Abstract
Objective:
p21 (Cdc42/Rac1) activated Kinase 1 (PAK1) is a candidate susceptibility factor for type 2 diabetes (T2D). PAK1 is depleted in the islets from T2D donors, compared to control individuals. In addition, whole-body PAK1 knock out (PAK1-KO) in mice worsens the T2D-like effects of high-fat diet. The current study tested the effects of modulating PAK1 levels only in β-cells.
Materials/methods:
β-cell-specific inducible PAK1 KO (βPAK1-iKO) mice were generated and used with human β-cells and T2D islets to evaluate β-cell function.
Results:
βPAK1-iKO mice exhibited glucose intolerance and elevated β-cell apoptosis, but without peripheral insulin resistance. β-cells from βPAK-iKO mice also contained fewer mitochondria per cell. At the cellular level, human PAK1-deficient β-cells showed blunted glucose-stimulated insulin secretion and reduced mitochondrial function. Mitochondria from human PAK1-deficient β-cells were deficient in the electron transport chain (ETC) subunits CI, CIII, and CIV; NDUFA12, a CI complex protein, was identified as a novel PAK1 binding partner, and was significantly reduced with PAK1 knockdown. PAK1 knockdown disrupted the NAD+/NADH and NADP+/NADPH ratios, and elevated ROS. An imbalance of the redox state due to mitochondrial dysfunction leads to ER stress in β-cells. PAK1 replenishment in the β-cells of T2D human islets ameliorated levels of ER stress markers.
Conclusions:
These findings support a protective function for PAK1 in β-cells. The results support a new model whereby the PAK1 in the β-cell plays a required role upstream of mitochondrial function, via maintaining ETC protein levels and averting stress-induced β-cell apoptosis to retain healthy functional β-cell mass.
Keywords: β-Cell mass, Diabetes, Mitochondrial number, Electron transport chain, Redox imbalance
1. Introduction
Diabetes was the seventh leading cause of death in the United States in 2015 [1]. Type 2 diabetes (T2D) is characterized by peripheral insulin resistance, dysregulated function of the pancreatic β-cells, and eventual β-cell death [2,3]. Pancreatic islet β-cells are central regulators of glucose-stimulated insulin secretion (GSIS). Since approximately 50% of islet β-cells from T2D patients fail to appropriately release insulin (dysfunction) or lack sufficient insulin-containing β-cells at the time of diagnosis [4], it is presumed that loss of functional β-cell mass occurs during pre-diabetes, prior to the development of frank T2D. Therefore, it is crucial to identify mechanisms causing β-cell dysfunction and destruction.
Mitochondrial-derived coupling factors serve a critical role in the control of β-cell insulin secretory function and the preservation of β-cell mass. The production of insulin by the β-cell requires a tremendous amount of ATP. During the pre-diabetic stage, the β-cell attempts to respond to the increased ‘workload’ caused by deficits in peripheral insulin response to manage glucose homeostasis, thereby increasing mitochondrial dysfunction [5]. Mitochondrial dysfunction results in altered mitochondrial enzymatic activity, diminished ATP production, impaired redox balance, and excessive generation of reactive oxygen species (ROS) [6]. In mitochondria, the electron transport chain (ETC) is the primary site of ATP production [7], and diabetogenic stimuli, such as high glucose, dysregulate the normal flow of electrons, further increasing ROS formation [8].
In β-cells, elevated ROS levels reduce mitochondrial function and erode mitochondrial structure, ultimately disrupting the mitochondrial membrane potential (ΔΨm) and initiating a cascade of events leading to β-cell death [9–11]. Mechanisms that may be therapeutically targeted to protect β-cells from this diabetogenic stimulus-induced ROS ‘storm’ have remained elusive.
Mitochondrial dysfunction is linked to endoplasmic reticulum (ER) stress. Impaired mitochondrial structure and function activates AMPK in β-cells [12], thereby increasing levels of ER stress. ER stress is sensed by three ER transmembrane proteins: Inositol Requiring 1 (IRE1), PKR-like ER kinase (PERK), and Activating Transcription Factor 6 (ATF6), which induce an ER-to-nucleus signaling cascade referred to as unfolded protein response (UPR) [13]. Excessive ER stress induces β-cell dysfunction and causes β-cell apoptosis [14]. Mitochondrial dysfunction and ER stress are implicated as early events and may participate in β-cell loss. However, the molecular mechanisms involved remain unclear.
Previous studies from our laboratory and others have implicated the serine/threonine p21-activated kinase 1 (PAK1) as a positive regulator of both β-cell function [15–18] and maintenance of β-cell mass [19,20]. In addition, the PAK1 protein expression level is reduced by 80% in islets from T2D donors, suggesting that PAK1 deficiency might contribute to T2D pathogenesis [15,16]. In islet β-cells, PAK1 serves as an important signaling hub, triggering signaling cascades in response to metabolic stimuli that dually support insulin release and β-cell survival. For example, PAK1 can facilitate glucose-stimulated F-actin remodeling to facilitate insulin granule mobilization to the plasma membrane [18,21,22]. PAK1 also promotes β-cell survival by activating the anti-apoptotic factor Bcl2 [21] and inactivating the pro-apoptotic factor Bad [19]. PAK1 also regulates the abundance of Survivin [20], a protein involved in the replication of β-cells [23]. However, a role for PAK1 signaling to support β-cell mitochondrial function has yet to be evaluated.
In the current study, we investigated whether PAK1 is required to prevent β-cell dysfunction, including mitochondrial dysfunction, ER stress, and apoptosis. Our findings support a new model whereby the local supply of cytosolic PAK1 in the β-cell is required for normal mitochondrial function by supporting ETC protein levels and averting stress-induced β-cell apoptosis to retain healthy functional β-cell mass.
2. Materials and methods
2.1. Dispersed human islet culture and GSIS assay
Human islets were obtained through the Integrated Islet Distribution Program (IIDP) or City of Hope Islet Core; islets with at least 80% purity and 75% viability were accepted for use. The human islets used in the current study are described in Supplementary Table 1. Upon receipt, the human islets were allowed to recover in complete CMRL medium (Gibco, Grand Island, NY) containing 10% fetal bovine serum (HyClone, South Logan, UT) and 1% penicillin/streptomycin (v/v, Gibco, Grand Island, NY) for 2 h. Islets were subsequently handpicked under a light microscope equipped with a green gelatin filter, which was used to detect and remove residual, non-islet material. The islets were dispersed into a monolayer cell suspension using 10 X TrypLE (Invitrogen, Carlsbad, CA) then seeded in ultra-low-attachment 24-well plates (Corning, Kennebunk, ME). The cells were cultured at 37 °C with 5% CO2 in complete CMRL medium. For each experimental group, approximately 1 × 106 cells were transfected with either siPAK1 (Qiagen, cat. #SI00605696, Valencia, CA) or siCON (Qiagen, cat. # 1027310) using RNAiMAX lipofectamine reagent (Invitrogen, Carlsbad, CA). The dispersed islets were maintained in the same culture conditions for 3 days. On the day of GSIS, dispersed islets were pre-incubated in Krebs-Ringer bicarbonate buffer (KRBB) in low-glucose (2.8 mM) for 2 h. Insulin secretory function was then assessed by static incubation of the dispersed islets for 30 min in KRBB in low glucose (2.8 mM), sampling of the supernatant for insulin, shifting the dispersed islets to high glucose (16.7 mM) for 30 min more, and resampling the supernatant. Released human insulin from the supernatant was quantified using a human insulin enzyme-linked immunosorbent assay (ELISA) kit (Mercodia, Uppsala, Sweden). Islets were harvested in 1% NP-40 lysis buffer (1% NP-40, 25 mM HEPES pH 7.4), 10% glycerol, 50 μM sodium fluoride, 10 mM sodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail (Roche, Indianapolis, IN) for evaluation of total protein and insulin content.
