Abstract
(1) Background: The etiology of orofacial cleft (OC) is not completely known but several genetic and environmental risk factors have been identified. Moreover, a knowledge gap still persists regarding neonatal characteristics. This study evaluated the effect of parental age and mothers’ body mass index on the risk of having an OC child, in a matched year and sex group (cleft/healthy control). Additionally, birth data were analyzed between groups. (2) Methods: 266 individuals born between 1995 to 2015 were evaluated: 133 OC individuals (85 males/48 females) and 133 control (85 males/48 females). A logistic model was used for the independent variables. ANOVA or Kruskal-Wallis tests were used for comparison between the OC phenotypes. (3) Results: Regarding statistically significant parental related factors, the probability of having a cleft child decreases for each maternal year increase (odds ratio = 0.903) and increases for each body mass index unit (kg/m2) increase (odds ratio = 1.14). On the child data birth, for each mass unit (kg) increase, the probability of having a cleft child decrease (odds ratio = 0.435). (4) Conclusions: In this study, only maternal body mass index and maternal age found statistical differences in the risk of having a cleft child. In the children’s initial data, the cleft group found a higher risk of having a lower birth weight but no relation was found regarding length and head circumference.
Keywords: cleft palate, cleft lip, environmental risk factors, parental age, body mass index, child birth data
1. Introduction
Orofacial clefts (OC) are one of the most common craniofacial malformations, with an international prevalence in newborns of 14 per 10,000 live births worldwide [1,2]. The prevalence of OC has been increasing over the years, perhaps as a result of improved surgical techniques, neonatal care resulting in reduced postnatal morbidity and mortality, more accurate documentation, and environmental factors such as smoking consumption [2,3,4]. According to the European Network for Epidemiological Surveillance of Congenital Anomalies (EUROCAT) report, the prevalence in 26 European countries was 14.5 per 10,000 births between 2011 and 2018 [5].
The etiology of OC is not completely known but several genetic and environmental risk factors have been identified. Previous studies have clarified the importance of epidemiologic knowledge, especially in (1) assessing the burden of OC in order to plan public health resources; (2) knowing the causes of OC, which stimulates research on primary prevention and treatments to improve the quality of care [6]. The genetic component plays an important role in the formation of isolated cleft. Some of the best-supported genes and genetic loci in the literature are IRF6, ch8q24, VAX1, and PAX7 [7,8]. Concerning environmental risk factors, previous researches, which have shown consistency, have proposed smoking consumption during pregnancy and gestational diabetes as maternal risk factors [4,9,10]. Other associations have been reported with less consistency, namely alcohol consumption during pregnancy, intrapartium interval, maternal and paternal age, and nutritional deficiencies (folic acid, vitamin A and B12, and zinc) [11,12,13,14,15,16,17,18]. Furthermore, both extremes of maternal body mass index (BMI) have been associated with birth defects, but with controversial results in the literature [10,19,20,21]. Recently, Kutbi et al. showed in a larger population-based study that obesity was associated with an increased risk of OC but no conclusion was drawn about maternal underweight [21].
Even though there are several studies about OC etiological factors in the literature, there is a gap regarding neonatal characteristics. Several studies showed that OC children are lighter and smaller than healthy controls but with inconsistent results [22,23]. Despite the growing literature about cleft etiology, the main limitation of these studies is the recruitment process, since there is often heterogeneity in relation to the sociodemographic factors and sex and age of patients evaluated. This approach is expected to produce more bias since it is already known that some of these factors are determinants of laterality and side of the cleft, such as sex [24]. Furthermore, the studies published on cleft birth data are focused on weight, leaving aside some anthropometric measurements (e.g., length and head circumference at birth) that are used in the evaluation of intrauterine growth deviations, overall health of the child, and early and long-term growth [22,25,26].
This study aims to evaluate the effect of possible parental related influencing factors on the development of cleft lip and/or palate as well as the differences in birth data. Therefore, this study used child recruitment with standardized methods in order to obtain a homogeneous sample regarding age, sex, and sociodemographic factors. Moreover, length and head circumference at birth will be measured in addition to birth weight. The purpose of this study was to test the hypothesis that the risk of having a cleft child increased with parental age or maternal BMI, and also to compare the differences in birth data (birth weight, birth length, and head circumference at birth) between cleft children and healthy controls (non-cleft group).
