Abstract
Objective: We aimed to examine the influence of increasing levels of discussion (both asked and advised, either asked or advised but not both, and neither asked nor advised) on quit behavior. Methods: We included 4133 adult current smokers from the 2015 National Health Interview Survey. The primary outcomes were quit intent and quit attempt, and the secondary outcomes were methods used for quitting. We used an instrumental variable analysis, as well as propensity score weighted and multivariable logistic regressions. Results: Compared to no discussion, having both or only one discussion, respectively, increased quit intent (OR = 1.65, 95% CI = 1.63–1.66 and OR = 1.02, 95% CI = 0.99–1.05), quit attempt (OR = 1.76, 95% CI = 1.75–1.77 and OR = 1.60, 95% CI = 1.57–1.63). Among those who attempted to quit (n = 1536), having both or only one discussion increased the use of pharmacologic (OR = 1.99, 95% CI = 1.97–2.02 and OR = 1.56, 95% CI = 1.49–1.63) or behavioral (OR = 2.01, 95% CI = 1.94–2.08 and OR = 2.91, 95% CI = 2.74–3.08) quit methods. Conclusions: Increasing levels of provider–patient discussion encourages quit behavior, and should be an integral part of reducing the health and economic burden of smoking. Strategies that promote the adherence and compliance of providers to communicate with patients may help increase the success of smoking cessation.
Keywords: provider–patient communication, intent to quit, attempt to quit, instrumental variable, smoking cessation
1. Introduction
While the prevalence of cigarette smoking has been falling and reached an all-time low of 14% among the U.S. adult population, smoking remains the leading preventable behavior for many major diseases including cancer and cardiovascular diseases [1]. To encourage behavior change, the U.S. Public Health Service’s Clinical Practice Guideline recommends healthcare professionals to use the 5A’s approach to help smokers quit: (1) ask about tobacco use at every visit; (2) advise all tobacco users to quit; (3) assess readiness to quit; (4) assist tobacco users with a quit plan; and (5) arrange follow-up visits [2,3,4].
Despite this five-step guideline, and studies showing positive effects of a provider’s discussion in helping smokers quit, the uptake of provider–patient discussion remains suboptimal [1,5]. Based on nationally representative samples from the National Health Interview Survey (NHIS), the prevalence of provider–patient discussion about smoking (the first A: ask) ranged from 51.3% in 2011 to 55.4% in 2015 [6,7]. The prevalence of patient–provider discussion on quitting (the second A: advise) ranged from 53.3% in 2000, and 58.9% in 2005, to 50.7% in 2010, and was 57.2% in 2015 [8,9]. Multiple factors influence the uptake of provider–patient discussion, including patient’s sex, age, race/ethnicity, education, health insurance, and health conditions [6,10]. For example, Hispanics were found to be less likely to receive advice to quit than non-Hispanic white people [8,11,12,13]. Older adults with smoking-related cancer were more likely to be advised to quit smoking by a healthcare provider than those without chronic diseases [14].
The levels of provider–patient discussion of the recommended 5A’s also vary in different settings, which in turn can affect quit behavior. In the National Adult Tobacco Survey (NATS), where all 5A’s were available, the proportion of ask, advise, assess, assist, and arrange was 88.3%, 66.4%, 43.4%, 38.6%, and 6.3%, respectively [15]. Corroborating results were reported in other studies showing that the compliance of provider–patient discussion decreased with subsequent 5A’s steps [5,16,17,18]. However, investigations on the combined usage of individual levels of the 5A’s using NATS data were limited by the small sample sizes available, where the proportion of patients who received any one of the 5A’s was 33.6%, any two was 18.3%, any three was 13.9%, any four was 28.1%, and all five was only 6.1% [15]. Existing studies that used NHIS data, where only the first two A’s of the five-step algorithm were available, focused on either the ask or advice aspect alone [6,13,14,19,20,21,22,23]. No study based on NHIS has examined the impact of increasing levels of provider–patient discussion (ask and advice, advice alone or ask alone, as compared to no discussion) on quit behavior.
In the current study, we aimed to investigate whether there was an increasing level of the positive influence of provider–patient discussion with the first 2A’s on quit behavior based on NHIS data. We also extended the existing studies with a rigorous statistical approach by using an instrumental variable (IV) analysis to reduce estimate bias due to unobserved confounders. To complement the IV analysis, we conducted multivariable logistic regressions and propensity score-based weighting regressions. Results from the study may help improve our understanding of the influence of provider–patient discussion and inform policy and practice on promoting smoking cessation.
2. Methods
2.1. Data Sources and Study Population
We used publicly available datasets from the 2015 NHIS, an ongoing cross-sectional survey. Each year, NHIS interviews a representative sample of the civilian non-institutionalized population in the U.S. through a multistage probability design [24]. We used both the core survey components and the latest available Tobacco Section in the Cancer Control Supplement, which was from 2015, to obtain comprehensive information about current smokers’ smoking and quit behavior, as well as their interactions with healthcare providers.
We included 4133 participants in the analysis following detailed selection steps outlined in the Supplementary Figure S1. Briefly, we first identified 5415 adult (age ≥ 18 years) current smokers in the 2015 NHIS data. Current smokers were defined as individuals who smoked at least 100 cigarettes in their lifetime and currently smoke cigarettes daily or on some days. We further restricted to those who had seen a doctor or other health professional in the past 12 months. We also excluded participants with missing values and those who responded “don’t know” or “refused” for the main outcomes, exposure, and covariates.
