The case
Mrs. C is an otherwise healthy, 58-year-old woman who consults her primary care physician after experiencing the acute onset of severe low thoracic pain. She reports having lifted her heavy grandson the previous day. X-ray films of the thoracic spine show a 50% compression fracture of the T11 vertebra. She has always had a reasonable intake of calcium and maintains an active lifestyle. She reached menopause at the age of 48 years, but she did not choose hormone replacement therapy because she was concerned that it might be associated with breast cancer. She continues to smoke 1 pack of cigarettes a day (despite counselling from her physician) and drinks alcohol socially. She has no family history of osteoporosis or cardiac disease. Mrs. C had a hysterectomy 10 years ago for reasons that are unclear. She is accompanied by her only daughter, a nurse, who is anxious that the appropriate tests be carried out and that treatment be started immediately.
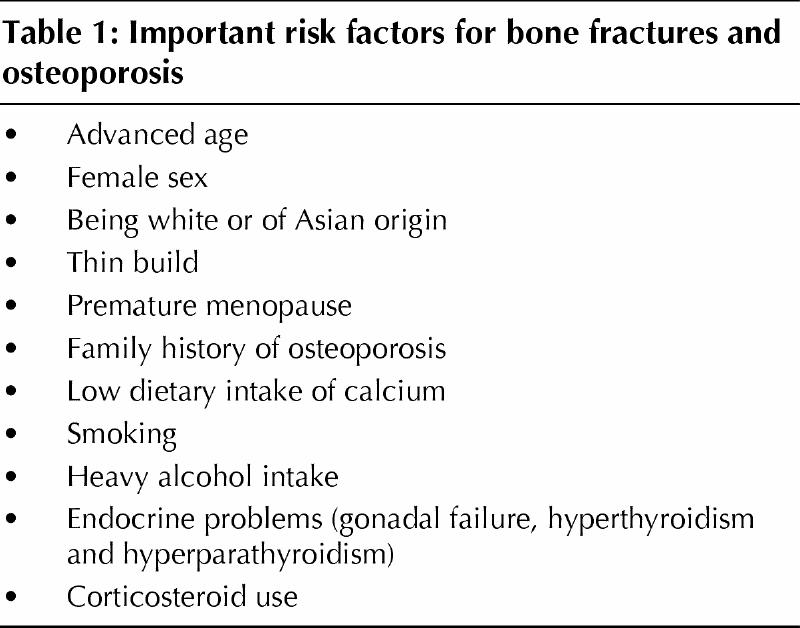
Osteoporosis is a common clinical problem that has been well reviewed.1,2,3 The lifetime risk of an osteoporotic fracture is 1 in 4 among women and 1 in 8 among men. Changing demographics will lead to a significantly increased risk as the population ages and to staggering health care costs in Canada and the United States.4,5,6,7,8 The ability to diagnose osteoporosis 10–20 years before clinically identifiable fractures occur has given the primary care physician the leading role in the prevention and treatment of this condition. The primary care physician can encourage the patient to make lifestyle changes and can provide other medical interventions early on, before there is irreversible bone loss or before clinically identifiable fractures occur. Identifying common risk factors (Table 1) can increase the likelihood of an early diagnosis of osteoporosis.
Table 1
Diagnosing osteoporosis
Osteoporosis may be suspected because of the presence of risk factors but is confirmed by the following: first, a history of fracture and a subsequent determination of low bone mineral density (BMD), second, low BMD without an earlier fracture and, third, blood tests to exclude other types of metabolic bone disease.
