Abstract
In Southeast Asia, cervical cancer is the second most common cancer in women. Low coverage for cervical cancer screening (CCS) becomes a roadblock to disease detection and treatment. Existing reviews on CCS have limited insights into the barriers and facilitators for SEA. Hence, this study aims to identify key barriers and facilitators among women living in SEA. A systematic literature review was conducted on Pubmed, Embase, PsycINFO, CINAHL, and SCOPUS. Primary qualitative and quantitative studies published in English that reported barriers and facilitators to CCS were included. The Mix Methods Appraisal Tool was used for the quality assessment of the included studies. Among the 93 included studies, pap smears (73.1%) were the most common screening modality. A majority of the studies were from Malaysia (35.5%). No studies were from Timor-Leste and the Philippines. The most common barriers were embarrassment (number of articles, n = 33), time constraints (n = 27), and poor knowledge of screening (n = 27). The most common facilitators were related to age (n = 21), receiving advice from healthcare workers (n = 17), and education status (n = 11). Findings from this review may inform health policy makers in developing effective cervical cancer screening programs in SEA countries.
Keywords: cervical cancer screening, barriers, facilitators, southeast asia, pap smear, HPV test, visual inspection with acetic acid
1. Introduction
In 2018, approximately 570,000 women developed cervical cancer and 311,000 women died from it [1]. Approximately 84% of all cervical cancers and 88% of all deaths caused by cervical cancer occurred in lower-resource countries [1]. Over the past four decades, a significant reduction in mortality and incidence of cervical cancer have been observed with preventive strategies such as cervical cancer screening (CCS) and vaccination against the human papilloma virus (HPV) [2]. Screening modalities for cervical cancer include a pap smear, HPV test, and visual inspection with acetic acid (VIA). Despite the proven effectiveness of screening, worldwide coverage of these preventive strategies remains poor, especially in developing countries [3].
The Southeast Asia (SEA) region comprises of 11 countries of diverse religions, cultures, and history: Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Singapore, Timor-Leste, and Vietnam. There are approximately 330 million women in SEA, equivalent to 4.3% of the world’s population [4]. Cervical cancer is the second most common cancer among women in the region [5]. In 2020, SEA was ranked seventh for cervical cancer incidence and sixth for mortality compared to other regions of the world [6]. Given the significant disease burden of cervical cancer in the presence of effective preventive strategies, a more detailed understanding of the barriers and facilitators to screening is needed to help in the planning of interventions to improve participation in screening.
Factors that influence CCS uptake include education status [7], health literacy [8], psychosocial factors [9], and contextual factors [10]. However, no study from SEA was included in these existing reviews. In addition, facilitators to CCS in countries with high disease incidence, including SEA nations, are not well characterized based on a systematic literature review [11]. Therefore, we aim to identify barriers to and facilitators of cervical cancer screening among women living in SEA, as well as to generate country-specific insights.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
The systematic review process was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [12]. We searched in PubMed, Embase, CINAHL, SCOPUS, and PsycINFO to identify studies for inclusion without any date restrictions. The search strategies involved keywords and controlled vocabulary related to the concepts of CCS, SEA, barriers, and facilitators. The search was conducted on 3 November 2020 and full details of the search strategy can be found in Table S1.
Studies were included in this review if they met the following criteria: (1) Primary quantitative and qualitative research that reported barriers or facilitators of CCS uptake or intention, (2) involved participants living in SEA, (3) reported barriers or facilitators of CCS separately if more than one disease was analyzed, and (4) published in the English language. The exclusion criteria were studies that: (1) Compared diagnostic performance between different CCS modalities, (2) focused primarily on colposcopy, (3) reported screening as part of a screen-and-treat program, and (4) investigated an intervention to increase CCS uptake or intention without reporting any baseline barrier to or facilitator of CCS. Where the same cohort of patients were analyzed by different studies, only the latest publication was included for this review.
2.2. Data Collection and Analysis
Two independent reviewers (BC and AL) performed the search strategy and study selection, while two independent reviewers (BC and MM) executed the data extraction and quality assessment of the included studies. A manual search of the reference list for all included studies was done to identify additional studies for inclusion. All citations were uploaded on Endnote for the removal of duplicates and exported to Microsoft Excel for screening studies for inclusion.
The following information were abstracted from eligible articles: Study title, authors, publication year, study design, screening instrument used, population size, age of study participants, and proportion of patients with history of CCS. In addition, for quantitative studies, data extracted included barriers and facilitators that were statistically significantly associated (p < 0.05) with CCS intention or uptake, as well as proportions of participants reporting a barrier or facilitator. When univariate and multivariable analyses were both conducted, only results from multivariable analyses were extracted. For qualitative studies, data extracted included all reported barriers and facilitators.
Thematic analysis with an inductive approach was performed to classify barriers and facilitators into major categories [13], with a focus on context and commonalities across included studies. Data extracted from included studies were first assigned a code and patterns were searched amongst the coded data. Similar codes were subsequently categorized into descriptive themes, and themes were clustered into higher-ranking themes (major categories subsuming the themes). Finally, the number of studies supporting each theme were summed up for each SEA country. The Mixed Methods Appraisal tool (MMAT) was used to conduct a quality assessment of the included studies [14]. The MMAT has different scoring criteria for different types of studies: Mixed methods, qualitative, quantitative non-randomized, and quantitative descriptive. Each type of study was assessed based on five criteria with a “yes”, “no”, or “unsure” response, with a maximum score of 5. Any discrepancies for study inclusion, data extraction, analysis, and quality assessment were resolved through consensus or by referral to a third reviewer (VM or WHL).
3. Results
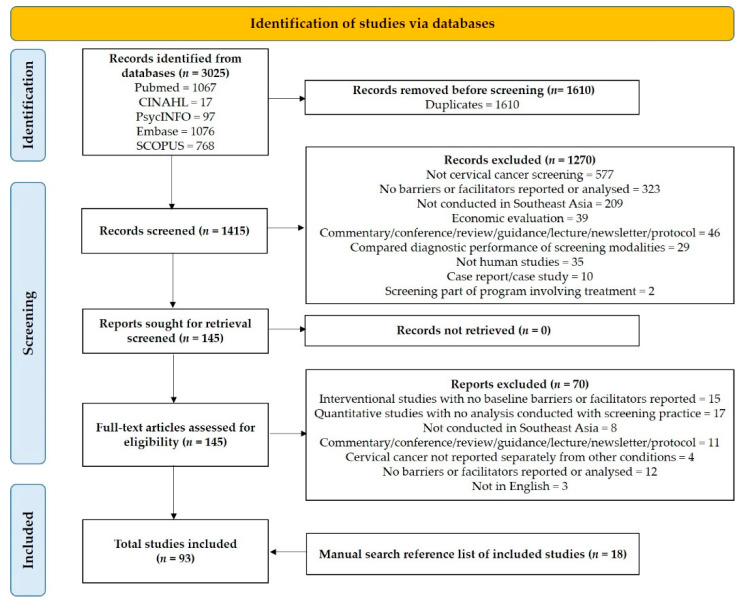
A total of 3025 records were retrieved from the databases. After the removal of 1610 duplicates, 1415 records underwent title and abstract screening resulting in the exclusion of 1270 articles (Figure 1). After a full text review of 145 studies, 75 articles met the inclusion criteria. In addition, we identified 18 studies that met inclusion criteria from manual search. Therefore, the final number of original research articles for data extraction, analysis, and synthesis was 93.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of included studies.
3.1. Study Characteristics
The study characteristics are detailed in Table 1. A total of 69 of the 93 studies were published within the past decade (2011 to 2020). A majority of the studies (n = 81) were cross-sectional or case-control studies, while the rest were qualitative (n = 9) or mixed-methods (n = 3) studies. Most of the studies were conducted in Malaysia (35.5%, n = 33) [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47], followed by Thailand (24.7%, n = 23) [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70], Indonesia (16.1%, n = 15) [71,72,73,74,75,76,77,78,79,80,81,82,83,84,85], and Singapore (15.1%, n = 14) [86,87,88,89,90,91,92,93,94,95,96,97,98,99]. None of the studies were conducted in the Philippines and Timor-Leste.
Table 1.
Characteristics of the included studies.
