understanding that airborne transmission may be responsible for the “super spread” of the virus, especially in the hospital environments where coronavirus patients receive treatments (Yao et al., 2020; Correia et al., 2020; Hadei et al., 2020). Since it has been proven that aerosol transmission of the virus is possible in indoor environments, the first line of defense against further spread of the virus indoors is through the HVAC systems (Augenbraunet al., 2020; Chirico et al., 2020; Azuma et al., 2020). A number of studies (Augenbraunet al., 2020; Borro et al., 2021/02) has demonstrated the role of HVAC systems in controlling or worsening the spread of the virus. For example, a group of researchers (Elias and Bar-Yam, 2020a) has speculated the likelihood of self-reinfection of the virus through aerosol transmission, aided by the airflow in the hospital environments. Thus, the researchers recommended air filtration around recuperating COVID-19 patients, and around those being transported in the ambulances, to reduce the surrounding atmosphere's viral loads. To reduce the likelihood of COVID-19 cross-infection inside the hospital environment due to the condition/design of the HVAC systems, Wang et al. (2020) recently simulated the optimization of air distribution in a three-bed general hospital ward in China. The study recommended displacement ventilation system with full fresh air supply as a means of reducing COVID-19 cross-infection in a hospital setting. The research question that urgently requires answers is “how should the new HVAC systems be effectively designed or the existing ones managed to provide protections against harmful microbial agents (such as SARS-CoV-2) in indoor atmosphere?”
In order to open a discussion on the research question raised above, in this work, we have reviewed the literature to demonstrate, by investigating historical and present circumstances, that indoor spread of infectious diseases may be assisted by the design and operating conditions of the HVAC systems. The methodology section describes how and where the articles reviewed are sourced. This is followed by a section on the history of infectious diseases, which strategically reviews the available literature to report our perspective on the HVAC systems as possible conduits that aid the spread and eventual transmission of aerosolized particulate matters of infectious diseases in indoor environments. The next part of this section deals with the vulnerability of the HVAC systems, which explains why the colony of indoor microbial populations are usually multiplied by the conditions of the HVAC systems. This is followed by a section on discussion, in which the findings are analyzed to reflect the aim of this article. The next section (containment and prevention recommendations) offers recommendations to the general stakeholders in the built environment and specifically to the operators of transportation (airlines, cruise ships, metros, and other public transport modes) on public safety in the face of global COVID-19 pandemic.
2. Methodology
To select articles for this review paper, emphasis was placed on the various types of buildings/spaces served by central HVAC system, be it hospitals, offices, shopping malls, restaurants, private clinics, aircraft cabins, cruise ships or metros. These buildings or conditioned spaces may be prone to the spread of SARS-CoV-2 and other harmful microbes, mainly because of central air circulation system serving the whole building/space. The method used for the articles for review was based on (1) strong connection to the theme of the review article (Contagion of COVID-19 and other microbes through the HVAC Systems), (2) strong correlation with sub-topics therein (i.e. different modes of ventilation, effect of air filtration and humidity on the spread of microbes etc.), (3) different modes of virus spread in the built environment (airborne nature of the virus and its survival time in the air), (4) air filtration/purification and disinfection methods, (5) vulnerability of HVAC systems to microbial invasion, history of infectious diseases spread through the HVAC system and different methods to prevent the spread of contagion in conditioned spaces. We reviewed 128 articles in total, covering different fields from 1972 to 2021, with themes related to the topic of this review paper. The article search was carried out through the traditional peer review articles' search engines, with many articles redirecting the search to the publishers’ websites. In the end, the articles were distributed among the authors to critique, evaluate, and review based on the topic and sub-topics of this review article.
3. History of infectious diseases through the HVAC systems
There have been several reported accounts of suspected airborne transmission of different infectious diseases (including coronavirus) in confined places; this work outlines a few of them to build arguments for airborne transmission. In 1979, Moser et al. (1979) reported airborne Influenza transmission onboard an airline in Alaska. Precisely on March 14, 1977, a commercial airplane (Boeing 737) was traveling to Kodiak from Anchorage with a scheduled stopover in Homer. The journey from Anchorage started with a total of 29 individuals onboard (24 passengers and five crew members). On getting to Homer, six passengers alighted, and 31 additional individuals boarded the airplane, bringing the total number of persons onboard to 54, consisting of 5 crewmembers and 49 passengers. During departure from Homer, the plane left engine developed some fault and takeoff was delayed for four and a half hours. During the delay, some passengers left for the terminal building, whereas others remained seated on board. A smaller aircraft, capable of carrying 34 passengers, was later flown in to complete the journey to Kodiak for 34 passengers, returned to Homer, and then took the remaining 15 passengers together with the crewmembers back to Anchorage. The next day (precisely March 15) in Kodiak, seven of the passengers started showing symptoms of severe respiratory disease, accompanied by headache, fever, severe cough, shaking chill and myalgia, and two of them were hospitalized. At about 60 h later, two crewmembers were equally hospitalized in Anchorage with related symptoms. The news was transferred to the public health authorities, and an investigation commenced immediately, which revealed an outbreak of A/Texas/1/77 (H3N2) virus. The epidemiological study later identified an index case to be a 21-year old female, who joined the flight at Homer, became very ill 15 min after getting on the plane, and remained seated when the takeoff was delayed with some passengers on board. None of the six passengers that alighted initially at Homer became sick. During the delayed takeoff, the ventilation system of the disabled plane was turned off. Thirty individuals (including the index case) remained on board throughout the delayed period. Thirty-eight individuals were infected, and those that were on the faulty plane with the index case during the delay had the most severe attacks.
In 2004, Yu et al. (Yuet al., 2004) reported another likely airborne spread of severe acute respiratory syndrome (SARS) —a coronavirus family that causes a severe form of pneumonia. The outbreak occurred in a high-rise building in the Amoy Gardens in Hong Kong. The index case was a 33-year old male, who started showing symptoms of SARS on March 14, 2003. He lived in Shenzhen, and traveled regularly to Amoy Garden to visit his brother, who lived on block E in the Amoy Garden housing units. He visited his brother twice in March of 2003; his first visit was on March 14 and the second visit was March 19. During his visits, he had diarrhea and frequently used his brother's toilet. As a result, he infected his brother, his brother's wife and the nurses that attended to him at the hospital. The disease however spread to other parts of the Amoy Garden. The suspected built environment contagion point, according to World Health Organization (WHO) epidemiological team (Organization, 2003), was a dried-out floor drains that was carried by winds and the exhaust fan of a toilet. During their investigation, WHO experts discovered that water traps in the floor drains in the inspected housing units were empty, obviously not been filled for a long time, and as a result, the seals in the traps had dried out. Consequently, there was a direct link from the floor drains to the vertical soil stacks, thereby allowing the bathroom exhaust fan to draw in aerosols and droplets from the contaminated unit into the air shaft. The most significant part of the study was the pattern of spread of the disease from one building to the other, especially on the upwind side of a building, which clearly points to airborne transmission as the only possible route of infection.
A recent account of coronavirus transmission possibly propagated by the HVAC systems involves three families (A, B and C). According to a study reported by Lu et al. (Luet al., 2020), the three families had lunch at a restaurant in Guangzhou, China on January 24, 2020. The index case, A1 (from family A), who traveled from Wuhan (the ground zero of COVID-19 outbreak) to Guangzhou on January 23, ate lunch with three other members of her family (A2, A3 and A4), wherein families B and C were also present at the restaurant at the time, having their lunch at nearby tables, more than 1 m apart. In the evening of that day, A1 became symptomatic and decided to visit a hospital, which revealed COVID-19 disease. About two weeks later (February 5 precisely), an additional nine individuals, involving four people from family A, three from family B and two from family C, had come down with coronavirus. The epidemiological study later revealed that; A1 contracted the virus from Wuhan, and was asymptomatic throughout her stay at the restaurant in Guangzhou. There were no physical contacts among families A, B and C. The study thus theorized that the possible routes of transmission from A1 to families B and C are droplet and aerosol transmissions, although transmissions within families A, B and C could have been from physical contacts or fomites. The stronger argument points to aerosol transmission, especially in a closed restaurant indoor atmosphere with strong airflow from the air conditioning system, in which the families were more than 1 m apart. Interesting inferences that can be drawn from the study fall into three categories: (1) A1 sneezed during the lunch, released large droplets between 60 and 100 μm in size most of which fell within 2 m away, the remaining droplets reduced to aerosols by evaporation and are carried away by the force of the sneeze at a velocity of 50 m/s, which are then carried more than 6 m away, and likely spent a considerable amount of time in the air by the strong airflow of the air conditioning system. (2) A1 coughed or shouted during the lunch, released large droplets and aerosols, which follow the aerodynamics of sneeze explained in (1) above, but are carried more within 2 m away at a velocity of 10 m/s (3) A1 breathed continuously during the lunch, and only exhaled aerosols that are carried away less than 1 m at a velocity of 1 m/s, which is very unlikely to have constituted the contagion point.
As reported nearly 20 years ago by James Chin (2000), nearly all pathogens, which colonizes/or replicates in the upper respiratory tract, have the potential to be transmitted through airborne droplets. However, the understanding of aerosol or droplet classification of infectious diseases in indoor environments requires some exposition into the vulnerability of HVAC systems to microbial spreads.