Estimating fracture risk using BMD studies
Although bone quality is normal in osteoporosis, the quantity of bone is reduced. The net balance between the formation of bone (a function of osteoblasts) and its resorption (a function of osteoclasts) determines whether the amount of bone increases or decreases over time. Bone mass, which is determined by the net effect of these 2 processes, increases from birth until the third decade of life, when it begins to decline in both men and women. A more rapid decline occurs in women during their early postmenopausal years.1
Currently, BMD is the best independent risk factor for fractures.4,5 BMD can be partly quantified by histomorphometric analysis of bone biopsy specimens, but this direct means of analysis is expensive and not useful in clinical settings. The widespread use of simple noninvasive bone densitometry has resulted in significant improvement in the early detection of osteoporosis. Dual-energy x-ray absorptiometry (DEXA) is a precise way of measuring BMD. The radiation risk of DEXA is extremely low (10% of the radiation of a chest radiograph). Assessment of BMD using radiographs of the hand and ultrasound attenuation are methods that are less expensive than DEXA and might be used to screen patients, but their clinical role, and whether these methods will replace DEXA, remains uncertain. Heel ultrasound can be used as a screening test for osteoporosis, but up to 10% of patients will have false “normal” results, in spite of having significant osteoporosis.
Because degenerative changes in the lumbar spine or calcification of the aorta may result in false elevations of BMD in elderly patients, it is more reasonable to assess the BMD of the proximal femur. In younger individuals, however, measurement of the BMD of the lumbar spine is more accurate and preferable. Studies of cost-effectiveness need to be carried out to determine whether a single site or 2 sites should be measured.
A BMD measurement is useful if it will alter the clinical decision-making process. Determination of BMD in women at menopause provides the primary care physician and the patient with useful information about whether treatment is needed.
Once a baseline level of BMD is obtained, the physician must decide whether future BMD tests will be helpful. The Osteoporosis Society of Canada recommends a follow-up measurement of BMD in 2–3 years, but this recommendation can be tailored to the individual patient. A repeated test will provide reassurance that treatment has been effective. Local guidelines may limit the frequency of repeated BMD testing.
In the future
Markers of bone formation and resorption might be useful in addition to BMD measurement in determining response to treatment. Current clinical urinary markers are not accurate, but if sensitive serum markers become clinically available in the next few years, these will need to be evaluated.
Defining osteoporosis
The World Health Organization (WHO) categorizes bone loss as osteopenia, osteoporosis or severe osteoporosis.7 Current BMD is compared with a patient's theoretical peak BMD. BMD between 1 and 2.5 standard deviations (SDs) below the peak value is defined as osteopenia. A density that is more than 2.5 SDs below the peak value confirms the diagnosis of osteoporosis; severe osteoporosis includes the presence of a clinical fracture. It is important to recognize that the WHO classification was primarily designed to assign patients to categories for clinical trials. In clinical practice, this strict criterion is less useful, because the physician often wishes to initiate treatment for a patient before the presence of osteoporosis is confirmed.
Treatment
Dietary calcium and vitamin D
Dietary calcium intake or calcium supplements should be recommended for all women and men, both young and old. Clinical studies have shown that calcium can reduce bone loss by up to 0.5% per year compared with placebo. In addition, most clinical trials of osteoporosis treatments use calcium and vitamin D in the placebo control group, and such studies often show no bone loss or even slight improvement in BMD over 3 years.9
Common calcium supplements include calcium carbonate and calcium citrate. Calcium carbonate is an effective and inexpensive form of calcium supplement that is readily available (e.g., 1 Tums tablet contains 200 mg calcium compared with 300 mg in a 250-mL glass of skim milk or whole milk). Patients with previous renal calculi should avoid excess calcium supplements and should consider calcium citrate if necessary.
Studies have revealed decreased levels of vitamin D in elderly people, providing a further reason to suggest that patients take vitamin D (800 IU/d).
A European study that considered levels of calcium and vitamin D in elderly nursing-home patients found that providing calcium citrate (1200 mg calcium) and vitamin D (800 IU) daily resulted in a reduction in hip fractures of up to 50%.10 These studies have resulted in the recommendation that all patients should have a good dietary calcium intake and should take vitamin D supplements (Table 2).
Table 2

Lifestyle factors
Women who smoke should be encouraged to stop. Smoking results in earlier menopause and significant bone loss in women. In addition, postmenopausal women who are smokers benefit less from oral conjugated estrogens in terms of maintaining BMD.11 If estrogen is required, smokers might be candidates for oral or transdermal estradiol.
