Abstract
Aim
The naturally fermented yak yogurt of pastoralists in the Tibetan Plateau, China, because of its unique geographical environment and the unique lifestyle of Tibetan pastoralists, is very different from other kinds of sour milk, and the microorganisms it contains are special. Lactococcus lactis subsp. lactis HFY14 (LLSL-HFY14) is a new lactic acid bacterium isolated from naturally fermented yak yogurt. The purpose of this study was to study the inhibitory effect of the bacterium on constipation.
Methods
Constipation was induced in ICR mice with diphenoxylate, and the constipated mice were treated with LLSL-HFY14. The weight and feces of the mice were visually detected. Colonic tissues were observed on hematoxylin and eosin-stained sections. Serum indices were detected with kits. mRNA expression in the colon was determined by quantitative polymerase chain reaction assay.
Results
Constipation caused weight loss, the number of defecation granules, defecation weight, fecal water content decreased, and the first black stool excretion time increased. LLSL-HFY14 alleviated these symptoms, and the effects were similar to those of lactulose (drug). The pathological examination revealed that constipation caused pathological changes in the colon, and LLSL-HFY14 effectively alleviated the disease. LLSL-HFY14 increased serum levels of motilin, gastrin, endothelin, substance P, acetylcholinesterase, and vasoactive intestinal peptide (VIP) and decreased serum levels of somatostatin in constipated mice. In addition, LLSL-HFY14 upregulated VIP, cAMP, protein kinase A, and aquaporin 3 expression in colonic tissues of constipated mice in a dose-dependent manner.
Conclusion
LLSL-HFY14 inhibited constipation, similar to lactulose, and has the potential to become a biological agent.
Keywords: yak yogurt, lactic acid bacterium, diphenoxylate, constipation, mice
Introduction
Pastoralists in the Qinghai Tibetan Plateau, China, have a long-term habit of drinking fermented yak yogurt. Yak yogurt is a natural green food in which lactose is transformed into lactic acid, organic acids, and ethanol by microbial fermentation. Due to the special climatic conditions and a special lifestyle for Tibetan pastoralists of the Tibetan Plateau, the composition of microorganisms during fermentation of the yogurt is unique, in which there are a large number of lactic acid bacteria.1 This class of yak yogurts contains lactic acid bacteria, which are relatively different from those used commercially, and studies have confirmed that this class of acid milks also has different bioactive effects, such as constipation inhibition effect. Some studies have confirmed that lactic acid bacteria can reduce intestinal injury, improve intestinal activity and inhibit constipation.2,3 More extensive isolation and identification work needs to be performed to accumulate additional strain resources, and develop rich probiotics that can be used in health foods and medicine.
In addition to probiotics, harmful bacteria also inhabit the gut, and the two are in balance under normal conditions.4 Gut probiotics also participate in digestion, as dyspepsia and gastrointestinal dysfunction occur without probiotics.5 Lactic acid bacteria effectively inhibit the growth and reproduction of harmful bacteria in the gastrointestinal tract by metabolizing lactic acid and maintaining the ecological balance and normal function of the gastrointestinal tract. Chronic diarrhea, constipation, abdominal distension, dyspepsia, and other symptoms are related to an imbalance of intestinal lactic acid bacteria.6,7 Lactic acid bacteria not only activate macrophage phagocytosis, but also play an active role in intestinal colonization. Lactic acid bacteria stimulate peritoneal macrophages, induce interferon production, promote cell division, produce antibodies, promote cellular immunity, enhance the non-specific and specific immune responses, and improve disease resistance.8,9 Lactic acid bacteria also promote peristalsis and defecation in a variety of ways.10
Constipation is manifested as difficult defecation with dry stool. Constipation slows down intestinal peristalsis, increases harmful bacteria in the intestine, and can cause other intestinal diseases.11 The expression of vasoactive intestinal peptide (VIP) in the colonic tissues of patients with esophagitis decreases, which leads to intestinal motility disorder.12 VIP can regulate intestinal water metabolism by affecting the expression of aquaporin 3 (AQP3) in intestinal epithelial cells.13 Cyclic adenosine monophosphate protein kinase A (camp PKA) pathway plays an important role in the biological effect of VIP, and the expression of AQP3 is also regulated by cAMP PKA pathway.14 The VIP-cAMP-PKA-AQP3 signal pathway is related to the regulation of intestinal motility to improve intestinal water metabolism, so as to significantly improve constipation symptoms, therefore, the disorder of water metabolism in constipation may be related to VIP-cAMP-PKA-AQP3 signaling pathway. Therefore, the disorder of water metabolism in cases of constipation is related to the VIP-cAMP-PKA-AQP3 signaling pathway. Lactococcus lactis subsp. lactis HFY14 was a lactic acid bacteria isolated and identified by our team from naturally fermented yak yogurt. Our team preliminarily screened the growth rate of gastric acid resistance and bile salt resistance in vitro, and found that this lactic acid bacteria had better in vitro resistance than common commercial lactic acid bacteria, and had the potential to be developed as a probiotic. In this study, we first further investigated the effect of Lactococcus lactis subsp. lactis HFY14 (LLSL-HFY14) on the VIP-CAMP-PKA-AQP3 signaling pathway in colonic tissues during constipation, and explored its mechanism of improved intestinal water metabolism.
Materials and Methods
Experimental Strain
LLSL-HFY14 was isolated and identified by our group from naturally fermented yogurt in Hongyuan, Sichuan, China. This strain is stored at the China General Microbiological Culture Collection Center (Beijing, China) under storage number CGMCC No. 16647.
