Abstract
Extracellular vesicles (EVs) are membranous structures, which are secreted by almost every cell type analyzed so far. In addition to their importance for cell-cell communication under physiological conditions, EVs are also released during pathogenesis and mechanistically contribute to this process. Here we summarize their functional relevance in asthma, one of the most common chronic non-communicable diseases. Asthma is a complex persistent inflammatory disorder of the airways characterized by reversible airflow obstruction and, from a long-term perspective, airway remodeling. Overall, mechanistic studies summarized here indicate the importance of different subtypes of EVs and their variable cargoes in the functioning of the pathways underlying asthma, and show some interesting potential for the development of future therapeutic interventions. Association studies in turn demonstrate a good diagnostic potential of EVs in asthma.
Keywords: airway, allergy, asthma, epigenetic(-s), exosome, extracellular vesicle (EV), inflammation, microRNA (miRNA), microvesicle (MV)
1. Introduction
Chronic non-communicable diseases (NCDs) are inflammatory conditions, which are not caused by infectious agents (e.g., bacteria, viruses, parasites). To name a few, these diseases include respiratory disorders such as asthma or chronic obstructive pulmonary disease (COPD), chronic inflammatory bowel diseases, cardiovascular disorders such as coronary artery disease/ischemic heart disease, peripheral vascular disease or stroke, all based on atherosclerosis, inflammatory disease conditions in the skin (e.g., atopic dermatitis, psoriasis), metabolic diseases such as obesity, metabolic syndrome, and diabetes, different forms of cancer, adverse mental outcomes, etc. Especially after the development of effective prevention (vaccines) and treatment (e.g., antibiotics) options against infectious diseases over the last decades, NCDs became the most significant cause of death in the world. According to the World Health Organization (WHO), in 2019 the three top causes of death in the World were ischemic heart disease accounting for about 9 million, stroke for more than 6 million deaths, and COPD for more than 3 million deaths in this single year only [1]. For comparison, as of mid-April 2021, the worldwide number of COVID-19-related deaths since the very beginning of the pandemic was approaching 3 million [2]. The burden of NCDs is high in western countries and still rising, in particular in less developed areas [3,4,5,6]. To effectively face this challenge, novel diagnostic and therapeutic approaches should be established based on the growing knowledge on pathobiological mechanisms underlying the development and the clinical course of NCDs.
This specifically applies also to asthma as one of the most prominent NCDs, for which, despite substantial progress, current diagnostic and therapeutic approaches remain suboptimal. One of the major reasons behind this is the heterogeneity of asthma, with a complex etiology and multiple clinical representations, requiring the development of stratified diagnosis and treatment strategies [7,8,9,10]. These can only be achieved on the basis of novel cellular and molecular insights based on innovative methods. In this review, we summarize the current knowledge on extracellular vesicle (EV)-mediated cell-cell communication obtained in the context of pathobiology and clinical pathology of asthma.
2. Asthma
Asthma is a chronic inflammatory disease of the airways characterized by recurrent symptoms of varying intensity and severity, including wheezing, shortness of breath, cough, feeling of tightness in the chest, and others. The symptoms of asthma are underlain by reversible airway obstruction resulting from easily triggered bronchospasm and enhanced mucus secretion. In a longer perspective, disease progression is associated with, or rather results from, airway remodeling including changes in structural cell composition and extracellular fibrosis [11,12,13,14].
Clinically, asthma is a very heterogeneous disorder with considerable differences in the symptomatology, factors triggering exacerbations, severity, time of onset, demographics, body weight, and other features. Characteristic clinical representations of asthma form so-called phenotypes that are associated with a variety of distinct pathomechanisms named endotypes. Several endotypes have been proposed, which can be roughly grouped into those related to T helper cell type-2 (Th2) and those related to non-Th2 (e.g., Th1/Th17) immune mechanisms. Since it became evident that Th cytokines can be secreted also by other cell types, e.g., innate lymphoid cells (ILCs), asthma forms are divided into those of a type-2 (mostly allergic) and those of a non-type-2 character, respectively. However, even this paradigm may not cover all possible mechanisms underlying different forms of asthma [8,9,13,15,16].
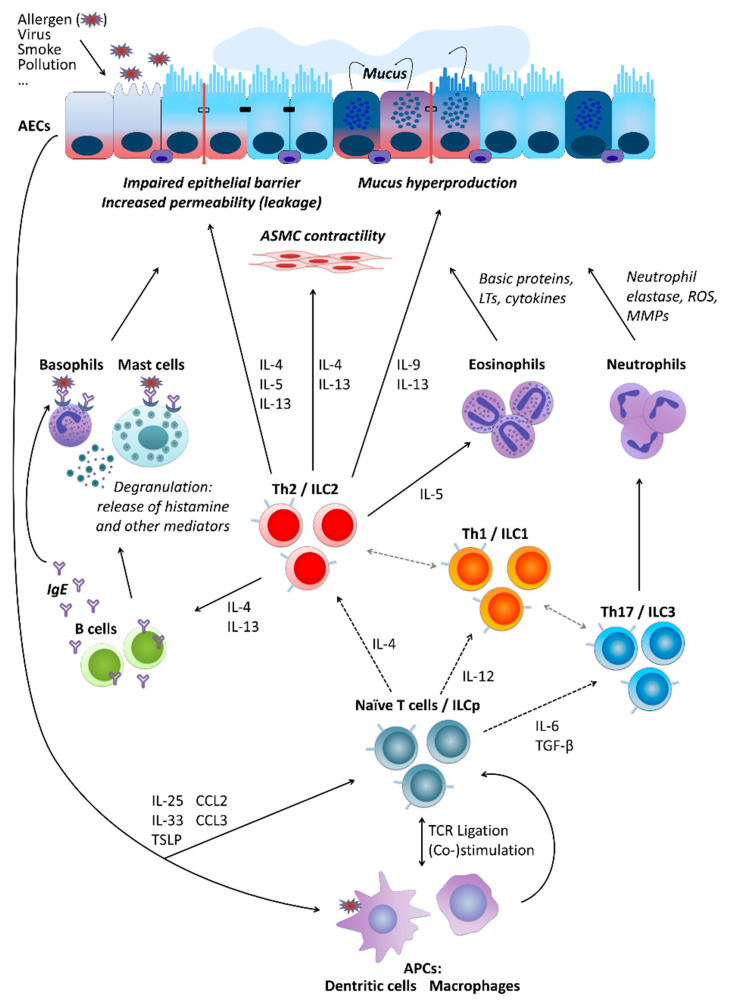
Partly independently of the pathomechanism behind it, several types of cells are crucially involved in asthma pathogenesis. These include airway epithelial cells (AECs) forming together with local macrophages the first point of contact for external influences entering the airways, for instance, allergens (type-2/atopic forms of asthma) or cigarette smoke (neutrophilic asthma belonging to non-type-2 disease forms). Cytokines secreted by AECs (e.g., thymic stromal lymphopoietin, TSLP; interleukin-25, IL-25; and IL-33) in response to stimulation influence of downstream cells including, among others, antigen-presenting cells (APCs) and T cells. Depending on the type of stimulation, T cells differentiate towards Th2 cells secreting cytokines driving allergic forms of the disease or Th17 and Th1 cells contributing to non-type-2 asthma endotypes. As type-2 cytokines, IL-4 triggers the differentiation of further Th2 cells and the production of immunoglobulin E (IgE) by B cells, IL-13 activates mast cells and basophils as well as stimulates airway smooth muscle (ASM) cell contractility and thus airway hyper-responsiveness (AHR) and hyperplasia of goblet cells and mucus production, IL-5 activates eosinophils, IL-9 further contributes to increased mucus production and enhanced proliferation of mast cells. Mediators secreted by mast cells and basophils result in allergic inflammation of the respiratory tract accompanied by a respective clinical picture. IL-17 released by Th17 cells, in turn, stimulates neutrophil activation, which leads to severe endothelial injury typical for non-type-2 neutrophilic asthma (Figure 1) [9,14,17,18,19].
Figure 1.
Basic cellular mechanisms underlying type-2 and non-type-2 asthma. For a more detailed description, please refer to the main text, Section 2. “Asthma”. AECs, airway epithelial cells; ASMC, airway smooth muscle cells; LTs, leukotrienes; ROS, reactive oxygen species; MMPs, matrix metalloproteinases; IL, interleukin; ILC, innate lymphoid cells; ILCp, ILC precursors; Th (cells), T helper (cells); IgE, immunoglobulin E; TGF-β, transforming growth factor beta; TSLP, thymic stromal lymphopoietin; CCL, C-C motif chemokine ligand; TCR, T cell receptor; APCs, antigen-presenting cells.
3. Extracellular Vesicles
A key feature that has significantly contributed to the evolution of multicellular organisms and especially higher levels of complexity is represented by the ability of intercellular communication such as transfer of soluble molecules between cells and/or direct cell-cell contact. After the discovery that cells release so-called apoptotic bodies during programmed cell death, it was shown already in the mid-60s that physiologically active cells also release extracellular particles, at that time referred to as the so-called “platelet dust” [20]. However, within the last two decades, EVs have turned out to be more prominent and functionally important than initially expected and emerged as an interesting and promising research field. Virtually all cell types analyzed so far release EVs, which can roughly be classified into two major groups: endosomal derived exosomes and microvesicles (MVs; also referred to as microparticles or ectosomes), the latter directly budding from the plasma membrane [21,22,23,24,25]. Although exosomes and MVs show differences, such as their biogenesis and release pathways, they also share many bio-physicochemical properties, including size range, density as well as certain surface proteins (for a summary of the differential characteristics of EVs see Table 1) [26,27,28,29,30,31,32,33]. These features barely allow distinguishing between the individual subpopulations in detail. Instead of referring to the individual subpopulations, the term EV should therefore be preferred in the nomenclature, which encompasses vesicles released by cells in their entirety [34], however, in the current review we will retain the terminology used in the original publications to which we refer.
Table 1.
| Exosomes | Microvesicles | Apoptotic Bodies | |
|---|---|---|---|
| Alternative nomenclature | - | Microparticles, ectosomes | - |
| Size | 10–150 nm | 100–1000 nm | 800–5000 nm |
| Origin | Intraluminal vesicles within multivesicular bodies | Plasma membrane and cellular content | Plasma membrane, fragmented cell |
| Formation mechanism | Fusion of multivesicular bodies with plasma membrane | Outward blebbing of plasma membrane | Shrinkage and programmed death of the cell |
| Release | Constitutive and/or cell activation | Constitutive and/or cell activation | Apoptosis |
| Time of release | ≥10 min | <1 s | - |
| Composition | Protein, lipids, coding RNA, noncoding RNA, DNA | Protein, lipids, cell organelles, coding RNA, noncoding RNA, DNA | Cell organelles, proteins, nuclear fractions, coding RNA, noncoding RNA, DNA |
| Enriched protein markers | CD81, CD63, Alix, Tsg101 | Selectins, integrin, CD40 | Caspase 3, histones |
Since EVs play an important role in cell-cell communication, they are not simply empty lipid bins but rather contain various biomolecules such as diverse RNA types, proteins, lipids and metabolites by which they have the potential to regulate the function of recipient cells. With respect to EV-mediated signaling, non-coding RNAs were studied in depth during the last decade. In particular, the role of microRNAs (miRNAs) turned into the focus of research, due to their well-established role in the regulation of gene expression [35,36,37]. Interestingly, the way in which EVs avoid degradation while entering the cell compartment by endocytosis and subsequent cargo release via membrane fusion suggests that EVs exploit mechanisms similar to those observed in certain viral infections, such as endosomal acidification [38,39].