The source and circulation pathways of building ventilation systems affect the distribution and colony of indoor microbial populations (Dietz et al., 2020). HVAC systems have been relied on for ages to deliver outside air directly into a spatial volume through the perimeter of the building. However, the HVAC systems are vulnerable to microbial spreads, especially in enhancing the diversity of indoor fungal, viral and bacterial populations. In most cases, energy-saving considerations have made the design of buildings airtight— windows stay shut, devoid of daylighting and natural ventilation, and HVAC systems continue to recirculates the same air, with a small percentage of fresh air-makeup. In indoor environment, there are certain factors that are essential in guaranteeing occupants' comfort. In a review on indoor cleaner air production, Mujan et al. (2019) examined those factors that affect human health and productivity in any given indoor environment. The review recommended the use of sensors to gather data on the four key factors (indoor air quality and ventilation, thermal, visual, and acoustic comfort) that influence occupants' comfort in an indoor built environment in order to advance research activities to attain higher standards in occupants’ comfort.
Indoor airborne diseases have been associated with inefficient and badly managed HVAC systems: wrong air distribution (Nazarian and Kleissl, 2016), filtration, humidity, and temperature controls of HVAC systems often lead to disastrous outcomes (Cabral, 2010; Cristina et al., 2009). An outbreak of Aspergillus in an operating room, which infected patients who have been operated on in the same operating theater within a 12-day period, is a classic example of the danger of poorly managed HVAC systems. As reported by Lutz et al., air sampling in the operating room later revealed ≥3 μm aerosols of the surrogate marker of Aspergillus conidia (Lutz et al., 2003). During the study, the group of investigators also cultured samples from ductworks, diffusers and other duct materials for Aspergillus, and discovered that they were all contaminated with the fungi. The study thus concluded that no case of Aspergillus infection was reported after the HVAC system was remediated. Other reported outbreaks of airborne infectious diseases transmission aided by the HVAC system involved methicillin-resistant Staphylococcus aureus in a burn unit at the North Carolina Memorial Hospital (Rutala et al., 1983), and tuberculosis at Jackson Memorial Hospital in Miami (EHRENKRANZ and KICKLIGHTER, 1972). The ongoing outbreak of SARS-CoV-2 has equally been aided by the operation and conditions of the HVAC systems. The first example involved the 37 confirmed cases out of 68 individuals who shared the same bus together on 100-min trips in China (Shenet al., 2020a). The second important example is that of the Diamond Princess cruise ship, in which 712 confirmed cases were reported out of 3711 individuals voyaging together on the ship (Azimi et al., 2021). The third example involved nine confirmed cases; an index case (had previously travelled to Wuhan, the ground zero of COVID-19) who resided in the apartment 15b, infected four other occupants in the apartments 25b and 27b in a vertically aligned housing complex in China (Linet al., 2021).
Table 1 shows reported studies in the literature on the role of HVAC systems in the spread of harmful microbes in the built environment. Fifteen different studies are presented in Table 1. One study reported the spread of H3N2 virus in an aircraft cabin that affected 38 individuals (Moser et al., 1979). Another study highlighted the spread of Aspergillus in a hospital in which six individuals were infected (Lutz et al., 2003). Two studies reported the spread of SARS-CoV-1, the first study involved 156 individuals in a hospital (Leeet al., 2003), and the second study involved 300 people in an apartment complex hospital (Yuet al., 2004). One study involved the spread of MERS in a hospital that affected three individuals (Kimet al., 2016). Ten studies involved the spread of the current SARS-CoV-2 outbreak at different built environments, such as departmental stores (Jianget al., 2021), hospitals (Onget al., 2020; Santarpiaet al., 2020), restaurants (Luet al., 2020; Liet al., 2020), public transport (Shenet al., 2020b), etc.
Table 1.
Reported studies on the role of HVAC systems in the spread of virus/fungus in the built environment.
| S/N | Reference | Year | Virus/Fungus type | Country | Building type | Cases | Comments/Role of HVAC systems |
|---|---|---|---|---|---|---|---|
| 1 | Moser et al. (1979) | 1979 | H3N2 | USA | Aircraft | 38 | The HVAC system of the aircraft was turned off due to a faulty aircraft engine, and this was suspected to have aided the spread of the virus. |
| 2 | Lutz et al. (2003) | 2003 | Aspergillus | USA | Hospital | 6 | HVAC contaminated ductwork and air supply to operating room was observed. |
| 3 | Leeet al. (2003) | 2003 | SARS-CoV-1 | Hong Kong | Hospital | 156 | Airborne aerosol was suspected in the study to aid the spread of the virus. |
| 4 | Yuet al. (2004) | 2004 | SARS-CoV-1 | China | Community housing | 300 | Site visit and observation. CFD Modelling |
| 5 | Kimet al. (2016) | 2016 | MERS | South Korea | Hospitals | 3 | Analysis of air samples from patients' rooms and toilets, and one common corridor. Contamination of HVAC air supply was confirmed. |
| 6 | Van Doremalenet al. (2020) | 2020 | SARS-CoV-2 | – | Experimental study in laboratory | NA | SARS-CoV-2 remained viable in aerosols throughout the duration of 3 h experiment. |
| 7 | Luet al. (2020) | 2020 | SARS-CoV-2 | China | Restaurant | 10 | The airflow of the air conditioning system was suspected to have aided the spread of the virus in the restaurant. |
| 8 | Santarpiaet al. (2020) | 2020 | SARS-CoV-2 | USA | Medical center | 13 | Analysis of air samples from individual rooms, toilets and hallways confirmed the presence of viable SARS-CoV-2 in the air. |
| 9 | Liet al. (2020) | 2020 | SARS-CoV-2 | China | Restaurant | 10 | Aerosol transmission of SARS-CoV-2 due to poor ventilation was supported by simulation. |
| 10 | Milleret al. (2020) | 2020 | SARS-CoV-2 | USA | Choir rehearsal | 53 | The pattern of the number of attendees, the duration of the event and other technical factors contributing towards the airborne transmission of SARS-CoV-2 were analyzed. There is scientific evidence from the study supporting that the source of this outbreak is airborne transmission. |
| 11 | Onget al. (2020) | 2020 | SARS-CoV-2 | Singapore | Hospital | NA | Air samples of 13 rooms and 3 toilet areas were found with viable SARS-CoV-2. |
| 12 | Shenet al. (2020b) | 2020 | SARS-CoV-2 | China | Public transportation | 24 | 24 of 68 people were infected at a bus in Ningbo City, Zhejiang Province. Study confirmed that compared to individuals in the non-exposed bus, those in the exposed bus were 41.5 times more likely to be infected with COVID-19. |
| 13 | Moriartyet al. (2020) | 2020 | SARS-CoV-2 | USA | Cruise ship | 103 | Two died and at least 103 people were infected among 1111 crew and 2460 passengers in Grand Princess cruise ship |
| 14 | Wuet al. (2020) | 2020 | SARS-CoV-2 | China | Shopping mall | 40 | 40 people were infected at a shopping mall in Tianjin. The evidence from the study supports the airborne transmission of SARS-CoV-2. |
| 15 | Jianget al. (2021) | 2021 | SARS-CoV-2 | China | Department store/shop | 12 | Analysis of exposure history of the infected individuals (customer) who visited the store. Aerosol Transmission Modelling |
From the historical perspectives of the nature of airborne pathogens, and in the light of emerging understanding of SARS-CoV-2, it is appropriate to state that the design, operating condition of the HVAC systems and the location of air supply/extract points would play a critical role in the spread or control/prevention of harmful microbial agents in a conditioned space.
4. Discussion
The current statistics (Bluyssen, 2019) reveals that, on average, people spend 90% of their time indoors (a combination of the time spent at work and at home). As climate change challenges increase now more than ever (although it has been reported that COVID-19 pandemic has slowed down greenhouse gas emission (Wang and Su, 2020; Hepburn et al., 2020)), this statistics is expected to increase because more energy will be expended to either cool or heat buildings to achieve occupants' thermal comfort. A large proportion of sick building syndrome (SBS) emanates from conditioned air through the HVAC systems, especially due to the accumulation of microbes on the cooling coils or passage of microbes along the ducts and plenum. The SBS is identified as ill health symptoms that are associated with; asthma symptoms (e.g. wheezing), mucous membrane irritation (e.g. sore throat and nasal congestion), gastrointestinal disturbances, neurotoxic effect (fatigue and headache), sensitivity to odors, and dry skin. Moreover, severe toxicosis and cancer have also been linked to continuous exposure to mycotoxin in HVAC systems (Croft et al., 1967). It is now becoming rampant to have these symptoms appeared among occupants in school buildings, office buildings, hospitals, recreational facilities and public buildings. If the symptoms persist in most cases, it often leads to the closure of the facility (Takeda et al., 2009). It is still not entirely clear whether SBS is solely acquired through the outdoor atmospheric condition or through indoor occupants’ state of health or the conditions of the conduits that transport the conditioned air. However, the unexplained spread of SARS-CoV-2, especially in high occupant density built environments (such as hospitals, nursing homes, cruise ships, etc.) does not require critical thinking to conclude that the HVAC systems aid the spread of the virus (Nissenet al., 2020; Azimi et al., 2021).