| Author (Year) | Country | Study Design/Instrument Used | Sample Size | Age of Participants (Years) | Type of Screening Methods Used | Prevalence of Ever Receiving Screening in the Past | MMAT Score |
|---|---|---|---|---|---|---|---|
| Suhaimi et al. (2020) [100] | Brunei | Cross-sectional/interview with structured questionnaire | 3808 (2131 females) |
Mean (SD): 41.9 (14.5) |
Pap smear | 56.5% | 5 |
| Touch et al. (2018) [101] | Cambodia | Cross-sectional/interview with structured questionnaire | 440 |
Distribution: 20–29: 88 (20.0%) 30–39: 88 (20.0%) 40–49: 88 (20.0%) 50–59: 88 (20.0%) 60–69: 88 (20.0%) |
Pap smear | 7.0% | 5 |
| Kim et al. (2012) [74] | Indonesia | Qualitative/interview and focus group discussion | 87 |
Range: 25–50 |
VIA | Unspecified | 5 |
| Anggraeni et al. (2016) [72] | Indonesia | Cross-sectional/survey questionnaire | 96 |
Distribution: <20 years: 2 (2.1%) 20–35 years: 44 (45.8%) >35 years: 50 (52.1%) |
Pap smear | 33.3% | 4 |
| Nurhasanah et al. (2017) [76] | Indonesia | Cross-sectional/unspecified | 176 |
Distribution: 20–29 years: 35 (19.9%) 30–39 years: 51 (29.0%) 40–49 years: 71 (40.3%) 50–59 years: 19 (10.8%) |
VIA | 33.0% | 3 |
| Sidabutar et al. (2017) [79] | Indonesia | Cross-sectional/survey questionnaire | 80 | Unspecified | VIA | Unspecified | 2 |
| Wakhidah et al. (2017) [83] | Indonesia | Case-control/survey questionnaire | 150 | Unspecified | VIA | NA | 4 |
| Anwar et al. (2018) [73] | Indonesia | Cross-sectional/interview with structured questionnaire | 5397 |
Mean: 52.9 |
Pap smear | 5.5% | 5 |
| Winarti et al. (2018) [85] | Indonesia | Case-control/survey questionnaire | 410 | Unspecified | VIA | NA | 3 |
| Sidabutar et al. (2018) [80] | Indonesia | Cross-sectional/interview with structured questionnaire | 245 | Unspecified | VIA | 15.5% | 4 |
| Aprina et al. (2018) [71] | Indonesia | Cross-sectional/survey questionnaire | 361 | Unspecified | VIA | 26.9% | 3 |
| Rahmawati et al. (2018) [77] | Indonesia | Cross-sectional/survey questionnaire | 188 |
Range: 20–55 |
VIA | Desire to screen: 57.4% | 4 |
| Saptowati et al. (2018) [78] | Indonesia | Cross-sectional/survey questionnaire | 200 | Unspecified | VIA | Unspecified | 3 |
| Sutarti et al. (2018) [82] | Indonesia | Cross-sectional/survey questionnaire | 369 | Unspecified | Pap smear, VIA | Unspecified | 3 |
| Spagnoletti et al. (2019) [81] | Indonesia | Qualitative/focus group discussion and interview | Focus group: 17 Interview: 22 |
Range: Focus group female: 28–40 Focus group male: 35–45 Interview female: 22–57 |
Pap smear, VIA | 31.8% | 4 |
| Widayanti et al. (2020) [84] | Indonesia | Cross-sectional/interview | 126 |
Distribution: <20: 9 (7.1%) 20-30: 72 (57.1%) 30-40: 45 (35.7%) |
VIA | Willingness to screen: 45.2% | 2 |
| Muhith et al. (2020) [75] | Indonesia | Cross-sectional/survey questionnaire | 393 |
Range: 20–50 |
VIA | Unspecified | 2 |
| Phongsavan et al. (2010) [102] | Laos | Cross-sectional/interview with structured questionnaire | 800 |
Mean (SD): 34.0 (9.4) |
Pap smear | 4.5% | 5 |
| Sichanh et al. (2014) [103] | Laos | Case-control/interview with structured questionnaire | 640 |
Mean (SD): 36.2 (8.0) |
Pap smear | 3.9% | 5 |
| Hando et al. (2018) [104] | Laos | Cross-sectional/interview with structured questionnaire | 356 |
Mean (SD): 38.2 (9.8) |
Pap smear | 46.3% | 3 |
| Chee et al. (2003) [42] | Malaysia | Cross-sectional/survey questionnaire | 486 |
Mean (SD): 26.7 (6.2) |
Pap smear | 6.4% Within past 3 years: 4.3% |
2 |
| Chee et al. (2003) [24] | Malaysia | Cross-sectional/survey questionnaire | 1720 |
Mean (SD): 30.1 (7.9) |
Pap smear | 25.3% Within past 3 years: 18.4% |
4 |
| Asmani et al. (2007) [39] | Malaysia | Cross-sectional/survey questionnaire | 280 |
Distribution: <30: 49 (17.5%) 30–39: 79 (28.2%) 40–49:78 (27.9%) >50: 74 (26.4%) |
Pap smear | 51.4% | 4 |
| Moy et al. (2007) [40] | Malaysia | Cross-sectional/survey questionnaire | 112 |
Mean (SD): 35.8 (9.1) |
Pap smear | 61.6% | 4 |
| Wong et al. (2008) [30] | Malaysia | Qualitative/interview | 20 |
Mean (range): 32.2 (21–56) |
Pap smear | 0% | 4 |
| Othman et al. (2009) | Malaysia | Cross-sectional/survey questionnaire | 221 |
Mean (range): 51 (41–61) |
Pap smear | 51.8% | 2 |
| Wong et al. (2009) [45] | Malaysia | Qualitative/semi-structured interview | 20 |
Mean (range): 32.2 (21–56) |
Pap smear | 0% | 5 |
| Abdullah et al. (2010) [16] | Malaysia | Qualitative/semi-structured interview | 11 (providers) |
Range: 37–57 |
Pap smear | Unspecified | 5 |
| Al-Naggar et al. (2010) [18] | Malaysia | Cross-sectional/survey questionnaire | 285 |
Mean (SD): 20.9 (1.9) |
Pap smear | 6.0% | 3 |
| Al-Naggar et al. (2010) [17] | Malaysia | Qualitative/focus group discussion | 23 (17 females) |
Range: 22–26 |
Pap smear | Unspecified | 5 |
| Dunn et al. (2010) [46] | Malaysia | Cross-sectional/survey questionnaire | 1013 |
Mean (SD): 42.9 (10.1) |
Pap smear | 63.0% | 4 |
| Oon et al. (2010) [47] | Malaysia | Qualitative/interview with structured questionnaire | 52 (44 females) |
Range: 23–70 |
Pap smear | 79.5% | 5 |
| Abdullah et al. (2011) [15] | Malaysia | Cross-sectional/survey questionnaire | 403 |
Distribution: <35: 203 >35: 200 |
Pap smear | 38.0% | 5 |
| Al-Naggar et al. (2012) [33] | Malaysia | Cross-sectional/survey questionnaire | 142 |
Mean (SD): 31.6 (8.2) |
Pap smear | 46.5% | 3 |
| Aziz et al. (2013) [32] | Malaysia | Cross-sectional/interview with structured questionnaire | 3693 | Mean (SD): 36.7 years (7.7) |
Pap smear | Within past 3 years: 52.2% | 4 |
| Baskaran et al. (2013) [20] | Malaysia | Cross-sectional/interview with structured questionnaire | 369 |
Mean (SD): 37.5 years (10.0) |
Pap smear | 75.6% | 3 |
| Gan et al. (2013) [44] | Malaysia | Cross-sectional/interview with structured questionnaire | 959 |
Mean (SD): 45.2 (12.2) |
Pap smear | 48.9% Within past 3 years: 18.4% |
5 |
| Wong et al. (2013) [43] | Malaysia | Cross-sectional/survey questionnaire | 231 |
Median (IQR): Tested: 46 (37–53) Not tested: 30.5 (25–43) |
Pap smear | 55.8% | 4 |
| Azrai et al. (2015) [29] | Malaysia | Cross-sectional/interview with structured questionnaire | 98 |
Mean (SD): 42.9 (12.9) |
Self-HPV test | 78.6% (Pap smear) | 3 |
| Abdullah et al. (2016) [27] | Malaysia | Cross-sectional/interview with structured questionnaire | 515 |
Mean (SD): 58.8 (7.1) |
Pap smear | 39.2% | 5 |
| Danial et al. (2016) [21] | Malaysia | Cross-sectional/survey questionnaire | 337 |
Distribution: 18-30: 148 (43.9%) 31-40: 118 (35.0%) ≥41: 71 (21.1%) |
Pap smear | 32.9% | 4 |
| Ma’som et al. (2016) [25] | Malaysia | Cross-sectional/survey questionnaire | 839 |
Median (IQR): 38 (30–48) |
Pap smear | 63.1% | 3 |
| Indra et al. (2017) [37] | Malaysia | Cross-sectional/interview with structured questionnaire | 147 |
Range: 18–63 |
Pap smear | 77.6% | 4 |
| Razi et al. (2017) [38] | Malaysia | Cross-sectional/survey questionnaire | 187 |
Distribution: 20–29: 18 (9.6%) 30–39: 106 (56.7%) 40–49: 57 (30.5%) 50: 6 (3.2%) |
Pap smear | 65.2% Within past 3 years: 42.8% |
4 |
| Abdullah et al. (2018) [34] | Malaysia | Cross-sectional/survey questionnaire | 164 |
Mean (SD): 40.6 (8.4) |
Self-HPV test | 73.2% | 4 |
| Nwabichie et al. (2018) [22] | Malaysia | Cross-sectional/survey questionnaire | 320 |
Distribution: 18–30: 100 (31.3%) 31–50: 218 (68.1%) 51–69: 2 (0.6%) |
Pap smear | 27.5% Within past 3 years: 3.8% |
5 |
| Rubini et al. (2018) [31] | Malaysia | Cross-sectional/interview with structured questionnaire | 305 (males and females) |
Range: >18 |
Pap smear | Unspecified | 4 |
| Sundraraj et al. (2018) [26] | Malaysia | Cross-sectional/survey questionnaire | 246 | Unspecified | Pap smear | 28.5% | 2 |
| Yunus et al. (2018) [36] | Malaysia | Cross-sectional/survey questionnaire | 316 |
Mean (SD): 41.2 (9.2) |
Pap smear | Every 3 years: 9.5% Within past 3 years: 41.8% |
4 |
| Romli et al. (2019) [41] | Malaysia | Cross-sectional/survey questionnaire | 210 |
Mean (SD): 43.0 (10.3) |
Pap smear | 55.2% Within past 5 years: 38.6% |
5 |
| Siraj et al. (2019) [28] | Malaysia | Cross-sectional/survey questionnaire | 300 |
Distribution 17–40: 71 (23.7%) 41–50: 64 (21.3%) 51–60: 80 (26.7%) >60: 85 (28.3%) |
Pap smear | 57.0% | 4 |
| Baharum et al. (2020) [19] | Malaysia | Cross-sectional/survey questionnaire | 417 |
Mean (SD): 24.9 (3.6) |
Pap smear | Unspecified | 5 |
| Ting et al. (2020) [35] | Malaysia | Cross-sectional/self-administered online questionnaire | 246 |
Distribution: 20–30: 141 (57.3%) 31–40: 65 (26.4%) 41–50: 18 (7.3%) 51–60: 16 (6.5%) 61–70: 6 (2.4%) |
Pap smear | 48.0% | 4 |
| Nandar et al. (2015) [105] | Myanmar | Cross-sectional/interview with structured questionnaire | 666 |
Distribution: 30–39: 421 (64.1%) 40–49: 236 (35.9%) |
Unspecified | Within past 3 years: 19.1% | 5 |
| Seow et al. (1994) [89] | Singapore | Cross-sectional/survey questionnaire | 568 |
Distribution: 21–29: 65 (11.7%) 30–39: 189 (33.4%) 40–49: 152 (26.9%) 50–59: 126 (22.1%) 60–65: 34 (6.0%) |
Pap smear | 54.4% | 5 |
| Seow et al. (1995) [90] | Singapore | Cross-sectional/interview with structured questionnaire | 568 |
Distribution: 21- 29: 65 (11.7%) 30–39: 189 (33.4%) 40–49: 152 (26.9%) 50–59: 126 (22.1%) 60–65: 34 (6.0%) |
Pap smear | Unspecified | 5 |
| Seow et al. (2000) [88] | Singapore | Cross-sectional/interview with structured questionnaire | 447 |
Range: 45–69 |
