Abstract
Background
Patients with diabetes are one of the most high-risk group to become infected with SARS-CoV-2. Current study was designed to evaluate the risk of other complications in COVID-19 patients with diabetes.
Methods
In this cross-sectional study (25 February to July 10, 2020), 458 patients with diabetes were enrolled based on their characteristics, symptoms and signs, laboratory data and presence of other underlying diseases. Multiple logistic regression and Chi-square test analysis were used to check the effectiveness of other comorbidities on the mortality outcome among patients with diabetes.
Results
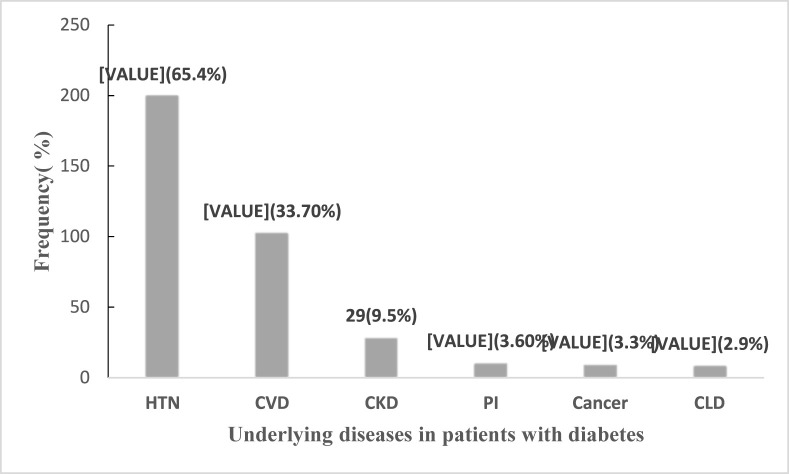
Of 458 patients with diabetes, 306 (67%) had other underlying diseases, such as 200 (65.4%) hypertension, 103 (33.7%) cardiovascular diseases and 29 (9.5%) kidney diseases. The rate of fatality was significantly high in patients with chronic kidney and liver diseases. The odds of mortality were increased 3.1-fold for patients over 55 years as compared to those under 55 years (P = 0.011), and the odds of mortality outcome were more than 5.1-fold for those who had chronic kidney disease (P < 0.001).
Conclusions
The presentation of SARS-CoV-2 in older patients with diabetes with other comorbidities such as chronic kidney and liver diseases is more severe in risk of mortality.
Keywords: COVID-19, Diabetes mellitus, Mortality, Kidney disease, Liver disease
1. Introduction
From the end of December 2019, a novel respiratory viral disease outbreak was reported from Wuhan city of China, which was caused by a novel coronavirus and was officially named COVID-19 in Mar 11 by the World Health Organization (WHO) (Mohammadi, 2020). Based on previous studies, it has been proven that the presence of different underlying diseases may play a key role in increasing the threat of COVID-19 in this group of individuals (Javanmardi et al., 2020). Uncontrolled diabetes is one of the most important underlying diseases with a high prevalence in the world (>463 million people) (Wu and McGoogan, 2020). Therefore, it is important to understand the special aspects of COVID-19 infection in people with this underlying comorbidity. From the first of the pandemic, extensive data about the association between diabetes and COVID-19 from various parts of the world have been accumulated. Based on these data, management of diabetes in cases with COVID-19 infection, and innovative strategies for medical consultation in view of limited access to healthcare facilities for patients with chronic diseases have been formulated. According to the critical role of diabetes mellitus (DM) as one of the leading causes of morbidity worldwide and its anticipated increase over the next decades, it is highly important to evaluate the role of DM in COVID-19 infected patients in order to manage the risks of this disease in the recent crisis (Knapp, 2013). In different meta-analysis studies, it has been proven that a significant correlation exists between severity of COVID-19 and diabetes, so that diabetes-related complications can increase the risk of mortality rate due to suppressed innate and humoral immune functions (Guo et al., 2020a). Obviously, in a study conducted by Li et al., another important point has been mentioned that new diabetes constitutes the highest percentage to be admitted to the ICU and requires more invasive mechanical ventilation (6). In some other studies, it has been pointed that the COVID-19 risk of mortality in pre‐existing diabetes is 5‐fold higher than individuals with normal glucose levels, while this is 10-fold higher in newly diagnosed patients (Sathish and de Mello, 2021). Although diabetes has been shown to be an important underlying disease in increasing risks associated with the COVID-19 crisis, the presence of other comorbidities, such as hypertension, cardiovascular, liver and kidney diseases, outweighs the immunosuppression status in these patients, and it may increase the risk of mortality (Emami et al., 2020a, 2020b). Recent investigations have indicated that kidney and liver are two organs with more vulnerability due to angiotensin-converting enzyme-2 (ACE-2) expression (Guo et al., 2020b). In addition, patients with diabetes suffering from pre-existing kidney and liver diseases beyond immune system impairment and chronic systemic inflammation are more prone to infectious diseases. In the current study, we reviewed the risk role of different underlying diseases other than diabetes in patients with COVID-19 in Fars Province in the south of Iran.
