Abstract
Background and Aims
Existing evidence for a link between alcohol use and memory impairments in adolescents and young adults is largely correlational. We aimed to determine whether associations between drinking and episodic memory were consistent with a causal effect of drinking or accounted for by familial factors confounding such associations. Because cannabis use is associated with a similar pattern of performance on episodic memory measures, we assessed whether any associations might be attributable to concurrent cannabis use.
Design
Observational study of individuals aged approximately 20 or 29 comprising two independent population-based cohorts of twins. A cotwin-control design permitted an estimate of alcohol exposure effects free of shared genetic and environmental confounding influences. Significant associations were followed up with twin-difference analyses. Propensity scores derived from measures collected at age 11 were used to adjust for unshared confounders.
Setting and Participants
Participants in both cohorts were assessed from the age of 11 (N = 1,251) under the auspices of the Minnesota Center for Twin and Family Research.
Measurements
Regression analyses with cumulative alcohol use as the predictor of interest. Multiple measures of attention, learning and memory from a widely used episodic memory task constituted dependent variables.
Findings
Drinking was associated with poorer attention (p ≤ .003) and learning (p ≤ .008). Results were similar across the two cohorts. The within-pair effect in twin-difference analyses was significant only for measures of learning (p-values ≤ .004). Results were not due to measured unshared confounders or cannabis use. Drinking in adolescence (to age 20) and early adulthood (between 20 and 29) exerted independent effects on learning.
Conclusions
There appears to be a robust and specific association between drinking and learning that can be reproduced across cohorts, is not easily accounted for by confounding factors or concurrent cannabis use, and is consistent with a causal influence of drinking.
Keywords: Alcohol, adolescence, verbal learning, short-term memory, cotwin-control
Concern has grown in recent years about adverse effects of alcohol use during adolescence and early adulthood. Experimentation with alcohol and other drugs typically begins during this period, which is also when substance use peaks on average. Data from the National Survey on Drug Use and Health (NSDUH) for 2017 indicate that 27% of 12to 17year-old youth have consumed alcohol and fully 44% of 21to 25-year-olds report having binged in the past month [48]. During the animal analog of adolescence, exposure to alcohol produces harmful and perhaps lasting effects not observed in fully mature animals [9, 47], including deficits in learning and memory [26]. A seminal study demonstrated impaired recall of words on a list learning task among alcohol-dependent adolescents relative to a control group [5]. Follow-up of this sample as well as subsequent studies provide further evidence of a link between adolescent drinking and task performance [7, 13, 30, 31, 32, 33, 34, 44, 45, 46, 49, 50, 57].
However, determining whether the association between alcohol exposure and learning and memory reflects a causal effect of exposure, rather than confounding by aspects of a pre-existing liability, remains a major challenge. It is sometimes assumed that significant associations between alcohol use and outcome measures reflect a causal, neurotoxic effect of drinking, even when the evidence comes primarily from cross-sectional studies (cf. [18]). Designs that disentangle potentially causal effects from confounding of statistical associations due to unmeasured factors are therefore necessary. Longitudinal studies can establish temporal sequence, a prerequisite for identifying causality, but are not immune from confounding, and they can be vulnerable to attrition and its attendant bias. In addition, individuals who use alcohol often use other drugs. Cannabis in particular has similar associations with learning and memory (reviewed in [6]), and its use is both common and commonly co-occurs with alcohol use [23]. Knowing whether associations between drinking and learning and memory might actually be due to co-occurring cannabis use becomes particularly important in light of increasing societal permissiveness toward the latter.
Compounding uncertainty about whether associations reflect a causal effect of drinking or a preexisting liability, the precise nature of the association between drinking and learning and memory is unclear. This is due to several factors: small samples; null findings; conflicting and contradictory results; and variation among studies in specific aspects of task performance examined. Many studies have used selected samples (e.g., treatment samples or samples selected for binge drinking). Conditioning sample selection on subject characteristics may bias estimates of the drinking–task performance association. In addition, the putative vulnerability of the brain in adolescence makes understanding effects of alcohol exposure across its full range important. Even normative levels of alcohol consumption appear to adversely affect structure of the hippocampus [56], which is central to learning and memory [3, 29], and a linear relationship between amount of alcohol consumed and list learning has been observed [32]
The present study examined multiple measures of learning and memory using a cotwin-control (CTC) design [27] to address these issues. We assessed associations between drinking and performance on a widely used list learning task in two large, independent population-based samples of young adult twins assessed longitudinally from age 11, with a total sample size of 1251. Our prospective design allowed for careful characterization of cumulative use during adolescence and early adulthood. We combined four aspects of alcohol exposure into a quasi-continuous measure of drinking at each wave. There were no sample exclusions and study attrition was minimal. The CTC in effect relates naturally occurring variation within twin pairs in overall levels of drinking to differences in outcome measures. Twin differences in exposure control for shared genetic and environmental influences on drinking that would otherwise be confounded with measures of learning and memory. They therefore represent a more appropriate measure of exposure than the raw drinking score. Because the CTC cannot control for unshared confounders, however, we drew on data from the initial, age-11 assessment, prior to any meaningful substance use, to estimate each individual’s propensity to drink and thus twin differences in these measured unshared risk factors. We also assessed independent effects of drinking during adolescence and in one’s 20s in order to determine whether adolescence constitutes a period of particular vulnerability to alcohol exposure. Finally, we used two methods to determine whether drinking–task performance associations might be due to concurrent cannabis use. We thus explicitly considered potential confounders, which is typically not done [8].
Method
Participants comprised two independent population-based cohorts of twins followed longitudinally through the Minnesota Center for Twin and Family Research (MCTFR). One cohort (MTFS) consisted of all live twin births in 1978–1984, the other (ES), births in 1988–1994. Twins became eligible upon turning 11 if the families met minimal eligibility criteria. (ES determined eligibility somewhat differently than MTFS; see the Supporting Information.) Families are racially, ethnically and socioeconomically representative of the population of Minnesota from those birth cohorts [15, 19]. Participants provided informed consent or assent, as appropriate for their age, at each assessment. Both projects were reviewed and approved by the Institutional Review Board at the University of Minnesota.
Twins completed comprehensive assessments at all waves, with target ages of 11, 14 17 and 20 for both cohorts and 24 and 29 for the MTFS cohort, that included questions about alcohol, cannabis and other substance use. The Rey Auditory-Verbal Learning Test (RAVLT), a list learning task widely used to assess episodic memory, was introduced into a recent follow-up assessment. The present investigation consisted of all 1251 participants completing the RAVLT as part of their in-person assessment: 254 ES participants (166 females; mean age, 20.8, range = 20.1–21.9) and 997 MTFS participants (554 females; mean age, 29.1, range = 28.2–33.1). Participation rates were high: 91.7% and 87.0% for the ES and MTFS cohorts, respectively.
