To the Editor:
In March 2020, the World Health Organization declared COVID-19 a global pandemic.1 Initially, hospitals canceled numerous elective and outpatient medical services, including nonurgent pulmonary function tests (PFTs).2 Gradually, these services were resumed in accordance with national guidelines.3 Organizations including the American Thoracic Society (ATS) and others4 , 5 have since published recommendations regarding provision of respiratory services in the era of COVID-19. We aimed to evaluate which practices were being most widely adopted by PFT laboratories across the United States and to examine how community COVID-19 prevalence shaped those practices.
Methods
In August 2020, we invited all members of the ATS PFT Laboratory Registry to complete an online survey, and we asked the American Association for Respiratory Care to post an invitation to members of the American Association for Respiratory Care Diagnostics Section. The questionnaire collected information on how PFT laboratories were responding to the COVID-19 pandemic including the following: demographics including local COVID-19 prevalence according to the Centers for Disease Control and Prevention coronavirus map,6 clinical utility and aerosol-generating potential (AGP) of specific PFTs, infection prevention, patient screening protocols, and laboratory functional status. We assessed associations by contingency analysis, with χ2 P < .05 indicating statistical significance (JMP Pro 15; SAS Institute Inc). The study did not require institutional review board review because it was considered a quality-related investigation.
Results
Respondents completed the survey between August 17 and October 31, 2020. From the ATS Registry, the response rate was 41%. Most of the 132 respondents indicated their clinical role as laboratory manager (48.8%) or PFT technology staff (35.1%). Most laboratories were in community and academic hospitals. Most laboratories were small (63%) and medium (31%) sized (Table 1 ). Most academic laboratories were medium or large, whereas community, private, and other laboratories were primarily small (P < .01). Fifty-one percent of laboratories were located in low COVID-19 prevalence areas, 28% in medium prevalence areas, and 21% in high prevalence areas. The results of the survey categorized by local prevalence of COVID-19 are shown in Table 1.
Table 1.
Summary of Survey Responses by Local COVID-19 Prevalence (Cases per 100,000)
| Survey Item | Low Prevalence (< 5,000) | Medium Prevalence (5,000-20,000) | High Prevalence (> 20,000) |
|---|---|---|---|
| Laboratory type (% of responses) | |||
| Community | 61 | 31 | 21 |
| Academic | 26 | 40 | 57 |
| Private/other | 13 | 29 | 21 |
| Laboratory size (% of responses) | |||
| Small (< 20 tests per day) | 73 | 57 | 43 |
| Medium (20-50 tests per day) | 24 | 37 | 43 |
| Large (> 50 tests per day) | 3 | 6 | 14 |
| PFT classification (% of responses) | |||
| PFT considered high AGP | 88 | 69 | 82 |
| PFT not considered high AGP | 12 | 31 | 18 |
| PPE practices (% utilization) | |||
| Patient mask | 94 | 94 | 93 |
| Provider mask | 100 | 97 | 93 |
| Surgical mask | 66 | 69 | 61 |
| N95 | 87 | 81 | 82 |
| PAPR | 19 | 17 | 21 |
| Face shield | 94 | 94 | 96 |
| Gown | 70 | 75 | 79 |
| Gloves | 84 | 89 | 82 |
| Hat | 19 | 17 | 7 |
| Shoe covers | 4 | 3 | 0 |
| Disinfection practices (% utilization) | |||
| Disinfect equipment and surface between patients | 100 | 100 | 100 |
| In-line antimicrobial filter | 93 | 89 | 93 |
| Change flow sensor between patients | 28 | 28 | 25 |
| Mean air exchange time, min | 33 | 24 | 32 |
| HEPA filter in at least some rooms | 31 | 47 | 39 |
| UV sanitization in at least some rooms | 15 | 25 | 14 |
| Negative pressure in at least some rooms | 40 | 33 | 29 |
| Waiting room precautions | 96 | 92 | 96 |
| Patient hand hygiene available | 84 | 78 | 75 |
| Patient screening practices (% utilization) | |||
| Symptoms screening | 97 | 100 | 96 |
| Temperature screening | 88 | 92 | 89 |
| SARS-CoV-2 PCR test requireda | 51 | 39 | 74a |
| Mean wait time if previous positive COVID-19 test, d | 20 | 22 | 21 |
| Operational status (% of responses) | |||
| Fully operational | 75 | 69 | 64 |
| Partially operational | 25 | 31 | 32 |
| Fully closed | 0 | 0 | 4 |
AGP = aerosol generating potential; HEPA = high-efficiency particulate air; PAPR = powered air-purifying respirator; PCR = polymerase chain reaction; PFT = pulmonary function test; PPE = personal protective equipment; UV = ultraviolet.