2. Materials and methods
In this cross-sectional retrospective study (25 February to July 10, 2020), 4585 patients with confirmed SARS-CoV-2 infection were evaluated according to the inclusion criteria of the study. These patients were admitted to the referral hospitals affiliated with Shiraz University of Medical Sciences (SUMS), Fars Province, Iran. The data for this study were obtained from the electronic base registry containing all the related data due to hospitalized patients with confirmed COVID-19 (Ethical code: IR-SUMS-REC.1399.022). Data contained demographic data, including age, gender, and baseline characteristics, such as symptoms and signs, laboratory data and underlying diseases.
The included patients were considered according to the following specifications: I). Positive for COVID-19 according to the qRT-PCR test; II). Confirmed for chronic diabetes; III). Presence of abnormalities in their computerized tomography (CT) scan. To investigate the impact of comorbidities in COVID-19 patients with diabetes, all diabetics with or without other comorbidities were considered in the study. The demographic and comorbidities data of each patient were gathered by self-declaration or first-degree family members’ statement. Based on these criteria, 458 patients were included. In the following, the effects of age, gender, smoking, opium, length of hospitalization stay and underlying diseases, such as hypertension, cardiovascular, and chronic kidney and liver diseases, cancer, and primary immunodeficiency, were considered in all included patients and were compared.
Categorical variables were expressed as frequency and percentages (%), and continuous variables were expressed as mean. Categorical variables between the groups were compared by using the χ2 test. Continuous variables were analyzed by employing Student's t-test. Multiple logistic regression analysis was used to determine the most powerful factors, such as demographic data, including age and gender, and underlying diseases affecting the mortality outcome among COVID-19 patients with diabetes, and with or without other comorbidities. All statistical analyses were conducted using the SPSS 18.0 software. P-value <0.05 was considered statistically significant.
3. Results
During the study time, of 4585 hospitalized confirmed positive patients for COVID-19, 458 (9.9%) individuals with diabetes criteria were considered. The included patients were categorized in two groups: I). with underlying diseases; 306 (67%); II). with no underlying diseases; 152 (33.2%). The rate of mentioned underlying diseases in Group I was as follows: 200 (65.4%) with hypertension, 103 (33.7%) with cardiovascular diseases, 29 (9.5%) with chronic kidney diseases, 11 (3.6%) with immunodeficiency, 10 (3.3%) with cancer, and 9 (2.9%) patients with chronic liver disease (Fig. 1 ). Comparing two groups (with and without other comorbidities), have showed that patients with other comorbidities have older ages than group without any comorbidities (62.9(61.7–64.3) vs 57.9(55.8–59.9)), which was statistically significant (p-value<0.001). The gender distribution was the same between the two groups. Although there was no difference between the two groups in smoking, no significant difference was found in the average of their length of hospitalization stay either (Table 1 ). A total of 238 (51.96) cases were cared in the Intensive Care Unit (ICU), while 32 (7%) cases were intubated. Among the cases, 13 patients were found with smoking, while 11 individuals needed to be hospitalized in the ICU (p-value = 0.017. The most common symptoms among the included patients were as follows: respiratory distress 273 (59.6%), cough 229 (50%), fever 179 (39.1%), muscular pain 144 (31.4%) and chest pain 13 (2.8%) (Table 1).
Fig. 1.
Underlying diseases in patients with diabetes
Hypertension (HTN), Chronic Cardiovascular Disease (CVD), Chronic Kidney Disease (CKD), Chronic Liver Disease (CLD), Primary Immunodeficiency (PI).
Table 1.