2.2. Human EndoC-βH1 cell culture and GSIS assay
Human EndoC-βH1 cells obtained from Dr. Roland Stein (Vanderbilt University) were cultured as described previously [24]. siPAK1- or siCON-transfected cells were incubated in low glucose (2.8 mM) media overnight, then pre-incubated in KRBB containing 2.8 mM glucose for 1 h. Subsequently, the buffer was changed to either 2.8 mM or 16.7 mM glucose and incubated for 30 min. The supernatant was collected for quantification of insulin released, using the human insulin ELISA kit (Mercodia, Cat. # 10–1113-01) or radioimmunoassay (Millipore, Cat. # HI-14K, Billerica, MA).
2.3. Oxygen consumption rate (OCR) measurements
Human EndoC-βH1, MIN6 and INS1–832/13 cells were cultured as previously described [21,24,25], and seeded in a 24-well Agilent Seahorse eXF24 plate. Cells were then transfected with either siPAK1 (Human EndoC-βH1 and MIN6; cat. # SI00605696, INS1–832/13; cat. # SI03082926) or siCON for 48 h (human EndoC-βH1 and INS1–832/13) or 72 h (MIN6). OCR and ECAR were measured using the Seahorse XF Cell Mito Stress test kit (Agilent Technology, Cat. # 103015–100, Santa Clara, CA). Prior to the assay, the growth medium in the well of the XF cell plate was replaced with XF assay medium (Seahorse XF DMEM medium, pH 7.4 (Agilent Technology, Cat. # 103334–100) containing 1 mM sodium pyruvate, 4 mM L-glutamate and 2.8 mM glucose). The sensor cartridge was calibrated, and the cell plate was incubated at 37 °C for 30 min. To estimate the proportion of the basal OCR coupled to ATP synthesis using oligomycin (1 μM), an ATP synthase (Complex V) inhibitor was used. The maximal OCR that the cells could sustain was determined by use of the proton ionophore FCCP (2 μM). Rotenone and antimycin A (RA,0.5 μM) were used to inhibit electron flux through CI and CIII, respectively. The cells were analyzed in the Agilent extracellular flux analysis machine (eXF24) at timed intervals to assess OCR. INS1–832/13 and MIN6 cells were then lysed with 1% NP-40 lysis buffer to quantify protein content for normalization of OCR.
2.4. Mitochondrial membrane potential and ROS analyses
At appropriate time points after transfection, human EndoC-βH1 cells in complete medium were incubated with 500 nM of MitoTracker (Thermo Scientific, cat. # M22426, Rockford, IL) for 30 min, to measure mitochondrial membrane potential (ΔΨm). The medium was removed, and the cells were rinsed with PBS before mounting on coverslips. Images were captured using a Keyence system (BZ-X710) and fluorescence intensity was analyzed using BZ-X Analyzer (Keyence, Itasca, IL). To measure ROS, the CellRox assay was used. In brief, β-cells were seeded in 12-well glass bottom plates, then transfected with either siCON or siPAK1, and incubated in complete medium for 48 h. Cells were stained by adding 5 μM of CellROX Orange Reagent (Thermo Scientific, cat. # C10443) and NucBlue Live Cell Stain (Thermo Scientific, cat. # R37605) to the complete medium for 30 min at 37 °C. Cells were then quickly washed with PBS and imaged using the Keyence microscope with a 20× objective.
2.5. NADH and NADPH measurements
The human EndoC-βH1 cells were transfected with siPAK1 or siCON and incubated in complete medium for 48 h. Cells were quickly rinsed with PBS prior to analysis. Intracellular NAD+/NADH and NADP+/NADPH ratios were determined using the NAD+/NADH assay kit (Abcam, cat. # ab221821, Cambridge, MA) and NADP+/NADPH assay kit (Abcam, cat. # ab65349) according to the manufacturer’s instructions.
2.6. Co-immunoprecipitation and immunoblot analysis
INS1–832/13 cells were transduced with adenoviral rat insulin promoter-driven human PAK1 (AdRIP-hPAK1) or transfected with siPAK1 for 48 h, then were harvested in 1% NP-40 lysis buffer after GSIS. Antibodies to PAK1 (10 μg, cat. # HPA003565, Sigma, St. Louis, MO) and NDUFA12 (NADH: Ubiquinone Oxidoreductase Subunit A12; 10 μg, Abcam, cat. # ab192617) were coupled to Pierce Protein A/G Magnetic Beads with disuccinimidyl suberate (DSS) crosslinking. The antibody-crosslinked beads were incubated with 2 mg (for PAK1) or 1 mg (for NDUFA12) of lysate for 1 h at room temperature (RT). The beads were washed twice with IP Lysis/Wash buffer and once with water. PAK1 and NDUFA12 were eluted from the beads with elution buffer for 5 min at RT and then neutralized with Neutralization buffer (Pierce Crosslink Magnetic IP/Co-IP Kit, Thermo Scientific, cat. #88805, Rockford, IL). The eluates were resolved by 15% SDS-PAGE and analyzed by western blotting for PAK1 (1:1000, Cell Signaling Technology, cat. # 2602, Danvers, MA) and NUDFA12 (1:1000, Abcam, cat. # ab192617).
For other immunoblot analyses, lysate proteins were resolved using 10–15% SDS-PAGE and transferred to polyvinylidene difluoride membranes (PVDF, Bio-Rad, Irvine, CA) for immunoblotting. Membranes were immunoblotted using rabbit anti-PAK1 (1:1000, Cell Signaling Technology, cat. # 2602), OXPHOS (1:2000, Abcam, cat. # ab110343), p-eIF2α (1:1000, Cell Signaling, cat. # 3398), eIF2α (1:1000, Cell Signaling, cat # 2103), CHOP (1:1000, Cell Signaling, cat. # 2895), GAPDH (1:5000, Abcam, cat. # 8245) and tubulin (1:5000, Sigma, cat. # T9026). Goat anti-mouse and anti-rabbit horseradish peroxidase secondary antibodies were obtained from Bio-Rad and used at 1:10,000 or 1:20,000. Immunoreactive bands were visualized with enhanced chemiluminescence (ECL) or ECL prime (GE Healthcare, Buckinghamshire, UK) reagents and imaged using a Chemi-Doc Touch system (Bio-Rad).