P = .02 for positive testing relative to low and medium prevalence groups.
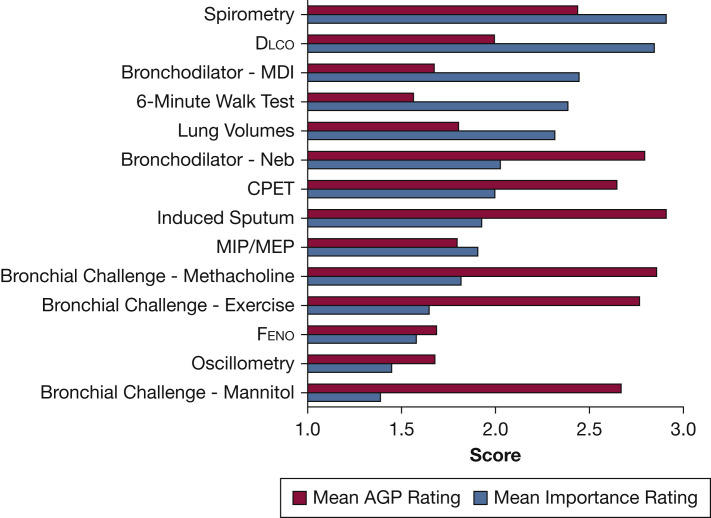
Respondents indicated that they thought spirometry and diffusing capacity of the lung for carbon monoxide had the highest utility for patient care (Fig 1 ). Most laboratories (82%) reported that most PFTs were considered by their institution to be high AGP (Fig 1), with no differences between types of laboratories (P = .34). Several respondents commented that they were working to convince their administration that PFTs should be considered high AGP.
Figure 1.
Mean rating of AGP and clinical utility of common pulmonary function tests. Procedures were rated individually based on subjective measures using a numerical interval scale (1 = low, 2 = medium, 3 = high). AGP = aerosol-generating potential; CPET = cardiopulmonary exercise testing; Dlco = diffusing capacity of the lung for carbon monoxide; Feno = fractional exhaled nitric oxide; MDI = metered-dose inhaler; MIP/MEP = maximal inspiratory pressure/maximal expiratory pressure; Neb = nebulizer.
With respect to personal protective equipment, 98% of laboratories required that staff wear a facemask during PFTs, and that patients wear one except during testing. There were no differences between the use of N95 respirators and local COVID-19 prevalence (P = .22), with 84% of laboratories using N95 respirators for at least some procedures. In addition to masks, 95% of laboratories used eye protection, 85% used gloves, and 70% used protective gowns during PFTs.
All laboratories disinfected surfaces and equipment in PFT rooms between patients, and 83% provided time for air exchange, with a mean duration ± SD of 31 ± 28 min. Almost all laboratories (92%) reported using in-line antimicrobial filters in PFT equipment. Additionally, 95% of laboratories implemented waiting room precautions (eg, physical distancing, mask-wearing policies, hand hygiene). We found that high-efficiency particulate air filters and negative pressure systems were used to similar extents (37% and 36%, respectively), both being more common than ultraviolet sanitization (17%). No significant variation was observed among room and equipment disinfection protocols based on local prevalence of COVID-19.
Screening for symptoms of COVID-19 and temperature checks prior to PFT appointments were implemented at rates of 99% and 96%, respectively. Testing for SARS-CoV-2 by polymerase chain reaction (PCR) was adopted by 54% of laboratories, of which 58% required patients to have a negative test result within 5 days of their appointment. We also found that implementation of PCR testing differed significantly between high prevalence areas (74%) and low or medium prevalence areas (51% and 39%, respectively) (P = .02). For patients with a positive COVID-19 diagnosis, 41% of laboratories required a period of at least 14 days symptom-free prior to testing, and 43% required a period of at least 30 days. Only 9% of laboratories indicated wait times of ≤ 10 days.