Experimental Animals and Groupings
Fifty 6-week-old female ICR mice were purchased from the Experimental Animal Center of Chongqing Medical University (Chongqing, China). The mice were reared in a clean room with a temperature of 20–25°C and moderate humidity of 50–60%. The mice were divided into five groups of ten mice each: the normal group, the model group, the low concentration LLSL-HFY14 (LLSL-HFY14-L) group, the high concentration LLSL-HFY14 (LLSL-HFY14-H) group, and the lactulose (positive drug control) group. This study was approved by the Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (202003058B, Chongqing, China) and adhered to the National Standards of the People’s Republic of China (GB/T 35892-2018) laboratory animal guidelines for ethical review of animal welfare.
Animal Experiment Treatment
Diphenoxylate was dissolved in distilled water to make a suspension with a concentration of 2.5 mg/mL and shock before use. The mice in all groups, except the normal group, were intragastrically administered 20 mg/kg diphenoxylate (Jiangsu Changzhou Kangpu Pharmaceutical Co., Ltd., Changzhou, Jiangsu, China) every day for 4 weeks to induce functional constipation.15 The diphenoxylate gavage was stopped 4 weeks later, and the LLSL-HFY14-L and LLSL-HFY14-H groups of mice were gavaged with 1×108 and 1×109 CFU/kg of LLSL-HFY14 for 4 weeks. The lactulose group of mice was gavaged with 3 g/kg lactulose (Solarbio, Beijing, China) for 4 weeks, and the model group of mice was gavaged with 0.2 mL distilled water every day. Mice in the normal group were fed 0.2 mL distilled water every day throughout the 8 weeks of the experiment (Table 1). The mice were monitored weekly for weight, fecal pellet count, fecal weight, and fecal water content throughout the experiment.
Table 1.
Animal Experiment Planning Process
| Group | 1–4 Week (Every Day Gavage) | 5–8 Week (Every Day Gavage) |
|---|---|---|
| Normal | 0.2 mL distilled water | 0.2 mL distilled water |
| Model | Diphenoxylate (20 mg/kg) | 0.2 mL distilled water |
| LLSL-HFY14-L | Diphenoxylate (20 mg/kg) | 1×108 CFU/kg LLSL-HFY14 |
| LLSL-HFY14-H | Diphenoxylate (20 mg/kg) | 1×109 CFU/kg LLSL-HFY14 |
| Lactulose | Diphenoxylate (20 mg/kg) | 3 g/kg lactulose |
Discharge Time of the First Black Stool
From the first week to the eighth week, every weekend, 0.1 mL/10 g of ink was given by gavage 30 min after the last day of administration, the feces were collected within 6 hours after the first black stool was discharged, and the number, weight, and water content of the fecal pellets were recorded and determined. At the end of eighth week, 30 min after ink treatment, the discharge time of the first black stool was recorded.
Hematoxylin and Eosin (H&E) Staining of Colonic Tissues
About 5 cm2 of colonic tissue was immediately fixed in 10% formalin solution for 48 h. Staining was performed after dehydration, transparency, wax immersion, embedding, and sectioning. The morphological changes in the colonic tissues were observed under a light microscope (BX43; Olympus, Tokyo, Japan).
Serum Biomarkers
The mouse blood was collected and centrifuged at 4000 r/min for 10 min at 4°C to collect the serum. Serum levels of motilin (MTL), gastrin (GAS), endothelin (ET), somatostatin (SS), acetylcholinesterase (AChE), substance P (SP), and VIP were determined according to the kit instructions (ABCAM, Cambridge, MA, USA).
Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-qPCR) Assay
Total RNA of the mouse colon was extracted according to the Trizol instructions (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Then, Then, 1 μL (oligo) of primer DT, 10 μL of sterile ultrapure water and 1 μg/μL RNA sample (1 μL) were mixed and reacted at 65°C for 5 min; 1 μL of ribolock RNase inhibitor, 2 μL of 100 mM dNTP mix, 4 μL of 5× reaction buffer, and 1 μL of Revert Aid M-mu/v RT (Thermo Fisher Scientific, Inc.) was added in above solution for synthesizing cDNA. The target gene was reverse transcribed and amplified (SteponePlus PCR instrument, Thermo Fisher Scientific, Inc.). β-actin was used as the housekeeping gene, and the relative expression of the target gene (Table 2) was calculated by the 2−∆∆CT method.16
Table 2.
Sequences of the Primers Used in This Study
| Gene | Sequences |
|---|---|
| VIP | F:5ʹ-TCTGCAAGGGTAGCAATCGA-3’ |
| R:5-GGTGGAGTCCCTATCACTGG-3’ | |
| cAMP | F:5ʹ-TGAGGACCCAGATACTCCCA-3’ |
| R:5ʹ-CAAAAGACTCTGCAGCCTGG-3’ | |
| PKA | F:5ʹ-GGGTAGCCTCAGTGCTTACA-3’ |
| R:5ʹ-CCACGGCAACATTAGACCAG-3’ | |
| AQP3 | F:5ʹ-AGTGAGTCAGGAAAGTGCCA-3’ |
| R:5ʹ-TGAGCTACGCCCCTCTTATG-3’ | |
| β-actin | F:5ʹ-CATGTACGTTGCTATCCAGGC-3’ |
| R:5ʹ-CTCCTTAATGTCACGCACGAT-3’ |
Statistical Analysis
SPSS software (SPSS Inc., Chicago, IL, USA) was used to analyze the experimental data. Differences were detected by one-way analysis of variance and Duncan’s multiple range test. A P-value < 0.05 was considered significant.