Heavy alcohol intake is another significant risk factor for osteoporosis. In men, this is one of the most common reasons for the development of osteoporosis and bone fractures. To minimize the adverse effect on bones, alcohol should be limited to 1–2 drinks daily.
Weight-bearing activity reduces bone loss and improves bone strength.12 People who exercise regularly have greater muscle bulk and are less likely to fall, resulting in a decreased number of fractures of the proximal femur and the upper extremities. Weight-bearing exercises include walking, dancing, climbing stairs and performing a low-impact exercise program. Using small ankle or wrist weights can further augment BMD in weight-bearing exercise regimes. Patients should be encouraged to walk briskly for 1 hour, 3 times a week. Nonweight-bearing activities can be useful in improving cardiopulmonary fitness but are less helpful in maximizing benefit to bone.
Patients with established fractures who undertake physiotherapy and strengthening exercises will further improve their muscle strength and reduce pain associated with fractures. Patients with significant osteoporosis should be cautioned to avoid active forward flexion of the thoracolumbar spine, because this can result in anterior wedging of vertebrae. Patients should be advised to flex at the hips and knees rather than bending forward to pick up objects from the floor (Table 2).
Approved treatments for osteoporosis
Currently, there are 4 approved treatments for postmenopausal osteoporosis: estrogen replacement therapy (ERT), selective estrogen receptor modulators (SERMs), calcitonin and bisphosphonates (Table 2). Traditionally, ERT has been the treatment of choice, but over the last 5 years large studies of the other treatment options, particularly bisphosphonates and SERMs, have demonstrated a reduction in the number of fractures, whereas there are no large, prospective studies that show that ERT reduces fractures.
Hormone replacement therapy
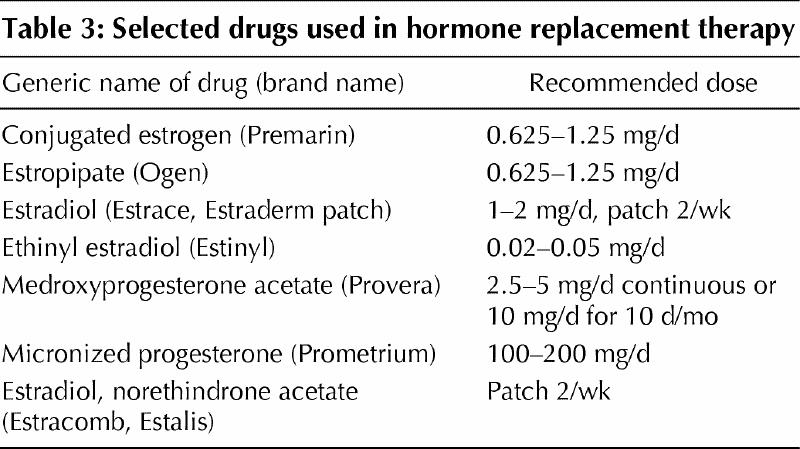
ERT during menopause relieves menopausal symptoms and prevents osteoporosis.13,14,15,16 Estrogen inhibits osteoclasts and slows bone loss and will actually increase bone quantity in the first few years following cessation of normal ovarian function.12 Estrogen can be provided orally, intramuscularly or transdermally. Estrogens and their esters are easily absorbed through the skin, the mucous membranes and the gastrointestinal tract. Oral estrogens, including conjugated equine estrogens, estrone sulfate and micronized estradiol-17β, are the most widely prescribed drugs for the treatment of postmenopausal osteoporosis (Table 3). Transdermal estrogen is effective in treating bone loss but may be less beneficial than oral estrogens regarding lipid metabolism.