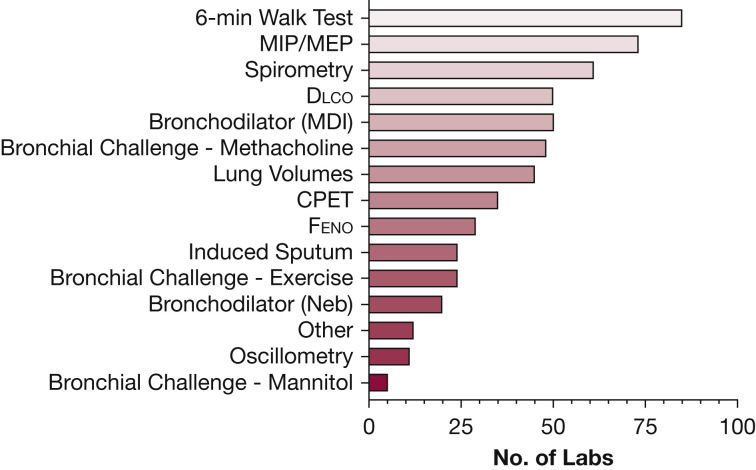
Pulmonary services had largely been restored by the close of the survey, with 71% of laboratories reporting as fully operational, 28% as partially operational, and 1% as fully closed. Many respondents commented that testing volume was markedly reduced because of cleaning protocols required to maintain a safe testing environment. The number of laboratories conducting various tests during the survey period is illustrated in Figure 2 . Only 70% of laboratories were conducting spirometry, which suggests that concern over the high AGP of spirometry was influencing laboratories not to perform it despite its high clinical utility.
Figure 2.
Distribution of procedures being conducted by laboratories at the time of survey. (n = 85 because of missing data). CPET = cardiopulmonary exercise testing; Dlco = diffusing capacity of the lung for carbon monoxide; Feno = fractional exhaled nitric oxide; MDI = metered-dose inhaler; MIP/MEP = maximal inspiratory pressure/maximal expiratory pressure; Neb = nebulizer.
Discussion
Our survey has demonstrated that PFT laboratories have acted in accordance with ATS4 or European Respiratory Society5 recommendations. Common practices include implementation of physical distancing and mask-wearing in common spaces, easy access to hand hygiene, appropriate personal protective equipment usage by staff, thorough disinfection of equipment and surfaces, and patient screening protocols for fever and other COVID-19 symptoms. Most laboratories’ institutional policies considered PFTs collectively to be high AGP, in line with recent evidence.7 , 8
Implementation of some practices varied depending on local prevalence, whereas others did not. For example, PCR testing was more likely to be adopted in high prevalence areas in comparison with low or medium prevalence areas, whereas N95 respirator usage among laboratories was relatively uniform. Some more resource-intensive measures were used to a lesser degree independent of prevalence. Despite all these challenges, nearly three-quarters of laboratories in our sample were fully operational. We acknowledge important limitations to these results, which include small sample size, estimated to be < 5% of all PFT laboratories, the timing of the survey, incomplete responses for Figure 2 data, and that nonoperational laboratories or those following less stringent protective measures may not have responded. Nevertheless, these results demonstrate the resilience and adaptability necessary to cope with the ever-changing demands of safely providing respiratory care during the COVID-19 pandemic.
Acknowledgments
Role of sponsors: The sponsor had no specific role in the development of the study or the manuscript.
Footnotes
FINANCIAL/NONFINANCIAL DISCLOSURES: The authors have reported to CHEST the following: J. M. H. is a consultant for Morgan Scientific Inc. D. A. K. is a speaker for MGC Diagnostics Inc. None declared (M. J. S., M. C. M., S. S.).
FUNDING/SUPPORT: This study was funded by the Vermont Lung Center, University of Vermont Larner College of Medicine.
References
- 1.World Health Organization WHO director-general’s opening remarks at the media briefing on COVID-19 - 11 March 2020. World Health Organization. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 2.Kouri A., Gupta S., Yadollahi A. Addressing reduced laboratory-based pulmonary function testing during a pandemic. Chest. 2020;158(6):2502–2510. doi: 10.1016/j.chest.2020.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Medicare & Medicaid Services Re-opening facilities to provide non-emergent non-COVID-19 healthcare: phase I. https://www.cms.gov/files/document/covid-flexibility-reopen-essential-non-covid-services.pdf
- 4.Wilson K.C., Kaminsky D.A., Michaud G. Restoring pulmonary and sleep services as the COVID-19 pandemic lessens: from an Association of Pulmonary, Critical Care, and Sleep Division Directors and American Thoracic Society-coordinated task force. Ann Am Thorac Soc. 2020;17(11):1343–1351. doi: 10.1513/AnnalsATS.202005-514ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ERS Group 9.1. ERS 9.1 Statement on lung function during COVID-19. https://ers.app.box.com/s/zs1uu88wy51monr0ewd990itoz4tsn2h
- 6.Centers for Disease Control and Prevention COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- 7.Helgeson S.A., Lim K.G., Lee A.S., Niven A.S., Patel N.M. Aerosol generation during spirometry. Ann Am Thorac Soc. 2020;17(12):1637–1639. doi: 10.1513/AnnalsATS.202005-569RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Jing G., Fink J.B., McLaughlin R., Vines D.L., Dhand R. Airborne particulate concentrations during and after pulmonary function testing. Chest. 2021;159(4):1570–1574. doi: 10.1016/j.chest.2020.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]