Results
Mice Body Weight
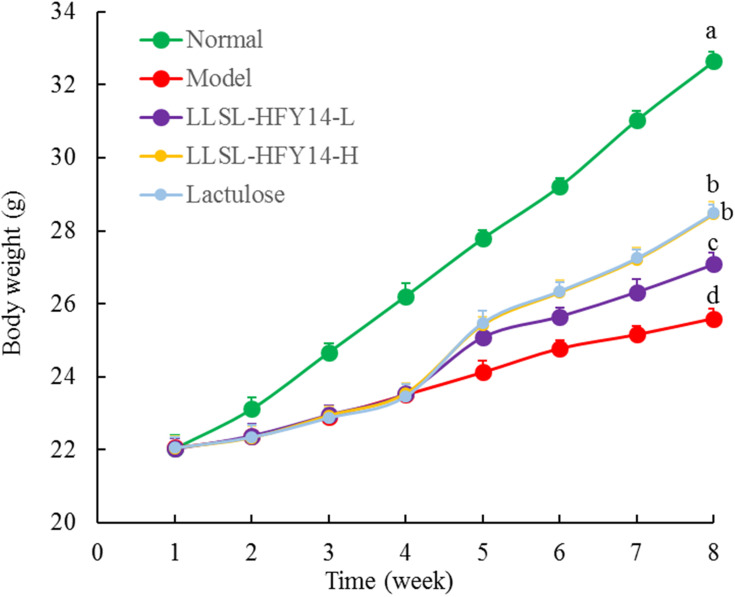
As shown in Figure 1, weight gain by the constipation-induced groups was slower than the normal group during the first 4 weeks. The weight gain of mice in the model group was slowest compared with the other groups beginning on week 5, and the weight gain of mice in the LLSL-HFY14-H and lactulose groups increased and was closest to the normal group.
Figure 1.
Body weights of the constipated mice. a–dMean values with different letters are significantly different (P < 0.05) based on Tukey’s test.
Fecal Status
As shown in Table 3, the number of fecal pellets, fecal weight, and water content of the groups began to decrease during the first 4 weeks compared to the normal group. The number of fecal pellets, fecal weight, and water content of mice in each group improved beginning on week 5 compared with the model group, and the improvement of mice in the LLSL-HFY14-H and lactulose groups was the largest and was closest to the normal group.
Table 3.
The Number, Weight, and Water Content of the Feces from Constipated Mice
| Fecal status | Normal | Model | LLSL-HFY14-L | LLSL-HFY14-H | Lactulose | |
|---|---|---|---|---|---|---|
| 1–4 weeks | Number | 38±3a | 13±4b | 14±4b | 14±3b | 13±3b |
| Weight | 0.92±0.13a | 0.21±0.07b | 0.22±0.06b | 0.21±0.06b | 0.21±0.08b | |
| Water content | 48.6±3.1a | 14.1±2.8b | 14.4±3.3b | 13.8±3.5b | 13.9±3.0b | |
| 5–8 weeks | Number | 40±4a | 14±3d | 21±4c | 33±4b | 35±4ab |
| Weight | 1.12±0.19a | 0.27±0.09d | 0.64±0.10c | 0.87±0.11b | 0.90±0.09ab | |
| Water content | 48.8±2.7a | 14.4±2.8d | 27.6±3.0c | 38.7±2.6b | 39.1±3.1b | |
Note: a–dMean values with different letters over the same row are significantly different (P < 0.05) according to Tukey’s test.
Discharge Time of the First Black Stool
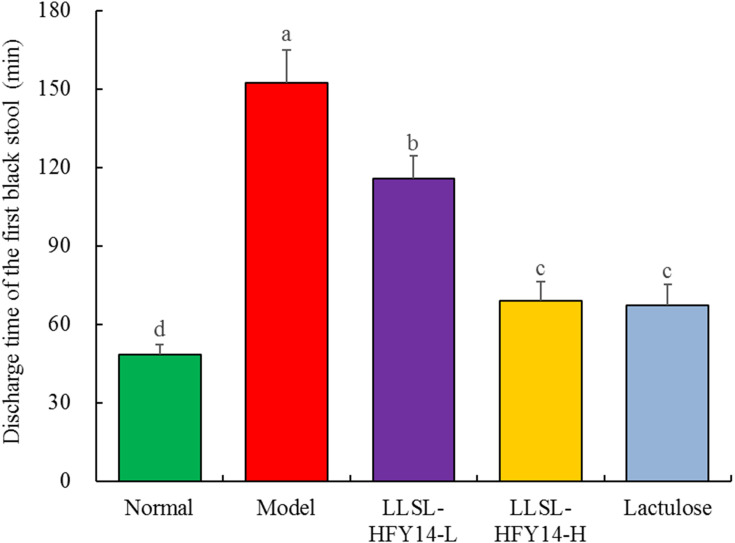
As shown in Figure 2, the time of the first black stool excretion was the shortest in the normal group at end of the eighth week, and that in the other groups was significantly longer than in the normal group. The time of the first black stool in the model group was the longest, while the times in the LLSL-HFY14-H and lactulose groups were significantly shorter than that in the LLSL-HFY14-L group, which was closest to the normal group.
Figure 2.
Discharge time of the first black stool by constipated mice at the end of eighth week. a–dMean values with different letters are significantly different (P < 0.05) based on Tukey’s test.