2.2. Outcome Variables
We focused on two binary (yes/no) primary outcomes: intent to quit (would like to completely quit smoking cigarettes) and attempt to quit (stopped smoking for more than one day because of trying to quit smoking during the past 12 months). Among those who attempted to quit, we further grouped the quit methods into pharmacological and behavioral methods, which were secondary outcomes. The pharmacological cessation method included self-reported use of any of the following: Chantix or Varenicline, Zyban, Bupropion, or Wellbutrin, nicotine patch, nicotine gum or lozenge, or nicotine containing nasal spray or inhaler. The behavioral cessation method included self-reported use of any the following: telephone help/quit line, one-on-one counseling, stop smoking clinic, class, or support group.
2.3. Exposure Variables
From the same cross-sectional survey, we defined the exposure variable as a 3-level provider–patient discussion being both asked and advised, either asked or advised but not both, or neither asked nor advised, based on participants’ responses to the following two questions: “DURING THE PAST 12 MONTHS, has a doctor or other health professional talked to you about your smoking?”, and “In the PAST 12 MONTHS, has a medical doctor, dentist, or other health professional ADVISED you to quit smoking, or to quit using other kinds of tobacco?”. We grouped only asked and only advised into the same category, assuming they had equivalent impact on quit intent and attempt.
2.4. Covariates
We included the following commonly adjusted socio-demographic factors: age, sex, race/ethnicity, marital status, and region. We also included education, employment status, health insurance type, and the ratio of family income to the applicable federal poverty thresholds. We then included health status variables that are indicative of the following smoking-related comorbidities: lung disease, cardiovascular diseases, and cancer [25]. Similar to previous studies [14,26], we included an indicator for serious psychological distress using the Kessler Scale. In addition, we included two measures of smoking exposure: years smoked, and numbers of cigarettes smoked daily. The former was calculated by subtracting age at smoking initiation from age at interview, while the latter was estimated based on answers to the following question: “On the average, when you smoked during the past 30 days, about how many cigarettes did you smoke a day?”. In total, we included 16 covariates for the analysis.
2.5. Instrumental Variable
Cross-sectional studies are prone to biases and confounders. While adjusting for potential confounders in multivariable regression models can ameliorate the problem, unobserved confounders may still exist. One approach to overcome this limitation is the use of an instrumental variable (IV). Different from a confounding variable, which is associated with both the outcome and the exposure, IV is a factor that is associated with the exposure but not with the outcome, and the effect of IV on the outcome is through the exposure. Due to the infeasibility of randomly assigning the subjects to groups as in randomized control trials (RCTs), an IV analysis is often applied in observational studies to yield an unbiased estimate of the effect of exposure on the outcome [27,28].
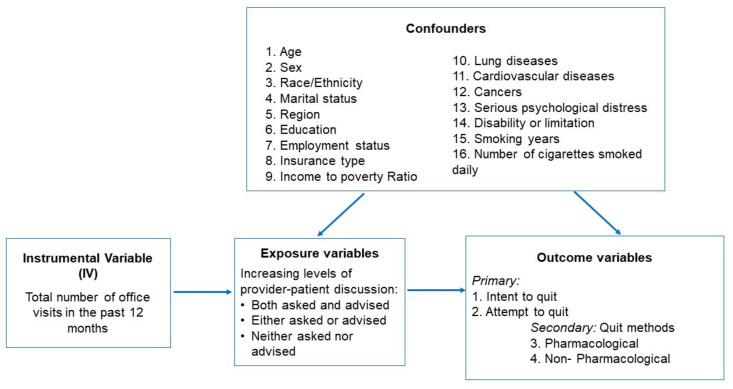
We considered the number of office visits in the past 12 months as the IV (Figure 1), with the rationale that the more office visits a patient has, the higher chance of him or her having a provider–patient discussion about smoking/quitting, which in turn can lead to a higher likelihood of smoking cessation.
Figure 1.
A graphic representation of the instrumental variable (IV) analysis.
2.6. Statistical Analysis
All analyses were performed using the SAS Enterprise Guide (version 7.1, SAS Institute Inc., Cary, NC, USA) and R version 3.6.2, during January to May 2020. Odds ratio (ORs) and 95% confidence interval (CI) were reported for the exposure–outcome association. All p-values were two-sided and a value less than 0.05 was considered statistically significant. Unless otherwise specified, all the analyses took into account the complex NHIS survey design to provide weighted population-level estimates.
In the descriptive analysis, we compared the respondents’ characteristics by the 3-level patient–provider discussion on smoking and quitting, using the Rao–Scott Chi-squared test for categorical variables and the F test for continuous variables [29]. Supplementary Table S1 lists the unweighted sample characteristics.
2.6.1. Instrumental Variable (IV) Analysis
We applied a two-stage residual inclusion (2SRI) method, an established method for producing consistent estimators for nonlinear parametric models [30,31]. In the first stage, the provider–patient discussion was modeled as a function of the IV and the aforementioned covariates using a multinomial logistic regression. In the second stage, the individual dichotomized outcome variable was predicted as a function of the exposure variable, all covariates, and the standardized residuals obtained from the first stage using a binomial logistic regression. The standard errors of two-stage estimators were calculated by implementing a bootstrap method with 500 repetitions [30,31].
We also performed the first-stage F test and falsification test to check the validity of two assumptions for IV analysis. First, an IV must be correlated with the exposure variable; second, the IV must not be correlated with the outcome variable or any other unmeasured confounders such that the effect of the IV on the outcome is only through the exposure variable [32]. The first assumption is often tested using the first-stage F test, where an IV is not considered a weak instrument if the value of the F statistic is greater than 10 [33]. Since the second assumption cannot be directly tested through a confirmatory test, a falsification test is performed as an alternative to show empirically a lack of violation [34]. A p-value of the coefficient of IV < 0.05 indicates that the IV may have direct effect on the outcome.