Pap smear | 52.5% Within past 3 years: 34.9% |
5 |
| Lee et al. (2002) [87] | Singapore | Cross-sectional/interview with structured questionnaire | 726 |
Range: 30–59 |
Pap smear | 62.1% Every 3 years: 41.6% |
5 |
| Wee et al. (2010) [94] | Singapore | Cross-sectional/survey questionnaire | 213 (125 females) |
Median (IQR): 63.0 (49–76) |
Pap smear | Eligible for screening every 3 years and went: 2.9% | 4 |
| Wee et al. (2012) [95] | Singapore | Cross-sectional/survey questionnaire | 1081 (623 females) |
Distribution: 40–50: 257 (23.8%) 50–60: 301 (27.8%) 60–70: 192 (17.8%) ≥70: 331 (30.6%) |
Pap smear | Eligible for screening every 3 years and went: 31.3% | 4 |
| Shea et al. (2013) [91] | Singapore | Cross-sectional/survey questionnaire | 393 |
Mean (SD): 21.1 (1.4) |
Pap smear | 2.8% | 3 |
| Chirayil et al. (2014) [86] | Singapore | Cross-sectional/survey questionnaire | 206 |
Range: 18–26 |
Pap smear | 5.3% Within past 3 years: 3.9% |
3 |
| Wong et al. (2015) [98] | Singapore | Cross-sectional/survey questionnaire | 4337 (1993 eligible for cervical cancer screening) |
Range: 18–79 |
Pap smear | 69.2% Every 3 years: 43.8% |
5 |
| Tay et al. (2015) [92] | Singapore | Cross-sectional/survey questionnaire | 1622 |
Distribution: Not stated: 22 (1.4%) <25: 445 (27.4%) 25–29: 456 (28.1%) 30–34: 220 (13.6%) 35–39: 121 (7.5%) 40–44: 112 (6.9%) 45–49: 86 (5.3%) 50–54: 86 (5.3%) >54: 74 (4.6%) |
Unspecified | 50.2% Within past 3 years: 21.2% |
3 |
| Wee et al. (2016) [93] | Singapore | Mixed method/survey questionnaire and interview | 1996 (1154 females) /20 interviewed |
Distribution: <60 years: 964 (48.3%) ≥60 years: 1032 (51.7%) |
Pap smear | Every 3 years (rental flats): 18.0% | 4 |
| Wee et al. (2016) [96] | Singapore | Qualitative/interview | 29 (20 patients, 9 provider) |
Range of patient group: 40–59: 11 (55.0%) ≥60: 9 (45.0%) |
Pap smear | Unspecified | 5 |
| Wee et al. (2017) [97] | Singapore | Mixed method/survey questionnaire and interview | 2037 (855 females)/12 (6 females) |
Distribution: 40–50: 412 (20.2%) 51–60: 1625 (79.8%) |
Pap smear | Every 3 years: 24.9% | 5 |
| Yeo et al. (2018) [99] | Singapore | Cross-sectional/survey questionnaire | 268 |
Distribution: 21–34: 208 (77.9%) 35–50: 59 (22.1%) |
Pap smear | 38.7% | 4 |
| Boonmongkon et al. (2002) [68] | Thailand | Cross-sectional/survey questionnaire | 1028 | Unspecified | Pap smear | Within past 2 years: 34.6% | 2 |
| Kritpetcharat et al. (2003) [51] | Thailand | Cross-sectional/interview with structured questionnaire | 1199 |
Distribution: 20–29: 185 (15.4%) 30–39: 345 (28.8%) 40–49: 315 (26.3%) 50–59: 207 (17.3%) ≥60: 147 (12.3%) |
Pap smear | 66.9% | 3 |
| Boonpongmanee et al. (2007) [64] | Thailand | Cross-sectional/survey questionnaire | 189 |
Mean (SD): 36.8 (7.69) |
Pap smear | 51.9% | 3 |
| Chalapati et al. (2007) [49] | Thailand | Prospective quasi-experimental/interview | 200 |
Mean Intervention: 47.0 Control: 47.4 |
Pap smear | 72.5% | 5 |
| Kietpeerakool et al. (2009) [61] | Thailand | Cross-sectional/interview with structured questionnaire | 402 |
Mean (SD): 27.1 (6.6) |
Pap smear | 85.8% | 3 |
| Oranratanaphan et al. (2010) [59] | Thailand | Cross-sectional/survey questionnaire | 78 |
Mean: 32.5 |
Pap smear | 79.5% | 2 |
| Srisakul et al. (2011) [55] | Thailand | Cross-sectional/interview with structured questionnaire | 450 |
Mean (SD): 45.3 (4.2) |
Unspecified | Unspecified | 3 |
| Chesun et al. (2012) [56] | Thailand | Case-control/interview with structured questionnaire | 400 |
Mean (SD): Case: 42.1 (5.3) Control: 42.5 (5.0) |
Pap smear | Unspecified | 4 |
| Thanapprapasr et al. (2012) [50] | Thailand | Cross-sectional/survey questionnaire | 1365 |
Distribution: <30: 713 (53.6%) 31–40: 384 (28.9%) 41–50: 159 (12.0%) ≥50: 73 (5.5%) |
Pap smear | 36.6% | 4 |
| Budkaew et al. (2014) [54] | Thailand | Case-control/survey questionnaire and interview | 195 |
Mean (range): 46.0 (30–60) |
Pap smear | NA | 3 |
| Oranratanaphan et al. (2014) [66] | Thailand | Cross-sectional/survey questionnaire | 100 |
Mean (SD): 40.6 (9.3) |
Self-HPV test, pap smear | 81.0% | 2 |
| Wongwatcharanukul et al. (2014) [53] | Thailand | Cross-sectional/interview with structured questionnaire | 547 |
Mean (SD): 43.0 (8.6) |
Pap smear | 64.9% | 4 |
| Mukem et al. (2015) [62] | Thailand | Cross-sectional/survey questionnaire | Health and Welfare Survey: 11,046,520 |
Mean: Health and Welfare Survey: 51.3 |
Pap smear, VIA | Health and Welfare Survey: 46.3% | 5 |
| Polrit et al. (2015) [65] | Thailand | Case-control/interview with structured questionnaire | 452 |
Mean (SD) Case: 45.8 (7.8) Controls: 44.8 (9.8) |
Pap smear, VIA | NA | 5 |
| Srisuwan et al. (2015) [60] | Thailand | Cross-sectional/survey questionnaire and interview | 128 village health volunteers/ 10 patients |
Mean: Village health volunteer: 46.8 |
Pap smear | Unspecified | 3 |
| Visanuyothin et al. (2015) [52] | Thailand | Cross-sectional/survey questionnaire | 595 |
Distribution: 30–39: 154 (25.9%) 40–49: 219 (36.8%) 50–60: 222 (37.3%) |
Pap smear | Within 5-year interval: 65.4% | 5 |
| Chaowawanit et al. (2016) [58] | Thailand | Cross-sectional/survey questionnaire | 4339 |
Mean (SD): 46.6 (9.9) |
Unspecified | 65.3% Within 5-year interval: 42.8% |
5 |
| Kittisiam et al. (2016) [67] | Thailand | Cross-sectional/survey questionnaire | 2810 |
Mean (SD): 46.9 (9.9) |
Self-HPV test | Unspecified | 5 |
| Mongsawaeng et al. (2016) [57] | Thailand | Cross-sectional/survey questionnaire | 265 |
Distribution: 30–40: 63 (23.8%) 41–50: 113 (42.6%) 51–60: 89 (33.6%) |
Pap smear | 89.8% | 5 |
| Chongthawonsatid et al. (2017) [70] | Thailand | Cross-sectional/interview with structured questionnaire | 15,074,126 |
Distribution: 30–39: 5,561,306 (36.9%) 40–49: 5,487,316 (36.4%) 50–59: 4,025,504 (26.7%) |
Pap smear | 68.4% | 5 |
| Gottschlich et al. (2019) [48] | Thailand | Cross-sectional/survey questionnaire | 267 |
Mean (SD): 50.4 (5.8) |
Pap smear | 82.0% | 5 |
| Bunkarn et al. (2020) [63] | Thailand | Prospective quasi-experimental/survey questionnaire | 130 |
Mean (SD): 44.5 (8.3) |
Unspecified | Unspecified | 4 |
| Songsiriphan et al. (2020) [69] | Thailand | Cross-sectional/survey questionnaire | 300 |
Mean (SD): 45.0 (9.5) |
Pap smear | 89.3% | 4 |
| Vo et al. (2018) [106] | Vietnam | Qualitative/interview | 86 |
Distribution: 20–24: 15 (17.4%) 25–29: 12 (14.0%) 30–34: 32 (37.2%) 35–39: 27 (31.4%) |
Pap smear, VIA | 3.1% | 4 |
| Hoang et al. (2018) [107] | Vietnam | Mixed method/survey questionnaire and interview | 130 (113 females) |
Mean (SD): Residents: 21 (2.0) Migrants: 22 (1.7) |
Pap smear | 7.1% | 4 |
HPV: Human papilloma virus; MMAT: Mixed Methods Appraisal Tool; VIA: Visual inspection with acetic acid.
The number of study participants ranged from 7 to 15,074,126. Data were primarily obtained via the administration of questionnaires or interview or a combination of both. Pap smear was the screening modality in a majority of the studies (73.1%, n = 68), followed by VIA (11.8%, n = 11). A small number of studies involved self-sampled HPV tests alone (n = 3), and a combination of pap smears and VIA/self-sampled HPV tests (n = 6). Excluding studies that only recruited patients with no history of CCS, the prevalence of patients reporting a screening history varied widely from 3.9% in Laos [103] to 89.8% in Thailand [57]. In the two studies, none of the factors investigated were statistically significant [21,36], and thus were not included in the categorization of barriers and facilitators. As two studies in Thailand referred to the Reproduction Health Survey 2009 [62,70], data were only extracted from the more recent publication [70]. Two studies from Singapore analyzed the same study cohort but reported unique barriers and facilitators separately for each study [89,90]. Hence, both studies were included in this analysis.
3.2. Quality Assessment
A majority of the studies (68.8%, n = 64) scored 4 or 5 out of 5 based on the MMAT. All qualitative (n = 9) and mixed-methods studies (n = 3) scored either 4 or 5 points. Among 73 quantitative non-randomized studies, the lack of description of the sampling process or population (n = 38), and the lack of description of tools for an outcome and exposure measurement (n = 20) were common. Four studies were not clear on the completeness of screening data. Among eight quantitative descriptive studies, five studies lack description on sampling or target population, where non-response bias becomes difficult to assess. The complete quality appraisal can be found in Table S2.
3.3. Factors Associated with Cervical Cancer Screening in Southeast Asia (n = 91)
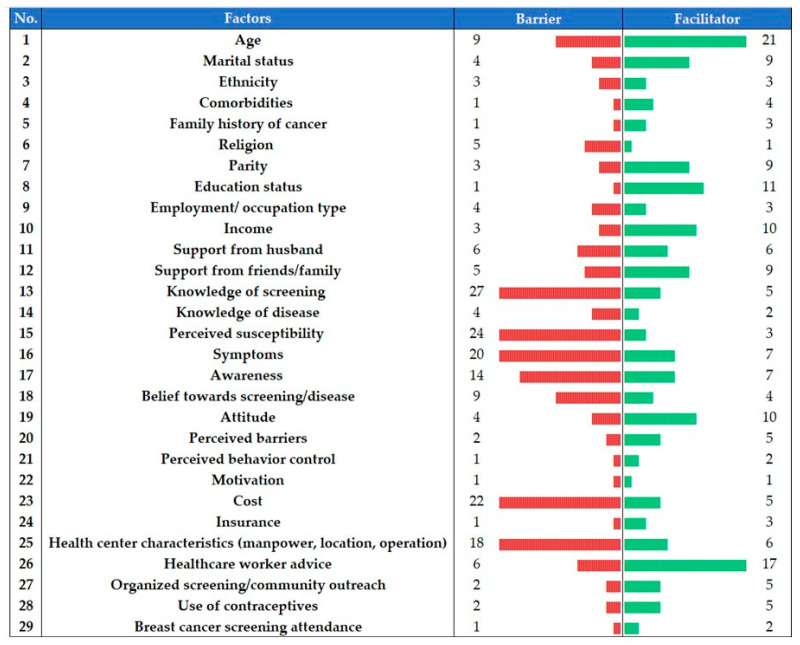
A total of 63 barriers from 63 studies were reported across seven countries in SEA (Table S3), while 71 facilitators from 73 studies were reported across nine countries (Table S4). The top three barriers to CCS in SEA by the number of publications include embarrassment (n = 33), busyness or time constraints (n = 27), and poor knowledge of screening (n = 27). The top three CCS facilitators include age (n = 21), healthcare workers’ advice for CCS (n = 17), and higher education status (n = 11). Figure 2 summarizes 29 factors that were described both as barriers and facilitators of CCS across studies in SEA (full details by country level are available in Figure S1).