Histopathological Analysis of Colonic Tissues
As shown in Figure 3, the colonic mucosal epithelial cells were intact, the inflammatory cells were normal, no infiltration was evident, the goblet cells were arranged in an orderly manner, and there was no congestion or edema in the normal group. In the model group, the colonic epithelial cells were significantly damaged, the intestinal wall was thickened, and edema, inflammatory cell infiltration, and reduced goblet cell size were observed. The hyperemia, edema, and cell infiltration decreased after treatment with LLSL-HFY14 or lactulose compared with the model group. LLSL-HFY14-H and lactulose had the most obvious improving effects on the colonic tissues, indicating that LLSL-HFY14 alleviated the constipation damage in the colon. The effect of LLSL-HFY14-H was enhanced as concentration was increased, and the effect of the high concentration sample was closest to that of lactulose.
Figure 3.
Hematoxylin and eosin (H&E) pathological staining status of colonic tissues from constipated mice. The black arrow indicates the damaged tissue.
Serum Levels of MTL, GAS, ET, SS, AChE, SP, and VIP
The levels of MTL, GAS, ET, AChE, and VIP were highest in the normal group, while the SS level was the lowest (Table 4). The serum levels of MTL, GAS, ET, SP, AChE, and VIP were lowest in the model group, while SS was the highest. The levels of MTL, GAS, ET, SP, AChE, and VIP in the LLSL-HFY14 and lactulose groups increased significantly compared with the model group (P < 0.05), but the SS level decreased significantly (P < 0.05). The serum levels of LLSL-HFY14-H and lactulose were closest to the normal group, and there was no significant (P > 0.05) difference between the two groups.
Table 4.
Serum Levels of Motilin (MTL), Gastrin (GAS), Endothelin (ET), Somatostatin (SS), Acetylcholinesterase (AChE), Substance P (SP), and Vasoactive Intestinal Peptide (VIP) in Constipated Mice
| Level (pg/mL) | Normal | Model | LLSL-HFY14-L | LLSL-HFY14-H | Lactulose |
|---|---|---|---|---|---|
| MTL | 225.16±20.33a | 74.63±5.32d | 127.66±14.31c | 175.30±14.47b | 178.62±15.87b |
| GAS | 123.02±14.17a | 30.85±4.34d | 66.03±7.12c | 94.63±6.32b | 96.34±7.11b |
| ET | 30.05±3.17a | 4.23±0.63d | 14.21±0.78c | 24.15±1.61b | 25.03±1.77b |
| SS | 24.15±3.49d | 89.60±5.36a | 61.03±4.02b | 40.15±3.96c | 39.12±3.89c |
| AChE | 40.65±2.79a | 8.30±0.79d | 21.03±2.30c | 33.17±2.03b | 34.19±3.13b |
| SP | 86.23±5.11a | 24.16±1.96d | 44.16±4.82c | 67.98±5.10b | 69.11±5.56b |
| VIP | 87.26±4.20a | 20.17±3.08d | 45.15±4.37c | 68.39±5.97b | 70.35±4.83b |
Notes: a–dMean values with different letters over the same row are significantly different (P < 0.05) according to Tukey’s test.
VIP, cAMP, PKA, and AQP3 mRNA Expression in Colonic Tissues
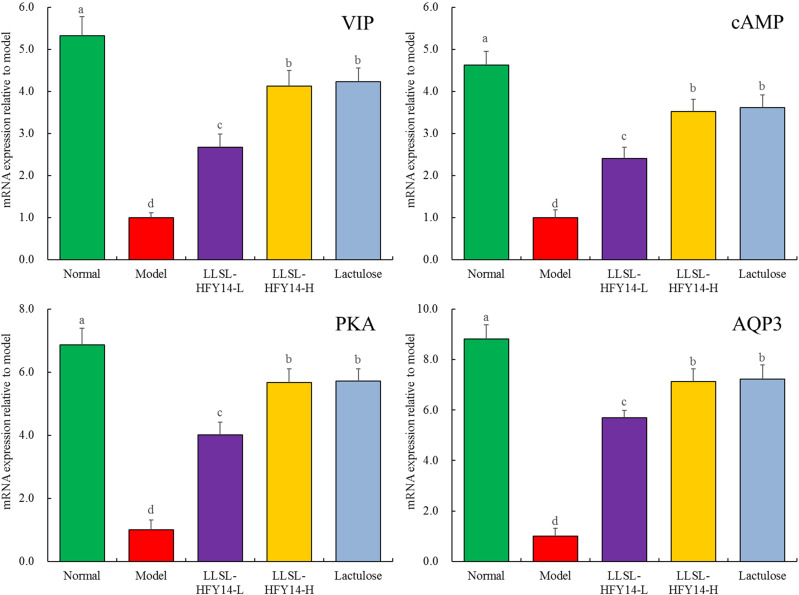
The expression levels of VIP, cAMP, PKA, and AQP3 were highest in the normal group, while the expression levels of VIP, cAMP, PKA, and AQP3 were lowest in the model group (Figure 4). LLSL-HFY14 and lactulose significantly upregulated the expression levels of VIP, cAMP, PKA, and AQP3 in constipated mice (model group), and lactulose and LLSL-HFY14-H enhanced these expression levels, but there was no significant difference between LLSL-HFY14-H and lactulose (P > 0.05).
Figure 4.
Vasoactive intestinal peptide (VIP), cAMP, protein kinase A (PKA), and aquaporin 3 (AQP3) mRNA expression in colonic tissues from constipated mice. a–dMean values with different letters are significantly different (P < 0.05) based on Tukey’s test.