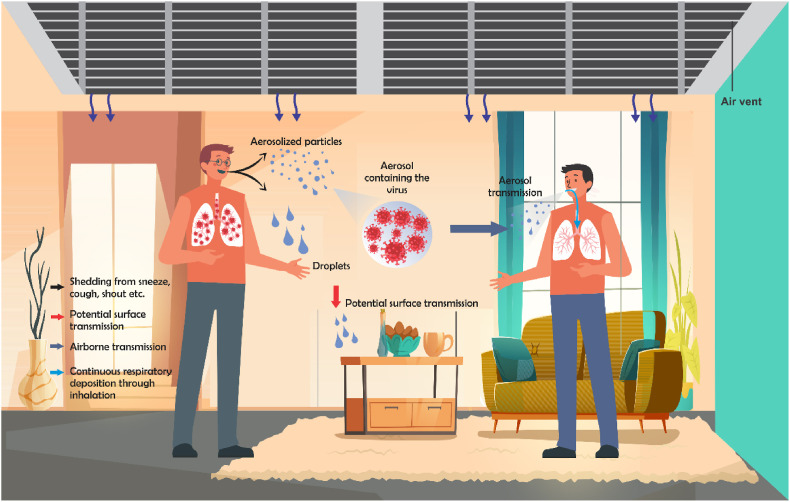
What makes a particular HVAC system vulnerable to the spread or harboring of microbial agents? In order to answer the question adequately, it is important to explain how and what makes a particular infectious microbe airborne especially in an indoor environment. A tiny particle that qualifies to be termed airborne is classified as that with an average aerodynamic diameter up to 2.5–10 μm (Mandell et al., 2009; Shaman and Kohn, 2009). While a large droplet is in the rage of 50–100 μm (Xie et al., 2007). Coronavirus families are in the range of 80–120 nm (Fung and Liu, 2019), which is about a factor of between 20 and 80 tinier than the classified airborne particles. There are two possible exposure routes of an infectious disease traveling through the air (see Fig. 1 ); (1) as droplets that are released by an infectious individual, which fall to the ground about 1 m from the point of release, and may be released as coughs, sneezes or shouts; (2) as tiny particles, which due to their relative weight continue to stay airborne for hours. These tiny particles are then transported and circulated through the HVAC systems, thereby constituting a contagion. The contagion fate of the first route (which involves large droplets) is not covered in this work, unless the droplets reduce in size by evaporation due to the surrounding atmosphere (dry air), and become aerosols like the second route.
Fig. 1.
Schematic representation of potential transmission routes of COVID-19 in an indoor environment.
Although there may be insufficient data to describe the detailed particle size distribution of sneezes, coughs, and shouts emanating from patients with infectious diseases, there have been researched works reported on particle size distributions of some coughed materials. Fennelly et al. (2004) measured the size distribution of coughs emanating from patients with tuberculosis, the results ranged from 0.65 to 3.3 μm. The obtained aerosol size distribution is similar to the one reported by Wainwright et al. (Wainwrightet al., 2009) from fibrosis patients’ coughed materials, which was ≤3.3 μm. While there is no reported work, to the best of our knowledge, on the size distribution of coughed materials emanating from coronavirus patients, high infectious viral load (39% precisely) was recovered in aerosols generated from coughed materials of influenza patients (Yanet al., 2018). Moreover, Xie et al. (2007) have comprehensively explored infectious droplet/aerosol conditions, movements and velocities in indoor environments, which have given more supports in favor of our perspective that COVID-19 is likely largely indoor airborne.
There is a mathematical model that predicts the probability of airborne infection. This model is termed Well-Riley's equation (see Eq. (1)), named after Riley and Nardell (1989) who proposed it.
| (1) |
Where C is the number of new infection; S is the number of those who are susceptible, i is the number of infectors, q is the number of doses or quantum of airborne infection and it is measured in quanta per hour (qph). p is the pulmonary ventilation per susceptible and it is measure in volume per hour, t is the exposure time measured in hour and Q is the volumetric flow rate of fresh or disinfected air and it is measured in volume per hour.
This model has been used in the past for certain infectious diseases like tuberculosis (Catanzaro, 1982) and measles (Riley et al., 1978). The exponential term (1-e-iqpt/Q) is the probability of infecting an individual that is susceptible to a particular infection. Although it is problematic to estimate q because the infectious doses of many infectious diseases are difficult to determine, Catanzaro et al. (Catanzaro, 1982) reported q to be in the range of 1.25–249 qph for tuberculosis while Riley et al. (1978) reported q to be 5480 qph for measles. However, for the current SARS-CoV-2 outbreak, Buonanno et al. (2020) has reported the value of q to be in the range of 10.5–1030 qph, which depends on the level of respiratory activity, for example, 320 qph was reported for singing, while 10.5 qph was reported for speaking (Amoatey et al., 2020). Another important variable in Eq. (1) that is relevant to this work is Q; it is the volumetric flow rate of injected disinfected or fresh air into a contaminated enclosure. For illustration, it is mathematically significant from Eq. (1) that when Q is large, the size of -iqpt/Q shrinks until it approaches zero, which ultimately turns C to zero. The objective of any HVAC expert working on the epidemiological pattern of any infectious disease is to achieve zero C in Eq. (1). Although the value of S can also drive down the value of C significantly due of their direct relationship, controlling S is more of a policy framework that is rooted in social distancing approach rather than an engineering principle. As a result, the only essential variable in Eq. (1) that requires HVAC experts’ inputs is Q. Raising the value of Q reduces exposure by diluting contaminated air with fresh or recycled decontaminated air. Q can equally be affected by other engineering control strategies, which include air filtration and the use of ultraviolet germicidal irradiation (UVGI). Hence, a more thorough analysis of Q should ensure total removal of infectious particles by filtration, ventilation, natural deactivation, agglomeration and/or any form of technological deactivation.
5. Containment and prevention recommendations
Buildings' HVAC systems are essential for maintaining indoor air quality, and play an important role in occupants’ thermal comfort and health (Cheet al., 2019). The primary infectious disease containment approach in buildings, which deals with the removal of infectious agents and dilution of air within an enclosed space, is always represented by an effective ventilation system (Liu et al., 2018). However, poorly managed HVAC systems have been reported (Borro et al., 2021/02) to be responsible for the transmission of airborne infectious diseases, for example, wrong air distribution or filtration or humidity or temperature controls may lead to “super spread” of harmful microbes (Megahed and Ghoneim, 2021). To reduce the vulnerability of an individual within a confined space to harmful microbes in the air or the exposure time, the main approach is to increase the air dilution rate (Nembhard et al., 2020). This strategy generally decreases pathogen concentrations in the air, and further minimizes the exposure time of objects, occupants, passengers, staff or patients to airborne microorganisms within the space. It is now confirmed that infectious disease aerosols stay viable up in the air within a confined space for a considerable amount of time (hours). HVAC system that is supplying, conditioning, extracting, and recirculating air can transmit airborne diseases and must be carefully designed, operated and maintained to avoid such health hazards (Capolongo et al., 2017; Saranet al., 2020; Dose). A combination of carefully designed air-handling units and fan-coil units (terminal heating/cooling devices inside a building) as part of a central HVAC system with full fresh air supply would also help minimize the spread of SARS-CoV-2 in a conditioned space (Chirico et al., 2020; Lu et al., 2009).
In general, there are three basic parts of an HVAC system (Saranet al., 2020)— (1) Outside/Fresh air intake and filtration, and air exhaust ducts with associated systems, (2) Air Handling Units (AHU) with heating, cooling coils and humidity control devices, and (3) Indoor air distribution systems. The following HVAC system/ventilation options are of interest to reduce the transmission of disease in the built environment (Dietz et al., 2020).
-
•
Increase fresh air supply, avoid recirculation/transfer of air from one room/zone to another
-
•
Dilution ventilation - mixing the air in a space with fresh air from outside and/or in-room filtered air
-
•
Consider increasing ventilation rates in toilets and communal/circulation areas of the building as per recommended guidelines by consultants/designers
-
•
Air filtration (central or within the room via portable air purifier) with HEPA or ULPA filter system
-
•
Laminar in-room or localized flow regimes will help minimize the risk of microbes and infectious air spread in the entire building
-
•
Moisture control to prevent mould growth and the spread of contamination (relative humidity of air in the range of 40–60% is recommended)
-
•
Displacement ventilation preferred over mixing ventilation system (where possible to implement)
-
•
Differential room pressurization, local source capture or personalized ventilation system will help containing the contaminated air and discharging it to the outside atmosphere
-
•
The use of UVGI (in the airstream and/or in-room upper level)
-
•
Central HVAC system - AHUs with full fresh air supply and indoor fan coil units as terminal space conditioning devices.
HEPA filters can capture 99.97% of aerosols with particle diameter that are 0.3 μm or larger, while the ULPA (Ultra-Low Particulate Air) filters can capture 99.99% of aerosols with particle diameter that are 0.12 μm and above, which makes them better than HEPA filters for the containment of airborne disease transmission (Joppolo and Romano, 2017). The deployment of industrial or commercial scale portable air filtration devices, such as ULPA or HEPA filters can reduce the likelihood of COVID-19 spread in locations where interaction amongst individuals is desirable on a regular basis (offices, laboratories, schools, shopping malls, hospitals, restaurants, cruise ships and metros etc.) (Elias and Bar-Yam, 2020b). Thus, the use of local air purifiers can help minimize the risks of infection (Dietz et al., 2020; Saranet al., 2020). The three characteristics that determine the effectiveness of an in-room air cleaner in reducing a room's aerosol level under typical operating scenarios are: (1) single-pass filtration efficiency, (2) filtration airflow rate, and (3) the airflow pattern that the cleaner induces in the room. These characteristics should be carefully investigated while applying an in-room air cleaner for a specific application (Vladimir Kogan et al., 2008).