2.7. RNA extraction and quantitative real-time PCR analysis
RNA was extracted using RNeasy Plus Mini kit according to the manufacturer’s protocol (Qiagen, cat. #74136). RNA (50 ng) was used for quantitative real-time PCR using the QuantiTect SYBR Green one-step qRT-PCR kit (Qiagen, cat. # 204243). The following primers were used: human PAK1 (Qiagen Cat. #QT00068306), rat GAPDH (Qiagen Cat. # QT00188633), rat PAK (forward: 5′-GGAGTTTACGGGGATGCCA GAA-3′, reverse: 5′-CAGCCTGTGGGTTTTTGTTC-3′), rat NDUFA12 (forward: 5′-CATTCTGGGATGTGGATGGAAG-3′, reverse 5′-GCAGTCAGTGG TTTTGTTGTTGG-3′). Real-time PCR was carried out for 40 cycles using the Quant Studio 3 (ThermoFisher). cDNA synthesis was carried out at 50 °C for 30 min and 95 °C for 2 min, then each cycle was run at 95 °C for 15 s, 56 °C for 30 s and 72 °C for 30 s.
2.8. β-Cell-specific PAK1 knock out mice (βPAK1-iKO)
Mice carrying a floxed site in the PAK1 gene (PAK1fl/fl mice) [26] were crossed with mouse insulin promoter (MIP)-Cre+/ER™ transgenic mice (kind gift from Dr. Lou Philipson, University of Chicago), all on the C57Bl/6 J background. Progeny of this cross express a tamoxifen-dependent CRE protein exclusively in insulin-producing β-cells. The mouse genotypes were determined by PCR. After weaning, the mice had free access to water and a standard chow diet ad libitum. Adult mice (9 to 10 weeks old, male and female) were gavaged with tamoxifen (TM; 2 mg/40 g of body weight dissolved in corn oil as the vehicle, Sigma, St. Louis, MO) for 5 consecutive days to activate the Cre in islet β-cells. Control mice carried floxed PAK1 and MIP-Cre but were provided vehicle only (corn oil) to prevent induction. All animal procedures/protocols were approved by the Institutional Animal Care and Use Committees at Indiana University School of Medicine (Indianapolis, IN) and City of Hope (Duarte, CA).
2.9. Intraperitoneal glucose and insulin tolerance tests
Three or four weeks after TM administration, the mice were fasted for 6 h prior to a 2 g/kg body weight intraperitoneal glucose tolerance test (IPGTT) as described [20]. The following week, the mice were fasted for 6 h prior to being injected intraperitoneally with insulin (Humulin R, Eli Lilly and company, Indianapolis, IN) at 0.75 U/kg body weight for insulin tolerance testing (ITT). Glucose measurements were performed using blood sampled from the tail vein and a HemoCue Glucose 201 Analyzer (Angelholm, Sweden).
2.10. Plasma insulin response to an acute glucose challenge
All mice were fasted for 6 h prior to an acute challenge of 2 g/kg body weight glucose injected intraperitoneally. To measure the plasma insulin levels, blood was collected from the tail vein prior to and 10 min after the glucose injection, and the resultant plasma was analyzed using a sensitive rat insulin radioimmunoassay kit (Millipore, cat. # SRI-13K).
2.11. Mouse islet isolation, immunoblotting and transmission electron microscopy (TEM)
Mouse pancreatic islets were isolated as previously described from 16 to 18-week-old male and female mice, tabulated separately. The islets were subsequently harvested in 1% NP-40 lysis buffer for evaluation of protein content for immunoblotting. For TEM, freshly isolated mouse pancreatic islets were placed immediately in 0.1 M cacodylate-buffer, pH 7.3, 2.5% glutaraldehyde, and 4% paraformaldehyde. After fixation for 2 h RT, the specimens were stored at 4 °C and later post-fixed for 1 h in 0.1 M cacodylate buffer, 1% OsO4. After rinsing in distilled water, the specimens were en bloc stained for 1 h in 1% uranyl acetate in distilled water. The specimens were dehydrated as follows: 30%, 50%, 60%, 70%, 80%, and 95% ethanol for 10 min each, then three changes of 100% dry ethanol for 10 min each, followed by transfer into 100% propylene oxide (PPO) for 10 min. For resin infiltration, the specimens were transferred to 2:1 PPO/Epon 812 resin for 2 h, then to 1:1 PPO/resin overnight, then to 1:2 PPO/resin for 2 h, and to 100% resin for 3–4 h RT. The specimens were transferred to resin molds and placed at 60 °C for 48 h. Ultrathin (70-nm) sections were cut with a Leica Ultracut, picked up on copper grids, post-stained with lead citrate and viewed with a FEI Tecnai T12 microscope operating at 120 KV. Mitochondrial numbers were counted in a blinded fashion.
2.12. TUNEL staining
The TUNEL In Situ Cell Death Detection Kit, Fluorescein (Roche, Cat. # 11684795910, Mannheim, Germany) was used to stain the apoptotic cells. The pancreatic sections were immunostained with guinea pig anti-insulin (1:100, Agilent DAKO, Cat. # A0564, Santa Clara, CA) and Alexa Fluor 488 goat anti-guinea pig (1:100, Invitrogen, Cat. # A-11073) secondary antibody was used for detection of insulin. All sections were scanned using a Keyence microscope. Results are expressed as the percentage of positive cells from TUNEL staining relative to the total number of insulin-positive cells. TUNEL stained cells were counted in a blinded fashion.
2.13. hPAK1 transduction using adenovirus in T2D human islets
T2D human islets were transduced with 100 MOI of AdRIP-hPAK1 or AdRIP-CON adenovirus for 1 h, washed with PBS, and incubated in complete CMRL media for 48 h [25]. These islets were subsequently used in perifusion analyses as previously described [27] and retrieved for assessment of insulin content (RIA, Millipore, Cat. # HI-13K) and PAK1 protein levels (immunoblotting).
2.14. Statistical analysis
All datasets were evaluated for statistical significance using Student’s t-test, or ANOVA with Tukey’s post-hoc test for multiple comparisons, in GraphPad Prism (GraphPad Software, Version 8, La Jolla, CA). The results are expressed as the mean ± S.E.M.