2.6.2. Sensitivity Analysis
We performed two sensitivity analyses using a propensity score weighting (PSW) model and a multivariable logistic model to ensure the robustness of the estimated effects from the IV analysis. The PSW approach is often used for an unbiased estimate when a RCT is not feasible [35,36]. We first derived a propensity score using a multinomial logistic regression, where the propensity score was the probability of having a provider–patient discussion conditioned on the covariates. Then, we modeled the association between the provider–patient discussion and quit behavior outcomes using a survey logistic regression, where the weight variable was the product of survey weight and the propensity score weight [37,38].
3. Results
3.1. Sample Characteristics
Out of 4133 adult current smokers who visited a doctor or a healthcare provider during the past 12 months, 735 (weighted prevalence: 17.61%) reported either being asked by the provider about smoking or advised to quit smoking, and 2219 (weighted prevalence: 54.64%) reported being both asked and advised (Table 1). Compared to those who had neither discussion, respondents who had both or only one level discussion were more likely to be older (mean age: 47.69 years and 44.23 years vs. 40.88 years, p-value < 0.001), female (51.26% and 55.21% vs. 43.14%, p-value < 0.001), married (56.16% and 50.36% vs. 48.79%, p-value = 0.007), and residing in the Northeast of the U.S. (19.07% and 13.67% vs. 12.23%, p-value < 0.001). They were less likely to be Hispanic (6.22% and 8.88% vs. 14.61%, p-value < 0.001), or without health insurance (10.23% and 13.28% vs. 21.70%, p-value < 0.001), and less likely to be employed (54.53% and 56.79% vs. 66.43%, p-value < 0.001). They were also more likely to have smoking-related comorbidities (p-value < 0.001), serious psychological distress (13.43% and 9.69% vs. 6.61%, p-value < 0.001), and disability or activity limitations (31.38% and 23.75% vs. 12.90%, p-value < 0.001). Moreover, they had a higher level of smoking exposure (p-value < 0.001).
Table 1.
Characteristics of the study sample by the level of provider–patient discussion, 2015 NHIS.
| Levels of Discussion | Asked and Advised (n = 2219) |
Asked or Advised (n = 735) |
Neither Asked or Advised (n = 1179) |
|
|---|---|---|---|---|
| Categorical Variables | N (weighted%) | N (weighted%) | N (weighted%) | p-value a |
| Sex | <0.001 | |||
| Female | 1202 (51.26) | 414 (55.2) | 563 (43.14) | |
| Male | 1017 (48.74) | 321 (44.8) | 616 (56.86) | |
| Race/Ethnicity | <0.001 | |||
| White Non-Hispanic | 1591 (78.09) | 490 (71.5) | 753 (65.36) | |
| Black Non-Hispanic | 310 (11.29) | 135 (15.58) | 150 (12.68) | |
| Hispanic | 197 (6.22) | 68 (8.88) | 172 (14.61) | |
| Others | 121 (4.4) | 42 (4.03) | 104 (7.34) | |
| Marital Status b | 0.007 | |||
| Yes | 958 (56.16) | 287 (50.36) | 464 (48.79) | |
| No | 1261 (43.84) | 448 (49.64) | 715 (51.21) | |
| Region | <0.001 | |||
| Northeast | 405 (19.07) | 95 (13.67) | 148 (12.23) | |
| Midwest | 535 (29.11) | 173 (24.47) | 305 (26.8) | |
| South | 772 (35.05) | 287 (41.69) | 408 (38.03) | |
| West | 507 (16.77) | 180 (20.18) | 318 (22.94) | |
| Education | 0.706 | |||
| Less than High School | 538 (24.03) | 205 (28.37) | 284 (25.3) | |
| High School Graduate | 598 (26.65) | 191 (26.69) | 300 (26.21) | |
| Some College | 519 (23.29) | 170 (22.93) | 292 (23.43) | |
| College or Above | 564 (26.03) | 169 (22.01) | 303 (25.07) | |
| Employment | <0.001 | |||
| Yes | 1083 (54.53) | 397 (56.79) | 737 (66.43) | |
| No | 1136 (45.47) | 338 (43.21) | 442 (33.57) | |
| Insurance | <0.001 | |||
| Private | 806 (44.17) | 288 (42.54) | 505 (47.36) | |
| Medicaid | 434 (17.54) | 153 (21.62) | 215 (17.18) | |
| Medicare | 555 (19.5) | 147 (17.61) | 134 (8.76) | |
| Others | 210 (8.56) | 52 (4.95) | 61 (5) | |
| Uninsured | 214 (10.23) | 95 (13.28) | 264 (21.7) | |
| Income/poverty ratio | 0.860 | |||
| <1.00 | 576 (19.42) | 191 (22.56) | 307 (21.51) | |
| 1.00–1.99 | 569 (23.92) | 194 (23.5) | 288 (23.14) | |
| 2.00–3.99 | 633 (31.27) | 206 (29.95) | 343 (31.71) | |
| 4.00 and over | 441 (25.38) | 144 (23.99) | 241 (23.65) | |
| Lung Disease c | <0.001 | |||
| Yes | 1279 (53.09) | 312 (38.24) | 386 (29.27) | |
| No | 940 (46.91) | 423 (61.76) | 793 (70.73) | |
| CVD d | <0.001 | |||
| Yes | 310 (12.07) | 65 (7.83) | 63 (4.29) | |
| No | 1909 (87.93) | 670 (92.17) | 1116 (95.72) | |
| Cancer e | <0.001 | |||
| Tobacco Related | 87 (3.09) | 17 (2.13) | 20 (0.93) | |
| Non-tobacco Related | 152 (6.3) | 43 (4.66) | 33 (2.68) | |
| None | 1980 (90.61) | 675 (93.21) | 1126 (96.39) | |
| Serious psychological distress f | <0.001 | |||
| Yes (Kessler score ≥ 13) | 272 (13.43) | 76 (9.69) | 82 (6.61) | |
| No (Kessler score < 13) | 1947 (86.57) | 659 (90.31) | 1097 (93.39) | |
| Disability/limitation g | <0.001 | |||