Table 3
Although ERT treatment to alleviate menopausal symptoms can be tapered off over months or a few years,17 long-term treatment is required to inhibit bone loss. If prevention of bone loss is to be maintained, estrogen can be continued well past the traditional 10-year period that used to be recommended. Estrogen also influences lipoprotein metabolism, which results in decreased low-density lipoprotein (LDL) cholesterol and increased high-density lipoprotein (HDL) cholesterol. This improvement in lipoprotein profile may result in significant protection against cardiovascular disease. Observational studies have shown significant reduction rates in myocardial infarctions and decreased mortality from cardiovascular disease, in addition to overall reductions in total mortality, for postmenopausal women. However, a recent randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women (HERS trial) did not show this benefit.15 In the first year of the study, there was an increased risk of coronary heart disease. This study demonstrates that large clinical randomized controlled trials (RCTs) with cardiovascular end points (myocardial infarct and stroke) are needed. Large prospective trials are underway, but the results will not be available for a few years.
The risks of using ERT include the increased risk of endometrial cancer (when ERT is not accompanied by progesterone), frequent vaginal bleeding and the potential for thrombosis in some individuals. Although the association between ERT and breast cancer is controversial, cross-sectional studies support an association between estrogen and breast cancer with a risk ratio of approximately 1.25, that is, a 25% increased risk of breast cancer.18 The relative risk is higher in individuals with first-degree relatives who have a history of breast cancer. Primary care physicians must discuss the potential benefits and risks of using ERT. If ERT is to be initiated, physicians must be confident that patients are well versed in breast self-examination and undergo screening mammography as recommended by local guidelines.
Oral conjugated estrogens can be started at a dose of 0.3–0.625 mg daily to prevent osteoporosis, but the dose can be increased up to 1.25 mg daily for patients with severe osteoporosis.
Progesterone should be considered for all patients who have started ERT and who have not had a hysterectomy. Progesterone minimally diminishes the favourable effect of estrogens on the lipoprotein profile, however, it also significantly reduces the increased risk of endometrial cancer associated with ERT. Unopposed estrogens should be given to women who have had a hysterectomy and, therefore, have no risk of developing endometrial hyperplasia.
Progesterone can be given in a cyclic fashion, resulting in monthly menstruation, but physicians are increasingly recommending lower doses of continuous progesterone. Continuous use of progesterone is effective in inhibiting endometrial proliferation and reducing endometrial cancer; however, it will occasionally cause breakthrough bleeding. Like estrogen, progesterone can be given orally, transdermally or by injection. Common oral progesterones include medroxyprogesterone, norethindrone and micronized progesterone.
SERMs
SERMs are available as an alternative to estrogen for the prevention and treatment of osteoporosis. These drugs were developed when it was observed in clinical trials for the treatment of breast cancer that tamoxifen increased BMD.19 Despite the fact that tamoxifen is a partial estrogen agonist and thereby acts as an estrogen antagonist, tamoxifen had beneficial effects on bone.20 Raloxifene, which was developed to treat osteoporosis, is similar to tamoxifen, but it appears to have different effects on tissues possessing estrogen receptors. As a result, raloxifene has beneficial effects on bone as well as endometrial and breast tissue.21,22
A clinical trial (the MORE trial) has demonstrated a 2%–3% increase in BMD of the lumbar spine and hip over 2–3 years with raloxifene and an approximately 50% reduction in vertebral fractures.21 In addition, raloxifene inhibits endometrial proliferation and thereby eliminates the need for progesterone. Raloxifene has beneficial effects similar to estrogen in reducing LDL-cholesterol and increasing HDL-cholesterol, but to a slightly lower degree. It further decreases fibrinogen levels, and high fibrinogen levels are a known independent risk factor for cardiovascular disease.