3. Results
3.1. Impaired GSIS is associated with PAK1 depletion
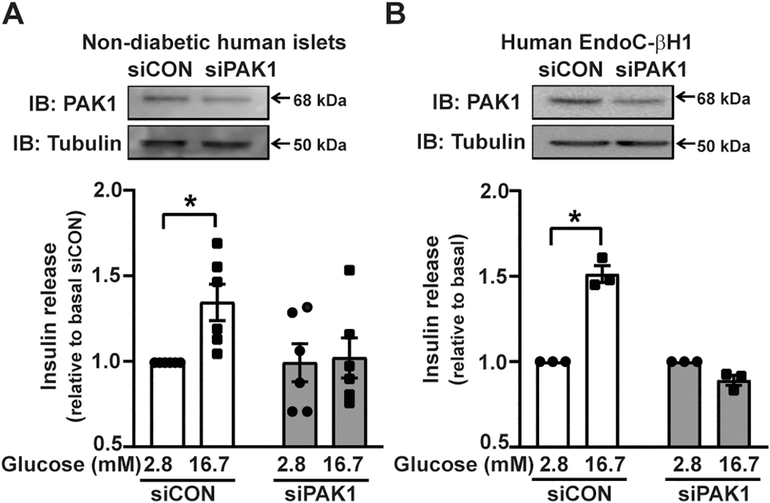
To test whether PAK1 is required for β-cell function in human islets and human EndoC-βH1 cells, we transiently transfected siPAK1 into dispersed non-diabetic human islets or human EndoC-βH1 cells and cultured them for 3 or 2 days, respectively, to generate PAK1 knockdown (PAK1-kd) cells. Depletion of PAK1 by siRNA was confirmed by immunoblot analysis (Human islets: 0.6 ± 0.1 = ~40% kd; Human EndoCβH1: 0.6 ± 0.1 = ~40% kd) and resulted in blunted GSIS compared to cells transfected with siCON (Fig. 1A and B). These data suggest that human EndoC-βH1 cells replicate the requirement for PAK1 observed for primary human islets.
Fig. 1.
PAK1 depletion in human β-cells ablates glucose stimulated insulin secretion (GSIS). (A) Dispersed human islets were transfected with siRNA (siPAK1 or siCON) and insulin release was measured in response to 2.8 mM or 16.7 mM glucose exposure. Insulin released into the buffer was normalized to total protein content in the corresponding cell lysates to obtain ng/insulin released/mg total protein for each data point per set of independent human islet donor batches (n = 6 donor batches). (B) Human EndoC-βH1 cells (n = 3 experiments using independent passages of cells), relative to cells expressing control siRNA (siCON). Each experiment is normalized to protein concentration, to obtain ng insulin/mg total protein. For comparisons between groups, data are shown as the stimulation index, glucose-stimulated insulin secretion/basal insulin release, for each independent experiment/cell passage. Data are shown as the mean ± SEM; *p < 0.05 using Student’s t-test.
3.2. PAK1 deficiency decreases mitochondrial respiration in pancreatic β-cells
The β-cell mitochondria generates ATP, which facilitates insulin exocytosis [28]. In addition, PAK1 kd in MIN6 cells resulted in increased cytochrome C [19], implicating PAK1 in/upstream of mitochondrial function. To expand upon this, we assessed mitochondrial bioenergetics in PAK1-kd β-cells by determining oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) following the sequential addition of pharmacological inhibitors to probe the function of individual components of the respiratory chain. PAK1-kd significantly decreased the basal respiration, spare respiratory capacity and ATP production, as compared to control siRNA, in human EndoC-βH1 cells in XF assay medium cells under basal conditions (Fig. 2A–B). Rat INS1–832/13 (Fig. 2C–D) and mouse MIN6 β-cell lines (Fig. 2E–F) showed similar decreases in basal respiration and ATP production. PAK1-kd β-cells showed a slight reduction in ECAR compared to control cells but the change in area under the curve was not significant (Supplementary Fig. 1), consistent with the conditions used [29]. Human EndoC-βH1 cell recovery following Seahorse analyses for protein normalization was impeded by cell non-adherence during the final PBS washes. Nevertheless, the OCR results from the human EndoC-βH1 cells showed the same pattern of effect as the INS1–832/13 and MIN6 cells for samples where protein normalization was able to be performed.
Fig. 2.
PAK1 depletion consistently disrupts mitochondrial function in multiple species of β-cells. Mitochondrial function was measured in human EndoC-βH1 (A–B), INS1–832/13 (C–D) and MIN6 (E–F) β-cells transfected with siPAK1 or siCON. Individual OCR parameters (Basal respiration, Proton Leak, Spare respiratory capacity and ATP Production) were calculated and expressed relative to the siCON. Data are shown as mean ± SEM (human EndoC-βH1, n = 5; INS1–832/13, n = 5; MIN6, n = 3; with all replicates representing independent passages of cells). Statistical significance was evaluated using ANOVA (A, C and E), or paired two-tailed Student’s t-test (B, D and F); *p < 0.05, **p < 0.01, ***p < 0.005 and ****p < 0.0001, versus siCON.
In addition, PAK1-kd in human EndoC-βH1 cells resulted in mitochondrial dysfunction, as determined by reduced staining intensity of MitoTracker (Fig. 3A–B), which labels the mitochondria within live cells. To determine if the observed reduction in mitochondrial respiration was caused by defective oxidative phosphorylation (OXPHOS) by the ETC, we immunoblotted for potential changes in the levels of mitochondrial complexes. The total OXPHOS antibody cocktail recognizes epitopes on CI (NDUFB8; NADH: Ubiquinone Oxidoreductase Subunit B8), complex II (CII, SDHB; Succinate Dehydrogenase Complex Iron Sulfur Subunit B), CIII (UQCRC2; Ubiquinol-Cytochrome C Reductase Core Protein 2), complex IV (CIV, MTCO1; Mitochondrially Encoded Cytochrome C Oxidase 1) and complex V (CV, ATP5α; ATP synthase F1 subunit alpha, mitochondrial). PAK1-kd cells showed significantly reduced protein levels of CI, CIII and CIV, as compared to control cells (Fig. 3C–D). Reductions in some of the same ETC proteins were detected in siPAK1-kd INS1–832/13 and MIN6 β-cells (Supplementary Fig. 2A–B). PAK1-kd reduced the PAK1 levels by ~30–50% (Fig. 3D, Suppl. Fig. 2A–B). These data suggest that PAK1 in human and rodent β-cells is required for regulating the protein levels of select ETC components to maintain mitochondrial function.
Fig. 3.