Discussion
Constipation is primarily caused by bad living and eating habits and serious cases can lead to other gastrointestinal diseases. Proper physical exercise, good defecation habits, and a reasonable diet relieve constipation when symptoms are mild. Drugs are useful when the symptoms are serious, but the drugs are easy to become dependent and some intestinal side effects can occur, such as rheum, cassia et al17 Constipation is clinically characterized by reduced frequency of defecation, difficult defecation, and dry stool. The number, weight, and water content of the fecal pellets are the most intuitive indicators to evaluate constipation under laboratory conditions.18 At the same time, constipation can lead to the decline of intestinal motility and water content, thus slowing the intestinal peristalsis, making it difficult to excrete the intestinal contents. Therefore, the time of the first black stool excreted after intragastric administration of ink is an index to evaluate the degree of constipation.19 In this study, weight gain of the mice decreased after constipation was induced for 4 weeks, and the number, weight, and water content of the fecal pellets decreased significantly. The time to the first black stool discharge was extended. LLSL-HFY14 effectively alleviate these symptoms, and the effect was similar to that of lactulose.
A clinical-pathological examination is an important diagnostic method that provides the basis for clinical treatment. A pathological examination of animal tissues under laboratory conditions is an important technical way to judge experimental animal disease.20 In this study, H&E staining was used for the pathological examination. The H&E stained sections revealed that the LLSL-HFY14 alleviated the damage in colonic tissue lesions caused by long-term constipation.
The levels of neurotransmitters (eg, MTL, GAS, ET, SS, AChE, SP, and VIP) in patients with constipation will change. MTL, GAS, AChE, SP, and VIP are excitatory neurotransmitters, whereas ET and SS are inhibitory neurotransmitters, intestinal motility was enhanced by acute neurotransmitters, but decreased by inhibitory neurotransmitters.21 MTL is an important index to evaluate gastrointestinal peristalsis. Most authors believe that MTL promotes movement in various parts of the gastrointestinal tract and that a reduction of MTL release reduces gastrointestinal peristalsis.22 GAS is an important gastrointestinal hormone that promotes the secretion of gastric juice, increases peristalsis of the gastrointestinal tract, accelerates gastric emptying, and promotes relaxation of the pyloric sphincter.23 Acetylcholine (ACh) is one of two neurotransmitters that play an important role in intestinal motility by binding to its receptor; the acetyl cholinesterase (AChE) level is positively correlated with the ACh level.24 SP is an excitatory transmitter of gastrointestinal motor neurons. It strongly promotes contraction of smooth muscle in the digestive tract, stimulates the small intestinal and colonic mucosa to secrete water and electrolytes, and promotes gastrointestinal motility.25 In this study, the serum levels of MTL, GAS, AChE, and SP were significantly lower in mice from the model group than those from the normal group, while the levels of these neurotransmitters increased significantly in the LLSL-HFY14 group, indicating that constipation was related to decreased levels of MTL, GAS, AChE, and SP and that LLSL-HFY14 increased the levels of these neurotransmitters and played a role in relieving constipation.
ET is a multifunctional peptide with cardiovascular and intestinal functions.26 SS inhibits the release of gastrointestinal hormones, decreases the gastric emptying rate, and decreases smooth muscle contraction, all of which can contribute to constipation.27 VIP is an inhibitory neurotransmitter secreted by motor and secretory neurons. VIP motor neurons mainly inhibit gastrointestinal muscle tone, thereby inhibiting gastrointestinal motility.28 The results of this study show that the SS level was highest in the model group, while ET and VIP levels were lowest, and compare with the model group, the SS level in the LLSL-HFY14 groups decreased significantly, but the ET and VIP levels in the LLSL-HFY14 groups increased. ET and VIP levels in all groups increased significantly, suggesting that LLSL-HFY14 has an interventional effect on constipation.
VIP and its specific receptor activate the cAMP/PKA signaling pathway, which causes hyperpolarization and relaxation of gastrointestinal smooth muscle cells.29 VIP receptors are mainly distributed on the basement membrane of intestinal epithelial cells.30 VIP activates the guanylate binding protein on cell membranes through a cAMP-dependent protein kinase pathway.31 The activated guanylate binding protein activates adenylate cyclase on the cell membrane and catalyzes ATP to remove a pyrophosphate to produce cAMP.32 cAMP acts as a second messenger to activate cAMP protein kinase (PKA), which phosphorylates the target protein and regulates the cellular response.33 At the same time, the rapid transmembrane transfer of water molecules is realized through aquaporin (AQP) residing on the cell membrane. AQP is mainly involved in the secretion and absorption of water and regulates the balance of water inside and outside cells.34 AQP3 is highly expressed in the digestive tract epithelial cells, which is mainly involved in the transfer and metabolism of digestive tract fluids and electrolytes. VIP plays an important role in the biological effect of the cAMP/PKA pathway, and AQP3 expression is regulated by the cAMP-PKA pathway.35 Phosphorylation of AQP3 is cAMP-dependent. The activation of adenylate cyclase increases intracellular cAMP level; thus, activating PKA and increasing permeability of the membrane to water.36 Abnormal expression of AQP3 leads to excessive intestinal water in the cells or intestinal mucus secretion, leading to constipation.37 VIP regulates intestinal motility and intestinal water metabolism, which is closely related to constipation.38–40 VIP regulates intestinal water metabolism by affecting AQP3 expression in intestinal epithelial cells.39 cAMP-PKA is a VIP-AQP3 intermediate link. As an upstream neurotransmitter, VIP regulates expression of the AQP3 protein through the cAMP/PKA signaling pathway.35 This study also confirmed that LLSL-HFY14 inhibited constipation by regulating mRNA expression of the VIP-cAMP-PKA-AQP3 pathway.
