Abstract
The nervous system is a significant part of the human body, and peripheral nerve injury caused by trauma can cause various functional disorders. When the broken end defect is large and cannot be repaired by direct suture, small gap sutures of nerve conduits can effectively replace nerve transplantation and avoid the side effect of donor area disorders. There are many choices for nerve conduits, and natural materials and synthetic polymers have their advantages. Among them, the nerve scaffold should meet the requirements of good degradability, biocompatibility, promoting axon growth, supporting axon expansion and regeneration, and higher cell adhesion. Polymer biological scaffolds can change some shortcomings of raw materials by using electrospinning filling technology and surface modification technology to make them more suitable for nerve regeneration. Therefore, polymer scaffolds have a substantial prospect in the field of biomedicine in future. This paper reviews the application of nerve conduits in the field of repairing peripheral nerve injury, and we discuss the latest progress of materials and fabrication techniques of these polymer scaffolds.
Keywords: peripheral nerve injury, nerve conduits, nerve reconstruction, polymeric scaffold, natural and synthetic polymers
1. Introduction
Peripheral nerve injury (PNI) is a frequently occurring disease, which means that the peripheral nerve plexus, nerve trunk, or its branches are damaged by an external force. The incidence of new peripheral nerve injury is increasing worldwide, and about one million patients need peripheral nerve surgery every year [1]. Peripheral nerve injury is usually caused by traumatic lesions such as penetrating wounds, squeezing, stretching, and also ischemia [2]. From 1% to 3% of trauma patients will have an injury involving a peripheral nerve [3,4]. Although peripheral nerves have a significant ability to regenerate after injury, long-term nerve defects after peripheral nerve injury (PNI) are a challenge to entire recovery [5]. Functional recovery of peripheral nerve injury is a slow process. In order to achieve better neural function quickly, a variety of nerve suture methods and new polymer nerve conduits have been widely proposed. The development of polymer nerve conduits to enhance nerve repair remains an important research area, and future clinical developments in the area of nerve regeneration and repair could potentially improve patient outcomes.
This paper reviews the applications of different polymer scaffold nerve conduits in peripheral nerve injury, discusses the possible reasons, advantages, and disadvantages of each material in promoting nerve functional rehabilitation, and puts forward a possible direction for developing nerve repair materials in the future.
2. Use of Conduits
Direct tensionless end-to-end repair is considered a significant factor in the successful recovery of sensorimotor function. When there is a long peripheral nerve defect that cannot be directly repaired without tension, autologous nerve transplantation is considered the gold standard [6,7]. However, this approach often leads to obvious donor site complications. With the development of new materials, the effect of nerve conduit repair is close to, or even better than, that of nerve graft. Besides, some new materials have absorbability and good biocompatibility. No axon escape, promotes axon growth and maximizes the amplification and crossing domination of nerve innervation, making it possible for small axons to repair large axons quickly. Research into tissue engineering scaffold materials widely use natural scaffold materials, synthetic degradable polymers, and composite scaffold scaffolds.
3. Natural Materials
The natural biofunctional scaffold is composed of biomaterials full of promoting elements, such as nerve growth factor, vascular endothelial growth factor, and neurotrophic factor, providing a promising strategy for the regeneration of peripheral nerve defects. Various natural, synthetic, and biological materials have been applied to fabricate artificial nerve conduits. Natural-based materials are widely available, although their histocompatibility differs from that of acellular nerve grafts. For example, chitosan can be used as an absorbable natural material when used in nerve regeneration. Extracellular matrix (ECM) components have mostly been used for nerve reconstruction. Among these natural polymers are laminin, fibronectin, and collagen. Nerve conduits made of different materials have different biophysical characteristics, but there are still challenges in promoting functional rehabilitation.
3.1. Chitosan
As a kind of linear polysaccharide, chitosan has many similarities with the extracellular matrix and has specific tension resistance and plasticity. However, physically guided nerve catheters may not be sufficient to promote optimal recovery [8]. After implantation, the conduit should degrade as the nerve regenerates. Nerve conduits made using biodegradable materials have been considered especially promising. Water-soluble, biocompatible and non-toxic chitosan is a natural substitute and can be used as a conduit for axonal regeneration. The design of chitosan catheters can provide an environment conducive to axon regeneration and nerve regeneration, until the vascularization of regenerated tissue, and the degradation of catheter materials [9]. Many studies have shown that chitosan catheters can be used as a suitable scaffold biomaterial to promote nerve regeneration with minimum cytotoxicity and high biodegradability [10]. Another study showed a large number of axons in the chitosan embedded nerve graft group, compared with the nerve graft group alone. The purpose of an absorbable nerve conduit is to quickly provide a tranquil environment for nerve regeneration, and then degrade without an inflammatory reaction after nerve reconstruction. For defects with long nerve stump distance, studies have shown that a chitosan catheter can induce significant motor and sensory axon regeneration in a mouse model with a 10–15 mm nerve injury [11,12]. Even in the study of Ding, neural scaffolds made of chitosan and its copolymers combined with stem cells, showed certain target muscle reinnervation in the repair of a 50 mm defect model [13].
In addition to the separate application of chitosan catheters, there are many experiments in which chitosan is combined with a variety of cells, nerve growth factors, or other implants to achieve a better effect in promoting nerve regeneration. For example, Azizi et al. used a chitosan catheter to load α-lipoic acid to promote sciatic nerve regeneration in rats [14]. Similarly, a chitosan catheter combined with hyaluronic acid prevents sciatic nerve scar in rats with a peripheral nerve crush injury. Artificial nerve grafts composed of a mixture of chitosan and synthetic polymers have a more remarkable ability to regenerate when combined with Sertoli cells. In some studies, a chitosan conduit and SF fibers were used as fundamental biomaterials for reconstituting SC-derived ECM, and to generate a hybrid nerve scaffold [15]. It is suggested that the transplantation into an injured 10 mm sciatic nerve of tissue-engineered nerve, constructed by chitosan nerve conduit, can increase the diameter and area of axons, improve motor function and improve the electrical conductivity of regenerated axons [16].