Results of recent studies (Zhao et al., 2020; Mousavi et al., 2020) have revealed that at the beginning of an outbreak, such as the present COVID-19 disease, hospitals, particularly those positioned around the epicenter of the pandemic, would be busy providing treatments to the patients as they are brought in. Pressure would mount on the hospital due to shortage of time, space and work force. There may not be enough isolation rooms available to contain the influx. The design of isolation rooms is done to allow quick removal or containment of pathogenic materials. As a result, innovative actions must be taken to prepare the hospital system for such an emergency. To prevent the spread of harmful microbial agents in the general areas of the hospitals order than quarantine or isolation wards, portable HEPA air purifier and plastic sheets are a temporary solution that is recommended to be deployed (Christopherson et al., 2020). The plastic sheet barrier can prevent the spread of nearly 80% of contagious particles from penetrating the adjacent spaces even when portable HEPA air purifiers are too expensive to be deployed (Mousavi et al., 2020). Together with a portable HEPA air purifier, the use of plastic sheets can raise the level of infectious aerosol containment to 99% or above (Mousavi et al., 2020). Moreover, for confirmed or suspected COVID-19 patients who are isolated at home, the deployment of air purifiers can decrease the chance of exposure to individuals in their households (Elias and Bar-Yam, 2020c). To prevent reinfection or secondary contamination, the used filter should be properly disposed of, or for total prevention, air purifier filter with disinfection capacity should be preferred.
A particle counter can be used from time to time to assess the presence of contaminants' aerosols (viable and non-viable) in the indoor air. For this purpose, a calibrated optical particle counter (OPC) can be used, to monitor the particulates sizes by taking a sample of the air in a specified space (Capolongo et al., 2017). The OPC has the capability to measure different particle sizes, most commonly 0.5 μm and 5.0 μm, but the latest technology and more sophisticated techniques can help detect particulate sizes smaller than 0.5 μm (Capolongo et al., 2017). An active microbial air sampler (AMAS) can be used to measure the viable aerosolized particles in a defined air volume (Sandle, 2010). The AMAS carries out quantitative microbial analysis of the air, and account for the number of colony-forming units (CFU) per volume of the air sample. The colonies of microorganisms are counted from the incubated air sample that contains the viable airborne particulates. The OPC measurements give immediate results and are quite simple to perform (Jaenicke, 1972). The OPC is widely used for quantitative assessment of hospitals and other types of buildings’ ventilation systems to verify the dilution effects, filtration efficiency, HVAC system performance and indoor air quality (Mirhoseini et al., 2015). To determine cleanliness of space, the OPC measurements for indoor and outdoor space should be compared (Capolongo et al., 2017; Saranet al., 2020).
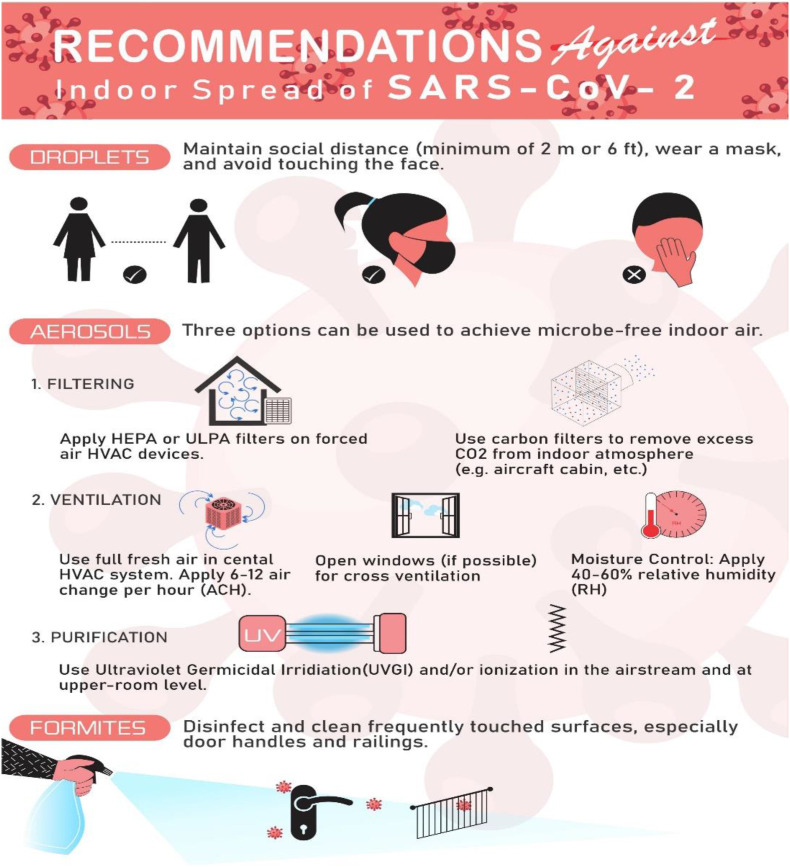
It is noteworthy that infectious air dilution and dispersion through effective ventilation systems are more important in healthcare buildings’ context compared to other types of buildings (Capolongo et al., 2017; Saranet al., 2020). Addressing the goals of IAQ and prevention of infectious diseases in buildings are accompanied by the need for ventilation as an effective way to control infections in confined spaces, and there is a challenge of high-energy consumption of HVAC systems. There should be a comprehensive strategy in place for energy efficient HVAC system to reduce the carbon footprint of buildings, which does not aid the transmission of infectious disease agents (Capolongo et al., 2017; Saranet al., 2020). In HVAC systems, effective air filtration plays an essential role in trapping particulate contaminants and microbiological pathogens from the circulating air. To achieve different grades of cleanliness, various grades of filtration systems can be used. An appropriate air filtration system for healthcare buildings is recommended to be two to three stages of filtration (Joppolo and Romano, 2017). The final filter shall be placed at the air diffuser side before discharging the supply air into space and with at least 90% efficiency (Joppolo and Romano, 2017). In critical areas like operating rooms and airborne infection isolation rooms (AIIRs), the final filter should be capable of filtering nearly all the particles of 0.1–0.2 μm diameter and larger sizes (Joppolo and Romano, 2017). Fig. 2 below summarizes the major prevention recommendations obtained from reviewed articles.
Fig. 2.
Recommended actions against indoor spread of SARS-CoV-12 either as droplets or as aerosols.
To reduce the infections in hospitals, World Health Organization (WHO) and the Centre for Disease Control and Prevention (CDC) recommend a minimum ventilation rate of 12 ACH (Memarzadeh and Xu, 2012) and isolating suspected COVID-19 patients in airborne infection isolation rooms (AIIRs) (Organization, 2020). The isolation facility should be negatively pressurized (Chinn and Sehulster, 2003) and supplied with 100% fresh air combined with HEPA filtration of the incoming fresh air (Villafruela et al., 2019). Where the local weather conditions and design of the building allow, natural ventilation through windows, doors, wind catchers and other vents can be used to bring fresh outside air to the building. The increased natural ventilation rate will help dilute the room/building air around a source of infectious agents and remove any airborne virus particles from the building (Capolongo et al., 2017; Saranet al., 2020). However, to control the spread of infection inside hospitals and healthcare buildings, natural ventilation through openable windows is not recommended by most ventilation standards and guidelines (Joppolo and Romano, 2017; E and "-19 Ve, 2020).
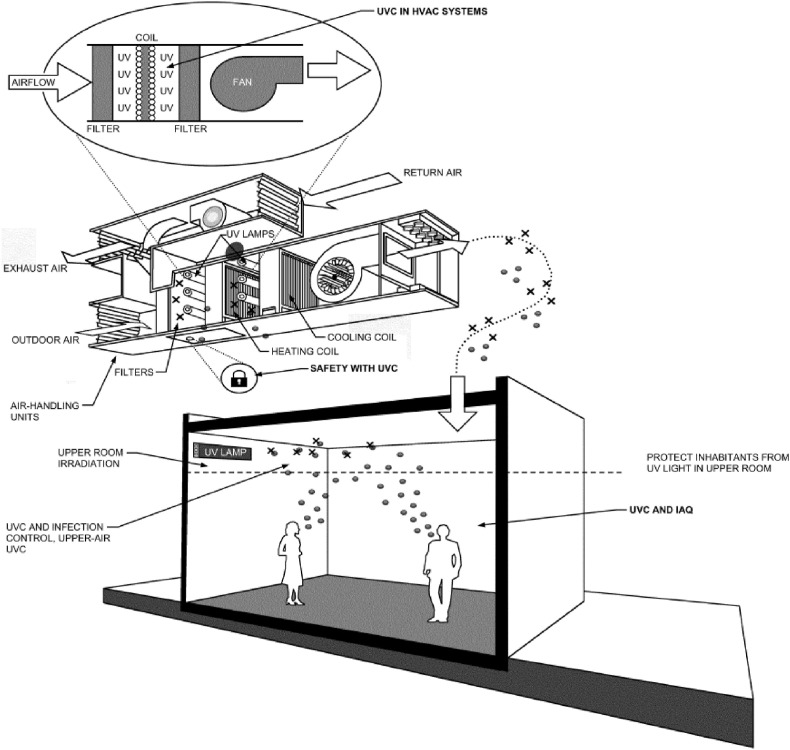
For the purpose of air and surface disinfection (see Fig. 3 ), UVC lamp devices are recommended to be placed in air handling units and at the upper-room level of space to deactivate microbes within (Edition, 2011). In-duct UVC systems inside air handling units can help prevent microorganisms and mould growth on cooling coils and other surfaces (Santos et al., 2020). This will improve the cooling coil efficiency and help reduce the pressure drop across air handling units, resulting in lower energy cost for air supply and removal of infection sources inside air handling units. To optimize the cost and energy use for conditioning space, the application of UVC can be combined with conventional air quality control methods, including particulate filtration, carbon filtration (carbon capture from recirculation air) and dilution ventilation (Dose; Edition, 2011).