The characteristics of patients with diabetes and covid-19 with or without other comorbidities.
| variables | No. (%) |
Without other comorbidities (n = 152) | With comorbidities (n = 306) | P-valuea | |
|---|---|---|---|---|---|
| Total (n = 458) | |||||
| Age | Mean age | 61.3(60.2–62.4) | 57.9(55.8–59.9) | 62.9(61.7–64.3) | <0.001 |
| ≤55 years | 136 (29.7%) | 60 (39.5%) | 76 (24%) | 0.001 | |
| >55 years | 322 (70.3%) | 92 (60.5%) | 230 (75.2%) | ||
| Gender | Male | 227(49.6) | 85(55.9) | 142(46.4) | 0.055 |
| Female | 231(50.4) | 67(44.1) | 164(53.6) | ||
| Base line Characteristics | Smoking | 13 (2.8) | 5(3.3) | 8 (2.6) | 0.767 |
| Opium | 7 (1.5) | 4 (2.6) | 3 (Mohammadi, 2020) | 0.227 | |
| Length of Stay (days) | 6.9(6.3–7.5) | 6.2 (5.4–7) | 7.3 (6.5–8.03) | 0.087 | |
| Intubation | 32(7) | 2 (6.2) | 30 (93.8) | 0.001 | |
| Signs and symptoms | Fever | 179(39.1) | 60(39.5) | 119(38.9) | 0.904 |
| Cough | 229(50) | 78 (51.3) | 151 (49.3) | 0.691 | |
| Headache | 58(12.7) | 17 (11.2) | 41 (13.4) | 0.512 | |
| Chest pain | 13(2.8) | 2 (1.3) | 11 (3.6) | 0.249 | |
| Muscular Pain | 144(31.4) | 57 (37.5) | 87 (28.4) | 0.049 | |
| Respiratory-distress | 273(59.6) | 91 (59.9) | 182 (59.5) | 0.936 | |
| Diarrhea | 24(5.2) | 4 (2.6) | 20 (6.5) | 0.107 | |
| Laboratory data | Neutrophil count, × 109/L | 3.50(2.23–6.45) | 7.25(4.33–11.81) | 3.94(2.54–7.71) | 0.210 |
| Lymphocyte count, × 109/L | 1.14(0.75–2.5) | 0.54(0.42–0.93) | 0.81(0.56–1.24) | 0.14 | |
| Platelet count, × 109/L | 220(180–273.5) | 161.0(126.5–232.5) | 173.0(130.0–230.0) | 0.303 | |
| D-dimer, μg/mL FEU | 0.8(0.4–3.5) | 2.6(1.0–21.10) | 1.2(0.4–10.7) | 0.012 | |
| Alanine aminotransferase, U/L | 15.3(12.64–35.12) | 22.5(16.3–40) | 30.12(15.0–38.3) | 0.026 | |
| Aspartate aminotransferase, U/L | 24.32(19.74–28.36) | 31.0(21.3–58.3) | 34.0(22.0–47.0) | 0.011 | |
| Albumin, mean ± SD, g/L | 41.16(38.36–45.47) | 32.2(27.12–45.65) | 38.9(28.64–40.12) | 0.009 | |
| Creatine kinase, U/L | 151.14(83.14–185.63) | 106.0(70.0–290.0) | 132.5(63.8–233.3) | 0.329 | |
| Creatinine, μmol/L | 65.41(58.14–78.36) | 78.5(65.8–102.3) | 83.0(58.0–100.0) | 0.26 | |
| ESR, mm/H | 32.19 (28.16–45.48) | 38.0(15.12–65.8) | 27.0(14.5–43.5) | 0.14 | |
| Lactic dehydrogenase, U/L | 184.12(173.41–220.32) | 330.12(306–645) | 465.14(231–535) | 0.011 | |
| Outcome | Mortality | 50 (10.9) | 13 (8.6) | 37 (12.1) | 0.253 |
| Intensive Care Units(ICU) | 238(51.96) | 81(53.3) | 157(51.3) | 0.734 |
In analyzing the symptoms between the two groups (with and without comorbidities), it has been deduced that frequency of muscular pain was significant different between two groups (28.4% vs. 37.5%; P-value = 0.049). It was interesting that muscular pain was the frequent symptom in the included patients with hypertension (P-value = 0.027). Comparison of the percentages of mortality between the two groups (with and without other comorbidities) showed no significant differences (37 (12.1%) vs. 13 (8.6%); P-value = 0.253) (Table 1). Among died cases, (50; 10.92%) total of 37 (74%) of them had at least one of the mentioned comorbidities (Table 1). The high frequency of underlying diseases in dead cases was as following respectively: hypertension, 21 (42%); chronic kidney disease, 10 (20%); cardiovascular disease, 9 (18%); and 3 (6%) of them had other underlying diseases. Analysis of underlying diseases among the dead cases indicated that the rate of fatality was significantly high in patients with chronic kidney (20% vs 4.9%) and liver (6% vs 1.5%) diseases, respectively (p-value<0.001, p-value = 0.029) (Table 2 ). Furthermore, the case fatality rate in patients with chronic kidney disease was more observed at ages over 55 years (p-value<0.001). As Table 1 shows, various biomarkers were evaluated in the studied groups. D-dimer, alanine aminotransferase, aspartate aminotransferase, albumin and lactic dehydrogenase were significant in individuals between two studied groups. Obviously, patients with other comorbidities have higher liver enzyme and also more creatinine, and creatine kinas level.