3.2. EMC and Its Derivatives
Many extracellular matrix components (EMC) and their derivatives can be made into nerve scaffolds after a series of processes, including laminin, collagen, glycosaminoglycan, elastin, and fibronectin. Remarkable progress has been made in many studies on applying these components alone, or in combination with other synthetic fibers. Besides, some natural proteins, polysaccharides can also be used to manufacture biological scaffolds, such as silk fibroin, spider silk protein, keratin, and alginate, which are similar to nerve conduits made by extracellular matrix components.
3.2.1. Collagen
Collagen is the main structural protein in the human body, collagen-rich nerve bridging scaffolds and collagen-binding NGF could be useful biomaterials for the repair of severe nerve injuries [17]. Compared with autogenous nerve transplantation, bridging a 30-mm nerve defect using collagen filaments achieved a good result [18]. Although the collagen nerve guide channel has been successful in nerve repair, collagen is relatively expensive, and because of the lack of mechanical strength, challenging to deal with in the suturing process [19]. In terms of combining cytokines, a comparative experiment of artificial nerve catheters has been carried out, including collagen extract alone and autologous nerve transplantation. Cui et al. used a functional collagen nerve conduit combined with nerve cytokines to bridge a 35 mm long facial nerve gap. A miniature pig model was used to evaluate facial nerve regeneration at a longer distance than the rodent model [20]. They concluded that the collagen tubules filled with BDNF-rich collagen appeared to be at least as effective as autologous nerve grafts in compensating for the short facial nerve gap [21]. Furthermore, the rats repaired by Waitayawinyu and his team using biological scaffolds made of collagen polymers, showed better isometric muscle contractility, axon count, wet muscle weight, and axial protruding bud tissue [22].
3.2.2. Laminin
In addition to collagen, laminin present in the vascular basal lamina can also act as a conduit for axonal growth [23,24]. It is expressed in the basement membranes surrounding peripheral nerves, capillaries, brain, and skeletal muscle [25]. Because it can regulate the proliferation, differentiation, myelin formation, and differentiation of Schwann cells, it is considered a potential material for nerve repair [26,27]. In addition, a laminin catheter with multi-layer design is appropriate for surgical implantation. Laminin and laminin-polycaprolactone (PCL) blend nanofibers, can mimic peripheral nerve basement membrane [28]. Improvement of sciatic nerve regeneration has been shown using laminin-binding human NGF-beta [29]. Furthermore, the improvement of axon growth was also observed in nerve guides filled with laminin gel [30]. These results prove the powerful effect of laminin in promoting nerve regeneration.
3.2.3. Alginate Keratin Silk Fibroin Protein
Alginate is a kind of polysaccharide widely distributed in brown algae’s cell wall. Alginate can promote Schwann cells’ survival and growth and promote the neurite germination of embryonic dorsal root ganglion neurons [31]. Fannon et al. demonstrated that the aggregation and differentiation of mouse embryonic stem cells encapsulated in an alginate scaffold, could achieve aggregates similar to embryonic stem cells in the standard environment [32]. Keratin-derived nerve scaffolds can also effectively repair 4-mm tibial nerve defects in mice and 10–15 mm sciatic nerve defects in rats [33,34,35]. In addition to alginate and keratin, silk fibroin is also widely used in biological scaffolds, and stem cells on silk fibroin scaffolds can be induced faster, and promote axonal regeneration. These non-extracellular matrix components can be polymerized or modified naturally, to produce scaffolds that are more in line with requirements.
4. Synthetic Polymers
Because synthetic polymers can better simulate the physical and chemical properties of nerve tissue, the polymerization or esterification between different materials may have more advantages in tissue engineering. Therefore, it has attracted significant attention in the field of biological regeneration. Like natural polymers, synthetic nerve conduits connect nerve posts’ gaps, to guide and support nerve regeneration. They can be broadly classified into degradable and non-degradable materials. However, experiments have shown that biodegradable nerve catheters are more effective in promoting the regeneration of peripheral nerves than silicone catheters [36]. This means soluble polymer nerve conduits have become a promising research direction in modern tissue engineering.
4.1. Synthetic Non-Degradable Polymers
The synthesized non-degradable polymer, organosilicon, is one of the earliest nerve repair materials. When, in rats, a sciatic nerve defect of 10 mm was repaired with a silicone conduit, the unmyelinated nerve fibers could be regenerated on the 16th day, and the muscle strength of gastrocnemius muscle was partially restored on the 120th day [37]. Clinical studies have shown that when, at the midpoint, the nerve is removed to form a 10 mm gap and bridged with the silicone tube, the repair effect is still considerable after 3–5 years [38]. Because silicon conduits have achieved good results in repairing peripheral nerves, it is still meaningful to undertake repair based on single-compartment silicon derivatives, or combined with nerve growth factor. However, these synthetic non-degradable scaffolds will remain in the body after axonal regeneration, causing foreign bodies or space-occupation, and limiting their development.
4.2. Synthetic Degradable Polymers
The ideal synthetic degradable scaffold should be biocompatible, display a macrostructure that supports cell growth and nutrient and waste exchange, and a surface that allows for cell migration, attachment, and differentiation. This yields a fully degradable, porous, highly interconnected scaffold. Comparing degradable and non-degradable scaffolds reveals that degradable scaffolds promote vascularization, in terms of the amount and invasion depth [39].
4.2.1. PLA, PGA, and Its Derivatives
The nerve conduit fabrication method can also be applied to various polymers, such as polylactic acid (PLA), polyglycolic acid (PGA), and its derivatives. PLA can be extracted from renewable resources such as potatoes and sugarcane. A variety of methods can synthesize it, including ring-opening polymerization of lactide dimer in the presence of suitable catalysts, lactic acid polycondensation, or enzymatic synthesis of lipases in the absence of metal catalysts [40,41]. Because PLA has good shaping and molding properties, it is the preferred base material for nerve regeneration pipelines [42]. PLA conduits facilitate nerve regeneration in rats with sciatic nerve injury and are considered a promising alternative for autologous nerve grafts [43,44]. Lu et al. used a polylactic acid (PLA) fiber-reinforced catheter to evaluate peripheral nerve regeneration in a 10 mm rat sciatic nerve model. The nerve bundle of the PLA group was significantly more extensive than that of other groups. The reason may be that the artificial catheter provides an essential microenvironment for peripheral nerve regeneration, especially the effect of nerve amplification. However, PLA itself does not cater to any nerve regeneration activity. Compared with PGA, PLA has stronger hydrophobicity, and its degradation by-product is lactic acid, which is easily eliminated by metabolism [45]. Polyglycolic acid is a linear aliphatic polyester that can be extracted from glycolide synthesized from sugarcane or pineapple. The ester group is responsible for stabilizing the structure of the polymer. The degradation product is the glycolic acid group, which can be excreted in urine. This polymer can be prepared by ring-opening polymerization of GA [46]. Poly (α-hydroxy ester) polymer, is a polymer formed by polylactic acid (PLA) and polyglycolic acid (PGA). Compared with its components, poly (α-hydroxy ester) polymer, when used in biological scaffolds and nano-drug delivery systems, is more easily processed and shows high biocompatibility, high mechanical strength, low toxicity, biodegradability, and non-immunogenicity. Ester bond cleavage can be reduced to polylactic acid and polyglycolic acid to continue its metabolism. PLGA is a copolymer formed by PLA and PGA, and because its surface has a more microporous structure, it can better load and slowly release growth factors and other complexes, and may be more promising.