| Yes | 854 (31.38) | 196 (23.75) | 205 (12.9) | |
| No | 1365 (68.62) | 539 (76.25) | 974 (87.1) | |
| Continuous variables | Weighted Mean (SD) | Weighted Mean (SD) | Weighted Mean (SD) | p-value a |
| Age (years) | 47.69 (14.74) | 44.23 (15.9) | 40.88 (15.12) | <0.001 |
| Smoking length h (year) | 29.9 (15.19) | 26.06 (16.12) | 22.59 (15.37) | <0.001 |
| Number of cigarettes smoked daily | 13.37 (9.32) | 11.08 (9.25) | 9.57 (8.07) | <0.001 |
Notes:a The analysis took into account the NHIS survey design. Comparison of categorical variables by the 3-level provider–patient discussion was conducted using the Rao–Scott Chi-square test in SURVEYFREQ procedure, and using the F-test in SURVEYREG procedure. b Married included those who are married or living with a partner. c Lung disease types included COPD, emphysema, and chronic bronchitis. d Cardiovascular diseases (CVD) included coronary heart disease, angina, stroke hypertension, heart attack, and other heart disease. e Tobacco-related cancer types included 12 tobacco-associated cancers as defined by the CDC: lip, oral cavity, pharynx, esophagus, stomach, colon and rectum, liver, pancreas, larynx, trachea, lung, bronchus, cervix uteri, kidney and renal pelvis, urinary bladder, and acute myeloid leukemia. f: The Kessler Psychological Distress Scale consists of six questions that ask about feelings of sadness, nervousness, restlessness, worthlessness, hopelessness, and feeling like everything is an effort during the past 30 days. Participants were asked to respond on a Likert Scale ranging between ‘none of the time’ (score = 0) to ‘all of the time’ (score = 4), and a cutoff of 13 was used to dichotomize the status of serious psychological distress. g Defined as having any functional limitations inclusive of all physical conditions. h Calculated as the difference between age at interview and age when smoking regularly.
3.2. Associations between Provider-Patient Discussion and Quit Behavior
The population-weighted proportions of having quit intent were 65%, 66%, and 74% among those who were neither asked nor advised, either asked only or advised only, and both asked and advised, respectively; of having quit attempts were 46%, 47%, and 54%, respectively; for using pharmacological quit methods were 18%, 31%, and 40%, respectively; and, for using non-pharmacological quit methods were 4%, 11%, and 10%, respectively.
Compared to receiving neither asked nor advised discussion, being both asked and advised was associated with greater odds for quit intent (OR 1.65, 95% CI (1.63−1.66)) and quit attempt (OR 1.76, 95% CI (1.75–1.77)); while being either asked or advised was only statistically significantly associated with quit attempt (OR 1.60, 95% CI (1.57–1.63)), but not with quit intent (OR 1.02, 95% CI (0.99–1.05)) (Table 2).
Table 2.
The association between provider–patient discussion and quit behavior outcomes: quit intent, quit attempt, and quit methods from an instrumental variable analysis.
| Outcome Variable |
Exposure Variable: Level of Discussion |
Outcome Events/Total | Weighted % of Outcome Events |
Odds Ratio (95% CI) |
|---|---|---|---|---|
| Intent to Quit (Yes vs. No) |
Both Asked and Advised | 1611/2184 | 74% | 1.65 (1.63–1.66) Funding: |
| Either Asked or Advised | 479/727 | 66% | 1.02 (0.99–1.05) | |
| Neither | 726/1162 | 65% | Reference | |
| Attempt to Quit (Yes vs. No) |
Both Asked and Advised | 1177/2219 | 54% | 1.76 (1.75–1.77) |
| Either Asked or Advised | 359/735 | 47% | 1.60 (1.57–1.63) | |
| Neither | 549/1179 | 46% | Reference | |
| Pharmacological quit methods (Yes vs. No) |
Both Asked and Advised | 488/1177 | 40% | 1.99 (1.97–2.02) |
| Either Asked or Advised | 109/359 | 31% | 1.56 (1.49–1.63) | |
| Neither | 111/549 | 18% | Reference | |
| Non-pharmacological quit methods (Yes vs. No) |
Both Asked and Advised | 137/1177 | 10% | 2.01 (1.94–2.08) |
| Either Asked or Advised | 32/359 | 11% | 2.91 (2.74–3.08) | |
| Neither | 24/549 | 4% | Reference |
Notes: Instrumental variable (IV) analysis was implemented using the two-stage residual inclusion (2SRI) method. The number of doctor office visits in the past 12 months was used as the IV. The analysis took into account the complex NHIS survey design. All models adjusted for the 16 covariates listed in Table 1.
Among those who tried to quit, having both or only one level discussion was significantly associated with the use of pharmacological or non-pharmacological quit methods, as compared to having no discussion (Table 2). The association in pharmacological quit methods was slightly higher for having both rather than only one discussion (OR = 1.99, 95% CI (1.97–2.02) vs. 1.56, 95% CI (1.49–1.63)). The opposite trend was found for non-pharmacological quit methods, where the association was lower for having both rather than one discussion (OR 2.01, 95 % CI (1.94–2.08) vs. OR 2.91, 95% CI (2.74−3.08)).