Fig. 3.
Potential Applications of UVC to Control Microorganisms in Air and on Surfaces. UVC system, in-duct and upper-room level. Source: ASHRAE. Ultraviolet Air and Surface Treatment. ASHRAE Handbook-HVAC Appl 2011. (Edition, 2011).
To maintain a comfortable and healthy environment inside commercial aircrafts' cabins for the passengers and crewmembers, the air distribution system plays a vital role (Desai et al., 2020). Compared to other means of public transportation, the aircrafts’ cabins indoor environment is one of the most complex among all.
To control the spread of airborne contaminants while at the same time maintaining passengers' thermal comfort, suitable ventilation strategies for ensuring air quality must be employed. Different strategies have been investigated for controlling and improving air quality and thermal comfort inside aircrafts' cabins (You et al., 2019; Du et al., 2017; Fang et al., 2018). Various types of contaminants and gases are produced in aircrafts' cabins environment (Li et al., 2014). The important ones considered are VOCs, carbon dioxide, ozone, ethanol and disinfection pesticides. The source of main particulate contaminants, associated with the risk of spreading infectious air inside an aircraft cabin, originates from coughing, sneezing and breathing (Gupta et al., 2012). The main three categories of airflow distribution for the ventilation system of an aircraft are mixing, displacement and personalized airflow distribution systems (Zhang and Chen, 2007). A Personalized displacement ventilation system is widely considered to provide improved air quality while at the same time ensures occupants’ thermal comfort compared to the conventional ventilation systems (Youet al., 2018).
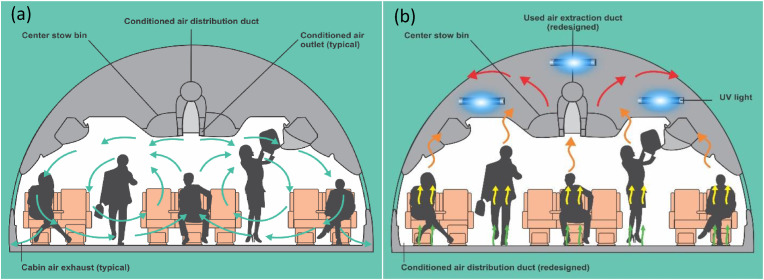
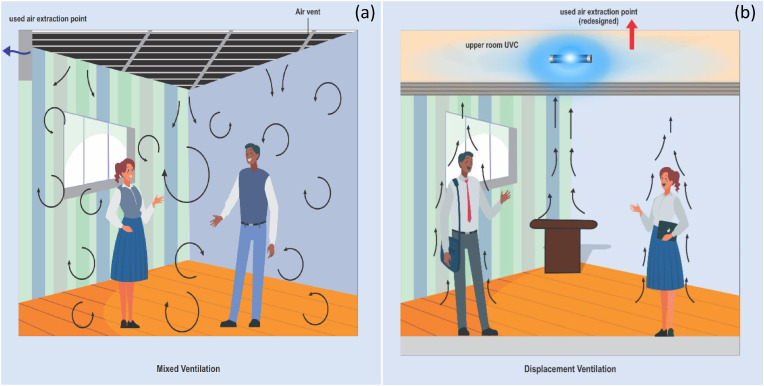
The current ventilation arrangement (mixed-air ventilation system see Fig. 4 a) inside the airplane cabin is designed for passengers to receive conditioned air from the top (over the head of the passenger), and then the air is extracted at the bottom (at the feet of the passengers) (Zhang and Chen, 2007). This approach allows infectious diseases to be transmitted on board the airplane if a passenger with infectious disease is onboard (Zhang and Chen, 2007). Cold air (from the aircraft air-conditioning system) is supplied from the top, cools down the passengers and it is collected at the feet of the passengers. Having cooled down the passengers, the air becomes warm, and from ideal gas law (which air obeys to certain extents), warm air is lighter than cold air and is expected to travel to the top naturally. As a result, a part of the infectious air will escape the suction system at the feet of the passengers, travels to the top and then spreads to other parts of the cabin (which is the main issue with the mixed-air ventilation).
Fig. 4.
Schematic representation of aerodynamic differences between (a) typical mixed-air ventilation system and (b) personalized displacement ventilation system inside the aircraft cabin.
In order to prevent this, we recommend a different approach (personalized displacement ventilation system see Fig. 4 b) (Youet al., 2018) that will change the current aerodynamics in the airplane cabin; fresh air is supplied from the lower level of the cabin (as shown in Fig. 4 b), or from the side (at the level of the armrest) or in front (where the entertainment screen is located) of the passengers, cools down the passengers and is collected at the top of the cabin following natural convection. This approach is similar in principle to underfloor ventilation concept reported by Alajmi and El-Amer (2010). With this, any infectious agent released by the infected passenger(s) will be summarily localized and collected at the upper roof of the cabin. At the point of collection of the used air at the upper roof of the cabin, germicidal ultraviolet (UVC) light will be stationed there to deactivate any microbe therein (Welchet al., 2018).
There have been reports that high concentration of CO2 in an enclosed space is responsible for wheezing attacks in children with asthma history (Kim et al., 2002), tiredness and muscular pains (Jung et al., 2009), increase in airborne bacterial markers (Fox et al., 2003), sicknesses (cough, headache, rhinitis, wheezing and irritation of mucus membrane) (Ferreira and Cardoso, 2014), especially where fresh air circulation may be poor (eter.com. "High2 L, 2019).
The use of CO2 carbon filter to reduce the concentration of CO2 in the aircraft/building/metro is another recommendation (Rosenberger, 2018). Used air by the passengers is collected from the top of the cabin (following displacement ventilation system) and treated with UVGI as explained in Fig. 4b. Thereafter, the air passes through the embedded carbon filter in the air duct, which is treated with adsorbents capable of selectively capturing CO2 from the air stream at ppm level (Lakhiet al., 2015; Wilson and Tezel, 2020; Amhamed et al., 2014). The filter material may be modified with nanomaterials (e.g. carbon nitride or metal-organic frameworks, etc.) that have high CO2 uptake in ambient air (Wilson and Tezel, 2020; Altamash et al., 2020). The deployment of nanoporous CO2 adsorption filters in indoor atmosphere (for example inside the aircraft cabin) would enhance the indoor air quality as well as increase the aircraft HVAC energy efficiency. This is because the use of CO2 adsorption filters would reduce the percentage of fresh air makeup needed to dilute the CO2-ladened atmosphere inside the aircraft, thereby contributing to the energy efficiency (Kim et al., 2015, 2020).
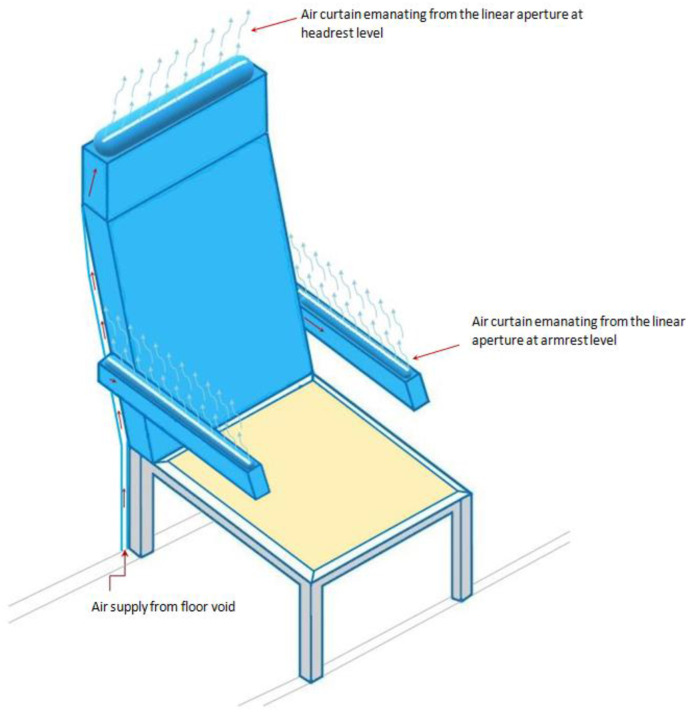
Another important technique to localize the infectious air in order to minimize the risk of contagion in an aircraft's cabin is the use of noiseless and invisible air barrier (air-curtains/air-blades) around the passengers' seats. With this, droplets and aerosols (produced when passengers, seated next to each other, cough, sneeze, snore, shout, or talk) will be largely contained within a space of a single passenger, and the risk of inhalation by the nearby passengers will be minimized. The air curtain follows similar air-blade principles found in high-pressure hand dryer (Drever, 2012) or those of air curtains used at the entrance of buildings (Goubran et al., 2016) for energy saving purposes. However, the newly proposed air-curtain is noiseless (with embedded muffler) and less pressurized (remotely located air supply fan away from the cabin environment). The air curtains extend from the armrest level (for the left and right sides of a seat) and at the headrest level (for the back side) to the crest (ceiling) of the cabin (see Fig. 5 ), where the air is extracted as part of an active displacement ventilation system, in which air is supplied from the lower level and extracted at the upper level of the cabin. This is in contrast to the state-of-the-art (TEAGUE, 2020), in which a 3D-printed component is mounted on the gasper vent to apply the air-curtain from top to bottom as part of a mixed-air ventilation system.