In line with this, recent studies also implicated EVs in the progression of human disease, including cancer and infectious diseases (for a summary see [40,41]).
4. MicroRNA
Epigenetic mechanisms alter the expression of genes without affecting DNA nucleotide sequences. There are different types of epigenetic control, which can be roughly divided into classical and non-classical epigenetic mechanisms. Classical epigenetic mechanisms include DNA methylation and posttranslational histone modifications, such as acetylation, methylation, phosphorylation, and others [17,42]. Non-classical epigenetic mechanisms are mediated by post-transcriptional control elements including non-coding transcripts, such as miRNAs, approximately twenty-two nucleotides-long RNA molecules that can also be detected as cargoes of EVs [17,43].
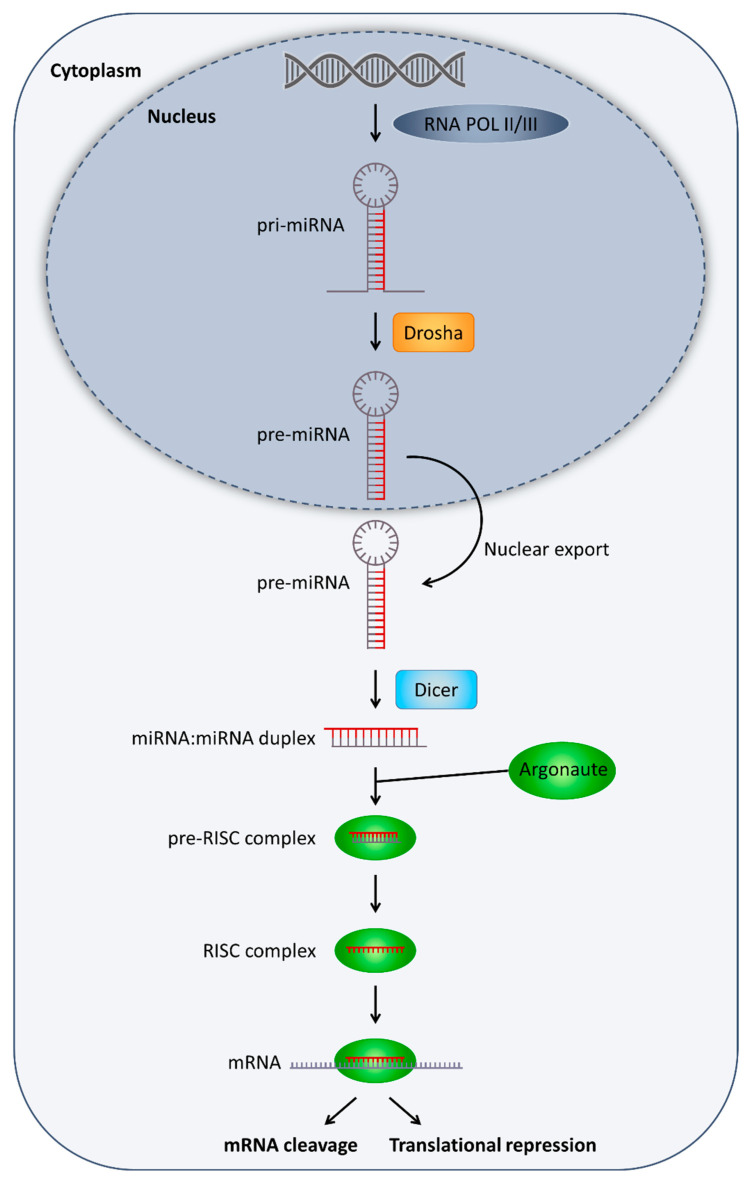
With the advent of high-throughput sequencing technologies, the number of known miRNAs in the human genome has steadily increased during recent years, so that currently approximately 2500 miRNA sequences are known. The canonical miRNA molecules are located within non-coding introns, coding exons as well as intergenic regions. The transcription of miRNAs is commonly mediated by RNA Polymerase II (RNA Pol II), but in a few cases also by RNA Pol III. All miRNAs undergo a series of maturation steps, in which the immature transcripts are processed by type III RNases. More precisely, primary (pri)-miRNAs are processed by Drosha within the nucleus to pre-miRNAs and after export into the cytoplasm, further by Dicer into active mature approximately 22-nt long miRNAs (Figure 2) [44,45,46,47].
Figure 2.
Basic processes involved in microRNA (miRNA) biogenesis and function. For detailed description, please refer to the main text, Section 4. “MicroRNA”. RISC, RNA-induced silencing complex; mRNA, messenger RNA.
After processing, one strand of the mature miRNA is loaded into the RNA-induced silencing complex (RISC), where it ensures the specific recognition of a target mRNA. Gene expression is mainly regulated by binding of RISC to the 3′ untranslated region (UTR) of the mRNA, which can lead to translation inhibition or even degradation of the mRNA. The two mechanisms are mainly regulated by the complementarity of the miRNA to the target sequence. Thus, complete binding of the miRNA results in the fragmentation of the mRNA (Figure 2), which is induced by Argonaute 2 (Ago2) a component of the RISC complex [44,45,46,47].
Importantly, miRNAs are considered as one of the key regulators of literally all cellular pathways [17,45,48,49].
5. Extracellular Vesicles and Asthma: Cellular Level
5.1. Airway Epithelial Cells and Fibroblasts
EVs are involved in asthma-related interactions between different cell types. Additionally, for AECs, the exchange of EV cargo seems to be an important way of communicating with each other, as well as with other cell types. For example, in primary human tracheobronchial cells and cultured Calu-3 cells, a respiratory epithelial cell line, the reciprocal transfer of EV-associated proteins and miRNAs was shown to be sufficient to qualitatively and quantitatively alter the profiles of airway secretions including miRNA cargo of EVs of the target cells and cause mucin hypersecretion [50]. This mechanism may play an important role in epithelial remodeling and other pathologic processes in the airways involved in chronic inflammatory disorders of the respiratory tract, such as asthma, cystic fibrosis, and bronchogenic carcinoma [50]. In a mouse study, it was shown that the composition of the pool of extracellular miRNAs in the lung was very similar to that of the airway epithelium, with 80% of the EVs detected in bronchoalveolar lavage fluid (BALF) being of epithelial origin [51]. However, the number of miRNAs selectively expressed by immune cells, including miR-223 and miR-142a, and hematopoietic cell-derived EVs increased significantly following the induction of allergic airway inflammation (AAI), showing the importance of alterations in the EV miRNA pool for the development of allergic inflammation [51]. Another group reported that EV secretion and production of EV-associated proteins were both higher in the lungs of mice in which AAI was induced compared to the control animals [52]. These EVs, which were released during asthma/AAI by AECs under the influence of type-2 cytokines such as IL-13, triggered the proliferation and chemotaxis of undifferentiated macrophages [52]. Not surprisingly, the use of GW4869, an inhibitor of exosome production, resulted in a reduction in the population of proliferating monocytes in the AAI mouse model and the alleviation of various asthmatic features [52].
In another in vitro study, EV-associated miRNAs were released to the basal and apical sides by normal human bronchial epithelial cells (NHBECs) undergoing IL-13 treatment to mimic a type-2 inflammatory condition [53]. Candidate miRNAs identified were subsequently validated in EVs isolated from nasal lavages obtained from children with non-severe or severe asthma compared to healthy controls. It was shown that miR-92b, miR-210, and miR-34a expression levels correlated with the changes in lung function [53]. Treatment with IL-13 was also able to increase the expression of tissue factor (TF) and a release of TF-positive EVs from NHBECs, both induced by compressive stress mimicking asthmatic bronchoconstriction [54,55]. Besides, allergen challenge increased the release of TF into BALF in both mice and humans [54]. Although TF is best known for its role as a cellular initiator of coagulation [56,57], this multifunctional protein has also been implicated in a number of pathophysiologic conditions such as asthma [58,59,60].
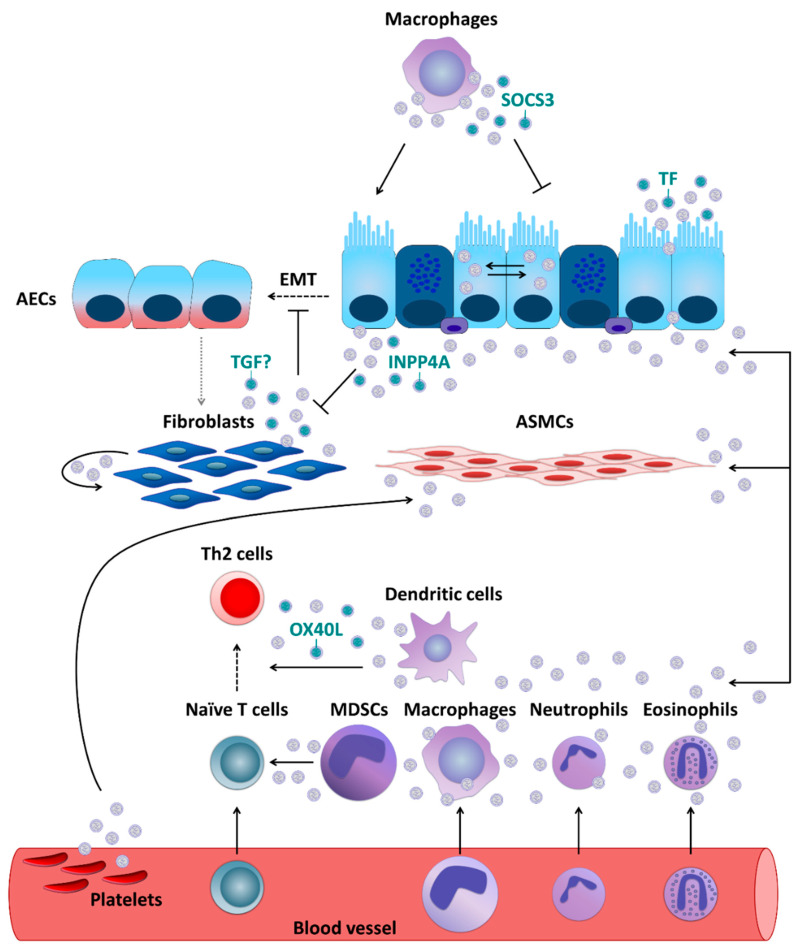
Additionally, primary human fibroblasts were demonstrated to secrete exosomes, which undergo subsequent internalization by NHBECs [61]. Moreover, compared to healthy controls, exosomes derived from fibroblasts which were obtained from severe asthmatics showed lower levels of transforming growth factor beta 2 (TGF-β2) and significantly increased the proliferation of NHBECs [61]. These results are intriguing, given that TGF-β is considered to be a major driver of abnormal epithelial-mesenchymal transition (EMT) [9,62]. During EMT, epithelial cells demonstrate enhanced motility and invasive capacity through the downregulation of epithelial markers and higher expression of mesenchymal proteins, being this way a source of migrating myofibroblasts and fibroblasts. In turn, these cells promote extracellular matrix deposition and subepithelial fibrosis, which strongly contributes to the establishment of a persistent asthma phenotype [63,64]. Moreover, fibroblasts themselves can also be recipients of EVs. In vitro experiments using cell lines demonstrated that AECs were able to secrete enzymatically active inositol polyphosphate 4-phosphatase type I A (INPP4A) in EVs and as a soluble free form. INPP4A was then transferred to lung fibroblasts, and inhibition of such transfer resulted in increased fibroblast proliferation [65]. Moreover, in mice with or without AAI neutralization of extracellular INPP4A-induced AHR, with prominent airway remodeling, subepithelial fibroblast proliferation, and collagen deposition [65]. EV-mediated interactions between major cellular players involved in asthma/AAI (reported in Section 5.1, Section 5.2, Section 5.3, Section 5.4 and Section 5.5) are summarized in Figure 3.