In human β-cells, PAK1 depletion disrupts mitochondria membrane potential, electron transport chain protein expression and redox balance, and elevates ROS. (A) siRNA-transfected human EndoC-βH1 cells were incubated with MitoTracker and fixed. The mitochondria (shown pseudocolored white) were visualized using Keyence fluorescence microscopy. (B) Quantitation of MitoTracker staining intensity in human EndoC-βH1 cells transfected with either siCON or siPAK1 (PAK1-kd). At least three randomly selected microscopic fields were used to calculate staining in each of the three independent cell passages. Values are given as mean ± SEM, *p < 0.05 by Student’s t-test. (C) Protein expression of ETC CI (NUDFB8), CII (SDHB), CIII (UQCRC2), CIV (MTCO1) and CV (ATP5A) were determined by immunoblot in human EndoC-βH1 cells transfected with either siCON or siPAK1. (D) The bar graph quantitation of ETC immunoblots represents the mean ± SEM, for PAK1-kd and siCON human EndoC-βH1 cells. Each ETC factor in siPAK1-kd lysates was normalized to the paired siCON band set equal to one in each of the five independent sets of cell passages. (E) CellRox intensity (shown, pseudocolored white) was visualized by Keyence fluorescence microscopy in three randomly selected fields in each of the independent passages of INS1–832/13 cells transfected with siPAK1 or siCON. H2O2 treatment serves as positive control in each passage for CellRox detection of ROS. (F) Quantitation of CellRox staining intensity in at least three randomly selected microscopic fields were used to calculate staining. Values are given as mean ± SEM, *p < 0.05 by Student’s t-test. (G) The total NAD+/NADH levels, as well as the total NADPH and NADP+ levels (H–I), and the NADP+/NADPH ratio (J), are shown for siPAK1 and siCON cells. The NADP+/NADPH ratio was calculated (NADPtotal-NADPH)/NADPH. *p < 0.05, **p < 0.01, ***p < 0.005 and ****p < 0.0001 vs siCON by Student’s t-test.
Moreover, PAK1-kd in INS1–832/13 cells resulted in elevated ROS levels, as detected by increased CellRox intensity (Fig. 3E–F). The levels of NAD+/NADH and NADP+/NADPH which are critical in regulating the cellular redox state, energy metabolism, mitochondrial function, are significantly impaired in diabetic tissues [30–32]. We found that the cellular NAD+/NADH ratios and NADPH levels were significantly reduced, and the NADP+ and NADP+/NADPH ratios were significantly increased, in PAK1-kd human EndoC-βH1 cells compared to control cells (Fig. 3G–J). These results suggest that deficient PAK1 results in elevated ROS, with redox imbalance resulting from the elevated production of NADH and decreased NADPH.
3.3. β-Cell specific PAK1 knock out mice (βPAK1-iKO) have blunted islet responsiveness
To evaluate the in vivo ramifications of β-cell-selective PAK1 deletion, we generated an inducible βPAK1-iKO mouse model, which allowed the evaluation of acute PAK1 deficiency only in adult mice, circumventing any developmental effects of PAK1 loss. This new model was required because of the confounding effect of peripheral insulin resistance in the previously studied whole body PAK1 knockout mice [15,21], which prevented clear interpretation of whether PAK1 is required for β-cell function. PAK1 levels were vastly reduced in TM-induced βPAK1-iKO mouse islets relative to islets from uninduced (CON: PAK1fl/fl; Cre/+) mice (Fig. 4A); PAK1 levels were unaffected in the cerebellum or hypothalamus, indicating lack of off-target effects.
Fig. 4.
βPAK1-iKO mice show defective islet response to glucose in vivo. (A) Efficient PAK1 deletion in β-cells was determined by western blot, with nooff-target effects in hypothalamus or cerebellum. (B) βPAK1-iKO mice showed an impaired insulin response following an acute 10 min glucose challenge compared to control mice (CON = fl/fl;cre/+, vehicle-gavaged). (C) βPAK1-iKO mice show an impaired stimulation index (glucose-stimulated/fasted insulin) compared to control (CON) mice. (D) Intraperitoneal glucose tolerance test (IPGTT) results and area under the IPGTT curve (AUC) for (D, E) male and (G, H) female βPAK1-iKO or control mice. Intraperitoneal insulin tolerance test (IPITT) results for male (F) and female (I) βPAK1-iKO or control mice. Data are shown as mean ± SEM for 4–7 mice. Statistics were calculated using two-way ANOVA (B,D,F,G and I), or Student’s t-test (C, E and H) *p < 0.05, **p < 0.01, ***p < 0.005 and ****p < 0.0001.
βPAK1-iKO mice showed significantly blunted plasma insulin levels following an acute glucose challenge, compared with control mice (Fig. 4B), as demonstrated by reduction of the stimulation index (glucose-stimulated plasma insulin level at 10 min/basal plasma insulin level at 0 min) (Fig. 4C). βPAK1-iKO mice were glucose intolerant (Fig. 4D–E, male; and G–H, female), but insulin tolerant (Fig. 4F, male; and I, female). No significant differences in overall body weight or weights of organs/tissues were detected between βPAK1-iKO and control mice (Supplementary Table 2). These data confirm the requirement for PAK1 in the β-cells of adult mice in vivo, and support further mechanistic evaluation of the requirement for PAK1 in regulating β-cell responsiveness.
3.4. Increased β-cell death correlates with decreased mitochondrial number in islet β-cells of the βPAK1-iKO mice
To determine the mechanism underlying the putative loss of β-cell glucose responsiveness in the βPAK1-iKO mice, we used TUNEL staining to detect apoptotic β-cells. βPAK1-iKO islets (from fed mice) contained significantly more TUNEL-positive insulin-positive cells (yellow arrows pointing to red nuclei), indicative of apoptotic β-cells, than islets from control mice (Fig. 5A). Because mitochondrial dysfunction/loss is known to underlie β-cell failure and enhanced β-cell apoptosis [33], we evaluated mitochondrial numbers in βPAK1-iKO islet β-cells using TEM. βPAK1-iKO mice were evaluated 8–9 weeks post-TM injection to simulate chronic PAK1 loss in the β-cells. Islet β-cells from the βPAK1-iKO mice contained significantly fewer mitochondria as compared to control mouse islets (Fig. 5B), both cumulatively across cohorts, as well as within cohorts (Supplementary Fig. 3). These data suggest that PAK1 in the β-cell is required to support mitochondrial content and that the loss of mitochondrial content may underscore the β-cell apoptosis observed in the βPAK1-iKO mice.
Fig. 5.
PAK1-deficient β-cells show increased apoptosis and reduced mitochondrial number. (A) TUNEL immunofluorescence staining (left) and quantification of TUNEL-positive cells as a percentage of total β-cells (right) from pancreata of fed control (CON) or βPAK1-iKO mice. Yellow arrows denote TUNEL-positive: insulin-positive β-cells. Scale bar = 50 μm. (B) TEM of isolated islets from βPAK1-iKO and control mice. Mitochondria numbers were counted in a blinded manner, from 10 to 18 sections/cohort (2 pancreata per group per cohort). TEM scale bar = 2 μm. *p < 0.05, ****p < 0.0001 by Student’s t-test.