Both the F test and the falsification test for assumption checking indicated that the number of doctor office visits was a reasonable instrumental variable (Supplementary Table S2).
Results from the PSW and multivariable logistic regression were consistent with those observed in the main IV analysis (Table 3). Supplementary Table S3 shows the comparison of sample characteristics before and after PSW.
Table 3.
The association between provider–patient discussion and quit behavior from a propensity score weighted model and a multivariable logistic model.
| Outcome | Exposure Variable: Level of Discussion |
Propensity Score Weighted Model ORs (95% CI) a | Multivariable Logistic Model OR (95% CI) b |
|---|---|---|---|
| Intent to Quit (Yes vs. No) |
Both Asked and Advised | 1.74 (1.31–2.30) | 1.68 (1.32–2.14) |
| Either Asked or Advised | 1.04 (0.77–1.414) | 0.97 (0.75–1.25) | |
| Neither | Reference | Reference | |
| Attempt to Quit (Yes vs. No) |
Both Asked and Advised | 1.88 (1.49–2.38) | 1.80 (1.47–2.21) |
| Either Asked or Advised | 1.24 (0.94–1.64) | 1.19 (0.92–1.55) | |
| Neither | Reference | Reference | |
| Pharmacological quit methods (Yes vs. No) |
Both Asked and Advised | 2.01 (1.44–2.82) | 1.95 (1.41–2.70) |
| Either Asked or Advised | 1.72 (1.12–2.64) | 1.54 (1.00–2.36) | |
| Neither | Reference | Reference | |
| Non-pharmacological quit methods (Yes vs. No) |
Both Asked and Advised | 1.92 (1.01–3.66) | 1.97 (0.97–3.99) |
| Either Asked or Advised | 2.57 (1.17–5.67) | 2.40 (1.09–5.30) | |
| Neither | Reference | Reference |
4. Discussion
The use of 5A’s has long been recognized as an evidence-based guideline for healthcare providers to promote smoking cessation among current smokers; however, its effectiveness varies [10,39]. Using a nationally representative sample of non-institutionalized adults, we found that current smokers who reported being both asked and advised by a healthcare provider had significantly increased odds (65%) of quit intent, compared to those who reported receiving neither discussion, while the increase was not significant among smokers who reported being only asked or only advised. Having both discussions also had a higher point estimate for quit attempt than having one discussion. For those who tried to quit, the odds of trying to quit with the assistance of pharmacological or non-pharmacological methods also increased among those with a provider–patient discussion, which is consistent with the known effectiveness of these two cessation methods [40,41]. In addition, we found a higher association in choosing pharmacological quit methods among those who received both rather than single discussion. It was also encouraging to find that about 50% of current smokers had tried to quit.
The finding of a growing influence between increasing levels of provider–patient discussion on quit behavior is new, as no NHIS studies have examined such a relationship. This finding suggests that delivery of both ask and advise may encourage more quit attempts among current smokers than delivery of only one of these 2A’s, and even having one of the 2A’s is better than no discussion at all. Our result supports the notion that each provider–patient discussion presents an opportunity to promote smoking cessation, and combined discussions may achieve a greater effect. This study provides concrete evidence that improving provision of even just one of the first 2A’s of the physician–patient discussion guideline can increase the probability of smoking cessation.
Regardless of the use of both of the first 2A’s or just one, having any level of provider–patient discussion was statistically significantly associated with increased quit attempts and greater use of either pharmacological or behavioral quit methods. Our results agreed with previous findings based on NHIS data that did not differentiate between the degrees of discussion [6,13,14,19,20,21,22,23]. Our result is also consistent with studies based on NATS data where all 5 A’s were surveyed, which showed that smokers who received any three or four components of the 5A’s were associated with greater use of cessation treatments [15]. Different from the previous studies, we detected some discrepancies in the method chosen by the levels of provider–patient discussion. Current smokers who received both 2A’s tended to choose pharmacological quit methods, while those who received either of the 2A’s tended to choose non-pharmacological quit methods. It is possible that those who received 2A’s might have received other A’s which were not asked in NHIS, though another study indicated a progressive decline in reports of receiving the 5A’s from first to last [15]. Given the evidence that using cessation medication approved by the FDA alone or in conjunction with behavioral counseling increases the chance of cessation [40,41], our results highlight the importance of having a comprehensive provider–patient discussion in helping smokers quit.
This study had several methodological strengths. Our IV analysis reduced the bias in estimating the association between provider–patient discussion and quit behavior compared to the estimates from commonly used multivariable logistic regressions. Biased estimates resulting from unobserved confounders are a major concern in observational studies [42], and their impact can be mitigated through an IV analysis. Candidates for IV in the context of this and similar studies can be distance-, time-, or preference-based [10,43]. Previously, Bao et al. used physicians’ advice about diet and physical activity as the IV to address the selection bias of a provider in giving smoking cessation advice [10]. Different from that study, we used the number of office visits as the IV, with the rationale that patients with more visits tend to be asked/advised more, and this patient behavior pattern is not directly correlated with quit behavior. Results from our IV analysis were consistent with that from the PSW regression, another sophisticated method to reduce confounding and unobserved bias [36]. The comparison of three approaches demonstrated the consistency and strong internal validity of our results.