Alcohol use.
Participants were asked about substance use on a computerized questionnaire, completed in privacy at in-person visits until the age-17 (ES) or age-20 assessment (MTFS), and in age-appropriate semi-structured diagnostic interviews [36, 37, 54], administered by trained individuals with at least a college degree in Psychology or related field. Both instruments included similar questions about frequency of use, typical quantity consumed, density of use (maximum number of drinks in a 24-hr period) and misuse (number of times intoxicated) [25, 28]. The distribution of responses to these questions tended to be sparse and positively skewed. We therefore used a procedure developed previously at the MCTFR to derive five and six-point ordinal scales from each question and summed scores on the resulting scales. We averaged scales derived from the two different instruments when both were available (see the Supporting Information for additional detail). These were subsequently aggregated across waves in the form of the mean score. The distribution of scores is plotted separately for the two cohorts in Figure 1. Because less than 1% of participants in either cohort reported any drinking at the age-11 assessment, we did not include this wave when cumulating scores across assessments. Approximately 88% of ES participants had data for all three follow-ups and 93% of MTFS participants had data for at least four of five follow-ups.
Fig 1.
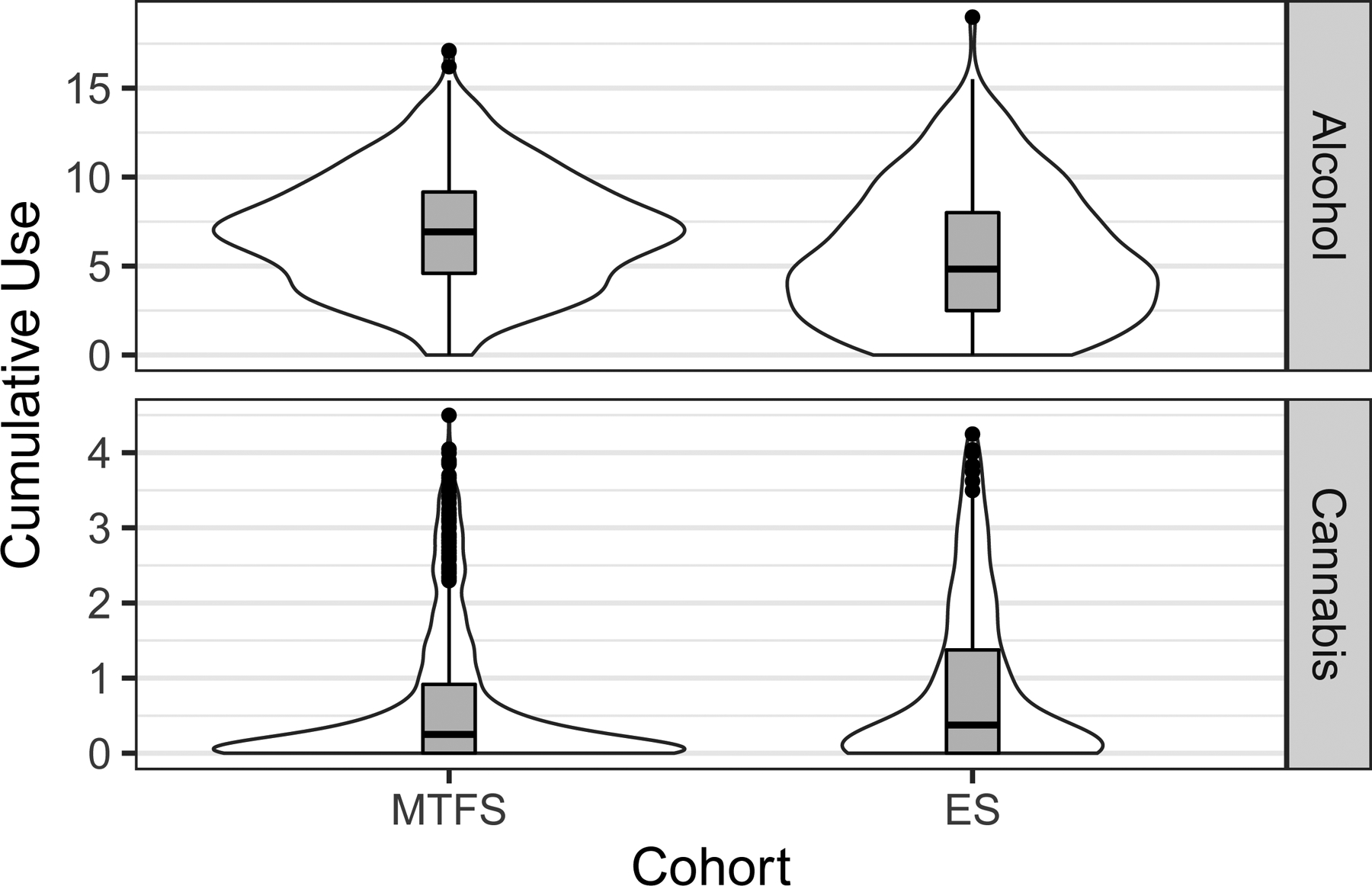
The distribution of cumulative drinking and cannabis scores. These are provided separately for the two cohorts (MTFS and ES) and in two forms, with box plots inside violin plots. The box spans the first and third quartiles of the distribution, while the interior line of the box plot represents the median.
Cannabis use.
Measures of cannabis consisted of the number of uses since the previous assessment and frequency of use. After deriving ordinal scales from these questions in a similar manner as for the drinking questions, we averaged them into a single scale and aggregated scales across assessment by computing the mean (see Figure 1 for a depiction of the distribution of scores).
Learning and memory.
In the RAVLT, subjects listen to list of 15 simple words spoken at a pace of one word per second (List A), then are asked to repeat as many words as possible from this list. There are five learning trials (Trials 1-–5) followed by a trial with 15 new words (List B, or Trial B). Participants are then asked without warning to recall as many words from List A as possible (Trial 6, immediate recall). After 30 min, during which time participants performed other tasks structured to avoid interference with verbal memory, participants were asked, again without warning, to recall as many words as possible from List A (Trial 7, or delayed recall).