4.2.2. Polyethylene Glycol (PEG)
Polyethylene glycol (PEG) has a low adverse immune reaction, which is considered to prolong the release time of the complex, and as a filler, it is commonly used in nerve cannulas. Being a hydrogel, it can be affected by polyethylene glycol and change its mechanical properties, so is widely used in the field of cosmetics.
4.2.3. Polycaprolactone (PCL)
Polycaprolactone (PCL) is a kind of aliphatic polyester. There are two main methods for the synthesis of polycaprolactone. One is the polycondensation of hydroxycarboxylic acid; the other is the ring-opening polymerization of lactone [47]. PCL polymer will be hydrolyzed into low molecular weight oligolactone (OCL) under in vivo conditions. Next, macrophages and giant cells eliminate larger fragments by phagocytosis, and finally, esterase degrades smaller fragments in cells [48]. The degradation of PCL is prolonged and takes up to 24 months [49]. Therefore, some studies prefer to classify it as a non-degradable biomaterial.
4.2.4. Composite Material
The hydrophobicity of PLA or the non-adhesion of PCL make more and more scholars devote themselves to the research and development of composite materials. Luis et al. used a neural tube made of DL-lactide-ε-caprolactone copolyester to bridge the sciatic nerve’s 10 mm defect in rats [50]. Polymeric polymers can also be mixed to obtain better properties and higher surface adhesion, such as PCL-PLGA scaffolds. Alternatively, synthetic biomaterial scaffolds are becoming convenient because of their excellent mechanical properties, pure chemical composition, and degradation rate. However, these synthetic scaffolds lack biometric recognition. The major challenge in developing a scaffold, lies primarily in the choice of a blend of biomaterials with the correct combination of properties [51]. If these composites can better combine bioactive factors such as stem cells, they may promote better repair.
5. Other Material
Many other materials, such as fiberglass, ceramics, and metals, have been implanted into damaged peripheral nerves to achieve regeneration. For example, some mixtures of metal magnesium are implanted into the nerve conduit, which can satisfy degradability and increase electrical conductivity, in an attempt to achieve a better effect on nerve repair.
6. Conduit Spatial Structure and Fillers
Using natural or synthetic polymers as raw materials, various techniques are used to prepare peripheral nerve regeneration scaffolds. If the production process is different, its biological and physicochemical properties are also different. Melt extrusion is one of the earliest processes used in the manufacture of neural scaffolds. However, this process is only suitable for some thermoplastic materials. The high temperature in the production process can lead to denaturation and deactivation of materials, and now that more attention is paid to the biocompatibility of materials, this process has been phased out [52,53]. Another method, is the freeze-drying integrated molding process, in which active materials, such as proteins, or easily degradable materials, such as polysaccharides, can be processed [54]. The products produced in this way have higher porosity and are conducive to cytokine infiltration, vascular component exchange, and nutrient transmission. Other processes such as enzyme digestion, or chemical treatment can meet different needs. Given the discovery of more and more excellent materials and the maturity of technology, 3D printing has gradually become the mainstream mode of catheter production. In this process, ducts can be arbitrarily edited into the structure they need, or a specific pore structure can be mixed with a variety of materials such as living cells [55,56]. In addition, some techniques incorporate nanofibers into printed structures to simulate extracellular matrix to achieve higher biocompatibility.
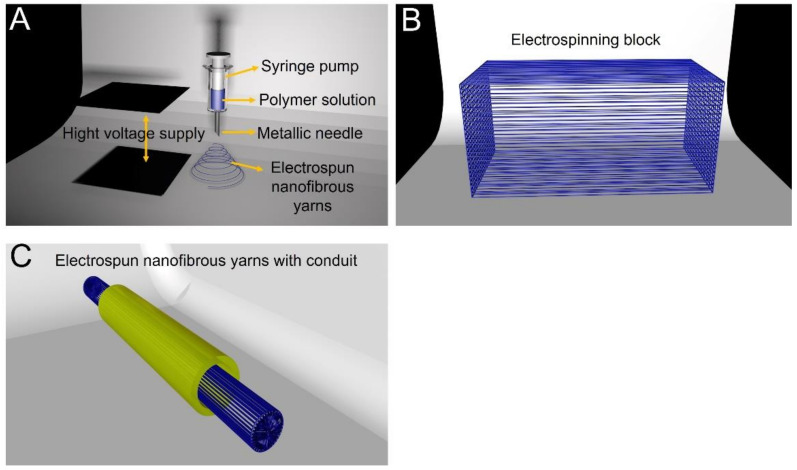
6.1. Electrospun Nanofibrous Yarns and Conduits
Electrospinning is a multi-purpose and cost-effective process for producing non-woven micro/nanofibers, which can provide an excellent ECM-like environment for tissue and cell growth [57,58]. Electrospun fibers can meet various structural requirements for tissue engineering scaffolds, such as porosity, pores that communicate with each other, adjustable void size, functional filaments, and adjustable morphology [59,60,61]. Electrospinning is a standard method for the preparation of micro-nano fiber [62]. The electric field strength can adjust the diameter of the fiber, mainly by adjusting three parameters in the spinning process: the applied voltage, the defined flow rate, and the distance between the needle and the grounding electrode [63]. The material, shape, and the size of the receiving device, can also change the electric field’s strength and shape, affecting the fiber’s diameter; Figure 1A clearly describes this process. Electrospun micro/nanofilaments can produce a high specific surface area, so that the slow release and diffusion of therapeutic drugs can be achieved through the slow degradation of polymers. The therapeutic drug used in electrospinning, when released, reaches the therapeutic concentration locally and is released continuously; avoiding the damage to other organs that can be caused by systematic administration [64,65]. It is reported that the fiber-containing NGF, can be obtained by electrospinning polycaprolactone and ethyl ethylene copolymer directly, mixed with human β nerve growth factor (NGF) [66]. Due to phase separation, NGF encapsulated in the electrospinning solution is uniformly distributed in the fiber during aggregation. Bioactive NGF can be released in vitro for at least three months, especially when the spun fiber is made to have a unique three-dimensional configuration, as shown in Figure 1B. Some researchers show that, because of their special potential, blends of PHAs may be used in the manufacture of multichannel and electrospun NGCs [67]. From in vivo studies, it was also found that sustained-release could be achieved. Results of the total number of myelinated axons, a cross-sectional area of a regenerated nerve, and the G ratio, confirmed that the directional electrospinning fiber conduit, encapsulated by glial cell line-derived neurotrophic factor (GDNF), could significantly promote the regeneration of rat sciatic nerve [68]. Bovine serum albumin (BSA) was added to the spinning solution of PCL and NGF for electrospinning. The addition of BSA not only increased the total amount of NGF released from the filaments, but also improved the biological activity of the released NGF and promoted the neurite formation of PC12 cells. As shown in Figure 1C, the nerve sleeve made of this electrospinning structure can be compounded with a variety of nutritional factors to promote the growth of axons. Therefore, electrospinning technology has been successfully applied to the preparation of peripheral nerve injury conduits [69].