3.5. PAK1 enrichment reduces ER stress in T2D human islets
Since T2D human islets are deficient in PAK1, we evaluated whether restoration of PAK1 would ameliorate β-cell dysfunction. To test this, T2D human islets were transduced with AdRIP-hPAK1 to drive the βcell-selective expression of human PAK1 or the control vector, and levels of the ER stress markers p-eIF2α and CHOP were determined by immunoblotting. eIF2α is phosphorylated by PERK, a kinase sensor of stress in the ER lumen, while CHOP is elevated with prolonged activation of the UPR. AdRIP-hPAK1-expressing T2D human islets showed significantly reduced ratios of phosphorylated (p)-eIF2α/total eIF2α, resulting from reduced p-eIF2α, and CHOP expression (n = 4 independent human donor islet batches) (Fig. 6A–B). AdRIP-hPAK1-expressing T2D human islets also showed improved GSIS in perifusion analyses (Fig. 6C–E). Notably, in the human T2D islets available for these analyses to date, detection of ETC proteins has revealed increased levels of CI, CIII and CIV in AdRIP-PAK1- vs control (CON)-transduced T2D islets (Supplementary Fig. 4), the same ETC proteins that were reduced in siPAK1-kd human β-cells (Fig. 3C–D). These results suggest that the increase of PAK1 in T2D human islet β-cells can ameliorate ER stress and improve T2D human islet β-cell GSIS function.
Fig. 6.
PAK1 enrichment in T2D human islets reduces endoplasmic reticulum (ER) stress markers and enhances insulin secretion. (A) ER stress was assessed using immunoblotting analysis of phospho- and total eIF2α, along with CHOP, normalized to tubulin in AdRIP-human (h)PAK1 or -CON transduced T2D human islets. (B) The bar graphs represent immunoblot quantification of four independent batches of human T2D donor islets. **p < 0.01 vs AdRIP-CON by Student’s t-test. AdRIP-hPAK1 overexpression in three independent batches of T2D human islets results in increased insulin release in perifusion analyses. Insulin was measured by radioimmunoassay in cases I (C), II (D) and III (E).
3.6. PAK1 associates with the mitochondria ETC complex 1 subunit NDUFA12 and regulates its cellular protein abundance in β-cells
To determine how PAK1 regulates mitochondria and ETC proteins, mass spectrometry analysis was used to identify potential PAK1 binding partners in clonal β-cells. MIN6 cells were transduced to express hPAK1 or GFP (control) and anti-GFP- or anti-PAK1 antibodies used to capture interacting proteins from the cell lysates by co-immunoprecipitation. Of 151 proteins identified, 22 proteins were detected in PAK1-overexpressing lysates that were not in GFP-overexpressing lysates, with 16 of these being related to mitochondria (Supplementary Table 3). NDUFA12, a CI subunit protein, was identified as a candidate PAK1 interaction partner; this PAK1NDUFA12 association was validated using PAK1-conjugated magnetic beads in co-immunoprecipitation studies (Fig. 7A). siPAK1-kd lysates served as controls for the specificity of the NDUFA12 signal, as did the IgG control reaction. Interestingly, siPAK1-kd input lysates showed a consistent attenuation of NDUFA12 protein level relative to that in siCON lysates (Fig. 7B); hPAK1 expression was without effect (Fig. 7C). The siPAK1-related attenuation of NDUFA12 protein was determined to be a post-transcriptional change, given that the NDUFA12 mRNA levels remained unchanged in cells with depleted or increased PAK1 expression levels (Fig. 7D, Supplementary Fig. 5). These data suggest that PAK1 is required for maintaining normal NDUFA12 protein levels in β-cells. This result is consistent with a model wherein PAK1 depletion suppresses NDUFA12 levels, thereby reducing mitochondrial function, leading to ER stress and β-cell death.
Fig. 7.
PAK1 interacts with NDUFA12 and regulates its protein abundance in β-cells. (A) INS1–832/13 cells were transduced with AdRIP-hPAK1 or AdRIP control vector (CON) and left unstimulated (2.8 mM glucose) or stimulated with 20 mM glucose for 30 min; cells transfected with siPAK1 were used as control for specificity. AdRIP-hPAK1 and siPAK1 lysates were divided into two coimmunoprecipitation reactions containing anti-PAK1 or anti-NDUFA12. IgG control immunoprecipitation reaction used pooled un/stimulated AdRIP-hPAK1 lysates. Results are representative of three independent sets of cell lysates used in immunoprecipitation experiments. Vertical dashed lines indicate splicing of lanes from within the same gel exposure. (B) Bar graph quantitation of NDUFA12 protein levels detected in siPAK1 or siCon lysates, *p < 0.05. (C) Bar graph quantitation of NDUFA12 protein levels in the hPAK1 overexpressing input lysates used in (A); no significant differences were detected using ANOVA. (D) NDUFA12 mRNA levels detected by qRT-PCR. Cells transfected with siPAK1 (with siCon as control), or transduced with AdRIP-hPAK1 (with AdRIP-vector as control) for 48 h were harvested from complete medium, washed with PBS, and RNA extracted. N.S., not significant.
4. Discussion
In the current study, we have demonstrated that β-cell-specific loss of PAK1 is sufficient to induce glucose intolerance and β-cell dysfunction in the absence of peripheral insulin resistance. Furthermore, PAK1 is required for GSIS locally in human islet β-cells and human EndoC-βH1 cells. PAK1-deficient β-cells show reduced mitochondrial number, reduced mitochondrial respiration, disrupted redox balance, elevated ROS, as well as reduced levels of the ETC proteins CI, CIII and CIV and increased apoptosis. The same three ETC proteins were increased by PAK1-enrichment in β-cells of T2D human islets, concordant with the decreased appearance of ER stress markers, and improved GSIS. Mechanistic analyses revealed that PAK1 binds to and regulates NDUFA12, the ETC CI assembly and stabilizing subunit, identifying a key proximal role for PAK1 in the formation of mitochondrial ETC super complexes (CI-CIII and CI-CIII-CIV) and mitochondrial ETC function [34,35]. Downstream disturbances in OCR, ROS, and cytochrome C release observed in PAK1-deficient β-cells is consistent with this new role, and these are further associated with loss of functional β-cell mass and apoptosis.
A role for PAK1 in proliferation and survival has been reported in various cell types [36]. For example, PAK1 can down-regulate pro-apoptotic factors such as FKHR, Bad, and BimL [37,38]. In early β-cell studies, PAK1 was implicated in proliferation and survival since PAK1 depletion was shown to increase Bad protein levels, increase release of mitochondrial cytochrome C into the cytosolic fraction, and reduce Survivin levels, consistent with an anti-apoptotic role for PAK1 in the β-cell [19,20]. Later studies performed using classic PAK1 knockout mice fed a western diet (45% palmitate) supported this concept, showing decreased β-cell mass [21]. However, evaluating the role of PAK1 in the β-cell was confounded by two factors: 1) the mice were overtly insulin resistant due to the diet, and 2) PAK1 deletion during development may have influenced the adult phenotype observed [21]. Our current work with the new β-cell-specific inducible βPAK1-iKO mice clearly demonstrates that PAK1 depletion increases β-cell apoptosis, and that this occurs concurrently with decreased mitochondrial number in islet β-cells, in vivo.