2. Materials and Methods
2.1. Study Design
This retrospective case-control study was approved by the Ethics Committee of the Faculty of Medicine of University of Coimbra (Reference: CE-072/2020). The study was handled in accordance with the Declaration of Helsinki.
2.2. Data Collection Procedure
The study group included parents from children (born between 1995–2015), with non-syndromic OC, being rehabilitated at the Institute of Orthodontics, Faculty of Medicine, University of Coimbra (FMUC), regardless of sex or place of birth. The control group included individuals without clinical alterations or craniofacial defects. To minimize bias, controls were matched according to year of birth and sex. Both groups had similar socio-economic backgrounds.
Data were collected through two sources: (1) medically recorded data—parents were requested to send the pre-natal care logbook and all medical record during the pregnancy; (2) maternal self-reported information.
A structured questionnaire was also distributed to the parents of both groups after the clinical visit, avoiding any harm to the patients. The questionnaires were deployed always by the same examiner (I.F.) after being properly trained for the activity. Before fulfilling the questionnaire, parents were asked to read the enclosed list as a memory aid with the most common maternal diseases during pregnancy and pregnancy supplements. From both groups, the parents who did not consent to participate in the study or who did not answer the questionnaire sufficiently, parents with consanguineous marriages, and children with syndromic OC were excluded.
The questionnaire included retrospective parent and patient information. Variables were divided in three groups:
-
-
Sociodemographic—age of parents and child; child’s sex.
-
-
Obstetrical data—alcohol intake, smoking, drugs, and pregnancy supplements used, diseases diagnosed before and during pregnancy and exposure to carbon monoxide, recorded by gestational month; early pregnancy body mass index.
-
-
Child birth data—presence and phenotype of cleft, birth weight, birth length, and head circumference at birth.
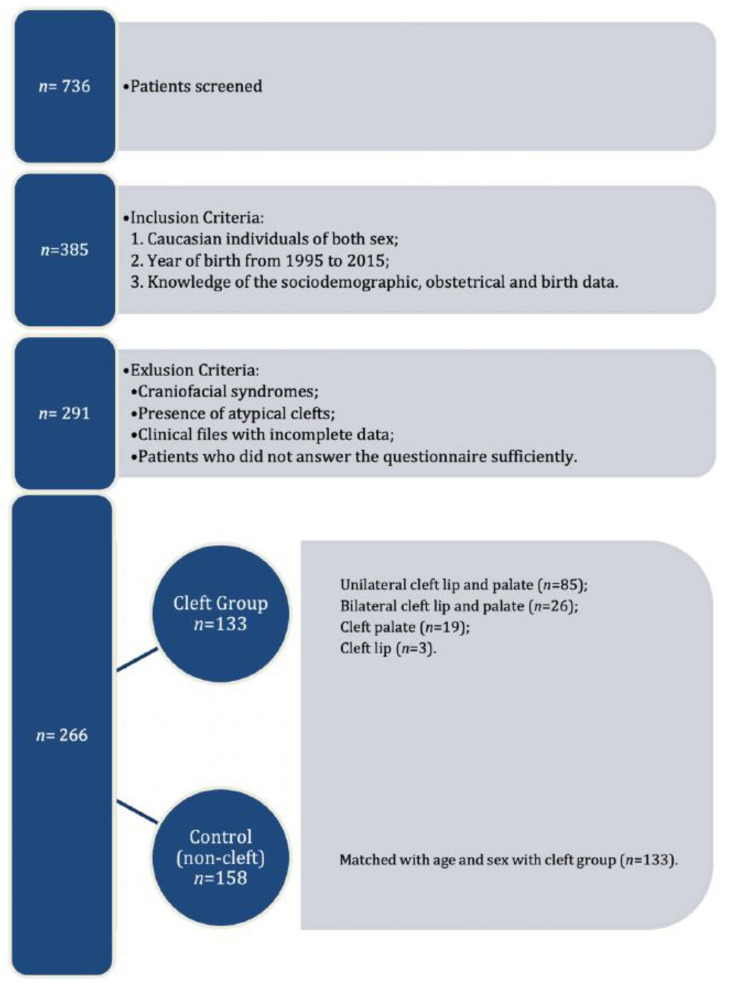
After matching the control group to the OC group, the final sample consisted of 266 individuals (Figure 1). All participants gave their written informed consent for secondary use of their records.