Figure 3.
Extracellular vesicle- (EV-) mediated communication between cells crucial for asthma pathobiology. If not otherwise stated, EVs are thought to carry their usual content such as microRNAs, proteins, lipids, etc. For a more detailed description, please refer to the main text, Section 5.1 “Airway Epithelial Cells and Fibroblasts”, Section 5.2 “Antigen-Presenting Cells”, Section 5.3 “Granulocytes and Mast Cells”, and Section 5.4 “Platelets”. SOCS3, suppressor of cytokine signaling 3; TF, tissue factor; EMT, epithelial-mesenchymal transition; INPP4A, inositol polyphosphate 4-phosphatase type I A; OX40L, OX40 ligand; MDSCs, myeloid-derived suppressor cells; otherwise, please, refer to the legend to Figure 1.
5.2. Antigen-Presenting Cells
APCs, such as dendritic cells (DCs), macrophages, monocytes, and others can communicate through EVs with other types of cells involved in asthma development. A study performed in primary human macrophages and DCs demonstrated that they can secrete exosomes which contain enzymes for leukotriene biosynthesis and thus contribute to chronic inflammation, for example through granulocyte recruitment [66]. Primary human DCs activated with TSLP, an epithelial cell-derived cytokine [67], release exosomes expressing OX40 ligand (OX40L), which was able to promote proliferation and differentiation of CD4+ T cells towards a Th2 phenotype [68]. Resident alveolar macrophages were, in turn, demonstrated to dampen inflammatory signaling in AECs [69] and thus AAI in a mouse model [70] through transcellular delivery of suppressor of cytokine signaling 3 (SOCS3) within EVs. Air pollutants such as particulate matter are well-known contributors to the pathogenesis of chronic inflammatory airway diseases including asthma [17,71]. In vitro exposure to particulate matter stimulated human macrophages to release more EVs in a dose-dependent manner. Moreover, those EVs were able to induce secretion of pro-inflammatory cytokines, such as IL-6 and tumor necrosis factor-α (TNF-α), by pulmonary epithelial cells [72].
5.3. Granulocytes and Mast Cells
Likewise, human eosinophils were found to be able to secrete exosomes, the production of which was higher by cells deriving from asthmatics [73]. In addition, exosomes secreted by the eosinophils of patients with asthma could, in an autocrine manner, modify several specific eosinophil functions related to asthma pathogenesis including an increase in reactive oxygen species and nitric oxide synthesis and an augmentation of eosinophil migration and adhesion, suggesting that they could fundamentally contribute to the development and maintenance of asthma [74]. Further, asthmatic eosinophil-derived exosomes could enhance the apoptosis of primary AECs and delay the repair of established epithelial damage, as well as increase the proliferation of primary bronchial smooth muscle cells and perpetuate airway inflammation status [75]. Upon in vitro stimulation with LPS, horse neutrophil-derived exosomes, carrying proteins associated with immune response and positive regulation of cell communication, were rapidly internalized by equine ASM cells and enhanced their proliferative capabilities [76]. The effects of neutrophil-derived exosomes on ASM proliferation [76] might play an important role in the neutrophil-mediated progression of asthma and promotion of airway remodeling in severe and corticosteroid-insensitive patients with asthma [8]. Based on in vitro data obtained using human cells, exosomes were also suggested to partially contribute to mast cell-mediated pro-inflammatory modulation of ASM cells, although it was undisputed that soluble, extra-exosomal factors such as IL-8 were critical for the effect [77].
5.4. Platelets
Moreover, platelets, which are known to contribute to the pathophysiology of asthma as well [78,79], can exert their effects through EVs [80]. Plasma EVs, a substantial portion of which is of platelet origin, isolated from asthmatics were found to be able to reduce the endothelium-dependent relaxation in response to bradykinin and increase the acetylcholine-induced contraction of the mouse trachea, which is suggestive of their potential role in ASM dysfunction typical for asthma [81,82]. Moreover, the levels of circulating platelet microparticles (PMPs) were reported to be increased in asthmatics [83].
5.5. Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) comprise a heterogeneous group of immature myeloid cells that are capable of regulating T cell function through various mechanisms. It is hypothesized that they are involved in the regulation of respiratory inflammation in asthma [84]. Interestingly, EVs isolated from primary MDSCs of asthmatics and non-asthmatics have been reported to contain mitochondria, with a higher mitochondrial content in EVs derived from asthmatics [85]. Beyond that, these EVs could be transferred ex vivo to T cells, where they co-localized with the cellular mitochondrial network [85]. Mitochondrial transfer via EVs from MDSCs to T cells could represent an important novel regulatory mechanism contributing to the pathophysiology of asthma [85].
5.6. Mesenchymal Stem Cells and Adipose Tissue
Mesenchymal stem cells (MSCs) are multipotent stromal cells that possess self-renewing capacity and give rise to many specialized cell types such as chondrocytes, adipocytes, myocytes, and osteoblasts. MSCs are primarily located within the bone marrow (BM) stroma, but they can be isolated from almost any organ [86,87,88]. MVs produced by horse amniotic MSCs reduced the secretion of TNF-α and, to a certain extent, TGF-β and IL-6 from primary equine alveolar macrophages [89]. Exosomes secreted by human BM-derived MSCs were, in turn, able to upregulate secretion of IL-10 and TGF-β1 from peripheral blood mononuclear cells (PBMCs) of asthmatic patients, thus promoting proliferation and immunosuppressive capacity of regulatory T cells (Tregs). This effect was most probably mediated by APCs, shown to directly interact with exosomes produced by MSCs [90]. Furthermore, EVs deriving from mouse and human BM-MSCs turned out to ameliorate the mixed Th2/Th17 phenotype in an Aspergillus-induced mouse model of AAI reflective of severe refractory asthma [91]. In another in vitro study, miR-1470-containing exosomes from human MSCs were found to be able to promote the differentiation of Tregs among CD4+ T cells separated from PBMCs of acute asthmatic patients [92].
Exosomes from mouse adipose tissue-derived MSCs could effectively suppress the maturation of BM-derived DCs as reflected by decreased IL-6 release but augmented IL-10 and TGF-β secretion. In addition, lymphocyte proliferation was diminished in the presence of DCs treated with MSC-derived exosomes [93]. Treatment with EVs secreted by human adipose tissue-derived MSCs reduced the symptoms as well as cellular and molecular features of ovalbumin (OVA)-induced AAI in mice; lung TGF-β levels were also reduced [94]. Another study demonstrated that the attenuating effect of exosomes secreted by mouse adipose tissue-derived MSCs on airway remodeling observed in a mouse model of OVA-induced AAI could be further augmented by genetic modifications of the cells affecting the composition of the secreted exosomes [95]. Finally, adipocytes were found to be able to secrete fatty acid-binding protein 4 (FABP4; also called aP2) in exosome-like vesicles [96]. FABP4 was found to be necessary for AAI development in an OVA-based mouse model [97], which might represent a further link connecting (fat) metabolism and asthma [8]. Table 2 summarizes asthma/AAI-related cellular effects of EVs released by MSCs.
Table 2.
Major asthma-related cellular and systemic effects of extracellular vesicles (EVs) released by mesenchymal stem cells (MSCs).
| EVs | Source Cells/Tissue | Recipient | Main Effect(-s) | Publication |
|---|---|---|---|---|
| MVs | Equine amniotic MSC | Horse | Reduction in TNF-α secretion and, to a lesser degree, TGF-β and IL-6 from primary alveolar macrophages | [89] |
| Exosomes | Human BM-derived MSCs | Human | Upregulation of IL-10 and TGF-β1 secretion from PBMCs of asthmatics and promotion of proliferation and immunosuppressive capacity of Tregs | [90] |
| EVs | Human/mouse BM-derived MSCs | Mouse | Amelioration of Aspergillus extract-induced AAI in sensitized animals | [91] |
| miR-1470-containing exosomes | Human MSCs | Human | Promotion of Tregs differentiation from CD4+ T cells isolated from PBMCs of acute asthmatics | [92] |
| Exosomes | Mouse adipose tissue-derived MSCs | Mouse | Effective suppression of the maturation of BM-derived DCs as reflected by decreased IL-6 release but augmented IL-10 and TGF-β secretion | [93] |
| EVs | Human adipose tissue-derived MSCs | Mouse | Reduced symptoms and cellular and molecular features of OVA-induced AAI as well as lung TGF-β levels in OVA-sensitized animals | [94] |
| Exosomes | Mouse adipose tissue-derived MSCs | Mouse | Attenuating effect on airway remodeling in a model of OVA-induced AAI could be further augmented by genetic modifications of MSCs | [95] |
MVs, microvesicles; BM, bone marrow; IL-10, interleukin-10; TGF-β1, transforming growth factor beta 1; PBMCs, peripheral blood mononuclear cells; Tregs, regulatory T cells; AAI, allergic airway inflammation; DCs, dendritic cells; OVA, ovalbumin.
6. Extracellular Vesicles and Asthma: Higher Levels of Organization
6.1. Lower Respiratory Tract
Substantial differences in BALF exosomal miRNA profiles were observed between healthy subjects and patients with unprovoked, mild, stable asthma. Those referred to twenty-four miRNAs including members of the let-7 and miRNA-200 families, with a subset of sixteen miRNAs driving the robust separation between healthy control subjects and asthmatic patients [98]. Another study reported altered small RNA cargo with a lower load of miRNA in BALF exosomes obtained from severe asthmatics compared to healthy controls. Moreover, altered miRNA content of exosomes from severe asthmatics was predicted to be involved in the regulation of cellular pathways participating in airway inflammation and remodeling and correlated with lung function and the magnitude of BAL eosinophil and neutrophil infiltrations [99]. In contrast, a study performed in a mouse model of house dust mite (HDM)-induced AAI demonstrated the amount of airway-secreted EVs to be much higher in BALF from HDM-exposed mice compared to that from control animals [100]. HDM stimulated the secretion of exosomes containing increased levels of miRNAs inhibiting key molecules of type-2 inflammation, including the IL-5 receptor and IL-13. While the expression of those miRNAs was reduced in lung tissues, miRNAs that were less abundant in the exosomes were overrepresented in the lungs [100]. Pretreatment with the above-mentioned exosome secretion inhibitor GW4869 was able to diminish the number of exosomes secreted into BALF. Furthermore, the application of GW4869 also decreased type-2 cytokines and eosinophil counts in BALFs and reduced eosinophil accumulation in airway walls and mucosa [100]. Taken together, the results of this study suggest a very interesting mechanism in which selective sorting of miRNA, predominantly miRNAs inhibiting type-2 molecules, into airway-secreted EVs and their increased release to the airways after HDM exposure, i.e., removing them from the cells, could be involved in the pathogenesis of AAI [100]. Interestingly, analysis of BALF exosomes in mice subjected to an OVA-based model of AAI treated with scorpion and centipede demonstrated that BALF exosomal miRNAs might be involved in mediating the anti-asthmatic effects of these traditional Chinese insect medicines [101].