Our study also had a few limitations. First, the responses were based on self-reported data, such as comorbidities, which were not confirmed by medical record. The outcomes of interest were also subject to recall bias [6]. Due to the nature of cross-sectional NHIS data, we were not able to establish temporality or draw causal inference. For instance, the survey lacked information about whether the intent/attempt to quit smoking occurred after being asked/advised by a provider. We also lacked the detailed information about the content and timing of the provider–patient discussion or about the provider’s characteristics, all of which could affect smokers’ quit behavior and choice of cessation methods. We were only able to assess the first 2A’s of the five-step algorithm, as other components (assess, assist, and arrange) were not surveyed in the NHIS. We could not rule out the possibility that some participants who had one or both of the first 2A’s might also have some or all of the other A’s, though the portion of these patients was likely to be small as shown in a national survey where data on all 5A’s were available [15]. Lastly, as the landscape of tobacco use has changed rapidly in recently years, [44] so has the provider–patient communication about smoking. While we used the most up-to-date publicly available data, more recent population-based data on the 5A’s are needed.
5. Conclusions
Smoking remains the leading cause of preventable diseases and deaths in the United States. Approximately 34 million adults currently smoke cigarettes, 70% of whom say they want to quit [1]. Our findings shed light on understanding the influence of varying levels in provider–patient discussion on the patient’s smoking cessation behavior. Being asked and/or advised has consistently increased smokers’ quit intent and quit attempt, as well as their use of pharmacological or behavioral cessation methods, compared to no provider–patient discussion. Individuals who currently smoke cigarettes may benefit more from increased levels of being asked and advised by a provider than either asked or advised alone.
Strategies that facilitate the adherence and compliance of providers to communicate with patients, such as continued education/training, embedding the 5A’s algorithm or three-step methods (e.g., ask-advise-refer or ask-advise-connect [45,46,47]) in the electronic health record, and adding incentives for both parties to promote provider–patient discussion, may help increase the success of smoking cessation [18,41,48,49].
Acknowledgments
The authors would like to acknowledge the support of the Biostatistics Shared Resource Facility, Icahn School of Medicine at Mount Sinai.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18094593/s1, Figure S1: Flow chart of the study population selection; Table S1: Unweighted characteristics of study sample by the 3-level of provider-patient discussion, 2015 NHIS; Table S2: Falsification test of total number of office visits in the past 12 months as an Instrumental Variable; Table S3: Characteristics of study sample by the level of discussion (survey and PS weights).
Author Contributions
B.L., L.L. and S.Z. conceived and designed the study, and drafted the manuscript. S.Z. carried out the statistical analysis under the supervision of L.L. and B.L., B.L., L.L., S.Z., K.M.W. and M.M. interpreted the results. M.M. and K.M.W. critically revised the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
B.L. receives support from a grant unrelated to this work from the National Cancer Institute (NCI): 1R21CA235153–02. L.L and S.Z. receives support related to this work from NCI Cancer Center Grant P30CA196521; M.M. receives grant funding paid to her institution for grants related to this work NCI (P30CA196521, CA220491, U24CA224319-01, DK124165) and unrelated to this work from NCATS (TR002997) and NIA (AG028741, AG066605, P30AG028741, R01AG054540).
Institutional Review Board Statement
This secondary data analysis project based on publicly available data is considered not human subjects research.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used for this study were publicly available from the NIHS website, https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm (accessed on January 2020).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Public Health Service, Office of the Surgeon General . Smoking Cessation: A Report of the Surgeon General. U.S. Department of Health and Human Services; Rockville, MD, USA: 2020. [(accessed on 31 January 2020)]. Available online: https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf. [Google Scholar]
- 2.O’Connor R.J. Non-cigarette tobacco products: What have we learnt and where are we headed? Tob. Control. 2012;21:181–190. doi: 10.1136/tobaccocontrol-2011-050281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toll B.A., Rojewski A.M., Duncan L.R., Latimer-Cheung A.E., Fucito L.M., Boyer J.L., Malley S.S., Salovey P., Herbst R.S. “Quitting Smoking Will Benefit Your Health”: The Evolution of Clinician Messaging to Encourage Tobacco Cessation. Clin. Cancer Res. 2014;20:301. doi: 10.1158/1078-0432.CCR-13-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacek L.R., Villanti A.C., McClernon F.J. Not Quite the Rule, But No Longer the Exception: Multiple Tobacco Product Use and Implications for Treatment, Research, and Regulation. Nicotine Tob. Res. 2019;22:2114–2117. doi: 10.1093/ntr/ntz221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chase E.C., McMenamin S.B., Halpin H.A. Medicaid Provider Delivery of the 5A’s for Smoking Cessation Counseling. Nicotine Tob. Res. 2007;9:1095–1101. doi: 10.1080/14622200701666344. [DOI] [PubMed] [Google Scholar]