Figure 1.
Flow chart of the inclusion and exclusion criteria.
2.3. Statistical Analysis
All analyses were performed using the Statistical Package for the Social Sciences, version 26.0 for Windows (SPSS Inc., Chicago, IL, USA). The significance level chosen was 0.05.
Descriptive statistics for the quantitative variables were obtained using mean, standard deviation values, and 75th and 25th percentiles. Categorical variables were expressed as absolute and relative frequency.
The comparison between the groups was performed using Fisher’s exact test for gender (nominal variable), and for the quantitative variables, the Mann-Whitney test was used when a violation of the normality assumption was verified through the Shapiro-Wilk test.
To assess the risk factors, a logistic model was adjusted using as independent variables parental age, maternal BMI, and birth data (weight, length, and head circumference at birth). The logistic model was evaluated using the Naguelkerke’s R2 and by measuring the area under the ROC curve. The null hypothesis of the statistical analysis (the logistic regression) is that no risk factors exist.
Comparison between the different phenotypes of cleft was performed using the ANOVA test or the Kruskal-Wallis test when the normality assumption was not observed. Tukey’s post-hoc tests were performed, after verifying the significant ANOVA test.
3. Results
In total, 266 eligible cases were identified in the FMUC between 1995 and 2015, among them 133 were orofacial cleft and 133 were control group.
Table 1 presents descriptive statistics concerning sex distribution, parental age, maternal BMI, and birth data of the subjects studied. The distribution of age and sex is homogenous (p = 1.00) between groups (Control vs. Orofacial Cleft).
Table 1.
Descriptive analysis by each group.
| Variables | Control (133) | Orofacial Cleft (133) | p |
|---|---|---|---|
| Sex (M/F) | 85/48 (63.9%/36.1%) | 85/48 (63.9%/36.1%) | 1.000 # |
| Maternal age (years) a | 31 (4.2) 28.0/34.0 | 28 (5.6) 25.0/32.0 | <0.001 § |
| Paternal age (years) a | 33 (4.9) 29.0/36.0 | 31 (6.3) 27.0/35.0 | 0.016 § |
| Maternal BMI (kg/m2) a | 23 (3.9) 20.8/25.1 | 25 (4.3) 21.9/28.3 | <0.001 § |
| Birth Length (cm) a | 49 (2.1) 48.0/50.0 | 48 (2.9) 47.0/50.0 | 0.519 § |
| Birth Weight (kg) a | 3 (0.5) 3.0/3.6 | 3 (0.5) 2.8/3.4 | 0.033 § |
| Birth Head circumference (cm) a | 35 (2.2) 33.5/35.0 | 34 (2.3) 33.5/35.0 | 0.457 § |
a mean (standard deviation) 25–75 percentiles; # Fisher’s exact test; § Mann-Whitney, M-male, F-female, BMI- body mass index.
Three independent variables (maternal age, maternal BMI, and child birth weight) found statistical significance using the logistic model (p < 0.001). The model obtained explains about 17% of the variance (R2Naguelkerke = 0.169) and presents an accuracy of 63.2% which is compared with 50% obtained in the null model. Table 2 shows the regression coefficients obtained with the corresponding adjusted odds ratios (OR) and their confidence intervals (CI).
Table 2.