Moreover, other components of asthmatic EVs were also studied. Exosomal proteomics was analyzed in BALF obtained from patients with asthma, cystic fibrosis, or primary ciliary dyskinesia [102]. Mass spectrometry analysis made it possible to quantify 665 proteins, 14 of which were significantly differentially expressed depending on the disease condition. Furthermore, hierarchical analysis proved those 14 proteins to nicely distinguish the three diseases, with cystic fibrosis and primary ciliary dyskinesia having more similar protein profiles than had any of the two conditions compared with asthma [102]. Another study contrasted the lipid composition and presence of specific lipid mediators in EVs obtained from the BALF of healthy controls and asthmatic subjects exposed or not exposed to second-hand smoke [103]. Concentrations of BALF exosomes were higher in asthmatics, where they correlated with serum IgE levels and blood eosinophil counts. Phosphatidylglycerol, ceramide-phosphates, and ceramides were significantly more abundant in exosomes obtained from asthmatics compared to the non-exposed control groups. On the other hand, levels of sphingomyelin 34:1 were lower in exosomes of asthmatics with second-hand smoke exposure compared to healthy controls [103]. BALF exosomes were also compared between patients with mild asthma with an allergy against birch pollen and healthy individuals with exosomes obtained from asthmatics, and were characterized by higher levels of exosome-associated surface markers such as the tetraspanins CD63 and CD81 compared to those isolated from healthy controls [104]. Allergen provocation exerted no major effects on the characteristics of the exosomes. BALF exosomes contained enzymes for leukotriene biosynthesis and, especially if obtained from asthmatics, were able to promote the secretion of leukotriene C4 and IL-8 from the human bronchial epithelial cell line 16HB14o [104].
Asthma-related EV content has also been studied in sputum. It was demonstrated that exosomes could be detected in induced sputum of mild allergic asthmatics both before and after the challenge with an allergen [105]. The presence of RNA, especially short RNA species, and immune-related proteins in the samples as well as easy accessibility of induced sputum compared with BALF suggested sputum EV to be potential candidates for biomarker research in asthma [105].
6.2. Upper Respiratory Tract
Nasal exosomes have also been investigated in the context of asthma. Exosomal proteins and their potential functional properties were analyzed in nasal lavage fluid (NLF) obtained from asthmatics, subjects with asthma accompanied by chronic rhinosinusitis (CRS), and healthy individuals [106]. Strong associations were observed between the exosomal proteome and immune-related functions of NFL such as immune cell trafficking. Furthermore, while barrier-related proteins and those with antimicrobial functions were less abundant, serum-associated proteins and mucins were found to be present at higher levels in the exosomes obtained from patients suffering from respiratory diseases compared to healthy individuals. Finally, nasal exosomes were able to in vitro induce migration of various immune cells, including monocytes, neutrophils, and natural killer (NK) cells [106]. In another study, MV types and levels were comparatively analyzed in NLF samples obtained from healthy individuals and subjects with CRS without nasal polyps, CRS with nasal polyps, and aspirin-exacerbated respiratory disease (AERD), which is a syndrome comprising CRS with nasal polyps, asthma, and aspirin sensitivity [107]. Analysis of the released MVs demonstrated that mast cells, platelets, and basophils were more activated in subjects with AERD compared to patients with CRS, whereas epithelial injury was lower in CRS subjects with nasal polyps than in those with CRS without nasal polyps or AERD. These findings show that typing of NLF MVs might help to identify phenotypes of CRS and in distinguishing AERD from CRS with nasal polyps [107]. It was also observed that, independently of the presence or absence of concomitant asthma, exosomes released by primary human nasal epithelial cells obtained from patients suffering from CRS with nasal polyposis carried proteins influencing cell proliferation pathways and potentially leading to remodeling of the sinonasal mucosa [108].
6.3. Blood
Several studies focused on serum EVs and their importance for asthma. Mice subjected to an OVA-based model of AAI were first pretreated with either serum or exosomes isolated from the serum of either OVA-tolerized mice or control animals [109]. The mice receiving serum from OVA-tolerized mice displayed reduced airway eosinophilia as well as lower serum levels of total or OVA-specific IgE compared to mock-pretreated animals [109]. The developing tolerance was associated with a significantly higher number of activated Tregs in both mediastinal and celiac lymph nodes. In addition, other studies comparing the influence of pretreatment with serum and serum-isolated exosomes showed that the tolerogenic effect of the serum was mediated by exosomes [109]. In an equine study on recurrent airway obstruction, a severe asthma-like disease similar to human asthma [110], another group identified serum miRNA patterns having potential as biomarkers of severe asthma [111]. Eleven miRNAs were found to be differentially expressed between the case and control horses. Subsequent bioinformatic analysis using experimentally validated target genes of the human homologous miRNAs demonstrated significant enrichment in the pathways regulating EMT and modulating maturation and function of CD4+ T cells. Moreover, the bioinformatic patterns were in line with a Th2/Th17 type immune response present in severe equine “asthma” [111]. Compared to healthy controls, the expression of miRNA-125b in serum-EVs was higher in asthmatics and correlated with the severity of the disease [112]. Additionally, serum exosomes miRNA-126 levels were higher in allergic asthmatics than in healthy controls [113]. Quite comparably, lung tissue miRNA-126 levels in mice subjected to an OVA-based AAI model were also higher than those in control animals [113]. Another study started with the identification of a set of miRNAs demonstrating differences in expression between eosinophils obtained from asthmatics and those isolated from healthy individuals [114]. Some of these miRNAs demonstrated differences also when analyzed in serum, such as miR-185-5p discriminating asthmatic from healthy subjects. Moreover, in combination with two other miRNAs, miR-185-5p made it possible to create models even better discriminating both conditions and capable of allocating the asthmatic patients into those with intermittent, mild persistent, moderate persistent, and severe persistent disease [114]. Interestingly, serum levels of miR-185-5p and other differentiating miRNAs remained stable over time in asthmatics, in whom no changes in therapy and clinical parameters or health status occurred [115]. Another human study revealed the potential of miR-122-5p in EVs isolated from plasma and sputum to differentiate between healthy controls and (severe) asthmatics. Besides, considering that miR-122-5p correlated with blood immune cell types and that this miRNA was predicted in the pathway analysis to contribute to the differentiation and the function of lymphocytes, it was speculated that miR-122-5p can drive sub-differentiation of various asthma forms, including neutrophilic from eosinophilic asthma [116]. Finally, some circulating miRNAs also demonstrated the potential to discriminate between healthy people and those with asthma or COPD (miR-146a-5p) [117] or between asthma, asthma-COPD overlap, and COPD (miR-320a) [118].
Selected human studies on asthma demonstrating the biomarker potential of EVs are summarized in Table 3.
Table 3.
Selected human studies on asthma demonstrating biomarker potential of extracellular vesicle (EV) analysis.
| Analyte | Biological Material | Study Subjects | Major result | Publication |
|---|---|---|---|---|
| Exosomal miRNAs | BALF | Patients with unprovoked, mild, stable asthma and healthy subjects | A subset of miRNAs allowed a robust separation between patients and controls | [98] |
| Exosomal proteins | BALF | Patients with asthma, cystic fibrosis, or primary ciliary dyskinesia | A subset of proteins allowed accurate separation of the three diseases | [102] |
| EV lipids | BALF | Asthmatic subjects and healthy controls exposed or not to second-hand smoke | Levels of several lipids different between asthmatics and control groups | [103] |
| EV RNA, EV proteins | Inducedsputum | Mild allergic asthmatics both before and after allergen challenge | Presence of diverse RNA, especially short RNA species, and immune-related proteins in the samples | [105] |
| Exosomal proteins | NLF | Asthmatics, asthmatics with chronic rhinosinusitis, and healthy individuals | Levels of several proteins different between patients with respiratory diseases and controls | [106] |
| Exosomal miRNA | Serum | Untreated asthmatics with various grades of disease severity and healthy controls | Levels of miRNA-125b higher in patients and correlating with disease severity | [112] |
| Exosomal miRNA | Serum | Allergic asthma patients and healthy controls | Levels of miRNA-126 higher in asthmatics | [113] |
| (Exosomal) miRNAs | Serum | Asthmatics and healthy individuals | A set of miRNAs capable of discriminating between asthmatics and controls and ranking disease severity; no changes in miRNA levels over time in patients with stable disease | [100,114] |
| EV miRNA | Plasma, sputum | Mild-to-moderate or severe asthmatics and healthy controls | Levels of miR-122-5p higher in patients with (severe) asthma | [116] |
miRNA, microRNA; BALF, Bronchoalveolar Lavage Fluid; NLF, Nasal Lavage Fluid.
7. Extracellular Vesicles and Asthma: Microorganisms and Other External Influences
7.1. Viruses
Viral and bacterial infections of the respiratory tract are known to induce the release of EVs [119,120,121]. Human rhinoviruses (HRV) are responsible for a significant proportion of the exacerbations of chronic inflammatory diseases of the respiratory tract, such as asthma or COPD. It has also been hypothesized that early childhood HRV infection, especially in combination with an atopic pre-disposition, can lead to the establishment of the allergic asthma phenotype, which can persist into adulthood [120,122,123]. Interestingly, HRV infection in young children has been found to be associated with alterations in the airway secretory miRNAome, including a highly specific additional presence of miR-155 in the EVs of nasal secretions [124]. Since miR-155 plays an important role in EV-mediated immune regulation and fine-tuning of type-1/type-2 homeostasis, changes in respiratory tract expression may represent a mechanism by which HRV infection contributes to asthma pathology [124]. In another study, experimental infection with HRV type 16 could induce procoagulatory changes in the respiratory tract of asthmatics, mediated by increased activity of TF-exposing EVs. In addition, these EV-related pro-coagulant alterations were shown to be dependent on HRV load and associated with both neutrophilic and eosinophilic inflammation [125].
7.2. Bacteria
Like eukaryotic cells, gram-negative and gram-positive bacteria also release vesicles, referred to as outer membrane vesicles (OMVs) and bacterial membrane vesicles (BMVs), respectively [126,127]. Repeated intranasal application of indoor dust obtained from bed mattresses or EVs isolated from that dust led in mice to the development of neutrophilic pulmonary inflammation accompanied by lung infiltration of both Th1 and Th17 cells, the changes mimicking a typical picture of neutrophilic asthma or COPD in human [128]. In addition, serum levels of IgG1 against dust were found in atopic asthmatic children to be significantly higher than in atopic but otherwise healthy children or those with rhinitis or dermatitis [128]. Subsequent studies conducted in a larger group of individuals suggested that the presence of IgG antibodies against indoor dust EVs in serum is a major risk factor for the development of asthma, COPD, and lung cancer [129]. On the other hand, intranasal pretreatment with exosomes isolated from Pseudomonas aeruginosa was able to ameliorate AHR, allergic inflammation, and atopic sensitization levels in mice subjected to OVA- and HDM-based models of AAI [130]. Furthermore, these protective effects were accompanied by an enhancement of Treg responses and a simultaneous decrease in type-2-driven immune mechanisms [130].