Figure 1.
(A) Electrospinning fiber production process. (B) Electrospinning fiber is made into an electrospinning block with a specific structure. (C) The electrospinning block is filled into the nerve conduit to suture the broken end of the peripheral nerve.
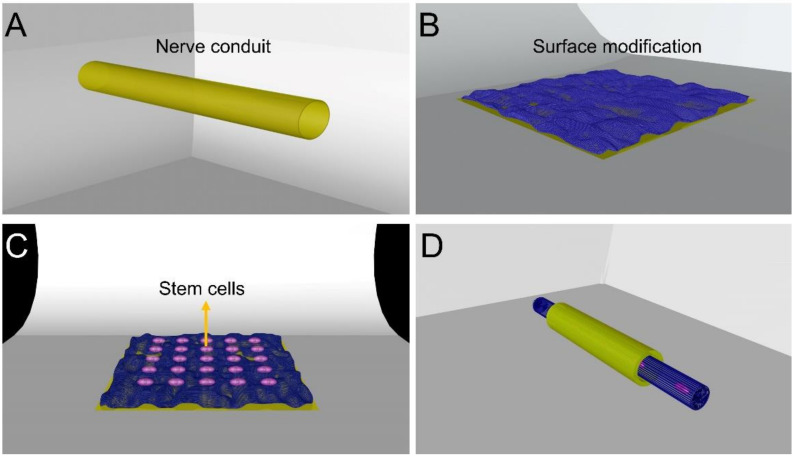
6.2. Surface Modification Technology
The interaction between cells and carriers is primarily limited by the surface characteristics and structure of the vessel. Although nanofibers can mimic the morphology of extracellular matrix, some modifications are still needed [70,71]. These are necessary to obtain essential characteristics that can respond to specific biological signals and improve cell attachment, cell proliferation, cell differentiation, and tissue regeneration [72,73,74]. The adhesion of cells can be improved by changing the catheter’s inner surface roughness; Figure 2A,B shows the process of this surface modification. For example, García-Gareta et al.’s data showed that CaP deposits would promote initial attachment, proliferation, and osteogenic differentiation of BM-MSCs [75]. The graphene oxide layer can be sprayed on the surface to improve the roughness, wrinkling, hydrophilicity, and electrical conductivity of nanofibers. Studies have shown that electrical stimulation can guide axon orientation and axon extension, thus promoting nerve regeneration, and Ghasemi-Mobarakeh et al. have summarized the different modification methods that can be carried out to modify conductive biomaterials [76]. Therefore, coating or mixing conductive compounds in scaffolds can improve cell activity, neural signal transduction, extensive dendritic branches, axonal growth, nerve proliferation, migration, and differentiation, resulting in functional recovery. Therefore, it may be a promising direction to imitate neural conductivity with biomaterials [77,78,79]. As mentioned above, the use of nerve repair cells, as shown in Figure 2C, and surface-modified nerve conduit materials, can promote cell attachment and proliferation. These can then be made into a tube; refer to the legend for Figure 2D. Surface modification technology is of great significance in polymerizing polymer repair materials. Whether it is through surface spraying to improve the roughness of the casing surface and promote the attachment of growth factors and cells, or through multi-layer three-dimensional structures to increase the specific surface area of the casing and change the electrical conductivity; all provide potential possibilities for the repair of injured nerves.
Figure 2.
(A) Common nerve conduit model surface. (B) Use of specific surface modification, to change the roughness of the surface or the three-dimensional surface area. (C) This surface-modified nerve conduit material can promote cell attachment and proliferation. (D) Nerve catheter made of cells that promote peripheral nerve regeneration such as Schwann cells.
7. Conclusions and Prospects
As peripheral nerve injures caused by various injuries can seriously reduce patients’ quality of life, polymerized nerve stents can be used to promote the injury repair, especially when the defect is large. Therefore, researchers worldwide are working hard to promote efficient medical devices and biomaterials to promote the effectiveness of neural rehabilitation. Some synthetic polymer materials have been widely used in the clinic, with a high efficiency of nerve repair, few side effects, and some characteristics that traditional nerve repair materials do not have. These include an appropriate degradation rate, designable material structure, variable surface characteristics, and variable electrical conductivity. On the other hand, synthetic scaffolds face many challenges because of their high hydrophobicity, low cell adhesion, and potential rejection. The application of electrospinning inner plant and surface modification technology, has dramatically expanded its application to change these defects. In the clinic, in order to improve the repair effect of peripheral nerve injury, polymer stenting is only one of the links, and other techniques, such as finding suitable electrical stimulation, are also essential. In the future, polymer materials with better biocompatibility may have a high prospect in biological tissue engineering for nerve repair.