Table 2.
The relationship between demographics, underlying disease and mortality rate in patients with diabetes and COVID-19.
| Variables | No. (%) |
Alive |
Dead |
P Value | |
|---|---|---|---|---|---|
| Total (n = 458) | N = 408 | N = 50 | |||
| Age | ≤55 | 136 (29.7%) | 129 (31.6%) | 7 (14%) | 0.01 |
| >55 | 322 (70.3%) | 279 (68.4%) | 43 (86%) | ||
| Gender | Male | 227 (49.6) | 197 (48.3%) | 30 (13%) | <0.001 |
| Female | 231 (50.4) | 211 (51.7%) | 20 (8%) | ||
| Comorbidities | HTN | 200 (43.7%) | 179 (43.9%) | 21 (42%) | 0.801 |
| CVD | 103 (22.5%) | 94 (23%) | 9 (18%) | 0.421 | |
| Cancer | 10 (2.2%) | 8 (2%) | 2 (4%) | 0.352 | |
| CKD | 30 (6.6%) | 20 (4.9%) | 10 (20%) | <0.001 | |
| CLD | 9 (2%) | 6 (1.5%) | 3 (6%) | 0.029 | |
| PI | 11(2.4%) | 8 (2%) | 3 (6%) | 0.108 |
Hypertension (HTN), Chronic Cardiovascular Disease (CVD), Chronic Kidney Disease (CKD), Chronic Liver Disease (CLD), Primary Immunodeficiency (PI).
All of the above results were confirmed by the multiple logistic regression analysis. In this analysis, it was showed that age and chronic kidney disease were the most powerful factors affecting the mortality outcome among COVID-19 patients with diabetes (Table 3 ). In the proposed model, it was revealed that the odds of mortality were 3.1-fold for patients over 55 years (P-value = 0.011), and the odds of mortality for those who had chronic kidney diseases were 5.1-fold more than others (P-value <0.001).
Table 3.
Multiple logistic regression analysis of risk factors leading to mortality.
| variable | subgroup | β estimate | SE | Wald | Odds Ratio | CI(OR) | P value |
|---|---|---|---|---|---|---|---|
| Age | ≤55 | Baseline | 0.44 | 6.54 | 3.1 | (1.3–7.3) | 0.011 |
| >55 | 1.12 | ||||||
| Gender | Male | 0.355 | 0.323 | 1.2 | 1.43 | (0.76–2.69) | 0.273 |
| Female | Baseline | ||||||
| HTN | Yes | 0.317 | 0.334 | 0.9 | 1.37 | (0.71–2.64) | 0.343 |
| No | Baseline | ||||||
| CVD | Yes | 0.62 | 0.419 | 2.2 | 1.9 | (0.82–4.23) | 0.138 |
| No | Baseline | ||||||
| Cancer | Yes | 0.908 | 0.845 | 1.16 | 2.5 | (0.47–12.99) | 0.282 |
| No | Baseline | ||||||
| CKD | Yes | 1.63 | 0.461 | 12.55 | 5.1 | (2.07–6.6) | <0.001 |
| No | Baseline | ||||||
| CLD | Yes | 1.31 | 0.794 | 2.72 | 3.7 | (0.78–7.61) | 0.099 |
| No | Baseline | ||||||
| PI | Yes | 0.521 | 0.773 | 0.454 | 1.6 | (0.37–7.7) | 0.501 |
| No | Baseline |
Hypertension (HTN), Chronic Cardiovascular Disease (CVD), Chronic Kidney Disease (CKD), Chronic Liver Disease (CLD), Primary Immunodeficiency (PI).