In another study, bacterial EVs in the urine of healthy children as well as children with chronic rhinitis, allergic rhinitis, or atopic asthma were analyzed. The distinct bacterial composition of EVs in urine observed between the groups of the study participants suggested that urinary EVs could be an indicator for the assessment of allergic airway diseases in children [131].
7.3. Diet
Mouse macrophages treated with purified bovine milk-derived EVs were found to express classically-activated M1 phenotype rather than an alternatively-activated M2 phenotype [132], the latter known to promote type-2 responses and to be associated with allergic asthma [133]. M1 shift was also observed for peritoneal macrophages obtained from mice first fed with EV-enriched diet and then subjected to intranasal installation of agricultural dust extract, while M2 polarization was seen in animals receiving a normal diet and then exposed to organic dust extract [132].
EVs such as exosomes, carrying miRNA and other types of RNA as well as cytosolic and membrane-bound proteins, are also present in human breast milk [134,135,136,137]. Breast milk EVs possess immunomodulatory features and, together with human milk oligosaccharides, seem to play a crucial role in the development of the infant microbiome, which is very important for the appropriate maturation of the immune system [134,135,138,139]. Interestingly, it has been demonstrated that exosomes in human breast milk show phenotypic differences depending on the maternal sensitization status and lifestyle, which might have consequences for the development of allergy in the offspring [140].
7.4. Smoking
Differences in miRNA profiles were observed between EVs isolated from BALF of smokers and non-smokers [141]. Furthermore, treatment with smoker and non-smoker BALF EVs exerted differential effects on the function of human bronchial epithelial BEAS-2B cells, including higher IL-6 secretion when smoker EVs were applied [141].
8. Conclusions and Perspectives
The studies presented in this review, whatever their nature, i.e., in vitro, in vivo on animals, in human studies, etc., clearly demonstrate the existence of EV communication between cells known as the crucial players in asthma pathology and, moreover, they also strongly suggest an importance of EV-mediated communication mechanisms for the pathobiology of the disease. This includes the mediation of etiopathogenic effects of environmental factors, e.g., microbes or pollutants, and the role of EVs from external origins, e.g., those present in cow’s milk or secreted by certain bacteria. Some of the studies already characterized components of the cargo of EVs and identified molecules responsible for asthma-related effects, mainly small RNAs, proteins, and lipid mediators. Based on this continuously expanding knowledge, the high diagnostic potential of EVs has been highlighted in a variety of studies. It has been shown that EV-based asthma diagnostics effectively targeting miRNA, proteins, or lipids could be performed in different types of biomaterials such as BALF, sputum, NLF, or serum. Considering the access to biomaterials and the methodological rationale, the analytical approach based on the analysis of serum miRNAs seems particularly promising, irrespective of whether and how the miRNAome patterns in blood and lung correspond to each other. These approaches will certainly be expanded in the future to more precisely identify asthma phenotypes particularly by means of non- or minimally-invasive diagnostic sampling techniques [142]. Finally, several in vitro and animal studies reviewed in this article already show that EVs, not only, but especially those secreted by MSCs, can exert beneficial immunomodulatory effects with anti-asthmatic capacity, suggesting a promising potential for EV-based therapeutic approaches. Although procedures regarding targeting specific cell types and the level of EV (cargo) degradation still need to be further optimized, it seems that modified or designed EVs with a higher propensity to fuse with (endosomal) membranes will offer even better therapeutic abilities [38,39]. As also briefly outlined here, another approach being developed as a possible anti-asthmatic therapeutic strategy may involve the use of inhibitors of EV production, which have been shown to exhibit anti-AAI effects in some studies. However, further basic and clinical studies are needed, which undoubtedly will lead to diagnostic and therapeutic innovations based on their results.
Acknowledgments
We are grateful to Viviane Ponath, Christian Preußer and Elke Pogge von Strandmann for their critical reading and constructive comments.
Author Contributions
Conceptualization, B.A.A., D.P.P. and H.G.; Funding acquisition, B.A.A., D.P.P. and H.G.; Investigation, B.A.A. and D.P.P.; Supervision, D.P.P. and H.G.; Visualization, S.M.; Writing—original draft, B.A.A., D.P.P., S.M., F.A., A.M. and L.H.; Writing—review & editing, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
B.A.A. and A.M. were funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; Grant 416910386–GRK 2573/1). F.A. was supported by the Deutscher Akademischer Austauschdienst (DAAD, German Academic Exchange Service; personal reference number 91726294), the HessenFonds by the Hessen State Ministry for Higher Education, Research and the Arts (HMWK), and World University Service (WUS).
Conflicts of Interest
All authors report no potential conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO The Top 10 Causes of Death. [(accessed on 18 April 2021)]; Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 2.WHO WHO Coronavirus (COVID-19) Dashboard: With Vaccination Data. [(accessed on 18 April 2021)]; Available online: https://covid19.who.int/
- 3.Camps J., García-Heredia A. Introduction: Oxidation and inflammation, a molecular link between non-communicable diseases. Adv. Exp. Med. Biol. 2014;824:1–4. doi: 10.1007/978-3-319-07320-0_1. [DOI] [PubMed] [Google Scholar]
- 4.Pahwa R., Goyal A., Bansal P., Jialal I. Chronic Inflammation. StatPearls Publishing; Treasure Island, FL, USA: 2021. [PubMed] [Google Scholar]
- 5.Phillips C.M., Chen L.-W., Heude B., Bernard J.Y., Harvey N.C., Duijts L., Mensink-Bout S.M., Polanska K., Mancano G., Suderman M., et al. Dietary Inflammatory Index and Non-Communicable Disease Risk: A Narrative Review. Nutrients. 2019;11:1873. doi: 10.3390/nu11081873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prynn J.E., Kuper H. Perspectives on Disability and Non-Communicable Diseases in Low- and Middle-Income Countries, with a Focus on Stroke and Dementia. Int. J. Environ. Res. Public Health. 2019;16:3488. doi: 10.3390/ijerph16183488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray A., Oriss T.B., Wenzel S.E. Emerging molecular phenotypes of asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308:L130–L140. doi: 10.1152/ajplung.00070.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miethe S., Guarino M., Alhamdan F., Simon H.-U., Renz H., Dufour J.-F., Potaczek D.P., Garn H. Effects of obesity on asthma: Immunometabolic links. Pol. Arch. Intern. Med. 2018;128:469–477. doi: 10.20452/pamw.4304. [DOI] [PubMed] [Google Scholar]
- 9.Potaczek D.P., Miethe S., Schindler V., Alhamdan F., Garn H. Role of airway epithelial cells in the development of different asthma phenotypes. Cell. Signal. 2020;69:109523. doi: 10.1016/j.cellsig.2019.109523. [DOI] [PubMed] [Google Scholar]
- 10.Tost J. A translational perspective on epigenetics in allergic diseases. J. Allergy Clin. Immunol. 2018;142:715–726. doi: 10.1016/j.jaci.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Quirt J., Hildebrand K.J., Mazza J., Noya F., Kim H. Asthma. Allergy Asthma Clin. Immunol. 2018;14:50. doi: 10.1186/s13223-018-0279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bush A. Pathophysiological Mechanisms of Asthma. Front. Pediatr. 2019;7:68. doi: 10.3389/fped.2019.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenzel S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 14.Alashkar Alhamwe B., Miethe S., Pogge von Strandmann E., Potaczek D.P., Garn H. Epigenetic Regulation of Airway Epithelium Immune Functions in Asthma. Front. Immunol. 2020;11:1747. doi: 10.3389/fimmu.2020.01747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuruvilla M.E., Lee F.E.-H., Lee G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019;56:219–233. doi: 10.1007/s12016-018-8712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr T.F., Zeki A.A., Kraft M. Eosinophilic and Noneosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2018;197:22–37. doi: 10.1164/rccm.201611-2232PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potaczek D.P., Harb H., Michel S., Alhamwe B.A., Renz H., Tost J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics. 2017;9:539–571. doi: 10.2217/epi-2016-0162. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi N.A., Bennett B.L., Graham N.M.H., Pirozzi G., Stahl N., Yancopoulos G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016;15:35–50. doi: 10.1038/nrd4624. [DOI] [PubMed] [Google Scholar]
- 19.Jeong J.S., Kim J.S., Kim S.R., Lee Y.C. Defining Bronchial Asthma with Phosphoinositide 3-Kinase Delta Activation: Towards Endotype-Driven Management. Int. J. Mol. Sci. 2019;20:3525. doi: 10.3390/ijms20143525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 21.Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neurooncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kowal J., Tkach M., Théry C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Raposo G., Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 26.Benedikter B.J., Wouters E.F.M., Savelkoul P.H.M., Rohde G.G.U., Stassen F.R.M. Extracellular vesicles released in response to respiratory exposures: Implications for chronic disease. J. Toxicol. Environ. Health B Crit. Rev. 2018;21:142–160. doi: 10.1080/10937404.2018.1466380. [DOI] [PubMed] [Google Scholar]
- 27.Burger D., Schock S., Thompson C.S., Montezano A.C., Hakim A.M., Touyz R.M. Microparticles: Biomarkers and beyond. Clin. Sci. 2013;124:423–441. doi: 10.1042/CS20120309. [DOI] [PubMed] [Google Scholar]
- 28.Jan A.T., Rahman S., Khan S., Tasduq S.A., Choi I. Biology, Pathophysiological Role, and Clinical Implications of Exosomes: A Critical Appraisal. Cells. 2019;8:99. doi: 10.3390/cells8020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson C., Vicencio J.M., Yellon D.M., Davidson S.M. Microvesicles and exosomes: New players in metabolic and cardiovascular disease. J. Endocrinol. 2016;228:R57–R71. doi: 10.1530/JOE-15-0201. [DOI] [PubMed] [Google Scholar]