The regression coefficients for maternal age, maternal BMI, and weight at birth.
| Variables | B | p | OR | CI95% |
|---|---|---|---|---|
| Maternal age (years) | −0.102 | <0.001 | 0.903 | [0.856; 0.953] |
| Maternal BMI (kg/m2) | 0.131 | <0.001 | 1.140 | [1.068; 1.216] |
| Birth Weight (kg) | −0.833 | 0.003 | 0.435 | [0.253; 0.746] |
| 2.477 | 0.062 | 11.904 |
B—regression coefficient; OR—odds ratio; CI—confidence interval.
Regarding parental related factors, it was found that (1) for each maternal year increase, the probability of having a cleft child decreases 0.9 (OR = 0.903) and (2) for each BMI unit (kg/m2) increase, the probability of having a cleft child increases 1.14 (OR = 1.14). The child-birth data showed that for each mass unit (kg) increase at birth, the probability of having a cleft child decreases 0.4 (OR = 0.435). The area under the ROC curve for the probabilities obtained by the logistic model was 0.699 (IC95% [0.637; 0.761], p < 0.001).
No association between maternal age and BMI index on the risk of having a child with a cleft was found (p = 0.573).
The final sample of OC group consisted of 85 unilateral cleft lip and palate, 26 bilateral cleft lip and palate, 19 cleft palate, and 3 cleft lip. Table 3 presents descriptive statistics concerning phenotype of cleft. It was found that maternal BMI affects the risk of having cleft in the four clefts types (p = 0.023) with a statistical difference in cleft lip (Table 4).
Table 3.
Descriptive statistics concerning phenotype of cleft.
| Variables | Unilateral Cleft Lip and Palate (n = 85) | Bilateral Cleft Lip and Palate (n = 26) | Cleft Palate (n = 19) | Cleft Lip (n = 3) | p |
|---|---|---|---|---|---|
| Maternal age (years) a | 28 (5.4) 25.0/31.0 | 28 (5.8) 24.0/31.0 | 30 (5.8) 26.0/37.0 | 30 (9.1) 23.0/40.0 | 0.4271 § |
| Paternal age (years) a | 31 (6.2) 27.0/34.0 | 32 (6.9) 28.0/34.0 | 34 (5.4) 29.0/38.0 | 27 (3.2) 25.0/31.0 | 0.121 £ |
| Maternal BMI (kg/m2) a | 25 (4.2) 22.3/27.6 | 25 (5.0) 20.3/29.1 | 25 (3.6) 21.8/26.4 | 33 (1.7) 30.8/34.2 | 0.023 £ |
| Birth Length (cm) a | 48 (2.7) 47.0/50.0 | 48 (2.6) 47.0/50.0 | 49 (4.3) 48.0/51.0 | 48 (1.2) 47.0/49.0 | 0.452 § |
| Birth Weight (kg) a | 3 (0.5) 2.8/3.4 | 3 (0.5) 2.9/3.5 | 3 (0.4) 2.9/3.4 | 3 (0.7) 2.2/3.6 | 0.505 £ |
| Birth Head circumference (cm) a | 34 (1.7) 33.0/35.0 | 34 (1.2) 33.5/35.0 | 36 (4.3) 33.5/36.0 | 33 (2.6) 30.0/35.0 | 0.702 § |
a mean (standard deviation) 25–75 percentiles; § Kruskal-Wallis; £ ANOVA.
Table 4.
Correlation between maternal BMI and cleft phenotype.
| Phenotype of Cleft | p |
|---|---|
| Unilateral cleft lip and palate vs. bilateral cleft lip and palate | p = 0.990 |
| Unilateral cleft lip and palate vs. cleft palate | p = 0.947 |
| Unilateral cleft lip and palate vs. cleft lip | p = 0.016 |
| Bilateral cleft lip and palate vs. cleft palate | p = 0.995 |
| Bilateral cleft lip and palate vs. cleft lip | p = 0.016 |
| Cleft palate vs. cleft lip | p = 0.014 |
4. Discussion
The purpose of this study was to evaluate the effect of parental age and mothers’ BMI on the risk of having a child with OC. Possible interaction of mother risk factors is also investigated. Moreover, birth data of cleft children were analyzed to assess the differences between patients with OC and healthy controls. Of the six study variables, three variables (maternal age, maternal body mass index, and birth weight) found statistically significant differences using the logistic model (p < 0.001).