The RAVLT taps several psychological processes – verbal learning, attention, interference and recall – all reported to be associated with drinking. We used multiple measures of each when feasible (Table 1). This included three measures of learning: overall learning (“learning efficiency”), a robust measure with good discriminant validity [20]; learning over trials (LOT) [17], which adjusts the total number of words recalled on the five learning trials for the number expected based on performance on the first trial; and performance on the final learning trial, a criterion of learning success (“final learning” [51]). Because Trial 1 and Trial B represent one’s initial exposure to a word list, they yield a measure of attention and immediate memory uncontaminated by rehearsal [14, 51], although Trial B performance reflects interference effects as well. We defined proactive interference as the difference between number of words recalled on Trial B relative to Trial 1. Measures of memory consisted of the difference between the number of words recalled on each recall trial (Trials 6 and 7, respectively) and Trial 5, the final learning trial. They are thus adjusted for overall learning. We refer to these as measures of immediate and delayed retention (cf. [32]) to distinguish them from raw recall scores.
Table 1.
RAVLT measures used and their definitions.
| Psychological process | Definition |
|---|---|
| Overall learning (“learning capacity”) | |
| Learning over trials (LOT) | |
| Trial 5 total (“final learning”) | y5 |
| Attention and immediate memory: Trial 1 total | y1 |
| Attention and immediate memory: Trial B total | yB |
| Proactive interference | yB−y1 |
| Immediate retention | y6−y5 |
| Delayed retention (after 30 min) | y7−y5 |
Note: y represents the number of words recalled on a given trial, with the subscript indicating which trial (Trials 1–7 or Trial B). The first two measures of learning involve summing the number recalled on the five learning trials, indicated by the summation operator.
General intellectual ability.
To determine whether any associations obtained between drinking and RAVLT performance could be attributed to overall intellectual ability rather than reflect a specific possible effect of alcohol use, we included IQ, assessed with the WISC-R [53] at the intake, age-11 assessment, as a covariate in a follow-up analysis of our primary individual-level and CTC analyses.
Statistical analysis
All analyses were conducted using the svyglm function for general(ized) linear models in the survey package [21, 22] in the R computing environment [35]. svyglm accommodates the nesting of individuals within pairs as well as probability weights used in secondary analyses. Analyses were not preregistered, and results should be considered exploratory.
Preliminary analyses: Cohort and sex effects.
Because MTFS subjects might have experienced more years of exposure to alcohol than ES subjects as well as greater opportunity to moderate their consumption, we tested for significant differences between cohorts in the association between cumulative drinking and task performance. Absent any such differences, we combined cohorts. We also examined results of primary analyses in the two cohorts separately to assess consistency of findings (see Supporting Information). Because women may be especially susceptible to adverse effects of drinking [16], we also assessed interactions between drinking and sex.
Associations between cumulative drinking and task performance.
All analyses included cohort, sex and zygosity as covariates. The cluster-robust sandwich estimator in svyglm provided appropriate standard errors of parameter estimates given that individual twins are nested within pairs (families).
Cotwin-control analyses.
Significant associations between drinking and task performance were followed up with CTC analyses. Each individual twin’s score on the drink index was decomposed into two orthogonal parts: the twin pair’s mean score and each twin’s deviation from the twin-pair mean. We focus on the latter, which reflects levels of drinking unconfounded by familial influences shared with the co-twin, which we refer to as the twin-difference or within-pair effect.
Propensity score adjustment.
Although the CTC design controls all shared confounders, including those that are unmeasured, it cannot control for unshared confounders. We used inverse probability of treatment weighting (ITPW) [1] to adjust for twin differences in potential confounders, creating a propensity score [38] from the many measures collected at the age-11 assessment, prior to any real substance exposure. (A detailed description of the approach, including propensity score indicators, is provided in the Supporting Information.) We estimated a generalized propensity score (GPS) using the Covariate Balancing Propensity Score approach in the CBPS package [11], which estimates the propensity score while simultaneously optimizing balance across levels of drinking in the sample. Weights were computed as twin deviations from the twin pair mean so as to retain the desired interpretation of the within-pair effect in the CTC design [40, 43] and trimmed at the 1st and 99th quantiles to avoid numerical instability due to extreme values. This procedure yielded propensity score-adjusted estimates of the within-pair effect that are, in principle, free from unshared confounding, albeit limited to available measured characteristics.
Specificity of findings to adolescence.
Recent definitions treat adolescence as spanning approximately 10 to 20 (e.g., World Health Organization) or even 24 [41] years of age. Significant associations between drinking and task performance in the ES cohort at age 20 implicate adolescent drinking by definition. For the older MTFS cohort, we computed cumulative drinking to age 20, providing an analog to the ES cohort, as well as drinking between this assessment and the age-29 assessment, which is effectively a raw change score. This allowed us to determine whether drinking in adolescence and change in, or moderation of, drinking during early adulthood are independently associated with task performance. We included both measures in individual-level and CTC analyses.
Specificity of findings to drinking.
Cannabis use shows associations with episodic memory performance similar to those observed in relation to drinking [6], and many individuals who drink also use cannabis [23]. We therefore assessed potential confounding of alcohol use–performance associations by concurrent cannabis use in two ways. First, we used a second GPS in IPT-weighted regression analyses of the association between cumulative cannabis use and task performance. This permitted an assessment of cannabis use–task performance associations with propensity to drink approximately equated in the sample. Second, we assess mediation at the individual level in a within-pair framework [58], disaggregating each participant’s cannabis use score as for the drinking score and using drinking and cannabis use deviation scores as joint predictors of task performance.
Results
Preliminary analyses.
RAVLT performance was comparable across cohorts. Both cohorts showed the expected increase in number of words recalled across learning trials and forgetting on recall trials (Trials 6 and 7) (Figure 2). Interactions between cumulative drinking and age cohort, adjusted for sex and zygosity effects, were not significant for any performance measure (p-values ≥ 0.173). Interactions between cumulative drinking and sex, with age cohort and zygosity as covariates, were also not significant (p-values ≥ 0.144). We therefore combined cohorts for subsequent analyses. (Figure S3 in the Supporting Information provides a graphic examination of results in each cohort.)
Fig 2.