- 30.Stahl P.D., Raposo G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology. 2019;34:169–177. doi: 10.1152/physiol.00045.2018. [DOI] [PubMed] [Google Scholar]
- 31.Ståhl A.-L., Johansson K., Mossberg M., Kahn R., Karpman D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr. Nephrol. 2019;34:11–30. doi: 10.1007/s00467-017-3816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todorova D., Simoncini S., Lacroix R., Sabatier F., Dignat-George F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017;120:1658–1673. doi: 10.1161/CIRCRESAHA.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W., Zhou X., Zhang H., Yao Q., Liu Y., Dong Z. Extracellular vesicles in diagnosis and therapy of kidney diseases. Am. J. Physiol. Renal Physiol. 2016;311:F844–F851. doi: 10.1152/ajprenal.00429.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witwer K.W., Théry C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles. 2019;8:1648167. doi: 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bélanger É., Madore A.-M., Boucher-Lafleur A.-M., Simon M.-M., Kwan T., Pastinen T., Laprise C. Eosinophil microRNAs Play a Regulatory Role in Allergic Diseases Included in the Atopic March. Int. J. Mol. Sci. 2020;21:9011. doi: 10.3390/ijms21239011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pegtel D.M., Cosmopoulos K., Thorley-Lawson D.A., van Eijndhoven M.A.J., Hopmans E.S., Lindenberg J.L., de Gruijl T.D., Würdinger T., Middeldorp J.M. Functional delivery of viral miRNAs via exosomes. Proc. Natl Acad. Sci. USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 38.Joshi B.S., de Beer M.A., Giepmans B.N.G., Zuhorn I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano. 2020;14:4444–4455. doi: 10.1021/acsnano.9b10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becker A., Thakur B.K., Weiss J.M., Kim H.S., Peinado H., Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coakley G., Maizels R.M., Buck A.H. Exosomes and Other Extracellular Vesicles: The New Communicators in Parasite Infections. Trends Parasitol. 2015;31:477–489. doi: 10.1016/j.pt.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alashkar Alhamwe B., Alhamdan F., Ruhl A., Potaczek D.P., Renz H. The role of epigenetics in allergy and asthma development. Curr. Opin. Allergy Clin. Immunol. 2020;20:48–55. doi: 10.1097/ACI.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 43.Baskara-Yhuellou I., Tost J. The impact of microRNAs on alterations of gene regulatory networks in allergic diseases. Adv. Protein Chem. Struct. Biol. 2020;120:237–312. doi: 10.1016/bs.apcsb.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Peng Y., Croce C.M. The role of MicroRNAs in human cancer. Signal. Transduct. Target. Ther. 2016;1:15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piletič K., Kunej T. MicroRNA epigenetic signatures in human disease. Arch. Toxicol. 2016;90:2405–2419. doi: 10.1007/s00204-016-1815-7. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z., Zhuang L., Lin C.-P. Roles of MicroRNAs in Establishing and Modulating Stem Cell Potential. Int. J. Mol. Sci. 2019;20:3643. doi: 10.3390/ijms20153643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuzic M., Rojo Arias J.E., Wohl S.G., Busskamp V. Retinal miRNA Functions in Health and Disease. Genes. 2019;10:377. doi: 10.3390/genes10050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vidigal J.A., Ventura A. The biological functions of miRNAs: Lessons from in vivo studies. Trends Cell Biol. 2015;25:137–147. doi: 10.1016/j.tcb.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Treiber T., Treiber N., Meister G. Regulation of microRNA biogenesis and function. Thromb. Haemost. 2012;107:605–610. doi: 10.1160/TH11-12-0836. [DOI] [PubMed] [Google Scholar]
- 50.Gupta R., Radicioni G., Abdelwahab S., Dang H., Carpenter J., Chua M., Mieczkowski P.A., Sheridan J.T., Randell S.H., Kesimer M. Intercellular Communication between Airway Epithelial Cells Is Mediated by Exosome-Like Vesicles. Am. J. Respir. Cell Mol. Biol. 2019;60:209–220. doi: 10.1165/rcmb.2018-0156OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pua H.H., Happ H.C., Gray C.J., Mar D.J., Chiou N.-T., Hesse L.E., Ansel K.M. Increased Hematopoietic Extracellular RNAs and Vesicles in the Lung during Allergic Airway Responses. Cell Rep. 2019;26:933–944.e4. doi: 10.1016/j.celrep.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kulshreshtha A., Ahmad T., Agrawal A., Ghosh B. Proinflammatory role of epithelial cell-derived exosomes in allergic airway inflammation. J. Allergy Clin. Immunol. 2013;131:1194–1203.e14. doi: 10.1016/j.jaci.2012.12.1565. [DOI] [PubMed] [Google Scholar]
- 53.Bartel S., La Grutta S., Cilluffo G., Perconti G., Bongiovanni A., Giallongo A., Behrends J., Kruppa J., Hermann S., Chiang D., et al. Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development. Allergy. 2020;75:346–356. doi: 10.1111/all.14008. [DOI] [PubMed] [Google Scholar]
- 54.Mitchel J.A., Antoniak S., Lee J.-H., Kim S.-H., McGill M., Kasahara D.I., Randell S.H., Israel E., Shore S.A., Mackman N., et al. IL-13 Augments Compressive Stress-Induced Tissue Factor Expression in Human Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2016;54:524–531. doi: 10.1165/rcmb.2015-0252OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park J.-A., Sharif A.S., Tschumperlin D.J., Lau L., Limbrey R., Howarth P., Drazen J.M. Tissue factor-bearing exosome secretion from human mechanically stimulated bronchial epithelial cells in vitro and in vivo. J. Allergy Clin. Immunol. 2012;130:1375–1383. doi: 10.1016/j.jaci.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Undas A., Stepien E., Potaczek D.P., Tracz W. Tissue factor +5466AG polymorphism determines thrombin formation following vascular injury and thrombin-lowering effects of simvastatin in patients with ischemic heart disease. Atherosclerosis. 2009;204:567–572. doi: 10.1016/j.atherosclerosis.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Potaczek D.P., Pieculewicz M., Mazur M., Branicka A., Nishiyama C., Okumura K., Podolec P., Undas A. Tissue factor +5466AG polymorphism predicts plasma TF levels in subjects with cryptogenic ischaemic stroke. Thromb. Haemost. 2009;102:173–175. doi: 10.1160/TH09-02-0099. [DOI] [PubMed] [Google Scholar]
- 58.Schouten M., van de Pol M.A., Levi M., van der Poll T., van der Zee J.S. Early activation of coagulation after allergen challenge in patients with allergic asthma. J. Thromb. Haemost. 2009;7:1592–1594. doi: 10.1111/j.1538-7836.2009.03523.x. [DOI] [PubMed] [Google Scholar]
- 59.Potaczek D.P., Owczarek D., Cibor D., Nishiyama C., Okumura K., Mach T., Undas A. Tissue factor -1208DI polymorphism is associated with D-dimer levels in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2010;16:1095–1096. doi: 10.1002/ibd.21149. [DOI] [PubMed] [Google Scholar]
- 60.Shinagawa K., Ploplis V.A., Castellino F.J. A severe deficiency of coagulation factor VIIa results in attenuation of the asthmatic response in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296:L763–L770. doi: 10.1152/ajplung.90638.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haj-Salem I., Plante S., Gounni A.S., Rouabhia M., Chakir J. Fibroblast-derived exosomes promote epithelial cell proliferation through TGF-β2 signalling pathway in severe asthma. Allergy. 2018;73:178–186. doi: 10.1111/all.13234. [DOI] [PubMed] [Google Scholar]
- 62.Yang Z.-C., Qu Z.-H., Yi M.-J., Shan Y.-C., Ran N., Xu L., Liu X.-J. MiR-448-5p inhibits TGF-β1-induced epithelial-mesenchymal transition and pulmonary fibrosis by targeting Six1 in asthma. J. Cell Physiol. 2019;234:8804–8814. doi: 10.1002/jcp.27540. [DOI] [PubMed] [Google Scholar]
- 63.Yang N., Zhang H., Cai X., Shang Y. Epigallocatechin-3-gallate inhibits inflammation and epithelial-mesenchymal transition through the PI3K/AKT pathway via upregulation of PTEN in asthma. Int. J. Mol. Med. 2018;41:818–828. doi: 10.3892/ijmm.2017.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu X., Li Q., Hu G., Wang J., Hu Q., Liu Z., Wu G., Zhong Y. BMS-345541 inhibits airway inflammation and epithelial-mesenchymal transition in airway remodeling of asthmatic mice. Int. J. Mol. Med. 2018;42:1998–2008. doi: 10.3892/ijmm.2018.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khanna K., Chaudhuri R., Aich J., Pattnaik B., Panda L., Prakash Y.S., Mabalirajan U., Ghosh B., Agrawal A. Secretory Inositol Polyphosphate 4-Phosphatase Protects against Airway Inflammation and Remodeling. Am. J. Respir. Cell Mol. Biol. 2019;60:399–412. doi: 10.1165/rcmb.2017-0353OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esser J., Gehrmann U., D’Alexandri F.L., Hidalgo-Estévez A.M., Wheelock C.E., Scheynius A., Gabrielsson S., Rådmark O. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J. Allergy Clin. Immunol. 2010;126:1032–1040.e4. doi: 10.1016/j.jaci.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 67.Fu Z., Akula S., Thorpe M., Hellman L. Highly Selective Cleavage of TH2-Promoting Cytokines by the Human and the Mouse Mast Cell Tryptases, Indicating a Potent Negative Feedback Loop on TH2 Immunity. Int. J. Mol. Sci. 2019;20:5147. doi: 10.3390/ijms20205147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang L., Zhang X., Wang M., Chen Z., Yan Y., Gu W., Tan J., Jiang W., Ji W. Exosomes from Thymic Stromal Lymphopoietin-Activated Dendritic Cells Promote Th2 Differentiation through the OX40 Ligand. Pathobiology. 2019;86:111–117. doi: 10.1159/000493013. [DOI] [PubMed] [Google Scholar]
- 69.Bourdonnay E., Zasłona Z., Penke L.R.K., Speth J.M., Schneider D.J., Przybranowski S., Swanson J.A., Mancuso P., Freeman C.M., Curtis J.L., et al. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J. Exp. Med. 2015;212:729–742. doi: 10.1084/jem.20141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Draijer C., Speth J.M., Penke L.R.K., Zaslona Z., Bazzill J.D., Lugogo N., Huang Y.J., Moon J.J., Peters-Golden M. Resident alveolar macrophage-derived vesicular SOCS3 dampens allergic airway inflammation. FASEB J. 2020;34:4718–4731. doi: 10.1096/fj.201903089R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harb H., Alashkar Alhamwe B., Garn H., Renz H., Potaczek D.P. Recent developments in epigenetics of pediatric asthma. Curr. Opin. Pediatr. 2016;28:754–763. doi: 10.1097/MOP.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 72.Martin P.J., Héliot A., Trémolet G., Landkocz Y., Dewaele D., Cazier F., Ledoux F., Courcot D. Cellular response and extracellular vesicles characterization of human macrophages exposed to fine atmospheric particulate matter. Environ. Pollut. 2019;254:112933. doi: 10.1016/j.envpol.2019.07.101. [DOI] [PubMed] [Google Scholar]