The current study suggests that for each maternal year increase, the probability of having a cleft child decreases 0.9 (OR = 0.903) but no statistical differences were found regarding paternal age. The association between the risk of having cleft and parental age does not have a consensus in the literature [27,28,29]. Herkrath et al. suggested that mothers 35 years of age or older and parents 40 years of age or older had an increased risk of 20% and 58% of having a child with a cleft, respectively [27]. In contrast, Carvalho et al. did not find an association between maternal age and orofacial clefts (p = 0.747) in all age groups study, including mothers 35 years of age or older [28]. Nevertheless, some studies reported that the risk of having a cleft is also related to the interaction of the age of both parents [30,31]. Berg et al. showed that the risk of having cleft lip only increases when the age of both parents was high [31].
A positive association between BMI and orofacial cleft was also found: for each BMI unit (kg/m2) increase, the probability of having a cleft child increases 1.14 (OR = 1.14). These findings are consistent with the results of three meta-analyses that found an association between overweight women and orofacial clefts with a similar odds ratio for cleft palate and for unilateral cleft lip and palate [32,33,34]. However, the main limitation of these studies is that they did not analyze the interaction of BMI in cleft lip and bilateral cleft lip and palate. The present study proved that BMI affects the risk of having cleft in the four cleft phenotypes (p = 0.023) with a statistical difference in cleft lip (Table 4). A cohort study from United Kingdom, investigating the association between BMI and the majority of structural congenital anomalies, included three types of cleft (cleft lip; cleft lip and palate; and cleft palate) and found that only the cleft lip shows a statistical difference (aOR = 3.71, 95% CI: 1.05, 13.10; p = 0.04) [35]. The mechanism that explains how obesity acts as teratogenic factor has not been fully elucidated. Still, some authors suggested a possible explanation based on the relationship between obesity and gestational diabetes since hyperglycemia can modify the fetal expression of developmental genes, such as bone morphogenetic protein 4 [34].
Despite the average associations regarding maternal age and maternal BMI, no exploration of possible interaction in both risk factors has been done. Thus, this is the first study that intends to verify this relation, revealing that no association can be established between these two factors (p = 0.573). This result is unexpected because ageing is associated with an increase in abdominal white adipose tissue and fat deposition in skeletal muscle, which significantly affect insulin sensitivity [36]. Moreover, changes in gametes through life due to environmental exposures or chromosomal alterations and increased permeability to teratogenic agents in the placenta in older mothers may also increase the risk of having a child with OC [37].
Regarding child birth data (weight, length, and head circumference), all variables presented a lower mean in the cleft group compared with non-cleft individuals. However, only birth weight found statistical differences using the logistic model (p < 0.001), suggesting that for each increase in mass unit (kg) at birth, the probability of having a cleft decreases 0.4 (OR = 0.435). This finding is consistent with the Wyszynski et al. study, which verified that cleft palate patients with or without lip involvement had slightly lower birth weight (mean difference 159 and 197, respectively) [23]. Becker et al. also showed that patients with cleft lip with or without palate involvement were lighter and shorter than control subjects [22]. These authors attempted to hypothesize several explanations for the lower weight in cleft patients at birth: (1) incomplete development of facial tissues; (2) environment factors associated with the risk of having cleft (such as smoking and maternal dietary intake) may slow the rate of fetal development [22,23]. Despite this, children with cleft palate with or without cleft lip presented spontaneous recovery that starts at approximately 5 months of age. In addition, cleft lip children showed similar growth to healthy children [38].
The main limitation of the present study was the analysis of the different types of orofacial clefts because the division of data into subgroups results in very small numbers, especially in cleft lip where the prevalence is lower. This may explain why only BMI showed differences statically significant among the different types of cleft studied. Additionally, unmeasured confounding factors cannot be excluded even though the model obtained presents an accuracy of 63.2%. This study has several strengths compared with the published literature: (1) both groups had similar socio-economic backgrounds; (2) controls (non-cleft group) were individually matched to each case regarding age and sex; (3) a geneticist participated in the diagnosis of the cleft group, avoiding including syndromic patients; and (4) data birth were recorded by medical professionals.