Figure 2.
Factors described as barriers and facilitators of cervical cancer screening in Southeast Asia, according to publication numbers.
The barriers to and facilitators of CCS were broadly organized into 11 categories: (1) Demographics, (2) socio-economic status, (3) healthcare utilization, (4) social support, (5) psychological, emotional, (6) knowledge, (7) risk perception, (8) perception, attitude, belief, (9) motivation, preference, (10) financial access, and (11) health system. Factors that do not fall under the 11 categorizes were classified as “others”.
3.4. Common Barriers and Facilitators in Cervical Cancer Screening Across Countries in Southeast Asia
The barriers to and facilitators of CCS were also summarized by the number of countries in Tables S3 and S4, respectively, to allow for commonalities across countries to be drawn. The barrier categories reported by most countries (n = 6) were demographics, knowledge, risk perception, and health system. Poor awareness to screening was the most common barrier reported by countries in SEA (n = 6). Other common barriers (n = 5) include poor knowledge of screening, poor perceived susceptibility, having no symptoms, factors related to health center characteristics (manpower, operations, and location), embarrassment, fear of results, fear of pain, and costs related to CCS. Most countries in SEA (n = 7) reported facilitators in the category of demographics. This is followed by knowledge, financial access, and health system-related facilitators (n = 6). Specific facilitators common across countries include age (n = 6), followed by good awareness of screening, and the receipt of healthcare worker advice (n = 5).
The commonalities among the top three barriers and facilitators for each country were also assessed. Embarrassment (n = 4) and poor knowledge to screening (n = 3) were the two most common barriers in SEA. Other common barriers (n = 2) include time constraints, having no symptoms, and the cost of screening. Age and advice from healthcare workers were the most common facilitators of CCS (n = 3), followed by health center characteristics (manpower, operations, and location), and support from friends or family members (n = 2).
3.5. Factors Associated with Cervical Cancer Screening (Country Level Analyses)
3.5.1. Brunei (n = 1)
Based on a population health survey among adults aged 18 to 69 years old, 56.5% of female participants had a history of having a pap smear [100]. Barriers to CCS include older age, employment type, and breast cancer screening attendance [100]. Facilitators to CCS include being married, the presence of comorbidities or family members with comorbidities, diet, and alcohol intake [100].
3.5.2. Cambodia (n = 1)
Among women aged 20 to 69 in a rural district, only 7.0% have ever received a pap smear [101]. Only facilitators were reported in this cross-sectional study, where younger women, and those with good awareness towards screening expressed greater willingness to CCS [101].
3.5.3. Indonesia (n = 15)
Two qualitative studies and thirteen quantitative studies were published after 2010, 11 of which reported VIA as the screening modality for CCS. Two studies reported pap smears for CCS [72,73], while two studies reported both pap smear and VIA [81,82]. The prevalence of having a screening done in the past ranged from 5.5% to 33.3% [71,72,73,76,80,81,85], while participants’ desire to screen was 45.2% and 57.4% based on two studies [77,84].
Of the seven studies reporting barriers to CCS in Indonesia, four studies reported facilitators in the categories of knowledge as well as perception, attitude, and belief. No studies reported barriers related to demographics or socio-economic status. Embarrassment [74,81], knowledge deficits in CCS [81,84], having no symptoms [74,81], and fear of result [76,81], were among the top barriers in Indonesia. Two studies described the lack of knowledge of cervical cancer as a barrier to CCS, which was only reported in Indonesia [74,81].
Facilitators were more commonly described in the categories of perception, attitude, and beliefs (n = 7) as well as motivation and perception (n = 5) among 13 studies from Indonesia. There were no facilitators in the classification of psychological or emotional factors, and healthcare utilization. Intentions to screening was among the top facilitators of CCS [72,78,80], which was reported more commonly in Indonesia compared to other SEA countries. In addition, support from family or friends was another crucial facilitator of CCS based on three publications [71,74,81].
3.5.4. Laos (n = 3)
All three quantitative studies from Laos described pap smears as the screening modality of interest, two of which were published after 2010 [103,104]. Among working women, a higher prevalence of ever receiving a pap smear was reported (46.3%) [104], compared to village residents and women with HIV (3.9% to 4.5%) [102,103].
All three studies reported barriers in the category of psychological or emotional factors, knowledge, risk perception, financial access, and the health system. No studies reported barriers in the category of socio-economic status. Barriers reported by at least two studies include embarrassment [102,103,104], fear of pain [102,103,104], fear of results [102,103], poor awareness [103,104], poor perceived susceptibility [102,103], being not at risk for cancer [103,104], and having no symptoms [103,104]. Other barriers include concerns with screening cost [102,103,104], an inaccessible screening location [102,103], and the lack of CCS advice from a healthcare worker [103,104]. Only one study described a knowledge-related facilitator, where higher scores for knowledge of cervical cancer and prevention were reported among women who received CCS in the past [104].
3.5.5. Malaysia (n = 31)
Nineteen studies were published from 2011, five of which were qualitative studies. The take up of pap smear in different female populations varied largely, from 6.0% among university students [18], to 79.5% among patients from an obstetrics and gynecology clinic [47]. Similarly, screening in the past three years varied largely from 3.8% among African immigrants to Malaysia [22], to 42.8% among university staff [38].
Across 21 studies in Malaysia reporting barriers to CCS, a majority of the studies reported barriers in the categories of psychological or emotional factors (n = 16) and knowledge (n = 13). Embarrassment was the most common reason cited for not attending CCS [15,17,18,20,23,29,30,33,38,39,46,47] followed by having no time or busyness [16,17,20,23,25,30,31,33,38,39,46]. Other top barriers include fear of pain [17,18,19,20,29,31,33,47], and poor knowledge of screening [16,18,20,31,33,38,39,46,47]. Barriers relating to health center characteristics were reported in eight studies [17,20,30,31,33,38,39,46]. This includes a long wait time for screening [30,31,38], an inconvenient screening location [17,33,46], difficulty in securing an appointment for screening [31], inconvenient clinic hours [30,31], having no adequate facility for screening [46], no transport [39], and no female health provider [17,20,30,33]. Compared to other nations in SEA, worry [18,20,33] and the lack of support from their husband [18,20,23], friends, or family [30,33,38,46], were prominent barriers to CCS in Malaysia.
A total of 26 studies reported facilitators to CCS in Malaysia, a majority of which involved facilitators categorized under demographics (n = 15) and knowledge (n = 11). Among the top facilitators were demographic factors such as age [24,25,32,34,37,39,40,43,44,46], marriage status [22,24,33,37,40,43], and parity [15,32,38,43,44]. Receiving advice from healthcare workers [19,27,29,30,33,47], and a good attitude towards CCS [26,35,37,38,41], were also common facilitators reported in Malaysia. Other facilitators, which were also more commonly reported in Malaysia than other SEA nations, include good knowledge of screening or cervical cancer [22,24,26,32,35,37,41,43,44], and the use of contraceptives [24,27,37,41,44].
3.5.6. Myanmar (n = 1)
A total of 666 migrant women were surveyed in a study, where 19.1% had CCS in the past three years [105]. Screening in the past three years was more likely among older women, those with a family history of cancer, had expressed willingness to pay for screening, received encouragement from nurses, had low perceived barriers, and had good disease and screening knowledge [105].
3.5.7. Singapore (n = 14)
Of the 11 quantitative, one qualitative and two mixed-methods studies, nine were published between 2011 to 2020. The prevalence of ever being screened for cervical cancer varied widely from 2.8% among undergraduates [91], to 69.2% among participants in the National Health Survey [98]. Similarly, the proportion of patients who received CCS within the past three years varied widely from 2.9% to 44.3% [86,87,88,89,92,93,94,95,97,98].
Seven of the ten studies reporting barriers to CCS in Singapore described barriers classified under risk perception and motivation or preference. Six studies reported barriers in the categories of financial access and psychological or emotional factors. Perceived susceptibility was the most common barrier to CCS, where patients do not care about screening [87,92,93,94,95,96,98]. Other top barriers to CCS include busyness or lack of time [91,92,93,94,95,96], cost concerns relating to screening [87,93,94,95,96,98], embarrassment [92,93,94,95], and the fear of receiving unfavorable results [87,93,95,96]. Amongst the top barriers was the poor knowledge of screening which involved knowledge of a screening location [94,95], screening frequency [95], and screening criteria [87,94,95,96]. Barriers more commonly reported in Singapore compared to other SEA nations, include having a fatalistic attitude [88,94,95,96], inconvenience [94,95,96], and the lack of companions to attend screening with [94,95,96].
Of the 12 studies reporting facilitators in Singapore, five reported facilitators within the category of perception, attitude, and belief, while four described facilitators in the categories of knowledge, demographics, and healthcare utilization. Top facilitating factors were related to age [89,92,99], support from family and friends [88,89,92], good awareness of screening [89,92,96], and high perceived benefit [90,92,99]. Other top facilitators include healthcare worker advice for screening [89,92,96] and health center characteristics such as a convenient screening location [87,93,96] and the availability of female health workers or nurses for the conduct of screening [87,96].
3.5.8. Thailand (n = 23)
A majority of the studies (n = 17) were published between 2011 to 2020. The prevalence of ever having pap smear screening ranged between 36.6% to 89.8% [48,49,50,51,53,57,59,64,66,69,70], while screening with pap smear or VIA in the past five years ranged from 32.5% to 85.8% [52,54,58,61,62]. Most of the studies described pap smears as the screening modality while one study explored the willingness to screen with a self-HPV test [67].