- 6.Huo J., Chung T.H., Kim B., Deshmukh A.A., Salloum R.G., Bian J. Provider-Patient Discussions About Smoking and the Impact of Lung Cancer Screening Guidelines: NHIS 2011–2015. J. Gen. Intern. Med. 2020;35:43–50. doi: 10.1007/s11606-019-05111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huo J., Hong Y.-R., Bian J., Guo Y., Wilkie D.J., Mainous A.G. Low Rates of Patient-Reported Physician–Patient Discussion about Lung Cancer Screening among Current Smokers: Data from Health Information National Trends Survey. Cancer Epidemiol. Biomarks Prev. 2019;28:963–973. doi: 10.1158/1055-9965.EPI-18-0629. [DOI] [PubMed] [Google Scholar]
- 8.Kruger J., Shaw L., Kahende J., Frank E. Health care providers’ advice to quit smoking, National Health Interview Survey, 2000, 2005, and 2010. Prev. Chronic Dis. 2012;9:E130. doi: 10.5888/pcd9.110340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan A.S.L., Young-Wolff K.C., Carter-Harris L., Salloum R.G., Banerjee S.C. Disparities in the Receipt of Tobacco Treatment Counseling within the US Context of the Affordable Care Act and Meaningful Use Implementation. Nicotine Tob. Res. 2018;20:1474–1480. doi: 10.1093/ntr/ntx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao Y., Duan N., Fox S.A. Is some provider advice on smoking cessation better than no advice? An instrumental variable analysis of the 2001 National Health Interview Survey. Health Serv. Res. 2006;41:2114–2135. doi: 10.1111/j.1475-6773.2006.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanton C.A., Sharma E., Edwards K.C., Halenar M.J., Taylor K.A., Kasza K.A., Day H., Anic G., Gardner L.D., Hammad H.T., et al. Longitudinal transitions of exclusive and polytobacco electronic nicotine delivery systems (ENDS) use among youth, young adults and adults in the USA: Findings from the PATH Study Waves 1–3 (2013–2016) Tob. Control. 2020;29(Suppl 3):s147. doi: 10.1136/tobaccocontrol-2019-055574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cokkinides V.E., Halpern M.T., Barbeau E.M., Ward E., Thun M.J. Racial and Ethnic Disparities in Smoking-Cessation Interventions: Analysis of the 2005 National Health Interview Survey. Am. J. Prev. Med. 2008;34:404–412. doi: 10.1016/j.amepre.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Quintero C., Crum R.M., Neumark Y.D. Racial/ethnic disparities in report of physician-provided smoking cessation advice: Analysis of the 2000 National Health Interview Survey. Am. J. Public Health. 2006;96:2235–2239. doi: 10.2105/AJPH.2005.071035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henley S.J., Asman K., Momin B., Gallaway M.S., Culp M.B., Ragan K.R., Richards T.B., Babb S. Smoking cessation behaviors among older U.S. adults. Prev. Med. Rep. 2019;16:100978. doi: 10.1016/j.pmedr.2019.100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruger J., O’Halloran A., Rosenthal A.C., Babb S.D., Fiore M.C. Receipt of evidence-based brief cessation interventions by health professionals and use of cessation assisted treatments among current adult cigarette-only smokers: National Adult Tobacco Survey, 2009–2010. BMC Public Health. 2016;16:141. doi: 10.1186/s12889-016-2798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quinn V.P., Hollis J.F., Smith K.S., Rigotti N.A., Solberg L.I., Hu W., Stevens V.J. Effectiveness of the 5-As Tobacco Cessation Treatments in Nine HMOs. J. Gen. Intern. Med. 2008;24:149. doi: 10.1007/s11606-008-0865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinn V.P., Stevens V.J., Hollis J.F., Rigotti N.A., Solberg L.I., Gordon N., Ritzwoller D., Smith K.S., Hu W., Zapka J. Tobacco-Cessation Services and Patient Satisfaction in Nine Nonprofit HMOs. Am. J. Prev. Med. 2005;29:77–84. doi: 10.1016/j.amepre.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 18.King B.A., Dube S.R., Babb S.D., McAfee T.A. Patient-reported recall of smoking cessation interventions from a health professional. Prev. Med. 2013;57:715–717. doi: 10.1016/j.ypmed.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L., Babb S., Schauer G., Asman K., Xu X., Malarcher A. Cessation Behaviors and Treatment Use Among U.S. Smokers by Insurance Status, 2000–2015. Am. J. Prev. Med. 2019;57:478–486. doi: 10.1016/j.amepre.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Malarcher A.M., Ford E.S., Nelson D.E., Chrismon J.H., Mowery P., Merritt R.K., Herman W.H. Trends in Cigarette Smoking and Physicians’ Advice to Quit Smoking Among People with Diabetes in the U.S. Diabetes Care. 1995;18:694. doi: 10.2337/diacare.18.5.694. [DOI] [PubMed] [Google Scholar]
- 21.Tong E.K., Ong M.K., Vittinghoff E., Pérez-Stable E.J. Nondaily Smokers Should Be Asked and Advised to Quit. Am. J. Prev. Med. 2006;30:23–30. doi: 10.1016/j.amepre.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 22.Coups E.J., Dhingra L.K., Heckman C.J., Manne S.L. Receipt of Provider Advice for Smoking Cessation and Use of Smoking Cessation Treatments Among Cancer Survivors. J. Gen. Intern. Med. 2009;24:480. doi: 10.1007/s11606-009-0978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomar S.L., Husten C.G., Manley M.W. Do dentists and physicians advise tobacco users to quit? J. Am. Dent. Assoc. 1996;127:259–265. doi: 10.14219/jada.archive.1996.0179. [DOI] [PubMed] [Google Scholar]
- 24.NCHS, National Health Interview Survey (NHIS). U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. [(accessed on 5 February 2020)];2016 Available online: https://www.cdc.gov/nchs/nhis/1997-2018.htm.