4. Discussion
Based on the results of the current study, it has been shown that patients with diabetes with other comorbidities are more at risk of progression of COVID-19. According to the main results, it has been concluded that comorbidities in patients with diabetes are a key risk factor for the progression and prognosis of COVID-19. Awareness in this regard has significant benefits for treatment, decreases the complications and mortality rate, and increases the quality of life of this group of patients. According to many studies conducted during the recent crisis, it has been reported that diabetes plays a critical role in the outcome of SARS-CoV-2 pneumonia (Guo et al., 2020b; Bloomgarden, 2020). Furthermore, according to the results of few studies, it has been documented that patients with diabetes are more prone to certain bacterial and viral infections and their complications (Knapp, 2013; Shah and Hux, 2003; Muller et al., 2005; Das and Barai, 2017; Yang et al., 2006). Therefore, it is necessary to pay considerable attention to this group of patients, particularly in the current new crisis. In a study in China, it has been reported that COVID-19 complications are more in patients with diabetes (9.7%) (Yang et al., 2020).
In the current study, it has been indicated that hypertension (65.4%), cardiovascular (33.7%) and chronic kidney diseases (6.6%) are the most prevalent comorbidities in infected patients with diabetes. Except for chronic kidney diseases, the same results for hypertension and cardiovascular have been reported in other studies around the world (Guan et al., 2020; Singh et al., 2020; Alanazi et al., 2020). Comparison of the comorbidities in coronavirus infections, such as Severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome Coronavirus (MERS), shows the same results (Simonson, 1988).
In one meta-analysis study report, hypertension, cardiovascular, and chronic kidney diseases were respectively the most prevalent underlying diseases among hospitalized patients with COVID-19 (Emami et al., 2020a). The results of one study in Saudi Arabia (2020) showed that diabetes was associated with common comorbidities, such as ischemic heart disease, hypertension, and dyslipidemia (Gazzaz et al., 2020); therefore, in the recent infection crisis (COVID-19), we must consider all patients with diabetes with other underlying diseases and manage their treatment totally.
Based on our study results, we found no significant difference between male and female infected patients with diabetes (49.6% vs 50.4%). This is while in a study by Jin et al., the rate of mortality reported higher in males (males who died from COVID-19 was 2.4 times that of females) (Jin et al., 2020). Furthermore, in Italy report, higher risks have also been reported in males than females (Livingston and Bucher, 2020). According to the results of another study, 54.1% and 45.9% of COVID-19 patients with diabetes were male and female, respectively (Guo et al., 2020b). In our study results, we found no significant difference between tobacco consumption and average length of hospitalization stay. Although some studies indicated the increased risk of ICU admission in patients with diabetes, in our study, showed that this risk is increase with smoking (Cao et al., 2020; Huang et al., 2020; Wang et al., 2020; Shi et al., 2020).
In the current study, among patients with COVID-19, the rate of mortality was 3.5% higher in patients with diabetes who suffer from other underlying diseases. The mortality rate in patients with MERS who had diabetes was reported 35% (Al-Tawfiq et al., 2014; Alraddadi et al., 2016). In another study was conducted in China, the case fatality rate was reported 7.3% in patients with diabetes in China (Wu and McGoogan, 2020).
According to our study, it was found that D-dimer, alanine aminotransferase, aspartate aminotransferase, albumin and lactic dehydrogenase were significant in patients with diabetes suffering from other comorbidities. This finding revealed that patients with diabetes and with other comorbidities had higher alanine aminotransferase and aspartate aminotransferase. To date, there is no evidence about direct mechanism of kidney and liver involvement in COVID-19. Thus, it seems that due to this system impairment, special attention must be paid to the uremic state, excessive oxidative stress status due to retention of a plethora of toxins, and accumulation of oxidative products that could worsen once the patient is infected (D'Marco et al., 2020). Moreover, pre-existence of comorbidities accompanied by diabetes creates serious complications when become infected with SARS-CoV-2, thereby it increase the risk of acute and chronic syndrome due to the pro-inflammatory process (Ceriello et al., 2020).