A majority of the 19 studies reporting barriers in Thailand described barriers classified under psychological or emotional factors (n = 15) and risk perception (n = 13). Similar to Malaysia, embarrassment was the most common reason for not receiving CCS in Thailand [48,49,50,51,53,57,58,59,60,62,69,70]. This is followed by poor knowledge of screening [48,50,51,53,61,62,63,67,68,70], having no symptoms [48,49,50,53,54,58,59,61,62,68,69], busyness or the lack of time [50,53,54,57,58,59,62,63,70], fear of pain [48,49,50,53,58,63,69,70], and poor perceived susceptibility [48,50,51,54,58,62,69,70]. Besides that, religion [48,53,62,70], and having a poor impression of the health system [56,58,60,63,69], were reported more frequently as barriers to CCS in Thailand.
Of the 17 studies reporting facilitators, nine described facilitators in the health system category, while eight reported demographic-related facilitators. The top facilitator of CCS was receiving advice from healthcare workers [48,53,54,56,61,69]. This is followed by demographic and socio-economic factors such as age [50,64,65,69,70], income level [54,56,68,70], and education status [53,62,64,70]. Having gynecological symptoms were amongst the top facilitators and was also more commonly reported in Thailand compared to other countries [50,53,54,66,68]. Other facilitators more commonly reported in Thailand include CCS being part of a routine health check [50,53,54,56,66], advice from employers [50,54,61], occupation type [52,70], and the fear or suspicion of cervical cancer [50,53,61].
3.5.9. Vietnam (n = 2)
A qualitative and a mixed methods study with a total of 216 participants revealed a low prevalence of prior pap smear or VIA ranging from 3.1% to 7.1% [106,107]. Barriers to CCS were related to religious beliefs, low risk perception, and the lack of healthcare worker advice [106]. Both studies reported poor awareness as a barrier to screening [106,107], while the beliefs of health workers that CCS is only for married women was reported in one study [107]. Facilitators to CCS include spousal support, low cost of screening, and having healthcare workers’ advice for CCS [106].
4. Discussion
To our knowledge, this is the first systematic review to focus on barriers to and facilitators of CCS among women in SEA, a region with high disease incidence yet poor screening uptake. Over 60 barriers from seven countries and over 70 barriers from nine countries were identified and categorized into 11 broad categories. We presented the findings at the country level to provide insights on how screening uptake can be improved in each country. We also compared the top barriers and facilitators between countries, to provide guidance for countries that have limited information on factors affecting CCS. A majority of the studies in this review were published within the past 10 years, which highlights the growing interest and challenges faced in the area of cervical cancer screening within SEA. A broad search strategy was employed in this study to maximize findings by including both quantitative and qualitative studies without any date restrictions. This allowed for the study of a broader perspective from patients, family members, healthcare providers, and health officials, which provides valuable insight to guide the design of public health programs.
The barriers and facilitators identified from this study are related to demographics, socio-economic status, social support, knowledge, attitudes, perceptions, financial access, health system, and psychological or emotional factors. We found that the top barrier category to CCS is psychological or emotional factors (n = 44), namely embarrassment and fear. This is followed by knowledge (n = 38), which includes the lack of knowledge and awareness to cervical cancer and CCS. The top facilitator categories are predominantly factors related to demographics (n = 33), as well as perception, attitudes, and beliefs to screening (n = 29). These are consistent with prior research in lower-middle income countries [108,109,110], and interestingly in developed countries as well, such as the United States [111] and Australia [112]. Our findings also support a previously demonstrated relationship between higher education status and higher CCS uptake [7]. Similarly, psychosocial and contextual factors described in prior systematic literature reviews [9,10] were also reported by women in SEA. These include factors associated with the health system, cost, time constraints, screening attitudes, knowledge, awareness, emotional factors, social support, and experiences with healthcare professionals. However, our findings differed from a review among high cervical cancer incidence countries where the top barriers to CCS were fatalism, and negative attitudes and beliefs towards non-traditional healthcare [11]. A possible reason is that a majority of the studies included in that review are from Africa, where traditional healers are likely sought prior to or in conjunction with medical care [113].
Barriers common to SEA countries, reported by five to six countries, include poor awareness and knowledge of screening, poor perceived susceptibility to cervical cancer, having no symptoms, and factors related to health center characteristics. Facilitators common across countries include the influence of age, receiving advice from healthcare workers, and good awareness of screening. These common factors identified can also provide guidance for countries with limited insights into barriers and facilitators to CCS, such as the Philippines, Timor-Leste, Cambodia, Myanmar, Vietnam, and Laos. Several factors were also unique in certain countries, which reported certain barriers and facilitators more frequently than other countries. For example, religion and poor impression of the health system were more frequently reported barriers in Thailand while occupation type, advice from employers, and receiving CCS as part of a structured health program were more frequently reported facilitators. In Malaysia, unique barriers include the lack of support from husband, family members, and friends, while unique facilitators include the knowledge of screening and the use of contraceptives. Reasons for these differences between countries could be driven by the social, cultural, religious, and health system differences of SEA countries [114], as well as researchers’ interests in specific factors affecting CCS in the country. Hence, existing CCS programs should consider addressing the country-specific barriers and facilitators in the design of interventions to increase screening uptake.
Broadly, our findings suggest that patient education-based interventions are key to increasing CCS uptake in SEA, as key barriers to CCS such as fear, embarrassment, and the lack of knowledge can be addressed. Through the use of community health workers, brochures, phone counselling, and multimedia, educational interventions alone has been found to increase the odds of CCS uptake by more than 2 times [115], compared to routine care. Equally important, facilitators of CCS should be considered in the design of such interventions to increase CCS uptake, as barriers and facilitators are often two sides of the same coin. For example, knowledge of screening is one of the 29 factors that was described both as a barrier and facilitator across studies in SEA. Improving knowledge to screening among women facilitates CCS uptake while the lack of it represents a barrier. While these factors may represent key targets for intervention design, their differential impact across various contexts have been carefully considered [116]. Furthermore, the experience of different facilitators may have a greater influence on CCS uptake in a setting where barriers are commonly experienced by women. In a UK study, women with up-to-date CCS prioritized the following facilitators of CCS compared to those who had overdue or never had CCS: (1) Perceived benefits of CCS and (2) perceived self-responsibility over one’s health [117]. On the other hand, the ranking of common barriers, such as fear and embarrassment did not differ by participants’ screening history. The interplay of barriers and facilitators to CCS warrants further research, and the findings can be harnessed to guide interventions to increase CCS uptake. Therefore, we have presented barriers to CCS alongside its facilitators at the country level in this study.
The current systemic literature review has a few limitations. Firstly, the availability of evidence varies in SEA countries. A majority of the studies identified in the current review are from upper-middle income countries according to the World Bank criteria (Indonesia, Malaysia, and Thailand) [118], and fewer are published from lower-middle income countries (Myanmar, Cambodia, Vietnam, and Laos). In addition, there was no study from the Philippines and Timor-Leste. Although the common themes identified across SEA countries can provide insights into barriers to and facilitators of CCS in lower-middle income countries in the region, we do think that country-specific studies are still necessary, owing to the cultural differences between countries. However, technical expertise may be lacking in some countries, which highlights the need for support from foundations or non-governmental organizations with interests in women’s health. Secondly, we have applied an English language restriction in this review, while relevant articles may have been published in local languages. However, we assess its impact to be low as few studies (n = 3) were not published in English. Thirdly, the top barriers and facilitators identified in this review was based on publication numbers, which are dependent on previous researchers’ study interest, as well as questionnaire or interview design, where selected factors may be explored more frequently. Nevertheless, this remains a common methodology employed to assess the importance of factors identified in barriers and facilitator reviews, especially when no acceptable standard practice guideline is available at present [116]. Lastly, the heterogeneity among studies limits us from assessing the effect size of each barrier or facilitator on CCS uptake. Such heterogenicity is anticipated and necessary, which highlights the need for culture-specific studies and interventions to address the diverse cultural contexts in SEA countries.
5. Conclusions
A significant number of barriers affect the uptake of cervical cancer screening. Coupled with a lack of resources and possibly low prioritization of cervical cancer, challenges to address these barriers remain. This is perhaps why women in SEA continue to face a high risk of cervical cancer mortality and morbidity while significant progress has been made in the developed world. This review of studies published in the SEA region from 1994 to 2020 identified that psychological-, emotional-, and knowledge-related factors remain a significant concern among women in SEA. Facilitating factors include receiving advice from healthcare workers for CCS, and factors associated to patient demographics. Future studies can explore the interplay of the barriers and facilitators on CCS and generate more insights into low-middle income countries in the region. Nevertheless, findings from this review may inform health policy makers in developing effective cervical cancer screening programs in SEA countries.
Acknowledgments
We would like to thank the Economic Development Board (Singapore) for providing their support to B.C. as a Doctor of Philosophy candidate in the Industrial Postgraduate Programme with the National University of Singapore.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18094586/s1, Table S1: Search strategy in each database. Table S2: Quality assessment of included studies using the Mixed Methods Appraisal Tool. Table S3: Barriers to cervical cancer screening in Southeast Asia. Table S4: Facilitators of cervical cancer screening in Southeast Asia. Figure S1: Factors described as barriers to and facilitators of cervical cancer screening in Southeast Asia, according to country and publication numbers.
Author Contributions
Conceptualization, B.C., V.M., C.A. and H.L.W.; Methodology, B.C., A.L., M.M., V.M., C.A. and H.L.W.; Writing—original draft preparation, B.C.; Writing—review and editing, B.C., V.M., C.A. and H.L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arbyn M., Weiderpass E., Bruni L., de Sanjosé S., Saraiya M., Ferlay J., Bray F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre L.A., Islami F., Siegel R.L., Ward E.M., Jemal A. Global cancer in women: Burden and trends. Cancer Epidemiol. Biomark. Prev. 2017;26:444–457. doi: 10.1158/1055-9965.EPI-16-0858. [DOI] [PubMed] [Google Scholar]
- 3.Gakidou E., Nordhagen S., Obermeyer Z. Coverage of cervical cancer screening in 57 countries: Low average levels and large inequalities. PLoS Med. 2008;5:e132. doi: 10.1371/journal.pmed.0050132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Population, Total—Timor-Leste, Singapore, Indonesia, Malaysia, Vietnam, Cambodia, Philippines, Thailand, Lao PDR, Myanmar, Brunei Darussalam, World. [(accessed on 20 October 2020)]; Available online: https://data.worldbank.org/indicator/SP.POP.TOTL?end=2019&locations=TL-SG-ID-MY-VN-KH-PH-TH-LA-MM-BN-1W&start=2019.
- 5.Cervical Cancer, Human Papillomavirus (HPV), and HPV Vaccines in Southeast Asia. [(accessed on 3 March 2020)]; Available online: https://www.amfar.org/uploadedFiles/_amfarorg/Articles/Around_The_World/TreatAsia/2016/hpva4(2).pdf.
- 6.Cervix uteri. [(accessed on 10 January 2021)]; Available online: https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf.