This study highlights the complexity in identifying the risk factors for having orofacial cleft, especially when trying to detect differences between phenotypes. In some countries all over the world, healthcare systems cannot afford treatment for cleft lip and palate. Identification of strategies to modify risk factors for non-syndromic OC (such as being overweight) is the first step toward primary prevention [39]. As strong evidence of some of the prevention factors is still lacking, the next reasonable step for research might be the observational studies that consider the interactions of several risk factors, namely maternal family history of diabetes.
5. Conclusions
In this homogenous population-based study, maternal body mass index and maternal age was found to affect the risk of having a cleft child. However, the association of these two factors does not increase the risk. In the children’s initial data, the cleft group found a higher risk of having a lower birth weight but no relation was found regarding length and head circumference. This trial and further studies will allow the incorporation of the findings into preconception counseling health care programs for women of reproductive age.
Author Contributions
I.F.: conceptualization, methodology, investigation, and writing—original draft preparation; F.C.: methodology, formal analysis, and data curation; M.H.F.: methodology and writing—review and editing; F.V.: writing—review and editing and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Faculty of Medicine of University of Coimbra (Reference: CE-072/2020, 27 July 2020). The study was handled in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vieira A.R., Orioli I.M. Birth order and oral clefts: A meta analysis. Teratology. 2002;66:209–216. doi: 10.1002/tera.10088. [DOI] [PubMed] [Google Scholar]
- 2.Mossey P.A., Castilla E. Global Registry and Database on Craniofacial Anomalies. Report of a WHO Registry Meeting on Craniofacial Anomalies. Geneva, Switzerland: WHO. [(accessed on 23 March 2021)];2003 Available online: https://apps.who.int/iris/bitstream/handle/10665/42840/9241591102.pdf?sequence=1&isAllowed=y.
- 3.Rintala A., Stegars T. Increasing incidence of clefts in Finland: Reliability of hospital records and central register of congenital malformations. Scand. J. Plast Reconstr. Surg. 1982;16:35–40. doi: 10.3109/02844318209006568. [DOI] [PubMed] [Google Scholar]
- 4.Little J., Cardy A., Munger R.G. Tobacco smoking and oral clefts: A meta-analysis. Bull. World Health Organ. 2004;82:213–218. [PMC free article] [PubMed] [Google Scholar]
- 5.European Surveillance of Congenital Anomalies EUROCAT Data and Surveillance—Prevalence (per 10,000 Births) for the Following Registries: All Full Registries, Oro-Facial Clefts, from 2011–2018. [(accessed on 27 January 2021)]; Available online: https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence_en.
- 6.Mossey P. Epidemiology underpinning research in the aetiology of orofacial clefts. Orthod. Craniofac. Res. 2007;10:114–120. doi: 10.1111/j.1601-6343.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- 7.Leslie E.J., Marazita M.L. Genetics of Cleft Lip and Cleft Palate. Am. J. Med. Genet. C Semin. Med. Genet. 2013;163:246–258. doi: 10.1002/ajmg.c.31381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahimov F., Jugessur A., Murray J.C. Genetics of nonsyndromic orofacial clefts. Cleft Palate Craniofac. J. 2012;49:73–91. doi: 10.1597/10-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spilson S.V., Kim H.J., Chung K.C. Association between maternal diabetes mellitus and newborn oral cleft. Ann. Plast. Surg. 2001;47:477–481. doi: 10.1097/00000637-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L., Wang X.H., Zheng X.M., Liu T.Z., Zhang W.B., Zheng H., Chen M.F. Maternal gestational smoking, diabetes, alcohol