Fig. 5.
Inflatable jacket (seat cover) mounted on a typical cabin seat showing air-curtain's air distribution around the passenger.
A similar concept can be replicated in buildings. It is important to note that the work environment during and after COVID-19 pandemic will change dramatically to conform to the new world created by the spread of the virus (E and "-19 Ve, 2020). In order to maintain trust and confidence among colleagues at work, certain precautionary conditions must be met to assure individuals of their safety against viral infections. The application of upper-room and in-duct UVC system (Edition, 2011), when combined with HEPA/ULPA filters in a displacement ventilation system (Zhang and Chen, 2005, 2007), are equally recommended for minimizing the risk of spreading infectious and virus laden air inside confined spaces (Bosbach et al., 2013; Elmaghraby et al., 2018). Just as explained in Fig. 4 a above, most buildings use mixed-air ventilation system (see Fig. 6 a) that allows the spread of microbes in indoor atmosphere.
Fig. 6.
Schematic representation of aerodynamic differences between (a) typical mixed-air ventilation system and (b) personalized displacement ventilation system in occupied buildings.
In order to avert this, we recommend a similar concept (displacement ventilation system) in Fig. 6 b, just as that recommended in Fig. 4 b, that will change the current aerodynamics inside the buildings; fresh air is supplied from the bottom (as shown in Fig. 6 b), mimicking personalized underfloor ventilation reported by Cermak and Melikov (2007), cools down the occupants and is collected in the upper room following natural convection. With this, any infectious agent released by infected occupant(s) will be transported and collected at the upper room. At the point of collection of the used air at the upper room, germicidal ultraviolet (UVC) light (Welchet al., 2018) will be positioned there to deactivate any microbe therein for the used air to be recycled.
6. Conclusion
This work has reviewed the existing literature on infectious diseases' spread patterns in order to report our perspective on the indoor airborne nature of COVID-19. We have been able to show, from the historical perspective of the outbreak of communicable diseases and the current emerging understanding of microbes’ nature, that SARS-CoV-2 is airborne in indoor environments. We have equally been able to show, also from reported works, that social distancing policy within a confined space may only stop the virus from being transmitted through the droplets, however, aerosol transmission is still possible through the airflow of the HVAC systems. It should be noted that little attention has been paid to this viewpoint by governments and the public, our perspective however rests on the evidences presented in the literature, which further reveal that the spread may be aided by the conditions of the HVAC systems. We have recommended an innovative air circulation concept, which localizes a communicable disease to the infected individual(s) alone, in addition to the use of UVGI, in combination with a nanoporous air filter in enclosed spaces, such as onboard the airplanes, inside the metro tubes, in the staterooms on cruise ships, inside the hospitals, in commercial and government buildings. For future work, we believe research efforts should focus on achieving the stated proposed personalized displacement ventilation system with simulations that can be validated by experimental work. This will add an effective ventilation strategy to the pool of existing ventilation systems. Research effort should equally consider the capital investment and operating cost of the required number of UVC devices within an enclosed space for effective disinfection of the surrounding air. These recommendations are not conceived to override the current protective/preventive measures, such as social distancing, personal hygiene, and the use of facemasks, however, lasting engineering solutions must be sought to prevent future occurrences.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors acknowledge the financial support from Qatar Environment and Energy Research institute (QEERI), Hamad bin Khalifa University (HBKU) (member of Qatar Foundation), and Qatar National Research Fund, NPRP12C-0821–190017, Doha, Qatar.
References
- The Economic Times. The Economic Times; 2020. WHO acknowledges 'evidence emerging' of airborne spread of COVID-19. [Google Scholar]
- Al Huraimel K., Alhosani M., Kunhabdulla S., Stietiya M.H. SARS-CoV-2 in the environment: modes of transmission, early detection and potential role of pollution. Sci. Total Environ. 2020:140946. doi: 10.1016/j.scitotenv.2020.140946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajmi A., El-Amer W. Saving energy by using underfloor-air-distribution (UFAD) system in commercial buildings. Energy Convers. Manag. 2010;51(8):1637–1642. [Google Scholar]
- Altamash T., Amhamed A., Aparicio S., Atilhan M. Effect of hydrogen bond donors and acceptors on CO2 absorption by deep eutectic solvents. Processes. 2020;8(12):1533. [Google Scholar]
- Amhamed A., Atilhan M., Berdiyorov G. Permeabilities of CO2, H2S and CH4 through choline-based ionic liquids: atomistic-scale simulations. Molecules. 2014;24(10) doi: 10.3390/molecules24102014. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoatey P., Omidvarborna H., Baawain M.S., Al-Mamun A. Eng), Sci Total Environ. vol. 733. 2020. Impact of building ventilation systems and habitual indoor incense burning on SARS-CoV-2 virus transmissions in Middle Eastern countries. 139356-139356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augenbraun B.L., et al. Assessment and mitigation of aerosol airborne SARS-CoV-2 transmission in laboratory and office environments. J. Occup. Environ. Hyg. 2020;17(10):447–456. doi: 10.1080/15459624.2020.1805117. [DOI] [PubMed] [Google Scholar]
- Azimi P., Keshavarz Z., Laurent J.G.C., Stephens B., Allen J.G. Mechanistic transmission modeling of COVID-19 on the Diamond Princess cruise ship demonstrates the importance of aerosol transmission. Proc. Natl. Acad. Sci. Unit. States Am. 2021;118(8) doi: 10.1073/pnas.2015482118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma K., Yanagi U., Kagi N., Kim H., Ogata M., Hayashi M. Environmental factors involved in SARS-CoV-2 transmission: effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ. Health Prev. Med. 2020;25(1):1–16. doi: 10.1186/s12199-020-00904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluyssen P.M. Towards an integrated analysis of the indoor environmental factors and its effects on occupants. Intell. Build. Int. 2019:1–9. [Google Scholar]
- Booth T.F., et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191(9):1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borro L., Mazzei L., Raponi M., Piscitelli P., Miani A., Secinaro A. The role of air conditioning in the diffusion of Sars-CoV-2 in indoor environments: a first computational fluid dynamic model, based on investigations performed at the Vatican State Children's hospital. Environ. Res. 2021/02/01/2021;193:110343. doi: 10.1016/j.envres.2020.110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosbach J., Lange S., Dehne T., Lauenroth G., Hesselbach F., Allzeit M. Alternative ventilation concepts for aircraft cabins. CEAS Aeronautical Journal. 2013;4(3):301–313. [Google Scholar]
- Buonanno G., Stabile L., Morawska L. Estimation of airborne viral emission: quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020;141:105794. doi: 10.1016/j.envint.2020.105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral J.P. Can we use indoor fungi as bioindicators of indoor air quality? Historical perspectives and open questions. Sci. Total Environ. 2010;408(20):4285–4295. doi: 10.1016/j.scitotenv.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Capolongo S., Settimo G., Gola M. Springer; 2017. Indoor Air Quality in Healthcare Facilities. [Google Scholar]
- Catanzaro A. Nosocomial tuberculosis. Am. Rev. Respir. Dis. 1982;125(5):559–562. doi: 10.1164/arrd.1982.125.5.559. [DOI] [PubMed] [Google Scholar]
- Cermak R., Melikov A.K. Protection of occupants from exhaled infectious agents and floor material emissions in rooms with personalized and underfloor ventilation. HVAC R Res. 2007;13(1):23–38. [Google Scholar]
- Che W.W., et al. Energy consumption, indoor thermal comfort and air quality in a commercial office with retrofitted heat, ventilation and air conditioning (HVAC) system. Energy Build. 2019;201:202–215. [Google Scholar]
- Chen C., Zhao B. Makeshift hospitals for COVID-19 patients: where health-care workers and patients need sufficient ventilation for more protection. J. Hosp. Infect. 2020;105(1):98–99. doi: 10.1016/j.jhin.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Hao X., Zhang X., Chen F. Have traffic restrictions improved air quality? A shock from COVID-19. J. Clean. Prod. 2020;279:123622. doi: 10.1016/j.jclepro.2020.123622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P.Y., et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020;11(1):1–7. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. 2000. Control of Communicable Diseases Manual. [Google Scholar]
- Chinn R.Y., Sehulster L. HICPAC); 2003. Guidelines for Environmental Infection Control in Health-Care Facilities; Recommendations of CDC and Healthcare Infection Control Practices Advisory Committee. [PubMed] [Google Scholar]
- Chirico F., Sacco A., Bragazzi N.L., Magnavita N. Can air-conditioning systems contribute to the spread of SARS/MERS/COVID-19 infection? Insights from a rapid review of the literature. Int. J. Environ. Res. Publ. Health. 2020;17(17):6052. doi: 10.3390/ijerph17176052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson D.A., Yao W.C., Lu M., Vijayakumar R., Sedaghat A.R. High-efficiency particulate air filters in the era of COVID-19: function and efficacy. Otolaryngology-Head Neck Surg. (Tokyo) 2020;163(6):1153–1155. doi: 10.1177/0194599820941838. [DOI] [PubMed] [Google Scholar]
- Correia G., Rodrigues L., Da Silva M.G., Gonçalves T. Airborne route and bad use of ventilation systems as non-negligible factors in SARS-CoV-2 transmission. Med. Hypotheses. 2020;141:109781. doi: 10.1016/j.mehy.2020.109781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristina M.L., Sartini M., Spagnolo A.M. Health care-acquired aspergillosis and air conditioning systems. J Prev Med Hyg. 2009;50(1):3–8. [PubMed] [Google Scholar]
- Croft W.A., Jarvis B.B., Yatawara C. Airborne outbreak of trichothecene toxicosis. Atmos. Environ. 1967;20(3):549–552. 1986. [Google Scholar]
- Desai P.S., Sawant N., Keene A. medRxiv; 2020. On COVID-19-Safety Ranking of Seats in Intercontinental Commercial Aircrafts: A Preliminary Multiphysics Computational Perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz L., Horve P.F., Coil D., Fretz M., Van Den Wymelenberg K. 2019. Novel Coronavirus (COVID-19) Outbreak: A Review of the Current Literature and Built Environment (BE) Considerations to Reduce Transmission; p. 2020. [Google Scholar]
- Dietz L., Horve P.F., Coil D.A., Fretz M., Eisen J.A., Van Den Wymelenberg K. 2019 novel coronavirus (COVID-19) pandemic: built environment considerations to reduce transmission. mSystems. 2020;5(2) doi: 10.1128/mSystems.00375-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U. Dose, "Ultraviolet air and surface treatment".