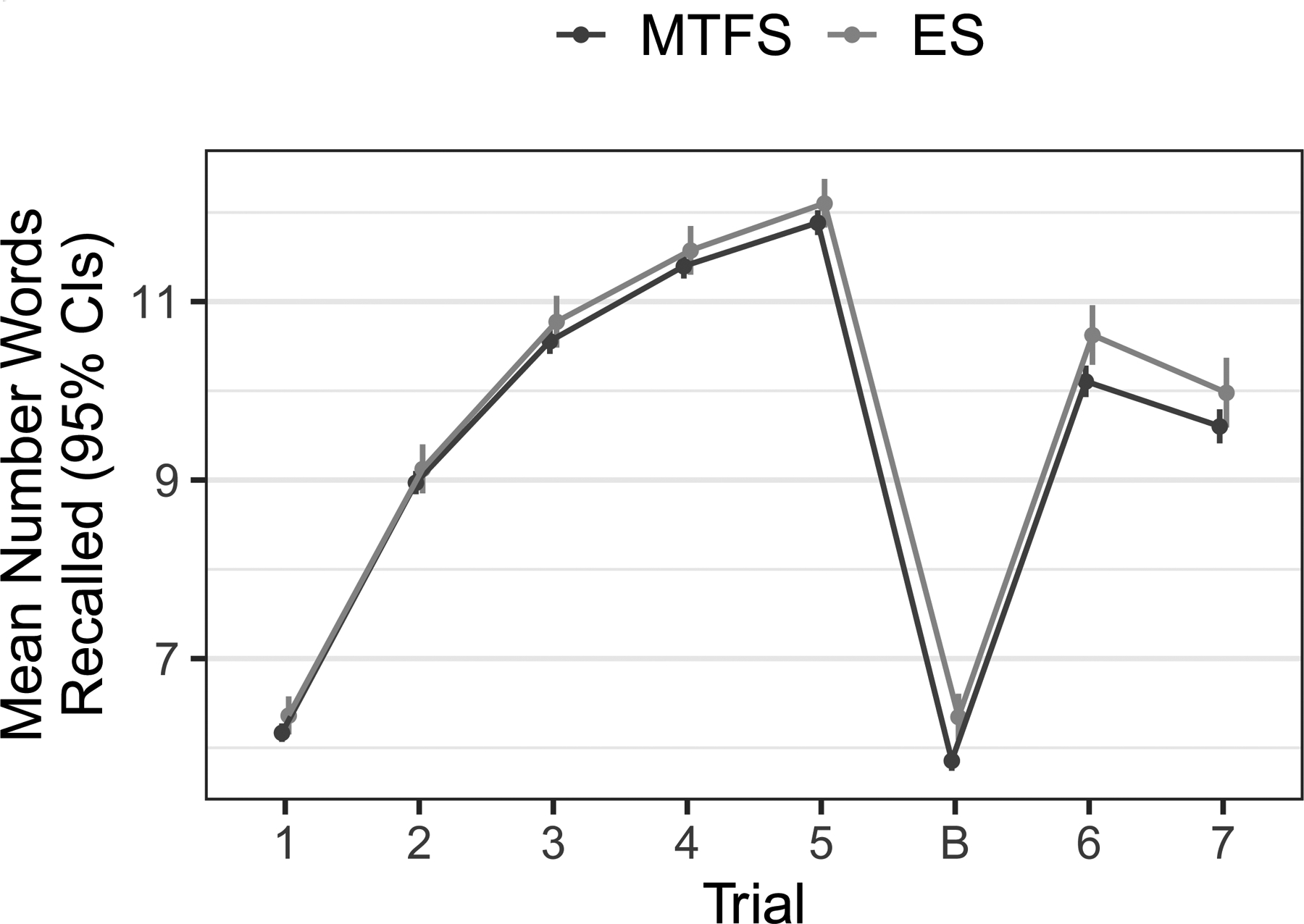
Words recalled on each trial plotted separately for the ES and MTFS cohorts. The figure indicates the expected and initially rapid improvement in performance across the five learning trials. A decrement in performance to approximately the level of the second to third learning trial is evident on the two recall trials (Trials 6 and 7), indicating forgetting.
Cumulative drinking and task performance.
The left-hand columns of Table 2 indicate a significant inverse association between cumulative drinking and the three measures of learning. There was also a significant inverse association between cumulative drinking and measures of attention/immediate memory (number of words recalled on Trial 1 and Trial B).
Table 2.
Individual-level, phenotypic associations between drinking and learning and memory in RAVLT.
| Measure | Unadjusted | Adjusted for Effects of IQ | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | t-statistic | p-value | Estimate | 95% CI | t-statistic | p-value | |
| Overall Learning | −0.381 | [−0.544— −0.219] | 4.60 | <.001 | −0.293 | [−0.443— −0.144] | 3.84 | <.001 |
| Learning Over Trials | −0.160 | [−0.278— −0.042] | 2.66 | .008 | −0.148 | [−0.265— −0.030] | 2.45 | .014 |
| Trial 5 Total | −0.094 | [−0.133— −0.054] | 4.60 | <.001 | −0.079 | [−0.117— −0.041] | 4.05 | <.001 |
| Trial 1 Total | −0.044 | [−0.074— −0.015] | 2.93 | .003 | −0.029 | [−0.057— −0.002] | 2.07 | .039 |
| Trial B Total | −0.077 | [−0.112— −0.042] | 4.29 | <.001 | −0.061 | [−0.093— −0.028] | 3.64 | <.001 |
| Trial B--Trial 1 | −0.032 | [−0.067— 0.002] | 1.84 | .067 | −0.031 | [−0.066— 0.003] | 1.77 | .077 |
| Immediate Retention | −0.010 | [−0.043— 0.023] | 0.60 | .550 | −0.010 | [−0.043— 0.023] | 0.01 | .991 |
| Delayed Retention | 0.004 | [−0.034— 0.042] | 0.22 | .823 | 0.015 | [−0.024— 0.053] | 0.74 | .457 |
Note: Estimate is the parameter estimate and 95% CI the 95% confidence interval around this estimate, obtained by means of the cluster-robust sandwich estimator of standard errors in svyglm. All parameter estimates are adjusted for any effects of age cohort, sex and zygosity. Estimates in the right-hand columns have been adjusted for effects of IQ measured at age 11.
To determine whether these associations might simply be due to individual differences in general intellectual ability, rather than reflecting specific alcohol effects on learning and memory, we repeated all analyses with full scale IQ as a covariate. Results indicated that IQ was significantly and positively associated with all measures of task performance except proactive interference (see Supporting Information), despite having been assessed many years earlier. However, associations between cumulative drinking and task performance adjusted for IQ remained significant (p < .05) (see the right-hand columns of Table 2).
Cotwin-control analyses.
Within-pair estimates were significant for all three measures of learning but not the two measures of attention (left-hand columns of Table 3). Thus, associations between cumulative drinking and learning are not due to shared confounding factors and are consistent with a causal effect of drinking, whereas associations between cumulative drinking and attention reflect confounding factors, such as a preexisting liability. Tests of the interaction between cohort and the twin-difference measure were not significant (p-values ≥ 0.284), indicating consistency of the within-pair effect across cohorts. Adjusting for individual differences in IQ did not affect the significance of within-pair effects on learning, all p-values ≤ .001.
Table 3.