Intestinal health is affected by constipation, as a large number of harmful bacteria accumulate in the intestine and toxic substances are absorbed into the body, affecting health.41 Some lactic acid bacteria can be used as probiotics, which can improve intestinal health, inhibit the number of harmful bacteria, and remove toxic substances from the intestine.42 In addition, lactic acid bacteria produce acidic substances during metabolism, such as lactic acid and acetic acid. These acids stimulate intestinal secretion of a large amount of intestinal fluid to help soften the feces. The acidic intestinal internal environment produced by probiotics enhances peristalsis, which is also conducive to excretion of feces.43 The LLSL-HFY14 in this study may have similar efficacy. In further research, we will conduct an in-depth study of related mechanisms.
Previous studies have shown that constipation is related to VIP-cAMP-PKA-AQP3 signaling pathway. Regulating VIP-cAMP-PKA-AQP3 signaling pathway by active substances can inhibit constipation.44,45 At the same time, the study also confirmed that Lactobacillus belongs to a probiotic bacteria, which can be used to regulate the function of the intestine, improve the internal environment of the intestine, regulate the balance of the intestinal microecology, promote the intestinal peristalsis function, and relieve constipation.46,47 None of these studies have yet correlated the effects of probiotics on intestinal water metabolism. This study proposed lactic acid bacteria (LLSL-HFY14) as a new action point of probiotics from water metabolism pathway to inhibit constipation and preliminarily elucidated part of the mechanism. At the same time, the lack of nutrients may also be responsible for constipation,48 lactic acid bacteria can act as probiotics, but also as nutrients to improve intestinal health, play a role in protecting the intestine and improving constipation. In this study, LLSL-HFY14 has been proved to be able to interfere with the VIP-cAMP-PKA-AQP3 signaling pathway and play a role in inhibiting constipation, in addition to this new function of lactic acid bacteria.
Conclusions
LLSL-HFY14 is a type of lactic acid bacteria isolated from naturally fermented yogurt in the Tibetan Plateau area. Animal experiments show that LLSL-HFY14 has a dose-dependent inhibitory effect on diphenoxylate-induced constipation in mice, which is similar to that of lactulose. LLSL-HFY14 inhibits constipation through the VIP-cAMP-PKA-AQP3 signaling pathway. Taken together, LLSL-HFY14 has the potential to be developed into a biological agent to inhibit constipation and is a potentially effective drug.
Acknowledgments
This research was funded by the Chongqing University Innovation Research Group Project (CXQTP20033), the Science and Technology Project of the Chongqing Education Commission (KJQN202001604) and Scientific and Technological Innovation Project of “Construction of Double City Economic Circle in Chengdu-Chongqing Area” of Chongqing Education Commission (KJCX2020052), China.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Guo X, Long R, Kreuzer M, et al. Importance of functional ingredients in yak milk-derived food on health of tibetan nomads living under high-altitude stress: a review. Crit Rev Food Sci Nutr. 2014;54(3):292–302. doi: 10.1080/10408398.2011.584134 [DOI] [PubMed] [Google Scholar]
- 2.Zhao X, Qian Y, Li G, Yi R, Park KY, Song JL. Lactobacillus plantarum YS2 (yak yogurt Lactobacillus) exhibited an activity to attenuate activated carbon-induced constipation in male Kunming mice. J Dairy Sci. 2019;102(1):26–36. doi: 10.3168/jds.2018-15206 [DOI] [PubMed] [Google Scholar]
- 3.Zhao X, Yi R, Qian Y, Park KY. Lactobacillus plantarum YS-3 prevents activated carbon-induced constipation in mice. J Med Food. 2018;21(6):575–584. doi: 10.1089/jmf.2017.4109 [DOI] [PubMed] [Google Scholar]
- 4.Xue L, He J, Gao N, et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci Rep. 2017;7(1):45176. doi: 10.1038/srep45176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherner JA, Jensen RT, Dubois A, O’Dorisio TM, Gardner JD, Metcalfe DD. Gastrointestinal dysfunction in systemic mastocytosis. A prospective study. Gastroenterology. 1988;95(3):657–667. doi: 10.1016/s0016-5085(88)80012-x [DOI] [PubMed] [Google Scholar]
- 6.Reyna-Figueroa J, Barrón-Calvillo E, García-Parra C, et al. Probiotic supplementation decreases chemotherapy-induced gastrointestinal side effects in patients with acute leukemia. J Pediatr Hematol Oncol. 2019;41(6):468–472. doi: 10.1097/MPH.0000000000001497 [DOI] [PubMed] [Google Scholar]
- 7.Haller D, Colbus H, Gänzle MG, Scherenbacher P, Bode C, Hammes WP. Metabolic and functional properties of lactic acid bacteria in the gastro-intestinal ecosystem: a comparative in vitro study between bacteria of intestinal and fermented food origin. Syst Appl Microbiol. 2001;24(2):218–226. doi: 10.1078/0723-2020-00023 [DOI] [PubMed] [Google Scholar]
- 8.Chon H, Choi B. The effects of a vegetable-derived probiotic lactic acid bacterium on the immune response. Microbiol Immunol. 2010;54(4):228–236. doi: 10.1111/j.1348-0421.2010.00202.x [DOI] [PubMed] [Google Scholar]