- 7.Damiani G., Basso D., Acampora A., Bianchi C.B., Silvestrini G., Frisicale E.M., Sassi F., Ricciardi W. The impact of level of education on adherence to breast and cervical cancer screening: Evidence from a systematic review and meta-analysis. Prev. Med. 2015;81:281–289. doi: 10.1016/j.ypmed.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Kim K., Han H.R. Potential links between health literacy and cervical cancer screening behaviors: A systematic review. Psychooncology. 2016;25:122–130. doi: 10.1002/pon.3883. [DOI] [PubMed] [Google Scholar]
- 9.Bukowska-Durawa A., Luszczynska A. Cervical cancer screening and psychosocial barriers perceived by patients. A systematic review. Contemp. Oncol. 2014;18:153. doi: 10.5114/wo.2014.43158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plourde N., Brown H.K., Vigod S., Cobigo V. Contextual factors associated with uptake of breast and cervical cancer screening: A systematic review of the literature. Women Health. 2016;56:906–925. doi: 10.1080/03630242.2016.1145169. [DOI] [PubMed] [Google Scholar]
- 11.Driscoll S.D. Barriers and facilitators to cervical cancer screening in high incidence populations: A synthesis of qualitative evidence. Women Health. 2016;56:448–467. doi: 10.1080/03630242.2015.1101742. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun V., Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 14.Hong Q.N., Fàbregues S., Bartlett G., Boardman F., Cargo M., Dagenais P., Gagnon M.-P., Griffiths F., Nicolau B., O’Cathain A., et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 2018;34:285–291. doi: 10.3233/EFI-180221. [DOI] [Google Scholar]
- 15.Abdullah F., Aziz N.A., Tin T.S. Factors related to poor practice of pap smear screening among secondary school teachers in Malaysia. Asian Pac. J. Cancer Prev. 2011;12:1347–1352. [PubMed] [Google Scholar]
- 16.Abdullah F., Su T.T. Enhancement of the cervical cancer screening program in Malaysia: A qualitative study. Asian. Pac. J. Cancer Prev. 2010;11:1359–1366. [PubMed] [Google Scholar]
- 17.Al-Naggar R.A., Isa Z.M. Perception and opinion of medical students about Pap smear test: A qualitative study. Asian. Pac. J. Cancer Prev. 2010;11:435–440. [PubMed] [Google Scholar]
- 18.Al-Naggar R.A., Low W., Isa Z.M. Knowledge and barriers towards cervical cancer screening among young women in Malaysia. Asian. Pac. J. Cancer Prev. 2010;11:867–873. [PubMed] [Google Scholar]
- 19.Baharum N.N., Ariffin F., Isa M.R., Tin S.T. Health Literacy, Knowledge on Cervical Cancer and Pap Smear and Its Influence on Pre-Marital Malay Muslim Women Attitude towards Pap Smear. Asian Pac. J. Cancer Prev. 2020;21:2021–2028. doi: 10.31557/APJCP.2020.21.7.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baskaran P., Subramanian P., Rahman R.A., Ping W.L., Taib N.A.M., Rosli R. Perceived susceptibility, and cervical cancer screening benefits and barriers in Malaysian women visiting outpatient clinics. Asian Pac. J. Cancer Prev. 2013;14:7693–7699. doi: 10.7314/APJCP.2013.14.12.7693. [DOI] [PubMed] [Google Scholar]
- 21.Danial M., Sivasangari S., Arulappen A., Ong L. Journey of the human papillomavirus (HPV) in a developing country over 5 Years (2010–2015) Asian Pac. J. Cancer Prev. 2016;17:1363–1368. doi: 10.7314/APJCP.2016.17.3.1363. [DOI] [PubMed] [Google Scholar]
- 22.Nwabichie C.C., Manaf R.A., Ismail S.B. Factors affecting uptake of cervical cancer screening among African women in Klang Valley, Malaysia. Asian Pac. J. Cancer Prev. 2018;19:825. doi: 10.22034/APJCP.2018.19.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Othman N.H., Devi B.C., Halimah Y. Cervical cancer screening: Patients understanding in major hospitals in Malaysia. Asian Pac. J. Cancer Prev. 2009;10:569–574. [PubMed] [Google Scholar]
- 24.Chee H., Rashidah S., Shamsuddin K., Intan O. Factors related to the practice of breast self examination (BSE) and Pap smear screening among Malaysian women workers in selected electronics factories. BMC Womens Health. 2003;3:3. doi: 10.1186/1472-6874-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma’som M., Bhoo-Pathy N., Nasir N.H., Bellinson J., Subramaniam S., Ma Y., Yap S.-H., Goh P.-P., Gravitt P., Woo Y.L. Attitudes and factors affecting acceptability of self-administered cervicovaginal sampling for human papillomavirus (HPV) genotyping as an alternative to Pap testing among multiethnic Malaysian women. BMJ Open. 2016;6:e011022. doi: 10.1136/bmjopen-2015-011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundraraj Y.A., Murad N.S.A., Ismail N.A., Rahman N.I.A. The prevalence of pap smear screening and association with knowledge, attitude and practice in Kota Kinabalu, Sabah, Malaysia. e–Acad. J. 2018:153–158. [Google Scholar]
- 27.Abdullah N.N., Daud S., Al-Kubaisy W., Saari I.S., Saad S.R. Cervical cancer screening after 50: Near extinction? Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;206:136–140. doi: 10.1016/j.ejogrb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Siraj F., Radzijohari M., Bakar N.A.A., Sahazudin F. Health Belief Model and Its Association with Cervical Cancer Screening Among Malaysian Women. Indian J. Public Health Res. Dev. 2019;10:1145–1151. doi: 10.5958/0976-5506.2019.01738.8. [DOI] [Google Scholar]
- 29.Azrai A., Nirmala C.K., Nur Azurah A.G., Hatta M.D., Lim P.S., Omar M.H., Zainuddin A.A., Yulianty A., Sulaiman A.S. Knowledge towards human papilloma virus (hpv) and acceptability of hpv dna self sampling testing for cervical cancer prevention in rural population. Int. J. Curr Res. 2015;7:12052–12056. [Google Scholar]
- 30.Wong L., Wong Y., Low W., Khoo E., Shuib R. Cervical cancer screening attitudes and beliefs of Malaysian women who have never had a pap smear: A qualitative study. Int. J. Behav. Med. 2008;15:289–292. doi: 10.1080/10705500802365490. [DOI] [PubMed] [Google Scholar]
- 31.Rubini G., Fatini A., Siti Khadijah M., Hamsa Laxmee R., Noorhasriyantie H., RiniAzmeera K., NurSyarafina H., UmmiAtiqah S., Thivya B., Sabariah A. Barrier and belief towards pap smear screening in Sepang, Selangor, Malaysia: Gender perspective. Int. J. Educ. Res. 2018;6:269–278. [Google Scholar]
- 32.Aziz A., Azman N.A.A., Mahmud A., Abdul Hamid R., Khairuddin L. Socio-economic determinants of pap smear screening among married women in Peninsular Malaysia. [(accessed on 25 April 2021)];Int. J. Humanit. Soc. Sci. 2013 3 Available online: http://familyrepository.lppkn.gov.my/303/ [Google Scholar]
- 33.Al-Naggar R. Practice and barriers towards pap smear test from a public hospital in malaysia. J. Commun. Med. Health Educ. 2012;2:2. doi: 10.4172/2161-0711.1000132. [DOI] [Google Scholar]
- 34.Abdullah N.N., Daud S., Wang S.M., Mahmud Z., Mohd Kornain N.K., Al-Kubaisy W. Human Papilloma Virus (HPV) self-sampling: Do women accept it? J. Obstet. Gynaecol. 2018;38:402–407. doi: 10.1080/01443615.2017.1379061. [DOI] [PubMed] [Google Scholar]
- 35.Ting N.P., Ismail N.A., Abd Rahman N.I., Sundraraj Y.A. Knowledge, attitude and practice of pap smear screening among women in Gombak District, Selangor. Malays. J. Med. Health Sci. 2020;16:82–87. [Google Scholar]
- 36.Yunus N., Yusoff H.M., Draman N. Non-adherence to recommended pap smear screening guidelines and its associated factors among women attending health clinic in Malaysia. Malays. Fam. Phys. 2018;13:10. [PMC free article] [PubMed] [Google Scholar]
- 37.Indra I.S., Mahamud M.Z., Ling N.Z., Manaf R.A., Ismail S. Pap smear uptake and its associated factors among Orang Asli women in Selangor. Malays. J. Med. Health Sci. 2017;13:3–10. [Google Scholar]
- 38.Razi N.A.M., Manaf R.A., Ismail S. Prevalence and predictors of pap smear practice among staff of a public university in Nilai, Negeri Sembilan. Malays. J. Med. Health Sci. 2017;13:33–42. [Google Scholar]
- 39.Asmani A., Aziah D. Pap smear screening practice among women in Mukim Jaya Setia, Kelantan. Malays. J. Public Health Med. 2007;7:20–24. [Google Scholar]
- 40.Moy F., Lim C., Tan C., Tay S. Factors influencing the uptake of pap smear screening among malay women in a public university in Kuala Lumpur-a pilot study. Malays. J. Public Health Med. 2007;7:23–28. [Google Scholar]
- 41.Romli R., Shahabudin S.a., Saddki N., Mokhtar N., Mohamad M. Cervical cancer and pap smear screening: Knowledge, attitude and practice among working women in northern state of Malaysia. Med. J. Malays. 2019;74:9. [PubMed] [Google Scholar]
- 42.Chee H., Rashidah S., Shamsuddin K., Sharifah S. Knowledge and practice of breast self examination and Pap smear screening among a group of electronics women workers. Med. J. Malays. 2003;58:320–329. [PubMed] [Google Scholar]
- 43.Wong Y.-L., Chinna K., Mariapun J., Shuib R. Correlates between risk perceptions of cervical cancer and screening practice. Prev. Med. 2013;57:S24–S26. doi: 10.1016/j.ypmed.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Gan D.E.H., Dahlui M. Cervical screening uptake and its predictors among rural women in Malaysia. Singap. Med. J. 2013;54:163–168. doi: 10.11622/smedj.2013047. [DOI] [PubMed] [Google Scholar]
- 45.Wong L., Wong Y., Low W., Khoo E., Shuib R. Knowledge and awareness of cervical cancer and screening among Malaysian women who have never had a Pap smear: A qualitative study. Singap. Med. J. 2009;50:49. [PubMed] [Google Scholar]
- 46.Dunn R.A., Tan A.K. Cervical cancer screening in Malaysia: Are targeted interventions necessary? Soc. Sci. Med. 2010;71:1089–1093. doi: 10.1016/j.socscimed.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 47.Oon S.W., Shuib R., Ali S.H., Hussain N.H.N., Shaaban J., Yusoff H.M. Knowledge and Attitude among Women and Men in Decision Making on Pap Smear Screening in Kelantan, Malaysia. Int. J. Soc. Behav. Educ. Econ. Bus. Ind. Eng. 2010;4:1384–1391. [Google Scholar]