Within-pair associations between drinking and measures of learning and attention with and without propensity score adjustment.
| Measure | Unadjusted | Propensity Score-Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | t-statistic | p-value | Estimate | 95% CI | t-statistic | df | p-value | |
| Overall Learning | −0.624 | [−0.964 — −0.283] | 3.59 | <.001 | −0.609 | [−0.949 — − 0.268] | 3.50 | 48.1 | .001 |
| Learning Over Trials | −0.449 | [−0.753 — −0.145] | 2.90 | .004 | −0.447 | [−0.752 — − 0.141] | 2.87 | 48.1 | .006 |
| Trial 5 Total | −0.161 | [−0.259 — −0.063] | 3.23 | .001 | −0.158 | [−0.257 — − 0.060] | 3.16 | 48.1 | .003 |
| Trial 1 Total | −0.035 | [−0.107 — 0.037] | 0.95 | .342 | −0.032 | [−0.104 — 0.040] | 0.88 | 48.1 | .383 |
| Trial B Total | −0.063 | [−0.139 — 0.013] | 1.63 | .103 | −0.061 | [−0.136 — 0.015] | 1.56 | 48.1 | .124 |
Note: Estimate is the parameter estimate for the within-pair effect and 95% the 95% confidence interval around it, obtained by means of the cluster-robust sandwich estimator of standard errors in svyglm, adjusted for any effects of age cohort, sex and zygosity. Estimates in the right-hand portion of the table are propensity score-adjusted estimates of the within-pair effect. Propensity score indicators were all from the age-11 assessment (see Table S2). Missing values were imputed 50 times, a propensity score estimated for each imputation set, and cotwin-control analyses were conducted on each set using inverse probability of treatment weighting (IPTW). Results of the 50 sets of IPTW-weighted analyses were combined as using the procedure outlined by Rubin [39]. df are corrected as recommended by Barnard and Rubin [2].
In separate CTC analyses of learning for MZ and DZ twins, the within-pair estimate was significant for DZ twins but not MZ twins for all three measures of learning (Figure 3), consistent with partial genetic confounding of the drinking–learning association. Interactions with zygosity were not significant (p-values 0.154), however, cautioning against an unqualified interpretation of results as reflecting partial genetic confounding. In addition, although the wide confidence intervals around the within-pair estimates in MZ and DZ twins signal that they are estimated imprecisely, the within-pair effects in MZ twins were comparable in magnitude to the individual-level effects, which is expected if confounding is absent [27].
Fig 3.
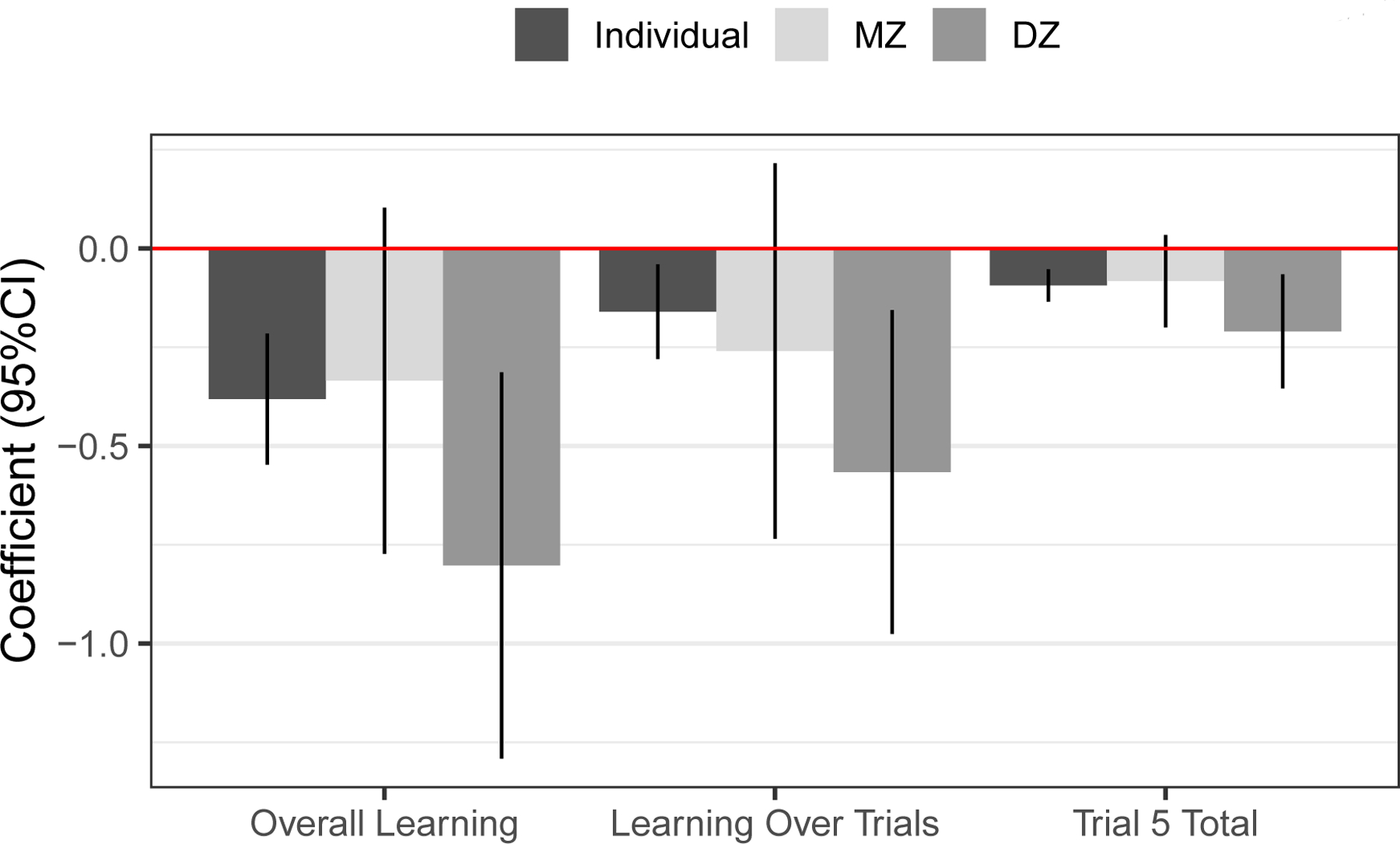
Within-pair estimates for MZ and DZ twins separately. Within-pair (deviation score) regression coefficients with 95% CIs around them are plotted for the three measures of learning, which yielded significant within-pair effects in the combined cohorts. For comparison, the individual-level estimate for these three measures is included as well. These correspond to the first three values in the “Estimate” column of Table 2.
Propensity score adjustment.
Propensity score indicators predicted cumulative exposure equally well in the two cohorts (see Supporting Information). The CBPS procedure achieved excellent propensity score balance (see Supporting Information). Adjusting the within-pair effect for propensity to drink via IPTW weighting did not substantively change the original results (right-hand columns of Table 3). The within-pair estimate remained significant for all measures of learning and thus does not appear to reflect unshared confounding due to measured characteristics.