- 73.Mazzeo C., Cañas J.A., Zafra M.P., Rojas Marco A., Fernández-Nieto M., Sanz V., Mittelbrunn M., Izquierdo M., Baixaulli F., Sastre J., et al. Exosome secretion by eosinophils: A possible role in asthma pathogenesis. J. Allergy Clin. Immunol. 2015;135:1603–1613. doi: 10.1016/j.jaci.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 74.Cañas J.A., Sastre B., Mazzeo C., Fernández-Nieto M., Rodrigo-Muñoz J.M., González-Guerra A., Izquierdo M., Barranco P., Quirce S., Sastre J., et al. Exosomes from eosinophils autoregulate and promote eosinophil functions. J. Leukoc. Biol. 2017;101:1191–1199. doi: 10.1189/jlb.3AB0516-233RR. [DOI] [PubMed] [Google Scholar]
- 75.Cañas J.A., Sastre B., Rodrigo-Muñoz J.M., Fernández-Nieto M., Barranco P., Quirce S., Sastre J., Del Pozo V. Eosinophil-derived exosomes contribute to asthma remodelling by activating structural lung cells. Clin. Exp. Allergy. 2018;48:1173–1185. doi: 10.1111/cea.13122. [DOI] [PubMed] [Google Scholar]
- 76.Vargas A., Roux-Dalvai F., Droit A., Lavoie J.-P. Neutrophil-Derived Exosomes: A New Mechanism Contributing to Airway Smooth Muscle Remodeling. Am. J. Respir. Cell Mol. Biol. 2016;55:450–461. doi: 10.1165/rcmb.2016-0033OC. [DOI] [PubMed] [Google Scholar]
- 77.Xia Y.C., Harris T., Stewart A.G., Mackay G.A. Secreted factors from human mast cells trigger inflammatory cytokine production by human airway smooth muscle cells. Int. Arch. Allergy Immunol. 2013;160:75–85. doi: 10.1159/000339697. [DOI] [PubMed] [Google Scholar]
- 78.Potaczek D.P. Links between allergy and cardiovascular or hemostatic system. Int. J. Cardiol. 2014;170:278–285. doi: 10.1016/j.ijcard.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 79.Nastałek M., Potaczek D.P., Wojas-Pelc A., Undas A. Plasma platelet activation markers in patients with atopic dermatitis and concomitant allergic diseases. J. Dermatol. Sci. 2011;64:79–82. doi: 10.1016/j.jdermsci.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 80.Pan Y., Liang H., Liu H., Li D., Chen X., Li L., Zhang C.-Y., Zen K. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J. Immunol. 2014;192:437–446. doi: 10.4049/jimmunol.1301790. [DOI] [PubMed] [Google Scholar]
- 81.Gao J., Xu X., Ying Z., Jiang L., Zhong M., Wang A., Chen L.-C., Lu B., Sun Q. Post-Effect of Air Quality Improvement on Biomarkers for Systemic Inflammation and Microparticles in Asthma Patients After the 2008 Beijing Olympic Games: A Pilot Study. Inflammation. 2017;40:1214–1224. doi: 10.1007/s10753-017-0564-y. [DOI] [PubMed] [Google Scholar]
- 82.Aatonen M.T., Ohman T., Nyman T.A., Laitinen S., Grönholm M., Siljander P.R.-M. Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicles. 2014;3:24692. doi: 10.3402/jev.v3.24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duarte D., Taveira-Gomes T., Sokhatska O., Palmares C., Costa R., Negrão R., Guimarães J.T., Delgado L., Soares R., Moreira A. Increased circulating platelet microparticles as a potential biomarker in asthma. Allergy. 2013;68:1073–1075. doi: 10.1111/all.12190. [DOI] [PubMed] [Google Scholar]
- 84.Deshane J.S., Redden D.T., Zeng M., Spell M.L., Zmijewski J.W., Anderson J.T., Deshane R.J., Gaggar A., Siegal G.P., Abraham E., et al. Subsets of airway myeloid-derived regulatory cells distinguish mild asthma from chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2015;135:413–424.e15. doi: 10.1016/j.jaci.2014.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hough K.P., Trevor J.L., Strenkowski J.G., Wang Y., Chacko B.K., Tousif S., Chanda D., Steele C., Antony V.B., Dokland T., et al. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol. 2018;18:54–64. doi: 10.1016/j.redox.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abughanam G., Elkashty O.A., Liu Y., Bakkar M.O., Tran S.D. Mesenchymal Stem Cells Extract (MSCsE)-Based Therapy Alleviates Xerostomia and Keratoconjunctivitis Sicca in Sjogren’s Syndrome-Like Disease. Int. J. Mol. Sci. 2019;20:4750. doi: 10.3390/ijms20194750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han Y., Li X., Zhang Y., Han Y., Chang F., Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8:886. doi: 10.3390/cells8080886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seo Y., Shin T.-H., Kim H.-S. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int. J. Mol. Sci. 2019;20:3827. doi: 10.3390/ijms20153827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zucca E., Corsini E., Galbiati V., Lange-Consiglio A., Ferrucci F. Evaluation of amniotic mesenchymal cell derivatives on cytokine production in equine alveolar macrophages: An in vitro approach to lung inflammation. Stem Cell Res. Ther. 2016;7:137. doi: 10.1186/s13287-016-0398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Du Y.-M., Zhuansun Y.-X., Chen R., Lin L., Lin Y., Li J.-G. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp. Cell Res. 2018;363:114–120. doi: 10.1016/j.yexcr.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 91.Cruz F.F., Borg Z.D., Goodwin M., Sokocevic D., Wagner D.E., Coffey A., Antunes M., Robinson K.L., Mitsialis S.A., Kourembanas S., et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles Ameliorates Aspergillus Hyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem Cells Transl. Med. 2015;4:1302–1316. doi: 10.5966/sctm.2014-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhuansun Y., Du Y., Huang F., Lin L., Chen R., Jiang S., Li J. MSCs exosomal miR-1470 promotes the differentiation of CD4+CD25+FOXP3+ Tregs in asthmatic patients by inducing the expression of P27KIP1. Int. Immunopharmacol. 2019;77:105981. doi: 10.1016/j.intimp.2019.105981. [DOI] [PubMed] [Google Scholar]
- 93.Shahir M., Mahmoud Hashemi S., Asadirad A., Varahram M., Kazempour-Dizaji M., Folkerts G., Garssen J., Adcock I., Mortaz E. Effect of mesenchymal stem cell-derived exosomes on the induction of mouse tolerogenic dendritic cells. J. Cell Physiol. 2020;235:7043–7055. doi: 10.1002/jcp.29601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Castro L.L., Xisto D.G., Kitoko J.Z., Cruz F.F., Olsen P.C., Redondo P.A.G., Ferreira T.P.T., Weiss D.J., Martins M.A., Morales M.M., et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res. Ther. 2017;8:151. doi: 10.1186/s13287-017-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shang Y., Sun Y., Xu J., Ge X., Hu Z., Xiao J., Ning Y., Dong Y., Bai C. Exosomes from mmu_circ_0001359-Modified ADSCs Attenuate Airway Remodeling by Enhancing FoxO1 Signaling-Mediated M2-like Macrophage Activation. Mol. Ther. Nucleic Acids. 2020;19:951–960. doi: 10.1016/j.omtn.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ertunc M.E., Sikkeland J., Fenaroli F., Griffiths G., Daniels M.P., Cao H., Saatcioglu F., Hotamisligil G.S. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J. Lipid Res. 2015;56:423–434. doi: 10.1194/jlr.M055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shum B.O.V., Mackay C.R., Gorgun C.Z., Frost M.J., Kumar R.K., Hotamisligil G.S., Rolph M.S. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J. Clin. Investig. 2006;116:2183–2192. doi: 10.1172/JCI24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levänen B., Bhakta N.R., Torregrosa Paredes P., Barbeau R., Hiltbrunner S., Pollack J.L., Sköld C.M., Svartengren M., Grunewald J., Gabrielsson S., et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J. Allergy Clin. Immunol. 2013;131:894–903. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Francisco-Garcia A.S., Garrido-Martín E.M., Rupani H., Lau L.C.K., Martinez-Nunez R.T., Howarth P.H., Sanchez-Elsner T. Small RNA Species and microRNA Profiles are Altered in Severe Asthma Nanovesicles from Broncho Alveolar Lavage and Associate with Impaired Lung Function and Inflammation. Noncoding RNA. 2019;5:51. doi: 10.3390/ncrna5040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gon Y., Maruoka S., Inoue T., Kuroda K., Yamagishi K., Kozu Y., Shikano S., Soda K., Lötvall J., Hashimoto S. Selective release of miRNAs via extracellular vesicles is associated with house-dust mite allergen-induced airway inflammation. Clin. Exp. Allergy. 2017;47:1586–1598. doi: 10.1111/cea.13016. [DOI] [PubMed] [Google Scholar]
- 101.Tang B., Wu Y., Fang H., Wu Y., Shi K. Small RNA Sequencing Reveals Exosomal miRNAs Involved in the Treatment of Asthma by Scorpio and Centipede. BioMed Res. Int. 2020;2020:1061407. doi: 10.1155/2020/1061407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rollet-Cohen V., Bourderioux M., Lipecka J., Chhuon C., Jung V.A., Mesbahi M., Nguyen-Khoa T., Guérin-Pfyffer S., Schmitt A., Edelman A., et al. Comparative proteomics of respiratory exosomes in cystic fibrosis, primary ciliary dyskinesia and asthma. J. Proteom. 2018;185:1–7. doi: 10.1016/j.jprot.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 103.Hough K.P., Wilson L.S., Trevor J.L., Strenkowski J.G., Maina N., Kim Y.-I., Spell M.L., Wang Y., Chanda D., Dager J.R., et al. Unique Lipid Signatures of Extracellular Vesicles from the Airways of Asthmatics. Sci. Rep. 2018;8:10340. doi: 10.1038/s41598-018-28655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Torregrosa Paredes P., Esser J., Admyre C., Nord M., Rahman Q.K., Lukic A., Rådmark O., Grönneberg R., Grunewald J., Eklund A., et al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy. 2012;67:911–919. doi: 10.1111/j.1398-9995.2012.02835.x. [DOI] [PubMed] [Google Scholar]
- 105.Sánchez-Vidaurre S., Eldh M., Larssen P., Daham K., Martinez-Bravo M.-J., Dahlén S.-E., Dahlén B., van Hage M., Gabrielsson S. RNA-containing exosomes in induced sputum of asthmatic patients. J. Allergy Clin. Immunol. 2017;140:1459–1461.e2. doi: 10.1016/j.jaci.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 106.Lässer C., O’Neil S.E., Shelke G.V., Sihlbom C., Hansson S.F., Gho Y.S., Lundbäck B., Lötvall J. Exosomes in the nose induce immune cell trafficking and harbour an altered protein cargo in chronic airway inflammation. J. Transl. Med. 2016;14:181. doi: 10.1186/s12967-016-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takahashi T., Kato A., Berdnikovs S., Stevens W.W., Suh L.A., Norton J.E., Carter R.G., Harris K.E., Peters A.T., Hulse K.E., et al. Microparticles in nasal lavage fluids in chronic rhinosinusitis: Potential biomarkers for diagnosis of aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2017;140:720–729. doi: 10.1016/j.jaci.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhou M., Tan K.S., Guan W.-J., Jiang L.-J., Deng J., Gao W.-X., Lee Y.M., Xu Z.-F., Luo X., Liu C., et al. Proteomics profiling of epithelium-derived exosomes from nasal polyps revealed signaling functions affecting cellular proliferation. Respir. Med. 2020;162:105871. doi: 10.1016/j.rmed.2020.105871. [DOI] [PubMed] [Google Scholar]