- 9.Hatcher GE, Lambrecht RS. Augmentation of macrophage phagocytic activity by cell-free extracts of selected lactic acid-producing bacteria. J Dairy Sci. 1993;76(9):2485–2492. doi: 10.3168/jds.S0022-0302(93)77583-9 [DOI] [PubMed] [Google Scholar]
- 10.Mojgani N, Shahali Y, Dadar M. Immune modulatory capacity of probiotic lactic acid bacteria and applications in vaccine development. Benef Microbes. 2020;11(3):213–226. doi: 10.3920/BM2019.0121 [DOI] [PubMed] [Google Scholar]
- 11.Schiller LR. Review article: the therapy of constipation. Aliment Pharmacol Ther. 2001;15(6):749–763. doi: 10.1046/j.1365-2036.2001.00982.x [DOI] [PubMed] [Google Scholar]
- 12.King SK, Sutcliffe JR, Ong SY, et al. Substance P and vasoactive intestinal peptide are reduced in right transverse colon in pediatric slow-transit constipation. Neurogastroenterol Motil. 2010;22(8):883–892. doi: 10.1111/j.1365-2982.2010.01524.x [DOI] [PubMed] [Google Scholar]
- 13.Itoh A, Tsujikawa T, Fujiyama Y, Bamba T. Enhancement of aquaporin-3 by vasoactive intestinal polypeptide in a human colonic epithelial cell line. J Gastroenterol Hepatol. 2003;18(2):203–210. doi: 10.1046/j.1440-1746.2003.02949.x [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Wang Y, Zhang H, Yan S, Wang B, Xie P. Effect of vasoactive intestinal peptide on defecation and VIP-cAMP-PKA-AQP3 signaling pathway in rats with constipation. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016;41(11):1175–1180. doi: 10.11817/j.issn.1672-7347.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Zhang J, Suo H, et al. Preventive effects of different fermentation times of Shuidouchi on diphenoxylate-induced constipation in mice. Foods. 2019;8(3):86. doi: 10.3390/foods8030086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan Y, Liang J, Diao W, et al. Lactobacillus plantarum KSFY06 and geniposide counteract montmorillonite-induced constipation in Kunming mice. Food Sci Nutr. 2020;8(9):5128–5137. doi: 10.1002/fsn3.1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karabudak E, Koksal E, Macit MS. The relationship between body weight, fiber and fluid intake status and functional constipation in young adults. Nutr Food Sci. 2019;49(1):129–140. doi: 10.1108/NFS-03-2018-0083 [DOI] [Google Scholar]
- 18.Rasmussen LS, Pedersen PU. Constipation and defecation pattern the first 30 days after thoracic surgery. Scand J Caring Sci. 2010;24(2):244–250. doi: 10.1111/j.1471-6712.2009.00713.x [DOI] [PubMed] [Google Scholar]
- 19.Suo H, Zhao X, Qian Y, et al. Therapeutic effect of activated carbon-induced constipation mice with Lactobacillus fermentum Suo on treatment. Int J Mol Sci. 2014;15(12):21875–21895. doi: 10.3390/ijms151221875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Wang B, Yuan X, et al. Vitexin prevents colitis-associated carcinogenesis in mice through regulating macrophage polarization. Phytomedicine. 2021;83:153489. doi: 10.1016/j.phymed.2021.153489 [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Xiao X, Zhou X, et al. Effects of Lactobacillus plantarum CQPC0-fermented soybean milk on activated carbon-induced constipation through its antioxidant activity in mice. Food Sci Nutr. 2019;7(6):2068–2082. doi: 10.1002/fsn3.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Zhou X, Chen B, et al. Preventive effect of Lactobacillus plantarum CQPC10 on activated carbon induced constipation in institute of cancer research (ICR) mice. Appl Sci. 2018;8(9):1498. doi: 10.3390/app8091498 [DOI] [Google Scholar]
- 23.Yin J, Liang Y, Wang D, et al. Naringenin induces laxative effects by upregulating the expression levels of c-Kit and SCF, as well as those of aquaporin 3 in mice with loperamide-induced constipation. Int J Mol Med. 2018;41(2):649–658. doi: 10.3892/ijmm.2017.3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R, Sun P, Zhou Y, Zhao X. Preventive effect of Dendrobium candidum Wall. ex Lindl. on activated carbon-induced constipation in mice. Exp Ther Med. 2015;9(2):563–568. doi: 10.3892/etm.2014.2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian Y, Suo H, Du M, et al. Preventive effect of Lactobacillus fermentum Lee on activated carbon-induced constipation in mice. Exp Ther Med. 2014;9(1):272–278. doi: 10.3892/etm.2014.2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X, Qian Y, Suo H, et al. Preventive effect of Lactobacillus fermentum Zhao on activated carbon-induced constipation in mice. J Nutr Sci Vitaminol. 2015;61(2):131–137. doi: 10.3177/jnsv.61.131 [DOI] [PubMed] [Google Scholar]
- 27.Li G, Wang Q, Qian Y, Zhou Y, Wang R, Zhao X. Component analysis of Pu-erh and its anti-constipation effects. Mol Medi Rep. 2014;9(5):2003–2009. doi: 10.3892/mmr.2014.2009 [DOI] [PubMed] [Google Scholar]
- 28.Li G, Qian Y, Sun P, Feng X, Zhu K, Zhao X. Preventive effect of polysaccharide of Larimichthys Crocea swimming bladder on activated carbon-induced constipation in mice. J Kor Soc Appl Biol Chem. 2014;57(2):167–172. doi: 10.1007/s13765-014-4024-1 [DOI] [Google Scholar]