Author Contributions
The literature survey and first draft writing were performed by M.Z., L.-P.Z., and C.L. W.P. designed and drew the picture, including the revisions, which were accomplished by P.-X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China, No. 31771322; Beijing Natural Science Foundation, No. 7212121; Major R&D Program of National Ministry of Science and Technology of China, No. 2018YFB1105504.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability:
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Daly W.T., Yao L., Abu-rub M.T., O’Connell C., Zeugolis D.I., Windebank A.J., Pandit A.S. The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials. 2012;33:6660–6671. doi: 10.1016/j.biomaterials.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Watson J.C., Huntoon M.A. Neurologic Evaluation and Management of Perioperative Nerve Injury. Reg. Anesth. Pain Med. 2015;40:491–501. doi: 10.1097/AAP.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 3.Zuckerman S.L., Kerr Z.Y., Pierpoint L., Kirby P., Than K.D., Wilson T.J. An 11-year analysis of peripheral nerve injuries in high school sports. Phys. Sportsmed. 2019;47:167–173. doi: 10.1080/00913847.2018.1544453. [DOI] [PubMed] [Google Scholar]
- 4.Kouyoumdjian J.A., Graça C.R., Ferreira V.F.M. Peripheral nerve injuries: A retrospective survey of 1124 cases. Neurol. India. 2017;65:551–555. doi: 10.4103/neuroindia.NI_987_16. [DOI] [PubMed] [Google Scholar]
- 5.Gu X., Ding F., Williams D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35:6143–6156. doi: 10.1016/j.biomaterials.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 6.Griffin J.W., Hogan M.V., Chhabra A.B., Deal D.N. Peripheral nerve repair and reconstruction. J. Bone Jt. Surg. Am. 2013;95:2144–2151. doi: 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- 7.Braga Silva J., Marchese G.M., Cauduro C.G., Debiasi M. Nerve conduits for treating peripheral nerve injuries: A systematic literature review. Hand Surg. Rehabil. 2017;36:71–85. doi: 10.1016/j.hansur.2016.10.212. [DOI] [PubMed] [Google Scholar]
- 8.Chaw J.R., Liu H.W., Shih Y.C., Huang C.C. New designed nerve conduits with a porous ionic cross-linked alginate/chitisan structure for nerve regeneration. Biomed. Mater. Eng. 2015;26(Suppl. 1):S95–S102. doi: 10.3233/BME-151294. [DOI] [PubMed] [Google Scholar]
- 9.Ao Q., Fung C.K., Tsui A.Y.-P., Cai S., Zuo H.C., Chan Y.S., Shum D.K.-Y. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials. 2011;32:787–796. doi: 10.1016/j.biomaterials.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh Y.Y., Chang Y.J., Huang T.C., Fan S.C., Wang D.H., Chen J.J., Wu C.C., Lin S.C. Functional recoveries of sciatic nerve regeneration by combining chitosan-coated conduit and neurosphere cells induced from adipose-derived stem cells. Biomaterials. 2014;35:2234–2244. doi: 10.1016/j.biomaterials.2013.11.081. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Perez F., Cobianchi S., Geuna S., Barwig C., Freier T., Udina E., Navarro X. Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurgery. 2015;35:300–308. doi: 10.1002/micr.22362. [DOI] [PubMed] [Google Scholar]
- 12.Muheremu A., Chen L., Wang X., Wei Y., Gong K., Ao Q. Chitosan nerve conduits seeded with autologous bone marrow mononuclear cells for 30 mm goat peroneal nerve defect. Sci. Rep. 2017;7:44002. doi: 10.1038/srep44002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porzionato A., Stocco E., Barbon S., Grandi F., Macchi V., De Caro R. Tissue-Engineered Grafts from Human Decellularized Extracellular Matrices: A Systematic Review and Future Perspectives. Int. J. Mol. Sci. 2018;19:4117. doi: 10.3390/ijms19124117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azizi S., Heshmatian B., Amini K., Raisi A., Azimzadeh M. Alpha-lipoic acid loaded in chitosan conduit enhances sciatic nerve regeneration in rat. Iran. J. Basic Med. Sci. 2015;18:228–233. [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Y., Zhu J., Xue C., Li Z., Ding F., Yang Y., Gu X. Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials. 2014;35:2253–2263. doi: 10.1016/j.biomaterials.2013.11.087. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L., Qu W., Wu Y., Ma H., Jiang H. Dorsal root ganglion-derived Schwann cells combined with poly(lactic-co-glycolic acid)/chitosan conduits for the repair of sciatic nerve defects in rats. Neural Regen. Res. 2014;9:1961–1967. doi: 10.4103/1673-5374.145374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rbia N., Bulstra L.F., Saffari T.M., Hovius S.E.R., Shin A.Y. Collagen Nerve Conduits and Processed Nerve Allografts for the Reconstruction of Digital Nerve Gaps: A Single-Institution Case Series and Review of the Literature. World Neurosurg. 2019;127:e1176–e1184. doi: 10.1016/j.wneu.2019.04.087. [DOI] [PubMed] [Google Scholar]
- 18.Altinova H., Möllers S., Führmann T., Deumens R., Bozkurt A., Heschel I., Damink L.H.H.O., Schügner F., Weis J., Brook G.A. Functional improvement following implantation of a microstructured, type-I collagen scaffold into experimental injuries of the adult rat spinal cord. Brain Res. 2014;1585:37–50. doi: 10.1016/j.brainres.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 19.Mu L., Sobotka S., Chen J., Nyirenda T. Nerve growth factor and basic fibroblast growth factor promote reinnervation by nerve-muscle-endplate grafting. Muscle Nerve. 2018;57:449–459. doi: 10.1002/mus.25726. [DOI] [PubMed] [Google Scholar]