- Drever J.L. 2012. Sanitary Ambiance: the Noise Effects of High Speed Hand Dryers. [Google Scholar]
- Du X., Li B., Liu H., Wu Y., Cheng T. The appropriate airflow rate for a nozzle in commercial aircraft cabins based on thermal comfort experiments. Build. Environ. 2017;112:132–143. [Google Scholar]
- CIBSE . 2020. CIBSE COVID-19 Ventilation Guidance Version 4 October 2020,".https://www.cibse.org/knowledge/knowledge-items/detail?id=a0q3Y00000HsaFtQAJ [Online]. Available: [Google Scholar]
- S. Edition, "2011 ASHRAE HANDBOOK HVAC Applications-gearju. com".
- Ehrenkranz N.J., Kicklighter J.L. Tuberculosis outbreak in a general hospital: evidence for airborne spread of infection. Ann. Intern. Med. 1972;77(3):377–382. doi: 10.7326/0003-4819-77-3-377. [DOI] [PubMed] [Google Scholar]
- Elias B., Bar-Yam Y. New England Complex Systems Institute; 2020. Could Air Filtration Reduce COVID-19 Severity and Spread? [Google Scholar]
- Elias B., Bar-Yam Y. New England Complex Systems Institute; Cambridge, MA: 2020. Could Air Filtration Reduce COVID-19 Severity and Spread. [Google Scholar]
- Elias B., Bar-Yam Y. vol. 9. New England Complex Systems Institute; 2020. Could Air Filtration Reduce COVID-19 Severity and Spread. [Google Scholar]
- Elmaghraby H.A., Chiang Y.W., Aliabadi A.A. Ventilation strategies and air quality management in passenger aircraft cabins: a review of experimental approaches and numerical simulations. Science and Technology for the Built Environment. 2018;24(2):160–175. [Google Scholar]
- C. Meter.com . 2019. High CO2 Levels Indoors Will Surprise You.https://www.co2meter.com/blogs/news/1114862-high-co2-levels-indoors [Google Scholar]
- Fang Z., Liu H., Li B., Cheng Y. Thermal comfort and skin temperature responses to the supplied air from personal air nozzles in aircraft cabins. Indoor Built Environ. 2018;27(6):831–845. [Google Scholar]
- Faridi S., et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total Environ. 2020;725:138401. doi: 10.1016/j.scitotenv.2020.138401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennelly K.P., Martyny J.W., Fulton K.E., Orme I.M., Cave D.M., Heifets L.B. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am. J. Respir. Crit. Care Med. 2004;169(5):604–609. doi: 10.1164/rccm.200308-1101OC. [DOI] [PubMed] [Google Scholar]
- Ferreira A.M.d.C., Cardoso M. Indoor air quality and health in schools. J. Bras. Pneumol. 2014;40(3):259–268. doi: 10.1590/S1806-37132014000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Harley W., Feigley C., Salzberg D., Sebastian A., Larsson L. Increased levels of bacterial markers and CO 2 in occupied school rooms. J. Environ. Monit. 2003;5(2):246–252. doi: 10.1039/b212341j. [DOI] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73:529–557. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- Goubran S., Qi D., Saleh W.F., Wang L.L., Zmeureanu R. Experimental study on the flow characteristics of air curtains at building entrances. Build. Environ. 2016;105:225–235. [Google Scholar]
- Gupta J.K., Lin C.H., Chen Q. Risk assessment of airborne infectious diseases in aircraft cabins. Indoor Air. 2012;22(5):388–395. doi: 10.1111/j.1600-0668.2012.00773.x. [DOI] [PubMed] [Google Scholar]
- Hadei M., Hopke P.K., Jonidi A., Shahsavani A. A letter about the airborne transmission of SARS-CoV-2 based on the current evidence. Aerosol and Air Quality Research. 2020;20(5):911–914. [Google Scholar]
- Hadei M., et al. Presence of SARS-CoV-2 in the air of public places and transportation. Atmospheric Pollution Research. 2021 doi: 10.1016/j.apr.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn C., O'Callaghan B., Stern N., Stiglitz J., Zenghelis D. Will COVID-19 fiscal recovery packages accelerate or retard progress on climate change? Oxf. Rev. Econ. Pol. 2020;36 [Google Scholar]
- Jaenicke R. The optical particle counter: cross-sensitivity and coincidence. J. Aerosol Sci. 1972;3(2):95–111. [Google Scholar]
- Jiang G., et al. Aerosol transmission, an indispensable route of COVID-19 spread: case study of a department-store cluster. Front. Environ. Sci. Eng. 2021;15(3):1–12. doi: 10.1007/s11783-021-1386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joppolo C.M., Romano F. Indoor Air Quality in Healthcare Facilities. Springer; 2017. HVAC system design in healthcare facilities and control of aerosol contaminants: issues, tools, and experiments; pp. 83–94. [Google Scholar]
- Jung K.-S., Kim N.-S., Lee J.-D., HwangBo Y., Son B.-S., Lee B.-K. The association of subjective symptoms of students and indoor air quality in private academic facilities. Korean Journal of Environmental Health Sciences. 2009;35(6):468–477. [Google Scholar]
- Kim C., Lim Y., Yang J., Hong C., Shin D. Effects of indoor CO2 concentrations on wheezing attacks in children. Indoor Air. 2002;2 [Google Scholar]
- Kim M.K., Baldini L., Leibundgut H., Wurzbacher J.A., Piatkowski N. A novel ventilation strategy with CO2 capture device and energy saving in buildings. Energy Build. 2015;87:134–141. doi: 10.1016/j.enbuild.2014.11.017. [DOI] [Google Scholar]
- Kim M.K., Baldini L., Leibundgut H., Wurzbacher J.A. Evaluation of the humidity performance of a carbon dioxide (CO2) capture device as a novel ventilation strategy in buildings. Appl. Energy. 2020;259:112869. doi: 10.1016/j.apenergy.2019.03.074. [DOI] [Google Scholar]
- Kim S.-H., et al. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Rev. Infect. Dis. 2016;63(3):363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klontz K.C., Hynes N.A., Gunn R.A., Wilder M.H., Harmon M.W., Kendal A.P. An outbreak of influenza A/Taiwan/1/86 (H1N1) infections at a naval base and its association with airplane travel. Am. J. Epidemiol. 1989;129(2):341–348. doi: 10.1093/oxfordjournals.aje.a115137. [DOI] [PubMed] [Google Scholar]
- Lakhi K.S., et al. Cage type mesoporous carbon nitride with large mesopores for CO2 capture. Catal. Today. 2015;243:209–217. [Google Scholar]
- Lednicky J.A., et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Leung N.H., et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020:1–5. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. Is the coronavirus airborne? Experts can't agree. Nature. 2020;580(7802):175. doi: 10.1038/d41586-020-00974-w. [DOI] [PubMed] [Google Scholar]
- Li F., Liu J., Pei J., Lin C.-H., Chen Q. Experimental study of gaseous and particulate contaminants distribution in an aircraft cabin. Atmos. Environ. 2014;85:223–233. doi: 10.1016/j.atmosenv.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., et al. MedRXiv; 2020. Evidence for Probable Aerosol Transmission of SARS-CoV-2 in a Poorly Ventilated Restaurant. [Google Scholar]
- Lin G., et al. Community evidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission through air. Atmos. Environ. 2021;246:118083. doi: 10.1016/j.atmosenv.2020.118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Ma S., Cao G., Meng C., He B.-J. Distribution characteristics, growth, reproduction and transmission modes and control strategies for microbial contamination in HVAC systems: a literature review. Energy Build. 2018;177:77–95. [Google Scholar]
- Liu Y., et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Lu Z., Lu W., Zhang J., Sun D. Microorganisms and particles in AHU systems: measurement and analysis. Build. Environ. 2009;44(4):694–698. [Google Scholar]
- Lu J., et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China. Emerg. Infect. Dis. 2020;26(7) doi: 10.3201/eid2607.200764. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B.D., Jin J., Rinaldi M.G., Wickes B.L., Huycke M.M. Outbreak of invasive Aspergillus infection in surgical patients, associated with a contaminated air-handling system. Clin. Infect. Dis. 2003;37(6):786–793. doi: 10.1086/377537. [DOI] [PubMed] [Google Scholar]
- Mandell G., Dolin R., Bennett J. Elsevier; 2009. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (No. Ed. 7) [Google Scholar]
- Megahed N.A., Ghoneim E.M. Indoor Air Quality: rethinking rules of building design strategies in post-pandemic architecture. Environ. Res. 2021;193:110471. doi: 10.1016/j.envres.2020.110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memarzadeh F., Xu W. vol. 5. Springer; 2012. Role of air changes per hour (ACH) in possible transmission of airborne infections; pp. 15–28. (Building Simulation). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.L., et al. Transmission of SARS‐CoV‐2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air. 2020 doi: 10.1111/ina.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirhoseini S.H., Nikaeen M., Khanahmad H., Hatamzadeh M., Hassanzadeh A. Monitoring of airborne bacteria and aerosols in different wards of hospitals-Particle counting usefulness in investigation of airborne bacteria. Ann. Agric. Environ. Med. 2015;22(4) doi: 10.5604/12321966.1185772. [DOI] [PubMed] [Google Scholar]