Drinking in adolescence versus early adulthood.
Table 4 presents associations in the MTFS cohort between adolescent and early adult drinking, respectively, and task performance. Results for use through age 20 are in the left-hand columns and the same associations with change in use between age 20 and age 29 are in the right-hand columns. Significant inverse associations with all three measures of learning are evident for both measures of drinking. There was also a significant inverse association between the two measures of attention and adolescent drinking specifically.
Table 4.
Phenotypic associations between task performance and adolescent-limited or continuing alcohol use, respectively.
| Measure | Effects of Adolescent Drinking | Effects of Change in Drinking | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | z-statistic | p-value | Estimate | 95% CI | z-statistic | p-value | |
| Overall Learning | −0.410 | [−0.608 — −0.213] | 4.07 | <.001 | −0.723 | [−1.152 — −0.293] | 3.30 | .001 |
| Learning Over Trials | −0.180 | [−0.320 — −0.040] | 2.52 | .012 | −0.405 | [−0.722 — −0.087] | 2.50 | .013 |
| Trial 5 Total | −0.099 | [−0.147 — −0.051] | 4.02 | <.001 | −0.160 | [−0.271 — −0.049] | 2.83 | .005 |
| Trial 1 Total | −0.046 | [−0.080 — −0.012] | 2.69 | .007 | −0.064 | [−0.140 — 0.013] | 1.62 | .105 |
| Trial B Total | −0.088 | [−0.129 — −0.047] | 4.17 | <.001 | −0.091 | [−0.184 — 0.002] | 1.92 | .055 |
| Trial B —Trial 1 | −0.042 | [−0.083 — 0.000] | 1.96 | .050 | −0.027 | [−0.123 — 0.068] | 0.56 | .573 |
| Immediate Retention | −0.015 | [−0.053 — 0.022] | 0.80 | .422 | −0.105 | [−0.194 — −0.017] | 2.34 | .020 |
| Delayed Retention | <0.001 | [−0.043 — 0.042] | 0.01 | .989 | −0.048 | [−0.145 — 0.050] | 0.96 | .336 |
Note: Estimate is the parameter estimate and 95% CI the 95% confidence interval around it, obtained by means of the cluster-robust sandwich estimator of standard errors in svyglm. Parameter estimates are adjusted for any effects of sex and zygosity. In addition, estimates for each drinking measure are adjusted for effects of the other. “Effects of Adolescent Drinking” give associations between task performance measures and cumulative drinking through the age-20 assessment whereas “Effects of Change in Drinking” give associations for the change in drinking between the age-20 and age-29 assessments (the magnitude of increase or decrease) and the same performance measures. The p-value for the effect of adolescent drinking on the Trial B—Trial 1 difference score appears marginally significant but this is due to rounding.
Follow-up CTC analyses using twin deviations in both drinking measures yielded significant within-pair estimates for all measures of learning (Table 5), suggesting that drinking during adolescence and in early adulthood have independent effects on learning that cannot be ascribed to confounding factors. By contrast, the adolescent drinking–attention association was confounded with familial factors.
Table 5.
Within-pair effects of adolescent-limited and continuing alcohol use.
| Effects of Adolescent Drinking | Effects of Change in Drinking | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Estimate | 95% CI | z-statistic | p-value | Estimate | 95% CI | z-statistic | p-value |
| Overall Learning | −0.599 | [−0.944 — −0.254] | 3.41 | .001 | −0.807 | [−1.390 — −0.223] | 2.71 | .007 |
| Learning Over Trials | −0.436 | [−0.775 — −0.126] | 2.75 | .006 | −0.684 | [−1.338 — −0.030] | 2.05 | .041 |
| Trial 5 Total | −0.155 | [−0.254 — −0.056] | 3.07 | .002 | −0.197 | [−0.366 — −0.029] | 2.29 | .022 |
| Trial 1 Total | −0.033 | [−0.105 — 0.040] | 0.89 | .376 | −0.025 | [−0.169 — 0.120] | 0.33 | .739 |
| Trial B Total | −0.063 | [−0.140 — 0.013] | 1.62 | .106 | −0.076 | [−0.247 — 0.094] | 0.88 | .380 |
Note: Estimate is the parameter estimate for the within-pair effect and 95% CI the 95% confidence interval around it, obtained by means of the cluster-robust sandwich estimator of standard errors in svyglm. Parameter estimates are adjusted for any effects of sex and zygosity. In addition, estimates for each twin-difference drinking measure are adjusted for effects of the other. “Effects of Adolescent Drinking” give associations between task performance measures and cumulative drinking through the age-20 assessment whereas “Effects of Change in Drinking” give associations for the change in drinking between the age-20 and age-29 assessments and the same performance measures.
Are associations specific to alcohol use?
Associations between cumulative cannabis use and task performance are provided in Table S3 in the Supporting Information. As expected, they were similar to the pattern observed for drinking: cannabis use was inversely associated with two of three measures of learning (Overall Learning and Trial 5 Total) as well as both measures of attention/immediate memory. After adjusting for propensity to drink using IPTW weighting, however, none of these associations remained significant (right-hand columns of Table S3). In addition, within-pair mediation analyses using twin differences in both drinking and cannabis use failed to yield significant cannabis within pair estimates for any measure, whereas within-pair estimates for drinking were significant for all measures of learning (Table S4).
Discussion
In this investigation, we examined associations between cumulative drinking in adolescence and early adulthood (through one’s 20s) and measures of verbal learning, attention, memory and proactive interference. Drinking was significantly associated with all three measures of learning, whether adjusted for general intellectual ability or not. Follow-up analyses using a CTC design indicated that the drinking–learning association was consistent with a causal influence of drinking on learning, although we caution that the CTC cannot definitively establish causality. Drinking was also significantly associated with our two measures of attention/immediate memory. By contrast with the drinking—learning associations, these associations were confounded with familial factors and therefore likely reflect an underlying liability for drinking.
We derived propensity scores reflecting variation in propensity for different levels of drinking from measures collected at the age-11 assessment, before any real drinking began. CTC analyses of learning combined with IPTW weighting with this propensity score did not affect significance of within-pair estimates. Measured unshared confounders thus do not account for our results. We also determined the degree to which drinking during adolescence in particular might have driven our results. Findings in the younger ES cohort explicitly implicate adolescent drinking. In the older MTFS cohort, cumulative use through the age-20 assessment and drinking between 20 and 29 independently predicted our three measures of learning, and this was due to the within-pair effect.