- 20.Cui Y., Lu C., Meng D., Xiao Z., Hou X., Ding W., Kou D., Yao Y., Chen B., Zhang Z., et al. Collagen scaffolds modified with CNTF and bFGF promote facial nerve regeneration in minipigs. Biomaterials. 2014;35:7819–7827. doi: 10.1016/j.biomaterials.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 21.Małczyńska P., Piotrowicz Z., Drabarek D., Langfort J., Chalimoniuk M. The role of the brain-derived neurotrophic factor (BDNF) in neurodegenerative processes and in the neuroregeneration mechanisms induced by increased physical activity (Rola mózgowego czynnika neurotroficznego (BDNF) w procesach neurodegeneracji oraz w mechanizmach neuroregeneracji wywołanej wzmożoną aktywnością fizyczną) Postepy Biochem. 2019;65:2–8. doi: 10.18388/pb.2019_251. [DOI] [PubMed] [Google Scholar]
- 22.Itai S., Suzuki K., Kurashina Y., Kimura H., Amemiya T., Sato K., Nakamura M., Onoe H. Cell-encapsulated chitosan-collagen hydrogel hybrid nerve guidance conduit for peripheral nerve regeneration. Biomed. Microdevices. 2020;22:81. doi: 10.1007/s10544-020-00536-x. [DOI] [PubMed] [Google Scholar]
- 23.Korn C., Augustin H.G. Mechanisms of Vessel Pruning and Regression. Dev. Cell. 2015;34:5–17. doi: 10.1016/j.devcel.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Tran K.A., Partyka P.P., Jin Y., Bouyer J., Fischer I., Galie P.A. Vascularization of self-assembled peptide scaffolds for spinal cord injury repair. Acta Biomater. 2020;104:76–84. doi: 10.1016/j.actbio.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 25.Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 26.Chang W., Shah M.B., Zhou G., Walsh K., Rudraiah S., Kumbar S.G., Yu X. Polymeric nanofibrous nerve conduits coupled with laminin for peripheral nerve regeneration. Biomed. Mater. 2020;15:035003. doi: 10.1088/1748-605X/ab6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haggerty A.E., Bening M.R., Pherribo G., Dauer E.A., Oudega M. Laminin polymer treatment accelerates repair of the crushed peripheral nerve in adult rats. Acta Biomater. 2019;86:185–193. doi: 10.1016/j.actbio.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neal R.A., Tholpady S.S., Foley P.L., Swami N., Ogle R.C., Botchwey E.A. Alignment and composition of laminin-polycaprolactone nanofiber blends enhance peripheral nerve regeneration. J. Biomed. Mater. Res. A. 2012;100:406–423. doi: 10.1002/jbm.a.33204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu D., Raafat A., Pak E., Clemens S., Murashov A.K. Dicer-microRNA pathway is critical for peripheral nerve regeneration and functional recovery in vivo and regenerative axonogenesis in vitro. Exp. Neurol. 2012;233:555–565. doi: 10.1016/j.expneurol.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X.F., Liu H.X., Ortiz L.S., Xiao Z.D., Huang N.P. Laminin-modified and aligned poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/polyethylene oxide nanofibrous nerve conduits promote peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2018;12:e627–e636. doi: 10.1002/term.2355. [DOI] [PubMed] [Google Scholar]
- 31.Rahmati M., Ehterami A., Saberani R., Abbaszadeh-Goudarzi G., Rezaei Kolarijani N., Khastar H., Garmabi B., Salehi M. Improving sciatic nerve regeneration by using alginate/chitosan hydrogel containing berberine. Drug Deliv. Transl. Res. 2020;7:1–11. doi: 10.1007/s13346-020-00860-y. [DOI] [PubMed] [Google Scholar]
- 32.Fannon O.M., Bithell A., Whalley B.J., Delivopoulos E. A Fiber Alginate Co-Culture Platform for the Differentiation of mESC and Modeling of the Neural Tube. Front. Neurosci. 2020;14:524346. doi: 10.3389/fnins.2020.524346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalho C.R., Costa J.B., Costa L., Silva-Correia J., Moay Z.K., Ng K.W., Reis R.L., Oliveira J.M. Enhanced performance of chitosan/keratin membranes with potential application in peripheral nerve repair. Biomater. Sci. 2019;7:5451–5466. doi: 10.1039/C9BM01098J. [DOI] [PubMed] [Google Scholar]
- 34.Pace L.A., Plate J.F., Smith T.L., Van Dyke M.E. The effect of human hair keratin hydrogel on early cellular response to sciatic nerve injury in a rat model. Biomaterials. 2013;34:5907–5914. doi: 10.1016/j.biomaterials.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 35.Gao J., Zhang L., Wei Y., Chen T., Ji X., Ye K., Yu J., Tang B., Sun X., Hu J. Human hair keratins promote the regeneration of peripheral nerves in a rat sciatic nerve crush model. J. Mater. Sci Mater. Med. 2019;30:82. doi: 10.1007/s10856-019-6283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi J., Kim J.H., Jang J.W., Kim H.J., Choi S.H., Kwon S.W. Decellularized sciatic nerve matrix as a biodegradable conduit for peripheral nerve regeneration. Neural Regen. Res. 2018;13:1796–1803. doi: 10.4103/1673-5374.237126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbasipour-Dalivand S., Mohammadi R., Mohammadi V. Effects of Local Administration of Platelet Rich Plasma on Functional Recovery after Bridging Sciatic Nerve Defect Using Silicone Rubber Chamber; An Experimental Study. Bull. Emerg. Trauma. 2015;3:1–7. [PMC free article] [PubMed] [Google Scholar]
- 38.Mohammadi R., Masoumi-Verki M., Ahsan S., Khaleghjoo A., Amini K. Improvement of peripheral nerve defects using a silicone conduit filled with hepatocyte growth factor. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013;116:673–679. doi: 10.1016/j.oooo.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Mehdizadeh H., Bayrak E.S., Lu C., Somo S.I., Akar B., Brey E.M., Cinar A. Agent-based modeling of porous scaffold degradation and vascularization: Optimal scaffold design based on architecture and degradation dynamics. Acta Biomater. 2015;27:167–178. doi: 10.1016/j.actbio.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Li R., Chen L., Fu J., Liu Z., Wang S., Pan Y. Promoting peripheral nerve regeneration with biodegradable poly (DL-lactic acid) films. Int. J. Clin. Exp. Pathol. 2015;8:8057–8065. [PMC free article] [PubMed] [Google Scholar]