- Morawska L., Cao J. Environment International; 2020. Airborne transmission of SARS-CoV-2: the world should face the reality; p. 105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty L.F., et al. Public health responses to COVID-19 outbreaks on cruise ships—worldwide, February–March 2020. MMWR (Morb. Mortal. Wkly. Rep.) 2020;69(12):347–352. doi: 10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M.R., Bender T.R., Margolis H.S., Noble G.R., Kendal A.P., Ritter D.G. An outbreak of influenza aboard a commercial airliner. Am. J. Epidemiol. 1979;110(1):1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- Mousavi E.S., Pollitt K.J.G., Sherman J., Martinello R.A. Performance analysis of portable HEPA filters and temporary plastic anterooms on the spread of surrogate coronavirus. Build. Environ. 2020;183:107186. doi: 10.1016/j.buildenv.2020.107186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujan I., Anđelković A.S., Munćan V., Kljajić M., Ružić D. Influence of indoor environmental quality on human health and productivity-A review. J. Clean. Prod. 2019;217:646–657. [Google Scholar]
- Nazarian N., Kleissl J. Realistic solar heating in urban areas: air exchange and street-canyon ventilation. Build. Environ. 2016;95:75–93. [Google Scholar]
- Nembhard M.D., Burton D.J., Cohen J.M. Ventilation use in nonmedical settings during COVID-19: cleaning protocol, maintenance, and recommendations. Toxicol. Ind. Health. 2020;36(9):644–653. doi: 10.1177/0748233720967528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen K., et al. Long-distance airborne dispersal of SARS-CoV-2 in COVID-19 wards. Sci. Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-76442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323(16):1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. World Health Organization; 2003. Consensus Document on the Epidemiology of Severe Acute Respiratory Syndrome (SARS) [Google Scholar]
- Organization W.H. World Health Organization; 2020. Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID-19 Disease Is Suspected: Interim Guidance, 13 March 2020. [Google Scholar]
- Repici A., et al. Gastrointestinal endoscopy; 2020. Coronavirus (COVID-19) Outbreak: what the Department of Endoscopy Should Know. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R., Nardell E. Clearing the air. Am. Rev. Respir. Dis. 1989;139:1286–1294. doi: 10.1164/ajrccm/139.5.1286. [DOI] [PubMed] [Google Scholar]
- Riley E., Murphy G., Riley R. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol. 1978;107(5):421–432. doi: 10.1093/oxfordjournals.aje.a112560. [DOI] [PubMed] [Google Scholar]
- Rosenberger W. Effect of charcoal equipped HEPA filters on cabin air quality in aircraft. A case study including smell event related in-flight measurements. Build. Environ. 2018;143:358–365. [Google Scholar]
- Rutala W., Katz E., Sherertz R., Sarubbi F. Environmental study of a methicillin-resistant Staphylococcus aureus epidemic in a burn unit. J. Clin. Microbiol. 1983;18(3):683–688. doi: 10.1128/jcm.18.3.683-688.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandle T. Selection of active air samplers. Eur. J. Parenter. Pharm. Sci. 2010;15(4):119–124. [Google Scholar]
- Santarpia J.L., et al. University of Nebraska Medical Center; MedRxiv: 2020. Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the. [Google Scholar]
- Santos A.F., Gaspar P.D., Hamandosh A., Aguiar E.B.d., Guerra Filho A.C., Souza H.J. L.d. Best practices on HVAC design to minimize the risk of COVID-19 infection within indoor environments. Braz. Arch. Biol. Technol. 2020;63 [Google Scholar]
- Saran S., et al. Heating, ventilation and air conditioning (HVAC) in intensive care unit. Crit. Care. 2020;24:1–11. doi: 10.1186/s13054-020-02907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen L., et al. ASHRAE; Atlanta, GA, USA: 2009. ASHRAE Position Document on Airborne Infectious Diseases. [Google Scholar]
- Shaman J., Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106(9):3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., et al. Community outbreak investigation of SARS-CoV-2 transmission among bus riders in eastern China. JAMA internal medicine. 2020;180(12):1665–1671. doi: 10.1001/jamainternmed.2020.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., et al. 2020. Airborne Transmission of COVID-19: Epidemiologic Evidence from Two Outbreak Investigations. [Google Scholar]
- Takeda M., Saijo Y., Yuasa M., Kanazawa A., Araki A., Kishi R. Relationship between sick building syndrome and indoor environmental factors in newly built Japanese dwellings. Int. Arch. Occup. Environ. Health. 2009;82(5):583–593. doi: 10.1007/s00420-009-0395-8. [DOI] [PubMed] [Google Scholar]
- Teague . 2020. "In Air Travel, Social Distancing Is Not Enough.https://teague.com/insights/aviation/airshield-concept [Google Scholar]
- Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19(1):101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafruela J.M., Olmedo I., Berlanga F.A., Ruiz de Adana M. Assessment of displacement ventilation systems in airborne infection risk in hospital rooms. PloS One. 2019;14(1) doi: 10.1371/journal.pone.0211390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimir Kogan C.H., Hesse David J., Hofacre Kent C. 2008. Evaluation of In-Room Particulate Matter Air Filtration Devices.www.epa.gov/ord EPA/600/R-08/012. [Google Scholar]
- Wainwright C.E., et al. Cough-generated aerosols of Pseudomonas aeruginosa and other Gram-negative bacteria from patients with cystic fibrosis. Thorax. 2009;64(11):926–931. doi: 10.1136/thx.2008.112466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Su M. A preliminary assessment of the impact of COVID-19 on environment–A case study of China. Sci. Total Environ. 2020:138915. doi: 10.1016/j.scitotenv.2020.138915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-X., Cao X., Chen Y.-P. An air distribution optimization of hospital wards for minimizing cross-infection. J. Clean. Prod. 2020;279:123431. doi: 10.1016/j.jclepro.2020.123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wat D. The common cold: a review of the literature. Eur. J. Intern. Med. 2004;15(2):79–88. doi: 10.1016/j.ejim.2004.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch D., et al. Far-UVC light: a new tool to control the spread of airborne-mediated microbial diseases. Sci. Rep. 2018;8(1):1–7. doi: 10.1038/s41598-018-21058-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.M., Tezel F.H. Direct dry air capture of CO2 using VTSA with faujasite zeolites. Ind. Eng. Chem. Res. 2020;59(18):8783–8794. [Google Scholar]
- Wu W., et al. Investigation and analysis on characteristics of a cluster of COVID-19 associated with exposure in a department store in Tianjin. Zhonghua liu xing bing xue za zhi= Zhonghua liuxingbingxue zazhi. 2020;41(4):489–493. doi: 10.3760/cma.j.cn112338-20200221-00139. [DOI] [PubMed] [Google Scholar]
- Xie X., Li Y., Chwang A., Ho P., Seto W. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17(3):211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Yan J., et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. Unit. States Am. 2018;115(5):1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Zhang L., Ma J., Zhou L. On airborne transmission and control of SARS-Cov-2. Sci. Total Environ. 2020;731:139178. doi: 10.1016/j.scitotenv.2020.139178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You R., Lin C.H., Wei D., Chen Q. Evaluating the commercial airliner cabin environment with different air distribution systems. Indoor Air. 2019;29(5):840–853. doi: 10.1111/ina.12578. [DOI] [PubMed] [Google Scholar]
- You R., et al. An innovative personalized displacement ventilation system for airliner cabins. Build. Environ. 2018;137:41–50. doi: 10.1016/j.buildenv.2018.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T., et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350(17):1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Zhang T., Chen Q. Comparison of different ventilation systems for commercial aircraft cabins. Proceedings of indoor air. 2005;4:3205–3210. [Google Scholar]
- Zhang T., Chen Q.Y. Novel air distribution systems for commercial aircraft cabins. Build. Environ. 2007;42(4):1675–1684. doi: 10.1016/j.buildenv.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Liu Y., Chen C. Building and Environment; 2020. Air Purifiers: A Supplementary Measure to Remove Airborne SARS-CoV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]