Three-year clinical data for 7705 postmenopausal women who took either raloxifene or placebo showed 13 cases of breast cancer in 5129 women assigned to raloxifene and 27 cases in 2576 women assigned to placebo. Raloxifene decreased the risk of estrogen receptor–positive breast cancer by 90%.22
Calcitonin
Calcitonin is probably the least potent of the available treatments for osteoporosis. Calcitonin is a 32-amino-acid peptide that directly inhibits osteoclasts and can slow bone loss. There is a similarity between the receptor on osteoclasts for calcitonin and the parathyroid hormone receptor on osteoblasts.23 Calcitonins are presently ineffective orally, because they are broken down by aminopeptidases and proteases in the gastrointestinal tract. Injectable calcitonin is well absorbed, but the route of administration is often inconvenient. Although intranasal calcitonin, 200 IU/d, is widely used and has wide patient acceptance, it is not as well absorbed as injectable calcitonin. It has been reported that in RCTs intranasal calcitonin improved BMD by 1%–2% at 2 years.23 A prospective study of the use of salmon calcitonin, 200 IU/d, in the form of a nasal spray in postmenopausal women with osteoporosis (PROOF study) over 5 years demonstrated statistically significant vertebral fracture reduction.24 However, a further arm of the study using a salmon calcitonin nasal spray, 400 IU/d, showed a positive trend but did not meet statistical significance. The results of this study have been discussed widely.
Daily doses of 50–100 IU of injectable calcitonin result in higher blood levels, have analgesic properties in addition to an anti–bone absorption effect and can be recommended for patients with severe pain who have had recent vertebral compression fractures.25,26 Marine calcitonins (salmon or eel) are often used because they are many times more potent than human forms. Minor adverse effects of calcitonin include flushing and gastrointestinal symptoms (nausea, vomiting, diarrhea). Potential new oral formulations are being studied and, if effective, should significantly increase patient compliance.
Bisphosphonates
Bisphosphonates are currently the most potent treatments for osteoporosis based on BMD and fracture studies. Bisphosphonates are analogues of pyrophosphate but have a carbon atom in place of oxygen. Their phosphate-carbon-phosphate structure allows for many variations in the side chains on the carbon atom. Small changes in the structure of the side chains result in significant physicochemical, biologic and therapeutic differences.
Bisphosphonates have a strong affinity for calcium pyrophosphate and act exclusively in bone tissue. Unlike calcitonin, which has an immediate effect on osteoclasts, bisphosphonates take 48 hours to block resorption. Different bisphosphonates have significantly different antiresorptive potency: for example, the relative strengths of etidronate, clodronate, pamidronate, alendronate and risedronate are 1, 10, 100, 1000 and 5000 respectively.
Oral bisphosphonates are poorly absorbed (at most 1%–10% of a given dose). Physicians should remind patients that bisphosphonates should never be taken with calcium supplements, food or milk products because they significantly inhibit absorption.
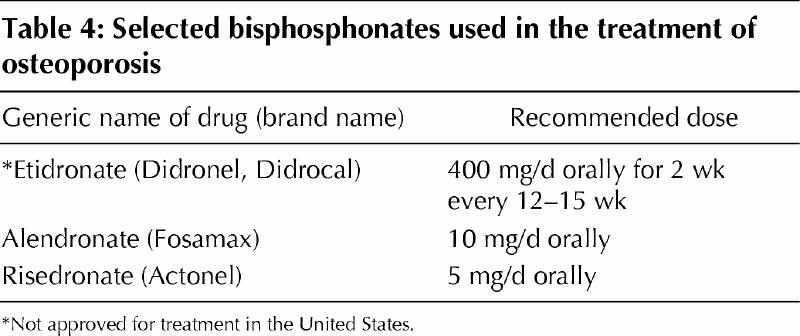
Large prospective RCT treatment studies27,28,29,30,31 of osteoporosis with 3 bisphosphonates (etidronate, alendronate and risedronate) have demonstrated increased BMD of 4%–8% over the first 3 years of treatment and at least 50% fewer vertebral fractures. There is some concern that etidronate can cause osteomalacia if given continuously, and it is recommended that it be administered intermittently with periods of several weeks to 3 months without the medication. Other bisphosphonates, such as alendronate and risedronate, do not have this effect on bone osteoblasts and can be given continuously (Table 4). Etidronate has not been as well studied for osteoporosis and, although it is used in Canada, it has not been approved in the United States.