- 48.Gottschlich A., Nuntadusit T., Zarins K.R., Hada M., Chooson N., Bilheem S., Navakanitworakul R., Nittayaboon K., Virani S., Rozek L. Barriers to cervical cancer screening and acceptability of HPV self-testing: A cross-sectional comparison between ethnic groups in Southern Thailand. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-031957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalapati W., Chumworathayi B. Can a home-visit invitation increase Pap smear screening in Samliem, Khon Kaen, Thailand? Asian Pac. J. Cancer Prev. 2007;8:119. [PubMed] [Google Scholar]
- 50.Thanapprapasr D., Deesamer S., Sujintawong S., Udomsubpayakul U., Wilailak S. Cervical cancer screening behaviours among Thai women: Results from a cross-sectional survey of 2112 healthcare providers at Ramathibodi Hospital, Thailand. Eur. J. Cancer Care. 2012;21:542–547. doi: 10.1111/j.1365-2354.2012.01333.x. [DOI] [PubMed] [Google Scholar]
- 51.Kritpetcharat O., Suwanrungruang K., Sriamporn S., Kamsa-Ard S., Kritpetcharat P., Pengsaa P. The coverage of cervical cancer screening in Khon Kaen, northeast Thailand. Asian Pac. J. Cancer Prev. 2003;4:103–106. [PubMed] [Google Scholar]
- 52.Visanuyothin S., Chompikul J., Mongkolchati A. Determinants of cervical cancer screening adherence in urban areas of Nakhon Ratchasima Province, Thailand. J. Infect. Public Health. 2015;8:543–552. doi: 10.1016/j.jiph.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 53.Wongwatcharanukul L., Promthet S., Bradshaw P., Jirapornkul C., Tungsrithong N. Factors affecting cervical cancer screening uptake by Hmong hilltribe women in Thailand. Asian Pac. J. Cancer Prev. 2014;15:3753–3756. doi: 10.7314/APJCP.2014.15.8.3753. [DOI] [PubMed] [Google Scholar]
- 54.Budkaew J., Chumworathayi B. Factors associated with decisions to attend cervical cancer screening among women aged 30–60 years in Chatapadung Contracting Medical Unit, Thailand. Asian Pac. J. Cancer Prev. 2014;15:4903–4907. doi: 10.7314/APJCP.2014.15.12.4903. [DOI] [PubMed] [Google Scholar]
- 55.Srisakul S., Nirattharadorn M., Suwannarurk K. Factors predicting intention for cervical cancer screening among women aged 30 to 60 years in Ratchaburi province, Thailand: Population-based study. Thammasat Med. J. 2011;11:521–527. [Google Scholar]
- 56.Chesun A., Harncharoen K., Taechaboonsermsak P., Siri S. Factors related with cervical cancer screening test among Thai muslim women in Satun province. Asia J. Public Health. 2012;3:79–85. [Google Scholar]
- 57.Mongsawaeng C., Kokorn N., Kujapun J., Norkaew J., Kootanavanichpong N., Chavenkun W., Ponphimai S., Kaewpitoon S.J., Tongtawee T., Padchasuwan N. Knowledge, attitude, and practice regarding cervical cancer among rural community women in Northeast Thailand. Asian Pac. J. Cancer Prev. 2016;17:85–88. doi: 10.7314/APJCP.2016.17.1.85. [DOI] [PubMed] [Google Scholar]
- 58.Chaowawanit W., Tangjitgamol S., Kantathavorn N., Phoolcharoen N., Kittisiam T., Khunnarong J., Supawattanabodee B., Srijaipracharoen S., Thavaramara T., Pataradool K. Knowledge, attitudes and behavior of Bangkok metropolitan women regarding cervical cancer screening. Asian Pac. J. Cancer Prev. 2016;17:945–952. doi: 10.7314/APJCP.2016.17.3.945. [DOI] [PubMed] [Google Scholar]
- 59.Oranratanaphan S., Amatyakul P., Iramaneerat K., Srithipayawan S. Knowledge, attitudes and practices about the Pap smear among medical workers in Naresuan University Hospital. Asian Pac. J. Cancer Prev. 2010;11:1727–1730. [PubMed] [Google Scholar]
- 60.Srisuwan S., Puapornpong P., Srisuwan S., Bhamarapravatana K., Suwannarurk K. Knowledge, attitudes and practices regarding cervical cancer screening among village health volunteers. Asian Pac. J. Cancer Prev. 2015;16:2895–2898. doi: 10.7314/APJCP.2015.16.7.2895. [DOI] [PubMed] [Google Scholar]
- 61.Kietpeerakool C., Phianmongkhol Y., Jitvatcharanun K., Siriratwatakul U., Srisomboon J. Knowledge, awareness, and attitudes of female sex workers toward HPV infection, cervical cancer, and cervical smears in Thailand. Int. J. Gynaecol. Obstet. 2009;107:216–219. doi: 10.1016/j.ijgo.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 62.Mukem S., Meng Q., Sriplung H., Tangcharoensathien V. Low coverage and disparities of breast and cervical cancer screening in Thai women: Analysis of national representative household surveys. Asian Pac. J. Cancer Prev. 2016;16:8541–8551. doi: 10.7314/APJCP.2015.16.18.8541. [DOI] [PubMed] [Google Scholar]
- 63.Bunkarn O., Kusol K., Eksirinimit T. The Outcome of a Self-Efficacy Enhancement Program for Cervical Cancer Screening among Women in Phrasaeng District, Suratthani Province, Thailand. Asian Pac. J. Cancer Prev. 2020;21:2075–2081. doi: 10.31557/APJCP.2020.21.7.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boonpongmanee C., Jittanoon P. Predictors of Papanicolaou testing in working women in Bangkok, Thailand. Cancer Nurs. 2007;30:384–389. doi: 10.1097/01.NCC.0000290804.38335.32. [DOI] [PubMed] [Google Scholar]
- 65.Polrit K., Kamsa-ard S., Jirapornkul C., Promthet S. Proximity of Health Care Center and Cervical Cancer Screening Uptake in Thailand. Asian Pac. J. Cancer Prev. 2015;16:2899–2902. doi: 10.7314/APJCP.2015.16.7.2899. [DOI] [PubMed] [Google Scholar]
- 66.Oranratanaphan S., Termrungruanglert W., Khemapech N. Acceptability of self-sampling HPV testing among Thai women for cervical cancer screening. Asian Pac. J. Cancer Prev. 2014;15:7437–7441. doi: 10.7314/APJCP.2014.15.17.7437. [DOI] [PubMed] [Google Scholar]
- 67.Kittisiam T., Tangjitgamol S., Chaowawanit W., Khunnarong J., Srijaipracharoen S., Thavaramara T., Pataradool K. Knowledge and Attitudes of Bangkok Metropolitan Women towards HPV and Self-Sampled HPV Testing. Asian Pac. J. Cancer Prev. 2016;17:2445–2451. [PubMed] [Google Scholar]
- 68.Boonmongkon P., Nichter M., Pylypa J., Sanhajariya N., Saitong S. Women’s health in northeast Thailand: Working at the interface between the local and the global. Women Health. 2002;35:59–80. doi: 10.1300/J013v35n04_05. [DOI] [PubMed] [Google Scholar]
- 69.Songsiriphan A., Salang L., Somboonpha W., Eamudomkarn N., Nhokaew W., Kuchaisit C., Harnlakorn P. Knowledge, Attitudes, and Practices Regarding Cervical Cancer Screening among HIV-infected Women at Srinagarind Hospital: A Cross-Sectional Study. Asian Pac. J. Cancer Prev. 2020;21:2979–2986. doi: 10.31557/APJCP.2020.21.10.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chongthawonsatid S. Inequity of healthcare utilization on mammography examination and Pap smear screening in Thailand: Analysis of a population-based household survey. PLoS ONE. 2017;12:e0173656. doi: 10.1371/journal.pone.0173656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aprina, Agustina L., Rajiani I. The behavior of fertile women in rural areas toward the acetic acid visual inspection. Indian J. Public Health Res. Dev. 2018;9:143–148. doi: 10.5958/0976-5506.2018.01329.3. [DOI] [Google Scholar]
- 72.Anggraeni F.D., Murti B., Dharmawan R. Path analysis and theory of planned behavior on using PAP smear as early detection of cervical cancer in Sewon I community health center, Bantul, Yogyakarta, Indonesia. J. Health Promot. Behav. 2016;1:1–8. doi: 10.26911/thejhpb.2016.01.01.01. [DOI] [Google Scholar]
- 73.Anwar S.L., Tampubolon G., Van Hemelrijck M., Hutajulu S.H., Watkins J., Wulaningsih W. Determinants of cancer screening awareness and participation among Indonesian women. BMC Cancer. 2018;18:208. doi: 10.1186/s12885-018-4125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim Y.-M., Ati A., Kols A., Lambe F.M., Soetikno D., Wysong M., Tergas A.I., Rajbhandari P., Lu E. Influencing women’s actions on cervical cancer screening and treatment in Karawang District, Indonesia. Asian Pac. J. Cancer Prev. 2012;13:2913–2921. doi: 10.7314/APJCP.2012.13.6.2913. [DOI] [PubMed] [Google Scholar]
- 75.Muhith A., Winarti E., Perdana S.I., Haryuni S., Rahayu K.I.N., Mallongi A. Internal locus of control as a driving factor of early detaction behavior of servical cancer by inspection visual of acetic acid method. Open Access Maced. J. Med. Sci. 2020;8:113–116. doi: 10.3889/oamjms.2020.4341. [DOI] [Google Scholar]
- 76.Nurhasanah N., Afiyanti Y. Factors affecting behaviors of cervical cancer screening using VIA (Visual Inspection with Acetic Acid) method on women in Srengseng Sawah Jakarta Indonesia. UI Proc. Health Med. 2017;2 doi: 10.7454/uiphm.v2i0.157. [DOI] [Google Scholar]
- 77.Rahmawati N.A., Dewanti L. Direct Experience with Cervical Cancer Patient, Husband Support, and Self-Perception as Determinant Factors of Women’s Desire to Take VIA Screening Test. Kesmas Natl. Public Health J. 2018;13:36–42. doi: 10.21109/kesmas.v13i1.1617. [DOI] [Google Scholar]
- 78.Saptowati D., Mudigdo A., Murti B. Biopsychosocial Determinants of Visual Inspection Acetic-Acid Test Uptake in Sragen, Central Java. J. Matern. Child. Health. 2018;3:197–206. doi: 10.26911/thejmch.2018.03.03.04. [DOI] [Google Scholar]
- 79.Sidabutar S., Martini S., Wahyuni C.U. Analysis of factors affecting women of childbearing age to screen using visual inspection with acetic acid. Osong Public Health Res. Perspect. 2017;8:61. doi: 10.24171/j.phrp.2017.8.1.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sidabutar S., Suwandi T., Martini S., Hargono R. Factors influencing decisions to conduct early detection of cervical cancer. Health Notions. 2018;2:630–636. [Google Scholar]