- 25.Linda M., Collins S.T.L. Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences. Wiley; Hoboken, NJ, USA: 2009. [Google Scholar]
- 26.Babb S., Malarcher A., Schauer G., Asman K., Jamal A. Quitting Smoking Among Adults—United States, 2000–2015. MMWR Morb. Mortal W. 2017;65:1457–1464. doi: 10.15585/mmwr.mm6552a1. [DOI] [PubMed] [Google Scholar]
- 27.Lousdal M.L. An introduction to instrumental variable assumptions, validation and estimation. Emerg. Themes Epidemiol. 2018;15:1. doi: 10.1186/s12982-018-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rassen J.A., Schneeweiss S., Glynn R.J., Mittleman M.A., Brookhart M.A. Instrumental Variable Analysis for Estimation of Treatment Effects with Dichotomous Outcomes. Am. J. Epidemiol. 2008;169:273–284. doi: 10.1093/aje/kwn299. [DOI] [PubMed] [Google Scholar]
- 29.Rao J.N.K., Scott A.J. On Simple Adjustments to Chi-Square Tests with Sample Survey Data. Ann. Stat. 1987;15:385–397. doi: 10.1214/aos/1176350273. [DOI] [Google Scholar]
- 30.Geraci A., Fabbri D., Monfardini C. Testing Exogeneity of Multinomial Regressors in Count Data Models: Does Two Stage Residual Inclusion Work? J. Econom. Methods. 2018;7:1–19. doi: 10.1515/jem-2014-0019. [DOI] [Google Scholar]
- 31.Terza J.V., Basu A., Rathouz P.J. Two-stage residual inclusion estimation: Addressing endogeneity in health econometric modeling. J. Health Econ. 2008;27:531–543. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angrist J.D., Imbens G.W., Rubin D.B. Identification of Causal Effects Using Instrumental Variables. J. Am. Stat. Assoc. 1996;91:444–455. doi: 10.1080/01621459.1996.10476902. [DOI] [Google Scholar]
- 33.Stock J., Yogo M. Testing for Weak Instruments in Linear IV Regression. In: Andrews D.W.K., editor. Identification and Inference for Econometric Models. Cambridge University Press; New York, NY, USA: 2005. pp. 80–108. [Google Scholar]
- 34.Keele L., Zhao Q., Kelz R.R., Small D. Falsification Tests for Instrumental Variable Designs with an Application to Tendency to Operate. Med. Care. 2019;57:167–171. doi: 10.1097/MLR.0000000000001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 36.Austin P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Austin P.C., Jembere N., Chiu M. Propensity score matching and complex surveys. Stat. Methods Med. Res. 2016;27:1240–1257. doi: 10.1177/0962280216658920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridgeway G., Kovalchik Stephanie A., Griffin Beth A., Kabeto Mohammed U. Propensity Score Analysis with Survey Weighted Data. J. Causal Inference. 2015;3:237–249. doi: 10.1515/jci-2014-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallaway M.S., Glover-Kudon R., Momin B., Puckett M., Lunsford N.B., Ragan K.R., Rohan E.A., Babb S. Smoking cessation attitudes and practices among cancer survivors—United States, 2015. J. Cancer Surviv. 2019;13:66–74. doi: 10.1007/s11764-018-0728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villanti A.C., Johnson A.L., Ambrose B.K., Cummings K.M., Stanton C.A., Rose S.W., Feirman S.P., Tworek C., Glasser A.M., Pearson J.L., et al. Flavored Tobacco Product Use in Youth and Adults: Findings from the First Wave of the PATH Study (2013-2014) Am. J. Prev. Med. 2017;53:139–151. doi: 10.1016/j.amepre.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aveyard P., Begh R., Parsons A., West R. Brief opportunistic smoking cessation interventions: A systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction. 2012;107:1066–1073. doi: 10.1111/j.1360-0443.2011.03770.x. [DOI] [PubMed] [Google Scholar]
- 42.Newhouse M.B.M.J.P. Overview of the special supplement issue. Health Serv. Res. 2000;35:1061–1069. [PMC free article] [PubMed] [Google Scholar]
- 43.Huang H.H., Cagle P.J., Jr., Mazumdar M., Poeran J. Statistics in Brief: Instrumental Variable Analysis: An Underutilized Method in Orthopaedic Research. Clin. Orthop. Relat. Res. 2019;477:1750–1755. doi: 10.1097/CORR.0000000000000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hyland A., Kasza K.A., Borek N., Kimmel H.L., Taylor K.A., Compton W.M., Day H., Donaldson E.A., Sharma E., Anic G., et al. Overview of tobacco use transitions for population health. Tob. Control. 2020;29:S134–S138. doi: 10.1136/tobaccocontrol-2019-055367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vidrine J.I., Shete S., Cao Y., Greisinger A., Harmonson P., Sharp B., Miles L., Zbikowski S.M., Wetter D.W. Ask-Advise-Connect: A new approach to smoking treatment delivery in health care settings. JAMA Intern. Med. 2013;173:458–464. doi: 10.1001/jamainternmed.2013.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon J.S., Andrews J.A., Crews K.M., Payne T.J., Severson H.H. The 5A’s vs 3A’s plus proactive quitline referral in private practice dental offices: Preliminary results. Tob. Control. 2007;16:285–288. doi: 10.1136/tc.2007.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schroeder S.A. What to do with a patient who smokes. JAMA J. Am. Med. Assoc. 2005;294:482–487. doi: 10.1001/jama.294.4.482. [DOI] [PubMed] [Google Scholar]
- 48.Kalkhoran S., Appelle N.A., Napoles A.M., Munoz R.F., Lum P.J., Alvarado N., Gregorich S.E., Satterfield J.M. Beyond the Ask and Advise: Implementation of a Computer Tablet Intervention to Enhance Provider Adherence to the 5As for Smoking Cessation. J. Subst. Abus. Treat. 2016;60:91–100. doi: 10.1016/j.jsat.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Notley C., Gentry S., Livingstone-Banks J., Bauld L., Perera R., Hartmann-Boyce J. Incentives for smoking cessation. Cochrane Database Syst. Rev. 2019 doi: 10.1002/14651858.CD004307.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for this study were publicly available from the NIHS website, https://www.cdc.gov/nchs/nhis/data-questionnaires-documentation.htm (accessed on January 2020).