drinking, pre-pregnancy obesity and the risk of cryptorchidism: A systematic review and meta-analysis of observational studies. PLOS ONE. 2015;10:e0119006. doi: 10.1371/journal.pone.0119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida S., Takeuchi M., Kawakami C., Kawakami K., Ito S. Japan Environment and Children’s Study Group. Maternal multivitamin intake and orofacial clefts in offspring: Japan Environment and Children’s Study (JECS) cohort study. BMJ Open. 2020;10:e035817. doi: 10.1136/bmjopen-2019-035817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva H.P.V., Arruda T.T.S., Souza K.S.C., Bezerra J.F., Leite G.C.P., Brito M.E.F., Lima V.M.G.D.M., Luchessi A.D., Bortolin R.H., Ururahy M.A.G., et al. Risk factors and comorbidities in Brazilian patients with orofacial clefts. Braz. Oral Res. 2018;32:e24. doi: 10.1590/1807-3107bor-2018.vol32.0024. [DOI] [PubMed] [Google Scholar]
- 13.Cheshmi B., Jafari Z., Naseri M.A., Davari H.A. Assessment of the correlation between various risk factors and orofacial cleft disorder spectrum: A retrospective case-control study. Maxillofac. Plast. Reconstr. Surg. 2020;42:26. doi: 10.1186/s40902-020-00270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martelli D.R.B., Cruz K.W., Barros L.M., Silveira M.F., Swerts M.S.O., Júnior H.M. Maternal and paternal age, birth order and interpregnancy interval evaluation for cleft lip-palate. Braz. J. Otorhinolaryngol. 2010;76:107–112. doi: 10.1590/S1808-86942010000100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamilian A., Sarkarat F., Jafari M., Neshandar M., Amini E., Khosravi S., Ghassemi A. Family history and risk factors for cleft lip and palate patients and their associated anomalies. Stomatologija. 2017;19:78–83. [PubMed] [Google Scholar]
- 16.Zhou Y., Sinnathamby V., Yu Y., Sikora L., Johnson C.Y., Mossey P., Little J. Folate intake, markers of folate status and oral clefts: An updated set of systematic reviews and meta-analyses. Birth Defects Res. 2020;112:1699–1719. doi: 10.1002/bdr2.1827. [DOI] [PubMed] [Google Scholar]
- 17.Johansen A.M.W., Lie R.T., Wilcox A.J., Andersen L.F., Drevon C.A. Maternal dietary intake of vitamin A and risk of orofacial clefts: A population-based case-control study in Norway. Am. J. Epidemiol. 2008;167:1164–1170. doi: 10.1093/aje/kwn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw G.M., Carmichael S.L., Laurent C., Rasmussen S.A. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–291. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- 19.Cedergren M., Källén B. Maternal obesity and the risk for orofacial clefts in the offspring. Cleft Palate Craniofac. J. 2005;42:367–371. doi: 10.1597/04-012.1. [DOI] [PubMed] [Google Scholar]
- 20.Block S.R., Watkins S.M., Salemi J.L., Rutkowski R., Tanner J.P., Correia J.A., Kirby R.S. Maternal pre-pregnancy body mass index and risk of selected birth defects: Evidence of a dose-response relationship. Paediatr. Perinat. Epidemiol. 2013;27:521–531. doi: 10.1111/ppe.12084. [DOI] [PubMed] [Google Scholar]
- 21.Kutbi H., Wehby G.L., Uribe L.M.M., Romitti P.A., Carmichael S., Shaw G.M., Olshan A.F., DeRoo L., Rasmussen S.A., Murray J.C., et al. Maternal underweight and obesity and risk of orofacial clefts in a large international consortium of population-based studies. Int. J. Epidemiol. 2017;46:190–199. doi: 10.1093/ije/dyw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker M., Svensson H., Källén B. Birth weight, body length, and cranial circumference in newborns with cleft lip or palate. Cleft Palate Craniofac. J. 1998;35:255–261. doi: 10.1597/1545-1569_1998_035_0255_bwblac_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 23.Wyszynski D.F., Sarkozi A., Vargha P., Czeizel A.E. Birth weight and gestational age of newborns with cleft lip with or without cleft palate and with isolated cleft palate. J. Clin. Pediatr. Dent. 2003;27:185–190. doi: 10.17796/jcpd.27.2.475367q2601u3x4w. [DOI] [PubMed] [Google Scholar]