Furthermore, the prevalence of signs and symptoms, such as cough, fever, headache, chest pain, and respiratory distress in patients with diabetes with or without comorbidities were not different significantly. Another study verified that signs and symptoms among patients with diabetes and non-diabetes had no significant difference (Guo et al., 2020b). Meanwhile, some studies have confirmed that various signs and symptoms (nonproductive cough, fever, diarrhea, and nausea/vomiting) are generally associated with COVID-19 (Zhou et al., 2020).
The results of our research indicated that the prevalence of diabetes was significantly high among patients over 55 years. In addition, the mortality rate in patients with diabetes over 55 years was detected significantly high, being 3.1 times higher than that in younger patients. Based on another study, the mean case fatality rate for people aged under 60 is estimated to be less than 0.2%, while this range in people aged over 80 is 9.3% (Ferguson et al., 2020). Based on our results, among all patients with diabetes, hypertension, chronic kidney disease, and cardiovascular were the most important factors influencing the mortality rate in these patients, and the mortality rate in patients with chronic kidney disease was approximately 5.1-fold as compared to those who had not this underlying disease.
Although the main result of the severity of COVID-19 is unknown mainly in people with diabetes, chronic kidney disease, or other chronic diseases, it may be explainable with the expression of angiotensin-converting enzyme-2 (ACE2) in other organs, such as liver and kidney tissues (Ma and Holt, 2020). Overall, it appears that presentation of SARS-CoV-2 in patients with diabetes is more severe, and those who have comorbidities are at a higher risk of mortality. Chronic kidney and liver diseases are two major factors affecting the increasing mortality rate of diabetics with COVID-19. Comparing the current result and other studies revealed that patients with diabetes who had pre-existing liver and kidney diseases are more severe in aspect of COVID-19 and so worsen outcome (Li and Tian, 2020; Sathish et al., 2021). Therefore, this group of patients needs to be strictly kept under surveillance for blood glucose screening. Based on, new data and in a systematic review it is also important to note that COVID-19 may increase the risk of diabetes in infected individuals (Sathish et al., 2021; Sathish and Kapoor, 2021). These include damage to pancreatic β cells, exaggerated response to proinflammatory cytokines, activation of the renin-angiotensin (RAS) system, and changes in health behaviors during the epidemic. None of these possibilities is unique in themselves, and it is discussable that several factors may be combined to contribute to the development or progression of type 1 or type 2 diabetes, or in fact, lead to a new form of diabetes.
5. Conclusion
From the recent study, we can conclude that older-aged patients with diabetes and some comorbidities like chronic kidney diseases are more at risk of mortality during the COVID-19 crisis. Since the role of symptom screening of underlying diseases in the current new crisis is vital, there is a need to further study COVID-19 in patients with diabetes and to understand individual, regional and ethnic variations in the disease prevalence.
6. Limitations
According to the acute condition of the patients at the time of admission and the presence of crisis in the referral centers we had some limitations as follows:
1- Due to the patient's condition and the impossibility of examining some factors such as Body mass index (BMI) we couldn't add this factor to our analysis. It must be mentioned that there is enough evidence about the risk of obesity in COVID-19 severity.
2- According to that the demographic and comorbidities data of each patient were collected by self-declaration or first-degree family members' statements; we didn't have data on their medications and fasting/random blood glucose/HbA1c taken at the admission time.
Funding
No funding was received.
Declaration of competing interest
No conflict of interest.