- 29.Gilmont RR, Somara S, Bitar KN. VIP induces PKA-mediated rapid and sustained phosphorylation of HSP20. Biochem Biophys Res Commun. 2008;375(4):552–556. doi: 10.1016/j.bbrc.2008.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toumi F, Neunlist M, Denis MG, et al. Vasoactive intestinal peptide induces IL-8 production in human colonic epithelial cells via MAP kinase-dependent and PKA-independent pathways. Biochem Biophys Res Commun. 2004;317(1):187–191. doi: 10.1016/j.bbrc.2004.03.033 [DOI] [PubMed] [Google Scholar]
- 31.Fernández M, Sánchez-Franco F, Palacios N, Sánchez I, Cacicedo L. IGF-Iand vasoactive intestinal peptide (VIP) regulate cAMP-response element-binding protein (CREB)-dependent transcription via the mitogen-activated protein kinase (MAPK) pathway in pituitary cells: requirement of Rap1. J Mol Endocrinol. 2005;34(3):699–712. doi: 10.1677/jme.1.01703 [DOI] [PubMed] [Google Scholar]
- 32.Liu DY, Gorrod JW. Effects of cAMP-dependent protein kinase and ATP on N1-oxidation of 9-benzyladenine by animal hepatic microsomes. Life Sci. 2000;66(1):77–88. doi: 10.1016/s0024-3205(99)00563-9 [DOI] [PubMed] [Google Scholar]
- 33.Yaniv Y, Juhaszova M, Lyashkov AE, Spurgeon HA, Sollott SJ, Lakatta EG. Ca2+-regulated-cAMP/PKA signaling in cardiac pacemaker cells links ATP supply to demand. J Mol Cell Cardiol. 2011;51(5):740–748. doi: 10.1016/j.yjmcc.2011.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinigarzadeh A, Muniandy S, Salleh N. Combinatorial effect of genistein and female sex-steroids on uterine fluid volume and secretion rate and aquaporin (AQP)-1, 2, 5, and 7 expression in the uterus in rats. Environ Toxicol. 2017;32(3):832–844. doi: 10.1002/tox.22283 [DOI] [PubMed] [Google Scholar]
- 35.Hua Y, Ding S, Zhang W, et al. Expression of AQP3 protein in hAECs is regulated by Camp-PKA-CREB signalling pathway. Front Biosci. 2015;20(7):1047–1055. doi: 10.2741/4357 [DOI] [PubMed] [Google Scholar]
- 36.Wang W, Zheng M. Role of cAMP-PKA/CREB pathway in regulation of AQP 5 production in rat nasal epithelium. Rhinology. 2011;49(4):464–469. doi: 10.4193/Rhino10.107 [DOI] [PubMed] [Google Scholar]
- 37.Zhan Y, Tang X, Xu H, Tang S. Maren pills improve constipation via regulating AQP3 and NF-κB signaling pathway in slow transit constipation in vitro and in vivo. Evid Based Complement Alternat Med. 2020;2020(1):1–12. doi: 10.1155/2020/9837384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma L, Li C, Wang Y. Effects of zengye decoction on the expression of AQP3 and VIP in colon tissues of mice with slow transit constipation. J Beijing Univ Tradit Chin Med. 2015;2015(8):532–534. doi: 10.3969/j.issn.1006-2157.2015.08.006 [DOI] [Google Scholar]
- 39.Ding Y, Zheng P, Li F, et al. Effects of lactitol and Bifidobacterium infantis on AQP3 and ICC in a rat model of constipation. Chin J Microbiol Immunol. 2015;35(12):890–895. doi: 10.3760/cma.j.issn.0254-5101.2015.12.006 [DOI] [Google Scholar]
- 40.Zhou Y, Wang Y, Yan S, Xie P, Li S, Wang D. Effect of Xiaofu Tongjie recipe on VIP-cAMP-PKA-AQP3 signaling pathway in colon tissues of rats with functional constipation. Chin J Exp Tradit Med Form. 2016;22(24):99–104. doi: 10.13422/j.cnki.syfjx.2016240099 [DOI] [Google Scholar]
- 41.Indrio F, Neu J. The intestinal microbiome of infants and the use of probiotics. Curr Opin Pediatr. 2011;23(2):145–150. doi: 10.1097/MOP.0b013e3283444ccb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.An HM, Baek EH, Jang S, et al. Efficacy of lactic acid bacteria (LAB) supplement in management of constipation among nursing home residents. Nutr J. 2010;9:5. doi: 10.1186/1475-2891-9-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee DK, Jang S, Baek EH, et al. Lactic acid bacteria affect serum cholesterol levels, harmful fecal enzyme activity, and fecal water content. Lipids Health Dis. 2009;8:21. doi: 10.1186/1476-511X-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Wang Y, Zhang H, Yan S, Wang B, Xie P. Effect of vasoactive intestinal peptide on defecation and VIP-cAMP-PKA-AQP3 signaling pathway in rats with constipation. J Central South Univ Med Sci. 2016;41(11):1175–1180. doi: 10.11817/j.issn.1672-7347.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 45.Cong L, Duan LW, Su WP, Hao S, Li DF. Efficacy of high specific volume polysaccharide - a new type of dietary fiber - on molecular mechanism of intestinal water metabolism in rats with constipation. Med Sci Monit. 2019;25:5028–5035. doi: 10.12659/MSM.916526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Nie SP, Zhu KX, et al. Effect of Lactobacillus plantarum NCU116 on loperamide-induced constipation in mice. Int J Food Sci Nut. 2015;66(5):533–538. doi: 10.3109/09637486.2015.1024204 [DOI] [PubMed] [Google Scholar]
- 47.Wojtyniak K, Horvath A, Dziechciarz P, Szajewska H. Lactobacillus casei rhamnosus Lcr35 in the management of functional constipation in children: a randomized Trial. J Pediatr. 2017;184:101–105. doi: 10.1016/j.jpeds.2017.01.068 [DOI] [PubMed] [Google Scholar]
- 48.Chinese Clinical Trial Registry [homepage on the Internet]. Investigation on nutrition status and its clinical outcome of common cancers; 2018. Available from: http://www.chictr.org.cn/showproj.aspx?proj=31813. Accessed December24, 2018.