- 24.Kruse T., Mangold E., Braumann B. Impact of Maternal Smoking on Nonsyndromic Clefts: Sex-Specific Associations with Side and Laterality. Cleft Palate Craniofac. J. 2021;58:181–188. doi: 10.1177/1055665620951099. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia J. Growth curves: How to best measure growth of the preterm infant. J. Pediatr. 2013;162(Suppl. 3):S2–S6. doi: 10.1016/j.jpeds.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 26.Pereira-da-Silva L., Virella D., Fusch C. Nutritional Assessment in Preterm Infants: A Practical Approach in the NICU. Nutrients. 2019;11:1999. doi: 10.3390/nu11091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herkrath A.P.C.Q., Herkrath F.J., Rebelo M.A.B., Vettore M.V. Parental age as a risk factor for non-syndromic oral clefts: A meta-analysis. J. Dent. 2012;40:3–14. doi: 10.1016/j.jdent.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho P.H.P., Machado R.A., Reis S.R.A., Martelli D.R.B., Dias V.O., Martelli-Júnior H. Parental age is related to the occurrence of cleft lip and palate in Brazilian populations. Braz. J. Oral Sci. 2016;15:167–170. doi: 10.20396/bjos.v15i2.8648758. [DOI] [Google Scholar]
- 29.Figueiredo A.C., Ly S., Magee K.S., Ihenacho U., Baurley J.W., Sanchez-Lara P.A., Brindopke F., Nguyen T.H.D., Nguyen V., Tangco M.I., et al. Parental risk factors for oral clefts among Central Africans, Southeast Asians, and Central Americans. Birth Defects Res. A Clin. Mol. Teratol. 2015;103:863–879. doi: 10.1002/bdra.23417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermann N.V., Darvann T.A., Munch A., Kreiborg S. Parental age in relation to the severity of cleft lip and/or palate. Orthod. Craniofac. Res. 2018;21:236–241. doi: 10.1111/ocr.12241. [DOI] [PubMed] [Google Scholar]
- 31.Berg E., Lie R.T., Sivertsen A., Haaland Ø.A. Parental age and the risk of isolated cleft lip: A registry-based study. Ann. Epidemiol. 2015;25:942–947.e1. doi: 10.1016/j.annepidem.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Stothard K.J., Tennant P.W.G., Bell R., Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 33.Izedonmwen O.M., Cunningham C., Macfarlane T.V. What is the Risk of Having Offspring with Cleft Lip/Palate in Pre-Maternal Obese/Overweight Women When Compared to Pre-Maternal Normal Weight Women? A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Res. 2015;6:e1. doi: 10.5037/jomr.2015.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanco R., Colombo A., Suazo J. Maternal obesity is a risk factor for orofacial clefts: A meta-analysis. Br. J. Oral Maxillofac. Surg. 2015;53:699–704. doi: 10.1016/j.bjoms.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 35.Rankin J., Tennant P.W.G., Stothard K.J., Bythell M., Summerbell C.D., Bell R. Maternal body mass index and congenital anomaly risk: A cohort study. Int. J. Obes. 2010;34:1371–1380. doi: 10.1038/ijo.2010.66. [DOI] [PubMed] [Google Scholar]
- 36.Jura M., Kozak L.P. Obesity and related consequences to ageing. Age. 2016;38:23. doi: 10.1007/s11357-016-9884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauk K. Gerontological Nursing: Competencies for Care. 2nd ed. Jones and Bartlett Publishers; London, UK: 2009. p. 869. [Google Scholar]
- 38.Miranda G.S., Marques I.L., Barros S.P., Arena E.P., Souza L. Weight, Length, and Body Mass Index Growth of Children under 2 Years of Age with Cleft Lip and Palate. Cleft Palate Craniofac. J. 2016;53:264–271. doi: 10.1597/14-003. [DOI] [PubMed] [Google Scholar]
- 39.Mossey P.A., Little J., Munger R.G., Dixon M.J., Shaw W.C. Cleft lip and palate. Lancet. 2009;374:1773–1785. doi: 10.1016/S0140-6736(09)60695-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.