- 41.Jahromi H.K., Farzin A., Hasanzadeh E., Barough S.E., Mahmoodi N., Najafabadi M.R.H., Farahani M.S., Mansoori K., Shirian S., Ai J. Enhanced sciatic nerve regeneration by poly-L-lactic acid/multi-wall carbon nanotube neural guidance conduit containing Schwann cells and curcumin encapsulated chitosan nanoparticles in rat. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;109:110564. doi: 10.1016/j.msec.2019.110564. [DOI] [PubMed] [Google Scholar]
- 42.Liu H., Lv P., Zhu Y., Wu H., Zhang K., Xu F., Zheng L., Zhao J. Salidroside promotes peripheral nerve regeneration based on tissue engineering strategy using Schwann cells and PLGA: In vitro and in vivo. Sci. Rep. 2017;7:39869. doi: 10.1038/srep39869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nune M., Subramanian A., Krishnan U.M., Sethuraman S. Peptide nanostructures on nanofibers for peripheral nerve regeneration. J. Tissue Eng. Regen. Med. 2019;13:1059–1070. doi: 10.1002/term.2860. [DOI] [PubMed] [Google Scholar]
- 44.Goulart C.O., Lopes F.R., Monte Z.O., Dantas S.V., Jr., Souto A., Oliveira J.T., Almeida F.M., Tonda-Turo C., Pereira C.C., Borges C.P., et al. Evaluation of biodegradable polymer conduits—poly(L-lactic acid)—for guiding sciatic nerve regeneration in mice. Methods. 2016;99:28–36. doi: 10.1016/j.ymeth.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Lasprilla A.J., Martinez G.A., Lunelli B.H., Jardini A.L., Filho R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012;30:321–328. doi: 10.1016/j.biotechadv.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Vojtova L., Michlovska L., Valova K., Zboncak M., Trunec M., Castkova K., Krticka M., Pavlinakova V., Polacek P., Dzurov M., et al. The Effect of the Thermosensitive Biodegradable PLGA⁻PEG⁻PLGA Copolymer on the Rheological, Structural and Mechanical Properties of Thixotropic Self-Hardening Tricalcium Phosphate Cement. Int. J. Mol. Sci. 2019;20:391. doi: 10.3390/ijms20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samadian H., Ehterami A., Sarrafzadeh A., Khastar H., Nikbakht M., Rezaei A., Chegini L., Salehi M. Sophisticated polycaprolactone/gelatin nanofibrous nerve guided conduit containing platelet-rich plasma and citicoline for peripheral nerve regeneration: In vitro and in vivo study. Int. J. Biol. Macromol. 2020;150:380–388. doi: 10.1016/j.ijbiomac.2020.02.102. [DOI] [PubMed] [Google Scholar]
- 48.Chang S.H., Lee H.J., Park S., Kim Y., Jeong B. Fast Degradable Polycaprolactone for Drug Delivery. Biomacromolecules. 2018;19:2302–2307. doi: 10.1021/acs.biomac.8b00266. [DOI] [PubMed] [Google Scholar]
- 49.Vijayavenkataraman S., Kannan S., Cao T., Fuh J.Y.H., Sriram G., Lu W.F. 3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 2019;7:266. doi: 10.3389/fbioe.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luis A.L., Rodrigues J.M., Amado S., Veloso A.P., Armada-Da-Silva P.A., Raimondo S., Fregnan F., Ferreira A.J., Lopes M.A., Santos J.D., et al. PLGA 90/10 and caprolactone biodegradable nerve guides for the reconstruction of the rat sciatic nerve. Microsurgery. 2007;27:125–137. doi: 10.1002/micr.20317. [DOI] [PubMed] [Google Scholar]
- 51.Subramanian A., Krishnan U., Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: Biomaterial mediated neural regeneration. J. Biomed. Sci. 2009;16:108. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohamadi F., Ebrahimi-Barough S., Nourani M.R., Ahmadi A., Ai J. Use new poly (ε-caprolactone/collagen/NBG) nerve conduits along with NGF for promoting peripheral (sciatic) nerve regeneration in a rat. Artif. Cells Nanomed. Biotechnol. 2018;46(Suppl. 2):34–45. doi: 10.1080/21691401.2018.1451339. [DOI] [PubMed] [Google Scholar]
- 53.Gordon T. Peripheral Nerve Regeneration and Muscle Reinnervation. Int. J. Mol. Sci. 2020;21:8652. doi: 10.3390/ijms21228652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yi S., Xu L., Gu X. Scaffolds for peripheral nerve repair and reconstruction. Exp. Neurol. 2019;319:112761. doi: 10.1016/j.expneurol.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 55.Ciardelli G., Chiono V. Materials for peripheral nerve regeneration. Macromol. Biosci. 2006;6:13–26. doi: 10.1002/mabi.200500151. [DOI] [PubMed] [Google Scholar]
- 56.Osidak E.O., Karalkin P.A., Osidak M.S., Parfenov V.A., Sivogrivov D.E., Pereira F.D.A.S., Gryadunova A.A., Koudan E.V., Khesuani Y.D., Kasyanov V.A., et al. Viscoll collagen solution as a novel bioink for direct 3D bioprinting. J. Mater. Sci. Mater. Med. 2019;30:31. doi: 10.1007/s10856-019-6233-y. [DOI] [PubMed] [Google Scholar]
- 57.Capulli A.K., MacQueen L.A., Sheehy S.P., Parker K.K. Fibrous scaffolds for building hearts and heart parts. Adv. Drug Deliv. Rev. 2016;96:83–102. doi: 10.1016/j.addr.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Entekhabi E., Haghbin Nazarpak M., Shafieian M., Mohammadi H., Firouzi M., Hassannejad Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J. Biomed. Mater. Res. A. 2021;109:300–312. doi: 10.1002/jbm.a.37023. [DOI] [PubMed] [Google Scholar]
- 59.Jaswal R., Shrestha S., Shrestha B.K., Kumar D., Park C.H., Kim C.S. Nanographene enfolded AuNPs sophisticatedly synchronized polycaprolactone based electrospun nanofibre scaffold for peripheral nerve regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;116:111213. doi: 10.1016/j.msec.2020.111213. [DOI] [PubMed] [Google Scholar]