One key finding of the present study is that PAK1 is required to maintain normal levels of ETC proteins. This loss of ETC proteins may underlie the observed reduction in OCR in PAK1-kd cells since it has been shown that decreased ETC capability can also increase ROS production [39]. High levels of ROS impairs vital enzymes such as NADH dehydrogenase, cytochrome c oxidase, and ATP synthase, resulting in the shutdown of mitochondrial energy production [40]. Excess ROS generation over time impairs the cell’s ability to mount an effective antioxidant response [41], and β-cells are already more susceptible to ROS attack due to inherent lack of antioxidant enzymes [42]. Recent interest has been placed on the role of reducing equivalent NADPH. For example, stimulatory glucose increases the NADPH level and, in parallel, enhances insulin secretion [43,44]. By contrast, our studies here show that PAK1-kd cells had decreased NADPH levels and increased NADP+, resulting in an elevated NADP+/NADPH ratio. PAK1-kd cells also had reduced CI and CIII levels, and low levels of CI and CIII are known to create an environment wherein a reduced ATP production and respiration rate are reported to contribute to ROS generation [39].
Mitochondrial dysfunction and ER stress are associated with loss of GSIS in pancreatic β-cells [45,46]. The mitochondrion and ER are tightly interconnected through the mitochondria-associated ER membrane [47]. Mitochondria-ER miscommunication can promote a perpetuated state of oxidative stress, unfolded protein accumulation, and mitochondrial and ER dysfunction, which progressively impairs β-cell function and increases β-cell death [48]. In support of this interconnection between mitochondria and the ER, we found that the restoration of PAK1 in T2D human islets reduces markers of ER stress; pilot results suggest that this may co-occur along with increasing the three ETC proteins that were diminished in PAK1-kd cells (Supplemental Fig. 4).
Despite the advances made in the current study expanding our knowledge of PAK1 playing a required role in supporting ETC levels and redox status, and linkage of PAK1 to ameliorating ER stress in human T2D islets, one limitation is in determining how PAK1 controls levels of NDUFA12, a mitochondrial ETC protein, given that PAK1 is not reported as a mitochondrial protein. One possibility is that PAK1 serves as a transport scaffold for NDUFA12, consistent with other reports of protein scaffolding by PAK1 in other cell types [49]. For example, PAK1 may facilitate the delivery of newly biosynthesized NDUFA12 protein to the mitochondria where it is involved in the assembly of CI. This concept is supported by our data showing that NDUFA12 mRNA levels were unchanged by PAK1 depletion, whereas protein levels of NDUFA12 were attenuated. Moreover, RNAseq analyses of PAK1 overexpression or depletion failed show evidence of its directive control of ETC genes, despite a reported ability of PAK1 to translocate to the nucleus in other cell types [50]. A second limitation is in obtaining enough T2D human islets for detection and correlation of the amelioration of ER stress and dysfunctional GSIS following PAK1 enrichment, with improved mitochondrial ETC protein levels and mitochondrial function. T2D human islets are rarely available for study, and when they are available, have inherently variable phenotypes, due to the differing stages of disease of the donors and the genetic/environmental diversity affiliated with T2D.
In summary, we have demonstrated the importance of β-cell-localized PAK1 in maintaining functional β-cell mass. The mechanism of impaired GSIS by PAK1 depletion in β-cells involves the need for PAK1 in a proximal role supporting mitochondrial function and maintaining the cellular redox balance via ETC complex proteins I, III and IV. – These data provide the first evidences for the following new concept: PAK1 → NDUFA12 mitochondrial and CI complex assembly → mitochondrial structure/function → GSIS and β-cell mass. Future studies are underway to discern the PAK1-linked signaling/scaffolding mechanism underlying these beneficial effects as a means to derive new targets for diabetes treatment and prevention.
Supplementary Material
Acknowledgements
We thank Dr. Lou Philpson for sharing the MIP-CreER™ mouse breeding stock and Dr. Roland Stein for providing the human EndoC-βH1 cells, on behalf of P. Ravassard. Human islets were supplied by the Southern California Islet Cell Resource Center (City of Hope), and by the Integrated Islet Distribution Program (IIDP). Nancy Linford, PhD, provided editing assistance.
Funding
This study was supported by grants from the National Institutes of Health (DK067912, DK112917 and DK102233 to D.C.T.). Research reported in this publication also includes work performed in the City of Hope Islet Service Center, the Proteomics Core and the Electron Microscopy Core, supported by the National Cancer Institute of the National Institutes of Health under award number P30CA33572.
Abbreviations:
- PAK1-KO
whole-body Pak1 knock out mice
- βPAK1-iKO
β-cell-specific inducible PAK1 KO mice
- TM
tamoxifen
- MIP
mouse insulin promoter
- RIP
rat insulin promoter
- GSIS
glucose-stimulated insulin secretion
- OCR
oxygen consumption rate
- ITT
insulin tolerance test
- IPGTT
intraperitoneal glucose tolerance test
- NDUFA12
NADH Ubiquinone Oxidoreductase Subunit A12
Footnotes
Declaration of competing interest
The authors declare no conflicts of interest.
References
- [1].CDC. Centers for disease control and prevention: national diabetes statistics report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. [Google Scholar]
- [2].Tripathy D, Eriksson KF, Orho-Melander M, Fredriksson J, Ahlqvist G, Groop L. Parallel manifestation of insulin resistance and beta cell decompensation is compatible with a common defect in type 2 diabetes. Diabetologia. 2004;47:782–93. [DOI] [PubMed] [Google Scholar]
- [3].Cohrs CM, Panzer JK, Drotar DM, Enos SJ, Kipke N, Chen C, et al. Dysfunction of persisting β cells is a key feature of early type 2 diabetes pathogenesis. Cell Rep. 2020;31:107469. [DOI] [PubMed] [Google Scholar]
- [4].Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52: 102–10. [DOI] [PubMed] [Google Scholar]
- [5].Salunkhe VA, Veluthakal R, Kahn SE, Thurmond DC. Novel approaches to restore beta cell function in prediabetes and type 2 diabetes. Diabetologia. 2018;61: 1895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 1863;2017:1066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Alexandre A, Reynafarje B, Lehninger AL. Stoichiometry of vectorial H+ movements coupled to electron transport and to ATP synthesis in mitochondria. Proc Natl Acad Sci U S A. 1978;75:5296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Scialo F, Fernandez-Ayala DJ, Sanz A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front Physiol. 2017;8: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rocha M, Apostolova N, Diaz-Rua R, Muntane J, Victor VM. Mitochondria and T2D: role of autophagy, ER stress, and inflammasome. Trends Endocrinol Metab. 2020; 10:725–41. [DOI] [PubMed] [Google Scholar]
- [10].Kennedy ED, Maechler P, Wollheim CB. Effects of depletion of mitochondrial DNA in metabolism secretion coupling in INS-1 cells. Diabetes. 1998;47:374–80. [DOI] [PubMed] [Google Scholar]