- 81.Spagnoletti B.R.M., Bennett L.R., Wahdi A.E., Wilopo S.A., Keenan C.A. A qualitative study of parental knowledge and perceptions of human papillomavirus and cervical cancer prevention in rural central Java, Indonesia: Understanding community readiness for prevention interventions. Asian Pac. J. Cancer Prev. 2019;20:2429. doi: 10.31557/APJCP.2019.20.8.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sutarti T., Abdul M., Mallongi A., Saputra M.H., Darmawati L. Analysis of educational factors, interest and motivation towards the behavior of early detection of cancer cerviks in women of fertile age in the Upt Health Center Kembangbahu Iamongan. Indian J. Public Health Res. Dev. 2018;9:1371–1375. doi: 10.5958/0976-5506.2018.02044.2. [DOI] [Google Scholar]
- 83.Wakhidah M.S., Hastuti U.R.B., Dewi Y.L.R. The influence of personal factor, husband’s support, health workers and peers toward the use of IVA Screening among women of reproductive age in the regency of Karanganyar. J. Health Promot. Behav. 2017;2:124–137. doi: 10.26911/thejhpb.2017.02.02.03. [DOI] [Google Scholar]
- 84.Widayanti D.M., Qomaruddin M.B., Irawandi D. Mother’s knowledge and attitudes towards Visual Acetate Acid Inspection test in Surabaya. J. Public Health Res. 2020;9:113–116. doi: 10.4081/jphr.2020.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Winarti E., Santoso B., Suhatno S., Hargono R. Trigger, self efficacy and motivation in the implementation of cervical cancer screening. Health Notions. 2018;2:494–499. [Google Scholar]
- 86.Chirayil E.I., Thompson C.L., Burney S. Predicting human papilloma virus vaccination and pap smear screening intentions among young Singaporean women using the theory of planned behavior. Sage Open. 2014;4:2158244014554961. doi: 10.1177/2158244014554961. [DOI] [Google Scholar]
- 87.Lee J., Seow A., Ling S.-L., Peng L.H. Improving adherence to regular pap smear screening among Asian women: A population-based study in Singapore. Health Educ. Behav. 2002;29:207–218. doi: 10.1177/1090198102029002006. [DOI] [PubMed] [Google Scholar]
- 88.Seow A., Huang J., Straughan P.T. Effects of social support, regular physician and health-related attitudes on cervical cancer screening in an Asian population. Cancer Causes Control. 2000;11:223–230. doi: 10.1023/A:1008954606992. [DOI] [PubMed] [Google Scholar]
- 89.Seow A., Lee H. Prevalence and determinants of cervical cancer screening: A community-based study in Singapore. Ann. Acad. Med. Singap. 1994;23:342. [PubMed] [Google Scholar]
- 90.Seow A., Wong M., Smith W., Lee H. Beliefs and attitudes as determinants of cervical cancer screening: A community-based study in Singapore. Prev. Med. 1995;24:134–141. doi: 10.1006/pmed.1995.1026. [DOI] [PubMed] [Google Scholar]
- 91.Shea J., Klainin-Yobas P., Mackey S. Young Singaporean women’s knowledge of cervical cancer and pap smear screening: A descriptive study. J. Clin. Nurs. 2013;22:3310–3319. doi: 10.1111/jocn.12420. [DOI] [PubMed] [Google Scholar]
- 92.Tay K., Tay S.K., Tesalona K.C., Rashid N.M., Tai E.Y., Najib S.J. Factors affecting the uptake of cervical cancer screening among nurses in Singapore. Int. J. Gynaecol. Obstet. 2015;130:230–234. doi: 10.1016/j.ijgo.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 93.Wee L.E., Cher W.Q., Sin D., Li Z.C., Koh G.C.-H. Primary care characteristics and their association with health screening in a low-socioeconomic status public rental-flat population in Singapore-a mixed methods study. BMC Fam Pract. 2016;17:16. doi: 10.1186/s12875-016-0411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wee L.E., Koh G.C., Toh Z.J. Multi-disease health screening in an urban low-income setting: A community-based study. Ann. Acad. Med. Singap. 2010;39:750. [PubMed] [Google Scholar]
- 95.Wee L.E., Koh G.C.-H., Chin R.T., Yeo W.X., Seow B., Chua D. Socioeconomic factors affecting colorectal, breast and cervical cancer screening in an Asian urban low-income setting at baseline and post-intervention. Prev. Med. 2012;55:61–67. doi: 10.1016/j.ypmed.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 96.Wee L.E., Lim L.Y., Koh G.C.-H. Two sides of the coin: A qualitative study of patient and provider perspectives on colorectal, breast and cervical cancer screening in a low-income Asian community. Proc. Singap. Healthc. 2016;25:80–91. doi: 10.1177/2010105815616404. [DOI] [Google Scholar]
- 97.Wee L.E., Sin D., Cher W.Q., Li Z.C., Tsang T., Shibli S., Koh G. “I’m healthy, I don’t have pain”-health screening participation and its association with chronic pain in a low socioeconomic status Singaporean population. Korean J. Pain. 2017;30:34. doi: 10.3344/kjp.2017.30.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wong H.Z., Lim W.Y., Ma S.S., Chua L.A., Heng D.M. Health screening behaviour among Singaporeans. Ann. Acad. Med. Singap. 2015;44:326–334. [PubMed] [Google Scholar]
- 99.Yeo C., Fang H., Koh S.S.L., Shorey S. Factors affecting Pap smear uptake in a maternity hospital: A descriptive cross-sectional study. J. Adv. Nurs. 2018;74:2533–2543. doi: 10.1111/jan.13769. [DOI] [PubMed] [Google Scholar]
- 100.Suhaimi A.M.A.P., Rahman H.A., Ong S.K., Koh D. Predictors of non-communicable diseases screening behaviours among adult population in Brunei Darussalam: A retrospective study. J. Public Health. 2020 doi: 10.1007/s10389-020-01240-z. [DOI] [Google Scholar]
- 101.Touch S., Oh J.-K. Knowledge, attitudes, and practices toward cervical cancer prevention among women in Kampong Speu Province, Cambodia. BMC Cancer. 2018;18:294. doi: 10.1186/s12885-018-4198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Phongsavan K., Phengsavanh A., Wahlström R., Marions L. Women’s perception of cervical cancer and its prevention in rural Laos. Int. J. Gynecol. Cancer. 2010;20:821–826. doi: 10.1111/IGC.0b013e3181daaefb. [DOI] [PubMed] [Google Scholar]
- 103.Sichanh C., Fabrice Q., Chanthavilay P., Diendere J., Latthaphasavang V., Longuet C., Buisson Y. Knowledge, awareness and attitudes about cervical cancer among women attending or not an HIV treatment center in Lao PDR. BMC Cancer. 2014;14:161. doi: 10.1186/1471-2407-14-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hando K., Vilayvong S., Vongdala C., Vongsonephet K., Xayaphet P., Soejima Y. Knowledge and attitude toward cervical cancer and its prevention among female workers in the Vientiane Capital, Lao PDR. J. Med. Dent. Sci. 2018;65:9–18. [Google Scholar]
- 105.Nandar C.S., Laosee O. Determinants of cevical cancer screening among migrants in northern district of Yangon, Myanmar. J. Public Health Dev. 2015;13:3–17. [Google Scholar]
- 106.Vo T.Q., Vo N.X. Knowledge, attitude and practice of cervical cancer screening among female residents in southern Vietnam. Eur. J. Anal. Chem. 2018;13:620–629. [Google Scholar]
- 107.Hoang A., Nguyen C.Q., Duong C.D. Youth experiences in accessing sexual healthcare services in Vietnam. Cult. Health Sex. 2018;20:545–559. doi: 10.1080/13691058.2017.1360945. [DOI] [PubMed] [Google Scholar]
- 108.Devarapalli P., Labani S., Nagarjuna N., Panchal P., Asthana S. Barriers affecting uptake of cervical cancer screening in low and middle income countries: A systematic review. Indian J. Cancer. 2018;55:318. doi: 10.4103/ijc.IJC_253_18. [DOI] [PubMed] [Google Scholar]
- 109.Islam R.M., Billah B., Hossain M.N., Oldroyd J. Barriers to cervical cancer and breast cancer screening uptake in low-income and middle-income countries: A systematic review. Asian Pac. J. Cancer Prev. 2017;18:1751. doi: 10.22034/APJCP.2017.18.7.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Williams-Brennan L., Gastaldo D., Cole D.C., Paszat L. Social determinants of health associated with cervical cancer screening among women living in developing countries: A scoping review. Arch. Gynecol. Obstet. 2012;286:1487–1505. doi: 10.1007/s00404-012-2575-0. [DOI] [PubMed] [Google Scholar]
- 111.Scarinci I.C., Garcia F.A., Kobetz E., Partridge E.E., Brandt H.M., Bell M.C., Dignan M., Ma G.X., Daye J.L., Castle P.E. Cervical cancer prevention: New tools and old barriers. Cancer. 2010;116:2531–2542. doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nagendiram A., Bougher H., FRACGP C.H.M.D.D. Australian women’s self-perceived barriers to participation in cervical cancer screening: A systematic review. Health Promot. J. Austr. 2020;31:343–353. doi: 10.1002/hpja.280. [DOI] [PubMed] [Google Scholar]
- 113.Nelson J.A., Francis S.A., Liverpool J., Soogun S., Mofammere N. Healers in a non-traditional role; a focus group study of Sangoma’s knowledge of and attitudes to cervical cancer prevention and screening in Johannesburg, South Africa. Sex. Reprod. Healthc. 2010;1:195–196. doi: 10.1016/j.srhc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 114.Chongsuvivatwong V., Phua K.H., Yap M.T., Pocock N.S., Hashim J.H., Chhem R., Wilopo S.A., Lopez A.D. Health and health-care systems in southeast Asia: Diversity and transitions. Lancet. 2011;377:429–437. doi: 10.1016/S0140-6736(10)61507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Musa J., Achenbach C.J., O’Dwyer L.C., Evans C.T., McHugh M., Hou L., Simon M.A., Murphy R.L., Jordan N. Effect of cervical cancer education and provider recommendation for screening on screening rates: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0183924. doi: 10.1371/journal.pone.0183924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bach-Mortensen A.M., Verboom B. Barriers and facilitators systematic reviews in health: A methodological review and recommendations for reviewers. Res. Synth. Methods. 2020;11:743–759. doi: 10.1002/jrsm.1447. [DOI] [PubMed] [Google Scholar]
- 117.Wilding S., Wighton S., Halligan D., West R., Conner M., O’Connor D.B. What factors are most influential in increasing cervical cancer screening attendance? An online study of UK-based women1. Health Psychol. Behav. Med. 2020;8:314–328. doi: 10.1080/21642850.2020.1798239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.World Bank Country and Lending Groups. [(accessed on 7 December 2020)]; Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.