References
- Al-Tawfiq J.A., Hinedi K., Ghandour J., Khairalla H., Musleh S., Ujayli A., et al. Middle East respiratory syndrome coronavirus: a case-control study of hospitalized patients. Clin. Infect. Dis. 2014;59(2):160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanazi K.H., Abedi G.R., Midgley C.M., Alkhamis A., Alsaqer T., Almoaddi A., et al. Diabetes mellitus, hypertension, and death among 32 patients with MERS-CoV infection, Saudi Arabia. Emerg. Infect. Dis. 2020;26(1):166. doi: 10.3201/eid2601.190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alraddadi B.M., Watson J.T., Almarashi A., Abedi G.R., Turkistani A., Sadran M., et al. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi Arabia. Emerg. Infect. Dis. 2016;22(1):49. doi: 10.3201/eid2201.151340. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomgarden Z.T. Diabetes and COVID‐19. J. Diabetes. 2020;12(4):347–348. doi: 10.1111/1753-0407.13027. [DOI] [PubMed] [Google Scholar]
- Cao J., Hu X., Cheng W., Yu L., Tu W.-J., Liu Q. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A., Standl E., Catrinoiu D., Itzhak B., Lalic N.M., Rahelic D., et al. Issues for the management of people with diabetes and COVID-19 in ICU. Cardiovasc. Diabetol. 2020;19(1):1–7. doi: 10.1186/s12933-020-01089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Marco L., Puchades M.J., Romero-Parra M., Gorriz J.L. Diabetic kidney disease and COVID-19: the crash of two pandemics. Front. Med. 2020;7:199. doi: 10.3389/fmed.2020.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Barai A. A possible alternate pathway for intravascular thrombosis-Investigation of the circumstantial evidence by microfluidics. Medical Science and Discovery. 2017;4(1):1–11. [Google Scholar]
- Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1) [PMC free article] [PubMed] [Google Scholar]
- Emami A., Javanmardi F., Akbari A., Moghadami M., Bakhtiari H., Falahati F., et al. Characteristics of deceased patients with CoVID-19 after the first peak of the epidemic in Fars province, Iran. Infect. Ecol. Epidemiol. 2020;10(1):1781330. doi: 10.1080/20008686.2020.1781330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N., Laydon D., Nedjati Gilani G., Imai N., Ainslie K., Baguelin M., et al. 2020. Report 9: Impact of Non-pharmaceutical Interventions (NPIs) to Reduce COVID19 Mortality and Healthcare Demand. [Google Scholar]
- Gazzaz Z.J., Iftikhar R., Jameel T., Baig M., Murad M.A. Association of dyslipidemia and comorbidities with risk factors among diabetic patients: a retrospective analysis. Diabetes, Metab. Syndrome Obes. Targets Ther. 2020;13:935. doi: 10.2147/DMSO.S235546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W-j, Liang W-h, Zhao Y., Liang H-r, Chen Z-s, Li Y-m, et al. Comorbidity and its impact on 1590 patients with covid-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55(5) doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Shi Z., Zhang Y., Wang C., Moreira N.C.D.V., Zuo H., et al. Comorbid diabetes and the risk of disease severity or death among 8807 COVID-19 patients in China: a meta-analysis. Diabetes Res. Clin. Pract. 2020:108346. doi: 10.1016/j.diabres.2020.108346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes/metabolism research and reviews. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanmardi F., Keshavarzi A., Akbari A., Emami A., Pirbonyeh N. Prevalence of underlying diseases in died cases of COVID-19: a systematic review and meta-analysis. PloS One. 2020;15(10) doi: 10.1371/journal.pone.0241265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.-M., Bai P., He W., Wu F., Liu X.-F., Han D.-M., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Frontiers in Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S. Diabetes and infection: is there a link?-A mini-review. Gerontology. 2013;59(2):99–104. doi: 10.1159/000345107. [DOI] [PubMed] [Google Scholar]
- Li H., Tian S. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. 2020;22(10):1897–1906. doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston E., Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. Jama. 2020;323(14):1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- Ma R.C.W., Holt R.I.G. COVID-19 and diabetes. Diabet. Med. : a journal of the British Diabetic Association. 2020;37(5):723–725. doi: 10.1111/dme.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M.T. Psychological impacts of covid-19 outbreak on mental health status of society individuals: a narrative review. Journal Mil Med. 2020;22(2):184–192. [Google Scholar]
- Muller L., Gorter K., Hak E., Goudzwaard W., Schellevis F., Hoepelman A., et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 2005;41(3):281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- Sathish T., Kapoor N. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. 2021;23(3):870–874. doi: 10.1111/dom.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish T., de Mello G.T. Is newly diagnosed diabetes a stronger risk factor than pre-existing diabetes for COVID-19 severity? 2021;13(2):177–178. doi: 10.1111/1753-0407.13125. [DOI] [PubMed] [Google Scholar]
- Sathish T., Tapp R.J., Cooper M.E., Zimmet P. Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes & metabolism. 2021;47(2):101204. doi: 10.1016/j.diabet.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah B.R., Hux J.E. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson D.C. Etiology and prevalence of hypertension in diabetic patients. Diabetes Care. 1988;11(10):821–827. doi: 10.2337/diacare.11.10.821. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Gupta R., Ghosh A., Misra A. Diabetes & Metabolic Syndrome. Clinical Research & Reviews; 2020. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yang J., Feng Y., Yuan M., Yuan S., Fu H., Wu B., et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. 10229. [DOI] [PMC free article] [PubMed] [Google Scholar]