- [11].Simmons RA, Suponitsky-Kroyter I, Selak MA. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to beta-cell failure. J Biol Chem. 2005;280:28785–91. [DOI] [PubMed] [Google Scholar]
- [12].Lee JW, Kim WH, Yeo J, Jung MH. ER stress is implicated in mitochondrial dysfunction-induced apoptosis of pancreatic beta cells. Mol Cells. 2010;30:545–9. [DOI] [PubMed] [Google Scholar]
- [13].Ghosh R, Colon-Negron K, Papa FR. Endoplasmic reticulum stress, degeneration of pancreatic islet β-cells, and therapeutic modulation of the unfolded protein response in diabetes. Molecular metabolism. 2019;27s (S60-s8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fonseca SG, Burcin M, Gromada J, Urano F. Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr Opin Pharmacol. 2009;9:763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang Z, Oh E, Clapp DW, Chernoff J, Thurmond DC. Inhibition or ablation of p21-activated kinase (PAK1) disrupts glucose homeostatic mechanisms in vivo. J Biol Chem. 2011;286:41359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yoder SM, Dineen SL, Wang Z, Thurmond DC. YES, a Src family kinase, is a proximal glucose-specific activator of cell division cycle control protein 42 (Cdc42) in pancreatic islet beta cells. J Biol Chem. 2014;289:11476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chiang YA, Shao W, Xu XX, Chernoff J, Jin T. P21-activated protein kinase 1 (Pak1) mediates the cross talk between insulin and beta-catenin on proglucagon gene expression and its ablation affects glucose homeostasis in male C57BL/6 mice. Endocrinology. 2013;154:77–88. [DOI] [PubMed] [Google Scholar]
- [18].Kalwat MA, Yoder SM, Wang Z, Thurmond DC. A p21-activated kinase (PAK1) signaling cascade coordinately regulates F-actin remodeling and insulin granule exocytosis in pancreatic beta cells. Biochem Pharmacol. 2013;85:808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang Z, Thurmond DC. PAK1 limits the expression of the pro-apoptotic protein bad in pancreatic islet β-cells. FEBS OpenBio. 2012:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen YC, Fueger PT, Wang Z. Depletion of PAK1 enhances ubiquitin-mediated Survivin degradation in pancreatic beta-cells. Islets. 2013;5:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ahn M, Yoder SM, Wang Z, Oh E, Ramalingam L, Tunduguru R, et al. The p21-activated kinase (PAK1) is involved in diet-induced beta cell mass expansion and survival in mice and human islets. Diabetologia. 2016;59:2145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kowluru A, Veluthakal R, Rhodes CJ, Kamath V, Syed I, Koch BJ. Protein farnesylationdependent Raf/extracellular signal-related kinase signaling links to cytoskeletal remodeling to facilitate glucose-induced insulin secretion in pancreatic beta-cells. Diabetes. 2010;59:967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu X, Zhang Q, Wang X, Zhu J, Xu K, Okada H, et al. Survivin is required for beta-cell mass expansion in the pancreatic duct-ligated mouse model. PloS one. 2012;7: e41976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ravassard P, Hazhouz Y, Pechberty S, Bricout-Neveu E, Armanet M, Czernichow P, et al. A genetically engineered human pancreatic beta cell line exhibiting glucose-inducible insulin secretion. J Clin Invest. 2011;121:3589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Oh E, Kalwat MA, Kim MJ, Verhage M, Thurmond DC. Munc18–1 regulates first-phase insulin release by promoting granule docking to multiple syntaxin isoforms. J Biol Chem. 2012;287:25821–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu W, Zi M, Naumann R, Ulm S, Jin J, Taglieri DM, et al. Pak1 as a novel therapeutic target for antihypertrophic treatment in the heart. Circulation. 2011;124:2702–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem. 2007;282:9536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wollheim CB, Maechler P. Beta-cell mitochondria and insulin secretion: messenger role of nucleotides and metabolites. Diabetes. 2002;51(Suppl. 1):S37–42. [DOI] [PubMed] [Google Scholar]
- [29].Mookerjee SA, Goncalves RLS, Gerencser AA, Nicholls DG, Brand MD. The contributions of respiration and glycolysis to extracellular acid production. Biochim Biophys Acta. 1847;2015:171–81. [DOI] [PubMed] [Google Scholar]
- [30].Wu J, Luo X, Thangthaeng N, Sumien N, Chen Z, Rutledge MA, et al. Pancreatic mitochondrial complex I exhibits aberrant hyperactivity in diabetes. Biochem Biophys Rep. 2017;11:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–54. [DOI] [PubMed] [Google Scholar]
- [32].Wu J, Jin Z, Yan LJ. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol. 2017;11:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ma ZA, Zhao Z, Turk J. Mitochondrial dysfunction and beta-cell failure in type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rak M, Rustin P. Supernumerary subunits NDUFA3, NDUFA5 and NDUFA12 are required for the formation of the extramembrane arm of human mitochondrial complex I. FEBS Lett. 2014;588:1832–8. [DOI] [PubMed] [Google Scholar]
- [35].Koopman WJ, Nijtmans LG, Dieteren CE, Roestenberg P, Valsecchi F, Smeitink JA, et al. Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation. Antioxid Redox Signal. 2010;12:1431–70. [DOI] [PubMed] [Google Scholar]
- [36].Bokoch GM. Biology of the P21-activated kinases. Annu Rev Biochem. 2003;72: 743–81. [DOI] [PubMed] [Google Scholar]
- [37].Mazumdar A, Kumar R. Estrogen regulation of Pak1 and FKHR pathways in breast cancer cells. FEBS Lett. 2003;535:6–10. [DOI] [PubMed] [Google Scholar]
- [38].Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, et al. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000;20:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–14. [DOI] [PubMed] [Google Scholar]
- [40].Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kawagishi H, Finkel T. Unraveling the truth about antioxidants: ROS and disease: finding the right balance. Nat Med. 2014;20:711–3. [DOI] [PubMed] [Google Scholar]
- [42].Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463–6. [DOI] [PubMed] [Google Scholar]
- [43].Attie AD. How do reducing equivalents increase insulin secretion? J Clin Invest. 2015;125:3754–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, in’t Veld P, Renstrom E, et al. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–42. [DOI] [PubMed] [Google Scholar]
- [45].Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, et al. beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes care. 2014;37:1751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hasnain SZ, Prins JB, McGuckin MA. Oxidative and endoplasmic reticulum stress in beta-cell dysfunction in diabetes. J Mol Endocrinol. 2016;56:R33–54. [DOI] [PubMed] [Google Scholar]
- [47].Marchi S, Patergnani S, Pinton P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta. 1837; 2014:461–9. [DOI] [PubMed] [Google Scholar]
- [48].Thivolet C, Vial G, Cassel R, Rieusset J, Madec AM. Reduction of endoplasmic reticulum- mitochondria interactions in beta cells from patients with type 2 diabetes. PLOS ONE. 2017;12:e0182027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008;10:1356–64. [DOI] [PubMed] [Google Scholar]
- [50].Singh RR, Song C, Yang Z, Kumar R. Nuclear localization and chromatin targets of p21-activated kinase 1. J Biol Chem. 2005;280:18130–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.