Table 4
Clinical studies with alendronate have shown a significant 8% improvement in BMD at 3 years and a 50% reduction in the rate of single fractures.28,29 Up to a 90% reduction in the rate of 2 or more spinal fractures has also been demonstrated with alendronate. In a prevention study in 1609 postmenopausal women under the age of 60 years, alendronate, 5 mg/d, administered orally was as well tolerated and as effective as hormone replacement therapy (estrogen and progesterone) in preventing bone loss at the spine.30
Clinical studies with risedronate in North America and Europe show very similar improvement in BMD and reduction in vertebral fractures over 3 years.31 Analysis at 1 year showed the bone fracture reduction effect to be early. A study of risedronate that was designed to look at hip fractures showed significant fracture reduction in high-risk groups with established osteoporosis.
Studies of bisphosphonates such as alendronate suggest that they can be given once weekly to improve convenience.
Treatment for Mrs. C
Mrs. C's physician orders laboratory blood tests including a complete blood count, measurement of calcium, alkaline phosphatase and creatinine levels, serum protein electrophoresis and measurement of thyroid-stimulating hormone. The physician and Mrs. C agree that a BMD determination is not going to make a difference (at least in the short term) to the choice of treatment, but they decide that Mrs. C should have a follow-up BMD determination.
Mrs. C is keen to take advantage of as many treatment options as possible. She plans to take calcium carbonate (1000 mg elemental calcium) and vitamin D 800 IU/d (2 multivitamins) and has decided to stop smoking. She plans to start a daily walking program over the next few months.
After some discussion, she also plans to take a conjugated estrogen, starting at 0.625 mg/d and increasing to 1.25 mg/d if she can tolerate it. Her physician advises her against taking progesterone because she has had a hysterectomy. She is going to consider taking raloxifene, 60 mg/d, especially if she has any breast tenderness. There is some discussion about her taking a bisphosphonate concurrently. She and her physician agree that if she does not tolerate estrogen, she will start taking a bisphosphonate (alendronate 10 mg/d orally or risedronate 5 mg/d orally) or a SERM (raloxifene 60 mg/d).
The physician also suggests that if Mrs. C's pain is not controlled by analgesics, she should begin taking calcitonin (50–100 IU/d subcutaneously). Her daughter has agreed to give the injections if needed. Calcitonin has been shown to be effective in 2 studies at reducing pain if given subcutaneously. Large studies need to be carried out to measure the effectiveness of intranasal salmon calcitonin in reducing pain.
Key points .
The ability to diagnose osteoporosis, based on risk factors, 10–20 years before fractures occur has given the primary care physician the leading role in the prevention and treatment of this condition.
Primary care physicians can encourage patients to make lifestyle changes and can provide medical intervention before irreversible bone loss occurs.
Although the quality of bone in osteoporosis is normal, the net balance between bone formation and resorption is negative; a rapid decline in bone mass occurs in women during their early postmenopausal years.
The widespread use of simple, noninvasive bone densitometry has resulted in significant improvement in the early detection of osteoporosis.
Treatment of osteoporosis includes lifestyle changes, such as taking more exercise, increasing calcium and vitamin D intake, giving up smoking and reducing alcohol intake.
Medical treatments include estrogen replacement therapy, selective estrogen receptor modulators, calcitonin and bisphosphonates.
Estrogen slows bone loss and will actually increase bone quantity in the first few years following cessation of normal ovarian function.
Although estrogen improves the lipoprotein profile and may result in protection against cardiovascular disease, recent studies have not supported this conclusion.
Estrogen replacement therapy may increase the risk of endometrial cancer (when it is not accompanied by progesterone), vaginal bleeding and the potential for thrombosis in some individuals; the association between estrogen replacement therapy and breast cancer remains controversial.
Selective estrogen receptor modulators, although acting as estrogen antagonists, have been shown to increase bone mineral density.
Calcitonin directly inhibits osteoclasts and can slow bone loss; a disadvantage of calcitonin is that it cannot be taken orally.
Bisphosphonates are currently the most potent treatment for osteoporosis based on bone mineral density and fracture studies.
Articles to date in the rheumatology series .