Thus, drinking in general affects learning, and results of the CTC analyses are consistent with a causal effect even if we cannot rule out partial genetic confounding of the drinking—learning association. Results indicate a persisting, residual effect of adolescent drinking, consistent with the notion that adolescents are especially vulnerable to adverse effects of alcohol consumption [18]. However, adolescent drinking can be partially offset by a decrease in drinking in early adulthood (or exacerbated by an increase in drinking). In fact, this moderation effect was larger in magnitude than the adolescent drinking effect, although estimated with less precision (larger SEs). Amount of drinking closest in time to assessment of learning appears to have a greater effect than earlier consumption.
Finally, we examined effects of cannabis use, which commonly co-occurs with drinking and is associated with similar performance deficits on list learning tests. Indeed, the pattern of associations with task performance was virtually identical for the two substances. However, follow-up analyses, adjusting for propensity for drinking via IPTW weighting in one and using a within-pair mediation approach in the other, indicated that associations between drinking and learning were not due to co-occurring cannabis use. In fact, the reverse was true: associations between cannabis use and learning were due to co-occurring drinking.
Our finding that drinking was robustly associated with poorer learning has precedents in the literature on adolescent [32] and adult drinking [10, 42]. It has been suggested that a verbal learning deficit is specific to alcoholism that is not predominantly antisocial in nature [52]. Acute alcohol administration is associated with impaired verbal learning and short-term memory [24]. This effect is likely mediated by inhibitory effects on N-methyl-D-aspartic acid (NMDA) receptors for glutamate in the hippocampus and disruption of long-term potentiation [4], widely considered a cellular mechanism underlying learning in the hippocampus. Experimental administration of alcohol to rats produces deficits in hippocampus-dependent learning and memory [26] that may endure beyond acute exposure [55]. Our results are broadly consistent with these findings.
The CTC design: Properties and limitations.
The CTC design cannot definitively establish that a relationship between drinking and an outcome variable reflects a causal influence of drinking on the outcome. However, it is one of the best available tools for controlling confounding factors, whether measured or not, and thus ruling them in or out as potential causal influences. Centering an individual’s drinking score on the twin-pair mean, as in the twin-deviation score, removes confounders shared by twins, thereby producing a stronger test of the hypothesis that it is drinking per se that affects the outcome. Despite the inferential power this provides, the design has several limitations. Although unlikely here given the prospective nature of our design, it cannot rule out reverse causality. The within-pair estimate is unbiased only if exposure is measured without error and all confounders are completely shared [43]. Neither is likely to be true. Error in measurement of exposure attenuates estimates of within-pair effects, more so than individual-level effects, leading to an underestimate of the true effect [27]. The CTC design cannot control for unshared confounders – those influences that are unique to one or the other member of a twin pair. Our use of a propensity score was an attempt to control for such confounding. However, this is by definition limited to those characteristics that are measured (and precede exposure). Furthermore, we cannot know the full extent of unshared confounders. Therefore, a significant within-pair effect can only be consistent with a causal effect of drinking rather than determinative.
Including covariates to account for unshared confounders (or weighting by propensity score) can reduce bias in the within-pair estimate relative to the individual-level estimate, but it can also increase it. The degree and direction of bias depends on three factors: the twin correlation on exposure, the twin correlation on covariate and the sensitivity of the covariate to the unknown confounder [40]. The latter is unknown. The relative magnitudes of the two twin correlations suggest that upward bias is likely (for exposure, r = 0.74; for propensity score weight, r = 0.44). Thus, the estimated within-pair effect we observed may well be both biased upward and attenuated, the degree of attenuation being greater for MZ twins than DZ twins, making the magnitude of estimate difficult to ascertain. In addition, the wide confidence interval around these estimates in Figure 3 makes clear that they are not estimated precisely. Nevertheless, our results are most consistent with a substantively important within-pair effect.
Conclusions.
This investigation has many strengths, including use of two large, populationbased samples, multiple measures of attention, learning and memory, the CTC design and explicit treatment of potential confounding factors. The consistency of results -within and between the domains of learning and attention and between the two cohorts -is compelling and provides a strong measure of confidence in our findings. The overall pattern of results yields four specific conclusions: poorer auditory-verbal learning is a robust and specific correlate of drinking, a finding that replicated in independent samples; poorer learning may in fact be a consequence of drinking; it is not due to shared familial factors, those unshared confounders for which we could control statistically, nor to co-occurring cannabis use; and drinking in early adulthood as well as adolescence leads to poorer auditory-verbal learning performance.
Supplementary Material
Acknowledgements
Work on this paper was supported by grants R37DA005147, R37AA009367, R01DA036216, K01DA03720 and R21AA02969. We are grateful to Tasha Walvig for her gracious help verifying some of the interview data used and to Gretchen Saunders for helpful discussions about bias in the cotwin-control design.
Footnotes
The authors have no competing interests to declare.