- 109.Almqvist N., Lönnqvist A., Hultkrantz S., Rask C., Telemo E. Serum-derived exosomes from antigen-fed mice prevent allergic sensitization in a model of allergic asthma. Immunology. 2008;125:21–27. doi: 10.1111/j.1365-2567.2008.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pacholewska A., Jagannathan V., Drögemüller M., Klukowska-Rötzler J., Lanz S., Hamza E., Dermitzakis E.T., Marti E., Leeb T., Gerber V. Impaired Cell Cycle Regulation in a Natural Equine Model of Asthma. PLoS ONE. 2015;10:e0136103. doi: 10.1371/journal.pone.0136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pacholewska A., Kraft M.F., Gerber V., Jagannathan V. Differential Expression of Serum MicroRNAs Supports CD4⁺ T Cell Differentiation into Th2/Th17 Cells in Severe Equine Asthma. Genes. 2017;8:383. doi: 10.3390/genes8120383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao M., Juanjuan L., Weijia F., Jing X., Qiuhua H., Hua Z., Fuhe L., Hao P. Expression Levels of MicroRNA-125b in Serum Exosomes of Patients with Asthma of Different Severity and its Diagnostic Significance. Curr. Drug Metab. 2019;20:781–784. doi: 10.2174/1389200220666191021100001. [DOI] [PubMed] [Google Scholar]
- 113.Zhao M., Li Y.-P., Geng X.-R., Zhao M., Ma S.-B., Yang Y.-H., Deng Z.-H., Luo L.-M., Pan X.-Q. Expression Level of MiRNA-126 in Serum Exosomes of Allergic Asthma Patients and Lung Tissues of Asthmatic Mice. Curr. Drug Metab. 2019;20:799–803. doi: 10.2174/1389200220666191011114452. [DOI] [PubMed] [Google Scholar]
- 114.Rodrigo-Muñoz J.M., Cañas J.A., Sastre B., Rego N., Greif G., Rial M., Mínguez P., Mahíllo-Fernández I., Fernández-Nieto M., Mora I., et al. Asthma diagnosis using integrated analysis of eosinophil microRNAs. Allergy. 2019;74:507–517. doi: 10.1111/all.13570. [DOI] [PubMed] [Google Scholar]
- 115.Rial M.J., Rodrigo Muñoz J.M., Sastre B., Sastre J., Del Pozo V. Stability of Asthma Control Implies No Changes in microRNAs Expression. J. Investig. Allergol. Clin. Immunol. 2019;29:388–389. doi: 10.18176/jiaci.0410. [DOI] [PubMed] [Google Scholar]
- 116.Bahmer T., Krauss-Etschmann S., Buschmann D., Behrends J., Watz H., Kirsten A.-M., Pedersen F., Waschki B., Fuchs O., Pfaffl M.W., et al. RNA-seq-based profiling of extracellular vesicles in plasma reveals a potential role of miR-122-5p in asthma. Allergy. 2021;76:366–371. doi: 10.1111/all.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsai M.-J., Tsai Y.-C., Chang W.-A., Lin Y.-S., Tsai P.-H., Sheu C.-C., Kuo P.-L., Hsu Y.-L. Deducting MicroRNA-Mediated Changes Common in Bronchial Epithelial Cells of Asthma and Chronic Obstructive Pulmonary Disease-A Next-Generation Sequencing-Guided Bioinformatic Approach. Int. J. Mol. Sci. 2019;20:553. doi: 10.3390/ijms20030553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodrigo-Muñoz J.M., Rial M.J., Sastre B., Cañas J.A., Mahíllo-Fernández I., Quirce S., Sastre J., Cosío B.G., Del Pozo V. Circulating miRNAs as diagnostic tool for discrimination of respiratory disease: Asthma, asthma-chronic obstructive pulmonary disease (COPD) overlap and COPD. Allergy. 2019;74:2491–2494. doi: 10.1111/all.13916. [DOI] [PubMed] [Google Scholar]
- 119.Eltom S., Dale N., Raemdonck K.R.G., Stevenson C.S., Snelgrove R.J., Sacitharan P.K., Recchi C., Wavre-Shapton S., McAuley D.F., O’Kane C., et al. Respiratory infections cause the release of extracellular vesicles: Implications in exacerbation of asthma/COPD. PLoS ONE. 2014;9:e101087. doi: 10.1371/journal.pone.0101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mills J.T., Schwenzer A., Marsh E.K., Edwards M.R., Sabroe I., Midwood K.S., Parker L.C. Airway Epithelial Cells Generate Pro-inflammatory Tenascin-C and Small Extracellular Vesicles in Response to TLR3 Stimuli and Rhinovirus Infection. Front. Immunol. 2019;10:1987. doi: 10.3389/fimmu.2019.01987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alipoor S.D., Adcock I.M., Folkerts G., Garssen J., Mortaz E. A bioinformatics analysis of exosomal microRNAs released following mycobacterial infection. Int. J. Mycobacteriol. 2019;8:218–222. doi: 10.4103/ijmy.ijmy_88_19. [DOI] [PubMed] [Google Scholar]
- 122.Potaczek D.P., Garn H., Unger S.D., Renz H. Antisense molecules: A new class of drugs. J. Allergy Clin. Immunol. 2016;137:1334–1346. doi: 10.1016/j.jaci.2015.12.1344. [DOI] [PubMed] [Google Scholar]
- 123.Potaczek D.P., Unger S.D., Zhang N., Taka S., Michel S., Akdağ N., Lan F., Helfer M., Hudemann C., Eickmann M., et al. Development and characterization of DNAzyme candidates demonstrating significant efficiency against human rhinoviruses. J. Allergy Clin. Immunol. 2019;143:1403–1415. doi: 10.1016/j.jaci.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 124.Gutierrez M.J., Gomez J.L., Perez G.F., Pancham K., Val S., Pillai D.K., Giri M., Ferrante S., Freishtat R., Rose M.C., et al. Airway Secretory microRNAome Changes during Rhinovirus Infection in Early Childhood. PLoS ONE. 2016;11:e0162244. doi: 10.1371/journal.pone.0162244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Majoor C.J., van de Pol M.A., Kamphuisen P.W., Meijers J.C.M., Molenkamp R., Wolthers K.C., van der Poll T., Nieuwland R., Johnston S.L., Sterk P.J., et al. Evaluation of coagulation activation after rhinovirus infection in patients with asthma and healthy control subjects: An observational study. Respir. Res. 2014;15:14. doi: 10.1186/1465-9921-15-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim S.W., Lee J.S., Park S.B., Lee A.R., Jung J.W., Chun J.H., Lazarte J.M.S., Kim J., Seo J.-S., Kim J.-H., et al. The Importance of Porins and β-Lactamase in Outer Membrane Vesicles on the Hydrolysis of β-Lactam Antibiotics. Int. J. Mol. Sci. 2020;21:2822. doi: 10.3390/ijms21082822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uddin M.J., Dawan J., Jeon G., Yu T., He X., Ahn J. The Role of Bacterial Membrane Vesicles in the Dissemination of Antibiotic Resistance and as Promising Carriers for Therapeutic Agent Delivery. Microorganisms. 2020;8:670. doi: 10.3390/microorganisms8050670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim Y.-S., Choi E.-J., Lee W.-H., Choi S.-J., Roh T.-Y., Park J., Jee Y.-K., Zhu Z., Koh Y.-Y., Gho Y.S., et al. Extracellular vesicles, especially derived from Gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clin. Exp. Allergy. 2013;43:443–454. doi: 10.1111/cea.12085. [DOI] [PubMed] [Google Scholar]
- 129.Kim Y.S., Choi J.P., Kim M.H., Park H.K., Yang S., Kim Y.S., Kim T.B., Cho Y.S., Oh Y.M., Jee Y.K., et al. IgG Sensitization to Extracellular Vesicles in Indoor Dust is Closely Associated with the Prevalence of Non-Eosinophilic Asthma, COPD, and Lung Cancer. Allergy Asthma Immunol. Res. 2016;8:198–205. doi: 10.4168/aair.2016.8.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ding F.-X., Liu B., Zou W.-J., Li Q.-B., Tian D.-Y., Fu Z. Pseudomonas aeruginosa-derived exosomes ameliorates allergic reactions via inducing the Treg response in asthma. Pediatr. Res. 2018;84:125–133. doi: 10.1038/s41390-018-0020-1. [DOI] [PubMed] [Google Scholar]
- 131.Samra M., Nam S.K., Lim D.H., Kim D.H., Yang J., Kim Y.-K., Kim J.H. Urine Bacteria-Derived Extracellular Vesicles and Allergic Airway Diseases in Children. Int. Arch. Allergy Immunol. 2019;178:150–158. doi: 10.1159/000492677. [DOI] [PubMed] [Google Scholar]
- 132.Nordgren T.M., Heires A.J., Zempleni J., Swanson B.J., Wichman C., Romberger D.J. Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J. Nutr. Biochem. 2019;64:110–120. doi: 10.1016/j.jnutbio.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feketea G., Bocsan C.I., Popescu C., Gaman M., Stanciu L.A., Zdrenghea M.T. A Review of Macrophage MicroRNAs’ Role in Human Asthma. Cells. 2019;8:420. doi: 10.3390/cells8050420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Admyre C., Johansson S.M., Qazi K.R., Filén J.-J., Lahesmaa R., Norman M., Neve E.P.A., Scheynius A., Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 135.Le Doare K., Holder B., Bassett A., Pannaraj P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018;9:361. doi: 10.3389/fimmu.2018.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Esch B.C.A.M., Porbahaie M., Abbring S., Garssen J., Potaczek D.P., Savelkoul H.F.J., van Neerven R.J.J. The Impact of Milk and Its Components on Epigenetic Programming of Immune Function in Early Life and Beyond: Implications for Allergy and Asthma. Front. Immunol. 2020;11:2141. doi: 10.3389/fimmu.2020.02141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou Q., Li M., Wang X., Li Q., Wang T., Zhu Q., Zhou X., Wang X., Gao X., Li X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012;8:118–123. doi: 10.7150/ijbs.8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alsaweed M., Hartmann P.E., Geddes D.T., Kakulas F. MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother. Int. J. Environ. Res. Public Health. 2015;12:13981–14020. doi: 10.3390/ijerph121113981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Acevedo N., Alashkar Alhamwe B., Caraballo L., Ding M., Ferrante A., Garn H., Garssen J., Hii C.S., Irvine J., Llinás-Caballero K., et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients. 2021;13:724. doi: 10.3390/nu13030724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Torregrosa Paredes P., Gutzeit C., Johansson S., Admyre C., Stenius F., Alm J., Scheynius A., Gabrielsson S. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy. 2014;69:463–471. doi: 10.1111/all.12357. [DOI] [PubMed] [Google Scholar]
- 141.Héliot A., Landkocz Y., Roy Saint-Georges F., Gosset P., Billet S., Shirali P., Courcot D., Martin P.J. Smoker extracellular vesicles influence status of human bronchial epithelial cells. Int. J. Hyg. Environ. Health. 2017;220:445–454. doi: 10.1016/j.ijheh.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 142.Baumann R., Untersmayr E., Zissler U.M., Eyerich S., Adcock I.M., Brockow K., Biedermann T., Ollert M., Chaker A.M., Pfaar O., et al. Noninvasive and minimally invasive techniques for the diagnosis and management of allergic diseases. Allergy. 2021;76:1010–1023. doi: 10.1111/all.14645. [DOI] [PubMed] [Google Scholar]