Esdaile JM. Rheumatology: introduction to the series. CMAJ 2000;162(7):1007.
Ensworth S. Rheumatology: 1. Is it arthritis? CMAJ 2000; 162 (7): 1011-6.
Shojania K. Rheumatology: 2. What laboratory tests are needed? CMAJ 2000;162(8):1157-63.
Reid G, Esdaile JM. Rheumatology: 3. Getting the most out of radiology. CMAJ 2000;162(9):1318-25.
Cibere J. Rheumatology: 4. Acute monoarthritis. CMAJ 2000; 162(11):1577-83.
Klinkhoff A. Rheumatology: 5. Diagnosis and management of inflammatory polyarthritis. CMAJ 2000;162 (13): 1833-8.
Price GE. Rheumatology: 6. Localized therapy. CMAJ 2000; 163(2):176-83.
Huang SHK. Rheumatology: 7. Basics of therapy. CMAJ 2000; 163(4):417-23.
Lacaille D. Rheumatology: 8. Advanced therapy. CMAJ 2000; 163(6):721-8.
Clark BM. Rheumatology: 9. Physical and occupational therapy in the management of arthitis. CMAJ 2000; 163 (8):999-1005.
Brady OH, Masri BA, Garbuz DS, Duncan CP. Rheumatology: 10. Joint replacement of the hip and knee — when to refer and what to expect. CMAJ 2000;163(10):1285-91.
Puttick MPE. Rheumatology: 11. Evaluation of the patient with pain all over. CMAJ 2001;164(2):223-7.
Tsang I. Rheumatology: 12. Pain in the neck. CMAJ 2001; 164 (8): 1182-7.
Wing PC. Rheumatology: 13. Minimizing disability in patients with low-back pain. CMAJ 2001;164(10):1459-68.
Taunton JE, Wilkinson M. Rheumatology: 14. Diagnosis and management of anterior knee pain. CMAJ 2001; 164(11):1595-601.
Footnotes
This series has been reviewed and endorsed by the Canadian Rheumatology Association.
The Arthritis Society salutes CMAJ for its extensive series of articles on arthritis. The Society believes that this kind of information is crucial to educating physicians about this devastating disease.
This article has been peer reviewed.
Competing interests: Dr. Wade has received honoraria for Continuing Medical Education talks and travel assistance to attend medical meetings for educational purposes from Procter & Gamble, Aventis, Merck Frosst and Eli Lilly.
Correspondence to: Dr. John P. Wade, Mary Pack Arthritis Centre, 895 West 10th Ave., Vancouver BC V5Z 1L7; fax 604 875-1569
References
- 1.Riggs BL, Melton LJ III. The prevention and treatment of osteoporosis. N Engl J Med 1992;327:620-7. [DOI] [PubMed]
- 2.Prevention and management of osteoporosis: consensus statements from the Scientific Advisory Board of the Osteoporosis Society of Canada. CMAJ 1996;155(7 Suppl):921-65. Available: www.cma.ca/disease/osteo_p1/no1.htm [PMC free article] [PubMed]
- 3.Eastall R. Treatment of postmenopausal osteoporosis. N Engl J Med 1998; 338: 736-45. [DOI] [PubMed]
- 4.Ross PH, David JW, Epstein RS, Wasnich RD. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 1991;114:919-23. [DOI] [PubMed]
- 5.Paradimitropoulos E, Coyte PC, Josse RG, Greenwood CE. Current and projected rates of hip fracture in Canada. CMAJ 1997;157(10):1347-63. Available: www.cma.ca/cmaj/vol-157/issue-10/1357.htm [PMC free article] [PubMed]
- 6.Black DM, Arden NK, Palermo L, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1999;14(5):821-8. [DOI] [PubMed]
- 7.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Geneva: The Organization; 1994. Report no 843. [PubMed]
- 8.Looker AC, Johnston CC Jr, Wahner HW, Dunn WL, Calvo MS, Harris TB, et al. Prevalence of low femoral bone density in older US women from NHANES III. J Bone Miner Res 1995;10(5):796-802. [DOI] [PubMed]