References
- [1].Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research 46, 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barnard J, Rubin DB. Miscellanea. small-sample degrees of freedom with multiple imputation. Biometrika 86, 948–955. [Google Scholar]
- [3].Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361, 31–9. [DOI] [PubMed] [Google Scholar]
- [4].Blitzer RD, Gil O, Landau EM. Long-term potentiation in rat hippocampus is inhibited by low concentrations of ethanol. Brain Research 537, 203–208. [DOI] [PubMed] [Google Scholar]
- [5].Brown SA et al. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res 24, 164–71. [PubMed] [Google Scholar]
- [6].Broyd SJ et al. Acute and chronic effects of cannabinoids on human cognition—a systematic review. Biological psychiatry 79, 557–567. [DOI] [PubMed] [Google Scholar]
- [7].Carbia C et al. Binge drinking during adolescence and young adulthood is associated with deficits in verbal episodic memory. PLoS One 12, e0171393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carbia C et al. A systematic review of neuropsychological studies involving young binge drinkers. Neurosci Biobehav Rev 90, 332–349. [DOI] [PubMed] [Google Scholar]
- [9].Crews FT et al. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism-Clinical and Experimental Research 24, 1712–1723. [PubMed] [Google Scholar]
- [10].Daig I et al. Decreased verbal learning but not recognition performance in alcoholdependent individuals during early abstinence. Ir J Psychol Med 29, 96–101. [DOI] [PubMed] [Google Scholar]
- [11].Fong C, Ratkovic M, Imai K. CBPS: Covariate Balancing Propensity Score. R package version 0.14 2017. [Google Scholar]
- [12].Funder DC, Ozer DJ. Evaluating effect size in psychological research: sense and nonsense. Advances in Methods and Practices in Psychological Science 2, 156–168. [Google Scholar]
- [13].Hanson KL et al. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychol Addict Behav 25, 127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hoffman R, Al’absi M. Concurrent use of khat and tobacco is associated with verbal learning and delayed recall deficits. Addiction 108, 1855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Holdcraft LC, Iacono WG. Cross-generational effects on gender differences in psychoactive drug abuse and dependence. Drug Alcohol Depend 74, 147–58. [DOI] [PubMed] [Google Scholar]
- [16].Hommer D et al. Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry 158, 198–204. [DOI] [PubMed] [Google Scholar]
- [17].Ivnik RJ et al. The Auditory-Verbal Learning Test (AVLT): norms for ages 55 years and older. Psychological Assessment: A Journal of Consulting and Clinical Psychology 2, 304–312. [Google Scholar]
- [18].Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annu Rev Clin Psychol 9, 703–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Keyes MA et al. The Enrichment Study of the Minnesota Twin Family Study: increasing the yield of twin families at high risk for externalizing psychopathology. Twin research and human genetics: the official journal of the International Society for Twin Studies 12, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lezak MD. Neuropsychological Assessment. 4th ed. New York: Oxford University Press, 2004. [Google Scholar]
- [21].Lumley T. Analysis of complex survey samples. Journal of Statistical Software 9, 1–19. [Google Scholar]
- [22].Lumley T. survey: analysis of complex survey samples. R package version 3.35–1. 2019. [Google Scholar]
- [23].Lynskey MT, Fergusson DM, Horwood LJ. The origins of the correlations between tobacco, alcohol, and cannabis use during adolescence. J Child Psychol Psychiatry 39, 995–1005. [PubMed] [Google Scholar]
- [24].Magrys SA, Olmstead MC. Alcohol intoxication alters cognitive skills mediated by frontal and temporal brain regions. Brain Cogn 85, 271–6. [DOI] [PubMed] [Google Scholar]
- [25].Malone SM et al. Adolescent drinking and motivated decision-making: a cotwincontrol investigation with monozygotic twins. Behavior Genetics 44, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Markwiese BJ et al. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res 22, 416–21. [PubMed] [Google Scholar]
- [27].McGue M, Osler M, Christensen K. Causal inference and observational research: the utility of twins. Perspectives on Psychological Science 5, 546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McGue M et al. Parent-offspring similarity for drinking: a longitudinal adoption study. Behavior Genetics 44, 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morris RG et al. Selective impairment of learning and blockade of long-term potentiation by an n-methyl-d-aspartate receptor antagonist, ap5. Nature 319, 774–6. [DOI] [PubMed] [Google Scholar]
- [30].Mota N et al. Binge drinking trajectory and neuropsychological functioning among university students: a longitudinal study. Drug Alcohol Depend 133, 108–14. [DOI] [PubMed] [Google Scholar]
- [31].Nguyen-Louie TT et al. Effects of emerging alcohol and marijuana use behaviors on adolescents’ neuropsychological functioning over four years. J Stud Alcohol Drugs 76, 738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nguyen-Louie TT et al. Learning and memory in adolescent moderate, binge, and extreme-binge drinkers. Alcohol Clin Exp Res 40, 1895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nguyen-Louie TT et al. Earlier alcohol use onset predicts poorer neuropsychological functioning in young adults. Alcohol Clin Exp Res 41, 2082–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Parada M et al. Binge drinking and declarative memory in university students. Alcohol Clin Exp Res 35, 1475–84. [DOI] [PubMed] [Google Scholar]
- [35].R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria, 2017. [Google Scholar]
- [36].Reich W. Diagnostic Interview for Children and Adolescents (DICA). Journal of the American Academy of Child and Adolescent Psychiatry 39, 59–66. [DOI] [PubMed] [Google Scholar]
- [37].Robins LN, Babor TF, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis: Authors, 1987. [Google Scholar]
- [38].Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 70, 41–55. [Google Scholar]
- [39].Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons, 2004. [Google Scholar]
- [40].Saunders GRB, McGue M, Malone SM. Sibling comparison designs: addressing confounding bias with inclusion of measured confounders. Twin Res Hum Genet 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sawyer SM et al. The age of adolescence. Lancet Child Adolesc Health 2, 223–228. [DOI] [PubMed] [Google Scholar]
- [42].Sherer M et al. Performance of alcoholic and brain-damaged subjects on the Luria Memory Words test. Arch Clin Neuropsychol 7, 499–504. [PubMed] [Google Scholar]
- [43].Sjölander A, Frisell T, Öberg S. Causal interpretation of between-within models for twin research. Epidemiologic Methods 1, 217–237. [Google Scholar]
- [44].Smith JL et al. Verbal learning and memory in cannabis and alcohol users: an event-related potential investigation. Front Psychol 8, 2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sneider JT et al. Differential effects of binge drinking on learning and memory in emerging adults. J Addict Res Ther Suppl 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Solowij N et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl) 216, 131–44. [DOI] [PubMed] [Google Scholar]
- [47].Spear LP. Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci 19, 197–214. [DOI] [PubMed] [Google Scholar]
- [48].Substance Abuse and Mental Health Services Administration. Results from the 2017 National Survey on Drug Use and Health: Detailed tables. Report. 2018.
- [49].Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: four-year outcomes. J Int Neuropsychol Soc 5, 481–93. [DOI] [PubMed] [Google Scholar]
- [50].Tapert SF et al. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society 8, 873–883. [DOI] [PubMed] [Google Scholar]
- [51].Vakil E, Blachstein H. Rey auditory-verbal learning test: structure analysis. J Clin Psychol 49, 883–90. [DOI] [PubMed] [Google Scholar]
- [52].Waldstein SR et al. Predictors of neuropsychological impairment in alcoholics: antisocial versus nonantisocial subtypes. Addict Behav 21, 21–7. [DOI] [PubMed] [Google Scholar]
- [53].Wechsler D. Manual for the Wechsler Intelligence Scale for Children–Revised. San Antonio, TX: The Psychological Corporation, 1981. [Google Scholar]
- [54].Welner Z et al. Reliability, validity, and parent-child agreement studies of the Diagnostic Interview for Children and Adolescents (DICA). Journal of the Academy of Child and Adolescent Psychiatry 26, 649–653. [DOI] [PubMed] [Google Scholar]
- [55].White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann N Y Acad Sci 1021, 206–20. [DOI] [PubMed] [Google Scholar]
- [56].Wilson S et al. Problematic alcohol use and hippocampal volume in a female sample: disentangling cause from consequence using a co-twin control study design. Psychol Med 48, 1673–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Winward JL et al. Adolescent heavy episodic drinking: neurocognitive functioning during early abstinence. J Int Neuropsychol Soc 20, 218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang Z, Zyphur M, Preacher K. Testing multilevel mediation using hierarchical linear models. Organizational Research Methods 12, 695–719. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