- 60.da Silva M.A., Crawford A., Mundy J., Martins A., Araújo J.V., Hatton P.V., Reis R.L., Neves N.M. Evaluation of extracellular matrix formation in polycaprolactone and starch-compounded polycaprolactone nanofiber meshes when seeded with bovine articular chondrocytes. Tissue Eng. Part A. 2009;15:377–385. doi: 10.1089/ten.tea.2007.0327. [DOI] [PubMed] [Google Scholar]
- 61.Rezvani Z., Venugopal J.R., Urbanska A.M., Mills D.K., Ramakrishna S., Mozafari M. A bird’s eye view on the use of electrospun nanofibrous scaffolds for bone tissue engineering: Current state-of-the-art, emerging directions and future trends. Nanomedicine. 2016;12:2181–2200. doi: 10.1016/j.nano.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 62.Liu S., Zhao J., Ruan H., Tang T., Liu G., Yu D., Cui W., Fan C. Biomimetic sheath membrane via electrospinning for antiadhesion of repaired tendon. Biomacromolecules. 2012;13:3611–3619. doi: 10.1021/bm301022p. [DOI] [PubMed] [Google Scholar]
- 63.Frost H.K., Andersson T., Johansson S., Englund-Johansson U., Ekström P., Dahlin L.B., Johansson F. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci. Rep. 2018;8:16716. doi: 10.1038/s41598-018-34699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wnek G.E. 3. Electrospun polymer fibers as a versatile delivery platform for small molecules and macromolecules: Original research article: Release of tetracycline hydrochloride from electrospun poly (ethylene-co-vinylacetate), poly (lactic acid), a blend, 2002. J. Control. Release. 2014;190:34–36. [PubMed] [Google Scholar]
- 65.Suzuki K., Tanaka H., Ebara M., Uto K., Matsuoka H., Nishimoto S., Okada K., Murase T., Yoshikawa H. Electrospun nanofiber sheets incorporating methylcobalamin promote nerve regeneration and functional recovery in a rat sciatic nerve crush injury model. Acta Biomater. 2017;53:250–259. doi: 10.1016/j.actbio.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Zech J., Leisz S., Göttel B., Syrowatka F., Greiner A., Strauss C., Knolle W., Scheller C., Mäder K. Electrospun Nimodipine-loaded fibers for nerve regeneration: Development and in vitro performance. Eur. J. Pharm. Biopharm. 2020;151:116–126. doi: 10.1016/j.ejpb.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 67.Lizarraga-Valderrama L.R., Nigmatullin R., Taylor C., Haycock J.W., Claeyssens F., Knowles J.C., Roy I. Nerve tissue engineering using blends of poly (3-hydroxyalkanoates) for peripheral nerve regeneration. Eng. Life Sci. 2015;15:612–621. doi: 10.1002/elsc.201400151. [DOI] [Google Scholar]
- 68.Zhang Q., Tong Z., Chen F., Wang X., Ren M., Zhao Y., Wu P., He X., Chen P., Chen Y. Aligned soy protein isolate-modified poly(L-lactic acid) nanofibrous conduits enhanced peripheral nerve regeneration. J. Neural Eng. 2020;17:036003. doi: 10.1088/1741-2552/ab8d81. [DOI] [PubMed] [Google Scholar]
- 69.Valmikinathan C.M., Defroda S., Yu X. Polycaprolactone and bovine serum albumin based nanofibers for controlled release of nerve growth factor. Biomacromolecules. 2009;10:1084–1089. doi: 10.1021/bm8012499. [DOI] [PubMed] [Google Scholar]
- 70.Kun M., Chan C., Ramakrishna S., Kulkarni A., Vadodaria K. 12—Textile-Based Scaffolds for Tissue Engineering. In: Rajendran S., editor. Advanced Textiles for Wound Care. 2nd ed. Woodhead Publishing; Sawston, Cambridge, UK: 2019. pp. 329–362. [Google Scholar]
- 71.Carvalho C.R., Reis R.L., Oliveira J.M. Fundamentals and Current Strategies for Peripheral Nerve Repair and Regeneration. Adv. Exp. Med. Biol. 2020;1249:173–201. doi: 10.1007/978-981-15-3258-0_12. [DOI] [PubMed] [Google Scholar]
- 72.Zander N.E., Orlicki J.A., Rawlett A.M., Beebe T.P., Jr. Surface-modified nanofibrous biomaterial bridge for the enhancement and control of neurite outgrowth. Biointerphases. 2010;5:149–158. doi: 10.1116/1.3526140. [DOI] [PubMed] [Google Scholar]
- 73.Wang J., Cheng Y., Chen L., Zhu T., Ye K., Jia C., Wang H., Zhu M., Fan C., Mo X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019;84:98–113. doi: 10.1016/j.actbio.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 74.Das S., Sharma M., Saharia D., Sarma K.K., Muir E.M., Bora U. Electrospun silk-polyaniline conduits for functional nerve regeneration in rat sciatic nerve injury model. Biomed. Mater. 2017;12:045025. doi: 10.1088/1748-605X/aa7802. [DOI] [PubMed] [Google Scholar]
- 75.García-Gareta E., Hua J., Orera A., Kohli N., Knowles J., Blunn G. Biomimetic surface functionalization of clinically relevant metals used as orthopaedic and dental implants. Biomed. Mater. 2017;13:015008. doi: 10.1088/1748-605X/aa87e6. [DOI] [PubMed] [Google Scholar]
- 76.Ghasemi-Mobarakeh L., Prabhakaran M., Morshed M., Nasr-Esfahani M., Baharvand H., Kiani S., Al-Deyab S., Ramakrishna S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 2011;5:e17–e35. doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- 77.Günter C., Delbeke J., Ortiz-Catalan M. Safety of long-term electrical peripheral nerve stimulation: Review of the state of the art. J. Neuroeng. Rehabil. 2019;16:13. doi: 10.1186/s12984-018-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qian Y., Zhao X., Han Q., Chen W., Li H., Yuan W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018;9:323. doi: 10.1038/s41467-017-02598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang J., Zheng W., Chen L., Zhu T., Shen W., Fan C., Wang H., Mo X. Enhancement of Schwann Cells Function Using Graphene-Oxide-Modified Nanofiber Scaffolds for Peripheral Nerve Regeneration. ACS Biomater. Sci. Eng. 2019;5:2444–2456. doi: 10.1021/acsbiomaterials.8b01564. [DOI] [PubMed] [Google Scholar]