- 9.NIH Consensus conference. Optimal calcium intake. NIH Consensus Development Panel on Optimal Calcium Intake. JAMA 1994;272(24):1942-8. [PubMed]
- 10.Chapuy MC, Arlot ME, Duboeuf F, Brun J, Crouzet B, Arnaud S, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992;327(23):1637-42. [DOI] [PubMed]
- 11.Kiel DP, Felson DT, Anderson JJ, Wilson PW, Moskowitz MA. Hip fracture and the use of estrogens in postmenopausal women. The Framingham Study. N Engl J Med 1987;317(19):1169-74. [DOI] [PubMed]
- 12.Prince R, Devine A, Dick I, Criddle A, Kerr D, Kent N, et al. The effects of calcium supplementation (milk powder or tablets) and exercise on bone density in postmenopausal women. J Bone Miner Res 1995;10(7):1068-75. [DOI] [PubMed]
- 13.Lindsay R, Hart DM, Aitken JM, MacDonald EB, Anderson JB, Clarke AC. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet 1976;1(7968):1038-41. [DOI] [PubMed]
- 14.Ettinger B, Genant HK, Cann CE. Postmenopausal bone loss is prevented by treatment with low-dosage estrogen with calcium. Ann Intern Med 1987;106:40-5. [DOI] [PubMed]
- 15.Hulley SB, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998;280(7):605-13. [DOI] [PubMed]
- 16.Cauley JA, Seeley DG, Ensrud K, Ettinger B, Black D, Cummings SR. Estrogen replacement therapy and fractures in older women. Study of Osteoporotic Research Group. Ann Intern Med 1995;122(1):9-16. [DOI] [PubMed]
- 17.Byyny RL, Speroff L. A clinical guide for the care of older women; primary and preventative care. 2nd ed. Baltimore: Williams and Wilkins; 1996. p. 548.
- 18.Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. Lancet 1997;350(9084):1047-59. [PubMed]
- 19.Love RR, Mazess RB, Barden HS, Epstein S, Newcomb PA, Jordan VC, et al. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 1992;326(13):852-6. [DOI] [PubMed]
- 20.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997;389(6652):753-8. [DOI] [PubMed]
- 21.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999;282(7):637-45. [DOI] [PubMed]
- 22.Cummings SR, Eckert S, Krueger KA. The effects of raloxifene on risk of breast cancer in postmenopausal women. Results from the MORE randomized trial. JAMA 1999;281:2189-97. [DOI] [PubMed]
- 23.Ellerington MC, Hillard TC, Whitcroft SI, Marsh MS, Lees B, Banks LM, et al. Intranasal salmon calcitonin for the prevention and treatment of postmenopausal osteoporosis. Calcif Tissue Int 1996;59(1):6-11. [DOI] [PubMed]
- 24.Chesnut CH III, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 2000;109(4):267-76. [DOI] [PubMed]
- 25.Pun KK, Chan LWL. Analgesic effect of intranasal salmon calcitonin in the treatment of osteoporotic vertebral fractures. Clin Ther 1989;11:205-9. [PubMed]
- 26.Gennari C, Agnusdei D, Comporeale A. Use of calcitonin in the treatment of bone pain associated with osteoporosis. Calcif Tissue Int 1991;49(Suppl 2):S9-13. [DOI] [PubMed]
- 27.Storm T, Thamsborg G, Steiniche T, Genant HK, Sorensen OH. Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. N Engl J Med 1990;322(18):1265-71. [DOI] [PubMed]
- 28.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996;348(9041):1535-41. [DOI] [PubMed]
- 29.Cummings SR, Black DM, Thompson DE. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures. JAMA 1998;280:2077-82. [DOI] [PubMed]
- 30.Hosking D, Chilvers CE, Christiansen C, Ravn P, Wasnich R, Ross P, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med 1998;338(8):485-92. [DOI] [PubMed]
- 31.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999;282(14):1344-52. [DOI] [PubMed]


